Abstract
This guideline presents recommendations for the diagnosis and management of patients with celiac disease. Celiac disease is an immune-based reaction to dietary gluten (storage protein for wheat, barley and rye) that primarily affects the small intestine in those with a genetic predisposition and resolves with exclusion of gluten from the diet. There has been a substantial increase in the prevalence of celiac disease over the last 50 years and an increase in the rate of diagnosis in the last 10 years. Celiac disease can present with many symptoms, including typical gastrointestinal symptoms (e.g. diarrhea, steatorrhea, weight loss, bloating, flatulence, abdominal pain) and also non-gastrointestinal abnormalities (e.g. abnormal liver function tests, iron deficiency anemia, bone disease, skin disorders, and many other protean manifestations). Indeed, many individuals with celiac disease may have no symptoms at all. Celiac disease is usually detected by serologic testing of celiac-specific antibodies. The diagnosis is confirmed by duodenal mucosal biopsies. Both serology and biopsy should be performed on a gluten-containing diet. The treatment for celiac disease is primarily a gluten-free diet (GFD), which requires significant patient education, motivation, and follow-up. Non-responsive celiac disease occurs frequently, particularly in those diagnosed in adulthood. Persistent or recurring symptoms should lead to a review of the patient’s original diagnosis to exclude alternative diagnoses, a review of the GFD to ensure there is no obvious gluten contamination, and serologic testing to confirm adherence with the GFD. In addition, evaluation for disorders associated with celiac disease that could cause persistent symptoms, such as microscopic colitis, pancreatic exocrine dysfunction, and complications of celiac disease, such as enteropathy-associated lymphoma or refractory celiac disease, should be entertained. Newer therapeutic modalities are being studied in clinical trials, but are not yet approved for use in practice. Given the incomplete response of many patients to a GFD free diet as well as the difficulty of adherence to the GFD over the long term, development of new effective therapies for symptom control and reversal of inflammation and organ damage are needed. The prevalence of celiac disease is increasing worldwide and many patients with celiac disease remain undiagnosed, highlighting the need for improved strategies in the future for the optimal detection of patients.
INTRODUCTION
This clinical guideline addresses the diagnosis, treatment, and overall management of patients with celiac disease, including an approach to the evaluation of non-responsive celiac disease. While it is primarily directed at the care of adult patients, variations pertinent to the pediatric population have been included.
Each section will provide specific recommendations based on the current literature and a summary of the evidence supporting those recommendations. The GRADE system was used to evaluate the quality of supporting evidence.1 (Table 1) A “strong” recommendation was made when the benefits clearly outweigh the negatives and the result of no action. “Conditional” was used when some uncertainty remained about the balance of benefit/potential harm. The quality of the evidence was graded from high to low. “High” quality evidence indicates that further research is unlikely to change the authors’ confidence in the estimate of effect. “Moderate” quality evidence indicates that further research would be likely to have an impact on the confidence of the estimate, whereas “Low” quality evidence indicates that further study would likely have an important impact on the confidence in the estimate of the effect and would likely change the estimate.
Table 1.
Criteria for assigning grade of evidence
| Type of Evidence |
| Randomized trial = high |
| Observational study = low |
| Any other evidence = very low |
Decrease grade if:
|
Increase grade if:
|
Definition of grades of evidence:
|
Reprinted with permission from Camilleri M, et al. Am J Gastroenterol 2013; 108(1): 18–37
WHEN TO TEST FOR CELIAC DISEASE
Recommendations
Patients with symptoms, signs, or laboratory evidence suggestive of malabsorption, such as chronic diarrhea with weight loss, steatorrhea, postprandial abdominal pain and bloating, should be tested for CD. (Strong recommendation, high level of evidence)
Patients with symptoms, signs, or laboratory evidence for which CD is a treatable cause should be considered for testing for CD. (Strong recommendation, moderate level of evidence)
Patients with a first degree family member who has a confirmed diagnosis of CD should be tested if they show possible signs or symptoms or laboratory evidence of CD. (Strong recommendation, high level of evidence)
Consider testing of asymptomatic relatives with a first degree family member who has a confirmed diagnosis of CD (Conditional recommendation, high level of evidence)
Celiac disease should be sought among the explanations for elevated serum aminotransferase levels when no other etiology is found, (Strong recommendation, high level of evidence)
Patients with type I diabetes mellitus should be tested for CD if there are any digestive symptoms, or signs, or laboratory evidence suggestive of celiac disease. (Strong recommendation, high level of evidence)
Summary of Evidence
Celiac disease is one of the most common causes of chronic malabsorption.2 This results from injury to the small intestine with loss of absorptive surface area, reduction of digestive enzymes, and consequential impaired absorption of micronutrients such as fat-soluble vitamins, iron and potentially B12 and folic acid. 3 In addition, the inflammation exacerbates symptoms of malabsorption by causing net secretion of fluid that can result in diarrhea. The failure of absorption of adequate calories leads to weight loss, and the malabsorption results in abdominal pain and bloating.3 These are common symptoms associated with CD.4, 5
Celiac disease remains under-diagnosed in the US.6 CD may present in many ways.7 Currently, active case-finding (serologic testing for CD in patients with symptoms or conditions closely associated with CD) is the favored strategy to increase detection of CD.8 Active case-finding may increase detection of CD among patients with symptoms attending a primary care office, although this strategy is insufficient to detect most patients with CD.7 There is no consensus regarding which symptoms, laboratory abnormalities, and/or associated diseases require evaluation for CD. The frequency of CD in common clinical scenarios varies from modestly elevated, such as irritable bowel syndrome, to substantially elevated, such as unexplained iron deficiency anemia (Table 2).9, 10, 11
Table 2.
Conditions in which CD occurs more frequently than in the general population and/or for whom a GFD may be beneficial.
| CD Common (>2 times prevalence of general population) | CD less Common but treatable |
|---|---|
| Symptomatic malabsorption | Pulmonary hemosiderosis |
| Diarrhea with weight loss | Unexplained male or female infertility |
| Chronic diarrhea with or without abdominal pain | Dyspepsia |
| Chronic iron deficiency and anemia | Amenorrhea |
| Metabolic bone disease and premature osteoporosis | Chronic fatigue |
| Postprandial bloating and gaseousness | Apparent malabsorption of thyroid replacement medication |
| Unexplained weight loss | Epilepsy or Ataxia |
| Abnormal elevated liver enzymes | Constipation |
| Incidental discovery of villous atrophy endoscopically or histologically | Recurrent abdominal pain |
| Dermatitis herpetiformis | |
| Peripheral neuropathy | |
| Oral aphthous ulcers | |
| Growth failure | |
| Discolored teeth or developmentally synchronous enamel loss | |
| Thyroid disease | |
| Irritable bowel syndrome | |
| Down’s and Turner’s syndromes |
The complexity of deciding who to test is exemplified by patients with dyspepsia. The prevalence of biopsy-proven CD in patients with dyspepsia is 1%, similar to that of the general population12 and therefore, systematic screening for CD is not recommended based on disease prevalence alone. However, treatment for dyspepsia can be a clinical challenge13 and dyspepsia as a symptom of CD, will readily respond to the gluten free diet. 4, 14 Thus, mucosal biopsies of the duodenum should be considered in patients with dyspepsia who undergo investigation with upper endoscopy because of persistent symptoms despite initial therapy, are aged >55 years old, and/or present alarm symptoms (e.g., weight loss or clinical evidence of anemia).15
The frequency of CD is substantially increased in patients who have a first degree family member affected with CD. 16, 17 The precise risk is highest in monozygous twins, next in human leucocyte antigen (HLA)-matched siblings, siblings, and then finally parents and children of patients with CD.16 A lower rate probably applies to second degree relatives.18 Members of families who have more than one individual already identified with CD are at higher risk of CD and recommendations for screening should extend to all other family members including second degree relatives.19 The estimates of prevalence of CD in family members varies substantially with one large multicenter study in the U.S. showing a rate as low as 5% in both first degree relatives and second relatives.18 Other studies, especially those that are community-based, show a rate that is substantially higher affecting up to 20% in siblings and 10% in other 1st degree relatives.16 The clinical implications are that newly-diagnosed patients with CD should inform their first degree family members of the potential increased risk for CD and the recommendation for testing. In addition, health care providers should determine whether there is a family history of CD in patients with symptoms or signs suggestive of CD and if so consider screening the patient.
Testing of truly symptomless first degree relatives is reasonable but controversial. Even those patients who initially thought themselves to be without symptoms on direct questioning at the time of detection, often report improved health after adapting to the gluten free diet because disappearance of symptoms that may not have been previously explained.20 Others may have symptoms that they did not consider abnormal until after they initiated a gluten-free diet (GFD) and these symptom resolve.21 Asymptomatic patients detected by screening do not experience new symptoms after onset of a GFD.22 The majority of patients with CD identified on the basis of screening reported dietary adherence and improvements in quality of life on the GFD.20 A small proportion of patients, however, report increased health-related anxiety after diagnosis. 23 Overall satisfaction with the diagnosis was high (93%).20
Abnormal liver blood tests, in particular elevations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are commonly seen in clinical care, although the prevalence of clinically significant liver disease is low.24 In CD, hypertransaminasemia is often a subclinical finding that is gluten dependent.25 Patients with unexplained elevation of liver enzymes should be assessed for CD. 26 There is reasonable data to show that gluten-dependent hypertransaminasemia will normalize in most patients (>95%) on a GFD.27 Rarely, CD can be associated with severe liver disease.28, 29
There is evidence that CD is substantially more common in patients with type I diabetes mellitus (DM) than in the general Caucasian population. The estimates vary between 3% and 10%.30–32 In children, it has been suggested that yearly or every-other-year screening for CD be undertaken utilizing serology. Patients with type I DM who are undergoing upper endoscopy should undergo duodenal biopsies to rule out CD if they have never been tested previously.
After gastrointestinal symptoms, the second most common manifestation of CD in patients with type I DM is diminished or impaired bone mineralization. There is some evidence suggesting that there is added disease burden to patients already struggling with the management of type I DM. In addition, there is good evidence that gastrointestinal symptoms present at diagnosis will respond to a GFD with overall improvement in quality of life related to GI symptoms. The impact of the treatment of CD on the management of type I DM is mixed. 33 Some data suggest an increase in absorption, leading to the need for increased insulin doses. Other data suggests improvement of DM controlled by reduction of hypoglycemic events, especially postprandial.
Testing for CD in asymptomatic patients with type I DM is controversial. No significant adverse outcomes were identified in children with type 1 DM identified by screening who delay therapy with a GFD for up to 2 years.34 However, it is necessary to look at the potential long term impact of CD in type I DM as well. 35 A large study from Sweden showed an increased risk of diabetic retinopathy in patients with coexistent type I DM and CD. 36 Patients with undiagnosed CD and type 1 DM had a higher prevalence of retinopathy (58% v. 25%) and nephropathy (42% vs. 4%).37 Treatment with a GFD for 1 year was safe in patients with coexistent type I DM and CD.37 The effect (if any) of a GFD on DM-related complications requires further investigation.
Parents of children with Type 1 DM or the children of parents with type 1 DM are at increased risk of CD, estimated to be ~4%. 38, 39, 40 Because many patients with unrecognized CD may actually have symptoms that improve on a gluten free diet, informing such parents of the risk of CD is suggested. Also, a family history of either CD or type 1 DM indicates an increased risk of CD in the patient and CD should be considered. There is no data to support a recommendation about when to stop screening for CD in children with type 1 DM but screening is not necessary in the absence of HLA DQ2 and DQ8.
DIAGNOSIS OF CELIAC DISEASE
Recommendations
IgA anti-tissue transglutaminase antibody is the preferred single test for detection of CD in individuals over the age of 2 years. (Strong recommendation, high level of evidence)
When there exists a high probability of CD the possibility of IgA deficiency is considered, total IgA should be measured. An alternative approach is to include both IgA and IgG based testing, such as IgG deamidated gliadin peptides [DGP], in these high probability patients. (Strong recommendation, moderate level of evidence)
In patients in whom low IgA or selective IgA deficiency is identified, IgG-based testing (IgG deamidated gliadin peptides [DGP] and IgG tissue transglutaminase [TTG]) should be performed. (Strong recommendation, moderate level of evidence)
If the suspicion of CD is high, intestinal biopsy should be pursued even if serologies are negative. (Strong recommendation, moderate level of evidence)
All diagnostic serologic testing should be done with patients on a gluten-containing diet. (Strong recommendation, high level of evidence)
Antibodies directed against native gliadin are not recommended for the primary detection of CD. (Strong recommendation, high level of evidence)
Combining several tests for CD in lieu of TTG IgA alone may marginally increase sensitivity for CD but reduces specificity therefore are not recommended in low risk populations. (Conditional recommendation, moderate level of evidence)
When screening children younger than 2 years of age for CD, the IgA TTG test should be combined with deamidated gliadin peptide (IgA and IgG). (Strong recommendation, moderate level of evidence)
Summary of Evidence
The use of TTG-IgA testing and its accuracy in the primary care setting and referral cohorts has been extensively studied.9 The sensitivity of the TTG-IgA for untreated CD is about 95%. 41 The specificity is also 95% or greater. The higher the titer of the test, there is greater likelihood of a true positive result. 9 The test is most commonly based on an enzyme-linked immunosorbent assay test and less commonly on radioimmunoassay.42 There are recognized differences in test performance between the various commercially available test kits, but overall there is consistency in the sensitivity and specificity of the test.42–44
In the past, several antibody tests have been developed to detect CD. 45 Antibodies may be directed against native or altered cereal derived peptides. Anti-gliadin antibodies (AGA) have been used for decades and are reasonably accurate when there is a high pretest prevalence of CD.46 However, it was with the advent of auto-antibodies, first directed against reticulin and then endomysium antibodies (EMA) and finally TTG antibodies that the truly celiac specific testing was developed.47 The identification of TTG immunoglobulin (Ig) A antibody as the target antigen for IgA EMA antibodies was a major advance.48 This antigen was initially produced by extraction from the liver or purification from human red cells and most recently, by recombinant protein production. Tissue transglutaminase based assays have brought accurate serology for CD into the reach of most doctors and hospitals. The College of American Pathology laboratory proficiency survey has included TTG antibody testing for several years and most laboratories in the US that provide TTG testing participate. Other similar systems are in place outside the US.
IgA deficiency is more common in CD than in the general population. It affects anywhere between 1 in 400 to 1 in 800 members of the general population, but in 2–3% of patients with CD and 1% of those getting tested for CD.49, 50 In patients in whom there is a high pre-test prevalence of CD, the measurement of IgA levels should be considered, especially if IgA-based celiac serology test is negative. One approach is to measure total IgA at the beginning of testing to determine whether IgA levels are sufficient and, if not, to incorporate IgG based testing into the serology testing cascade. Deamidated gliadin peptides IgG and/or TTG IgG would then be the preferred test in this circumstance.51, 52 EMA IgG is not widely available. It has been suggested that IgA deficiency should be considered if the TTG-IgA levels are undetectable.53, 54 However not all assays can detect this with any accuracy or the result is merely reported as negative. While there is limited data on the sensitivity of each of these tests for CD in an IgA deficient person this may be about 80%–90% individually and higher if tests are combined. If the suspicion for CD is high, intestinal biopsy should be pursued even when serologies are negative. Finding IgA deficiency should prompt evaluation for other diseases that may cause villous atrophy such as giardiasis, small-bowel bacterial overgrowth, or common variable immunodeficiency.55
The antibodies directed against gliadin or its deamidated products as well as the self-antigen TTG are dependent on the ingestion of gluten. The reduction or cessation of dietary gluten leads to a decrease in the levels of all these celiac-associated antibodies to normal concentrations. While little is known about the precise dynamics of the reduction, a weakly positive individual may become negative within weeks of strict adherence to GFD. 56 After 6–12 months of adhering to a GFD, 80% of subjects will test negative by serology.57 By 5 years, more than 90% of those adhering to the GFD will have negative serologies. 58
Whilst antibodies directed against native gliadin (anti-gliadin antibodies (AGA)) have been in use for several decades, there is a wide variability in their diagnostic accuracy.43 Both IgA and IgG AGA antibodies have sensitivities and specificities inferior to those of the TTG-IgA and DGP-IgA assays57 and should no longer be included in the routine testing strategy for CD.
No one test for CD has a perfect sensitivity or specificity. Thus, individual tests may be combined in commercially available panels. This strategy may increase the sensitivity if any positive test is regarded as an overall positive result; however, the increased sensitivity comes at the expense of a reduction of specificity. 59 Unless all patients who test positive in the panel undergo histological confirmation of CD, this practice may lead to incorrect and over diagnosis followed by unnecessary treatment with GFD. Conversely, if the threshold is set that all tests within the panel must be positive for a “positive” panel test, then the specificity and hence positive predictive value (PPV) for CD will be increased, but at the expense of sensitivity.9 One diagnostic approach is shown in Figure 1.
Figure 1.

Celiac disease diagnostic testing algorithm
There is some evidence that both TTG and EMA are less sensitive in young children (less than 2 years of age).60, 61 In this age group the sensitivity of AGA and DGP antibodies is higher than both the TTG and EMA. 61–63 In general, AGA have a low sensitivity and specificity and are not recommended as a screening test for CD. 64, 65 Although DGP tests perform less well than TTG and EMA tests, they are superior to the AGA. 66 For this reason it is preferable to combine the TTG with DGP tests when screening young children.
