Abstract
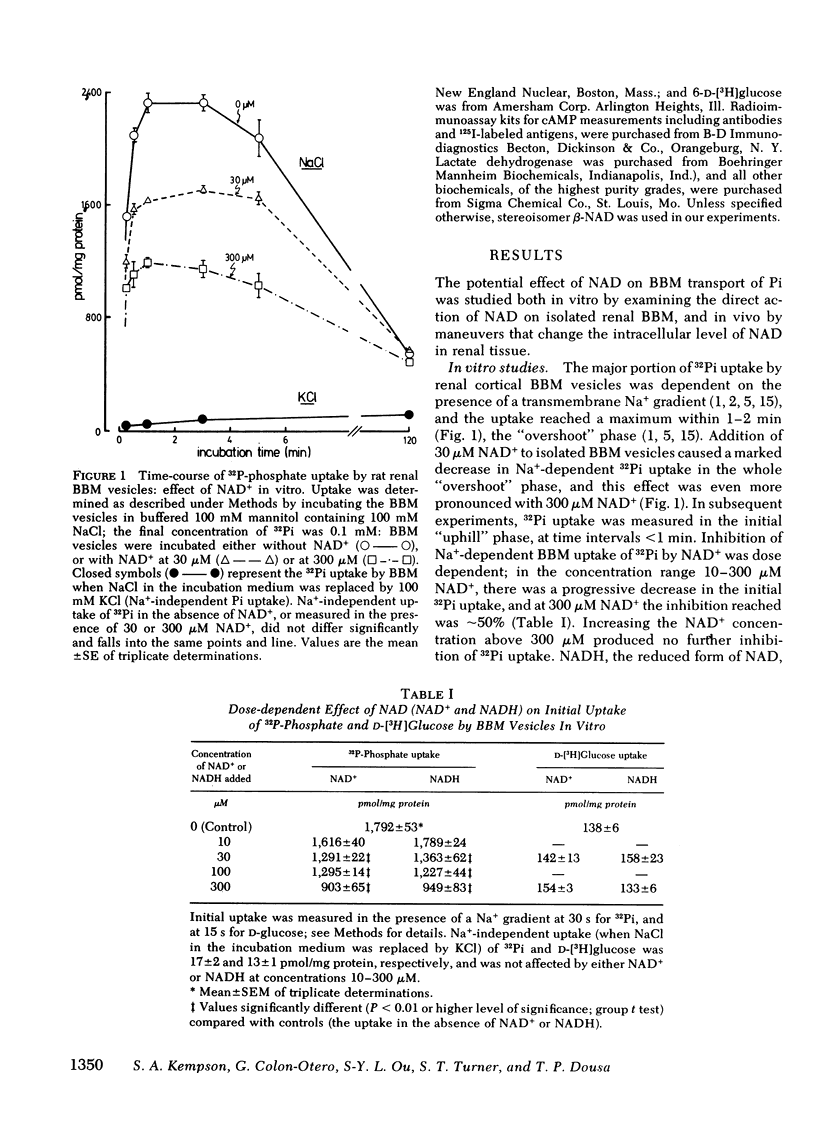
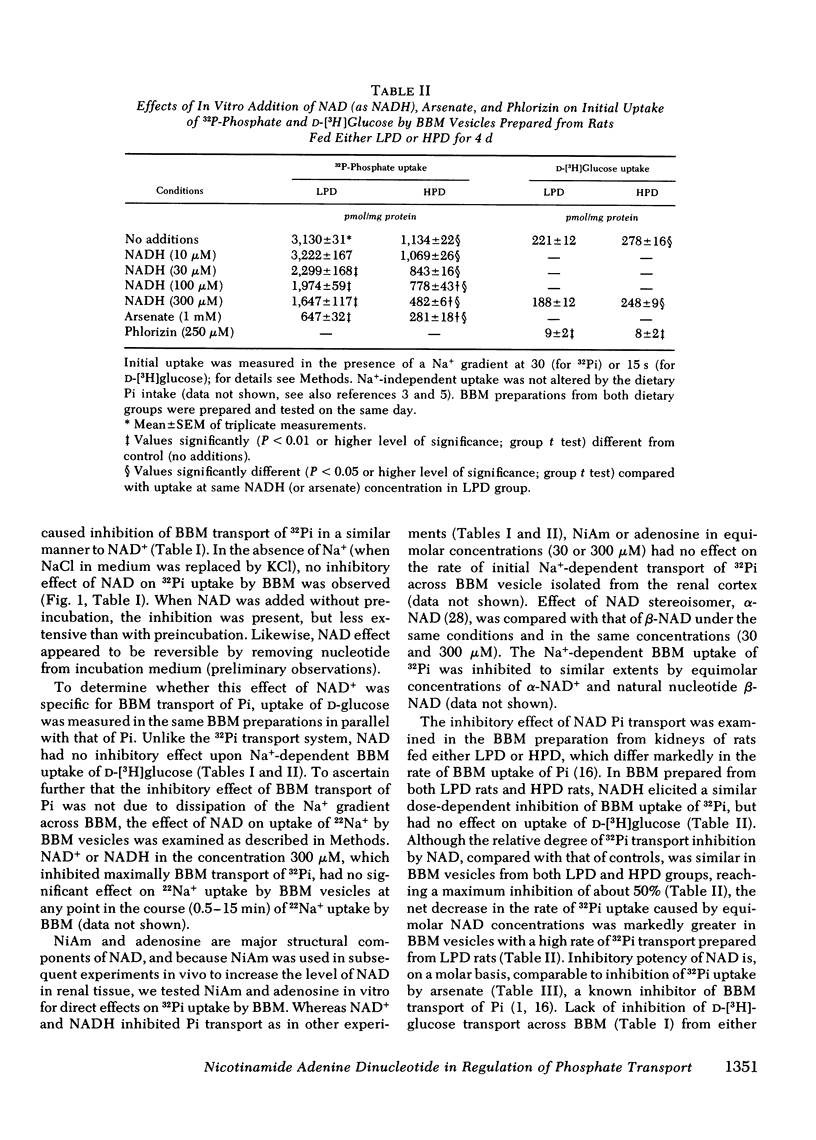
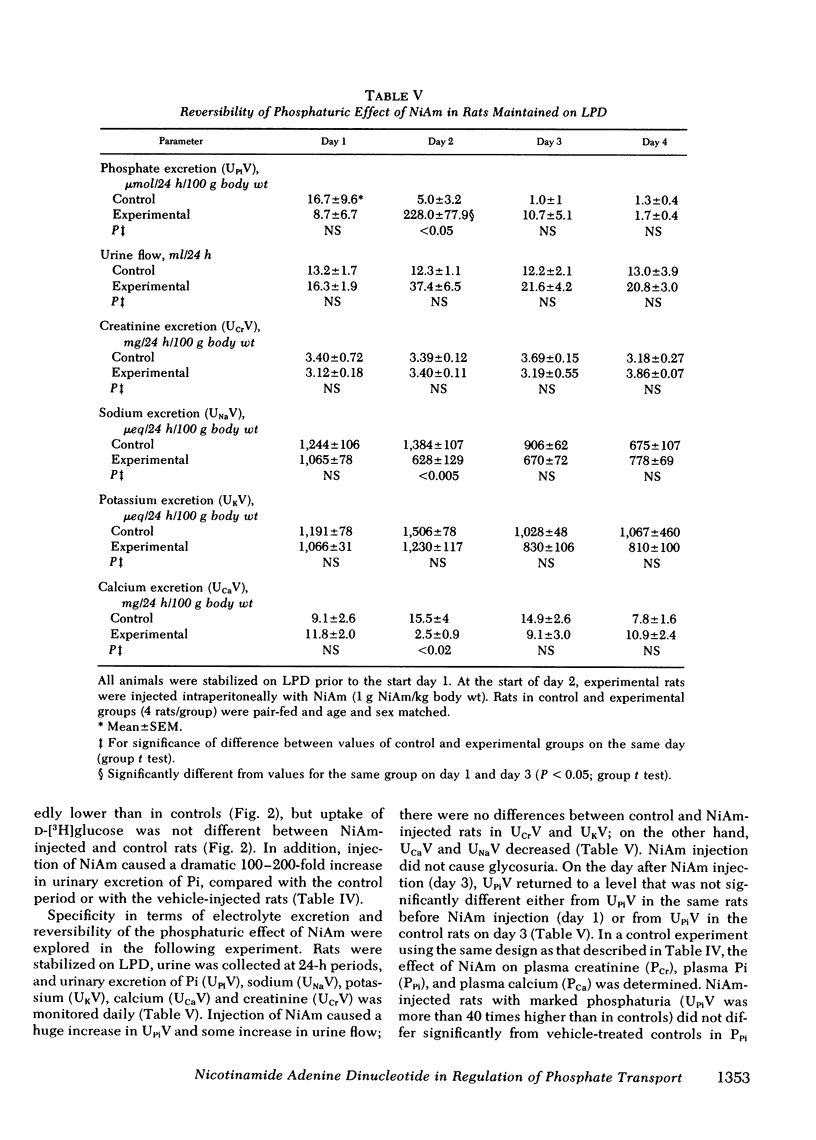
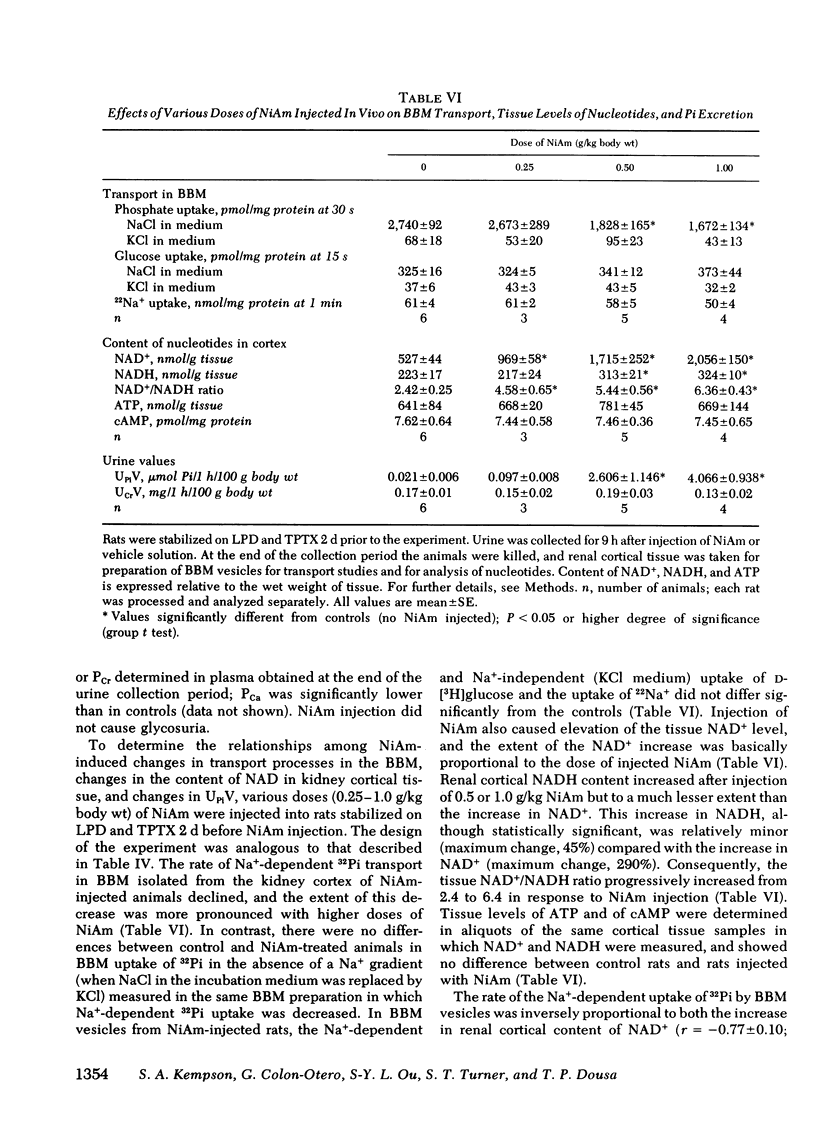
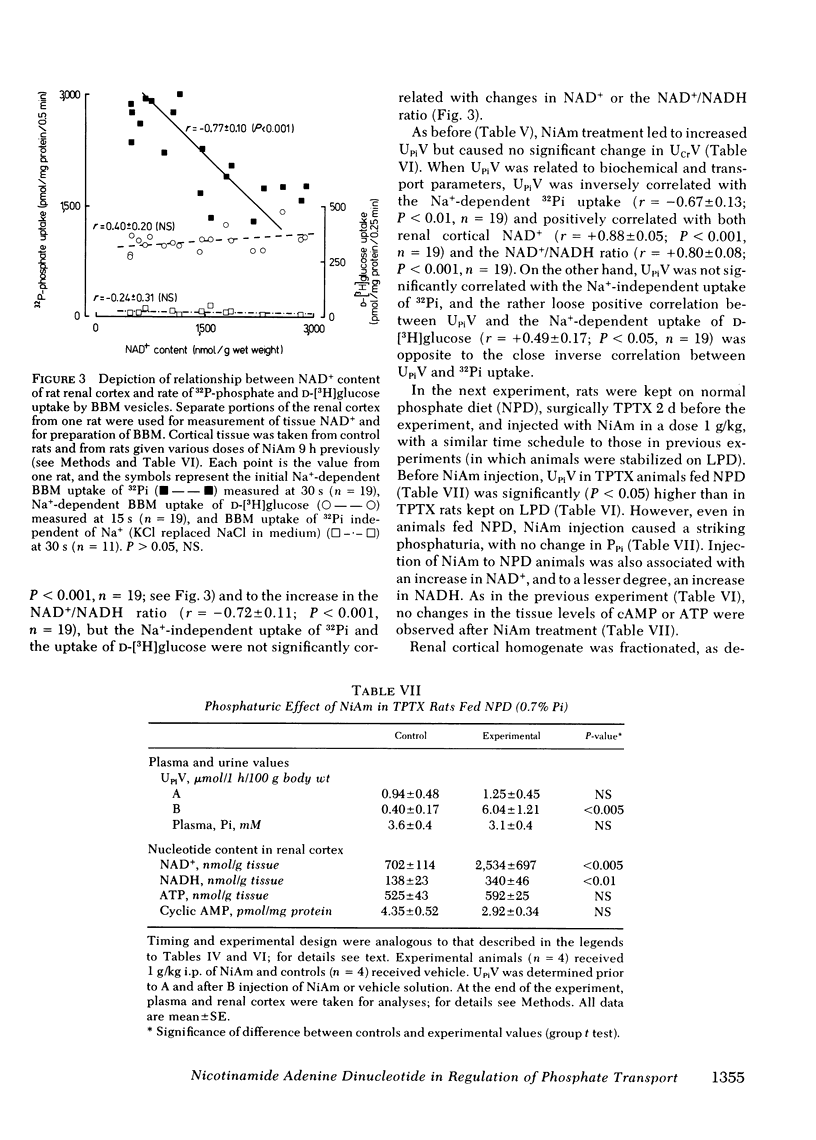
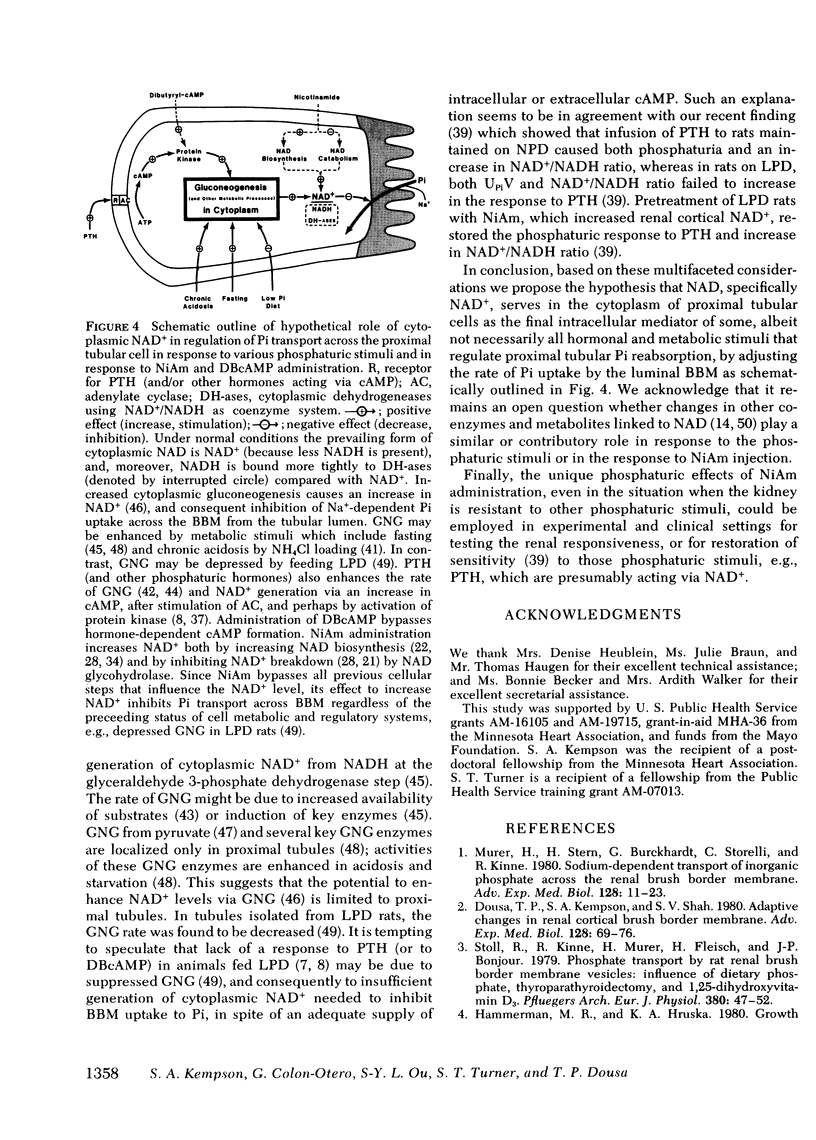
In these experiments we investigated whether NAD could serve as an intracellular modulator of the brush border membrane (BBM) transport of inorganic phosphate (Pi). NAD, both oxidized (NAD+) and reduced (NADH) form, inhibited the Na+-dependent uptake of 32Pi in the concentration range of 10-300 microM NAD when added in vitro to BBM vesicles isolated from rat kidney cortex, but did not inhibit BBM uptake of D-[3H]glucose or BBM uptake of 22Na+. Neither nicotinamide (NiAm) nor adenosine alone influenced BBM uptake of 32Pi. NAD had a similar relative effect (percent inhibition) in BBM from rats stabilized on low Pi diet (0.07% Pi), high Pi diet (1.2% Pi), or normal Pi diet (0.7% Pi). Subsequently, we examined the renal effects of changing the tissue NAD level in vivo. Rats stabilized on low Pi diet were injected intraperitoneally with NiAm (0.25-1.0 g/kg body wt); urinary excretions of Pi (UPiV), of fluid, and of other solutes were measured before and after NiAm