Abstract
Objective
This study was designed to investigate the potential beneficial effects of bone marrow stromal cell (MSC) treatment of traumatic brain injury (TBI) in mice.
Methods
Twelve female C57BL/6J mice (weight, 21–26 g) were injured with controlled cortical impact and divided into 2 groups (n = 6 each). The experimental group was injected with MSCs (0.3 × 106) intravenously one day after TBI, whereas the control group was injected with saline. MSCs were harvested from male mice, and male to female transplantation performed to identify male donor cells within female recipient animals. This was achieved by localizing Y chromosomes within the female mice. Neurological function was assessed using the Morris water maze and Foot Fault tests. All mice were sacrificed 35 days after TBI. Brain sections were stained using in situ hybridization and immunohistochemistry to identify MSCs as well as to analyze vascular density following MSC treatment.
Results
Both modalities of testing demonstrated significant improvement in neurological function in the MSC-treated group compared to the saline-treated control group (p < 0.05). Histologically, Y-chromosome labeled MSCs were easily identified in the injured brain, localized primarily around the lesion boundary zone. There was also significant increase in vascular density in the lesion boundary zone and hippocampus of MSC-treated mice compared to control mice.
Conclusion
This is the first study to show beneficial effects of MSC treatment after TBI in mice.
Keywords: Traumatic brain injury (TBI), marrow stromal cells (MSCs), mice
INTRODUCTION
Traumatic brain injury (TBI) continues to be a major health problem in the United States. The incidence of patient’s with closed head injuries admitted annually to hospitals is 200 per 100,000 (Narayan et al., 2002). Currently, there is no effective treatment to repair the biostructural damage that occurs following TBI. We have utilized neurorestorative treatments (Lu et al., 2004; Lu et al., 2003; Lu et al., 2001; Mahmood et al., 2003; Mahmood et al., 2005; Mahmood et al., 2001) as a means of invoking neural plasticity to reduce neurological deficits after TBI. One agent that has shown promise in improving outcome after TBI is bone marrow derived marrow stromal cells (MSCs) (Lu et al., 2003; Lu et al., 2001; Mahmood et al., 2005; Mahmood et al., 2001). However, all of the research on MSCs to date has focused on rat models of TBI. To test the efficacy of MSCs across another species, we have investigated the effects of MSCs in mice after TBI. The expansion of MSC research to mice will make it easier to conduct future studies on the mechanism of action of MSCs. Genetic as well as molecular studies are easier to conduct in mice than in some other species such as the rat. Although MSCs have shown great promise in improving outcome after TBI, the factors linking molecular and biochemical events to therapeutic benefit still need to be identified and clarified. Further research using the mouse model of TBI will help us address many of these questions.
RESULTS
Spatial learning functional response
Significant MSC effect and time effect was observed with p<0.01 for MSC effect and p=0.02 for time effect, respectively. No treatment by time interaction was observed (p=0.55).
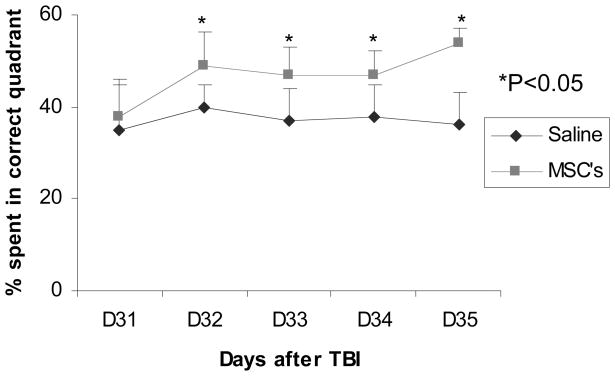
On Day 31 post TBI, the mean percentage of time spent in the correct quadrant (containing the platform) was approximately 37%, for both the MSC- and saline-treated groups. On Day 32 post TBI, the percentage of time the animal spent in the correct quadrant increased significantly in the MSC-treated group compared to that of the saline-treated group (49 ± 7.5% versus 40 ± 5.0%) (p<0.05). The difference between the two groups remained consistent during the subsequent days with p<0.05 (Fig. 1). These data demonstrate that transplantation of MSCs enhances the restoration of spatial learning function damaged by TBI in the female wild type C57BL/J6 mouse.
