Abstract
Eudistomin Y class compounds are a series of β-carbolines which was originally isolated from a marine turnicate or ascidian near the South Korea Sea. These compounds contain bromo-substituted groups, which is one of the typical characters of marine natural products. We report herein the chemical synthesis and biological evaluation of seven new β-carboline-based metabolites, Eudistomins Y1–Y7, and their hydroxyl-methylated phenyl derivatives. Using bromo-substituted tryptamines and bromo-substituted phenylglyoxals as the key intermediates, Eudistomins Y1–Y7 and their derivatives were synthesized via the acid-catalyzed Pictet-Spengler reaction and fully characterized by 1H- and 13C-NMR and mass spectroscopy. Biological studies revealed that all of the compounds showed moderate growth inhibitory activity against breast carcinoma cell line MDA-231 with IC50 of 15–63 μM and the inhibitory activities of hydroxyl-methylated phenyl products were higher than that of the corresponding natural products Eudistomins Y1–Y7.
Keywords: marine alkaloid, Eudistomins Y1–Y7, β-carboline, Pictet-Spengler reaction
1. Introduction
The diversity of marine compounds offers a great advantage for being developed into new drugs because of their unique and complex structures, developed through old and underexplored species evolution. However, most marine alkaloids were usually isolated in very small quantities, hindering further studies to establish their biological activities as well as structure modifications [1]. Therefore, chemical synthesis of marine alkaloids in larger quantities and by sufficient means is necessary to investigate their mode of action and their biological implications. β-carbolines are a large group of natural and synthetic indole alkaloids with different substitutions at the C-1, C-6 and C-7 position, some of which are widely distributed in many plants and mammals and exhibit a wide spectrum of biological activities [2,3,4].
Eudistomin Y class β-carboline compounds are different from previously isolated marine metabolites due to the presence of a benzoyl group attached to the β-carboline nucleus at C-1. These compounds contain bromo-substituted groups, which is one of the typical characters of the marine natural products. Seven eudistomins compounds, Eudistomins Y1–Y7, were isolated from a tunicate of the genus Eudistoma collected near Tong-Yeong City, South Sea, Korea in 2008 [5], and another six Eudistomins Y8–Y13 were isolated from Korean ascidian Synoicum sp. in 2012 [6]. From bioactivity evaluation results, several of these natural compounds exhibited moderate to significant antibacterial, antimicrobial activity and weak cytotoxic activity [5,6].
Kennedy et al. have reported the synthetic methods of Eudistomins Y1–Y7 with 3 steps in a two-pot process; overall yields ranged from 6% to 25% [7]. In the present paper, we report the total chemical syntheses of the marine alkaloid Eudistomins Y1–Y7 23–29 and their hydroxyl-methylated phenyl derivatives (16–22) with modified one-pot oxidation via the acid-catalyzed Pictet-Spengler reaction, as well as their biological activities against breast carcinoma cell line MDA-231. All structures of target compounds were confirmed by 1H NMR, 13C NMR and HRMS. The spectra data of synthetic compounds 23–29 were consistent with those of natural β-carboline alkaloids Eudistomins Y1–Y7 in the literature [5].
2. Results and Discussion
2.1. Chemistry
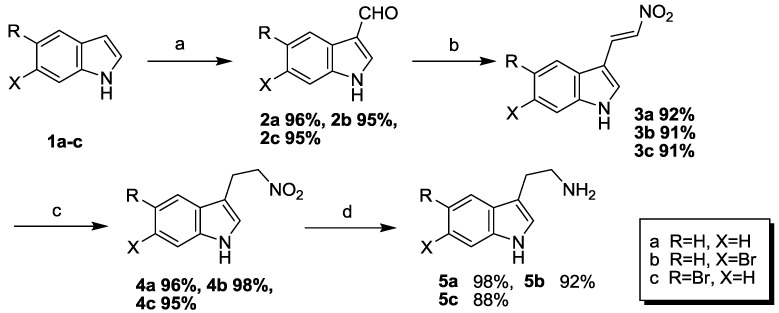
The particularity of the marine environment leads to the rich bromine in the marine natural products, so the syntheses of bromo-substituted indole compounds are the key step in the preparations of marine alkaloid Eudistomins Y1–Y7 23–29. The target compounds were started from the commercially available indole. Substituted indole-3-carboxaldehyde 2a–c was synthesized from 1a–c by Vilsmeier-Hacck reaction with phosphorus oxychloride (POCl3) and N,N-dimethylformamide (DMF) according to the reference [8]. Intermediates 2a–c was condensed with nitromethane (CH3NO2) based on the classical Henry reaction to form the corresponding vinyl nitro compounds 3a–c [9], reduction of which by NaBH4 at room temperature gave compounds 4a–c in high yields. Subsequently, 4a was reduced by Pd/C in methanol at room temperature to give 5a. 4b–c were reduced by LiAlH4 and refluxed in THF to give bromo-substituted tryptamines 5b–c [10]. The reaction routes were outlined in Scheme 1.
Scheme 1.
Reagents and conditions: (a) POCl3/DMF/NaOH; (b) CH3NO2/CH3COONH4/benzene, reflux; (c) NaBH4/THF/CH3OH, rt.; (d) Pd/C, H2, rt., for 5a; LiAlH4/THF, refulx, for 5b and 5c.
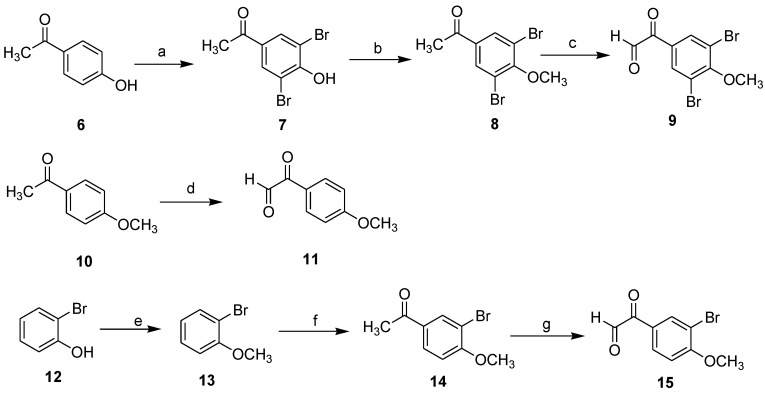
Methylation of bromophenol by dimethyl sulfate in acetone, 7 and 12 was converted to substituted methoxybenzene 8 and 13. Intermediate 14 was prepared by 1-bromo-2-methoxybenzene 13 using acetyl chloride by Friedel–Craft acylation. The substituted acetophenones 8, 10 and 14 were converted to substituted phenylglyoxals 9, 11 and 15 by oxidation with SeO2 in dioxane–water mixture [11]. The reaction routes were outlined in Scheme 2.
