Abstract
The blood-brain barrier (BBB) is not simply a physical barrier but a regulatory interface between the central nervous system (CNS) and immune system. The BBB both affects and is affected by the immune system and connects at many levels with the CNS, including the following: (1) the BBB transports cytokines and secretes various substances with neuroinflammatory properties; (2) transporters are altered in disease states including traumatic injury, Alzheimer's disease and inflammatory processes; (3) cytokines and other immune secretions from the cells comprising the BBB are both constitutive and inducible; (4) immune cells are transported across the BBB by the highly regulated process termed diapedesis, which involves communication and interactions between the brain endothelial cells and the immune cells; (5) the neuroimmune system has various effects on the BBB, including modulation of important transport systems and in extreme pathological conditions even disruption of the BBB, and (6) the brain-to-blood efflux transporter P-glycoprotein is altered in inflammatory conditions, thus affecting drug delivery to the brain. In summary, the BBB is an interactive interface that regulates and defines many of the ways that the CNS and the immune system communicate with one another.
Key Words: Blood-brain barrier, Neuroinflammation, Cytokines, Transport, Alzheimer's disease, P-glycoprotein, Drug delivery
Introduction
Inflammatory processes are involved in a wide variety of diseases and conditions that involve the central nervous system (CNS). These range from neurodegenerative diseases such as Alzheimer's disease (AD) to obesity. The study of the interactions between the immune system and CNS has given rise to a rich field often referred to as neuroimmunology or psychoneuroimmunology. The CNS was once felt to be an immune-privileged area. A major rationale for this notion of sequestration was the blood-brain barrier (BBB), which was believed to prevent immune cells and mediators of immunity from accessing the CNS. Currently, the separation of the immune system and the CNS is appreciated to be a qualified one with cross-talk between these two systems occurring both in disease and physiological states. Definition of the role of the BBB has also shifted in that it is now appreciated to mediate, and in some cases to define, the interactions between the CNS and the immune system.
The ways in which the CNS and the immune system interact are numerous (fig. 1). Having participated in the dichotomy of the neuro- and immune systems by separating the CNS and the circulation, the BBB does not immediately appear to be involved in their interactions. For example, a great deal of communication between the CNS and immune systems occurs through vagal mediation [1,2]. However, a number of other mechanisms have been discovered through which the CNS and immune systems communicate, and the BBB is involved in many of them. As examples, immune cell trafficking into the CNS and the exchange of cytokines between the circulation and the CNS each involve the BBB. Additionally, the immune system influences the functioning of the BBB, which in turn affects CNS function in health and disease. Hence, there is a dynamic interplay between the CNS, the BBB and the immune system. This review will examine in two sections some of the established mechanisms that involve the BBB and neuroimmune interactions. The first section will concentrate on physiologic processes and regulation of those processes, although occasionally disease states will be discussed that help to illustrate underlying physiologic processes. The second section will then examine how these processes can contribute to and even produce diseases.
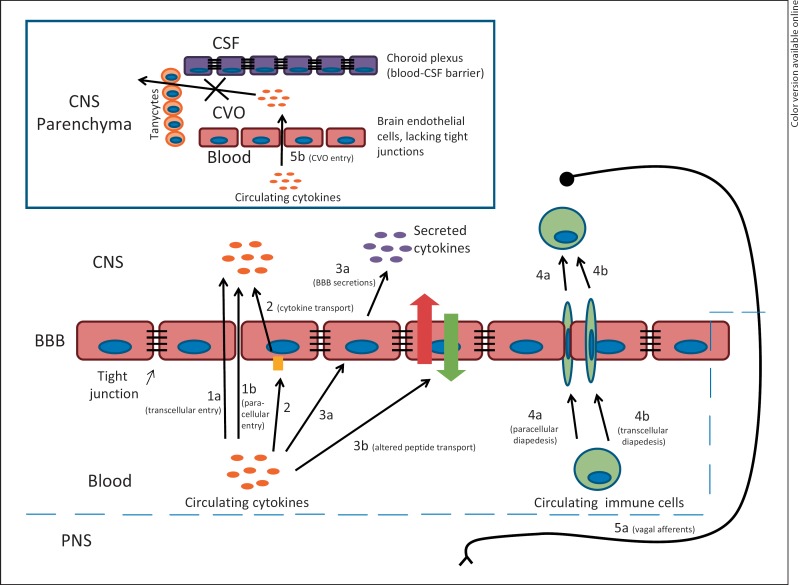
Fig. 1.
An endocentric view of neuroinflammation. (1) Disruption of BBB integrity and disassembly of tight junctions permits transcellular (la) and paracellular (lb) entry of circulating components. (2) Circulating cytokines are transported across the BBB into the brain. (3) BBB endothelial cells are activated by circulating cytokines and other circulating inflammatory mediators, causing secretion of inflammatory mediators including cytokines into the brain parenchyma (3a) and alterations in the transport of substances across the BBB (3b). (4) Circulating immune cells cross the BBB via diapedesis by paracellular (4a) and transcellular (4b) pathways. (5) Circulating cytokines bypass the BBB by inflammatory activation of vagai afférents (5a) or inflammatory mediators crossing the leaky vasculature into circumventricular organs (CVOs; 5b), which are compartmentalized from the rest of the CNS by tanycytic barriers. PNS = Peripheral nervous system.
