Abstract
Background
Apelin-13 (APLN) through apelin receptor (APJ) exerts peripheral vasodilatory and potent positive inotropic effects. We examined the effects of exogenous intravenous infusion of APLN on left ventricular (LV) systolic function in dogs with heart failure (HF, LV ejection fraction, EF~30%).
Methods and Results
Studies were performed in 7 dogs with microembolization-induced HF. Each dog received an intravenous infusion of low dose and high dose APLN followed by washout period. LV end-diastolic volume (EDV), end-systolic volume (ESV) and LV EF were measured at specified time points. APLN protein level was determined in plasma at all time points. mRNA and protein levels of APLN and APJ in LV tissue were also measured in 7 normal (NL) and 7 heart failure (HF) dogs. APLN reduced EDV only at the high dose, significantly reduced ESV and increased EF with both doses. In plasma of HF dogs, APLN levels were reduced significantly compared to NL dogs. APLN treatment in HF dogs significantly increased the plasma APLN levels at both low and high doses. Expression of APLN, but not of APJ, was reduced in LV tissue of HF dogs compared to NL.
Conclusion
Exogenous administration of APLN improved LV systolic function in dogs with advanced HF.
INTRODUCTION
Numerous endogenous peptides exist in eukaryotic cells and each exerts its effect by binding to G-protein coupled receptors (GPCR). In some cases, the endogenous peptide and its counterpart GPCR has been identified but many GPCRs are yet undiscovered. These unknown GPCRs are referred to as “orphan receptors” and can be potential targets for drug discovery. A decade ago, a human gene was identified with significant homology to the gene encoding the angiotensin receptor. The novel receptor encoded by this gene was named apelin receptor (APJ) 1.APJ shares 40% to 50% homology with the angiotensin type 1 (AT1) receptor in the hydrophobic transmembrane regions, but does not bind angiotensin II1. Five years later, a ligand of the APJ receptor was isolated from bovine stomach extracts and named apelin-36. Subsequently, shorter sequences of the C-terminus of apelin-36 were found to be more potent than apelin-36 with an N-terminal pyroglutamate form ([Pyr1] apelin-13) (APLN)2. Further studies reported that apelin is the only known ligand for APJ and is synthesized as a pre-propeptide consisting of 77 amino acids with shorter biologically active forms including the 36, 17, 16, 13, and 12 amino acids encoded in the COOH-terminal region 2–4. Although each of these isoforms has biological activity, the predominant isoform in the cardiovascular system appears to be APLN5. Since then, numerous studies have reported the presence of APJ and APLN in both the adult rat heart and lung6, but the precise function of APLN has not yet been established.
The APLN-APJ signaling pathway regulates systemic perfusion and has effects both on the heart and the vasculature. Exogenous APLN is a potent activator of cardiac contractility when added to isolated rat cardiomyocytes or intact heart preparations and when administered in vivo to rats or mice7–9. In one study, APLN administered systemically to rats immediately lowered blood pressure, with a small increase in heart rate6. In the vasculature, APLN is a vasodilator in both the arterial and venous circulations, and these effects appear to be mediated at least, in part, by nitric oxide10–13. APLN has been shown to have a positive inotropic effect on failing myocardium5,12. It has previously been reported that chronic infusion of APLN to mice increases cardiac output without causing hypertrophy9. Plasma APLN levels tend to increase in early stages of heart failure (HF) but are presumably diminished during functional decompensation, suggesting that endogenous APLN may be secreted in an attempt to increase contractility 13. Consistent with this hypothesis, it has been found that APJ is one of the most differentially expressed genes in the human failing heart13. To understand the role of endogenous APLN-APJ signaling in cardiovascular physiology, mice lacking either the ligand (APLN_−/−) or the receptor (APJ_−/−) of this pathway were created. It was reported that both APLN −/− and APJ −/− mice have decreased basal cardiac contractility at the whole animal level with a more dramatic decrement at the cellular level, suggesting a direct role for endogenous APLN-APJ signaling in basal contractile function with the potential for autocrine signaling in vitro14. In the present study, we examined the expression of APLN and APJ in left ventricular (LV) myocardium of normal dogs and dogs with HF and examined the effects of exogenous APLN on LV function in dogs with advanced HF produced by multiple sequential intracoronary microembolizations15.
METHODS
Experimental Model
The study was approved by Henry Ford Hospital Institutional Animal Care and Use Committee (IACUC) and adhered to the American Physiological Society’s Guiding Principles in the Care and Use of Animals. The canine model of HF used in this study was previously described in detail15–19. The model manifests many of the sequelae of HF observed in humans including marked and progressive depression of LV systolic and diastolic function, reduced cardiac output, and increased LV filling pressures. In the present study, 7 healthy mongrel dogs weighing 19.1–26.2 kg underwent serial coronary microembolizations with polystyrene Latex microspheres to produce HF as previously described in detail15,16,18. Coronary microembolizations were performed during cardiac catheterization under general anesthesia and sterile conditions. Anesthesia was induced using a combination of intravenous injections of oxymorphone hydrochloride (0.22 mg/kg) and diazepam (0.2–0.6 mg/kg). Plane of anesthesia was maintained throughout the study using 1% to 2% isoflurane. Left and right heart catheterizations were performed via a femoral arteriotomy and venotomy. Coronary microembolizations were discontinued when LV ejection fraction (EF), determined angiographically, was approximately 30%. A period of 2 weeks was allowed after the last microembolization to ensure that infarctions produced by the last microembolizations have completely healed and HF was established before the study was undertaken.
