Abstract
Most acute and chronic neurodegenerative conditions are accompanied by neuroinflammation; yet the exact nature of the inflammatory processes and whether they modify disease progression is not well understood. In this review, we discuss the key epidemiological, clinical, and experimental evidence implicating inflammatory processes in the progressive degeneration of the dopaminergic (DA) nigrostriatal pathway and their potential contribution to the pathophysiology of Parkinson's disease (PD). Given that interplay between genetics and environment are likely to contribute to risk for development of idiopathic PD, recent data showing interactions between products of genes linked to heritable PD that function to protect DA neurons against oxidative or proteolytic stress and inflammation pathways will be discussed. Cellular mechanisms activated or enhanced by inflammatory processes that may contribute to mitochondrial dysfunction, oxidative stress, or apoptosis of dopaminergic (DA) neurons will be reviewed, with special emphasis on tumor necrosis factor (TNF) and interleukin-1-beta (IL-1β) signaling pathways. Epigenetic factors which have the potential to trigger neuroinflammation, including environmental exposures and age-associated chronic inflammatory conditions, will be discussed as possible ‘second-hit’ triggers that may affect disease onset or progression of idiopathic PD. If inflammatory processes have an active role in nigrostriatal pathway degeneration, then evidence should exist to indicate that such processes begin in the early stages of disease and that they contribute to neuronal dysfunction and/or hasten neurodegeneration of the nigrostriatal pathway. Therapeutically, if anti-inflammatory interventions can be shown to rescue nigral DA neurons from degeneration and lower PD risk, then timely use of anti-inflammatory therapies should be investigated further in well-designed clinical trials for their ability to prevent or delay the progressive loss of nigral DA neurons in genetically susceptible populations.
Keywords: Parkinson's disease, Neuroinflammation, Microglia activation, Cytokines, Neurodegeneration, Oxidative stress, Dopamine neuron death, Anti-inflammatory therapy, TNF, IL-1β
Overview
Although the etiology of idiopathic Parkinson's disease (PD) is unknown, this neurodegenerative disease is characterized by the loss of dopamine (DA)-producing neurons in the ventral midbrain with cell bodies in the substantia nigra pars compacta (SNpc) that project to the striatum (nigrostriatal pathway), with a lesser effect on DA neurons in the ventral tegmental area (VTA) (Uhl et al., 1985; Moore et al., 2005). PD prevalence is age-associated, with approximately 1% of the population being affected at 65–70 years of age, increasing to 4–5% in 85-year-olds (Fahn, 2003). Epidemiological studies and pathological analyses demonstrate a mean age of onset of 70 in sporadic PD, which accounts for about 95% of patients (Tanner, 2003; Farrer, 2006); but familial forms of the disease linked to mutations in a restricted number of genes account for 4% and these patients develop early-onset disease before the age of 50 (Mizuno et al., 2001; Van Den Eeden et al., 2003). Over the past decade, a definitive link has been demonstrated between mutations in specific genes and heritable forms of PD (for an in-depth review see Farrer, 2006). Mutations in Parkin, DJ-1 and PINK1 have been linked to recessively inherited parkinsonism (Kitada et al., 1998; Bonifati et al., 2003a,b; Valente et al., 2004b,a) whereas mutations in α-synuclein and LRRK2 (also known as Dardarin) have been linked to dominantly inherited parkinsonism (Polymeropoulos et al., 1997; Paisan-Ruiz et al., 2004; Zimprich et al., 2004). Although some of these mutations can be found in higher frequency among certain ethnic populations, together they account for only a small percentage (perhaps up to 15%) of all PD cases.
For the idiopathic or non-familial forms of PD, the prevailing view is that the causes are multifactorial and genetic predispositions, environmental toxins, and aging are likely to be important factors in disease initiation and progression (Nagatsu and Sawada, 2006). The finding that the single greatest risk factor for developing PD is age, implicates cumulative CNS damage as a causative mechanism. However, nigral lesions in PD and aged individuals vary considerably, raising the possibility that aging and the disease process underlying PD may be occurring independently. At the cellular level, cumulative evidence supports an “oxidative stress hypothesis” for initiation of nigral dopamine neuron loss (for in-depth reviews see Owen et al., 1996, 1997; Jenner and Olanow, 1998; Beal, 2005; Lin and Beal, 2006). Oxidative stress occurs when there is an intracellular accumulation of reactive oxygen and nitrogen species (ROS/RNS) due to reduced endogenous anti-oxidant capacity and/or overproduction of ROS within the cell. Clearly, all aerobic organisms are susceptible to oxidative stress because ROS (primarily superoxide and hydrogen peroxide) are produced by mitochondria during respiration. However, the brain is considered to be abnormally sensitive to oxidative damage in part because oxygen consumption by the brain constitutes 20% of the total oxygen consumption in the body; and the brain is enriched in the more easily peroxidizable fatty acids (20:4 and 22:6) while its anti-oxidant defenses (such as catalase, superoxide dismutase, glutathione, and glutathione peroxidase) are relatively sparse (Floyd, 1999). Within the midbrain, the SN appears to be among the most vulnerable regions primarily because it operates under a pro-oxidative state relative to other parts of the brain even in healthy individuals. Specifically, the substantia nigra has a high metabolic rate combined with a high content of oxidizable species, including DA, DA-derived ROS, neuromelanin, polyunsaturated fatty acids, iron, and a low content of antioxidants (glutathione in particular) all of which render this brain region highly vulnerable to the effects of peroxynitrite and sulfite (Marshall et al., 1999); when combined with the high levels of ascorbate in the brain, the iron/ascorbate mixture is a potent pro-oxidant for brain membranes (Floyd, 1999). Further support for this claim is the finding that carbonyl modifications (indicative of protein oxidation) in the SN of normal individuals are present at twice the level present in the basal ganglia and prefrontal cortex (Floor and Wetzel, 1998). The extent of oxidative damage measured by the presence of the nucleoside oxidation product 8-hydroxyguanosine is approximately 16-fold greater and that of the aldehyde 4-hydroxy-2,3-nonenal (HNE) is about 6-fold greater in SN of PD brains compared to that of healthy control subjects (Yoritaka et al., 1996; Zhang et al., 1999). Biochemical studies indicate HNE can covalently modify proteins, block mitochondrial respiration (Picklo et al., 1999), and induce caspase-dependent apoptosis (Liu et al., 2000b). In summary, evidence of enhanced oxidative stress in the brains of PD patients includes increased oxidation of lipids, DNA and proteins and has been documented in a large number of studies (Owen et al., 1996; Spencer et al., 1998; Halliwell, 2006; Waragai et al., 2006).
Extensive evidence also supports the involvement of impaired mitochondrial function in PD (Schapira et al., 1989; Schapira, 1994; Jenner and Olanow, 1996; Sherer et al., 2002a; Keeney et al., 2006; Schapira, 2006). Mitochondria generate ROS as by-products of molecular oxygen consumption in the electron transport chain. Mitochondria are not only sources of ROS, they have been shown to be subcellular targets of cytokines such as TNF that promote over-production of ROS in mitochondria (Fernandez-Checa et al., 1997). Glutathione is the only anti-oxidant ion in the cell available to metabolize hydrogen peroxide and a small fraction of the total cellular GSH pool is sequestered in mitochondria (mGSH) by the action of a carrier that transports it from the cytosol to the mitochondrial matrix. Reduced levels of GSH, discussed in more detail under the TNF section, and increased levels of oxidized GSH (GSSG) are detectable in the surviving neurons of the SN of PD patients compared to age-matched controls (Sofic et al., 1992; Pearce et al., 1997), reflecting an increase in oxidative stress presumably from DA oxidation. The physiological consequences of mGSH depletion will be discussed in more detail in the TNF section as they have been extensively studied in TNF-induced hepatotoxicity (Fernandez-Checa et al., 1997; Fernandez-Checa and Kaplowitz, 2005). Other evidence consistent with an important role of mitochondrial function in the etiology of PD includes the observation that inhibition of the mitochondrial respiratory complex-I after exposure to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) gives rise to PD-like pathologies in humans, non-human primates, mice, and rats (Tetrud et al., 1989; Langston et al., 1999; Yazdani et al., 2006; Jackson-Lewis and Przedborski, 2007). Clinically, untreated PD patients display decreased mitochondrial activity of complexes I and I/III (Krige et al., 1992). It has been proposed that DA neurons may have an intrinsic sensitivity to complex I defects or agents that compromise its function on the basis of studies that demonstrate selective toxicity of the pesticide rotenone for DA neurons despite the fact that rotenone inhibits mitochondrial complex I throughout the brain (for in-depth review see Sherer et al., 2002a). For more in-depth reviews on the role of oxidative stress and mitochondrial dysfunction in PD, the reader should consult elsewhere (Beal, 2005; Schapira, 2006).
Another recent and important discovery in support of the mitochondrial hypothesis underlying dysfunction in PD was the identification of the first PD-linked gene encoding a kinase enriched in mitochondria: PTEN-induced kinase 1, PINK1, a 581-amino acid protein with both mitochondrial targeting and serine/threonine kinase domains underlying certain forms of autosomal recessive parkinsonism (Valente et al., 2004b,a). PINK1 localizes to Lewy bodies in sporadic PD and in inclusions present in alpha-synucleinopathies (Gandhi et al., 2006; Murakami et al., 2007). Homozygous mutations linked to familial PD affect the activity of its kinase domain which has high homology to that of Ca2+/calmodulin-dependent serine/threonine kinases and prevent its ability to preserve mitochon-drial membrane potential in conditions known to compromise mitochondrial integrity (Gandhi et al., 2006). PINK1(−/−) mice have normal numbers of nigral DA neurons, striatal DA levels and DA receptors but display deficits in striatal plasticity (decreases in evoked DA release, reductions in synaptic vesicle quantal size and release frequency, and impairments in corticostriatal long-term potentiation and long-term depression) that are l-3,4-dihydroxyphenylalanine (l-DOPA) responsive (Kitada et al., 2007).
Evidence of neuroinflammation in PD
Hallmarks of neuroinflammation
Long considered to be an immune-privileged site because of the presence of the blood–brain barrier (BBB) and the lack of a lymphatic system, it is now well established that the brain is fully capable of mounting an inflammatory response. Invading pathogens, trauma, infection, and stroke can trigger activation of brain resident macrophages known as microglia, local invasion of circulating immune cells, and production of reactive oxygen and nitrogen species (ROS/RNS), cytokines, chemokines, and other immune factors that can contribute to brain edema. Inflammation in the central nervous system (CNS) has been appropriately described as a two-edged sword (Wyss-Coray and Mucke, 2002; McGeer and McGeer, 2004). In acute situations and when short-lived, inflammatory mechanisms limit injury and promote healing; however, when chronically sustained at high levels, neuroinflammation can seriously damage viable host tissue.
Within the microenvironment of the brain, microglia serve a critical role in normal CNS function by mediating innate immune responses to invading pathogens (Wersinger and Sidhu, 2002; Wyss-Coray and Mucke, 2002). Resting microglia can mount a graded response to many forms of disturbances and become rapidly activated when pathological events occur (reviewed in Wojtera et a l., 2005). In their resting state and in the healthy brain, microglial cells display a ramified morphology and a low expression of surfacer eceptors that mediate their normal macrophage functions, including leucocyte common antigen (LCA) also known as complement receptor (CD)45, CD14 and Mac-1 (CD11b/CD18) (Kreutzberg, 1996). It has been shown that within 24 h of stimulus exposure, microglia display elevated levels of immunoglobulin (Ig)G reactivity, upregulation of CD1 and cell adhesion molecules such as lymphocyte function-associated antigen 1 (LFA-1), Mac-1, intercellular adhesion molecule (ICAM)-1 (also known as CD54), and vascular cell adhesion molecule (VCAM)-1 (also known as CD106). Moderately activated microglia are believed to play a homeostatic role in the CNS by scavenging excess neurotoxins, by removing dying cells and cellular debris (see reviews by Nakamura, 2002; Orr et al., 2002), and by releasing trophic factors that promote axonal sprouting of DA neurons such as brain-derived neurotrophic factor (BDNF) (see review by Aloisi, 1999; Batchelor et al., 1999). In the continued presence of the activating stimulus, microglia are attracted by chemokines such as monocyte chemoattractant protein-1 (MCP-1) and interferon (IFN)-inducible protein-10 produced by neurons (Aloisi et al., 2000; Aloisi, 2001). These neuron-derived mediators permit regulation of microglia and facilitate microglial adherence to neurons (Kreutzberg, 1996) and microglial scavenger functions which are critical in the event of infection, inflammation, trauma, ischaemia and neurodegeneration in the CNS (Beyer et al., 2000). Although microglia are the key mediators of neuroinflammatory responses, astrocytes and oligodendrocytes can also participate in the process. Astrocytes provide homeostatic control of the extracellular environment in the CNS by regulating glutamate uptake (reviewed in Tilleux and Hermans, 2007) but they can also become activated by chemicals, physical damage, or ischemia in a process termed reactive gliosis that is characterized by up-regulation of the glial fibrillary acidic protein (GFAP) and the gap-junction protein Connexin 43 (Haupt et al., 2007). Activation of oligodendrocytes results secretion of inflammatory molecules, such as nitric oxide (NO), cytokines, and prostaglandins and most notably in upregulation of several chondroitin sulfate proteoglycans, including NG2, which contributes to the growth-inhibitory environment that prevents regeneration of axons in the injured CNS (Rhodes et al., 2006). Clinically, immune-mediated damage to oligodendrocytes as a result of innate and adaptive immune system attack results in extensive demyelination, loss of oligodendrocytes and axonal degeneration in patients with chronic neurodegenerative multiple sclerosis (MS) (McQualter and Bernard, 2007); however, the extent to which oligodendrocytes become damaged in other chronic neuroinflammatory degenerative conditions and their contribution to the neuroinflammatory response associated with those diseases are less well defined. An initial physical or pathogenic event in the central nervous system (CNS) is expected to elicit activation of many glial cell types and secretion of factors shown in Table 1, including growth factors, ROS and RNS, prostaglandins, chemokines, and cytokines. Some of these factors have neuroprotective and trophic activities and aid in the repair process; while others enhance oxidative stress and trigger apoptotic cascades. To resolve the inflammatory response cell trafficking, phagocytosis and production of endogenous mediators with anti-inflammatory properties are elicited to balance or suppress pro-inflammatory gene expression. In short, pro- and anti-inflammatory responses must be in balance to prevent the potential detrimental effects of prolonged or unregulated inflammation.
Table 1. Glial-derived mediators that modulate neuronal survival.