CONFIRMATORY TESTING IN CELIAC DISEASE
Recommendations
The confirmation of a diagnosis of CD should be based on a combination of findings from the medical history, physical examination, serology, and upper endoscopy with histological analysis of multiple biopsies of the duodenum. (Strong recommendation, high level of evidence)
Upper endoscopy with small bowel biopsy is a critical component of the diagnostic evaluation for persons with suspected CD and is recommended to confirm the diagnosis. (Strong recommendation, high level of evidence)
Multiple biopsies of the duodenum (one or two biopsies of the bulb and at least 4 biopsies of the distal duodenum) are recommended to confirm the diagnosis CD. (Strong recommendation, high level of evidence)
Lymphocytic infiltration of the intestinal epithelium in the absence of villous atrophy is not specific for CD and other causes should also be considered. (Strong recommendation, high level of evidence)
Summary of Evidence
Gastrointestinal symptoms alone cannot accurately differentiate CD from other common gastrointestinal disorders (e.g., 20–50% of patients with CD fulfilled Rome criteria for irritable bowel syndrome).4, 67 A meta-analysis showed a pooled prevalence of irritable bowel syndrome-type symptoms of 38% (95% confidence interval, 27%–50%) in patients with CD.68 Improvement of gastrointestinal symptoms or clinical exacerbation after re-introduction of gluten have very low PPV for CD (36% and 28%, respectively) and should not be used for diagnosis in the absence of other supportive evidence.69 Moreover, ingestion of gluten can cause gastrointestinal symptoms including abdominal pain and bloating in the absence of CD.70 A GFD improved gastrointestinal symptoms in about 60% of patients with diarrhea-predominant irritable bowel syndrome, especially those with HLA-DQ2.71
A positive CD specific serology (TTG, DGP, and EMA) in patients with villous atrophy confirms the diagnosis of CD.43 TTG-IgA may be negative in 5%–16% of patients with biopsy-confirmed CD tested when following a gluten-containing diet.41, 57 IgA EMA negative CD has been described in patients with normal IgA.72 Thus, a negative CD specific serology in patients with villous atrophy does not completely exclude the diagnosis of CD though it does make it much less likely. Other causes of villous atrophy are summarized in Table 3
Table 3.
Other causes of villous atrophy in duodenum
| Tropical sprue |
| Small-bowel bacterial overgrowth |
| Autoimmune enteropathy |
| Hypogammaglobulinemic sprue |
| Drug-associated enteropathy (e.g. olmesartan) |
| Whipple disease |
| Collagenous sprue |
| Crohn’s disease |
| Eosinophilic enteritis |
| Intestinal lymphoma |
| Intestinal tuberculosis |
| Infectious enteritis (e.g. giardiasis) |
| Graft versus host disease |
| Malnutrition |
| Acquired immune deficiency syndrome enteropathy |
Histological response to GFD in patients with villous atrophy strongly supports a diagnosis CD. HLA typing and histological response may help to rule out or confirm the diagnosis of CD in patients with sero-negative CD.73, 74
Small intestinal biopsy has been central to the confirmation of the diagnosis of CD since the late 50’s. 75 Traditionally, the diagnosis of CD required 3 intestinal biopsies: a biopsy on a gluten-containing diet (diagnosis), a biopsy after a period on GFD, and a biopsy after a gluten challenge.76 Subsequent studies demonstrated that a biopsy at the time of diagnosis in children without subsequent intestinal biopsies was able to correctly diagnose 95% of cases.77 The availability of CD specific serological tests facilitated the recognition of many CD patients and the wide spectrum of clinical manifestations. 6, 18 A positive serological test is supportive of the diagnosis but no single test is 100% specific for CD and the diagnostic accuracy varies dramatically between laboratories.43 Indeed, a large international study found that laboratory sensitivity ranged from 63% to 93% and specificity ranged from 96% to 100% when comparing TTG assays among various research and clinical laboratories.42 Serological tests may perform less well in the clinical setting than research (a positive result of both TTG and EMA had sensitivity of 81%).78 A diagnosis of CD requires the demonstration of histological changes associated with the disease which can be classified according Marsh, Marsh modified (Oberhuber), or more recently the simplified Corazza classification.79–81 Table 4 Small bowel biopsy is also useful for the differential diagnosis of malabsorptive disorders.82, 83
Table 4.
Summary of histologic classifications frequently used for celiac disease
| Marsh modified (Oberhuber) | Histologic Criterion | Corazza | ||
|---|---|---|---|---|
| Increased intraepithelial lymphocytes* | Crypt hyperplasia | Villous atrophy | ||
| Type 0 | No | No | No | None |
| Type 1 | Yes | No | No | Grade A |
| Type 2 | Yes | Yes | No | |
| Type 3a | Yes | Yes | Yes (partial) | Grade B1 |
| Type 3b | Yes | Yes | Yes (subtotal) | |
| Type 3c | Yes | Yes | Yes (total) | Grade B2 |
>40 intraepithelial lymphocytes per 100 enterocytes for Marsh modified (Oberhuber); >25 intraepithelial lymphocytes per 100 enterocytes for Corazza
A recent guideline promulgated by the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) proposed that it may be possible to avoid any intestinal biopsy in children who meet the following criteria: characteristic symptoms of CD, TTG IgA levels >10x upper limit of normal (confirmed with a positive EMA in a different blood sample), and positive HLA-DQ2.84 A TTGA IgA >5x upper limit of normal was observed in 9% of 236 adult patients with suspected CD and had a PPV for CD of 86.4%.85 PPV was 97.4% among 150 symptomatic children whom met the “triple test” ESPGHAN criteria.86 Among 3031 family members (25% younger than 18 years old) of patients with CD, TTGA IgA was abnormal in 336 (11%), of these 336, 88 (26%) had TTGA IgA ≥100U.87 Population-based data are not available to know how frequent the “triple test” criteria are meet on unselected populations. In the absence of standardization of TTG assays, use of a predefined threshold to select a population to avoid intestinal biopsy may not be the optimal strategy.88 Prospective data to validate ESPGHAN recommendation in children or adults are lacking.
Histological abnormalities associated with CD can be patchy. 89–93 Multiple biopsies of duodenum should be performed if the diagnosis of CD is considered. Among 132,352 patients without known CD who underwent duodenal biopsy in the United States, the probability of a new diagnosis of CD was significantly increased when ≥4 specimens were submitted (1.8% vs. 0.7%, p<0.0001).94 Unfortunately, 4 or more biopsies were taken in only 39% of patients undergoing biopsy for evaluation of malabsorption/suspicion of CD.94 The rate of duodenal biopsy was significantly lower among black, older (70 years and older), and male patients.95 In children and adults with positive CD specific serologies, adding biopsies of the duodenal bulb increases the diagnostic yield because 9%–13% had villous atrophy exclusively in the bulb.96–98 A targeted duodenal bulb biopsy from either the 9- or 12-o’clock position in addition to biopsies of the distal duodenum have a sensitivity of 96% for the diagnosis of CD.99 Care must be taken when interpreting duodenal bulb biopsies to allow for the normal surface architectural changes that overlie Brunner’s glands and the acute inflammatory changes of peptic duodenitis. Expert opinion suggests that only a single biopsy specimen should be obtained with each pass of the biopsy forceps,5 however, there is no evidence that supports that recommendation. We recommend multiple biopsies of the duodenum including one or two biopsies of the bulb (either 9- or 12-o’clock position) and at least 4 biopsies of post-bulbar duodenum. There is insufficient data to guide practice in patients who have not yet been tested serologically o in whom the pre-test prevalence is much lower. The added yield of duodenal bulb biopsies is likely to be small in such circumstances.
Lymphocytic infiltration (≥25 intraepithelial lymphocytes per 100 epithelial cells) also known as lymphocytic duodenosis is common in the general population (prevalence of 5.4%).100 Most patients with lymphocytic duodenosis do not belong to the spectrum of CD and other causes should be sought including work-up to rule out CD.101, 102 The frequency of diarrhea and weight loss was similar among patients with lymphocytic duodenosis and those with CD.102 Anemia, skin disorders, positive TTG, and human leukocyte antigen (HLA) DQ2 were more frequent among patients with CD. 102 Other disorders have been associated with lymphocytic duodenosis including Helicobacter pylori (H. pylori) infection, medications (e.g., non-steroidal anti-inflammatory drugs), small-bowel bacterial overgrowth, and systemic autoimmune disorders.103 Persistent intraepithelial lymphocytosis was observed in 56% patients with treated CD despite evidence of normal villous architecture, the only factor associated with this finding was oat consumption.104
Among 56 children without a prior diagnosis of CD and lymphocytic duodenosis evaluated at a referral center, CD was diagnosed in only 9% of these cases.105 GFD may be beneficial in children and adults with either lymphocytic duodenosis or Marsh II lesions and positive EMA. 106, 107
ROLE OF ANCILLARY TESTING IN CELIAC DISEASE
Recommendations
-
1
Human leukocyte antigen DQ2/DQ8 testing should not be used routinely in the initial diagnosis of CD. (Strong recommendation, moderate level of evidence)
-
2
Human leukocyte antigen DQ2/DQ8 genotyping testing should be used to effectively rule out the disease in selected clinical situations. (Strong recommendation, moderate level of evidence)
Examples of such clinical situations include but are not limited to:
Equivocal small bowel histological finding (Marsh I–II) in seronegative patients
Evaluation of patients on a GFD in whom no testing for CD was done before GFD
Patients with discrepant celiac specific serology and histology
Patients with suspicion of refractory CD where the original diagnosis of celiac remains in question
Patients with Down’s syndrome
-
3
Capsule endoscopy should not be used for initial diagnosis except when positive celiac specific serology who are unwilling or unable to undergo upper endoscopy with biopsy. (Strong recommendation, moderate level of evidence)
-
4
Capsule endoscopy should be considered for the evaluation of small bowel mucosa in patients with complicated CD. (Strong recommendation, moderate level of evidence)
-
5
Intestinal permeability tests, D-xylose, and small bowel follow-through are neither specific nor sensitive and are not recommended for CD diagnosis. (Strong recommendation, moderate level of evidence)
-
7
Stool studies or salivary tests are neither validated nor recommended for use in the diagnosis of CD. (Strong recommendation, weak level of evidence)
Summary of Evidence
The most important genetic risk factor for CD is the presence of HLA-DQ heterodimers DQ2 (encoded by alleles A1*05 and B1*02) and DQ8 (encoded by alleles A1*03 and B1*0302).108–110 In a prospective study that included 463 symptomatic patients referred for small-bowel biopsy due to suspicion of CD, the addition of HLA-DQ typing to serological tests (TTG and EMA) did not improve accuracy of serologic tests alone for diagnosis of CD.78
HLA-DQ2 (~95%) or HLA-DQ8 (~5%) are present in almost all patients with CD. 111, 112 Testing negative for both HLA-DQ types make CD diagnosis very unlikely (NPV >99%).78 Among rare patients not carrying these heterodimers, the majority encoded half of the HLA-DQ2 heterodimer.113 Because HLA-DQ2 is present in approximately 25%–30% of the white population,111, 114 testing for CD with either HLA-DQ type is not useful because the PPV is only about 12%.78
HLA DQ2 and DQ8 testing has been useful for exclusion of CD in patients with either equivocal small bowel histological finding or those following a GFD.74 HLA DQ2 and DQ8 testing has been used to exclude a diagnosis of CD in patients with unexplained sprue.115, 116 The prevalence of CD among persons affected by Down’s syndrome was 10% in the United States.117 HLA-DQ2 was present in 88% of persons with both Down’s syndrome and positive EMA, but only 16% of those with Down’s and negative EMA.117 In a prospective study including 155 children with Down’s syndrome, all children with CD tested positive for either HLA-DQ2 or DQ8.118 Testing negative for both HLA-DQ2 and DQ8 can reassure most parents of children with Down’s syndrome about the absence of genetic risk for CD development. The utility of HLA testing in other at-risk groups (such as Type I diabetics or family members), is more limited because a high proportion of these subjects carry the CD susceptibility alleles (e.g., 73% of first degree family members carry HLA-DQ2).16
Capsule endoscopy allows non-invasive visualization of the whole small-bowel mucosa.119 Capsule endoscopy can be performed in patients who are unable or unwilling to undergo upper endoscopy.120, 121 A meta-analysis showed that capsule endoscopy had a pooled sensitivity of 89% and specificity of 95% for diagnosis of CD.122 Capsule endoscopy had better overall sensitivity for detection of macroscopic features of atrophy compared with regular upper endoscopy (92% vs. 55%).123 The sensitivity of capsule endoscopy is less when there is partial villous atrophy and all non-atrophic lesions (Marsh I–II) may elude visual detection.123 In addition, markers of villous atrophy were not observed by capsule endoscopy among 8 patients with positive TTG or EMA and normal duodenal biopsy.124
Capsule endoscopy can detect severe complications associated with CD.87, 125–127 Extensive mucosal damage detected by capsule endoscopy was associated with low albumin and refractory CD type II.125 Macroscopic features of atrophy found in 31% of the cases was the most frequent finding by capsule endoscopy in patients with non-responsive celiac disease (NRCD).127 Other capsule findings among patients with NRCD include stenosis, erosions, ulcers, and lymphoma.125, 127 Erosions or ulcerations are frequent findings among NRCD patients often associated with the use of non-steroidal anti-inflammatory drugs.127 Capsule findings in complicated CD may be used to assess the need for further evaluation with deep enteroscopy, especially among these patients with clinical suspicion of lymphoma, adenocarcinoma, or ulcerative jejunitis.128 Other diagnostic modalities that may be of value in complicated CD include computed tomography enterography and magnetic resonance imaging enterography or enteroclysis.115, 129, 130
D-xylose is a pentose absorbed unchanged from the small-bowel. 131 The D-xylose test involves measurement of serum xylose or measurement of excreted xylose in urine after ingestion of D-xylose.132 The test is abnormal in patients with malabsorption due to mucosal disorders but remains normal in those with maldigestion of pancreatic origin.132 Sensitivity (<65%) and specificity (<74%) for either 1-hr plasma test or 4-hr urine excretion test are both lower than those obtained with IgA-TTG or IgA-EMA and the accuracy of the test is suboptimal for diagnosis of CD.133, 134
Intestinal permeability is altered in CD.135 Although permeability tests (e.g., sucrose, lactulose-mannitol ratio) can detect the gross changes on intestinal permeability associated with CD, their sensitivity and specificity are quite variable and these tests are not recommended for diagnosis of CD.136–138 Small-bowel follow-through does not have a role in the initial evaluation of patients with suspicion of CD and may have a limited role for evaluation of chronic diarrhea (e.g., suspicion of small bowel diverticulosis).139 Jejunoileal fold pattern reversal had a sensitivity of 86% for CD in a retrospective study.140 Other radiological signs of malabsorption (e.g., dilation, flocculation and segmentation of barium) are nonspecific (rarely seen with modern barium preparations) and can be seen in subjects with normal fecal fat analysis.141 Salivary tests for detection of TTG antibodies are under active investigation but there is not enough evidence to make a recommendation for their use.142, 143 Sensitivity of fecal IgA antibodies against TTG was as low as 10%, not suitable for accurate screening for CD.144
DIFFERENTIATION OF CELIAC DISEASE FROM NON-CELIAC GLUTEN SENSITIVITY
Recommendations
Symptoms or symptom response to a GFD alone should not be used to diagnosis CD as these do not differentiate CD for from non-celiac gluten sensitivity. (Strong recommendation, moderate level of evidence)
A diagnosis of non-celiac gluten sensitivity should be considered only after CD has been excluded with appropriate testing. (Strong recommendation, moderate level of evidence)
Summary of Evidence
Non-celiac gluten sensitivity, a condition in which individuals do not have the diagnostic features of CD but nonetheless develop celiac-like symptoms upon exposure to dietary gluten, is important to consider in the differential diagnosis of CD.70, 145, 146 Symptoms alone cannot reliably differentiate CD from non-celiac gluten sensitivity as there is often substantial overlap in symptoms between the two conditions.70, 146 Objective tests including celiac serology and small intestinal histology (both obtained while the patient is consuming a gluten-rich diet) and HLA-DQ typing (to rule out CD if negative) are needed to differentiate between the two disorders.70, 146
Knowledge of the pathogenesis, epidemiology and natural history of non-celiac gluten sensitivity is quite rudimentary.142, 146–148 However, at this time, it appears that non-celiac gluten sensitivity does not have a strong hereditary basis, is not associated with malabsorption or nutritional deficiencies and is not associated with any increased risk for auto-immune disorders or intestinal malignancy. Given these major differences in natural history and outcomes, the differentiation of CD and non-celiac gluten sensitivity is important for advising patients regarding the importance of ongoing disease monitoring, the required duration and strictness of adherence to the gluten free diet, and for counseling and testing of family members.
DIAGNOSIS AMONG PATIENTS ON A GLUTEN FREE DIET
Recommendations
While standard diagnostic tests (specific serology and intestinal biopsy) have a high positive predictive value for CD, they should not be relied upon to exclude CD in patients already adhering to a GFD. (Strong recommendation, high level of evidence)
Human leukocyte antigen DQ2/DQ8 genotyping should be used to try to exclude celiac disease prior to embarking on a formal gluten challenge. (Strong recommendation, high level of evidence)
CD should be differentiated from non-celiac gluten sensitivity in order to identify risk for nutritional deficiency states, complications of CD, risk for CD and associated disorders in family members and to influence the degree and duration of adherence to the GFD. (Conditional recommendation, moderate level of evidence)
Formal gluten challenge should be considered, where necessary, to diagnose or exclude CD in patients already adhering to a GFD. (Strong recommendation, high level of evidence)
Despite the disadvantages of neither confirming nor excluding a diagnosis of CD, some patients will opt to continue on a strictly GFD without undergoing formal gluten challenge; such patients should be managed in a similar fashion to those with known CD. (Conditional recommendation, low level of evidence)
Summary of Evidence
The specific serologic and histologic features of CD do not normalize immediately upon the initiation of a gluten free diet.8, 43, 149, 150 If the duration of GFD has been brief (less than one month), serology and histology are often still abnormal and can be used to diagnose CD in patients already on GFD. Conversely, given that the degree of serologic and histologic abnormality varies substantially in untreated CD, some patients will quickly revert to normal on a gluten free diet. Hence, normal serologic and histologic findings on a gluten free diet cannot be used to exclude CD definitively.8, 43, 149, 150
As discussed above, the required genotypes, encoding HLA-DQ2 or DQ8, are not influenced by diet and can be used to evaluate the likelihood of CD in patients either on a normal or on a gluten free diet.8, 151 HLA DQ2/DQ8 testing should be performed prior to embarking on a formal gluten challenge as a negative result will obviate the need for further workup.