Overview of Interactions between the BBB and the Neuroimmune System
BBB Disruption
That the BBB can be disrupted during infection and inflammation has long been known [3,4,5,6,7]. BBB disruption can be catastrophic to an organism, removing the protective function that the BBB provides to the CNS. Early studies with cytokines suggested that disruption occurred quite readily, although later studies showed that this in part may have been because solvents such as SDS were not totally removed from the early preparations of cytokines [8,9]. Treatment with lipopolysaccharide (LPS) can also result in BBB disruption [6]. LPS is derived from the cell wall of Gram-negative bacteria and induces a robust increase in blood and brain levels of many cytokines. Subsequent studies have shown that it can be difficult to consistently disrupt the BBB with individual cytokines and even with LPS [10,11]. This makes teleological sense as mechanisms would be expected to have evolved under evolutionary pressures to protect the brain by preventing BBB disruption.
The mechanisms by which neuroimmune events disrupt the BBB are not fully understood. It is also increasingly speculated that the restrictive aspects of the BBB are not always maximal, but may be modulated within limits as part of physiological processes. It is clear that some cytokines have effects on brain endothelial cells that could lead to either disruption or modulation of the restrictive aspects of the BBB. These effects include alterations of the actin cytoskeleton and tight junction expression [12,13].
Immune Cell Trafficking
Leukocytes were once thought to enter the CNS only during disease and as a result of BBB disruption. More recent studies have shown that immune cell surveillance is a physiologic aspect of neuroimmunity [14,15,16]. The detailed mechanisms by which immune cells enter the CNS have been extensively described [for a review, see [17,18]] and are beyond the scope of this review. Instead, this section will provide a brief overview of the process of immune cell trafficking under physiological conditions, and later sections will describe how trafficking is altered under neuroinflammatory conditions. Because the rate of leukocytes entering the CNS is relatively low under physiological conditions [15], inflammatory models have been critical in delineating mechanisms by which leukocytes cross the BBB in vivo. One example of such a model is experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis where high levels of leukocyte infiltration into the CNS are observed [14]. In EAE, cross-talk between activated myelin-specific CD4+ T lymphocytes, endothelial cells of a healthy BBB and other components of the neurovascular unit is required both for entry of the activated lymphocytes into the CNS as well as further recruitment of leukocytes from the circulation [17]. In vivo studies following fluorescently labeled encephalitogenic T cells have shown that the capture and subsequent diapedesis of these cells across a noninflamed BBB is dependent on the interaction of α4-integrin with vascular cell adhesion molecule-1 expressed on brain endothelial cells [19,20]. Interestingly, these studies showed that the initial capture of immune cells at the BBB in spinal cord and retinal microvessels happens abruptly without rolling, which may be due to a lack of P-selectin in Weibel-Palade bodies of parenchymal endothelial cells [21,22]. Furthermore, diapedesis in these vessels occurs slowly, requiring between 4 and 16 h for complete passage across the BBB [19,20]. This delayed response is likely due to further communication between the immune and endothelial cells [23], which is necessary for the recruitment of other factors to facilitate diapedesis, such as intercellular adhesion molecule-1 [17,24]. Although a small amount of serum protein accompanies immune cells crossing the BBB [25], under the conditions outlined above, the BBB has been shown to remain intact [26]. When the BBB is inflamed, additional interactions occur, which will be discussed in the disease section of this review.
BBB Transporters for Cytokines
Many cytokines are capable of crossing the BBB, thus providing a direct link between the circulating and CNS compartments of the immune system [27,28]. Many of these cytokines, including interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, fibroblast growth factor, epidermal growth factor and leukemia inhibitory factor [29,30,31,32,33,34], are transported across the BBB by distinctive unidirectional saturable transport systems. Additionally, cytokines can cross from cerebrospinal fluid into blood with the reabsorption of cerebrospinal fluid; by this mechanism, the CNS can make significant contributions to blood levels of cytokines [35,36]. Only one cytokine, IL-2, has to date been found to be transported by a saturable system in the brain-to-blood direction [37].
In some cases, the transporter across the BBB has been identified. In the case of TNF, the transporters are known to be the same proteins as its receptors, with both p55 and p75 being involved in a transcytotic (i.e. vesicle-dependent) mechanism [38,39]. In other cases, such as for epidermal growth factor, the transporter protein is not the same protein as that forming the receptor [31].
Cytokine transporters at the BBB provide one mechanism by which circulating cytokines can affect the CNS. For example, transport of IL-1α into the posterior division of the septum mediates in large part the cognitive impairments associated with sickness behavior [40,41]. It is likely that IL-1α crossing into the brain from the blood both acts directly at receptors at the posterior division of the septum and also stimulates release of IL-1α and IL-1β from brain sources [42]. TNF-α transport mediates neurotoxicity at the substantia nigra, providing a pathway by which this cytokine could be involved in promoting Parkinson's disease [43]. The transport of fibroblast growth factor across the BBB explains its ability after peripheral administration to promote neurogenesis and to protect the hippocampus from ischemic injury [44,45].
Immune Events Affect BBB Transporters
Several of the classic saturable transporters at the BBB are altered or modulated by neuroimmune-related events. For example, tryptophan levels increase in the brain of animals exposed to TNF, likely because of increased transport across the BBB [46]. Insulin transport across the BBB is enhanced in mice treated with LPS and is mediated through induction of nitric oxide synthase [47]. The rate of transport of TNF-α from blood to brain is enhanced in mice with experimental allergic encephalopathy. Although these mice also have disruption of the BBB, the increased entry of TNF-α is caused by an enhancement of its saturable transporter [48]. In contrast, the transport of IL-15 is selectively abolished in the brain but not in the spinal cord of mice with experimental allergic encephalopathy [49].
Another BBB efflux transporter whose expression and function are modulated by inflammatory processes is P-glycoprotein (P-gp), a member of the ATP-binding cassette protein family [50]. P-gp is predominantly located on the luminal side of brain endothelial cells [50]; this is consistent with its primary function to restrict the passage of circulating amphipathic molecules, including many xenobiotics, into the brain [50,51]. Multiple studies reporting the effects of proinflammatory cytokines on P-gp expression and function in cultured brain endothelial cells show varying results. For example, increases, decreases or no changes in P-gp mRNA, protein expression and function have been reported for TNF-α, IL-1β and IL-6 [52,53,54,55,56,57,58,59,60]. Possible explanations for these disparate findings include the use of brain endothelial cells from different species, the use of primary versus immortalized cells and the time between cytokine application and analysis.
