Abstract
In this work, we investigated the spasmolytic effect of caulerpine, a bisindole alkaloid isolated from marine algae of the Caulerpa genus, on guinea pig ileum. Our findings indicated that caulerpine inhibited phasic contractions induced by carbachol (IC50 = 7.0 ± 1.9 × 10−5 M), histamine (IC50 = 1.3 ± 0.3 × 10−4 M) and serotonin (IC50 = 8.0 ± 1.4 × 10−5 M) in a non-selective manner. Furthermore, caulerpine concentration-dependently inhibited serotonin-induced cumulative contractions (pD′2 = 4.48 ± 0.08), shifting the curves to the right with Emax reduction and slope of 2.44 ± 0.21, suggesting a noncompetitive antagonism pseudo-irreversible. The alkaloid also relaxed the ileum pre-contracted by KCl (EC50 = 9.0 ± 0.9 × 10−5 M) and carbachol (EC50 = 4.6 ± 0.7 × 10−5 M) in a concentration-dependent manner. This effect was probably due to inhibition of Ca2+ influx through voltage-gated calcium channels (CaV), since caulerpine slightly inhibited the CaCl2-induced contractions in depolarizing medium without Ca2+, shifting the curves to the right and with Emax reduction. According to these results, the spasmolytic effect of caulerpine on guinea pig ileum seems to involve inhibition of Ca2+ influx through CaV. However, other mechanisms are not discarded.
Keywords: caulerpine, guinea pig ileum, spasmolytic effect, Ca2+ channel
1. Introduction
Natural products are a traditional source of drug molecules to treat infectious diseases, pain, and cancer, among others [1,2]. Marine organisms have played a relevant role among the natural products. They make many secondary metabolites with broad chemical diversity and complexity and with great pharmaceutical potential [3,4,5], such as conotoxins from cone snails [6] and compounds from algae [7] and tunicates [8]. Nowadays, there are four marine natural product-derived drugs approved for marketing: cytarabine and vidarabine, nucleosides with anticancer and antiviral properties, respectively; ziconotide, a peptide with potent analgesic activity; and trabectedin, a tetrahydroisoquinoline alkaloid approved for the treatment of soft tissue sarcoma and ovarian carcinoma [9].
Among the class of secondary metabolites in marine organisms, alkaloids are the second most abundant [10]. Indole alkaloids represent a quarter of all alkaloids [11], which are regarded as promising compounds in new drug discovery since they possess novel and complex frameworks [11,12]. Several activities are related to these compounds, including antiviral [13], cytotoxic [14], anti-inflammatory [15,16], antinociceptive [16], calmodulin antagonist [17], muscle relaxant [18], spasmolytic [19] and others.
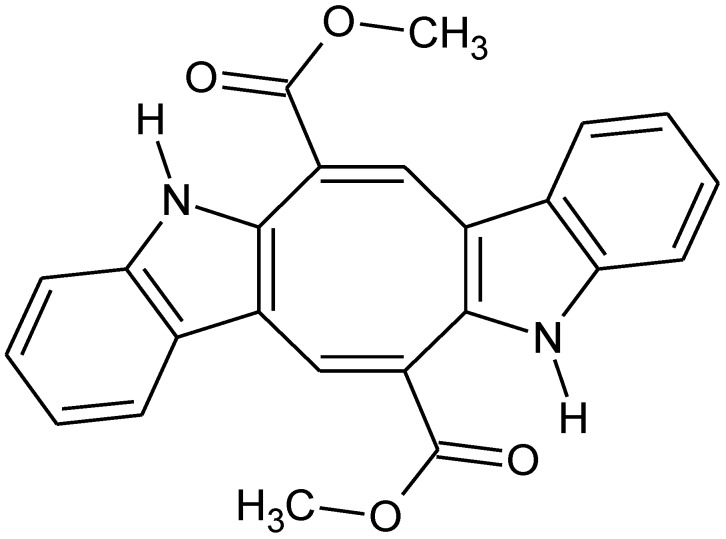
5,12-dihydro-cycloocta[1,2-b;5,6-b′]diindole-6,13-dicarboxylic acid dimethyl ester, called caulerpine or caulerpin (Figure 1), is a bisindole alkaloid which is isolated mainly from green algae of the genus Caulerpa [20,21]. Additionally, its presence was also reported in other green (i.e., Codium decorticatum and Halimeda incrassate) [22,23] and red (i.e., Chondria armata) algae [24]. Since its first isolation in 1968 [20], caulerpine has shown some activities such as antitumor [25], antibacterial [26], inhibitor of human protein tyrosine phosphatase-1B (hPTP1B) [27], inhibitor of hypoxia-inducible factor (HIF) [28], antiviral [29], antinociceptive and anti-inflammatory [16].
Figure 1.
Structure of caulerpine.
