Abstract
A large cohort of patients participated in a longitudinal study of early glaucoma progression. During follow up, six eyes of three patients displayed a relatively rapid deterioration of pattern electroretinogram (PERG) signal compared to changes in visual acuity, IOP, Standard Automated Perimetry, and Retinal Nerve Fiber Layer thickness measured by OCT. This deterioration prompted further testing including magnetic resonance imaging (MRI), which revealed pituitary tumors in all three patients, two of which were abutting but not compressing the chiasm. Following tumor resection, the PERG signal gradually recovered to baseline values in all six eyes. Results indicate that pituitary tumors may cause retrograde dysfunction of retinal ganglion cells (RGC) even in the absence of visible mechanical compression of the visual pathway, and such dysfunction may be reversed by tumor reduction. The results suggest that PERG is a useful tool in the early diagnosis and management of patients with chiasmal mass lesions.
Keywords: Pattern Electroretinogram (PERG), Optic nerve, Optic chiasm, Pituitary tumors
Introduction
Secreting pituitary tumors often cause hormonal imbalance and a variety of non-visual symptoms [1]. These symptoms may alert the physician to pursue specific investigation, including brain imaging, which may result in early medical and/or surgical intervention. Non-secreting pituitary tumors are often asymptomatic until relatively moderate stages when visual field defects typically appear, as the tumor becomes large enough to compress and elevate the chiasm [1,2].
Different hypotheses have been proposed to explain the pathophysiological mechanism of insult to chiasmal fibers and loss of vision. These include ischemic damage of crossing nasal retinal fibers [3] and/or mechanical damage of nasal fibers [4]. Early detection of chiasmal dysfunction is under active investigation to facilitate timely treatment of the condition and to prevent irreversible visual loss. Several studies have demonstrated substantial alterations of both contrast sensitivity and visually evoked potentials (VEP) [5–7] even in the presence of normal visual acuity and fields, thereby indicating a more widespread and insidious abnormality of the visual pathway than expected based on tumor size. Damage to the visual pathway includes retrograde degeneration of RGC axons, resulting in a reduction of retinal nerve fiber layer thickness [8]. The pattern electroretinogram (PERG) is a noninvasive and objective method that can be used to detect retinal ganglion cell (RGC) dysfunction in a variety of disorders [9–11]. The PERG signal has been previously shown to be impaired in relatively advanced stages of chiasmal compression [12, 13]. Previous studies concluded that a reduced PERG amplitude represented a predictor of unfavorable post-surgical outcome [12, 13]. In contrast, here, we describe three cases of early PERG deterioration associated with the presence of pituitary tumors, with recovery after surgical removal of the tumor.
Materials and methods
The three subjects described were among a large cohort (n > 250) of patients with suspicion for glaucoma who participated in a longitudinal study monitoring for glaucomatous progression [14]. All subjects received serial testing every 6 months, which included the Stratus OCT to measure RNFL thickness (FASTRNFL program), Humphrey 24-2 white-on-white standard automated perimetry, a baseline set of stereo disk photographs, and PERG to measure RGC function.
A steady-state PERG was recorded simultaneously from both eyes of each subject in response to contrast reversal of high-contrast gratings (1.7 cy/deg, contrast 98%, 16.28 rev/s, circular field size 24 deg diameter, mean luminance 50 cd/m2, 600 sweeps averaged) according to the PERGLA paradigm [15, 16]. The PERGLA uses skin electrodes taped over the lower eyelids. Waveforms are automatically analyzed by Discrete Fourier Transform, and amplitude (in µV) and phase (in π rad) are displayed (example shown in Fig. 1). A phase decrease (delay) of 0.1 π rad corresponds to a latency delay of 3.14 ms. Both PERG amplitude loss and phase delay were considered indicators of impaired PERG signal. A reduction of the PERG amplitude may be due to lack of activity from dead or missing RGCs, reduced activity of viable RGCs, or a combination of both conditions. A delay of PERG phase may be due to reduced excitability of RGCs [11, 17].
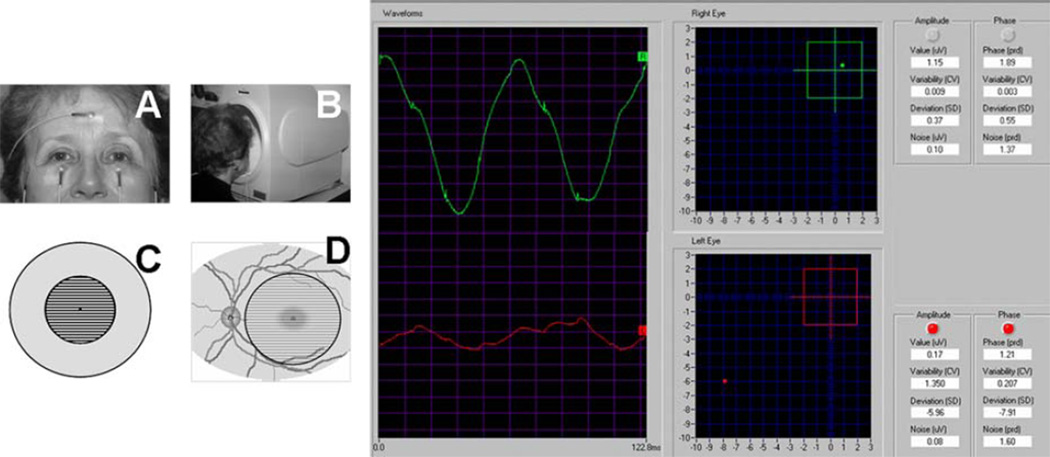
Fig. 1.