In support of the latter explanation, studies using freshly isolated rat brain capillaries demonstrated that TNF-α induces a time-dependent modulation of P-gp activity. An initial decrease in activity lasting for 3 h is followed by an increase in P-gp activity that doubles control values by 6 h [54,57,58]. The effects of TNF mediated directly at brain endothelial cells occur via nuclear factor (NF)-κB [61,62]. Interestingly, multiple in vitro as well as in vivo studies have also found that P-gp activity does not always correlate with protein levels of P-gp [54,58,63,64]. Furthermore, induction of systemic inflammation by intraperitoneal administration of LPS has been found to result in decreased P-gp activity [63], although another group found that this change was only significant in mice lacking the p50 subunit of NF-κB [64]. In both of these studies, P-gp protein expression was increased, further suggesting that inflammatory regulation of P-gp relies on posttranslational mechanisms. Given that decreases in P-gp activity at the BBB affect the bioavailability of many drugs in the brain [51], a better understanding of inflammatory regulation of P-gp function could have important applications in a clinical setting.
BBB Cell Secretions
Brain endothelial cells form the vascular BBB, and epithelial cells form the cerebrospinal fluid-blood barrier. Both of these cell types secrete cytokines and other substances associated with immune cell activation, such as nitric oxide and prostaglandins [52,65,66,67]. Thus, the cells that form the BBB can themselves become activated in a fashion similar to that of circulating immune cells. BBB cells secrete some cytokines constitutively, but secretion can also be induced or modulated. For example, several workers have shown that IL-6 release from brain endothelial cells is stimulated by LPS [65,67,68]. Another example is the release of endothelin-1, IL-6 and IL-8 from brain endothelium in response to HIV-1, its cell surface glycoprotein gp120 and TNF [69,70]. Release of gp120-induced endothelin-1 from brain endothelial cells is inhibited by N-acetylcysteine through a pathway dependent on mitogen- and stress-activated protein kinase [71].
A unique feature of cytokine release relies on the polarity of the brain endothelial cell, the only cell of the neuroimmune system that is simultaneously both in the CNS (the abluminal cell membrane faces brain interstitial fluid) and in the periphery (the luminal cell membrane faces the circulation). As such, the brain endothelial cell can receive input from one membrane surface that modulates the release of a cytokine from the other membrane surface. For example, LPS applied to the abluminal surface greatly increases the release of IL-6 from the luminal surface, whereas adiponectin applied to the luminal surface reduces IL-6 release from the abluminal surface [68,72]. This polarity likely underlies one mechanism by which immune signaling can be relayed between the CNS and the circulation.
Some cytokines produced by brain endothelial cells are apparently not secreted but have intracellular roles. IL-32, for example, is associated with endoplasmic reticulum and its expression is under the influence of Akt [73].
Diseases Involving the BBB and Neuroimmune Mechanisms
Immune Cell Trafficking
Under pathological conditions involving neuroinflammation, an inflamed BBB presents additional signals that lead to increased levels of immune cell recruitment to the CNS. For example, whereas initial capture of encephalitogenic T cells by a healthy BBB in EAE relies solely on the interaction between vascular cell adhesion molecule-1 and α4-integrin, interactions with the inflamed BBB persist in spite of α4-integrin blockade and likely involve a synergistic interaction with selectins [17]. Upregulation of adhesion molecules also occurs in response to increased levels of cytokines and chemokines in the brain [74,75]. Initiation of neuroinflammation by injection of cytokines into the brain also recruits immune cells to the parenchyma, but interestingly this process may be blocked if there is concurrent systemic inflammation [76]. Immune cell trafficking to the CNS has also been found to occur in other neurodegenerative conditions such as AD, Parkinson's disease and neuroAIDS and is thought to contribute to varying extents to disease progression. In Parkinson's disease, T lymphocytes are present in the midbrain, and leukocyte infiltration occurs in brains of mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [77]. Furthermore, mice deficient in CD4 are resistant to MPTP-induced neurotoxicity in the substantia nigra [77]. In the context of AD, evidence suggests that circulating immune cells of monocyte lineage enter the CNS and can effectively clear amyloid-β (Aβ) from the brain [78]. Although it has been disputed that monocyte entry into the CNS is an artifact from BBB disruption that occurs following irradiation after adoptive transfer [79,80], it is noteworthy that BBB disruption does occur in later stages of AD [81]. Therefore, additional studies are necessary to elucidate whether leukocyte trafficking occurs in earlier stages of AD when the BBB remains intact. In neuroAIDS, lentiviral-infected macrophages are recruited to the CNS, acting as Trojan horses that carry HIV across the BBB [82]. Once in the CNS, HIV causes inflammatory reactions in the brain that can result in CD8+ lymphocyte infiltration [83], as found in cases of HIV encephalitis [84]. Together, these findings suggest that immune cell trafficking across the BBB is an adaptive physiological process, but that loss of fine-tuned regulation has serious consequences which can manifest as neurodegenerative disease.
