Abstract
Tooth bleaching agents may weaken the tooth structure. Therefore, it is important to minimize any risks of tooth hard tissue damage caused by bleaching agents. The aim of this study was to evaluate the effects of applying 45S5 bioglass (BG) before, after, and during 35% hydrogen peroxide (HP) bleaching on whitening efficacy, physicochemical properties and microstructures of bovine enamel. Seventy-two bovine enamel blocks were prepared and randomly divided into six groups: distilled deionized water (DDW), BG, HP, BG before HP, BG after HP and BG during HP. Colorimetric and microhardness tests were performed before and after the treatment procedure. Representative specimens from each group were selected for morphology investigation after the final tests. A significant color change was observed in group HP, BG before HP, BG after HP and BG during HP. The microhardness loss was in the following order: group HP>BG before HP, BG after HP>BG during HP>DDW, BG. The most obvious morphological alteration of was observed on enamel surfaces in group HP, and a slight morphological alteration was also detected in group BG before HP and BG after HP. Our findings suggest that the combination use of BG and HP could not impede the tooth whitening efficacy. Using BG during HP brought better protective effect than pre/post-bleaching use of BG, as it could more effectively reduce the mineral loss as well as retain the surface integrity of enamel. BG may serve as a promising biomimetic adjunct for bleaching therapy to prevent/restore the enamel damage induced by bleaching agents.
Keywords: Bioglass, enamel, hydrogen peroxide, tooth bleaching
Introduction
Tooth discoloration may compromise the beauty of smile and thus boost the demand of patients for esthetic dental assistance. Among various therapies, tooth bleaching has rapidly gained prevalence as an easy, affordable and conservative way of treating discolored teeth.1 The underlying mechanism of tooth whitening is not fully understood.2 It is commonly accepted that peroxide can penetrate into the hard tissue where it generates free radicals to oxidize organic chromophores, leading to a reduction of color particularly in dentine.3 Despite the absence of dispute about its efficacy, general concerns still remain regarding the negative impact of hydrogen peroxide (HP) on enamel. Numerous studies found bleaching agents could induce chemical, structural and mechanical changes of enamel.4,5,6 Besides, the mineral reduction of bleached enamel is not only limited in the enamel surface, but also extends to the enamel subsurface.7 Therefore, it is still necessary to minimize potential damages on enamel caused by HP exposure.
To achieve this, previous studies have incorporated fluoride into bleaching regimens to decrease demineralization and hasten rehardening of enamel.8,9,10 However, other studies reported that the fluoride uptake was not enhanced in bleached enamel,11 and fluoridated bleaching gels could not promote remineralization of predemineralized enamel.12 Despite the conflicting results, a major problem with fluoride is its low solubility, leading to a rapid deposition of fluorapatite in the enamel surface layer, thus blocking deeper ionic penetration and full-layer remineralization.13 Besides, for a unit cell stoichiometry of Ca10(PO4)6F2, 10 mol of calcium and 6 mol of phosphate ions are required for every 2 mol fluoride ions, thus making calcium and phosphate ions the limiting factor for remineralization.13 Apart from topical fluoridation, biomimetic materials, such as nanohydroxyapatite and calcium phosphopeptide-amorphous calcium phosphate (CPP-ACP), were also investigated to reduce mineral loss, maintain structural integrity and remineralize the white spot lesions.14,15,16 These agents were used mostly after bleaching or mixed with bleaching agents. A recent study showed that using CPP-ACP before bleaching could also bring protective effect.16 However, the rationale of such application sequences has been rarely scrutinized, and which application sequence would bring optimum protective effect has not been evaluated in the literature.
45S5 bioglass (BG), originally developed by Hench et al.,17 consists of 45% SiO2, 24.5% Na2O, 24.5% CaO and 6% P2O5 in weight. It is a highly biocompatible material possessing remarkable osteoconductivity, osteoinductivity and controllable biodegradability.18 In aqueous media, this material is capable of forming hydroxycarbonate apatite that resembles biological mineral, and thus was widely used in bone regeneration and tissue engineering.18 Specializing for dental application, the commercial products derived from 45S5 bioglass include PerioGlas (NovaBone Osteobiologics, Jacksonville, FL, USA) and NovaMin (NovaMin Technology, Alachua, FL, USA). Previous studies suggested that BG can occlude dentine tubules, inhibit dentine demineralization and promote dentine remineralization via interfacial apatite precipitation.19,20 This apatite layer was reported tightly adherent to dentine tubules and resistant to acid and brushing-abrasion wear challenge.21 Furthermore, BG has also been shown capable of inhibiting and reversing initial caries progression in enamel.22,23 These studies indicate the potential of BG in preventing and restoring the enamel defect induced by bleaching agents. In principle, BG may form a protective layer on the enamel surface to inhibit demineralization when used before HP bleaching, or enhance remineralization when used after HP bleaching. Moreover, BG, as an alkaline salt, may buffer the acidity of HP and reduce demineralization when mixed with HP.
Therefore, the present study aimed to investigate whether BG, when used before and after HP bleaching, or mixed with HP for bleaching, can benefit the bleaching therapy as evaluated in terms of color, microhardness and morphology of bovine enamel. The null hypothesis was that incorporating BG into bleaching regimen can reduce the whitening efficacy and does not protect enamel demineralization induced by acid HP.
