Abstract
Mechanical factors play a crucial role in the development of articular cartilage in vivo. In this regard, tissue engineers have sought to leverage native mechanotransduction pathways to enhance in vitro stem cell-based cartilage repair strategies. However, a thorough understanding of how individual mechanical factors influence stem cell fate is needed to predictably and effectively utilize this strategy of mechanically-induced chondrogenesis. This article summarizes some of the latest findings on mechanically stimulated chondrogenesis, highlighting several new areas of interest, such as the effects of mechanical stimulation on matrix maintenance and terminal differentiation, as well as the use of multifactorial bioreactors. Additionally, the roles of individual biophysical factors, such as hydrostatic or osmotic pressure, are examined in light of their potential to induce mesenchymal stem cell chondrogenesis. An improved understanding of biomechanically-driven tissue development and maturation of stem cell-based cartilage replacements will hopefully lead to the development of cell-based therapies for cartilage degeneration and disease.
Keywords: Osteoarthritis, Cartilage defect, Scaffold, Tissue engineering, Mesenchymal stem cell, Adipose stem cell, Regeneration, Synovial joint, Ion channel, Mechanical signal transduction
Introduction
Musculoskeletal tissues are highly sensitive to their mechanical environment, allowing them to adapt to the physical demands of their environment. Mechanical factors can influence the structure and function of these tissues at all stages of life, including development, growth, remodeling, injury and disease, and repair [1-4]. In particular, chondrocytes and chondroprogenitors have been shown to transduce and respond to a wide array of mechanical stimuli both during development as well as throughout adulthood, including deformation, shear, fluid flow, streaming potentials, hydrostatic pressure, and osmotic pressure [1,2,5-7].
Similarly, there is growing interest in understanding the mechanobiology of multipotent stem cells, which are abundant, expandable, and available from various tissue depots including bone marrow, fat, and synovium [8-10]. The cells are capable of chondrogenic differentiation, and provide a potential cell source for the regeneration and replacement of damaged articular cartilage resulting from injury or diseases such as osteoarthritis [11-14]. However, an effective cell-based tissue replacement requires a stably differentiated cell population capable of producing and maintaining a functional neo-tissue. There is great interest in leveraging these native mechanical and biophysical cues to enhance the current strategies for stem cell-based cartilage tissue repair. This review summarizes recent progress on the use of mechanical and biophysical signals to influence the chondrogenic differentiation of stem cell-based cartilage replacements. Furthermore, we discuss several candidate transduction mechanisms that may play a role in the process of biomechanically-induced chondrogenesis, in the hope that a better understanding of the pathways behind the mechanosensitivity of stem cells will lead to more practical, reliable, and effective methods of leveraging these pathways for cartilage tissue engineering [15].
Mechanical stimulation of chondrogenesis
The current literature on in vitro mechanical stimulation of chondrogenesis encompasses a broad variety of scaffolds, cell types, and bioreactors, making it challenging to draw definitive conclusions across studies. This section focuses primarily on the most common model system of dynamic compressive loading of bone marrow-derived mesenchymal stem cells (MSCs) supported by synthetic foam and/or hydrogel scaffolds (summarized in Table 1). Of course, future comparative analyses of studies involving other bioreactors and scaffold systems will probably be needed to further understand the mechanisms behind mechanical signals and chondrogenesis. Furthermore, investigations into the mechanical responses of stem cells sourced from other tissues of interest (adipose, synovium) will also be necessary to move them towards clinical applications, as it is currently unclear whether these stem cells utilize the same mechanisms of mechanical signal transduction as bone marrow-derived MSCs.
Table 1.
Summary of recent dynamic compressive loading studies evaluating chondrogenesis in mesenchymal stem cells
| Bioreactor | Compressive loading regimen | Scaffold | Species (age) | Medium supplementation | Preculture (days) | Reference |
|---|---|---|---|---|---|---|
| Compression |
1 Hz, 10%, 1 to 20 hours |
Agarose |
Bovine |
± Dex ± TGFβ1 |
8 or 16 |
[16] |
| Compression |
0.33 to 3 Hz, 10%, 1 to 3 hours |
Agarose |
Bovine (3 to 6 months) |
Dex ± TGFβ3 |
3 |
[17] |
| Compression (semipermeable) |
0.3 Hz, 7.5%, 45 minutes on/45 minutes rest |
Agarose |
Horse (2 to 5 years) |
Dex ± TGFβ1 |
0 |
[18] |
| Compression |
0.01 to 1 Hz, 10%, 1 to 4 hour/day |
Agarose |
Bovine (3 to 6 months) |
Dex ± TGFβ3 |
0 or 21 |
[19] |
| Compression + shear |
0.1 to 1 Hz, 5 to 20%, 1 hour/day |
Fibrin/PU |
Human |
Dex ± TGFβ1 |
7 |
[20] |
| Compression |
1 Hz, 10%, 1 hour/day |
Agarose |
Porcine (4 months) |
Dex ± TGFβ3 |
0 or 21 |
[21] |
| Compression + shear |
1 Hz, 10 to 20%, 1 hour/day |
Fibrin/PU |
Human |
None |
2 to 4 |
[22] |
| Compression |
1 Hz, 10%, 1 hour |
Agarose |
Porcine (4 months) |
Dex + TGFβ3 |
0, 7, 14, 21 |
[23] |
| Compression + shear |
0.1 Hz, 5 to 20%, 3 hours/day |
Agarose |
Bovine (3 to 6 months) |
Dex ± TGFβ3 |
21 |
[24] |
| Compression | 1 Hz, 10%, 4 hours/day | Hyaluronic acid | Human | Dex + TGFβ1 | 3 | [25] |
Dex, dexamethasone; PU, polyurethane; TGFβ, transforming growth factor beta.
Mechanical stimulation can directly influence the fate of undifferentiated stem cells [1-3,5-7,26]. Dynamic compressive loading, specifically cyclic unconfined compression, has been one of the most highly utilized model systems of mechanical stimulation in cartilage tissue engineering and mechanobiology [7]. This system has also been used to investigate the potential of mechanical stimulation for use in MSC-based cartilage regeneration and repair, and the results of these studies can be summarized by four main findings: growth factor treatment is a more potent stimulus than mechanical stimulation for initiating MSC differentiation; provided there is a period of predifferentiation and other specific loading parameters, mechanical stimulation can be effective at enhancing growth-factor induced MSC differentiation and tissue neo-formation; the mechanoresponsiveness of differentiated MSC constructs appears to persist over time to direct matrix remodeling and maintain a stable chondrogenic phenotype; and bioreactors that impart multifactorial mechanical stimulation, such as compression–shear loading, further enhance mechanically-induced chondrogenesis.
Mechanically-induced chondrogenesis in the absence of exogenous growth factors
In general, the effects of dynamic compressive loading alone (that is, in the absence of exogenous growth factors) on MSC chondrogenesis appear to be minimal and transient, particularly in comparison with growth factor treatment alone. For example, although dynamic loading of MSC-laden constructs increased aggrecan promoter activity and sulfated glycosaminoglycan (sGAG) accumulation, exposure to transforming growth factor beta (TGFβ) alone led to far greater sGAG accumulation compared with disks loaded in the absence of growth factors [17]. Kisiday and colleagues similarly observed that while loading without growth factors led to increased sGAG compared with the unloaded control, TGFβ alone led to a much greater increase in sGAG content, as well as an increase in collagen content [18]. The chondrogenic effects of loading on MSCs in the absence of growth factors also appear to be transient. For example, while loading in the absence of growth factors increased Col2α1 and aggrecan gene expression after 1 and 2 weeks of loading, expression of these chondrogenic markers returned to baseline levels after an additional week of continued loading [19].