The BBB in Traumatic Brain Injury
Traumatic brain injury (TBI) is a serious condition in emergency medicine, and its pathophysiological profile is varied and complicated [85]. Neuroinflammation is an important component of TBI, contributing to many aspects of its CNS pathology. BBB disruption caused by TBI leads to neutrophil influx [86]. Leukocytes release proinflammatory cytokines, cytotoxic proteases and reactive oxygen species, in turn initiating the immune functions of native glia [87,88]. Activated glial cells, especially classically activated microglia, then propagate many of the same inflammatory processes as invading neutrophils and contribute to neuroinflammatory processes after TBI [89]. Key contributing factors to secondary brain damage are inflammation, metabolic disturbances and cerebrovascular dysfunction that further increase injury-induced tissue ischemia and brain edema resulting from BBB disruption [90]. The control of neutrophil influx following TBI might attenuate secondary brain injury. At sites of inflammation, neutrophils adhere to endothelial cells by binding to adhesion molecules such as intercellular adhesion molecule-1, vascular cell adhesion molecule and platelet endothelial cell adhesion molecule-1 [91]. The central role of matrix metalloproteinase-mediated pathological processes has been demonstrated in several models of neuroinflammation [92]. Hypoxia-inducible factor-1α, an upstream transcription factor induced by hypoxia, regulates the subsequent expression of many kinds of proteins responding to the various pathophysiological conditions induced by hypoxia [93]. Expression of matrix metalloproteinase-9 and hypoxia-inducible factor-1α is enhanced after TBI and likely plays an important role in BBB disruption, as inhibition of this expression suppresses BBB disruption and brain edema following TBI [92,94]. Symptomatic epilepsy and neurodegenerative diseases after TBI are also very serious secondary complications and affect the quality of life in TBI patients. Recently, these complications have also been thought to be closely related to neuroinflammation. A relationship between BBB dysfunction and these diseases has been reported [95]. Thus, BBB disruption and neuroinflammation are important therapeutic targets in every stage of TBI.
BBB Secretions in Disease
Secretion of cytokines from the cells that comprise the BBB may also mediate disease processes. Deane et al. [96] have outlined a pathway by which Aβ protein activates the BBB receptor transporter receptor for advanced glycation end products. In turn, the receptor for advanced glycation end products induces the release of endothelin-1 from brain endothelial cells, which then mediates vasoconstriction. Endothelin-1 release from brain endothelial cells is also induced by HIV-1 proteins [69]. The endothelin-1 is released from the abluminal side of the brain endothelial cells, thus directly accessing neurons and other cells within the CNS. Endothelin-1 levels in cerebrospinal fluid correlate with the severity of the neuroAIDS syndrome, and endothelin-1 also affects the functioning of BBB transporters, including P-gp, which regulate the retention of antiviral drugs [54,57,97,98].
BBB Transporters
The section above on transporters (“BBB Transporters for Cytokines”) has already outlined how IL transport can mediate the cognitive changes associated with sickness behavior, an adaptive mechanism that likely enhances survival in the short term [99]. That section also briefly considered work showing how transport of TNF-α can mediate toxicity at the substantia nigra and thus contribute to Parkinson's disease.
One mechanism by which neuroinflammation could contribute to AD is by altering the ability of the BBB to remove Aβ from the CNS. Low-density lipoprotein receptor-related protein-1 (LRP-1) is a multifunctional protein expressed in a variety of cell types throughout the body [100]. At the BBB, it is best characterized as an efflux transporter for Aβ [102,103,104] located at the abluminal surface [101] (fig. 2), whose accumulation in the brain is linked to AD pathogenesis [105]. Additionally, studies using animal models as well as human tissue support a role for an LRP-1 defect at the BBB in the onset and progression of AD [102,103,106]. The neurovascular hypothesis as stated by Zlokovic [107] posits that this defect in the brain-to-blood efflux of Aβ contributes to the progression of AD.
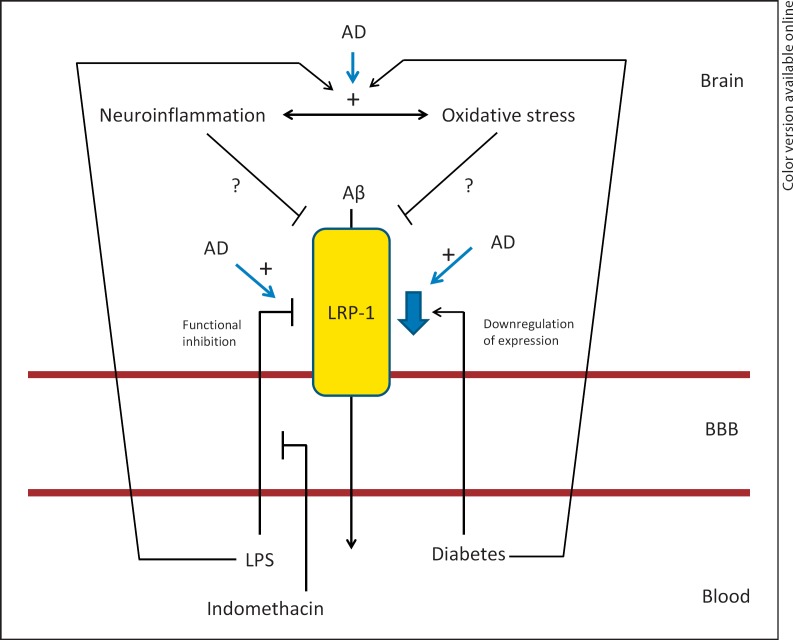
Fig. 2.
Inflammatory regulation of LRP-1. Aß is transported across the BBB in the brain-to-blood direction (efflux) by the transporter LRP-1. In AD, LRP-1 is down-regulated at the BBB and decreased efflux of Aß occurs, contributing to Aß accumulation in the CNS. Inflammation induced by peripheral administration of LPS reduces Aß efflux via LRP-1, and this is inhibited by indomethacin. Diabetes causes downregulation of LRP-1 at the BBB. Both LPS and diabetes cause inflammation and oxidative stress in the CNS, which could potentially contribute to LRP-1 dysfunction.