The steady-state PERG is recorded from skin electrodes in response to fine horizontal bars reversing 16.28 times per second. The pattern stimulus covers a circular area of 25 degrees diameter centered on the fovea. A normal PERG waveform has a sinusoidal-like shape (green tracing) that is automatically analyzed in its amplitude and phase. Amplitude and phase are also expressed as SD deviations from age-predicted normal values. The red tracing is an example of abnormal PERG in glaucoma. Note the reduced amplitude and the delayed phase
The methods applied in the study adhered to the tenets of the Declaration of Helsinki for the use of human subjects in biomedical research. Institutional Review Board/Ethics Committee approval was obtained for the study, and informed consent was obtained from each subject before recording.
Results
Case 1
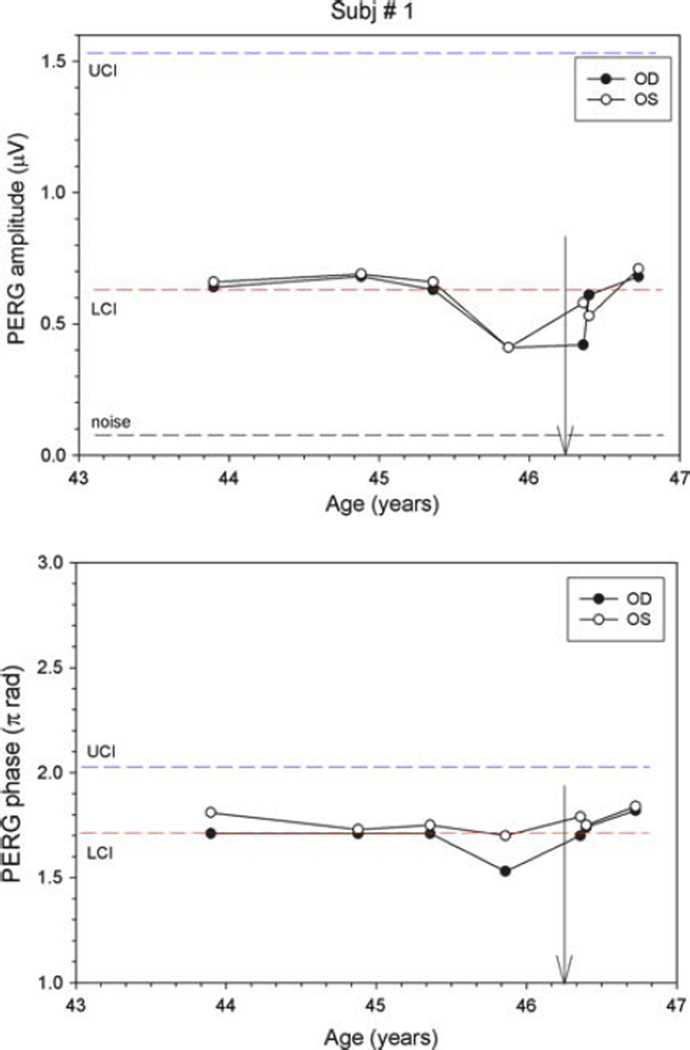
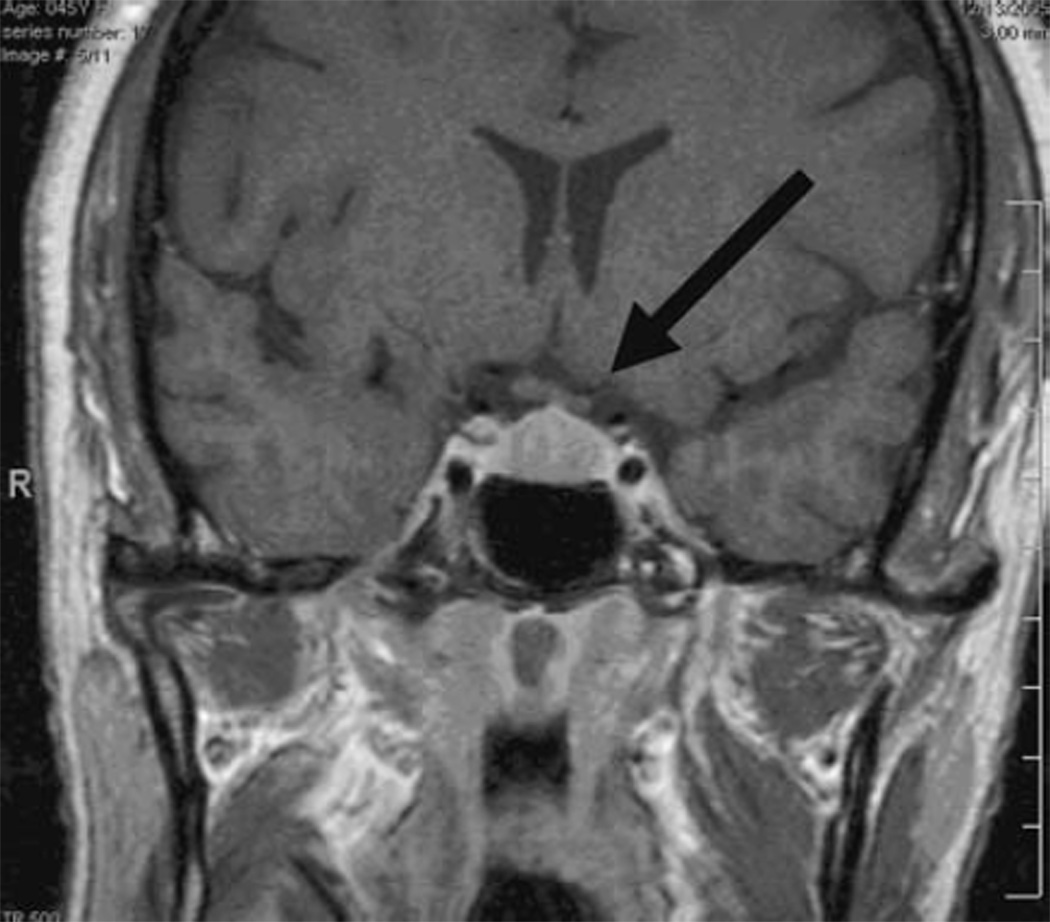