| Abbreviation | Name | Function/Effect |
|---|---|---|
| IL-1β | Interleukin-1β | Proinflammatory |
| IL-6 | Interleukin-6 | Induction of iNOS and ↑ oxidative stress |
| IL-18 | Interleukin-18 | Induction of iNOS and ↑ oxidative stress |
| TNF | Tumor necrosis factor | Neurotoxic |
| MCP-1 | Monocyte chemoattractant protein 1 | Chemotaxic |
| MIP-1α | Macrophage inflammatory protein 1α | Chemotaxic |
| MIP-1β | Macrophage inflammatory protein 1β | Chemotaxic |
| MIP-2 | Macrophage inflammatory protein 2 | Chemotaxic |
| MDC | Macrophage-derived chemokine | Chemotaxic |
| IL-3 | Interleukin-3 | Proliferation |
| IL-15 | Interleukin-15 | T cell regulation |
| M-CSF | Monocyte colony-stimulating factor | Proliferation |
| PGE2 | Prostaglandin E2 Eicosanoids (prostaglandins, leukotrienes, thromboxanes) | Proinflammatory, proliferative; may impair glutamate uptake by astrocytes and ↑ excitotoxicity |
| AA | Arachidonic acid | Potentiate NMDA receptors → excitotoxicity; calcium overload |
| PAF | Platelet-activating factor | |
| QA | Quinolinic acid | |
| NO | Nitric oxide | Neurotoxic, oxidative stress; inhibition of glutamate uptake by |
| O•− | Superoxide | astrocytes |
| H2O2 | Hydrogen peroxide | |
| OH− | Hydroxyl radical | |
| NOO− | Peroxynitrite | |
| TGFβ | Transforming growth factor beta | Anti-inflammatory |
| IL-10 | Interleukin-10 | Immunosuppression |
| NGF | Nerve growth factor | Neurotrophic factor |
| BDNF | Brain-derived neurotrophic factor | Neurotrophic factor |
| GDNF | Glial-derived neurotrophic factor | Neurotrophic factor |
| NT-3 | Neurotrophin-3 | Neurotrophic factor |
| NT-4 | Neurotrophin-4 | Neurotrophic factor |
Molecular mechanisms that contribute to proteolytic and oxidative stress, inflammation, and death of DA neurons
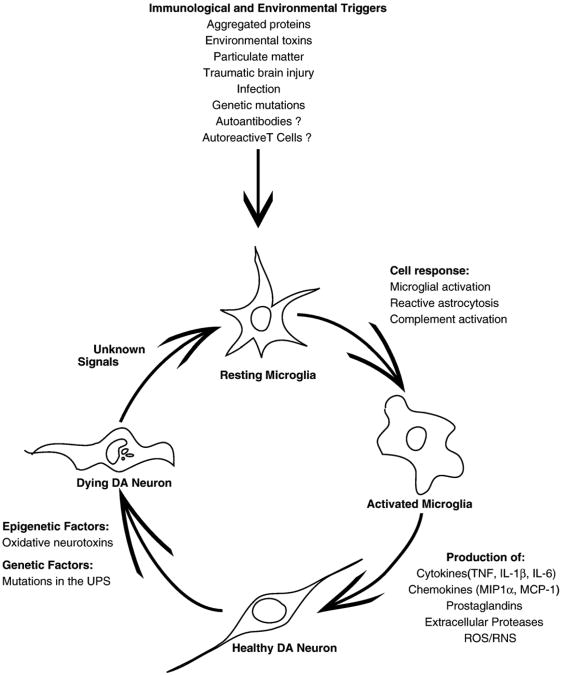
Neurodegenerative diseases are characterized by the loss of specific neuronal populations and often by intraneuronal as well as extracellular accumulation of fibrillary materials, which have been shown to promote neuroinflammation. Formation of intracellular inclusion bodies may result from abnormal protein–protein interactions, aberrant protein folding, and/or dysregulation of the ubiquitin–proteasome system (UPS). These conditions, often referred to as ‘proteinopathies,’ are now thought to play a principal role in neuronal dysfunction and death of neurons that characterizes several common neurodegenerative diseases (Ross and Poirier, 2004; Selkoe, 2004; Moore et al., 2005; Lansbury and Lashuel, 2006). Although the key molecular and cellular events underlying development of neurodegenerative diseases such as Alzheimer's (AD), Parkinson's (PD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS) are clearly divergent, one common way in which a number of divergent molecular or cellular events (e.g., mutations, oxidation, protein misfolding, truncation, or aggregation) may all contribute over time to death of neurons is via activation of resident microglial populations in specific brain regions. If the initial trigger that elicited microglial activation is not resolved (as in the case of a genetic mutation or a prolonged or repeated environmental exposure), a self-sustaining cycle of neuroinflammation is likely to ensue and contribute to neuronal dysfunction and eventual death of vulnerable neuronal populations. In support of this idea, experimental, clinical, and epidemiological studies indicate that activation of resident microglial populations may be occurring in parallel with the neuronal dysfunction underlying the disease process in certain neurodegenerative diseases. For example, chronic inflammation is associated with a broad spectrum of neurodegenerative diseases of aging, including diseases that affect the CNS such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), all of the tauopathies, and age-related macular degeneration (McGeer and McGeer, 2004; Block and Hong, 2005; Mrak and Griffin, 2005; Nagatsu and Sawada, 2006). If future studies support the notion that neuroinflammation promotes or facilitates the neurodegenerative effects of proteinopathies, early intervention with anti-inflammatory therapies in populations identified to be at risk due to genetic mutations may represent a unique opportunity to establish a causal role for inflammation in progression of neurodegenerative disease. In addition to formation of intracellular and extracellular protein aggregates, other cellular and molecular processes that activate microglia in the CNS and promote acute or chronic neuroinflammation have been identified in recent years and found to be present in a number of chronic neurodegenerative conditions. These include accumulation of abnormally modified cellular components, molecules released from or associated with injured neurons or synapses, and deregulation of inflammatory control mechanisms such as those that occur with aging.
While acute inflammation in the CNS is often accompanied by secretion of microglial-derived neuroprotective factors which act to limit tissue damage and promote repair (Wyss-Coray and Mucke, 2002; Marchetti and Abbracchio, 2005), chronic neuroinflammation is more likely to increase the susceptibility of vulnerable neurons to toxic injury because it can induce oxidative stress. The two ways neuroinflammation induces oxidative stress are via production of high levels of ROS by activated glia such as microglia and astrocytes and via arachidonic acid signaling through the activation of cyclo-oxygenase (COX) and lipoxygenase (LOX) pathways. Although it may be reasonable to propose that CNS infections, encephalitis, brain trauma, heavy metal and pesticide exposure, and other environmental factors associated with PD risk may do so in part by eliciting a neuroinflammatory response in which activated microglia overproduce neurotoxic inflammatory mediators, the mechanistic basis to explain toxic selectivity for the nigrostriatal pathway remains unclear. The brain expresses both COX-1 and COX-2; COX-1 is the key cyclo-oxygenase in microglia that becomes upregulated during inflammatory responses (Ju and Neufeld, 2002; Schwab et al., 2002) whereas COX-2 levels are dynamically regulated by pro-inflammatory mediators under physiological conditions (Yamagata et al., 1993). In addition, COX-2 has been shown to catalyze oxidation of cytosolic DA (Hastings, 1995) and its expression is upregulated in neurons and astrocytes in response to CNS injury (Consilvio et al., 2004). Consistent with these observations, examination of brain samples from humans or mice exposed to MPTP confirms the presence of elevated COX-2 levels and extensive DA-quinone formation as well as formation of protein-bound 5-cysteinyl-DA adducts (Teismann et al., 2003b,a). COX-2 ablation or pharmacological inhibition protects against MPTP- (Feng et al., 2002) and 6-OHDA-induced nigral DA neuron loss (Sanchez-Pernaute et al., 2004). PGE2, produced by COX-2, can induce an intraneuronal toxic effect directly on DA neurons (Gao et al., 2003). Prostaglandins of the J2 series also induce oxidative stress by causing a decrease in glutathione and gluta-thione peroxidase activity, decreases in mitochondrial membrane potential and over-production of protein-bound lipid peroxidation products including acrolein and 4-hydroxy-2-nonenal (HNE). These effects suggest that prostaglandins of the J2 series are either a source of markedly increased ROS generation or modulators of ROS sensitivity. Another source of oxidative stress associated with arachidonic acid signaling in the CNS is the synthesis of eicosanoids via the lipoxygenase (LOX) pathway, but much less is known about the contribution of this pathway to oxidative stress induced by neuroinflammation. LOXs are a family of monomeric non-heme, non-sulfur iron dioxygenases, which catalyze the conversion of polyunsaturated fatty acids into conjugated hydroperoxides and are known to activate programmed cell death pathways (for review see Maccarrone et al., 2001). Lastly, the high density of microglia in midbrain relative to other parts of the brain (Lawson et al., 1990) coupled with expression of MHC1 and beta2-micro-globulin in SN DA neurons (Linda et al., 1999) may promote microglial-derived oxidative stress in this region of the brain by facilitating presentation of misfolded, aggregated, or oxidized proteins or protein fragments to microglia, thereby enhancing their phagocytic activity against DA neurons. Neuromelanin (NM), a by-product of DA biosynthesis, is another potent trigger of microglia phagocytic activity (Wilms et al., 2003b; Zucca et al., 2004; Kim and Joh, 2006; Zecca et al., 2006) that is released from the dying melanized DA neurons of the SNpc. In short, all of the above characteristics of the midbrain environment may act in concert to confer increased susceptibility to nigral DA neurons to inflammatory stimuli.
Evidence thatneuroinflammation compromises dopaminergic neuron survival
Clinical and epidemiological evidence
In the last decade, a great wealth of new information has emerged to suggest that inflammation-derived oxidative stress and cytokine-dependent toxicity may contribute to nigrostriatal pathway degeneration and hasten progression of disease in humans with idiopathic PD (reviewed in Hald and Lotharius, 2005; Hirsch et al., 2005; Nagatsu and Sawada, 2005, 2006; Wersinger and Sidhu, 2006; Whitton, 2007). The existence of ongoing inflammatory processes that may contribute to progression of PD is supported by evidence of activated microglia, accumulation of cytokines, nuclear factor kappa B (NF-κB) pathway activation, and oxidative damage to proteins in the CSF and brains of live PD patients as well as in post-mortem brain samples (McGeer et al., 1988; Vawter et al., 1996; Banati et al., 1998; Hunot et al., 1999; Gerhard et al., 2006) and most experimental models of PD (Czlonkowska et al., 1996; Cassarino et al., 1997; Hirsch et al., 1997; Castano et al., 1998; Kohutnicka et al., 1998; Liberatore et al., 1999; Dehmer et al., 2000; Herrera et al., 2000; Mandel et al., 2000; Mogi et al., 2000a; Vila et al., 2001; Gayle et al., 2002; Nakagawa and Schwartz, 2004; De Pablos et al., 2005). In PD, the lateral tier of the substantia nigra (SNL) degenerates earlier and more severely than the more medial nigral component (SNm), yet the cause of this brain regional vulnerability has remained unclear. Consistent with a role for inflammation-derived oxidative stress and mitochondrial dysfunction, a recent microarray study of PD and control brains indicated increased expression of genes encoding pro-inflammatory cytokines and subunits of the mitochondrial electron transport chain and decreased expression of several glutathione-related genes in the more vulnerable lateral tier region of SN (Duke et al., 2007). Furthermore, because many of the genes differentially regulated in this region are known to be expressed at high levels and predominantly in glial cells, these findings support the idea that glial dysregulation may be an important mechanism underlying PD pathogenesis. Some have postulated that the course of PD may spiral out of control with excessive activation of microglia, over production of cytokines and other inflammatory mediators, as well as reactive oxygen species (ROS) (Whitton, 2007). Further support for this comes from recent studies of in vivo imaging of microglial activation with the peripheral benzodiazepine receptor binding ligand [11C]-(R) PK11195 in positron emission tomography (PET) scans. Specifically, patients with idiopathic PD have markedly elevated neuroinflammation in the pons, basal ganglia, striatum, and frontal and temporal cortical regions compared to age-matched healthy controls irrespective of the number of years with the disease, indicating that changes in microglia activation in the affected nigrostriatal pathway in early PD are likely to occur in parallel with loss of dopaminergic terminals (Gerhard et al., 2006). It is possible that the neuroinflammatory activity in the CNS of PD patients is partly a result of abnormal infiltration of peripheral blood-borne macrophages from the systemic circulation due to a dysfunctional BBB, as reduced function of efflux pumps that regulate BBB permeability has been reported for PD patients (Kortekaas et al., 2005). Taken together, these studies strongly suggest that brain microglia may become activated early in the disease process and remain activated, possibly allowing them to contribute to disease progression via cytokine release. An important feature of the oxidative stress hypothesis of PD is that a transient initiation factor (i.e., toxins, bacterial, or viral infections, particulate matter, pesticides, etc.) may trigger an active, self-perpetuating cycle of chronic neuroinflammation (i.e., overproduction of chemokines, cytokines, ROS/RNS and adhesion molecules by activated microglia) which may serve to promote clustering of activated microglia around DA neurons (Bronstein et al., 1995; Banati et al., 1998; McGeer and McGeer, 1998b) and contribute to irreversible neuronal dysfunction. Even if neuroinflammation does not occur in the early stages of DA neuron dysfunction, the release of chemoattractants by the dying DA neurons (Aloisi, 2001; Kim and De Vellis, 2005; Sriram et al., 2006) would be expected to lead to greater infiltration of the region by activated microglia coming to remove neuronal debris and may be a contributing factor in progression of disease as the respiratory bursts associated with phagocytic activities would further enhance oxidative stress for the remaining population of DA neurons.
Perhaps the most convincing and compelling evidence to support the claim that inflammatory mechanisms are likely to contribute to PD risk comes from epidemiological studies (McGeer and McGeer, 1998a; Chen et al., 2003, 2005). Specifically, a large prospective study of hospital workers indicated that the incidence of idiopathic Parkinson's disease (PD) in chronic users of over-the-counter non-steroidal anti-inflammatory drugs (NSAIDs) which scavenge free oxygen radicals and inhibit cyclo-oxygenase (COX) activity was 46% lower than that of age-matched non-users (Chen et al., 2003). Similar findings were reported for chronic users of the non-selective COX inhibitor ibuprofen in a follow-up study involving a large (∼180,000) cohort of U.S. men and women (Chen et al., 2005). Inhibition of COX-mediated DA oxidation (Teismann et al., 2003b), as well as inhibition of microglial-derived toxic mediator production, are likely to be among the mechanisms that contribute to decreased incidence of PD in chronic NSAID users (Chen et al., 2003; Chen et al., 2005). This and other evidence relating to the protective effects of aspirin or other NSAIDs on DA neurons in animal models of PD as well as epidemiological data exploring the effectiveness of NSAIDs in the prevention of PD has been reviewed recently (Esposito et al., 2007). Results of these studies are less surprising in light of the fact that it is well-documented that certain neuron–glia interactions can lead to neuronal death (Wersinger and Sidhu, 2002; Barcia et al., 2003; Hirsch et al., 2003, 2005; McGeer et al., 2003; Herrera et al., 2005; Mrak and Griffin, 2005; Zhang et al., 2006). Mechanistically, these PD risk-lowering effects of NSAIDs strongly suggests that neuroinflammatory processes contribute to DA neuron loss and development of PD in humans. Although the protective effect of NSAIDs are likely to be primarily mediated by COX inhibition, multiple mechanisms, including the Rho kinase pathway (Zhou et al., 2003; Tang and Liou, 2007), cannot be ruled out at this time in mediating the beneficial effects of NSAIDs. Therapeutically, these findings raise the possibility that early intervention with NSAIDs or similar anti-inflammatory therapy may be neuroprotective and could delay or prevent onset of PD.
Dopaminergic neurotoxins
Direct evidence of the susceptibility of nigral DA neurons to inflammatory stimuli comes from development of endotoxin-based in vivo models of nigrostriatal pathway degeneration. Almost a decade ago, intranigral delivery of lipopolysaccharide (LPS) was first shown to induce an inflammatory reaction that activated microglia and induced selective and irreversible damage to nigral DA neurons while sparing serotonergic neurons (Castano et al., 1998). Mechanistically, LPS-induced nigral DA neuron death was shown to be independent of nitric oxide and inhibitable by dexamethasone (Castano et al., 2002). Most importantly, administration of alpha-methyl-p-tyrosine (alpha-MPT), an inhibitor of tyrosine hydroxylase, prevented LPS-induced nigral DA neuron loss and strongly supported the notion that DA neurons are intrinsically more susceptible to inflammatory stimuli (De Pablos et al., 2005). In recent years, two additional bacteriotoxin-induced inflammatory models of PD consisting of chronic low-dose LPS infusion into SNpc of rats (Gao et al., 2002b) or intrauterine exposure to LPS (Carvey et al., 2003) were reported and shown to induce delayed, chronic, and progressive loss of DA neurons in the adult SNpc or in the offspring, respectively. Together, these inflammogen models of PD lend further support for a role of toxin-induced inflammation in the degeneration of the nigrostriatal pathway.
Several exogenous compounds that inhibit specific protein complexes along the mitochondrial electron transport chain and cause DA neurotoxicity have been used to generate animal models of PD and all of them have a robust associated glial reaction. The best characterized of these models include MPTP, 6-hydroxydopamine (6-OHDA), N-methyl(R)salsolinol, rote-none and paraquat (Kirik et al., 1998; Betarbet et al., 2000; Naoi et al., 2000; Blum et al., 2001; Carvey et al., 2003; Przedborski et al., 2004). The enzyme monoamine oxidase B is present in astrocytes and microglia where it converts MPTP into MPP+ [1-methyl-4-phenyl-pyridinium], a free radical oxidant that is taken up by DA transporters in neurons. MPP+ enters the mitochondria where it inhibits complex I function, thereby disrupting the first step in the electron transport chain required to sustain oxidative phosphorylation and triggering a circular cascade of oxidative stress that culminates in activation of the mitochondrial cell death machinery (Dauer and Przedborski, 2003; Przedborski and Vila, 2003; Przedborski et al., 2004).
Although each neurotoxin may trigger different initial cascades of events, they all consistently involve oxidative stress as the critical mechanism that elicits the death of DA neurons. Thus, general anti-oxidants (Ling et al., 1999; Gao et al., 2002a, 2003; Isacson, 2002; Lin et al., 2003) are being intensely investigated for their ability to offer DA neuroprotec-tion in experimental models of PD. Evidence that the cycle of neuroinflammation triggered by exogenous neurotoxins persists long after the initial insult abates and may contribute to the progressive degeneration is compelling (McGeer et al., 1988, 2003; Langston et al., 1999; Hald and Lotharius, 2005; Hirsch et al., 2005). Loss of DA neurons in MPTP models is associated with a glial response that peaks prior to the death of neurons (Czlonkowska et al., 1996; Kohutnicka et al., 1998; Liberatore et al., 1999; Dehmer et al., 2000; Vila et al., 2001). MPTP is capable of inducing massive and prolonged microglia activation after single exposure in mice (Sugama et al., 2003), monkeys (McGeer et al., 2003), and humans (Langston et al., 1999). Consistent with a critical role of the glial reaction in MPTP-mediated nigral neurotoxicity, anti-inflammatory drugs such as pioglitazone (a PPARγ agonist) and minocycline (a immuno-suppressive and anti-inflammatory tetracycline derivative) have been shown to provide clinical benefit in MPTP-intoxicated mice (Hirsch et al., 2003). Numerous other anti-inflammatory compounds are under intense investigation as potential neuroprotective agents in experimental models of PD (Gao et al., 2003; Sairam et al., 2003; Cleren et al., 2005; Lund et al., 2005; Marchetti and Abbracchio, 2005; Youdim and Bucca-fusco, 2005).
