Abstract
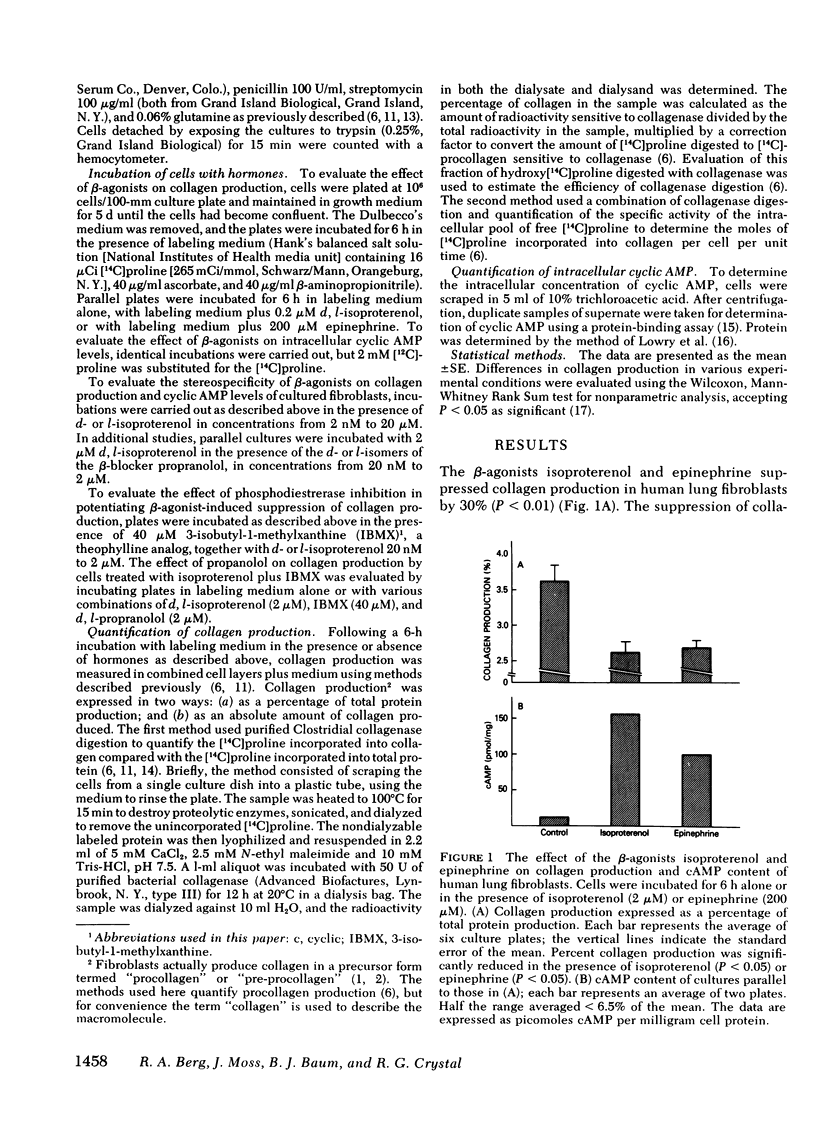
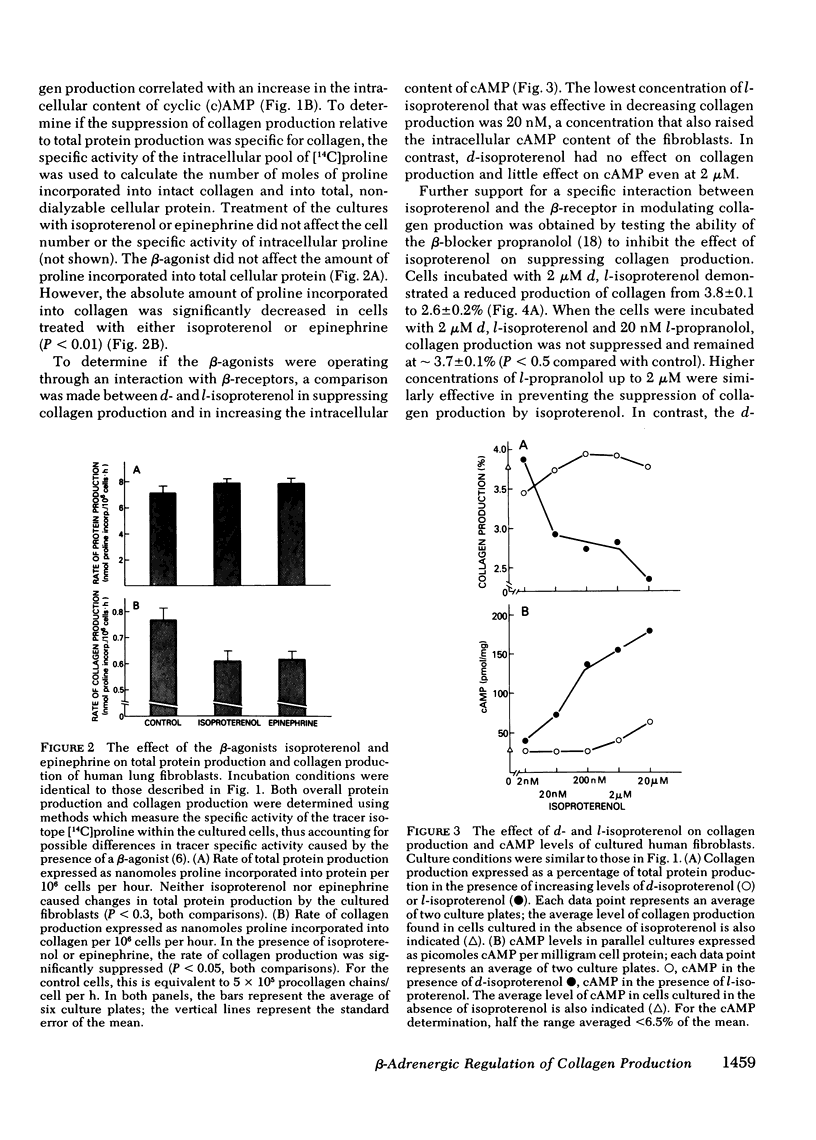
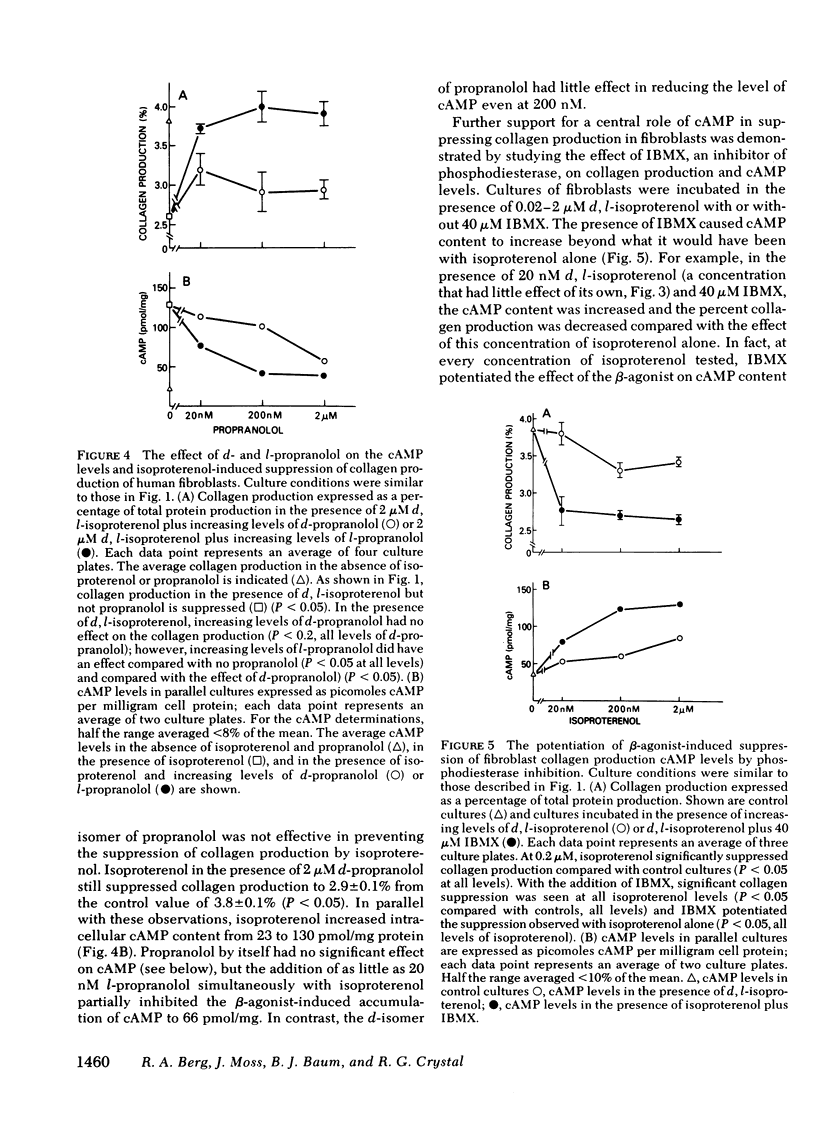
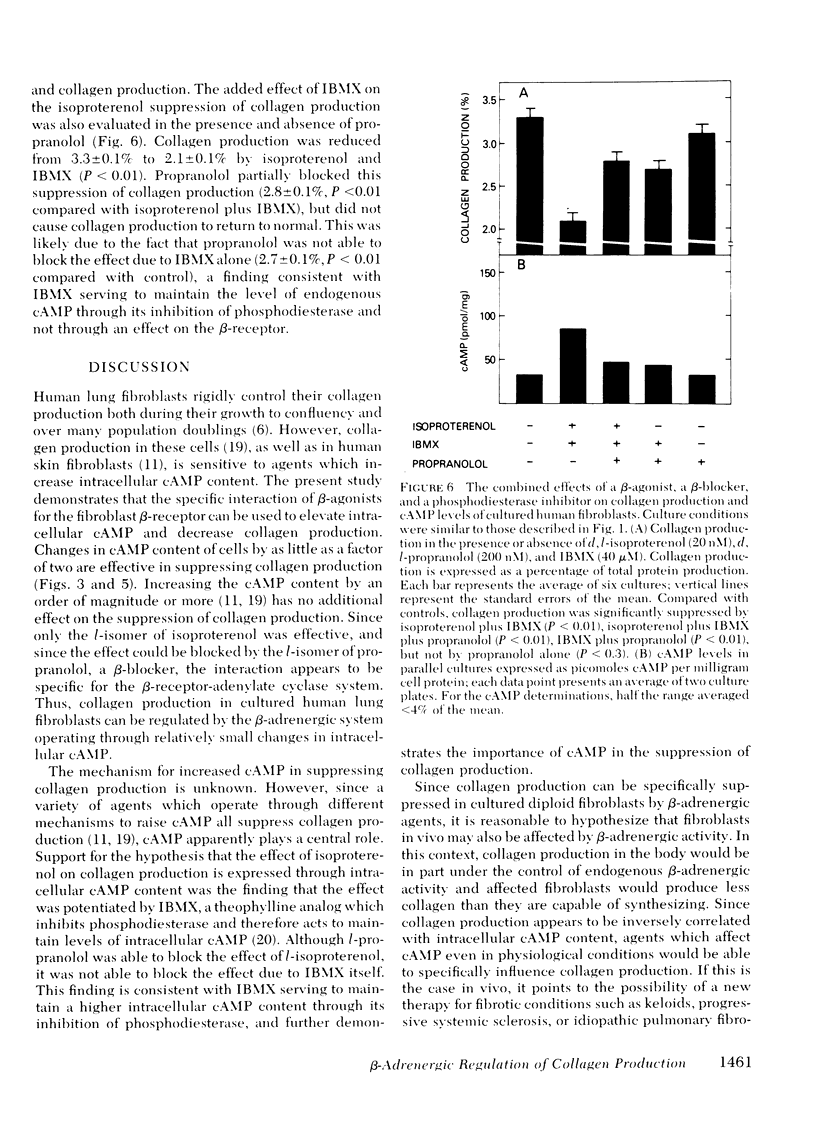
The suppression of collagen production by increasing the cyclic (c) AMP content of cultured cells was examined vis-à-vis the β-adrenergic system. Cultured human fetal lung fibroblasts incubated for 6 h with the β-agonists isoproterenol or epinephrine produced ∼30% less collagen per cell than in the absence of the hormones. To demonstrate that the β-agonists were operating by their interaction with the β-receptor to stimulate adenylate cyclase to increase the intracellular content of cAMP, d- and l-isoproterenol were incubated separately with the cultured cells. Only l-isoproterenol increased intracellular cAMP and decreased collagen production. While 20 nM l-isoproterenol was effective, the d-isomer was ineffective even at 2μM. An increase in cAMP from 40 to 73 pmol/mg protein was effective in suppressing collagen production; increasing the cAMP content to much higher levels had little additional effect on collagen production. 3-Isobutyl-1-methylxanthine, an analog of theophylline that inhibits phosphodiesterase, potentiated the effect of isoproterenol in suppressing collagen production. Further support for the concept that isoproterenol suppressed collagen production by acting through the β-receptor was provided by the finding that only the l-isomer of propranolol, a β-blocker, was effective in blocking