Primary chondrocytes, in comparison, maintain a stable chondrogenic phenotype in three-dimensional culture, and can produce an appreciable amount of functional matrix [27,28] even in the absence of growth factors or serum [29,30]. However, the literature on the effects of loading on chondrocytes cultured in growth factor and serum-free conditions is limited, and therefore difficult to compare with what is known about growth factor-free and serum-free loading of MSC-laden constructs. Loading of chondrocyte-laden disks in the presence of low levels (0.2 and 2%) of fetal bovine serum produced no change in sGAG production, but decreased functional properties of the constructs [31]. In contrast, a large positive effect on matrix accumulation and functional properties was observed in another study with chondrocyte-laden constructs loaded in serum-free conditions [30]. Interestingly, both bovine MSCs and chondrocytes embedded in agarose and precultured for 3 days in growth-factor-free medium were able to respond to dynamic loading with an increase in aggrecan promoter activity [17], suggesting that a similar mechanism of mechanotransduction in chondrocytes may be present, to some degree, in undifferentiated stem cells. Nevertheless, in the case of MSCs, dynamic compressive loading alone appears to be insufficient for inducing appreciable differentiation and matrix production in the absence of growth-factor stimulation, and therefore is not, as yet, a suitable substitute for growth-factor-induced stem cell differentiation.
Culture conditions that support mechanically-induced chondrogenesis
Dynamic loading in the presence of growth factors can significantly enhance MSC chondrogenic differentiation, particularly if a chondrogenic preculture period is provided. For example, while loading of MSCs after 8 days of preculture in TGFβ and dexamethasone-supplemented medium did not increase aggrecan or Col2α1 gene expression or sGAG or protein synthesis, loading after an additional 8 days (16 days in total) of preculture increased all of these measures [16]. Of note, the effects of loading were more limited when dexamethasone was not added. By preculturing constructs for 0, 7, 14, and 21 days in the presence of TGFβ and dexamethasone and then assessing the gene response immediate following 1 hour of loading, Haugh and colleagues observed that generally later time points (7 and 21 days) of preculture resulted in the greatest relative increases in core aggrecan and Col2α1 expression compared with unloaded controls [23]. Likewise, the annular cell population also demonstrated a dependence on 14 or 21 days of preculture to exhibit a loading-induced enhancement of Col2α1 and aggrecan expression. In a similarly designed study, loading was initiated both immediately following construct creation as well as after 3 weeks of preculture, all in the presence of TGFβ and dexamethasone. While continuous loading elicited negative effects on DNA, sGAG, and collagen content, 3 weeks of chondrogenic preculture completely abrogated these negative effects, as well as leading to improvements in functional properties and extracellular matrix distribution (Figure 1) [19]. Yet another study observed similar detrimental effects of loading without preculture on sGAG content and dynamic modulus, as well the attenuation of this negative effect with delayed loading [21].
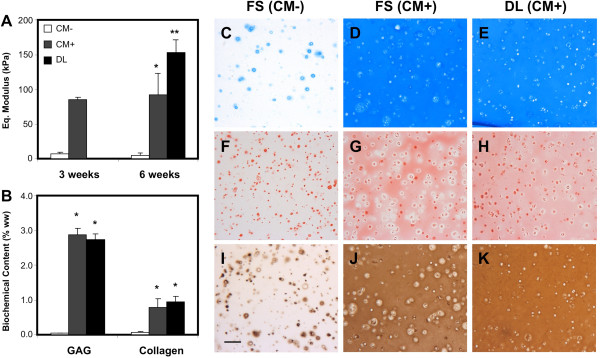
Figure 1.
Delayed dynamic compressive loading improves mechanical properties and extracellular matrix distribution without increasing biochemical content. Following 3 weeks of chondrogenic preculture, dynamic compressive loading was applied daily to human mesenchymal stem cell (MSC)–agarose constructs for 3 weeks. (A) The equilibrium modulus of MSC-seeded constructs was higher in medium containing transforming growth factor beta (TGFβ; CM+) compared with culture without TGFβ (CM–) at 3 and 6 weeks; dynamic loading (DL) in CM + for 3 weeks further improved mechanical properties. (B) Biochemical content of dynamically loaded constructs at week 6 was not different compared with CM + controls. (C) to (E) Alcian Blue staining at week 6 showed equal distribution of proteoglycans between CM + controls and loaded constructs with weak staining in CM– controls. (F) to (H) Picrosirius Red staining and (I) to (K) collagen type II immunostaining showed more homogeneous distribution of collagen in loaded constructs compared with controls. Scale bar: 100 μm. *Greater than CM– controls (P <0.05). **Greater than CM + controls (P <0.05). FS, free swelling. Reproduced from [19] with kind permission from eCM journal [32].
Beyond the application of growth factors and the duration of preculture, the response of MSCs to dynamic loading has been found dependent on a number of other factors as well, including duty cycle and loading frequency. An extended loading regime totaling 12 hours of loading per day reduced sulfate and protein incorporation [18]. A separate study observed no effect of 20 hours of continuous loading on sGAG or protein synthesis after an 8-day preculture [16]. Interestingly, significant increases in these measures were observed after 16 days of chondrogenic preculture, indicating a dependence on differentiation status and construct maturation on the response to mechanical factors. The frequency of dynamic loading is also a critical parameter that may influence chondrogenic responses. For example, increases in the functional properties of MSC-laden constructs were observed with 1 Hz dynamic compressive loading, but were absent at lower frequencies of mechanical stimulation [19]. Given that chondrocytes also exhibit a high sensitivity to duty cycle and loading frequency [33,34], further optimization of loading parameters may be able to improve mechanically-driven MSC chondrogenesis and matrix accumulation.
Mechanical regulation of neo-cartilage maintenance and turnover
While current methods of mechanical stimulation do not appear to have an especially large effect on bulk matrix accumulation during growth factor-induced maturation of MSC-laden constructs, there have been stronger indications for the importance of mechanical signals on MSC-based neo-cartilage maintenance. Normal matrix metabolism, such as aggrecan turnover [35] and collagen reorganization and remodeling in response to loading, has probably been an underappreciated metric for cartilage tissue engineering. For example, a gene array analysis found that 413 genes associated with chondrogenesis were upregulated, versus 139 that were downregulated, with 3 weeks of dynamic loading of MSC constructs that had been precultured for 3 weeks [19]. Furthermore, mechanical loading differentially regulated genes specifically involved in matrix remodeling and organization, such as matrix metalloproteinases, tissue inhibitors of metalloproteinases, and cross-linking proteins, suggesting that this coordinated response may have led to the enhanced pericellular and extracellular matrix distribution and organization, as well as the improved functional properties, observed in the loaded constructs [19]. Again, we can compare this matrix remodeling response of MSCs with the native response of chondrocytes undergoing delayed, long-term loading, where functional property increases are elicited with delayed loading in the absence of any changes in bulk matrix content [36]. Indeed, the similarity of MSCs to chondrocytes in terms of their long-term response to loading may represent an additional characteristic of the sustained chondrogenic differentiation in this system. However, since the conventional assays performed in the majority of cartilage mechanobiology and tissue engineering papers are largely insensitive to long-term matrix remodeling and turnover, more work will be needed to confirm mechanically-driven matrix remodeling and maintenance in MSC-laden constructs.
