Abstract
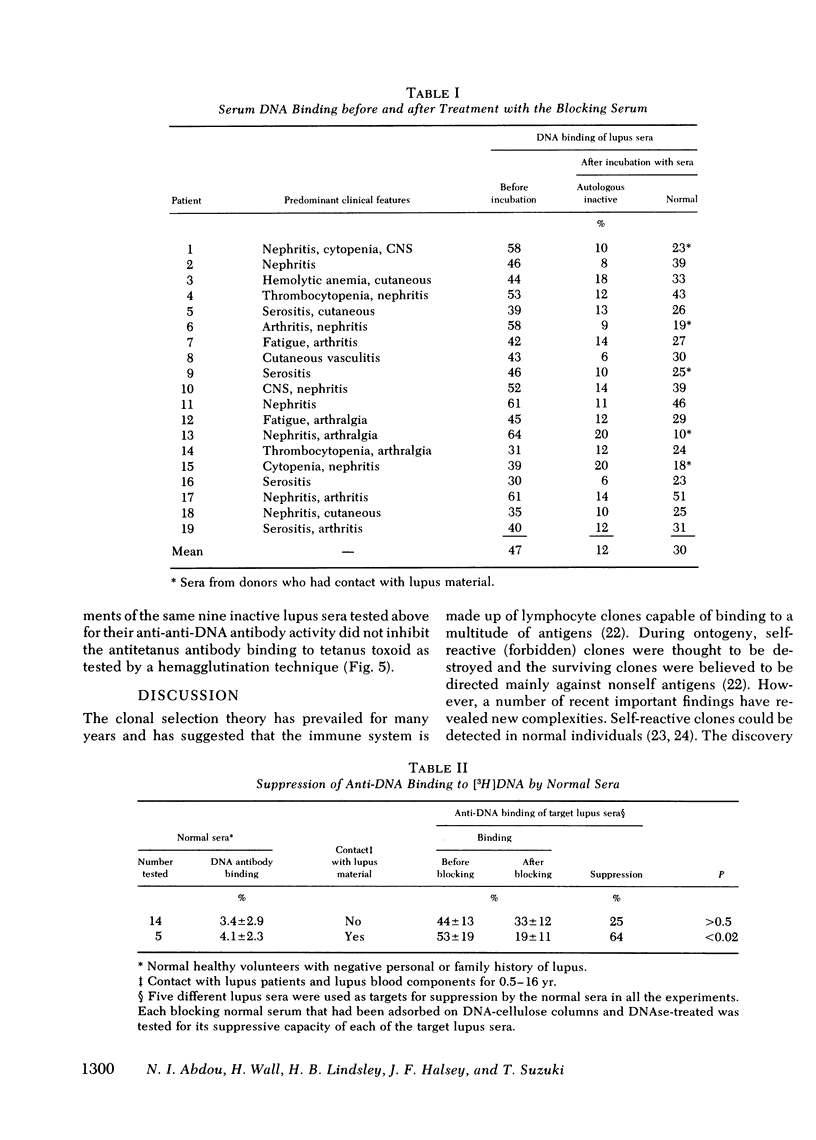
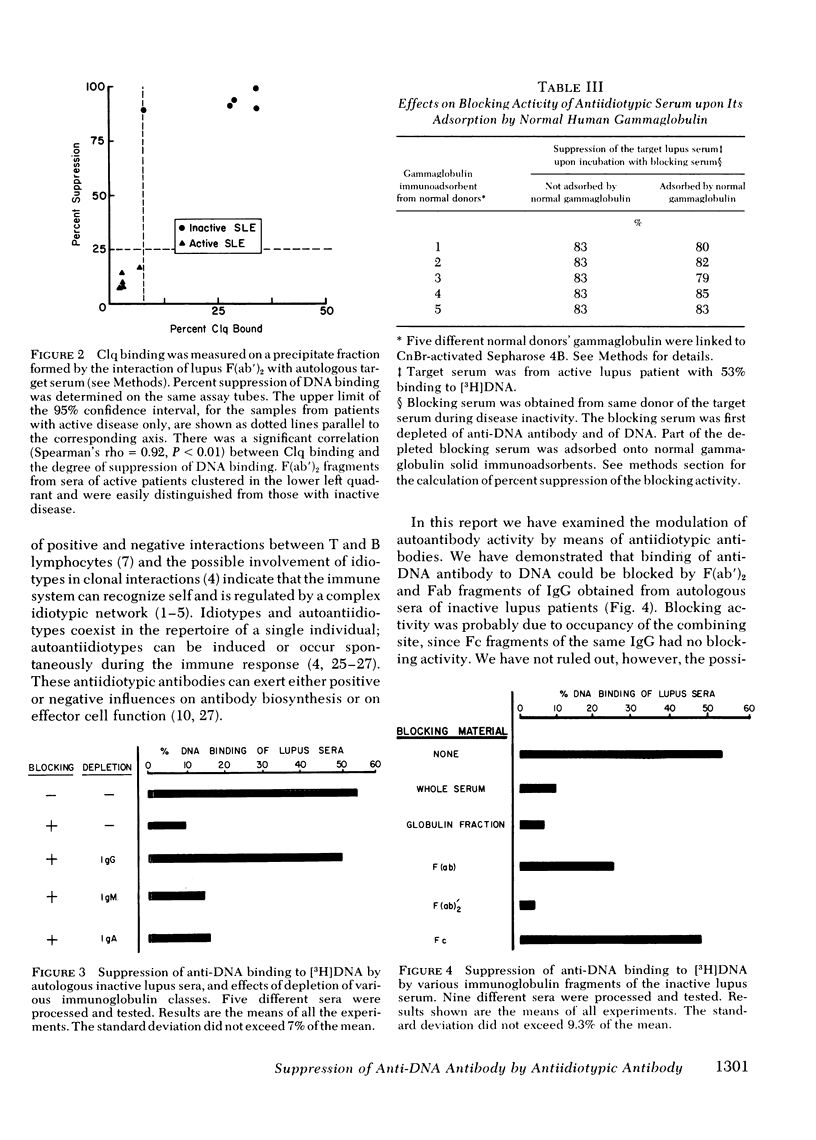
Regulation of serum anti-DNA antibody in systemic lupus erythematosus (SLE) by an antiidiotypic antibody was evaluated. Various sera from SLE patients in active and inactive states of their disease, as well as sera from normal individuals, were first completely depleted of anti-DNA and of DNA by affinity chromatography. The suppressive capacity of equimolar concentrations of the various depleted sera (blocking sera) on target lupus sera were determined. The target sera were from lupus patients with known DNA-binding capacity. Blocking sera from inactive SLE suppressed the binding of autologous anti-DNA antibody to [3H]DNA (n = 19,P < 0.01). Blocking sera from active SLE (n = 19), as well as human serum albumin, did not suppress. Sera from normal donors who had no contact with lupus patients or with lupus sera did not suppress (n = 14, P > 0.5), whereas those from normal donors who had contact with lupus patients or sera did suppress the binding (n = 5,P < 0.02). The anti-anti-DNA antibody suppressive activity in the inactive lupus serum was shown to be localized within the F(ab′)2 portion of immunoglobulin (Ig)G and could not be removed upon adsorption by normal human gammaglobulin. Furthermore, immune complexes could be detected by a Clq binding assay when the inactive lupus blocking sera were incubated with the anti-DNA antibody containing target sera. The specificity of the suppressive serum factor was shown by its inability to block the binding of tetanus toxoid to antitetanus antibody and its ability to block the binding of DNA to F(ab′)2 fragments of active lupus IgG.
Regulation of serum anti-DNA antibody levels by anti-antibodies could induce and maintain disease remission in lupus patients and prevent disease expression in normals.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Adams D. D., Knight J. G. H gene theory of inherited autoimmune disease. Lancet. 1980 Feb 23;1(8165):396–398. doi: 10.1016/s0140-6736(80)90946-0. [DOI] [PubMed] [Google Scholar]
- Allison A. C. The roles of T and B lymphocytes in self-tolerance and autoimmunity. Contemp Top Immunobiol. 1974;3:227–242. doi: 10.1007/978-1-4684-3045-5_9. [DOI] [PubMed] [Google Scholar]
- Bankhurst A. D., Williams R. C., Jr Cellular origins of autoantibody--a preplexing question. Am J Med. 1976 Sep;61(3):303–307. doi: 10.1016/0002-9343(76)90364-8. [DOI] [PubMed] [Google Scholar]
- Bankhurst A. D., Williams R. C., Jr Identification of DNA-binding lymphocytes in patients with systemic lupus erythematosus. J Clin Invest. 1975 Dec;56(6):1378–1385. doi: 10.1172/JCI108218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz H., Wigzell H. Antiidiotype antibodies: induction of specific transplantation tolerance in adult animals. Transplant Proc. 1979 Mar;11(1):914–918. [PubMed] [Google Scholar]