In addition to its dysfunction in AD, impairment of LRP-1 at the BBB occurs with systemic inflammation (fig. 2). Peripheral administration of LPS decreases the efflux of Aβ from the brain [108]. Furthermore, that study demonstrated that LRP-1 impairment was reversible when mice were treated with indomethacin prior to LPS administration. Interestingly, of 22 cytokines measured in serum in that study, none were found to correlate with the effects of indomethacin on Aβ transport following LPS administration [108]. This suggests that BBB LRP-1 dysfunction in response to LPS results from cytokines that are produced locally in the CNS and/or from autocrine/paracrine actions of other inflammatory mediators on brain endothelial cells.
AD is associated with increased neuroinflammation as well as increased oxidative stress in the CNS. Given that systemic inflammation also causes increased CNS inflammation and oxidative stress, it is possible that these processes may contribute to LRP-1 dysfunction at the BBB (fig. 2). Elevated levels of 4-hydroxy-2-nonenal (HNE)-bound LRP-1 have been found in the hippocampus of individuals with AD compared to age-matched controls [109], indicating that oxidative damage in AD contributes to LRP-1 dysfunction. Whether these changes occur at the BBB or in other cell types in the CNS has yet to be determined. Multiple cell types in the brain express LRP-1 [100], and in addition to Aβ transport, LRP-1 has other defined roles in neurons such as lipid homeostasis [110] and neuronal survival [111]. Therefore, determining which cell types contribute to observed changes in LRP-1 oxidative modifications will provide additional insight into the role of LRP-1 dysfunction in AD.
Other disease models associated with neuroinflammation, such as streptozotocin-induced diabetes [112], also cause decreases in LRP-1 expression at the BBB [113]. Together, these findings suggest that neuroinflammation as a result of multiple pathological conditions causes LRP-1 dysfunction at the BBB and therefore could contribute to the onset and progression of AD through this mechanism.
Penetration of HIV-1 and its proteins across the BBB is enhanced by treatment with LPS [114,115] and is consistent with the many interactions among HIV-1, the BBB and the neuroimmune system. As noted above, these other interactions include release of endothelin-1, a cytokine whose levels in the CNS correlate with the severity of neuroAIDS and enhanced interactions of immune cells with the BBB.
In summary, the BBB interacts in a variety of ways that connect the immune system and CNS. In some cases, the BBB separates the immune system and CNS, in other cases it acts as a mediator of neuroimmune interactions and in still other cases the BBB itself can be a target of neuroimmune interactions. These diverse interactions are not only important in normal or physiologic interactions but also in mediation of disease processes.
Acknowledgements
This work was supported by the Veterans Affairs Merit Review Program (W.A.B.) and R01 AG029839 (W.A.B.).
References
- 1.Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1 beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- 3.Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Bales RA, Ethisham A, Greenberg RN, Berger JR. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J Neurovirol. 2004;10:223–232. doi: 10.1080/13550280490463532. [DOI] [PubMed] [Google Scholar]
- 4.Chou S, Dix RD. Viral infections and the blood-brain barrier. In: Neuwelt EA, editor. Implications of the Blood-Brain Barrier and Its Manipulation, vol 2: Clinical Aspects. New York: Plenum; 1989. pp. 449–468. [Google Scholar]
- 5.Rapoport SI. Pathological Alterations of the Blood-Brain Barrier; Blood-Brain Barrier in Physiology and Medicine. New York: Raven; 1976. pp. 129–152. [Google Scholar]
- 6.Wispelwey B, Lesse AJ, Hansen EJ, Scheld WM. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest. 1988;82:1339–1346. doi: 10.1172/JCI113736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quagliarello VJ, Wispelwey B, Long WJ Jr, Scheld WM. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat: characterization and comparison with tumor necrosis factor. J Clin Invest. 1991;87:1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobiler D, Lustig S, Gozes Y, Ben Nathan D, Akov Y. Sodium dodecylsulphate induces a breach in the blood-brain barrier and enables a West Nile virus variant to penetrate into mouse brain. Brain Res. 1989;496:314–316. doi: 10.1016/0006-8993(89)91079-2. [DOI] [PubMed] [Google Scholar]
- 9.Ellison MD, Povlishock JT, Merchant RE. Blood-brain barrier dysfunction in cats following recombinant interleukin-2 infusion. Cancer Res. 1987;47:5765–5770. [PubMed] [Google Scholar]
- 10.Waguespack PJ, Banks WA, Kastin AJ. Interleukin-2 does not cross the blood-brain barrier by a saturable transport system. Brain Res Bull. 1994;34:103–109. doi: 10.1016/0361-9230(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 11.Banks WA, Kastin AJ. The interleukins-1 alpha, −1 beta, and −2 do not acutely disrupt the murine blood-brain barrier. Int J Immunopharmacol. 1992;14:629–636. doi: 10.1016/0192-0561(92)90124-4. [DOI] [PubMed] [Google Scholar]
- 12.Deli MA, Descamps L, Dehouck MP, Cecchelli R, Joo F, Abraham CS, Torpier G. Exposure of tumor necrosis factor-alpha to luminal membrane of bovine brain capillary endothelial cells cocultured with astrocytes induces a delayed increase of permeability and cytoplasmic stress fiber formation of actin. J Neurosci Res. 1995;41:717–726. doi: 10.1002/jnr.490410602. [DOI] [PubMed] [Google Scholar]
- 13.Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, Sawada Y, Kataoka Y. Transforming growth factor-beta1 upregulates the tight junction and p-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol. 2004;24:491–497. doi: 10.1023/B:CEMN.0000022776.47302.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelhardt B. The blood-central nervous system barriers actively control immune cell entry into the central nervous system. Curr Pharm Des. 2008;14:1555–1565. doi: 10.2174/138161208784705432. [DOI] [PubMed] [Google Scholar]