Study Protocol and End-points
During cardiac catheterization, each dog received an intravenous infusion of APLN 500 ng/kg/min for 2 hours followed by a higher infusion of 50 μg/kg/min of APLN for another 2 hours. APLN infusion was then stopped and dogs were monitored for a one hour washout period. The escalating dose of APLN was used to assess whether the potential benefits of exogenous APLN was dose-dependent. Hemodynamic, ventriculographic, echocardiographic, and electrocardiographic measurements were made at baseline, prior to administration of APLN and repeated at the end of infusion of each dose (2 hours and 4 hours respectively) and at the end of the washout period (washout). In addition, myocardial oxygen consumption (MVO2) was also measured at the time points described above. Venous blood samples (10 ml) were collected in ethylene diamine tetra acetic acid (EDTA)-tube at baseline and at the end of each dose and washout. Blood samples were centrifuged at 3000 rpm for 10 minutes and plasma withdrawn and placed in cryostorage tubes stored upright at −70°C for subsequent measurements of plasma APLN. The study primary end-points were: 1) Change in LV EF determined from ventriculography; 2) Change in LV end-systolic volume (ESV) and end-diastolic volume (EDV) determined from ventriculography and 3) Change in MVO2. Fresh, rapidly frozen heart tissue specimens (~ 2 grams) were obtained from the LV free wall at mid-ventricular level from 6 normal (NL) dogs and from 6 dogs with coronary microembolization-induced HF (EF~30%). The tissue was stored at −70°C and used to assess mRNA and protein expression of APLN and APJ.
Hemodynamic and Angiographic Measurements
All hemodynamic measurements were made during left and right heart catheterizations in anesthetized dogs at each specified study time point. Heart rate (HR), systolic aortic pressure, mean aortic pressure (mAoP), LV end-diastolic pressure (LVEDP), peak LV +dP/dt, peak LV −dp/dt, time constant of isovolumic relaxation (Tau), stroke volume (SV), cardiac output (CO) and systemic vascular resistance (SVR) were measured at each study time point. Left ventriculograms were obtained a single plane 30° right anterior oblique projection and recorded on digital media at 30 frame/sec during the injection of 20 ml of contrast material (ISOVU-300, Bracco Diagnostics, Inc., Princeton, NJ). Correction for image magnification was made with a radiopaque calibrated grid placed at the level of the LV. LV ESV and EDV were calculated from LV silhouettes using the area-length method and LV EF was calculated as previously described20. Extrasystolic and post-extrasystolic beats were excluded from any of the angiographic analyses.
Echocardiographic and Doppler Measurements
Echocardiographic studies were performed in all dogs at all specified study time points using a General Electric VIVID-7 ultrasound system. All echocardiographic measurements were made with the dog placed in the right lateral decubitus position and recorded on digital media for subsequent off-line analysis using the General Electric Digital PC analytical system. Short-axis 2-dimensional echocardiograms obtained at the level of the papillary muscle, were used to calculate global LV fractional area of shortening (FAS). LV major and minor semiaxes were measured and used for calculation of LV end-diastolic circumferential wall stress (EDWS). Wall stress was calculated as follows: EDWS = Pb/h(1-h/2b)(1-hb/2a2), where ‘P’ is LV EDP, ‘a’ is LV major semiaxis, ‘b’ is LV minor semiaxis, and ‘h’ is LV wall thickness. Mitral inflow velocity was measured by pulsed-wave Doppler echocardiography. The velocity waveforms were used to calculate 1) peak mitral inflow velocity during early active filling (PE), 2) peak mitral inflow velocity during left atrial contraction (PA), 3) time-velocity integral of the mitral inflow velocity waveform representing early filling (Ei), 4) time-velocity integral representing LA contraction (Ai), 5) ratio Ei/Ai, and 6) deceleration time (DT) of early mitral inflow velocity as previously described 16,18.
Myocardial Oxygen Consumption Measurements
Measurements of MVO2 were made at all study time-points. The methods used to calculate MVO2 were previously described21. Briefly, coronary artery blood flow velocity was measured using a Doppler flow velocity wire placed in the proximal segment of the circumflex coronary artery distal to the first marginal branch. Blood flow was estimated by calculating the cross-sectional area of the circumflex coronary artery at the site of the catheter-tip using coronary arteriograms. Total LV coronary blood flow was estimated as twice that measured in the circumflex artery. MVO2 was determined as the product of total coronary blood flow and aorta to coronary sinus O2 difference.
