Abstract
Recent studies in secretory pathway calcium ATPases (SPCA) revealed novel functions of SPCA2 in interacting with store-operated Ca2+ channel Orai1 and inducing Ca2+ influx at the cell surface. Importantly, SPCA2-mediated Ca2+ signaling is uncoupled from its conventional role of Ca2+-ATPase and independent of store-operated Ca2+ signaling pathway. SPCA2-induced store-independent Ca2+ entry (SICE) plays essential roles in many important physiological processes, while unbalanced SICE leads to enhanced cell proliferation and tumorigenesis. Finally, we have summarized the clinical implication of SICE in oral cancer prognosis and treatment. Inhibition of SICE may be a new target for the development of cancer therapeutics.
Keywords: Ca2+ signaling, human cancers, store-independent Ca2+ entry, store-operated Ca2+ entry
Introduction
In vertebrates, there are three families of P-type Ca2+-ATPases regulating intracellular Ca2+ homeostasis. Functions and physiological roles of two, the plasma membrane Ca2+-ATPase (PMCA)1 and the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA),2 have been well established. Located in the plasma and endoplasmic reticulum membrane respectively, PMCA and SERCA transport Ca2+ out of the cell or into the internal stores, maintaining relatively low cytosolic Ca2+ and thus a steep inward gradient directed to the cytosol that enables rapid transduction of Ca2+ signaling by transient elevation of intracellular Ca2+ levels.1,2
The third family of Ca2+-ATPases identified more recently, secretory pathway calcium ATPase (SPCA), is essential for protein sorting, processing and glycosylation in the Golgi and post-Golgi compartments.3,4 The SPCAs are unique in transporting not only Ca2+, but also Mn2+ with submicromolar affinity into secretory pathway, utilizing the energy of ATP hydrolysis.3,4 Whereas a single SPCA isoform is found in lower eukaryotes from yeast to fish, two isoforms of SPCA proteins, SPCA1 and SPCA2, have been identified in higher eukaryotes including human.5,6
Until recently, the physiological role of SPCA2 has been puzzling. The expression of SPCA2 seems to be redundant along with SPCA1 in mammals. First, SPCA1 is ubiquitously distributed while the expression of SPCA2 is limited to gastrointestinal, genitourinary and nervous systems, as well as mammary and salivary glands.5,6 Second, SPCA proteins are highly conserved in the critical regions responsible for their Ca2+-ATPase activity suggesting that they share similar and redundant catalytic activity.6 Third, SPCA2 appears to have a lower affinity of Ca2+ binding and lower turnover in transporting Ca2+ into the secretory pathway.6 Therefore, one might expect SPCA1 alone to be sufficient to carry out SPCA functions and the expression of SPCA2 to be dispensable. Indeed, mice that are homozygous null for SPCA1 display embryonic lethality, indicating that SPCA2 cannot compensate for loss of SPCA1 (ref. 7). However, recent progress has revealed a novel function of SPCA2 superseding its conventional roles as a Golgi Ca2+ pump which is to directly interact with cell surface Ca2+ channels and elicit Ca2+ entry.8 The investigation of this function which is unique to SPCA2 but not SPCA1 shed light to the understanding of many physiological and pathophysiological processes.
Store-independent Ca2+ signaling mediated by SPCA2
The paradigm for Ca2+ entry in non-excitable cells is store-operated Ca2+ entry (SOCE), responsible for refilling the endoplasmic reticulum (ER) store and maintaining a number of important biological processes involved in cell proliferation, differentiation and mobilization.9 Translocation of ER-localized Ca2+ sensor STIM1 to ER-plasma membrane junctions is a key event in SOC signaling connecting depletion of ER store and activation of cell surface Ca2+-release-activating-Ca2+ channel (CRAC) Orai1 (ref.10–13). Named after a keeper of the gates of heaven in Greek mythology, Orai1 is the core subunit of SOC channels, mediating rapid and highly selective Ca2+ influx to induce a range of downstream signaling events.14,15,16 Interestingly, there is new evidence for store-independent opening of Orai1, termed store-independent Ca2+ entry (SICE), mediated by SPCA2 (ref. 8). In contrast to SPCA1 whose expression is limited to Golgi and secretory pathway, SPCA2 is able to traffic to the plasma membrane. Interaction between SPCA2 and Orai1 on the cell surface, detected by co-immunoprecipitation and pull-down methods, resulted in activation of Orai1 and elevation of cytosolic Ca2+. SICE