Scheme 2.
Reagents and conditions: (a) NBS/H2SO4/H2O, 92%; (b) (CH3)2SO4/K2CO3/acetone, 95%; (c) SeO2/dioxane, 80 °C, 71%; (d) SeO2/dioxane, 80 °C, 75%; (e) (CH3)2SO4/K2CO3/acetone; (f) acetyl chloride, AlCl3/CS2, 76%; (g) SeO2/dioxane, 80 °C, 74%.
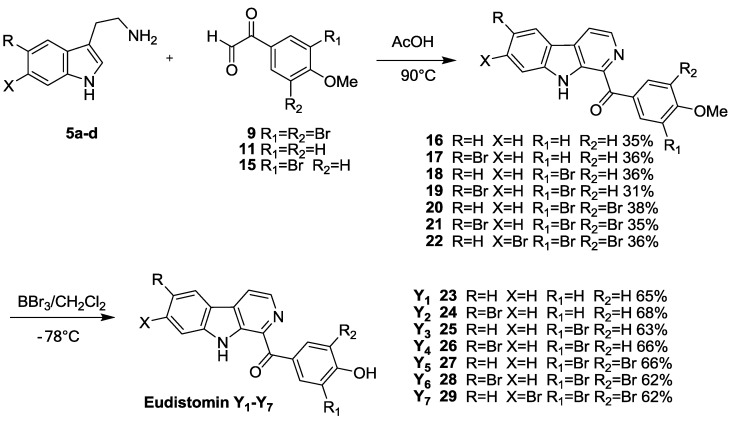
The classical method to prepare β-carboline alkaloids through Pictet-Spengler reaction was a two-step method, and involved the acid-catalyzed condensation of an aliphatic amine attached to a sufficiently reactive aromatic nucleus with aldehydes. Specifically, in the first step, an imine was formed, which can be activated by acids, while in the second step, the endo cyclization occurred between a carbon nucleophile of a sufficiently reactive aromatic moiety and the activated iminium ion, resulting in tetrahydro-β-carboline by the formation of N-heterocyclic ring through a new C–C bond. After dehydrogenation, tetrahydro-β-carboline was converted to β-carboline [12,13,14,15]. In our experiments, the treatment of substituted tryptamines with substituted phenylglyoxal [16,17] under the acidic conditions did not produce the expected tetrahydro-β-carboline but rather directly generated a dehydrogenated β-carboline product 16–22 as reported by Zhang et al. [18]. It is possible that the acidity and polarity of glacial acetic acid promote the reaction of cyclization and dehydrogenation aromatizing to completion continuously. The reaction routes were outlined in Scheme 3. Pictet-Spengler cyclization of substituted tryptamine 5a–d with substituted phenylglyoxal 9, 11 or 15 in acetic acid afforded compounds 16–22, which was transformed into the target compounds Eudistomins Y1–Y7 23–29 in the presence of BBr3 in CH2Cl2 at −1278 °C with the yields of 62%–68%. The reaction routes were outlined in Scheme 3.
Scheme 3.
The synthesis routes of Eudistomins Y1–Y7.
The modified one-pot oxidation reaction shown as the Scheme 3 was more efficient and convenient in preparing 1-substituted β-carbolines without the need of aromatization step or decarboxylation. Using this modified Pictet-Spengler reaction, the target compounds Eudistomins Y1–Y7 23–29 were synthesized.
2.2. Biological Results and Discussion
The in vitro anti-proliferative activity of select compounds 16–29 was evaluated against the breast carcinoma cell line MDA-231 using the 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) metabolic assay. Briefly, exponentially growing cells (1 × 103 cells) were seeded in 96-well plates. After 18 h, cells were continuously treated with compounds 16–29. Following this, after 96 h, cell survival was evaluated. The inhibitory activity (IC50) of the various compounds on cell proliferation was determined (Table 1). Moderate anti-proliferative activity was observed with all the compounds tested. Surprisingly, Eudistomins Y1–Y3 and Y5–Y7 with a hydroxyl group were found to have a poor cytotoxic activity compared with their precursors 16–18 and 20–22. In summary, in vitro inhibitory activities of methylated products were preferable to the demethylated products except for compounds 19 and 26.
Table 1.
Cytotoxicity of eudistomins Y1–Y7 23–29 and derivatives 16–22 (Scheme 3) in vitro a (μM).
| Compound | IC50 (μM) b | Compound | IC50 (μM) b |
|---|---|---|---|
| MDA-231 c | MDA-231 c | ||
| 16 | 31.1 | 23 | 56.3 |
| 17 | 15.8 | 24 | 51.1 |
| 18 | 30.2 | 25 | 63.6 |
| 19 | 31.2 | 26 | 15.6 |
| 20 | 28.1 | 27 | 32.1 |
| 21 | 20.1 | 28 | 28.1 |
| 22 | 25.9 | 29 | 37.2 |
a Data represent the mean values of three independent determinations; b Cytotoxicity as IC50 for each cell line, is the concentration of compound that causes a 50% growth inhibition to untreated cells using the MTT assay; c Breast carcinoma cell lines MDA-231.
In order to determine the intracellular target of these compounds, the inhibitory activity of compound 26 was tested against various kinases in vitro. Briefly, human recombinant full-length kinases were incubated in kinase buffer containing ATP and substrate (Poly Glu:Tyr) for 4 h at room temperature with or without the presence of compounds 26 at 10 μM final concentration. Remaining ATP in solution was then quantified utilizing the Kinase-Glo-luminescence kit (Promega). The ability of compound 26 to inhibit the kinase activity of these kinases was evaluated (Table 2). As such, the anti-proliferative activity of compound 26 does not seem to be significantly modulated by any of the kinases tested.
Table 2.