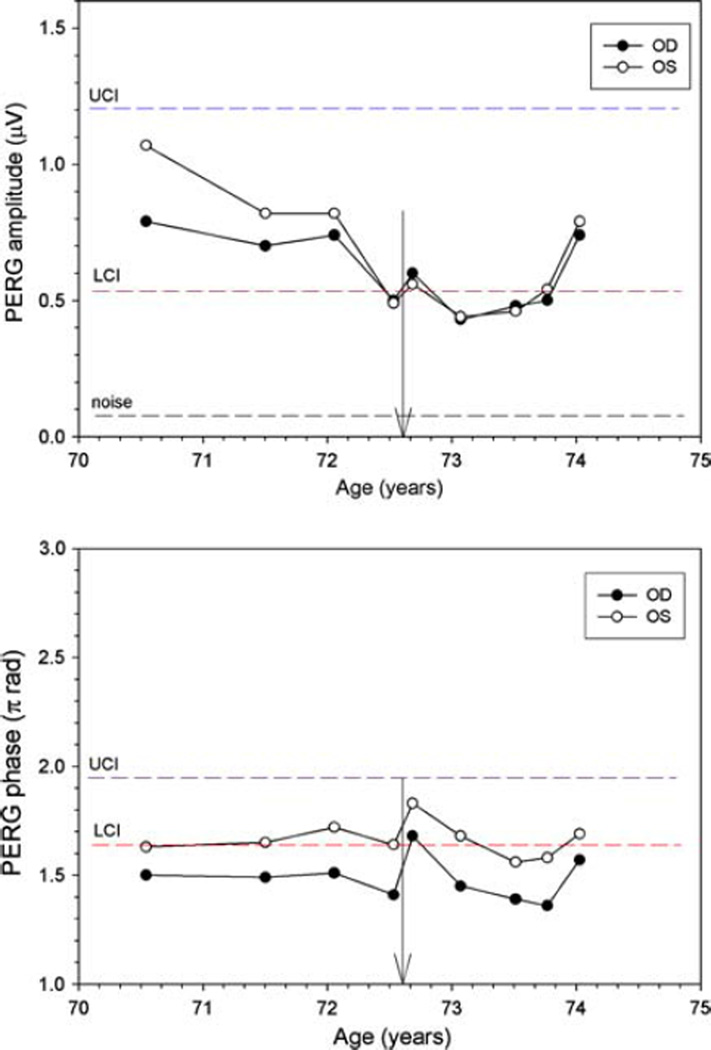
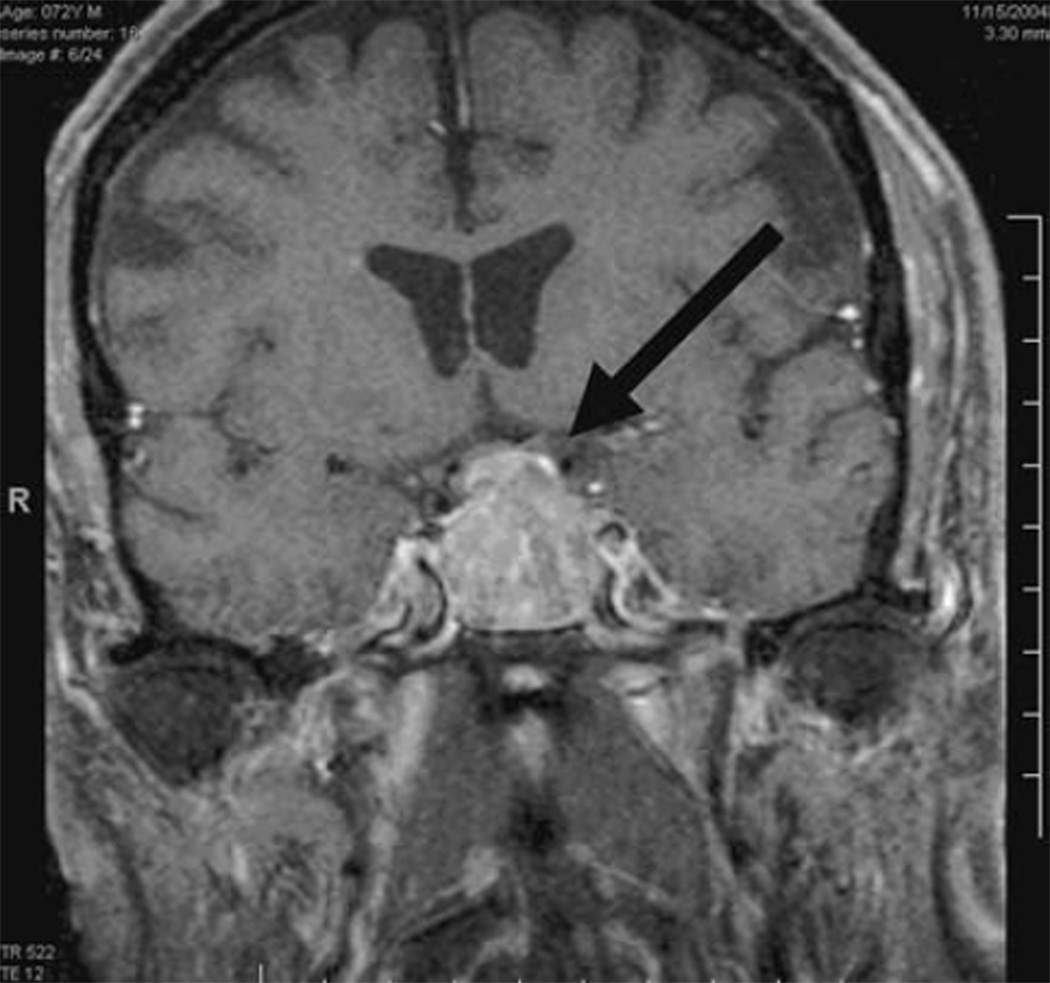
Subject 1 is a 47-year-old, Black woman with normal IOPs who was followed as a glaucoma suspect due to an increased cup to disk ratio that showed no sign of change over 3 years. Two years after inclusion into our study, she presented for a routine 6-month visit with complaints of bitemporal headaches and development of menopause over the previous 2 months. While Humphrey visual fields and OCT RNFL were normal, the PERG amplitudes had deteriorated notably in both eyes. Compared to three previous visits, amplitudes dropped considerably in both eyes (~38%), with the phase decreasing (latency delayed by ~5.5 ms) in the right eye (Fig. 2). MRI showed a pituitary tumor measuring 1.3 by 1.7 by 1.0 cm abutting the chiasm with no visible compressive effect (Fig. 3). Following transphenoidal tumor resection, both PERG amplitudes and phase returned to baseline.