Mechanical stimulation and the maintenance of chondrogenesis
Another critical issue of long-term MSC culture is the ossification and hypertrophic differentiation of chondrogenically-induced MSC constructs upon implantation [37]. Establishing a stable, articular chondrocytic phenotype is therefore another critical design goal of cartilage tissue engineering. Col1 gene expression is one marker of hypertrophic or osteogenic differentiation, and has been examined in response to loading [23,25]. For example, the accumulation of Col1 in the annulus of constructs was found to decrease with loading after 21 days of preculture [23], while other studies showed that dynamic compressive loading suppressed a number of other hypertrophic markers such as collagen type 10, matrix metalloproteinase-13, and alkaline phosphatase gene expression, as well as the calcium content of constructs exposed to hypertrophic factors [25]. Few studies have examined the effects of loading on terminal differentiation of chondrogenically-induced MSCs, so these preliminary findings will need to be followed up with more comprehensive studies.
Multimodal bioreactors for enhancing mechanically-induced chondrogenesis
Mechanical loading of the joint produces a complex environment in articular cartilage in vivo, consisting of a diverse array of tensile, shear, and compressive stresses and strains, in addition to other physicochemical effects [7,38]. Accordingly, bioreactors that impose additional components of mechanical stimulation have been examined for their potential to further induce and support chondrogenic differentiation. Adding a component of shear to compressive loading has been shown to be superior to single-factor loading regimens at inducing matrix biosynthesis in chondrocytes [39], and this multifactorial strategy has more recently been applied to the study of MSC chondrogenesis. For example, a compression–shear bioreactor consisting of a rotating ceramic ball in contact with the surface of a construct that also transverses perpendicular to the surface was shown to enhance both Col2α1 and aggrecan expression in the absence of exogenous growth factors, and also increased sGAG production, with or without exogenous TGFβ [20]. In addition, endogenous production of TGFβ was induced by this compression–shear loading in the absence of exogenous growth factors. Furthermore, a synergistic effect of compression and shear on Sox9 and Col2α1 upregulation was observed (Figure 2) when this system was used to compare the effects of compression–shear with each component individually [22].
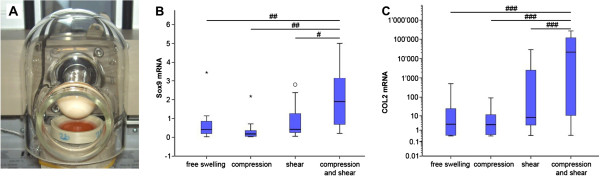
Figure 2.
Mechanical loading using a multimodal bioreactor enhances mesenchymal stem cell chondrogenesis. (A) The bioreactor can apply both compression and shear to the cell-seeded construct through rotation of the ceramic hip ball in contact with the surface of the construct and through vertical movement of the ball perpendicular to the construct surface. Relative (B) Sox9 and (C) Col2 mRNA expression of human mesenchymal stem cells after culture for 21 days in fibrin/polyurethane constructs without exogenous growth factors. Although either compression or shear loading alone increased these chondrogenic markers above free swelling levels, the combination of shear and compression loading further enhanced the response. #P <0.05, ##P <0.01, ###P <0.001. Reproduced from [22] with kind permission from eCM journal [32].
In other studies, another compression plus shear bioreactor, utilizing a spherical indenter that travels across the surface of the construct, enhanced Col2α1 and aggrecan expression, in the presence or absence of TGFβ, after 21-day chondrogenic preculture [24]. Furthermore, 21 days of additional slide-contact loading led to increases in sGAG and collagen content, a more uniform collagen distribution, and enhanced tensile properties. In addition, loaded constructs demonstrated a depth-dependent distribution of sGAG. In addition to their ability to promote anisotropy that is more similar to that of native cartilage, multifactorial bioreactors will help to further elucidate the roles of different mechanical stimuli, as well as their interactions. These systems also move a step closer to recreating the in vivo physical environment, and therefore may provide a system for predicting how cartilage tissue replacements will perform within a joint.
Mechanisms of mechanically-induced chondrogenesis
Physical transduction of mechanical loading
There are obvious practical limitations to mechanically conditioning anatomically-shaped tissue replacements, including the spatial constraints and inhomogeneity of the stress and strain fields. Furthermore, these inhomogeneities can also act to obscure the detailed understanding of the cellular effects on mechanical signals. As such, there is a growing interest in studying the individual biophysical components present during deformational loading, which practically are much simpler to deliver in a repeatable and uniform manner. A thorough understanding of the mechanisms by which individual components of mechanical stimulation leads to cartilage development, maintenance, and disease should also allow for more practical and predicable strategies for enhancing the maturation of stem cell-based cartilage replacement tissues.
Joint loading leads to complex tissue strains, including components of compression, tension, and shear, producing direct cellular and nuclear deformation [40]. In addition, indirect biophysical factors are also generated (Figure 3) as a result of the exudation of interstitial water and ions from cartilage, including streaming potentials, changes in local pH and osmolarity, and hydrostatic pressure [38]. While application of dynamic compression to isolated chondrocytes or MSCs seeded into hydrogels or polymeric scaffolds will recapitulate many of these biophysical changes that occur in native cartilage, it is important to appreciate that the amount of extracellular matrix relative to the original scaffold or hydrogel present within the constructs, as well as the mechanical properties of these scaffolds, will influence the range of biophysical stimuli generated by loading (for example [41]). A number of studies have examined the response of chondrocytes and MSCs to individual biophysical stimuli generated during loading of intact articular cartilage. In this respect, future studies comparing donor-matched responses among chondrocytes, undifferentiated MSCs, and differentiated MSCs to these stimuli would be highly useful in further elucidating the mechanisms involved in mechanotransduction in different cell types.
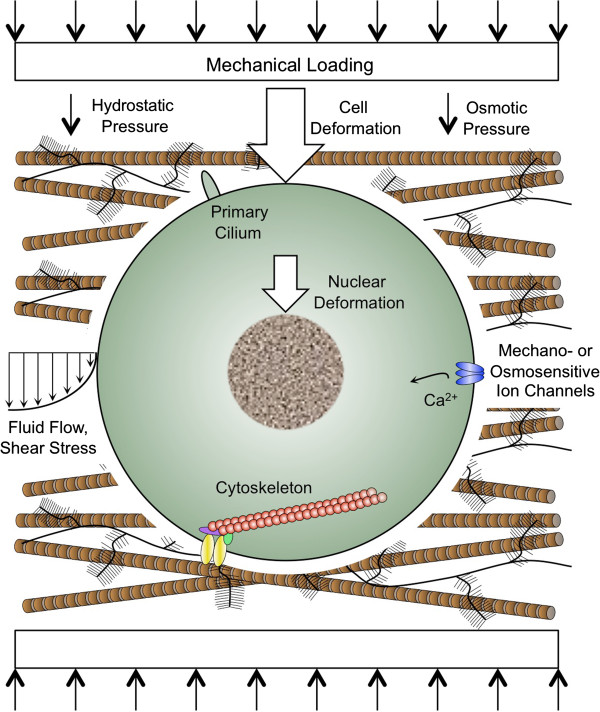
Figure 3.
Mechanisms of mechanically-induced chondrogenesis. Joint loading produces complex tissue strains, which lead to direct cellular and nuclear deformation, and generates indirect biophysical factors, including osmotic and hydrostatic pressure and fluid flow. Mechanical loading of isolated chondrocytes or mesenchymal stem cells (MSCs) seeded into hydrogels or polymeric scaffolds may recapitulate many of the changes that occur in native cartilage. Candidate mechanical signal transducers in chondrocytes and MSCs include ion channels, the primary cilium, the nucleus, and the cytoskeleton.