Fig. 1.
Graph showing time spent in the correct quadrant in the MSC- and saline-treated groups during the Morris water maze test. The percentage time spent in the correct quadrant was significantly more in the MSC-treated group (n=6) than in the saline-treated (n=6) group from 32–35 days post TBI. * = p<0.05.
Foot Fault Test
Significant overall MSC effect was observed (p<0.05) on improvement of forelimb as well as hind limb function (Fig 2). This improvement was seen on Days 5, 8, 14 and 21 after TBI. These data demonstrate that MSCs reduce the neuromotor deficits from TBI.
Fig. 2.
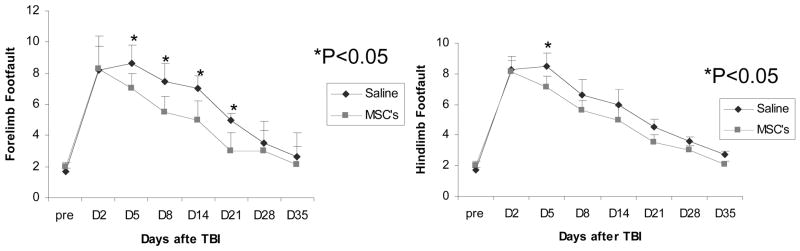
Graph showing the results of Foot Fault test in the right forelimb (left, n=6) and right hind limb (right, n=6). There was significant improvement seen on Days 5, 8, 14 and 21 post TBI. * = p<0.05.
Lesion Volume
Using the image analyzer system, the lesion volume, including both the cavity and the lesion boundary zone in the cortex, was calculated in both groups. There was no significant difference in lesion volume between the MSC- and the saline-treated groups (8.55 ± 3.4% versus 10.89 ± 4.3%, respectively). These data demonstrate that the transplanted MSCs do not significantly reduce lesion volume in the female mouse after TBI.
Distribution and identification of Y chromosome-positive cells
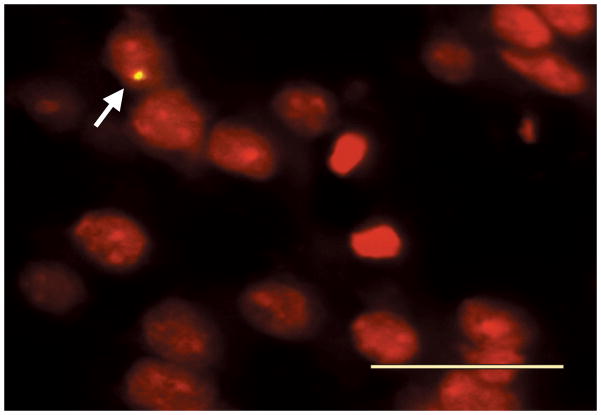
No Y chromosome-positive cells were observed in the slides from the saline-treated group. In the brains of the MSC-treated animals, Y chromosome-positive cells were detected in the boundary zone of the injured area (Fig. 3). The average number of cells was 35.2 ± 5.58 (mean ± SD) per millimeter square. The results show that the intravenously transplanted MSCs can reach the area damaged by TBI.
Fig. 3.
Microphotograph showing Y chromosome positive MSC (arrow) within the lesion boundary zone. The digoxigenin-labeled Y chromosome is clearly visible within the nucleus stained by propidium iodide (red). Bar = 25 μm, original magnification × 400.
Vascular Changes after TBI
The number of vessels was measured in the lesion boundary zone of the injured cortex and CA3 region of the hippocampus by counting von Willebrand Factor (VWF)-positive vessels per square millimeter, regardless of the vessel size (Fig. 4). Vessel density was significantly greater in the MSC-treated group compared to the saline-treated group in the lesion boundary zone (465±43/mm2 compared with 361±41/mm2, p<0.05) as well as in the hippocampal CA3 region (294±34/mm2 compared with 210±27/mm2, p<0.05; Fig 5). These data demonstrate that MSCs increase the vascular density in the injured brain in female mouse after TBI.
Fig. 4.