Fig. 2.
PERG amplitude (upper panel) and phase (lower panel) at different ages for subject #1. LCI (red dashed lines) and UCI (blue dashed line) represent the lower and upper confidence intervals, respectively, for age-matched normal population. The black dashed line represents the noise amplitude, obtained by recording a response with the stimulus occluded. Vertical arrows on the x axis represent subject’s age at the time of surgery
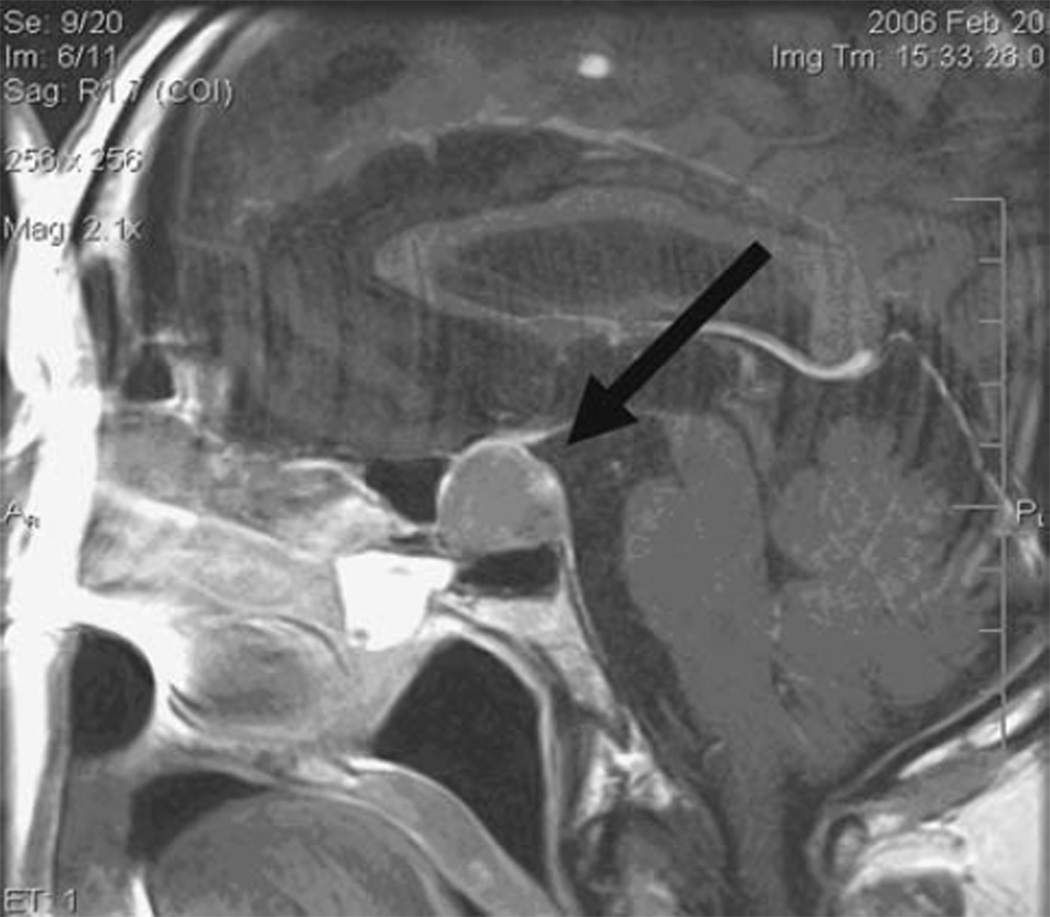
Fig. 3.