Patients with CD treated by a strictly GFD may yield negative results on celiac serology testing and small intestinal histology.8, 43, 149, 151 HLA-DQ2 or DQ8 positivity will persist but is not sufficiently specific to be useful for positive diagnosis.8 Gluten challenge is the process whereby a patient with suspected but unproven CD and already treated with a GFD reverts to a normal, gluten-rich diet, under medical supervision, to enable diagnostic testing.152, 153 Gluten challenge was routine for CD diagnosis in the past, but is now less frequently used because of the high PPV of specific celiac serology testing.
Gluten challenge remains the gold standard for CD diagnosis in HLA DQ2 or DQ8 positive patients who have normal serologic and histologic findings when tested on a GFD. It must be noted that patients who develop severe symptoms following gluten ingestion are not suitable candidates for gluten challenge. Although gluten challenge with a diet containing at least 10 grams of gluten per day for 6 to 8 weeks has long been the norm, there are few data to indicate the diagnostic efficacy of this approach or the optimum dose or duration of challenge.154, 155 A recent study found that even if a patient can only tolerate lower doses of gluten (3 grams per day), diagnostic changes are seen in most CD patients after as little as 2 weeks of gluten ingestion. 152 An approach to gluten challenge is presented in Figure 2.152
Figure 2.
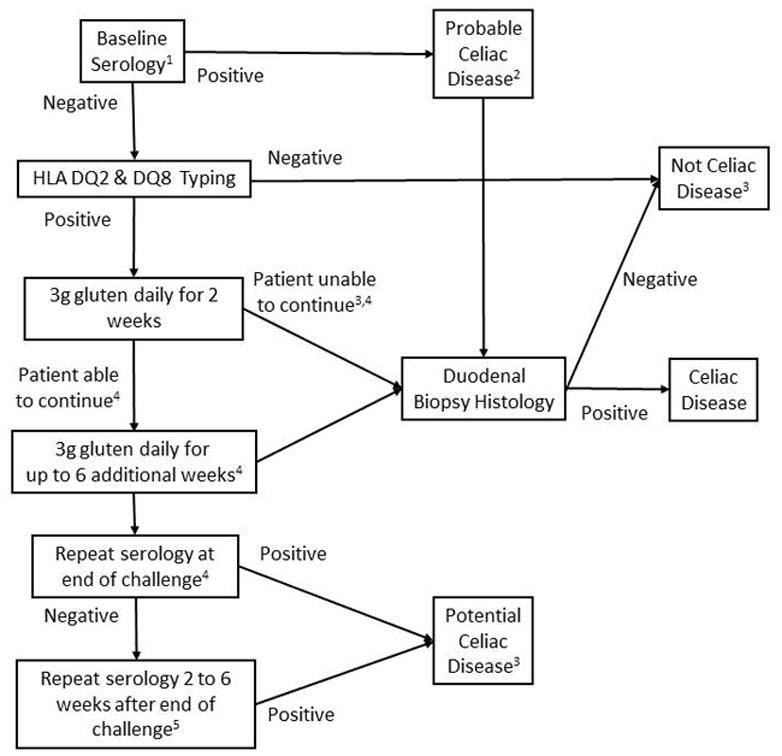
An approach to gluten challenge for the diagnosis or exclusion of CD in patients maintained on a gluten free diet without prior definitive diagnostic testing. [Adapted from reference Leffler D, Gut 2012]
1. Tissue transglutaminase, endomysium and/or deamidated gliadin peptide antibody serology.
2. Normal or non-diagnostic histology in a patient with positive serology while maintaining a GFD requires gluten challenge and repeat biopsy for definitive diagnosis or exclusion of CD.
3. Those with positive celiac serology but a normal biopsy have potential CD and should be evaluated and monitored further depending upon their clinical circumstances
4. In one study of subjects receiving a gluten challenge for 14 days Marsh III histology was seen in 68%, positive celiac serology in 75% and either Marsh III histology or positive serology in 90%. Thus, a 2 week gluten challenge may yield false negative results in 10% of patients. The added diagnostic sensitivity of extending the challenge to 8 weeks is unknown.
5. Celiac serology antibody concentrations may continue to rise after a gluten challenge ends. In one study positive tTG serology was seen in 25% of subjects and positive DGP serology in 30% at the end of a 14 day gluten challenge; 50% had at least one positive serology on day 14. Positivity rates rose to 55% and 45% respectively 14 days later, despite the fact that subjects had resumed a GFD; 75% had at least one positive serology on day 28, 14 days after the gluten challenge ended.
The importance of differentiating CD from non-celiac gluten sensitivity is outlined above. If a patient is unwilling or unable to undergo testing to make this distinction then their further management becomes less well-defined. The management of non-celiac gluten sensitivity is symptom-based without data to elicit major concerns for long term sequel of inadequate therapy.146, 147 The ongoing management of CD is more complex, as described elsewhere in this document. It is reasonable to manage patients with a moderate to high suspicion for (unproven) CD in a similar fashion to those with known CD. However, this approach will of necessity include unnecessary monitoring, therapy and expense. Therefore the patient should be aware of the ongoing availability of definitive testing should they so desire.
MANAGEMENT OF CELIAC DISEASE
Recommendations
People with CD should adhere to a gluten free diet for life. A gluten free diet entails strict avoidance of all products containing the proteins from wheat, barley and rye. (Strong recommendation, high level of evidence)
While pure oats appear to be safely tolerated by the majority of people with CD, oats should be introduced into the diet with caution and patients should be monitored closely for evidence of adverse reaction. (Strong recommendation, moderate level of evidence)
People with CD should be referred to a registered dietitian who is knowledgeable about CD in order to receive a thorough nutritional assessment and education on the gluten free diet. (Strong recommendation, moderate level of evidence)
People with newly diagnosed CD should undergo testing and treatment for micronutrient deficiencies. Deficiencies to be considered for testing should include, but not be limited to, iron, folic acid, vitamin D, and vitamin B12. (Conditional recommendation, low level of evidence)
Summary of Evidence
A gluten free diet is the only effective treatment for CD as there are currently no medications that can reliably and safely prevent the mucosal damage caused by exposure to gluten. The principle sources of dietary gluten are wheat, barley and rye. While the term “gluten free” implies complete elimination of all sources of gluten, in reality this is not possible due to contamination of foods with trace amounts of gluten. Hence the term “gluten free” indicates a diet that contains gluten at such a low level as to be considered harmless. The exact level below which gluten is harmless is not known but a recent review suggests less than 10 mg per day is unlikely to cause damage in most patients. 156. The current international Codex Alimentarius defines gluten free foods as having less than 20 ppm of gluten.
A GFD will result in resolution of symptoms and repair of the intestinal damage over time in most people with CD. Failure to adhere to the GFD carries risk for adverse health consequences and increased mortality. There is an increased risk for malignancies (e.g., small bowel adenocarcinoma, cancer of esophagus, B-cell and T-cell Non-Hodgkin lymphomas), and in particular intestinal T-cell lymphomas, in people with CD.157 Evidence suggests the risk for increased mortality and malignancies is reduced in those who adhere to the diet.158–160 There is evidence that a GFD improves nutritional parameters in symptomatic adults and children with CD. This includes increases in body weight, body mass index and bone mineralization.161–163
Untreated CD is associated with an increased prevalence of low bone mineral density and risk for fractures. Treatment of CD with a GFD improves bone mineral density in both adults and children. 45, 164–176 Women with CD have an increased risk of infertility, spontaneous abortions, preterm deliveries and delivery of low birth weight infants. Treatment of women with CD with GFD reduces these risks to that of the general population. 177–181
Consumption of oats improves the nutrient content of the diets of people on a GFD by increasing the intake of fiber, vitamin B, magnesium and iron.182 While in the past there has been concern that oats can cause intestinal mucosal damage in people with CD, recent evidence suggests oats that are pure and uncontaminated by other gluten containing grains can be safely ingested by most people with CD provided they are taken in limited quantities.183–190 However there is still need for caution when introducing oats into the diet of people with CD as there is a high likelihood that commercial oats may be contaminated with gluten from other grains. 191, 192 There is also evidence that a small number of people with CD may be intolerant to pure oats and can develop an immunological response to oat avenins. Based on in vitro studies this may in part be related to a variation in toxicity of oat cultivars.193, 194 Commercial oats should only be introduced into the diet of people with CD provided the oats are guaranteed to be pure and uncontaminated by other gluten containing grains. Even if confirmed to be pure, if oats are introduced into the diet of people with CD there should be careful follow up to monitor for signs of both clinical and serological relapse.
Following a GFD can be cumbersome and strict avoidance of gluten is difficult because there are many hidden sources of gluten in commercial food products. There is evidence that compliance with the GFD is improved in those who are more knowledgeable about CD and the diet. 195–197 Most physicians do not have the knowledge about the diet to adequately counsel patients. Registered dietitians are trained to evaluate patients for potential current and future dietary nutrient deficiencies and advise and educate them on how to maintain a strict GFD with provision of healthy alternatives to gluten. The Academy of Nutrition and Dietetics has published evidence based guidelines for treatment of CD and it is recommended these are followed (available at http://www.adaevidencelibrary.com/topic.cfm?cat=3677). In addition to providing initial counseling and education, once the relationship with a dietitian is established the patient can be monitored for compliance with the diet and undergo repeated assessments for potential dietary nutrient deficiencies, inadequate fiber intake, and excess weight gain each of which may be associated with adherence to the GFD.
There is some evidence that people with untreated CD are more frequently deficient in a number of micronutrients compared to those without CD. Micronutrient deficiencies identified include iron, 198–203, folic acid,198, 204 and Vitamin B12 and B6.205–207 Low bone mineral density in people with untreated CD is believed to be partly due to vitamin D deficiency. Other deficiencies described in CD include copper, zinc, and carnitine. 199, 208, 209 Some deficiencies may persist even after a prolonged period on a gluten free diet.210, 211 In addition to testing for micronutrient deficiencies, dietary review by a registered dietitian, both at the time of initial diagnosis and after starting a GFD, is helpful for identifying potential nutrient deficiencies.
MONITORING OF CELIAC DISEASE
Recommendations
People with CD should be monitored regularly for residual or new symptoms, adherence to GFD, and assessment for complications. In children, special attention to assure normal growth and development is recommended.. (Strong recommendation, moderate level of evidence)
Periodic medical follow-up should be performed by a health care practitioner with knowledge of CD. Consultation with a dietitian should be offered if gluten contamination is suspected. (Strong recommendation, moderate level of evidence)
Monitoring of adherence to GFD should be based on a combination of history and serology (IgA TTG or IgA [or IgG] deamidated gliadin peptide antibodies). (Strong recommendation, moderate level of evidence)
Upper endoscopy with intestinal biopsies is recommended for monitoring in cases with lack of clinical response or relapse of symptoms despite a GFD. (Strong recommendation, moderate level of evidence)
Monitoring of people with CD should include verification of normalization of laboratory abnormalities detected during initial laboratory investigation. (Strong recommendation, moderate level of evidence)
Summary of Evidence
There is universal agreement on the necessity of long-term monitoring of patients with CD.212 The number of patients with CD who receive follow-up is unknown. In the United States, follow-up appears to be suboptimal in practice.213 A systematic review supports the role of strict adherence to the GFD to control symptoms, improve quality of life, and decrease the risk of complications.214 Normal growth and development are achievable on a GFD and should be goals for monitoring children with CD.215 Control of symptoms (if present), facilitation of adherence to GFD, and avoidance or early detection of complications should be the general goals of monitoring after diagnosis of CD (Figure 3).
Figure 3.
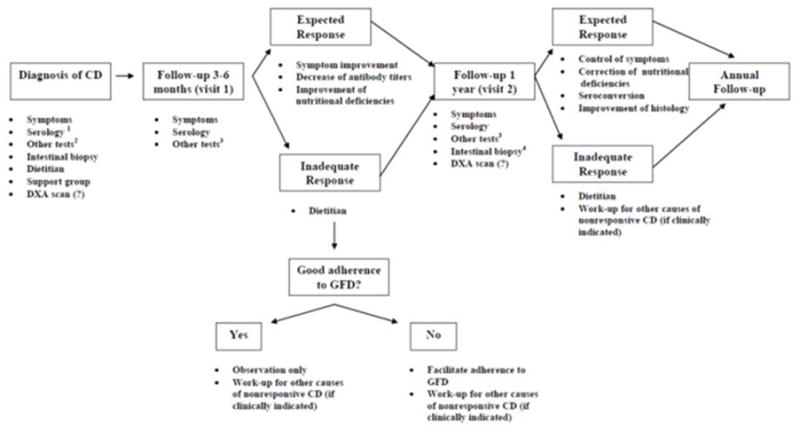
An approach to monitoring CD [Adapted from Rubio-Tapia A. Seguimiento Médico del Paciente Celiaco. En Rodrigo L. editor. Enfermedad Celiaca. Barcelona, España. OmniaScience, 2012. In press]
1. TTG and DGP can be used for monitoring CD
2. Other tests may include complete blood count, ALT, vitamins (A, D, E, B12), copper, zinc, carotene, folic acid, ferritin, iron
3. Blood tests at follow-up should be individualized to verify correction of laboratory tests that were abnormal at baseline
4. The role of biopsy for monitoring CD is discussed in detail in the text
It is not clear who should perform follow-up of patients with CD and at what frequency. In a survey of patients in the United Kingdom, the health care practitioner preferred by patients for follow-up was a dietitian with a doctor available if needed.216 In a population-based cohort of 122 patients from the Midwest in the United States, there were 314 follow-up visits over a period of 5 years. Of these visits, 175 (56%) were conducted with primary care providers and 122 (39%) with gastroenterologists. 213 A nationwide study from Finland showed that medical follow-up by primary care providers was effective (average adherence rate was 88%).217 Annual follow-up with serology (TTG IgA) was associated with increasing rate of seroconversion of the TTG antibody (99%) among 2245 patients who underwent systematic follow-up.58 Until more evidence is available, annual follow-up seems reasonable.
There is extensive evidence to support the central role of consultation with a dietitian in patients with NR CD or if gluten contamination is suspected.218, 219 There is no evidence to suggest that medical follow-up by a dietitian and a doctor together is better (or worse) in terms of outcome than follow-up done by either provider alone.
There are several methods to assess adherence to GFD: visits with the doctor and/or dietitian, serology, biopsy of intestine, and structured surveys. The gold standard for monitoring adherence to GFD is consultation with a skilled dietitian.220 All serologic markers associated with celiac autoimmunity are gluten-dependent. A decrease from baseline values is expected within months of strict adherence to the GFD.221, 222 A gluten challenge produces increasing values of antibodies.222 Lack of declining values and/or persistently positive serology one year after starting a GFD strongly suggest gluten contamination.219 Persistently positive serology was seen in only 1% of patients who underwent annual follow-up during a 5 year period.58 Serology is not accurate to detect lesser degrees of gluten contamination. Seroconversion after GFD does not necessarily imply healing of the intestine.73, 223, 224 The only accurate method available to verify intestinal healing is biopsy. Structured short surveys have been explored as an alternative to dietitian consultation for quick assessment of adherence to the diet.225–227 More studies are needed to examine the role of survey instruments for assessment of adherence in practice.
Patients with persistent or recurrent symptoms despite GFD require additional work-up to investigate the presence of disorders commonly associated with NRCD (see “Evaluation of nonresponsive celiac disease” for details).228 Observational experience from referral centers support the role of upper endoscopy with intestinal biopsies for evaluation of NRCD.218, 219, 229 Intestinal biopsies are the only way to document healing of the intestine. In adults, the intestine will often fail to heal despite negative serology and absence of symptoms.73, 224, 230 This lack of healing may increase the risk of lymphoma, bone disease and ultimately the development of refractory CD.73, 231 A large Swedish study demonstrated no risk of lymphoma (HR = 0.97; 95% CI = 0.44 to 2.14) among patients with normal histology suggesting that mucosal healing could be the goal to consider during follow-up.232 Among a group 381 patients with baseline and follow-up biopsy after GFD, mucosal healing was associated with a borderline lower risk of death (HR=0.13, 95% CI: 0.02–1.06, P=0.06) adjusted for age and sex.73 A much larger study from Sweden failed to confirm a protective role of mucosal healing on mortality risk, yet mortality risk was significantly lower among patients who underwent follow-up biopsy.233 Follow-up biopsy could be considered for assessment of mucosal healing in adults with negative serology and absence of symptoms. In a US study, the median time from onset of GFD to achieve mucosal healing was 3 years.73 It is reasonable to do follow-up biopsy in adults after 2 years of starting a GFD to assess for mucosal healing. Mucosal healing was observed in 95% children within 2 years of starting a GFD.230 Follow-up biopsy is not recommended as a routine in children, although the evidence for mucosal healing after GFD in children is limited.