Biochemical Studies
Sodium dodecyl sulfate (SDS) extract of approximately 100 mg frozen LV tissue specimens from 6 NL and 6 HF dogs was prepared and protein was assayed as previously described in detail22,23. For Western blotting, approximately 50 μg protein was loaded on precast 4–20% SDS-PAGE gels (Bio-Rad) for APJ or 150 μg protein loaded on 10–20% Tris-Tricine precast gels (Bio-Rad) for APLN after denaturation in reducing sample buffer for 10 min at 95°C. Proteins from gels were transferred to Immobolin-P PVDFmembranes (Millipore, Billerica, MA) and subsequently developed as previously described 23 with slight modification. Immunoblotting was performed using antibodies against APJ [rabbit anti-rat APJ (355–375): Phoenix Pharmaceuticals, Belmont, CA] and APLN (rabbit anti-human apelin; Phoenix Pharmaceuticals, Belmont, CA). The Supersignal West Pico Chemillumenescent Substrate (Pierce, Rockford, IL) was used for detection and band intensity was quantified using a Bio-Rad gel densitometer and expressed in densitometric units. To confirm equal loading of total protein into each lane, the blot was also probed with an internal control calsequestrin (CSQ) antibody (abcam, Cambridge, MA). The total concentration of myocardial APLN and APJ proteins was expressed as densitometric units normalized to CSQ and thereby providing a relative comparison of protein concentration between NL and HF animals. Total APLN concentration in cardiac tissue and plasma samples was determined using an enzyme immunoassay (EIA) kit for apelin (Phoenix Pharmaceuticals, Belmont, CA). A standard logarithmic curve was plotted and used to calculate protein concentration in the samples using apelin-12 as a standard and expressed in pg/ml in plasma or pg/mg in cardiac tissue sample.
mRNA expression for APLN, APJ and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was determined in RNA isolated from LV tissue as previously described 23. Product formation was examined by agarose gel electrophoresis and intensity of the bands was quantified in densitometric units. APLN and APJ mRNA level was expressed in densitometric units normalized to GAPDH. Product formation for all in-study genes was linear from 25–35 cycles but saturated at 40 cycles. For quantitative purpose, gene amplification was carried out at 32 cycles. Primers used for APLN (gene ID NM_017413, amplicon size 151 bp, forward: 5′-GGCGGTTATGTCTCCTCCATAG-3′; reverse: 5′-TCCAAACTACAGCCAGGAGCAC-3′), APJ (gene ID NM_005161, amplicon size 118 bp, forward: 5′-CCTGCCATCTACATGTTGGTCT-3′, reverse: 5′ –GGCTAGCAATGAAGATATCAGCTG-3′), and GAPDH (gene ID NM_002046, amplicon size 528 bp, forward: 5′-ACCACCATGGAGAAGGCTGG-3′, reverse: 5′-CTCAGTGTAGCCCAGGATGC-3′) were synthesized from Operon Biotechnologies, Inc (Huntsville, AL).
Statistical Analysis
Repeated measures analysis of variance (ANOVA) was used to examine hemodynamic, ventriculographic, echocardiographic and Doppler measures obtained during APLN infusion and washout with alpha set at 0.05. If significance was achieved, pair wise comparisons were performed using the Student-Neuman-Keuls test. For this test, a probability value <0.05 was considered significant. Comparison of the biochemical changes between the NL dogs and dogs with HF were made using a t-statistic for two means with significance set at p<0.05. All data are expressed as means ± SEM.
RESULTS
None of the study dogs developed acute decompensation or died during the study. In addition, none of the dogs developed de-novo ventricular or atrial arrhythmias, sinus tachycardia or hypotension at anytime during either drug infusions or saline infusion.
APLN Levels in LV Tissue and Plasma of Normal Dogs and Dogs with Heart Failure
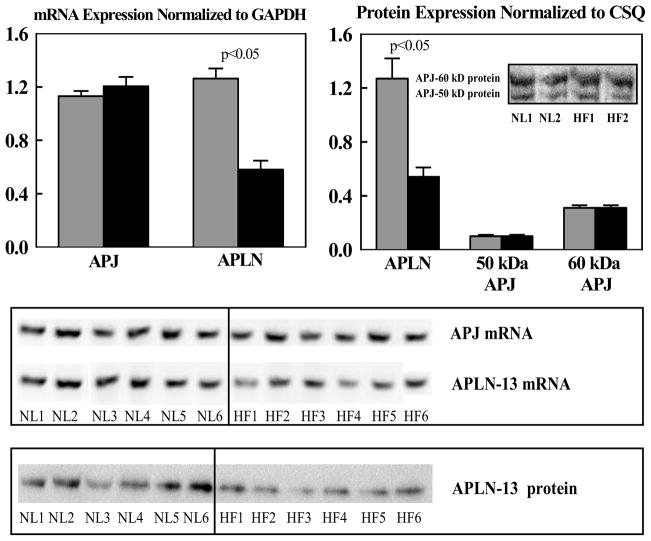
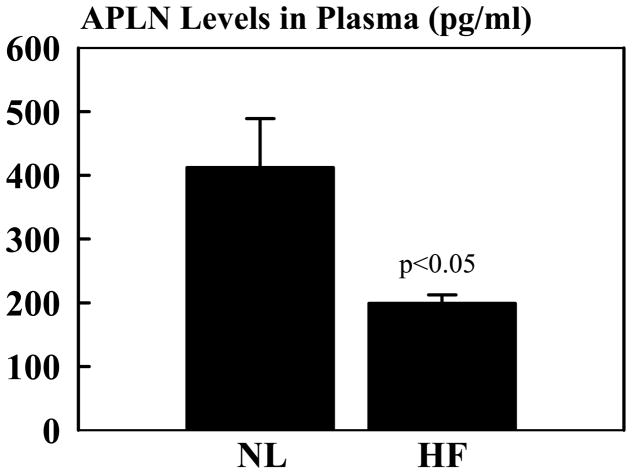
Compared to NL dogs, mRNA and protein levels of APLN were significantly reduced in dogs with advanced HF compared to NL dogs (Fig. 1). APLN antibody recognized a major band near 1.50 kDa region on tris-tricine gel. In contrast to APLN, APJ mRNA or protein levels determined in LV tissue were essentially the same in NL dogs compared to dogs with advanced HF (Fig. 1). The antibody to APJ identified 2 major bands at 50 kDa (not glycosylated) and 60 kDa (glycosylated) in LV extracts of NL and HF dogs. The band at 60 kDa was approximately 3 fold as intense as the band at 50 kDa protein. When the intensity of both bands was quantified, the intensity of either band of NL dogs was not different from HF dogs. Consistent with observations in LV tissue, plasma levels of APLN were significantly lower in dogs with untreated advanced HF compared to NL dogs (Fig. 2).