had two noteworthy features: (i) independence from the ER stores and SOC pathway, and (ii) uncoupling from Ca2+ pump activity of SPCA2. Thus knockdown of the STIM sensors or mutational inactivation of the Ca2+-ATPase activity of SPCA2 did not affect SICE (ref. 8). An investigation of the molecular mechanism for SPCA2–Orai1 interaction and activation revealed that the short 26 amino acid C-terminal domain of SPCA2 mediated Ca2+ entry, possibly by gating the Orai1 channel through electrostatic interactions. The SPCA2 N-terminus also interacted directly with both N- and C-terminal domains of Orai1, and this interaction appeared to induce the exposure of SPCA2 C-terminal activating domain. Although C-terminus of SPCA1 was also able to activate Orai1, lack of the ability to bind to Orai1 through its N-terminal binding domain led to a failure to expose the C-terminus and activate Ca2+ entry.8
Physiological functions of SICE in transcellular Ca2+ transport
It is worth noting that SPCA2 expression is correlated with highly active Ca2+ absorbance and secretion in tissues such as salivary gland, mammary gland and intestine. This is reminiscent of the constitutive Ca2+ entry pathway mediated by SPCA2. Interestingly, a transcript of SPCA2 encoding exons 24–27, corresponding to the membrane-anchored C-terminal domain, was identified in exocrine pancreas. The expression of this transcript was under the regulation of a basic helix–loop–helix transcription factor MIST1, and appeared to be independent of the transcription of full-length SPCA2 (ref. 17) A significant upregulation of the Ca2+ entry-activating domain may correlate with effectively managed Ca2+ transport and secretion in pancreas and other secretory tissue including the mammary glands. During lactation, high-throughput transcellular transport of Ca2+ is carried out from maternal blood to milk, which is essential for maintaining total Ca2+ levels up to 100 m mol·L−1 in the milk.18 Despite the physiological importance of this process, its molecular mechanism has only begun to be elucidated by recent studies. Consistent with the secretory pathway playing an essential role in Ca2+ secretion in mammary gland, expression of both isoforms of SPCA was induced during lactation. Whereas the change in SPCA1 was more modest and relatively late, the expression of SPCA2 was dramatically enhanced during midpregnancy and upon parturition and stayed high through lactation.19 Similarly, in vitro treatment of mammary epithelial cells with prolactin, a polypeptide hormone essential for milk production and secretion, induced an increase of SPCA2 expression, as well as a change of intracellular Ca2+ mobilization.20 In lactating mammary tissues, SPCA1 was localized to Golgi apparatus of all mammary gland cells, while SPCA2 and Orai1 coexpressed in luminal epithelial cells (unpublished results). Investigation using a three-dimensional mammosphere differentiation model of mouse mammary epithelial cell line SCp2 suggested colocalization of SPCA2 and Orai1 at the basal membrane. SICE mediated by SPCA2 and Orai1 was elevated in mammospheres which was critical for mammosphere differentiation and formation. Further study suggested that knockdown of SPCA2 in mammospheres impaired trafficking of Orai1 to the cell surface and C-terminal Ca2+ entry-activating domain or full length SPCA2 both were able to rescue this defect and restore SICE (unpublished results). Taken together, SICE mediated by SPCA2–Orai1 is essential for maintaining transcellular Ca2+ transport during lactation by inducing elevated Ca2+ entry from maternal blood side for secretion into the milk by secretory pathways and efflux transporters on the apical membrane of mammary gland (Figure 1).
Figure 1.
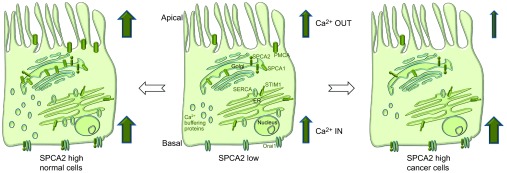
Function and regulation of SPCA2 in normal physiological conditions and cancerous cells. In normal physiological conditions, enhanced Ca2+ entry induced by upregulation of SPCA2 and its interaction with Orai1 is balanced by increased expression of Ca2+ pumps and buffering proteins mediating Ca2+ OUT. When transcellular Ca2+ is augmented, cytosolic Ca2+ is maintained at a low level. While in cancer cells, this orchestration is disrupted. Inhibited Ca2+ OUT synergizes with upregulated Ca2+ IN elicited by SPCA2–Orai1 to yield sustained elevation of cytosolic Ca2+ and thus constitutive activation of downstream signaling events controlling cell growth and survival which promote cell proliferation and tumorigenesis. SPCA, secretory pathway calcium ATPase.
