Abstract
Purpose
To investigate the long-term (6- and 12-month) effects of the Strong Healthy Women intervention on health-related behaviors, weight and body mass index (BMI), and weight gain during pregnancy. Strong Healthy Women is a small-group behavioral intervention for pre- and interconceptional women designed to modify key risk factors for adverse pregnancy outcomes; pretest–posttest findings from a randomized, controlled trial have been previously reported. The following questions are addressed: 1) were significant pretest–posttest changes in health-related behaviors (previously reported) maintained over the 12-month follow-up period; 2) did the intervention impact weight and BMI over the 12-month follow-up period; and 3) did the intervention impact pregnancy weight gain for those who gave birth during the follow-up period?
Methods
Data are from 6- and 12-month follow-up telephone interviews of women in the original trial of the Strong Healthy Women intervention (n = 362) and from birth records for singleton births (n = 45) during the 12-month follow-up period. Repeated measures regression was used to evaluate intervention effects.
Main Findings
At the 12-month follow-up, participants in the Strong Healthy Women intervention were significantly more likely than controls to use a daily multivitamin with folic acid and to have lower weight and BMI. The intervention’s effect on reading food labels for nutritional values dropped off between the 6- and 12-month follow-up. Among those who gave birth to singletons during the follow-up period, women who participated in the intervention had lower average pregnancy weight gain compared with controls. Although the intervention effect was no longer significant when controlling for pre-pregnancy obesity, the adjusted means show a trend toward lower weight gain in the intervention group.
Conclusion
These findings provide important evidence that the Strong Healthy Women behavior change intervention is effective in modifying important risk factors for adverse pregnancy outcomes and may improve an important pregnancy outcome, weight gain during pregnancy. Because the intervention seems to help women manage their weight in the months after the intervention and during pregnancy, it may be an effective obesity prevention strategy for women before, during, and after the transition to motherhood.
Introduction
The U.S. Centers for Disease Control and Prevention (CDC) have called for clinical and community-based programs to improve women’s health before pregnancy as a strategy for reducing adverse pregnancy outcomes (CDC, 2006). This shift to encouraging pre-pregnancy intervention from focusing on prenatal intervention alone is based on the recognition that, once a woman has become pregnant, it may be too late to reduce risks to early fetal development and to healthy outcomes for the mother and baby. The shift is also based on a growing body of evidence that women’s preconceptional health affects pregnancy-related outcomes including birthweight, fetal growth, and pregnancy weight gain and its sequelae (Institute of Medicine [IOM], 2009; Weisman et al., 2009, 2010). To date, however, few preconceptional interventions have been subjected to rigorous testing, and there is little evidence of the effectiveness of pre-pregnancy health promotion programs (Korenbrot, Steinberg, Bender, & Newberry, 2002; Wahabi, Alziedan, Bawazeer, Al-Ansary, & Esmaiel, 2010; Whitworth & Dowsell, 2009).
The Strong Healthy Women intervention was developed by the investigator team of the Central Pennsylvania Women’s Health Study, based on the social cognitive approach to behavior change, and it was designed to improve the health-related attitudes and behaviors and health status of pre- and interconceptional women (Downs et al., 2009). The six-session, small-group intervention, conducted over a 12-week period in community settings, focuses on modifying behaviors related to key risks for adverse pregnancy outcomes such as preterm birth and low birthweight. These risks include poor nutrition, low physical activity, tobacco and alcohol use and exposure, unhealthy coping with stress, gynecologic infections, and inadequate pregnancy planning or spacing (Downs et al., 2009; Weisman et al., 2006). The group format was intended to motivate women through social support from peers and the lay group facilitators. Strong Healthy Women was tested in a randomized, controlled trial in which nonpregnant women ages 18 to 35 were recruited from 15 low-income rural communities in Central Pennsylvania (Velott, Baker, Hillemeier, Weisman, 2008), and significant pretest–posttest findings have been reported previously (Hillemeier et al., 2008). In short, the intent-to-treat pre–post analyses revealed that participants in the Strong Healthy Women intervention, compared with controls, had significantly greater improvements in self-efficacy for eating healthy foods; perceived internal control of birth outcomes; intent to eat healthy foods; intent to be more physically active; reading food labels for nutritional values; physical activity levels consistent with recommended levels; and daily use of a multivitamin containing folic acid. Significant dose–response effects also were found: Each additional intervention session attended was associated with higher perceived internal control of preconceptional control of birth outcomes, reading food labels, engaging in relaxation exercises or meditation for stress management, and daily use of a multivitamin with folic acid (Hillemeier et al., 2008). No significant effects of the intervention were found for the immediate posttest anthropometric measures (e.g., body mass index [BMI]) or biomarkers (e.g., nonfasting blood glucose). Null findings on these physiologic outcomes may have been due to the relatively short 14-week period between pretest and posttest data collection.