- Bona C., Paul W. E. Cellular basis of regulation of expression of idiotype. I. T-suppressor cells specific for MOPC 460 idiotype regulate the expression of cells secreting anti-TNP antibodies bearing 460 idiotype. J Exp Med. 1979 Mar 1;149(3):592–600. doi: 10.1084/jem.149.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Rodkey L. S. Autoregulation of an antibody response via network-induced auto-anti-idiotype. J Exp Med. 1979 Jul 1;150(1):67–85. doi: 10.1084/jem.150.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza H., Köhler H. Specific suppression of the antibody response by antibodies to receptors. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2701–2705. doi: 10.1073/pnas.69.9.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHoratius R. J., Rubin R. L., Messner R. P., Carr R. I. Lymphocytotoxic antibodies in laboratory personnel exposed to SLE sera. Lancet. 1979 Nov 24;2(8152):1141–1142. doi: 10.1016/s0140-6736(79)92547-9. [DOI] [PubMed] [Google Scholar]
- Doll N. J., Wilson M. R., Salvaggio J. E. Prevalence of Sm antibody in family members of Sm positive patients with systemic lupus erythematosus. J Rheumatol. 1980 May-Jun;7(3):334–338. [PubMed] [Google Scholar]
- Eichmann K., Rajewsky K. Induction of T and B cell immunity by anti-idiotypic antibody. Eur J Immunol. 1975 Oct;5(10):661–666. doi: 10.1002/eji.1830051002. [DOI] [PubMed] [Google Scholar]
- Ginsberg B., Keiser H. A Millipore filter assay for antibodies to native DNA in sera of patients with systemic lupus erythematosus. Arthritis Rheum. 1973 Mar-Apr;16(2):199–207. doi: 10.1002/art.1780160210. [DOI] [PubMed] [Google Scholar]
- Halsey J. F., Woolery W. A., Oleinick S., Kahaleh M. B., Leroy E. C. DNA binding activity of serum from patients with systemic lupus erythematosus. Clin Exp Immunol. 1979 Mar;35(3):356–363. [PMC free article] [PubMed] [Google Scholar]
- Herbert J., Sadeghee S., Schumacher H. R., Zweiman B., Zmijewski C., Abdou N. I. Null cells in peripheral blood of normals and systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Nov;6(3):347–358. doi: 10.1016/0090-1229(76)90088-x. [DOI] [PubMed] [Google Scholar]
- Locker J. D., Medof M. E., Bennett R. M., Sukhupunyaraksa S. Characterization of DNA used to assay sera for anti-DNA antibodies; determination of the specificities of anti-DNA antibodies in SLE and non-SLE rheumatic disease states. J Immunol. 1977 Feb;118(2):694–701. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Miller K. B., Schwartz R. S. Familial abnormalities of suppressor-cell function in systemic lupus erythematosus. N Engl J Med. 1979 Oct 11;301(15):803–809. doi: 10.1056/NEJM197910113011502. [DOI] [PubMed] [Google Scholar]