both the increase in intracellular cAMP and the suppression of collagen production caused by isoproterenol. These results demonstrate that collagen production in human fibroblasts can be regulated by the β-adrenergic system and indicate that when the cAMP content is increased beyond a threshold value, collagen production is suppressed. Since collagen production is sensitive to the small changes of cAMP content of cells brought about by β-stimulation in cultured cells, the results point to a possibly important mechanism for the regulation of collagen production in the body.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas D., Steer M. L., Levitzki A. Stereospecific binding of propranolol and catecholamines to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4246–4248. doi: 10.1073/pnas.71.10.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B. J., Moss J., Breul S. D., Berg R. A., Crystal R. G. Effect of cyclic AMP on the intracellular degradation of newly synthesized collagen. J Biol Chem. 1980 Apr 10;255(7):2843–2847. [PubMed] [Google Scholar]
- Baum B. J., Moss J., Breul S. D., Crystal R. G. Association in normal human fibroblasts of elevated levels of adenosine 3':5'-monophosphate with a selective decrease in collagen production. J Biol Chem. 1978 May 25;253(10):3391–3394. [PubMed] [Google Scholar]
- Bienkowski R. S., Baum B. J., Crystal R. G. Fibroblasts degrade newly synthesised collagen within the cell before secretion. Nature. 1978 Nov 23;276(5686):413–416. doi: 10.1038/276413a0. [DOI] [PubMed] [Google Scholar]
