Abstract
Ion channel targeted drugs have always been related with either the central nervous system (CNS), the peripheral nervous system, or the cardiovascular system. Within the CNS, basic indications of drugs are: sleep disorders, anxiety, epilepsy, pain, etc. However, traditional channel blockers have multiple adverse events, mainly due to low specificity of mechanism of action. Lately, novel ion channel subtypes have been discovered, which gives premises to drug discovery process led towards specific channel subtypes. An example is Na+ channels, whose subtypes 1.3 and 1.7-1.9 are responsible for pain, and 1.1 and 1.2 – for epilepsy. Moreover, new drug candidates have been recognized. This review is focusing on ion channels subtypes, which play a significant role in current drug discovery and development process. The knowledge on channel subtypes has developed rapidly, giving new nomenclatures of ion channels. For example, Ca2+ channels are not any more divided to T, L, N, P/Q, and R, but they are described as Cav1.1-Cav3.3, with even newer nomenclature α1A-α1I and α1S. Moreover, new channels such as P2X1-P2X7, as well as TRPA1-TRPV1 have been discovered, giving premises for new types of analgesic drugs.
Keywords: ASIC, central nervous system, CNS, KCNQ, ion channels, NMDA, P2X, TRP.
I. INTRODUCTION
Ion channels have been always related with drug discovery process. Their types, primarily recognized as Na+, K+, Ca2+, Cl-, have been basically associated with neuronal processes. Therefore, drugs targeted to them influence all organs or systems related with neuronal activity: the central nervous system (CNS), the peripheral nervous system, and the cardiovascular system. Within the CNS, basic indications of drugs are: sleep disorders, anxiety, epilepsy, pain, etc.
Due to the fact that many CNS diseases are related to variable etiology, many drugs have been developed by in vivo screening, where pharmacological tests performed on animals resemble a state of disease in a human brain. Therefore, the drugs reveal selectivity towards certain channel types, eg. lamotrigine, a known and quite modern anticonvulsant, acts mostly via Na+ and Ca2+ channels.
Lately, novel ion channel subtypes have been discovered, which give premises to drug discovery process which lead towards specific channel subtypes. An example is Na+ channels, whose subtypes 1.3 and 1.7-1.9 are responsible for pain, and 1.1 and 1.2 – for epilepsy. Currently hardly any drug is specific to a single channel, which contributes to drug toxicity. However, new drug candidates have been recognized.
This review is focusing on ion channels subtypes, which play a significant role in current drug discovery and development process. Nowadays, there are no specific drugs targeted to a single channel subtype. Moreover, the knowledge on channel subtypes has developed rapidly, giving new nomenclatures of ion channels. For example, Ca2+ channels are not any more divided into T, L, N, P/Q, and R, but they are described as Cav1.1-Cav3.3, with even newer nomenclature α1A-α1I and α1S. Moreover, new channels such as P2X1-P2X7, as well as TRPA1-TRPV1 have been discovered, giving premises for new types of analgesic drugs.
The review has been divided by channels families, subfamilies, and drugs in various stages of development. Structural diversity of channel subtypes has been shown. The biological activity of drugs has been described and structure-activity relationship, where possible, has been commented.
II. ION CHANNELS
1. Voltage-gated Sodium Channels
The voltage-gated sodium channels (VGSCs) are heteromeric transmembrane proteins which open in response to alteration in membrane potential to provide selective permeability for sodium ions [1].
Volted-gated sodium channels as drug targets in CNS disorders were recently deeply reviewed by Mantegazza et al. [2], Chahine et al. [3] and Tarnawa et al. [4]. In the current review we would like to summarize up to date information regarding their use in CNS disorders.
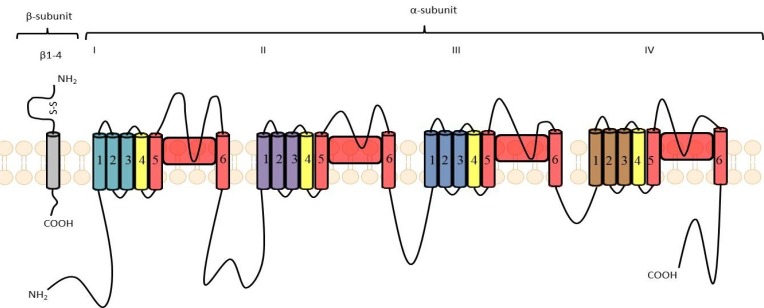
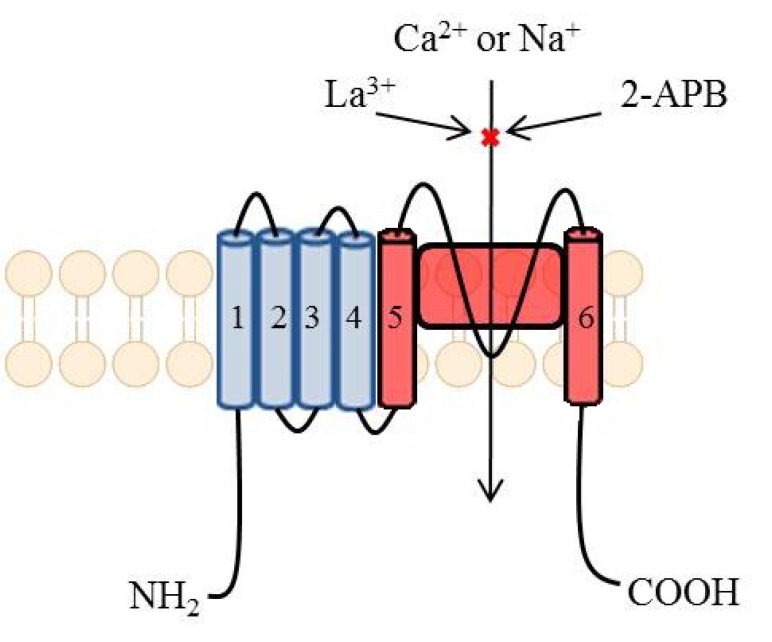
Sodium channels are built by several subunits. Subunit α forms a Na+ selective pore. It has molecular mass of 260 kDa. It consists of four homologous domains (I-IV or D1-D4), of which each contains six α-helical transmembrane segments (S1-S6) and one non-helical relatively short reentrant segment (SS1/SS2), known also as the P-segment, located between S5 and S6. All segments and domains are connected by internal or external polypeptide loops (Fig. 2). The S4 segments are positively charged due to presence of positively charged amino acid residues and their role is to initiate the voltage-dependent activation of sodium channels by moving outward while influenced by the electric field. Therefore, S4 segments serve as voltage sensors. The short intracellular loop connecting domains III and IV occlude the cytoplasmic end of the pore when channel inactivates. The membrane reentrant loops between S5 and S6, which are the part of P-segment form the ion selectivity filter and ion pathway as well as the outer region of the pore. Sodium channels possess also one or more β-subunits of about 35 kDa [5, 6]. The role of β-subunits is influencing the properties of α-subunits including modulation of sodium currents. Moreover, they function as cell adhesion molecules and play role in aggregation, migration as well as cell surface expression. β-subunits typically possess a large extracellular immunoglobulin-like N-terminal domain, a single transmembrane region and intracellular C-terminal region. α- and β-subunits are associated non-covalently (α with β1 or β3) or covalently, e.g. by means of disulfide bond (α with β2 or β4) [6, 7].
Fig. (2).
Schematic arrangement of the α-and β-subunits of the VGSC. The pore is colored in red, the voltage sensors are yellow [according to: 2, 5].
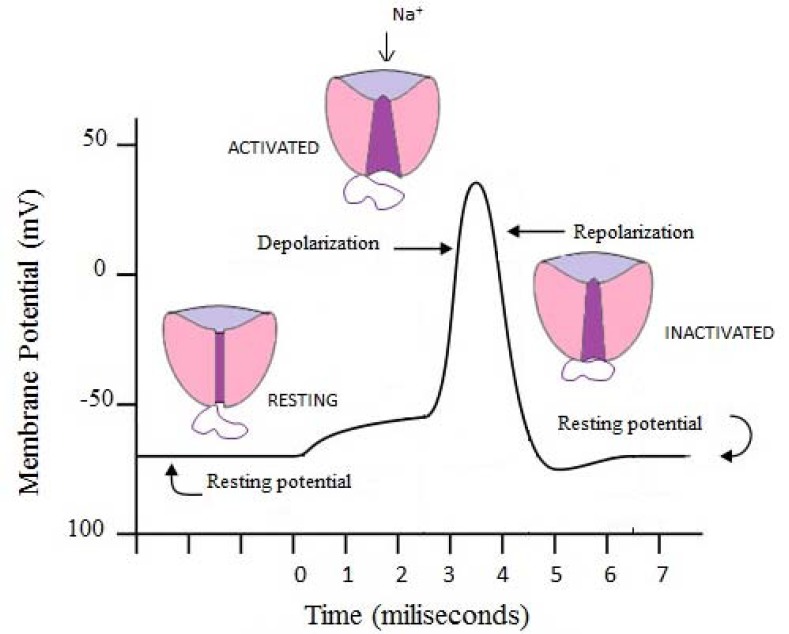
There are at least three functional states of sodium channels (Fig. 3). The “resting” (closed, activateable) state emerges from conformational change that requires repolarization of the membrane (membrane potentials below -60 mV). In that state the channels are ready to open. In response to membrane depolarization they become “open” (or “activated”) and allow rapid influx of sodium ions. Then they are converted to “inactivated”, when the channels undergo conformational changes in which isoleucine, phenylalanine, and methionine between domains III and IV play an important role. When inactivation takes a time of around 1 ms it is called “fast-inactivation” and channels shift into a fast-inactivated state. On the other hand, some channels undergo slow-inactivation, which takes considerably longer time (seconds to minutes). As a result “slow-inactivation” state, a fourth possible functional state is created [1, 6].
Fig. (3).
Action potential of neuronal membrane in different functional states of VGSC.
Moreover, functional changes and increased permeability of Na+ ions result in generation of sodium currents, which can be measured. Those currents are responsible for the upstroke of action potential. Many neurons possess two types of sodium currents: transient and persistent. Transient Na+ current (INaT) is related with opening of the channel when sodium ions passively move through the channel on the basis of its electrochemical gradient [8]. On the other hand, persistent Na+ current (INaP) is a small slowly inactivating sodium current with relatively long kinetics of inactivation (tens of seconds), which appears when inactivation of channels is incomplete [2]. It activates as potentials close to or slightly more negative than resting membrane potential and hardly inactivates. Its amplitude is relatively small, it constitutes about 1% of the peak amplitude of the transient sodium current [8, 9].
First classification of sodium channels divided them into two groups, either sensitive or non-sensitive to the puffer fish toxin, tetrodotoxin (TTX). The existing names of voltage-gated sodium channels are derived from the kind of α-subunit that have been cloned. Channels named as NaV1.1 to NaV1.9 refer to proteins and differ in functional form of α-subunit. Moreover, a unique α-subunit known as NaX has been also recognized. The subunit is lacking amino acids needed for proper voltage gating and it has been shown to be gated by extracellular sodium concentration [6]. TTX-resistance is characteristic for NaV1.5, NaV1.8 and NaV1.9 (blockade in micromolar concentration), while NaV1.1, NaV1.4, NaV1.6 and Nav1.7 are sensitive to nanomolar concentrations of TTX [6]. Expression of different subtypes shows cell and tissue specificity (Table 1).
Table 1.
Localization of Different Types of Voltage-gated Sodium Channels in Human Organism
| Type of Sodium Channel α-Subunit | Human Gene | Localization | Disease |
|---|---|---|---|
| NaV1.1 | SCN1A | Brain: neuronal somata, proximal dendrites [2, 12] | Epilepsy |
| NaV1.2 | SCN2A | Brain: predominantly along axons: unmyelinated and myelinated [2], axon initial segments [13] | Epilepsy |
| NaV1.3 | SCN3A | Brain: neuronal somata, proximal dendrites; [2, 12], upregulated expression in epileptic hippocampal neurons [14] | Epilepsy, Central neuropathic pain [15] |
| NaV1.4 | SCN4A | Skeletal muscle [2] | |
| NaV1.5 | SCN5A | Cardiac muscle; some neurons [2], microglial cells [16] | Atrial fibrillation, ventricular fibrillation [10] |
| NaV1.6 | SCN8A | Brain: axon initial segments, nodes of Ranvier, neuronal somata, dendrites of projection neurons [2] proximal dendrites [12], unmyelinated and myelinated axons [17] | Ataxia |
| NaV1.7 | SCN9A | Peripheral primary sensory neurons [2] Trigeminal ganglion neurons [18] |
Neuropathic pain |
| NaV1.8 | SCN10A | ||
| NaV1.9 | SCN11A |
Names SCN1A-SCN5A and SCN8A-SCN11A refer to genes which code proteins building different α-subunits of sodium channels, and SCN6A/SCN7A codifies Nax subunit [2]. SCN1B-SCN4B are genes responsible for encoding of β-subunit, β1-β4, respectively [7]. SCN1B is expressed as two splice variants, β1 and β1B, both characteristic for human brain and heart [10]. Four sodium channel genes are primarily expressed in CNS: SCN1A, SCN2A, SCN3A, and SCN8A [11].
Epilepsy
Epilepsy is one of the most common neurological disorder. In general, it is a tendency to the occurrence of unprovoked epileptic seizures which are a result of synchronous discharges of large number of neurons [19, 20]. It is now known that abnormal expression or function of VGSCs may be involved in the pathophysiology of both acquired and inherited epilepsy [2]. Great evidence of the role of some VGSCs in epileptogenesis emerges from the identification of several mutations in VGSCs genes leading to inherited epileptic syndromes. Some examples are listed in (Table 2).
Table 2.
Epilepsy Syndromes As Result of Mutations in Sodium Channels Genes
| Gene with Mutation | Epilepsy Syndrome |
|---|---|
|
| |
| SCN1A | Severe myoclonic epilepsy of infancy (SMEI) [21] |
| Generalized epilepsy with febrile seizures plus (GEFS+) [21] | |
| Benign febrile seizures (FS) [13] | |
| SCN2A | Benign neonatal-infantile familial seizures (BNIFS) [11] |
| Generalized epilepsy with febrile seizures plus (GEFS+) [11] | |
| Idiopathic generalized epilepsy [22] | |
| SCN1B | Severe myoclonic epilepsy of infancy (SMEI) [10] |
| Generalized epilepsy with febrile seizures plus (GEFS+) [10] | |
| Childhood absence epilepsy [22] | |
| Temporal lobe epilepsy [22] | |
The disease in also thought to be associated with significantly increased persistent Na+ current, 2-5 times larger than under physiological conditions, which is observed in models of temporal lobe epilepsy and in neurons obtained from the resected temporal lobe of epileptic patients [2]. This current may result in enhancing of synaptic potentials, generation of subthreshold oscillations, facilitation of repetitive firing, and prolongation of depolarized potentials [23]. Persistent sodium current is especially interested when observed in neocortical pyramidal neurons [24].
For any compound, including sodium channel blockers, anticonvulsant activity is not possible without penetration of blood-brain barrier, because chemical molecule must reach its target NaV1.1, NaV1.2, NaV1.3, and/or NaV1.6 channels localized in brain [12, 11]. Moreover, state-dependent affinity to open and/or inactivated channels is beneficial with respect to pathomechanism of epilepsy. Thus, anticonvulsants possess little effect on normal brain activity but affect pathological discharges during seizures. Inhibition of open and/or inactivated channels results in strong delaying the recovery from that state and reduction of sodium conductance [25]. Sodium channel blockers used as antiepileptic drugs do not show significant species or subtypes differences in their potency, which may be result of highly conserved nature of the binding site of those drugs [12]. Phenytoin and carbamazepine belong to antiepileptic drugs (AEDs) which exert their action mainly through inhibition of Na+ current. Their clinical efficacy in partial and generalized tonic-clonic seizures and lack of activity against absence seizures stay in agreement with their activity profile in animal seizure models. They are effective in maximal electroshock seizure (MES) test which is thought to be a predictive model for generalized tonic-clonic seizures, but they are not active in subcutaneous pentetrazole test (scMet) which is considered as predictive for drug’s activity against nonconvulsive seizures [22, 26]. On the other hand, many currently used AEDs have a mixed mechanism of action and sodium channels inhibition or modulation only accompanies influencing additional targets in brain. Those drugs are clinically effective against different types of seizure. There are still many concerns about contribution of VGSCs inhibition to their anticonvulsant efficacy [26]. Valproic acid (2-propylpentanoic acid) (Fig. (4) may serve as an example. It is a one of the most widely used AEDs in the treatment of generalized and partial seizures both in adults and children [27]. Several studies reported its possibility of reducing sodium currents in neocortical rat neurons (at concentration 0.2-2mM) [28], and especially persistent Na+ current (with high potency IC50 of 13.87±0.36 µM) [24] as well as in recombinant human NaV1.2 channels (IC50 514 µM) [12]. The proposed mechanism of VGSCs alteration is that valproate, being a fatty acid, may modulate the channels by influencing the biophysical properties of the channel’s membrane but it does not explain the whole activity [27, 29].
Fig. (4).
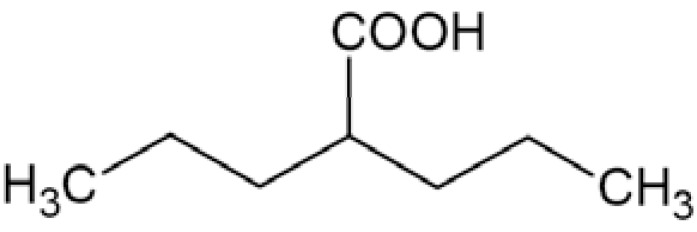
Chemical structure of valproic acid.
Neuronal Cell Damage (Neurodegeneration)
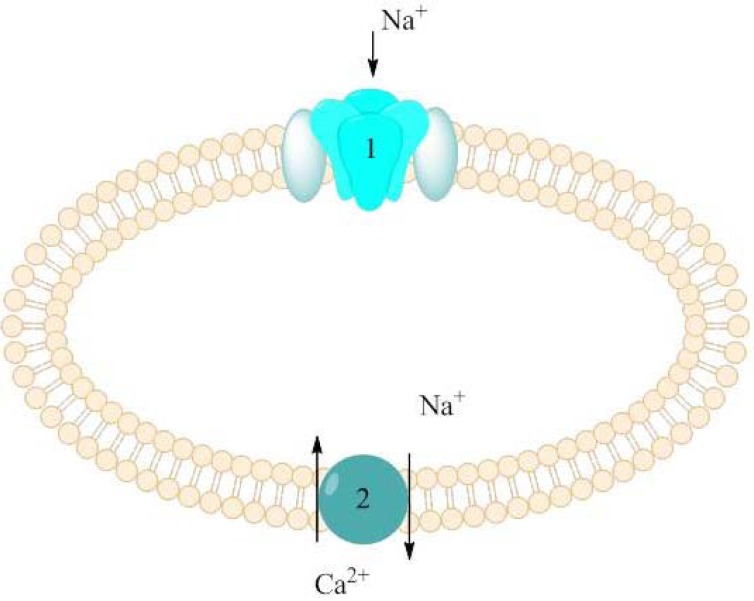
Neuronal cell damage may be caused among others by hypoxia which appears in case of decreased or interrupted oxygen delivery. Persistent sodium current was identified as early and fundamental event in hypoxia. Other mechanisms involved in central axons injury are Na+-K+ ATPase failure and K+ efflux. As a result, increased concentration of sodium ions as well as depolarization of cell membrane were observed, among other in rat hippocampal neurons [30, 31]. Raised level of Na+ concentration triggers action of the Na+/Ca2+ exchanger (Fig. 5). As a consequence Ca2+ concentration significantly increases generating Ca-dependent injury mechanism. Thus, drugs which inhibit persistent sodium current might reduce cell damage in CNS neurons during ischaemia or hypoxia, be means of secondary decreasing of Ca2+ concentration in cells [9].
Fig. (5).
Raised Ca2+ concentration in cell as a consequence of increased Na+ influx.
NaV1.6 were found to produce larger sodium persistent current than other types of sodium channels which may be responsible for increasing action of the Na+/Ca2+ exchanger. As a result, injury of demyelinated axons in spinal cord is likely to occur [17]. Persistent sodium current may also constitute an important factor contributing to neuronal damage in Amyotrophic Lateral Sclerosis (ALS) [32]. It is worth to mention that multiple sclerosis (MS), a prototypical white matter disorder is not only neuroinflammatory condition but may be also caused by mitochondrial dysfunction and its molecular mechanism of tissue damage is similar to that of hypoxic CNS injury. It has been proposed that neuroprotecting agents designed for ischemia may be effective in MS as adjuvant therapeutics [33]. Increased influx of sodium ions into the cells has been also postulated as a key early event in the pathogenesis of secondary traumatic central nervous system injury. Some VGSCs blockers like phenytoin, or riluzole showed neuroprotective activity in many experimental spinal cord injury studies in aspects of motor, neurobehavioral and histopathological recovery [34].
Migraine
Migraine is a common episodic CNS disorder, which is characterized by recurrent attacks of disabling headaches and associated symptoms [35]. Several sodium channels blockers have been implicated in the therapy of migraine, however, their precise mechanism of action in that condition is not fully understood [2]. It is generally accepted that inhibition of sodium currents can decrease cortical hyperexcitability predisposing to migraine. Some hypotheses say also that VGSCs blockers may influence cortical spreading depression (CSD), defined as slowly propagating wave of neuronal and glial depolarization. There is growing evidence that migraine aura and headache are triggered by CSD. Moreover, familial hemiplegic migraine, an autosomal dominant migraine syndrome, may be caused among others by mutation in SCN1A gene [36].
Neuropathic Pain
Neuropathic pain is a kind of pain caused by functional abnormality of neurons, related with their damage [37]. Voltage-gated sodium channels located in peripheral sensory neurons play an important role in its pathophysiology because of their hyperexcitability and generation of spontaneous action potential firings. Blockade of VGSC contributes to analgesic activity [38]. Most beneficial in pharmacotherapy of neuropathic pain could be some selective NaV1.7, NaV1.8 or NaV1.9 channels blockers, till now not available at the pharmaceutical market. In that situation non-selective blockers are used which were initially evaluated in different disorders e.g. epilepsy [37]. NaV1.3 sodium channels have also been implicated in peripheral neuropathic pain. They are expressed at relatively high level in rat embryonic dorsal root ganglion (DRG) neurons but in adult rodent their expression in DRG is very limited [39]. However, after either chronic inflammation or nerve injury they are upregulated in second-order dorsal horn sensory neurons. These findings suggest that NaV1.3 might be related with central neuropathic pain [39, 40]. Thus not only NaV1.7, NaV1.8 or NaV1.9 blockers, but also NaV1.3 blockers may be used in the treatment of neuropathic pain.
Ataxia
Ataxia is another condition associated with sodium channels, and more specific with NaV1.6. The reason of that assumption rose form genetic studies which showed that mutations in mice Scn8a gene result in a variety of symptoms ranging from mild ataxia to dystonia, paralysis, and juvenile lethality [41]. Other research proved the role of their mutations in epileptic syndromes. They might serve as genetic modifiers of SMEI and GEFS+ [11]. Studies in human genome selected one mutation in the SCN8A gene expressed NaV1.6 channels which may be responsible for motor and cognitive deficits of variable expressivity. This heterozygous null mutation leads to cerebellar atrophy, ataxia and mental retardation, but an epileptic phenotype in humans was not seen [42]. Selective ligands for NaV1.6, if arise, may find a place among therapeutics for ataxia.
Sodium Channels/Sodium Currents Blockers
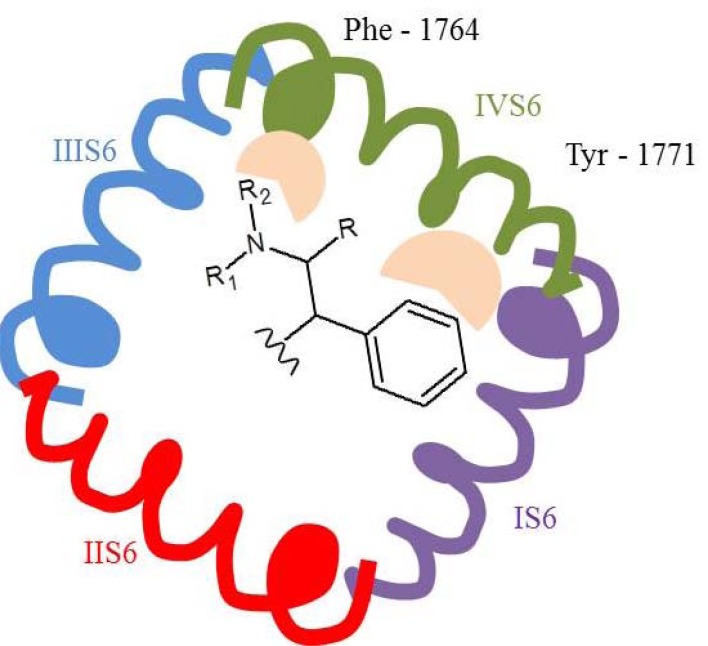
Sodium channel blockers were used in therapy even before their pharmacological targets had been identified or cloned. For example, the first local anaesthetic agent, cocaine, was introduced in 1884 for perioperative analgesia [6]. With the development of science, detailed information about structure of sodium channels has become available. At first, simple models were derived, showing how subunits are embedded in membrane (see (Fig. 2)), then schematic pore-forming regions were created using known potassium channels as model channel [1]. Finally, crystal structure of Arcobacter butzleri voltage-gated sodium channel (NaVAb) was obtained [43, 44]. Parallel with structural studies on sodium channels, functional assays have been developed. For that purpose, cloning of individual sodium channels, including human recombinant, both wild-type or even mutant, in different cell lines have been implicated. That enabled detailed studies on targeting a particular population of channels. Moreover, many technologies have been implicated to investigate biological properties of sodium channels blockers, e.g. radioligand binding assays, radioactive flux assays, fluorescence-based assays, usage of voltage-sensitive dyes [1]. Several binding sites on VGSCs have already been identified. Sodium channels are the molecular target of many neurotoxins which may serve as tools in studying the function and the structure of channels. For example, tetrodotoxin and saxitoxin bind to site 1 (domains I SS2-S6, II SS2-S6, III SS2-S6, and IV SS2-S6), batrachotixin, veratridine and aconitine to site 2 (domain IS6), α-scorpion toxins to site 3 (I S5-S6), β-scorpion toxins to site 4, DDT site 7, last but not least, common anticonvulsants and local anesthetics bind to site 9. Binding to specific site of the receptor is related with a defined physiologal effect, direct affecting of ion transport is characteristic for sites 1 and 9 and modification of gating process with sites 2-8 [5]. Phenytoin, carbamazepine and lamotrigine, despite different chemical structures, seem to bind to the same site of the receptor. The model of interaction is bimolecular which means one-to-one binding process. Moreover, anticonvulsant drug action’s site is thought to be located on the extracellular side of channel [45]. Molecular modeling has been also implicated in evaluation of binding of different sodium channels blockers but it uses homology models with other voltage-gated ion channels: calcium and potassium [25, 46]. Both molecular modeling and site-directed mutagenesis studies helped in indentifying residues which are important for binding to the channel (Fig. (6). A pharmacophore model of the AED binding to VGSCs suggests that presence of aromatic ring and polar amide, imide or amine group in special spatial arrangement is required [46]. In the field of sodium currents research invention of whole-cell patch clamp recordings enabled measurement of transient and persistent currents and thus activity of particular compounds on specific currents.
Fig. (6).
Simple presentation of binding sites of AEDs acting as sodium channels blockers in the NaV1.2 homology model [46]. Aromatic ring has an aromatic-aromatic interaction with Tyr-1771, and amide, imide or amine moiety interacts with the aromatic ring of Phe-1764 by a low-energy amino-aromatic bond.
Currently, sodium channels blockers serve as drugs in several common disorders. They are also extensively investigated, especially with respect to their selectivity. Many sodium channels blockers are state-dependent which means that they have preferential affinity for the open and/or inactivated channel’s state when compared to its resting state [6]. The same situation refers to both anticonvulsant drugs as well as local anesthetics. Although both groups significantly differ in chemical structure, they seem to bind to the same site of the channel’s pore with similar affinity showing different therapeutic effect [46]. VGSCs blockers act through different sites of α-subunit, no drugs are known to interact directly with β-subunit [1].
Phenytoin (PHT)
Phenytoin (5,5-diphenylhydantoin), while tested in animals is active in MES test but not in scMet [26]. It is considered as a prototype sodium channel blocker among antiepileptic drugs. PHT preferentially binds to fast-inactivated rather than to the resting sodium channels [45], which has a beneficial role in inhibition of action potential spread.
Preclinical evaluation showed also beneficial role of phenytoin in mice model of multiple sclerosis – experimental autoimmune encephalomyelitis (EAE). Administration of PHT provided improved clinical status, preservation of axons, enhanced action potential conduction and reduced immune infiltrates, so it definitely acted as neuroprotectant [47].
It is been shown that PHT binds to inactivated VGSCs with Ki 7 µM (tested in rat hippocampal neurons) [48] and 19 µM (tested in wild type sodium channels expressed in Xenopus oocyte) [49].
Molecular docking to NaV1.2 channel using homology model with crystal structures of potassium channels, showed that it might bind to the site located in domain IV-S6. Aromatic ring is responsible for aromatic-aromatic interaction with Tyr-1771, and polar amide or imide group interacts with the aromatic ring of Phe-1764 by a low-energy amino-aromatic hydrogen bond [46]. Several phenytoin derivatives have been synthesized and evaluated for affinity to Na+ channels [50] resulting in conclusion that the second aromatic ring in position 5 is not obligatory for VGSCs binding, it can be replaced with aliphatic pentyl, hexyl or heptyl chain. Moreover, phenytoin is effective in partial and generalized tonic-clonic seizures but not nonconvulsive seizures [26].
Carbamazepine (CBZ)
Carbamazepine (5H-dibenzo[b,f]azepine)-5-carboxa mide) Fig. (8) is and old AED, developed in late 1950s - 1960s. While tested in vivo CBZ showed effectiveness in MES test (ED50s 7.81 mg/kg b.w. (body weight) mice, i.p. and 17 mg/kg b.w. mice p.o.) as well as amygdala-kindled rats model but it did not show activity in subcutaneous pentetrazole test [26, 37].
Fig. (8).
Chemical structure of carbamazpine.
Although well established position in pharmaceutical market, its mechanism of action is not yet completely understood. One of the fact is that it inhibits VGSCs in a voltage-dependent and frequency-dependent manner [2]. It has higher affinity to fast-inactivated that to resting VGSC. CBZ also blocked persistent Na+ current in concentration dependent manner in NaV1.3 channels expressed in HEK293 cells with EC50 16±4 µM and maximum block (Emax) 46±4%. Inactivation of persistent sodium current seems to be parallel with inhibition of transient Na+ current [8]. Molecular modeling showed that the pharmacophore segments of CBZ and PHT are similar, and CBZ is also able to develop interaction with binding site in domain IV-S6 [46].
CBZ is used in the treatment of simple partial, complex partial and generalized tonic-clonic seizures but it is not effective against absence seizures [26, 37].
Eslicarbazepine Acetate (ESL)
Eslicarbazepine acetate ((S)-(-)-10-acetoxy-10,11-dihyd ro-5H-dibenz[b,f]azepine-5-carboxamide, BIA 2-093) (Fig. 9) is an AED second-generation to carbamazepine. It showed efficacy in several animal seizure models both electrically (MES) and chemically (pentetrazole, bicuculline, picrotoxin, 4-aminopyridine) evoked [51].
Fig. (9).
Chemical structure of eslicarbazepine acetate.
Although its precise mechanism of action is not yet explained, ESL proved to inhibit Na+ current in a voltage-dependent manner while tested in NIE-115 mouse neuroblastoma cells. It preferably binds to VGSCs in inactivated state what may result in limitation of repetitive firing and seizure spread. In tests performed in rat brain membranes ESL bonds in a competitive manner to neurotoxin site 2 of the channel, but not site 1, with IC50 of 222 µM (138-358) in displacing 3H-BTX [52]. ESL has been licensed in Europe (not in the USA) as adjunctive treatment for partial seizures with or without secondary generalization in adults [53].
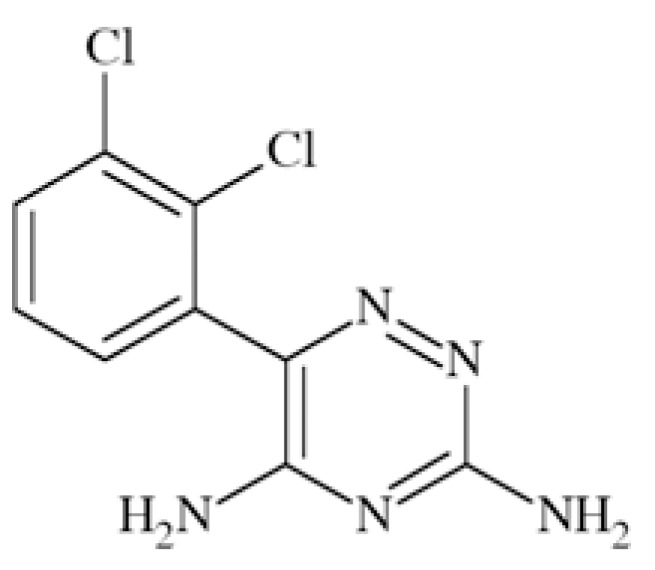
Lamotrigine (LTG) and Related Compounds
Lamotrigine (6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine) Fig. (10) proved efficacy in several animal seizure test: MES, 6Hz test, corneal kindled mice, hippocampal kindled rats, sound induced seizures. Its mechanism of action involves inhibition of sodium and calcium channels [22, 37].