The neurotoxin of choice for inducing nigrostriatal degeneration in rats is the neurotoxic dopamine analog 6-hydroxydopamine (6-OHDA) and several studies support the importance of glial activity and inflammatory mediators in the neurodegenerative activity of this DA neurotoxin. 6-OHDA can be injected directly into the SN, forebrain bundle or the striatal terminals where it induces a retrograde degeneration and leads to the apoptotic death of DA neuron cell bodies in the SNpc (Kirik et al., 1998). Evidence indicates that the toxic effects of 6-OHDA are in part mediated through the activation of microglia and cytokines with toxic effects on DA neurons. PET studies with the ligand (11)C-PK11195 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3 isoquinoline carboxamide) that binds to the peripheral benzodiazepine receptor which is upregulated in activated microglia (Wilms et al., 2003a), showed increased binding in the striatum and SN following 6-OHDA administration (Cicchetti et al., 2002). The microglial activation and delayed loss of DA neurons induced by direct administration of 6-OHDA into the SN of mice can be blocked by the potent anti-inflammatory tetracycline derivative minocycline (He et al., 2001). Similarly, the COX-2 inhibitor celecoxib attenuated microglia activation induced by intrastriatal 6-OHDA administration and the delayed and progressive phase of DA neuron loss that occurs between 12 and 21 days after the lesion (Sanchez-Pernaute et al., 2004). Although 6-OHDA-lesioned rats have been demonstrated to have increased levels of cytokines, in particular TNF, in both SN and striatum (Mogi et al., 1999; Nagatsu and Sawada, 2005), the identity of the inflammatory mechanisms that mediate 6-OHDA-induced DA neurodegeneration has not been clearly established. However, pharmacologic evidence implicates TNF-dependent events in death of DA neurons. Specifically, chronic pharmacological inhibition of soluble TNF signaling for 2 weeks with a dominant negative TNF inhibitor attenuated 6-OHDA-induced DA neuron loss detected at 3 weeks post-lesion by approximately 50% (McCoy et al., 2006). Lastly, microglia activation following exposure to the pesticide rotenone has also been demonstrated to occur in vivo and to precede degeneration of dopaminergic terminals (Sherer et al., 2003b); inhibitors of NADPH oxidase block microglial-derived superoxide production and attenuate rotenone-induced death of DA neurons in vitro (Gao et al., 2002a), suggesting a critical role for microglia in mediating rotenone-induced degeneration.
Consistent with the prevailing view that the interplay between genetics and environment influences etiology and progression of PD, several recent studies indicate that the function of several proteins encoded by genes linked to familial forms of PD can be placed in biochemical pathways in relation to each other (reviewed in Cookson, 2003) and may be modulated by environmental factors (Corti et al., 2005; Lin and Beal, 2006). PINK1 and DJ-1 interact with mitochondria to regulate responses to oxidative stress and, Parkin is likely to have a crucial role in proteasomal degradation of cellular substrates of importance to DA neurons some of which may interact with PINK1 or DJ-1 to interfere with their respective functions. Given the long-standing appreciation that neurotoxin-induced nigrostriatal degeneration or parkinsonism by 6-OHDA and MPTP occur via mitochondrial complex I inhibition, it seems highly likely that mitochondrial dysfunction may be the common link between the seemingly disparate genetic and environmental causes underlying PD pathogenesis. Although the precise functions of α-synuclein have not been fully elucidated, its distribution is ubiquitous throughout the brain and its association with membranes and vesicular structures (Irizarry et al., 1996; Kahle et al., 2000) support a role in exocytotic vesicle trafficking. The propensity of mutated α-synuclein to misfold, nucleate protein aggregates, and contribute to formation of Lewy bodies in neurites underlies its role in monogeneic forms of PD in humans (Lansbury and Brice, 2002) and triplication of the α-synuclein locus can also lead to Parkinson's disease in humans (Singleton et al., 2003). On the basis of evidence from cell culture and animal models, it has been proposed that the ability of environmental toxins to promote α-synuclein aggregation and deposition into Lewy bodies is a downstream consequence of mitochondrial dysfunction. Specifically, chemical products used in herbicides and pesticides, including paraquat and rotenone, are structurally similar to MPTP and have been shown to inhibit mitochondrial complex I and promote α-synuclein aggregation in vitro (Betarbet et al., 2000; Sherer et al., 2002b, 2003c,a).
In addition to exogenous toxic compounds, brain tissue contains endogenous toxins that may also be contributing to nigral DA neuron loss and risk of developing PD. These compounds include tetrahydroisoquinolines (TIQs) which are produced via the reaction of DA with aldehydes and include salsolinol and its metabolic products which can have adverse effects in the nigrostriatal pathway by decreasing dopaminergic neurotransmission, generating ROS, and triggering apoptosis (Naoi et al., 2000; Jung and Surh, 2001; Maruyama et al., 2001). The molecule tetrahydrobiopterin (BH4), a cofactor of phenylalanine hydroxylase, is essential for endothelial nitric oxide synthase (eNOS) activity but can also inhibit mitochondrial complexes I and IV and lead to a breakdown in the function of the electron transport train, thus creating oxidative stress and ultimately PD-like symptoms (Choi et al., 2006). NO also contributes to DA neuron death through mechanisms that, while not completely understood, are likely to involve mediation of excitotoxicity, activation of PARP-1 (poly-ADP ribose polymerase), DNA damage, activation of caspase-dependent and independent cell death, and/or nitrosylation of proteins including α-synuclein and Parkin (Zhang et al., 2006).
Evidence implicating cytokines in nigrostriatal pathway degeneration
Post-mortem analyses indicate that the levels of several cytokines including TNF and interleukin 1-beta (IL-1β) are significantly elevated in the area of substantia nigra where maximal destruction of vulnerable melanin-containing dopamine-producing neurons occurs in PD patients (Hirsch et al., 1998). Although the genes for various cytokines, chemokines and acute phase proteins have been surveyed and individual reports show significant genetic linkage of single-nucleotide polymorphisms and increased risk for PD (Kruger et al., 2000; Nishimura et al., 2001, 2005; Hakansson et al., 2005a,b; Wahner et al., 2007), these findings need to be confirmed. Moreover, meta-analyses of multiple such association studies are needed to assess overall genetic effect. Nevertheless, substantial evidence implicates several cytokines in the pathophysiology of PD. Here we discuss how the downstream signaling pathways activated by TNF and IL-1β may represent common pathways on which divergent stimuli converge to elicit death of vulnerable neuronal populations through mechanisms that include mitochondrial toxicity (as described for TNF below) and caspase-dependent apoptosis or other forms of programmed cell death.
TNF
Strong evidence implicates TNF as an early and late player in the pathophysiology of PD. TNF signals through one of two receptors. Tnfrsf1a (formerly TNFR1) is expressed in many cell types (Tartaglia et al., 1993; Boka et al., 1994) including nigrostriatal DA neurons (Aloe and Fiore, 1997; Ling et al., 1998; McGuire et al., 2001; Gayle et al., 2002; Carvey et al., 2005). Tnfrsf1b (formerly TNFR2) is expressed primarily by cells of the immune system including microglia (Dopp et al., 1997), but its expression has also been reported in heart and endothelial cells, as well as dopaminergic (McGuire et al., 2001), cortical (Marchetti et al., 2004), and hippocampal (Bernardino et al., 2005; Heldmann et al., 2005) neurons. Levels of TNF in the healthy adult brain are generally very low and produced primarily by neurons (Breder et al., 1993). In contrast, high levels of TNF and soluble Tnfrsf1a (which is cleaved in response to local elevations of soluble TNF protein) have been detected in CSF and in the SN of postmortem brains of PD patients (Boka et al., 1994; Mogi et al., 1994; Bessler et al., 1999; Hunot et al., 1999; Hasegawa et al., 2000; Mogi et al., 2000b; Nagatsu et al 2000b.,). SN dopaminergic neurons are extremely sensitive to TNF (Aloe and Fiore, 1997; Ling et al., 1998; McGuire et al., 2001; Gayle et al., 2002; Carvey et al., 2005). In addition, TNF can activate the abundant numbers of microglia in the midbrain (Lawson et al., 1990; Kim et al., 2000), potentiating inflammatory responses that lead to auto-amplification of ROS, NO, and superoxide radicals to form highly oxidizing peroxynitrite species (Beckman et al., 1990; Dawson et al., 1994). TNF-dependent microglia activation in the SN creates an environment of oxidative stress through activation of NADPH oxidase (Hunot et al., 1999; Wyss-Coray and Mucke, 2002) which further hastens oxidative damage of DA neurons (Wyss-Coray and Mucke, 2002). In experimental models of PD, significantly elevated levels of TNF mRNA and protein were detectable in rodent midbrain substantia nigra (SN) within hours of in vivo administration of 6-OHDA (Nagatsu et al., 2000a) or MPTP intoxication (Rousselet et al., 2002; Sriram et al., 2002; Ferger et al., 2004). Consistent with a role of TNF in contributing to dopaminergic neuron death in chronic parkinsonism, plasma TNF levels were shown to remain elevated in MPTP-treated non-human primates 1 year after administration of the neurotoxin (Barcia et al., 2005). In addition, mice lacking TNF or both TNF receptors have been reported to have altered dopamine metabolism (Rousselet et al., 2002), reduced microglial responsiveness to MPTP, and reduced sensitivity to MPTP-induced DA neuron loss (Sriram et al., 2002, 2006; Ferger et al., 2004). Confirmation that soluble TNF signaling has an important role in degeneration of DA neurons induced in vivo was shown using a pharmacological approach in non-genetically manipulated animals. Specifically, engineered dominant negative TNF inhibitors selective for soluble TNF displayed significant neuroprotective properties in the 6-OHDA oxidative neurotoxin and chronic LPS rat models of PD (McCoy et al., 2006). Lastly, single-nucleotide polymorphisms (SNPs) in the promoter of the TNF gene at positions −1031, −863, and −857, have been studied in patients with sporadic PD. Interestingly, a polymorphic allele (−1031 C) that drives transcriptional activity and results in higher than normal TNF production was found at higher frequency in a cohort of Japanese PD patients with early onset PD compared to late-onset PD and unaffected controls (Nishimura et al., 2001). Another polymorphism in the TNF gene promoter (−308 G/A) that influences basal TNF levels has also been shown to be differentially represented in PD patients with sporadic PD (Kruger et al., 2000; Wahner et al., 2007). Confirmation of these findings by other investigators as well as meta-analyses from a larger number of studies will be needed to establish the validity and significance of these associations. Together, findings obtained from histopathologic, genetic, and pharmacologic studies strongly implicate a role for TNF-dependent mechanisms and downstream targets in neurotoxin- and bacteriotoxin-induced loss of nigral DA neurons and suggest that high TNF levels in the midbrain may increase susceptibility for PD in humans.
One important biological question that remains unanswered is ‘what are the molecular and cellular mechanisms by which TNF mediates the neuroinflammatory and neurotoxic effects that contribute to nigrostriatal degeneration?’ Since the original identification of TNF receptors approximately 15 years ago, modern molecular techniques have revealed a complex array of potential proteins that interact with TNF receptors to modulate activation of a number of downstream signaling pathways (for in-depth reviews see Locksley et al., 2001; MacEwan, 2002; Liu, 2005; Shen and Pervaiz, 2006). Depending on cellular context, activation of TNF-dependent pathways contributes to mitochondrial toxicity, ROS production, apoptosis, or new gene transcription for promotion of cell survival, proliferation and differentiation.
Although less well studied compared to Tnfrsf1a signaling, Tnfrsf1b signaling involves recruitment of the adaptor proteins TRAF1 and 2 and cellular Inhibitor of Apoptosis proteins (cIAPs) to activate the NF-κB pathway which is a key regulator of immunity, inflammation and cell survival. Cytotoxic ROS signaling appears to be mediated by TNF in part by activation of the c-Jun N-terminal kinase (JNK)/p38 mitogen-activated protein kinase (MAPK) cascade. However, the key signaling pathways downstream of TNF, the NF-κB, the stress-induced p38MAPK, and the c-Jun N-terminal kinase (JNK) pathways have now been shown to regulate ROS signaling events in the cell via cross-talk at the level of two NF-κB target genes XIAP and GADD45β/Myd118, an inhibitor of the MKK7/JNKK2 kinase (De Smaele et al., 2001; Papa et al., 2004). Their crosstalk has been shown to involve a series of complex positive feedback loops between JNK and ROS resulting in potentiation of ROS production as well as negative feedback loops to dampen ROS production and activate expression of key antioxidant enzymes such as MnSOD. This NF-κB-imposed restraint on ROS and JNK signaling is crucial for antagonism of programmed cell death elicited by TNF and likely other proinflammatory triggers (Papa et al., 2006). In addition to the well-established role of its downstream effectors RIP and TRAF2 in mediating programmed cell death, TNF-dependent ROS production may also mediate non-apoptotic (necrotic) cell death in a RIP and TRAF2-dependent manner at the level of NF-κB and JNK/ASK-1 (Apoptosis Signal-regulating Kinase-1) activation (Liu et al., 2000a; Liu, 2005).
The Tnfrsf1a receptor is a canonical ‘death receptor’ which triggers production of ceramide and sphingomyelinase (Luberto et al., 2002) as well as a cellular apoptotic response the activation of which has been extensively studied in hepatic injury during endotoxemia. Activation of Tnfrsf1a is intimately connected with the mitochondrial apoptosis pathway via Bax and Bid, the BH3-only pro-death Bcl-2 family proteins that are independently activated by TNF receptor signaling. Bax becomes activated by TNF and translocates to mitochondria where its insertion depends on Bid. The latter is also required for mitochondrial release of cytochrome c whereas Bax is not, suggesting that Bid can activate additional downstream molecules other than Bax (Zhao et al., 2001). Both Bid-dependent and Bid-independent mitochondrial activation occurs early in response to TNF and while the Bid-mediated mechanism is rapid and potent, the Bid-independent mechanism progresses gradually and involves multiple players, including ROS, JNK, and mitochondria permeability transition (MPT) (Jurewicz et al., 2003; Chen et al., 2007). JNK2 promotion of caspase-8 activation promotes Bid cleavage and the TNF mitochondrial death pathway (Gross et al., 1999; Wang et al., 2006). Ceramide is a breakdown product of sphingomyelin, a key cellular membrane phospholipid that is hydrolyzed by sphingomyelinases. Ceramide has been shown to act as a cellular second messenger and has been reported to induce apoptosis in a range of cell systems including TNF signaling (Dbaibo et al., 1993; Hannun et al., 1993; Obeid et al., 1993). Consistent with its potent activation of apoptosis through the mitochondrial death pathway and ROS production, release of TNF in vivo following transplantation of fetal ventral mes-encephalic tissue into striatum was shown to compromise survival of transplanted fetal DA neurons unless TNF activity was blocked with an anti-TNF antibody (Clarke and Branton, 2002). The relative expression of TNF receptors and their contribution to DA neuron survival is less well understood. It is possible that activation of the NF-κB pathway by Tnfrsf1b counteracts the apoptotic activity of Tnfrsf1a by activating transcription of pro-survival genes; alternatively, each may independently contribute to rendering SN DA neurons to inflammatory and oxidative stimuli. In support of this idea, mice deficient in either Tnfrsf1a or 1b display sensitivity to MPTP-induced toxicity whereas mice deficient in both receptors or the TNF ligand display reduced sensitivity (Sriram et al., 2002, 2006; Ferger et al., 2004; Leng et al., 2005). In primary cortical neurons, a TNF stimulus results in activation of both Tnfrsf1a and 1b receptors and induction of the NF-κB pathway, yet with distinguishable kinetics and upstream activating components. Tnfrsf1a only induced transient NF-κB activation, whereas Tnfrsf1b facilitated long term phosphatidylinositol 3-kinase-dependent NF-κB activation and was essential for neuronal survival (Marchetti et al., 2004). Thus, the duration of NF-κB activation may be a critical determinant for sensitivity toward a TNF stimulus and is dependent on differential upstream signal pathway usage of the two TNF receptors. In summary, the survival pathways activated by TNF generally lead to the activation NF-κB, which in turn controls transcription of various pro-survival genes. It should be noted that this activation of NF-κB is dependent on the NF-κB inhibitor protein IKKα, being degraded by the 26S proteasome. Given that sufficient evidence exists to indicate that there is decreased proteasomal function in PD, this raises the possibility that the functional outcome of TNF signaling in the nigrostriatal pathway is more likely to favor apoptosis rather than pro-survival of DA neurons with compromised proteaso-mal function (Duke et al., 2007).