Inhibitory kinase activity of compound 26 (10 μM) against various kinases.
| Kinase | Aurora B | EGFR | MEK1 | FAK | Src |
|---|---|---|---|---|---|
| Inhibition (%) | 10 | 0 | 3 | 9 | 2 |
3. Experimental Section
3.1. Materials and Methods
The starting materials and reagents, purchased from commercial suppliers, were used without further purification. All reactions were monitored by thin-layer chromatography (TLC), on aluminium sheets (Silica gel 60-F254, E. Merck). Compounds were visualized by UV light. Column chromatography was carried out using silica gel (200–300 mesh). All reaction solvents were dried prior to use according to standard procedures. All primary reagents were commercially available. Silica gel chromatography solvents were of analytical grade. Melting points were recorded on a micro melting point apparatus MP-500D and were uncorrected. NMR spectra were recorded on a Jeol JNM-ECP spectrometer at 600 MHz for 1H NMR and 150 MHz for 13C NMR with TMS as the internal standard. Chemical shifts are expressed in δ (ppm) and coupling constants (J) in Hz. Multiplicity is indicated as follows: s (singlet), d (doublet), t (triplet), dd (doublet of doublets), brs (broad singlet), etc. Mass spectra were recorded using a Q-TOF Ultima™ Global by chemical ionization.
3.2. General Procedure for Compounds 2a–c
In a separate tear-shaped flask under a nitrogen atmosphere, 40 mL of DMF was cooled to 0 °C, and then POCl3 (150 mmol) was added dropwise within 0.5 h under nitrogen. After the addition, stirring was continued for 1.5 h at 0 °C. It was followed by addition of a solution of indole 1a–c (100 mmol) in DMF (20 mL) below 0 °C within 1.5 h. Then the reaction solution was heated to 40 °C for another 2 h and cooled to 0 °C. Subsequently, crushed ice (50 g) was added, and then the pH was adjusted to 8–9 by adding NaOH solution (20%, w/w). The resulting reaction mixture was then heated at reflux for 12 h. And then, the resulting suspension solution was cooled naturally to the room temperature and filtered through celite to afford a white crude product. The crude product was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/hexane (1:3) as the mobile phase to provide the compounds 2a–c [8].
1H-Indole-3-carbaldehyde (2a): White solid; yield 96%; 1H NMR (600 MHz, CDCl3) δ 11.21 (brs, 1H), 10.04 (s, 1H), 8.24 (d, J = 7.8 Hz, 1H), 8.21 (s, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.30–7.23 (m, 2H); 13C NMR (151 MHz, CDCl3) δ 184.6, 137.3, 124.7, 123.7, 122.2, 121.4, 119.2, 112.2.
6-Bromo-1H-indole-3-carbaldehyde (2b): White solid; yield 95%; 1H NMR (600 MHz, CDCl3) δ 11.33 (brs, 1H), 10.03 (s, 1H), 8.24 (s, 1H), 8.15 (d, J = 8.3 Hz, 1H), 7.76 (d, J = 1.8 Hz, 1H), 7.39 (dd, J = 8.3, 1.8 Hz, 1H); 13C NMR (151 MHz, CDCl3) δ 184.7, 137.9, 125.3, 123.6, 122.9, 119.0, 116.5, 115.2.
5-Bromo-1H-indole-3-carbaldehyde (2c): White solid; yield 95%; 1H NMR (600 MHz, CDCl3) δ 11.36 (brs, 1H), 10.02 (s, 1H), 8.39 (d, J = 1.8 Hz, 1H), 8.26 (s, 1H), 7.53 (d, J = 8.7 Hz, 1H), 7.40 (dd, J = 8.7 Hz, 1.8 Hz, 1H); 13C NMR (151 MHz, CDCl3) δ 184.6, 138.2, 136.1, 126.5, 126.4, 123.8, 118.5, 115.3, 114.2.
3.3. General Procedure for Compounds 3a–c
To a solution of 1H-indole-3-carbaldehyde 2a–c (80 mmol) in CH3NO2 (80 mL), was added ammonium acetate (40 mmol) and benzene (1.1 mL, 12.4 mmol). The reaction mixture was heated at reflux for 12 h. After cooling to room temperature, some solvent was removed under reduced pressure and filtered through Celite to afford a yellow crude product. The crude product was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/hexane (1:2) as the mobile phase to provide the compounds 3a–c [9].
(E)-3-(2-Nitrovinyl)-1H-indole (3a): Deep yellow solid; yield 92%; 1H NMR (600 MHz, CDCl3) δ 11.33 (brs, 1H), 8.39 (d, J = 13.3 Hz, 1H), 8.16 (d, J = 3.2 Hz, 1H), 7.97 (dd, J = 5.9, 2.3 Hz, 1H), 7.92 (d, J = 13.3 Hz, 1H), 7.59 (dd, J = 5.9, 2.3 Hz, 1H), 7.32–7.29 (m, 2H); 13C NMR (151 MHz, CDCl3) δ 138.2, 135.1, 133.9, 131.9, 125.1, 123.6, 122.1, 120.5, 112.8, 108.8.
(E)-6-Bromo-3-(2-nitrovinyl)-1H-indole (3b): Deep yellow solid; yield 91%; 1H NMR (600 MHz, CDCl3) δ 11.39 (brs, 1H), 8.36 (d, J = 13.3 Hz, 1H), 8.18 (s, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.91 (d, J = 13.3 Hz, 1H), 7.78 (d, J = 1.8 Hz, 1H), 7.41 (dd, J = 8.3, 1.8 Hz, 1H); 13C NMR (151 MHz, CDCl3) δ 138.9, 135.4, 133.2, 132.7, 124.9, 124.1, 122.0, 116.5, 115.7, 108.8.
(E)-5-Bromo-3-(2-nitrovinyl)-1H-indole (3c): Deep yellow solid; yield 91%; 1H NMR (600 MHz, CDCl3) δ 11.41 (brs, 1H), 8.35 (d, J = 13.7 Hz, 1H), 8.19 (s, 1H), 8.16 (d, J = 1.8 Hz, 1H), 7.97 (d, J = 13.7 Hz, 1H), 7.54 (d, J = 8.7 Hz, 1H), 7.42 (dd, J = 8.7, 1.8 Hz, 1H); 13C NMR (151 MHz, CDCl3) δ 136.7, 135.5, 133.0, 132.7, 126.8, 126.3, 122.9, 115.0, 114.5, 108.3.