Our research group reported some pharmacological activities of marine algae and their secondary metabolites [16,30,31,32]. Recently, we described the antinociceptive activity of caulerpine [16]. Since, Ca2+ is involved in the genesis of the nociceptive process [33] and Ca2+ signaling triggers smooth muscle contraction [34], besides the fact that other indole compounds have a spasmolytic effect [35,36,37], the aim of this work was to investigate the spasmolytic effect of caulerpine on guinea pig ileum.
2. Results and Discussion
In this work, the spasmolytic effect of caulerpine was evaluated in ileal smooth muscle of guinea pig. We demonstrated for the first time a non-selective spasmolytic effect of caulerpine, and that this effect is due in part to the inhibition of Ca2+ influx through voltage-gated calcium channels (CaV).
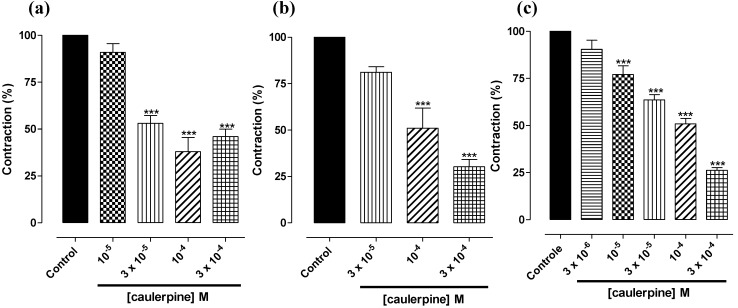
Initially, the caulerpine effect on phasic contractions induced by three different agonists was evaluated. It was observed that caulerpine antagonized phasic contractions induced by 10−6 M carbachol (CCh) (IC50 = 7.0 ± 1.9 × 10−5 M, Emax = 62.0 ± 7.6%), 10−6 M histamine (IC50 = 1.3 ± 0.3 × 10−4 M, Emax = 71.0 ± 4.9%) and 10−5 M serotonin (IC50 = 8.0 ± 1.4 × 10−5 M, Emax = 73.8 ± 1.4%) (Figure 2). A comparison of IC50 values showed that caulerpine had a non-selective effect. Silva et al. [35] has also reported a spasmolytic effect of another indole alkaloid, bisnordihydrotoxiferine, on rat uterus and guinea pig ileum.
Figure 2.
Effect of caulerpine on phasic contractions induced by 10−6 M CCh (a); 10‑6 M histamine (b); and 10−5 M serotonin (c); on guinea pig ileum (n = 5). Columns and vertical bars represent the means ± S.E.M., respectively. Significant differences are indicated by *** p < 0.001.
On guinea pig ileum, the contractile effect of histamine and CCh are mediated by H1 [38] and M3 [39] receptors, respectively. Both receptors are coupled to the heterotrimeric Gq/11 protein, which functions as a transducer to relay information to the inositol 1,4,5-trisphosphate (IP3)/diacylglycerol (DAG) signaling pathway, which in turn triggers an elevation in cytosolic calcium and consequent smooth muscle contraction [38,39,40]. On the other hand, serotonin-induced contraction is mediated by 5-HT2A and 5-HT3 receptors [41,42]. The first is a G protein-coupled receptor (GPCR) that shares the same second messenger system with H1 and M3 receptors. 5-HT3 receptor is a ligand-gated ion channel and it is prone to rapid desensitization. The stimulation of 5-HT3 receptors on enteric cholinergic neurons induces acetylcholine release, resulting in smooth muscle contraction [41,42,43].
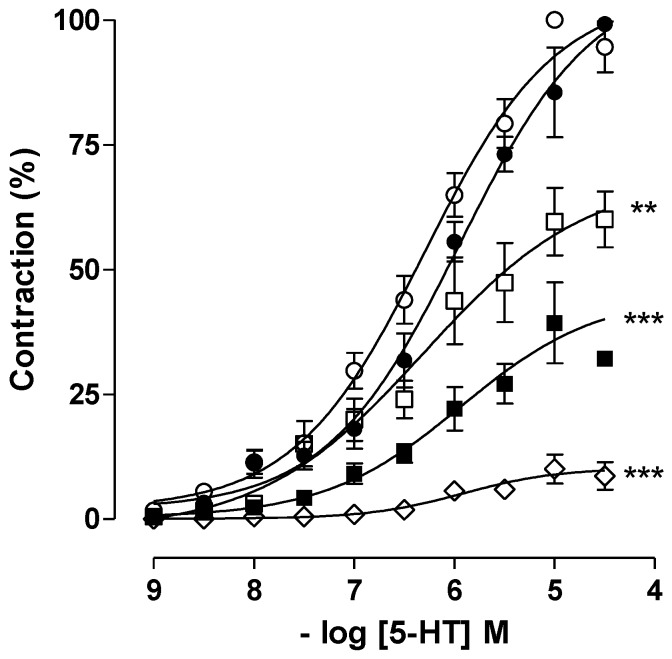
Caulerpine antagonized phasic contractions induced by different agonists in an equipotent manner, which could suggest that this alkaloid does not act at the receptor level to inhibit contraction on guinea pig ileum. To confirm this hypothesis, serotonin-induced cumulative contractions were studied. As observed in Figure 3, caulerpine concentration-dependently inhibited serotonin-induced cumulative contractions and shifted the curves to the right with Emax reduction and slope of 2.13 ± 0.13, discarding thus a competitive type antagonism. The relaxation potency expressed as pD′2 was 4.48 ± 0.04 (Figure 2). Thus, by the antagonism pattern shown in Figure 3, the spasmolytic effect induced by caulerpine is characteristic of noncompetitive antagonism pseudo-irreversible. Furthermore, this result could explain the non-selective effect of caulerpine in relation to the agonists tested. Therefore, it is probable that caulerpine acts in another step of the cascade of events that leads to smooth muscle contraction. In addition, the spasmolytic effect on gastrointestinal smooth muscles through noncompetitive antagonism by the indole alkaloid trinervine was reported by Diniz et al. [36].