Materials and methods
Characterization of BG
45S5 BG was provided by the Nuohuamin Bio Sci. & Tech Co., Ltd (Wuhan, China). The powder was characterized by scanning electron microscopy coupled with electron-dispersive X-ray spectroscopy (SEM-EDXS), X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR). SEM-EDXS (S-2460N; Hitachi, Tokyo, Japan) was used to examine the surface morphology and constituents of BG. The crystalline phase was detected by XRD (X'Pert PRO; PANalytical, Almelo, Netherlands) operating at 10°–70° 2θ, 40 kV and 40 mA under Cu Kα radiation. The absorption spectra were recorded using FTIR (Nicolet 6700 spectrometer; Nicolet, Madison, WI, USA) on pellet samples obtained by pressing BG with KBr.
Specimen preparation
Permanent bovine incisors were obtained from a local slaughterhouse and stored at 4 °C in 0.1% thymol solutions until use. Cubic enamel blocks (5×5×4 mm3) were prepared with a diamond-coated band saw under continuous water cooling (Struers Minitom; Struers, Copenhagen, Denmark) and embedded in Orthodontic Resin (L.D. Caulk, Milford, CT, USA). The natural labial surfaces were serially smoothed with waterproof SiC abrasive papers (800–5 000 grit; Struers, Copenhagen, Denmark), followed by polishing on a felt cloth impregnated with diamond paste (1–5 µm; Struers, Copenhagen, Denmark), thus removing the outermost parts of enamel layer (approximately 200 µm as measured by a vernier caliper). Subsequently the specimens with mirror-like surfaces were ultrasonically cleaned for 5 min in distilled water bath, and inspected under a stereomicroscope (SMZ1000; Nikon, Tokyo, Japan) to exclude those with cracks or defects. Finally, the specimens were stored at Hank's balanced salts solution until use.
Treatment procedure
The specimens were randomly assigned to six treatment groups (Table 1). During the treatment period, the specimens were stored in an incubator with 95%±5% relative humidity at 37 °C. At treatment intervals, the specimens were thoroughly rinsed with air-water spray for 15 s and coated with cotton tissues containing distilled deionized water (DDW) to avoid dehydration. The treatment procedure was repeated in triplicate so that the total application time of 35% HP (Jingxin Chemcial Reagent Co. Ltd, Hangzhou, China) was 60 min. A mass fraction of 7.5% was used to make the BG+DDW and BG+HP suspensions.
Table 1. Treatment procedure.
| Groups (n=12) | Treatment on each specimen |
|---|---|
| DDW | 2 g DDW for 20 min |
| BG | 2.16 g BG+DDW suspension for 20 min |
| HP | 2 g HP for 20 min |
| BG before HP | 2.16 g BG+DDW suspension for 20 min followed by 2 g HP for 20 min |
| BG after HP | 2 g HP for 20 min followed by 2.16 g BG+DDW suspension for 20 min |
| BG during HP | 2.16 g BG+HP suspension for 20 min |
BG, bioglass; DDW, distilled deionized water; HP, hydrogen peroxide.
Color change
The colorimetric values were obtained using a spectrophotometer (SpectraScan PR-650; Photo Research, Los Angeles, CA, USA) in a darkened laboratory. The instrument, using wave length from 380 nm to 780 nm at 4 nm intervals, was warmed up for 30 min and pre-calibrated with a white reflectance standard (WS-1; Ocean optics, Dunedin, FL, USA). The 1.5 mm circular center of each specimen was recorded in terms of the Commission Internationale De L'Eclairage system under conditions of standard illuminant D65, 95 mm working distance, 10° observer and 45-0 geometry. Commission Internationale De L'Eclairage L*a*b* system provides a numeric three-dimensional color space, with L* representing dark-to-light, a* green-to-red and b* blue-to-yellow. Each specimen was measured in triplicate and rotated by 120° between two consecutive measurements. The overall color difference ΔE was determined according to the formula: ΔE=(ΔL*2+Δa*2+Δb*2)1/2, where ΔL*, Δa* and Δb* represented the difference between L*, a* and b* at baseline and endpoint of the experiment.
Surface and cross-section microhardness analysis
Surface microhardness (SMHbaseline) of the sound enamel was evaluated with a Knoop diamond indenter (Duramin-1/-2; Struers, Copenhagen, Denmark) at 50 g load for 15 s dwell time. Five indentations spaced 50 µm perpendicularly from each other were made at the center of the exposed window. After the treatment procedure, the specimens were ultrasonically cleaned for 5 min, and reassessed for final surface microhardness (SMHfinal) in Knoop hardness number (KNH). Another five indentations was averaged parallel (space=150 µm) to the baseline indentations. The percentage of surface microhardness loss was calculated as follows: SMHL=[(SMHbaseline–SMHfinal)/SMHbaseline]×100%
Subsequently, five blocks in each group were randomly selected to perform cross-sectional microhardness tests (CSMH) according to Cury et al.24 Briefly, the specimens were longitudinally sectioned through the center and two lanes of indentations each were made at the depths of 25, 50, 75, 100, 150 and 200 µm from the outer enamel surface. Microhardness indentations were made using a Knoop diamond indenter under a 25-g load for 15 s. CSMH values were converted to mineral content according to Featherstone et al.:25 volume percentage of mineral=4.3 KHN1/2+11.3.
SEM-EDXS analysis
After the SMH analysis, representative specimens from each group were selected for SEM preparation. These specimens were ultrasonically cleaned for 5 min, air-dried and gold sputter-coated. Morphological alterations of enamel surface were observed using scanning electron microscope (S-2460N; Hitachi, Tokyo, Japan). To observe the BG precipitation on enamel surface from BG+DDW and BG+HP suspensions, additional four specimens were prepared and treated with the same procedures as those in either group BG or group BG during HP. Then without ultrasonically cleaning, these specimens were directly air-dried and submitted to SEM preparation.