Pathophysiological roles of SICE in cell proliferation and tumorigenesis
In normal physiological conditions, the enhancement of Ca2+ ‘IN' by SPCA2 upregulation is well balanced by a hyperactive Ca2+ ‘OUT' process mediated by increased expression of a number of components in Ca2+ signaling system, including PMCA pumps and Ca2+ buffering proteins, to ensure high rate transcellular Ca2+ transport while maintaining normal cytosolic Ca2+ levels. However, when this orchestration is disrupted, high levels of SPCA2 induce unbalanced Ca2+ influx and constitutive activation of downstream signaling cascades that lead to enhanced cell proliferation and transformation (Figure 1). Many breast cancer tumor cell lines show upregulation of SPCA2 but not SPCA1 as compared to the normal breast epithelial cells.8 Suppression of SPCA2 expression in breast cancer cells lowered basal Ca2+ levels, which led to the attenuation of cell growth and tumorigenicity in vivo. Ectopic expression of full length SPCA2 or the C-terminal Ca2+ entry-activating domain in normal mammary gland epithelial cells significantly elevated cytosolic Ca2+ level which was sufficient to drive transformation of these cells. Further studies revealed that SPCA2 interacted with Orai1 to induce constitutive SICE in cancer cells, while SOC signaling mediated by STIM proteins appeared to play a minor role in tumor proliferation.8 In contrast, STIM1-Orai1-mediated SOC was shown to be indispensable for invasion and colonization of metastatic cancer cells in which the expression of SPCA2 was undetectable, suggesting an interesting possibility that SICE and SOCE may contribute to different stages of cancer progression. Importantly, SPCA2-induced elevation of cytosolic Ca2+ is correlated with constitutive activation of RAS–ERK signaling pathway, by eliciting ERK1/2 phosphorylation and Cyclin D1 expression.8
Therapeutic implications of SICE in oral malignancy
Oral cancer is one of the most common malignancies in the world, with 350 000–400 000 new cases of oral squamous cell carcinoma identified each year.21 Despite its prevalence, the prognosis and development of therapies for the treatment of oral cancers still remains in its infancy. Importantly, salivary gland was one of the tissues in which both full-length and the membrane-anchored C-terminal Ca2+ entry-activating domain of SPCA2 was expressed, under the regulation of MIST1, suggesting a potential role of SICE in oral tissue development and homeostasis.17 In oral epithelial cells, dysregulation of the Ca2+-signaling components for Ca2+ ‘OUT' may synergize with the effects of enhanced Ca2+ influx induced by SPCA2, to yield sustained increase of cytosolic Ca2+ level and constitutive activation of signaling controlling cell growth and survival which promotes transformation and tumorigenesis (Figure 1). Indeed, heterozygous deletion of SERCA2, an isoform of SERCA pumps in mice led to the spontaneous development of squamous cell tumors in oral cavities.22 More importantly, studies comparing patient samples from human oral premalignant lesion and oral squamous cell carcinoma indicated downregulation of SERCA2 and PMCA1 in oral cancer cells which contributed to the increase of cytosolic Ca2+ concentration.23,24 In addition, a carcinogen safrole was shown to induce cell proliferation of human oral cancer cells by augmenting cytosolic Ca2+ level.25
Identification of the pathophysiological role of Ca2+ signaling highlighted its clinically implications in prognosis and treatment of oral malignancy. A systemic examination of the expression of SICE regulators in normal, premalignant and tumor samples from oral tissues will reveal specific expression patterns of SICE components at different stages of cancer development, establish the role of SICE in oral cancer initiation and progression, and provide molecular signatures for oral cancer prognosis. The critical role of SICE in cancer cells revealed the therapeutic potential of SPCA2 and Orai1 as druggable targets for cancer treatment. There are potentially three strategies for targeting SPCA2–Orai1 complex. First, we may screen for specific antibodies or small molecules which directly bind to and inhibit C-terminal Ca2+ entry-activating domain of SPCA2. Second, disruption of the interaction between SPCA2 and Orai1 would diminish SICE. A polypeptide of 40 amino acids in the N-terminus of SPCA2 was identified to be the minimal binding domain to Orai1. Overexpression of this domain showed a dominant-negative effect by competitively binding to Orai1 and inhibiting activation of Orai1 by full-length SPCA2 (ref. 8). Therefore, this polypeptide may be delivered into the cancer cells to disrupt SPCA2–Orai1 complex and block SICE. Third, we may directly disrupt the transcripts of SPCA2 through molecular biological methodology.
Conclusion and perspectives
The novel function of SPCA2 in mediating SICE underscores the importance of Ca2+ signaling in normal physiological processes and human diseases. Highly active SICE is important for maintaining regular Ca2+ secretion and absorption, but constitutive activation of SICE in a microenvironment not well orchestrated may also leads to uncontrolled cell growth and enhanced tumorigenecity. Despite the exciting progress toward physiological and pathophysiological functions of SPCA2-mediated SICE, many questions remain unexplored. First, SPCA2 is localized to both Golgi and plasma membrane while SPCA1 is mostly restricted to Golgi and post Golgi vesicles. What is the signaling event triggering the trafficking of SPCA2 between Golgi apparatus and cell surface? Second, what are the differences between pump-functional and SICE-activating populations SPCA2 and is additional post-translational modification processed for SPCA2 to carry out this ‘moonlighting' function of SICE induction? Third, Orai1 is involved in both SOCE and SICE. How are Orai1 channels distributed between these two processes and are they undergoing similar mechanisms of membrane docking and activation? Fourth, how is the expression of SPCA2 regulated at different stages of cancer progression and is cell surface expression of SPCA2 on the primary tumors a poor-prognosis signature? Further investigation of SPCA2-induced SICE should shed light to the understanding of the basic principles of Ca2+ signaling in many important biological processes, and stimulate the development of new strategies for druggable targets selection and cancer treatment.