A significant decrease (or normalization) of markers of malabsorption such as fat-content of the stools should be expected after GFD.215 Verification of either declining antibody levels or seroconversion of CD specific antibodies is critical during monitoring follow-up.221 A persistently positive TTG antibody after GFD was significantly associated with abnormal duodenal histology, low ferritin, and poor adherence to GFD.234 Among a heterogenous group of patients with refractory iron-deficiency anemia, anemia improved in 92% of patients with CD after treatment with a GFD.235 Copper deficiency has been described in association with CD. 208, 236 Copper levels normalize within a month of adequate supplementation and a GFD, although reversibility of established neurological manifestations is unclear.208 Copper deficiency appears to be a very rare cause of peripheral neuropathy.237 Long-term adherence to GFD leads to significant improvement in bone density, especially among patients with strict adherence to the diet238 Although it is well accepted that CD is associated with an increased risk of bone fractures,239, 240, 241 the protective role of GFD on subsequent fracture risk may not be universal. Low serum vitamin B12 was present in about 12% of patients with CD, correction should be expected with adequate replacement and GFD.205
NON-RESPONSIVE OR REFRACTORY CELIAC DISEASE
Recommendations
Patients with non-responsive celiac disease should be evaluated carefully to identify and treat the specific etiology in each patient. (Strong recommendation, high level of evidence)
Early steps in the evaluation should include measurement of celiac serologies and a thorough review of the patient’s diet by a dietician who is experienced in CD management. (Strong recommendation, high level of evidence)
Differentiation should be made between Type I and Type II refractory CD as this is important for management and prognosis. (Strong recommendation, moderate level of evidence)
Treatment with medication, as an adjunct to the GFD, should be considered in refractory CD. (Conditional recommendation, moderate level of evidence)
Patients with RCD should be monitored closely and receive aggressive nutritional support including parenteral nutrition whenever indicated. (Strong recommendation, high level of evidence)
Summary of Evidence
Non-responsive celiac disease may be defined as persistent symptoms, signs or laboratory abnormalities typical of CD despite 6 to 12 months of dietary gluten avoidance.218, 219, 242, 243 NRCD is common affecting from 7% to 30% of patients treated with a gluten free diet for CD.218, 219, 242 There are many distinct etiologies including inadvertent gluten ingestion (the most common cause), other food intolerances (including lactose and fructose intolerance), small intestinal bacterial overgrowth, microscopic colitis, pancreatic insufficiency, irritable bowel syndrome and refractory CD.218, 219, 242–247 Thus, careful evaluation is needed to identify and treat the specific source in any given patient.218, 219, 242, 243 The first step in evaluation is to re-confirm the initial diagnosis of CD by review of small intestinal histology and serology obtained at the time of diagnosis.(Figure 4) If the diagnosis of CD is not correct then response to a GFD is not to be expected and alternative diagnoses and treatments must be considered.248 In those with confirmed CD the ingestion of gluten, either purposeful or inadvertent, is the most common causes of NRCD being identified in 35% to 50% of cases.218, 219 Thus, a careful evaluation of the patient’s diet by a dietician who is experienced in CD management is the next important assessment. This evaluation should also seek other food intolerances, for example to lactose or fructose. Celiac serologies are helpful if positive as this points to probable gluten exposure as the cause for NRCD.218 However, normal serologies do not exclude intermittent or low-level gluten ingestion sufficient to cause persistent CD activity. Once dietary causes of NRCD have been excluded, small intestinal biopsy should be repeated and the findings compared to the diagnostic biopsy. Ongoing inflammatory enteropathy with villous atrophy is consistent with refractory CD, gluten exposure or possibly small intestinal bacterial overgrowth and other causes of villous atrophy.115, 219, 242, 245 Normal or near-normal small intestinal histology suggests other etiologies such as irritable bowel syndrome, microscopic colitis, food intolerances or pancreatic insufficiency.218, 219, 242 CD and microscopic colitis do overlap.249, 250 There is no sufficient data to make a recommendation for routine testing of CD in patients with microscopic colitis. However, CD should be considered in patients with unresponsive microscopic colitis or those with microscopic colitis and other symptoms or signs suggestive of CD.251
Figure 4.
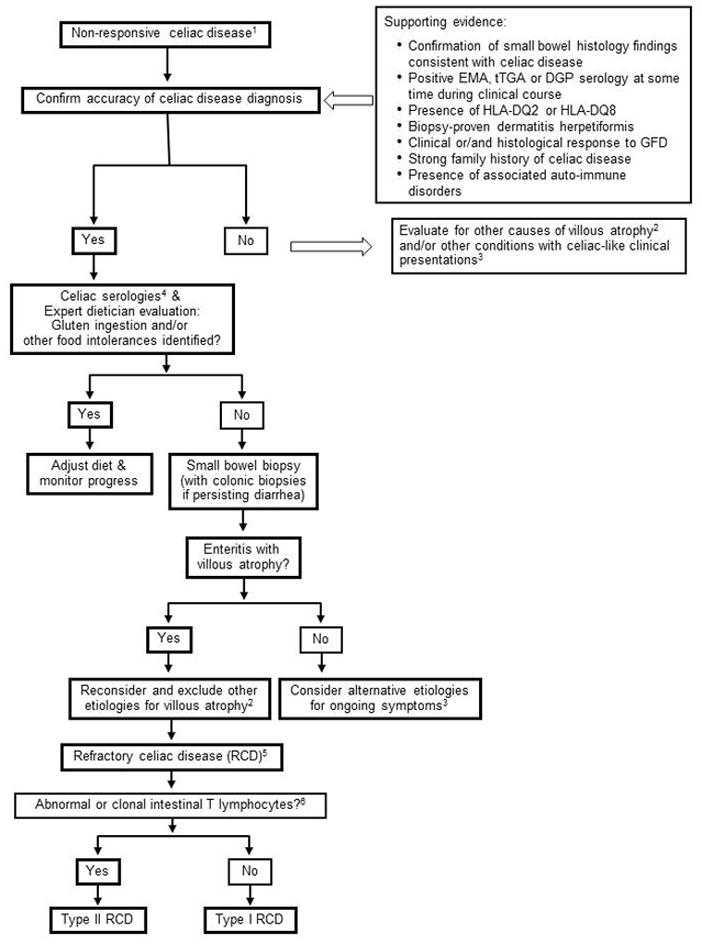
An approach to the investigation of NRCD and RCD. [Adapted from references Rubio-Tapia A Gut 2010 and Abdallah H Curr Gastroenterol Rep 2007]
1. Non-responsive celiac disease [NRCD] may be defined as persistent symptoms, signs or laboratory abnormalities typical of CD despite 6 to 12 months of dietary gluten avoidance.
2. Causes of non-celiac, small intestinal villous atrophy that may be misdiagnosed as CD include autoimmune enteropathy, tropical sprue, small intestinal bacterial overgrowth, hypogammaglobulinemia and combined variable immunodeficiency (CVID), collagenous sprue, eosinophilic enteritis, Crohn’s disease, and peptic duodenitis.
3. Conditions that present clinically in a similar fashion to CD but where villous atrophy is not evident include irritable bowel syndrome, food intolerances, small intestinal bacterial overgrowth, eosinophilic enteritis, Crohn’s disease, and microscopic colitis.
4. Positive celiac serologies despite 12 months of treatment with a GFD suggest that there may be ongoing gluten ingestion.
5. Refractory celiac disease (RCD) may be defined as persistent or recurrent malabsorptive symptoms and signs with small intestinal villous atrophy despite a strict GFD for more than 12 months and in the absence of other disorders including overt lymphoma.
6. Abnormal intestinal lymphocytes may be identified by immunohistochemistry of IELs or by flow cytometry showing an increased number of CD3 positive cells lacking CD8 or by the identification of clonal T-cell receptor gene rearrangement by molecular analysis.
Refractory celiac disease (RCD) may be defined as persistent or recurrent symptoms and signs of malabsorption with small intestinal villous atrophy despite a strict GFD for more than 12 months and in the absence of other disorders including overt lymphoma.145, 218, 252 RCD is uncommon, affecting 1–2% of patients with CD.115, 244, 245 In Type I RCD lymphocyte infiltration of the small intestinal mucosa is similar to that seen in untreated CD.244, 246, 253, 254 In Type II RCD, CD3-positive intraepithelial T cells exhibit an abnormal immunophenotype with lack of expression of normal cell surface differentiation markers such as CD8.246, 253, 254 Furthermore, T-cell receptor analyses may reveal oligoclonal T cell expansion within the small bowel mucosa.244, 246, 253, 254 These T cell abnormalities in Type II RCD are associated with a significantly less favorable prognosis as compared to Type I RCD.244, 246 In the United States, Type I RCD appears to be more common than Type II RCD.245
Management of Type I RCD includes excluding inadvertent gluten exposure as a cause of ongoing disease activity and evaluation for and treatment of nutritional deficiencies that may result from enteropathy with malabsorption.115, 218, 245 Symptomatic treatment to reduce diarrhea is often required. There are no published randomized, controlled trials of therapy for Type I RCD. Traditional medical treatment in severe cases consists of systemic steroid therapy with prednisone or a similar agent. In patients with an incomplete response to steroid treatment or who recur when the steroid dose is reduced, immunosuppressive agents such as azathioprine can be used. Recent reports indicate that budesonide or small intestinal release mesalamine may be effective and carry the potential advantage of causing fewer side-effects.255–257
The general approach to management of Type II RCD is the same as for Type I RCD.115, 244–246 However, symptoms and signs of disease are more severe in Type II RCD and are less likely to respond to therapy. Malnutrition in Type II RCD may be profound and require parenteral nutritional support. In one study, the 5 year survival of patients with Type II RCD was 44% compared to 93% for Type I RCD.244 Causes of death included lymphoma, malnutrition and sepsis.
There are no published randomized, controlled trials of therapy for Type II RCD and there are no treatments of proven efficacy. Agents that are used for treatment include systemic corticosteroids, enteric-coated budesonide, azathioprine or 6-mercaptopurine, methotrexate, cyclosporine, anti-TNF antibodies or cladribine.6, 115, 116, 244, 252, 255, 258–261 Transformation to enteropathy-associated T cell lymphoma (EATCL) is a prominent risk and may require treatment by surgery, chemotherapy or bone marrow transplantation. 262, 263 In some patients EATCL may run a prolonged, non-aggressive course but the overall prognosis remains poor.
SUMMARY OF RECOMMENDATIONS
Patients with symptoms, signs, or laboratory evidence suggestive of malabsorption, such as chronic diarrhea with weight loss, steatorrhea, postprandial abdominal pain and bloating, should be tested for CD. (Strong recommendation, high level of evidence)
Patients with symptoms, signs, or laboratory evidence for which CD is a treatable cause should be considered for testing for CD. (Strong recommendation, moderate level of evidence)
Patients with a first degree family member who has a confirmed diagnosis of CD should be tested if they show possible signs or symptoms or laboratory evidence of CD.
Consider testing of asymptomatic relatives with a first degree family member who has a confirmed diagnosis of CD (Conditional recommendation, high level of evidence)
Celiac disease should be sought among the explanations for elevated serum aminotransferase levels when no other etiology is found, (Strong recommendation, high level of evidence)
Patients with type I diabetes mellitus should be tested for CD if there are any digestive symptoms, or signs, or laboratory evidence suggestive of celiac disease. (Strong recommendation, high level of evidence)
IgA anti-tissue transglutaminase antibody is the preferred single test for detection of CD in individuals over the age of 2 years. (Strong recommendation, high level of evidence)
When there exists a high probability of CD the possibility of IgA deficiency is considered, total IgA should be measured. An alternative approach is to include both IgA and IgG based testing, such as IgG deamidated gliadin peptides [DGP], in these high probability patients. (Strong recommendation, moderate level of evidence)
In patients in whom low IgA or selective IgA deficiency is identified, IgG-based testing (IgG deamidated gliadin peptides [DGP] and IgG tissue transglutaminase [TTG]) should be performed. (Strong recommendation, moderate level of evidence)
If the suspicion of CD is high, intestinal biopsy should be pursued even if serologies are negative. (Strong recommendation, moderate level of evidence)
All diagnostic serologic testing should be done with patients on a gluten-containing diet. (Strong recommendation, high level of evidence)
Antibodies directed against native gliadin are not recommended for the primary detection of CD. (Strong recommendation, high level of evidence)
Combining several tests for CD in lieu of TTG IgA alone may marginally increase sensitivity for CD but reduces specificity therefore are not recommended in low risk populations. (Conditional recommendation, moderate level of evidence)
When screening children younger than 2 years of age for CD, the IgA TTG test should be combined with deamidated gliadin peptide (IgA and IgG). (Strong recommendation, moderate level of evidence)
The confirmation of a diagnosis of CD should be based on a combination of findings from the medical history, physical examination, serology, and upper endoscopy with histological analysis of multiple biopsies of the duodenum. (Strong recommendation, high level of evidence)
Upper endoscopy with small bowel biopsy is a critical component of the diagnostic evaluation for persons with suspected CD and is recommended to confirm the diagnosis. (Strong recommendation, high level of evidence)
Multiple biopsies of the duodenum (one or two biopsies of the bulb and at least 4 biopsies of the distal duodenum) are recommended to confirm the diagnosis CD. (Strong recommendation, high level of evidence)
Lymphocytic infiltration of the intestinal epithelium in the absence of villous atrophy is not specific for CD and other causes should also be considered. (Strong recommendation, high level of evidence)
Human leukocyte antigen DQ2/DQ8 testing should not be used routinely in the initial diagnosis of CD. (Strong recommendation, moderate level of evidence)
-
Human leukocyte antigen DQ2/DQ8 genotyping testing should be used to effectively rule out the disease in selected clinical situations. (Strong recommendation, moderate level of evidence)
Examples of such clinical situations include but are not limited to:
Equivocal small bowel histological finding (Marsh I–II) in seronegative patients
Evaluation of patients on a GFD in whom no testing for CD was done before GFD
Patients with discrepant celiac specific serology and histology
Patients with suspicion of refractory CD where the original diagnosis of celiac remains in question
Patients with Down’s syndrome
Capsule endoscopy should not be used for initial diagnosis except when positive celiac specific serology who are unwilling or unable to undergo upper endoscopy with biopsy. (Strong recommendation, moderate level of evidence)
Capsule endoscopy should be considered for the evaluation of small bowel mucosa in patients with complicated CD. (Strong recommendation, moderate level of evidence)
Intestinal permeability tests, D-xylose, and small bowel follow-through are neither specific nor sensitive and are not recommended for CD diagnosis. (Strong recommendation, moderate level of evidence)
Stool studies or salivary tests are neither validated nor recommended for use in the diagnosis of CD. (Strong recommendation, weak level of evidence)
Symptoms or symptom response to a GFD alone should not be used to diagnosis CD as these do not differentiate CD for from non-celiac gluten sensitivity. (Strong recommendation, moderate level of evidence)
A diagnosis of non-celiac gluten sensitivity should be considered only after CD has been excluded with appropriate testing. (Strong recommendation, moderate level of evidence)
While standard diagnostic tests (specific serology and intestinal biopsy) have a high positive predictive value for CD, they should not be relied upon to exclude CD in patients already adhering to a GFD. (Strong recommendation, high level of evidence)
Human leukocyte antigen DQ2/DQ8 genotyping should be used to try to exclude celiac disease prior to embarking on a formal gluten challenge. (Strong recommendation, high level of evidence)
CD should be differentiated from non-celiac gluten sensitivity in order to identify risk for nutritional deficiency states, complications of CD, risk for CD and associated disorders in family members and to influence the degree and duration of adherence to the GFD. (Conditional recommendation, moderate level of evidence)
Formal gluten challenge should be considered, where necessary, to diagnose or exclude CD in patients already adhering to a GFD. (Strong recommendation, high level of evidence)
Despite the disadvantages of neither confirming nor excluding a diagnosis of CD, some patients will opt to continue on a strictly GFD without undergoing formal gluten challenge; such patients should be managed in a similar fashion to those with known CD. (Conditional recommendation, low level of evidence)
People with CD should adhere to a gluten free diet for life. A gluten free diet entails strict avoidance of all products containing the proteins from wheat, barley and rye. (Strong recommendation, high level of evidence)
While pure oats appear to be safely tolerated by the majority of people with CD, oats should be introduced into the diet with caution and patients should be monitored closely for evidence of adverse reaction. (Strong recommendation, moderate level of evidence)
People with CD should be referred to a registered dietitian who is knowledgeable about CD in order to receive a thorough nutritional assessment and education on the gluten free diet. (Strong recommendation, moderate level of evidence)
People with newly diagnosed CD should undergo testing and treatment for micronutrient deficiencies. Deficiencies to be considered for testing should include, but not be limited to, iron, folic acid, vitamin D, and vitamin B12. (Conditional recommendation, low level of evidence)
People with CD should be monitored regularly for residual or new symptoms, adherence to GFD, and assessment for complications. In children, special attention to assure normal growth and development is recommended.. (Strong recommendation, moderate level of evidence)
Periodic medical follow-up should be performed by a health care practitioner with knowledge of CD. Consultation with a dietitian should be offered if gluten contamination is suspected. (Strong recommendation, moderate level of evidence)
Monitoring of adherence to GFD should be based on a combination of history and serology (IgA TTG or IgA [or IgG] deamidated gliadin peptide antibodies). (Strong recommendation, moderate level of evidence)
Upper endoscopy with intestinal biopsies is recommended for monitoring in cases with lack of clinical response or relapse of symptoms despite a GFD. (Strong recommendation, moderate level of evidence)
Monitoring of people with CD should include verification of normalization of laboratory abnormalities detected during initial laboratory investigation. (Strong recommendation, moderate level of evidence)
Patients with non-responsive celiac disease should be evaluated carefully to identify and treat the specific etiology in each patient. (Strong recommendation, high level of evidence)
Early steps in the evaluation should include measurement of celiac serologies and a thorough review of the patient’s diet by a dietician who is experienced in CD management. (Strong recommendation, high level of evidence)
Differentiation should be made between Type I and Type II refractory CD as this is important for management and prognosis. (Strong recommendation, moderate level of evidence)
Treatment with medication, as an adjunct to the GFD, should be considered in refractory CD. (Conditional recommendation, moderate level of evidence)
Patients with RCD should be monitored closely and receive aggressive nutritional support including parenteral nutrition whenever indicated. (Strong recommendation, high level of evidence)
Acknowledgments
Financial support: The authors are supported by National Institutes of Health DK-57982 (JAM), 1K08 DK090150 (AHC), and American College of Gastroenterology Junior Faculty Development Award (ART).
Abbreviations
- AGA
anti-gliadin antibodies
- CD
celiac disease
- DGP
deamidated gliadin peptides
- EMA
endomysium antibody
- ESPGHAN
European Society of Pediatric Gastroenterology, Hepatology, and Nutrition
- GFD
gluten-free diet
- HLA
human leukocyte antigen
- Ig
immunoglobulin
- NRCD
non-responsive celiac disease
- NPV
negative predictive value
- PPV
positive predictive value
- RCD
refractory celiac disease
- TTG
tissue transglutaminase
Footnotes
Specific author contributions: All authors were involved in writing the manuscript and providing critical revision of the manuscript for important intellectual content.