Most of the significant pre–post results were related to the domains of nutrition and physical activity. Therefore, it is plausible that, although the intervention had not significantly reduced women’s short-term weight or BMI by the time of the postintervention assessment, such outcomes might be observed at a later time point, particularly if observed nutritional and physical activity behavior changes were maintained in the intervention group. For those women who became pregnant during the follow-up period and delivered newborns, the intervention would also be expected to impact pregnancy weight gain based on this same assumption. That is, women participating in the intervention would be expected to gain less weight during pregnancy, or to gain an appropriate amount of weight based on their pre-pregnancy BMI (i.e., within the IOM 2009 guidelines), compared with women in the control group.
In this paper, we report the longer-term effects of the intervention using data collected at 12-month follow-up with both intervention and control participants. Specifically, we address the following questions: (1) were significant pretest–posttest changes in health-related behaviors maintained over the 12-month follow-up period; (2) did the intervention impact weight and BMI over the 12-month follow-up period; and (3) did the intervention impact pregnancy weight gain for those women who gave birth during the follow-up period? The latter research question is exploratory, as the original trial was not powered to examine pregnancy outcomes.
Methods
Study Design and Sample
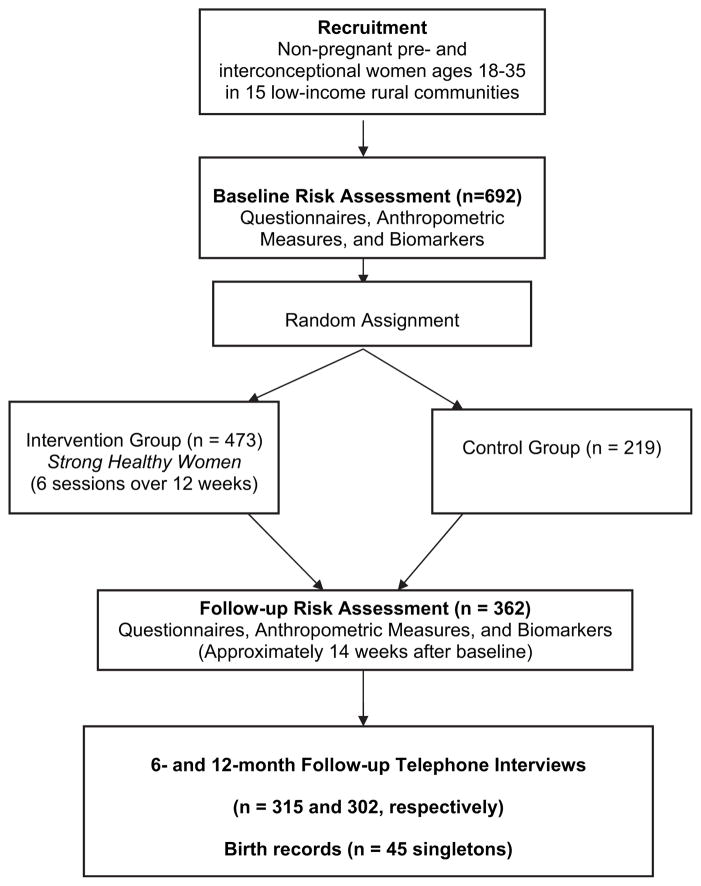
The study was approved by the Institutional Review Board of the Pennsylvania State University College of Medicine. The design of the randomized controlled trial of Strong Healthy Women is displayed in Figure 1. Non-pregnant pre- and interconceptional women ages 18–35 were recruited in 15 low-income rural communities using a variety of recruitment techniques; the 692 women recruited, compared with women in their communities, were significantly more likely to be poor or near poor, non-white, not married or partnered, and to have poorer health care access (Velott et al. 2008). Pretest–posttest analyses were conducted on 362 women who completed both the baseline and followup risk assessment and therefore had complete questionnaire data, anthropometric measures, and biomarkers for pretest–posttest analyses (Hillemeier et al. 2008). Because this analysis focuses on whether pretest–posttest effects were maintained over time, the analytic sample is the same 362 women who were included in the pretest–posttest analyses.