Fig. (10).
Chemical structure of lamotrigine.
Common pharmacophore concept for PTH enables to chose elements in the structure of LTG responsible for binding to the receptor site IV-S6. They are phenyl ring and amino group in position five of the triazine, which is moved from aromatic ring at the distance of three chemical bonds [46]. The binding affinity of LTG to rat brain NaV1.2 expressed in Xenopus oocytes in inactivated state was reported to be 31.9 µM [54] while compound’s affinity to the closed state of the channel was very low (IC50 641 µM; inhibition of peak current at - 90 mV in human NaV1.2 α-subunit expressed in Chinese hamster ovary (CHO) cells) [55]. Many compounds with LTG related structure were synthesized. Examples of three, which had markedly different biological properties, helped in explanation of relationship of binding site of sodium channel with pharmacological effects. All compounds blocked sodium channel in a voltage-dependent manner but differed in their affinities for inactivated sodium channels (Table 3). By means of assays with mutant sodium channels it was proved that all compounds bind to site located in S6 in domain IV, and more detailed to amino acid residues 1764, 1771 and in some extents also 1760. In fact, 1764 seems to be essential in voltage-dependent sodium channel blocking, whereas interaction with other residues is more variable [54].
Table 3.
Binding Affinities, Pharmacological Properties and Proposed Biding Sites of LTG and Similar Compounds [54]
| KD [µM] Rat Brain Na+ Channels (NaV1.2) Expressed in Xenopus oocytes in Inactivated State (- 50 mV) | Pharmacological Properties in Preclinical Evaluation | Position of Amino Acid Residues in IV- S6 Binding Site | |
|---|---|---|---|
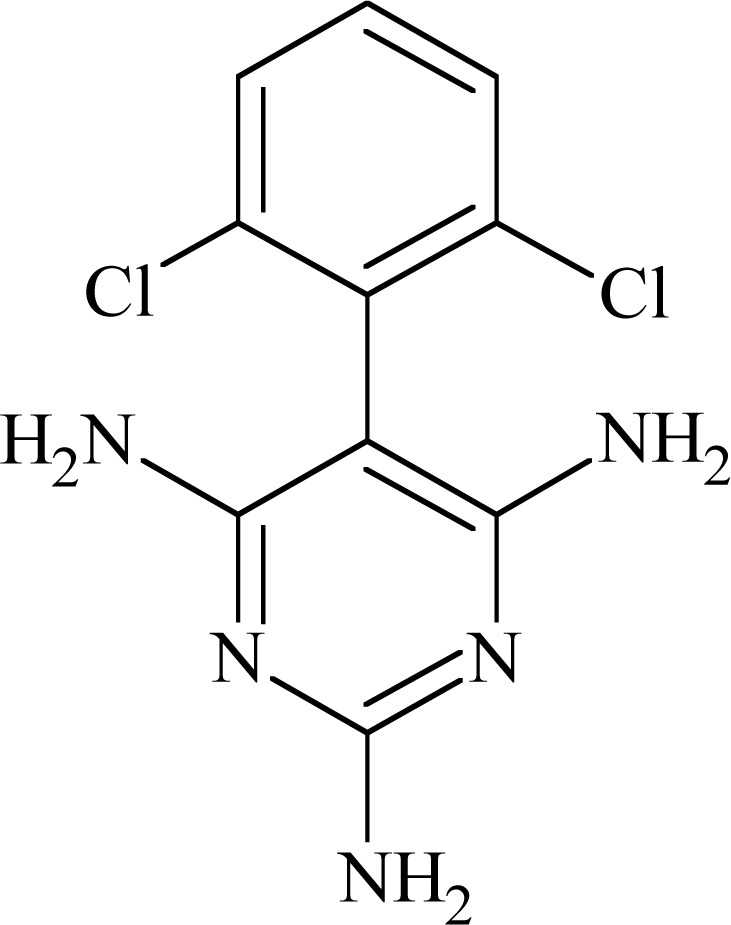 |
17.3 | Analgesic but no anticonvulsant activity | 1764 – strong interaction |
| 1771 – strong interaction | |||
| 1760 – weaker interaction | |||
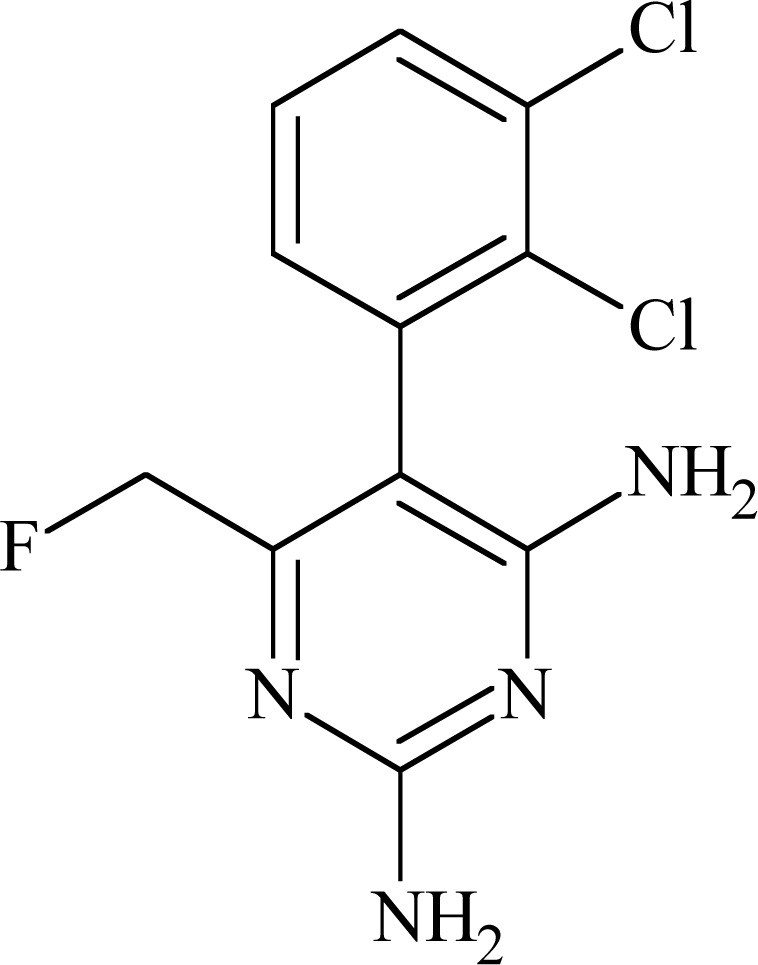 |
3.7 | Analgesic and anticonvulsant activity | 1764 – much stronger interaction; |
| 1771 – modest interaction; | |||
| 1760 weaker interaction | |||
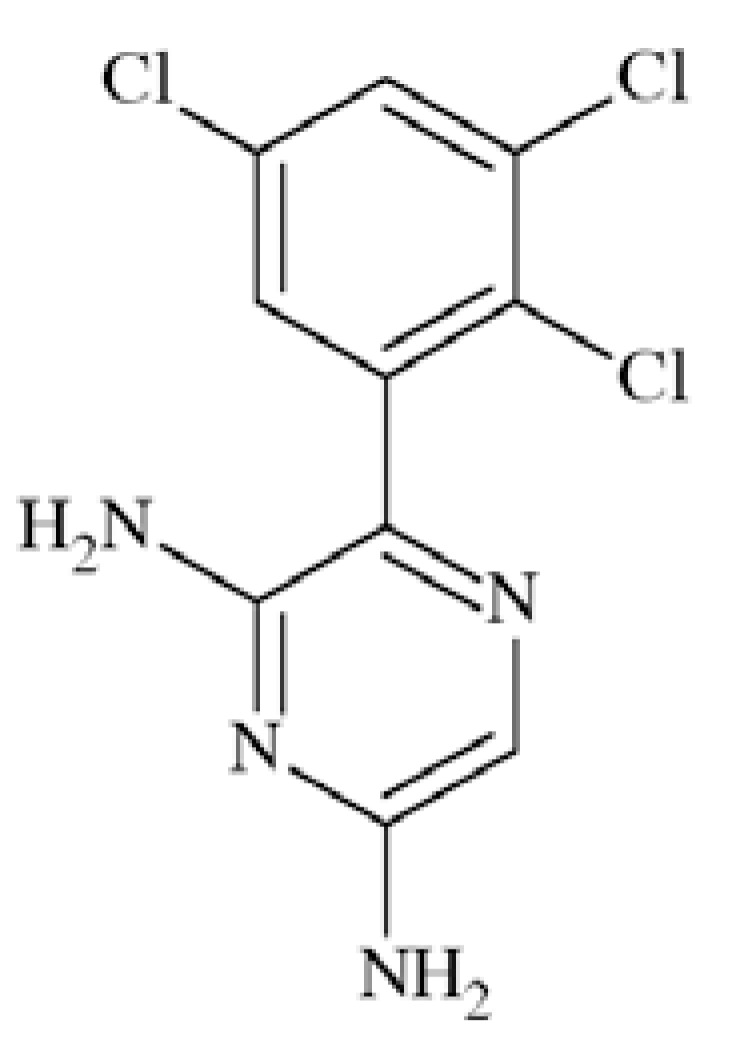
 |
10.3 | Prevention of neuronal toxicity following stroke | 1764 – strong interaction; |
| 1771 – weak interaction; | |||
| 1760 – weak interaction; | |||
| Possible interactions with amino acid | |||
| Residues in other transmembrane segments | |||
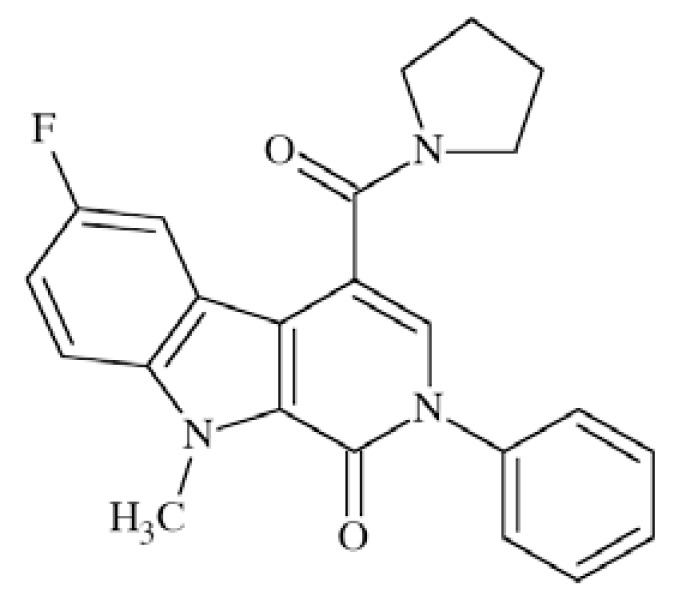
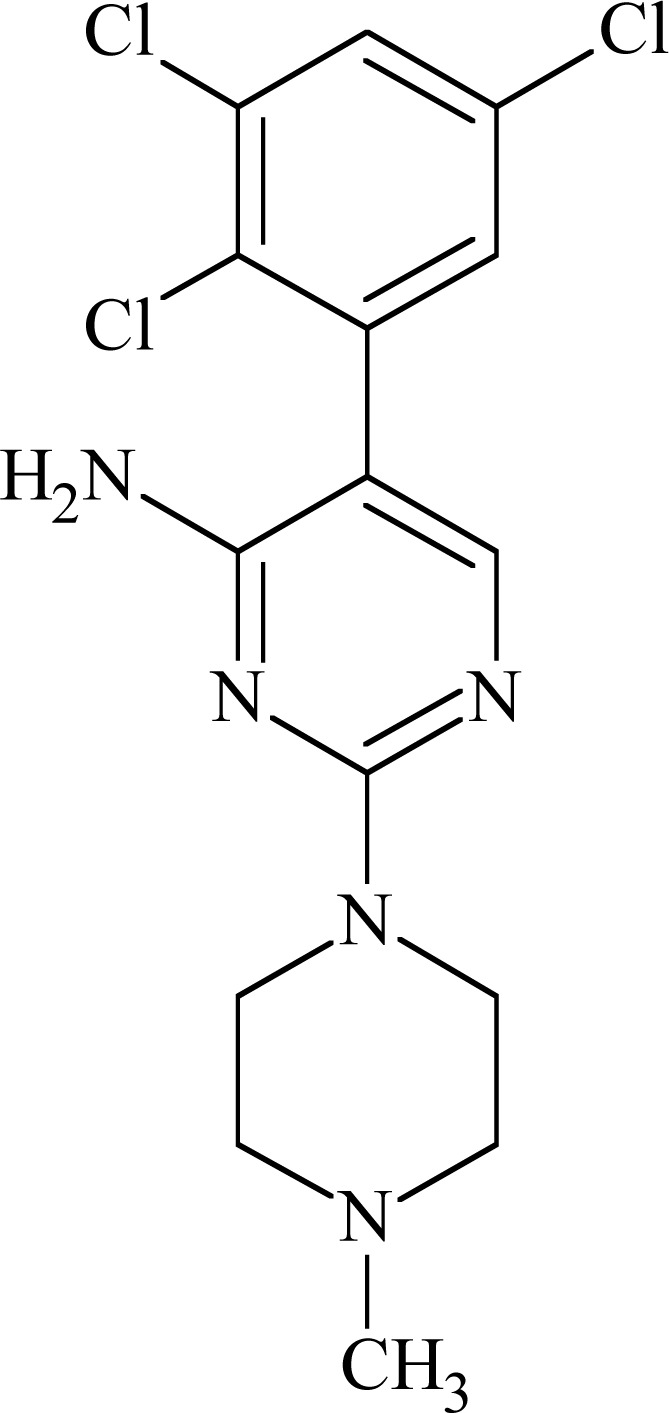
JZP-4
JZP-4 (3-(2,3,5-trichloro-phenyl)-pyrazine-2,6-diamine) Fig. (11) is a second generation drug to lamotrigine, with only slightly altered structure. It possesses broad spectrum of anticonvulsant activity in preclinical evaluation (MES, rat hippocampal and amygdala kindling, 6 Hz). It has inhibitory effect on both sodium and calcium channels [56].
Fig. (11).
Chemical structure of JZP-4.
Tests on human NaV1.2 sodium channels proved its voltage- and use-dependent inhibition, as well as possibility of hyperspolarizing shift in the inactivated state. IC50 values for JZP-4 were 165 and 6 µM at −90 and −60mV, respectively. While tested in NaV1.3 its IC50 were 333, 43 and 7 µM at −120, −90 and −70mV, respectively. In addition to the effect on NaV1.2A and 1.3 channels, JZP-4 also showed a weaker inhibition of NaV1.8/1.9 from rat dorsal root ganglia tissue. JZP-4 is in the Phase II of clinical evaluation in epilepsy.
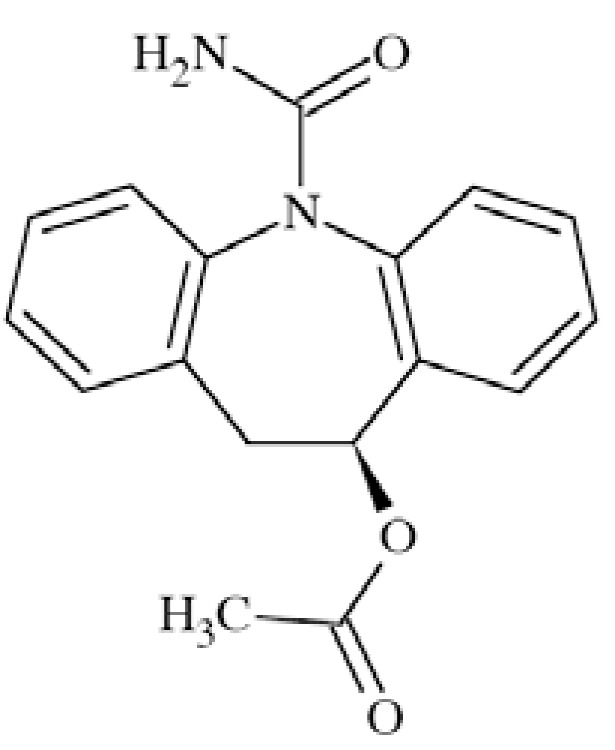
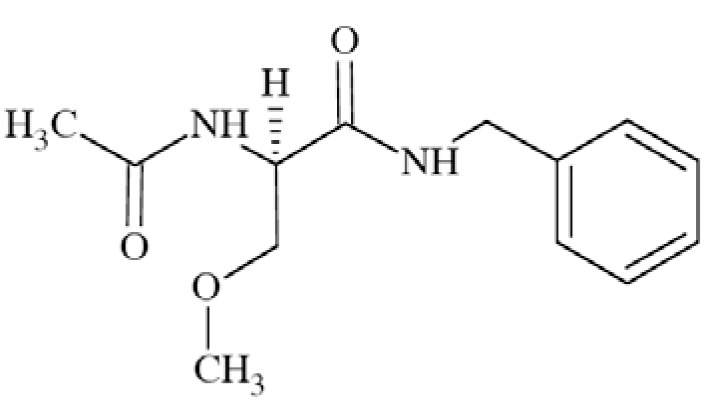
Lacosamide (LCM)
Lacosamide ((2R)-2-acetylamido-N-benzyl-3-methoxy propanamide; formerly harkoseride) Fig. (12) demonstrates broad anticonvulsant activity in several seizure models, like maximal electroshock seizure (MES) test, hippocampal kindling, audiogenic seizures, self-sustaining status epilepticus (SSSE), amygdala kindling, and 6 Hz test. Activity in 6-Hz test is unlike for other AEDs affecting sodium channels [57].
Fig. (12).
Chemical structure of lacosamide.
Lacosamide does not exhibit high-affinity for a range of receptors and ion channels. However, it showed weak affinity for site 2 of rat cortex VGSC (displacement of [3H]-batrachotixin – 25% of inhibition at 10 µM). After further test, it was proved that LCM’s anticonvulsant and analgesic effects are due to attenuation of Na+ currents. Lacosamide tested in recombinant human NaV1.3 and NaV1.7 sodium channels possessed higher affinity for inactivated channels than for resting but still less than carbamazepine. In contrary to other AEDs like carbamazepine, LCM probably affects sodium channel slow inactivation with no effect on fast inactivation. This is a unique mechanism of action which results in preferentially block the electrical activity of neurons that are chronically depolarized but not those possessing more normal resting potentials [15, 58].
LCM has been licensed for the treatment of partial-onset seizures. It has properties that may be also useful for a broad range of neuropathic pain patients e.g. in painful diabetic neuropathy [15, 57].
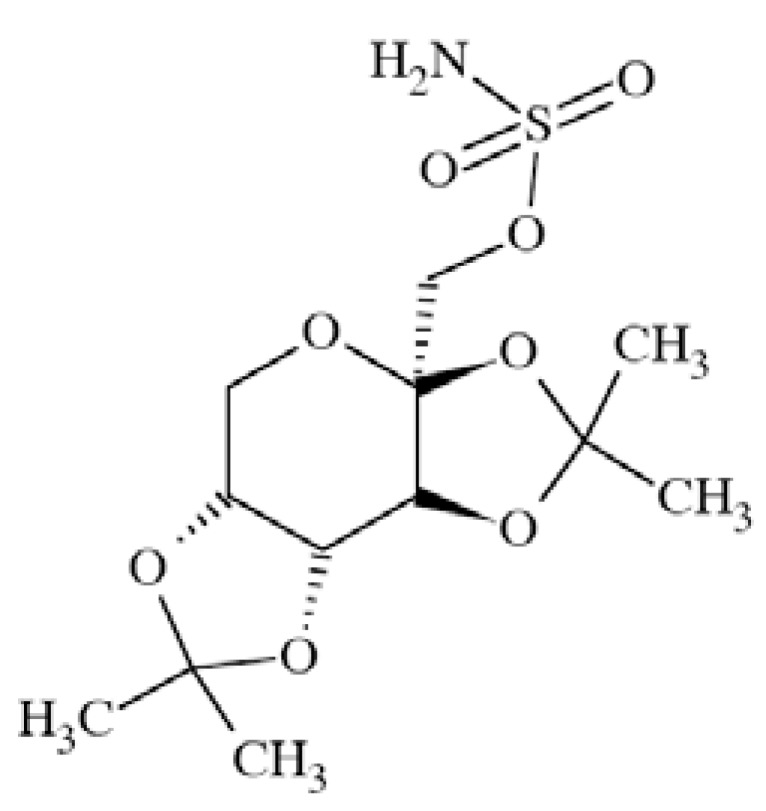
Topiramate (TPM)
Topiramate (2,3:4,5-bis-O-(1-methylethylidene)-β-D-fruc topyranosesulfamate) Fig. (13) exerts its antiepileptic activity by means of different mechanisms of action like VGSCs blockage, potentiation of GABAergic transmission, and AMPA receptor sites modulation. It shows relatively broad spectrum of anticonvulsant properties in animal studies like MES (mice and rats), genetically seizure-prone DBA/2 mice, amygdala kindled rats. Topiramate is inactive/weakly active in chemically-induced seizure models including pentetrazole, picrotoxin, bicuculline, strychnine [59].
Fig. (13).
Chemical structure of topiramate.
It was proved that TPM blocks persistent Na+ current in NaV1.3 expressed in HEK293 cells with EC50 61±37 nM and maximum block Emax 30±4%. At the same time, its ability to shift transient sodium current to negative potentials is much lower (ED50 3.2 µM). This finding suggests that topiramate acts mainly by means of inhibition of persistent Na+ current on the contrary to popular AEDs like carbamazepine and phenytoin, which inhibit mainly transient Na+ current [8]. Its clinical indication is the management of refractory partial and secondarily generalized seizures [59]. At the doses of 25 up to 100 mg/day TMP was also approved for prophylaxis of migraine [60].
Carisbamate
Carisbamate (S-2-O-carbamoyl-1-o-chlorophenyletanol, RWJ-333369) Fig. (14) showed a broad spectrum of activity in preclinical anticonvulsant evaluation, like in MES, scMET, bicuculine picrotoxin induced seizures, and audiogenic seizures. Moreover, it proved efficacy in amygdala kindled rats and lamotrigine-resistant kindled rats. Its proposed mechanism of action includes inhibition of VGSCs and modest inhibition of calcium channels [56, 22].
Fig. (14).
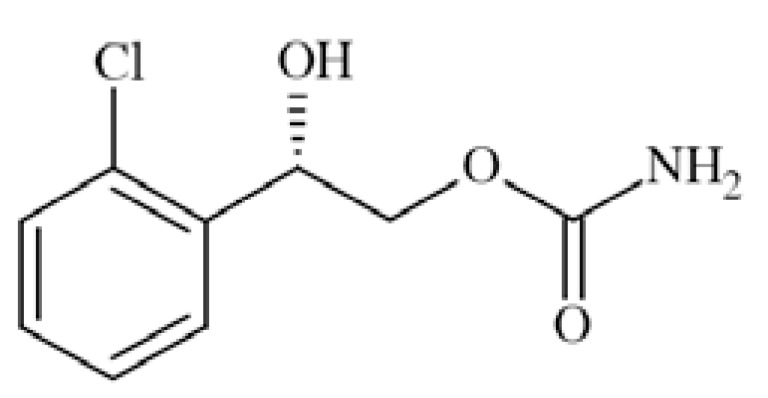
Chemical structure of carisbamate.
Carisbamate showed a concentration-, voltage- and use-dependent inhibition of rat NaV1.2 expressed in CHL1610 cells, with an IC50 value of 68 µM (at −67mV). In rat hippocampal neurons, carisbamate similarly blocked VGSCs, with an IC50 value of 89 µM (at −67mV), and inhibited repetitive firing of action potentials in a concentration-dependent manner (by 46% at 30 µM and 87% at 100 µM) [61].
Rufinamide
Rufinamide (1-(2,6-difluoro-phenyl)methyl-1H-1,2,3-tria zole-4-carboxamide, CGP 33101) (Fig. 15) showed activity in MES and pentetrazole-induced test in rodents, as well as in bicuculline- and picrotoxin clonus in mice [53].
Fig. (15).
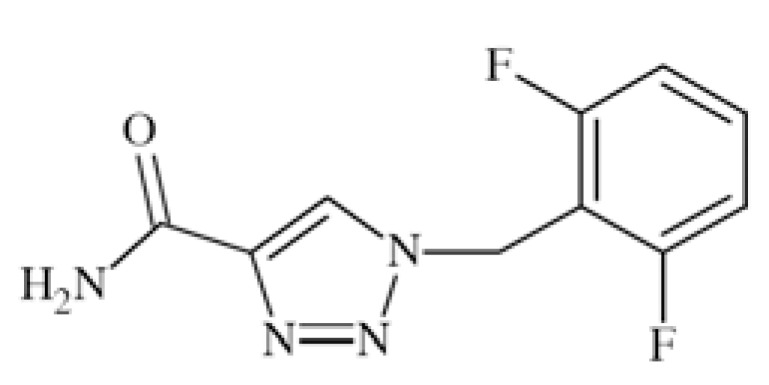
Chemical structure of rufinamide.
Modulation of sodium channels seems the main mechanism of action of that compound. At concentration 1 µM or higher it inhibited VGSCs recovery from inactivation in cortical neurons from immature rats. With EC50 of 3.8 µM it limited sustained repetitive firing of sodium dependent action potentials [62].
Rufinamide is available in Europe and the USA as efficacious and well-tolerated adjunctive treatment for patients with partial seizures and Lennox-Gastaut syndrome [53].
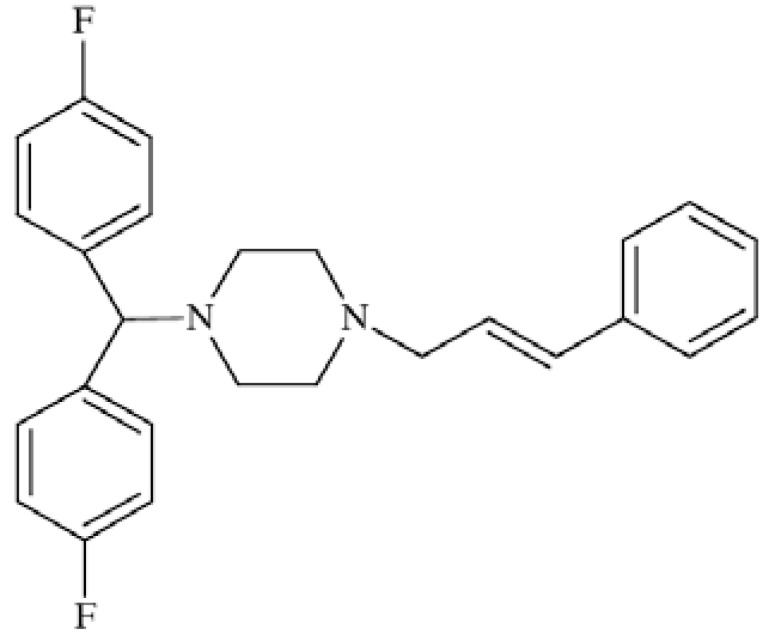
Flunarizine (FLN)
Flunarizine ((E)-1-[bis(4-fluorophenyl)methyl]-4-(3-phenyl-2-propenyl)piperazine) Fig. (16) acts by means of various mechanisms of action although not yet fully understood [63]. Recently it has been proved that the compound efficiently blocks cortical neuronal sodium current in a concentration- and use-dependent manner with IC50 value of 0.94 µM. Moreover, it delays the recovery from fast inactivated state INa. This finding suggests that inhibition of sodium current which can decrease cortical hyperexcitability may may be one of the brain targets of FLN [64]. It antagonizes T-type calcium channels, exhibiting affinity towards N-type channels of IC50=0.08 µM and towards L-type with IC50=0.31 µM [65]. Flunarizine has been successfully used for migraine prophylaxis [63].
Fig. (16).
Chemical structure of flunarizine.
Riluzole
Riluzole (2-amino-6-trifluoromethoxybenzothiazole, RP 54274) Fig. (17) is considered as neuroprotective agent with anticonvulsant properties. Its mechanism of action involves primary inhibition of VGSC but also reduction of glutamate release [32].
Fig. (17).
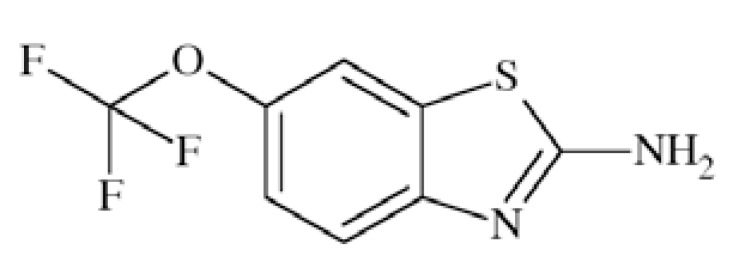
Chemical structure of riluzole.
It inhibited persistent sodium current in dose-dependent manner with EC50 of 2 µM while tested in rat brain neurons [32]. Other studies conducted in bovine adrenal chromaffin cells showed blockade of Na+ channels by riluzole in concentration-dependent manner with IC50=5.3 μM and binding to the veratridine site 2 of channels. In the test using rat brain sodium channel α-subunits expressed in Xenopus oocytes the compounds blocked the close state of the channel with IC50 ranging from 30 to 90 µM, but inhibited the inactivated state of Na channels 150–300 times more effectively [66]. Riluzole in the only drug currently approved for the treatment of Amyotrophic Lateral Sclerosis (ALS) [32]. It is also a non-competitive NMDA antagonist. In a progressive stratial degeneration the compound was able to reduce motor symptoms associated with striatal lesions [67].
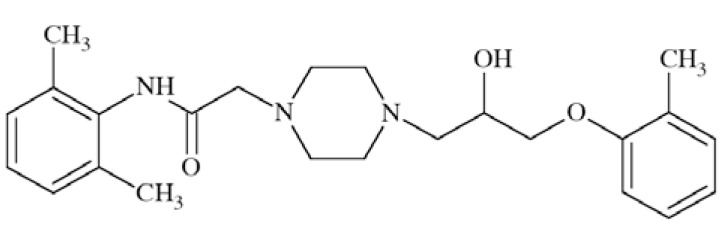
Ranolazine
Ranolazine ((+)-N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-1-piperazineacetamide) (Fig. 18), a drug for the treatment of chronic angina [68], was previously evaluated as modulator of function of different sodium channels like NaV1.4, NaV1.5, NaV1.7, NaV1.8, and recently also NaV1.1. In the study, human wild-type and mutant NaV1.1 channels expressed heterologously in human tsA201 cells were used. The compound was able to block persistent current 16-fold more selective in comparison to tonic block of peak current and 3.6-fold more selective than use-dependent block of peak current. Interestingly, similar selectivity was observed for ranolazine in blockage of increased persistent current exhibited by NaV1.1 mutant channels. Ranalozine proved also ability to cross the blood-brain barrier while tested in rats, which together with inhibition of persistent current in mutant NaV1.1 channels give rise for possible providing a new useful therapeutic strategy for SCN1A-associated epilepsy and some migraine syndroms [69].
Fig. (18).
Chemical structure of ranolazine.
2. POTASSIUM CHANNELS
Voltage-gated Potassium Channels
Voltage-gated potassium channels (VGKCs, KV) belong to broad family of potassium channels, including besides them Ca2+-activated (KCa, ligand-gated), inward-rectifying (KIR), and two-pore (K2P) channels, which all contribute to the excitability of neurons, signaling in the nervous system as well as ion homeostasis. Potassium ion channels play critical role in repolarization of action potentials and regulation of firing frequency [70]. VGKCs were for the first time successfully cloned from Drosophila melanogaster cDNAs [71]. Tempel et al. [72] isolated mammalian cDNA of KV1.1 from mouse brain, later research has led to encoding of cDNA of other members of KV1 family, and finally to isolation of cDNAs of all currently known potassium channels [73]. In 2005 Gutman et al. [74] provided an opinion of International Union of Pharmacology concerning classification as well as deep review of pharmacology, regulation of expression, and disease association of voltage-gated potassium channels. In the current paper we would like to focus on VGKCs as targets in CNS disorders.