Subject #1. Coronal T1 MRI with gadolinium showing pituitary tumor (1.3 cm anterior posterior (AP) × 1.7 cm Transverse (T) × 1.0 cm Craniocaudal (CC) abutting the optic chiasm without visible compressive effect
Case 2
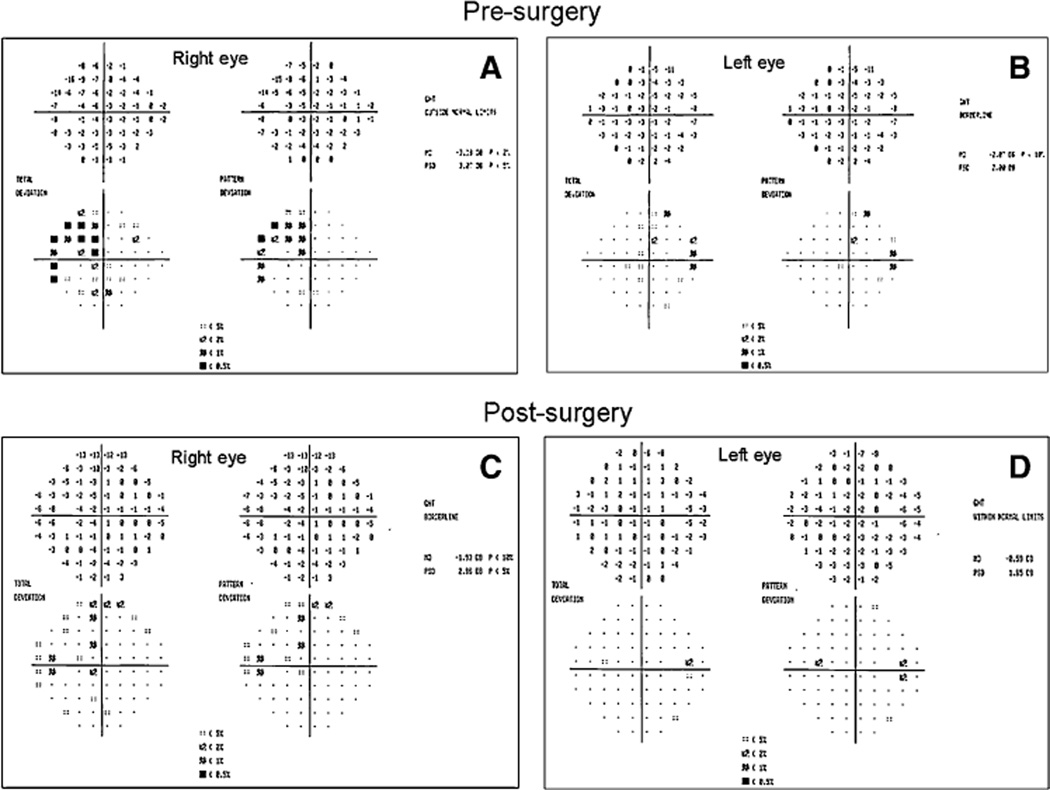
Subject 2 is a 76-year-old white male with POAG (maximum recorded IOP of 23 mm Hg), and a family history of glaucoma in his mother. Between age 65 and 67, he developed progressive optic disk cupping without pallor OU and a fluctuating nasal step on Humphrey perimetry OS, and Xalatan was begun. At age 73, the patient developed a two-point temporal cluster depression on SAP of the right eye (MD −2.07 DB) and a supero-temporal visual field defect in the left eye respecting midline, which was confirmed (MD −3.38 DB) (Fig. 4). OCT did not show thinning of the nasal or temporal RNFL. Over the observation period, the treated IOP was 13 ± 1.9 in OD and 14 ± 2.1 mm Hg in OS; SAP Mean Deviations were −1.9 ± 0.6 in OD and −2.7 ± 1.2 DB in OS. At baseline, PERG amplitude was within the normal limits in each eye (OD had relatively smaller values); the PERG phase had borderline value in OS and was delayed in OD (Fig. 5). The marked deterioration of the PERG amplitude from baseline values over a 2-year period (by ~54% in OD and by ~37% in OS) alerted the clinician to order an MRI. A large pituitary macroadenoma (3.4 cm × 2.5 cm × 3.0 cm) compressing the chiasm was visualized (Fig. 6). The tumor was incompletely resected due to its large size and extension into the left cavernous sinus. Post-operative MRI on 12/17/2004 showed decompression of the chiasm despite residual tumor. MD improved in the right eye and the temporal defect in the left eye cleared dramatically on 1/10/2005, within 3 months of resection (MD −0.59 DB in the right eye and MD −1.93 DB in the left eye) (Fig. 4). PERG abnormalities, however, persisted for nearly 1 year in both the eyes before substantial improvement was seen. At the last visit, the PERG values recovered to the range of those of the initial visit. The phase remained relatively stable over the entire observation period.
Fig. 4.
Visual fields of subject # 2. (a, b) Presurgical condition at age 73, showing a two-point cluster depression on SAP of the right eye and a superotemporal visual field defect in the left eye respecting midline. (c, d) Post-surgical condition, 1 month after incomplete tumor resection. The field is markedly improved compared to baseline
Fig. 5.