Figure 1.
Design of the Strong Healthy Women intervention trial.
Two types of data were collected for long-term follow-up: (1) telephone surveys were conducted 6 and 12 months after the follow-up risk assessment, and (2) birth records were obtained from the Pennsylvania Department of Health for live births occurring during the 12-month follow-up period. The 6-month telephone interview was completed by 315 women in the pretest–posttest analysis, and the 12-month telephone interview was completed by 302 women (83.4% response rate at 12 months). The primary reason for loss-to-followup was failure to locate women; refusals were rare (4.7%). Response bias analyses showed that women who responded to the follow-up surveys tended to be older (p = 0.002) compared with women who did not respond, which is consistent with greater residential stability. No differences between responders and non-responders were found for other sociodemographics (educational level, race/ethnicity, poverty, residence along the urban-rural continuum).
Measures
In the follow-up interviews, questions pertained to the same behavioral domains measured in the follow-up risk assessment, current weight status, and pregnancy incidence and outcomes; repeat measures of behavioral intent and self-efficacy were not included in the follow-up interviews. Therefore, the long-term impact of the intervention was assessed for the following outcome measures: reading food labels for nutritional values (all or most of the time versus some of the time, rarely or never); using a daily multivitamin with folic acid (yes/no); meeting prevailing recommended physical activity levels (30 minutes or more of moderate or strenuous physical activity on 4 or more days per week [American College of Sports Medicine 2000]; yes/no); consuming fruits and vegetables at least daily (at least one serving per day of fruit and of vegetables in a typical week, based on measures from the Behavioral Risk Factor Surveillance System (yes/no); weight (in pounds, measured at baseline and self-reported at follow-up); and BMI (computed using baseline measured height and weight, and self-reported weight at follow-up, depending upon the point in time).
For those women who gave birth during the 12-month follow-up period, birth records provided information on pre-pregnancy weight and weight at delivery, from which we computed pregnancy weight gain. Outcome measures included both total pregnancy weight gain in pounds and adherence to IOM (2009) recommendations for gestational weight gain: Specifically, whether or not the woman’s pregnancy weight gain exceeded or did not exceed the upper limit for recommended weight gain for her pre-pregnancy BMI category. This translates to weight gain exceeding 40 pounds for underweight women (BMI < 18.5 kg/m2), 35 pounds for normal weight women (BMI = 18.5–24.9), 25 pounds for overweight women (BMI = 25–29.9), and 20 pounds for obese women (BMI ≥ 30; IOM, 2009). The pregnancy weight gain variables were analyzed for full-term singleton births that occurred during the follow-up period and for which birth records were available. Analysis is limited to full-term singletons because the IOM guidelines refer to total pregnancy weight gain and are only provisional for women with multiple fetuses.
Analysis
Repeated measures regression was performed to evaluate the 6- and 12-month intervention effects. Depending on the response variable being analyzed, either a general linear or ordinary logistic model was used within a generalized estimating equations framework. The generalized estimating equations framework accounts for the within-subject correlation resulting from multiple measurements per subject, which are the responses for the follow-up risk assessment, namely, 6- and 12-month follow-up interviews in this instance. This method uses all available data, including all 362 women who completed both the baseline and follow-up risk assessment (Figure 1), regardless of whether they completed the 6- or 12-month follow-up interview (Beunckens, Molenberghs, & Kenward, 2005). For all models, the baseline measure of the response variable was included as a covariate to adjust for any differences in baseline measures. In addition to treatment condition and baseline measure, age and educational level at baseline were included in each model, as was done in the original pre–post analyses, because age and education were associated with study retention (Hillemeier et al., 2008). Finally, models included an indicator for time (follow-up risk assessment, 6- or 12-month follow-up interview) and the time by treatment condition interaction to estimate the intervention effect at 6- and 12-month follow-up. Because of the effect of pregnancy on weight, weight and BMI were set to missing if the woman was pregnant at the time of the follow-up risk assessment, or 6- or 12-month follow-up interview, respectively. (No women were pregnant at baseline owing to exclusion criteria.) Likewise, a covariate was included in the weight and BMI models to adjust for incident pregnancy between the follow-up risk assessment and 12-month follow-up interview.