Figure 1.
Top Left: Bar graph (mean ± SEM) depicting mRNA gene expression normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for apelin-13 (APLN) and apelin receptor (APJ) in left ventricular myocardium of normal dogs (gray bars) and untreated dogs with coronary microembolization-induced heart failure (black bars). Top Right: Bar graph (mean ± SEM) depicting protein levels normalized to calsequestrin (CSQ) for apelin-13 (APLN) and apelin receptor (APJ) for bands at 50 kDa and 60 kDa in left ventricular myocardium of normal dogs (gray bars) and untreated dogs with coronary microembolization-induced heart failure. Insert shows sample of bands from Western blots in 2 normal (NL) dogs and 2 dogs with heart failure (HF). P-value denotes significant difference between normal dogs (gray bars) and heart failure dogs (black bars). Bottom: Immunoblots for mRNA and protein expression of apelin (APLN) and APJ receptor from all 6 normal (NL) and all 6 heart failure (HF) dogs reported in the study. P-value denotes significant difference between normal dogs and heart failure dogs (black bars).
Figure 2.
Bar graph (mean ± SEM) depicting apelin-13 (APLN) level in plasma from normal dogs (NL) and untreated dogs with coronary microembolization-induced heart failure (HF). P-values denote significant difference between normal dogs and heart failure dogs.
Effect of Intravenous Infusion of APLN on Hemodynamic, Ventriculographic, Echocardiographic and Doppler Measures in Dogs with Advanced HF
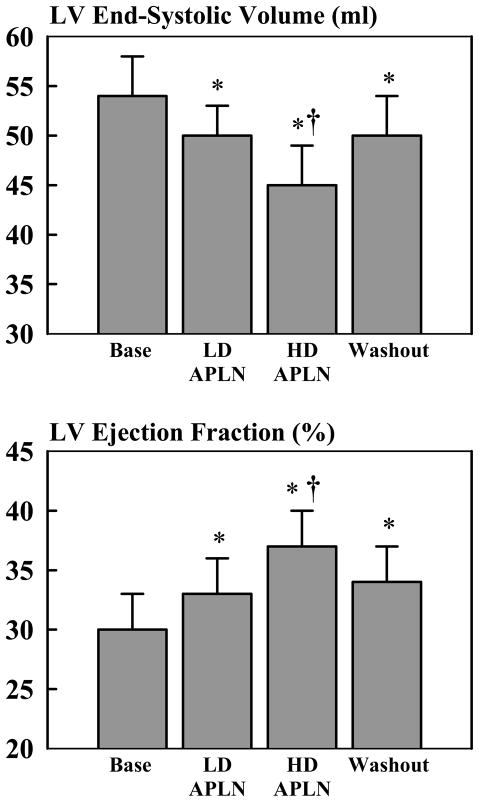
The hemodynamic, ventriculographic, echocardiographic and Doppler measures obtained at baseline and during administration of exogenous APLN and at end of the washout period are shown in Table 1. Low and high dose APLN had no significant effect on HR, systolic aortic pressure or mAoP and only modestly reduced LV EDP. Both doses of APLN significantly decreased LV ESV and increased LV EF in a dose-dependent manner (Fig. 3). A significant reduction of LV EDV occurred only at the high dose of APLN. APLN significantly increased SV, CO, peak LV +dP/dt, FAS and modestly decreased SVR. These improvements in LV systolic function occurred in the absence of a significant increase of MVO2 (Table 1). Infusion of APLN had limited beneficial effects on indices of LV diastolic function. Ei/Ai increased significantly with APLN infusion in a dose-dependent fashion but PE and PA did not change significantly. Both peak LV −dP/dt and Tau tended to improve, but the improvement did not reach statistical significance. High dose APLN significantly increased DT and lowered EDWS but the latter did not reach statistical significance. Discontinuation of APLN infusion for one hour (washout period) was associated with a partial return of all measures toward baseline values (Table 1, Fig. 3). As expected, plasma levels of APLN increased significantly during infusion of exogenous APLN in a dose dependent manner but decreased dramatically to near baseline levels one hour after discontinuation of APLN infusion (Table 1).