Chemical analysis of BG dissolution
BG+DDW and BG+HP suspensions were stirred at a constant rate of 75 r·min−1 at 37 °C for 20 min. The pH value was measured with a calibrated digital electrode (Orion 4-Star Plus Benchtop pH/ISE Meter; Thermo Fisher Scientific, Beverly, CA, USA). The filtrates of the suspensions for ionic release profiling were obtained by sequential passage through 0.45 µm and 0.2 µm Whatman filter (Puradisc 13 syringe filter; GE Healthcare, Waukesha, WI, USA). The free Ca, Na, Si and P were determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES, optima 2000DV; Perkin-Elmer, Waltham, MA, USA). Each element was measured in triplicate.
Statistical analysis
Statistical analysis was performed using the SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Data normality and variance homogeneity were tested by the Kolmogorov–Smirnov test and Levene's test. The SMHL and ΔE among groups were analyzed by one-way analysis of variance followed by least significant difference test and Tamhane's T2 test, respectively. CSMH and ionic release profiles were evaluated by two-way analysis of variance and post hoc multiple comparison Games–Howell tests. The significance level was set at 0.05.
Results
Characterization of BG
Figure 1a shows the SEM image of BG. The morphology of the powder was characterized by micrometric particles with irregular shape. EDXS identified the constituents of Ca, Si, Na, O and C, while the presence of C was attributed to the carbon-tabs on which the sample was mounted (Figure 1b). The XRD pattern exhibited a peak centered at 2θ≈32° with a broad contour and low intensity, indicating the amorphous state of BG (Figure 1c). The FTIR spectrum is showed in Figure 1d. The bands at 1 026, 934 cm−1 were attributed to Si–O–Si asymmetric, symmetric stretching modes and 496 cm−1 to Si–O–Si bending mode, respectively.
Figure 1.
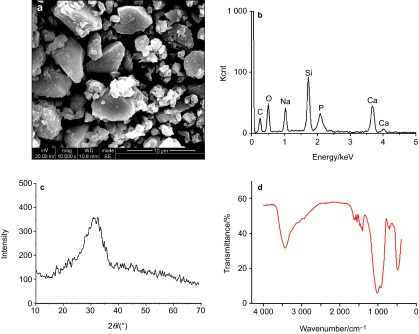
Characterization of BG. (a) SEM image; (b) EDXS; (c) XRD pattern; (d) FTIR spectrum. BG, bioglass; EDXS, electron-dispersive X-ray spectroscopy; FTIR, Fourier-transform infrared spectroscopy; SEM, scanning electron microscopy; XRD, X-ray diffraction.
Color change
The mean colorimetric change according to different treatments is illustrated in Table 2. The ΔE of group HP, BG before HP, BG after HP and BG during HP were significantly higher than those of group DWW (P=0.000, P=0.000, P=0.002, P=0.002) and BG (P=0.000, P=0.000, P=0.001, P=0.001). No statistically significant difference of the ΔE was found among group HP, and BG before HP, BG after HP, BG during HP (P=1.000, P=1.000, P=1.000), and between group DWW and BG (P=1.000).
Table 2. Effect of different treatments on enamel color parameters.
| Groups | ΔL | Δa | Δb | ΔE |
|---|---|---|---|---|
| DDW | −0.32 (1.10) | −0.41 (0.47) | −1.23 (0.85) | 1.85 (0.61)a |
| BG | −0.12 (1.41) | −0.49 (0.36) | −0.70 (0.84) | 1.59 (0.94)a |
| HP | 4.04 (2.91) | −0.83 (0.46) | −2.56 (1.57) | 5.54 (1.83)b |
| BG before HP | 3.79 (1.71) | −0.84 (0.57) | −3.01 (1.21) | 5.08 (1.71)b |
| BG after HP | 3.82 (2.20) | −0.79 (0.75) | −2.87 (2.05) | 5.32 (2.10)b |
| BG during HP | 3.24 (2.93) | −0.55 (1.22) | −2.74 (1.78) | 5.15 (2.06)b |
BG, bioglass; DDW, distilled deionized water; HP, hydrogen peroxide.
Mean values (standard deviations).
Different lower case superscript letters (a-b) denote statistical significant difference for ΔE in different groups (P<0.05).
Surface and cross-section microhardness analysis
Surface microhardness evaluations are illustrated in Table 3. The baseline data were equivalent among all groups (P=0.841). The SMHL of group BG before HP, BG after HP and BG during HP was significantly higher than that of group DWW (P=0.000, P=0.000, P=0.000) and group BG (P=0.000, P=0.000, P=0.000), but lower that of group HP (P=0.003, P=0.043, P=0.000). Group BG during HP showed significantly lower SMHL than group BG before HP and BG after HP (P=0.015, P=0.001). No statistically significant difference of SMHL was found between group BG before HP and BG after HP (P=0.308).
Table 3. Surface microhardness analysis according to the treatments.
| Groups | SMHbaseline | SMHfinal | SMHL/% |
|---|---|---|---|
| DDW | 383.11 (28.76) | 380.00 (26.85) | 0.66 (3.33)a |
| BG | 373.47 (24.91) | 369.89 (26.76) | 0.64 (3.80)a |
| HP | 370.04 (28.70) | 299.97 (22.76) | 19.60 (5.45)b |
| BG before HP | 382.53 (33.57) | 328.80 (33.41) | 14.00 (4.46)c |
| BG after HP | 372.73 (25.83) | 313.14 (22.76) | 15.87 (4.48)c |
| BG during HP | 373.22 (36.66) | 336.58 (19.13) | 9.48 (4.86)d |
BG, bioglass; DDW, distilled deionized water; HP, hydrogen peroxide; SMH, surface microhardness.