Among women who gave birth during the follow-up period, weight gain during pregnancy (in pounds) and weight gain during pregnancy relative to IOM guidelines were analyzed to assess the effect of the intervention. Due to skewness, robust regression with M estimation was used to evaluate weight gain during pregnancy (in pounds) adjusted for age and education (Huber, 1973). Robust regression is a method to provide stable estimates in the presence of outlying observations. Pre-pregnancy obesity (BMI ≥ 30.0) was controlled because it is the strongest predictor of less weight during pregnancy (Chu, Callaghan, Bish, & D’Angelo, 2009), and there were more obese women giving birth in the intervention group than in the control group. Logistic regression was used to measure the intervention effect on weight gain during pregnancy relative to the IOM recommendations while adjusting for age and education. Only women with full-term births (≥37 weeks gestation) were included in the weight gain analyses. All analyses were performed using SAS software, Version 9.2 of the SAS System for Windows (SAS Institute Inc., Cary, NC) and a significance level of .05.
Results
Table 1 shows the sociodemographic characteristics of the intervention and control groups at the time of the 12-month follow-up interview. The only significant difference between the groups was age: Intervention participants were 1.9 years older than control participants, on average. This is consistent with baseline demographic comparisons of the study groups, previously reported (Hillemeier et al., 2008).
Table 1.
Baseline Sociodemographic Characteristics of Intervention and Control Groups at 12-Month Follow-up (n = 302)
| Intervention (n = 218) | Control (n = 84) | p-Value* | |
|---|---|---|---|
| Marital status | .170 | ||
| Married or living with partner | 63.4% (135) | 54.8% (46) | |
| Not married | 36.6% (78) | 45.2% (38) | |
| Mean age in years (standard deviation) | 28.2 (5.0) | 26.3 (4.7) | .003 |
| Education | |||
| High school graduate or less | 36.7% (80) | 30.9% (26) | |
| Some college | 30.7% (67) | 32.1% (27) | |
| College graduate or more | 32.6% (71) | 36.9% (31) | |
| Race/ethnicity | .214 | ||
| White, non-Hispanic | 92.6% (200) | 88.1% (74) | |
| Other (African American, Hispanic, Asian) | 7.4% (16) | 11.9% (10) | |
| Rural-urban residence† | .081 | ||
| Urban-focused | 43.6% (95) | 54.8% (46) | |
| Rural | 56.4% (123) | 45.2% (38) | |
| Poverty status‡ | .777 | ||
| Poor | 27.4% (51) | 28.6% (18) | |
| Near poor | 34.9% (65) | 30.2% (19) | |
| Not poor | 37.6% (70) | 41.3% (26) | |
Based on chi-square or t-test, as appropriate.
Based on zip code approximation of Rural-Urban Commuting Area (RUCA) codes.
U.S. Census definitions based on household income and composition.
Table 2 shows the findings for the first two research questions: 1) Were significant pretest–posttest changes in health-related behaviors maintained over the 12-month follow-up period? and 2) Did the intervention impact weight and BMI over the 12-month follow-up period? The intervention’s effects on reading food labels for nutritional values and using a daily multivitamin with folic acid, which were observed in pretest–posttest analyses (Hillemeier et al., 2008), were maintained at the 6-month follow-up interview, but the effect on reading food labels had become nonsignificant by the time of the 12-month interview. At 12 months, the intervention more than doubled the odds of using a daily multivitamin containing folic acid (adjusted odds ratio = 2.15), indicating that this behavior change was maintained in the intervention group, compared with the control group, over the 12-month follow-up period, but with a smaller effect size (the pretest–posttest odds ratio = 6.59 [Hillemeier et al., 2008]). The intervention effect on meeting physical activity level guidelines observed in the pre–post analyses was not maintained at either the 6- or 12-month follow-up. The intervention did not significantly impact consumption of fruits and vegetables during the follow-up period, consistent with previously reported pre–post findings. Significant effects of the intervention emerged for both weight in pounds and BMI at 12 months, with intervention participants having significantly lower weight (mean difference of 4.33 pounds) and lower BMI (mean difference of 0.75) at the time of the 12-month follow-up interview.