- 15.Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- 16.Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 17.Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNSs castle. Fluids Barriers CNS. 2011;8:4. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812:220–230. doi: 10.1016/j.bbadis.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vajkoczy P, Laschinger M, Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–565. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Manivannan A, Liversidge J, Sharp PF, Forrester JV, Crane IJ. Requirements for passage of T lymphocytes across non-inflamed retinal microvessels. J Neuroimmunol. 2003;142:47–57. doi: 10.1016/s0165-5728(03)00258-3. [DOI] [PubMed] [Google Scholar]
- 21.Doring A, Wild M, Vestweber D, Deutsch U, Engelhardt B. E- and P-selectin are not required for the development of experimental autoimmune encephalomyelitis in C57BL/6 and SJL mice. J Immunol. 2007;179:8470–8479. doi: 10.4049/jimmunol.179.12.8470. [DOI] [PubMed] [Google Scholar]
- 22.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 23.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood J, Etienne-Manneville S, Adamson P, Couraud PO. Lymphocyte migration into the central nervous system: implication of ICAM-1 signalling at the blood-brain barrier. Vascul Pharmacol. 2002;38:315–322. doi: 10.1016/s1537-1891(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 25.Toborek M, Lee YW, Flora G, Pu H, Andras IE, Wylegala E, Hennig B, Nath A. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005;25:181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 27.Banks WA. Neuroimmune networks and communication pathways: the importance of location. Brain Behav Immun. 2004;18:120–122. doi: 10.1016/j.bbi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Pan W, Kastin AJ. Cytokine transport across the injured blood-spinal cord barrier. Curr Pharm Des. 2008;14:1620–1624. doi: 10.2174/138161208784705450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the blood-brain barrier. Neurosci Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 30.Cuevas P, Fermamdez-Ayerdi A, Carceller F, Colin S, Mascarelli F, Munoz-Willery I, Gimenez-Gallego G. Central nervous system distribution of fibroblast growth factor injected into the blood stream. Neurol Res. 1996;18:267–272. doi: 10.1080/01616412.1996.11740418. [DOI] [PubMed] [Google Scholar]
- 31.Pan W, Kastin AJ. Entry of EGF into brain is rapid and saturable. Peptides. 1999;20:1091–1098. doi: 10.1016/s0196-9781(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 32.Pan W, Kastin AJ, Brennan JM. Saturable entry of leukemia inhibitory factor from blood to the central nervous system. J Neuroimmunol. 2000;106:172–180. doi: 10.1016/s0165-5728(00)00241-1. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 34.Banks WA, Kastin AJ, Durham DA. Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull. 1989;23:433–437. doi: 10.1016/0361-9230(89)90185-8. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Castro WL, Chow HH, Reichlin S. Clearance of 125I-labeled interleukin-6 from brain into blood following intracerebroventricular injection in rats. Endocrinology. 1997;138:4830–4836. doi: 10.1210/endo.138.11.5533. [DOI] [PubMed] [Google Scholar]
- 36.Chen G, Reichlin S. Clearance of [125I]-tumor necrosis factor-alpha from the brain into the blood after intracerebroventricular injection in rats. Neuroimmunomodulation. 1998;5:261–269. doi: 10.1159/000026346. [DOI] [PubMed] [Google Scholar]
- 37.Banks WA, Niehoff ML, Zalcman SS. Permeability of the mouse blood-brain barrier to murine interleukin-2: predominance of a saturable efflux system. Brain Behav Immun. 2004;18:434–442. doi: 10.1016/j.bbi.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Pan W, Kastin AJ. TNFα transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- 39.Pan W, Kastin AJ, Daniel J, Yu C, Baryshnikova LM, von Bartheld CS. TNF alpha trafficking in cerebral vascular endothelial cells. J Neuroimmunol. 2007;185:47–56. doi: 10.1016/j.jneuroim.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maness LM, Banks WA, Zadina JE, Kastin AJ. Selective transport of blood-borne interleukin-1α into the posterior division of the septum of the mouse brain. Brain Res. 1995;700:83–88. doi: 10.1016/0006-8993(95)00913-b. [DOI] [PubMed] [Google Scholar]
- 41.Banks WA, Farr SA, La Scola ME, Morley JE. Intravenous human interleukin-1α impairs memory processing in mice: dependence on blood-brain barrier transport into posterior division of the septum. J Pharmacol Exp Ther. 2001;299:536–541. [PubMed] [Google Scholar]
- 42.Rachal Pugh C, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 43.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuevas P, Carceller F, Munoz-Willery I, Gimenez-Gallego G. Intravenous fibroblast growth factor penetrates the blood-brain barrier and protects hippocampal neurons against ischemia-reperfusion injury. Surg Neurol. 1998;49:77–83. doi: 10.1016/s0090-3019(97)00193-6. [DOI] [PubMed] [Google Scholar]
- 46.Ando T, Dunn AJ. Mouse tumor necrosis factor-alpha increases brain tryptophan concentrations and norepinephrine metabolism while activating the HPA axis in mice. Neuroimmunomodulation. 1999;6:319–329. doi: 10.1159/000026391. [DOI] [PubMed] [Google Scholar]
- 47.Banks WA, Dohgu S, Nakaoke R, Lynch JL, Fleegal-DeMotta MA, Erickson MA, Vo TQ. Nitric oxide isoenzymes regulate LPS-enhanced insulin transport across the blood-brain barrier. Endocrinology. 2008;149:1514–1523. doi: 10.1210/en.2007-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan W, Banks WA, Kennedy MK, Gutierrez EG, Kastin AJ. Differential permeability of the BBB in acute EAE: enhanced transport of TNF-α. Am J Physiol. 1996;271:E636–E642. doi: 10.1152/ajpendo.1996.271.4.E636. [DOI] [PubMed] [Google Scholar]