Figure 3.
Cumulative concentration-response curves for 5-HT in the absence (○) and presence of caulerpine: 3 × 10−5 (●), 10−4 (□), 3 × 10−4 (■) and 10−3 M (◇) (n = 5). Symbols and vertical bars represent the means and S.E.M., respectively. One-way ANOVA followed by Bonferroni’s test, significant differences are indicated by ** p < 0.01 and *** p < 0.001.
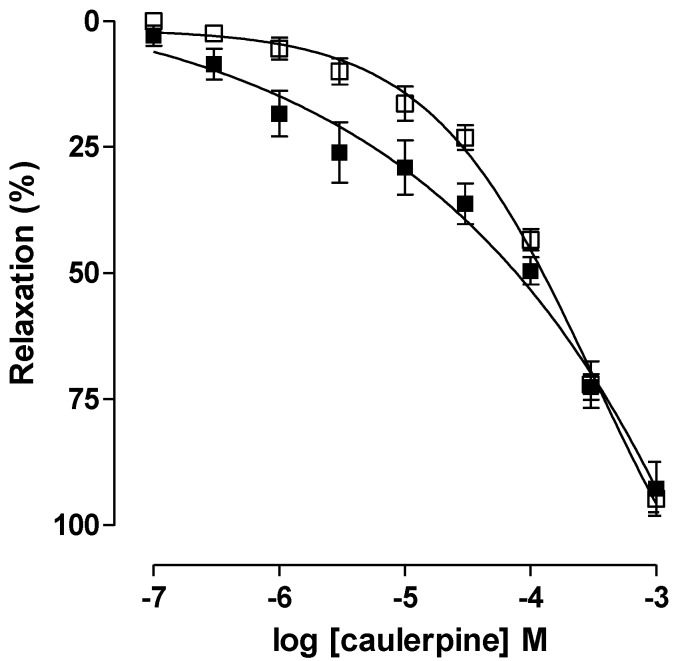
Guinea pig ileum shows biphasic contraction, comprising an initial phase in which the muscle exhibits a fast and transient contraction followed by a long-lasting second phase characterized by a maintained tonic contraction [44,45]. Both phasic and tonic contractions are dependent on extracellular calcium since both are inhibited in its absence [46]. The removal of extracellular Ca2+ prevents contraction induced by depolarizing agents, such as KCl, or by agonists, such as CCh and serotonin, in few seconds, indicating that intracellular Ca2+ does not contribute significantly to the tension level [47]. However, the influence of extracellular Ca2+ is relatively greater in tonic contractile response than in the phasic one [48]. Furthermore, the mechanisms involved in the maintenance of tonic contraction components are different from the phasic ones in guinea pig ileum [46]. Thus, the effect of caulerpine on the tonic contraction component induced by KCl (electromechanical coupling) and CCh (pharmacomechanical and electromechanical coupling) was investigated.
Caulerpine relaxed in a significant and concentration-dependent manner the ileum pre-contracted by 40 mM KCl (EC50 = 9.2 ± 0.9 × 10−5 M, Emax = 94.8 ± 2.6%) and 10−5 M CCh (EC50 = 5.2 ± 0.4 × 10−5 M, Emax = 92.7 ± 5.4) (Figure 4). An analysis of EC50 values indicated that caulerpine was slightly more potent in relax guinea pig ileum contracted by CCh than KCl, only 1.7-fold. The presence of an indole nucleus may be responsible for the relaxant effect, since a study performed with a series of derivatives analogous to Nb-benzoyltryptamine (N-[2-(1H-Indol-3-yl)ethyl]benzamide), sharing the indole nucleus, showed non-selective relaxant activity on guinea pig ileum [37].