PERG amplitude and phase at different ages for subject #2. Descriptions as for Fig. 2
Fig. 6.
Subject #2. Coronal T1 MRI with gadolinium showing a large pituitary macroadenoma (3.4 cm × 2.5 cm × 3.0 cm) compressing the chiasm
Case 3
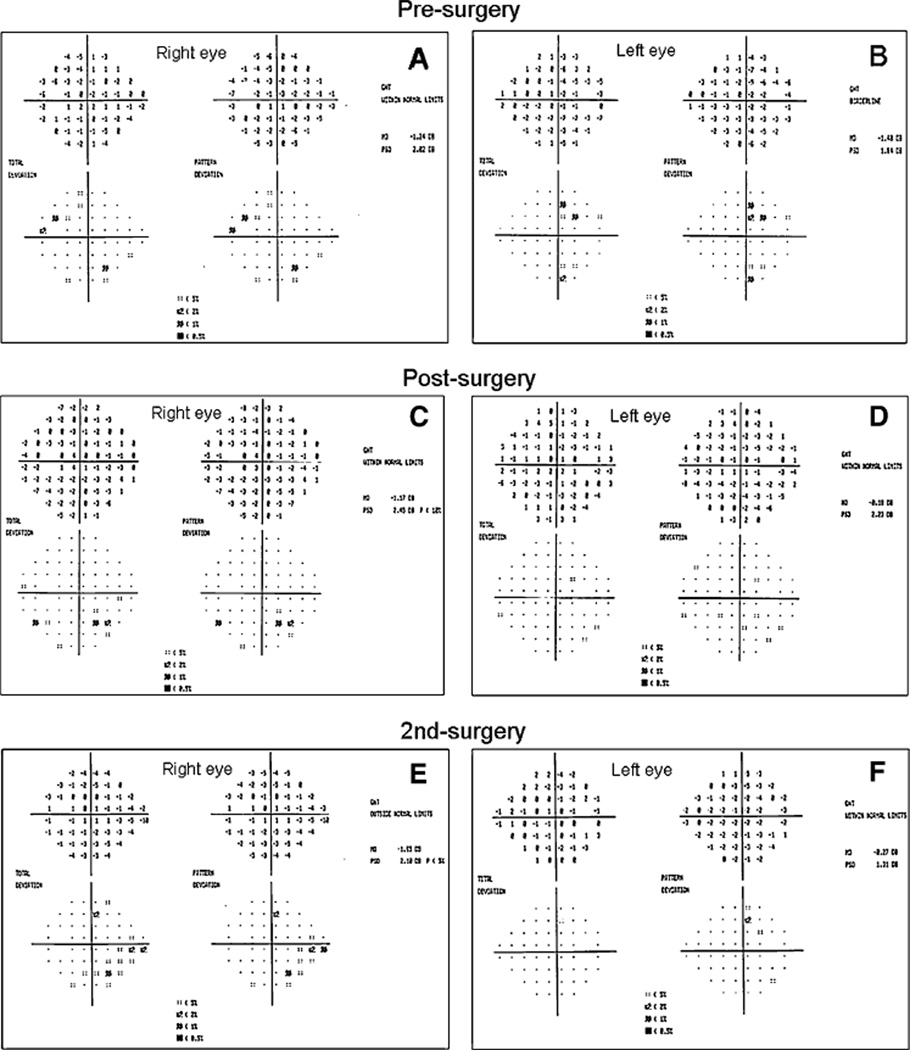
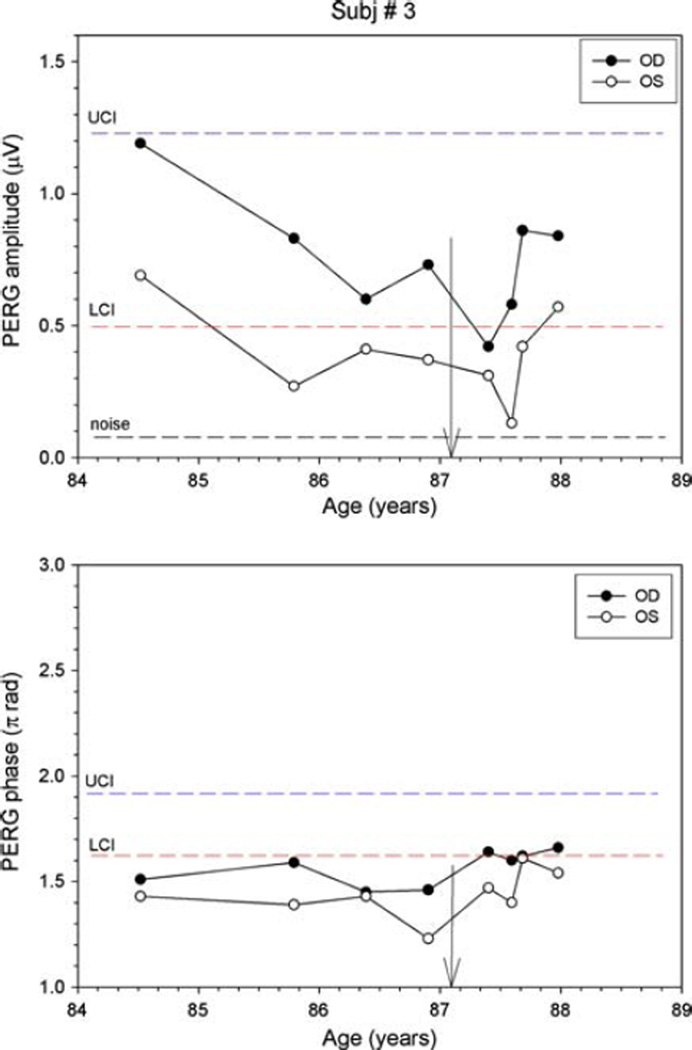
Subject 3 is an 87-year-old gentleman who was followed as a glaucoma suspect for 12 years with ocular hypertension (maximum recorded IOP 27 mm Hg) and consistently normal visual fields. At age 83, subtle bitemporal field changes developed with a superotemporal three-point cluster (P < 5%) depression OD (MD −1.48 DB) and a two-point temporal cluster depression in the left eye (MD −1.24 DB) (Fig. 7). MRI revealed a large pituitary adenoma measuring 2 cm × 2.4 cm × 2 cm, which was compressing the chiasm, and was incompletely resected. A post-op MRI showed that the residual tumor mass had decreased to 1.6 cm × 1.5 cm × 1.1 cm and visual fields returned to normal within 4 months of surgical excision, indicating that chiasmal compression had been alleviated. Five months after incomplete resection, the patient was enrolled in our PERG study as a glaucoma suspect at age 84. Baseline PERG amplitudes were within normal limits, but phase values were below normal limits. Despite the initial reduction in tumor size, PERG amplitudes deteriorated from baseline over the course of two and one-half years (Fig. 8) Compared to the initial visits, amplitudes dropped by 65% in the right eye, and by 81% in the left eye; the phase remained relatively stable in each eye. Despite PERG deterioration, neither visual fields nor OCT RNFL showed signs of chiasmal compromise in the form of temporal changes. Over the 3-year observation period, SAP Mean Deviations were −0.8 ± 0.4 in OD and −0.9 ± 0.7 dB in OS; IOP was 16 ± 2.5 in OD and 16 ± 2.5 mm Hg in OS (Fig. 7). Deterioration of the PERG prompted the ophthalmologist to contact the neurosurgeon who ordered a repeat MRI, which showed that the tumor had increased in size to 1.9 cm × 1.7 T × 1.6 CC and was now abutting the chiasm(Fig. 9). The tumor was re-excised using gamma knife radiation at age 87. The only visual field defect that developed was a glaucomatous-like inferonasal step in the left eye, which was reproduced on subsequent fields after tumor re-excision (Fig. 7). Clinically, the patient recovered uneventfully, and PERG amplitudes of each eye progressively recovered to values close to those of the initial visit (−29% and −17% of initial amplitude in OD and OS, respectively).