injection, then renal cortical tissue nucleotide content was determined, and a BBM fraction was isolated for transport measurements. In BBM from NiAm-treated rats, the Na+-dependent uptake of 32Pi was decreased, but BBM uptake of D-[3H]glucose and BBM uptake of 22Na+ were not changed. NiAm injection elicited an increase in NAD+ (maximum change, 290%), a lesser increase in NADH (maximum change, +45%), but no change in the content of ATP or cyclic AMP in the renal cortex. Na+-dependent BBM uptake of 32Pi ws inversely correlated with NAD+ content in renal cortex (r = -0.77 +/- 0.1; P less than 0.001) and with UPiV (r = -0.67 +/- 0.13; P less than 0.01). NAD+ in renal cortex was positively correlated with UPiV (r = 0.88 +/- 0.05; P less than 0.001). Injection of NiAm elicited a marked increase in UPiV, but no change in excretions of creatinine or K+, or in urine flow; excretion of Na+ and Ca declined. NiAm injection caused similar renal responses, in normal and in thyroparathyroidectomized rats, as well as in rats on normal Pi diet and low Pi diet. We conclude that NAD can serve as an intracellular modulator (inhibitor) of Na+-dependent transport of Pi across the renal luminal BBM and across the proximal tubular wall by its direct interaction with BBM. We propose that at least some hormonal and/or metabolic stimuli elicit phosphaturia by increasing NAD+ in cytoplasm of proximal tubular cells.