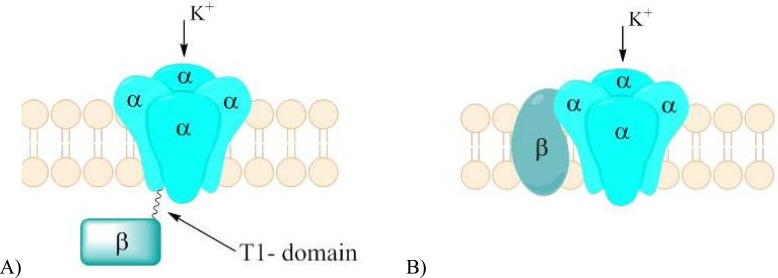
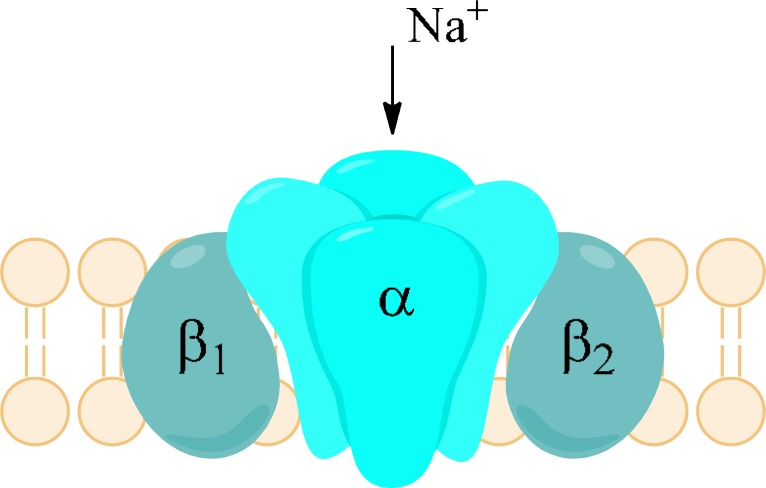
Voltage-gated potassium channels contain four transmembrane pore-forming voltage-sensing α-subunits. Individual α-subunit of KV channel consists of six segments (S1-S6). Four transmembrane S4 segments carry multiple positive charges and act as main voltage-sensing component, which responds to changes of membrane potential resulting in conformational alterations in the channel (voltage gating). Segments S5 and S6 from each of the four α-subunits form a pore, which is in fact an ionic conductance pathway. At the narrowest part of the pore, S5 and S6 connecting loop form a selectivity filter (P). Voltage-sensing segments surround the pore. Thus, the structure is similar to described above voltage-gated sodium channel. Although most of KV channels are homotertamers (four α-subunits are the identical), they may also form heteroteramers, in which two or more distinct types of α-subunits occur (e.g. KV7.2/KV7.3). Those heteroteramers may possess different properties in comparison to homotetramers [70, 73]. Native neuronal potassium channels complexes contain both cytoplasmic and transmembrane auxiliary β-subunits Fig. (19). Several genes that code β-subunits were identified. Their alternative splicing can generate the number of functionally distinct isoforms. Moreover, although called auxiliary, they proved to be able to dramatically alter α-subunits expression and localization as well as functions by means of changing gating properties of channels [73].
Fig. (19).
Schematic arrangement of the α-and β-subunits of KV channel characteristic for KV1 and KV4 subfamily. There are differences in position of β-subunit.
3D reconstruction of the KV α-subunit suggested that it is arranged in two distinct domains: the larger membrane-embedded and smaller cytoplasmic domain. Both domains are connected together by thin linker. The structure of the membrane-embedded domain is highly homologous among all KV families [70]. On the other hand, the cytoplasmic domain differs both in structure and function between different KV types. The very important part in cytoplasmic domain is so called tetramerization domain (T1), which is responsible for promoting the tetramerization of potassium channels subunits. T1 may be connected with cytoplasmic N-terminal fragment of the polypeptide chain, like in KV1- KV4 [75]. On the other hand KV7 and KV11 possess a unique tetramerization domain in the C-terminal region, which also form the binding sites for various ligands (calmodulin, phosphadiylinozytol-4,5-bisphosphate - PIP2, and cyclic nucleotide) that can modulate the channel’s function [76-78].
Currently KV channels are divided based on the relative sequence homology into twelve subfamilies: KV1-KV12. KV1-KV4 channels were classified in one group, KV7 in another, KV5, KV6, KV8, KV9 in different ,and KV10-KV12 in the last. Among the subfamilies, KV5, KV6, KV8 and KV9 did not prove to yield functional expression in forming ion channels, that is why they are also called “electrically silent” α-subunit-like polypeptides. Undoubtedly they are able to modulate the expression and/or gating of channels formed from bona fide α-subunits which is similar to β-subunits functions. Names beginning with KCN refer to genes which code proteins of different α-subunits of potassium channels [73]. Early classification provided a division of voltage-gated potassium channels into three families: Shaker, KvLQT and ether-a-go-go. Ether-a-go-go family was further divided onto three subfamilies: eag, elk and erg. Those names still exist in the literature, they were created before providing the current classification and identification of broad range of currently known voltage-gated potassium channels (Table 4).
Table 4.
Classification of Voltage Gated Potassium Channels [According to 74]
| Other Name | Type of Potassium Channel | Human Gene | Other Name | Type of Potassium Channel | Human Gene |
|---|---|---|---|---|---|
| Shaker-related family | KV1.1 | KCNA1 | KvLQT | KV7.1 | KCNQ1 |
| KV1.2 | KCNA2 | KQT2 | KV7.2 | KCNQ2 | |
| KV1.3 | KCNA3 | - | KV7.3 | KCNQ3 | |
| KV1.4 | KCNA4 | - | KV7.4 | KCNQ4 | |
| KV1.5 | KCNA5 | - | KV7.5 | KCNQ5 | |
| KV1.6 | KCNA6 | KV2.3rc | KV8.1 | KCNV1 | |
| KV1.7 | KCNA7 | - | KV8.2 | KCNV2 | |
| KV1.8 | KCNA10 | Modifiers | KV9.1 | KCNS1 | |
| Shab-related family | KV2.1 | KCNB1 | KV9.2 | KCNS2 | |
| KV2.2 | KCNB2 | KV9.3 | KCNS3 | ||
| Shaw-related family | KV3.1 | KCNC1 | eag1 | KV10.1 | KCNH1 |
| KV3.2 | KCNC2 | eag2 | KV10.2 | KCNH5 | |
| KV3.3 | KCNC3 | erg1 | KV11.1 | KCNH2 | |
| KV3.4 | KCNC4 | erg2 | KV11.2 | KCNH6 | |
| Shal-related family | KV4.1 | KCND1 | erg3 | KV11.3 | KCNH7 |
| KV4.2 | KCND2 | elk1, elk3 | KV12.1 | KCNH8 | |
| KV4.3 | KCND3 | elk2 | KV12.2 | KCNH3 | |
| Modifier | KV5.1 | KCNF1 | elk1 | KV12.3 | KCNH4 |
| Modifiers | KV6.1 | KNCG1 | |||
| KV6.2 | KCNG2 | ||||
| KV6.3 | KCNG3 | ||||
| KV6.4 | KCNG4 | ||||
Voltage-gated potassium channels are present in whole organism, in different organs like: brain, spinal cord, skeletal and smooth muscle, heart, retina, lung, islets, spleen, thymus and many more (see Gutman et al. [74]). Expression of different subtypes throughout brain and spinal cord show specific patterns of subcellular localization, for example KV1 and KV3 are situated mostly in axons and in some dendrites (KV1.1, KV3.1, KV3.2), while KV2 and KV4 in neuronal somata and dendrites [73]. A great majority of known VGKCs are located in CNS but only few of them proved the ability to serve as drug targets in disorders like epilepsy (KV7.2-KV7.5), psychosis (KV7), ataxia type I (KV1.1), multiple sclerosis (KV1.3). There is also growing evidence that auto-antibodies reactive to voltage-gated potassium channels play a pathogenic role in a broad spectrum of CNS disorders. Raised level of voltage-gated potassium channels antibodies (VGKCs-Ab) have been reported in cases of limbic encephalitis, while the improvement in neuropsychological functioning in some patients correlated with the fall in antibodies. Thus, a term autoimmune limbic encephalitis (ALE) was introduced, for indistinguishable from other encephalopathies. Limbic encephalitis is commonly associated with syndromes like episodic memory impairment, disorientation, seizures, hallucinations, and sleep disturbance [79]. Raised level of VGKCs-Ab was also confirmed in patients with long standing drug resistant epilepsy [80] and in case of adult-onset drug refractory seizure disorder [81].
Epilepsy
Voltage-gated potassium channels, because of their contribution to excitability of neurons, may serve as drug target in epilepsy. K+ currents occurring while opening the channels take part in reducing membrane excitability, thus VGKCs openers are of interest as antiepileptic drugs [82]. The especially interesting group of potassium channels while considering therapy of epilepsy is KV7 (KNCQ) family or sometimes even more specified KV7.2 and/or KV7.3. The members of KNCQ family, KV7.1 – KV7.5, are characteristic for different localizations in human organism, but only channels from KV7.2 to KV7.5 were found in brain (Table 5). KV7 channels in physiological condition are activated by membrane depolarization at subthreshold membrane potentials (from about -60 mV) and produce a sustained outward current at membrane voltages negative to the firing threshold of action potentials, thus act as efficient inhibitors of neuronal excitability [83, 84].
Table 5.
| Type of Potassium Channel α-subunit | Localization | Disease In Which Serves As Target | ||
|---|---|---|---|---|
| CNS | Subcellular | Other | ||
| KV7.1 | - | - | Heart, kidney, rectum, lung, placenta, cochlea | Arhytmias |
| KV7.2 | Brain, ganglia | Axons | Lung, testis, heart, eye, small intestine, breast | Epilepsy |
| KV7.3 | Brain, ganglia | Axons, neuronal somata, dendrites | Testis, retina, colon, eye | Epilepsy |
| KV7.4 | Brain | Cochlea, vestibular hair cells, placenta | Epilepsy Psychosis |
|
| KV7.5 | Brain, ganglia | Neuronal somata, dendrites | Skeletal muscle | Epilepsy |
KV7 channels were originally termed “M-channels” as all members of those family generated so called M-like currents when expressed in oocytes or cell lines. It was proved, that in native neurons, M-channels are composed of heteromeric KV7.2/KV7.3 subunits or homomeric KV7.2 subunits, probably with a contribution of KV7.5 [83].
Activity of KV7 channels may be modulated by means of several ligands of G-protein coupled receptors (GPCRs), which produce several signaling pathways (hydrolysis of PIP2; changes in local Ca2+ concentration; phosphorylation by protein kinases), which finally inhibit activity of potassium channels. Exact mechanism of that modulation is not completely understood. It was also proved that M-like currents are inhibited by activation of muscarinic receptors [84]. On the other hand, synthetic activators of KV7 seem to cause conformational changes leading to channel opening by direct binding to the protein [82].
Identification of KCNQ2 and KCNQ3 genes mutations as the molecular cause of benign familial neonatal seizures (BFNS; an autosomal dominant idiopathic epilepsy syndrome of newborns, characterized by unprovoked partial seizures typically beginning at the age of around three days), supported the position of those channels as drug targets in epilepsy. Those mutations are located in C-terminal cytoplasmic region, but missense mutations within the transmembrane have also been described [85, 86]. More evidence were found in animals. Watanabe et al. provided results from test in Kcnq2-knockout mice. Homozygotus mutant died within first postnatal day, while heterozygotus animals characterized normal behavior and morphology compared with wild-type mice. Further studies showed that Kcnq2+/- mice had much higher sensitivity to the chemoconvulsant – pentetrazole, [87]. Yang et al. gave evidence that mutation in Kcnq2 gene led to the decreased seizure threshold in electric-induced convulsions. Kncq2 mutation was also proved to be characteristic for Szt1 mice, which serve as genetic model of epilepsy [88]. Peters et al. showed that suppression of the M-currents in mice was the cause of spontaneous seizures and M-channels were found to be critical determinants of cellular and neuronal network excitability [89]. Pharmacological effectiveness in epilepsy was proved for KV7 channels activators (like retigabine described below). On the other hand, their blockers produced pro-epileptic side effects like tremors, thus their utility as therapeutics in other diseases is limited [83].
Psychosis
KV7 (KCNQ) channels may represent a potential target for the treatment of psychosis, as some of them (KNCQ2 and KNCQ4) are expressed postsynaptically in dopaminergic neurons of mesolimbic and nigrostriatal pathways (e.g. in the ventral tegmental area). Opening of those channels is expected to decrease neuronal excitability, which can affect psychotic symptoms believed to be associated with an increased excitability of dopaminergic cells in the mesencephalon. The evidence that dopamine D2 receptors are functionally coupled with KV7 channels in dopamine neuron was proved by means of co-expressed channels and receptors in Xenopus oocytes and human neuroblastoma cells [90]. In other studies, the firing of dopaminergic neurons recorded in rat mesencephalic slices was significantly inhibited in a concentration-dependent manner by retigabine and that compound completely blocked the excitatory effect of dopamine D2 autoreceptor antagonists, suggesting that the modulation of dopaminergic activity by D2 autoreceptors would involve the activation of KCNQ2 and/or KCNQ4 channels [91]. More evidence came from in vivo electrophysiological studies conducted in anesthetized rats, in which KV7 channels activators (e.g. retigabine) suppressed burst firing activity in the ventral tegmental area, whereas XE-991, a selective KNCQ blocker, induced opposite effect. In the conditioned avoidance response paradigm one of in vivo rat models predictive for antipsychotic activity, retigabine was found to inhibit avoidance responses, moreover that effect was blocked by coadministration of XE-991. Furthermore, in phencyclidine (PCP)-sensitized rats, considered as a model for schizophrenia, retigabine was found to significantly inhibit the hyperlocomotor response to a phencyclidine challenge [92].
Above findings combined with immunocytochemistry test, which revealed that KV7.4 is the major KV7 channel type expressed in dopaminergic neurons [91] support for the hypothesis that those channels may serve as drug target in psychosis and that their activators could become a new class of antipsychotic drugs.
Episodic Ataxia
Episodic ataxia type I (EA1) belongs to paroxysmal movement disorders. It is an autosomal-dominant disorder, characterized by intermittent cerebellar dysfunction with otherwise essentially normal brain function [93]. It was identified as a channelopathy connected with mutations in KV1.1 gene (KCNA1 in chromosome 12p13) [94]. A common clinical features associated with EA1 are: from CNS - occurrence of seizures, from peripheral nerves – neuromyotonia, but no connection with headache during acute episode was proved [95]. On the other hand, episodic ataxia type II is caused most often by the mutation in calcium channel gene CACNA1A, but potassium channel blocker – 4-amino pyridine, is one of the efficient treatment option [96]. Currently no VGKCs ligand is used in the therapy of EA1.
Multiple Sclerosis
Multiple sclerosis (MS) is a disease of central nervous system, of which myelin damage is one of the pathological issues. There are various theories about the etiology of MS including immunologic, environmental, genetic, and infectious factors [97]. Myelin damage has been shown to cause abnormal K+ currents, thus potassium channels are believed to play an important role in multiple sclerosis, as they are responsible for conductance failure in that condition. Potassium channels blockade reduced this ionic leakage and improved conductance. Moreover, some VGKCs blockers were successfully used in many in vitro studies and animal models of neurodegenerative disorders as well as in clinical trials. Voltage-gated sodium channels, and especially KV1.3, were connected with several autoimmune diseases including MS. KV1.3 are expressed among others in immune system cells and organs like thymus, spleen, macrophages, lymphocytes. Blockage of those receptors localized in T-lymphocytes proved to be an effective approach in managing autoimmune disorders [98].
Voltage-gated Potassium Channels Modulators
Voltage-gated potassium channels constitute a wide and hererogenous group thus no common binding pattern could not be determined. Modulators of different type potassium channels were recently reviewed by Wulff and Zhorov [99]. Here we would like to focus on selected VGKCs ligands and their utility in CNS disorders.
Retigabine (RTG; Ezogabine)
Retigabine (N-[2-amino-4-(4-fluorobenzylamino)-phenyl]carbamic acid ethyl ester) Fig. (20) represents a unique mechanism of action among AEDs which is enhancing of the activity of KV7 channels in central nervous system resulting in reduction of neuronal excitability. The other mechanism constitutes enhancing of γ-aminobutyric acid transmission but only at supra-therapeutic concentration (while tested in vitro) [100]. The compound showed broad anticonvulsant activity in several animal seizure models: MES, scMet, 6-Hz test, hippocampal-kindled rats, amygdala-kindled rats ,and many more, including some genetic animal models of epilepsy [100]. Moreover, it had antipsychotic effect in an animal model of schizophrenia and mania [92]. It also showed activity in rat models of neuropathic pain [37].
Fig. (20).
Chemical structure of retigabine.
Retigabine is thought to bind selectively to KV7.2 – KV7.5 channels, shifting the current–voltage curve to the left, which results that the channels open at more hyperpolarized membrane potentials [101]. The lack of RTG effect on KV7.1 may be due to inefficient binding, which, however, has not been proved [82]. It increased the rate of channels activation while tested in Xenopus oocytes expressing KCNQ2 [102]. Retigabine, at concentration 0.1 – 10 µM, affected CHO cells expressing KV7.2/KV7.3 heteromeric channel (CHO-KNCQ2/Q3) resulting in increasing M-like potassium currents and hyperpolarization of the cell membrane. Moreover, it shifted the voltage dependence of channel activation with an EC50 value of 1.6±0.3 µM [103]. The compound also significantly influenced properties of Xenopus oocytes membrane expressing recombinant human KV7.2/KV7.3, as well as channel itself. At the concentration of 10 µM RTG shifted both the activation threshold and voltage for half-activation by approximately 20 mV in the hyperpolarizing direction. It also affected kinetics of the channels, causing increased rate of activation and slowing deactivation. Membrane potential recordings showed that the compound caused a concentration-dependent hyperpolarization of the oocyte (IC50 of 5.2 µM) [102].
The prove that retigabine acts through activation of KV7 channels came also from in vivo animal studies. In two of them anticonvulsant efficacy was reduced by the selective KV7 blocker, XE-991.That happened in MES test in mice and in the rapid kindling model in rats [100]. Other test were performed in the Szt1 mouse model, in which functionality of the KV7.2 channel is impaired, because of C-terminal deletion in the Kcnq2 gene. Research showed that anticonvulsant potential of retigabine was then reduced [104]. It is worth to mention, that neuroprotective effect of RTG proved in some in vitro studies was not mediated by KV7.2 [100].
Systematic mutagenesis studies identified segment S5 as a binding site for retigabine, and more detailed a crucial tryptofan residue, which is specific for KV7.2 – KV7.5, while in KV7.1 there is leucine in corresponding position. Additional residues have also been found as affected by retigabine, like glycine residue in S6 [82].
Retigabine has recently been approved as adjunctive therapy in adults with partial-onset seizures [100].
ICA-27243
ICA-27243 (N-(6-chloro-pyridin-3-yl)-3,4-difluoro-ben zamide) Fig. (21), considered as a second-generation structure to retigabine, is a selective activator of KV7.2/7.3. It showed in in vivo animal tests a broad spectrum of anticonvulsant activity including the MES, scPTZ, 6 Hz and kindling models [22].
Fig. (21).
Chemical structure of ICA-27243.
ICA-27243 is a potent and selective activator of KV7.2/7.3 showing EC50=0.20±0.03 µM, determined in 86RB+-efflux assay conducted on recombinant human channels expressed in CHO cells. It the same test, EC50 for activation of KV7.4 was 7.1±0.1 µM and for KV7.3/7.5 was not able to be determined but estimated at about 10 µM. ICA-27243 shifted the voltage-dependent activation of KV7.2/7.3 to more hyperpolarized potentials in concentration-depen dent manner. The halfmaximal shift in the midpoint of the activation curve (ΔV1/2) was observed at 4.8±1.6 µM. The compound had no effect on GABAA-activated chloride channels, NaV1.2, or CaV channels.
Preclinical tests with ICA-27243 provided the evidence that KV7.2/7.3 activation alone is sufficient for broad-spectrum anticonvulsant activity in rodents. The finding that ICA-27243 can discriminate among different KV7 subtypes suggests that it may interact at a novel binding site on KV7.2 and/or KV7.3 channels that is not present in other KV7 channels. The binding site has anyway not yet been identified [105, 106].
4-Aminopyridine
4-Aminopyridine (4-AP, fampridine) Fig. (22) inhibits in dose-dependent manner fast voltage-gated potassium channels including KV1.1, KV1.2, KV1.4- KV1.7, KV3.1- KV3.3, KV4.1. It also increases Ca2+ influx at presynaptic endings.
Fig. (22).
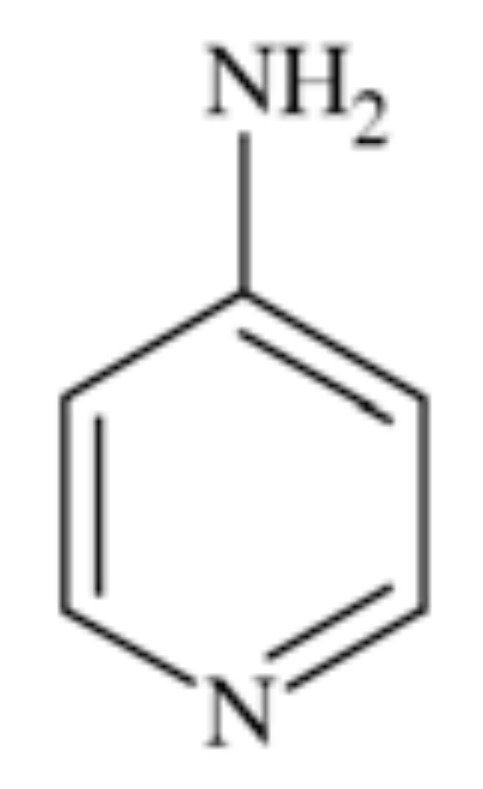
Chemical structure of 4-aminopyridine.
In in vitro experiments it proved to selectively inhibit so-called A-potassium current, which is characteristic for some members of KV. The sensitivity of different channels varied from micromolar to millimolar concentrations of 4-AP and in particular channel the sensitivity depended on its state (open or closed). It was proved that the compound more readily affected open than closed channels and did so from the cytoplasmic site and is not transferred with the pore [107]. KDs while inhibiting KV2.1 and KV3.1 expressed in Xenopus oocytes were 17 and 0.08 nM, respectively and the mechanism of action remained similar [108]. It also proved activity in several in vitro models of neuronal degeneration, significantly improving function of neurons [107].
4-Aminopyridine proved efficiency in few clinical trials in patients with multiple sclerosis [107]. The compound was also efficient in episodic ataxia type II, probably due to increasing the inhibitory influence of the Purkinje cells [96]. It was approved in January 2010 for patients with MS to improve their walking [97].
3. CALCIUM CHANNELS
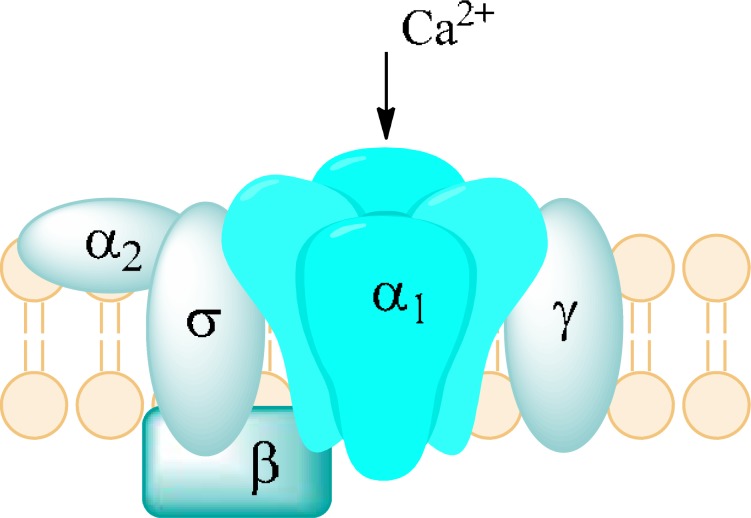
Calcium channels have been well known drug targets and still they are subject of intensive research. Lately, they have been reveiewed by Belardetti [109]. Voltage-gated calcium channels are present in membranes of excitable cells. They are opened during depolarization and this gives rise to an influx of Ca2+. Increase in concentration of this ions stimulates an ongoing depolarization by opening other voltage-gated ion channels. In addition they initiate a release of neurotransmitters [110]. Moreover, calcium ions act as a second messenger and activate enzymatic processes [111].
Calcium channels consist of two families: HVA (High Voltage-Activated) and LVA (Low Voltage-Activated). HVA family comprises L-, N-, P-, Q-, and R-type channels. They are heterotrimers of subunits α, β, and α2-δ. Subunit α forms a pore with ancillary subunit β. Subunit α2-δ forms a functional pore by linking with subunit α. LVA family consists of T-type channels which are monomers and are composed only of subunit α. LVA and HVA differ in function and electrophysiological activity. LVA requires a small amount of depolarization, close to resting membrane potential in order to be opened. In contrast to HVA, they are activated and inactivated very quickly. LVA also display low conductance and better resistance to blockers [112].
A role of Cav1 is to respond to depolarization by inducing intracellular processes. They occur in various sorts of tissues and are responsible for many intracellular activities. In skeletal, smooth muscles and myocytes an influx of Ca2+ leads to contraction. In neurons, Cav1 modulate genes’ transcription. Majority of them occur in a cell body and proximal part of dendrites to ease access for Ca2+ to nucleus. In addition they initiate release of neurotransmitters. This subtype of calcium channels have been a cardiovascular targets, however, due to their presence in neurons, they play some role in low-potassium triggered paralysis (CaV1.1), autism, depression, bipolar disorder, or schizophrenia (CaV1.2), and deafness (CaV1.3) [109].
Cav2 occur in presynaptic nerve terminals in most CNS synapses. Their main role is to mediate release of neurotransmitters to synapses. Depolarization results in an opening of Cav2 and an influx of Ca2+. Ions bind with proteins called synaptogamins, placed on a surface of synaptic vesicles which contain neurotransmitters. Then synaptic vesicles fuse with presynaptic membranes and neurotransmitter is released. The release of neurotransmitter occurs by exocytosis. After that the neurotransmitter binds to a postynaptic receptor, mediating an excitation [113]. Mutations of Cav2 channels can influence its functions. Changes in P/Q-type channels lead to appearance of absence epilepsy. By substitution particular amino acids, an activity of these channels are reduced. In consequence there is a diminished ability to excite neurons [114].
Cav3 mainly occur in neurons and the heart. In CNS, they are responsible for a process of dreaming and their mutation leads to appearance of absence epilepsy [114]. These channels are capable of activation when a membrane potential is close to the resting membrane potential -60mV. A small depolarization triggers an opening of channels and an influx of Ca2+. In consequence the depolarization continues by activation Na+ and Ca2+ channels. At the time of repolarization, these channels deactivate slowly (become closed) which allows to flow of a larger amount of Ca2+. In the end of discharge hyperpolarization appears, bringing about closure of inactivated calcium channels [112]. It is essential as depolarization is possible only when calcium channels are closed (deactivated) [115].
A feature of Cav3 channels is that they contribute to various models of excitability in neurons. There are 3 models:
-
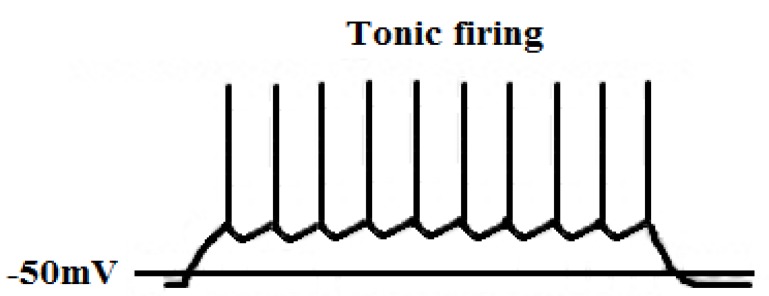
Tonic firing Fig. (24)
It is a single burst of action potential. If a cell is more depolarized than -60mV for 50 – 100 msec, calcium channels are inactivated, and the cell responds to an excitatory input in tonic mode [115, 116].
-
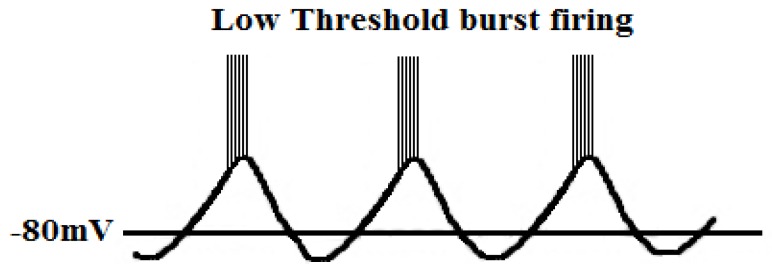
Low-threshold burst firing Fig. (25)
This type of firing occurs when a membrane is hyperpolarized below -65mV for >50 - 100 msec. The hyperpolarization is essential in order to make calcium channels shift from being inactivated to being closed. During depolarization calcium channels are activated and elicit a low-threshold Ca2+ spike (LTS) with a burst of 1–10 action potentials. Because of influx of calcium ions a membrane potential diminish to -40mV. Consequently it leads to an activation of voltage-gated sodium channels [115, 116].
-
Slow oscillations (<1Hz)
T-type calcium channels produce a large window current in the membrane potential’s range of -60 to -40 mV and are always open and do not inactivate. It is possible because there is an overlap between activation, inactivation, deactivation within this range of membrane potential. It enables a sustained influx of calcium ions and plays an important role in the process of dreaming [117].
Fig. (24).
Tonic firing of neurons.
Fig. (25).
Low-threshold burst firing of neurons.
Drug Dependency
L-type calcium channels play a necessary role in sensitization to psychostimulants, seen in an animal model of incentive/motivational aspect of cocaine and amphetamine dependency. Their block in the nucleus accumbens prevents cocaine reinstatement and self-administration in rats [120]. It would be interesting if clinical evidence was provided for use of a CaV1 channel antagonist in treatment of drug dependency.
Parkinson’s Disease
There is both molecular and clinical evidence that CaV1.3 activity contributes to cell death within substantia nigra, leading to deficiency of dopaminergic neurons in this area, which is a known cause for Parkinson’s Disease. From the clinical point of view, cardiovascular patients treated by L-type calcium blockers penetrating blood-brain barrier significantly reduced risk of PD in patients [109].
It has been noticed that mutation in CaV2.1 gene causes familial hemiplegic migraine [121]. Contrary to this fact, CaV2.1 mice are severely ataxic and display absence seizures, followed by death at the age of 4 weeks [122]. Both these facts discourage from targetting CaV2.1 in drug discovery process.
Pain
N-type (CaV2.2) calcium channels blockers have been a prime target for analgesics as they are expressed in the dorsal horn of the spinal cord. They transmit pain signals from afferent neurons to second order neurons projecting to higher CNS centers. They also are a target for descending activation of noradrenergic pathways by norepinephrine and for inhibition by opioid pathways [123]. The first analgesic drugs to be registered as antagonists of CaV2.2 was ziconotide, a peptide derivative of ω-conotoxin-MVIIA. Its toxicity, which probably resulted from small selectivity to the targets, narrowed the use of these compounds. Intrathecal administration was also a considerable disadvantage so far [109]. However, there are some efforts in drug discovery for analgesics acting by antagonism of CaV2.2 (see below).
Absence Epilepsy
Absence epilepsy is associated with generalized epileptic seizures which concerns 10% of population suffering from epilepsy. It is characterized by loss or impaired consciousness, which lasts up to 30 seconds. During an attack of epilepsy the patient displays absent impression on his face and does not respond. It is accompanied by quivering of the eyelids, turning pale and relaxation of facial muscles. Patient experiences amnesia and is not aware of having seizures.
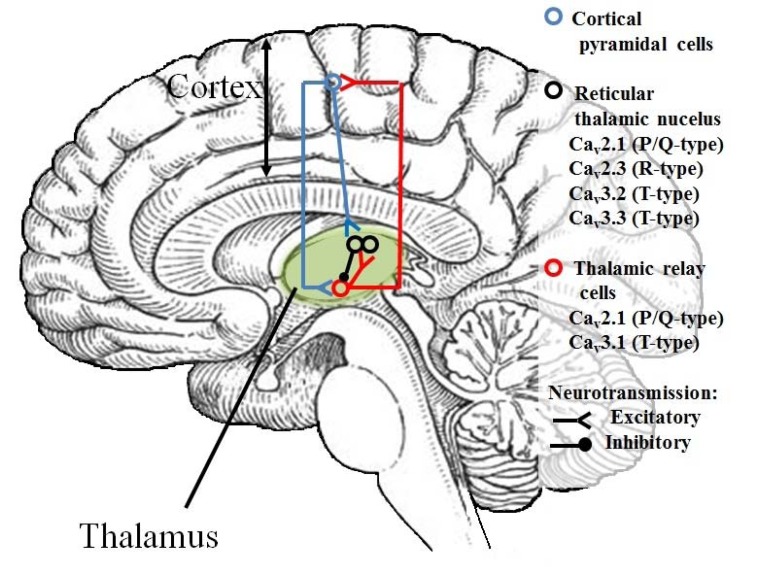
The cause of absence epilepsy is abnormal hyperexcitability of neurons in a thalamus-cortex circuitry. In EEG records occur oscillation consisted of multiple spike-waves and slow-waves at more than 2.5 Hz, predominantly 3–6 Hz. The thalamus-cortex circuitry comprises:
Thalamic relay cells of the ventroposterolateral and ventroposteromedial thalamic region
Pyramidal neurons in the cortex (layers III-VI)
Reticular thalamic nucleus (RTN) [124]
CaV2 and CaV3 types of calcium channels are present in thalamic relay cells and RTN Fig. (26). Thus in both structures two models of neurons’ excitability are possible – tonic and burst firing. The tonic firing mode is characteristic of stages of high vigilance. The burst firing is characteristic of dreaming process and some pathological states like absence epilepsy [124].
Fig. (26).
Thalamus-cortex circuitry.
In a properly working circuitry, thalamic relay cells exert glutamatergic projections on pyramidal neurons in layers III and IV. These pyramidal neurons project to pyramidal neurons in layer V and VI of the cortex. Then they re-innervate thalamic relay cells with glutamate as a neurotransmitter. Both thalamic relay cells and cortex are linked to reticular thalamic nucleus by glutamatergic neurons. RTN comprises only GABAergic interneurons. It is linked with thalamus and projects inhibitory neurotransmitter. GABA leads to hyperpolarization of memebrane of thalamic relay cells and this allows calcium channels to shift from inactivated state to deactivated state. Consequently calcium channels can be opened and the burst firing occurs [124]. In EEG record there are characteristic oscillations. Spikes represent an excitability of neurons during glutamatergic projections. Waves appear when RTN projects inhibitory neurotransmitter [125].