Mean values (standard deviations).
Different lower case superscript letters (a-d) denote statistical significant difference for SMHL in different groups (P<0.05).
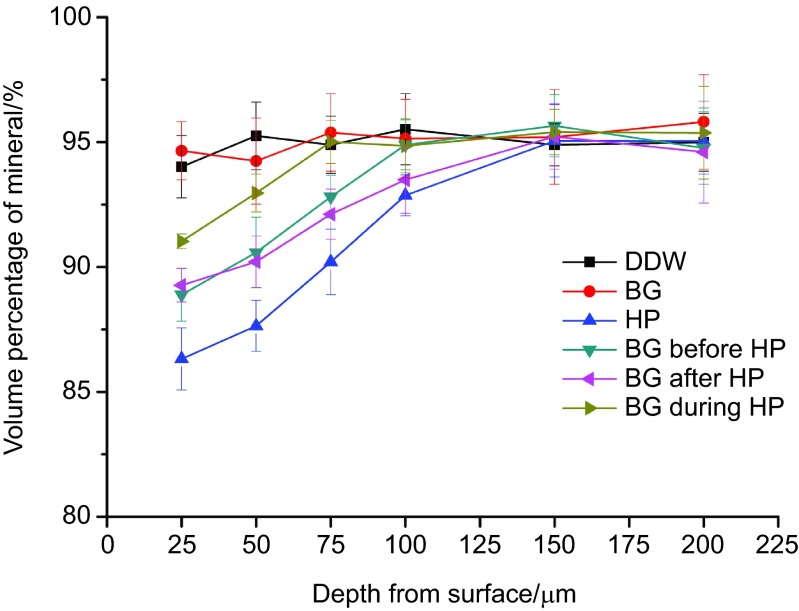
The profiles of volume percent mineral vs. individual depth from the surface are shown in Table 4 and Figure 2. Group HP showed mineral content reduction extending beyond 100 µm from enamel surface. The mineral content of group BG before HP and BG after HP was significantly lower group BG during HP, but higher than group HP within 25–75 µm from enamel surface (P<0.05). No statistically significant difference of mineral content was found between group BG before HP and BG after HP within 25–75 µm (P>0.05). However, in deeper depth of 100 µm, group BG after HP was not as effective as group BG before HP and BG during HP in maintaining the mineral content (P>0.05).
Table 4. Enamel mineral content values of different groups.
| Depth from surface/μm | Volume percentage of mineral/% | |||||
|---|---|---|---|---|---|---|
| DDW | BG | HP | BG before HP | BG after HP | BG during HP | |
| 25 | 94.01 (1.24)aA | 94.65 (1.16)aA | 86.32 (1.24)bA | 89.89 (1.06)cA | 89.27 (0.61)cA | 91.03 (1.29)dA |
| 50 | 95.25 (1.35)aA | 94.24 (1.72)aA | 87.64 (1.89)bA | 90.58 (1.41)cB | 90.21 (1.03)cA | 92.96 (0.76)dB |
| 75 | 94.89 (1.14)aA | 95.38 (1.55)aA | 90.20 (1.31)bB | 92.81 (0.85)cC | 92.51 (1.01)cB | 95.01 (0.86)aC |
| 100 | 95.51 (1.42)aA | 95.14 (1.57)aA | 92.87 (0.82)bC | 94.89 (1.00)aD | 93.49 (1.34)bC | 94.85 (1.08)aC |
| 150 | 94.89 (0.84)aA | 95.20 (1.90)aA | 95.05 (1.45)aD | 95.65 (1.24)aD | 95.22 (1.31)aD | 95.40 (1.14)aC |
| 200 | 94.99 (1.16)aA | 95.81 (2.12)aA | 95.04 (1.32)aD | 94.76 (1.46)aD | 94.60 (2.04)aD | 95.37 (1.86)aC |
BG, bioglass; DDW, distilled deionized water; HP, hydrogen peroxide; SMH, surface microhardness.
Different lower (a-d) and upper (A-D) case letters indicate statistically significant differences (P<0.05) for inter-group and intra-group comparison, respectively.
Figure 2.
Comparative cross-sectional mineral profiles for different groups.