Microphotographs showing DAB staining for vWF for evaluation of vascular density in the saline- (a) and MSC-treated (b) groups. Scale bar shown in b = 50 μ. Original magnification, × 200.
Fig. 5.
Bar graphs showing the vascular density to be significantly more in the boundary zone (BZ) and hippocampus (CA3) in the MSC-treated group (n=6). * = p<0.05.
DISCUSSION
Our results demonstrate that MSCs 1) migrate successfully into injured mouse brain, 2) improve functional outcome, and 3) increase vascular density.
The MSCs were injected intravenously with a dose of 0.3 × 106 cells 1 day after TBI. The dose was selected based on our previous experience with MSC treatment of rats (i.e., by calculating the number of cells per killogram of animal) (Lu et al., 2003; Lu et al., 2001; Mahmood et al., 2005; Mahmood et al., 2001). The cells were injected intravenously rather than directly transplanting them into the lesion cavity. Intravenous administration is a safer and easier mode of administration, and a larger number of cells can be injected via this route rather than by intracerebral transplantation. We have successfully used intravenous administration in TBI experiments in rats and have achieved therapeutic benefits (Lu et al., 2001; Mahmood et al., 2005; Mahmood et al., 2001). MSCs can engraft within mouse brain after systemic infusion and this engraftment is increased after TBI (Francois et al., 2006). In the present study, MSCs could be identified within the injured brain as late as 35 days after injection. This confirms their successful migration within the central nervous system after systemic administration. They were localized primarily within the lesion boundary zone which is consistent with our previous studies of TBI in rats (Lu et al., 2001; Mahmood et al., 2005; Mahmood et al., 2001).
We did not use any immunosuppression in this study since immune rejection has never been observed after MSC transplantation (Lu et al., 2001; Mahmood et al., 2003; Mahmood et al., 2005; Mahmood et al., 2001; Li et al., 2002). This lack of immune response to MSCs may be related to weak immunogenicity of these cells due to absence or low expression of major histocompatibility complex Classes I and II and costimulatory molecules (CD40, CD80, CD86) (Zinkernagel et al., 1979). There is also the possibility that MSCs secrete mediators that decrease the immune responses involved in xenograft rejection (Bernstein et al., 1988).
Brain injury in this model of controlled cortical impact is characterized by cystic cavity formation in the cortex that causes asymmetric sensorimotor deficits (Lu et al., 2003) and selective cell damage in hippocampal formation that causes spatial learning dysfunction (Lu et al., 2004). Therefore, sensorimotor (foot fault) and spatial function tests (Morris water maze) were utilized to evaluate functional response to injury and treatment after TBI, and we observed improvement with both modalities. Hippocampus and other basal forebrain systems play a major role in maintaining cognitive function and spatial learning (Shindo et al., 2006), and they are also the areas selectively susceptible to mechanically induced injury (Geddes et al., 2003). MSCs have been shown to promote cellular proliferation within the hippocampus and subventricular zone after TBI (Mahmood et al., 2004) and this effect may be at least partly responsible for the improvement in spatial learning seen in mice after TBI. In addition to inducing cellular proliferation MSC treatment has also been shown to suppress apoptosis and promote neuronal survival (Li et al., 2002).
Our study also showed that MSCs induced the increased vascular density indicative of angiogenesis. This neovascularity can improve perfusion within the injured brain and thereby promote neural recovery. Ischemia is an integral component of TBI (Hlatky et al., 2003) and adds significantly to the cascade of damaging events that follow it. Promotion of angiogenesis by MSCs may also be autoinductive since it will enhance the delivery as well as survival of MSCs within the target tissue.
EXPERIMENTAL PROCEDURE
All experimental procedures were approved by Henry Ford Hospital’s Institutional Animal Care and Use Committee.
Preparation of MSCs
MSCs from male mice were prepared, frozen in liquid nitrogen and transported to our laboratory by Theradigm, Inc. (Sunnyvale, CA). On the day of transplantation MSCs were restored and a sample was selected for cell counting by using trypan blue stain at 0.4%. Nucleated marrow cells were counted using a cytometer to ensure adequate cell number for transplantation. The cell survival rate ranged from 87%–97%. Dead cells were excluded from the injected cell number. MSCs suspended in phosphate-buffered saline (PBS) were injected into mice via the tail vein.