3.4. General Procedure for Compounds 4a–c
To a solution of (E)-3-(2-nitrovinyl)-1H-indole 3a–c (20mmol) in THF (60 mL) and CH3OH (9 mL), was added NaBH4 (40 mmol) in batch over 0.5 h. The above reaction solution was stirred at room temperature for about 1 h and the completion of the reaction was monitored by TLC. Then water (100 mL) and hydrochloric acid (100 mL, 10%, v/v) was added slowly. The resulting reaction mixture was extracted with CH2Cl2 (30 mL × 3). The combined organic phase was washed with H2O (20 mL × 3) and brine (20 mL × 3), dried over anhydrous MgSO4 and the solvent was removed under reduced pressure. The residue was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/hexane (1:3) as the mobile phase to provide the compounds 4a–c [10].
3-(2-Nitroethyl)-1H-indole (4a): Brown solid; yield 96%; 1H NMR (600 MHz, CDCl3) δ 8.09 (brs, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.38 (d, J = 8.3 Hz, 1H), 7.25 (t, J = 7.8, 7.3 Hz, 1H), 7.18 (t, J = 8.3, 7.3 Hz, 1H), 7.04 (s, 1H), 4.67 (t, J = 6.9 Hz, 2H), 3.49 (t, J = 6.9 Hz, 2H); 13C NMR (151 MHz, CDCl3) δ 136.3, 126.7, 122.7, 122.6, 119.9, 118.2, 111.6, 110.0, 75.8, 23.7.
6-Bromo-3-(2-nitroethyl)-1H-indole (4b): Brown solid; yield 98%; 1H NMR (600 MHz, CDCl3) δ 8.13 (brs, 1H), 7.49 (d, J = 1.8 Hz, 1H), 7.41 (d, J = 8.2 Hz, 1H), 7.24 (dd, J = 8.2, 1.8 Hz, 1H), 7.00 (d, J = 1.8 Hz, 1H), 4.64 (t, J = 6.9 Hz, 2H), 3.49 (t, J = 6.9 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 137.1, 125.7, 123.3, 123.2, 119.5, 116.1, 114.5, 110.3, 75.8, 23.5.
5-Bromo-3-(2-nitroethyl)-1H-indole (4c): Brown solid; yield 95%; 1H NMR (600 MHz, CDCl3) δ 8.18 (brs, 1H), 7.68 (d, J = 1.8 Hz, 1H), 7.29 (dd, J = 8.7, 1.8 Hz, 1H), 7.22 (d, J = 8.7 Hz, 1H), 7.03 (d, J = 1.8 Hz, 1H), 4.64 (t, J = 7.3 Hz, 2H), 3.41 (t, J = 7.3 Hz, 2H); 13C NMR (151 MHz, CDCl3) δ 134.9, 128.5, 125.4, 124.0, 120.8, 113.2, 113.1, 109.7, 75.7, 23.4.
2-(1H-indol-3-yl)ethanamine (5a): To a solution of 3-(2-nitroethyl)-1H-indole 4a (1.9 g, 10 mmol) in methanol (30 mL), was added 10% Pd/C (0.08 g). H2 was passed in at room temperature, then hydrogenated under normal pressure for 24 h. The completion of the reaction was monitored by TLC. The reaction mixture was then filtered and evaporated under reduced pressure. The residue was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/methanol (10:1) as the mobile phase to provide 2-(1H-indol-3-yl)ethanamine 5a as an white solid in a yield of 98%. 1H NMR (600 MHz, CDCl3) δ 11.04 (brs, 1H), 7.57 (d, J = 7.8 Hz, 1H), 7.42 (d, J = 7.8 Hz, 1H), 7.17 (s, 1H), 7.11 (t, J = 7.8 Hz, 1H), 7.02 (t, J = 7.8 Hz, 1H), 2.89 (t, J = 6.8 Hz, 2H), 2.83 (t, J = 6.8 Hz, 2H), 2.70 (s, 2H); 13C NMR (151 MHz, CDCl3) δ 136.9, 127.9, 123.2, 121.4, 118.9, 113.0, 111.9, 43.1, 29.8.
3.5. General Procedure for Compounds 5b–c
To a solution of substituted 3-(2-nitroethyl)-1H-indole 4b–c (10 mmol) in THF (30 mL), was added LiAlH4 (20 mmol) slowly. After the addition, it was heated at reflux for about 5 h. The completion of the reaction was monitored by TLC. The mixture was allowed to cool to room temperature and quenched by dropwise addition of saturated Na2SO4 solution. The resulting suspension mixture was filtered through Celite, and the filtrate was extracted with EtOAc (30 mL × 3). The combined organic phase was washed with H2O (20 mL × 3) and brine (20 mL × 3), dried over anhydrous MgSO4 and the solvent was removed under reduced pressure. The residue was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/methanol (10:1) as the mobile phase to provide the compounds 5b–c as a gray solid [10].
2-(6-Bromo-1H-indol-3-yl)ethanamine (5b): White solid; yield 92%; 1H NMR (600 MHz, CDCl3) δ 11.08 (brs, 1H), 7.52 (d, J = 1.8 Hz, 1H), 7.47 (d, J = 8.7 Hz, 1H), 7.18 (s, 1H), 7.09 (dd, J = 8.7, 1.8 Hz, 1H), 3.53 (s, 2H), 2.83 (t, J = 6.4 Hz, 2H), 2.77 (t, J = 6.4 Hz, 2H); 13C NMR (151 MHz, CDCl3) δ 137.7, 126.9, 124.4, 121.5, 120.7, 114.5, 114.2, 113.1, 42.6, 28.7.
2-(5-Bromo-1H-indol-3-yl)ethanamine (5c): White solid; yield 88%; 1H NMR (600 MHz, CDCl3) δ 11.17 (brs, 1H), 7.75 (d, J = 1.8 Hz, 1H), 7.31–7.21 (m, 2H), 7.16 (s, 1H), 3.51 (s, 2H), 2.83 (t, J = 6.4 Hz, 2H), 2.74 (t, J = 6.4 Hz, 2H); 13C NMR (151 MHz, CDCl3) δ 135.4, 129.7, 124.9, 124.0, 123.7, 121.1, 113.9, 111.2, 42.4, 28.5.
1-(3,5-Dibromo-4-hydroxyphenyl)ethanone (7): To a suspended mixture of 1-(4-hydroxyphenyl)ethanone (4.08 g, 30 mmol) and deionized water (200 mL), was added N-Bromosuccinimide (8.01 g, 45 mmol). The reaction mixture was heated to 60 °C, then 40% (v/v) H2SO4 (20 mL) was added. After stirred for about 10 h and the completion of the reaction was monitored by TLC, the reaction mixture was filtered through Celite to afford a white crude product. The crude product was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/hexane (1:3) as the mobile phase to provide a compound 1-(3,5-dibromo-4-hydroxyphenyl)ethanone (7) as a white solid in the yield of 92%.