Table 2.
Estimates of Intervention Effects on Behaviors, Weight, and BMI at 6- and 12-Month Follow-up (n = 362)*
| 6-Month Follow-Up
|
12-Month Follow-up
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention Effect† | p-Value | Intervention | Control | Intervention Effect† | p-Value | |
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |||||||
| Reads food labels for nutritional values‡ | 42.8% | 34.9% | 1.97 (1.07, 3.65) | .030 | 36.9% | 44.0% | 0.70 (0.40, 1.23) | .218 |
| Uses daily multivitamin with folic acid | 62.9% | 43.0% | 2.67 (1.56, 4.57) | <.001 | 57.9% | 40.5% | 2.15 (1.19, 3.88) | .011 |
| Meets recommended physical activity level§ | 37.0% | 40.7% | 0.99 (0.57, 1.71) | .970 | 36.9% | 39.8% | 1.00 (0.57, 1.76) | .992 |
| Daily fruit consumption¶ | 41.5% | 47.7% | 0.66 (0.38, 1.16) | .153 | 38.3% | 40.5% | 0.68 (0.39, 1.21) | .190 |
| Daily vegetable consumption¶ | 44.7% | 40.7% | 1.17 (0.67, 2.04) | .589 | 42.1% | 40.5% | 0.99 (0.57, 1.71) | .958 |
| Mean Difference (95% CI) | Mean Difference (95% CI) | |||||||
| Weight (in pounds)|| | 160.6 (158.7, 162.6) | 163.2 (160.5, 166.0) | −2.61 (−5.93, 0.71) | .123 | 162.1 (159.5, 164.6) | 166.4 (163.4, 169.4) | −4.33 (−8.16, −0.49) | .027 |
| BMI|| | 27.1 (26.7, 27.4) | 27.1 (27.1, 28.0) | −0.45 (−1.00, 0.10) | .110 | 27.3 (26.9, 27.7) | 28.0 (27.5, 28.6) | −0.75 (−1.39, −0.11) | .021 |
Abbreviation: CI, confidence interval.
All models control for baseline level on the dependent variable, age and educational level (see text).
Model-based estimates.
Defined as all or most of the time versus some of the time, rarely, or never.
Defined as ≥30 minutes of moderate or strenuous physical activity on ≥4 days per week.
Defined as ≥1 serving per day in a typical week.
Adjusted means and confidence intervals. For women pregnant at the time of the 6- or 12-month follow-up interview, weight, and BMI were set to missing. Additional covariate controls for incident pregnancy during follow-up period.
Table 3 shows the findings for the last research question: Did the intervention impact pregnancy weight gain for those women who gave birth during the 12-month follow-up period? Women in the intervention group who gave birth to full-term singletons gained significantly less total weight during their pregnancies compared with controls. The adjusted mean weight gain for women in the intervention group was 23.4 pounds, compared with 41.4 pounds for women in the control group. When pre-pregnancy obesity (BMI ≥ 30) was controlled, the intervention effect was no longer significant, although the adjusted means show a trend toward lower weight gain in the intervention group. The intervention did not reduce the odds of exceeding the IOM recommended weight gain for the woman’s pre-pregnancy BMI category, although again the trend is in the expected direction: 42.9% of women in the intervention group had pregnancy weight gain exceeding the IOM recommendation for their pre-pregnancy BMI category, compared with 55.6% in the control group.
Table 3.
Analysis of Intervention Effects on Pregnancy Weight Gain Among Women Who Gave Birth to Singletons Over the 12-Month Follow-up Period (n = 37)*
| Intervention | Control | Intervention Effect†
|
||
|---|---|---|---|---|
| Mean Difference (95% CI) | p-Value | |||
| Pregnancy weight gain, in pounds§ | 23.4 (16.5, 30.3) | 41.4 (28.9, 53.8) | −17.95 (−33.42, −2.49) | .023 |
| Pregnancy weight gain, controlling for pre-pregnancy obesity‡,§ | 23.8 (18.3, 29.2) | 34.2 (23.8, 44.7) | −10.46 (−24.27, 3.36) | .138 |
| Odds Ratio (95% CI)
|
||||
| Pregnancy weight gain exceeded IOM guidelines¶ | 42.9% | 55.6% | 0.685 (0.137, 3.431) | .645 |
Abbreviation: CI, confidence interval.