Animal Model
We employed a controlled cortical impact model of TBI (Smith et al., 1995; Xiong et al., 2005). Twelve female wild type C57BL/6J mice weighing 21 to 26 g were anesthetized with intraperitoneally administered chloral hydrate 400 mg/kg body weight. Rectal temperature was controlled at 37°C with a feedback-regulated water-heating pad. A controlled cortical impact device was used to induce the injury. Mice were placed in a stereotactic frame. One 4-mm-diameter craniotomy was performed adjacent to the central suture, midway between the lambda and bregma. The dura was kept intact over the cortex. Injury was induced by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 2.5-mm-diameter tip at a rate of 4 m/s and 0.8 mm of compression. Velocity was measured with a linear velocity displacement transducer.
Experimental Groups
Twelve female wild type mice C57BL/6J were randomly divided into two groups (6 animals per group): 1) MSC-treated group: 1 day after TBI, the mice were injected with MSCs from a male mouse via the tail vein (0.3 × 106 cells in 200 μl saline/each). The number of cells employed was based on our prior data in the rat (Lu et al., 2003; Lu et al., 2001; Mahmood et al., 2003; Mahmood et al., 2005; Mahmood et al., 2001) and was reduced using a weight scaling factor; and 2) saline-treated control group: 1 day after TBI, the mice were injected with saline. All mice were sacrificed 35 days after TBI.
Spatial Function Test Procedure
The testing procedure is a modification of the Morris water maze test (MWM) as described previously (Day and Schallert 1996; Day et al., 1999; Lu et al., 2004). The experimental apparatus consisted of a circular water tank (140 cm in diameter and 45 cm high). An invisible platform (15 cm in diameter and 35 cm high) was placed 1.5 cm below the surface of the water, with a temperature of 30°C. The pool was located in a large test room where there were many clues external to the maze (for example, pictures, lamps and so forth); these clues were visible from the pool and presumably used by the rats for spatial orientation. The position of the clues remained unchanged throughout the test. Data collection was automated using the HVS Image 2020 Plus Tracking System (US HVS Image, San Diego, CA). For descriptive data collection, the pool was subdivided into four equal quadrants formed by imaging lines.
Spatial learning was tested towards the end of the study (days 31–35) since the neurorestorative processes require certain time periods to be clinically evident (Lu et al., 2003). Each animal was subjected to five trials. One trial was performed each day from day 31 to 35 after TBI. At the start of a trial, the mouse was placed randomly at one of four fixed starting points, randomly facing either toward the wall or inwardly (designated North, South, East and West) and allowed to swim for 90 seconds or until they found the platform. The platform was located in a randomly changing position within the NE (correct) quadrant throughout the test period (e.g., sometimes equidistant from the center and edge of the pool, against the wall, near the center of the pool, and at the edges of the NE quadrant). If the animal found the platform, it was allowed to remain there for 15 seconds before being returned to its cage. If the animal was unable to find the platform within 90 seconds, the experiment was terminated and a maximum score of 90 seconds was assigned. The percentage of time traveled within the NE quadrant was calculated relative to the total amount of time spent swimming before reaching the platform.
Foot Fault Test
To evaluate coordination and fine motor control of the digits (Yelena et al., 2003) the footfault test was carried out before and at 2, 5, 8, 14, 21, 28, and 35 days after TBI by an investigator blind to the treatment groups. The animals were allowed to walk on a 12 cm × 57cm grid with 1.3 cm × 1.3 cm diameter openings. The animals’ fault steps were counted in 50 steps on right forelimbs and hind limbs, respectively, in each trial. The average duration of each trial was 5 minutes. Limb fault was defined as a limb missing the grid bars and placing it into one of the openings. And if this occurred, it was recorded as a footfault.
Tissue Preparation
Thirty-five days after TBI, the animals were euthanized with an overdose of chloral hydrate (~450 mg/kg body weight) administered intraperitoneally. All animals were perfused with intra-cardiac heparinized saline followed by 10% buffered formalin. The brains were removed and stored in 10% buffered formalin for two days at room temperature. Standard 2-mm-thick blocks were cut by use of rodent brain matrix and processed for paraffin sectioning. A series of adjacent 6-μm-thick sections were cut, and a section of each block was stained with hematoxylin and eosin (H&E). Standard H&E staining was employed to calculate lesion volume.