- 49.Hsuchou H, Pan W, Wu X, Kastin AJ. Cessation of blood-to-brain influx of interleukin-15 during development of EAE. J Cereb Blood Flow Metab. 2009;29:1568–1578. doi: 10.1038/jcbfm.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10:1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 51.Schinkel AH, Wagenaar E, Mol CAAM, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandi Y, Ocsovszki I, Szabo D, Nagy Z, Nelson J, Molnar J. Nitric oxide production and MDR expression by human brain endothelial cells. Anticancer Res. 1998;18:3049–3052. [PubMed] [Google Scholar]
- 53.Fernandez C, Buyse M, German-Fattal M, Gimenez F. Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality. J Pharm Pharm Sci. 2004;7:359–371. [PubMed] [Google Scholar]
- 54.Hartz AM, Bauer B, Fricker G, Miller DS. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol. 2004;66:387–394. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- 55.Poller B, Drewe J, Krahenbuhl S, Huwyler J, Gutmann H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the blood-brain barrier. Cell Mol Neurobiol. 2010;22:63–70. doi: 10.1007/s10571-009-9431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Wedel-Parlow M, Wolte P, Galla HJ. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem. 2009;111:111–118. doi: 10.1111/j.1471-4159.2009.06305.x. [DOI] [PubMed] [Google Scholar]
- 57.Bauer B, Hartz AMS, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 58.Hartz AMS, Bauer B, Fricker G, Miller DS. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–470. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- 59.Yu C, Kastin AJ, Tu H, Waters S, Pan W. TNF activates P-glycoprotein in cerebral microvascular endothelial cells. Cell Physiol Biochem. 2007;20:853–858. doi: 10.1159/000110445. [DOI] [PubMed] [Google Scholar]
- 60.Theron D, de Lagerie SB, Tardivel S, Pelerin H, Demeuse P, Mercier C, Mabondzo A, Farinotti R, Lacour B, Roux F, Gimenez F. Influence of tumor necrosis factor-alpha on the expression and function of P-glycoprotein in an immortalized rat brain capillary endothelial cell line, GPNT. Biochem Pharmacol. 2003;66:579–587. doi: 10.1016/s0006-2952(03)00340-x. [DOI] [PubMed] [Google Scholar]
- 61.Yu C, Argyropoulos G, Zhang Y, Kastin AJ, Hsuchou H, Pan W. Neuroinflammation activates Mdr1b efflux transport through NFkappaB: promoter analysis in BBB endothelia. Cell Physiol Biochem. 2008;22:745–756. doi: 10.1159/000185558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu C, Pan W, Tu H, Waters S, Kastin AJ. TNF activates Pp-glycoprotein in cerebral microvascular endothelial cells. Cell Physiol Biochem. 2007;20:853–858. doi: 10.1159/000110445. [DOI] [PubMed] [Google Scholar]
- 63.Salkeni MA, Lynch JL, Otamis-Price T, Banks WA. Lipopolysaccharide impairs blood-brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways. J Neuroimmune Pharmacol. 2009;4:276–282. doi: 10.1007/s11481-008-9138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan W, Yu C, Hsuchou H, Kastin AJ. The role of cerebrovascular NFkappaB in LPA-induced inflammation: differential regulation of efflux transporter and transporting cytokine receptors. Cell Physiol Biochem. 2010;25:623–630. doi: 10.1159/000315081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- 66.Macvilay S, Fabry Z. TGF cytokine production by murine brain microvessel endothelial cells. Neural Notes. 1997;3:21–23. doi: 10.1006/cyto.1999.0599. [DOI] [PubMed] [Google Scholar]
- 67.Reyes TM, Fabry Z, Coe CL. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Res. 1999;851:215–220. doi: 10.1016/s0006-8993(99)02189-7. [DOI] [PubMed] [Google Scholar]
- 68.Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: a polarized response to lipopolysaccharide. Brain Behav Immun. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Didier N, Banks WA, Creminon C, Dereuddre-Bosquet N, Mabondzo A. HIV-1-induced production of endothelin-1 in an in vitro model of the human blood-brain barrier. Neuroreport. 2002;13:1179–1183. doi: 10.1097/00001756-200207020-00022. [DOI] [PubMed] [Google Scholar]
- 70.Yang B, Akhter S, Chaudhuri A, Kanmogne GD. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood-brain barrier: modulatory effects of STAT1 signaling. Microvasc Res. 2009;77:212–219. doi: 10.1016/j.mvr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sury MD, Frese-Schaper M, Muhlemann MK, Schulthess FT, Blasig IE, Tauber MG, Shaw SG, Christen S. Evidence that N-acetylcysteine inhibits TNF-alpha-induced cerebrovascular endothelin-1 upregulation via inhibition of mitogen- and stress-activated protein kinase. Free Radic Biol Med. 2006;41:1372–1383. doi: 10.1016/j.freeradbiomed.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 72.Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, Pfeiffer A, Hileman SM, Tschop M, Banks WA. Adiponectin does not cross the blood-brain barrier, but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–147. [PubMed] [Google Scholar]
- 73.Kobayashi H, Lin PC. Molecular characterization of IL-32 in human endothelial cells. Cytokine. 2009;46:351–358. doi: 10.1016/j.cyto.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dietrich JB. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J Neuroimmunol. 2002;128:58–68. doi: 10.1016/s0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- 75.Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- 76.Ching S, Zhang H, Lai W, Quan N. Peripheral injection of lipopolysaccharide prevents brain recruitment of leukocytes induced by central injection of interleukin-1. Neuroscience. 2006;137:717–726. doi: 10.1016/j.neuroscience.2005.08.087. [DOI] [PubMed] [Google Scholar]
- 77.Chung YC, Ko HW, Bok E, Park ES, Huh SH, Nam JH, Jin BK. The role of neuroinflammation on the pathogenesis of Parkinson's disease. BMB Rep. 2010;43:225–232. doi: 10.5483/bmbrep.2010.43.4.225. [DOI] [PubMed] [Google Scholar]