Cell deformation
Mechanical loading of hydrogel scaffolds results in the transmission of strains to cells embedded within such constructs [28,42]. The relationship between ECM and cell-level strains in agarose-laden chondrocytes is also comparable with that of in situ chondrocytes in loaded cartilage explants [43] once a pericellular matrix has been elaborated. Lee and Bader observed that a 20% strain of day 0 chondrocyte-laden agarose constructs led to supraphysiological cell strains in chondrocytes cultured with or without serum, but that after 3 and 6 days of preculture the constructs cultured specifically with serum exhibited reduced cell strains, which were more similar with what occurs in situ[28]. They attributed this finding to enhanced pericellular matrix accumulation in the constructs cultured with serum, which was associated with increased pericellular sGAG staining with preculture. The requirement of a preculture period as well as growth factor treatment to facilitate loading-induced enhancement of MSC chondrogenesis suggests that the pericellular matrix is also an important transducer of deformational compressive loading in stem cells.
Dynamic compressive loading of MSCs in agarose and similarly compliant scaffolds would also be expected to induce cellular and nuclear deformation, and further investigation into the mechanical properties of stem cells, stem cell nuclei, and the neo-pericellular matrix prior to, during, and after stem cell differentiation may help elucidate the roles of cell and nuclear deformation in the transduction of compressive loading. Unconfined compression also allows for radial expansion, and generates a degree of radial and circumferential tensile strains. Tensile loading also modulates stem cell differentiation, although this loading regime in isolation appears to stimulate a fibrochondrocyte phenotype [44-46].
Hydrostatic pressure
While peak physiologic levels of fluid pressurization in articular cartilage are in the order of 10 to 20 MPa [47,48], compression of agarose hydrogels is predicted to generate only around 0.5 kPa of hydrostatic pressure, due to the scaffold’s high porosity [17]. With sufficient matrix elaboration, however, the fluid pressurization of agarose cylinders subjected to dynamic compressive loading [49] is predicted to resemble the increase in native loaded cartilage [50]. Nonetheless, fluid pressurization, even at high levels, is expected to induce little or no deformation of tissues and cells, due to the intrinsic incompressibility of water and the cartilage extracellular matrix [51]. To evaluate fluid pressurization as an isolated mechanical stimulus, in vitro bioreactors have been developed that directly pressurize the culture medium surrounding cell-seeded constructs, in the absence of cell or tissue deformation [6]. Alternatively, bioreactors can pressurize the gas phase above the culture medium, but one limitation of these types of bioreactors is that the partial pressures of gas in the culture medium are affected, and these bioreactors are less commonly used. Studies using systems that directly pressurize the culture medium have shown that isolated chondrocytes are responsive to hydrostatic pressure. Acute application of static hydrostatic pressure at 5 MPa for 4 hours enhanced Col2α1 and aggrecan expression by chondrocytes in agarose gels [52], while application of both dynamic and static hydrostatic pressure at 10 MPa to scaffoldless chondrocyte constructs for 1 hour/day on days 10 to 14 of culture led to increased sGAG production and compressive stiffness at day 28 [53].
Applying dynamic hydrostatic pressurization (3 to 10 MPa, 1 Hz) to human MSCs either seeded within scaffolds or in pellet culture in the presence of TGFβ increased expression of cartilage extracellular matrix genes and enhanced biochemical content compared with TGFβ alone [54-56]. In these studies, hydrostatic pressure was applied by directly pressurizing the culture medium for 1 to 4 hours/day beginning in the first week of culture, indicating that the MSC response to hydrostatic loading does not require a preculture period. Miyanishi and colleagues examined the dose dependency of hydrostatic loading with TGFβ supplementation, and found that while 0.1 MPa was sufficient to increase Sox9 expression, upregulation of Col2α1 expression only occurred with loading at 10 MPa [57]. Hydrostatic pressure also transiently increased cartilage-associated genes in the absence of TGFβ [55,58,59]. Recent studies with rat MSCs cultured in alginate applied hydrostatic pressure following an initial 8-day preculture in chondrogenic medium including TGFβ. Dynamic hydrostatic pressure applied by pressurization of the gas phase above the culture medium for 7 days at 13 to 36 kPa and 0.25 Hz – parameters lower than in previous studies – increased expression of Col2α1 and aggrecan, as well as sGAG accumulation, both in the absence and presence of TGFβ [60,61]. Furthermore, in the absence of exogenous TGFβ, hydrostatic pressure increased expression and secretion of TGFβ1, as well as the phosphorylation of Smad2/3 and p38 mitogen-activated protein kinase. However, pharmacologic inhibition of TGFβ signaling only modestly reduced the upregulation of Col2α1 by loading and had no influence on the upregulation of aggrecan by loading, suggesting the involvement of other signaling pathways in mediating the response to hydrostatic pressure [61].
Osmotic pressure
Healthy articular cartilage has an interstitial osmolarity ranging from 350 to 450 mOsm due to the high concentration of negatively charged proteoglycans in the tissue, which attracts counterions [62]. Extracellular matrix production by articular chondrocytes has been shown to be sensitive to the medium osmolarity. Culture for 48 hours in 550 mOsm medium increased sGAG synthesis by chondrocytes in alginate beads relative to culture in 380 mOsm medium, while culture in 270 mOsm medium decreased sGAG synthesis [63]. Chondrocytes cultured in medium at 370 mOsm for 6 days exhibited the greatest sGAG accumulation and sGAG synthesis by chondrocytes in alginate compared with culture in medium with either higher or lower osmolarity [64]. Recent longer-term studies have indicated that neo-tissue formation by articular chondrocytes in hydrogel systems is influenced by osmolarity of the culture medium, but the results have been contradictory [65,66]. Freshly isolated chondrocytes in alginate accumulated less sGAG at 270 mOsm compared with osmolarities ranging from 380 to 550 mOsm [65], while culture-expanded chondrocytes produced neo-tissue with superior mechanical properties when cultured in agarose at 300 mOsm compared with 400 mOsm [66].
Effects of osmolarity on extracellular matrix production may be due in part to regulation of the chondrocyte transcription factor Sox9. Treatment of freshly isolated chondrocytes from osteoarthritic human articular cartilage with hyperosmotic medium (550 mOsm vs. 380 mOsm) led to an increase in the levels of Sox9 mRNA and protein, an effect mediated in part by an increase in the half-life of Sox9 mRNA with hyperosmotic exposure [67]. However, the level of Col2α1 mRNA and its half-life were decreased by exposure to hyperosmotic conditions. Hyperosmotic medium also increased phosphorylation of p38 mitogen-activated protein kinase, and induction of Sox9 mRNA by hyperosmotic treatment was disrupted in the presence of a pharmacologic inhibitor to p38 mitogen-activated protein kinase. A similar study in equine articular chondrocytes showed that hyperosmotic treatment had varying effects on Sox9 mRNA levels dependent on whether treatment was applied in a static or cyclic manner and whether chondrocytes were from normal or osteoarthritic cartilage [68].
In these studies with isolated chondrocytes, the osmolarity of the culture medium was kept constant. However, articular chondrocytes in situ are exposed to cyclic changes in osmolarity due to joint loading and unloading during normal daily activity. Compression of articular cartilage causes extrusion of water relative to solutes due to fixed charges on the sulfated GAG chains, which leads to an increase in tissue osmolarity. High-frequency loading, such as walking, as well as prolonged joint loading resulting in diurnal strains [69], will produce a dynamic osmotic environment on the time scale ranging from seconds to hours. Similar to hydrostatic pressure, the osmotic changes in chondrocyte and MSC-laden constructs in response to dynamic compressive loading should be minimal initially, but should increase with sGAG accumulation. Although there is evidence that dynamic hypotonic loading at 0.1 Hz may enhance cartilage matrix gene expression in chondrocytes in monolayer culture after 2 hours of loading [70], little is known regarding the long-term effects of dynamic or repetitive daily osmotic loading on neo-tissue matrix content and mechanical properties.