SEM analysis
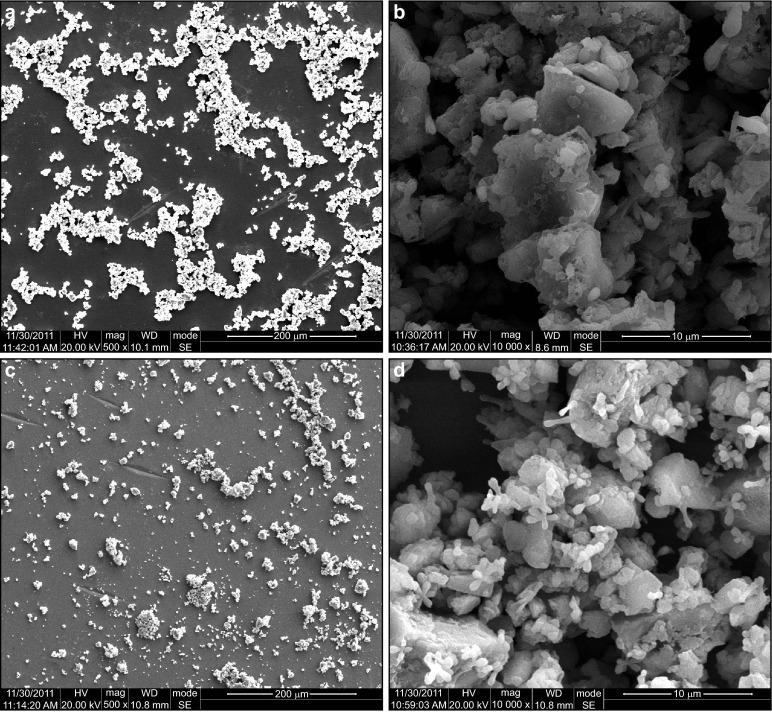
Representative SEM micrographs of enamel morphology are shown in Figure 3. The polished mirror-like enamel surfaces were unchanged in group DDW, BG and BG during HP. The most obvious morphological alteration was observed on enamel surfaces in group HP. The enamel prisms were eroded visible and the normal interprismatic structures were demolished with porosities. The slight morphological alteration was also detected in group BG before HP and BG after HP, with vague interprismatic destruction.
Figure 3.
The SEM image (×10 000) of enamel surfaces according to different treatments. (a) group DDW; (b) group BG; (c) group HP; (d) group BG before HP; (e) group BG after HP; (f) group BG during HP. BG, bioglass; DDW, distilled deionized water; HP, hydrogen peroxide; SEM, scanning electron microscopy.
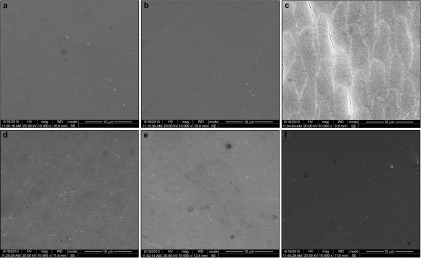
Figure 4 shows the retained BG on enamel surfaces treated with BG+DDW and BG+HP suspensions. The treated enamel surfaces were covered with disorderly packed BG particles, and BG particles from BG+HP suspension were more dispersed than those from BG+DDW suspension. Under higher magnification, unlike the irregular BG surfaces from BG+DDW suspension, remarkable dumbbell-like crystallites were formed and aggregated into flower-like appearance on BG surfaces from BG+HP suspension.
Figure 4.
The SEM image of retained BG on enamel surfaces. (a, b): Treated with BG+DDW; (c, d): Treated with BG+HP suspensions. At ×500 (left) and ×10 000 (right) magnifications. BG, bioglass; DDW, distilled deionized water; HP, hydrogen peroxide; SEM, scanning electron microscopy.
Chemical analysis of BG dissolution
After addition of BG, a rapid increase of pH (Figure 5a) occurred within 30 s of dissolution, and the pH of HP solution was elevated from 2.31 to 4.73. Ionic release profiles were shown in Figure 5b. Two-way analysis of variance analysis of Na, Ca, Si and P showed a statistically significant main effect for different suspensions and dissolution time (P=0.000), as well as time–suspensions interaction (P=0.000). The ionic release of BG was significantly faster in HP compared to that in DDW.
Figure 5.
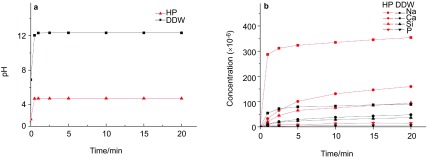
pH change and ionic release profile of BG+DDW and BG+HP suspensions. BG, bioglass; DDW, distilled deionized water; HP, hydrogen peroxide.
Discussion
The null hypothesis of this study was rejected because incorporating BG into bleaching regimen reduced the mineral loss and morphological alterations of enamel surface without impeding the whitening efficacy. The present study highlighted the beneficial effect of incorporating BG into bleaching regimen as well as the rationale of using BG during HP bleaching rather than before and after HP bleaching.
Dental enamel is the hardest and stiffest mineralized biological tissue containing approximately 96% mineral, 3% water and 1% organic matter by weight. In the present study, enamel specimens were obtained from bovine incisors. Bovine enamel was recognized as a reliable substitute for human enamel due to their similarity of physicochemical properties, such as composition, density, hardness and tensile strength.26,27 Despite a higher lightness with the shade of bovine enamel than in human enamel,28 use of young bovine teeth is considered a practicable model for evaluating bleaching regimens.8 The superficial aprismatic enamel is generally hypermineralized and rich in fluorapatite, and thus is more resistant to demineralization.29 This layer was removed and polished to retain a uniform mirror-like surface, minimizing the variance among teeth.
Currently, no standard protocol has been established to investigate the effects of bleaching regimens on dental hard tissues. It is generally designed based on whether the bleaching regimen is performed at home or in office. The at home bleaching regimens using low concentrations of HP or carbamide peroxides (CP) commonly requires application time for weeks, during which demineralization and remineralization will interact.30 Therefore an in office bleaching regimen using high concentration of HP was selected to avoid the influence of alternating de/remineralization on the effect of BG. Likewise, artificial saliva was also not used to store the enamel samples during treatment intermission, as it would remineralize the enamel and interact with BG, and thus interfere with the protective effect of BG against HP. In addition, the Knoop indenter penetrates about half as deep as the Vickers indenter;8 therefore, it is more sensitive than a Vickers indenter to differentiate superficial hardness changes. Overall, the simple set of present study offers a best-case scenario to effectively monitor the enamel change in a short time range.