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Hayslett J. P., Kashgarian M. Dissociation of proximal tubular glucose and Na+ reabsorption by amphotericin B. Am J Physiol. 1979 Apr;236(4):F392–F397. doi: 10.1152/ajprenal.1979.236.4.F392. [DOI] [PubMed] [Google Scholar]
- BURCH H. B., LOWRY O. H., VONDIPPE P. THE STABILITY OF TRIPHOSPHOPYRIDINE NUCLEOTIDE AND ITS REDUCED FORM IN RAT LIVER. J Biol Chem. 1963 Aug;238:2838–2842. [PubMed] [Google Scholar]
- Beck N., Webster S. K., Reineck H. J. Effect of fasting on tubular phosphorus reabsorption. Am J Physiol. 1979 Sep;237(3):F241–F246. doi: 10.1152/ajprenal.1979.237.3.F241. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. Proteins of the kidney microvillus membrane. Identification of subunits after sodium dodecylsullphate/polyacrylamide-gel electrophoresis. Biochem J. 1976 Nov;159(2):395–407. doi: 10.1042/bj1590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R. O., Fishman P. H. Biotransducers of membrane-mediated information. Adv Enzymol Relat Areas Mol Biol. 1979;50:303–323. doi: 10.1002/9780470122952.ch6. [DOI] [PubMed] [Google Scholar]