Immunoperoxidase Staining
To identify the vascular structure, deparaffinized brain tissue sections were incubated in 1% Bovine serum albumin/PBS at room temperature for 1 h and subsequently treated with mouse anti-vWF antibody (Dako Cytomation, Carpinteria, CA) diluted to 1:200 in PBS at 4°C overnight. vWF is a marker of endothelial cells and is routinely used to identify vessels in tissue sections (Zanetta et al., 2000). Following sequential incubation with biotin-conjugated anti-mouse immunoglobulin G (dilution 1:100; Dakopatts, Carpinteria, CA), the sections were treated with an avidin-biotin-peroxidase system (ABC kit; Vector Laboratories, Inc., CA). Diaminobenzidine was then used as sensitive chromogen for light microscopy. Negative control sections from each animal received identical staining preparation except that secondary anti-mouse immunoglobulin G was omitted.
In Situ Hybridization
Briefly, 6-μm-thick sections from brain were dewaxed and rehydrated with xylene and graded ethanol and subsequently digested with proteinase K (100 μg/ml) for 15 minutes at 37°C. The previously prepared DNA probe for Y chromosome-1 5′CATCGAAGGGTTAAAGTGCCA was labeled by a DIG labeling and Detection Kit (Roche Diagnostics USA). Hybridization was performed in a mixture consisting of 50% deionized formamide, 10% salmon testes DNA, 10% dextran sulfate, 10% 50 × Denhardt’s solution, 10% 20 × standard saline citrate, and 500 ng digoxigenin-labeled probe at 37°C overnight. The digoxigenin-labeled Y chromosome was visualized using a fluorescent antibody enhancer for DIG detection (Roche Diagnostics USA) under fluorescent microscopy, which resulted in fluorescein isothiocyanate fluorescence (green). The slides were then counterstained with 10 ng/ml of propidium iodide (red) for nuclear staining and mounted with Fluoromount-G (SouthernBiotech USA) and coverslipped. Negative control sections from each animal received identical staining preparation, except that the probe or the antidigoxigen antibodies were omitted.
Volumetric analysis
To estimate the lesion volume of the cortex, tissue sections were stained with H&E and analyzed using a X 10 objective lens and a computer imaging analysis system (Zhang et al., 2002). The lesion volume was calculated by measuring the area of the lesion, including the lesion cavity and boundary zone, from each section and multiplying the lesion area by the section thickness and the sampling intervals.
Measurement of Vascular Density
Five sections with 50-μm intervals through the dorsal dentate gyrus were stained for vWF and the images were digitized with a light microscope at magnification of X 200 (at the interaural 1.98-mm levels). The vWF-positive vessels were counted in the boundary zone of the lesion and the CA3 region of the hippocampus, using the Meta Imaging Series system (Nikon, Inc., Melville NY). The boundary zone was defined as the area surrounding the lesion cavity, which morphologically differs from surrounding intact brain. The vascular density in the boundary zone and CA3 region of the hippocampus was determined by dividing the number of the immunoreactive vessels by the corresponding area (Zhang et al., 2002).
Estimates of Y chromosome positive cell number
For measurement of Y chromosome-positive cells, three equally spaced slides, at intervals of approximately 100 μm, were obtained from the block encompassing the lesion and hippocampus (Block E or F). The number of the Y chromosome-positive cells was counted using fluorescent microscopy. To reduce the biases introduced by sampling parameters, all sections for Y chromosome identification from mice were stained simultaneously, and the criteria for Y chromosome-positive cells (i.e., yellow in the nuclei) were defined before the cells were counted by observers blinded to the individual treatment.
Statistical Analysis
Repeated measure analysis of variance (ANCOVA) was used to study the effect of MSC treatment on functional recovery. Analysis began testing for the treatment by time interaction, followed by testing the effects of treatment at each time point, if interaction or main effect of time or treatment was detected at the 0.05 level. The same analytic approach was used to test the effects of MSCs on vascular density in the lesion boundary zone and hippocampus. The mean and standard deviation (SD) by time were presented as data illustration.
