Abstract
Purpose
To evaluate fundus autofluorescence (FAF) patterns in patients with primary intraocular (vitreoretinal) lymphoma (PIOL/PVRL).
Methods
Records of all PIOL patients who underwent FAF imaging at the National Eye Institute (NEI) were reviewed. FAF patterns were evaluated with respect to clinical disease status and the findings on fluorescein angiography (FA) and spectral-domain optical coherence tomography (SD-OCT) images.
Results
There were 18 eyes (10 patients) with PIOL who underwent FAF imaging. Abnormal autofluorescence in the form of granular hyper- and hypoautofluorescence was seen in 11 eyes (61%) and blockage by mass lesion was seen in 2 eyes (11%). All eyes with granular pattern on FAF had active PIOL at the time of imaging, but there were 5 eyes with unremarkable FAF which were found to have active lymphoma. The most common pattern on FA was hypofluorescent round spots with a “leopard-spot” appearance (43%). These hypofluorescent spots on FA correlated to hyperautofluorescent spots on FAF in 5 (36%) eyes (inversion of FAF). Nodular hyperreflective spots at the level of RPE on OCT were noted in 43% of eyes. The hyperautofluorescent spots on FAF correlated with nodular hyperreflective spots on OCT in 6 eyes (43%).
Conclusion
Granularity on fundus autofluorescence was associated with active lymphoma in majority of cases. An inversion of FAF (hyperautofluorescent spots on FAF corresponding to hypofluorescent spots on FA) was observed in less than half of the eyes.
Keywords: Cytokines, diagnosis, fundus autofluorescence, primary intraocular lymphoma, primary vitreoretinal lymphoma, uveitis
Introduction
Primary intraocular lymphoma (PIOL), recently suggested to be renamed primary vitreoretinal lymphoma (PVRL) and a subset of primary central nervous system lymphoma (PCNSL), is an aggressive, diffuse large cell lymphoma, mostly of B cell origin. The median age of onset is in the sixth decade, and it commonly masquerades as a chronic intermediate uveitis that is initially partially steroid responsive but becomes less so with prolonged steroid use.1–4 The diagnosis of PIOL can be difficult and its delay may have serious consequences.3 The average time from the onset of ocular symptoms to diagnosis is 12 to 24 months.5 The majority of patients with PIOL develop CNS involvement, and the median survival period is 31 months.6,7
The diagnosis of PIOL is achieved with the identification of atypical lymphoid cells in the eye or in the cerebrospinal fluid (CSF).2 Since the diagnosis often requires multiple interventions, it is important to identify patients at most risk using ancillary clinical tools.8,9 In addition to a thorough clinical examination, fluorescein angiogram (FA), indocyanine green angiogram, and optical coherence tomography (OCT) have been shown to be helpful in identifying PIOL cases.10 Recently, fundus autofluorescence (FAF), another non-invasive method, has been increasingly used to aid in the diagnosis of various ocular diseases.11–14 Autofluorescence observed in FAF depends on lipofuscin, a molecular byproduct of oxidative stress that is known to accumulate in the retinal pigmented epithelium (RPE). Increased autofluorescence is seen with RPE dysfunction and decreased autofluorescence is seen with loss of RPE or blockage of autofluorescence.13,14 FAF takes advantage of the fluorophore nature of lipofuscin and has been helpful in assessing the function of the RPE in various retinal disorders such as age related macular degeneration, retinitis pigmentosa, posterior uveitis, and various choroidal tumors.15 Data regarding FAF changes associated with PIOL is still scarce.16 We describe the FAF findings in correlation with FA and OCT in patients with PIOL.
Methods
Medical records of patients who carried the diagnosis of PIOL and were seen under an institutional review board–approved protocol in the uveitis and ocular immunology clinic at the National Eye Institute, were reviewed. All patients with PIOL who had an available FAF image of adequate quality for analysis were included for review.