During absence epilepsy seizures, spike-wave discharges are observed in the cortex [126]. These discharges lead to an excitability of reticular thalamic nucleus. RTN projects GABA to thalamic relay cells and causes sustained inhibition of thalamic relay cells. This enables calcium channels to become deactivated enough. As a result, burst firing does not occur. It is claimed that inhibition of thalamus causes loss of consciousness during absence seizures [127].
Knock-out of CaV2.1 calcium channels gives rise not only to seizures, but also to ataxia, while gain of function mutations may be related with familial migraine [109].
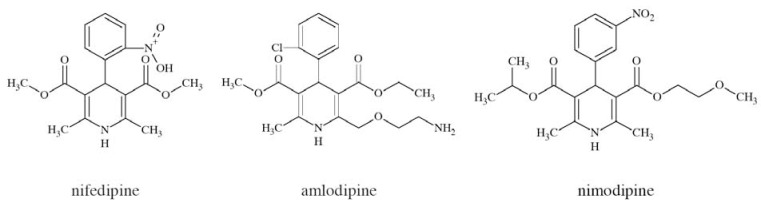
Nifedipine
Nifedipine, amlodipine, and nimodipine Fig. (27) are dihydropyridine derivatives. They have been developed as cardiovascular drugs, blocking L-type (CaV1) calcium channels. Amlodipine blocks CaV1.2 with IC50=100 nM and CaV2.1 with IC50=10 µM. Many drugs from the group of dihydropyridines crossing blood-brain barrier, including nifedipine and nimodipine (and excluding amlodipine), proved efficacy in preventing Parkinson’s disease in over 25% of patients [128]. However, in an animal study, nifedipine lowered anticonvulsant activity of topiramate in mice (it doubled its ED50). The authors suggest some pharmacodynamic interaction, e.g. L-type calcium channel binding of topiramate, rather than pharmacokinetic or any other interaction between nifedipine and topiramate [129]. These facts concerning derivatives of dihydropyridine are premises for drug discovery of more selective derivatives vs. CaV1 channels subtypes.
Fig. (27).
Chemical structures of nifedipine, amlodipine, and nimodipine.
Pregabalin
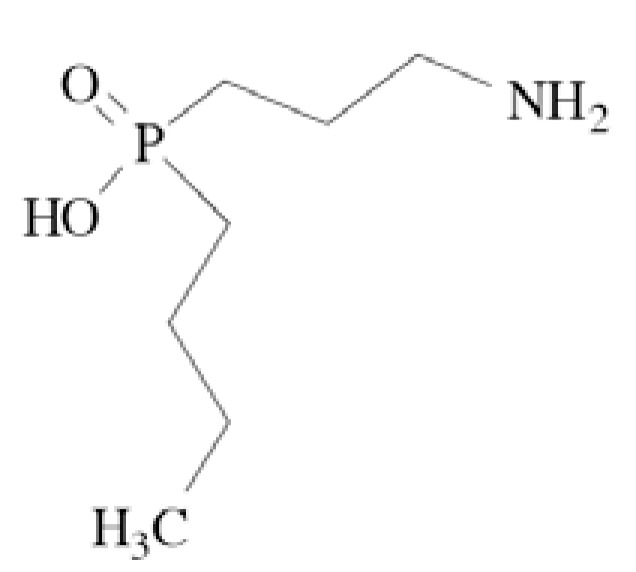
Pregabalin (CI-1008, (S)-3-aminomethyl-5-methylhexa noic acid) Fig. (28), is a derivative of GABA. It binds to the α2δ subunit of the CaV2 calcium channels. Moreover, it increases GABA concentration in neuronal tissues and enhances activity of glutamate decarboxylase. It is a well-known antiepileptic drug, exhibiting both anticonvulsant and analgesic activity in central and peripheral neuropathic pain. [130-132].
Fig. (28).
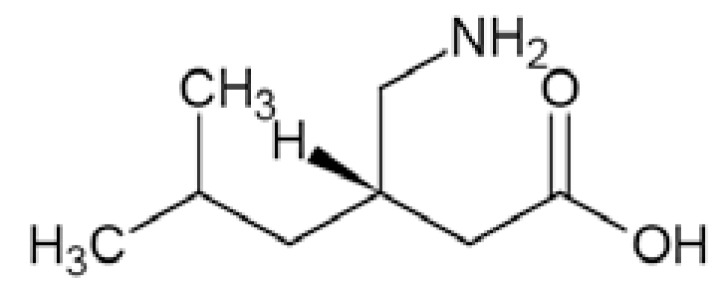
Chemical structure of pregabalin.
Gabapentin
Gabapentin (1-(aminomethyl)cyclohexaneacetic acid) Fig. (29) is a GABA derivative. It exhibits binding to α2δ subunit, causing inhibition of CaV2 calcium channels. The additional mechanism is related with the above, i.e. it inhibits release of glutamate, relieving neuronal hyperexcitability. It is a well known antiepileptic drug with proved preclinical and clinical efficacy in epilepsy and pain [133, 134].
Fig. (29).
Chemical structure of gabapentin.
Ziconotide
Ziconotide, SNX-111, this antagonist of CaV2.2 is registered for treatment of chronic pain, although it is administered intrathecally [118]. It is a synthetic analog of ω-conotoxin-MVIIA.
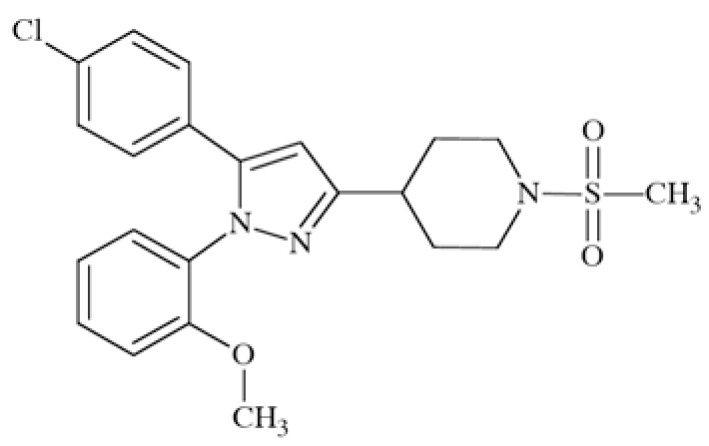
Pyrazolylpiperidine Derivative
This compound (Fig. (30) has been synthesized for screening vs. CaV2.2 channel, taking into account the need for an orally administered and metabolically stable anticonvulsant with a similar mechanism of action to ziconotide. It is active in a chronic constriction injury model for neuropathic pain (rats, i.p.) at 30 mg/kg b.w. (cold hypersensitivity) and in carrageenan-induced inflammatory pain (thermal hypersensitivity) [119].
Fig. (30).
Chemical structure of 1-(2-methoxyphenyl)-3-[4-(1-methylsulfonyl)piperidinyl]-5-(4-chlorophenyl)pyrazol.
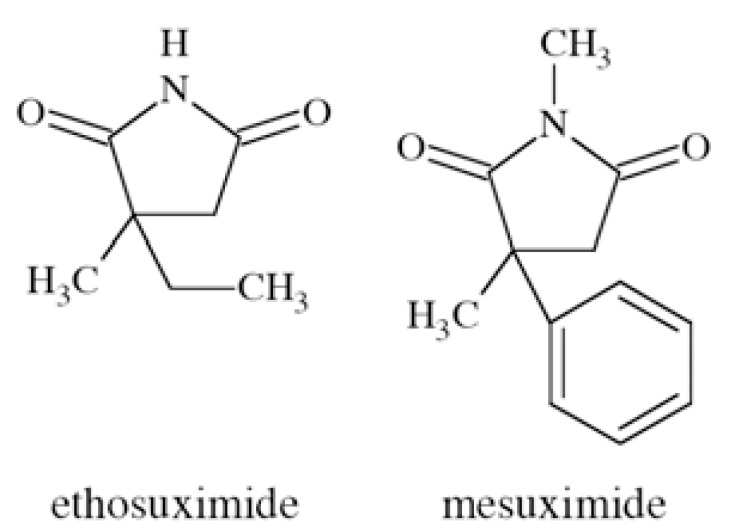
Ethosuximide and Mesuximide
Ethosuximide (3-ethyl-3-methylpyrrolidine-2,5-dione) and mesuximide (1,3-dimethyl-3-phenylpyrrolidine-2,5-dione) Fig. (31) are well established anticonvulsants, active in absence epilepsy. They show affinities towards CaV3 receptors. They are inactive in grand mal seizure models in rodents (e.g. MES), and active in petit mal epilepsy model – ethosuximide in scMET test with ED50=167 mg/kg b.w. (rats, p.o.) [56].
Fig. (31).
Chemical structures of ethosuximide and mesuximide.
4. P2X RECEPTORS
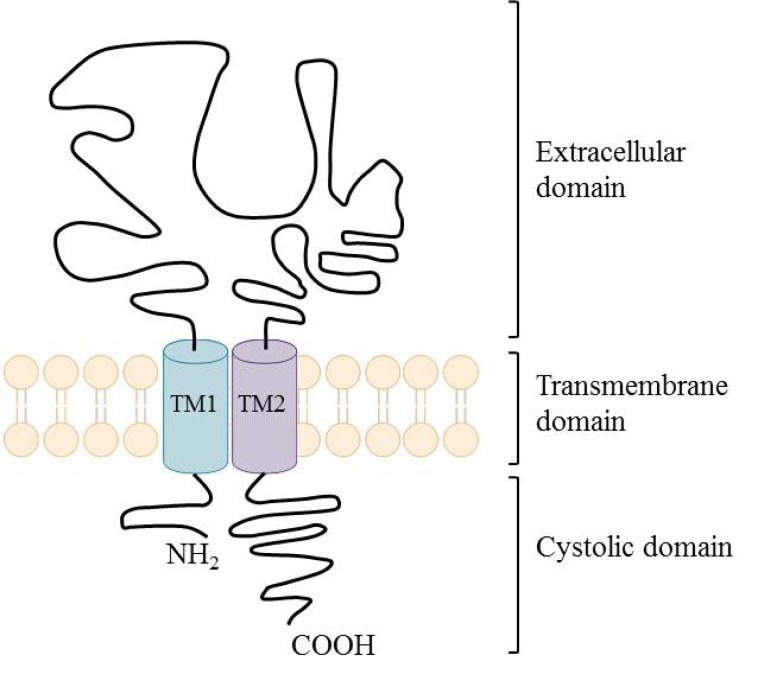
Extracellular adenosine 5’-triphosphate (ATP) is an important excitatory transmitter both in the peripheral and central nervous system [135]. P2X receptors are a distinct family of ligand-gated membrane ion channels activated by extracellular ATP. Human P2X receptors are a family of seven isoforms designated P2X1 to P2X7. The isoforms share 26–54% sequence identity and each subunit is between 379 and 595 amino acids in length [136-138]. P2X1, P2X2, P2X3, P2X4, P2X5, and P2X7 isoforms form functional homomeric receptors, however heteromeric assemblies such as P2X2/3 and P2X1/5 channels are also possible [139, 140]. P2X7 receptors are unique among the P2X receptor family because they fail to form heteromeric forms and are activated by high ATP concentrations (>100 µM) [141].
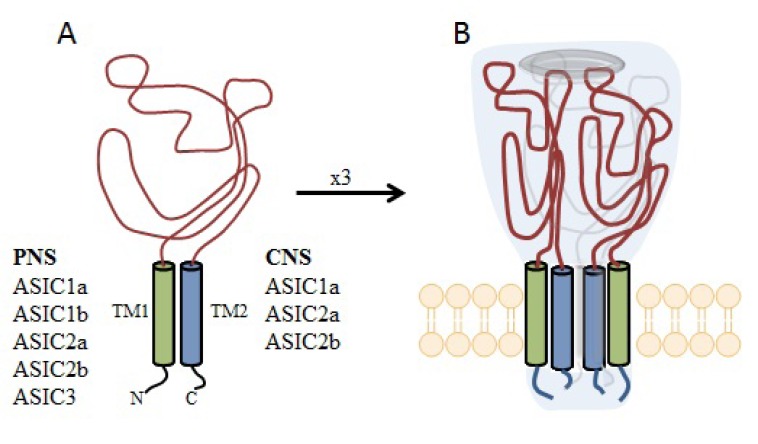
P2X receptors (Fig. (32) are organized as trimers [142] with the subunits arranged in a “head-to-tail” order [143]. All have two transmembrane domains (TM1 and TM2), an extracellular ligand binding loop that contains ten conserved cysteine residues that form disulphide bonds and intracellular amino and carboxy termini [139, 144]. Both TM1 and TM2 are thought to line an integral ion pore [145]. Thus, the resulting channel is composed of six transmembrane domains, two from each of three subunits, the structure that is unique in the ion channel literature.
Fig. (32).
Schematic structure of P2X receptor.
The primary agonist of all P2X receptors is ATP and at least 3 molecules of ATP are bound to the extracellular portions of open P2X channels [143]. P2X receptors undergo conformational changes leading to the opening of a cationic pore within milliseconds of binding ATP [146]. P2X receptors display significant calcium permeability. Moreover, they show sodium and potassium permeability [136] and some of them are also permeable to chloride [147]. Aside from ATP, most P2X receptors are also activated by diadenosine polyphosphates or related dinucleotides and some nucleoside triphosphates [140, 148].
P2X receptors are widely distributed in cell types of nearly every origin, including neuronal, epithelial, immune and muscular. They are involved in actions such as synaptic transmission in the peripheral and central nervous systems, contraction of smooth muscle, platelet aggregation, macrophage activation, cell death and immunomodulation [149, 150].
P2X receptors are widely expressed on central and peripheral neurons - with P2X2, P2X3, P2X4 and P2X7 being the most abundant in the nervous system [151]. P2X receptors have been explored as therapeutic targets, e.g., for chronic inflammatory diseases and pain. In particular, P2X3 and P2X7 receptor antagonists have been developed and exhibited antinociceptive or antiinflammatory activity in animal models of these diseases [152].
Apart from neuropathic pain conditions it has also been reported that the activation of P2X receptors may be involved in the process of excitotoxic neuronal injury caused by stroke [153]. It was proved that following ischemia especially P2X7 receptors are upregulated on neurons and glial cells in rat cerebral cortex [154]. The evidence from prior studies, however, showed that the deletion of P2X7 receptors (knock-out mice) and/or treatment with KN62, the antagonist of P2X7 receptors had minimal effect on ischemic cell death [155]. Another study showed that P2X receptors are involved in the mechanisms sustaining cell death evoked by metabolism impairment [156]. Additionally, both P2X4 and P2X7 receptors expressed on microglia might be involved in cortical damage produced by oxygen and/or glucose deprivation [157]. Several earlier studies have confirmed the idea as P2 receptors antagonists were able to abolish the cell death fate of primary neurons exposed to excessive glutamate [158], to hypoglycemia or chemical hypoxia [159, 160] and to serum/ potassium deprivation [161].
P2X7-mediated signaling is also implicated in neurodegenerative processes observed in CNS diseases, such as Parkinson’s and Alzheimer’s diseases and multiple sclerosis [162-166]. In case of Parkinson’s disease the release of ATP from disrupted cells might cause cell death in neighbouring cells expressing P2X7 receptors, consequently necrotic volume is increased [167]. Moreover, P2X7 receptors are specifically upregulated in the brain of patients with Alzheimer’s disease (AD) and in animal models of the disease [168].
In two different transgenic models of Huntington’s disease changes in P2X receptor-mediated neurotransmission in cortico-striatal projections have been identified. In addition, it was shown that P2X receptor antagonists might have potential as neuroleptic agents as microinjection of ATP analogues into the prepiriform cortex was able to induce generalized motor seizures [169]. Purinergic mechanisms and specific P2X receptor subtypes have also been involved in the pathogenesis of clinical conditions such as amyotrophic lateral sclerosis [170] and epilepsy [171, 172].
Suramin
Suramin (Fig. (33) the best known antagonist of P2X receptors [173] was assessed for its neuroprotective potential in a model of experimental stroke in rats. In the study focal brain ischemia was induced by unilateral occlusion and transection of the middle cerebral artery (MCAT) and bilateral occlusion of the common carotid arteries (CCA). It was shown that suramin at a dose of 100 mg/kg significantly decreased both infarct and edema volume. Thus, the compound is an effective pretreatment neuroprotective agent [153].
Fig. (33).
Chemical structure of suramin.
CE-224535
Pfizer’s compound CE-224535 (Fig. (34), a P2X7 receptor antagonist was recently evaluated in the clinic for the treatment of rheumatoid arthritis [174]. The compound is presently studied for the treatment of other conditions, such as pain and Alzheimer´ s disease [175].
Fig. (34).
Chemical structure of CE-224535.
5. NMDA RECEPTORS
N-methyl-D-aspartate (NMDA) receptors (Fig. (35) are a subtype of ionotropic glutamate receptors widely distributed in CNS. NMDA receptors play a crucial role in synaptic transmission, neural plasticity and neurodegeneration [176, 177], thus may serve as potential CNS therapeutic targets [178].
Fig. (35).
Schematic structure of NMDA receptor.
NMDA receptors are hetero-oligomeric ligand-gated cation channels comprising four NMDA subtypes: NR1, NR2, and NR3 [179]. The subtypes include the ubiquitously expressed NR1 subunit; a family of four distinct NR2 subunits (A, B, C, and D); and more rarely two NR3 subunits (NR3A and NR3B) [180, 181]. Most NMDA receptors are believed to assemble as tetramers, associating two NR1 and two NR2 subunits in a ‘dimer of dimers’ quaternary architecture [181-183]. The subunits differ in their permeation and block by divalent ions and sensitivity to various endogenous and exogenous ligands. It was discovered that NR1 and NR2A subunits are the most common and NR2D are the rarest. More heterogeneous receptors exist in CNS, comprising multiple types of NR1 and/or NR2 subunit in one receptor complex. Thus, NMDA receptors including NR1/NR2B/ NR2A and NR1/NR2B/NR2D can be distinguished in many areas of the CNS [184, 185].
NMDA receptors’ intrinsic ion channel is permeable to monovalent cations such as sodium and potassium, and bivalent ones including calcium. In order to open the channel pore by NMDA receptors the simultaneous binding of glutamate (neurotransmitter role) and the co-agonist – glycine (or D-serine, modulators) is needed. The glutamate binding site is situated on the NR2 subunit and the glycine binding site is situated on the NR1 subunit. NMDA receptor activation takes place after presynaptic glutamate release and with appropriate glycine concentration in the synaptic cleft [177, 178, 182].
In general, NMDA receptors are found in many but not all cerebral cortex neurons and some cortical astrocytes [178, 186] and play major roles in both physiological and pathological states of the CNS. Inappropriate activation of NMDA receptors has been stated in the etiology of various disorders.
Stroke
The first clinical indication considered for NMDA receptor antagonists was stroke. In such ischemic or hypoxic conditions the increased amount of glutamate is released into the synaptic clefts, thus the overstimulation of the NMDA receptor is observed [187]. It was proved that the blockade of NMDA receptors resulted in neuroprotective effect in animal models of stroke [188]. Also in traumatic brain injury (TBI) glutamate is released in large quantities into the extracellular fluid by neurons and glial cells. This was confirmed in both animal models and human studies [189, 190]. Thus, the application of NMDA antagonists might be of therapeutic benefit.
NMDA receptors via calcium fluxes are able to down-regulate the stream signaling pathways leading to short-term and long-term neuronal changes [191]. Thus, alternation in these receptors causes deficits in learning and memory [192].
Schizophrenia
Additionally, both genetic and pharmacological studies showed that glutamatergic dysfunction, particularly with involvement of NMDA receptors plays a vital role in the pathophysiology of schizophrenia [193, 194]. It was proved that mRNA encoding the NR2B subunit was increased in hippocampal and cortical regions in schizophrenia [195, 196]. Arias et al. [197] suggested that hyperphosphorylation of NR2B-containing receptors in vivo may cause increase of sensitivity to the endogenous transmitter, and induction of neuronal hyperexcitability and epilepsy.
Parkinson’s Disease
In the pathophysiology of Parkinson’s disease (PD) over-reactivity of the glutamatergic pathway may be stated. Thus, the manipulation of the glutamatergic system may show anti-parkinsonian effects [198]. Various studies indicated that the NR2B subunit is a important molecular component in the etiology of PD and therefore should be considered as a potential therapeutic target for the treatment of the disease [199-203].
Huntington’s Disease
Another severe disease related with NMDA receptors is Huntington’s disease (HD). It was confirmed that NR2B subtype NMDA receptors activation triggers the selective degeneration of the medium spinal striatal neurons in HD [204]. Mutant huntingtin may increase the number of functional NR1/NR2B-type receptors at the cell surface [205]. Moreover, the expression of polyglutamine-expanded huntingtin results in NMDA receptors sensitization and promotes neuronal apoptosis triggered by glutamate [206].
Gavestinel
Gavestinel (Fig. (36) a selective antagonist at the glycine site of the NMDA receptor and one of the indole derivatives was evaluated in various animal models of stroke. In the middle cerebral artery occlusion (MCAO) model of focal ischemia in rats administration of the compound (3 mg/kg i.v.) up to 6 h from occlusion resulted in a significant reduction of the infarct volume measured histologically 24 h later [207]. Moreover, gavestinel was characterized in terms of in vivo potency by inhibition of convulsions induced by N-methyl-D-aspartate in mice. The compound inhibited convulsions induced by NMDA when administered by both i.v. and p.o. routes (ED50= 0.06 and 6 mg/kg, respectively) [187]. Also magnetic resonance imaging confirmed the potential neuroprotective activity of gavestinel when administered either before or up to 6 h after ischemia [208]. Gavestinel was well tolerated in early human studies and showed no apparent significant CNS or hemodynamic effects [209, 210]. In a randomised, double-blind, placebo-controlled trial to test whether gavestinel could improve functional outcome after acute stroke in human beings the treatment with the compound within 6 h of acute ischemic stroke did not improve the outcome [211]. Additionally, in clinical trials of this compound in patients with intracerebral hemorrhage gavestinel was not of substantial benefit or harm to patients [212].
Fig. (36).
Chemical structure of gavestinel.
Selfotel
The competitive glutamate antagonist selfotel (CGS 19755) (Fig. (37) in preclinical animal tests produced anticonvulsant, anxiolytic and anti-ischemic effects [213]. The compound reduced neuronal damage in animal models of stroke [214-216]. In the rat model of global cerebral ischemia selfotel treatment (dose ranged from 10 to 30 mg/kg intraperitoneally) resulted in neuroprotection that was observed when administration was not delayed more than 30 minutes after the onset of ischemia [215]. The neuroprotective effect of selfotel was also confirmed in rats with occluded left middle cerebral and common carotid arteries. Intravenous infusion of selfotel (10 mg/kg) reduced infarct volume [214]. The compound was also effective in a rabbit model of reversible CNS ischemia [216]. Grotta et al. [215] evaluated the safety and tolerability of selfotel in patients with acute ischemic stroke. The study showed that although adverse effects are related with the treatment in a dose-dependent fashion, a single intravenous dose of 1.5 mg/kg is safe and tolerable with adverse experiences that are controllable. In Phase III efficacy trials conducted in patients with severe traumatic brain injury the compound (four doses of 5 mg/kg each, 24 h apart) failed to demonstrate a satisfactory risk/benefit ratio. One explanation is that the compound which is competitive with glutamate at the receptor may not influence events at the receptor site [217].
Fig. (37).
Chemical structure of selfotel.
Aptiganel
Aptiganel (Fig. 38) acts as a noncompetitive NMDA antagonist [218]. In a rat model of human ischemic stroke, aptiganel hydrochloride administered intravenously was able to reduce (by 40-70%) the volume of brain damage and the accompanying neurological dysfunction. Moreover, in a rat model of temporary focal ischemia the compound was able to protect both cerebral gray matter and white matter from ischemic injury [219]. The study on controlled cortical impact injury of the left temporoparietal cortex in rats demonstrated that the compound decreased contusion volume and hemispheric swelling as well as water content [220]. Neuroprotective activity of aptiganel HCl was also confirmed in controlled cortical impact injury (CCII) in rats. Compound’s administration was associated with decrease contusion volume, less hemispheric swelling, a lower intracranial pressure and increased cerebral perfusion pressure [221].
Fig. (38).
Chemical structure of aptiganel.
In a dose-ranging, placebo-controlled, phase I study Muir et al., [1994] confirmed that aptiganel’s pharmacokinetic effects are favourable for a potential neuroprotective therapy.
In human volunteers, however, dose-limiting effects of aptiganel were blood pressure increases and an excess of CNS. In another study both the safety and tolerability of aptiganel were assessed in patients with acute ischemic stroke. It was demonstrated that a 4.5-mg intravenous bolus of aptiganel HCl followed by infusion of 0.75 mg/h for 12 h is a tolerable dose that can produce plasma drug concentrations shown to be neuroprotective in animal models [222]. Albers et al. [223] determined whether aptiganel improves the clinical outcome in acute ischemic stroke patients. The compound administered in the tested doses within 6 h of symptom onset is not effective in the treatment of patients with acute ischemic stroke and may be harmful.
As non-selective NMDA receptor antagonists have impeding adverse side effects, attention has been directed towards compounds capable of modulating selectively certain subtypes of these receptors. In this field the NR2B-selective type of non-competitive antagonists has a strong potential, showing both analgesic and neuroprotective activity together with slight side effects [178, 224]. Many NR1/NR2B antagonists, including ifenprodil, eliprodil and the selective and potent congeners, traxoprodil and Ro 25-6981, offer promise in preclinical models of ischemia [225-229].
Ifenprodil
Ifenprodil (Fig. (39) NR2B selective NMDAR antagonist, when administered as a perfusion (0.3-3 mg/kg i.v.) over 3 hours after occlusion of the feline middle cerebral artery decreased the infarcted tissue volume (measured 4 days after occlusion) in a dose-related manner. At the highest dose a 42% reduction of infarcted volume was observed, substantially in cortical tissue [225]. The interaction of compound Ro 25-6981 (Fig. (39) with NMDA receptors proved that Ro 25–6981was more potent than ifenprodil [230].
Fig. (39).
Chemical structures of ifenprodil and Ro 25-6981.
Eliprodil
The neuroprotective activity of eliprodil (Fig. (40), NMDA receptor antagonist acting at the polyamine modulatory site, in brain trauma was examined in a rat model of lateral fluid-percussion brain injury. Eliprodil (10 mg/kg i.p.), reduced by 60% the volume of cortical damage. Moreover, the neuroprotective activity of the compound in experimental brain trauma using neuropathology was documented [226]. Eliprodil reduces lesion volume following traumatic brain injury [231].
Fig. (40).
Chemical structure of eliprodil.
Taxoprodil
The neuroprotective effect of traxoprodil (Fig. (41) - a competitive NMDA antagonist at NR2B binding subunit, was evaluated in a rat subdural hematoma (SDH) model. The drug was infused 30 min after induction of SDH. The reductions of ischemic brain damage achieved by traxoprodil was 29-37% [228]. Moreover, this drug exhibits a 69% reduction in infarct size in comparison to controls [232]. In humans it was well tolerated, penetrated the brain, and showed improvement in brain injury [232]. In a double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of traxoprodil in patients with a mild or moderate traumatic brain injury it had no psychotropic effects and was well-tolerated in patients [232]. Moreover, the compound had direct antiparkinsonian actions in rodents (decreased haloperidol-induced catalepsy with ED50=0.5 mg/kg) and monkeys (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MP TP)- treated monkeys, traxoprodil (1 mg/kg)) [233]. Moreover, no side effects were apparent at any dose of traxoprodil. The drug also reduced the maximum severity of levodopa-induced dyskinesia approximately by 30% but it did not improve Parkinsonism. Side effects were dose-related dissociation and amnesia [234], although in another study the drug was well tolerated, although no efficacy was seen in severe TBI [235].
Fig. (41).
Chemical structure of traxoprodil.
Ro 63-1908
Ro 63-1908 (Fig. (42) is a potent and highly selective antagonist of the NR2B subtype of NMDA receptors, active versus sound-induced seizures (ED50=4.5 mg/kg i.p., DBA/2 mice). It also inhibited NMDA-induced seizures with an ED50 =2.31 mg/kg i.v. In addition, Ro 63-1908 gave a dose-related neuroprotective effect against cortical damage in a model of permanent focal ischemia. Maximum protection of 39% was seen at a plasma concentration of 450 ng/ml. There were no adverse cardiovascular or CNS side-effects observed at these active doses [236].
Fig. (42).
Chemical structure of Ro 63-1908.
Amantadine
Amantadine (Fig. (43), a low affinity NMDA antagonist represents an approach for treating Huntington’s disease [237]. Amantadine is also used as an adjuvant therapy in Parkinson’s disease. The compound exhibited efficacious effects in Parkinson's disease patients suffering from dyskinesias [238].
Fig. (43).
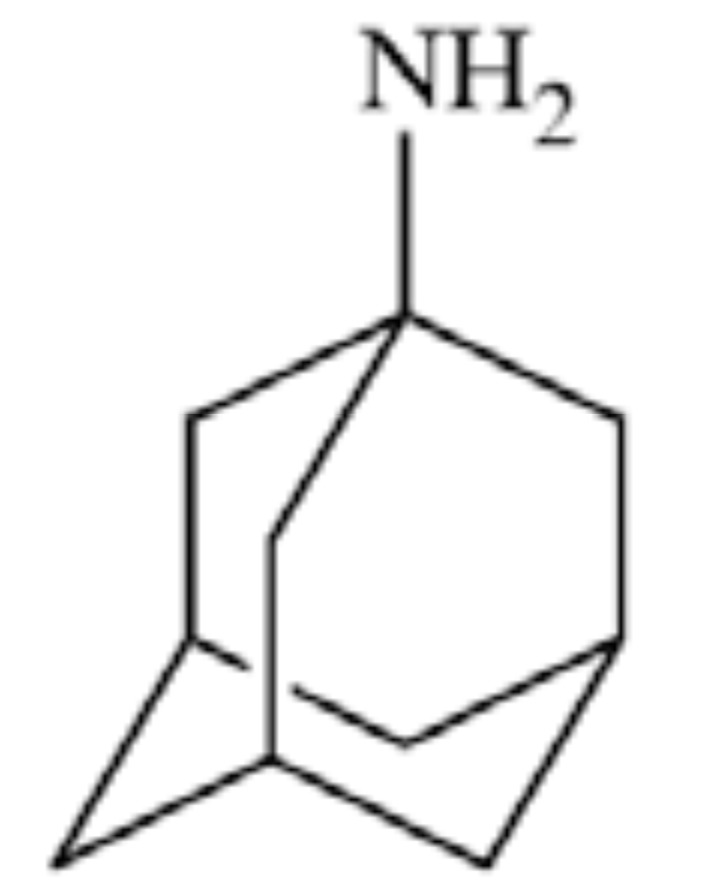
Chemical structure of amantadine.
Memantine
Memantine Fig. (44) is another low affinity NMDA receptor antagonist. The compound was tested for non-motor features of Parkinson’s disease and despite good tolerability the specific measures of sleepiness, fatigue, depression, and attention did not significantly improve [239]. It decreases probable REM sleep behavior disorder in patients with Parkinson's disease dementia [240]. It leads to improvements in cognitive functions, stabilization of motor impairments, and decreases in the severity of mental disorders, especially in patients with hyperhomocysteinemia [241, 242].
Fig. (44).
Chemical structure of memantine.
Memantine has also been studied as a treatment for Alzheimer’s disease (AD). It was associated with slowing of right hippocampal atrophy, and with improvement on one test of executive functioning as well as a test of confrontation naming ability [243].
Dexanabinol
Dexanabinol Fig. (45) belongs to non-competitive NMDA antagonists. Treated patients achieved significantly better intracranial pressure/cerebral perfusion pressure control without jeopardizing blood pressure. A trend toward faster and better neurologic outcome was also observed [244]. Further studies (phase III randomized, placebo-controlled clinical trial) showed that dexanabinol is safe, but not efficacious in treatment of TBI [245].
Fig. (45).
Chemical structure of dexanabinol.
Remacemide
Remacemide (Fig. (46) belongs to low affinity non-competitive NMDA antagonists. The drug exerts anticonvulsant activity both in various animal seizure models and in clinical studies. In addition, it seems to provide neuroprotection [246]. The antiepileptic effects include activity in genetic absence epilepsy rats from Strasbourg (GAERS), and in an audiogenic rat model. The study showed the effects of remacemide in tonic/clonic seizure, which was the first target of the drug, and confirm the effect of the anticonvulsant on absence seizures [247]. Moreover, no serious adverse events were observed [248]. Pretreatment with remacemide has a moderate neuroprotective effect against status epilepticus-induced neuronal damage [249].
Fig. (46).
Chemical structure of remacemide.
Remacemide hydrochloride might also improve symptoms of Parkinson’s disease by modulating glutamatergic overactivity in the basal ganglia or slow worsening by decreasing excitotoxicity. In rodent and primate models of Parkinson’s disease systemically administered compound has antiparkinsonian activity and low potential for side effects. It can be a safe and tolerable adjunct to dopaminergic therapy for patients with PD and motor fluctuations [250].
Neramexane
Neramexane (Fig. (47) similarly to memantine, is an open-channel NMDA receptor blocker. The compound displays a similar pharmacokinetic and comparable clinical tolerability. It enhanced a long-term spatial (hippocampus-dependent) memory in adult rats, thus it was concluded that may be helpful in treatment of dementia [251].