- Burch H. B., Narins R. G., Chu C., Fagioli S., Choi S., McCarthy W., Lowry O. H. Distribution along the rat nephron of three enzymes of gluconeogenesis in acidosis and starvation. Am J Physiol. 1978 Sep;235(3):F246–F253. doi: 10.1152/ajprenal.1978.235.3.F246. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Duarte C. G., Knox F. G. Effect of colchicine on urinary phosphate and regulation by parathyroid hormone. Am J Physiol. 1976 Jul;231(1):61–65. doi: 10.1152/ajplegacy.1976.231.1.61. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Kempson S. A., Shah S. V. Adaptive changes in renal cortical brush border membrane. Adv Exp Med Biol. 1980;128:69–76. doi: 10.1007/978-1-4615-9167-2_8. [DOI] [PubMed] [Google Scholar]
- GREENGARD P., QUINN G. P., REID M. B. PITUITARY INFLUENCE OF PYRIDINE NUCLEOTIDE METABOLISM OF RAT LIVER. J Biol Chem. 1964 Jun;239:1887–1892. [PubMed] [Google Scholar]
- Haase W., Schäfer A., Murer H., Kinne R. Studies on the orientation of brush-border membrane vesicles. Biochem J. 1978 Apr 15;172(1):57–62. doi: 10.1042/bj1720057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. F., Welt L. G. Adenosinetriphosphate in rat renal papilla: effects of vasopressin and of ischemia. Am J Physiol. 1967 Apr;212(4):939–944. doi: 10.1152/ajplegacy.1967.212.4.939. [DOI] [PubMed] [Google Scholar]