Data collected included demographics, laterality, clinical activity of lymphoma, diagnostic interventions to confirm the presence or recurrence of lymphoma, fundus examination findings, fundus photography (TRC-50EX; Topcon Medical Systems, Oakland, NJ, USA), fluorescein angiography, and spectral-domain optical coherence tomography (Cirrus HD, Carl Zeiss Meditec, Dublin, California, USA or Spectralis, Heidelberg Engineering, Germany). Any of these imaging modalities were considered concurrent with FAF findings if performed within six to eight weeks. FAF was performed with a 580nm excitation filter and a 695 nm emission filter using a Topcon fundus camera and the OIS WinStation software (Sacramento, CA, USA).
FAF images were evaluated for areas of abnormal granular hyper- or hypoautofluorescence, and blockage. All images were reviewed by 2 reviewers and adjudicated by a 3rd reviewer in case of disagreements. Granular pattern was defined as hyperautofluorescent spots ranging from 50–250 microns alternating with adjacent hypoautofluorescent spots in the posterior pole and periphery. The degree of granular pattern was graded as mild, moderate, or marked by two independent investigators. Blockage was defined as obstruction of autofluorescence by mass lesion leading to hypoautofluorescence. These features were correlated with clinical disease status (active vs. remission), fundus pictures, FA, and OCT features. PCNSL/PVRL was diagnosed based on vitreous cytology in 7 patients and brain biopsy (and/or CSF cytology) in 3 patients. In these 3 patients significantly elevated intraocular cytokines were demonstrated in addition to evidence of CNS lymphoma. Active lymphoma was defined as presence of a clinically visible mass, infiltrative lesion, or +1 or more cells in the anterior chamber or presence of any vitreous haze, and cytologic evidence (if imaging was done at the time of initial diagnosis) or elevated IL-10/IL-6 ratio (if imaging was done at the time of a recurrence) from intraocular fluid as described elsewhere.17 Fluorescein angiograms were evaluated for the presence of granular pattern, leopard-spot pattern, RPE mottling, and window defects (defined as granular areas of hyperfluorescence intermixed with hypofluorescence).10,18 Fundus photographs and records were examined for the presence of vitreous haze, mass lesions, areas of atrophy, and RPE mottling. OCT images were evaluated for areas of nodular hyperreflective spots at the level of the RPE.
Results
Ten patients (18 eyes) with PIOL who were seen at the National Eye Institute and had undergone FAF imaging concurrent with FA and/or OCT were identified. Of the 18 eyes, 14 had concurrent FA and 14 had concurrent OCT. Of the ten patients, one patient had unilateral disease and another (Patient 5) had a significant cataract preventing imaging in one eye. Nine patients (16 eyes) had active PIOL at the time of FAF imaging.
Abnormal fluorescence on FAF was identified in 11 eyes (61% of all eyes, 69% of eyes with active PIOL), whereas 7 eyes showed no remarkable findings on FAF. All eyes that showed abnormal FAF patterns had active PIOL at the time of imaging. Granular pattern on FAF was present in all 11 eyes with abnormal FAF, and additionally blockage by mass lesion was also seen in 2 of these eyes (2 patients). Of the 7 eyes with unremarkable FAF, 2 eyes (1 patient) were in remission and 5 eyes (4 patients) had active lymphoma. Granularity on FAF, when present, was observed in various locations in the retina and was not limited to where the visible tumor was located (Table 1).
Table 1.