Fig. (47).
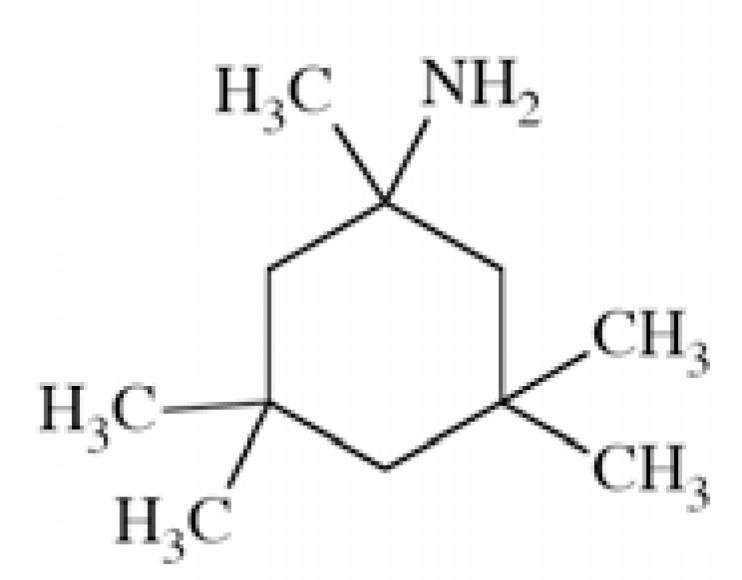
Chemical structure of neramexane.
Dimebon
Dimebon (Fig. (48) – an NMDA blocker at a site distinct from memantine, was initially classified as an antihistaminic drug. Systemic administration improves learning in animals with experimental model of Alzheimer's disease [252]. A randomised, double-blind, placebo-controlled study showed that dimebon was safe, well tolerated, and significantly improved the clinical course of patients with mild-to-moderate Alzheimer's disease [253].
Fig. (48).
Chemical structure of dimebon.
D-Cycloserine
The antibiotic D-cycloserine (Fig. (49) is a partial glycine site agonist that improves glutaminergic activity in schizophrenia. This preliminary evidence suggests that D-cycloserine may improve negative symptoms and cognitive deficits over a narrow dose range when added to conventional antipsychotic agents [254]. Studies confirmed potential role of D-cycloserine in the treatment of negative symptoms in schizophrenia. It was stated however that the degree of symptom reduction may be modest [255]. Preliminary studies with once-weekly administration showed that D-cycloserine may play potential new role in schizophrenia by demonstrating benefit for negative symptoms, memory consolidation, and facilitation of cognitive behavioral therapy for delusions [256].
Fig. (49).
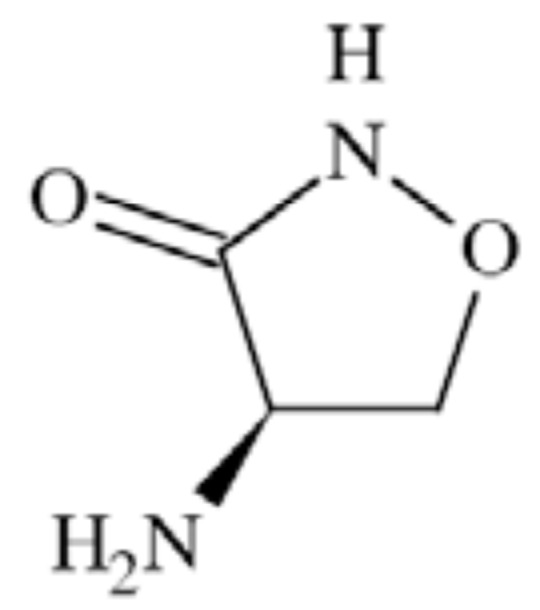
Chemical structure of cycloserine.
Felbamate
Felbamate (Fig. (50) a non-competitive NMDA antagonist, showed a broad anticonvulsant profile similar to valproic acid when tested in experimental models. The compound blocks NMDA receptors but fails to exhibit the neurobehavioral toxicity characteristic of other NMDA receptor antagonists. It exhibits modest selectivity for NMDA receptors composed of NR1a/NR2B subunits. This selectivity could, in part, account for the more favorable clinical profile of felbamate in comparison with NMDA receptor antagonists that do not show subunit selectivity [257]. The studies indicated that felbamate is an effective anticonvulsant drug in DBA/2 mice [258]. The in vitro antiepileptic activity of the novel anticonvulsant drug felbamate was tested in rat hippocampal slices on the CA1 epileptiform bursting induced by different chemical epileptogenic agents.
Fig. (50).
Chemical structure of felbamate.
6. ACID-SENSING ION CHANNELS
Acid-sensing ionic channels (ASICs) are almost ubiquitous in the mammalian nervous system, both at the periphery and in the brain [259]. They belong to H+-gated subgroup of the degenerin/epithelial Na+ channel (DEG/ENaC) family of cation channels [260]. In 1981, Krishtal and Pidoplichko described for the first time a receptor for H+ that carried inward Na+ currents in mammalian sensory neurons [261, 262]. The receptor was first cloned from the rat brain in 1997, by the group of Lazdunski [263]. Then, receptor was crystallized in 2007 [264].
ASICs appear to be the simplest of ligand-gated channels [263]. They are preferentially permeable to Na+, but to a lesser extent can also conduct other cations (e.g. Ca2+, K+, Li+ and H+). Individual subunits consist of two transmembrane domains, relatively short intracellular carboxy and amino termini and a large, cysteine-rich, extracellular domain [265, 260]. Investigation of the ASIC2a subunit showed that the pre-TM2 region is essential for pH sensitivity and gating [266]. ASICs are expressed in neurons and assemble into homomultimeric and heteromultimeric complexes. It is not known precisely how many subunits are required to form a channel. Some researchers, by the analogy with other DEG/ENaC channels suggested four to nine ASIC subunits [267] More recent studies concerning crystallization of a chicken ASIC1 in low-pH proved homotrimeric structure, where each subunit of the chalice-shaped homotrimer is composed of short amino and carboxy termini, two transmembrane helices, a bound chloride ion and a disulphide-rich, multidomain extracellular region enriched in acidic residues and carboxyl-carboxylate pairs within 3 Å, suggesting that at least one carboxyl group bears a proton. Between the acidic residues and the transmembrane pore there is a disulphide-rich ‘thumb’ domain poised to couple the binding of protons to the opening of the ion channel, thus demonstrating that proton activation involves long-range conformational changes [264, 268].
Individually expressed subunits form homomultimeric channels, whereas coexpression of 2 or more subunits allows formation of heteromultimeric channels. Surprisingly enough, ASIC2b does not form H+-gated channels when expressed alone but alters current properties when coexpressed with other ASICs. Coexpressing various subunits produces heteromultimers, with H+-gated currents revealing characteristics that in some cases reflect ‘average’ properties of contributing subunits and in other cases properties that lie outside the range of the individual subunits (e.g. rate of desensitization). Subunit composition also alters ion selectivity; for example ASIC1a homomultimeric complexes are permeable to Ca2+, whereas ASIC2-containing heteromultimeric complexes are not [260].
In heterologous cells, ASIC homomultimeric channels vary in terms of activation and desensitization kinetics as well as pH sensitivity. For example, in one study, the pH values required for half-maximal activation of murine ASIC subunits were 6.8 (ASIC1a), 6.2 (ASIC1b), 4.9 (ASIC2a) and 6.6 (ASIC3) [269, 270].
Activation and desensitization of ASICS occurs in response to extracellular acidification. The channels formed by the ASIC proteins are proton-gated cation channels, opening rapidly in the presence of extracellular acid. Opening of the ion pore could be accomplished by a concerted global rotation of the TM2 helices. Their fundamental role appears to be acid transduction, converting an acidic extracellular environment into a cellular signaling event, by allowing for the conduction of cations into the cell [271].
The underlying mechanism coupling proton binding in the extracellular region to pore gating is unknown, nevertheless the role of any ion channel in a disease process can often be distilled down into inappropriately increased or decreased activity.
In the peripheral nervous system (PNS), ASIC function usually as sensory receptors, for example in specialized nerve endings. They were found in neurons innervating skin [265, 273, 274], heart [275], intestines – especially ASIC3 [276], muscles [277], bones [278], eyes [279, 280], ears [281] and taste buds [282].
There are two suppositions about localization of ASICs in the brain. One of them locate ASICs at synapses but most likely they are situated in the post-synaptic membrane or throughout the neuron [283].
ASICs are associated with four genes (ASIC1, -2, -3, and -4) that encode for 6 subunits: ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4 [260]. When expressed in heterologous systems, all subunits except ASIC2b and ASIC4 can form functional homomultimeric channels with distinct electrophysiological and pharmacological properties. When co-expressed, the heteromultimeric channels may demonstrate characteristics dramatically different from their homomeric counterparts [284]. ASIC1 and -2; both have alternative splice transcripts: ASIC1a, -1b, -2a, and -2b. ASIC4 has not been shown to produce or modulate proton-evoked current and remains the least understood subunit.
The pluralism of nomenclature introduced by different groups of researchers can be confusing and should be systematized (Table 1). The terms involving ‘acid-sensing ionic channel’ (ASIC) could seem to be most advantageous. Sometimes, names indicate also the tissue specificity (e.g. the dorsal root ASIC, DRASIC) but it is still unclear whether H+ are, in fact, natural ligands of these peculiar receptor channels. ASICs belong to the family of amiloride-sensitive ENaC/DEG receptor channels, which determines their synonyms as ‘X-NaC’. The ‘NaC’ in this abbreviation means ‘sodium channel’ and ‘X’ is related to a basic feature of the protein (DEG – degenerin, B – brain, L – liver, I – intestine refers to the members of this family that are degenerin proteins). It is easy to anticipate that the ASIC nomenclature is still not final [259].
Anxiety
The primary function of the nervous system is to generate flexible behavior in a changing environment. The acid-sensing ion channel 1a (ASIC1a) is abundantly expressed in the amygdala complex and other brain regions associated with fear [283, 286]. Other work has shown that the amygdala, a collection of nuclei buried in the temporal lobe of the brain, is essential for both innate and learned fear in rodents and humans [300]. In 2009, group of Ziemann proved that ASIC1a in amygdala take part in the appearance of fear by detecting a decrease in extracellular pH and triggering cationic currents. They report that inhalation of carbon dioxide (CO2) – simulating suffocation - decreases the pH in the amygdala and yields freezing behavior in mice. Moreover, genetic deletion or pharmacological disruption of ASIC1a channel reduces fear associated with CO2 inhalation. This finding provides new insight into the mechanisms by which amygdala neurons detect threat and suggests a new role for amygdale chemosensation in learned fear [287].
Depression
Depression remains one of the most disabling medical diseases but the molecular pathways underlying depression are poorly understood, and existing treatments are too often ineffective. One of the brain part, the amygdala play a central role in mood regulation. ASIC1a is expressed widely in the central and peripheral nervous systems and is robustly expressed in structures associated with mood including the amygdale. ASIC1a is required for acid-evoked currents in central neurons, where it contributes to synaptic plasticity and in the regulation of dendritic spines. Some preclinical screenings showed that genetically disrupting ASIC1a in mice produced antidepressant-like effects in the forced swim test, the tail suspension test, and following unpredictable mild stress. Pharmacologically inhibiting ASIC1a also had antidepressant-like effects in the forced swim test. The effects of ASIC1a disruption in the forced swim test were independent of and additive to those of several commonly used antidepressants [289].
Epilepsy
There are some interesting insights in how ASICs interact epilepsy and seizures. One of them showed that acid-sensing ion channel 1a (ASIC1a) activation enhances neuronal excitability in the hippocampus and neocortex, indicating that ASIC1a might play a role in the generation and maintenance of epileptic seizures [292]. Other publication mentioned that ASICs’ inhibitor – amiloride neither altered the threshold for electroconvulsions, nor protected the animals against MES-induced seizures in mice but amiloride administered 75 and 100 mg/kg, i.p., 120 min prior to the test significantly enhanced the anticonvulsant effects of carbamazepine, oxcarbamazepine and phenobarbital, by reducing their ED50 values in the MES test [291]. On the other hand, there was also established that seizures reduce extracellular pH. Extracellular acidosis, in turn, activates ASIC1a, which terminates seizure activity [290].
Glioma
Gliomas are primary brain tumors with a complex biology characterized by antigenic and genomic heterogeneity and a propensity for invasion into normal brain tissue. High grade glioma cells possess a voltage-independent, amiloride-inhibitable, inward Na-current. This current does not exist in normal astrocytes or low grade tumor cells. Inhibition of this conductance decreases glioma growth and cell migration making it a potential therapeutic target. Some results have shown that the acid-sensing ion channels (ASICs especially ASIC2), are part of this current pathway [293].
Huntington’s Disease
Huntington's disease (HD) is a neurodegenerative genetic disorder that affects muscle coordination and leads to cognitive decline and psychiatric problems. Some HD models have been studied, where amiloride derivative benzamil (Ben), a chemical agent used to rescue acid-sensing ion channel (ASIC)-dependent acidotoxicity, was examined whether chronic acidosis is an important part of the HD pathomechanism and whether these drugs could be used as novel therapeutic agents. Ben markedly reduced the huntingtin-polyglutamine (htt-polyQ) aggregation in an inducible cellular system, and the therapeutic value of Ben was successfully recapitulated in the animal model of HD [294].
Multiple Sclerosis
Multiple sclerosis is one of the most common causes of progressive disability affecting young people. Multiple sclerosis is a neuroinflammatory disease associated with axonal degeneration. The neuronally expressed, proton-gated acid-sensing ion channel-1 (ASIC1) is permeable to Na+ and Ca2+, and excessive accumulation of these ions is associated with axonal degeneration. ASIC1 are hypothesized to contributes to axonal degeneration in inflammatory lesions of the central nervous system (CNS). In this context, ASIC1’s blockers could provide neuroprotection in multiple sclerosis [295]. According to some researches, blocking ASIC1 with amiloride protected both myelin and neurons from damage in the acute model, and when given either at disease onset or, more clinically relevant, at first relapse, ameliorated disability in mice with chronic-relapsing experimental autoimmune encephalomyelitis [288].
Pain
Tissue acidosis is a common feature of many painful conditions. Protons are indeed among the first factors released by injured tissues, inducing a local pH decreasing that depolarizes peripheral free terminals of nociceptors and leads to pain. ASICs are excitatory cation channels directly gated by extracellular protons that are expressed in the nervous system. At the peripheral level, ASIC3 is important for inflammatory pain. At the central level, ASIC1a is largely expressed in spinal cord neurons where it has been proposed to participate in the processing of noxious stimuli and in central sensitization. Blocking ASIC1a in the spinal cord also produces a potent analgesia. Targeting ASIC channels at different levels of the nervous system could therefore be an interesting strategy for the relief of pain [272]. Pharmacological block of ASIC1a activity by the PcTx1 injected intrathecally produces strong analgesic effects in several rodent models of acute and chronic pain. The genetic knockdown of ASIC1a expression by intrathecal delivery of antisense oligonucleotides produces similar analgesic effects [296, 297].
Amiloride
In the great majority of studies, amiloride has been used to block ASIC, but is a weak blocker, especially in contrast to its affinity for ENaCs (10–100-fold higher).
Aminoglycosides (AGs)
Aminoglycosides (AGs) are a group of antibiotics whose structure is based on aminocyclitol rings interconnected by glycosidic bonds. Some studies showed that AGs may constitute a valuable tool for the modulation of ASICs, and may be a feasible starting point for the development of new molecules structurally related to the AGs but with a greater affinity, specificity, and selectivity for the different ASIC subunits [306].
APETx2
APETx2 is a novel peptide toxin isolated from sea anemone venom, which selectively inhibits homomeric ASIC3 channels as well as the ASIC1+3, ASIC1b+3, and ASIC2b+3 heteromers. APETx2 is a basic peptide (pI = 9.59) of 42 amino acids, cross-linked by three disulfide bridges. The discovery of a toxin with ASIC3-blocking activity is of major significance given the substantial amount of evidence now implicating this channel in the transduction of acid-induced pain and hyperalgesia [303].
A-317567
A-317567 is a novel non-amiloride ASIC blocker with distinct mode of action and improved potency both in vitro and in vivo over amiloride. A-317567 blockes ASIC-like currents in cultured dorsal root ganglion neurons [301] and cultured cortical neurons [289]. While further evaluation of the mechanism of action of A-317567 is required to fully understand its mode of action, this compound will provide a new tool for investigating ASIC biology, physiology, and pathophysiology [301].
Diaminazene
Aromatic diamidines (Fig. 55) are DNA-binding agents and have long been used in the treatment of leishmaniasis, trypanosomiasis, pneumocystis pneumonia and babesiosis. Moreover, some aromatic diamidines are used as skin-care and baby products and others have potential to suppress tumor growth or to treat malaria. The application of aromatic diamidines is not limited to anti-parasitics. Recently, it was found that aromatic diamidines such as pentamidine, hydroxystilbamidine, and diminazene potently block ASICs [305]. Each of these compounds inhibited ASIC currents, indicating that the linker in each of these diamidines can be variable, while the amidine group linked with an aromatic moiety appears to be essential. Among them, diminazene blocks ASICs with an IC50 of 0.3 μM, being the most potent small molecule inhibitor of ASICs. Moreover, diminazene inhibits sensory neuron-specific ASIC1b more strongly than any other ASIC subunits. 3μM diminazene almost completely blocks ASIC1b [311].
Fig. (55).
Chemical structures of aromatic diamidines [305].
Nafamostat
Nafamostat (Futhan, FUT-175) is a synthetic, competitive, reversible serineprotease inhibitor. Therefore, nafamostat functions as an anti-inflammation and anti-coagulation agent. It is clinically used, mainly in the East Asia, for the treatment of disseminated intravascular coagulation, acute pancreatitis, and as an anticoagulant in extracorporeal circulation. Nafamostat, like aromatic diamidines, is also a highly polarized, linear di-cationic agent governed by an amidine group at one terminus and a guanidine group at the other. Recently, nafamostat blocks ASICs heterogeneously expressed in Xenopus oocytes with an IC50 of 2–70 μM depending on the ASIC subtype [307].
Nonsteroid Anti-inflammatory Drugs (NSAIDs)
Nonsteroid anti-inflammatory drugs (NSAIDs) (Fig. (57) are major drugs against inflammation and pain. They are well known inhibitors of cyclooxygenases (COXs). However, many studies indicate that they may also act on other targets such as ASICs. There were proposed that the two effects, i.e., direct channel block and inhibition of inflammation-induced ASIC expression, play an important role in the antinociceptive effects of NSAIDs in addition to their well known effects on COXs and more particularly in case of inflammation [304].
Fig. (57).
Chemical structures of some NSAIDs.
PcTx1
The first selective inhibitor of ASIC1a was Psalmo-toxin 1 (PcTx1). It was purified from the venom of the tarantula Psalmopoeus cambridge [308]. PcTx1 is a 40 amino acid peptide crosslinked by three disulfide bridges, it has a molecular mass of 4689.40 Da.
PcTx1 inhibit similarly homomeric ASIC1a channels in various cell expression systems with a very high affinity (see Table 9). PcTx1 inhibits ASIC1a by increasing its apparent affinity for H+. PcTx1 affinity for ASIC1a depends on extracellular pH and is the highest in pH=7.4. However, the mechanism by which PcTx1 increases the apparent H+ affinity remains unclear [312]. PcTx1 also interacts with ASIC1b, a splice variant of ASIC1a. However, PcTx1 does not inhibit ASIC1b but promotes its opening; under slightly acidic conditions, PcTx1 behaves like an agonist for ASIC1b [313]. At the molecular level, it was suggested that the ion pore structure, constituted by trans-membrane M1 and M2 segments and intracellular pre-M1 and post-M2 regions (Fig. (58), are not directly involved in either the PcTx1 binding site or in its mechanism of inhibition. Amiloride, a known pore blocker of ENaC/DEG/ASIC channels, does not inhibit PcTx1 binding, which also supports this conclusion. PcTx1 binds principally on both cysteine-rich domains I and II (CRDI and CRDII) of the extracellular loop; the post-M1 and pre-M2 regions, although not involved in the binding site, are crucial for the ability of PcTx1 to inhibit ASIC1a current by modification of channel gating; the linker domain between CRDI and CRDII is important for their correct spatial positioning to form the PcTx1 binding site [310].
Table 9.
Binding Affinities of Known ASIC Ligands
| Ligand | Subunit | IC50 |
|---|---|---|
|
| ||
| A-317567 [301] | ASIC1 | 2.0 µM |
| ASIC2 | 29.1 µM | |
| ASIC3 | 9.5 µM | |
| Amiloride [263, 301-302] | ASIC1a | 10 µM |
| ASIC1b | 21 µM | |
| ASIC2a | 0.15 µM | |
| ASIC3 | 37.2 µM | |
| ASIC3 | 63.0 µM | |
| APETx2 (Sea anemone toxin) [303] | ASIC3 | 63.0 nM |
| ASIC1a+3 | 2.0 µM | |
| ASIC1b+3 | 0.9 µM | |
| ASIC2b+3 | 117.0 nM | |
| Acetylsalicylic acid [304] | ASIC3 | 260 µM |
| DAPI [305] | ASIC in hippocampal neurons | 2.8 µM |
| Diclofenac [304] | ASIC3 | 92 µM |
| Diminazene [305] | ASIC in hippocampal neurons | 0.3 µM |
| Flurbiprofen [304] | ASIC1a | 350 µM |
| Gentamycin [306] | ASIC in dorsal root ganglion neurons | 44 µM |
| Hydroxystilbamidine [305] | ASIC in hippocampal neurons | 1.5 µM |
| Ibuprofen [304] | ASIC1a | 350 µM |
| Nafamostat [307] | ASIC depending on the subunit | 2-70 µM |
| Neomycin [306] | ASIC in dorsal root ganglion neurons | 32 µM |
| PcTx-1 (Tarantula toxin, psalmotoxin 1) [308-310] | ASIC1a | 0.9 nM |
| Pentamidine [305] | ASIC in hippocampal neurons | 38 µM |
| Synthalin [305] | ASIC in hippocampal neurons | 29 µM |
Fig. (58).
PcTx1 binding site on ASIC1a channel.
7. TRANSIENT RECEPTOR POTENTIAL (TRP) CATION CHANNELS
The superfamily of transient receptor potential (TRP) cation channels function as cellular sensors of various extra- and intracellular environmental changes. The first member of TRP channels was identifed in 1969 (by Cosens and Manning) in Drosophila mutants that displayed a transient response to light [314]. The family of transient receptor potential canonical (TRPC) proteins was the first group of TRP homologs cloned in mammals after discovery of the TRP protein in Drosophila [315].
The TRP channels comprises of five families (TRPA, TRPC, TRPM, TRPN and TRPV) and two subfamilies (TRPML and TRPP), which share several key properties: they are nonselective cation channels and most have six transmembrane domains [316, 317]. They are also divided into subtypes (Table 10) [318].
Table 10.
Classification of TRP Channels
| Type | Name | Subtype | |
|---|---|---|---|
| Families | TRPA | Ancyrin | TRPA1 |
| TRPC | Canonical | TRPC1-7 | |
| TRPM | Melastatin | TRP1-8 | |
| TRPN | No mechanorecepor potential | ||
| TRPV | Vanilloid | TRP1-6 | |
| Subfamilies | TRPP | Policystin | TRPP1-3, 5 |
| TRPML | Mucolipin | TRPML1-3 |
It was determined that, TRPs are tetrameric cation channels [319]. Each channel subunit contains six transmembrane segments (S1–S6) and a pore-forming region formed by a loop between the S5–S6 segments. Both the amino (N) terminus and the carboxyl (C) terminus are located intracellularly. Most of the TRP channels have an N-terminal ankyrin repeating domain and a C-terminal TRP domain. The long N and C termini of TRPs also contain several additional regulatory domains which are relatively conserved in most of the TRPs (Fig. (59) [314].
Fig. (59).
Schematic structure of TRP channels [320].
TRPA (ankyrin) family has one member: TRPA1 which contributes to the detection of intense mechanical stimuli. It has a characteristic large number of ankyrin protein repeats, thought to act as a spring and intracellular anchor, which may contribute to mechanosensory transduction. It responds to a range of environmental irritants e.g. formaline, extracts of mustard, cinnamon, garlic, oregano [321-323]. TRPA1 is localised in gastro-oesophageal vagal afferents, splanchnic colonic afferents and pelvic colonic afferents [316].
There are seven members in this family (TRPC1–7). The TRPC channels formed by homo- or heteromeric TRPC proteins are Ca2+-permeable nonselective cation channels. According to the similarity in amino acid sequence, the mammalian TRPCs can be classified into four subgroups: TRPC1, TRPC2, TRPC3/6/7, and TRPC4/5, while the TRPC2 is a pseudogene in primates. The TRPC proteins have six transmembrane domains, and both N- and C-termini of these proteins are intracellular, suggesting that these channels can be regulated by intra cellular signaling molecules [324]. These channels can be activated in various cell types by G-protein-coupled receptors (GPCR) and receptor tyrosine kinases (RTK) through a phospholipase C (PLC)-dependent mechanism. Therefore, TRPC channels may act as a sensor for environmental cues. In mammals, they were found in brain, heart, testis, ovaries, lung, retina, endothelia and adrenal glands. They partake in multiple physiological functions such as neuronal excitability in Purkinje cells, acrosome reaction in sperm cells, pheromone response, vasorelaxation and neurotransmitter release. They represent an important Ca2+ source for cells and have also been associated with Ca2+ oscillations [325].
TRPM subunits are localized in dorsal root ganglion neurons, taste buds, gut epithelium, brain, kidney, heart, prostate, intestine, liver and lung [325, 326]. TRPM family is now widely researched because it mediates the cooling and soothing sensation of e.g. menthol and eucalyptol. In this context, TRPM8 may be critical in alleviating pain [316].
The TRPN family has not been detected in mammals, but is present in Drosophila, zebrafish and worm. It has been proposed to be sensitive to mechanical stimuli and is expressed in mechanosensory neurons of the ear and eye [327].
The TRPV, also called vanilloid family, is probably the best known subfamily of TRP channels, notably TRPV1 which is activated by the vanilloid chilli extract capsaicin. They are localized in neurons innervating gut, colon, stomach, skin, urinary bladder, spleen, liver, placenta, kidney, lung and testis [317, 325, 316].
The polycystin, or TRPP family has four members, TRPP1, 2, 3 and 5. Their name is derived from an association between a TRPP mutation and polycystic kidney disease. They play roles in developmental guidance and ciliary function. They recently received interest in connection with a role in acid sensing [316].
The mucolipin, or TRPML family has three members, one of which is associated with mucolipidosis. They have an intracellular function putatively in vesicle formation [316].
Alzheimer’s Disease
Cognitive impairment and emotional disturbances in Alzheimer's disease (AD) result from the degeneration of synapses and neuronal death in the limbic system and associated regions of the cerebral cortex. Some indications suggest a role for TRPC6 in Alzheimer’s disease. Mutations in the presenilin genes are linked to the development of early-onset Alzheimer’s and transient expression of presenilin mutants (N141I, M239V) along with TRPC6 in human embryonic kidney - 293 cells results in a strong inhibition of agonist (1-oleoyl-2-acetyl-sn-glycerol (OAG) and angiotensin II (Ang II)) induced Ca2+ entry. Interestingly, the loss of function PS mutant D263A augments the activity of TRPC6 [328, 329].
Bipolar Disorder
The pathophysiology of bipolar disorder has been connected to a variety of TRP channels. Findings of Goedding and coworkers implicate the calcium-permeable TRPM2 and canonical TRPC3 in the pathogenesis of bipolar disorder (BD). These channels are involved in calcium and oxidative stress signaling, both of which are disrupted in BD. Thus, authors sought to determine if these channels were differentially affected by oxidative stress in cell lines of BD patient origin using B lymphoblast cell lines from bipolar I disorder (BD-I) patients, the oxidative stressor and TRPM2 and TRPC3 respective activators H2O2 and OAG. Data support an important role for TRPM2 and TRPC3 in sensing and responding to oxidative stress and in transducing oxidative stress signaling to intracellular calcium homeostasis and cellular stress responses, all of which have been implicated in the pathophysiology of BD [330]. It is also worth mentioning in connection with depressive mental disorders that leptin, which is connected to TRPC1 (and probably also TRPC3), has antidepressant effects and that impaired leptin production contributes to depression-like phenotypes in a rat model [331].
Ischemic Stroke
It is known that restoring extracellular Ca2+ following a period of low Ca2+ concentrations paradoxically causes an increase in intracellular Ca2+ levels that can lead to cell death. The mystery of this ‘Ca2+ paradox’ is made more intriguing by observations that lowering concentrations of extracellular Ca2+ and/or Mg2+ paradoxically enhances the entry of Ca2+ into hippocampal neurons. Some research concluded that extracellular Ca2+ and Mg2+ are important regulators of intracellular Ca2+, through their modulation of TRPM7 and potentially other non-selective cation channels such as TRPM2 [332, 333].
Parkinson’s Disease
1-Methy-4-phenylpyridium (MPP1) induces Parkinson disease-like syndromes. When applied to human dopaminergic SH-SY5Y neuroblastoma cells, MPP1 causes decreased expression and plasma membrane localization of TRPC1. In an opposite manner, TRPC1 overexpression reduces the neurotoxicity of MPP1. Some studies showed that activation of TRPC1 by thapsigargin or carbachol decreased MPP1 neurotoxicity Thus TRPC1 may execute a neuroprotective role in dopaminergic neurons. Furthermore, TRPC1 may inhibit degenerative apoptotic signaling to provide neuroprotection against Parkinson’s disease-inducing agents [334, 335].
Schizophrenia
Schizophrenia is a chronic, debilitating psychiatric disorder that afflicts people of all races and carries a life-time risk of the order of 1%. Schizophrenia is accompanied by morphological changes in the brain that include enlarged ventricles, a reduced cortical thickness, but an increased prefrontal neuron density. Patients also exhibit reduced pain sensitivity and a diminished niacin skin flare response. The latter symptoms hint towards a defect in TRPV1-expressing afferent nerve fibers. Direct evidence for links between schizophrenia and TRP channels is lacking. However, several aspects of the pathophysiology of the disorder point to a possible involvement of TRP channels. Links between TRP channels and schizophrenia with respect to neurodevelopment, dopaminergic and cannabinoid systems, thermoregulation, and sensory processes, are still discussed. Investigation of these links holds the prospect of a new understanding of schizophrenia with resultant therapeutic advances [336, 337].
Traumatic Brain Injury (TBI)
Traumatic brain injury classically results from mechanical injury induced by physical impact or a rapid change in acceleration/deceleration of the head. TBI can quickly lead to dysfunction of the microvessel endothelial cells in brain, including disruption of blood–brain barrier (BBB). It is now evident that elevation of cytosolic calcium levels [Ca2+]i can compromise the BBB integrity, however the mechanism by which mechanical injury can produce a [Ca2+]i increase in brain endothelial cells is unclear. Ca2+ is a ubiquitous second messenger that plays key roles in the regulation of cellular processes such as gene expression, secretion and apoptosis. Ca2+ channels are of particular interest in cell proliferation because of the profound anti-proliferative effect seen when extracellular Ca2+ is removed. Evidence from studies of many cell types indicates that Ca2+ entry mechanisms have an essential role in this effect. The concentration of Ca2+ is carefully controlled through the regulation of a variety of membrane channels and pumps including TRP channels [338]. It was stated that blockade of TRPC1 and TRPP2 both by chemical inhibitors (amiloride, LOE908) and by siRNA knockdown assays directed at TRPC1 and TRPP2 expression abolished the injury transient [Ca2+]i increase. Taking into account these fact it can be stated that mechanical injury of brain endothelial cells induces a rapid influx of calcium, mediated by TRPC1 and TRPP2 channels [339].
8. GABA RECEPTORS
GABA (γ-aminobutyric acid) is the most abundant inhibitory neurotransmitter found in the mammalian CNS, where about 20% of all neurons are GABAergic [342]. GABA acts by binding to two different types of receptors, ionotropic receptors (GABAA and GABAc) and metabotropic receptors (GABAB). Two GABA receptors subfamilies GABAA and GABAC differ in their ability to form endogenous heteromeric and homomeric receptors and in their physiological and pharmacological properties [343].
GABAA, as ligand-gated ion channels, facilitate rapid responses. Activation leads to two types of GABAergic inhibition: phasic and tonic inhibition. Classical phasic GABAA receptor-mediated inhibition is produced by brief exposure of postsynaptic GABAA receptors to high concentrations of GABA. On the other hand, tonic GABAA receptor-mediated inhibition results from the activation of extrasynaptic receptors by low concentrations of ambient GABA [344]. In many regions of the brain GABAA receptor-mediated currents are dominated by tonic currents, which account for about 75%-90% of total inhibitory currents [345].
GABAA receptors (Fig. (65) are a heterogeneous family of ligand-gated ion channels that mediate inhibitory neurotransmission in the CNS. They are assembled from five subunits in a heteropentameric chloride ion channels. Various GABAA receptor subunit subtypes as well as splice variants can be distinguished, such as α1-α6, β1-β3, γ1-γ3, δ, ε, π and θ. Each GABAA receptor subunit is composed of a long extracellular N terminus, four transmembrane domains (M1-M4), one extracellular M2-3 loop, two intracellular loops (M1-2 and M3-4) and a short extracellular C terminus.
Fig. (65).