- 78.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 79.Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turrin NP, Plante MM, Lessard M, Rivest S. Irradiation does not compromise or exacerbate the innate immune response in the brains of mice that were transplanted with bone marrow stem cells. Stem Cells. 2007;25:3165–3172. doi: 10.1634/stemcells.2007-0508. [DOI] [PubMed] [Google Scholar]
- 81.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;64:133–145. doi: 10.1016/j.neuron.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167:5429–5438. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- 84.Petito CK, Torres-Munoz JE, Zielger F, McCarthy M. Brain CD8+ and cytotoxic T lymphocytes are associated with, and may be specific for, human immunodeficiency virus type 1 encephalitis in patients with acquired immunodeficiency syndrome. J Neurovirol. 2006;12:272–283. doi: 10.1080/13550280600879204. [DOI] [PubMed] [Google Scholar]
- 85.Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 2006;148:255—268. doi: 10.1007/s00701-005-0651-y. discussion 268. [DOI] [PubMed] [Google Scholar]
- 86.Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol. 1997;61:279–285. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- 87.Morganti-Kossmann MC, Bye N, Nguyen P, Kossmann T. Influence of brain trauma on blood-brain barrier properties. In: De Vries E, Prat A, editors. The Blood Brain Barrier and Its Microenvironment: Basic Physiology to Neurological Disease. New York: Taylor & Francis; 2005. pp. 457–479. [Google Scholar]
- 88.Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 89.Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S, Shioda S, Aruga T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation. 2010;7:41. doi: 10.1186/1742-2094-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Margulies S, Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clausen F, Lorant T, Lewen A, Hillered L. T lymphocyte trafficking: a novel target for neuroprotection in traumatic brain injury. J Neurotrauma. 2007;24:1295–1307. doi: 10.1089/neu.2006.0258. [DOI] [PubMed] [Google Scholar]
- 92.Nag S, Manias JL, Stewart DJ. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol. 2009;118:197–217. doi: 10.1007/s00401-009-0541-0. [DOI] [PubMed] [Google Scholar]
- 93.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 94.Higashida T, Kreipke CW, Rafols JA, Peng C, Schafer S, Schafer P, Ding JY, Dornbos D 3rd, Li X, Guthikonda M, Rossi NF, Ding Y. The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg. 2011;114:92–101. doi: 10.3171/2010.6.JNS10207. [DOI] [PubMed] [Google Scholar]
- 95.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deane R, Yan SD, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;7:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 97.Rolinski B, Heigermoser A, Lederer E, Bogner JR, Loch O, Goebel FD. Endothelin-1 elevated in the cerebrospinal fluid of HIV-infected patients with encephalopathy. Infection. 1999;27:244–247. doi: 10.1007/s150100050020. [DOI] [PubMed] [Google Scholar]
- 98.Kim RB, Fromm MF, Wandel C, Leake B, Wood AJJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(suppl 1):S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 100.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid beta-peptide elimination from the brain. J Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer's amyloid-β1-40 peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 104.Jaeger LB, Dohgu S, Hwang MC, Farr SA, Murphy MP, Fleegal-DeMotta MA, Lynch JL, Robinson SM, Niehoff ML, Johnson SN, Kumar VB, Banks WA. Testing the neurovascular hypothesis of Alzheimer's disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J Alzheimers Dis. 2009;17:553–570. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 106.Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic BV. Substitution at codon 22 reduces clearance of Alzheimer's amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging. 2002;23:405–412. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 107.Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 108.Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfiled DA, Banks WA. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer's disease. Brain Behav Immun. 2009;23:507–517. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Owen JB, Sultana R, Aluise CD, Erickson MA, Price TO, Bu G, Banks WA, Butterfield DA. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free Radic Biol Med. 2010;49:1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, Bao J, Han X, Weeber EJ, Bu G. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci. 2010;30:17068–17078. doi: 10.1523/JNEUROSCI.4067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fuentealba RA, Liu Q, Kanekiyo T, Zhang J, Bu G. Low density lipoprotein receptor-related protein 1 promotes anti-apoptotic signaling in neurons by activating Akt survival pathway. J Biol Chem. 2009;284:34045–34053. doi: 10.1074/jbc.M109.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ugochukwu NW, Mukes JD, Figgers CL. Ameliorative effects of dietary caloric restriction on oxidative stress and inflammation in the brain of streptozotocin-induced diabetic mice. Clin Chim Acta. 2006;370:165–167. doi: 10.1016/j.cca.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 113.Hong H, Liu LP, Liao JM, Wang TS, Ye FY, Wu J. Downregulation of LRP1 at the blood-brain barrier in streptozotocin-induced diabetic mice. Neuropharmacology. 2009;56:1054–1059. doi: 10.1016/j.neuropharm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 114.Banks WA, Kastin AJ, Brennan JM, Vallance KL. Adsorptive endocytosis of HIV-1gp120 by blood-brain barrier is enhanced by lipopolysaccharide. Exp Neurol. 1999;156:165–171. doi: 10.1006/exnr.1998.7011. [DOI] [PubMed] [Google Scholar]
- 115.Dohgu S, Banks WA. Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by the p38 mitogen-activated protein kinase pathway. Exp Neurol. 2008;210:740–749. doi: 10.1016/j.expneurol.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]