CONCLUSION
In summary, MSCs are effective in improving functional outcome after TBI in mice. In addition, the mouse may be a valuable model in testing MSCs as potential therapy for TBI.
Acknowledgments
This work was supported by NIH grant #RO1NS42259.
References
- 1.Bernstein DC, Shearer GM. Suppression of human cytotoxic T lymphocyte responses by adherent peripheral blood leukocytes. Ann N Y Acad Sci. 1988;532:207–213. doi: 10.1111/j.1749-6632.1988.tb36339.x. [DOI] [PubMed] [Google Scholar]
- 2.Day LB, Schallert T. Anticholinergic effects on acquisition of place learning in the Morris water task: spatial mapping deficit or inability to inhibit nonplace strategies? Behav Neurosci. 1996;110:998–1005. doi: 10.1037//0735-7044.110.5.998. [DOI] [PubMed] [Google Scholar]
- 3.Day LB, Weisend M, Sutherland RJ, Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behav Neurosci. 1999;113:914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- 4.Francois S, Bensidhoum M, Mouiseddine M, Mazurier C, Allenet B, Semont A, Frick J, Sache A, Bouchet S, Thierry D, Gourmelon P, Gorin NC, Chapel A. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: A study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 5.Geddes DM, LaPlaca MC, Cargill RS. Susceptibility of hippocampal neurons to mechanically induced injury. Exp Neurol. 2003;184:420–427. doi: 10.1016/s0014-4886(03)00254-1. [DOI] [PubMed] [Google Scholar]
- 6.Hlatky R, Vladka A, Robertson C. Intracranial hypertension and cerebral ischemia after severe traumatic brain injury. Neurosurg Focus. 2003;14:104. doi: 10.3171/foc.2003.14.4.2. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiramin N, Chopp M. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 8.Lu D, Mahmood A, Chopp M. Biological transplantation and neurotrophin-induced neuroplasticity after traumatic brain injury (review) J Head Trauma Rehabil. 2003;18:357–376. doi: 10.1097/00001199-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lu D, Mahmood A, Goussev A, Schallert T, Qu C, Zhang ZG, Li Y, Lu M, Chopp M. Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvasular patency and integrity, and enhancement of spatial memory in rats subjected to traumatic brain injury. J Neurosurg. 2004;101:813–821. doi: 10.3171/jns.2004.101.5.0813. [DOI] [PubMed] [Google Scholar]
- 10.Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. NeuroReport. 2001;12:559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 11.Mahmood A, Lu D, Chopp M. MSC transplantation after traumatic brain injury promotes cellular proliferation with the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–703. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 13.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Human marrow stromal cell treatment provides long lasting benefits after traumatic brain injury in rats. Neurosurgery. 2005;57:1026–1031. doi: 10.1227/01.neu.0000181369.76323.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1204. [PubMed] [Google Scholar]
- 15.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SD, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heerderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh JK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shindo T, Matsumoto Y, Wang Q, Kawai N, Tamiya T, Nagao S. Differences in the neuronal stem cells survival, neuronal differentiation and neurological improvement after transplantation of neural stem cells between mild and severe experimental traumatic brain injury. J Med Invest. 2006;53:42–51. doi: 10.2152/jmi.53.42. [DOI] [PubMed] [Google Scholar]
- 17.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. Model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- 18.Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS. Prevention of mitochondrial dysfunction in post-traumatic mouse brain by superoxide dismutase. J Neurochem. 2005;95:732–744. doi: 10.1111/j.1471-4159.2005.03412.x. [DOI] [PubMed] [Google Scholar]
- 19.Yelena K, Baskin W, Dalton D, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 20.Zanetta L, Marcus SG, Vasile J, Dobryansky M, Cohen H, Eng K, Shamamian P, Mignatti P. Expression of von Willebrand factor, an endothelial cell marker is upregulated by angiogenesis factors: A potential method for objective assessment of tumor angiogenesis. Int J Cancer. 2000;85:281–288. doi: 10.1002/(sici)1097-0215(20000115)85:2<281::aid-ijc21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 22.Zinkernagel RM, Doherty PC. MHC-restricted cytotoxic T cells: Studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]