Growth and chondrogenic differentiation of MSCs are also influenced by culture medium osmolarity. High-osmolarity medium (485 mOsm) reduced proliferation of both rat MSCs and human adipose-derived stem cells [71,72]. Increasing the osmolarity of chondrogenic differentiation medium containing TGFβ by 100 mOsm enhanced Sox9, Col2α1, and aggrecan expression, as well as expression of the hypertrophic chondrocyte markers Col10 and Runx2, in day 21 monolayer cultures of human MSCs [73]. Whether osmolarity influences matrix accumulation or functional properties of MSC-laden constructs remains to be investigated.
Fluid flow
Mechanical loading of the cartilage layer results in large gradients in hydrostatic pressure, which subsequently induce flow of the interstitial fluid within the extracellular matrix. One way that mechanical loading is predicted to enhance tissue maturation is through this flow-mediated nutrient and growth factor exchange, as well as through physical activation of growth factors [74]. Loading may also influence tissue maturation through direct transduction of fluid shear stress across the cellular membrane. Fluid flow in response to joint loading is complex and challenging to recapitulate for isolated chondrocytes in three-dimensional cultures. However, controlled medium flow has been used to culture chondrocyte-seeded constructs, as culture with dynamic fluid flow provides several advantages over static culture including enhanced mass transport, a more controlled biochemical environment, and the application of hydrodynamic stimuli. Perfusion and rotating wall bioreactors have been shown to enhance extracellular matrix accumulation by chondrocytes seeded in porous polymeric scaffolds [75-77]. Perfusion bioreactors have similarly been found to enhance the biochemical content of MSC-seeded constructs grown in chondrogenic medium including TGFβ [78,79]. In another study, culture in an oscillating bioreactor that delivered slow, directional perfusion to MSC-woven poly(ϵ-caprolactone) constructs improved functional properties, increased type II collagen content, and supported more homogeneous matrix deposition (Figure 4) [80]. These studies indicate the importance of fluid flow on construct maturation, although it remains unclear whether flow-induced nutrient transport, growth factor activation, and/or direct cellular sensing are influencing cellular behavior.
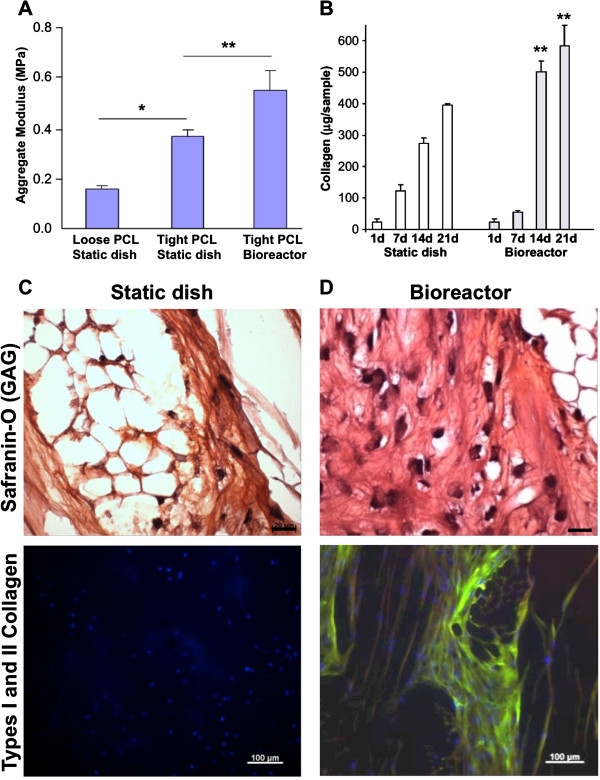
Figure 4.
Culture in an oscillating bioreactor enhances tissue mechanical properties and collagen content. (A) Aggregate modulus and (B) total collagen content in human mesenchymal stem cell–poly(ϵ-caprolactone) (hMSC-PCL) constructs. *Significant difference due to scaffold structure (P < 0.05); **Significant difference due to culture vessel (P < 0.05). (C), (D) Histological (top) and immunohistological (bottom) appearance of day 21 hMSC-PCL constructs cultured (C) statically or (D) in a bioreactor. Tissue sections were stained for safranin-O (top, scale bar: 20 μm) and double immunostained (bottom, cellular DNA counter-stained, scale bar: 100 μm) for collagen I (red, not seen) and collagen II (green). GAG, glycosaminoglycan. Adapted from [80].
Molecular mechanisms of transduction
The molecular mechanisms of mechanical signal transduction in chondrocytes and MSCs are not fully understood and represent an area of growing investigation. Ion channels, the primary cilium, the nucleus, and the cytoskeleton have all been proposed as candidate mechanical signal transducers in articular chondrocytes (Figure 3). Ion channels in chondrocytes include potassium channels, sodium channels, transient receptor potential (TRP) nonselective cation channels, and chloride channels [81]. Various ion channels in chondrocytes appear to be regulated by changes in osmolarity, as well as different forms of mechanical stimulation. For example, the TRPV4 ion channel has been identified as the major sensor of osmolarity in chondrocytes [82], and activation of this channel leads to an influx of calcium ions. Calcium signaling has also been observed in chondrocytes in response to hydrostatic pressurization [83] and compressive loading [84]. Changes in intracellular calcium downstream of ATP secretion and binding to purinergic receptors have also been proposed as a mechanical signaling pathway in chondrocytes [85]. Calcium signaling has been linked to the propagation of mechanical effects on gene expression in cartilage explants [86]. Treatment with nifedipine, a calcium channel inhibitor, or 4-aminopyridine, a potassium channel inhibitor, disrupted mechanical stimulation of sGAG synthesis by chondrocytes in agarose, suggesting possible roles for calcium and potassium signaling in mediating this loading effect [87]. A recent study showed that altering the intracellular sodium and calcium concentrations using the pharmacologic agents oubain and ionomycin for 1 hour daily on days 10 to 14 of culture increased the tensile modulus of neo-tissue produced by chondrocytes from young bovine donors in a scaffoldless culture system at 4 weeks of culture, providing evidence that ion channel regulation can also influence functional properties of neo-cartilage [88].
The primary cilium is a nonmotile organelle that extends from the cell surface and has been implicated in both sensory and signaling functions in a variety of cells [89]. Primary cilia were identified on articular chondrocytes more than three decades ago [90,91], and recent work indicates that the primary cilium may have an important role in chondrocyte mechanotransduction [92,93]. Primary cilia on sternal chondrocytes were shown to have α2, α3, and β1 integrins on their surface [94], allowing a direct linkage between the cilium and collagens in the surrounding pericellular matrix. As such, tissue compression during joint loading could lead to deformation of the cilium. Ion channels, including TRPV4, are also found on primary cilia. Interestingly, chemical disruption of the primary cilia on articular chondrocytes blocked the increase in intracellular calcium caused by exposure to hypo-osmotic stress or a TRPV4 channel agonist [82]. Acute compressive loading of sternal chondrocyte–agarose disks has been shown to induce an increase in calcium signaling, upregulation of aggrecan expression, and higher sGAG accumulation; these loading effects were absent in IFT88(orpk) mutant chondrocytes that lack primary cilium [95]. Together, these studies suggest that the primary cilium may contribute in multiple and complex ways to mechanical signal transduction in chondrocytes, and further investigations are needed to clarify the contributions of this proposed mechanosensory organelle.
Deformation of the nucleus in chondrocytes may be important in propagating the cellular response to biophysical stimuli [96]. The connections between the extracellular matrix, integrins, cytoskeleton, LINC complex, and nuclear lamina allow for direct transmission of biophysical forces from the cell exterior to the nucleus and potentially to subnuclear structures. The nucleus in chondrocytes deforms in response to compression of articular cartilage explants [40] and chondrocyte/agarose constructs [97]. Application of osmotic stress to chondrocytes also influences nuclear volume and structure [98], with changes in the nucleus probably reflecting alterations in intracellular macromolecular concentrations [99]. Studies are needed to define how these direct pathways by which biophysical stimuli influence the nucleus contribute to regulation of gene expression by mechanical loading in chondrocytes and stem cells.