Table 1.
Hemodynamic, angiographic, echocardiographic and Doppler measures at baseline, at the end of 2 hours infusion of low dose APLN, at the end of 2 hours infusion of high dose APLN and at one hour after discontinuation of APLN infusion (Washout) in HF Dogs (n=7).
| Measurements | Baseline | Low Dose APLN | High Dose APLN | Washout |
|---|---|---|---|---|
| HR (beats/min) | 80±3 | 80±3 | 79±2 | 76±2 |
| Systolic AoP (mmHg) | 97±4 | 95±4 | 93±5 | 91±5 |
| mAoP (mmHg) | 79±5 | 78±5 | 75±6 | 72±5* |
| LV EDP (mmHg) | 16±2 | 15±2 | 15±3* | 16±2 |
| Peak LV +dP/dt (mmHg/sec) | 1690±195 | 1854±212* | 1829±239* | 1616±169 |
| Peak LV −dP/dt (mmHg/sec) | 1811±209 | 1889±225 | 1927±261 | 1532±189* |
| Tau (msec) | 84±31 | 68±12 | 68±9 | 71±6 |
| LV EDV (ml) | 76±3 | 75±4 | 71±4* | 75±4 |
| LV ESV (ml) | 54±4 | 50±3* | 45±4*† | 50±4* |
| LV EF (%) | 30±3 | 33±3* | 37±3*† | 34±3* |
| CO (L/min) | 1.80±0.16 | 1.99±0.49* | 2.08±0.22* | 1.90±0.19 |
| SV (ml) | 23±2 | 25±2* | 26±2* | 25±2* |
| SVR (dynes-sec-cm−5) | 3594±296 | 3250±382* | 3033±442* | 3199±410* |
| FAS (%) | 32±4 | 39±3* | 43±3* | 39±4* |
| PE (cm/sec) | 46±3 | 48±2 | 52±3 | 44±2 |
| PA (cm/sec) | 26±3 | 24±2 | 26±3 | 23±1 |
| Ei/Ai | 3.67±0.9 | 4.58±0.9* | 5.84±1.4*† | 4.79±1.0* |
| DT (msec) | 72±4 | 82±5 | 100±10*† | 84±6 |
| EDWS (g/cm2) | 84±17 | 78±19 | 71±12 | 79±17 |
| MVO2 (pmols/min) | 142±25 | 178±37 | 180±45 | 177±37 |
| APLN (pg/ml) | 168±14 | 8,935±1,422* | 30,590±6,586*† | 569±32* |
HR = heart rate; AoP = aortic pressure; mAoP = mean aortic pressure; LV = left ventricular; EDP = end-diastolic pressure; EDV = end-diastolic volume; +dP/dt = rate of change of pressure during the isovolumic contraction; −dP/dt = rate of change of pressure during the isovolumic relaxation; Tau = time constant of isovolumic relaxation; ESV = end-systolic volume; EF = ejection fraction; SV = stroke volume; CO = cardiac output; SVR = systemic vascular resistance; FAS = fractional area of shortening; PE = peak early mitral inflow velocity; PA = peak mitral inflow velocity during atrial contraction; Ei = time-velocity integral of the mitral inflow velocity waveform representing early filling; Ai = time-velocity integral representing LA contraction; Ei/Ai = ratio of Ei to Ai; DT = deceleration time of early mitral inflow velocity; EDWS = LV end-diastolic circumferential wall stress; MVO2 = myocardial oxygen consumption; APLN = apelin-13;
p <0.05 vs. Baseline,
p <0.05 vs. Low Dose.
Figure 3.
Top: Bar graph (mean ± SEM) depicting change in left ventricular (LV) end-systolic volume at baseline (Base) prior to initiating drug infusion, at the end of low dose (LD) infusion of apelin-13 (APLN), at the end of infusion of high dose (HD) apelin-13 and at the at one hour after discontinuation of apelin-13 infusion (Washout). Bottom: Bar graph (mean ± SEM) depicting change in left ventricular (LV) ejection fraction at baseline (Base) prior to initiating drug infusion, at the end of low dose (LD) infusion of apelin-13 (APLN), at the end of infusion of high dose (HD) apelin-13 and at the at one hour after discontinuation of apelin-13 infusion (Washout). P-value denotes significant difference between normal dogs and heart failure dogs (black bars). * = p<0.05 versus Base; † = p<0.05 vs. LD APLN.
DISCUSSION
The results of this study indicate that in dogs with advanced HF, APLN infusion improves global LV systolic function in a dose-dependent manner and to a much lesser extent diastolic function. This observation of improved LV systolic function is supported by the finding that APLN infused systematically increased LV EF, FAS, peak LV +dP/dt, CO, SV, and decreased LV ESV, EDV and to a lesser extent, EDP. Not all indexes of LV performance showed an APLN dose-dependent effect suggesting the possibility that both doses of APLN used in the study may have fully saturated APJ receptor sites. The beneficial effects of APLN seen in this study likely reflect the dual actions of this peptide, namely both vasodilation, evidenced in the present study by a reduction in SVR, and increased contractility, evidenced by an increase of peak LV +dP/dt. The increase in peak +dP/dt in the face of a modest drop of blood pressure, augers well for the presence of a strong component of enhanced contractility. Unlike traditional vasodilators, APLN even when administered at a high dose did not elicit a significant drop of systemic blood pressure or an increase of heart rate even though SVR decreased modestly. The absence of a decline in blood pressure can reflect the increase in CO derived from increased contractility. Thus from a clinical perspective, APLN may be an attractive therapeutic modality based on its apparent ability to increase CO without lowering blood pressure in the patient with heart failure. Previous studies have focused on the effects of APLN as a potential therapeutic in the setting of AMI and chronic ischemia. Our study expands on these observations by exploring the acute benefits of APLN in large animals with advanced chronic HF and the potential for this therapeutic approach to favorably impact LV systolic and diastolic function.