- Breul S. D., Bradley K. H., Hance A. J., Schafer M. P., Berg R. A., Crystal R. G. Control of collagen production by human diploid lung fibroblasts. J Biol Chem. 1980 Jun 10;255(11):5250–5260. [PubMed] [Google Scholar]
- Chasin M., Harris D. N. Inhibitory and activators of cyclic nucleotide phosphodiesterase. Adv Cyclic Nucleotide Res. 1976;7:225–264. [PubMed] [Google Scholar]
- Dietrich J. W., Canalis E. M., Maina D. M., Raisz L. G. Hormonal control of bone collagen synthesis in vitro: effects of parathyroid hormone and calcitonin. Endocrinology. 1976 Apr;98(4):943–949. doi: 10.1210/endo-98-4-943. [DOI] [PubMed] [Google Scholar]
- Doherty C. C., McGeown M. G., Donaldson R. A. Retroperitoneal fibrosis after treatment with atenolol. Br Med J. 1978 Dec 23;2(6154):1786–1786. doi: 10.1136/bmj.2.6154.1786-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwteman T. M., Braat M. C., van Aken W. G. Interstitial pulmonary fibrosis: a new side effect of practolol. Br Med J. 1977 Jul 30;2(6082):297–298. doi: 10.1136/bmj.2.6082.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Crystal R. G. Rigid control of synthesis of collagen types I and III by cells in culture. Nature. 1977 Jul 14;268(5616):152–154. doi: 10.1038/268152a0. [DOI] [PubMed] [Google Scholar]