Schematic structure of GABAA receptor.
αβγ and αβδ receptors are the principal isoforms present in vivo [346]. The most omnipresent and predominant synaptic receptor in the brain is α1β2γ2 isoform [347]. The αβδ GABAA receptors are localized extra- or perisynaptically and mediate GABAergic tonic inhibition. In comparison with synaptically localized αβγ receptors, αβδ receptors are more sensitive to GABA, show relatively slower desensitization and display lower efficacy to GABA agonism [348]. A lot of studies have implicated the important role of tonic inhibition in neuronal excitability mediation under physiological conditions.
Recent studies have demonstrated that defects in αβδ receptor function or tonic inhibition resulted in neurological disorders such as epilepsy [349, 350]. Studies regarding the genetics of human epilepsy demonstrated that various heritable mutations in GABAA receptor subunits are associated with epilepsy [351]. It is well established that activation of GABAA receptors plays an important role in sleep [352]. It was indicated that especially β3 GABAA receptor subunit is involved in this condition [353].
Aside from epilepsy and sleep disturbances there are a number of pathological conditions for which GABAA receptors represent vital therapeutic targets such as anxiety disorders, cognitive disorders, moods disorders and schizophrenia [354].
GABAA receptor, apart from response to GABA, can be also allosterically regulated by an increasing number of agents such as benzodiazepines (BZs), barbiturates, anticonvulsants and anesthetics [355]. Three distinct binding sites present on GABAA receptors were identified: GABA/ muscimol, t-butylbicyclophosphorothionate (TBPS)/picrotoxin, and benzodiazepine [356].
GABAA receptors are omnipresent in the CNS. Thus, the current goal in neuropharmacology is to target drugs selectively to defined GABAA receptor subtypes. This new strategy may help to improve the therapeutic spectrum of the presently available drugs and indicate new therapeutic indications. Additionally, such subtype-specific drugs are expected to display fewer concomitant unwanted effects, as they influence only a small population of GABAA receptors.
As the number of compounds interacting with GABAA receptors is impressive a complete coverage of all of them within this review would be difficult. Then, only recently developed compounds showing efficacy at GABAA receptors is presented.
SL651498
SL651498 (Fig. 66) a pyridoindole derivative is a full agonist at GABAA receptors containing α2 and α3 subunits and a partial agonist at GABAA receptors containing α1 and α5 subunits. The compound showed anxiolytic-like activity similar to that of diazepam (minimal effective dose (MED): 1-10 mg/kg, i.p.) in conflict models such as the elevated plus-maze, the light/dark test, and the defense test battery in rats and mice. Additionally, the study indicated that SL651498 produced ataxia, muscle weakness or sedation at doses much higher than those producing anxiolytic-like activity. No appearance of tolerance to its anticonvulsant effects or physical dependence was observed in mice [357]. SL651498 elicits anxiolytic-like effects similar to conventional BZs. The compound also fully induced muscle relaxation but in contrast to classical BZs produced minimal ataxia in monkeys [358]. The highest dose of SL651498 demonstrated slight effects on saccadic peak velocity (SPV) and smooth pursuit performance, albeit to a much lesser extent than lorazepam. Additionally, in contrast to lorazepam, none of the SL651498 doses influenced visual analogue scale alertness, body sway, attention, or memory tests [359].
Fig. (66).
Chemical structure of SL651498.
MRK-409
MRK-409 (Fig. (37) also known as MK-0343 was originally designed to be a less sedating anxiolytic, based on reduced efficacy at the α1 subtype and significant efficacy at α2 and α3 subtypes of the GABAA receptor. The anxiolytic-like and sedative activity was observed at minimum effective doses corresponding to occupancies, depending on the model used, ranging from about 35% to 65% yet there were minimal overt signs of sedation at occupancies greater than 90% [360]. MK-0343 at 0.75 mg in humans was equipotent with lorazepam, on the other hand diminished effects on memory and postural stability were observed. 0.25 mg dose only influenced postural stability to a similar extent as MK-0343 0.75 mg [361]. Recent studies showed that treatment with MRK-409 leads to sedation in humans at a dose (2 mg) corresponding to the levels of occupancy considerably less than those predicted formerly in rodent models to be required for anxiolytic activity. It was concluded that the preclinical non-sedating anxiolytic profile of MRK-409 could not be translated into humans and further development of the compound was halted.
BL-1020
BL-1020 (Fig. (68) is a novel, orally bioavailable ester of perphenazine and GABA. Unlike first- and second-generation antipsychotics, it has agonist activity at GABAA. Compound’s efficacy in acute and subchronic schizophrenia rat models was evaluated by Geffen et al. [362]. The study showed that acute and subchronic treatment with BL-1020 antagonized amphetamine-induced hyperactivity, with significantly lower catalepsy and sedation compared to equimolar perphenazine. Phase-II, open-label study suggested that 20 to 40 mg/d of BL-1020 is associated with clinically relevant improvement of psychosis with no worsening of extrapyramidal symptoms (EPS) [363]. Fitzgerald [364] summarized clinical trials of BL-1020 and concluded that the compound was well tolerated in all trials conducted, and clinically meaningful improvements were demonstrated in phase II trials in patients with schizophrenia.
Fig. (68).
Chemical structure of BL-1020.
L-838417
L-838417 (Fig. (69) is the novel benzodiazepine site ligand, a partial agonist at GABAA receptors containing an α2, α3 or α5 subunit and an antagonist at α1 receptors, giving an anxiolytic profile devoid of sedation [365, 366]. It showed anxiolytic-like activity in wild-type rats as shown in the elevated plus-maze test and in a conditioned fear-potentiated startle protocol. Significant motor effects were not observed [367]. L-838417 was also anxiolytic in murine Vogel conflict test [368]. Additionally, the compound exhibited anticonvulsant activity [369].
Fig. (69).
Chemical structure of L-838417.
TPA-023
TPA-023 (Fig. (70) is an agonist selective for α2 and α3-containing GABAA receptors. In unconditioned (elevated plus maze) and conditioned (fear-potentiated startle and conditioned suppression of drinking) rat models of anxiety the compound showed anxiolytic-like activity with minimum effective doses (MED; 1–3 mg/kg) corresponding to 70 to 88% occupancy. No appreciable sedation was stated in a response sensitivity (chain-pulling) assay at a dose of 30 mg/kg. The compound elicited anxiolytic effect in the squirrel monkey conditioned emotional response assay (MED of 0.3 mg/kg), however, it was not able to produce any sedation in a lever-pressing test of sedation. Finally, TPA-023 showed anticonvulsant activity in a mouse pentylenetetrazole seizure model [370]. TPA-023 had significant dose dependent effects on saccadic peak velocity that approximated the effects of lorazepam [371]. However in contrast to lorazepam, TPA-023 had no detectable effects on saccadic latency or inaccuracy. In addition unlike lorazepam, TPA023 did not affect VAS alertness, memory or body sway.
Fig. (70).
Chemical structure of TPA-023.
Indiplon (Fig. (71) is a high-affinity allosteric potentiator of GABAA responses that demonstrates preference for α1 subunit-containing GABAA receptors. The compound is studied for the treatment of insomnia. A double-blind, placebo-controlled trial investigated the efficacy and safety of modified-release indiplon in elderly patients with chronic insomnia. The study demonstrated that indiplon (15 mg), was well tolerated and significantly improved all patient-reported measures of sleep during the of treatment [372]. Long-term (3 months) nightly treatment with the drug (10 mg and 20 mg) has confirmed its efficacy in sleep onset, maintenance, and duration [373]. Indiplon’s activity was also confirmed in healthy volunteers in a model of transient insomnia [374].
Fig. (71).
Chemical structure of indiplon.
Y-23684
Y-23684 (Fig. (72) is pyridazinone derivative and selective partial agonist at BZ receptors. The compound is highly suggested to be a potent and selective anxiolytic agent with fewer side effects than classical BZ-anxiolytics. Studies comparing the pharmacological properties of Y-23684 with a classical benzodiazepine agonist diazepam demonstrated that in rat conflict tests (Geller-Seifter and water-lick tests), Y-23684 showed an antipunishment action at doses 2-4 times lower than diazepam and was at least as efficacious as diazepam in rodent models of anxiety (social interaction and elevated plus-maze tests). In a mouse model of anxiety (exploration (light/dark box) test), Y-23684 was two fold less effective as diazepam. In these paradigms, the compound demonstrated a selective anxiolytic activity over a wide dose-range without loss of efficacy and sedative action. Further studies with Y-23684 proved that the impairment of motor coordination (rotarod) and the enhancement of CNS depressants action by Y-23684 was weaker than that of diazepam [375]. Behavioral profiles in the mouse defence test battery confirmed Y-23684 anxiolytic potential that was comparable to that of BZs in the MDTB [376].
Fig. (72).
Chemical structure of Y-23684.
Most recently the development of selective brain-penetrating GABAC receptor antagonists is of particular interest [377]. In this group of compounds there are conformationally restricted analogs of the orally active GABAB/C receptor antagonist (3-aminopropyl)-n-butylphosphinic acid (CGP36742 or SGS742) (Fig. 73). These cis- and trans-(3-aminocyclopentanyl)butylphosphinic acids (cis- and trans-3-ACPBPA) are highly potent and selective for ρ1 GABAC receptors, being greater than 100 times more potent at GABAC than GABAA receptors and 50 times more potent at GABAC than GABAB receptors. These compounds showed potential use in sleep disorders, preventing the development of experimental myopia. Additionally, the substances enhanced learning and memory in rats [378, 379].
Fig. (73).
Chemical structure of CGP36742 or SGS742.
3. CONCLUSIONS
This review shows that there is a significant progress in understanding etiopathogenesis and molecular biology also in CNS diseases, which have been a major challenge for decades. However, drug discovery process seems too slow to provide specific ligands for ion channels/receptors subtypes. Many receptors subtypes lack a ligand, and if an effect of agonism/antagonism would be beneficial, this fact indicates a strong need for drug discovery in this field.
Currently, many old drugs are tested for their potential use in diseases which are still defined as “unmet need”. This in turn implies side effects, which are rarely positive, as in the case of dihydropyridine derivatives binding to CaV2.1 (P/Q) channels. In most cases of drugs the side effects are negative, and this together with effectiveness of old drugs in new diseases, is the greatest premise for drug development in novel subtypes of receptors. Nifedipine can serve as an example of an old drug, which on one hand could prevent from Parkinson’s disease, and on the other hand – it diminishes anticonvulsant activity of topiramate.
Ion channels have been biological targets for the first synthesized drugs (e.g. phenytoin, carbamazepine, diazepam). Therefore, they seemed unattractive for some pharmaceutical companies, seeing no use in developing a “me-too” drug. However, this review shows that understanding of mechanisms and discovery of subtypes of ion channels may increase their involvement in therapies in the future (with some exceptions, of course, e.g. CaV2.1 channels). CNS diseases, which are particularly hard to treat, are waiting to be managed, and we hope to have proved that this process is possible with use of novel ion channels ligands developed by the pharmaceutical market.
Fig. (1).
Schematic structure of VGSC.
Fig. (7).
Chemical structure of phenytoin.
Fig. (23).
Schematic structure of calcium channels.
Fig. (51).
(A) Schematic concentration of an ASIC unit, (B) Trimeric organization of a functional ASIC channel (TM1 and TM2 tramsmembrane domains 1 and 2) [272].
Fig. (52).
Chemical structure of benzamil.
Fig. (53).
Chemical structure of amiloride.
Fig. (54).
Chemical structure of A -317567.
Fig. (56).
Chemical structure of nafamostat.
Fig. (60).
Chemical structure of OAG.
Fig. (61).
Chemical structure of angiotensin II.
Fig. (62).
Chemical structure of thapsigargin.
Fig. (63).
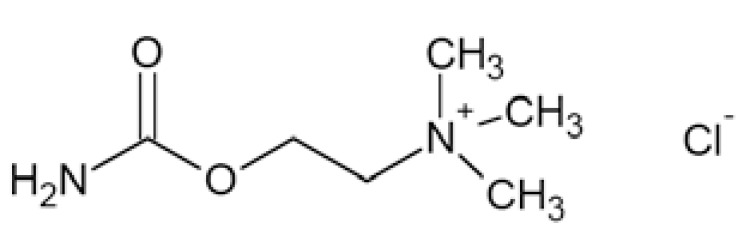
Chemical structure of carbachol.
Fig. (64).
Fig. (67).
Chemical structure of MRK-409.
Fig. (74).
Chemical structures of cis-3-ACPBPA and trans-3- ACPBPA.
Table 6.
| Family | Subfamily | Subtype/Type | Type | Antagonist |
|---|---|---|---|---|
| HVA | Cav1 | Cav1.1 | L | Nifedipine Amlodipine |
| Cav1.2 | L | |||
| Cav1.3 | L | |||
| Cav1.4 | L | |||
| Cav2 | Cav2.1 | P/Q | - | |
| Cav2.2 | N | ω-conotoxin-MVIIA,
ziconotide Pyrazolylpiperidine derivative |
||
| Cav2.3 | R | Lamotrigine | ||
| LVA | Cav3 | Cav3.1 | T | Ethosuximide Mesuximide |
| Cav3.2 | T | |||
| Cav3.3 | T |
Table 7.
Nomancletures of ASICs
| Subunit | Isoform | Synonym |
|---|---|---|
| ASIC1 | ASIC1a | ASICα, BNaC2α |
| ASIC1b | ASICα, BNaC2β | |
| ASIC2 | ASIC2a | MDEG1, BNaC1α, BNC1 |
| ASIC2b | MDEG2, BNaC1 β, BNC1 | |
| ASIC3 | DRASIC, TNaC | |
| ASIC4 | SPASIC |
Table 8.
Disorders in CNS Involving ASICs
ACKNOWLEDGEMENTS
This publication is supported by the European Regional Development Fund through the Innovative Economy Program.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Anger T, Madge DJ, Mulla M, Riddall D. Medicinal chemistry of neuronal voltage-gated sodium channel blockers. J. Med. Chem. 2001;44(2):115–137. doi: 10.1021/jm000155h. [DOI] [PubMed] [Google Scholar]
- 2.Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9(4):413–424. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- 3.Chahine M, Chatelier A, Babich O, Krupp JJ. Voltage-gated sodium channels in neurological disorders. CNS Neurol. Disord. Drug Targets. 2008;7(2):144–158. doi: 10.2174/187152708784083830. [DOI] [PubMed] [Google Scholar]
- 4.Tarnawa I, Bolcskei H, Kocsis P. Blockers of voltage-gated sodium channels for the treatment of central nervous system diseases. Recent Pat. CNS Drug Discov. 2007;2(1):57–78. doi: 10.2174/157488907779561754. [DOI] [PubMed] [Google Scholar]
- 5.Cestèle S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82(9-10):883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 6.Kyle DJ, Ilyin VI. Sodium channel blockers. J. Med. Chem. 2007;50(11):2583–2588. doi: 10.1021/jm061005v. [DOI] [PubMed] [Google Scholar]
- 7.Patino G, Isom LL. Electrophysiology and beyond: multiple roles of Na+ channel β subunits in development and disease. Neurosci. Lett. 2010;486(2):53–59. doi: 10.1016/j.neulet.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun GC, Werkman TR, Battefeld A, Clare JJ, Wadman WJ. Carbamazepine and topiramate modulation of transient and persistent sodium currents studied in HEK293 cells expressing the Na(v)1.3 alpha-subunit. Epilepsia. 2007;48(4):774–782. doi: 10.1111/j.1528-1167.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- 9.Hammarström AKM, Gage PW. Hypoxia and persistent sodium current. Eur. Biophys. J. 2002;31(5):323–330. doi: 10.1007/s00249-002-0218-2. [DOI] [PubMed] [Google Scholar]
- 10.Patino GA, Brackenbury WJ, Bao Y, Lopez-Santiago LF, O’Malley HA, Chen C, Calhoun JD, Lafrenière RG, Cossette P, Rouleau GA, Isom LL. Voltage-gated Na+ channel β1B: a secreted cell adhesion molecule involved in human epilepsy. J. Neurosci. 2011;31(41):14577–14591. doi: 10.1523/JNEUROSCI.0361-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum. Mol. Genet. 2007;16(23):2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- 12.Large CH, Kalinichev M, Lucas A, Carignani C, Bradford A, Garbati N, Sartori I, Austin NE, Ruffo A, Jones DNC, Alvaro G, Read KD. The relationship between sodium channel inhibition and anticonvulsant activity in a model of generalised seizure in the rat. Epilepsy Res. 2009;85(1):96–106. doi: 10.1016/j.eplepsyres.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Wimmer VC, Reid CA, So EY, Berkovic SF, Petrou S. Axon initial segment dysfunction in epilepsy. J. Physiol. 2010;588(Pt 11):1829–1840. doi: 10.1113/jphysiol.2010.188417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitaker WR, Faull RL, Dragunow M, Mee EW, Emson PC, Clare JJ. Changes in the mRNAs encoding voltage-gated sodium channel types II and III in human epileptic hippocampus. Neuroscience. 2001;106(2):275–285. doi: 10.1016/s0306-4522(01)00212-3. [DOI] [PubMed] [Google Scholar]
- 15.Sheets PL, Heers C, Stoehr T, Cummins TR. Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-3-methoxypropana mide], lidocaine, and carbamazepine. J. Pharmacol. Exp. Ther. 2008;326(1):89–99. doi: 10.1124/jpet.107.133413. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson E, Randall AD. Na(v)1.5 sodium channels in a human microglial cell line. J. Neuroimmunol. 2009;215(1-2):25–30. doi: 10.1016/j.jneuroim.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J. Physiol. 2005;564(Pt 3):803–815. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waxman SG, Cummins TR, Dib-Hajj S, Fjell J, Black JA. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle Nerve. 1999;22(9):1177–1187. doi: 10.1002/(sici)1097-4598(199909)22:9<1177::aid-mus3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Sander JW. The epidemiology of epilepsy revisited. Curr. Opin. Neurol. 2003;16(2):165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 20.Khan HN, Kulsoom S, Rashid H. Ligand based pharmacophore model development for the identification of novel antiepileptic compound. Epilepsy Res. 2012;98(1):62–71. doi: 10.1016/j.eplepsyres.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama J. Progress in searching for the febrile seizure susceptibility genes. Brain Dev. 2009;31(5):359–365. doi: 10.1016/j.braindev.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat. Rev. Drug Discov. 2010;9(1):68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 23.Yan B, Li P. An integrative view of mechanisms underlying generalized spike-and-wave epileptic seizures and its implication on optimal therapeutic treatments. PloS One. 2011;6(7):e22440. doi: 10.1371/journal.pone.0022440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taverna S, Mantegazza M, Franceschetti S, Avanzini G. Valproate selectively reduces the persistent fraction of Na+ current in neocortical neurons. Epilepsy Res. 1998;32(1-2):304–308. doi: 10.1016/s0920-1211(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 25.Lipkind GM, Fozzard HA. Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol. Pharmacol. 2010;78(4):631–638. doi: 10.1124/mol.110.064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20(5):359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16(10):669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 28.Zona C, Avoli M. Effects induced by the antiepileptic drug valproic acid upon the ionic currents recorded in rat neocortical neurons in cell culture. Exp. Brain Res. 1990;81(2):313–317. doi: 10.1007/BF00228121. [DOI] [PubMed] [Google Scholar]
- 29.Vreugdenhil M, Wadman WJ. Modulation of sodium currents in rat CA1 neurons by carbamazepine and valproate after kindling epileptogenesis. Epilepsia. 1999;40(11):1512–1522. doi: 10.1111/j.1528-1157.1999.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 30.Hammarstrom AK, Gage PW. Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J. Physiol. 1998;510(Pt 3):735–741. doi: 10.1111/j.1469-7793.1998.735bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79(4):1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 32.Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur. J. Neurosci. 2000;12(10):3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- 33.Stys PK. General mechanisms of axonal damage and its prevention. J. Neurol. Sci. 2005;233(1-2):3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Ates O, Cayli SR, Gurses I, Turkoz Y, Tarim O, Cakir CO, Kocak A. Comparative neuroprotective effect of sodium channel blockers after experimental spinal cord injury. J. Clin. Neurosci. 2007;14(7):658–665. doi: 10.1016/j.jocn.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Weir GA, Cader MZ. New directions in migraine. BMC Med. 2011;9:116–122. doi: 10.1186/1741-7015-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eikermann-Haerter K, Ayata C. Cortical spreading depression and migraine. Curr. Neurol. Neurosci. Rep. 2010;10(3):167–173. doi: 10.1007/s11910-010-0099-1. [DOI] [PubMed] [Google Scholar]
- 37.Waszkielewicz AM, Gunia A, Słoczyńska K, Marona H. Evaluation of anticonvulsants for possible use in neuropathic pain. Curr. Med. Chem. 2011;18(28):4344–4358. doi: 10.2174/092986711797200408. [DOI] [PubMed] [Google Scholar]
- 38.Dib-Hajj SD, Binshtok AM, Cummins TR, Jarvis MF, Samad T, Zimmermann K. Voltage-gated sodium channels in pain states: role in pathophysiology and targets for treatment. Brain Res. Rev. 2009;60(1):65–83. doi: 10.1016/j.brainresrev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 2003;23(26):8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108(3):237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 41.Smith MR, Smith RD, Plummer NW, Meisler MH, Goldin AL. Functional analysis of the mouse Scn8a sodium channel. J. Neurosci. 1998;18(16):6093–6102. doi: 10.1523/JNEUROSCI.18-16-06093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trudeau MM, Dalton JC, Day JW, Ranum LPW, Meisler MH. Heterozygosity for a protein truncation mutation of sodium channel SCN8A in a patient with cerebellar atrophy, ataxia, and mental retardation. J. Med. Genet. 2006;43(6):527–530. doi: 10.1136/jmg.2005.035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475(7356):353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Payandeh J, Gamal El-Din TM, Scheuer T, Zheng N, Catterall WA. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486(7401):135–139. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo CC. A common anticonvulsant binding site for phenytoin, carbamazepine, and lamotrigine in neuronal Na+ channels. Mol. Pharmacol. 1998;54(4):712–721. [PubMed] [Google Scholar]
- 46.Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol. Pharmacol. 2005;68(6):1611–1622. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- 47.Black JA, Waxman SG. Phenytoin protects central axons in experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2008;274(1-2):57–63. doi: 10.1016/j.jns.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Kuo CC, Bean BP. Slow binding of phenytoin to inactivated sodium channels in rat hippocampal neurons. Mol. Pharmacol. 1994;46(4):716–725. [PubMed] [Google Scholar]
- 49.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl. Acad. Sci. U S A. 1996;93(17):9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown ML, Brown GB, Brouillette WJ. Effects of log P and phenyl ring conformation on the binding of 5-phenylhydantoins to the voltage-dependent sodium channel. J. Med. Chem. 1997;40(4):602–607. doi: 10.1021/jm960692v. [DOI] [PubMed] [Google Scholar]
- 51.Almeida L, Soares-da-Silva P. Eslicarbazepine Acetate (BIA 2-093) Neurotherapeutics. 2007;4(1):88–96. doi: 10.1016/j.nurt.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonifácio MJ, Sheridan RD, Parada A, Cunha RA, Patmore L, Soares-da-Silva P. Interaction of the novel anticonvulsant, BIA 2-093, with voltage-gated sodium channels: comparison with carbamazepine. Epilepsia. 2001;42(5):600–608. doi: 10.1046/j.1528-1157.2001.43600.x. [DOI] [PubMed] [Google Scholar]
- 53.Stephen LJ, Brodie MJ. Pharmacotherapy of epilepsy: newly approved and developmental agents. CNS drugs. 2011;25(2):89–107. doi: 10.2165/11584860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.Liu G, Yarov-Yarovoy V, Nobbs M, Clare JJ, Scheuer T, Catterall WA. Differential interactions of lamotrigine and related drugs with transmembrane segment IVS6 of voltage-gated sodium channels. Neuropharmacology. 2003;44(3):413–422. doi: 10.1016/s0028-3908(02)00400-8. [DOI] [PubMed] [Google Scholar]
- 55.Xie X, Dale TJ, John VH, Cater HL, Peakman TC, Clare JJ. Electrophysiological and pharmacological properties of the human brain type IIA Na + channel expressed in a stable mammalian cell line. Pflugers Arch. 2001;441(4):425–433. doi: 10.1007/s004240000448. [DOI] [PubMed] [Google Scholar]
- 56.Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX) Epilepsy Res. 2009;83(1):1–43. doi: 10.1016/j.eplepsyres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Doty P, Rudd GD, Stoehr T, Thomas D. Lacosamide. Neurotherapeutics. 2007;4(1):145–148. doi: 10.1016/j.nurt.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Errington AC, Coyne L, Stöhr T, Selve N, Lees G. Seeking a mechanism of action for the novel anticonvulsant lacosamide. Neuropharmacology. 2006;50(8):1016–1029. doi: 10.1016/j.neuropharm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Perucca E. A pharmacological and clinical review on topiramate, a new antiepileptic drug. Pharmacol. Res. 1997;35(4):241–256. doi: 10.1006/phrs.1997.0124. [DOI] [PubMed] [Google Scholar]
- 60.Mohammadianinejad SE, Abbasi V, Sajedi SA, Majdinasab N, Abdollahi F, Hajmanouchehri R, Faraji A. Zonisamide versus topiramate in migraine prophylaxis: a double-blind randomized clinical trial. Clin. Neuropharmacol. 2011;34(4):174–177. doi: 10.1097/WNF.0b013e318225140c. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Yohrling GJ, Wang Y, Hutchinson TL, Brenneman DE, Flores CM, Zhao B. Carisbamate, a novel neuromodulator, inhibits voltage-gated sodium channels and action potential firing of rat hippocampal neurons. Epilepsy Res. 2009;83(1):66–72. doi: 10.1016/j.eplepsyres.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Arroyo S. Rufinamide. Neurotherapeutics. 2007;4(1):155–162. doi: 10.1016/j.nurt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abu-Arafeh I. Flunarizine for the prevention of migraine - a new look at an old drug. Dev. Med. Child Neurol. 2012;54(3):204–205. doi: 10.1111/j.1469-8749.2011.04152.x. [DOI] [PubMed] [Google Scholar]
- 64.Ye Q, Yan LY, Xue LJ, Wang Q, Zhou ZK, Xiao H, Wan Q. Flunarizine blocks voltage-gated Na(+) and Ca(2+) currents in cultured rat cortical neurons: A possible locus of action in the prevention of migraine. Neurosci. Lett. 2011;487(3):394–399. doi: 10.1016/j.neulet.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 65.Zamponi GW, Feng ZP, Zhang L, Pajouhesh H, Ding Y, Belardetti F, Pajouhesh H, Dolphin D, Mitscher LA, Snutch TP. Scaffold-based design and synthesis of potent N-type calcium channel blockers. Bioorg. Med. Chem. Lett. 2009;19(22):6467–6472. doi: 10.1016/j.bmcl.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Yokoo H, Shiraishi S, Kobayashi H, Yanagita T, Yamamoto R, Wada A. Selective inhibition by riluzole of voltage-dependent sodium channels and catecholamine secretion in adrenal chromaffin cells. Naunyn Schmiedebergs Arch. Pharmacol. 1998;357(5):526–531. doi: 10.1007/pl00005203. [DOI] [PubMed] [Google Scholar]
- 67.Palfi S, Riche D, Brouillet E, Guyot MC, Mary V, Wahl F, Peschanski M, Stutzmann JM, Hantraye P. Riluzole reduces incidence of abnormal movements but not striatal cell death in a primate model of progressive striatal degeneration. Exp. Neurol. 1997;146(1):135–141. doi: 10.1006/exnr.1997.6520. [DOI] [PubMed] [Google Scholar]
- 68.Abrams J, Jones CA, Kirkpatrick P. Ranolazine. Nat. Rev. Drug Discov. 2006;5(6):453–454. doi: 10.1038/nrd2069. [DOI] [PubMed] [Google Scholar]
- 69.Kahlig KM, Lepist I, Leung K, Rajamani S, George AL. Ranolazine selectively blocks persistent current evoked by epilepsy-associated Naν1.1 mutations. Br. J. Pharmacol. 2010;161(6):1414–1426. doi: 10.1111/j.1476-5381.2010.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pischalnikova AV, Sokolova OS. The domain and conformational organization in potassium voltage-gated ion channels. J. Neuroimmune Pharmacol. 2009;4(1):71–82. doi: 10.1007/s11481-008-9130-6. [DOI] [PubMed] [Google Scholar]
- 71.Jan LY, Jan YN. Cloned potassium channels from eukaryotes and prokaryotes. Annu. Rev. Neurosci. 1997;20:91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- 72.Tempel BL, Jan YN, Jan LY. Cloning of a probable potassium channel gene from mouse brain. Nature. 1988;332(6167):837–839. doi: 10.1038/332837a0. [DOI] [PubMed] [Google Scholar]
- 73.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol. Rev. 2008;88(4):1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, Mckinnon D, Pardo LA, Robertson G A, Rudy B, Sanguinetti MC, Stühmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005;57(4):473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 75.Lu J, Robinson JM, Edwards D, Deutsch C. T1-T1 interactions occur in ER membranes while nascent Kv peptides are still attached to ribosomes. Biochemistry. 2001;40(37):10934–10946. doi: 10.1021/bi010763e. [DOI] [PubMed] [Google Scholar]
- 76.Howard RJ, Clark KA, Holton JM, Minor DL., Jr Structural insight into KCNQ (Kv7) channel assembly and chennelopathy. Neuron. 2007;53(5):663–675. doi: 10.1016/j.neuron.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui J, Kagan A, Qin D, Mathew J, Melman YF, McDonald TV. Analysis of the cyclic nucleotide binding domain of the HERG potassium channel and interactions with KCNE2. J. Biol. Chem. 2001;276(20):17244–17251. doi: 10.1074/jbc.M010904200. [DOI] [PubMed] [Google Scholar]
- 78.Hernandez CC, Zaika O, Tolstykh GP, Shapiro MS. Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications. J. Physiol. 2008;586(7):1811–1821. doi: 10.1113/jphysiol.2007.148304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, Clover L, Parkinson A, Bien CG, Omer S, Lang B, Rossor MN, Palace J. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127(Pt3):701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 80.Majoie HJM, de Baets M, Renier W, Lang B, Vincent A. Antibodies to voltage-gated potassium and calcium channels in epilepsy. Epilepsy Res. 2006;71(2-3):135–41. doi: 10.1016/j.eplepsyres.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Barajas RF, Collins DE, Cha S, Geschwind MD. Adult onset drug refractory seizure disorder associated with anti voltage-gated potassium channel antibody. Epilepsia. 2010;51(3):473–477. doi: 10.1111/j.1528-1167.2009.02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiong Q, Gao Z, Wang W, Li M. Activation of Kv7 (KCNQ) voltage-gated potassium channels by synthetic compounds. Trends Pharmacol. Sci. 2008;29(2):99–107. doi: 10.1016/j.tips.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 83.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009;156(8):1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pongs O. Regulation of excitability by potassium channels. Results Probl. Cell Differ. 2008;44:145–161. doi: 10.1007/400_2007_032. [DOI] [PubMed] [Google Scholar]
- 85.Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat. Rev. Neurosci. 2004;5:400–408. doi: 10.1038/nrn1388. [DOI] [PubMed] [Google Scholar]
- 86.Richards MC, Heron S, Spendlove H, Scheffer I, Grinton B, Berkovic S, Mulley J, Davy A. Novel mutations in the KCNQ2 gene link epilepsy to a dysfunction of the KCNQ2-calmodulin interaction. J. Med. Genet. 2004;41(3):e35. doi: 10.1136/jmg.2003.013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watanabe H, Nagata E, Kosakai A, Nakamura M, Yokoyama M, Tanaka K, Sasai H. Disruption of the epilepsy KCNQ2 gene results in neural hyperexcitability. J. Neurochem. 2000;75(1):28–33. doi: 10.1046/j.1471-4159.2000.0750028.x. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y, Beyer BJ, Otto JF, O’Brien TP, Letts VA, White HS, Frankel WN. Spontaneous deletion of epilepsy gene orthologs in a mutant mouse with a low electroconvulsive threshold. Hum. Mol. Genet. 2003;12(9):975–984. doi: 10.1093/hmg/ddg118. [DOI] [PubMed] [Google Scholar]
- 89.Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat. Neurosci. 2005;8(1):51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- 90.Ljungstrom T, Grunnet M, Jensen BS, Olesen SP. Functional coupling between heterologously expressed dopamine D (2) receptors and KCNQ channels. Pflugers Arch. 2003;446(6):684–694. doi: 10.1007/s00424-003-1111-2. [DOI] [PubMed] [Google Scholar]
- 91.Hansen HH, Ebbesen C, Mathiesen C, Weikop P, Rønn LC, Waroux O, Scuvée-Moreau J, Seutin V, Mikkelsen JD. The KCNQ channel opener retigabine inhibits the activity of mesencephalic dopaminergic systems of the rat. J. Pharmacol. Exp. Ther. 2006;318(3):1006–1019. doi: 10.1124/jpet.106.106757. [DOI] [PubMed] [Google Scholar]