Nigral DA neurons may be uniquely vulnerable to neuroinflammatory insults that enhance oxidative stress and TNF may be a critical player required for activation of downstream apoptotic pathways that kill DA neurons. Alternatively, the higher sensitivity of substantia nigra DA neurons to injury induced by neuroinflammatory mediators may be secondary to reduced antioxidant capacity resulting from TNF-induced glutathione depletion (Jenner and Olanow, 1996; Liu et al., 1998) and TNF-induced sensitization to NO (Hemmer et al., 2001). Under normal conditions, DA neurons must withstand a high rate of oxidant species production because the dopamine biosynthetic pathway involves the creation of a number of reactive dopamine metabolites such as DA-quinone which are toxic to neurons (Graham, 1978; Stokes et al., 1999). In humans, cytokine-stimulated expression of the low-affinity IgE receptor CD23 participates in the activation of inducible nitric oxide synthetase (iNOS), with the subsequent release of NO. Moreover, expression of CD23 is upregulated in glial cells in the SN of PD patients compared with control groups, suggesting that cytokine-CD23-dependent iNOS activation in microglial cells may be part of a cascade that contributes to enhanced oxidative stress of DA neurons (Hunot et al., 1999). After an initial toxic insult, a self-perpetuating cycle of microglia activation would contribute to persistently elevated levels of toxic mediators in the nigral environment. Under either of these scenarios and independent of trigger that elicits its production, we hypothesize TNF action in the high oxidant environment of the SN is likely to result in (1) depletion of endogenous anti-oxidant capacity in vulnerable DA neurons, (2) potentiation of microglial production of ROS via NADPH oxidase-dependent pathways, and (3) activation of cell death pathways in oxidatively stressed neurons. The basis for this hypothesis is the observation that mGSH plays a central role in the control of mitochondrial ROS and modulates the sensitivity of many cell types to cell death pathways. Mechanistically, depletion of GSH has been linked to enhanced toxicity of DA and hydrogen peroxide in culture (Stokes et al., 1999; Shang et al., 2003) and increased sensitivity to TNF-induced death in glutathione-depleted hepatocytes (Fernandez-Checa, 2003; Garcia-Ruiz and Fernandez-Checa, 2006). Restoration of mGSH by the in vivo administration of S-adenosyl-L-methionine (SAM) or the in vitro use of GSH ethyl ester prevents the susceptibility of hepatocytes to TNF (Fernandez-Checa et al., 1998) and may represent a potential therapeutic intervention to protect GSH-depleted DA neurons against TNF-dependent inflammatory stimuli. The signaling of TNF through its membrane receptor Tnfrsf1a from mitochondrial complex I to complex II is similar in hepatocytes depleted or not depleted in mGSH after alcohol exposure, yet hepatocellular susceptibility to TNF occurs only if mGSH is depleted. Thus, depletion of mGSH is a critical factor in the development of sensitization of hepatocytes to inflammatory cytokines. It is therefore possible that toxins which deplete anti-oxidant stores and induce oxidative stress in the nigrostriatal pathway depend on TNF in an analogous manner to activate the final common apoptotic pathway to kill DA neurons.
IL-1β
The effects of IL-1β on neuronal function have been difficult to elucidate primarily because in vitro and in vivo studies have yielded contradictory findings (reviewed in Allan et al., 2005). There is also conflicting data regarding the role of IL-1β in dopaminergic neuron survival, but due to its high concentrations in CSF and post-mortem brains of PD patients it has been implicated in the pathophysiology of the disease (Blum-Degen et al., 1995; Mogi et al., 1996). Injection of IL-1β into various CNS regions evaluated at 1 and 6 days after the injection of the cytokine induced changes in neuronal integrity only when injected into the dentate gyrus of the hippocampus, but not in cortex, striatum, or SN (Depino et al., 2005). However, sustained expression of IL-1β via adenoviral vector delivery into SN for 60 days induced glial activation, progressive DA neuron death, and akinesia (Ferrari et al., 2006). IL-1β is able to induce mitochondrial dysfunction in pancreatic islets (Veluthakal et al., 2005) and chondrocytes (Yasuhara et al., 2005) through disruption of the mitochondrial membrane potential and energy depletion through formation of peroxynitrite. Interestingly, human cases of Alzheimer's disease/Lewy body dementia showed co-localization of IL-1β-expressing microglia with neurons that overexpressed βAPP and contained both Lewy bodies and neurofibrillary tangles (Grigoryan et al., 2000). Taken together, these findings raise the interesting possibility that IL-1β signaling may mediate the clinical and neuropathological overlap between AD and PD. On the other hand, a recent report suggests that IL-1β may also have a neuroprotective role by eliciting GDNF release from astrocytes under acute inflammatory conditions (Saavedra et al., 2007). Consistent with this idea, epidemiological studies suggest that IL-1β promoter polymorphisms may be protective in PD (Nishimura et al., 2001, 2005); additional studies will be needed to confirm these findings.
Interaction of PD-linked genes with inflammation pathways
The role of inclusions (in particular the role of α-synuclein in their formation) and how these contribute to etiology and/or progression of the disease is complex and remains somewhat controversial (Tran and Miller, 1999; Norris et al., 2003; Li et al., 2005; Liu et al., 2005; Norris and Giasson, 2005). Some propose that development of inclusions is a mechanism aimed at compartmentalizing accumulated proteins to prevent the obstruction of normal cell function. Other studies support the idea that inclusions confer cytotoxic effects that contribute to cellular damage and neurodegeneration in part by triggering proinflammatory responses. Regardless of which of these two possibilities is correct, if PD patients indeed have a dysfunctional ubiquitin–proteasome pathway, proteins that are normally turned over by this pathway will tend to aggregate and form inclusions. In terms of a link to inflammation, studies with agents that elicit abnormal accumulation and aggregation of ubiquitinated proteins in neuronal cultures support the idea that protein aggregation triggers a neuroinflammatory response characterized by increased expression and activity of the proinflammatory cyclooxygenase COX-2 and these in turn enhance oxidative stress and further prostaglandin production which can feed back in a positive toxic loop and affect UPS function (Rockwell et al., 2000; Figueiredo-Pereira et al., 2002; Li et al., 2003, 2004a,b). Misfolded or aggregated proteins in Lewy bodies of diseased SN DA neurons may elicit a self-propelling cycle of microglial activation and overproduction of inflammatory mediators in SN, thus providing a tertiary hit required for PD-associated dysfunction to spread to neighboring neurons (Zhang et al., 2005; Sulzer, 2007). In support of this idea, it has been shown that mice that overexpress wild-type α-synuclein display early microglia activation and that α-synuclein release from an α-synuclein-overexpressing dopaminergic neuron-like cell line triggers a cascade of proinflammatory mediators that include TNF, IL-1β, IL-6, COX-2 and iNOS (Su et al., 2007). Among the mechanisms mediating this microglia activation, the scavenger receptor CD36 (Su et al., 2007), and the prostaglandin E2 receptor subtype 2 (EP2) (Jin et al., 2007), have been implicated using ex vivo, in vivo, and in vitro experimental systems and genetic models. In addition, α-synuclein and in particular the A30P, E46K and A53T α-synuclein mutations linked to familial PD have been reported to potently activate human microglia and the human monocytic cell line THP-1 to secrete high levels of IL-1β and TNF, resulting in cytotoxic effects on human SH-SY5Y neuroblastoma cells (Klegeris et al., 2006). Interestingly but not surprisingly, α-synuclein may also have important roles in microglia in regulation of their activation state. Specifically, microglia from α-synuclein-deficient mice (Scna−/−) have been shown to display a reactive phenotype under basal culture conditions and a hyper-reactive phenotype (overproduction of proinflammatory cytokines TNF and IL-6) after stimulation compared with microglia from wild-type mice (Austin et al., 2006). Moreover, microglia from Scna−/− mice display significant morphologic differences (extremely large and ramified cells filled with vacuole-like structures) and increased levels of activation markers (CD68 and β1-integrin) compared with microglia from wild-type mice yet Scna−/− microglia exhibit impaired phagocytic ability (Austin et al., 2006).
Microglial ‘priming’ may be another mechanism by which neuroinflammation contributes to the death of dopaminergic neurons. If microglia become primed by a stimulus, they may respond to subsequent stimuli in a way that would be injurious to DA neurons. Specifically, a recent study reported that a single paraquat exposure induced microglia activation, including induction of NADPH oxidase. If this activation was blocked with the anti-inflammatory drug minocycline, subsequent exposures to the herbicide failed to cause oxidative stress and neurodegeneration (Purisai et al., 2007). However, if microglia were first primed by pre-treatment with LPS, a single paraquat exposure became capable of triggering loss of DA neurons. Consistent with the importance of microglial-derived oxidant stress, mutant mice lacking functional NADPH oxidase were spared from neurodegeneration caused by repeated paraquat exposures (Purisai et al., 2007). Therefore, microglial priming may in part regulate the microglial phenotype and shift microglial activities from neuroprotective to neurotoxic ones (i.e., from trophic factor production and debris removal to ROS/RNS, prostaglandin, cytokine, and chemokine overproduction) the outcome of which may be to hasten the death of vulnerable neuronal populations (Block and Hong, 2005; Mrak and Griffin, 2005; Ito et al., 2006; Kim and Joh, 2006; Nagatsu and Sawada, 2006; Sawada et al., 2006). Even in the absence of microglial priming, if microglia become activated normally during the sustained course of a disease, the resulting respiratory bursts and sustained elevation of cytokines, chemokines, and prostaglandins may act to compound neuronal dysfunction and aid in disease progression (Block and Hong, 2005; Minghetti, 2005; Minghetti et al., 2005; Zhang et al., 2006; Nagatsu and Sawada, 2006; Wersinger and Sidhu, 2006). Given the fact that microglia activation is not limited to end-stage PD but is likely to be occurring in parallel with DA neuron loss (Gerhard et al., 2006), moderate increases in oxidative species generation due to sustained microglial activation in the nigral microenvironment could easily overwhelm the natural defenses of the remaining DA neurons by contributing to enhanced oxidative stress. Therefore, targeted inhibition of the glial reaction and inflammatory processes triggered by environmental toxins may represent an attractive therapeutic approach to slow down or delay progression of PD.
In addition to the evidence demonstrating that α-synuclein can potently modulate microglia activation in vitro and in vivo when it is overexpressed, misfolded or truncated, there is also evidence that Parkin and DJ-1 activity may modulate cellular responses to inflammation. Specifically, recent studies suggest that Parkin is a novel activator of NF-κB signaling and activation of this cascade is a critical component of its neuroprotective effects through modulation of the ubiquitin–proteasome pathway (Henn et al., 2007). The proposed mechanism involves interaction of Parkin with two critical components of the NF-κB pathway, NF-κB Essential Modulator (NEMO) which modulates the two subunits of the I κ kinase (IKK) complex composed of IKKβ and IKKα, and the E3 ligase TRAF2 [TNF (tumor necrosis factor) receptor-associated factor 2] to promote their degradation-independent ubiquitinylation. Further support for the role of NF-κB in Parkin function was shown by demonstrating that inhibition of NF-κB activation by an IkB super-repressor or a kinase-inactive IKKβ interfered with the neuroprotective activity of Parkin, and Parkin mutants linked to autosomal recessive parkinsonism which have impaired neuroprotective capacity in vitro also failed to stimulate NF-κB-dependent transcription to any significant extent (Henn et al., 2007). Functionally, the impaired ability of Parkin mutants to activate the NF-κB pathway (due to abnormal NEMO/TRAF2 degradation) would be expected to influence the outcome of an inflammatory stimulus in the nigrostriatal pathway in a way that survival of DA neurons would be compromised. Specifically, if a neuron has abnormal Parkin function, its activation of the pro-survival NF-κB cascade downstream of TNF may not be robust enough to oppose the pro-apoptotic arm of the TNF cascade which is activated in parallel, resulting in a TNF signaling outcome that would compromise neuronal survival. Therefore, it is possible that environmental exposures and the degree to which the latter promote neuroinflammatory responses in the midbrain could account for the variability in the age-at-onset of parkinsonism in patients bearing Parkin mutations, even in families carrying the same mutant allele (Deng et al., 2006). In support of this idea, the effect of environmental exposures (pesticides, organic solvents, rural living) in individuals bearing parkin polymorphisms was recently reported to have strong effect in lowering the age of onset of PD through mechanisms that are likely to include increases in lipid peroxidation and oxidative stress (Ghione et al., 2007). Further studies will be needed to investigate the extent to which environmental triggers that converge on inflammation modulate Parkin function and to reveal additional mechanistic insight into the links between inflammation, the ubiquitin–proteasome pathway, and nigral DA neuron survival.
Given that the putative redox-sensor and chaperone DJ-1 has also been linked to recessive forms of PD, an attractive hypothesis is that DJ-1 may have important roles in protecting neurons against inflammation-induced oxidative stress. Consistent with this idea, the brain proteome of mice deficient in TNF signaling (Tnfrsf1a and 1b double knockouts) displays abnormal expression of DJ-1 (Pejovic et al., 2004), suggesting that at least developmentally, DJ-1 lies downstream of TNF in a signaling cascade and raising the possibility that another DJ-1 function may be to translocate to mitochondria and protect neurons from oxidative stress triggered by neuroinflammatory stimuli. However, since post-mortem analyses of brains from individuals bearing DJ-1 mutations have not been reported, the role of inflammation in triggering or contributing to DJ-1-linked parkinsonism is unclear. Future studies should help clarify the extent to which and the mechanisms through which diverse proteins linked to familial PD and the environmental toxins suspected to trigger sporadic PD participate in a set of common biochemical pathway(s) that converge upon mitochondria. If neuroinflammatory processes hasten neuronal dysfunction and contribute to degeneration, it may be possible to delay the onset of disease in patients bearing PD-linked mutations by modulating microglial activation with anti-inflammatory therapy.
Epigenetic factors that may contribute to neuroinflammation and risk for PD
Neuroinflammation may be triggered by immunological challenges (bacterial or viral infections), neuronal injury (brain trauma or stroke), and other epigenetic factors including chronic inflammatory syndromes (rheumatoid arthritis, arthrosclerosis, Crohn's disease, multiple sclerosis) and environmental toxins (pesticides, particulate matter) (Kreutzberg, 1996; Aloisi, 1999; Streit, 2000; Block and Hong, 2005; Hirsch et al., 2005; Minghetti, 2005; Minghetti et al., 2005; Mrak and Griffin, 2005). Many of these insults can increase the permeability of the blood–brain barrier (BBB) to allow infiltration of lymphocytes and macrophages into the brain parenchyma. Depending on context, duration, and type of inflammatory response, inflammation may be detrimental or beneficial to the individual. The relationship and potential mechanisms by which some of these factors may influence risk for development or progression of PD has been intensely investigated in recent years and is discussed below.
Several environmental triggers known to promote neuroinflammatory responses have been implicated in non-familial or idiopathic PD and include traumatic head injury, viral inflammation, exposure to heavy metals, organophosphate compounds, neurotoxins like MPTP, and certain pesticides such as paraquat and rotenone (Stern et al., 1991; Casals et al., 1998; Betarbet et al., 2000; Akhmedova et al., 2001; Thiruchelvam et al., 2002; Liu et al., 2003a; Sherer et al., 2003c; Caboni et al., 2004; Goldman et al., 2006; Kamel et al., 2007). In addition, epidemiological research indicates that rural living, pesticide use, well-water consumption and certain occupations, including mining and welding, are associated with an increased risk of PD (Priyadarshi et al., 2001; Firestone et al., 2005; Jankovic, 2005). However, it has not been possible to identify any causative environmental chemical agent in the etiology of Parkinson's disease despite intensive research. A number of mitochondrial and proteasomal toxins have been associated with clusters of atypical parkinsonism (i.e., MPTP) and as discussed earlier, acute administration of these compounds has proved useful in providing experimental models of disease; to date, toxin models have provided important clues about the potential mechanisms by which oxidative stress may contribute to nigrostriatal pathway degeneration. Although a comprehensive discussion of potential environmental triggers is beyond the scope of this review, we highlight here those epigenetic factors with strongest association to PD.
Viral encephalitis
The influenza pandemic towards the end of the First World War (1914–1918) was associated with a dramatic increase in post-encephalytic parkinsonism (PEP; ‘sleeping sickness’ or von Economo encephalitis) (Dale et al., 2004) in the 1920s and 30s with PEP accounting for about 50% of all parkinsonism cases (Josephs et al., 2002). Moreover, it is well known that human populations infected with Japanese encephalitis virus (JEV) in India, China, and Southeast Asia for longer than 1 year are likely to develop post-encephalitis parkinsonism which shows many of the same neuropathological and locomotor symptoms as those seen in patients with sporadic PD (Shoji et al., 1993). Experimentally, JEV has been used to create a preclinical model of post-encephalitic parkinsonism in rats (Ogata et al., 1997) in which JEV induces brain catecholamine (dopamine and norepinephrine) depletion and severe hypokinesia (Hamaue et al., 2006). Therefore, chronic inflammation in the brain, such as that which occurs in encephalitic syndromes, may induce neuroimmune dysregulation. The accompanying oxidative stress associated with this type of neuroinflammatory response would be expected to have a greater impact on neuronal populations that operate under high oxidant conditions, which would almost certainly include midbrain DA neurons.