APLN and LV Function in Heart Failure: Possible Mechanism(s) of Action
Consistent with our findings in dogs with chronic HF, previous studies in rats with AMI showed that infusion of APLN improves LV EF, increases CO and reduces LV EDP 5,24. In normal mice, APLN therapy was associated with increased myocardial LV circumferential shortening along with an increase of CO9. These findings, as well as those from the present study, point to the possibility that acute restoration of systemic APLN concentrations enhances ligand-receptor interaction (APLN-APJ receptor interaction) that lead to acute improvement of LV function.
The improvement in LV systolic function during acute exogenous infusion of APLN is likely the result of combined vasodilation and improved cardiac contractility25. In isolated rat aortas, the mechanism of arterial vasodilation mediated by APLN was shown to involve the L-arginine endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) pathway26. APJ receptors are expressed in vascular smooth muscle where APLN induces phosphorylation of the myosin light chains27. This strongly suggests that the predominant actions of APLN on peripheral vascular tissue are mediated by NO production from the endothelium25. In addition to eliciting NO-mediated arterial vasodilation, APLN exerts a positive inotropic action on cardiac muscle by increasing the availability of intracellular calcium rather than enhancing the calcium sensitivity of myofilaments7. The increase in contractility is even more potent in the failing heart than in the normal heart28. The exact pathway that leads to increased availability of intracellular calcium is not fully understood but may be due to improved sarcoplasmic reticulum (SR) calcium cycling mediated by improved SR calcium ATPase activity29. Phosphoilipase C, protein kinase C, sarcolemmal-H+ and sodium-calcium exchangers have also been shown to be involved in the APLN-mediated increase of intracellular calcium availability7. The canine model of HF used in the present study, manifest abnormalities of both the NOS/NO pathway as well as abnormalities of SR calcium cycling, including reduced calcium ATPase activity30,31, all or some of which, when partially corrected by APLN, can lead to improved global LV performance.
In addition to modulating peripheral vasomotor tone and contractility, APLN and its receptor have been suggested to influence cardiac performance in multiple ways. In mice with pressure overload cardiac hypertrophy produced by aortic constriction, chronic treatment with APLN prevented oxidative stress-linked cardiac hypertrophy32. In cardiomyocytes isolated from mice and exposed to H2O2, APLN treatment activated catalase and decreased plasma lipid hydroperoxide which is an index of oxidative stress32. APLN-APJ signaling has also been shown to increase the activity of 5′-AMP-activated protein kinase (AMPK), a recognized mediator of mitochondrial biogenesis33. In isolated cardiomyocytes, treatment with APLN increased sarcomere fractional shortening and the amplitude of the electrical-stimulated calcium concentration transients29. In addition, treatment with APLN increased the sarcoplasmic reticulum calcium ATPase activity29. In addition to reducing oxidative stress, APLN-APJ signaling was shown to prevent cardiomyocyte apoptosis induced by glucose deprivation via activation of PI3K/Akt pathway34. Some of these pathways, however, are not likely to have contributed to the acute benefits of exogenous APLN observed in the present study. Given the pleiotropy of actions of the APLN-APJ signaling pathway, additional studies are needed to clarify the exact mechanisms by which APLN improves LV function in HF.
Endogenous Expression of APLN and APJ in Failing Hearts
Endogenous APLN plays an important role in the regulation of myocardial function3,13,22,33,35. When endogenous APLN stores are reduced, exogenous administration of APLN has been shown to elicit therapeutic benefits manifested by enhanced myocardial performance, as recently reported by several groups 3,5,13,20,32,35–37 in small laboratory animals. The mechanisms that govern the regulation of APLN expression and its APJ receptors in the myocardium during normal and disease states, however, remain poorly understood. In the present study, we examined the changes in myocardial levels of APLN and its APJ receptors in the setting of advanced chronic HF in a well described dog model of ischemic cardiomyopathy with focus on the most potent apelin isotype namely, apelin-13 (APLN). Using Elisa and Western blotting techniques, we showed that APLN levels were depressed nearly 2 fold in LV tissue of untreated HF dogs compared to NL dogs. A similar reduction of APLN protein level was found in plasma of HF dogs compared to NL. A comparison of our findings with those in the literature is difficult due to sparsity of published reports. In rats with sepsis induced by cecal ligation and puncture, mRNA and protein levels of APLN were decreased38. Pre-treatment with APLN in these rats partially prevented the decline in LV performance assessed based on peak LV +dp/dt38. Contrary to our findings, studies in rats with HF produced by ligation of the left anterior descending coronary artery 5 showed that APLN levels as well as APJ concentration in LV tissue were increased and suggested that this increase represents a compensatory response to ischemia5. Despite these contradictory findings, infusion of APLN in these rats with AMI-induced HF also improved myocardial function5. Consistent with our findings, APLN levels were reduced in circulating plasma obtained from patients with AMI36. APLN levels were also reduced in blood plasma drawn from the coronary sinus in patients with LV systolic dysfunction suggesting reduced APLN concentrations in cardiac tissue39.