Potential competing interests:
Dr. Rubio-Tapia has nothing to declare.
Dr. Hill serves on the editorial boards of Journal of Pediatrics and Journal of Pediatric Gastroenterology and Nutrition.
Dr. Kelly acts or has acted as a scientific and medical advisor to Alba, Alvine and ImmunosanT and has received research funding support on celiac disease from Alba and Shire.
Dr. Calderwood has nothing to declare.
Dr. Murray has received grant support from Alba Therapeutics (>$50,000), served on the Advisory Board of Alvine Pharmaceuticals, Inc. (<$10,000), and served as consultant to Ironwood, Inc. (<$10,000), Flamentera (<$10,000), Actogenix (<$10,000), Bayer Healthcare Pharmaceuticals (<$10,000), Vysera Biomedical (<$10,000), 2G Pharma, Inc. (<$10,000), ImmunosanT, Inc (<$10,000), and Shire US Inc (<$10,000).
References
- 1.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW, Jr, Zaza S. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480–93. doi: 10.1016/S0140-6736(09)60254-3. [DOI] [PubMed] [Google Scholar]
- 3.Reilly NR, Fasano A, Green PH. Presentation of celiac disease. Gastrointest Endosc Clin N Am. 2012;22:613–21. doi: 10.1016/j.giec.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr. 2004;79:669–73. doi: 10.1093/ajcn/79.4.669. [DOI] [PubMed] [Google Scholar]
- 5.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59:547–57. doi: 10.1136/gut.2009.195131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catassi C, Kryszak D, Louis-Jacques O, Duerksen DR, Hill I, Crowe SE, Brown AR, Procaccini NJ, Wonderly BA, Hartley P, Moreci J, Bennett N, Horvath K, Burk M, Fasano A. Detection of Celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol. 2007;102:1454–60. doi: 10.1111/j.1572-0241.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 8.Lebwohl B, Rubio-Tapia A, Assiri A, Newland C, Guandalini S. Diagnosis of celiac disease. Gastrointest Endosc Clin N Am. 2012;22:661–77. doi: 10.1016/j.giec.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Windt DA, Jellema P, Mulder CJ, Kneepkens CM, van der Horst HE. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. Jama. 2010;303:1738–46. doi: 10.1001/jama.2010.549. [DOI] [PubMed] [Google Scholar]
- 10.Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BM, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009;169:651–8. doi: 10.1001/archinternmed.2009.22. [DOI] [PubMed] [Google Scholar]
- 11.Aziz I, Sanders DS. The irritable bowel syndrome-celiac disease connection. Gastrointest Endosc Clin N Am. 2012;22:623–37. doi: 10.1016/j.giec.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Ching E, Moayyedi P. Meta-analysis: yield of diagnostic tests for coeliac disease in dyspepsia. Aliment Pharmacol Ther. 2009;30:28–36. doi: 10.1111/j.1365-2036.2009.04008.x. [DOI] [PubMed] [Google Scholar]
- 13.Lacy BE, Talley NJ, Locke GR, 3rd, Bouras EP, DiBaise JK, El-Serag HB, Abraham BP, Howden CW, Moayyedi P, Prather C. Review article: current treatment options and management of functional dyspepsia. Aliment Pharmacol Ther. 2012;36:3–15. doi: 10.1111/j.1365-2036.2012.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norstrom F, Sandstrom O, Lindholm L, Ivarsson A. A gluten-free diet effectively reduces symptoms and health care consumption in a Swedish celiac disease population. BMC Gastroenterol. 2012;12:125. doi: 10.1186/1471-230X-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756–80. doi: 10.1053/j.gastro.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Rubio-Tapia A, Van Dyke CT, Lahr BD, Zinsmeister AR, El-Youssef M, Moore SB, Bowman M, Burgart LJ, Melton LJ, 3rd, Murray JA. Predictors of family risk for celiac disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:983–7. doi: 10.1016/j.cgh.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray JA. Celiac disease in patients with an affected member, type 1 diabetes, iron-deficiency, or osteoporosis? Gastroenterology. 2005;128:S52–6. doi: 10.1053/j.gastro.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 19.Book L, Zone JJ, Neuhausen SL. Prevalence of celiac disease among relatives of sib pairs with celiac disease in U.S. families. Am J Gastroenterol. 2003;98:377–81. doi: 10.1111/j.1572-0241.2003.07238.x. [DOI] [PubMed] [Google Scholar]
- 20.Kinos S, Kurppa K, Ukkola A, Collin P, Lahdeaho ML, Huhtala H, Kekkonen L, Maki M, Kaukinen K. Burden of illness in screen-detected children with celiac disease and their families. J Pediatr Gastroenterol Nutr. 2012;55:412–6. doi: 10.1097/MPG.0b013e31825f18ff. [DOI] [PubMed] [Google Scholar]
- 21.Vilppula A, Kaukinen K, Luostarinen L, Krekela I, Patrikainen H, Valve R, Luostarinen M, Laurila K, Maki M, Collin P. Clinical benefit of gluten-free diet in screen-detected older celiac disease patients. BMC Gastroenterol. 2011;11:136. doi: 10.1186/1471-230X-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paavola A, Kurppa K, Ukkola A, Collin P, Lahdeaho ML, Huhtala H, Maki M, Kaukinen K. Gastrointestinal symptoms and quality of life in screen-detected celiac disease. Dig Liver Dis. 2012;44:814–8. doi: 10.1016/j.dld.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Ukkola A, Maki M, Kurppa K, Collin P, Huhtala H, Kekkonen L, Kaukinen K. Diet improves perception of health and well-being in symptomatic, but not asymptomatic, patients with celiac disease. Clin Gastroenterol Hepatol. 2011;9:118–23. doi: 10.1016/j.cgh.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Kamath PS. Clinical approach to the patient with abnormal liver test results. Mayo Clin Proc. 1996;71:1089–94. doi: 10.4065/71.11.1089. quiz 1094–5. [DOI] [PubMed] [Google Scholar]
- 25.Korpimaki S, Kaukinen K, Collin P, Haapala AM, Holm P, Laurila K, Kurppa K, Saavalainen P, Haimila K, Partanen J, Maki M, Lahdeaho ML. Gluten-sensitive hypertransaminasemia in celiac disease: an infrequent and often subclinical finding. Am J Gastroenterol. 2011;106:1689–96. doi: 10.1038/ajg.2011.134. [DOI] [PubMed] [Google Scholar]
- 26.Rubio-Tapia A, Murray JA. The liver in celiac disease. Hepatology. 2007;46:1650–8. doi: 10.1002/hep.21949. [DOI] [PubMed] [Google Scholar]
- 27.Sainsbury A, Sanders DS, Ford AC. Meta-analysis: Coeliac disease and hypertransaminasaemia. Aliment Pharmacol Ther. 2012;34:33–40. doi: 10.1111/j.1365-2036.2011.04685.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaukinen K, Halme L, Collin P, Farkkila M, Maki M, Vehmanen P, Partanen J, Hockerstedt K. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology. 2002;122:881–8. doi: 10.1053/gast.2002.32416. [DOI] [PubMed] [Google Scholar]
- 29.Rubio-Tapia A, Abdulkarim AS, Wiesner RH, Moore SB, Krause PK, Murray JA. Celiac disease autoantibodies in severe autoimmune liver disease and the effect of liver transplantation. Liver Int. 2008;28:467–76. doi: 10.1111/j.1478-3231.2008.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dube C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, Macneil J, Mack D, Patel D, Moher D. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005;128:S57–67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Gillett PM, Gillett HR, Israel DM, Metzger DL, Stewart L, Chanoine JP, Freeman HJ. High prevalence of celiac disease in patients with type 1 diabetes detected by antibodies to endomysium and tissue transglutaminase. Can J Gastroenterol. 2001;15:297–301. doi: 10.1155/2001/640796. [DOI] [PubMed] [Google Scholar]
- 32.Holmes GK. Screening for coeliac disease in type 1 diabetes. Arch Dis Child. 2002;87:495–8. doi: 10.1136/adc.87.6.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amin R, Murphy N, Edge J, Ahmed ML, Acerini CL, Dunger DB. A longitudinal study of the effects of a gluten-free diet on glycemic control and weight gain in subjects with type 1 diabetes and celiac disease. Diabetes Care. 2002;25:1117–22. doi: 10.2337/diacare.25.7.1117. [DOI] [PubMed] [Google Scholar]
- 34.Simmons JH, Klingensmith GJ, McFann K, Rewers M, Ide LM, Taki I, Liu E, Hoffenberg EJ. Celiac autoimmunity in children with type 1 diabetes: a two-year follow-up. J Pediatr. 2011;158:276–81 e1. doi: 10.1016/j.jpeds.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rewers M, Eisenbarth GS. Autoimmunity: Celiac disease in T1DM-the need to look long term. Nat Rev Endocrinol. 2011;8:5–6. 7–8. doi: 10.1038/nrendo.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mollazadegan K, Kugelberg M, Montgomery SM, Sanders DS, Ludvigsson J, Ludvigsson JF. A Population-Based Study of the Risk of Diabetic Retinopathy in Patients With Type 1 Diabetes and Celiac Disease. Diabetes Care. 2013;36:316–21. doi: 10.2337/dc12-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leeds JS, Hopper AD, Hadjivassiliou M, Tesfaye S, Sanders DS. High prevalence of microvascular complications in adults with type 1 diabetes and newly diagnosed celiac disease. Diabetes Care. 2011;34:2158–63. doi: 10.2337/dc11-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hummel S, Hummel M, Banholzer J, Hanak D, Mollenhauer U, Bonifacio E, Ziegler AG. Development of autoimmunity to transglutaminase C in children of patients with type 1 diabetes: relationship to islet autoantibodies and infant feeding. Diabetologia. 2007;50:390–4. doi: 10.1007/s00125-006-0546-3. [DOI] [PubMed] [Google Scholar]
- 39.Hummel M, Bonifacio E, Stern M, Dittler J, Schimmel A, Ziegler AG. Development of celiac disease-associated antibodies in offspring of parents with type I diabetes. Diabetologia. 2000;43:1005–11. doi: 10.1007/s001250051483. [DOI] [PubMed] [Google Scholar]
- 40.Jaeger C, Hatziagelaki E, Petzoldt R, Bretzel RG. Comparative analysis of organ-specific autoantibodies and celiac disease--associated antibodies in type 1 diabetic patients, their first-degree relatives, and healthy control subjects. Diabetes Care. 2001;24:27–32. doi: 10.2337/diacare.24.1.27. [DOI] [PubMed] [Google Scholar]
- 41.Lewis NR, Scott BB. Meta-analysis: deamidated gliadin peptide antibody and tissue transglutaminase antibody compared as screening tests for coeliac disease. Aliment Pharmacol Ther. 2010;31:73–81. doi: 10.1111/j.1365-2036.2009.04110.x. [DOI] [PubMed] [Google Scholar]
- 42.Li M, Yu L, Tiberti C, Bonamico M, Taki I, Miao D, Murray JA, Rewers MJ, Hoffenberg EJ, Agardh D, Mueller P, Stern M, Bonifacio E, Liu E. A report on the International Transglutaminase Autoantibody Workshop for Celiac Disease. Am J Gastroenterol. 2009;104:154–63. doi: 10.1038/ajg.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010;105:2520–4. doi: 10.1038/ajg.2010.276. [DOI] [PubMed] [Google Scholar]
- 44.Klapp G, Masip E, Bolonio M, Donat E, Polo B, Ramos D, Ribes-Koninckx C. Coeliac Disease: The New Proposed ESPGHAN Diagnostic Criteria Do Work Well in A Selected Population. J Pediatr Gastroenterol Nutr. 2012 Oct 29; doi: 10.1097/MPG.0b013e318279887b. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Cranney A, Rostom A, Sy R, Dube C, Saloogee N, Garritty C, Moher D, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, MacNeil J. Consequences of testing for celiac disease. Gastroenterology. 2005;128:S109–20. doi: 10.1053/j.gastro.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Harewood GC, Murray JA. Diagnostic approach to a patient with suspected celiac disease: a cost analysis. Dig Dis Sci. 2001;46:2510–4. doi: 10.1023/a:1012396408370. [DOI] [PubMed] [Google Scholar]
- 47.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:516–25. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 48.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 49.Conrad K, Roggenbuck D, Ittenson A, Reinhold D, Buettner T, Laass MW. A new dot immunoassay for simultaneous detection of celiac specific antibodies and IgA-deficiency. Clin Chem Lab Med. 2012;50:337–43. doi: 10.1515/cclm.2011.760. [DOI] [PubMed] [Google Scholar]
- 50.McGowan KE, Lyon ME, Butzner JD. Celiac disease and IgA deficiency: complications of serological testing approaches encountered in the clinic. Clin Chem. 2008;54:1203–9. doi: 10.1373/clinchem.2008.103606. [DOI] [PubMed] [Google Scholar]
- 51.Villalta D, Alessio MG, Tampoia M, Tonutti E, Brusca I, Bagnasco M, Pesce G, Stella S, Bizzaro N. Testing for IgG class antibodies in celiac disease patients with selective IgA deficiency. A comparison of the diagnostic accuracy of 9 IgG anti-tissue transglutaminase, 1 IgG anti-gliadin and 1 IgG anti-deaminated gliadin peptide antibody assays. Clin Chim Acta. 2007;382:95–9. doi: 10.1016/j.cca.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 52.Villalta D, Tonutti E, Prause C, Koletzko S, Uhlig HH, Vermeersch P, Bossuyt X, Stern M, Laass MW, Ellis JH, Ciclitira PJ, Richter T, Daehnrich C, Schlumberger W, Mothes T. IgG antibodies against deamidated gliadin peptides for diagnosis of celiac disease in patients with IgA deficiency. Clin Chem. 2010;56:464–8. doi: 10.1373/clinchem.2009.128132. [DOI] [PubMed] [Google Scholar]
- 53.Lowbeer C, Wallinder H. Undetectable anti-tissue transglutaminase IgA antibody measured with EliA Celikey indicates selective IgA deficiency. Clin Chim Acta. 2010;411:612. doi: 10.1016/j.cca.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Sinclair D, Saas M, Turk A, Goble M, Kerr D. Do we need to measure total serum IgA to exclude IgA deficiency in coeliac disease? J Clin Pathol. 2006;59:736–9. doi: 10.1136/jcp.2005.031864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malamut G, Verkarre V, Suarez F, Viallard JF, Lascaux AS, Cosnes J, Bouhnik Y, Lambotte O, Bechade D, Ziol M, Lavergne A, Hermine O, Cerf-Bensussan N, Cellier C. The enteropathy associated with common variable immunodeficiency: the delineated frontiers with celiac disease. Am J Gastroenterol. 2010;105:2262–75. doi: 10.1038/ajg.2010.214. [DOI] [PubMed] [Google Scholar]
- 56.Casella S, Zanini B, Lanzarotto F, Villanacci V, Ricci C, Lanzini A. Celiac disease in elderly adults: clinical, serological, and histological characteristics and the effect of a gluten-free diet. J Am Geriatr Soc. 2012;60:1064–9. doi: 10.1111/j.1532-5415.2012.03997.x. [DOI] [PubMed] [Google Scholar]
- 57.Rashtak S, Ettore MW, Homburger HA, Murray JA. Comparative usefulness of deamidated gliadin antibodies in the diagnosis of celiac disease. Clin Gastroenterol Hepatol. 2008;6:426–32. doi: 10.1016/j.cgh.2007.12.030. quiz 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zanini B, Lanzarotto F, Mora A, Bertolazzi S, Turini D, Cesana B, Donato F, Ricci C, Lonati F, Vassallo F, Scarcella C, Lanzini A. Five year time course of celiac disease serology during gluten free diet: results of a community based “CD-Watch” program. Dig Liver Dis. 2010;42:865–70. doi: 10.1016/j.dld.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Rashtak S, Ettore MW, Homburger HA, Murray JA. Combination testing for antibodies in the diagnosis of coeliac disease: comparison of multiplex immunoassay and ELISA methods. Aliment Pharmacol Ther. 2008;28:805–13. doi: 10.1111/j.1365-2036.2008.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burgin-Wolff A, Gaze H, Hadziselimovic F, Huber H, Lentze MJ, Nussle D, Reymond-Berthet C. Antigliadin and antiendomysium antibody determination for coeliac disease. Arch Dis Child. 1991;66:941–7. doi: 10.1136/adc.66.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lagerqvist C, Dahlbom I, Hansson T, Jidell E, Juto P, Olcen P, Stenlund H, Hernell O, Ivarsson A. Antigliadin immunoglobulin A best in finding celiac disease in children younger than 18 months of age. J Pediatr Gastroenterol Nutr. 2008;47:428–35. doi: 10.1097/MPG.0b013e31817d80f4. [DOI] [PubMed] [Google Scholar]
- 62.Aberg AK, Olcen P. Serologic screening for celiac disease in children: a comparison between established assays and tests with deamidated gliadin-derived peptides plus conjugates for both IgA and IgG antibodies. Apmis. 2009;117:808–13. doi: 10.1111/j.1600-0463.2009.02541.x. [DOI] [PubMed] [Google Scholar]
- 63.Basso D, Guariso G, Fogar P, Meneghel A, Zambon CF, Navaglia F, Greco E, Schiavon S, Rugge M, Plebani M. Antibodies against synthetic deamidated gliadin peptides for celiac disease diagnosis and follow-up in children. Clin Chem. 2009;55:150–7. doi: 10.1373/clinchem.2008.110395. [DOI] [PubMed] [Google Scholar]