The cytoskeleton in articular chondrocytes is primarily composed of actin microfilaments, microtubules, and vimentin intermediate filaments [100]. Disruption of actin microfilaments with cytochalasin D was found to decrease viscoelastic mechanical properties of chondrocytes [101] and to alter chondrocyte nuclear deformation in response to compression of cartilage explants [40]. The actin cytoskeleton in articular chondrocytes has also been shown to undergo reorganization with osmotic stress [70,102], as well as compressive loading and hydrostatic pressure [103]. These studies suggest that the cytoskeleton is involved in the response of chondrocytes to mechanical loading, yet studies directly implicating the cytoskeleton are lacking. Prior work has shown that integrins are involved in responses of chondrocyte–hydrogel constructs to dynamic compressive loading [104,105]. A recent study demonstrated that, when chondrocytes were suspended in agarose and pretreated with a blocking antibody for αv or β1 integrin, the increases in sGAG synthesis and sGAG accumulation induced by 24 hours of dynamic compression were disrupted [106]. Linkages between integrins and cytoskeletal components are thought to be integral to mechanotransduction in various cell types [107], but such linkages in chondrocytes have not been well defined. How early signaling events downstream of changes in ion channels, the primary cilium, the nucleus, and the cytoskeleton are propagated into changes in gene expression and matrix synthesis that support chondrogenic differentiation and neo-tissue formation remain open questions for future investigations.
Conclusion
Mechanically-generated signals appear to play a critical role in the differentiation and maturation of MSCs into a chondrogenic phenotype. Compressive deformational loading of MSC-laden constructs produces a pro-chondrogenic and biosynthetic response that is advantageous for developing MSC-based neo-tissues for cartilage regeneration and repair, and this system can also be used as a model to better understand the mechanisms of MSC mechanotransduction. Use of more advanced bioreactors, such as those that also incorporate shear and other components of loading, further enhances the chondrogenic response of MSCs to mechanical loading, and better mimics the in vivo environment in which these cartilage neo-tissues are designed to reside. Knowledge about the mechanisms that transduce macroscopic mechanical forces into intracellular events is increasing with respect to both chondrocytes and chondrogenically-induced MSCs. Further delineations about these mechanisms will probably lead to controllable strategies for rapid and effective preconditioning of anatomically shaped MSC-based cartilage replacements.
Note
This article is part of a thematic series on Physical influences on stem cells edited by Gordana Vunjak-Novakovic. Other articles in the series can be found online at http://stemcellres.com/series/physical.
Abbreviations
MSC: Mesenchymal stem cell; sGAG: Sulfated glycosaminoglycan; TGFβ: Transforming growth factor beta; TRP: Transient receptor potential.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Christopher J O’Conor, Email: christopher.oconor@duke.edu.
Natasha Case, Email: natasha.case@dm.duke.edu.
Farshid Guilak, Email: guilak@duke.edu.
Acknowledgements
This work was supported by grants from the National Institutes of Health (AR48182, AR50245, AG15768, AR48852, AG40868, and GM08719) and by the Collaborative Research Center, AO Foundation, Davos, Switzerland.
References
- Estes BT, Gimble JM, Guilak F. Mechanical signals as regulators of stem cell fate. Curr Top Dev Biol. 2004;60:91–126. doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]
- Aaron RK, Ciombor DM, Wang S, Simon B. Clinical biophysics: the promotion of skeletal repair by physical forces. Ann N Y Acad Sci. 2006;1068:513–531. doi: 10.1196/annals.1346.045. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Jacobs CR. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today. 2010;90:75–85. doi: 10.1002/bdrc.20173. [DOI] [PubMed] [Google Scholar]
- McCullen SD, Haslauer CM, Loboa EG. Musculoskeletal mechanobiology: interpretation by external force and engineered substratum. J Biomech. 2010;43:119–127. doi: 10.1016/j.jbiomech.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Wescoe KE, Schugar RC, Chu CR, Deasy BM. The role of the biochemical and biophysical environment in chondrogenic stem cell differentiation assays and cartilage tissue engineering. Cell Biochem Biophys. 2008;52:85–102. doi: 10.1007/s12013-008-9029-0. [DOI] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev. 2009;15:43–53. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad S, Eglin D, Alini M, Stoddart MJ. Physical stimulation of chondrogenic cells in vitro: a review. Clin Orthop Relat Res. 2011;469:2764–2772. doi: 10.1007/s11999-011-1819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM. Nicolas Andry Award: multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 2010;2010(468):2530–2540. doi: 10.1007/s11999-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Tae SK, Lee SH, Park JS, Im GI. Mesenchymal stem cells for tissue engineering and regenerative medicine. Biomed Mater. 2006;1:63–71. doi: 10.1088/1748-6041/1/2/003. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Okabe T, Ikawa T, Iida T, Yasuda H, Nakamura H, Wakitani S. Articular cartilage repair with autologous bone marrow mesenchymal cells. J Cell Physiol. 2010;225:291–295. doi: 10.1002/jcp.22223. [DOI] [PubMed] [Google Scholar]
- Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Varshney RR, Ren L, Cai D, Wang DA. Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng Part B Rev. 2009;15:75–86. doi: 10.1089/ten.teb.2008.0586. [DOI] [PubMed] [Google Scholar]
- Guilak F, Butler DL, Goldstein SA. Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop Relat Res. 2001;391(Suppl):S295–S305. [PubMed] [Google Scholar]
- Mouw JK, Connelly JT, Wilson CG, Michael KE, Levenston ME. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells. 2007;25:655–663. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Byers BA, Yuan X, Tuan RS. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113–125. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- Kisiday JD, Frisbie DD, McIlwraith CW, Grodzinsky AJ. Dynamic compression stimulates proteoglycan synthesis by mesenchymal stem cells in the absence of chondrogenic cytokines. Tissue Eng Part A. 2009;15:2817–2824. doi: 10.1089/ten.tea.2008.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AH, Farrell MJ, Kim M, Mauck RL. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur Cell Mater. 2010;19:72–85. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yao SJ, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A. 2010;16:575–584. doi: 10.1089/ten.tea.2009.0262. [DOI] [PubMed] [Google Scholar]
- Thorpe SD, Buckley CT, Vinardell T, O’Brien FJ, Campbell VA, Kelly DJ. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-β3 induced chondrogenic differentiation. Ann Biomed Eng. 2010;38:2896–2909. doi: 10.1007/s10439-010-0059-6. [DOI] [PubMed] [Google Scholar]
- Schatti O, Grad S, Goldhahn J, Salzmann G, Li Z, Alini M, Stoddart MJ. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cell Mater. 2011;22:214–225. doi: 10.22203/ecm.v022a17. [DOI] [PubMed] [Google Scholar]
- Haugh MG, Meyer EG, Thorpe SD, Vinardell T, Duffy GP, Kelly DJ. Temporal and spatial changes in cartilage-matrix-specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng Part A. 2011;17:3085–3093. doi: 10.1089/ten.tea.2011.0198. [DOI] [PubMed] [Google Scholar]
- Huang AH, Baker BM, Ateshian GA, Mauck RL. Sliding contact loading enhances the tensile properties of mesenchymal stem cell-seeded hydrogels. Eur Cell Mater. 2012;24:29–45. doi: 10.22203/ecm.v024a03. [DOI] [PubMed] [Google Scholar]
- Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A. 2012;18:715–724. doi: 10.1089/ten.tea.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier E, Noailly J, Ito K. Directing bone marrow-derived stromal cell function with mechanics. J Biomech. 2010;43:807–817. doi: 10.1016/j.jbiomech.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- Lee DA, Bader DL. The development and characterization of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol Anim. 1995;31:828–835. doi: 10.1007/BF02634565. [DOI] [PubMed] [Google Scholar]
- Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- Kelly TA, Fisher MB, Oswald ES, Tai T, Mauck RL, Ateshian GA, Hung CT. Low-serum media and dynamic deformational loading in tissue engineering of articular cartilage. Ann Biomed Eng. 2008;36:769–779. doi: 10.1007/s10439-008-9476-1. [DOI] [PubMed] [Google Scholar]
- eCM Journal. http://www.ecmjournal.org.