Whereas some reports have described the regulation of APLN in hearts with compromised LV function, knowledge of the regulation of the APJ receptor is sparce indeed aside from the fact that such receptors are expressed throughout the cardiovascular system35. In the present study, we showed that expression of the APJ receptor is preserved in the failing heart compared to normal hearts. This was true for both mRNA as well as protein expression. In a study of rats with AMI secondary to ligation of the left anterior descending coronary artery, APJ protein levels were described as elevated compared to sham-operated rats5. This apparent species difference is not well understood and requires additional investigation. At present it suffices to state that both APLN and its APJ receptor are likely to be important in the overall regulation of cardiac function. Support for this is found in results of studies in mice lacking either the ligand APLN or APJ that manifest significant cardiac dysfunction12.
Conclusions
Results of the present study indicate that expression of APLN is markedly reduced in LV myocardium and plasma of dogs with advanced chronic HF; whereas APLN receptors are unaltered compared to LV myocardium of normal dogs. Furthermore, exogenous intravenous administration of APLN elicits significant improvements of LV systolic function in a dose-dependent manner. This latter observation provides a biological rationale for the potential use of APLN as a therapeutic in patients with exacerbation of HF in need to positive inotropic support.
Acknowledgments
Supported, in part, by research grants from Solvay Pharmaceuticals (Currently Abbott Laboratories, Inc.) and National Heart, Lung, and Blood Institute PO1 HL074237-07
Footnotes
Disclosures:
Dr. Sabbah received research grant from Solvay Pharmaceuticals (currently Abbott Laboratories, Inc.) and Drs. Hogie and Fischer, at the time the study was performed were employees of Solvay Pharmaceuticals (currently Abbott Laboratories, Inc.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–60. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 2.Tatemoto K, Hosoya M, Habata Y, Kumaki I, Zhang W, Kumano K, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 3.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochimica et biophysica acta. 1999;1452:25–35. doi: 10.1016/s0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 4.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. Journal of neurochemistry. 2003;84:1162–72. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 5.Atluri P, Morine KJ, Liao GP, Panlilio CM, Berry MF, Hsu VM, et al. Ischemic heart failure enhances endogenous myocardial apelin and APJ receptor expression. Cellular & molecular biology letters. 2007;12:127–38. doi: 10.2478/s11658-006-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, et al. Characterization of apelin, the ligand for the APJ receptor. Journal of neurochemistry. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 7.Szokodi I, Tavi P, Foldes G, Voutilainen-Myllyla S, Ilves M, Tokola H, et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circulation research. 2002;91:434–40. doi: 10.1161/01.res.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- 8.Farkasfalvi K, Stagg MA, Coppen SR, Siedlecka U, Lee J, Soppa GK, et al. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochemical and biophysical research communications. 2007;357:889–95. doi: 10.1016/j.bbrc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Ashley EA, Powers J, Chen M, Kundu R, Finsterbach T, Caffarelli A, et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovascular research. 2005;65:73–82. doi: 10.1016/j.cardiores.2004.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regulatory peptides. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 11.Lee DK, George SR, O’Dowd BF. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends in pharmacological sciences. 2006;27:190–4. doi: 10.1016/j.tips.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. The Journal of biological chemistry. 2004;279:26274–9. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 13.Chen MM, Ashley EA, Deng DX, Tsalenko A, Deng A, Tabibiazar R, et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 2003;108:1432–9. doi: 10.1161/01.CIR.0000091235.94914.75. [DOI] [PubMed] [Google Scholar]
- 14.Charo DN, Ho M, Fajardo G, Kawana M, Kundu RK, Sheikh AY, et al. Endogenous regulation of cardiovascular function by apelin-APJ. American journal of physiology Heart and circulatory physiology. 2009;297:H1904–13. doi: 10.1152/ajpheart.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–84. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi S, Guerrero M, Wang M, Ilsar I, Sabbah MS, Gupta RC, et al. Myocardial Transfection with Naked DNA Plasmid Encoding Hepatocyte Growth Factor Prevents the Progression of Heart Failure in Dogs. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00636.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabbah HN, Gupta RC, Imai M, Irwin ED, Rastogi S, Rossing MA, et al. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail. 4:65–70. doi: 10.1161/CIRCHEARTFAILURE.110.955013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. 