Systemic infections
Severe systemic infection associated with multiple organ failure, also known as sepsis, can affect the brain and is often studied in animal models using systemic injection of high concentrations of the bacterial endotoxin lipopolysaccharide (LPS). Systemic LPS injection results in functional breakdown of the BBB leading to granulocyte infiltration into the brain as well as in microglia activation in the brain parenchyma (Bohatschek et al., 2001; Kloss et al., 2001). Moreover, subthreshold systemic infections may also have deleterious effects on neuronal survival and prenatal infections in particular may be an under-appreciated risk factor for sporadic PD. Specifically, recent studies have demonstrated that LPS administered systemically to pregnant rats enters the chorioamniotic environment and this prenatal LPS exposure to the unborn pups induces delayed loss of DA neurons in the post-natal rat midbrain (Carvey et al., 2003) through mechanisms likely to involve inflammation-enhanced oxidative stress (Gayle et al., 2002; Ling et al., 2002, 2006). These observations further support a role for endotoxin-induced inflammation in nigrostriatal pathway degeneration and raise the interesting possibility that pre-natal infections (i.e., bacterial vaginosis) may be one of the triggers for sporadic PD. The permeability of the fetal BBB and the high sensitivity of nigral DA neurons to LPS (German et al., 1993; De Pablos et al., 2005) may act in concert to increase the probability that pre-natal neuroinflammation is a predisposing risk factor for development of PD later in life. Interestingly, bacterial vaginosis (BV) is a fairly common condition in humans that can occur during pregnancy and is associated with an excess of Gram-negative bacteria which produce the bacterial endotoxin LPS (Thorsen et al., 1998). Interms of causality, the delayed time-course may make it difficult (if not impossible) to establish a direct link between prenatal cerebral infection induced by BVand idiopathic PD later in life, but the possibility that it could explain the random epidemiology of idiopathic PD cannot be ruled out.
Gastrointestinal inflammation and infections
Because the triggering event for sporadic PD may be coming from environmental sources, it has been suggested that diseases of the gastrointestinal tract could contribute to enhanced vulnerability for PD (Przuntek et al., 2004; Weller et al., 2005a,b). In support of the concept that chronic inflammatory conditions may contribute to the risk of sporadic PD, three single-nucleotide polymorphisms arising from three independent mutations in the nucleotide-binding oligomerization domain (NOD) of the protein NOD2 encoded by the CARD15 gene and shown to be associated with a common chronic inflammatory disease of the intestinal tract known as Crohn's disease (Hugot et al., 2001; Ogura et al., 2001) have recently been shown to be over-represented in patients with sporadic PD (Bialecka et al., 2007). NOD1 and 2 are intracellular proteins that recognize bacterial components, mediate the activation of NF-κB, and induce or enhance apoptosis (Inohara and Nunez, 2003). The three pathogenic variants of NOD2 are thought to share a common molecular mechanism of uncontrolled activation of NF-κB, where patients inheriting a variant genotype display elevated NF-κB activity in vivo (Bonen and Cho, 2003). Most recently, CARD15 expression has been reported in brain tissue where it has been suggested that NOD2 protein expression may represent a mechanism by which astrocytes mediate chronic inflammation with damaging effects on vulnerable neuronal populations (Sterka et al., 2006). In addition, linking infectious diseases of the gastrointestinal (GI) tract and increased risk of PD is the evidence that parkinsonism has been loosely associated with prodromal peptic ulceration; and Helicobacter pylori is the most common bacterial infection in adults which is usually acquired in childhood and has been linked with peptic ulcer/non-ulcer dyspepsia, immunosuppression, and autoimmu-nity. Patients diagnosed with sporadic PD are more likely to be seropositive for H. pylori before age 75 [odds ratio 2.04 (95% CI: 1.04, 4.22); P<0.04] (Dobbs et al., 2000). Nevertheless, causality has been difficult to establish and the mechanisms are not well-understood. One possibility is that higher prevalence of H. pylori seropositivity in parkinsonism diagnosed before the 8th decade of life may be due to host susceptibility/interaction; alternatively, infection with particular H. pylori strain(s) may compromise catecholaminergic neuron function in the GI and predispose the individual to secondary triggers.
Traumatic brain injury (TBI)
The BBB is likely to be disrupted with brain trauma, infection, and stroke leading to extravasation of lymphocytes and inflammatory mediators that activate microglial cells and lead to the formation of ROS (Herrera et al., 2005). Although head injury is an inconsistently reported risk factor for PD perhaps because many related variables confound this association (Nayernouri, 1985; Factor and Weiner, 1991; Stern et al., 1991; Stern, 1991), recent twin studies provide compelling data to suggest that mild-to-moderate closed head injury may increasePDrisk decades later (Goldman et al., 2006). Specifically, it was reported that a prior head injury with amnesia or loss of consciousness was associated with an increased risk for PD [odds ratio 3.8 (95% CI: 1.3, 11); p=0.014] and the association was somewhat stronger in monozygotic than in dizygotic pairs. Moreover, in a subanalysis of 18 pairs concordant for PD, the twin with younger onset PD was more likely to have sustained a head injury. The mechanism (s) by which TBI contributes to increased risk for PD are still unknown, but an experimental model of TBI in mice suggests that one possibility may involve TBI-induced iNOS modifications of α -synuclein in aged mice triggered by the inflammatory sequelae associated with brain trauma. (Uryu et al., 2003). Although it is unclear how nitrative stress contributes to the development of α-synuclein pathology in human neurodegenerative diseases or TBI, previous studies suggest that oxidative/nitrative stress may play a role in the aggregation of α -synuclein (Paxinou et al., 2001; Norris et al., 2003) or in stabilizing aggregated forms of α-synuclein (Souza et al., 2000).
Atherosclerosis
Regulation of the brain neovascular unit – which consists of vascular endothelial cells, pericytes, closely juxtaposed astrocytes, and neurons – is critical for regulation of the BBB. Cholesterol plaque deposits in aging individuals with atherosclerosis is known to disrupt the function of the brain neovascular unit through a process of chronic inflammation that results from interactions between plasma lipoproteins, cellular components (monocyte/macrophages, T lymphocytes, endothelial cells and smooth muscle cells) and the extracellular matrix of the arterial wall (Fan and Watanabe, 2003). As such, atherosclerosis may be another age-associated disease that increases risk or accelerates development of sporadic PD by promoting neuroinflammation-dependent oxidative stress in the brain parenchyma. It has been demonstrated that atherogenic lipoproteins including oxidized low density lipoprotein (LDL), remnant lipoprotein (beta-VLDL) and lipoprotein play a critical role in inflammatory reactions because they activate scavenger receptors leading to increased cytokine production from macrophages; in contrast, high density lipoprotein (HDL) and anti-atherogenic lipoproteins exert anti-inflammatory functions (Osterud and Bjorklid, 2003). Additional research will be needed to conclusively establish a link between atherosclerosis-associated neuroinflammation and risk for sporadic PD.
Use of anti-inflammatory agents in PD: potential targets and therapies
Post-mortem examination of SN reveals the presence of activated microglial cells and elevated levels of inflammatory cytokines, including TNF, IL-1β, IL-2, IL-4 and IL-6 (Mogi et al., 1994; Mogi et al., 1996; Hunot et al., 1999). Therefore, an emerging area of pre-clinical investigation involves development of strategies to inhibit the glial reaction and/or target inflammatory cytokines (Nagatsu et al., 2000a; Barcia et al., 2003; Hirsch et al., 2005). IL-1β levels have been reported to increase rapidly after nigral administration of LPS in mice and neuroprotection can be achieved with nigral administration of an anti-IL-1β neutralizing antibody (Arai et al., 2004; Arai et al., 2006). In the case of TNF, approximately 50% of rat nigral DA neurons destined to die from oxidative neurotoxin and endotoxin-induced death can be rescued by transient (2 weeks) inhibition of soluble TNF signaling via nigral administration of the recombinant dominant negative TNF inhibitor XENP345 (McCoy et al., 2006).
Although numerous studies from animal models support a role for the NF-κB pathway in preventing neuronal death induced by oxidative stress, excitotoxicity, ischemia, or glucose deprivation (for review, see Mattson and Meffert, 2006; Kaltschmidt et al., 2005), there is also evidence supporting a dual role of NF-κB in neurodegenerative diseases perhaps in part due to the fact that activation of NF-κB in neurons generally promotes their survival, whereas NF-κB mediates proliferation and activation of glial cells some of which may promote pathological inflammatory processes (Mattson, 2005; Mattson and Meffert, 2006; Pizzi and Spano, 2006). In the anti-inflammatory phase of inflammation, NF-κB promotes apoptosis and expression of mediators such as TGFβ1 and cyclopente-none prostaglandins as well as transcription of genes such as Bax and p53 (Lawrence et al., 2001). Activation of the NF-κB pathway, as measured by nuclear translocation of the transacti-vating subunit NF-κB RelA (p65), has been reported to be as much as 70-fold higher in the SN of PD patients compared to that of age-matched healthy controls (Hunot et al., 1997). A possible relationship between the nuclear localization of NF-κB in nigral DA neurons of PD patients and oxidative stress in such neurons is supported by recent in vivo studies in which 6-OHDA-induced oxidative stress in rat DA neurons in SN was shown to be mediated through an NF-κB-dependent p53-signaling pathway (Liang et al., 2007). Additional research will be needed to conclusively demonstrate whether NF-κB itself or other pathways which modulate its activity are exerting oxidant-mediated apoptogenic transduction pathways in the nigrostriatal pathway that could contribute to development of PD.
Immunomodulatory effects have also been described in animal models and in vitro for two anti-parkinsonian drugs commonly used in humans: the monoamine oxidase B (MAO) inhibitor drug pargyline (Kohutnicka et al., 1998) and selegiline (Deprenyl) (Klegeris and McGeer, 2000). In addition, the immunosuppressants cyclosporine A and FK-506 (tacrolimus) as well as the non-immunosuppressant derivatives of FK-506 referred to as immunophilin ligands including pentoxifylline (Wersinger and Sidhu, 2002; Liu and Hong, 2003), and the non-selective COX-2 inhibitor sodium salicylate have all shown neuroprotective activity in either MPTP-induced nigral injury (Liu and Hong,2003)or the 6-OHDA rat modelofPD (Sanchez-Pernaute et al., 2004). Similarly, the glucocorticoid dexameth-asone was shown to be protective against MPTP (Kurkowska-Jastrzebska et al., 2004) and intranigral LPS (Castano et al., 2002). Other anti-inflammatory regimens such as steroids, which have been shown to be effective at arresting DA neuron loss in rodents are not likely to be suitable for long-term use in humans. Clinical trials in PD patients using short-term administration of immunophilin ligands (which lack the immuno-suppressive properties of the parent compounds) have had limited success (Gold and Nutt, 2002). The semi-synthetic tetracycline antibiotic derivative minocycline which readily crosses the BBB, has been shown to be neuroprotective against MPTP-, LPS- and 6-OHDA-induced nigral DA neuron loss (Du et al., 2001; He et al., 2001; Wu et al., 2002; Tomas-Camardiel et al., 2004). Because minocycline is well tolerated in humans, it was recently included in a randomized, double-blind, futility clinical trial in patients with early PD. The recommendations at the completion of Phase II was for advancement to Phase III clinical trials to determine if minocycline could alter long-term progression of PD (The National Institute of Neurological Disorders and Stroke Neuroprotective Exploratory Trials in Parkinson's Disease Investigators, 2006). In the future, chemical modification of tetracyclines derivatives such as minocycline using structure–activity relationship analysis may lead to creation of drugs that can neuroprotect nigral DA neurons at low doses.
Other compounds with anti-inflammatory actions that have been shown to rescue nigral DA neurons from a variety of neurotoxic insults (LPS, MPTP, etc.) include vasoactive intestinal peptide (VIP) (Delgado and Ganea, 2003b,a), the polyphenolic flavonoid silymarin (Wang et al., 2002), the NMDA receptor antagonist dextromethorphan commonly used in non-prescription anti-tussives (Liu et al., 2003b), and agonists of peroxisome proliferator-activated receptor-γ (PPARγ) (Breidert et al., 2002). The selective iNOS inhibitors S-methylisothiourea and L-N(G)-nitroarginine were shown to exert neuroprotecive effects on DA neurons in rats against LPS (Hemmer et al., 2001; Le et al., 2001; Iravani et al., 2002; Arimoto and Bing, 2003), suggesting that free radical scavengers or iNOS inhibitors may have potential therapeutic effects in PD. Copolymer-1 (Cop-1) immunization, which has been used effectively in patients with chronic neuroinflammatory disease such as relapsing–remitting MS, has recently been shown to have neuroprotective effects in the nigrostriatal pathway against MPTP by several immunomodulatory mechanisms, including promotion of CD4+ T cell accumulation within the SNpc, suppression of microglial activation, and increased local expression of the potent dopaminergic survival factor GDNF (Benner et al., 2004; Laurie et al., 2007).
In summary, the link between inflammation, oxidative stress and PD has become less controversial due to an overwhelming number of proof-of-principle studies that strongly implicate inflammatory processes in the progressive loss of nigral DA neurons. However, despite the promising data emerging from animal studies on neuroprotective effects of compounds with antiinflammatory properties, it remains to be determined whether anti-inflammatory therapy in humans could have a beneficial effect in preventing or slowing down progression of PD. Possible reasons for the failure of past clinical trials with antiinflammatory compounds may include the advanced state of the patients enrolled in the studies, the dosing regimens chosen for the trials, or simply the wrong anti-inflammatory compound; therefore, further clinical investigation in this area is warranted before dismissing the possibility of potential long-term benefits of antiinflammatory drugs. In addition, it will be important to identify other promising candidates as well as small molecules that can cross the blood–brain barrier or to devise safe and efficient modes of delivery for those that cannot cross. A successful outcome may indeed modify the course of the disease and afford therapeutic benefit in PD patients.
Importantly, it should be noted that most of the currently available drugs to treat inflammation, including NSAIDs, are not truly “anti-inflammatory” because they do not stimulate or trigger the anti-inflammatory response; rather, they halt the proinflammatory response. Moreover, given that the innate proinflammatory response often represents a beneficial event (Wyss-Coray, 2006), long-term global inhibition of proinflammatory responses may not be the most effective strategy to treat the inflammatory condition associated with neurodegenerative diseases (Wyss-Coray and Mucke, 2002; Marchetti and Abbracchio, 2005). Therefore, the challenge ahead lies in our ability to carefully dissect out which inflammatory mediators are beneficial and which are neurotoxic in specific parts of the brain where neuronal loss is occurring. Only such an approach will enable rational design and selection of anti-inflammatory therapy for targeted delivery to specific brain regions. Lastly, identification of cell type-specific differences in the proteins that activate complex signaling cascades that can either promote or interfere with neuronal survival, such as the NF-κB pathway, will be needed before such pathways can become successful pharmaceutical targets in the treatment of specific neurodegenerative diseases (reviewed in Camandola and Mattson, 2007).
Conclusions
An overwhelming amount of data from both animal and human studies has emerged over the past decade which strongly refutes the notion that neuroinflammation in PD patient brains at autopsy is merely the end-result of phagocytic microglia scavenging dead or dying DA neurons. As an individual ages, resident brain microglia within the SNpc are likely to become activated by any number of insults (Fig. 1) that include but are not limited to accumulation of protein aggregates, oxidized protein, exposure to bacterial or viral toxins, and environmental triggers (i.e., pesticides and particulate matter). In addition to the rare proteinopathies that arise from genetic mutations that disrupt the protein handling machinery of the cell, neuroinflammatory processes and chronic microglia activation may be triggered by a number of epigenetic factors (including the environmental exposures and chronic inflammatory conditions discussed in this review). As such, environmental influences may constitute life-long modifiers of PD risk. Recent imaging and epidemiological studies which demonstrate that microglial activation is clearly detectable in the brains of PD patients within a year or two of receiving a clinical diagnosis strongly support an active role of neuroinflammation in PD progression. In support of this, the PD risk-lowering effects of chronic NSAID use in humans strongly supports this link and should provide compelling rationale to re-examine different classes of anti-inflammatory agents and their modes of action in future clinical trials.
Fig. 1.
Mechanisms and triggers that initiate and sustain microglia activation and contribute to dopaminergic neuron degeneration. Divergent disease initiation steps including neurotoxin exposure, traumatic brain injury, infection, and genetic mutations may converge to activate various cell types resulting in microgliosis, reactive astrocytosis, complement activation and production of autotoxic substances. This neuroinflammatory response may also include the involvement of components of the acquired immune response with the recruitment of autoreactive T cells and the production of auto-antibodies. As a result of immune system involvement, through both glial proliferation and complement activation, there is increased production of cytokines (in particular TNF, IL-1β, and IL-6), chemokines, proteases, ROS, and RNS to create an environment of increased inflammatory and oxidative stress in the SNpc that becomes self-sustaining. Alternatively, a healthy neuron may become dysfunctional as a result of genetic mutations that affect the ubiquitin proteasome system (UPS) or mitochondrial function which may sensitize DA neurons to epigenetic factors (i.e., oxidative neurotoxins, neuroinflammation). Subsequent exposures to neurotoxic agents and inflammatory insults can also act to hasten the degeneration and death of DA neurons. Regardless of the factor that causes the initial cell death, the resulting immune response contributes to an auto-amplifying cycle of microglial activation sustained by microglial-derived autocrine factors. Lastly, the presence of dying or dead DA neurons may be accompanied by the generation of unknown signals which target a neuron for phagocytosis by activated microglia. Self-perpetuating cycles of neuroinflammation and neurotoxicity in the nigra would ensure the progressive destruction of DA neurons as an individual ages.