- 92.Sotty F, Damgaard T, Montezinho LP, Mørk A, Olsen CK, Bundgaard C, Husum H. Antipsychotic-like effect of retigabine [N-(2-Amino-4-(fluorobenzylamino)-phenyl) carbamic acid ester], a KCNQ potassium channel opener, via modulation of mesolimbic dopaminergic neurotransmission. J. Pharmacol. Exp. Ther. 2009;328(3):951–962. doi: 10.1124/jpet.108.146944. [DOI] [PubMed] [Google Scholar]
- 93.Ryan DP, Ptácek LJ. Episodic neurological channelopathies. Neuron. 2010;68(2):282–292. doi: 10.1016/j.neuron.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 94.Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat. Genet. 1994;8(2):136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- 95.Tomlinson SE, Hanna MG, Kullmann DM, Tan SV, Burke D. Clinical neurophysiology of the episodic ataxias: insights into ion channel dysfunction in vivo. Clin. Neurophysiol. 2009;120(10):1768–1776. doi: 10.1016/j.clinph.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 96.Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics. 2007;4(2):267–273. doi: 10.1016/j.nurt.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 97.McDonald S, Clements JN. Dalfampridine: a new agent for symptomatic management of multiple sclerosis. Am. J. Health Syst. Pharm. 2011;68(24):2335–2340. doi: 10.2146/ajhp110134. [DOI] [PubMed] [Google Scholar]
- 98.Wulff H, Pennington M. Targeting effector memory T-cells with Kv1. 3 blockers. Curr. Opin. Drug Discov. Devel. 2007;10(4):438–445. [PubMed] [Google Scholar]
- 99.Wulff H, Zhorov BS. K+ channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem. Rev. 2008;108(5):1744–1773. doi: 10.1021/cr078234p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Large CH, Sokal DM, Nehlig A, Gunthorpe MJ, Sankar R, Crean CS, Vanlandingham KE, White HS. The spectrum of anticonvulsant efficacy of retigabine (ezogabine) in animal models: implications for clinical use. Epilepsia. 2012;53(3):425–436. doi: 10.1111/j.1528-1167.2011.03364.x. [DOI] [PubMed] [Google Scholar]
- 101.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat. Rev. Neurosci. 2005;6(11):850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 102.Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol. Pharmacol. 2000;58(2):253–262. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- 103.Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol. Pharmacol. 2000;58(3):591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- 104.Otto JF, Yang Y, Frankel WN, Wilcox KS, White HS. Mice carrying the szt1 mutation exhibit increased seizure susceptibility and altered sensitivity to compounds acting at the m-channel. Epilepsia. 2004;45(9):1009–1016. doi: 10.1111/j.0013-9580.2004.65703.x. [DOI] [PubMed] [Google Scholar]
- 105.Wickenden AD, Krajewski JL, London B, Wagoner PK, Wilson WA, Clark S, Roeloffs R, McNaughton-Smith G, Rigdon GC. N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): a novel, selective KCNQ2 /Q3 potassium channel activator. Mol. Pharmacol. 2008;73(3):977–986. doi: 10.1124/mol.107.043216. [DOI] [PubMed] [Google Scholar]
- 106.Roeloffs R, Wickenden AD, Crean C, Werness S, McNaughton-Smith G, Stables J, McNamara JO, Ghodadra N, Rigdon GC. In vivo profile of ICA-27243 [N-(6-chloro-pyridin-3-yl)-3,4-difluorobenzamide], a potent and selective KCNQ2/Q3 (Kv7.2/Kv7.3) activator in rodent anticonvulsant models. J. Pharmacol. Exp. Ther. 2008;326(3):818–828. doi: 10.1124/jpet.108.137794. [DOI] [PubMed] [Google Scholar]
- 107.Kirsch GE, Shieh CC, Drewe JA, Vener DF, Brown AM. Segmental exchanges define 4-aminopyridine binding and the inner mouth of K+ pores. Neuron. 1993;11(3):503–512. doi: 10.1016/0896-6273(93)90154-j. [DOI] [PubMed] [Google Scholar]
- 108.Kirsch GE, Drewe JA. Gating-dependent mechanism of 4-aminopyridine block in two related potassium channels. J. Gen. Physiol. 1993;102(5):797–816. doi: 10.1085/jgp.102.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Belardetti F, Zamponi GW. Calcium channels as therapeutic targets. WIREs Membr. Transp. Signal. 2012;1(4):433–451. [Google Scholar]
- 110.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011;3(8):1–23. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nelson MT, Todorovic SM, Perez-Reyes E. The role of T-type calcium channels in epilepsy and pain. Curr. Pharm. Des. 2006;12(18):2189–2197. doi: 10.2174/138161206777585184. [DOI] [PubMed] [Google Scholar]
- 112.Iftinca MC. Neuronal T-type calcium channels: What’s new? Iftinca: T-type channel regulation. J. Med. Life. 2011;4(2):126–138. [PMC free article] [PubMed] [Google Scholar]
- 113.Wölfel M, Schneggenburger R. Presynaptic capacitance measurements and Ca2+ uncaging reveal submillisecond exocytosis kinetics and characterize the Ca2+ sensitivity of vesicle pool depletion at a fast CNS synapse. J. Neurosci. 2003;23(18):7059–7068. doi: 10.1523/JNEUROSCI.23-18-07059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zamponi GW, Lory P, Perez-Reyes E. Role of voltage-gated calcium channels in epilepsy. Pflügers Arch. 2010;460(2):395–403. doi: 10.1007/s00424-009-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cain SM, Snutch TP. Contributions of T-type calcium channel isoforms to neuronal firing. Channels. 2010;4(6):475–482. doi: 10.4161/chan.4.6.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhan XJ, Cox CL, Sherman SM. Dendritic depolarization efficiently attenuates low-threshold calcium spikes in thalamic relay cells. J. Neurosci. 2000;20(10):3909–3914. doi: 10.1523/JNEUROSCI.20-10-03909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chevalier M, Lory P, Mironneau C, Macrez N, Quignard JF. T-type CaV3.3 calcium channels produce spontaneous low-threshold action potentials and intracellular calcium oscillations. Eur. J. Neurosic. 2006;23(9):2321–2329. doi: 10.1111/j.1460-9568.2006.04761.x. [DOI] [PubMed] [Google Scholar]
- 118.Belardetti F. Evolving therapeutic indications for N-type calcium channel blockers: from chronic pain to alcohol abuse. Future Med. Chem. 2010;2(5):791–802. doi: 10.4155/fmc.10.30. [DOI] [PubMed] [Google Scholar]
- 119.Subasinghe NL, Wall MJ, Winters MP, Qin N, Lubin ML, Finley MF, Brandt MR, Neeper MP, Schneider CR, Colburn RW, Flores CM, Sui Z. A novel series of pyrazolylpiperidine N-type calcium channel blockers. Bioorg. Med. Chem. Lett. 2012;22(12):4080–4083. doi: 10.1016/j.bmcl.2012.04.075. [DOI] [PubMed] [Google Scholar]
- 120.Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JHJ, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat. Neurosci. 2008;11(3):344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 121.Tottene A, Conti R, Fabbro A, Vecchia D, Shapovalova M, Santello M, van den Maagdenberg AM, Ferrari M D, Pietrobon D. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca[v]2.1 knockin migraine mice. Neuron. 2009;61(5):762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 122.Fletcher CF, Tottene A, Lennon VA, Wilson SM, Dubel SJ, Paylor R, Hosford DA, Tessarollo L, McEnery MW, Pietrobon D, Copeland NG, Jenkins NA. Dystonia and cerebellar atrophy in Cacna1a null mice lacking P/Q calcium channel activity. FASEB J. 2001;15(7):1288–1290. doi: 10.1096/fj.00-0562fje. [DOI] [PubMed] [Google Scholar]
- 123.Perret D, Luo ZD. Targeting voltage-gated calcium channels for neuropathic pain management. Neurotherapeutics. 2009;6(4):679–692. doi: 10.1016/j.nurt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weiergräber M, Stephani U, Köhling R. Voltage-gated calcium channels in the etiopathogenesis and treatment of absence epilepsy. Brain Res. Rev. 2010;62(2):245–271. doi: 10.1016/j.brainresrev.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 125.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(Suppl 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 126.Sitnikova E. Thalamo-cortical mechanisms of sleep spindles and spike-wave discharges in rat model of absence epilepsy (a review) Epilepsy Res. 2010;89(1):17–26. doi: 10.1016/j.eplepsyres.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 127.Khosravani H, Zamponi GW. Voltage-gated calcium channels and idiopathic generalized epilepsies. Physiol. Rev. 2006;86(3):941–966. doi: 10.1152/physrev.00002.2006. [DOI] [PubMed] [Google Scholar]
- 128.Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann. Neurol. 2010;67(5):600–606. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Russo E, Constanti A, Ferreri G, Citraro R, De Sarro G. Nifedipine affects the anticonvulsant activity of topiramate in various animal models of epilepsy. Neuropharmacology. 2004;46(6):865–878. doi: 10.1016/j.neuropharm.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 130.Moon DE, Lee DI, Lee SC, Song SO, Yoon DM, Yoon MH, Kim HK, Lee YW, Kim C, Lee PB. Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter study. Clin. Ther. 2010;32(14):2370–2385. doi: 10.1016/j.clinthera.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 131.Guan Y, Ding X, Cheng Y, Fan D, Tan L, Wang Y, Zhao Z, Hong Z, Zhou D, Pan X, Chen S, Martin A, Tang H, Cui L. Efficacy of pregabalin for peripheral neuropathic pain: results of an 8-week, flexible-dose, double-blind, placebo-controlled study conducted in China. Clin. Ther. 2011;33(2):159–166. doi: 10.1016/j.clinthera.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 132.Semel D, Murphy TK, Zlateva G, Cheung R, Emir B. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam. Prac. 2010;11:85–97. doi: 10.1186/1471-2296-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Omori Y, Kagaya K, Enomoto R, Sasaki A, Andoh T, Nojima H, Takahata H, Kuraishi Y. A Mouse model of sural nerve injury–induced neuropathy: gabapentin inhibits pain-related behaviors and the hyperactivity of wide-dynamic range neurons in the dorsal horn. J. Pharmacol. Sci. 2009;109(4):532–539. doi: 10.1254/jphs.08319fp. [DOI] [PubMed] [Google Scholar]
- 134.Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: A summary of the Tenth Eilat Conference [EILAT X] Epilepsy Res. 2010;92(2-3):89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 135.Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J. Physiol. 1999;514(Pt2):351–367. doi: 10.1111/j.1469-7793.1999.351ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Séguéla P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53(1):107–118. [PubMed] [Google Scholar]
- 137.North RA. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 138.Kaczmarek-Hájek K, Lörinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors—recent progress and persisting challenges. Purinergic Signal. 2012;8(3):375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Torres GE, Egan TM, Voigt MM. Topological analysis of the ATP-gated ionotrophic P2X2 receptor subunit. FEBS Lett. 1998;425(1):19–23. doi: 10.1016/s0014-5793(98)00179-3. [DOI] [PubMed] [Google Scholar]
- 140.Coddou C, Stojilkovic SS, Huidobro-Toro JP. Allosteric modulation of ATP-gated P2X receptor channels. Rev. Neurosci. 2011;22(3):335–354. doi: 10.1515/RNS.2011.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jacobson KA, Jarvis MF, Williams M. Purine and Pyrimidine [P2] Receptors as Drug Targets. J. Med. Chem. 2002;45(19):4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nicke A, Rettinger J, Schmalzing G. Monomeric and dimeric byproducts are the principal functional elements of higher order P2X1 concatamers. Mol. Pharmacol. 2003;63(1):243–252. doi: 10.1124/mol.63.1.243. [DOI] [PubMed] [Google Scholar]
- 143.Jiang LH, Kim M, Spelta V, Bo X, Surprenant A, North RA. Subunit Arrangement in P2X Receptors. J. Neurosci. 2003;23(26):8903–8910. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Newbolt A, Stoop R, Virginio C, Surprenant A, North RA, Buell G, Rassendren F. Membrane topology of an ATP-gated ion channel (P2X receptor) J. Biol. Chem. 1998;273(24):15177–15182. doi: 10.1074/jbc.273.24.15177. [DOI] [PubMed] [Google Scholar]
- 145.Haines WR, Voigt MM, Migita K, Torres GE, Egan TM. On the contribution of the first transmembrane domain to whole-cell current through an ATP-gated ionotropic P2X receptor. J. Neurosci. 2001;21(16):5885–5892. doi: 10.1523/JNEUROSCI.21-16-05885.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jarvis MF, Khakh BS. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56(1):208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 147.Bo X, Jiang LH, Wilson HL, Kim M, Burnstock G, Surprenant A, North RA. Pharmacological and biophysical properties of the human P2X5 receptor. Mol. Pharmacol. 2003;63(6):1407–1416. doi: 10.1124/mol.63.6.1407. [DOI] [PubMed] [Google Scholar]
- 148.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 149.Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol. (Oxf) 2010;199(2):93–147. doi: 10.1111/j.1748-1716.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 150.Burnstock G, Kennedy C. P2X receptors in health and disease. Adv. Pharmacol. 2011;61:333–372. doi: 10.1016/B978-0-12-385526-8.00011-4. [DOI] [PubMed] [Google Scholar]
- 151.Rubio ME, Soto F. Distinct Localization of P2X receptors at excitatory postsynaptic specializations. J. Neurosci. 2001;21(2):641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jacobson K, Costanzi S, Joshi BV, Besada P, Shin DH, Ko H, Ivanov AA, Mamedova L. Agonists and antagonists for P2 receptors. Novartis Found Symp. 2006;276:58–68. doi: 10.1002/9780470032244.ch6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kharlamov A, Jones SC, Kim DK. Suramin reduces infarct volume in a model of focal brain ischemia in rats. Exp. Brain Res. 2002;147(3):353–359. doi: 10.1007/s00221-002-1251-1. [DOI] [PubMed] [Google Scholar]
- 154.Franke H, Günther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J. Neuropathol. Exp. Neurol. 2004;63(7):686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 155.LeFeuvre RA, Brough D, Touzani O, Rothwell NJ. Role of P2X 7 receptors in ischemic and excitotoxic brain injury. J. Cereb. Blood Flow Metab. 2003;23(3):381–384. doi: 10.1097/01.WCB.0000048519.34839.97. [DOI] [PubMed] [Google Scholar]
- 156.Cavaliere F, Florenzano F, Amadio S, Fusco FR, Viscomi MT, D'Ambrosi N, Vacca F, Sancesario G, Bernardi G, Molinari M, Volontè C. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120(1):85–98. doi: 10.1016/s0306-4522(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 157.Cavaliere F, Dinkel K, Reymann K. Microglia response and P2 receptor participation in oxygen/glucose deprivation-induced cortical damage. Neuroscience. 2005;136(3):615–623. doi: 10.1016/j.neuroscience.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 158.Volonté C, Merlo D. Selected P2 purinoceptor modulators prevent glutamate-evoked cytotoxicity in cultured cerebellar granule neurons. J. Neurosci. Res. 1996;45(2):183–193. doi: 10.1002/(SICI)1097-4547(19960715)45:2<183::AID-JNR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 159.Cavaliere F, D’Ambrosi N, Ciotti MT, Mancino G, Sancesario G, Bernardi G, Volonté C. Glucose deprivation and chemical hypoxia: neuroprotection by P2 receptor antagonists. Neurochem. Int. 2001;38(3):189–197. doi: 10.1016/s0197-0186(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 160.Cavaliere F, D’Ambrosi N, Sancesario G, Bernardi G, Volonté C. Hypoglycaemia-induced cell death: features of neuroprotection by the P2 receptor antagonist basilen blue. Neurochem. Int. 2001;38(3):199–207. doi: 10.1016/s0197-0186(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 161.Volonté C, Ciotti MT, D’Ambrosi N, Lockhart B, Spedding M. Neuroprotective effects of modulators of P2 receptors in primary culture of CNS neurones. Neuropharmacology. 1999;38(9):1335–1342. doi: 10.1016/s0028-3908(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 162.Romagnoli R, Baraldi PG, Cruz-Lopez O, Lopez-Cara C, Preti D, Borea PA, Gessi S. The P2X7 receptor as a therapeutic target. Expert Opin. Ther. Targets. 2008;12(5):647–661. doi: 10.1517/14728222.12.5.647. [DOI] [PubMed] [Google Scholar]
- 163.Hracskó Z, Baranyi M, Csölle C, Gölöncsér F, Madarász E, Kittel Á, Sperlágh B. Lack of neuroprotection in the absence of P2X7 receptors in toxin-induced animal models of Parkinson’s disease. Mol. Neurodegener. 2011;6:28. doi: 10.1186/1750-1326-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Matute C, Torre I, Pérez-Cerdá F, Pérez-Samartín A, Alberdi E, Etxebarria E, Arranz AM, Ravid R, Rodríguez-Antigüedad A, Sánchez-Gómez M, Domercq M. P2X 7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 2007;27(35):9525–9533. doi: 10.1523/JNEUROSCI.0579-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Díaz-Hernández M, Díez-Zaera M, Sánchez-Nogueiro J, Gómez-Villafuertes R, Canals JM, Alberch J, Miras-Portugal MT, Lucas JJ. Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23(6):1893–1906. doi: 10.1096/fj.08-122275. [DOI] [PubMed] [Google Scholar]
- 166.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc. Natl. Acad. Sci. U S A. 2009;106(30):12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Morales I, Dopico JG, Sabate M, Gonzalez-Hernandez T, Rodriguez M. Substantia nigra osmoregulation: taurine and ATP involvement. Am. J. Physiol. Cell Physiol. 2007;292(5):C1934–1941. doi: 10.1152/ajpcell.00593.2006. [DOI] [PubMed] [Google Scholar]
- 168.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2003;278(15):13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 169.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov. 2008;7(7):575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 170.Andries M, Van Damme P, Robberecht W, Van Den Bosch L. Ivermectin inhibits AMPA receptor-mediated excitotoxicity in cultured motor neurons and extends the life span of a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2007;25(1):8–16. doi: 10.1016/j.nbd.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 171.Rappold PM, Lynd-Balta E, Joseph SA. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089(1):171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 172.Nicolaidis R, Bruno AN, Sarkis JJ, Souza DO. Increase of adenine nucleotide hydrolysis in rat hippocampal slices after seizures induced by quinolinic acid. Neurochem. Res. 2005;30(3):385–390. doi: 10.1007/s11064-005-2613-4. [DOI] [PubMed] [Google Scholar]
- 173.Dunn PM, Blakeley AG. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br. J. Pharmacol. 1988;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stock TC, Bloom BJ, Wei N, Ishaq S, Park W, Wang X, Gupta P, Mebus CA. Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J. Rheumatol. 2012;39(4):720–727. doi: 10.3899/jrheum.110874. [DOI] [PubMed] [Google Scholar]
- 175.Duplantier AJ, Dombroski MA, Subramanyam C, Beaulieu AM, Chang SP, Gabel CA, Jordan C, Kalgutkar AS, Kraus KG, Labasi JM, Mussari C, Perregaux DG, Shepard R, Taylor TJ, Trevena KA, Whitney-Pickett C, Yoon K. Optimization of the physicochemical and pharmacokinetic attributes in a 6-azauracil series of P2X7 receptor antagonists leading to the discovery of the clinical candidate CE-224,535. Bioorg. Med. Chem. Lett. 2011;21(12):3708–3711. doi: 10.1016/j.bmcl.2011.04.077. [DOI] [PubMed] [Google Scholar]
- 176.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 177.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001;11(3):327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 178.Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat. Neurosci. 2002;5:1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- 179.Hawkins LM, Chazot PL, Stephenson FA. Biochemical evidence for the co-association of three N-methyl-D-aspartate (NMDA) R2 subunits in recombinant NMDA receptors. J. Biol. Chem. 1999;274(38):27211–27218. doi: 10.1074/jbc.274.38.27211. [DOI] [PubMed] [Google Scholar]
- 180.Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem. Biophys. Res. Commun. 1992;185(5):826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- 181.Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393(6683):377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- 182.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr. Opin. Pharmacol. 2007;7(1):39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 183.Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J. Neurosci. 2001;21(4):1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chazot PL, Stephenson FA. Molecular dissection of native mammalian forebrain NMDA receptors containing the NR1 C2 exon: direct demonstration of NMDA receptors comprising NR1, NR2A, and NR2B subunits within the same complex. J. Neurochem. 1997;69(5):2138–2144. doi: 10.1046/j.1471-4159.1997.69052138.x. [DOI] [PubMed] [Google Scholar]
- 185.Blahos J, 2nd, Wenthold RJ. Relationship between N-methyl-D-aspartate receptor NR1 splice variants and NR2 subunits. J. Biol. Chem. 1996;271(26):15669–15674. doi: 10.1074/jbc.271.26.15669. [DOI] [PubMed] [Google Scholar]
- 186.Conti F. Localization of NMDA receptors in the cerebral cortex : a schematic overview. Braz. J. Med. Biol. Res. 1997;30(5):555–560. doi: 10.1590/s0100-879x1997000500001. [DOI] [PubMed] [Google Scholar]
- 187.Di Fabio R, Capelli AM, Conti N, Cugola A, Donati D, Feriani A, Gastaldi P, Gaviraghi G, Hewkin CT, Micheli F, Missio A, Mugnaini M, Pecunioso A, Quaglia AM, Ratti E, Rossi L, Tedesco G, Trist DG, Reggiani A. Substituted indole-2-carboxylates as in vivo potent antagonists acting as the strychnine-insensitive glycine binding site. J. Med. Chem. 1997;40(6):841–850. doi: 10.1021/jm960644a. [DOI] [PubMed] [Google Scholar]
- 188.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(6738 Suppl):A7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 189.Folkersma H, Foster Dingley JC, van Berckel BN, Rozemuller A, Boellaard R, Huisman MC, Lammertsma AA, Vandertop WP, Molthoff CF. Increased cerebral [R]-[[11]C]PK11195 uptake and glutamate release in a rat model of traumatic brain injury: a longitudinal pilot study. J. Neuroinflammation. 2011;8:67. doi: 10.1186/1742-2094-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mellegård P, Sjögren F, Hillman J. The cerebral extracellular release of glycerol, glutamate, and FGF2 is increased in older patients following severe traumatic brain injury. J. Neurotrauma. 2012;29(1):112–118. doi: 10.1089/neu.2010.1732. [DOI] [PubMed] [Google Scholar]
- 191.Ghafari M, Patil SS, Höger H, Pollak A, Lubec G. NMDA-complexes linked to spatial memory performance in the Barnes maze in CD1 mice. Behav. Brain Res. 2011;221(1):142–148. doi: 10.1016/j.bbr.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 192.Gardoni F, Kamal A, Bellone C, Biessels GJ, Ramakers GM, Cattabeni F, Gispent WH, Di Luca M. Effects of streptozotocin-diabetes on the hippocampal NMDA receptor complex in rats. J. Neurochem. 2002;80(3):438–447. doi: 10.1046/j.0022-3042.2001.00713.x. [DOI] [PubMed] [Google Scholar]
- 193.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr. Bull. 2009;35(3):528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am. J. Psychiatry. 2000;157(7):1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 196.Grimwood S, Slater P, Deakin JF, Hutson PH. NR2B-containing NMDA receptors are up-regulated in temporal cortex in schizophrenia. Neuroreport. 1999;10(3):461–465. doi: 10.1097/00001756-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 197.Arias C, Montiel T, Peña F, Ferrera P, Tapia R. Okadaic acid induces epileptic seizures and hyperphosphorylation of the NR2B subunit of the NMDA receptor in rat hippocampus in vivo. Exp. Neurol. 2002;177(1):284–291. doi: 10.1006/exnr.2002.7988. [DOI] [PubMed] [Google Scholar]
- 198.Mitchell IJ, Carroll CB. Reversal of parkinsonian symptoms in primates by antagonism of excitatory amino acid transmission: potential mechanisms of action. Neurosci. Biobehav. Rev. 1997;21(4):469–475. doi: 10.1016/s0149-7634(96)00036-x. [DOI] [PubMed] [Google Scholar]
- 199.Gerlach M, Gsell W, Kornhuber J, Jellinger K, Krieger V, Pantucek F, Vock R, Riederer P. A post mortem study on neurochemical markets of glutamatergic neurons in basal ganglia-thalamocortical circuits in Parkinson syndrome. Brain Res. 1996;741(1-2):142–152. doi: 10.1016/s0006-8993(96)00915-8. [DOI] [PubMed] [Google Scholar]
- 200.Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J. Neurosci. 2002;22(9):3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Piggott MA, Perry EK, Perry RH, Court JA. [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res. 1992;588(2):277–286. doi: 10.1016/0006-8993(92)91586-4. [DOI] [PubMed] [Google Scholar]
- 202.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401(6748):63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 203.Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 2001;276(1):693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- 204.Zeron MM, Hansson O, Chen N, Wellington CL, Leavitt BR, Brundin P, Hayden MR, Raymond LA. Increased sensitivity to N-methyl-D-aspartate in a mouse model of Huntington’s disease. Neuron. 2002;33(6):849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 205.Chen N, Luo T, Wellington C, Metzler M, McCutcheon K, Hayden MR, Raymond LA. Subtype-specific enhancement of NMDA receptor currents by mutant huntingtin. J. Neurochem. 1999;72(5):1890–1898. doi: 10.1046/j.1471-4159.1999.0721890.x. [DOI] [PubMed] [Google Scholar]
- 206.Sun Y, Savanenin A, Reddy PH, Liu YF. Polyglutamine-expanded huntingtin promotes sensitization of N-methyl-D-aspartate receptors via post-synaptic density 95. J. Biol. Chem. 2001;276(27):24713–24718. doi: 10.1074/jbc.M103501200. [DOI] [PubMed] [Google Scholar]
- 207.Bordi F, Pietra C, Ziviani L, Reggiani A. The glycine antagonist GV150526 protects somatosensory evoked potentials and reduces the infarct area in the MCAo model of focal ischemia in the rat. Exp. Neurol. 1997;145:425–433. doi: 10.1006/exnr.1997.6442. [DOI] [PubMed] [Google Scholar]
- 208.Reggiani A, Pietra C, Arban R, Marzola P, Guerrini U, Ziviani L, Boicelli A, Sbarbati A, Osculati F. The neuroprotective activity of the glycine receptor antagonist GV150526: an in vivo study by magnetic resonance imaging. Eur. J. Pharmacol. 2001;419(2-3):147–153. doi: 10.1016/s0014-2999(01)00948-7. [DOI] [PubMed] [Google Scholar]
- 209.Dyker AG, Lees KR. Safety and tolerability of GV150526 (a glycine site antagonist at the N-methyl-D-aspartate receptor) in patients with acute stroke. Stroke. 1999;30(5):986–992. doi: 10.1161/01.str.30.5.986. [DOI] [PubMed] [Google Scholar]
- 210.Lees KR, Lavelle JF, Cunha L, Diener HC, Sanders EA, Tack P, Wester P. Glycine antagonist (GV150526) in acute stroke: a multicentre, double-blind placebo-controlled phase II trial. Cerebrovasc. Dis. 2001;11(1):20–29. doi: 10.1159/000047607. [DOI] [PubMed] [Google Scholar]
- 211.Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Kaste M, Orgogozo JM, Whitehead J. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. GAIN International Investigators. Lancet. 2000;355(9219):1949–1954. doi: 10.1016/s0140-6736(00)02326-6. [DOI] [PubMed] [Google Scholar]
- 212.Haley EC, Jr, Thompson JL, Levin B, Davis S, Lees KR, Pittman JG, DeRosa JT, Ordronneau P, Brown DL, Sacco RL. Gavestinel does not improve outcome after acute intracerebral hemorrhage: an analysis from the GAIN International and GAIN Americas studies. Stroke. 2005;36(5):1006–1010. doi: 10.1161/01.STR.0000163053.77982.8d. [DOI] [PubMed] [Google Scholar]
- 213.Bennett DA, Lehmann J, Bernard PS, Liebman JM, Williams M, Wood P, Boast CA, Hutchison AJ. CGS 19755: a novel competitive N-methyl-D-aspartate (NMDA) receptor antagonist with anticonvulsant, anxiolytic and anti-ischemic properties. Prog. Clin. Biol. Res. 1990;361:519–524. [PubMed] [Google Scholar]
- 214.Takizawa S, Hogan M, Hakim AM. The effects of a competitive NMDA receptor antagonist (CGS-19755) on cerebral blood flow and pH in focal ischemia. J. Cereb. Blood Flow Metab. 1991;11(5):786–793. doi: 10.1038/jcbfm.1991.136. [DOI] [PubMed] [Google Scholar]
- 215.Grotta JC, Picone CM, Ostrow PT, Strong RA, Earls RM, Yao LP, Rhoades HM, Dedman JR. CGS-19755, a competitive NMDA receptor antagonist, reduces calcium-calmodulin binding and improves outcome after global cerebral ischemia. Ann. Neurol. 1990;27(6):612–619. doi: 10.1002/ana.410270605. [DOI] [PubMed] [Google Scholar]
- 216.Madden KP, Clark WM, Zivin JA. Delayed therapy of experimental ischemia with competitive N-methyl-D-aspartate antagonists in rabbits. Stroke. 1993;24(7):1068–1071. doi: 10.1161/01.str.24.7.1068. [DOI] [PubMed] [Google Scholar]
- 217.Morris GF, Bullock R, Marshall SB, Marmarou A, Maas A, Marshall LF. Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. J. Neurosurg. 1999;91(5):737–743. doi: 10.3171/jns.1999.91.5.0737. [DOI] [PubMed] [Google Scholar]
- 218.Reddy NL, Hu LY, Cotter RE, Fischer JB, Wong WJ, McBurney RN, Weber E, Holmes DL, Wong ST, Prasad R, Keana JFW. Synthesis and structure-activity studies of N,N'- diarylguanidine derivatives. N-(1-naphthyl)-N'-(3-ethylphenyl)-N'- methylguanidine: a new, selective noncompetitive NMDA receptor antagonist. J. Med. Chem. 1994;37(2):260–267. doi: 10.1021/jm00028a009. [DOI] [PubMed] [Google Scholar]
- 219.Schäbitz WR, Li F, Fisher M. The N-methyl-D-aspartate antagonist CNS 1102 protects cerebral gray and white matter from ischemic injury following temporary focal ischemia in rats. Stroke. 2000;31(7):1709–1714. doi: 10.1161/01.str.31.7.1709. [DOI] [PubMed] [Google Scholar]
- 220.Kroppenstedt S, Schneider G. Protective effects of aptiganel HCl (Cerestat) following controlled cortical impact injury in the rat. J. Neutrotrauma. 1998;15(3):191–197. doi: 10.1089/neu.1998.15.191. [DOI] [PubMed] [Google Scholar]
- 221.Kroppenstedt SN, Schneider GH, Thomale UW, Unterberg AW. Neuroprotective properties of aptiganel HCl (Cerestat) following controlled cortical impact injury. J. Neurotrauma. 1998;15(3):191–197. doi: 10.1089/neu.1998.15.191. [DOI] [PubMed] [Google Scholar]
- 222.Dyker AG, Edwards KR, Fayad PB, Hormes JT, Lees KR. Safety and tolerability study of aptiganel hydrochloride in patients with an acute ischemic stroke. Stroke. 1999;30(10):2038–2042. doi: 10.1161/01.str.30.10.2038. [DOI] [PubMed] [Google Scholar]
- 223.Albers GW, Goldstein LB, Hall D, Lesko LM. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA. 2001;286(21):2673–2682. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- 224.Chizh BA, Headley PM, Tzschentke TM. NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol. Sci. 2001;22(12):636–642. doi: 10.1016/s0165-6147(00)01863-0. [DOI] [PubMed] [Google Scholar]
- 225.Gotti B, Duverger D, Bertin J, Carter C, Dupont R, Frost J, Gaudilliere B, MacKenzie ET, Rousseau J, Scatton B. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. I. Evidence for efficacy in models of focal cerebral ischemia. J. Pharmacol. Exp. Ther. 1988;247(3):1211–1221. [PubMed] [Google Scholar]
- 226.Toulmond S, Serrano A, Benavides J, Scatton B. Prevention by eliprodil (SL 82.0715) of traumatic brain damage in the rat. Existence of a large (18 h) therapeutic window. Brain Res. 1993;620(1):32–41. doi: 10.1016/0006-8993(93)90267-q. [DOI] [PubMed] [Google Scholar]
- 227.Okiyama K, Smith DH, White WF, Richter K, McIntosh TK. Effects of the novel NMDA antagonists CP-98,113, CP-101,581 and CP-101,606 on cognitive function and regional cerebral edema following experimental brain injury in the rat. J. Neurotrauma. 1997;14(4):211–222. doi: 10.1089/neu.1997.14.211. [DOI] [PubMed] [Google Scholar]
- 228.Tsuchida E, Rice M, Bullock R. The neuroprotective effect of the forebrain-selective NMDA antagonist CP101,606 upon focal ischemic brain damage caused by acute subdural hematoma in the rat. J. Neurotrauma. 1997;14(6):409–417. doi: 10.1089/neu.1997.14.409. [DOI] [PubMed] [Google Scholar]
- 229.Di X, Bullock R, Watson J, Fatouros P, Chenard B, White F, Corwin F. Effect of CP101,606, a novel NR2B subunit antagonist of the N-methyl-D-aspartate receptor, on the volume of ischemic brain damage off cytotoxic brain edema after middle cerebral artery occlusion in the feline brain. Stroke. 1997;28(11):2244–2251. doi: 10.1161/01.str.28.11.2244. [DOI] [PubMed] [Google Scholar]
- 230.Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J. Pharmacol. Exp. Ther. 1997;283(3):1285–1292. [PubMed] [Google Scholar]
- 231.Hogg S, Perron C, Barnéoud P, Sanger DJ, Moser PC. Neuroprotective effect of eliprodil: attenuation of a conditioned freezing deficit induced by traumatic injury of the right parietal cortex in the rat. J. Neurotrauma. 1998;15(7):545–553. doi: 10.1089/neu.1998.15.545. [DOI] [PubMed] [Google Scholar]
- 232.Bullock MR, Merchant RE, Carmack CA, Doppenberg E, Shah AK, Wildner KD, Ko G, Williams SA. An open-label study of CP-101,606 in subjects with a severe traumatic head injury or spontaneous intracerebral hemorrhage. Ann. N. Y. Acad. Sci. 1999;890:51–58. doi: 10.1111/j.1749-6632.1999.tb07980.x. [DOI] [PubMed] [Google Scholar]