- Homcy C. J., Strauss H. W., Kopiwoda S. Beta receptor occupancy. Assessment in the intact animal. J Clin Invest. 1980 May;65(5):1111–1118. doi: 10.1172/JCI109764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse N. J., Rowe D. W., Fujimoto W. Y., Bornstein P. Inhibitory effects of glucocorticoids on collagen synthesis by mouse sponge granulomas and granuloma fibroblasts in culture. Biochim Biophys Acta. 1978 Apr 19;540(1):101–116. doi: 10.1016/0304-4165(78)90439-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nancarrow J. F. Sclerosing peritonitis and timolol. Lancet. 1978 Sep 2;2(8088):525–526. doi: 10.1016/s0140-6736(78)92247-x. [DOI] [PubMed] [Google Scholar]
- Oikarinen A. Effect of betamethasone-17-valerate on synthesis of collagen and prolyl hydroxylase activity in chick embryo tendon cells. Biochem Pharmacol. 1977 May 1;26(9):875–879. doi: 10.1016/0006-2952(77)90403-8. [DOI] [PubMed] [Google Scholar]
- Osborne D. R. Propranolol and Peyronie's disease. Lancet. 1977 May 21;1(8021):1111–1111. doi: 10.1016/s0140-6736(77)92373-x. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Pryor J. P., Khan O. Beta blockers and Peyronie's disease. Lancet. 1979 Feb 10;1(8111):331–331. doi: 10.1016/s0140-6736(79)90748-7. [DOI] [PubMed] [Google Scholar]
- Schwarz R. I., Bissell M. J. Dependence of the differentiated state on the cellular environment: modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4453–4457. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin J. S. Peyronie's disease in association with metoprolol. Lancet. 1977 Dec 24;2(8052-8053):1355–1355. doi: 10.1016/s0140-6736(77)90399-3. [DOI] [PubMed] [Google Scholar]