Independent of the trigger that elicits neuroinflammation, persistently elevated levels of cytokines, chemokines, ROS/RNS, and prostaglandins resulting from chronically activated microglia would be expected to create a sustained environment of increased inflammatory stress in the CNS that may contribute to proteasomal dysfunction in the strong oxidant environment of the SNpc, hastening neuronal dysfunction and degeneration of DA neurons. In later stages of PD, as DA neurons begin to die, signals may be generated to further attract and activate microglia to promote phagocytosis and clean-up of neuronal debris. Additional basic science and translational studies will be needed to more fully understand the role of inflammation in normal and diseased CNS and to provide a clear mechanism-based understanding of the neuroinflammatory and neurotoxic processes that contribute to the progressive loss of DA neurons in PD. Because it is clear that inflammatory processes can play both beneficial and detrimental roles in neuronal survival, the critical challenge for our field within the next decade will be to place emphasis on development of therapeutic strategies to target in a temporal and region-specific manner microglial-derived mediators with neurotoxic properties against nigral DA neurons. Only this kind of approach will avoid global suppression of the immune system and allow development of safe and effective new anti-inflammatory drugs that could delay onset or slow down progression of this debilitating disease.
References
- Akhmedova SN, Yakimovsky AK, Schwartz EI. Paraoxonase 1 Met-Leu 54 polymorphism is associated with Parkinson's disease. J Neurol Sci. 2001;184:179–182. doi: 10.1016/s0022-510x(01)00439-7. [DOI] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev, Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Aloe L, Fiore M. TNF-alpha expressed in the brain of transgenic mice lowers central thyroxine hydroxylase immunoreactivity and alters grooming behavior. Neurosci Lett. 1997;238:65–68. doi: 10.1016/s0304-3940(97)00850-1. [DOI] [PubMed] [Google Scholar]
- Aloisi F. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. Adv Exp Med Biol. 1999;468:123–133. doi: 10.1007/978-1-4615-4685-6_10. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:65–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Serafini B, Adorini L. Glia-Tcell dialogue. J Neuroimmunol. 2000;107:111–117. doi: 10.1016/s0165-5728(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Arai H, Furuya T, Yasuda T, Miura M, Mizuno Y, Mochizuki H. Neurotoxic effects of lipopolysaccharide on nigral dopaminergic neurons are mediated by microglial activation, interleukin-1beta, and expression of caspase-11 in mice. J Biol Chem. 2004;279:51647–51653. doi: 10.1074/jbc.M407328200. [DOI] [PubMed] [Google Scholar]
- Arai H, Furuya T, Mizuno Y, Mochizuki H. Inflammation and infection in Parkinson's disease. Histol Histopathol. 2006;21:673–678. doi: 10.14670/HH-21.673. [DOI] [PubMed] [Google Scholar]
- Arimoto T, Bing G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol Dis. 2003;12:35–45. doi: 10.1016/s0969-9961(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord. 1998;13:221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- Barcia C, Fernandez Barreiro A, Poza M, Herrero MT. Parkinson's disease and inflammatory changes. Neurotox Res. 2003;5:411–418. doi: 10.1007/BF03033170. [DOI] [PubMed] [Google Scholar]
- Barcia C, de Pablos V, Bautista-Hernandez V, Sanchez-Bahillo A, Bernal I, Fernandez-Villalba E, Martin J, Banon R, Fernandez-Barreiro A, Herrero MT. Increased plasma levels of TNF-alpha but not of IL1-beta in MPTP-treated monkeys one year after the MPTP administration. Parkinsonism Relat Disord. 2005;11:435–439. doi: 10.1016/j.parkreldis.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, Nemachek C, Green SR, Przedborski S, Gendelman HE. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino L, Xapelli S, Silva AP, Jakobsen B, Poulsen FR, Oliveira CR, Vezzani A, Malva JO, Zimmer J. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J Neurosci. 2005;25:6734–6744. doi: 10.1523/JNEUROSCI.1510-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler H, Djaldetti R, Salman H, Bergman M, Djaldetti M. IL-1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson's disease. Biomed Pharmacother. 1999;53:141–145. doi: 10.1016/S0753-3322(99)80079-1. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Beyer M, Gimsa U, Eyupoglu IY, Hailer NP, Nitsch R. Phagocytosis of neuronal or glial debris by microglial cells: upregulation of MHC class II expression and multinuclear giant cell formation in vitro. Glia. 2000;31:262–266. doi: 10.1002/1098-1136(200009)31:3<262::aid-glia70>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bialecka M, Kurzawski M, Klodowska-Duda G, Opala G, Juzwiak S, Kurzawski G, Tan EK, Drozdzik M. CARD15 variants in patients with sporadic Parkinson's disease. Neurosci Res. 2007;57:473–476. doi: 10.1016/j.neures.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Bohatschek M, Werner A, Raivich G. Systemic LPS injection leads to granulocyte influx into normal and injured brain: effects of ICAM-1 deficiency. Exp Neurol. 2001;172:137–152. doi: 10.1006/exnr.2001.7764. [DOI] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology. 2003;124:521–536. doi: 10.1053/gast.2003.50045. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, Squitieri F, Krieger E, Vanacore N, van Swieten JC, Brice A, van Duijn CM, Oostra B, Meco G, Heutink P. DJ-1 (PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci. 2003a;24:159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003b;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB. Distribution and characterization of tumor necrosis factor-alpha-like immunoreactivity in the murine central nervous system. J Comp Neurol. 1993;337:543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson's disease. J Neurochem. 2002;82:615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Perez-Otano I, Sun V, Mullis Sawin SB, Chan J, Wu GC, Hudson PM, Kong LY, Hong JS, McMillian MK. Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Res. 1995;704:112–116. doi: 10.1016/0006-8993(95)01189-7. [DOI] [PubMed] [Google Scholar]
- Caboni P, Sherer TB, Zhang N, Taylor G, Na HM, Greenamyre JT, Casida JE. Rotenone, deguelin, their metabolites, and the rat model of Parkinson's disease. Chem Res Toxicol. 2004;17:1540–1548. doi: 10.1021/tx049867r. [DOI] [PubMed] [Google Scholar]
- Camandola S, Mattson MP. NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007;11:123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson's disease. Front Biosci. 2003;8:s826–s837. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Chen EY, Lipton JW, Tong CW, Chang QA, Ling ZD. Intra-parenchymal injection of tumor necrosis factor-alpha and interleukin 1-beta produces dopamine neuron loss in the rat. J Neural Transm. 2005;112:601–612. doi: 10.1007/s00702-004-0222-z. [DOI] [PubMed] [Google Scholar]
- Casals J, Elizan TS, Yahr MD. Postencephalitic parkinsonism—a review. J Neural Transm. 1998;105:645–676. doi: 10.1007/s007020050086. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Miller SW, Parks JP, Parker WD, Jr, Bennett JP., Jr Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson's disease. Biochim Biophys Acta. 1997;1362:77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh-TNF-alpha, IL-1beta and IFN-gamma. J Neurochem. 2002;81:150–157. doi: 10.1046/j.1471-4159.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk of Parkinson's disease. Ann Neurol. 2005;59:988–989. [Google Scholar]
- Chen X, Ding WX, Ni HM, Gao W, Shi YH, Gambotto AA, Fan J, Beg AA, Yin XM. Bid-independent mitochondrial activation in tumor necrosis factor alpha-induced apoptosis and liver injury. Mol Cell Biol. 2007;27:541–553. doi: 10.1128/MCB.01166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Lee SY, Cho Y, No H, Kim SW, Hwang O. Tetrahydrobiopterin causes mitochondrial dysfunction in dopaminergic cells: implications for Parkinson's disease. Neurochem Int. 2006;48:255–262. doi: 10.1016/j.neuint.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci. 2002;15:991–998. doi: 10.1046/j.1460-9568.2002.01938.x. [DOI] [PubMed] [Google Scholar]
- Clarke DJ, Branton RL. A role for tumor necrosis factor alpha in death of dopaminergic neurons following neural transplantation. Exp Neurol. 2002;176:154–162. doi: 10.1006/exnr.2002.7911. [DOI] [PubMed] [Google Scholar]
- Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- Consilvio C, Vincent AM, Feldman EL. Neuroinflammation, COX-2, and ALS—a dual role? Exp Neurol. 2004;187:1–10. doi: 10.1016/j.expneurol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Cookson MR. Pathways to Parkinsonism. Neuron. 2003;37:7–10. doi: 10.1016/s0896-6273(02)01166-2. [DOI] [PubMed] [Google Scholar]
- Corti O, Hampe C, Darios F, Ibanez P, Ruberg M, Brice A. Parkinson's disease: from causes to mechanisms. C R Biol. 2005;328:131–142. doi: 10.1016/j.crvi.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson's disease mice model. Neurodegeneration. 1996;5:137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- Dale RC, Church AJ, Surtees RA, Lees AJ, Adcock JE, Harding B, Neville BG, Giovannoni G. Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain. 2004;127:21–33. doi: 10.1093/brain/awh008. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Zhang J, Dawson VL, Snyder SH. Nitric oxide: cellular regulation and neuronal injury. Prog Brain Res. 1994;103:365–369. doi: 10.1016/s0079-6123(08)61150-4. [DOI] [PubMed] [Google Scholar]
- Dbaibo GS, Obeid LM, Hannun YA. Tumor necrosis factor-alpha (TNF-alpha) signal transduction through ceramide Dissociation of growth inhibitory effects of TNF-alpha from activation of nuclear factor-kappa B. J Biol Chem. 1993;268:17762–17766. [PubMed] [Google Scholar]
- De Pablos RM, Herrera AJ, Villaran RF, Cano J, Machado A. Dopamine-dependent neurotoxicity of lipopolysaccharide in substantia nigra. FASEB J. 2005;19:407–409. doi: 10.1096/fj.04-2153fje. [DOI] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB down-regulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000;74:2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson's disease by blocking microglial activation. FASEB J. 2003a;17:944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. FASEB J. 2003b;17:1922–1924. doi: 10.1096/fj.02-1029fje. [DOI] [PubMed] [Google Scholar]
- Deng H, Le WD, Hunter CB, Ondo WG, Guo Y, Xie WJ, Jankovic J. Heterogeneous phenotype in a family with compound heterozygous parkin gene mutations. Arch Neurol. 2006;63:273–277. doi: 10.1001/archneur.63.2.273. [DOI] [PubMed] [Google Scholar]
- Depino A, Ferrari C, Pott Godoy MC, Tarelli R, Pitossi FJ. Differential effects of interleukin-1beta on neurotoxicity, cytokine induction and glial reaction in specific brain regions. J Neuroimmunol. 2005;168:96–110. doi: 10.1016/j.jneuroim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Dobbs RJ, Charlett A, Dobbs SM, Weller C, Peterson DW. Parkinsonism: differential age—trend in Helicobacter pylori antibody. Aliment Pharmacol Ther. 2000;14:1199–1205. doi: 10.1046/j.1365-2036.2000.00815.x. [DOI] [PubMed] [Google Scholar]
- Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol. 1997;75:104–112. doi: 10.1016/s0165-5728(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke DC, Moran LB, Pearce RK, Graeber MB. The medial and lateral substantia nigra in Parkinson's disease: mRNA profiles associated with higher brain tissue vulnerability. Neurogenetics. 2007;8:83–94. doi: 10.1007/s10048-006-0077-6. [DOI] [PubMed] [Google Scholar]
- Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson's disease. Exp Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Factor SA, Weiner WJ. Prior history of head trauma in Parkinson's disease. Mov Disord. 1991;6:225–229. doi: 10.1002/mds.870060306. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10:63–71. doi: 10.5551/jat.10.63. [DOI] [PubMed] [Google Scholar]
- Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev, Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Feng ZH, Wang TG, Li DD, Fung P, Wilson BC, Liu B, Ali SF, Langenbach R, Hong JS. Cyclooxygenase-2-deficient mice are resistant to 1-methyl-4-phenyl 1, 2, 3, 6-tetrahydropyridine-induced damage of dopaminergic neurons in the substantia nigra. Neurosci Lett. 2002;329:354–358. doi: 10.1016/s0304-3940(02)00704-8. [DOI] [PubMed] [Google Scholar]
- Ferger B, Leng A, Mura A, Hengerer B, Feldon J. Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem. 2004;89:822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC. Alcohol-induced liver disease: when fat and oxidative stress meet. Ann Hepatol. 2003;2:69–75. [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A, Miranda M, Mari M, Ardite E, Morales A. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol. 1997;273:G7–G17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- Ferrari CC, Pott Godoy MC, Tarelli R, Chertoff M, Depino AM, Pitossi FJ. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol Dis. 2006;24:183–193. doi: 10.1016/j.nbd.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Li Z, Jansen M, Rockwell P. N-Acetylcysteine and celecoxib lessen cadmium cytotoxicity which is associated with cyclooxygenase-2 up-regulation in mouse neuronal cells. J Biol Chem. 2002;277:25283–25289. doi: 10.1074/jbc.M109145200. [DOI] [PubMed] [Google Scholar]
- Firestone JA, Smith-Weller T, Franklin G, Swanson P, Longstreth WT, Jr, Checkoway H. Pesticides and risk of Parkinson disease: a population-based case–control study. Arch Neurol. 2005;62:91–95. doi: 10.1001/archneur.62.1.91. [DOI] [PubMed] [Google Scholar]
- Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;70:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Muqit MM, Stanyer L, Healy DG, Abou-Sleiman PM, Hargreaves I, Heales S, Ganguly M, Parsons L, Lees AJ, Latchman DS, Holton JL, Wood NW, Revesz T. PINK1 protein in normal human brain and Parkinson's disease. Brain. 2006;129:1720–1731. doi: 10.1093/brain/awl114. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002a;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002b;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Novel anti-inflammatory therapy for Parkinson's disease. Trends Pharmacol Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival–death switch. J Gastroenterol Hepatol. 2006;21(Suppl 3):S3–S6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- Gayle DA, Ling Z, Tong C, Landers T, Lipton JW, Carvey PM. Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: roles of tumor necrosis factor-alpha, interleukin-1beta, and nitric oxide. Brain Res Dev Brain Res. 2002;133:27–35. doi: 10.1016/s0165-3806(01)00315-7. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- German DC, Speciale SG, Manaye KF, Sadeq M. Opioid receptors in midbrain dopaminergic regions of the rat: I. Mu receptor autoradiography. J Neural Transm Gen Sect. 1993;91:39–52. doi: 10.1007/BF01244917. [DOI] [PubMed] [Google Scholar]
- Ghione I, Di Fonzo A, Saladino F, Del Bo R, Bresolin N, Comi GP, Rango M. Parkin polymorphisms and environmental exposure: decrease in age at onset of Parkinson's disease. Neurotoxicology. 2007;28:698–701. doi: 10.1016/j.neuro.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Gold BG, Nutt JG. Neuroimmunophilin ligands in the treatment of Parkinson's disease. Curr Opin Pharmacol. 2002;2:82–86. doi: 10.1016/s1471-4892(01)00125-4. [DOI] [PubMed] [Google Scholar]
- Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson's disease risk in twins. Ann Neurol. 2006;60:65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Grigoryan GA, Gray JA, Rashid T, Chadwick A, Hodges H. Conditionally immortal neuroepithelial stem cell grafts restore spatial learning in rats with lesions at the source of cholinergic forebrain projections cholinergic forebrain projections. Restor Neurol Neurosci. 2000;17:1. [PubMed] [Google Scholar]
- Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- Hakansson A, Westberg L, Nilsson S, Buervenich S, Carmine A, Holmberg B, Sydow O, Olson L, Johnels B, Eriksson E, Nissbrandt H. Interaction of polymorphisms in the genes encoding interleukin-6 and estrogen receptor beta on the susceptibility to Parkinson's disease. Am J Med Genet B Neuropsychiatr Genet. 2005a;133:88–92. doi: 10.1002/ajmg.b.30136. [DOI] [PubMed] [Google Scholar]
- Hakansson A, Westberg L, Nilsson S, Buervenich S, Carmine A, Holmberg B, Sydow O, Olson L, Johnels B, Eriksson E, Nissbrandt H. Investigation of genes coding for inflammatory components in Parkinson's disease. Mov Disord. 2005b;20:569–573. doi: 10.1002/mds.20378. [DOI] [PubMed] [Google Scholar]
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Hamaue N, Ogata A, Terado M, Ohno K, Kikuchi S, Sasaki H, Tashiro K, Hirafuji M, Minami M. Brain catecholamine alterations and pathological features with aging in Parkinson disease model rat induced by Japanese encephalitis virus. Neurochem Res. 2006;31:1451–1455. doi: 10.1007/s11064-006-9197-5. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM, Wolff RA. The novel second messenger ceramide: identification, mechanism of action, and cellular activity. Adv Lipid Res. 1993;25:43–64. [PubMed] [Google Scholar]
- Hasegawa Y, Inagaki T, Sawada M, Suzumura A. Impaired cytokine production by peripheral blood mononuclear cells and monocytes/macro-phages in Parkinson's disease. Acta Neurol Scand. 2000;101:159–164. doi: 10.1034/j.1600-0404.2000.101003159.x. [DOI] [PubMed] [Google Scholar]