Clinical and imaging characteristics of patients with PIOL/PVRL.
| Patient | Eye | Clinical Disease Status |
Method of Diagnosis |
FAF Granularity |
FA | FA/FAF Inversion |
OCT |
|---|---|---|---|---|---|---|---|
| 1 | OD | active | Vitreous biopsy | marked | leopard spot pattern; window defects | Yes | nodular hyperreflective spots at the level of RPE |
| OS | active | marked | leopard spot pattern; window defects | Yes | nodular hyperreflective spots at the level of RPE; atrophy | ||
| 2 | OD | active | Lumbar puncture* | moderate | N/A | N/A | unremarkable |
| OS | active | mild | N/A | N/A | nodular hyperreflective spots at the level of RPE | ||
| 3 | OD | active | Brain biopsy and vitreous biopsy | mild | leopard spot pattern; window defects | Yes | N/A |
| OS | active | unremarkable | late small vessel leakage (no leopard spot pattern) | N/A | N/A | ||
| 4 | OD | active | Vitreous biopsy | unremarkable | small-medium (100–250µm) hypofluorescent spots; leopard spot pattern | N/A | unremarkable |
| OS | active | marked | peripapillary hypofluorescent spots | Yes | unremarkable | ||
| 5 | OD | active | Brain biopsy* | mild (surrounding mass lesion) | blockage by the mass lesion; leopard spot pattern | No | nodular hyperreflective spots at the level of RPE |
| OS | N/A (dense cataract)** | N/A | N/A | N/A | N/A | ||
| 6 | OD | active | Brain biopsy* | unremarkable | unremarkable | N/A | N/A |
| OS | active | unremarkable | unremarkable | N/A | N/A | ||
| 7 | OD | active | Vitreous biopsy | moderate (surrounding mass lesion) | blockage by the lesion with late staining (no leopard spot pattern) | No | large mass |
| OS | active | unremarkable | unremarkable | No | unremarkable | ||
| 8 | OD | active | Vitreous biopsy | moderate | N/A | N/A | unremarkable |
| OS | active | moderate | N/A | N/A | nodular hyperreflective spots at the level of RPE | ||
| 9 | OD | active | Vitreous biopsy | moderate | leopard spot pattern | Yes | nodular hyperreflective spots at the level of RPE |
| OS | no involvement | unremarkable | unremarkable | N/A | N/A | ||
| 10 | OD | quiet | Vitreous and subretinal biopsy | unremarkable | unremarkable: window defects (atrophy) | N/A | atrophic |
| OS | quiet | unremarkable | unremarkable: window defects (atrophy) | N/A | scar tissue; disrupted photoreceptor layer |
The diagnosis of PCNSL preceded the diagnosis of PIOL in these patients. The ocular involvement was confirmed with clinical findings and highly elevated IL-10/IL-6.
Left eye of this patient had a dense cataract that precluded quality imaging
N/A= not available/applicable, FAF= fundus autofluorescence, FA= fluorescein angiogram, OCT= optical coherence tomography
FA was available for 14 eyes (8 patients). Six eyes (5 patients) demonstrated a leopard spot-like pattern with a granular appearance (43% of eyes with FA imaging, 50% of active eyes with FA imaging) and all had active disease. Five eyes (3 patients) had granular RPE window defects, and 2 eyes (2 patients) displayed early blockage with late staining of mass lesions consistent with the blockage observed on FAF. In 5 eyes (4 patients), areas of hyperautofluorescence on FAF corresponded to the areas of hypofluorescence in the early to mid-phases of the FA (Figure 1). This concordance was observed in 62% of patients that had granular pattern on both FA and FAF. Importantly, while 11 eyes with active disease showed granular FAF pattern only 6 eyes showed granular or leopard-spot pattern on FA. In 6 eyes granular FAF pattern was the main reason for suspicion either because of lack of leopard pattern on FA or because an FA could not be obtained.