- Ng KW, Mauck RL, Wang CC, Kelly TA, Ho MM, Chen FH, Ateshian GA, Hung CT. Duty cycle of deformational loading influences the growth of engineered articular cartilage. Cell Mol Bioeng. 2009;2:386–394. doi: 10.1007/s12195-009-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder SH, Goldstein SA, Kimura JH, Soslowsky LJ, Spengler DM. Chondrocyte differentiation is modulated by frequency and duration of cyclic compressive loading. Ann Biomed Eng. 2001;29:476–482. doi: 10.1114/1.1376696. [DOI] [PubMed] [Google Scholar]
- Struglics A, Hansson M. MMP proteolysis of the human extracellular matrix protein aggrecan is mainly a process of normal turnover. Biochem J. 2012;446:213–223. doi: 10.1042/BJ20120274. [DOI] [PubMed] [Google Scholar]
- Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-β3. Osteoarthr Cartil. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech. 2000;33:1663–1673. doi: 10.1016/S0021-9290(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. Multi-axial mechanical stimulation of tissue engineered cartilage: review. Eur Cell Mater. 2007;13:66–73. doi: 10.22203/ecm.v013a07. discussion 73–64. [DOI] [PubMed] [Google Scholar]
- Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech. 1995;28:1529–1541. doi: 10.1016/0021-9290(95)00100-X. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Anseth KS, Lee DA, Bader DL. Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. J Orthop Res. 2004;22:1143–1149. doi: 10.1016/j.orthres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Freeman PM, Natarajan RN, Kimura JH, Andriacchi TP. Chondrocyte cells respond mechanically to compressive loads. J Orthop Res. 1994;12:311–320. doi: 10.1002/jor.1100120303. [DOI] [PubMed] [Google Scholar]
- Choi JB, Youn I, Cao L, Leddy HA, Gilchrist CL, Setton LA, Guilak F. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40:2596–2603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- Connelly JT, Vanderploeg EJ, Mouw JK, Wilson CG, Levenston ME. Tensile loading modulates bone marrow stromal cell differentiation and the development of engineered fibrocartilage constructs. Tissue Eng Part A. 2010;16:1913–1923. doi: 10.1089/ten.tea.2009.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Shah RP, Huang AH, Mauck RL. Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage. Tissue Eng Part A. 2011;17:1445–1455. doi: 10.1089/ten.tea.2010.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann Biomed Eng. 2000;28:150–159. doi: 10.1114/1.239. [DOI] [PubMed] [Google Scholar]
- Hall AC, Urban JP, Gehl KA. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991;9:1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- Park S, Krishnan R, Nicoll SB, Ateshian GA. Cartilage interstitial fluid load support in unconfined compression. J Biomech. 2003;36:1785–1796. doi: 10.1016/S0021-9290(03)00231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach NM, Mow VC, Guilak F. Incompressibility of the solid matrix of articular cartilage under high hydrostatic pressures. J Biomech. 1998;31:445–451. doi: 10.1016/S0021-9290(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Toyoda T, Seedhom BB, Kirkham J, Bonass WA. Upregulation of aggrecan and type II collagen mRNA expression in bovine chondrocytes by the application of hydrostatic pressure. Biorheology. 2003;40:79–85. [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21:451–457. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Miyanishi K, Trindade MC, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, Schurman DJ, Smith RL. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 2006;12:1419–1428. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- Wagner DR, Lindsey DP, Li KW, Tummala P, Chandran SE, Smith RL, Longaker MT, Carter DR, Beaupre GS. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng. 2008;36:813–820. doi: 10.1007/s10439-008-9448-5. [DOI] [PubMed] [Google Scholar]
- Miyanishi K, Trindade MC, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, Schurman DJ, Smith RL. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-beta3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 2006;12:2253–2262. doi: 10.1089/ten.2006.12.2253. [DOI] [PubMed] [Google Scholar]
- Finger AR, Sargent CY, Dulaney KO, Bernacki SH, Loboa EG. Differential effects on messenger ribonucleic acid expression by bone marrow-derived human mesenchymal stem cells seeded in agarose constructs due to ramped and steady applications of cyclic hydrostatic pressure. Tissue Eng. 2007;13:1151–1158. doi: 10.1089/ten.2006.0290. [DOI] [PubMed] [Google Scholar]
- Puetzer J, Williams J, Gillies A, Bernacki S, Loboa EG. The effects of cyclic hydrostatic pressure on chondrogenesis and viability of human adipose- and bone marrow-derived mesenchymal stem cells in three-dimensional agarose constructs. Tissue Eng Part A. 2013;19:299–306. doi: 10.1089/ten.tea.2012.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao Z, Yang J, Liu J, Wang J, Li X, Liu Y. p38 MAPK mediated in compressive stress-induced chondrogenesis of rat bone marrow MSCs in 3D alginate scaffolds. J Cell Physiol. 2009;221:609–617. doi: 10.1002/jcp.21890. [DOI] [PubMed] [Google Scholar]
- Li J, Wang J, Zou Y, Zhang Y, Long D, Lei L, Tan L, Ye R, Wang X, Zhao Z. The influence of delayed compressive stress on TGF-β1-induced chondrogenic differentiation of rat BMSCs through Smad-dependent and Smad-independent pathways. Biomaterials. 2012;33:8395–8405. doi: 10.1016/j.biomaterials.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- Hopewell B, Urban JP. Adaptation of articular chondrocytes to changes in osmolality. Biorheology. 2003;40:73–77. [PubMed] [Google Scholar]
- Negoro K, Kobayashi S, Takeno K, Uchida K, Baba H. Effect of osmolarity on glycosaminoglycan production and cell metabolism of articular chondrocyte under three-dimensional culture system. Clin Exp Rheumatol. 2008;26:534–541. [PubMed] [Google Scholar]
- Xu X, Urban JP, Tirlapur UK, Cui Z. Osmolarity effects on bovine articular chondrocytes during three-dimensional culture in alginate beads. Osteoarthr Cartil. 2010;18:433–439. doi: 10.1016/j.joca.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Oswald ES, Ahmed HS, Kramer SP, Bulinski JC, Ateshian GA, Hung CT. Effects of hypertonic (NaCl) two-dimensional and three-dimensional culture conditions on the properties of cartilage tissue engineered from an expanded mature bovine chondrocyte source. Tissue Eng Part C Methods. 2011;17:1041–1049. doi: 10.1089/ten.tec.2011.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew SR, Peffers MJ, McKay TR, Lowe ET, Khan WS, Hardingham TE, Clegg PD. Hyperosmolarity regulates SOX9 mRNA posttranscriptionally in human articular chondrocytes. Am J Physiol Cell Physiol. 2009;297:C898–C906. doi: 10.1152/ajpcell.00571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peffers MJ, Milner PI, Tew SR, Clegg PD. Regulation of SOX9 in normal and osteoarthritic equine articular chondrocytes by hyperosmotic loading. Osteoarthr Cartil. 2010;18:1502–1508. doi: 10.1016/j.joca.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Widmyer MR, Leddy HA, Utturkar GM, Spritzer CE, Moorman CT 3rd, Guilak F, Defrate LE. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech. 2013;46:541–547. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PH, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol. 2006;291:C718–C725. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- Wuertz K, Godburn K, Neidlinger-Wilke C, Urban J, Iatridis JC. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine (Phila Pa 1976) 2008;33:1843–1849. doi: 10.1097/BRS.0b013e31817b8f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Li H, Tao Y, Zhou X, Li F, Chen G, Chen Q. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med. 2012;10:49. doi: 10.1186/1479-5876-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron MM, van der Windt AE, Emans PJ, van Rhijn LW, Jahr H, Welting TJ. Osmolarity determines the in vitro chondrogenic differentiation capacity of progenitor cells via nuclear factor of activated T-cells 5. Bone. 2013;53:94–102. doi: 10.1016/j.bone.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Albro MB, Cigan AD, Nims RJ, Yeroushalmi KJ, Oungoulian SR, Hung CT, Ateshian GA. Shearing of synovial fluid activates latent TGF-beta. Osteoarthr Cartil. 2012;20:1374–1382. doi: 10.1016/j.joca.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- Pazzano D, Mercier KA, Moran JM, Fong SS, DiBiasio DD, Rulfs JX, Kohles SS, Bonassar LJ. Comparison of chondrogensis in static and perfused bioreactor culture. Biotechnol Prog. 2000;16:893–896. doi: 10.1021/bp000082v. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Obradovic B, Martin I, Freed LE. Bioreactor studies of native and tissue engineered cartilage. Biorheology. 2002;39:259–268. [PubMed] [Google Scholar]
- Goncalves A, Costa P, Rodrigues MT, Dias IR, Reis RL, Gomes ME. Effect of flow perfusion conditions in the chondrogenic differentiation of bone marrow stromal cells cultured onto starch based biodegradable scaffolds. Acta Biomater. 2011;7:1644–1652. doi: 10.1016/j.actbio.2010.11.044. [DOI] [PubMed] [Google Scholar]
- da Silva ML A, Martins A, Costa-Pinto AR, Correlo VM, Sol P, Bhattacharya M, Faria S, Reis RL, Neves NM. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J Tissue Eng Regen Med. 2011;5:722–732. doi: 10.1002/term.372. [DOI] [PubMed] [Google Scholar]
- Valonen PK, Moutos FT, Kusanagi A, Moretti MG, Diekman BO, Welter JF, Caplan AI, Guilak F, Freed LE. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(epsilon-caprolactone) scaffolds. Biomaterials. 2010;31:2193–2200. doi: 10.1016/j.biomaterials.2009.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Jolley R, Lewis R, Fallman R, Mobasheri A. The emerging chondrocyte channelome. Front Physiol. 2010;1:135. doi: 10.3389/fphys.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JA, Saunders K, Urban JP, Wilkins RJ. The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology. 2004;41:299–308. [PubMed] [Google Scholar]
- Han SK, Wouters W, Clark A, Herzog W. Mechanically induced calcium signaling in chondrocytes in situ. J Orthop Res. 2012;30:475–481. doi: 10.1002/jor.21536. [DOI] [PubMed] [Google Scholar]
- Pingguan-Murphy B, El-Azzeh M, Bader DL, Knight MM. Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. J Cell Physiol. 2006;209:389–397. doi: 10.1002/jcp.20747. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JB, Jin M, Grodzinsky AJ. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem. 2006;281:24095–24103. doi: 10.1074/jbc.M510858200. [DOI] [PubMed] [Google Scholar]
- Mouw JK, Imler SM, Levenston ME. Ion-channel regulation of chondrocyte matrix synthesis in 3D culture under static and dynamic compression. Biomech Model Mechanobiol. 2007;6:33–41. doi: 10.1007/s10237-006-0034-1. [DOI] [PubMed] [Google Scholar]
- Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, Athanasiou KA. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum. 2010;62:1097–1107. doi: 10.1002/art.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey DA, Downs ME, Jacobs CR. The mechanics of the primary cilium: an intricate structure with complex function. J Biomech. 2012;45:17–26. doi: 10.1016/j.jbiomech.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsman NJ. Cilia of adult canine articular chondrocytes. J Ultrastruct Res. 1978;64:270–281. doi: 10.1016/S0022-5320(78)90036-9. [DOI] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil. 1985;5:175–193. doi: 10.1002/cm.970050302. [DOI] [PubMed] [Google Scholar]
- Whitfield JF. The solitary (primary) cilium – a mechanosensory toggle switch in bone and cartilage cells. Cell Signal. 2008;20:1019–1024. doi: 10.1016/j.cellsig.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Muhammad H, Rais Y, Miosge N, Ornan EM. The primary cilium as a dual sensor of mechanochemical signals in chondrocytes. Cell Mol Life Sci. 2012;69:2101–2107. doi: 10.1007/s00018-011-0911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- Wann AK, Zuo N, Haycraft CJ, Jensen CG, Poole CA, McGlashan SR, Knight MM. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2012;26:1663–1671. doi: 10.1096/fj.11-193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RP, Finan JD, Guilak F, Lee DA. Mechanical regulation of nuclear structure and function. Annu Rev Biomed Eng. 2012;14:431–455. doi: 10.1146/annurev-bioeng-071910-124638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Knight MM, Bolton JF, Idowu BD, Kayser MV, Bader DL. Chondrocyte deformation within compressed agarose constructs at the cellular and sub-cellular levels. J Biomech. 2000;33:81–95. doi: 10.1016/S0021-9290(99)00160-8. [DOI] [PubMed] [Google Scholar]
- Finan JD, Chalut KJ, Wax A, Guilak F. Nonlinear osmotic properties of the cell nucleus. Ann Biomed Eng. 2009;37:477–491. doi: 10.1007/s10439-008-9618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan JD, Guilak F. The effects of osmotic stress on the structure and function of the cell nucleus. J Cell Biochem. 2010;109:460–467. doi: 10.1002/jcb.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain EJ. Involvement of the cytoskeletal elements in articular cartilage homeostasis and pathology. Int J Exp Pathol. 2009;90:1–15. doi: 10.1111/j.1365-2613.2008.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickey WR, Vail TP, Guilak F. The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes. J Orthop Res. 2004;22:131–139. doi: 10.1016/S0736-0266(03)0150-5. [DOI] [PubMed] [Google Scholar]
- Erickson GR, Northrup DL, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthr Cartil. 2003;11:187–197. doi: 10.1053/S1063-4584(02)00347-3. [DOI] [PubMed] [Google Scholar]
- Knight MM, Toyoda T, Lee DA, Bader DL. Mechanical compression and hydrostatic pressure induce reversible changes in actin cytoskeletal organisation in chondrocytes in agarose. J Biomech. 2006;39:1547–1551. doi: 10.1016/j.jbiomech.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Giannoni P, Siegrist M, Hunziker EB, Wong M. The mechanosensitivity of cartilage oligomeric matrix protein (COMP) Biorheology. 2003;40:101–109. [PubMed] [Google Scholar]
- Chowdhury TT, Appleby RN, Salter DM, Bader DA, Lee DA. Integrin-mediated mechanotransduction in IL-1 beta stimulated chondrocytes. Biomech Model Mechanobiol. 2006;5:192–201. doi: 10.1007/s10237-006-0032-3. [DOI] [PubMed] [Google Scholar]
- Chai DH, Arner EC, Griggs DW, Grodzinsky AJ. Alphav and beta1 integrins regulate dynamic compression-induced proteoglycan synthesis in 3D gel culture by distinct complementary pathways. Osteoarthr Cartil. 2010;18:249–256. doi: 10.1016/j.joca.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5:1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]