The tooth whitening is achieved by decomposing HP into active oxygen radicals, which can destroy the double bonds or oxidize other chemical moieties in the conjugated chain of chromophore.3 This decolourization process can be evaluated by visual shade and spectrophotometer, whilst the latter is more objective and reproducible for quantitative evaluation of tooth color change.31 In the present study, all bleaching groups showed typical color shift pattern of increased L* and decreased b* values, which was consistent with previous studies.14,30 Besides, because ΔE values of at least 3.3 are known to be visually perceptible,32 all bleaching treatments can be considered effective for tooth whitening with ΔE ranging from 5.08 to 5.54. Although it is conceivable that BG deposits may impair penetration of peroxide and impede the whitening efficacy, this speculation was not confirmed as ΔE values were equivalent among all bleaching groups. In addition, we expected that using BG during HP might enhance the whitening efficacy. However, this was not supported by our results. Previous studies showed similar results that increasing the pH value of HP from 3 to 5 could not brought significantly greater enamel color change, unless the pH was elevated above 7.30 It is possible that in moderate acid condition around 4.73 after addition of BG, the decomposition rate of HP is still limited to result in an enhanced whitening efficacy. In addition, our findings also suggest that short-term use of BG alone could not produce whitening effect. Therefore, the whitening ability of BG incorporated bleaching regimens should be mainly attributed to the HP.
Increased enamel mineral loss and pits, depressions and porosities of enamel surface were reported after HP bleaching.5,14,30 The oxide-reduction reaction of the bleaching agent can lead to dissolution of both organic and inorganic dental matrices up to the point that only carbon dioxide and water remain.33 In the present study, HP induced severe enamel SMH, CSMH loss and microstructure destruction, which was in agreement with these studies.5,14,30 It was suggested that morphological change of enamel is mainly due to the acidity of HP, while microhardness loss to the combined effects of demineralization and destruction of organic matter by HP (ref. 34). In contrast, pre/post-bleaching use of BG possessed much milder SMH, CSMH loss and morphological changes, indicating obvious protective effect of BG against HP. A recent study showed using BG after bleaching could remineralize enamel surface by increase the superficial Ca and P contents.35 The findings in this study corroborate that using BG after bleaching can enhance mineral gain in both enamel surface and subsurface layer and further reveal that pre-bleaching use of BG can also reduce mineral loss. It is important to consider that BG, as an inorganic compound in the class of highly biocompatible materials, reacts in aqueous environments to release Ca2+, Na+, and PO43– (ref. 23). More specifically, the rapid ionic exchange of Na+ with H+ or H3O+ at glass/liquid interface, as confirmed by initial rapid increase of pH and Na+, allowed Ca2+ and PO43– to be released to form supersaturated ionic reservoir for the enamel apatite. After BG network dissolution, silanols underwent rearrangement by polycondensation and served as the nucleation site. The disposition of free calcium and phosphate together with undissolved BG particles formed a protective calcium phosphate-rich layer on enamel surface. It also possible that the opening of diffusion channels on bleached enamel facilitated diffusion of calcium and phosphate into deeper enamel layers to favor remineralization over demineralization, as supported by the milder etched change after the pre/post bleaching use of BG. Considering saliva induced maximum apatite growth of BG (ref. 19), we further assume that BG mixed with saliva would bring even better protection when used before and after HP bleaching. This hypothesis will be investigated in future studies.
The most important finding of this study is that using BG during HP could more effectively reduce the mineral loss of enamel while retaining the enamel surface integrity, demonstrating optimum protective effect compared to pre/post-bleaching use of BG. BG, as an alkaline salt, elevated the pH of HP to 4.73 and reduced its acidity. Besides, the mineral ionic release of BG in HP was much faster in both magnitude and rate than that in DDW. Moreover, in acid condition, the released mineral ions may have a greater capacity to penetrate into deeper enamel layer compared to that in neutral condition, as supported by previous study.36 These effects together might account for the enhanced protection of using BG during HP. Generally, long reaction time has limited the application of BG as an efficient remineralization agent. Previous studies showed that the dentine remineralization can be improved by using nanometric BG particles, or supplying BG with phosphoric acid, nitric acid, and CO2 laser.19,20,37,38 In the present study, faster ionic release of BG indicates that HP can also catalyze the reaction of BG. Moreover, the unexpected formation of dumbbell-like and flower-like crystallites in BG+HP suspension supports this speculation. However, these crystallites were not detected by XRD (data not shown), possibly because that the mineral phases were not voluminous enough to result in significant diffraction intensity over the noisy amorphous background. It should be noticed that there was still slight microhardness reduction in BG during HP group. HP could alter the enamel organic matrix by protein oxidation,34 and denaturation of enamel organic matrix resulted in significant change of enamel hardness.39 Considering microhardness reflects not only mineral content but also its related organic matrix, it is reasonable to assume that oxidative degradation contributed to the microhardness loss observed in this study.
To maintain long-term stability, the majority of commercial HP products are made highly acidic and concentrated. It was also reported that carbopol, a frequently used stabilizer, caused a continuing demineralization of enamel even at neutral pH (ref. 40). Although the superficial changes of bleached enamel can be restored by salivary electrolytes,41 these initial bleached lesions might be further aggravated to form erosion-abrasion lesions. Therefore, a ‘milder' or even ‘non-invasive' bleaching approach brought by BG supplement may help to prevent the irreversible alterations of enamel surface. In addition, bleaching often produces an uneven, dull and lifeless appearance of tooth due to the decrease of enamel luster and translucency. Whether BG can prevent such overbleached tooth appearance has yet to be investigated. Therefore, further clinical trials are warranted to confirm the beneficial effect of incorporating BG into tooth bleaching therapy.