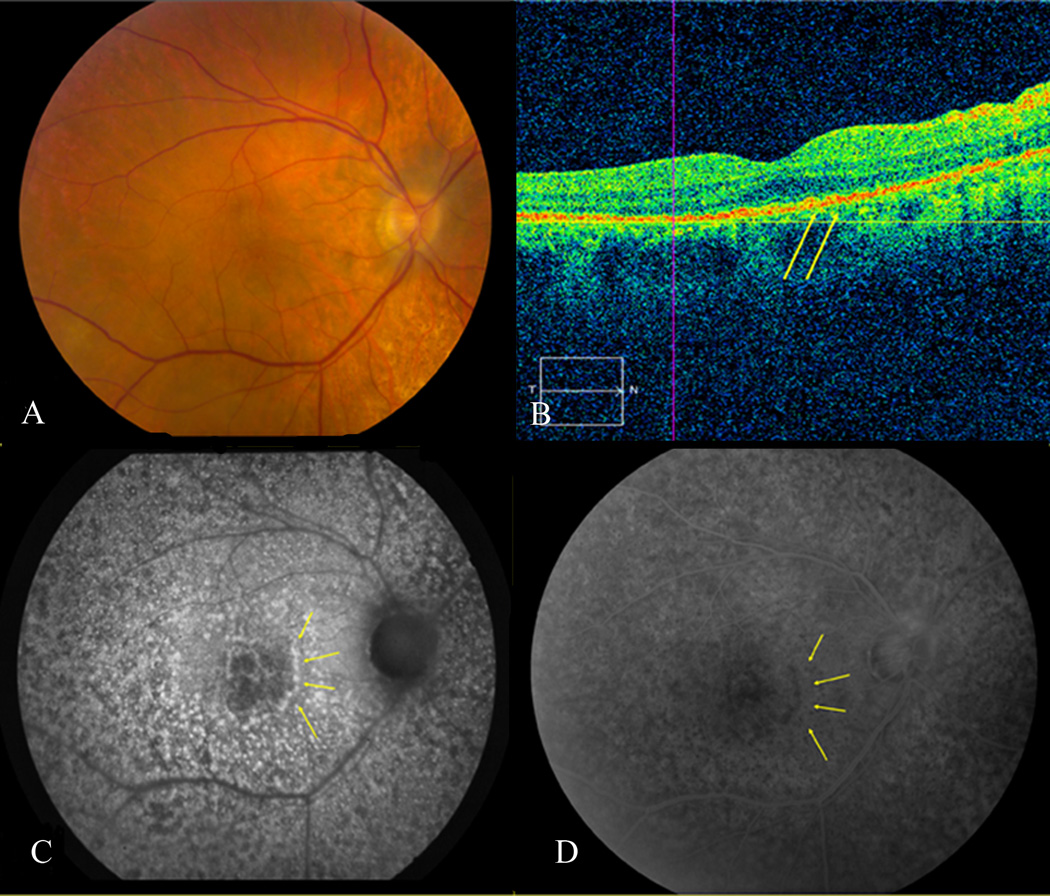
Figure 1.
Corresponding fundus photo (A), OCT (B), FAF (C), and FA (D) of patient #1. Despite relatively unremarkable color fundus photographs, except mild RPE mottling, FAF displays significant granular pattern. Hyperautofluorescent spots on FAF are hypofluorescent on FA (yellow arrowheads). Nodular hyperreflective spots at the level of RPE on OCT appear to correspond to granular areas on FAF (yellow arrowheads).
OCT was available in 14 eyes (8 patients). Nodular hyperreflective spots under the RPE that corresponded to areas of granular pattern on FAF were noted in 6 eyes (5 patients, 43% of all eyes with OCT, 50% of active eyes with OCT) (Figures 1 and 3). Other findings on OCT included an elevated hyperreflective area at the level of RPE, representing a fibrotic scar in one eye, a large macular mass in one eye, and a pigment epithelial detachment in another eye (Table 1).
Figure 3.