1-(3,5-Dibromo-4-methoxyphenyl)ethanone (8): To a solution of 7 (20 mmol) in anhydrous acetone (50 mL) were added potassium carbonate (30.0 mmol) and dimethyl sulfate (2.84 mL, 30.0 mmol), and refluxed for 2 h. After cooled to room temperature, the reaction mixture was filtered through Celite, and the filtrate was concentrated under vacuum. The residue was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/hexane (1:2) as the mobile phase to provide the desired compound 8 as a white solid in the yield of 95%. 1H NMR (600 MHz, CDCl3) δ 8.08 (s, 2H), 3.93 (s, 3H), 2.56 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 194.5, 158.1, 135.1, 133.0, 118.7, 60.9, 26.6.
3.6. General Procedure for Compounds 9, 11, 15
To a solution of dioxane/deionized water (20:1) (30 mL) was added SeO2 (12 mmol). The mixture was heated to 80 °C and stirred until the solid dissolved. It was followed by addition of substituted phenylethanone 8, 10, 14 (10 mmol), and was refluxed for about 12 h and the completion of the reaction was monitored by TLC. The hot solution was filtered through Celite, and the filtrate was concentrated under vacuum. The residue was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/hexane (1:3) as the mobile phase to provide the desired compound 9, 11, 15 [11].
2-(3,5-Dibromo-4-methoxyphenyl)-2-oxoacetaldehyde (9): Light brown solid; yield 71%; 1H NMR (600 MHz, CDCl3) δ 9.42 (s, 1H), 8.28 (s, 1H), 8.22 (s, 1H), 3.85 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 193.6, 191.9, 157.9, 134.5, 132.3, 118.3, 61.2.
2-(4-Methoxyphenyl)-2-oxoacetaldehyde (11): Light brown solid; yield 75%; 1H NMR (600 MHz, CDCl3) δ 9.53 (s, 1H), 8.06 (d, J = 8.7 Hz, 2H), 7.02 (d, J = 8.7 Hz, 2H), 3.83 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 195.2, 192.5, 164.0, 132.4, 126.7, 114.4, 56.1.
2-(3-Bromo-4-methoxyphenyl)-2-oxoacetaldehyde (15): Light brown solid; yield 74%; 1H NMR (600 MHz, CDCl3) δ 9.43 (s, 1H), 8.28 (s, 1H), 8.09 (d, J = 8.7 Hz, 1H), 7.21 (d, J = 8.7 Hz, 1H), 3.93 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 194.4, 192.6, 159.9, 134.8, 131.7, 127.8, 112.7, 110.9, 57.2.
1-(3-Bromo-4-methoxyphenyl)ethanone (14): To a suspension mixture of anhydrous AlCl3 (3.0 g, 22.5 mmol) in CS2 (50 mL) , was added 1-bromo-2-methoxybenzene (2.79 g, 15 mmol). The above mixture was maintained at room temperature and stirred for 0.5 h, and then acetyl chloride (1.6 mL, 22.5 mol) was added dropwise. After the addition, stirring was continued for another 0.5 h. And then, the reaction mixture was refluxed for 2 h. After cooled to room temperature, it was poured into 80 mL of ice-water containing 20 mL of concentrated hydrochloric acid and stirred to reach room temperature. The mixture was extracted with CH2Cl2 (20 mL × 3). The combined organic phase was washed with H2O (20 mL × 3), 10% aqueous NaOH (10 mL × 2), H2O (20 mL × 3) and brine (20 mL × 3), dried over anhydrous MgSO4 and the solvent was removed under reduced pressure. The residue was purified by recrystallization from petroleum ether (20 mL) to afford 1-(3-bromo-4-methoxyphenyl)ethanone (14) as a brown solid in the yield of 76%.
3.7. General Procedure for Compounds 16–22
To a solution of substituted phenylglyoxal 9, 11, 15 (3 mmol) in AcOH (30 mL), was added substituted tryptamine 5a–d (3 mmol). The above mixture was heated at 90 °C for 10 h, then cooled and adjusted pH to 5 by adding concentrated ammonium hydroxide. The resulting mixture was diluted with 100 mL of water and extracted with EtOAc (30 mL × 3). The combined organic phase was washed with H2O (20 mL × 3) and brine (20 mL × 3), dried over anhydrous MgSO4 and the solvent was removed under reduced pressure. The residue was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/hexane (1:3) as the mobile phase to provide the desired compounds 16–22.
(4-Methoxyphenyl)(9H-pyrido[3,4-b]indol-1-yl)methanone (16): Yellow solid; yield 35%; mp 182–183 °C; 1H NMR (600 MHz, CDCl3) δ 10.46 (brs, 1H), 8.60 (d, J = 5.0 Hz, 1H), 8.45 (d, J = 8.7 Hz, 2H), 8.17 (d, J = 7.8 Hz, 1H), 8.15 (d, J = 5.0 Hz, 1H), 7.62–7.58 (m, 2H), 7.34 (t, J = 7.8 Hz, 1H), 7.03 (d, J = 8.7 Hz, 2H), 3.91 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 193.7, 163.4, 141.1, 138.0, 137.4, 137.1, 134.0, 131.7, 130.4, 129.4, 122.0, 121.0, 120.8, 118.4, 113.6, 112.1, 55.7; HRMS: m/z calcd. for C19H15N2O2+, 303.1133; found: 303.1130.
(6-Bromo-9H-pyrido[3,4-b]indol-1-yl)(4-methoxyphenyl)methanone (17): Yellow solid; yield 36%; mp 194–195 °C; 1H NMR (600 MHz, CDCl3) δ 10.48 (brs, 1H), 8.62 (d, J = 5.0 Hz, 1H), 8.45 (d, J = 8.7 Hz, 2H), 8.29 (d, J = 1.8 Hz, 1H), 8.10 (d, J = 5.0 Hz, 1H), 7.68 (dd, J = 8.7, 1.8 Hz, 1H), 7.48 (d, J = 8.7 Hz, 1H), 7.04 (d, J = 8.7 Hz, 2H), 3.92 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 193.4, 163.5, 139.7, 138.3, 137.6, 137.4, 134.0, 132.1, 130.5, 130.1, 124.7, 122.8, 120.8, 118.5, 113.6, 113.5, 55.7; HRMS: m/z calcd. for C19H14N2O2Br+, 381.0239; found: 381.0237.