Conclusions
Within the limitation of this study, we may conclude that the 35% HP solution resulted in significant microhardness loss and morphological change of enamel. Using BG alone could not whiten the enamel, and the combination of BG and HP did not alter the whitening efficacy. Compared to pre/post-bleaching use of BG, using BG during HP was the optimal way to reduce the microhardness loss of enamel while retaining the enamel surface integrity. The alkalinity and accelerated ionic releasing of BG in HP make BG a promising biomimetic adjunct for bleaching therapy to ensure the lifelong integrity of tooth.
Acknowledgments
The authors thank Nuohuamin (Wuhan, China) for providing BG used in this study. This work was supported by the Research Fund from Science and Technology Department of Sichuan Province (No. 2009FZ0065), Key Project of the Science and Technology Department of Sichuan Province (No. 2011SZ0101), and Doctoral Fund of Ministry of Education of China (No. 20120181120002). Publication of this manuscript is supported by Open Fund of State Key Laboratory of Oral Diseases, Sichuan University.
References
- Kihn PW. Vital tooth whitening. Dent Clin North Am. 2007;51 2:319–331. doi: 10.1016/j.cden.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Sulieman M. An overview of bleaching techniques: I. History, chemistry, safety and legal aspects. Dent Update. 2004;31 10:608. doi: 10.12968/denu.2004.31.10.608. [DOI] [PubMed] [Google Scholar]
- Joiner A. The bleaching of teeth: a review of the literature. J Dent. 2006;34 7:412–419. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Severcan F, Gokduman K, Dogan A, et al. Effects of in-office and at-home bleaching on human enamel and dentin: an in vitro application of Fourier transform infrared study. Appl Spectrosc. 2008;62 11:1274–1279. doi: 10.1366/000370208786401554. [DOI] [PubMed] [Google Scholar]
- Josey AL, Meyers IA, Romaniuk K, et al. The effect of a vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil. 1996;23 4:244–250. doi: 10.1111/j.1365-2842.1996.tb00848.x. [DOI] [PubMed] [Google Scholar]
- da Silva AP, de Oliveira R, Cavalli V, et al. Effect of peroxide-based bleaching agents on enamel ultimate tensile strength. Oper Dent. 2005;30 3:318. [PubMed] [Google Scholar]
- Attin T, Vollmer D, Wiegand A, et al. Subsurface microhardness of enamel and dentin after different external bleaching procedures. Am J Dent. 2005;18 1:8. [PubMed] [Google Scholar]
- Chen HP, Chang CH, Liu JK, et al. Effect of fluoride containing bleaching agents on enamel surface properties. J Dent. 2008;36 9:718–725. doi: 10.1016/j.jdent.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Attin T, Betke H, Schippan F, et al. Potential of fluoridated carbamide peroxide gels to support post-bleaching enamel re-hardening. J Dent. 2007;35 9:755–759. doi: 10.1016/j.jdent.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Costa JB, Mazur RF. Effects of new formulas of bleaching gel and fluoride application on enamel microhardness: an in vitro study. Oper Dent. 2007;32 6:589–594. doi: 10.2341/06-166. [DOI] [PubMed] [Google Scholar]
- Burgmaier GM, Schulze IM, Attin T. Fluoride uptake and development of artificial erosions in bleached and fluoridated enamel in vitro. . J Oral Rehabil. 2002;29 9:799–804. doi: 10.1046/j.1365-2842.2002.00966.x. [DOI] [PubMed] [Google Scholar]
- Tschoppe P, Neumann K, Mueller J, et al. Effect of fluoridated bleaching gels on the remineralization of predemineralized bovine enamel in vitro. . J Dent. 2009;37 2:156–162. doi: 10.1016/j.jdent.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Cross KJ, Huq NL, Reynolds EC. Casein phosphopeptides in oral health-chemistry and clinical applications. Curr Pharm Design. 2007;13:793–800. doi: 10.2174/138161207780363086. [DOI] [PubMed] [Google Scholar]
- Jiang T, Ma X, Wang Z, et al. Beneficial effects of hydroxyapatite on enamel subjected to 30% hydrogen peroxide. J Dent. 2008;36 11:907–914. doi: 10.1016/j.jdent.2008.07.005. [DOI] [PubMed] [Google Scholar]
- de Vasconcelos AA, Cunha AG, Borges BC, et al. Enamel properties after tooth bleaching with hydrogen/carbamide peroxides in association with a CPP-ACP paste. Acta Odontol Scand. 2012;70 4:337–343. doi: 10.3109/00016357.2011.654261. [DOI] [PubMed] [Google Scholar]
- Gama Cunha AG, Meira de Vasconcelos AA, Dutra Borges BC, et al. Efficacy of in-office bleaching techniques combined with the application of a casein phosphopeptide-amorphous calcium phosphate paste at different moments and its influence on enamel surface properties. Microsc Res Techniq. 2012;75 8:1019–1025. doi: 10.1002/jemt.22026. [DOI] [PubMed] [Google Scholar]
- Hench LL, Splinter RJ, Allen WC, et al. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;5 6:117–141. [Google Scholar]