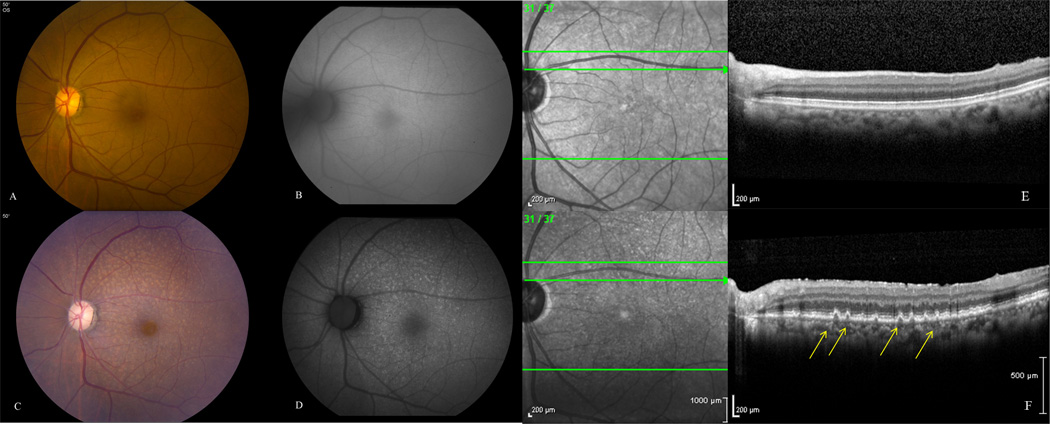
Left eye of patient #9 was asymptomatic (vision 20/20) with no infiltrates at presentation (A), normal fundus autofluorescence (B) and SD-OCT (E). 2 years later she noted slight vision decline (20/25) and fundus exam showed granular subretinal infiltrates (C) with corresponding areas of hyperautofluorescence (D) and nodular hyperreflective spots on SD-OCT (F). This was confirmed via cytokine analysis from the aqueous (elevated IL-10/IL-6) and MRI with concurrent CNS involvement.
Abnormal FAF findings were moderately sensitive (68.75%; 95% CI: 41.36 % to 88.87 %) and highly specific (100%; 95% CI: 19.29 % to 100.00 %) in distinguishing active disease from inactive. Positive predictive value was high (100%; 95% CI: 71.33 % to 100.00 %) with a very low negative predictive value (28.57%; 95% CI: 4.52 % to 70.73 %). However, these estimates had very large confidence intervals due to small sample size.
Discussion
The patterns demonstrated on FAF in eyes with PIOL were variable. A granular autofluorescence pattern was noted in the majority of the eyes, particularly those with active disease at the time of imaging. Eleven eyes with granular pattern on FAF had active disease, although 5 eyes with active disease revealed no granular pattern on FAF. Notably, granular FAF pattern was the main reason for clinical suspicion in 6 eyes where there were no evidence of leopard spot pattern on FA or an FA could not be obtained. Hyperautofluorescent spots appeared to correlate with the hypofluorescent spots on FA and the nodular hyperreflective spots on OCT in some patients. A granular pattern on FAF in eyes with PIOL has been reported in a case series. Contrary to our findings the authors reported abnormal FAF patterns in all eyes with active disease. They noted a similar correlation between hyperautofluorescent spots on fundus autofluorescence, hypofluorescent spots on FA, and hyperreflective spots on OCT.16
Our study demonstrated a moderate sensitivity and low negative predictive value despite relatively high specificity and positive predictive value of FAF, suggesting that while presence of abnormal FAF findings may indicate active disease, absence of such abnormality does not rule out the presence or recurrence of PIOL. Additionally, degree of granular pattern, as graded in this study, was not particularly helpful. Autopsied eyes with PIOL have demonstrated that lymphoma cells in PIOL typically reside between the RPE and Bruch’s membrane.8,19 It has also been reported that PIOL may directly affect the RPE.18 FAF depends on lipofuscin in RPE cells that accumulates in response to various types of damage. In many posterior uveitis active disease is often reflected by a hyperautofluorescent signal. An increased autofluorescence indicates RPE dysfunction and a decreased autofluorescence is typically seen with loss of the RPE or photoreceptors.20 We hypothesize that areas of lymphomatous infiltrates in the sub-RPE space can alter RPE metabolism leading to hyperautofluorescence on FAF and cause blockage leading to hypofluorescence on FA. Lipofuscin accumulation in the RPE occurs in choroidal melanoma resulting in hyperautofluorescence; however, there is no such evidence in PIOL.16 It is possible that hyperautofluorescence in PIOL may also be the result of lipofuscin accumulation in the RPE cells adjacent to the tumor. Hypoautofluorescence may be caused by blockage of the autofluorescence by the infiltrating tumor cells or RPE atrophy which can result from tumor resolution. Such areas of RPE atrophy can lead to hypofluorescence on FAF.16 Hence, an abnormal autofluorescence can be helpful in raising suspicion for the presence of lymphoma or recurrence in a patient with a known PIOL diagnosis.