(3-Bromo-4-methoxyphenyl)(9H-pyrido[3,4-b]indol-1-yl)methanone (18): Yellow solid; yield 36%; mp 192–193 °C; 1H NMR (600 MHz, CDCl3) δ 10.40 (brs, 1H), 8.69 (d, J = 1.8 Hz, 1H), 8.59 (d, J = 5.0 Hz, 1H), 8.49 (dd, J = 8.7, 2.2 Hz, 1H), 8.15 (m, 2H), 7.60 (m, 2H), 7.35–7.33 (m, 1H), 7.01 (d, J = 8.7 Hz, 1H), 3.99 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 192.2, 159.4, 141.1, 138.1, 137.5, 136.9, 136.5, 133.1, 131.8, 131.4, 129.5, 122.0, 120.9, 118.7, 112.2, 111.5, 110.9, 56.6; HRMS: m/z calcd. for C19H14N2O2Br+, 381.0239; found: 381.0220.
(3-Bromo-4-methoxyphenyl)(6-bromo-9H-pyrido[3,4-b]indol-1-yl)methanone (19): Yellow solid; yield 31%; mp 195–196 °C; 1H NMR (600 MHz, CDCl3) δ 10.50 (brs, 1H), 8.68 (d, J = 1.8 Hz, 1H), 8.59 (d, J = 5.0 Hz, 1H), 8.47 (dd, J = 8.7, 2.2 Hz, 1H), 8.26 (s, 1H), 8.08 (d, J = 5.0 Hz, 1H), 7.66 (dd, J = 8.7, 2.2 Hz, 1H), 7.46 (d, J = 8.7 Hz, 1H), 7.00 (d, J = 8.7 Hz, 1H), 3.99 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 191.9, 159.5, 139.6, 138.3, 137.5, 136.8, 133.2, 132.2, 131.1, 130.7, 124.7, 122.7, 118.7, 113.6, 111.5, 110.9, 56.6; HRMS: m/z calcd. for C19H13N2O2Br2+, 458.9344; found: 458.9327.
(3,5-Dibromo-4-methoxyphenyl)(9H-pyrido[3,4-b]indol-1-yl)methanone (20): Yellow solid; yield 38%; mp 197–198 °C; 1H NMR (600 MHz, CDCl3) δ 10.38 (brs, 1H), 8.62 (d, J = 5.0 Hz, 1H), 8.59 (s, 1H), 8.20–8.18 (m, 1H), 7.65–7.62 (m, 2H), 7.38–7.36 (m, 1H), 3.98 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 191.5, 157.5, 141.2, 138.4, 137.5, 136.0, 135.6, 132.1, 129.7, 122.1, 121.2, 120.9, 119.2, 118.1, 112.2, 60.9; HRMS: m/z calcd. for C19H13N2O2Br2+, 458.9344; found: 458.9333.
(6-Bromo-9H-pyrido[3,4-b]indol-1-yl)(3,5-dibromo-4-methoxyphenyl)methanone (21): Yellow solid; yield 35%; mp 234–235 °C; 1H NMR (600 MHz, CDCl3) δ 10.41 (brs, 1H), 8.64 (d, J = 5.0 Hz, 1H), 8.60 (s, 2H), 8.32 (d, J = 1.8 Hz, 1H), 8,16 (d, J = 5.0 Hz, 1H), 7.72 (dd, J = 8.7, 1.8 Hz, 1H), 7.52 (d, J = 8.7 Hz, 1H), 3.99 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 191.3, 157.6, 139.7, 138.7, 137.6, 136.1, 135.4, 134.1, 132.5, 131.0, 124.9, 122.7, 119.3, 118.1, 114.0, 113.8, 60.9; HRMS: m/z calcd. for C19H12N2O2Br3+, 536.8449; found: 536.8442.
(7-Bromo-9H-pyrido[3,4-b]indol-1-yl)(3,5-dibromo-4-methoxyphenyl)methanone (22): Yellow solid; yield 36%; mp 216–217 °C; 1H NMR (600 MHz, CDCl3) δ 10.38 (brs, 1H), 8.63 (d, J = 5.0 Hz, 1H), 8.59 (s, 2H), 8,16 (d, J = 5.0 Hz, 1H), 8.03 (d, J = 8.2 Hz, 1H), 7.78 (d, J = 1.8 Hz, 1H), 7.48 (dd, J = 8.7, 1.8 Hz, 1H), 3.98 (s, 3H); 13C NMR (151 MHz, CDCl3) δ 191.3, 157.7, 141.9, 138.9, 137.5, 136.1, 135.4, 131.5, 124.7, 123.5, 123.2, 119.9, 119.1, 118.1 115.4, 60.9; HRMS: m/z calcd. for C19H12N2O2Br3+, 536.8449; found: 536.8464.
3.8. General Procedure for Compounds 23–29
To a solution of the compounds 16–22 (0.5 mmol) in CH2Cl2 (10mL) at −78 °C under argon atmosphere, was slowly added dropwise BBr3 (5 mmol). The reaction mixture was stirred and warmed to r.t. and stirred for 24 h. NaOH solution (5 mL, 2 mol/L) was then slowly added dropwise. After addition, a short period of stirring was continued, and then the solution was acidified with hydrochloric acid (20 mL, 2 mol/L), followed by extraction with CH2Cl2 (30 mL × 3). The combined organic phase was washed with H2O (20 mL × 3) and brine (20 mL × 3), dried over anhydrous MgSO4 and the solvent was removed under reduced pressure. The residue was purified by flash column chromatography using silica gel as the stationary phase and using ethyl acetate/hexane (1:3) as the mobile phase to provide the desired compounds eudistomins Y1–Y7 23–29.