- 233.Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT. Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-D-aspartate receptors. Exp. Neurol. 2000;163(1):239–243. doi: 10.1006/exnr.2000.7374. [DOI] [PubMed] [Google Scholar]
- 234.Nutt JG, Gunzler SA, Kirchhoff T, Hogarth P, Weaver JL, Krams M, Jamerson B, Menniti FS, Landen JW. Effects of a NR2B Selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov. Disord. 2008;23(13):1860–1866. doi: 10.1002/mds.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yurkewicz L, Weaver J, Bullock MR, Marshall LF. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J. Neurotrauma. 2005;22(12):1428–1443. doi: 10.1089/neu.2005.22.1428. [DOI] [PubMed] [Google Scholar]
- 236.Gill R, Alanine A, Bourson A, Buttelmann B, Fischer G, Heitz MP, Kew JN, Lavet-Trafit B, Lorez HP, Malherbe P, Miss MT, Mutel V, Pinard E, Roever S, Schmitt M, Trube G, Wybrecht R, Wyler R, Kemp JA. Pharmacological characterization of Ro 63-1908 (1-[2-(4-hydroxy-phenoxy)-ethyl]-4-(4-methyl-benzyl)-piperidin-4-ol), a novel subtype-selective N-methyl-D-aspartate antagonist. J. Pharmacol. Exp. Ther. 2002;302(3):940–948. doi: 10.1124/jpet.102.034322. [DOI] [PubMed] [Google Scholar]
- 237.Verhagen Metman L, Morris MJ, Farmer C, Gillespie M, Mosby K, Wuu J, Chase TN. Huntington’s disease: a randomized, controlled trial using the NMDA-antagonist amantadine. Neurology. 2002;59(5):694–699. doi: 10.1212/wnl.59.5.694. [DOI] [PubMed] [Google Scholar]
- 238.Sawada H, Oeda T, Kuno S, Nomoto M, Yamamoto K, Yamamoto M, Hisanaga K, Kawamura T. Amantadine for dyskinesias in Parkinson’s disease: a randomized controlled trial. PloS One. 2010;5(12):e15298. doi: 10.1371/journal.pone.0015298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ondo WG, Shinawi L, Davidson A, Lai D. Memantine for non-motor features of Parkinson’s disease: a double-blind placebo controlled exploratory pilot trial. Parkinsonism Relat. Disord. 2011;17(3):156–159. doi: 10.1016/j.parkreldis.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 240.Larsson V, Aarsland D, Ballard C, Minthon L, Londos E. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson’s disease dementia. Int. J. Geriatr. Psychiatry. 2010;25(10):1030–1038. doi: 10.1002/gps.2506. [DOI] [PubMed] [Google Scholar]
- 241.Litvinenko IV, Odinak MM, Mogil'naya VI, Perstnev SV. Use of memantine (akatinol) for the correction of cognitive impairments in Parkinson’s disease complicated by dementia. Neurosci. Behav. Physiol. 2010;40(2):149–155. doi: 10.1007/s11055-009-9244-1. [DOI] [PubMed] [Google Scholar]
- 242.Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, Leroi I, Pozo-rodriguez F. Memantine in patients with Parkinson ’ s disease dementia or dementia with Lewy bodies : a double-blind , placebo-controlled , multicentre trial. Lancet Neurol. 8(7):613–618. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- 243.Weiner MW, Sadowsky C, Saxton J, Hofbauer RK, Graham SM, Yu SY, Li S, Hsu HA, Suhy J, Fridman M, Perhach JL. Magnetic resonance imaging and neuropsychological results from a trial of memantine in Alzheimer’s disease. Alzheimers Dement. 2011;7(4):425–435. doi: 10.1016/j.jalz.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 244.Knoller N, Levi L, Shoshan I, Reichenthal E, Razon N, Rappaport ZH, Biegon A. Dexanabinol (HU-211) in the treatment of severe closed head injury: a randomized, placebo-controlled, phase II clinical trial. Crit. Care Med. 2002;30(3):548–554. doi: 10.1097/00003246-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 245.Maas AI, Murray G, Henney H, 3rd, Kassem N, Legrand V, Mangelus M, Muizelaar JP, Stocchetti N, Knoller N. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5(1):38–45. doi: 10.1016/S1474-4422(05)70253-2. [DOI] [PubMed] [Google Scholar]
- 246.Małek R, Borowicz KK, Kimber-Trojnar Z, Sobieszek G, Piskorska B, Czuczwar SJ. Remacemide – a novel potential antiepileptic drug. Pol. J. Pharmacol. 2003;55(5):691–698. [PubMed] [Google Scholar]
- 247.Nehlig A, Boehrer A. Effects of remacemide in two models of genetically determined generalized epilepsy, the GAERS and the audiogenic Wistar AS. Epilepsy Res. 2003;52(3):253–261. doi: 10.1016/s0920-1211(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 248.Devinsky O, Vazquez B, Faught E, Leppik IE, Pellock JM, Schachter S, Alderfer V, Holdich TA. A double-blind, placebo-controlled study of remacemide hydrochloride in patients with refractory epilepsy following pre-surgical assessment. Seizure. 2002;11(6):371–376. doi: 10.1053/seiz.2001.0669. [DOI] [PubMed] [Google Scholar]
- 249.Halonen T, Nissinen J, Pitkänen A. Neuroprotective effect of remacemide hydrochloride in a perforant pathway stimulation model of status epilepticus in the rat. Epilepsy Res. 1999;34(2-3):251–269. doi: 10.1016/s0920-1211(98)00122-3. [DOI] [PubMed] [Google Scholar]
- 250.Shoulson I, Penney J, McDermott MM, Schwid S, Kayson E, Chase T, Fahn S, Greenamyre JT, Lang A, Siderowf A, Pearson N, Harrison M, Rost E, Colcher A, Lloyd M, Matthews M, Pahwa R, Mcguire D, Lew MF, Schuman S, Marek K, Broshjeit S, Factor S, Brown D, Feigin A, Mazurkiewicz J, Ford B, Jennings D, Dillon S, Comella S, Blasucci L, Janko K, Shulman L, Wiener W, Bateman-Rodriguez D, Carrion A, Suchowersky O, Lafontaine AL, Pantella C, Siemers E, Belden J, Davies R, Lannon M, Grimes D, Gray P, Martin W, Kennedy L, Adler C, Newman S, Hammerstad J, Stone C, Lewitt P, Bardram K, Mistura K, Miyasaki J, Johnston L, Cha JH, Tennis M, Panniset M, Hall J, Tetrud J, Friedlander J, Hauser R, Gauger L, Rodnitzky R, Deleo A, Dobson J, Seeberger L, Dingmann C, Tarsy D, Ryan P, Elmer L, Ruzicka D, Stacy M, Brewer M, Locke B, Baker D, Casaceli C, Day D, Florack M, Hodgeman K, Laroia N, Nobel R, Orme C, Rexo L, Rothenburgh K, Sulimowicz K, Watts A, Wratni E, Tariot P, Cox C, Leventhal C, Alderfer V, Craun AM, Frey J, McCree L, McDermott J, Cooper J, Holdich T, Read B. A randomized, controlled trial of remacemide for motor fluctuations in Parkinson's disease. Neurology. 2001;56(4):455–462. doi: 10.1212/wnl.56.4.455. [DOI] [PubMed] [Google Scholar]
- 251.Zoladz PR, Campbell AM, Park CR, Schaefer D, Danysz W, Diamond DM. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol. Biochem. Behav. 2006;85(2):298–306. doi: 10.1016/j.pbb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 252.Lermontova NN, Lukoyanov NV, Serkova TP, Lukoyanova EA, Bachurin SO. Dimebon improves learning in animals with experimental Alzheimer’s disease. Bull. Exp. Biol. Med. 2000;129(6):544–546. doi: 10.1007/BF02434871. [DOI] [PubMed] [Google Scholar]
- 253.Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372(9634):207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 254.Goff DC, Tsai G, Manoach DS, Coyle JT. Dose-finding trial of D-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am. J. Psychiatry. 1995;152(8):1213–1215. doi: 10.1176/ajp.152.8.1213. [DOI] [PubMed] [Google Scholar]
- 255.Heresco-Levy U, Javitt DC, Ermilov M, Silipo G, Shimoni J. Double-blind, placebo-controlled, crossover trial of D-cycloserine adjuvant therapy for treatment-resistant schizophrenia. Int. J. Neuropsychopharmacol. 1998;1(2):131–135. doi: 10.1017/S1461145798001242. [DOI] [PubMed] [Google Scholar]
- 256.Goff DC. D-Cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr. Bull. 2012;38(5):936–941. doi: 10.1093/schbul/sbs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Harty TP, Rogawski MA. Felbamate block of recombinant N-methyl-D-aspartate receptors: selectivity for the NR2B subunit. Epilepsy Res. 2000;39(1):47–55. doi: 10.1016/s0920-1211(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 258.De Sarro G, Ongini E, Bertorelli R, Aguglia U, De Sarro A. Anticonvulsant activity of 5, 7DCKA, NBQX, and felbamate against some chemoconvulsants in DBA/2 mice. Pharmacol. Biochem. Behav. 1996;55(2):281–287. doi: 10.1016/s0091-3057(96)00085-8. [DOI] [PubMed] [Google Scholar]
- 259.Krishtal O. The ASICs: Signaling molecules? Modulators? Trends Neurosci. 2003;26(9):477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 260.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29(10):578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 261.Krishtal OA, Pidoplichko VI. Receptor for protons in the membrane of sensory neurons. Brain Res. 1981;214(1):150–154. doi: 10.1016/0006-8993(81)90446-7. [DOI] [PubMed] [Google Scholar]
- 262.Krishtal OA, Pidoplichko VI. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6(12):2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- 263.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386(6621):173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 264.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449(7160):316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 265.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32(6):1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 266.Champigny G, Voilley N, Waldmann R, Lazdunski M. Mutations causing neurodegeneration in Caenorhabditis elegans drastically alter the pH sensitivity and inactivation of the mammalian H+-gated Na+ channel MDEG1. J. Biol. Chem. 1998;273(25):15418–15422. doi: 10.1074/jbc.273.25.15418. [DOI] [PubMed] [Google Scholar]
- 267.Eskandari S, Snyder PM, Kreman M, Zampighi GA, Welsh MJ, Wright EM. Number of subunits comprising the epithelial sodium channel. J. Biol. Chem. 1999;274(38):27281–27286. doi: 10.1074/jbc.274.38.27281. [DOI] [PubMed] [Google Scholar]
- 268.Carnally SM, Dev HS, Stewart AP, Barrera NP, Van Bemmelen MX, Schild L, Henderson RM, Edwardson JM. Direct visualization of the trimeric structure of the ASIC1a channel, using AFM imaging. Biochem. Biophys. Res. Commun. 2008;372(4):752–755. doi: 10.1016/j.bbrc.2008.05.100. [DOI] [PubMed] [Google Scholar]
- 269.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. U S A. 2002;99(4):2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Li M, Kratzer E, Inoue K, Simon RP, Xiong ZG. Developmental change in the electrophysiological and pharmacological properties of acid-sensing ion channels in CNS neurons. J. Physiol. 2010;588(Pt20):3883–3900. doi: 10.1113/jphysiol.2010.192922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tolino LA, Okumura S, Kashlan OB, Carattino MD. Insights into the mechanism of pore opening of acid-sensing ion channel 1a. J. Biol. Chem. 2011;286(18):16297–16307. doi: 10.1074/jbc.M110.202366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S, Lingueglia E. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol. Ther. 2010;128(3):549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 273.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407(6807):1007–1111. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 274.García-Añoveros J, Samad TA, Zuvela-Jelaska L, Woolf CJ, Corey DP. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. J. Neurosci. 2001;21(8):2678–2686. doi: 10.1523/JNEUROSCI.21-08-02678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons a possible mediator of myocardial ischemic sensation. Circ. Res. 1999;84(8):921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 276.Yiangou Y, Facer P, Smith JA, Sangameswaran L, Eglen R, Birch R, Knowles C, Williams N, Anand P. Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. Eur. J. Gastroenterol. Hepatol. 2001;13(8):891–896. doi: 10.1097/00042737-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 277.Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol. Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem. Biophys. Res. Commun. 2005;337(1):349–354. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 279.Tan J, Ye X, Xu Y, Wang H, Sheng M, Wang F. Acid-sensing ion channel 1a is involved in retinal ganglion cell death induced by hypoxia. Mol. Vis. 2011;17:3300–3308. [PMC free article] [PubMed] [Google Scholar]
- 280.Ettaiche M, Deval E, Pagnotta S, Lazdunski M, Lingueglia E. Acid-sensing ion channel 3 in retinal function and survival. Invest. Opthamol. Vis. Sci. 2009;50(5):2417–2426. doi: 10.1167/iovs.08-3028. [DOI] [PubMed] [Google Scholar]
- 281.Hildebrand MS, deSilva MG, Klockars T, Rose E, Price M, Smith RJ, McGuirt WT, Christopoulos H, Petit C, Dahl HH. Characterisation of DRASIC in the mouse inner ear. Hear. Res. 2004;190(1-2):149–160. doi: 10.1016/S0378-5955(04)00015-2. [DOI] [PubMed] [Google Scholar]
- 282.Shimada S, Ueda T, Ishida Y, Yamamoto T, Ugawa S. Acid-sensing ion channels in taste buds. Arch. Histol. Cytol. 2006;69(4):227–231. doi: 10.1679/aohc.69.227. [DOI] [PubMed] [Google Scholar]
- 283.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J. Neurosci. 2003;23(13):5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J. Biol. Chem. 2004;279(12):11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- 285.Bassilana F, Champigny G, Waldmann R, de Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J. Biol. Chem. 1997;272(46):28819–28822. doi: 10.1074/jbc.272.46.28819. [DOI] [PubMed] [Google Scholar]
- 286.Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc. Natl. Acad. Sci. U S A. 2004;101(10):3621–3626. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139(5):1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Vergo S, Craner MJ, Etzensperger R, Attfield K, Friese MA, Newcombe J, Esiri M, Fugger L. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134(Pt 2):571–584. doi: 10.1093/brain/awq337. [DOI] [PubMed] [Google Scholar]
- 289.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J. Neurosci. 2009;29(17):5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, 3rd, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. 2008;11(7):816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Luszczki JJ, Sawicka KM, Kozinska J, Dudra-Jastrzebska M, Czuczwar SJ. Amiloride enhances the anticonvulsant action of various antiepileptic drugs in the mouse maximal electroshock seizure model. J. Neural Trans. 2009;116(1):57–66. doi: 10.1007/s00702-008-0152-2. [DOI] [PubMed] [Google Scholar]
- 292.Lv RJ, He JS, Fu YH, Zhang YQ, Shao XQ, Wu LW, Lu Q, Jin LR, Liu H. ASIC1a polymorphism is associated with temporal lobe epilepsy. Epilepsy Res. 2011;96(1-2):74–80. doi: 10.1016/j.eplepsyres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 293.Vila-Carriles WH, Kovacs GG, Jovov B, Zhou ZH, Pahwa AK, Colby G, Esimai O, Gillespie GY, Mapstone TB, Markert JM, Fuller CM, Bubien JK, Benos DJ. Surface expression of ASIC2 inhibits the amiloride-sensitive current and migration of glioma cells. J. Biol. Chem. 2006;281(28):19220–19232. doi: 10.1074/jbc.M603100200. [DOI] [PubMed] [Google Scholar]
- 294.Wong HK, Bauer PO, Kurosawa M, Goswami A, Washizu C, Machida Y, Tosaki A, Yamada M, Knöpfel T, Nakamura T, Nukina N. Blocking acid-sensing ion channel 1 alleviates Huntington’s disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum. Mol. Genet. 2008;17(20):3223–3235. doi: 10.1093/hmg/ddn218. [DOI] [PubMed] [Google Scholar]
- 295.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat. Med. 2007;13(12):1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 296.Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, Xu L, Chen J, Xu TL. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J. Neurosci. 2007;27(41):11139–11148. doi: 10.1523/JNEUROSCI.3364-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gélot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat. Neurosci. 2007;10(8):943–945. doi: 10.1038/nn1940. [DOI] [PubMed] [Google Scholar]
- 298.Gu Q, Lee LY. Acid-sensing ion channels and pain. Pharmaceuticals. 2010;3(5):1411–1425. doi: 10.3390/ph3051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Deval E, Noël J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E. Acid-sensing ion channels in postoperative pain. J. Neurosci. 2011;31(16):6059–6066. doi: 10.1523/JNEUROSCI.5266-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Maren S. An acid-sensing channel sows fear and panic. Cell. 2009;139(5):867–869. doi: 10.1016/j.cell.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 301.Dubé GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honoré P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117(1-2):88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 302.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 2002;82(3):735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 303.Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004;23(7):1516–1525. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. 2001;21(20):8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chen X, Qiu L, Li M, Dürrnagel S, Orser BA, Xiong ZG, MacDonald JF. Diarylamidines: high potency inhibitors of acid-sensing ion channels. Neuropharmacology. 2010;58(7):1045–1053. doi: 10.1016/j.neuropharm.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Garza A, López-Ramírez O, Vega R, Soto E. The aminoglycosides modulate the acid-sensing ionic channel currents in dorsal root ganglion neurons from the rat. J. Pharmacol. Exp. Ther. 2010;332(2):489–499. doi: 10.1124/jpet.109.152884. [DOI] [PubMed] [Google Scholar]
- 307.Ugawa S, Ishida Y, Ueda T, Inoue K, Nagao M, Shimada S. Nafamostat mesilate reversibly blocks acid-sensing ion channel currents. Biochem. Biophys. Res. Commun. 2007;363(1):203–208. doi: 10.1016/j.bbrc.2007.08.133. [DOI] [PubMed] [Google Scholar]
- 308.Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Ménez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J. Biol. Chem. 2000;275(33):25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- 309.Escoubas P, Bernard D, Lambeau G, Lazdunski M, Darbon H. Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci. 2003;12(7):1332–1343. doi: 10.1110/ps.0307003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Diochot S, Salinas M, Baron A, Escoubas P, Lazdunski M. Peptides inhibitors of acid-sensing ion channels. Toxicon. 2007;49(2):271–284. doi: 10.1016/j.toxicon.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 311.Chen X, Orser BA, MacDonald JF. Design and screening of ASIC inhibitors based on aromatic diamidines for combating neurological disorders. Eur. J. Pharmacol. 2010;648(1-3):15–23. doi: 10.1016/j.ejphar.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 312.Chen X, Kalbacher H, Gründer S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J. Gen. Physiol. 2005;126(1):71–79. doi: 10.1085/jgp.200509303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chen X, Kalbacher H, Gründer S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J. Gen. Physiol. 2006;127(3):267–276. doi: 10.1085/jgp.200509409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Cheng W, Sun C, Zheng J. Heteromerization of TRP channel subunits: extending functional diversity. Protein Cell. 2010;1(9):802–810. doi: 10.1007/s13238-010-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. U S A. 1995;92(21):9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Blackshaw LA, Brierley SM, Hughes PA. TRP channels: new targets for visceral pain. Gut. 2010;59(1):126–135. doi: 10.1136/gut.2009.179523. [DOI] [PubMed] [Google Scholar]
- 317.Venkatachalam K, Montell C. TRP channels. Annu. Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J. Neurochem. 2010;113(2):313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.García-Sanz N, Fernández-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sánchez E, Fernández-Ballester G, Ferrer-Montiel A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J. Neurosci. 2004;24(23):5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat. Rev. Neurosci. 2001;2(6):387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 321.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427(6971):260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 322.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 323.McNamara C R, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl. Acad. Sci. U S A. 2007;104(33):13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Schaefer M. Homo- and heteromeric assembly of TRP channel subunits. Pflügers Arch. 2005;451(1):35–42. doi: 10.1007/s00424-005-1467-6. [DOI] [PubMed] [Google Scholar]
- 325.Guadalupe A, Bacigalupo J. TRP channels as biological sensors. Physiol. Mini-Rev. 2008;3:25–33. [Google Scholar]
- 326.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses. 2007;32(1):41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 327.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12(3):218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lessard CB, Lussier MP, Cayouette S, Bourque G, Boulay G. The overexpression of presenilin2 and Alzheimer’s-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cell. Signal. 2005;17(4):437–445. doi: 10.1016/j.cellsig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 329.Yamamoto S, Wajima T, Hara Y, Nishida M, Mori Y. Transient receptor potential channels in Alzheimer’s disease. Biochim. Biophys. Acta. 2007;1772(8):958–967. doi: 10.1016/j.bbadis.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 330.Roedding AS, Gao AF, Au-Yeung W, Scarcelli T, Li PP, Warsh JJ. Effect of oxidative stress on TRPM2 and TRPC3 channels in B lymphoblast cells in bipolar disorder. Bipolar Disord. 2012;14(2):151–161. doi: 10.1111/j.1399-5618.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 331.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc. Natl. Acad. Sci. U S A. 2006;103(5):1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.MacDonald JF, Xiong ZG, Jackson MF. Paradox of Ca2+ signaling, cell death and stroke. Trends Neurosci. 2006;29(2):75–81. doi: 10.1016/j.tins.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 333.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87(1):165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 334.Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J. Biol. Chem. 2005;280(3):2132–2140. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Selvaraj S, Watt JA, Singh BB. TRPC1 inhibits apoptotic cell degeneration induced by dopaminergic neurotoxin MPTP/MPP(+) Cell Calcium. 2009;46(3):209–218. doi: 10.1016/j.ceca.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Chahl LA. TRP’s: links to schizophrenia? Biochim. Biophys Acta. 2007;1772(8):968–977. doi: 10.1016/j.bbadis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 337.Chahl LA. TRP channels and psychiatric disorders. Adv. Exp. Med. Biol. 2011;704:987–1009. doi: 10.1007/978-94-007-0265-3_51. [DOI] [PubMed] [Google Scholar]
- 338.Li M, Chen C, Zhou Z, Xu S, Yu Z. A TRPC1-mediated increase in store-operated Ca2+ entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium. 2012;51(6):486–496. doi: 10.1016/j.ceca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 339.Berrout J, Jin M, O’Neil RG. Critical role of TRPP2 and TRPC1 channels in stretch-induced injury of blood-brain barrier endothelial cells. Brain Res. 2012;1436:1–12. doi: 10.1016/j.brainres.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 340.Encabo A, Romanin C, Birke FW, Kukovetz WR, Groschner K. Inhibition of a store-operated Ca2+ entry pathway in human endothelial cells by the isoquinoline derivative LOE 908. Br. J. Pharmacol. 1996;119(4):702–706. doi: 10.1111/j.1476-5381.1996.tb15729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Christensen T, Wienrich M, Ensinger HA, Diemer NH. The broad-spectrum cation channel blocker pinokalant (LOE 908 MS) reduces brain infarct volume in rats: a temperature-controlled histological study. Basic Clin. Pharmacol. Toxicol. 2005;96(4):316–324. doi: 10.1111/j.1742-7843.2005.pto960407.x. [DOI] [PubMed] [Google Scholar]
- 342.Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res. Brain Res. Rev. 1998;26(2-3):113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 343.Chebib M, Johnston GA. GABA-Activated ligand gated ion channels: medicinal chemistry and molecular biology. J. Med. Chem. 2000;43(8):1427–1447. doi: 10.1021/jm9904349. [DOI] [PubMed] [Google Scholar]
- 344.Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog. Biophys. Mol. Biol. 2005;87(1):33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J. Neurosci. 2005;25(50):11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends neurosci. 1996;19(4):139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 347.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101(4):815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 348.Feng HJ. Allosteric modulation of αβδ GABAA receptors. Pharmaceuticals. 2010;3:3461–3477. [Google Scholar]
- 349.Dibbens LM, Feng HJ, Richards M C, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum. Mol. Genet. 2004;13(13):1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- 350.Qi JS, Yao J, Fang C, Luscher B, Chen G. Downregulation of tonic GABA currents following epileptogenic stimulation of rat hippocampal cultures. J. Physiol. 2006;577(2):579–590. doi: 10.1113/jphysiol.2006.113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Jones-Davis DM, Macdonald RL. GABA(A) receptor function and pharmacology in epilepsy and status epilepticus. Curr. Opin Pharmacol. 2003;3(1):12–18. doi: 10.1016/s1471-4892(02)00015-2. [DOI] [PubMed] [Google Scholar]
- 352.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111(2):231–239. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 353.Laposky AD, Homanics GE, Basile A, Mendelson WB. Deletion of the GABA(A) receptor beta 3 subunit eliminates the hypnotic actions of oleamide in mice. Neuroreport. 2001;12(18):4143–4147. doi: 10.1097/00001756-200112210-00056. [DOI] [PubMed] [Google Scholar]
- 354.Taylor M, Bhagwagar Z, Cowen PJ, Sharp T. GABA and mood disorders. Psychol. Med. 2003;33(3):387–393. doi: 10.1017/s0033291702006876. [DOI] [PubMed] [Google Scholar]
- 355.Korpi ER, Gründer G, Lüddens H. Drug interactions at GABA(A) receptors. Prog. Neurobiol. 2002;67(2):113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 356.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol. Rev. 1995;47(2):181–234. [PubMed] [Google Scholar]
- 357.Griebel G, Perrault G, Simiand J, Cohen C, Granger P, Depoortere H, Françon D, Avenet P, Schoemaker H, Evanno Y, Sevrin M, George P, Scatton B. SL651498, a GABAA receptor agonist with subtype-selective efficacy, as a potential treatment for generalized anxiety disorder and muscle spasms. CNS Drug Rev. 2003;9(1):3–20. doi: 10.1111/j.1527-3458.2003.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Licata SC, Platt DM, Cook JM, Sarma PV, Griebel G, Rowlett JK. Contribution of GABAA receptor subtypes to the anxiolytic-like, motor, and discriminative stimulus effects of benzodiazepines: studies with the functionally selective ligand SL651498 [6-fluoro-9-methyl-2-phenyl-4-(pyrrolidin-1-yl-carbonyl)-2,9-dihydro-1H-pyridol[3,4-b]indol-1-one] J. Pharmacol. Exp. Ther. 2005;313(3):1118–1125. doi: 10.1124/jpet.104.081612. [DOI] [PubMed] [Google Scholar]
- 359.de Haas SL, Franson KL, Schmitt JA, Cohen AF, Fau JB, Dubruc C, van Gerven JM. The pharmacokinetic and pharmacodynamic effects of SL65.1498, a GABA-A alpha2,3 selective agonist, in comparison with lorazepam in healthy volunteers. J. Psychopharmacol. 2009;23(6):625–632. doi: 10.1177/0269881108092595. [DOI] [PubMed] [Google Scholar]
- 360. Atack JR, Wafford KA, Street LJ, Dawson GR, Tye S, Van Laere K, Bormans G, Sanabria-Bohórquez SM, De Lepeleire I, de Hoon JN, Van Hecken A, Burns HD, McKernan RM, Murphy MG, Hargreaves RJ. MRK-409 (MK-0343), a GABAA receptor subtype-selective partial agonist, is a non-sedating anxiolytic in preclinical species but causes sedation in humans. J. Psychopharmacol. 2011;25(3):314–328. doi: 10.1177/0269881109354927. [DOI] [PubMed] [Google Scholar]
- 361.de Haas SL, de Visser SJ, van der Post JP, Schoemaker RC, van Dyck K, Murphy MG, de Smet M, Vessey LK, Ramakrishnan R, Xue L, Cohen AF, van Gerven JM. Pharmacodynamic and pharmacokinetic effects of MK-0343, a GABA(A) alpha2,3 subtype selective agonist, compared to lorazepam and placebo in healthy male volunteers. J. Psychopharmacol. 2008;22(1):24–32. doi: 10.1177/0269881107082108. [DOI] [PubMed] [Google Scholar]
- 362.Geffen Y, Nudelman A, Gil-Ad I, Rephaeli A, Huang M, Savitsky K, Klapper L, Winkler I, Meltzer HY, Weizman A. BL-1020: a novel antipsychotic drug with GABAergic activity and low catalepsy, is efficacious in a rat model of schizophrenia. Eur. Neuropsychopharmacol. 2009;19(1):1–13. doi: 10.1016/j.euroneuro.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 363.Anand R, Geffen Y, Vasile D, Dan I. An open-label tolerability study of BL-1020 antipsychotic: a novel gamma aminobutyric acid ester of perphenazine. Clin. Neuropharmacol. 2010;33(6):297–302. doi: 10.1097/WNF.0b013e3181f8d501. [DOI] [PubMed] [Google Scholar]
- 364.Fitzgerald PB. BL-1020, an oral antipsychotic agent that reduces dopamine activity and enhances GABAA activity, for the treatment of schizophrenia. Curr. Opin. Investig. Drugs. 2010;11(1):92–100. [PubMed] [Google Scholar]
- 365.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat. Neurosci. 2000;3(6):587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 366.Licata SC, Platt DM, Rüedi-Bettschen D, Atack JR, Dawson GR, Van Linn ML, Cook JM, Rowlett JK. Discriminative stimulus effects of L-838,417 (7-tert-butyl-3-(2,5-difluoro-phenyl)-6-(2-methyl-2H-[1,2,4]triazol-3-ylmethoxy)-[1,2,4]triazolo[4,3-b]pyridazine): role of GABA(A) receptor subtypes. Neuropharmacology. 2010;58(2):357–364. doi: 10.1016/j.neuropharm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc. Natl. Acad. Sci. U S A. 2005;102(3):915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Mathiasen L, Mirza NR. A comparison of chlordiazepoxide, bretazenil, L838, 417 and zolpidem in a validated mouse Vogel conflict test. Psychopharmacology (Berl.) 2005;182(4):475–484. doi: 10.1007/s00213-005-0119-z. [DOI] [PubMed] [Google Scholar]
- 369.Rudolph U, Crestani F, Möhler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol. Sci. 2001;22(4):188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- 370.Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal B, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, Ragan I, Kerby J, Street L, Carling R, Castro JL, Whiting P, Dawson GR, McKernan RM. TPA023 [7-(1,1-dimethyl)-6-(2-ethyl-2H-1,2,4-triazol-3ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for alpha2- and alpha3-containing GABAA receptors , is a nonsedating anxiolytic in rodents and primates. J. Pharmacol. Exp. Ther. 2006;316(1):410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- 371.de Haas SL, de Visser SJ, van der Post JP, de Smet M, Schoemaker RC, Rijnbeek B, Cohen AF, Vega JM, Agrawal NG, Goel TV, Simpson RC, Pearson LK, Li S, Hesney M, Murphy MG, van Gerven JM. Pharmacodynamic and pharmacokinetic effects of TPA023, a GABA(A) alpha(2,3) subtype-selective agonist, compared to lorazepam and placebo in healthy volunteers. J. Psychopharmacol. 2007;21(4):374–383. doi: 10.1177/0269881106072343. [DOI] [PubMed] [Google Scholar]
- 372.Lydiard RB, Lankford DA, Seiden DJ, Landin R, Farber R, Walsh JK. Efficacy and tolerability of modified-release indiplon in elderly patients with chronic insomnia: results of a 2-week double-blind, placebo-controlled trial. J. Clin. Sleep Med. 2006;2(3):309–315. [PubMed] [Google Scholar]
- 373.Scharf MB, Black J, Hull S, Landin R, Farber R. Long-term nightly treatment with indiplon in adults with primary insomnia: results of a double-blind, placebo-controlled, 3-month study. Sleep. 2007;30(6):743–752. doi: 10.1093/sleep/30.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Rosenberg R, Roth T, Scharf MB, Lankford DA, Farber R. Efficacy and tolerability of indiplon in transient insomnia. J. Clin. Sleep Med. 2007;3(4):374–379. [PMC free article] [PubMed] [Google Scholar]
- 375.Yasumatsu H, Morimoto Y, Yamamoto Y, Takehara S, Fukuda T, Nakao T, Setoguchi M. The pharmacological properties of Y-23684, a benzodiazepine receptor partial agonist. Br. J. Pharmacol. 1994;111(4):1170–1178. doi: 10.1111/j.1476-5381.1994.tb14868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Griebel G, Perrault G, Sanger DJ. Differences in anxiolytic-like profile of two novel nonbenzodiazepine BZ (omega) receptor agonists on defensive behaviors of mice. Pharmacol. Biochem. Behav. 1999;62(4):689–694. doi: 10.1016/s0091-3057(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 377.Froestl W. An historical perspective on GABAergic drugs. Future Med. Chem. 2011;3(2):163–175. doi: 10.4155/fmc.10.285. [DOI] [PubMed] [Google Scholar]
- 378.Chebib M, Hinton T, Schmid KL, Brinkworth D, Qian H, Matos S, Kim HL, Abdel-Halim H, Kumar RJ, Johnston GAR, Hanrahan JR. Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J. Pharmacol. Exp. Ther. 2009;328(2):448–457. doi: 10.1124/jpet.108.146464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Ng CK, Kim HL, Gavande N, Yamamoto I, Kumar RJ, Mewett KN, Johnston GA, Hanrahan JR, Chebib M. Medicinal chemistry of ρ GABAC receptors. Future Med. Chem. 2011;3(2):197–209. doi: 10.4155/fmc.10.286. [DOI] [PubMed] [Google Scholar]