16:171–8. doi: 10.1007/s10741-010-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbah HN, Wang M, Gupta RC, Rastogi S, Ilsar I, Viole T, et al. Acute left ventricular unloading in dogs with chronic heart failure: continuous aortic flow augmentation versus intra-aortic balloon pumping. Journal of cardiac failure. 2009;15:523–8. doi: 10.1016/j.cardfail.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Zaca V, Rastogi S, Imai M, Wang M, Sharov VG, Jiang A, et al. Chronic monotherapy with rosuvastatin prevents progressive left ventricular dysfunction and remodeling in dogs with heart failure. Journal of the American College of Cardiology. 2007;50:551–7. doi: 10.1016/j.jacc.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 21.Chandler MP, Stanley WC, Morita H, Suzuki G, Roth BA, Blackburn B, et al. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circulation research. 2002;91:278–80. doi: 10.1161/01.res.0000031151.21145.59. [DOI] [PubMed] [Google Scholar]
- 22.Gupta RC, Mishra S, Rastogi S, Wang M, Rousso B, Mika Y, et al. Ca(2+)-binding proteins in dogs with heart failure: effects of cardiac contractility modulation electrical signals. Clinical and translational science. 2009;2:211–5. doi: 10.1111/j.1752-8062.2009.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta RC, Mishra S, Wang M, Jiang A, Rastogi S, Rousso B, et al. Cardiac contractility modulation electrical signals normalize activity, expression, and phosphorylation of the Na+-Ca2+ exchanger in heart failure. Journal of cardiac failure. 2009;15:48–56. doi: 10.1016/j.cardfail.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Berry MF, Pirolli TJ, Jayasankar V, Burdick J, Morine KJ, Gardner TJ, et al. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation. 2004;110:II187–93. doi: 10.1161/01.CIR.0000138382.57325.5c. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekaran B, Dar O, McDonagh T. The role of apelin in cardiovascular function and heart failure. European journal of heart failure. 2008;10:725–32. doi: 10.1016/j.ejheart.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH, Zhao J, et al. Apelin activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Peptides. 2007;28:2023–9. doi: 10.1016/j.peptides.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto T, Kihara M, Ishida J, Imai N, Yoshida S, Toya Y, et al. Apelin stimulates myosin light chain phosphorylation in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1267–72. doi: 10.1161/01.ATV.0000218841.39828.91. [DOI] [PubMed] [Google Scholar]
- 28.Dai T, Ramirez-Correa G, Gao WD. Apelin increases contractility in failing cardiac muscle. European journal of pharmacology. 2006;553:222–8. doi: 10.1016/j.ejphar.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Du JF, Wu F, Wang HC. Apelin decreases the SR Ca2+ content but enhances the amplitude of [Ca2+]i transient and contractions during twitches in isolated rat cardiac myocytes. American journal of physiology Heart and circulatory physiology. 2008;294:H2540–6. doi: 10.1152/ajpheart.00046.2008. [DOI] [PubMed] [Google Scholar]
- 30.Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart failure reviews. 2011;16:171–8. doi: 10.1007/s10741-010-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabbah HN, Sharov VG, Gupta RC, Mishra S, Rastogi S, Undrovinas AI, et al. Reversal of chronic molecular and cellular abnormalities due to heart failure by passive mechanical ventricular containment. Circulation research. 2003;93:1095–101. doi: 10.1161/01.RES.0000101932.70443.FE. [DOI] [PubMed] [Google Scholar]
- 32.Foussal C, Lairez O, Calise D, Pathak A, Guilbeau-Frugier C, Valet P, et al. Activation of catalase by apelin prevents oxidative stress-linked cardiac hypertrophy. FEBS letters. 2010;584:2363–70. doi: 10.1016/j.febslet.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Frier BC, Williams DB, Wright DC. The effects of apelin treatment on skeletal muscle mitochondrial content. American journal of physiology Regulatory, integrative and comparative physiology. 2009;297:R1761–8. doi: 10.1152/ajpregu.00422.2009. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Yu B, Tao GZ. Apelin protects against cardiomyocyte apoptosis induced by glucose deprivation. Chinese medical journal. 2009;122:2360–5. [PubMed] [Google Scholar]
- 35.Chong KS, Gardner RS, Ashley EA, Dargie HJ, McDonagh TA. Emerging role of the apelin system in cardiovascular homeostasis. Biomarkers in medicine. 2007;1:37–43. doi: 10.2217/17520363.1.1.37. [DOI] [PubMed] [Google Scholar]
- 36.Weir RA, Chong KS, Dalzell JR, Petrie CJ, Murphy CA, Steedman T, et al. Plasma apelin concentration is depressed following acute myocardial infarction in man. European journal of heart failure. 2009;11:551–8. doi: 10.1093/eurjhf/hfp043. [DOI] [PubMed] [Google Scholar]
- 37.Zeng XJ, Zhang LK, Wang HX, Lu LQ, Ma LQ, Tang CS. Apelin protects heart against ischemia/reperfusion injury in rat. Peptides. 2009;30:1144–52. doi: 10.1016/j.peptides.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Pan CS, Teng X, Zhang J, Cai Y, Zhao J, Wu W, et al. Apelin antagonizes myocardial impairment in sepsis. Journal of cardiac failure. 2010;16:609–17. doi: 10.1016/j.cardfail.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Chandrasekaran B, Kalra PR, Donovan J, Hooper J, Clague JR, McDonagh TA. Myocardial apelin production is reduced in humans with left ventricular systolic dysfunction. Journal of cardiac failure. 2010;16:556–61. doi: 10.1016/j.cardfail.2010.02.004. [DOI] [PubMed] [Google Scholar]