Excludes 4 preterm births and 4 women for whom weight gain at delivery was missing on the birth record. Models control for baseline age and educational level.
Model-based estimates.
Adjusted means and confidence intervals shown for intervention and control groups.
Obesity is defined as BMI ≥ 30.0.
Mother’s pregnancy weight gain exceeded IOM (2009) recommended weight gain for her pre-pregnancy BMI category (see text).
Discussion
These results provide evidence of the long-term effectiveness of the Strong Healthy Women behavior change intervention for pre- and interconceptional women. One positive behavior change attributable to the intervention in pre–post analyses was maintained over the 12-month follow-up period–use of a daily multivitamin containing folic acid–whereas the intervention effect on reading food labels for nutritional values dropped off after 6 months. In addition, women in the intervention group, compared with controls, had significantly lower weight and lower BMI by the time of the 12-month follow-up but not at the 6-month follow-up, suggesting that effects on weight and BMI take longer to appear.
Finally, among those women who became pregnant and delivered a full-term singleton during the 12-month follow-up period, the intervention was associated with significantly lower pregnancy weight gain, as derived from birth records. This effect seems to be explained by the higher prevalence of obese women giving birth in the intervention group, because obese women gained less during pregnancy compared with nonobese women (Chu et al., 2009). However, because the adjusted means show a trend toward less pregnancy weight gain in the intervention group after controlling for pre-pregnancy obesity, it is possible that the difference in weight gain by study group would have been significant in a larger sample of births. It is also possible that the intervention might have had some influence on the pregnancy timing decisions of obese women; in other words, the intervention might have increased obese women’s perceived internal control for birth outcomes or their confidence in having a healthy pregnancy. These are important issues to explore in future studies, because pregnancy weight gain in compliance with IOM recommendations is an important outcome that is related to cesarean delivery, to postpartum weight retention and subsequent obesity in the woman, and to fetal growth and child obesity (IOM, 2009). Nevertheless, it seems that the Strong Healthy Women preconceptional intervention has the potential to impact women’s attitudes and behaviors about weight, which in turn may translate into less overall weight gain in a subsequent pregnancy.
The long-term findings with regard to weight, BMI, and pregnancy weight gain are plausible given that the Strong Health Women intervention includes substantial content related to healthy nutrition and regular physical activity. Although the content of the Strong Healthy Women intervention does not focus on weight loss specifically, nutrition and physical activity are stressed throughout the six-session intervention with informational content as well as skills-building exercises including food preparation and time devoted to physical activity (e.g., walking, exercises, use of core balls). Interestingly, although our pre–post findings included significant intervention effects on intent to eat healthy foods, intent to be more physically active, and physical activity levels meeting recommended guidelines, neither the pre–post analyses nor the 12-month follow-up analysis found a significant intervention effect on daily consumption of fruits and vegetables.
It is possible that weight status and pregnancy weight gain were impacted by smaller improvements in healthy eating and physical activity than were captured by our 12-month follow-up measures. For example, we assessed physical activity as at least 30 minutes on 4 or more days per week, but participants may have increased exercise on all days but for a shorter period. In addition, the intervention recommended that participants increase physical activity through habits of daily life, such as walking up stairs, parking further from a destination, or engaging in yard work, but these outcomes were not measured. Weight status may also have been influenced by unmeasured changes in portion control or overall calorie intake. Slow but steady progress in controlling portion size or calories relative to physical activity levels also might explain the emergence of a significant intervention effect on weight and BMI by the time of the 12-month follow-up interview, but not by the time of the 6-month follow-up interview. With respect to pregnancy weight gain, the intervention’s information about weight management during pregnancy may have become more salient to participants once they became pregnant, prompting them to make substantial changes in their nutritional and/or physical activity habits at that time.