- 64.Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology. 2005;128:S25–32. doi: 10.1053/j.gastro.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Rostom A, Dube C, Cranney A, Saloojee N, Sy R, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, MacNeil J, Mack D, Patel D, Moher D. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology. 2005;128:S38–46. doi: 10.1053/j.gastro.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 66.Giersiepen K, Lelgemann M, Stuhldreher N, Ronfani L, Husby S, Koletzko S, Korponay-Szabo IR. Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J Pediatr Gastroenterol Nutr. 2012;54:229–41. doi: 10.1097/MPG.0b013e318216f2e5. [DOI] [PubMed] [Google Scholar]
- 67.O’Leary C, Wieneke P, Buckley S, O’Regan P, Cronin CC, Quigley EM, Shanahan F. Celiac disease and irritable bowel-type symptoms. Am J Gastroenterol. 2002;97:1463–7. doi: 10.1111/j.1572-0241.2002.05690.x. [DOI] [PubMed] [Google Scholar]
- 68.Sainsbury A, Sanders DS, Ford AC. Prevalence of Irritable Bowel Syndrome-type Symptoms in Patients With Celiac Disease: A Meta-analysis. Clin Gastroenterol Hepatol. 2012 Dec 13; doi: 10.1016/j.cgh.2012.11.033. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Campanella J, Biagi F, Ilaria Bianchi P, Zanellati G, Marchese A, Roberto Corazza G. Clinical response to gluten withdrawal is not an indicator of coeliac disease. Scand J Gastroenterol. 2008:1–4. doi: 10.1080/00365520802200036. [DOI] [PubMed] [Google Scholar]
- 70.Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, Shepherd SJ, Muir JG, Gibson PR. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–14. doi: 10.1038/ajg.2010.487. quiz 515. [DOI] [PubMed] [Google Scholar]
- 71.Wahnschaffe U, Schulzke JD, Zeitz M, Ullrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:844–50. doi: 10.1016/j.cgh.2007.03.021. quiz 769. [DOI] [PubMed] [Google Scholar]
- 72.Salmi TT, Collin P, Korponay-Szabo IR, Laurila K, Partanen J, Huhtala H, Kiraly R, Lorand L, Reunala T, Maki M, Kaukinen K. Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut. 2006;55:1746–53. doi: 10.1136/gut.2005.071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal Recovery and Mortality in Adults With Celiac Disease After Treatment With a Gluten-Free Diet. Am J Gastroenterol. 2010;105:1412–20. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaukinen K, Partanen J, Maki M, Collin P. HLA-DQ typing in the diagnosis of celiac disease. Am J Gastroenterol. 2002;97:695–9. doi: 10.1111/j.1572-0241.2002.05471.x. [DOI] [PubMed] [Google Scholar]
- 75.Shiner M. Small intestinal biopsy: diagnostic and research value. Proc R Soc Med. 1959;52:10–4. doi: 10.1177/003591575905200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McNeish AS, Harms HK, Rey J, Shmerling DH, Visakorpi JK, Walker-Smith JA. The diagnosis of coeliac disease. A commentary on the current practices of members of the European Society for Paediatric Gastroenterology and Nutrition (ESPGAN) Arch Dis Child. 1979;54:783–6. doi: 10.1136/adc.54.10.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guandalini S, Ventura A, Ansaldi N, Giunta AM, Greco L, Lazzari R, Mastella G, Rubino A. Diagnosis of coeliac disease: time for a change? Arch Dis Child. 1989;64:1320–4. doi: 10.1136/adc.64.9.1320. discussion 1324–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hadithi M, von Blomberg BM, Crusius JB, Bloemena E, Kostense PJ, Meijer JW, Mulder CJ, Stehouwer CD, Pena AS. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann Intern Med. 2007;147:294–302. doi: 10.7326/0003-4819-147-5-200709040-00003. [DOI] [PubMed] [Google Scholar]
- 79.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 80.Oberhuber G. Histopathology of celiac disease. Biomed Pharmacother. 2000;54:368–72. doi: 10.1016/S0753-3322(01)80003-2. [DOI] [PubMed] [Google Scholar]
- 81.Corazza GR, Villanacci V, Zambelli C, Milione M, Luinetti O, Vindigni C, Chioda C, Albarello L, Bartolini D, Donato F. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin Gastroenterol Hepatol. 2007;5:838–43. doi: 10.1016/j.cgh.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 82.Owens SR, Greenson JK. The pathology of malabsorption: current concepts. Histopathology. 2007;50:64–82. doi: 10.1111/j.1365-2559.2006.02547.x. [DOI] [PubMed] [Google Scholar]
- 83.Yantiss RK, Odze RD. Optimal approach to obtaining mucosal biopsies for assessment of inflammatory disorders of the gastrointestinal tract. Am J Gastroenterol. 2009;104:774–83. doi: 10.1038/ajg.2008.108. [DOI] [PubMed] [Google Scholar]
- 84.Husby S, Koletzko S, Korponay-Szabo IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, Lelgeman M, Maki M, Ribes-Koninckx C, Ventura A, Zimmer KP. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 85.Wakim-Fleming J, Pagadala MR, Lemyre MS, Lopez R, Kumaravel A, Carey WD, Zein NN. Diagnosis of Celiac Disease in Adults Based on Serology Test Results, Without Small-Bowel Biopsy. Clin Gastroenterol Hepatol. 2013 Jan 7; doi: 10.1016/j.cgh.2012.12.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 86.Klapp G, Masip E, Bolonio M, Donat E, Polo B, Ramos D, Ribes-Koninckx C. Coeliac Disease: The New Proposed ESPGHAN Diagnostic Criteria Do Work Well in A Selected Population. J Pediatr Gastroenterol Nutr. 2012 Oct 29; doi: 10.1097/MPG.0b013e318279887b. [Epub ahead of pring] [DOI] [PubMed] [Google Scholar]
- 87.Kurppa K, Salminiemi J, Ukkola A, Saavalainen P, Loytynoja K, Laurila K, Collin P, Maki M, Kaukinen K. Utility of the new ESPGHAN criteria for the diagnosis of celiac disease in at-risk groups. Journal of pediatric gastroenterology and nutrition. 2012;54:387–91. doi: 10.1097/MPG.0b013e3182407c6b. [DOI] [PubMed] [Google Scholar]
- 88.Swallow K, Wild G, Sargur R, Sanders DS, Aziz I, Hopper AD, Egner W. Quality not quantity for transglutaminase antibody 2: the performance of an endomysial and tissue transglutaminase test in screening coeliac disease remains stable over time. Clin Exp Immunol. 2013;171:100–6. doi: 10.1111/cei.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ravelli A, Bolognini S, Gambarotti M, Villanacci V. Variability of histologic lesions in relation to biopsy site in gluten-sensitive enteropathy. Am J Gastroenterol. 2005;100:177–85. doi: 10.1111/j.1572-0241.2005.40669.x. [DOI] [PubMed] [Google Scholar]
- 90.Ravelli A, Villanacci V, Monfredini C, Martinazzi S, Grassi V, Manenti S. How patchy is patchy villous atrophy? : distribution pattern of histological lesions in the duodenum of children with celiac disease. Am J Gastroenterol. 2010;105:2103–10. doi: 10.1038/ajg.2010.153. [DOI] [PubMed] [Google Scholar]
- 91.Weir DC, Glickman JN, Roiff T, Valim C, Leichtner AM. Variability of histopathological changes in childhood celiac disease. Am J Gastroenterol. 2010;105:207–12. doi: 10.1038/ajg.2009.557. [DOI] [PubMed] [Google Scholar]
- 92.Bonamico M, Mariani P, Thanasi E, Ferri M, Nenna R, Tiberti C, Mora B, Mazzilli MC, Magliocca FM. Patchy villous atrophy of the duodenum in childhood celiac disease. J Pediatr Gastroenterol Nutr. 2004;38:204–7. doi: 10.1097/00005176-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 93.Hopper AD, Cross SS, Sanders DS. Patchy villous atrophy in adult patients with suspected gluten-sensitive enteropathy: is a multiple duodenal biopsy strategy appropriate? Endoscopy. 2008;40:219–24. doi: 10.1055/s-2007-995361. [DOI] [PubMed] [Google Scholar]
- 94.Lebwohl B, Kapel RC, Neugut AI, Green PH, Genta RM. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74:103–9. doi: 10.1016/j.gie.2011.03.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lebwohl B, Tennyson CA, Holub JL, Lieberman DA, Neugut AI, Green PH. Sex and racial disparities in duodenal biopsy to evaluate for celiac disease. Gastrointest Endosc. 2012;76:779–85. doi: 10.1016/j.gie.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Evans KE, Aziz I, Cross SS, Sahota GR, Hopper AD, Hadjivassiliou M, Sanders DS. A prospective study of duodenal bulb biopsy in newly diagnosed and established adult celiac disease. Am J Gastroenterol. 2011;106:1837–742. doi: 10.1038/ajg.2011.171. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez S, Gupta A, Cheng J, Tennyson C, Lewis SK, Bhagat G, Green PH. Prospective study of the role of duodenal bulb biopsies in the diagnosis of celiac disease. Gastrointest Endosc. 2010;72:758–65. doi: 10.1016/j.gie.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 98.Bonamico M, Thanasi E, Mariani P, Nenna R, Luparia RP, Barbera C, Morra I, Lerro P, Guariso G, De Giacomo C, Scotta S, Pontone S, Carpino F, Magliocca FM. Duodenal bulb biopsies in celiac disease: a multicenter study. J Pediatr Gastroenterol Nutr. 2008;47:618–22. doi: 10.1097/mpg.0b013e3181677d6e. [DOI] [PubMed] [Google Scholar]
- 99.Kurien M, Evans KE, Hopper AD, Hale MF, Cross SS, Sanders DS. Duodenal bulb biopsies for diagnosing adult celiac disease: is there an optimal biopsy site? Gastrointest Endosc. 2012;75:1190–6. doi: 10.1016/j.gie.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 100.Walker MM, Murray JA, Ronkainen J, Aro P, Storskrubb T, D’Amato M, Lahr B, Talley NJ, Agreus L. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology. 2010;139:112–9. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jarvinen TT, Kaukinen K, Laurila K, Kyronpalo S, Rasmussen M, Maki M, Korhonen H, Reunala T, Collin P. Intraepithelial lymphocytes in celiac disease. Am J Gastroenterol. 2003;98:1332–7. doi: 10.1111/j.1572-0241.2003.07456.x. [DOI] [PubMed] [Google Scholar]
- 102.Vande Voort JL, Murray JA, Lahr BD, Van Dyke CT, Kroning CM, Moore SB, Wu TT. Lymphocytic duodenosis and the spectrum of celiac disease. Am J Gastroenterol. 2009;104:142–8. doi: 10.1038/ajg.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aziz I, Evans KE, Hopper AD, Smillie DM, Sanders DS. A prospective study into the aetiology of lymphocytic duodenosis. Alimentary pharmacology & therapeutics. 2010;32:1392–7. doi: 10.1111/j.1365-2036.2010.04477.x. [DOI] [PubMed] [Google Scholar]
- 104.Tuire I, Marja-Leena L, Teea S, Katri H, Jukka P, Paivi S, Heini H, Markku M, Pekka C, Katri K. Persistent duodenal intraepithelial lymphocytosis despite a long-term strict gluten-free diet in celiac disease. Am J Gastroenterol. 2012;107:1563–9. doi: 10.1038/ajg.2012.220. [DOI] [PubMed] [Google Scholar]
- 105.Shmidt E, Smyrk TC, Faubion WA, Oxentenko AS. Duodenal Intraepithelial Lymphocytosis with Normal Villous Architecture in Pediatric Patients, 2000–2009: The Mayo Clinic Experience. J Pediatr Gastroenterol Nutr. 2012 Jul 9; doi: 10.1097/MPG.0b013e318267c353. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 106.Kurppa K, Ashorn M, Iltanen S, Koskinen LL, Saavalainen P, Koskinen O, Maki M, Kaukinen K. Celiac disease without villous atrophy in children: a prospective study. J Pediatr. 2010;157:373–80. 380 e1. doi: 10.1016/j.jpeds.2010.02.070. [DOI] [PubMed] [Google Scholar]
- 107.Kurppa K, Collin P, Viljamaa M, Haimila K, Saavalainen P, Partanen J, Laurila K, Huhtala H, Paasikivi K, Maki M, Kaukinen K. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136:816–23. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 108.Lundin KE, Gjertsen HA, Scott H, Sollid LM, Thorsby E. Function of DQ2 and DQ8 as HLA susceptibility molecules in celiac disease. Hum Immunol. 1994;41:24–7. doi: 10.1016/0198-8859(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 109.Paulsen G, Lundin KE, Gjertsen HA, Hansen T, Sollid LM, Thorsby E. HLA-DQ2-restricted T-cell recognition of gluten-derived peptides in celiac disease. Influence of amino acid substitutions in the membrane distal domain of DQ beta 1*0201. Hum Immunol. 1995;42:145–53. doi: 10.1016/0198-8859(94)00086-6. [DOI] [PubMed] [Google Scholar]
- 110.Kim CY, Quarsten H, Bergseng E, Khosla C, Sollid LM. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc Natl Acad Sci U S A. 2004;101:4175–9. doi: 10.1073/pnas.0306885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989;169:345–50. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sollid LM, Thorsby E. The primary association of celiac disease to a given HLA-DQ alpha/beta heterodimer explains the divergent HLA-DR associations observed in various Caucasian populations. Tissue Antigens. 1990;36:136–7. doi: 10.1111/j.1399-0039.1990.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 113.Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, Ciclitira PJ, Sollid LM, Partanen J. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003;64:469–77. doi: 10.1016/s0198-8859(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 114.Wolters VM, Wijmenga C. Genetic background of celiac disease and its clinical implications. Am J Gastroenterol. 2008;103:190–5. doi: 10.1111/j.1572-0241.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- 115.Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99–107. doi: 10.1053/j.gastro.2008.10.013. quiz 352–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373–8. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Book L, Hart A, Black J, Feolo M, Zone JJ, Neuhausen SL. Prevalence and clinical characteristics of celiac disease in Downs syndrome in a US study. Am J Med Genet. 2001;98:70–4. [PubMed] [Google Scholar]
- 118.Wouters J, Weijerman ME, van Furth AM, Schreurs MW, Crusius JB, von Blomberg BM, de Baaij LR, Broers CJ, Gemke RJ. Prospective human leukocyte antigen, endomysium immunoglobulin A antibodies, and transglutaminase antibodies testing for celiac disease in children with Down syndrome. J Pediatr. 2009;154:239–42. doi: 10.1016/j.jpeds.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 119.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 120.Tennyson CA, Ciaccio EJ, Lewis SK. Video capsule endoscopy in celiac disease. Gastrointest Endosc Clin N Am. 2012;22:747–58. doi: 10.1016/j.giec.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 121.Chang MS, Rubin M, Lewis SK, Green PH. Diagnosing celiac disease by video capsule endoscopy (VCE) when esophogastroduodenoscopy (EGD) and biopsy is unable to provide a diagnosis: a case series. BMC Gastroenterol. 2012;12:90. doi: 10.1186/1471-230X-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rokkas T, Niv Y. The role of video capsule endoscopy in the diagnosis of celiac disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2012;24:303–8. doi: 10.1097/MEG.0b013e32834fa914. [DOI] [PubMed] [Google Scholar]
- 123.Murray JA, Rubio-Tapia A, Van Dyke CT, Brogan DL, Knipschield MA, Lahr B, Rumalla A, Zinsmeister AR, Gostout CJ. Mucosal atrophy in celiac disease: extent of involvement, correlation with clinical presentation, and response to treatment. Clin Gastroenterol Hepatol. 2008;6:186–93. doi: 10.1016/j.cgh.2007.10.012. quiz 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lidums I, Cummins AG, Teo E. The role of capsule endoscopy in suspected celiac disease patients with positive celiac serology. Dig Dis Sci. 2011;56:499–505. doi: 10.1007/s10620-010-1290-6. [DOI] [PubMed] [Google Scholar]
- 125.Barret M, Malamut G, Rahmi G, Samaha E, Edery J, Verkarre V, Macintyre E, Lenain E, Chatellier G, Cerf-Bensussan N, Cellier C. Diagnostic yield of capsule endoscopy in refractory celiac disease. Am J Gastroenterol. 2012;107:1546–53. doi: 10.1038/ajg.2012.199. [DOI] [PubMed] [Google Scholar]
- 126.Culliford A, Daly J, Diamond B, Rubin M, Green PH. The value of wireless capsule endoscopy in patients with complicated celiac disease. Gastrointest Endosc. 2005;62:55–61. doi: 10.1016/s0016-5107(05)01566-x. [DOI] [PubMed] [Google Scholar]
- 127.Atlas DS, Rubio-Tapia A, Van Dyke CT, Lahr BD, Murray JA. Capsule endoscopy in nonresponsive celiac disease. Gastrointest Endosc. 2011;74:1315–22. doi: 10.1016/j.gie.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hadithi M, Al-toma A, Oudejans J, van Bodegraven AA, Mulder CJ, Jacobs M. The value of double-balloon enteroscopy in patients with refractory celiac disease. Am J Gastroenterol. 2007;102:987–96. doi: 10.1111/j.1572-0241.2007.01122.x. [DOI] [PubMed] [Google Scholar]
- 129.Van Weyenberg SJ, Meijerink MR, Jacobs MA, van Kuijk C, Mulder CJ, van Waesberghe JH. MR enteroclysis in refractory celiac disease: proposal and validation of a severity scoring system. Radiology. 2011;259:151–61. doi: 10.1148/radiol.11101808. [DOI] [PubMed] [Google Scholar]