- Andersson Ö H, Kangasniemi I. Calcium phosphate formation at the surface of bioactive glass in vitro. J Biomed Mater Res. 1991;25 8:1019–1030. doi: 10.1002/jbm.820250808. [DOI] [PubMed] [Google Scholar]
- Curtis AR, West NX, Su B. Synthesis of nanobioglass and formation of apatite rods to occlude exposed dentine tubules and eliminate hypersensitivity. Acta Biomater. 2010;6 9:3740–3746. doi: 10.1016/j.actbio.2010.02.045. [DOI] [PubMed] [Google Scholar]
- Vollenweider M, Brunner TJ, Knecht S, et al. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007;3 6:936–943. doi: 10.1016/j.actbio.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Burwell A, Jennings D, Greenspan DC. NovaMin and dentin hypersensitivity—in vitro evidence of efficacy. J Clin Dent. 2010;21 3:66. [PubMed] [Google Scholar]
- Rehder Neto FC, Maeda FA, Turssi CP, et al. Potential agents to control enamel caries-like lesions. J Dent. 2009;37 10:786–790. doi: 10.1016/j.jdent.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Burwell AK, Litkowski LJ, Greenspan DC. Calcium sodium phosphosilicate (NovaMin®): remineralization potential. Adv Dent Res. 2009;21 1:35–39. doi: 10.1177/0895937409335621. [DOI] [PubMed] [Google Scholar]
- Cury JA, Rebelo M, del Bel Cury AA, et al. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res. 2000;34 6:491–497. doi: 10.1159/000016629. [DOI] [PubMed] [Google Scholar]
- Featherstone JD, ten Cate JM, Shariati M, et al. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 1983;17 5:385–391. doi: 10.1159/000260692. [DOI] [PubMed] [Google Scholar]
- Mellberg JR. Hard-tissue substrates for evaluation of cariogenic and anti-cariogenic activityi. J Dent Res. 1992;71:913–919. doi: 10.1177/002203459207100S25. [DOI] [PubMed] [Google Scholar]
- Urabe I, Nakajima S, Sano H, et al. Physical properties of the dentin-enamel junction region. Am J Dent. 2000;13 3:129. [PubMed] [Google Scholar]
- Kwon YH, Huo MS, Kim KH, et al. Effects of hydrogen peroxide on the light reflectance and morphology of bovine enamel. J Oral Rehabil. 2002;29 5:473–477. doi: 10.1046/j.1365-2842.2002.00856.x. [DOI] [PubMed] [Google Scholar]
- Kanemura N, Sano H, Tagami J. Tensile bond strength to and SEM evaluation of ground and intact enamel surfaces. J Dent. 1999;27 7:523–530. doi: 10.1016/s0300-5712(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Xu B, Li Q, Wang Y. Effects of pH values of hydrogen peroxide bleaching agents on enamel surface properties. Oper Dent. 2011;36 5:554–562. doi: 10.2341/11-045-1. [DOI] [PubMed] [Google Scholar]
- Paul S, Peter A, Pietrobon N, et al. Visual and spectrophotometric shade analysis of human teeth. J Dent Res. 2002;81 8:578. doi: 10.1177/154405910208100815. [DOI] [PubMed] [Google Scholar]
- Gomes MN, Francci C, Medeiors IS, et al. Effect of light irradiation on tooth whitening: enamel microhardness and color change. J Esthet Restor Dent. 2009;21 6:387–394. doi: 10.1111/j.1708-8240.2009.00295.x. [DOI] [PubMed] [Google Scholar]
- Soldani P, Amaral CM, Rodrigues JA. Microhardness evaluation of in situ vital bleaching and thickening agents on human dental enamel. Int J Periodontics Restorative Dent. 2010;30 2:203. [PubMed] [Google Scholar]
- Jiang T, Ma X, Wang Y, et al. Investigation of the effects of 30% hydrogen peroxide on human tooth enamel by Raman scattering and laser-induced fluorescence. J Biomed Opt. 2008;13 1:14019. doi: 10.1117/1.2870114. [DOI] [PubMed] [Google Scholar]
- Gjorgievska E, Nicholson JW. Prevention of enamel demineralization after tooth bleaching by bioactive glass incorporated into toothpaste. Aust Dent J. 2011;56 2:193–200. doi: 10.1111/j.1834-7819.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4 3:34104. doi: 10.1088/1748-6041/4/3/034104. [DOI] [PubMed] [Google Scholar]
- Lee BS, Tsai HY, Tsai YL, et al. In vitro study of DP-bioglass paste for treatment of dentin hypersensitivity. Dent Mater J. 2005;24 4:562–569. doi: 10.4012/dmj.24.562. [DOI] [PubMed] [Google Scholar]
- Bakry AS, Takahashi H, Otsuki M, et al. CO2 Laser improves 45S5 bioglass interaction with dentin. J Dent Res. 2011;90 2:246. doi: 10.1177/0022034510387793. [DOI] [PubMed] [Google Scholar]
- He L. Sydney; The University of Sydney; 2008. Mechanical behaviour of human enamel and the relationship to its structural and compositional characteristics [dissertation] [Google Scholar]
- Basting RT, Rodrigues AL, Jr, Serra MC. The effect of 10% carbamide peroxide, carbopol and/or glycerin on enamel and dentin microhardness. Oper Dent. 2005;30 5:608. [PubMed] [Google Scholar]
- Türkün M, Sevgican F, Pehlivan Y, et al. Effects of 10% carbamide peroxide on the enamel surface morphology: a scanning electron microscopy study. J Esthet Restor Dent. 2002;14 4:238–244. doi: 10.1111/j.1708-8240.2002.tb00169.x. [DOI] [PubMed] [Google Scholar]