- Raveche E. S., Steinberg A. D. Lymphocytes and lymphocyte functions in systemic lupus erythematosus. Semin Hematol. 1979 Oct;16(4):344–370. [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell antibody in systemic lupus erythematosus. Possible mechanism for suppressor-cell dysfunction. J Clin Invest. 1979 Mar;63(3):536–539. doi: 10.1172/JCI109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell dysfunction in systemic lupus erythematosus. Cells involved and in vitro correction. J Clin Invest. 1978 Oct;62(4):789–796. doi: 10.1172/JCI109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Ishida S., Onodera S., Saito T., Furuyama T., Yoshinaga K. Passive hemagglutination and hemolysis tests for the detection of anti-DNA antibody. J Immunol Methods. 1978;22(3-4):327–337. doi: 10.1016/0022-1759(78)90040-6. [DOI] [PubMed] [Google Scholar]
- Schrater A. F., Goidl E. A., Thorbecke G. J., Siskind G. W. Production of auto-anti-idiotypic antibody during the normal immune response to TNP-ficoll. I. Occurrence in AKR/J and BALB/c mice of hapten-augmentable, anti-TNP plaque-forming cells and their accelerated appearance in recipients of immune spleen cells. J Exp Med. 1979 Jul 1;150(1):138–153. doi: 10.1084/jem.150.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talal N. Autoimmunity and the immunologic network. Arthritis Rheum. 1978 Sep-Oct;21(7):853–861. doi: 10.1002/art.1780210719. [DOI] [PubMed] [Google Scholar]
- Urbain J., Collignon C., Franssen J. D., Mariamé B., Léo O., Urbain-Vansanten G., Van de Walle P., Wikler M., Wuilmart C. Idiotypic networks and self-recognition in the immune system. Ann Immunol (Paris) 1979 Mar-Apr;130(2):281–291. [PubMed] [Google Scholar]
- Weinberger J. Z., Germain R. N., Ju S. T., Greene M. I., Benacerraf B., Dorf M. E. Hapten-specific T-cell responses to 4-hydroxy-3-nitrophenyl acetyl. II. Demonstration of idiotypic determinants on suppressor T cells. J Exp Med. 1979 Oct 1;150(4):761–776. doi: 10.1084/jem.150.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Benacerraf B., Germain R. N. Feedback suppression of the immune response in vitro. II. IgVH-restricted antibody-dependent suppression. J Exp Med. 1980 Mar 1;151(3):681–694. doi: 10.1084/jem.151.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Nydegger U., Perrin L. H., Fehr K., McCormick J., Lambert P. H., Miescher P. A. Circulating and intra-articular immune complexes in patients with rheumatoid arthritis. Correlation of 125I-Clq binding activity with clinical and biological features of the disease. J Clin Invest. 1976 May;57(5):1308–1319. doi: 10.1172/JCI108399. [DOI] [PMC free article] [PubMed] [Google Scholar]