- 130.Tennyson CA, Semrad CE. Small bowel imaging in celiac disease. Gastrointest Endosc Clin N Am. 2012;22:735–46. doi: 10.1016/j.giec.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 131.Fordtran JS, Soergel KH, Ingelfinger FJ. Intestinal absorption of D-xylose in man. N Engl J Med. 1962;267:274–9. doi: 10.1056/NEJM196208092670602. [DOI] [PubMed] [Google Scholar]
- 132.Craig RM, Ehrenpreis ED. D-xylose testing. J Clin Gastroenterol. 1999;29:143–50. doi: 10.1097/00004836-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 133.Tveito K, Brunborg C, Bratlie J, Askedal M, Sandvik L, Lundin KE, Skar V. Intestinal malabsorption of D-xylose: comparison of test modalities in patients with celiac disease. Scand J Gastroenterol. 2010;45:1289–94. doi: 10.3109/00365521.2010.503969. [DOI] [PubMed] [Google Scholar]
- 134.Carroccio A, Iacono G, Montalto G, Cavataio F, Soresi M, Kazmierska I, Notarbartolo A. Immunologic and absorptive tests in celiac disease: can they replace intestinal biopsies? Scand J Gastroenterol. 1993;28:673–6. doi: 10.3109/00365529309098270. [DOI] [PubMed] [Google Scholar]
- 135.Heyman M, Abed J, Lebreton C, Cerf-Bensussan N. Intestinal permeability in coeliac disease: insight into mechanisms and relevance to pathogenesis. Gut. 2012;61:1355–64. doi: 10.1136/gutjnl-2011-300327. [DOI] [PubMed] [Google Scholar]
- 136.Smecuol E, Bai JC, Vazquez H, Kogan Z, Cabanne A, Niveloni S, Pedreira S, Boerr L, Maurino E, Meddings JB. Gastrointestinal permeability in celiac disease. Gastroenterology. 1997;112:1129–36. doi: 10.1016/s0016-5085(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 137.Juby LD, Rothwell J, Axon AT. Cellobiose/mannitol sugar test--a sensitive tubeless test for coeliac disease: results on 1010 unselected patients. Gut. 1989;30:476–80. doi: 10.1136/gut.30.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Juby LD, Rothwell J, Axon AT. Lactulose/mannitol test: an ideal screen for celiac disease. Gastroenterology. 1989;96:79–85. doi: 10.1016/0016-5085(89)90767-1. [DOI] [PubMed] [Google Scholar]
- 139.La Seta F, Buccellato A, Albanese M, Barbiera F, Cottone M, Oliva L, Agricola A, Costanzo GS, Lagalla R. Radiology and adult celiac disease. Current indications of small bowel barium examinations. Radiol Med. 2004;108:515–21. [PubMed] [Google Scholar]
- 140.Barlow JM, Johnson CD, Stephens DH. Celiac disease: how common is jejunoileal fold pattern reversal found at small-bowel follow-through? AJR Am J Roentgenol. 1996;166:575–7. doi: 10.2214/ajr.166.3.8623630. [DOI] [PubMed] [Google Scholar]
- 141.Weizman Z, Stringer DA, Durie PR. Radiologic manifestations of malabsorption: a nonspecific finding. Pediatrics. 1984;74:530–3. [PubMed] [Google Scholar]
- 142.Bonamico M, Ferri M, Nenna R, Verrienti A, Di Mario U, Tiberti C. Tissue transglutaminase autoantibody detection in human saliva: a powerful method for celiac disease screening. J Pediatr. 2004;144:632–6. doi: 10.1016/j.jpeds.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 143.Bonamico M, Nenna R, Luparia RP, Perricone C, Montuori M, Lucantoni F, Castronovo A, Mura S, Turchetti A, Strappini P, Tiberti C. Radioimmunological detection of anti-transglutaminase autoantibodies in human saliva: a useful test to monitor coeliac disease follow-up. Aliment Pharmacol Ther. 2008;28:364–70. doi: 10.1111/j.1365-2036.2008.03720.x. [DOI] [PubMed] [Google Scholar]
- 144.Kappler M, Krauss-Etschmann S, Diehl V, Zeilhofer H, Koletzko S. Detection of secretory IgA antibodies against gliadin and human tissue transglutaminase in stool to screen for coeliac disease in children: validation study. Bmj. 2006;332:213–4. doi: 10.1136/bmj.38688.654028.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, Lundin KE, Murray JA, Sanders DS, Walker MM, Zingone F, Ciacci C. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lundin KE, Alaedini A. Non-celiac Gluten Sensitivity. Gastrointest Endosc Clin N Am. 2012;22:723–34. doi: 10.1016/j.giec.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 147.Aziz I, Sanders DS. Emerging concepts: from coeliac disease to non-coeliac gluten sensitivity. Proc Nutr Soc. 2012;71:576–80. doi: 10.1017/S002966511200081X. [DOI] [PubMed] [Google Scholar]
- 148.Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, Brusca I, Florena AM, Ambrosiano G, Seidita A, Pirrone G, Rini GB. Non-Celiac Wheat Sensitivity Diagnosed by Double-Blind Placebo-Controlled Challenge: Exploring a New Clinical Entity. Am J Gastroenterol. 2012;107:1898–906. doi: 10.1038/ajg.2012.236. [DOI] [PubMed] [Google Scholar]
- 149.Bao F, Green PH, Bhagat G. An update on celiac disease histopathology and the road ahead. Arch Pathol Lab Med. 2012;136:735–45. doi: 10.5858/arpa.2011-0572-RA. [DOI] [PubMed] [Google Scholar]
- 150.Nasr I, Leffler DA, Ciclitira PJ. Management of celiac disease. Gastrointest Endosc Clin N Am. 2012;22:695–704. doi: 10.1016/j.giec.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 151.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 152.Leffler D, Schuppan D, Pallav K, Najarian R, Goldsmith JD, Hansen J, Kabbani T, Dennis M, Kelly CP. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2012 May 22; doi: 10.1136/gutjnl-2012-302196. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lahdeaho ML, Maki M, Laurila K, Huhtala H, Kaukinen K. Small- bowel mucosal changes and antibody responses after low- and moderate-dose gluten challenge in celiac disease. BMC Gastroenterol. 2011;11:129. doi: 10.1186/1471-230X-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rujner J, Socha J, Romanczuk W, Stolarczyk A, Wozniewicz B, Gregorek H, Madalinski K, Syczewska M. Individual sensitivity of jejunal mucosa to small doses of gluten in coeliac disease. Wiad Lek. 2002;55:554–60. [PubMed] [Google Scholar]
- 155.Ansaldi N, Tavassoli K, Faussone D, Forni M, Oderda G. Clinico-histological behavior of celiac patients after gluten load following the definitive diagnosis. Pediatr Med Chir. 1988;10:3–6. [PubMed] [Google Scholar]
- 156.Akobeng AK, Thomas AG. Systematic review: tolerable amount of gluten for people with coeliac disease. Aliment Pharmacol Ther. 2008;27:1044–52. doi: 10.1111/j.1365-2036.2008.03669.x. [DOI] [PubMed] [Google Scholar]
- 157.Ludvigsson JF. Mortality and malignancy in celiac disease. Gastrointestinal endoscopy clinics of North America. 2012;22:705–22. doi: 10.1016/j.giec.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 158.West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004;329:716–9. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Askling J, Linet M, Gridley G, Halstensen TS, Ekstrom K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428–35. doi: 10.1053/gast.2002.36585. [DOI] [PubMed] [Google Scholar]
- 160.Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, Sategna Guidetti C, Usai P, Cesari P, Pelli MA, Loperfido S, Volta U, Calabro A, Certo M. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358:356–61. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 161.Bardella MT, Fredella C, Prampolini L, Molteni N, Giunta AM, Bianchi PA. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am J Clin Nutr. 2000;72:937–9. doi: 10.1093/ajcn/72.4.937. [DOI] [PubMed] [Google Scholar]
- 162.Barera G, Mora S, Brambilla P, Ricotti A, Menni L, Beccio S, Bianchi C. Body composition in children with celiac disease and the effects of a gluten-free diet: a prospective case-control study. Am J Clin Nutr. 2000;72:71–5. doi: 10.1093/ajcn/72.1.71. [DOI] [PubMed] [Google Scholar]
- 163.Rea F, Polito C, Marotta A, Di Toro A, Iovene A, Collini R, Rea L, Sessa G. Restoration of body composition in celiac children after one year of gluten-free diet. J Pediatr Gastroenterol Nutr. 1996;23:408–12. doi: 10.1097/00005176-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 164.Kemppainen T, Kroger H, Janatuinen E, Arnala I, Kosma VM, Pikkarainen P, Julkunen R, Jurvelin J, Alhava E, Uusitupa M. Osteoporosis in adult patients with celiac disease. Bone. 1999;24:249–55. doi: 10.1016/s8756-3282(98)00178-1. [DOI] [PubMed] [Google Scholar]
- 165.Meyer D, Stavropolous S, Diamond B, Shane E, Green PH. Osteoporosis in a north american adult population with celiac disease. Am J Gastroenterol. 2001;96:112–9. doi: 10.1111/j.1572-0241.2001.03507.x. [DOI] [PubMed] [Google Scholar]
- 166.Kalayci AG, Kansu A, Girgin N, Kucuk O, Aras G. Bone mineral density and importance of a gluten-free diet in patients with celiac disease in childhood. Pediatrics. 2001;108:E89. doi: 10.1542/peds.108.5.e89. [DOI] [PubMed] [Google Scholar]
- 167.Sategna-Guidetti C, Grosso SB, Grosso S, Mengozzi G, Aimo G, Zaccaria T, Di Stefano M, Isaia GC. The effects of 1-year gluten withdrawal on bone mass, bone metabolism and nutritional status in newly-diagnosed adult coeliac disease patients. Aliment Pharmacol Ther. 2000;14:35–43. doi: 10.1046/j.1365-2036.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 168.Valdimarsson T, Lofman O, Toss G, Strom M. Reversal of osteopenia with diet in adult coeliac disease. Gut. 1996;38:322–7. doi: 10.1136/gut.38.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]
- 170.Fickling WE, McFarlane XA, Bhalla AK, Robertson DA. The clinical impact of metabolic bone disease in coeliac disease. Postgrad Med J. 2001;77:33–6. doi: 10.1136/pmj.77.903.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vasquez H, Mazure R, Gonzalez D, Flores D, Pedreira S, Niveloni S, Smecuol E, Maurino E, Bai JC. Risk of fractures in celiac disease patients: a cross-sectional, case-control study. Am J Gastroenterol. 2000;95:183–9. doi: 10.1111/j.1572-0241.2000.01682.x. [DOI] [PubMed] [Google Scholar]
- 172.West J, Logan RF, Card TR, Smith C, Hubbard R. Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology. 2003;125:429–36. doi: 10.1016/s0016-5085(03)00891-6. [DOI] [PubMed] [Google Scholar]
- 173.Ciacci C, Maurelli L, Klain M, Savino G, Salvatore M, Mazzacca G, Cirillo M. Effects of dietary treatment on bone mineral density in adults with celiac disease: factors predicting response. Am J Gastroenterol. 1997;92:992–6. [PubMed] [Google Scholar]
- 174.Mustalahti K, Collin P, Sievanen H, Salmi J, Maki M. Osteopenia in patients with clinically silent coeliac disease warrants screening. Lancet. 1999;354:744–5. doi: 10.1016/S0140-6736(99)01990-X. [DOI] [PubMed] [Google Scholar]
- 175.McFarlane XA, Bhalla AK, Robertson DA. Effect of a gluten free diet on osteopenia in adults with newly diagnosed coeliac disease. Gut. 1996;39:180–4. doi: 10.1136/gut.39.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mora S, Barera G, Beccio S, Menni L, Proverbio MC, Bianchi C, Chiumello G. A prospective, longitudinal study of the long-term effect of treatment on bone density in children with celiac disease. J Pediatr. 2001;139:516–21. doi: 10.1067/mpd.2001.116298. [DOI] [PubMed] [Google Scholar]
- 177.Choi JM, Lebwohl B, Wang J, Lee SK, Murray JA, Sauer MV, Green PH. Increased prevalence of celiac disease in patients with unexplained infertility in the United States. J Reprod Med. 2011;56:199–203. [PMC free article] [PubMed] [Google Scholar]
- 178.Ciacci C, Cirillo M, Auriemma G, Di Dato G, Sabbatini F, Mazzacca G. Celiac disease and pregnancy outcome. Am J Gastroenterol. 1996;91:718–22. [PubMed] [Google Scholar]
- 179.Norgard B, Fonager K, Sorensen HT, Olsen J. Birth outcomes of women with celiac disease: a nationwide historical cohort study. Am J Gastroenterol. 1999;94:2435–40. doi: 10.1111/j.1572-0241.1999.01370.x. [DOI] [PubMed] [Google Scholar]
- 180.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology. 2005;129:454–63. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 181.Khashan AS, Henriksen TB, Mortensen PB, McNamee R, McCarthy FP, Pedersen MG, Kenny LC. The impact of maternal celiac disease on birthweight and preterm birth: a Danish population-based cohort study. Hum Reprod. 2010;25:528–34. doi: 10.1093/humrep/dep409. [DOI] [PubMed] [Google Scholar]
- 182.Kemppainen TA, Heikkinen MT, Ristikankare MK, Kosma VM, Julkunen RJ. Nutrient intakes during diets including unkilned and large amounts of oats in celiac disease. Eur J Clin Nutr. 2010;64:62–7. doi: 10.1038/ejcn.2009.113. [DOI] [PubMed] [Google Scholar]
- 183.Sey MS, Parfitt J, Gregor J. Prospective study of clinical and histological safety of pure and uncontaminated Canadian oats in the management of celiac disease. JPEN J Parenter Enteral Nutr. 2011;35:459–64. doi: 10.1177/0148607110387800. [DOI] [PubMed] [Google Scholar]
- 184.Koskinen O, Villanen M, Korponay-Szabo I, Lindfors K, Maki M, Kaukinen K. Oats do not induce systemic or mucosal autoantibody response in children with coeliac disease. J Pediatr Gastroenterol Nutr. 2009;48:559–65. doi: 10.1097/MPG.0b013e3181668635. [DOI] [PubMed] [Google Scholar]
- 185.Kemppainen TA, Heikkinen MT, Ristikankare MK, Kosma VM, Sontag-Strohm TS, Brinck O, Salovaara HO, Julkunen RJ. Unkilned and large amounts of oats in the coeliac disease diet: a randomized, controlled study. Scand J Gastroenterol. 2008;43:1094–101. doi: 10.1080/00365520802014858. [DOI] [PubMed] [Google Scholar]
- 186.Guttormsen V, Lovik A, Bye A, Bratlie J, Morkrid L, Lundin KE. No induction of anti-avenin IgA by oats in adult, diet-treated coeliac disease. Scand J Gastroenterol. 2008;43:161–5. doi: 10.1080/00365520701832822. [DOI] [PubMed] [Google Scholar]
- 187.Maglio M, Mazzarella G, Barone MV, Gianfrani C, Pogna N, Gazza L, Stefanile R, Camarca A, Colicchio B, Nanayakkara M, Miele E, Iaquinto G, Giardullo N, Maurano F, Santoro P, Troncone R, Auricchio S. Immunogenicity of two oat varieties, in relation to their safety for celiac patients. Scand J Gastroenterol. 2011;46:1194–205. doi: 10.3109/00365521.2011.603159. [DOI] [PubMed] [Google Scholar]
- 188.Rashid M, Butzner D, Burrows V, Zarkadas M, Case S, Molloy M, Warren R, Pulido O, Switzer C. Consumption of pure oats by individuals with celiac disease: a position statement by the Canadian Celiac Association. Can J Gastroenterol. 2007;21:649–51. doi: 10.1155/2007/340591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Janatuinen EK, Kemppainen TA, Julkunen RJ, Kosma VM, Maki M, Heikkinen M, Uusitupa MI. No harm from five year ingestion of oats in coeliac disease. Gut. 2002;50:332–5. doi: 10.1136/gut.50.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hogberg L, Laurin P, Falth-Magnusson K, Grant C, Grodzinsky E, Jansson G, Ascher H, Browaldh L, Hammersjo JA, Lindberg E, Myrdal U, Stenhammar L. Oats to children with newly diagnosed coeliac disease: a randomised double blind study. Gut. 2004;53:649–54. doi: 10.1136/gut.2003.026948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koerner TB, Cleroux C, Poirier C, Cantin I, Alimkulov A, Elamparo H. Gluten contamination in the Canadian commercial oat supply. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:705–10. doi: 10.1080/19440049.2011.579626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thompson T. Gluten contamination of commercial oat products in the United States. N Engl J Med. 2004;351:2021–2. doi: 10.1056/NEJM200411043511924. [DOI] [PubMed] [Google Scholar]
- 193.Silano M, Dessi M, De Vincenzi M, Cornell H. In vitro tests indicate that certain varieties of oats may be harmful to patients with coeliac disease. J Gastroenterol Hepatol. 2007;22:528–31. doi: 10.1111/j.1440-1746.2006.04512.x. [DOI] [PubMed] [Google Scholar]
- 194.Comino I, Real A, de Lorenzo L, Cornell H, Lopez-Casado MA, Barro F, Lorite P, Torres MI, Cebolla A, Sousa C. Diversity in oat potential immunogenicity: basis for the selection of oat varieties with no toxicity in coeliac disease. Gut. 2011;60:915–22. doi: 10.1136/gut.2010.225268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Anson O, Weizman Z, Zeevi N. Celiac disease: parental knowledge and attitudes of dietary compliance. Pediatrics. 1990;85:98–103. [PubMed] [Google Scholar]
- 196.Jackson PT, Glasgow JF, Thom R. Parents’ understanding of coeliac disease and diet. Arch Dis Child. 1985;60:672–4. doi: 10.1136/adc.60.7.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lamontagne P, West GE, Galibois I. Quebecers with celiac disease: analysis of dietary problems. Can J Diet Pract Res. 2001;62:175–81. [PubMed] [Google Scholar]
- 198.Haapalahti M, Kulmala P, Karttunen TJ, Paajanen L, Laurila K, Maki M, Mykkanen H, Kokkonen J. Nutritional status in adolescents and young adults with screen-detected celiac disease. J Pediatr Gastroenterol Nutr. 2005;40:566–70. doi: 10.1097/01.mpg.0000154658.16618.f9. [DOI] [PubMed] [Google Scholar]