- Hastings TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem. 1995;64:919–924. doi: 10.1046/j.1471-4159.1995.64020919.x. [DOI] [PubMed] [Google Scholar]
- Haupt C, Witte OW, Frahm C. Up-regulation of Connexin43 in the glial scar following photothrombotic ischemic injury. Mol Cell Neurosci. 2007;35:89–99. doi: 10.1016/j.mcn.2007.02.005. [DOI] [PubMed] [Google Scholar]
- He Y, Appel S, Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 2001;909:187–193. doi: 10.1016/s0006-8993(01)02681-6. [DOI] [PubMed] [Google Scholar]
- Heldmann U, Thored P, Claasen JH, Arvidsson A, Kokaia Z, Lindvall O. TNF-alpha antibody infusion impairs survival of stroke-generated neuroblasts in adult rat brain. Exp Neurol. 2005;196:204–208. doi: 10.1016/j.expneurol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Hemmer K, Fransen L, Vanderstichele H, Vanmechelen E, Heuschling P. An in vitro model for the study of microglia-induced neurodegeneration: involvement of nitric oxide and tumor necrosis factor-alpha. Neurochem Int. 2001;38:557–565. doi: 10.1016/s0197-0186(00)00119-4. [DOI] [PubMed] [Google Scholar]
- Henn IH, Bouman L, Schlehe JS, Schlierf A, Schramm JE, Wegener E, Nakaso K, Culmsee C, Berninger B, Krappmann D, Tatzelt J, Winklhofer KF. Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J Neurosci. 2007;27:1868–1878. doi: 10.1523/JNEUROSCI.5537-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AJ, Castano A, Venero JL, Cano J, Machado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis. 2000;7:429–447. doi: 10.1006/nbdi.2000.0289. [DOI] [PubMed] [Google Scholar]
- Herrera AJ, Tomas-Camardiel M, Venero JL, Cano J, Machado A. Inflammatory process as a determinant factor for the degeneration of substantia nigra dopaminergic neurons. J Neural Transm. 2005;112:111–119. doi: 10.1007/s00702-004-0121-3. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y. Neuronal vulnerability in Parkinson's disease. J Neural Transm Suppl. 1997;50:79–88. doi: 10.1007/978-3-7091-6842-4_9. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson's disease: a role in neurodegeneration? Ann Neurol. 1998;44:S115–S120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S, Hartmann A. Neuroinflammatory processes in Parkinson's disease. Parkinsonism Relat Disord. 2005;11(Suppl 1):S9–S15. doi: 10.1016/j.parkreldis.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Dugas N, Faucheux B, Hartmann A, Tardieu M, Debre P, Agid Y, Dugas B, Hirsch EC. FcepsilonRII/CD23 is expressed in Parkinson's disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J Neurosci. 1999;19:3440–3447. doi: 10.1523/JNEUROSCI.19-09-03440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev, Immunol. 2003;3:371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Kashefi K, Mander P, Rose S, Jenner P. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience. 2002;110:49–58. doi: 10.1016/s0306-4522(01)00562-0. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Kim TW, McNamara M, Tanzi RE, George JM, Clayton DF, Hyman BT. Characterization of the precursor protein of the non-A beta component of senile plaques (NACP) in the human central nervous system. J Neuropathol Exp Neurol. 1996;55:889–895. doi: 10.1097/00005072-199608000-00004. [DOI] [PubMed] [Google Scholar]
- Isacson O. Models of repair mechanisms for future treatment modalities of Parkinson's disease. Brain Res Bull. 2002;57:839–846. doi: 10.1016/s0361-9230(01)00773-0. [DOI] [PubMed] [Google Scholar]
- Ito S, Sawada M, Haneda M, Ishida Y, Isobe KI. Amyloid-beta peptides induce several chemokine mRNA expressions in the primary microglia and Ra2 cell line via the PI3K/Akt and/or ERK pathway. Neurosci Res. 2006;56:294–299. doi: 10.1016/j.neures.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Searching for a relationship between manganese and welding and Parkinson's disease. Neurology. 2005;64:2021–2028. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996;47:S161–S170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Understanding cell death in Parkinson's disease. Ann Neurol. 1998;44:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- Jin J, Shie FS, Liu J, Wang Y, Davis J, Schantz AM, Montine KS, Montine TJ, Zhang J. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation. 2007;4:2. doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Parisi JE, Dickson DW. Alpha-synuclein studies are negative in postencephalic parkinsonism of von Economo. Neurology. 2002;59:645–646. doi: 10.1212/wnl.59.4.645. [DOI] [PubMed] [Google Scholar]
- Ju WK, Neufeld AH. Cellular localization of cyclooxygenase-1 and cyclooxygenase-2 in the normal mouse, rat, and human retina. J Comp Neurol. 2002;452:392–399. doi: 10.1002/cne.10400. [DOI] [PubMed] [Google Scholar]
- Jung Y, Surh Y. Oxidative DNA damage and cytotoxicity induced by copper-stimulated redox cycling of salsolinol, a neurotoxic tetrahydroisoquinoline alkaloid. Free Radic Biol Med. 2001;30:1407–1417. doi: 10.1016/s0891-5849(01)00548-2. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Tybor K, Selmaj K. TNF-induced death of adult human oligodendrocytes is mediated by c-jun NH2-terminal kinase-3. Brain. 2003;126:1358–1370. doi: 10.1093/brain/awg146. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha-synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta. 2005;1745:287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, Comyns K, Goldman S, Korell M, Langston J, Ross G, Sandler D. Pesticide exposure and self-reported Parkinson's disease in the agricultural health study. Am J Epidemiol. 2007;165:364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neuro-toxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. R-(−)-Deprenyl inhibits monocytic THP-1 cell neurotoxicity independently of monoamine oxidase inhibition. Exp Neurol. 2000;166:458–464. doi: 10.1006/exnr.2000.7517. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. alpha-Synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2006;20:2000–2008. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Kloss CU, Bohatschek M, Kreutzberg GW, Raivich G. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- Kohutnicka M, Lewandowska E, Kurkowska-Jastrzebska I, Czlonkowski A, Czlonkowska A. Microglial and astrocytic involvement in a murine model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Immunopharmacology. 1998;39:167–180. doi: 10.1016/s0162-3109(98)00022-8. [DOI] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH. Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AH. Platelet mitochondrial function in Parkinson's disease The Royal Kings and Queens Parkinson Disease Research Group. Ann Neurol. 1992;32:782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- Kruger R, Hardt C, Tschentscher F, Jackel S, Kuhn W, Muller T, Werner J, Woitalla D, Berg D, Kuhnl N, Fuchs GA, Santos EJ, Przuntek H, Epplen JT, Schols L, Riess O. Genetic analysis of immunomodulating factors in sporadic Parkinson's disease. J Neural Transm. 2000;107:553–562. doi: 10.1007/s007020070078. [DOI] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Litwin T, Joniec I, Ciesielska A, Przybylkowski A, Czlonkowski A, Czlonkowska A. Dexamethasone protects against dopaminergic neurons damage in a mouse model of Parkinson's disease. Int Immunopharmacol. 2004;4:1307–1318. doi: 10.1016/j.intimp.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lansbury PT, Jr, Brice A. Genetics of Parkinson's disease and biochemical studies of implicated gene products. Curr Opin Cell Biol. 2002;14:653–660. doi: 10.1016/s0955-0674(02)00377-0. [DOI] [PubMed] [Google Scholar]
- Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- Laurie C, Reynolds A, Coskun O, Bowman E, Gendelman HE, Mosley RL. CD4+ T cells from Copolymer-1 immunized mice protect dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neuroimmunol. 2007;183:60–68. doi: 10.1016/j.jneuroim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH. Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson's disease. J Neurosci. 2001;21:8447–8455. doi: 10.1523/JNEUROSCI.21-21-08447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng A, Mura A, Feldon J, Ferger B. Tumor necrosis factor-alpha receptor ablation in a chronic MPTP mouse model of Parkinson's disease. Neurosci Lett. 2005;375:107–111. doi: 10.1016/j.neulet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- Li Z, Jansen M, Pierre SR, Figueiredo-Pereira ME. Neurodegeneration: linking ubiquitin/proteasome pathway impairment with inflammation. Int J Biochem Cell Biol. 2003;35:547–552. doi: 10.1016/s1357-2725(02)00384-9. [DOI] [PubMed] [Google Scholar]
- Li Z, Jansen M, Ogburn K, Salvatierra L, Hunter L, Mathew S, Figueiredo-Pereira ME. Neurotoxic prostaglandin J2 enhances cyclooxygenase-2 expression in neuronal cells through the p38MAPK pathway: a death wish? J Neurosci Res. 2004a;78:824–836. doi: 10.1002/jnr.20346. [DOI] [PubMed] [Google Scholar]
- Li Z, Melandri F, Berdo I, Jansen M, Hunter L, Wright S, Valbrun D, Figueiredo-Pereira ME. Delta12-prostaglandin J2 inhibits the ubiquitin hydrolase UCH-L1 and elicits ubiquitin–protein aggregation without proteasome inhibition. Biochem Biophys Res Commun. 2004b;319:1171–1180. doi: 10.1016/j.bbrc.2004.05.098. [DOI] [PubMed] [Google Scholar]
- Li W, West N, Colla E, Pletnikova O, Troncoso JC, Marsh L, Dawson TM, Jakala P, Hartmann T, Price DL, Lee MK. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proc Natl Acad Sci U S A. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang ZQ, Li YL, Zhao XL, Han R, Wang XX, Wang Y, Chase TN, Bennett MC, Qin ZH. NF-kappaB contributes to 6-hydroxydo-pamine-induced apoptosis of nigral dopaminergic neurons through p53. Brain Res. 2007;1145:190–203. doi: 10.1016/j.brainres.2007.01.130. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin S, Wei X, Xu Y, Yan C, Dodel R, Zhang Y, Liu J, Klaunig JE, Farlow M, Du Y. Minocycline blocks 6-hydroxydopamine-induced neurotoxicity and free radical production in rat cerebellar granule neurons. Life Sci. 2003;72:1635–1641. doi: 10.1016/s0024-3205(02)02442-6. [DOI] [PubMed] [Google Scholar]
- Linda H, Hammarberg H, Piehl F, Khademi M, Olsson T. Expression of MHC class I heavy chain and beta2-microglobulin in rat brainstem motoneurons and nigral dopaminergic neurons. J Neuroimmunol. 1999;101:76–86. doi: 10.1016/s0165-5728(99)00135-6. [DOI] [PubMed] [Google Scholar]
- Ling ZD, Potter ED, Lipton JW, Carvey PM. Differentiation of mesencephalic progenitor cells into dopaminergic neurons by cytokines. Exp Neurol. 1998;149:411–423. doi: 10.1006/exnr.1998.6715. [DOI] [PubMed] [Google Scholar]
- Ling ZD, Robie HC, Tong CW, Carvey PM. Both the antioxidant and D3 agonist actions of pramipexole mediate its neuroprotective actions in mesencephalic cultures. J Pharmacol Exp Ther. 1999;289:202–210. [PubMed] [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, Hong JS, Carvey PM. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM. Progressive dopamine neuron loss following supra-nigral lipopolysaccha-ride (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006;199:499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15:24–27. doi: 10.1038/sj.cr.7290259. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J Biol Chem. 1998;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000a;293:607–617. [PubMed] [Google Scholar]
- Liu W, Kato M, Akhand AA, Hayakawa A, Suzuki H, Miyata T, Kurokawa K, Hotta Y, Ishikawa N, Nakashima I. 4-Hydroxynonenal induces a cellular redox status-related activation of the caspase cascade for apoptotic cell death. J Cell Sci. 2000b;113(Pt 4):635–641. doi: 10.1242/jcs.113.4.635. [DOI] [PubMed] [Google Scholar]
- Liu B, Gao HM, Hong JS. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003a;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Qin L, Li G, Zhang W, An L, Liu B, Hong J. Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J Pharmacol Exp Ther. 2003b;305:212–218. doi: 10.1124/jpet.102.043166. [DOI] [PubMed] [Google Scholar]
- Liu CW, Giasson BI, Lewis KA, Lee VM, Demartino GN, Thomas PJ. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J Biol Chem. 2005;280:22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2005;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, Hazen-Martin DJ, Obeid LM, Hannun YA, Smith GK. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem. 2002;277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- Lund S, Porzgen P, Mortensen AL, Hasseldam H, Bozyczko-Coyne D, Morath S, Hartung T, Bianchi M, Ghezzi P, Bsibsi M, Dijkstra S, Leist M. Inhibition of microglial inflammation by the MLK inhibitor CEP-1347. J Neurochem. 2005;92:1439–1451. doi: 10.1111/j.1471-4159.2005.03014.x. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Melino G, Finazzi-Agro A. Lipoxygenases and their involvement in programmed cell death. Cell Death Differ. 2001;8:776–784. doi: 10.1038/sj.cdd.4400908. [DOI] [PubMed] [Google Scholar]
- MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- Mandel S, Grunblatt E, Youdim M. cDNA microarray to study gene expression of dopaminergic neurodegeneration and neuroprotection in MPTP and 6-hydroxydopamine models: implications for idiopathic Parkinson's disease. J Neural Transm Suppl. 2000:117–124. doi: 10.1007/978-3-7091-6301-6_7. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Abbracchio MP. To be or not to be (inflamed) – is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation Essential role of a TNF receptor 2-mediated phosphatidylino-sitol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Marshall KA, Reist M, Jenner P, Halliwell B. The neuronal toxicity of sulfite plus peroxynitrite is enhanced by glutathione depletion: implications for Parkinson's disease. Free Radic Biol Med. 1999;27:515–520. doi: 10.1016/s0891-5849(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Teraoka H, Iwata H, Kazusaka A, Fujita S. Inhibitory effects of endogenous dopaminergic neurotoxin, norsalsolinol on dopamine secretion in PC12 rat pheochromocytoma cells. Neurochem Int. 2001;38:567–572. doi: 10.1016/s0197-0186(00)00121-2. [DOI] [PubMed] [Google Scholar]
- Mattson MP. NF-kappaB in the survival and plasticity of neurons. Neurochem Res. 2005;30:883–893. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative TNF inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol. 1998a;33:371–378. doi: 10.1016/s0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial cell reactions in neurodegenerative diseases: pathophysiology and therapeutic interventions. Alzheimer Dis Assoc Disord. 1998b;12(Suppl 2):S1–S6. [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- McGuire SO, Ling ZD, Lipton JW, Sortwell CE, Collier TJ, Carvey PM. Tumor necrosis factor alpha is toxic to embryonic mesencephalic dopamine neurons. Exp Neurol. 2001;169:219–230. doi: 10.1006/exnr.2001.7688. [DOI] [PubMed] [Google Scholar]
- McQualter JL, Bernard CC. Multiple sclerosis: a battle between destruction and repair. J Neurochem. 2007;100:295–306. doi: 10.1111/j.1471-4159.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Ajmone-Cat MA, De Berardinis MA, De Simone R. Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Res Brain Res Rev. 2005;48:251–256. doi: 10.1016/j.brainresrev.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Hattori N, Kitada T, Matsumine H, Mori H, Shimura H, Kubo S, Kobayashi H, Asakawa S, Minoshima S, Shimizu N. Familial Parkinson's disease Alpha-synuclein and parkin. Adv Neurol. 2001;86:13–21. [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Tanaka K, Ogawa N, Ichinose H, Nagatsu T. Increase in level of tumor necrosis factor (TNF)-alpha in 6-hydroxydopamine-lesioned striatum in rats without influence of systemic l-DOPA on the TNF-alpha induction. Neurosci Lett. 1999;268:101–104. doi: 10.1016/s0304-3940(99)00388-2. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Tanaka K, Ogawa N, Ichinose H, Nagatsu T. Increase in level of tumor necrosis factor-alpha in 6-hydroxydopamine-lesioned striatum in rats is suppressed by immunosuppressant FK506. Neurosci Lett. 2000a;289:165–168. doi: 10.1016/s0304-3940(00)01275-1. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J Neural Transm. 2000b;107:335–341. doi: 10.1007/s007020050028. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Murakami T, Moriwaki Y, Kawarabayashi T, Nagai M, Ohta Y, Deguchi K, Kurata T, Morimoto N, Takehisa Y, Matsubara E, Ikeda M, Harigaya Y, Shoji M, Takahashi R, Abe K. PINK1, a gene product of PARK6, accumulates in alpha-synucleinopathy brains. J Neurol Neurosurg Psychiatry. 2007;78:653–654. doi: 10.1136/jnnp.2006.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Inflammatory process in Parkinson's disease: role for cytokines. Curr Pharm Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Cellular and molecular mechanisms of Parkinson's disease: neurotoxins, causative genes, and inflammatory cytokines. Cell Mol Neurobiol. 2006;26:781–802. doi: 10.1007/s10571-006-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson's disease. J Neural Transm Suppl. 2000a:143–151. [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm, Suppl. 2000b:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Schwartz JP. Gene expression profiles of reactive astrocytes in dopamine-depleted striatum. Brain Pathol. 2004;14:275–280. doi: 10.1111/j.1750-3639.2004.tb00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y. Regulating factors for microglial activation. Biol Pharm Bull. 2002;25:945–953. doi: 10.1248/bpb.25.945. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Akao Y, Zhang J, Parvez H. Apoptosis induced by an endogenous neurotoxin, N-methyl(R)salsolinol, in dopamine neurons. Toxicology. 2000;153:123–141. doi: 10.1016/s0300-483x(00)00309-7. [DOI] [PubMed] [Google Scholar]