The intrinsic ability of the malignant B-cells to autofluoresce has been studied in vitro and compared to both normal and activated T-cells.21 Each cell population was effectively isolated based on their emissions when excited with different wavelengths. The intensity differences that allowed cell type differentiation were thought to be due to the relative increase in aerobic energy metabolism of the B-cell. This intrinsic ability of malignant B-cells to autofluoresce can therefore be appreciated in other organ systems affected by these cells, as it is not a property of PIOL but of the cells themselves. Interestingly, in a prospective gastrointestinal lymphoma study, lymphoma was successfully differentiated from lymphoid hyperplasia with the use of autofluorescent imaging.22 In theory, intrinsic ability of lymphocytes or macrophages may also contribute to abnormal autofluorescence in PIOL. This finding demonstrates the utility of autofluorescence imaging in different types of lymphoma and highlights the potential of this non-invasive tool.
The main limitations of this study are its retrospective nature and the small sample size. All diagnostic imaging techniques were not available on all patients. Additionally, ophthalmoscopy and imaging were limited in some patients with significant vitritis. The paucity of eyes with quiet disease also limited our ability to compare the utility of autofluorescence imaging in different clinical phases of PIOL. Also we did not have longitudinal follow-up to address the question of how the abnormal FAF pattern may change with treatment or multiple recurrences within the same patient. Nevertheless, we found characteristic patterns on FAF in patients with PIOL, particularly those with active disease. The two eyes with quiet disease demonstrated unremarkable FAF patterns.