(4-Hydroxyphenyl)(9H-pyrido[3,4-b]indol-1-yl)methanone (Eudistomins Y1 23): Yellow solid; yield 65%; mp 217–218 °C; 1H NMR (600 MHz, acetone-d6) δ 11.30(s, 1H), 9.26 (s, 1H), 8.56 (d, J = 5.0 Hz, 1H), 8.47 (d, J = 8.8 Hz, 2H), 8.38 (d, J = 5.0 Hz, 1H), 8.32 (d, J = 7.8 Hz, 1H), 7.88 (d, J = 7.8 Hz, 1H), 7.62 (t, J = 7.8 Hz, 1H), 7. 34 (t, J = 7.8 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H); 13C NMR (151 MHz, acetone-d6) δ 192.0, 163.1, 142.5, 139.0, 138.4, 137.6, 135.0, 131.0, 130.2, 129.4, 122.7, 122.1, 121.3, 119.7, 115.6, 113.5; HRMS: m/z calcd. for C18H11N2O2−, 287.0821; found: 287.0830.
(6-Bromo-9H-pyrido[3,4-b]indol-1-yl)(4-hydroxyphenyl)methanone (Eudistomins Y2 24): Yellow solid; yield 68%; mp 247–248 °C; 1H NMR (600 MHz, acetone-d6) δ 11.46 (s, 1H), 9.30 (s, 1H), 8.60 (d, J = 5.0 Hz, 1H), 8.53 (d, J = 1.8 Hz, 1H), 8.47 (d, J =8.8 Hz, 2H), 8.43 (d, J = 5.0 Hz, 1H), 7.88 (d, J = 8.8 Hz, 1H), 7.76 (dd, J = 8.8, 1.8 Hz, 1H), 7.08 (d, J = 8.8 Hz, 2H); 13C NMR (151 MHz, acetone-d6) δ 192.2, 162.8, 141.1, 139.0, 138.5, 137.5, 135.1, 132.3, 131.1, 130.0, 125.4, 123.5, 119.1, 115.5, 113.5, 112.0; HRMS: m/z calcd. for C18H10N2O2Br−, 364.9926; found: 364.9925.
(3-Bromo-4-hydroxyphenyl)(9H-pyrido[3,4-b]indol-1-yl)methanone (Eudistomins Y3 25): Yellow solid; yield 63%; mp 231–232 °C; 1H NMR (600 MHz, DMSO-d6) δ 11.99 (s, 1H), 11.28 (s, 1H), 8.57 (d, J = 2.2 Hz, 1H), 8.54 (d, J = 4.4 Hz, 1H), 8.43 (d, J = 4.4 Hz, 1H), 8.32 (d, J = 7.7 Hz, 1H), 8.24 (dd, J = 8.8, 2.2 Hz, 1H), 7.79 (d, J = 8.8 Hz, 1H), 7.60 (t, J = 7.7 Hz, 1H), 7.31 (t, J = 7.7 Hz, 1H), 7.13 (d, J = 7.7 Hz, 1H); 13C NMR (151 MHz, DMSO-d6) δ 190.1, 158.2, 141.6, 137.0, 136.7, 136.4, 135.8, 132.5, 131.0, 129.6, 128.9, 121.8, 120.1, 120.0, 118.7, 115.6, 112.9, 108.8; HRMS: m/z calcd. for C18H10N2O2Br−, 364.9926; found: 364.9932.
(3-Bromo-4-hydroxyphenyl)(6-bromo-9H-pyrido[3,4-b]indol-1-yl)methanone (Eudistomins Y4 26): Yellow solid; yield 66%; mp 265–266 °C; 1H NMR (600 MHz, DMSO-d6) δ 12.13 (s, 1H), 11.30 (s, 1H), 8.61 (s, 1H), 8.57 (d, J = 5.5 Hz, 1H), 8.56 (s, 1H), 8.50 (d, J = 5.5 Hz, 1H), 8.23 (dd, J = 8.8, 2.2 Hz, 1H), 7.75–7.72 (m, 1H), 7.13 (d, J = 8.8 Hz, 1H); 13C NMR (151 MHz, DMSO-d6) δ 189.9, 158.3, 140.3, 137.3, 137.1, 136.4, 135.9, 132.5, 131.4, 129.9, 129.4, 124.5, 122.0, 119.1, 115.6, 114.9, 112.2, 108.8; HRMS: m/z calcd. for C18H9N2O2Br2−, 442.9031; found: 442.9012.
(3,5-Dibromo-4-hydroxyphenyl)(9H-pyrido[3,4-b]indol-1-yl)methanone (Eudistomins Y5 27): Yellow solid; yield 66%; mp 267–268 °C; 1H NMR (600 MHz, DMSO-d6) δ 12.04 (s, 1H), 10.96 (s, 1H), 8.57 (d, J = 5.5 Hz, 1H), 8.54 (s, 2H), 8.47 (d, J = 5.5 Hz, 1H), 8.33 (d, J = 7.3 Hz, 1H), 7.81 (d, J = 8.2 Hz, 1H), 7.61 (t, J = 7.3 Hz, 1H), 7.32 (t, J = 7.3 Hz, 1H); 13C NMR (151 MHz, DMSO-d6) δ 188.9, 154.5, 141.7, 137.1, 136.0, 135.9, 135.4, 131.2, 131.0, 129.0, 121.9, 120.3, 120.0, 119.1, 113.0, 110.9; HRMS: m/z calcd. for C18H9N2O2Br2−, 442.9031; found: 442.9030.
(6-Bromo-9H-pyrido[3,4-b]indol-1-yl)(3,5-dibromo-4-hydroxyphenyl)methanone (Eudistomins Y6 28): Yellow solid; yield 62%; mp 277–278 °C; 1H NMR (600 MHz, DMSO-d6) δ 12.16 (s, 1H), 10.97 (brs, 1H), 8.61 (s, 1H), 8.59 (d, J = 4.4 Hz, 1H), 8.52 (s, 2H), 8.51 (d, J = 4.4 Hz, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.73 (dd, J = 8.8, 2.2 Hz, 1H); 13C NMR (151 MHz, DMSO-d6) δ 188.8, 154.6, 140.4, 137.4, 136.4, 136.0, 135.4, 131.5, 130.8, 130.1, 124.5, 122.0, 119.6, 115.0, 112.3, 110.9; HRMS: m/z calcd. for C18H8N2O2Br3−, 520.8136; found: 520.8134.