- 199.Botero-Lopez JE, Araya M, Parada A, Mendez MA, Pizarro F, Espinosa N, Canales P, Alarcon T. Micronutrient deficiencies in patients with typical and atypical celiac disease. J Pediatr Gastroenterol Nutr. 2011;53:265–70. doi: 10.1097/MPG.0b013e3181f988fc. [DOI] [PubMed] [Google Scholar]
- 200.Mody RJ, Brown PI, Wechsler DS. Refractory iron deficiency anemia as the primary clinical manifestation of celiac disease. J Pediatr Hematol Oncol. 2003;25:169–72. doi: 10.1097/00043426-200302000-00018. [DOI] [PubMed] [Google Scholar]
- 201.Corazza GR, Valentini RA, Andreani ML, D’Anchino M, Leva MT, Ginaldi L, De Feudis L, Quaglino D, Gasbarrini G. Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scand J Gastroenterol. 1995;30:153–6. doi: 10.3109/00365529509093254. [DOI] [PubMed] [Google Scholar]
- 202.Bottaro G, Cataldo F, Rotolo N, Spina M, Corazza GR. The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol. 1999;94:691–6. doi: 10.1111/j.1572-0241.1999.00938.x. [DOI] [PubMed] [Google Scholar]
- 203.Howard MR, Turnbull AJ, Morley P, Hollier P, Webb R, Clarke A. A prospective study of the prevalence of undiagnosed coeliac disease in laboratory defined iron and folate deficiency. J Clin Pathol. 2002;55:754–7. doi: 10.1136/jcp.55.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tikkakoski S, Savilahti E, Kolho KL. Undiagnosed coeliac disease and nutritional deficiencies in adults screened in primary health care. Scand J Gastroenterol. 2007;42:60–5. doi: 10.1080/00365520600789974. [DOI] [PubMed] [Google Scholar]
- 205.Dickey W. Low serum vitamin B12 is common in coeliac disease and is not due to autoimmune gastritis. Eur J Gastroenterol Hepatol. 2002;14:425–7. doi: 10.1097/00042737-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 206.Dahele A, Ghosh S. Vitamin B12 deficiency in untreated celiac disease. Am J Gastroenterol. 2001;96:745–50. doi: 10.1111/j.1572-0241.2001.03616.x. [DOI] [PubMed] [Google Scholar]
- 207.Reinken L, Zieglauer H. Vitamin B-6 absorption in children with acute celiac disease and in control subjects. J Nutr. 1978;108:1562–5. doi: 10.1093/jn/108.10.1562. [DOI] [PubMed] [Google Scholar]
- 208.Halfdanarson TR, Kumar N, Hogan WJ, Murray JA. Copper deficiency in celiac disease. J Clin Gastroenterol. 2009;43:162–4. doi: 10.1097/MCG.0b013e3181354294. [DOI] [PubMed] [Google Scholar]
- 209.Lerner A, Gruener N, Iancu TC. Serum carnitine concentrations in coeliac disease. Gut. 1993;34:933–5. doi: 10.1136/gut.34.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hallert C, Grant C, Grehn S, Granno C, Hulten S, Midhagen G, Strom M, Svensson H, Valdimarsson T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther. 2002;16:1333–9. doi: 10.1046/j.1365-2036.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- 211.Hallert C, Svensson M, Tholstrup J, Hultberg B. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Aliment Pharmacol Ther. 2009;29:811–6. doi: 10.1111/j.1365-2036.2009.03945.x. [DOI] [PubMed] [Google Scholar]
- 212.Pietzak MM. Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology. 2005;128:S135–41. doi: 10.1053/j.gastro.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 213.Herman ML, Rubio-Tapia A, Lahr BD, Larson JJ, Van Dyke CT, Murray JA. Patients with celiac disease are not followed up adequately. Clin Gastroenterol Hepatol. 2012;10:893–899 e1. doi: 10.1016/j.cgh.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Haines ML, Anderson RP, Gibson PR. Systematic review: The evidence base for long-term management of coeliac disease. Aliment Pharmacol Ther. 2008;28:1042–66. doi: 10.1111/j.1365-2036.2008.03820.x. [DOI] [PubMed] [Google Scholar]
- 215.Gerrard JW, Ross CA, Smellie JM. Coeliac disease; results of late treatment with gluten-free wheat diet. Lancet. 1955;268:587–9. doi: 10.1016/s0140-6736(55)91635-4. [DOI] [PubMed] [Google Scholar]
- 216.Bebb JR, Lawson A, Knight T, Long RG. Long-term follow-up of coeliac disease--what do coeliac patients want? Aliment Pharmacol Ther. 2006;23:827–31. doi: 10.1111/j.1365-2036.2006.02824.x. [DOI] [PubMed] [Google Scholar]
- 217.Kurppa K, Lauronen O, Collin P, Ukkola A, Laurila K, Huhtala H, Maki M, Kaukinen K. Factors Associated with Dietary Adherence in Celiac Disease: A Nationwide Study. Digestion. 2012;86:309–314. doi: 10.1159/000341416. [DOI] [PubMed] [Google Scholar]
- 218.Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5:445–50. doi: 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 219.Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97:2016–21. doi: 10.1111/j.1572-0241.2002.05917.x. [DOI] [PubMed] [Google Scholar]
- 220.Simpson S, Thompson T. Nutrition assessment in celiac disease. Gastrointest Endosc Clin N Am. 2012;22:797–809. doi: 10.1016/j.giec.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 221.Nachman F, Sugai E, Vazquez H, Gonzalez A, Andrenacci P, Niveloni S, Mazure R, Smecuol E, Moreno ML, Hwang HJ, Sanchez MI, Maurino E, Bai JC. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur J Gastroenterol Hepatol. 2011;23:473–80. doi: 10.1097/MEG.0b013e328346e0f1. [DOI] [PubMed] [Google Scholar]
- 222.Koop I, Ilchmann R, Izzi L, Adragna A, Koop H, Barthelmes H. Detection of autoantibodies against tissue transglutaminase in patients with celiac disease and dermatitis herpetiformis. Am J Gastroenterol. 2000;95:2009–14. doi: 10.1111/j.1572-0241.2000.02086.x. [DOI] [PubMed] [Google Scholar]
- 223.Kaukinen K, Sulkanen S, Maki M, Collin P. IgA-class transglutaminase antibodies in evaluating the efficacy of gluten-free diet in coeliac disease. Eur J Gastroenterol Hepatol. 2002;14:311–5. doi: 10.1097/00042737-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 224.Lanzini A, Lanzarotto F, Villanacci V, Mora A, Bertolazzi S, Turini D, Carella G, Malagoli A, Ferrante G, Cesana BM, Ricci C. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment Pharmacol Ther. 2009;29:1299–308. doi: 10.1111/j.1365-2036.2009.03992.x. [DOI] [PubMed] [Google Scholar]
- 225.Leffler DA, Dennis M, Edwards George JB, Jamma S, Magge S, Cook EF, Schuppan D, Kelly CP. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7:530–6. 536 e1–2. doi: 10.1016/j.cgh.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 226.Biagi F, Andrealli A, Bianchi PI, Marchese A, Klersy C, Corazza GR. A gluten-free diet score to evaluate dietary compliance in patients with coeliac disease. Br J Nutr. 2009;102:882–7. doi: 10.1017/S0007114509301579. [DOI] [PubMed] [Google Scholar]
- 227.Biagi F, Bianchi PI, Marchese A, Trotta L, Vattiato C, Balduzzi D, Brusco G, Andrealli A, Cisaro F, Astegiano M, Pellegrino S, Magazzu G, Klersy C, Corazza GR. A score that verifies adherence to a gluten-free diet: a cross-sectional, multicentre validation in real clinical life. Br J Nutr. 2012:1–5. doi: 10.1017/S0007114511007367. [DOI] [PubMed] [Google Scholar]
- 228.Lebwohl B, Granath F, Ekbom A, Montgomery S, Murray JA, Rubio-Tapia A, Green PH, Ludvigsson JF. Mucosal Healing and Mortality in Celiac Disease. Aliment Pharmacol Ther. 2013;37:332–9. doi: 10.1111/apt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dewar DH, Donnelly SC, McLaughlin SD, Johnson MW, Ellis HJ, Ciclitira PJ. Celiac disease: management of persistent symptoms in patients on a gluten-free diet. World J Gastroenterol. 2012;18:1348–56. doi: 10.3748/wjg.v18.i12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wahab PJ, Meijer JW, Mulder CJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol. 2002;118:459–63. doi: 10.1309/EVXT-851X-WHLC-RLX9. [DOI] [PubMed] [Google Scholar]
- 231.Kaukinen K, Peraaho M, Lindfors K, Partanen J, Woolley N, Pikkarainen P, Karvonen AL, Laasanen T, Sievanen H, Maki M, Collin P. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment Pharmacol Ther. 2007;25:1237–45. doi: 10.1111/j.1365-2036.2007.03311.x. [DOI] [PubMed] [Google Scholar]
- 232.Elfstrom P, Granath F, Ekstrom Smedby K, Montgomery SM, Askling J, Ekbom A, Ludvigsson JF. Risk of lymphoproliferative malignancy in relation to small intestinal histopathology among patients with celiac disease. J Natl Cancer Inst. 2011;103:436–44. doi: 10.1093/jnci/djq564. [DOI] [PubMed] [Google Scholar]
- 233.Lebwohl B, Granath F, Ekbom A, Montgomery SM, Murray JA, Rubio-Tapia A, Green PH, Ludvigsson JF. Mucosal healing and mortality in coeliac disease. Aliment Pharmacol Ther. 2013;37:332–9. doi: 10.1111/apt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dipper CR, Maitra S, Thomas R, Lamb CA, McLean-Tooke AP, Ward R, Smith D, Spickett G, Mansfield JC. Anti-tissue transglutaminase antibodies in the follow-up of adult coeliac disease. Aliment Pharmacol Ther. 2009;30:236–44. doi: 10.1111/j.1365-2036.2009.04039.x. [DOI] [PubMed] [Google Scholar]
- 235.Saez LR, Alvarez DF, Martinez IP, Mieres NA, Garcia PN, de Garcia RF, Menendez SR, Alegre SV, Goni JL. Refractory iron-deficiency anemia and gluten intolerance - Response to gluten-free diet. Rev Esp Enferm Dig. 2011;103:349–54. doi: 10.4321/s1130-01082011000700003. [DOI] [PubMed] [Google Scholar]
- 236.Halfdanarson TR, Litzow MR, Murray JA. Hematologic manifestations of celiac disease. Blood. 2007;109:412–21. doi: 10.1182/blood-2006-07-031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Patterson SK, Green PH, Tennyson CA, Lewis SK, Brannagan TH., 3rd Copper levels in patients with celiac neuropathy. J Clin Neuromuscul Dis. 2012;14:11–6. doi: 10.1097/CND.0b013e318260b455. [DOI] [PubMed] [Google Scholar]
- 238.Bai JC, Gonzalez D, Mautalen C, Mazure R, Pedreira S, Vazquez H, Smecuol E, Siccardi A, Cataldi M, Niveloni S, Boerr LA, Maurino E. Long-term effect of gluten restriction on bone mineral density of patients with coeliac disease. Aliment Pharmacol Ther. 1997;11:157–64. doi: 10.1046/j.1365-2036.1997.112283000.x. [DOI] [PubMed] [Google Scholar]
- 239.Sanchez MI, Mohaidle A, Baistrocchi A, Matoso D, Vazquez H, Gonzalez A, Mazure R, Maffei E, Ferrari G, Smecuol E, Crivelli A, de Paula JA, Gomez JC, Pedreira S, Maurino E, Bai JC. Risk of fracture in celiac disease: gender, dietary compliance, or both? World J Gastroenterol. 2011;17:3035–42. doi: 10.3748/wjg.v17.i25.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jafri MR, Nordstrom CW, Murray JA, Van Dyke CT, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Long-term fracture risk in patients with celiac disease: a population-based study in Olmsted County, Minnesota. Dig Dis Sci. 2008;53:964–71. doi: 10.1007/s10620-007-9976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ludvigsson JF, Michaelsson K, Ekbom A, Montgomery SM. Coeliac disease and the risk of fractures - a general population-based cohort study. Aliment Pharmacol Ther. 2007;25:273–85. doi: 10.1111/j.1365-2036.2006.03203.x. [DOI] [PubMed] [Google Scholar]
- 242.O’Mahony S, Howdle PD, Losowsky MS. Review article: management of patients with non-responsive coeliac disease. Aliment Pharmacol Ther. 1996;10:671–80. doi: 10.1046/j.1365-2036.1996.66237000.x. [DOI] [PubMed] [Google Scholar]
- 243.Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology. 1997;112:1830–8. doi: 10.1053/gast.1997.v112.pm9178673. [DOI] [PubMed] [Google Scholar]
- 244.Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, Bouhnik Y, Colombel JF, Delchier JC, Allez M, Cosnes J, Lavergne-Slove A, Meresse B, Trinquart L, Macintyre E, Radford-Weiss I, Hermine O, Brousse N, Cerf-Bensussan N, Cellier C. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90. doi: 10.1053/j.gastro.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 245.Roshan B, Leffler DA, Jamma S, Dennis M, Sheth S, Falchuk K, Najarian R, Goldsmith J, Tariq S, Schuppan D, Kelly CP. The incidence and clinical spectrum of refractory celiac disease in a north american referral center. Am J Gastroenterol. 2011;106:923–8. doi: 10.1038/ajg.2011.104. [DOI] [PubMed] [Google Scholar]
- 246.Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203–8. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 247.Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98:839–43. doi: 10.1111/j.1572-0241.2003.07379.x. [DOI] [PubMed] [Google Scholar]
- 248.Pallav K, Leffler DA, Tariq S, Kabbani T, Hansen J, Peer A, Bhansali A, Najarian R, Kelly CP. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther. 2012;35:380–90. doi: 10.1111/j.1365-2036.2011.04938.x. [DOI] [PubMed] [Google Scholar]
- 249.Stewart M, Andrews CN, Urbanski S, Beck PL, Storr M. The association of coeliac disease and microscopic colitis: a large population-based study. Alimentary pharmacology & therapeutics. 2011;33:1340–9. doi: 10.1111/j.1365-2036.2011.04666.x. [DOI] [PubMed] [Google Scholar]
- 250.Green PH, Yang J, Cheng J, Lee AR, Harper JW, Bhagat G. An association between microscopic colitis and celiac disease. Clin Gastroenterol Hepatol. 2009;7:1210–6. doi: 10.1016/j.cgh.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 251.Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011;140:1155–65. doi: 10.1053/j.gastro.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 252.Trier JS, Falchuk ZM, Carey MC, Schreiber DS. Celiac sprue and refractory sprue. Gastroenterology. 1978;75:307–16. [PubMed] [Google Scholar]
- 253.Cellier C, Patey N, Mauvieux L, Jabri B, Delabesse E, Cervoni JP, Burtin ML, Guy-Grand D, Bouhnik Y, Modigliani R, Barbier JP, Macintyre E, Brousse N, Cerf-Bensussan N. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998;114:471–81. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- 254.Daum S, Weiss D, Hummel M, Ullrich R, Heise W, Stein H, Riecken EO, Foss HD. Frequency of clonal intraepithelial T lymphocyte proliferations in enteropathy-type intestinal T cell lymphoma, coeliac disease, and refractory sprue. Gut. 2001;49:804–12. doi: 10.1136/gut.49.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Daum S, Ipczynski R, Heine B, Schulzke JD, Zeitz M, Ullrich R. Therapy with budesonide in patients with refractory sprue. Digestion. 2006;73:60–8. doi: 10.1159/000092639. [DOI] [PubMed] [Google Scholar]
- 256.Brar P, Lee S, Lewis S, Egbuna I, Bhagat G, Green PH. Budesonide in the treatment of refractory celiac disease. Am J Gastroenterol. 2007;102:2265–9. doi: 10.1111/j.1572-0241.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 257.Jamma S, Leffler DA, Dennis M, Najarian RM, Schuppan DB, Sheth S, Kelly CP. Small intestinal release mesalamine for the treatment of refractory celiac disease type I. J Clin Gastroenterol. 2011;45:30–3. doi: 10.1097/MCG.0b013e3181f42401. [DOI] [PubMed] [Google Scholar]
- 258.Maurino E, Niveloni S, Chernavsky A, Pedreira S, Mazure R, Vazquez H, Reyes H, Fiorini A, Smecuol E, Cabanne A, Capucchio M, Kogan Z, Bai JC. Azathioprine in refractory sprue: results from a prospective, open-label study. Am J Gastroenterol. 2002;97:2595–602. doi: 10.1111/j.1572-0241.2002.06029.x. [DOI] [PubMed] [Google Scholar]
- 259.Wahab PJ, Crusius JB, Meijer JW, Uil JJ, Mulder CJ. Cyclosporin in the treatment of adults with refractory coeliac disease--an open pilot study. Aliment Pharmacol Ther. 2000;14:767–74. doi: 10.1046/j.1365-2036.2000.00718.x. [DOI] [PubMed] [Google Scholar]
- 260.Tack GJ, Verbeek WH, Al-Toma A, Kuik DJ, Schreurs MW, Visser O, Mulder CJ. Evaluation of Cladribine treatment in refractory celiac disease type II. World J Gastroenterol. 2011;17:506–13. doi: 10.3748/wjg.v17.i4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Abdallah H, Leffler D, Dennis M, Kelly CP. Refractory celiac disease. Curr Gastroenterol Rep. 2007;9:401–5. doi: 10.1007/s11894-007-0049-5. [DOI] [PubMed] [Google Scholar]
- 262.Sieniawski M, Angamuthu N, Boyd K, Chasty R, Davies J, Forsyth P, Jack F, Lyons S, Mounter P, Revell P, Proctor SJ, Lennard AL. Evaluation of enteropathy associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplant. Blood. 2010;115:3664–70. doi: 10.1182/blood-2009-07-231324. [DOI] [PubMed] [Google Scholar]
- 263.Chandesris MO, Malamut G, Verkarre V, Meresse B, Macintyre E, Delarue R, Rubio MT, Suarez F, Deau-Fischer B, Cerf-Bensussan N, Brousse N, Cellier C, Hermine O. Enteropathy-associated T-cell lymphoma: a review on clinical presentation, diagnosis, therapeutic strategies and perspectives. Gastroenterol Clin Biol. 2010;34:590–605. doi: 10.1016/j.gcb.2010.09.008. [DOI] [PubMed] [Google Scholar]