- Kempson S. A., Dousa T. P. Phosphate transport across renal cortical brush border membrane vesicles from rats stabilized on a normal, high or low phosphate diet. Life Sci. 1979 Mar 5;24(10):881–887. doi: 10.1016/0024-3205(79)90337-0. [DOI] [PubMed] [Google Scholar]
- Kempson S. A., Shah S. V., Werness P. G., Berndt T., Lee P. H., Smith L. H., Knox F. G., Dousa T. P. Renal brush border membrane adaptation to phosphorus deprivation: effects of fasting versus low-phosphorus diet. Kidney Int. 1980 Jul;18(1):36–47. doi: 10.1038/ki.1980.108. [DOI] [PubMed] [Google Scholar]
- Kinne R. Current concepts of renal proximal tubular function. Contrib Nephrol. 1978;14:14–24. doi: 10.1159/000402347. [DOI] [PubMed] [Google Scholar]
- Kirsten R., Kirsten E. Redox state of pyridine nucleotides in renal response to aldosterone. Am J Physiol. 1972 Jul;223(1):229–235. doi: 10.1152/ajplegacy.1972.223.1.229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin L. F., Henderson L. M. Pyridinium precursors of pyridine nucleotides in perfused rat kidney and in the testis. J Biol Chem. 1972 Dec 25;247(24):8023–8030. [PubMed] [Google Scholar]