Fig. 7.
Visual fields of subject # 3. (a, b) At age 83, showing subtle bitemporal field changes, which were confirmed on a subsequent field. (c, d) Four months post-op showing return to normal. (e, f) After tumor re-excision, showing no signs of chiasmal compromise. However, glaucomatous inferonasal step in left eye reproduced on subsequent fields
Fig. 8.
PERG amplitude and phase at different ages for subject #2. Descriptions as for Fig. 2
Fig. 9.
Subject #3. Coronal T1 MRI with gadolinium showing that pituitary adenoma increased in size to 1.9 AP × 1.7 T × 1.6 CC
Discussion
Our results indicate that the presence of pituitary tumors may impair RGC electrical activity; RGC activity is restored by tumor reduction. Since the flow of electrical activity generated by RGCs is anterograde (from the retina to the brain), insults to the chiasm are expected to reduce electrical activity of the postchiasmatic visual pathway, resulting in both psychophysical (perimetry and contrast sensitivity) and VEP losses [5–7]. Reduced electrical activity of the prechiasmatic visual pathway (PERG loss) can only be explained by retrograde (from the brain to the retina) changes. PERG losses could be due to either degeneration of optic nerve fibers and RGCs or dysfunction of viable RGCs. Our results are consistent with the latter hypothesis, since PERG losses were reversible. A likely candidate for reversible RGC dysfunction is impairment of axoplasmic transport. RGCs are highly energy-dependent, and hence, very vulnerable to a range of metabolic and ischemic insults that can act along the course of the axon [18]. Axonal transport of molecules, vesicles, and organelles is instrumental in determining neuronal survival in disease [18]. Disruption of transport processes can result in RGC death [19]. Previous reports of irreversible PERG losses [12, 13] were associated with more advanced stages of tumor development, suggesting that the predominant pathophysiological mechanism was retrograde RGC death. This is consistent with the clinical observation that delayed diagnosis of pituitary tumors results in irreversible anatomical and functional loss.
Interestingly, reversible PERG losses were found in the absence of visible compressive effect of the optic chiasm on MRI in Cases 1 and 3. One possibility may be that micro-compression was missed by the MRI. Another possibility is that PERG loss is linked to interference in axoplasmic flow induced by local ischemia at the level of the optic chiasm [20]. A potential candidate for this hemodynamic perturbation is the potent vasoactive peptide endothelin-1 that is released by the pituitary tumor [21].
These cases demonstrate that MRI should be considered when PERG deterioration is not explained by glaucoma or any other ocular or systemic causes. The PERG may be altered in glaucoma, the magnitude of PERG loss being proportional to the severity of disease [17]. Since the natural progression of glaucoma is typically very slow [22], the marked PERG deterioration observed in these three individuals was likely due to other comorbid conditions. After excluding other potential ocular causes of PERG loss (such as cataract, ARMD, retinal detachment, retinoschisis, vascular occlusive phenomena, and retinal or vitreous hemorrhage), as well as systemic conditions that may cause deterioration of PERG, such as Diabetes Mellitus, Parkinson’s Disease, and Multiple Sclerosis [23–25], neuroimaging was undertaken.
As demonstrated by Case 2, when following visual fields for glaucoma, clinicians search for typical glaucomatous patterns of perimetric loss and may miss early subtle bitemporal changes. While Case 2 had several signs of early glaucoma, namely, a positive family history, elevated IOP, progression of cupping of the optic disk, and a fluctuating nasal step in the visual field, it was not until the PERG deteriorated markedly that the clinician recognized another disease process could be at play. Unfortunately, a high level of suspicion for pituitary tumors is often not sustained especially after years of following a patient with presumed glaucoma. Addition of the PERG to the battery of testing in following our study patients helped us to identify pituitary masses whose diagnosis may have been further delayed.
Case 3 demonstrates that the PERG may be a useful tool in monitoring patients for recurrence of residual pituitary masses. This gentleman initially presented with an early bitemporal visual field defect, which reverted to normal after primary incomplete resection of the tumor. Over the course of 3 years following incomplete tumor resection, PERG deteriorated with increasing size of the residual tumor. Since tumor regrowth did not apparently compress the chiasm on MRI and did not cause visual field changes, PERG deterioration might be explained as a reflection of an insidious process, which retrogradely affected the anterior visual pathway, preceding visual field changes.
The presence of pituitary tumor, even in the absence of compressive effect at the chiasm on MRI, may cause reversible RGC dysfunction, which precedes visual field loss and RGC death as supported by the unaffected RNFL-OCT. Reversible RGC dysfunction, measured by PERG, has also been demonstrated in early glaucoma after IOP lowering [26]. The difference between the two conditions is that in glaucoma, the presumed insult (elevated IOP and other factors) act at the retina and optic nerve head, whereas with pituitary tumors, the presumed insult acts at a retrobulbar location. It is possible that impairment of axoplasmic transport may contribute to both conditions. Furthermore, the delay in recovery of PERG in our cases indicates that PERG could be a useful parameter not only in the detection of pituitary tumor, but may also be sensitive measure in assessing the recovery of the visual system.
In conclusion, these cases suggest PERG has a role in the early diagnosis and management of patients with pituitary tumors. PERG may be of value not only as a diagnostic measure, but also to document the recovery of the visual system. Furthermore, PERG may be used to monitor the effects of recurrence of chiasmal mass lesions. In following patients presumed to have glaucoma, rapid PERG deterioration may alert the clinician to search for other comorbid conditions including an occult pituitary tumor.
Acknowledgements
We wish to express our appreciation to Dr. Byron Lam for his critical review of our manuscript.