Although unmeasured changes in behavior or the timing (e.g., during pregnancy) of behavioral changes may have led to impact on weight status, these results indicate that intervention impact on measured behaviors deteriorated over time. One behavior that was significantly improved in the pretest–posttest analyses but was not maintained over the follow-up period was physical activity level consistent with prevailing recommendations. It is possible that the intervention posttest effect on physical activity level was not sustained because the social support inherent in the group intervention format ended with the last group session. Maintaining physical activity levels consistent with the guidelines is challenging because it requires a person to plan, schedule, and integrate physical activity into everyday life. Although the Strong Healthy Women intervention had increased women’s physical activity levels in the initial pretest–posttest assessment, this was a relatively brief intervention without booster sessions. More sessions, a longer intervention period, or greater reinforcement may be beneficial for long-term maintenance of exercise behaviors in this population. Alternatively, it may not have been economically feasible for women to continue to make time for regular physical activity after the intervention ended or to join a gym to continue exercise on most days of the week. These possible explanations could inform future interventions.
Limitations of this study include the small sample size for pregnancy-related outcomes. The original trial was not powered for pregnancy outcomes, but rather for changes in risk factors for adverse pregnancy outcomes. Accordingly, there were insufficient incident pregnancies to confirm an intervention effect on pregnancy weight gain once other relevant covariates are controlled (e.g., parity) or to study the intervention’s impact on relatively rare outcomes such as preterm birth and low birth-weight. Another limitation is the lack of anthropometric measures and biomarkers at the 12-month follow-up (because women were not required to return to the research site) and the consequent reliance on self-reported measures of behavior change and follow-up weight status. There is some possibility of underestimation of follow-up BMI using self-reported weight. Although studies show that U.S. women tend to underreport their weight from 0.56 to >2 kg (Engstrom, Paterson, Doherty, Trabulsi, & Speer, 2003; Jain, 2010), self-reported height and weight have been found to accurately represent BMI abstracted from medical records for reproductive-age women (Huber, 2007). We have no evidence of differential underreport of weight by study group. Finally, about half of the participants in this trial had rural residences (Velott et al., 2008), and replication in urban settings is needed.
The Strong Healthy Women intervention trial was conducted in rural, low-income communities in a region that is largely White and non-Hispanic. This strategy was intended to target women who may not have optimal access to health services owing to both fewer financial resources and residence in medically underserved communities. To date, the intervention has not been tested in low-income urban areas or in a more race/ethnically diverse population. A subsequent focus group study (unpublished) with low-income urban women suggested some minor modifications to the content of Strong Healthy Women for implementation in urban communities; these modifications have been made in the intervention protocol in preparation for future studies. Further testing of the Strong Healthy Women intervention in other populations of pre- and interconceptional women are encouraged.
In conclusion, this study adds to the limited body of research on the effectiveness of preconception health promotion programs and is the first report, to our knowledge, of the long-term effectiveness of a preconception health promotion intervention in the United States. The results reported herein are promising for developing effective behavior change interventions to improve the health of women before conception and to address an important pregnancy outcome, weight gain during pregnancy. Because the intervention appears to help women effectively manage their weight in the months following the intervention and during pregnancy, it may be an effective obesity prevention strategy for women before, during, and after the transition to motherhood.
Acknowledgments
This research was funded, in part, under grant number 4100020719 with the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. The Penn State Survey Research Center conducted the telephone interviews and tracking of respondents. The Pennsylvania Department of Health provided birth records.
Footnotes
Author Descriptions
Carol S. Weisman, PhD, is a sociologist and health services researcher with a principal interest in women’s health care.
Marianne M. Hillemeier, PhD, MPH, is a sociologist/demographer with research interests in maternal and child health disparities.
Danielle Symons Downs, PhD, is an exercise psychology researcher whose expertise is in understanding the psychosocial and behavioral correlates of exercise in women and children.
Mark E. Feinberg, PhD, is a psychologist whose research focuses on family and community prevention programs.
Cynthia H. Chuang, MD, is a general internist with research interests in reproductive health care for women with chronic medical conditions.
John J. Botti, MD, is a maternal-fetal medicine specialist who cares for women with increased risks for adverse pregnancy outcomes.
Anne-Marie Dyer, MS, is a biostatistician and the data manager and chief data analyst for the Central Pennsylvania Women’s Health Study.
The authors have no conflicts of interest to report.