- Maleque A., Endou H., Koseki C., Sakai F. Nephron heterogeneity: gluconeogenesis from pyruvate in rabbit nephron. FEBS Lett. 1980 Jul 28;116(2):154–156. doi: 10.1016/0014-5793(80)80631-4. [DOI] [PubMed] [Google Scholar]
- Morrison A. R., Yates J., Klahr S. Effect of prostaglandin E on the adenyl cyclase-cyclic AMP system and gluconeogenesis in rat renal cortical slices. Biochim Biophys Acta. 1976 Feb 24;421(2):203–209. doi: 10.1016/0304-4165(76)90286-5. [DOI] [PubMed] [Google Scholar]
- Murer H., Stern H., Burckhardt G., Storelli C., Kinne R. Sodium-dependent transport of inorganic phosphate across the renal brush border membrane. Adv Exp Med Biol. 1980;128:11–23. doi: 10.1007/978-1-4615-9167-2_2. [DOI] [PubMed] [Google Scholar]
- Northrup T. E., Krezowski P. A., Palumbo P. J., Kim J. K., Hui Y. S., Dousa T. P. Insulin inhibition of hormone-stimulated protein kinase systems of rat renal cortex. Am J Physiol. 1979 Jun;236(6):E649–E654. doi: 10.1152/ajpendo.1979.236.6.E649. [DOI] [PubMed] [Google Scholar]
- Pfaller W., Rittinger M. Quantitative morphology of the rat kidney. Int J Biochem. 1980;12(1-2):17–22. doi: 10.1016/0020-711x(80)90035-x. [DOI] [PubMed] [Google Scholar]
- Preuss H. G. Pyridine nucleotides in renal ammonia metabolism. J Lab Clin Med. 1968 Sep;72(3):370–382. [PubMed] [Google Scholar]
- Ramasamy I., Butterworth P. J. The inhibition of pig kidney alkaline phosphatase by oxidized or reduced nicotinamide-adenine dinucleotide and related compounds. Biochem J. 1973 Feb;131(2):359–367. doi: 10.1042/bj1310359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobol A., Alleyne G. A. Regulation of renal gluconeogenesis by calcium ions, hormones and adenosine 3':5'-cyclic monophosphate. Biochem J. 1973 May;134(1):157–165. doi: 10.1042/bj1340157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. V., Kempson S. A., Northrup T. E., Dousa T. P. Renal adaptation to a low phosphate diet in rats. J Clin Invest. 1979 Oct;64(4):955–966. doi: 10.1172/JCI109562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll R., Kinne R., Murer H., Fleisch H., Bonjour J. P. Phosphate transport by rat renal brush border membrane vesicles: influence of dietary phosphate, thyroparathyroidectomy, and 1,25-dihydroxyvitamin D3. Pflugers Arch. 1979 May 15;380(1):47–52. doi: 10.1007/BF00582611. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]