Supported by NIH-NEI RO1 EY014957, NIH center grant P30-EY014801, and by an unrestricted grant to the University of Miami from Research to Prevent Blindness.
Footnotes
Conflict of interest: VP, Lace Elettronica, Italy
Contributor Information
Lori M. Ventura, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, 900 N.W. 17th Street, Miami, FL 33136, USA
Frank X. Venzara, III, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, 900 N.W. 17th Street, Miami, FL 33136, USA.
Vittorio Porciatti, Email: vporciatti@med.miami.edu, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, 900 N.W. 17th Street, Miami, FL 33136, USA.
References
- 1.Chanson P, Salenave S. Diagnosis and treatment of pituitary adenomas. Minerva Endocrinol. 2004;29(4):241–275. [PubMed] [Google Scholar]
- 2.Fujimoto N, et al. Criteria for early detection of temporal hemianopia in asymptomatic pituitary tumor. Eye. 2002;16(6):731–738. doi: 10.1038/sj.eye.6700165. [DOI] [PubMed] [Google Scholar]
- 3.Bergland R. The arterial supply of the human optic chiasm. J Neurosurg. 1969;31(3):327–334. doi: 10.3171/jns.1969.31.3.0327. [DOI] [PubMed] [Google Scholar]
- 4.McIlwaine GG, et al. A mechanical theory to account for bitemporal hemianopia from chiasmal compression. J Neuroophthalmol. 2005;25(1):40–43. doi: 10.1097/00041327-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Porciatti V, et al. Losses of hemifield contrast sensitivity in patients with pituitary adenoma and normal visual acuity and visual field. Clin Neurophysiol. 1999;110(5):876–886. doi: 10.1016/s1388-2457(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 6.Gutowski NJ, Heron JR, Scase MO. Early impairment of foveal magno- and parvocellular pathways in juxta chiasmal tumours. Vision Res. 1997;37(10):1401–1408. doi: 10.1016/s0042-6989(96)00306-9. [DOI] [PubMed] [Google Scholar]
- 7.Holder GE, Bullock PR. Visual evoked potentials in the assessment of patients with non-functioning chromophobe adenomas. J Neurol Neurosurg Psychiatry. 1989;52(1):31–37. doi: 10.1136/jnnp.52.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danesh-Meyer HV, et al. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci. 2006;47(11):4827–4835. doi: 10.1167/iovs.06-0327. [DOI] [PubMed] [Google Scholar]
- 9.Zrenner E. The physiological basis of the pattern electroretinogram. In: Osborne N, Chader G, editors. Progress in Retinal Research. Oxford: Pergamon Press; 1990. pp. 427–464. [Google Scholar]
- 10.Holder GE. Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res. 2001;20(4):531–561. doi: 10.1016/s1350-9462(00)00030-6. [DOI] [PubMed] [Google Scholar]
- 11.Ventura LM, Porciatti V. Pattern electroretinogram in glaucoma. Curr Opin Ophthalmol. 2006;17(2):196–202. doi: 10.1097/01.icu.0000193082.44938.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruther K, et al. Prognostic value of the pattern electroretinogram in cases of tumors affecting the optic pathway. Graefes Arch Clin Exp Ophthalmol. 1998;236(4):259–263. doi: 10.1007/s004170050074. [DOI] [PubMed] [Google Scholar]
- 13.Parmar DN, et al. Visual prognostic value of the pattern electroretinogram in chiasmal compression. Br J Ophthalmol. 2000;84(9):1024–1026. doi: 10.1136/bjo.84.9.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ventura L, Venzara F, Porciatti V. Rates of visual field and PERG changes over 4 years in early glaucoma. Invest. Ophthalmol. Vis. Sci. 2007;48 E-Abstract 221. [Google Scholar]
- 15.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111(1):161–168. doi: 10.1016/j.ophtha.2003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredette MJ, et al. Reproducibility of pattern electroretinogram in glaucoma patients with a range of severity of disease with the new glaucoma paradigm. Ophthalmology. 2007 doi: 10.1016/j.ophtha.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventura LM, et al. Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112(1):10–19. doi: 10.1016/j.ophtha.2004.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan JE. Circulation and axonal transport in the optic nerve. Eye. 2004;18(11):1089–1095. doi: 10.1038/sj.eye.6701574. [DOI] [PubMed] [Google Scholar]
- 19.Quigley HA, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41(11):3460–3466. [PubMed] [Google Scholar]
- 20.Cioffi GA. Ischemic model of optic nerve injury. Trans Am Ophthalmol Soc. 2005;103:592–613. [PMC free article] [PubMed] [Google Scholar]
- 21.Lange M, et al. Endothelin expression in normal human anterior pituitaries and pituitary adenomas. J Clin Endocrinol Metab. 1994;79(6):1864–1870. doi: 10.1210/jcem.79.6.7527415. [DOI] [PubMed] [Google Scholar]
- 22.Broman AT, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49(1):66–76. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falsini B, et al. Steady-state pattern electroretinogram in insulin-dependent diabetics with no or minimal retinopathy. Doc Ophthalmol. 1989;73(2):193–200. doi: 10.1007/BF00155037. [DOI] [PubMed] [Google Scholar]
- 24.Sartucci F, et al. Changes in pattern electroretinograms to equiluminant red-green and blue-yellow gratings in patients with early Parkinson’s disease. J Clin Neurophysiol. 2003;20(5):375–381. doi: 10.1097/00004691-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Porciatti V, Sartucci F. Retinal and cortical evoked responses to chromatic contrast stimuli Specific losses in both eyes of patients with multiple sclerosis and unilateral optic neuritis. Brain. 1996;119(3):723–740. doi: 10.1093/brain/119.3.723. [DOI] [PubMed] [Google Scholar]
- 26.Ventura LM, Porciatti V. Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction: a pilot study. Ophthalmology. 2005;112(1):20–27. doi: 10.1016/j.ophtha.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]