(7-Bromo-9H-pyrido[3,4-b]indol-1-yl)(3,5-dibromo-4-hydroxyphenyl)methanone (Eudistomins Y7 29): Yellow solid; yield 62%; mp 297–298 °C; 1H NMR (600 MHz, DMSO-d6) δ 12.11 (s, 1H), 10.96 (brs, 1H), 8.58 (d, J = 4.6 Hz, 1H), 8.53 (s, 2H), 8.46 (d, J = 4.6 Hz, 1H), 8.27 (d, J = 8.2 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H), 7.45 (dd, J = 8.2, 1.8 Hz, 1H); 13C NMR (151 MHz, DMSO-d6) δ 188.8, 154.6, 142.5, 137.7, 136.3, 136.0, 135.4, 130.8, 130.6, 123.7, 123.2, 121.8, 119.2, 115.6, 110.8; HRMS: m/z calcd. for C18H8N2O2Br3−, 520.8136; found: 520.8141.
4. Conclusion
In summary, we reported the synthesis of the natural β-carboline alkaloid Eudistomins Y1–Y7 using the modified Pictet-Spengler reaction in a one-pot process. The preliminary biological activities of the target marine alkaloids and their methylated productions 16–22 were studied.
Acknowledgments
This work was supported by Natural Science Foundation of China (Grant No. 21171154) and Special Fund for Marine Scientific Research in the Public Interest (01005024).
References
- 1.Panarese J.D., Waters S.P. Room-temperature aromatization of tetrahydro-β-carbolines by 2-iodoxybenzoic acid: Utility in a total synthesis of Eudistomin U. Org. lett. 2010;12:4086–4089. doi: 10.1021/ol101688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S.F., Zhang Y., Li Y., Li X.R., Kong L.M., Tan C.J., Li S.L., Di Y.T., He H.P., Hao X.J. β-Carboline alkaloids from the leaves of Trigonostemon lii Y.T. Chang. Bioorg. Med. Chem. Lett. 2012;22:2296–2299. doi: 10.1016/j.bmcl.2012.01.106. [DOI] [PubMed] [Google Scholar]
- 3.Wei L., Xiao S.L., Yang M. The syntheses of β-carboline-3-carboxamides derivatives and their interaction with DNA. J. Chin. Pharm. Sci. 2001;10:119–123. [Google Scholar]
- 4.Patel K., Gadewar M., Tripathi R., Prasad S.K., Patel D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of β-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012;2:660–664. doi: 10.1016/S2221-1691(12)60116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W.H., Nam S.J., Lee B.C., Kang H. β-Carboline alkaloids from a Korean tunicate Eudistoma sp. J. Nat. Prod. 2008;71:163–166. doi: 10.1021/np070064o. [DOI] [PubMed] [Google Scholar]
- 6.Won T.H., Jeon J., Lee S., Rho B.J., Oh K., Shin J. β-carboline alkaloids derived from the ascidian Synoicum sp. Bioorg. Med. Chem. 2012;20:4082–4087. doi: 10.1016/j.bmc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy J.P., Breininger M.L., Lindsley C.W. Total synthesis of Eudistomins Y1–Y6. Tetrahedron Lett. 2009;50:7067–7069. doi: 10.1016/j.tetlet.2009.09.180. [DOI] [Google Scholar]
- 8.Wager C.A.B., Miller S.A. Two robust, efficient syntheses of [phenyl ring-U-14C]indole through use of [phenyl ring-U-14C]aniline. J. Labelled Compd. Radiopharm. 2006;49:615–622. [Google Scholar]
- 9.Mor M., Rivara S., Silva C., Bordi F., Plazzi P.V. Melatonin receptor ligands: Synthesis of new melatonin derivatives and comprehensive comparative molecular field analysis (CoMFA) study. J. Med. Chem. 1998;41:3831–3844. doi: 10.1021/jm9810093. [DOI] [PubMed] [Google Scholar]
- 10.Magnus P., Gazzard L., Hobson L., Payne A.H., Rainey T.J., Westlund N., Lynch V. Synthesis of the Kopsia alkaloids (±)-pauciflorine B, (±)-lahadinine B, (±)-kopsidasine, (±)-kopsidasine-N-oxide, (±)-kopsijasminilam and (±)-11-methoxykopsilongine. Tetrahedron. 2002;58:3423–3443. doi: 10.1016/S0040-4020(02)00243-0. [DOI] [Google Scholar]
- 11.Bhella S.S., Elango M., Ishar M.P.S. Design, synthesis and conformational analysis of turn inducer cyclopropane scaffolds: Microwave assisted amidation of unactivated esters on catalytic solid support to obtain turn mimic scaffolds. Tetrahedron. 2009;65:240–246. doi: 10.1016/j.tet.2008.10.058. [DOI] [Google Scholar]
- 12.Pictet A., Spengler T. Formation of isoquinoline derivatives by the action of methylal on phenylethylamine, phenylalanine and tyrosine. Ber. Dtsch. Chem. Ges. 1911;44:2030–2036. doi: 10.1002/cber.19110440309. [DOI] [Google Scholar]
- 13.Cox E.D., Cook J.M. The Pictet-Spengler condensation: A new direction for an old reaction. Chem. Rev. 1995;95:1797–1842. doi: 10.1021/cr00038a004. [DOI] [Google Scholar]
- 14.Yang M.L., Kuo P.C., Damu A.G., Chang R.J., Chiou W.F., Wu T.S. A versatile route to the synthesis of 1-substituted β-carbolines by a single step Pictet-Spengler cyclization. Tetrahedron. 2006;62:10900–10906. doi: 10.1016/j.tet.2006.08.081. [DOI] [Google Scholar]
- 15.Duggineni S., Sawant D., Saha B., Kundu B. Application of modified Pictet-Spengler reaction for the synthesis of thiazolo- and pyrazolo-quinolines. Tetrahedron. 2006;62:3228–3241. doi: 10.1016/j.tet.2006.01.063. [DOI] [Google Scholar]
- 16.Kuivila H. Reduction of phthalyl and succinyl dichlorides with tri-n-butyltin hydride: Cyclization of γ-oxoacyl chlorides. J. Org. Chem. 1960;25:284–285. doi: 10.1021/jo01072a619. [DOI] [Google Scholar]
- 17.Garg N.K., Sarpong R., Stoltz B.M. The first total synthesis of Dragmacidin D. J. Am. Chem. Soc. 2002;124:13179–13184. doi: 10.1021/ja027822b. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P.Y., Sun X.F., Xu B., Bijian K., Wan S.B., Li G.G., Alaoui-Jamali M., Jiang T. Total synthesis and bioactivity of the marine alkaloid pityriacitrin and some of its derivatives. Eur. J. Med. Chem. 2011;46:6089–6097. doi: 10.1016/j.ejmech.2011.10.036. [DOI] [PubMed] [Google Scholar]