Acknowledgments
This work was supported by grant GM62142 from the National Institution of Health to Rajini Rao, and American Heart Association Pre-doctoral Fellowship 0815058E to Ming-Ye Feng. Publication of this manuscript is supported by Open Fund of State Key Laboratory of Oral Diseases, Sichuan University.
References
- Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81 1:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- East JM. Sarco(endo)plasmic reticulum calcium pumps: recent advances in our understanding of structure/function and biology (review) Mol Membr Biol. 2000;17 4:189–200. doi: 10.1080/09687680010009646. [DOI] [PubMed] [Google Scholar]
- Sorin A, Rosas G, Rao R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J Biol Chem. 1997;272 15:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- Durr G, Strayle J, Plemper R, et al. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9 5:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoevelen J, Dode L, Van Baelen K, et al. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J Biol Chem. 2005;280 24:22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- Xiang M, Mohamalawari D, Rao R. A novel isoform of the secretory pathway Ca2+,Mn2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J Biol Chem. 2005;280 12:11608–11614. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
- Okunade GW, Miller ML, Azhar M, et al. Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J Biol Chem. 2007;282 36:26517–26527. doi: 10.1074/jbc.M703029200. [DOI] [PubMed] [Google Scholar]
- Feng M, Grice DM, Faddy HM, et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143 1:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85 2:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Zhang SL, Yeromin AV, et al. Molecular basis of the CRAC channel. Cell Calcium. 2007;42 2:133–144. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, et al. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42 2:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446 7133:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- Vig M, Kinet JP. The long and arduous road to CRAC. Cell Calcium. 2007;42 2:157–162. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441 7090:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443 7108:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312 5777:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside VC, Kowalik AS, Johnson CL, et al. MIST1 regulates the pancreatic acinar cell expression of Atp2c2, the gene encoding secretory pathway calcium ATPase 2. Exp Cell Res. 2010;316 17:2859–2870. doi: 10.1016/j.yexcr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: targets for breast cancer. Biochim Biophys Acta. 2006;1765 2:235–255. doi: 10.1016/j.bbcan.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Faddy HM, Smart CE, Xu R, et al. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem Biophys Res Commun. 2008;369 3:977–981. doi: 10.1016/j.bbrc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantamongkol U, Takemura H, Suthiphongchai T, et al. Regulation of Ca2+ mobilization by prolactin in mammary gland cells: possible role of secretory pathway Ca2+-ATPase type 2. Biochem Biophys Res Commun. 2007;352 2:537–542. doi: 10.1016/j.bbrc.2006.11.055. [DOI] [PubMed] [Google Scholar]
- Jiang L, Zeng X, Yang H, et al. Oral cancer overexpressed 1 (ORAOV1): a regulator for the cell growth and tumor angiogenesis in oral squamous cell carcinoma. Int J Cancer. 2008;123 8:1779–1786. doi: 10.1002/ijc.23734. [DOI] [PubMed] [Google Scholar]
- Liu LH, Boivin GP, Prasad V, et al. Squamous cell tumors in mice heterozygous for a null allele of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump. J Biol Chem. 2001;276 29:26737–26740. doi: 10.1074/jbc.C100275200. [DOI] [PubMed] [Google Scholar]
- Endo Y, Uzawa K, Mochida Y, et al. Sarcoendoplasmic reticulum Ca2+ ATPase type 2 downregulated in human oral squamous cell carcinoma. Int J Cancer. 2004;110 2:225–231. doi: 10.1002/ijc.20118. [DOI] [PubMed] [Google Scholar]
- Saito K, Uzawa K, Endo Y, et al. Plasma membrane Ca2+ ATPase isoform 1 down-regulated in human oral cancer. Oncol Rep. 2006;15 1:49–55. [PubMed] [Google Scholar]
- Huang JK, Huang CJ, Chen WC, et al. Independent [Ca2+]i increases and cell proliferation induced by the carcinogen safrole in human oral cancer cells. Naunyn Schmiedebergs Arch Pharmacol. 2005;372 1:88–94. doi: 10.1007/s00210-005-1086-y. [DOI] [PubMed] [Google Scholar]