A high index of clinical suspicion is of utmost importance in the diagnosis and management of PIOL, and an abnormal FAF finding can help raise this index. While FAF cannot replace FA, our findings support the utility of FAF as a noninvasive adjunct in the diagnosis of PIOL or its recurrence. Wider use of fundus autofluorescence in posterior segment disorders may help improve our understanding of the disease process and ultimately produce a higher diagnostic yield.
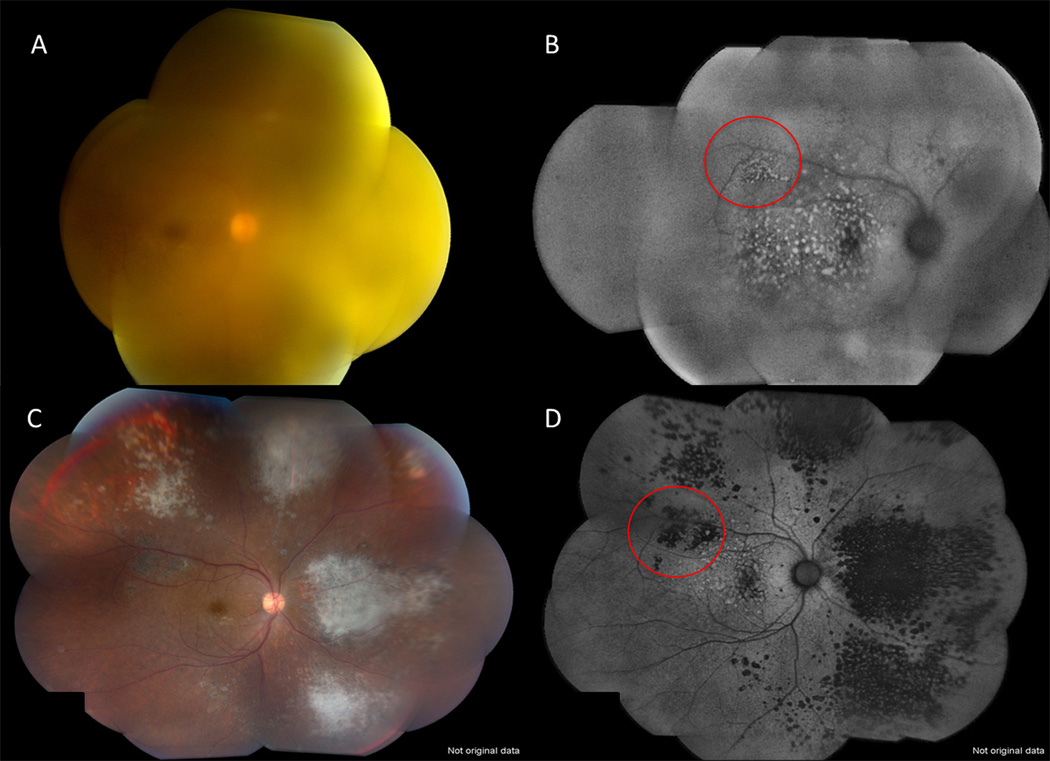
Figure 2.
Fundus photograph (A) and FAF (B) of the right eye a 62 year old female patient diagnosed with PIOL/PVRL through vitreous biopsy show suspicious areas of tumor infiltrates (hyperautofluorescence) on FAF despite significant vitreous haze on fundus photos. Images after treatment illustrate areas of atrophy with resolution of infiltrates (C) which correspond to areas of hypofluorescence on FAF (D). Note the corresponding changes from hyperautofluorescent spots to hypoautofluorescent spots after treatment (red circles).
Summary Statement.
This retrospective study evaluating FAF imaging in patients with primary intraocular (vitreoretinal) lymphoma (PIOL/PVRL) showed that abnormal autofluorescence in the form of granular hyperautofluorescence and hypoautofluorescence was seen in the majority of eyes. All eyes with granular pattern on FAF had active PIOL/PVRL, however all eyes with active disease did not demonstrate FAF abnormality. The hyperautofluorescent spots on FAF correlated with the hypofluorescent spots on FA and the nodular hyperreflective spots on OCT in approximately one third of cases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript was presented in part at the Association for Research in Vision and Ophthalmology (ARVO) meeting, 2011.
None of the authors have any conflict of interest in the materials presented in this manuscript.