- Nayernouri T. Posttraumatic parkinsonism. Surg Neurol. 1985;24:263–264. doi: 10.1016/0090-3019(85)90035-7. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Mizuta I, Mizuta E, Yamasaki S, Ohta M, Kaji R, Kuno S. Tumor necrosis factor gene polymorphisms in patients with sporadic Parkinson's disease. Neurosci Lett. 2001;311:1–4. doi: 10.1016/s0304-3940(01)02111-5. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Kuno S, Kaji R, Yasuno K, Kawakami H. Glutathione-S-transferase-1 and interleukin-1beta gene polymorphisms in Japanese patients with Parkinson's disease. Mov Disord. 2005;20:901–902. doi: 10.1002/mds.20477. [DOI] [PubMed] [Google Scholar]
- Norris EH, Giasson BI. Role of oxidative damage in protein aggregation associated with Parkinson's disease and related disorders. Antioxid Redox Signal. 2005;7:672–684. doi: 10.1089/ars.2005.7.672. [DOI] [PubMed] [Google Scholar]
- Norris EH, Giasson BI, Ischiropoulos H, Lee VM. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J Biol Chem. 2003;278:27230–27240. doi: 10.1074/jbc.M212436200. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Ogata A, Tashiro K, Nukuzuma S, Nagashima K, Hall WW. A rat model of Parkinson's disease induced by Japanese encephalitis virus. J Neurovirol. 1997;3:141–147. doi: 10.3109/13550289709015803. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Halliday GM. An inflammatory review of Parkinson's disease. Prog Neurobiol. 2002;68:325–340. doi: 10.1016/s0301-0082(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003;83:1069–1112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- Owen AD, Schapira AH, Jenner P, Marsden CD. Oxidative stress and Parkinson's disease. Ann N Y Acad Sci. 1996;786:217–223. doi: 10.1111/j.1749-6632.1996.tb39064.x. [DOI] [PubMed] [Google Scholar]
- Owen AD, Schapira AH, Jenner P, Marsden CD. Indices of oxidative stress in Parkinson's disease, Alzheimer's disease and dementia with Lewy bodies. J Neural Transm, Suppl. 1997;51:167–173. doi: 10.1007/978-3-7091-6846-2_14. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De Smaele E, Tang WJ, D'Adamio L, Franzoso G. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- Papa S, Bubici C, Zazzeroni F, Pham CG, Kuntzen C, Knabb JR, Dean K, Franzoso G. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–729. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. J Neural Transm. 1997;104:661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- Pejovic V, Soskic V, Pan W, Kastin AJ. Brain proteome of mice lacking the receptors for tumor necrosis factor alpha. Proteomics. 2004;4:1461–1464. doi: 10.1002/pmic.200300687. [DOI] [PubMed] [Google Scholar]
- Picklo MJ, Amarnath V, McIntyre JO, Graham DG, Montine TJ. 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J Neurochem. 1999;72:1617–1624. doi: 10.1046/j.1471-4159.1999.721617.x. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Spano P. Distinct roles of diverse nuclear factor-kappaB complexes in neuropathological mechanisms. Eur J Pharmacol. 2006;545:22–28. doi: 10.1016/j.ejphar.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrase-kharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson's disease: a meta-analysis. Environ Res. 2001;86:122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyr-idine mouse model: a tool to explore the pathogenesis of Parkinson's disease. Ann N Y Acad Sci. 2003;991:189–198. [PubMed] [Google Scholar]
- Przedborski S, Tieu K, Perier C, Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. J Bioenerg Biomembranes. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- Przuntek H, Muller T, Riederer P. Diagnostic staging of Parkinson's disease: conceptual aspects. J Neural Transm. 2004;111:201–216. doi: 10.1007/s00702-003-0102-y. [DOI] [PubMed] [Google Scholar]
- Purisai MG, McCormack AL, Cumine S, Li J, Isla MZ, Di Monte DA. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2007;25:392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Raivich G, Fawcett JW. The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience. 2006;140:87–100. doi: 10.1016/j.neuroscience.2006.01.055. [DOI] [PubMed] [Google Scholar]
- Rockwell P, Yuan H, Magnusson R, Figueiredo-Pereira ME. Proteasome inhibition in neuronal cells induces a proinflammatory response manifested by upregulation of cyclooxygenase-2, its accumulation as ubiquitin conjugates, and production of the prostaglandin PGE(2) Arch Biochem Biophys. 2000;374:325–333. doi: 10.1006/abbi.1999.1646. [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;(10 Supp):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Rousselet E, Callebert J, Parain K, Joubert C, Hunot S, Hartmann A, Jacque C, Perez-Diaz F, Cohen-Salmon C, Launay JM, Hirsch EC. Role of TNF-alpha receptors in mice intoxicated with the parkinsonian toxin MPTP. Exp Neurol. 2002;177:183–192. doi: 10.1006/exnr.2002.7960. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Baltazar G, Duarte EP. Interleukin-1beta mediates GDNF up-regulation upon dopaminergic injury in ventral midbrain cell cultures. Neurobiol Dis. 2007;25:92–104. doi: 10.1016/j.nbd.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Sairam K, Saravanan KS, Banerjee R, Mohanakumar KP. Nonsteroidal anti-inflammatory drug sodium salicylate, but not diclofenac or celecoxib, protects against 1-methyl-4-phenyl pyridinium-induced dopaminergic neurotoxicity in rats. Brain Res. 2003;966:245–252. doi: 10.1016/s0006-8993(02)04174-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J Neuroinflammation. 2004;1:6. doi: 10.1186/1742-2094-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson's disease. J Neural Transm, Suppl. 2006:373–381. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Evidence for mitochondrial dysfunction in Parkinson's disease—a critical appraisal. Mov Disord. 1994;9:125–138. doi: 10.1002/mds.870090202. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondrial disease. Lancet. 2006;368:70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Beschorner R, Meyermann R, Gozalan F, Schluesener HJ. Persistent accumulation of cyclooxygenase-1-expressing microglial cells and macrophages and transient upregulation by endothelium in human brain injury. J Neurosurg. 2002;96:892–899. doi: 10.3171/jns.2002.96.5.0892. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- Shang F, Lu M, Dudek E, Reddan J, Taylor A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic Biol Med. 2003;34:521–530. doi: 10.1016/s0891-5849(02)01304-7. [DOI] [PubMed] [Google Scholar]
- Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Greenamyre JT. Environment, mitochondria, and Parkinson's disease. Neuroscientist. 2002a;8:192–197. doi: 10.1177/1073858402008003004. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002b;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003a;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Kim JH, Greenamyre JT. Selective microglial activation in the rat rotenone model of Parkinson's disease. Neurosci Lett. 2003b;341:87–90. doi: 10.1016/s0304-3940(03)00172-1. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003c;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji H, Watanabe M, Itoh S, Kuwahara H, Hattori F. Japanese encephalitis and parkinsonism. J Neurol. 1993;240:59–60. doi: 10.1007/BF00838449. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson's disease. Neurosci Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Jenner P, Daniel SE, Lees AJ, Marsden DC, Halliwell B. Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: possible mechanisms of formation involving reactive oxygen species. J Neurochem. 1998;71:2112–2122. doi: 10.1046/j.1471-4159.1998.71052112.x. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. FASEB J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. FASEB J. 2006;20:670–682. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- Sterka D, Jr, Rati DM, Marriott I. Functional expression of NOD2, a novel pattern recognition receptor for bacterial motifs, in primary murine astrocytes. Glia. 2006;53:322–330. doi: 10.1002/glia.20286. [DOI] [PubMed] [Google Scholar]
- Stern MB. Head trauma as a risk factor for Parkinson's disease. Mov Disord. 1991;6:95–97. doi: 10.1002/mds.870060202. [DOI] [PubMed] [Google Scholar]
- Stern M, Dulaney E, Gruber SB, Golbe L, Bergen M, Hurtig H, Gollomp S, Stolley P. The epidemiology of Parkinson's disease A case–control study of young-onset and old-onset patients. Arch Neurol. 1991;48:903–907. doi: 10.1001/archneur.1991.00530210029018. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial response to brain injury: a brief synopsis. Toxicol Pathol. 2000;28:28–30. doi: 10.1177/019262330002800104. [DOI] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.006. May 28, Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Yang L, Cho BP, DeGiorgio LA, Lorenzl S, Albers DS, Beal MF, Volpe BT, Joh TH. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003;964:288–294. doi: 10.1016/s0006-8993(02)04085-4. [DOI] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Tang BL, Liou YC. Novel modulators of amyloid-beta precursor protein processing. J Neurochem. 2007;100:314–323. doi: 10.1111/j.1471-4159.2006.04215.x. [DOI] [PubMed] [Google Scholar]
- Tanner CM. Is the cause of Parkinson's disease environmental or hereditary? Evidence from twin studies. Adv Neurol. 2003;91:133–142. [PubMed] [Google Scholar]
- Tartaglia LA, Rothe M, Hu YF, Goeddel DV. Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF receptor. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V, Przedborski S. COX-2 and neurodegeneration in Parkinson's disease. Ann N Y Acad Sci. 2003a;991:272–277. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci U S A. 2003b;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetrud JW, Langston JW, Garbe PL, Ruttenber AJ. Mild parkinsonism in persons exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Neurology. 1989;39:1483–1487. doi: 10.1212/wnl.39.11.1483. [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke Neuroprotective Exploratory Trials in Parkinson's Disease Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Goodman BM, Baggs RB, Cory-Slechta DA. Developmental exposure to the pesticides paraquat and maneb and the Parkinson's disease phenotype. Neurotoxicology. 2002;23:621–633. doi: 10.1016/s0161-813x(02)00092-x. [DOI] [PubMed] [Google Scholar]
- Thorsen P, Jensen IP, Jeune B, Ebbesen N, Arpi M, Bremmelgaard A, Moller BR. Few microorganisms associated with bacterial vaginosis may constitute the pathologic core: a population-based microbiologic study among 3596 pregnant women. Am J Obstet Gynecol. 1998;178:580–587. doi: 10.1016/s0002-9378(98)70442-9. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Tomas-Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, Venero JL. Minocycline reduces the lipopolysaccha-ride-induced inflammatory reaction, peroxynitrite-mediated nitration of proteins, disruption of the blood–brain barrier, and damage in the nigral dopaminergic system. Neurobiol Dis. 2004;16:190–201. doi: 10.1016/j.nbd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Aggregates in neurodegenerative disease: crowds and power? Trends Neurosci. 1999;22:194–197. doi: 10.1016/s0166-2236(99)01409-5. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hedreen JC, Price DL. Parkinson's disease: loss of neurons from the ventral tegmental area contralateral to therapeutic surgical lesions. Neurology. 1985;35:1215–1218. doi: 10.1212/wnl.35.8.1215. [DOI] [PubMed] [Google Scholar]
- Uryu K, Giasson BI, Longhi L, Martinez D, Murray I, Conte V, Nakamura M, Saatman K, Talbot K, Horiguchi T, McIntosh T, Lee VM, Trojanowski JQ. Age-dependent synuclein pathology following traumatic brain injury in mice. Exp Neurol. 2003;184:214–224. doi: 10.1016/s0014-4886(03)00245-0. [DOI] [PubMed] [Google Scholar]
- Valente EM, Salvi S, Ialongo T, Marongiu R, Elia AE, Caputo V, Romito L, Albanese A, Dallapiccola B, Bentivoglio AR. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol. 2004a;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004b;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Dillon-Carter O, Tourtellotte WW, Carvey P, Freed WJ. TGFbeta1 and TGFbeta2 concentrations are elevated in Parkinson's disease in ventricular cerebrospinal fluid. Exp Neurol. 1996;142:313–322. doi: 10.1006/exnr.1996.0200. [DOI] [PubMed] [Google Scholar]
- Veluthakal R, Palanivel R, Zhao Y, McDonald P, Gruber S, Kowluru A. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis. 2005;10:841–850. doi: 10.1007/s10495-005-0431-4. [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, Korsmeyer SJ, Przedborski S. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Lin WW, Chen HL, Chang YH, Ou HC, Kuo JS, Hong JS, Jeng KC. Silymarin protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity by inhibiting microglia activation. Eur J Neurosci. 2002;16:2103–2112. doi: 10.1046/j.1460-9568.2002.02290.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waragai M, Wei J, Fujita M, Nakai M, Ho GJ, Masliah E, Akatsu H, Yamada T, Hashimoto M. Increased level of DJ-1 in the cerebrospinal fluids of sporadic Parkinson's disease. Biochem Biophys Res Commun. 2006;345:967–972. doi: 10.1016/j.bbrc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Weller C, Oxlade N, Dobbs SM, Dobbs RJ, Charlett A, Bjarnason IT. Role of inflammation in gastrointestinal tract in aetiology and pathogenesis of idiopathic parkinsonism. FEMS Immunol Med Microbiol. 2005a;44:129–135. doi: 10.1016/j.femsim.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Weller C, Charlett A, Oxlade NL, Dobbs SM, Dobbs RJ, Peterson DW, Bjarnason IT. Role of chronic infection and inflammation in the gastrointestinal tract in the etiology and pathogenesis of idiopathic parkinsonism: Part 3. Predicted probability and gradients of severity of idiopathic parkinsonism based on H. pylori antibody profile. Helicobacter. 2005b;10:288–297. doi: 10.1111/j.1523-5378.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Sidhu A. Inflammation and Parkinson's disease. Curr Drug Targets Inflamm Allergy. 2002;1:221–242. doi: 10.2174/1568010023344580. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Sidhu A. An inflammatory pathomechanism for Parkinson's disease? Curr Med Chem. 2006;13:591–602. doi: 10.2174/092986706776055760. [DOI] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms H, Claasen J, Rohl C, Sievers J, Deuschl G, Lucius R. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: evidence from activated microglial cells in vitro. Neurobiol Dis. 2003a;14:417–424. doi: 10.1016/j.nbd.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-κB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson's disease. Faseb J. 2003b;17:500–502. doi: 10.1096/fj.02-0314fje. [DOI] [PubMed] [Google Scholar]
- Wojtera M, Sikorska B, Sobow T, Liberski PP. Microglial cells in neurodegenerative disorders. Folia Neuropathol. 2005;43:311–321. [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease— a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yasuhara R, Miyamoto Y, Akaike T, Akuta T, Nakamura M, Takami M, Morimura N, Yasu K, Kamijo R. Interleukin-1beta induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J. 2005;389:315–323. doi: 10.1042/BJ20041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani U, German DC, Liang CL, Manzino L, Sonsalla PK, Zeevalk GD. Rat model of Parkinson's disease: chronic central delivery of 1-methyl-4-phenylpyridinium (MPP+) Exp Neurol. 2006;200:172–183. doi: 10.1016/j.expneurol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Buccafusco JJ. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol. 2005;26:27–35. doi: 10.1016/j.tips.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Zecca L, Zucca FA, Albertini A, Rizzio E, Fariello RG. A proposed dual role of neuromelanin in the pathogenesis of Parkinson's disease. Neurology. 2006;67:S8–S11. doi: 10.1212/wnl.67.7_suppl_2.s8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dawson VL, Dawson TM. Role of nitric oxide in Parkinson's disease. Pharmacol Ther. 2006;109:33–41. doi: 10.1016/j.pharmthera.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li S, Childs EE, Kuharsky DK, Yin XM. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-alpha-induced liver injury. J Biol Chem. 2001;276:27432–27440. doi: 10.1074/jbc.M102465200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Muller-Myhsok B, Farrer M, Leitner P, Sharma M, Hulihan M, Lockhart P, Strongosky A, Kachergus J, Calne DB, Stoessl J, Uitti RJ, Pfeiffer RF, Trenkwalder C, Homann N, Ott E, Wenzel K, Asmus F, Hardy J, Wszolek Z, Gasser T. The PARK8 locus in autosomal dominant parkinsonism: confirmation of linkage and further delineationof the disease-containing interval. Am J Hum Genet. 2004;74:11–19. doi: 10.1086/380647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca FA, Giaveri G, Gallorini M, Albertini A, Toscani M, Pezzoli G, Lucius R, Wilms H, Sulzer D, Ito S, Wakamatsu K, Zecca L. The neuromelanin of human substantia nigra: physiological and pathogenic aspects. Pigment Cell Res. 2004;17:610–617. doi: 10.1111/j.1600-0749.2004.00201.x. [DOI] [PubMed] [Google Scholar]