References
- American College of Sports Medicine (ACSM) ACSM’s guidelines for exercise testing and prescription. 6. Philadelphia: Lippincott, Williams & Wilkins; 2000. [Google Scholar]
- Beunckens C, Molenberghs G, Kenward MG. Direct likelihood analysis versus simple forms of imputation for missing data in randomized clinical trials. Clinical Trials. 2005;2:379–386. doi: 10.1191/1740774505cn119oa. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Recommendations to improve preconception health and health care–United States. MMWR. 2006;55:RR-6. [PubMed] [Google Scholar]
- Chu SY, Callaghan WM, Bish CL, D’Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2005–2005: Fueling future obesity. American Journal of Obstetrics and Gynecology. 2009;200(271):e1–271.e7. doi: 10.1016/j.ajog.2008.09.879. [DOI] [PubMed] [Google Scholar]
- Downs DS, Feinberg M, Hillemeier MM, Weisman CS, Chase GA, Chuang CH, et al. Design of the Central Pennsylvania Women’s Health Study (CePAWHS) Strong Healthy Women intervention: Improving preconceptional health. Maternal and Child Health Journal. 2009;13:18–28. doi: 10.1007/s10995-008-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom JL, Paterson SA, Doherty A, Trabulsi M, Speer KL. Accuracy of self-reported height and weight in women: An integrative review of the literature. Journal of Midwifery & Women’s Health. 2003;48:338–345. doi: 10.1016/s1526-9523(03)00281-2. [DOI] [PubMed] [Google Scholar]
- Hillemeier MM, Downs DS, Feinberg ME, Weisman CS, Chuang CH, Parrott R, et al. Improving women’s preconceptional health: Findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania Women’s Health Study. Women’s Health Issues. 2008;18S:S87–S96. doi: 10.1016/j.whi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LRB. Validity of self-reported height and weight in women of reproductive age. Maternal and Child Health Journal. 2007;11:137–144. doi: 10.1007/s10995-006-0157-0. [DOI] [PubMed] [Google Scholar]
- Huber PJ. Robust regression: Asymptotics, conjectures and Monte Carlo. Annals of Statistics. 1973;1:799–821. [Google Scholar]
- Institute of Medicine (IOM) Weight gain during pregnancy: Reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- Jain RB. Regression models to predict corrected weight, height and obesity prevalence from self-reported data: Data from BRFSS 1999–2007. International Journal of Obesity. 2010;34:1655–1664. doi: 10.1038/ijo.2010.80. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Steinberg A, Bender C, Newberry S. Preconception care: A systematic review. Maternal and Child Health Journal. 2002;6:75–88. doi: 10.1023/a:1015460106832. [DOI] [PubMed] [Google Scholar]
- Velott DL, Baker SA, Hillemeier MM, Weisman CS. Participant recruitment to a randomized trial of a community-based behavioral intervention for pre- and interconceptional women: Findings from the Central Pennsylvania Women’s Health Study. Women’s Health Issues. 2008;18:217–224. doi: 10.1016/j.whi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Wahabi HA, Alziedan RA, Bawazeer GH, Al-Ansary LA, Esmaiel SA. Preconception care for diabetic women for improving maternal and fetal outcomes: A systematic review and meta-analysis. BMC Pregnancy and Childbirth. 2010;10:63. doi: 10.1186/1471-2393-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman CS, Hillemeier MM, Chase GA, Dyer AM, Baker SA, Feinberg M, et al. Preconceptional health: Risks of adverse pregnancy outcomes be reproductive life stage in the Central Pennsylvania Women’s Health Study. Women’s Health Issues. 2006;16:216–224. doi: 10.1016/j.whi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Weisman CS, Misra DP, Hillemeier MM, Downs DS, Chuang CH, Camacho FT, et al. Preconception predictors of birth outcomes: Prospective findings from the Central Pennsylvania Women’s Health Study. Maternal and Child Health Journal. 2009 doi: 10.1007/s10995-009-0473-2. Published online 2009 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman CS, Hillemeier MM, Downs DS, Chuang CH, Dyer AM. Preconception predictors of weight gain during pregnancy: Prospective findings from the Central Pennsylvania Women’s Health Study. Women’s Health Issues. 2010;20:126–132. doi: 10.1016/j.whi.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth M, Dowsell T. The Cochrane collaboration. New York: John Wiley & Sons; 2009. Routine pre-pregnancy health promotion for improving pregnancy outcomes (review) [DOI] [PMC free article] [PubMed] [Google Scholar]