References
- 1.Coupland SE, Damato B. Understanding intraocular lymphomas. Clinical and Experimental Ophthalmology. 2008;36(6):564–578. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 2.Chan CC, Wallace DJ. Intraocular lymphoma: update on diagnosis and management. Cancer Control. 2004;11(5):285–295. doi: 10.1177/107327480401100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm SA, Pulido JS, Jahnke K, et al. Primary intraocular lymphoma: an international primary central nervous system lymphoma collaborative group report. Annals of Oncology. 2007;18(11):1851–1855. doi: 10.1093/annonc/mdm340. [DOI] [PubMed] [Google Scholar]
- 4.Choi JY, Kafkala C, Foster CS. Primary intraocular lymphoma: A review. Semin Ophthalmol. 2006 Jul-Sep;21(3):125–133. doi: 10.1080/08820530500350498. Review. [DOI] [PubMed] [Google Scholar]
- 5.Cassoux N, Giron A, Bodaghi B, et al. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Investigative Ophthalmology and Visual Science. 2007;48(7):3253–3259. doi: 10.1167/iovs.06-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm SA, McCannel CA, Omuro AM, et al. Primary CNS lymphoma with intraocular involvement: International PCNSL Collaborative Group Report. Neurology. 2008;71:1355–1360. doi: 10.1212/01.wnl.0000327672.04729.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan CC, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16(11):1589–1599. doi: 10.1634/theoncologist.2011-0210. Epub 2011 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular lymphoma: clinical and histopathologic diagnosis. Ophthalmology. 1993;100(9):1399–1406. doi: 10.1016/s0161-6420(93)31469-7. [DOI] [PubMed] [Google Scholar]
- 9.Moriarty E, Casady M, Faia LJ, et al. Association for Research in Vision and Ophthalmology. Fort Lauderdale, FL: 2011. Apr 22, Primary Intraocular Lymphoma: How Many Diagnostic Procedures Are Needed For A Definitive Diagnosis? [abstract] Abstract no. 4293/D1037. [Google Scholar]
- 10.Fardeau C, Lee CP, Merle-Béral H, et al. Retinal fluorescein, indocyanine green angiography, and optic coherence tomography in non-Hodgkin primary intraocular lymphoma. American Journal of Ophthalmology. 2009;147(5):886–894. doi: 10.1016/j.ajo.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Yannuzzi LA, Ober MD, Slakter JS, et al. Ophthalmic fundus imaging: today and beyond. American Journal of Ophthalmology. 2004;137:511–524. doi: 10.1016/j.ajo.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Yeh S, Forooghian F, Wong WT, et al. Fundus autofluorescence imaging of the white dot syndromes. Archives of Ophthalmology. 2010 Jan;128(1):46–56. doi: 10.1001/archophthalmol.2009.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparrow JR. Lipofuscin of the retinal pigment epithelium. In: Holz FG, Schmitz-Valckenberg S, Spaide RF, Bird AC, editors. Atlas of Fundus Autofluorescence Imaging. Heidelberg: Springer; 2007. pp. 1–16. [Google Scholar]
- 14.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008 Mar;28(3):385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 15.Chin K, Finger PT. Autofluorescence characteristics of suspicious choroidal nevi. Optometry. 2009;80(3):126–130. doi: 10.1016/j.optm.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Ishida T, Ohno-Matsui K, Kaneko Y, et al. Fundus autofluorescence patterns in eyes with primary intraocular lymphoma. Retina. 2010;30(1):23–32. doi: 10.1097/IAE.0b013e3181b408a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan CC. Molecular pathology of primary intraocular lymphoma. Transactions of the American Ophthalmological Society. 2003;101:275–292. [PMC free article] [PubMed] [Google Scholar]
- 18.Velez G, Chan CC, Csaky KG. Fluorescien Angiographic Findings in Primary Intraocular Lymphoma. Retina. 2002;22(1):37–43. doi: 10.1097/00006982-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Corriveau C, Easterbrook M, Payne D. Lymphoma stimulating uveitis (masquerade syndrome) Canadian Journal of Ophthalmology. 1986;21:144–149. [PubMed] [Google Scholar]
- 20.Meleth AD, Sen HN. Use of fundus autofluorescence in the diagnosis and management of uveitis. International Ophthalmology Clinics. 2012;52(4):45–54. doi: 10.1097/IIO.0b013e3182662ee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantanelli SM, Li Z, Fariss R, et al. Differentiation of malignant B-lymphoma cells from normal and activated T-cell populations by their intrinsic autofluorescence. Cancer Research. 2009;69(11):4911–4917. doi: 10.1158/0008-5472.CAN-08-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno N, Fujiya M, Moriichi K, et al. Endosopic autofluorescence imaging is useful for the differential diagnosis of intestinal lymphomas resembling lymphoid hyperplasia. Journal of Clinical Gastroenterology. 2011;45(6):507–513. doi: 10.1097/MCG.0b013e3181fbe22a. [DOI] [PubMed] [Google Scholar]