Figure 4.
Effect of caulerpine on the tonic contractions induced by 40 mM KCl (□) and 10‑5 M CCh (■) on guinea pig ileum (n = 5). Symbols and vertical bars represent the means and S.E.M., respectively.
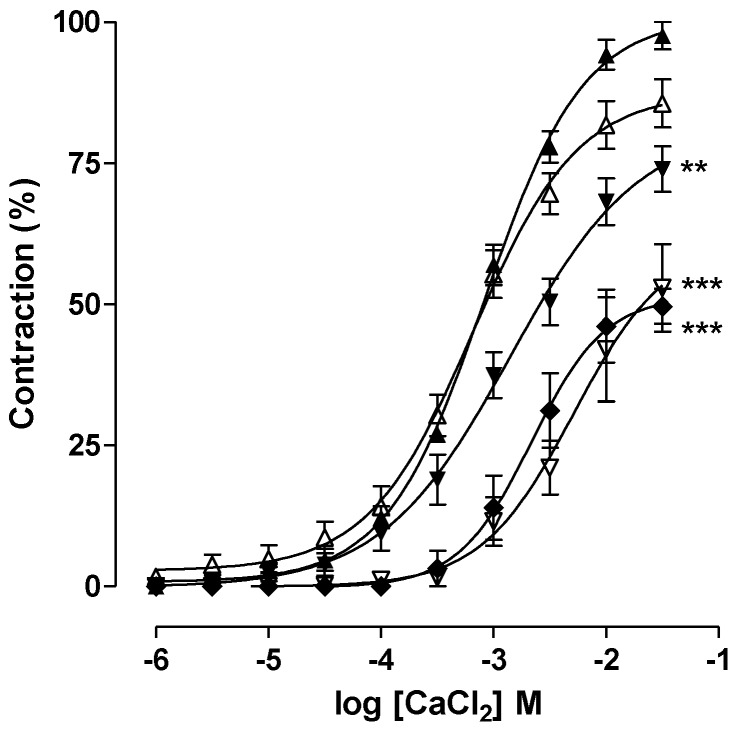
Independently of the contraction being evoked by either pharmacomechanical or electromechanical coupling, the maintenance of the tonic component involves the activation of CaV [49]. Therefore, we can postulate that caulerpine blocks these channels to produce non-selective spasmolytic effects. To evaluate this hypothesis, CaCl2-induced contraction in depolarizing medium nominally without Ca2+ was performed. This hypothesis was confirmed since CaCl2 cumulative concentration-response curves were shifted in a nonparallel way to the right and Emax was reduced from 100% (control) to 85.6 ± 4.2% (3 × 10−5 M), 73.9 ± 4.05% (10−4 M), 52.9 ± 7.7% (3 × 10−4 M) and 52.7 ± 4.5% (10−3 M) in the presence of caulerpine, which indicates a blockade of Ca2+ influx through CaV (Figure 5). Smooth muscle contractions are initiated when [Ca2+]c attains a threshold level [40]. Thus, a decrease in [Ca2+]c is a pivotal factor in smooth muscle relaxation.
Figure 5.
Cumulative concentration-response curves to CaCl2 in depolarizing medium nominally without Ca2+ in the absence (▲) and presence of caulerpine: 3 × 10−5 (△), 10−4 (▼), 3 × 10−4 (▽) and 10−3 M (♦). Symbols and vertical bars represent the means ± S.E.M., respectively. One-way ANOVA followed by Bonferroni’s test, significant differences are indicated by ** p < 0.01 and *** p < 0.001.
Over the last six decades an enormous number of marine natural products have been studied. They are a valuable source of pharmacological tools, since they act on different and specific targets, and are thereby potentially useful leads in the development of new therapies [3,4]. In conclusion, we showed for the first time that caulerpine has a spasmolytic effect on guinea pig ileum through Ca2+ influx blockade. This makes such an alkaloid a potential compound for treating gastrointestinal disorders or a prototype to obtain new compounds acting on Ca2+ signaling.
3. Experimental Section
3.1. Isolation
Caulerpa sertularioides and C. mexicana algae were collected from the coastal region of Cabo Branco, João Pessoa, Paraíba State, Brazil in March 2009. The specimens were identified by Dr. George Emmanuel Cavalcanti de Miranda. Voucher specimens of C. sertularioides (JPB 13983) and C. mexicana (13985) have been deposited in the Lauro Pires Xavier Herbarium at the Federal University of Paraíba (Universidade Federal da Paraíba), Brazil. The alga was extracted with MeOH at room temperature and the extract was partitioned between hexane, dichloromethane, ethyl acetate and metanol. In the ethyl acetate phase there was precipitation of a orange red pigment. On the basis of its NMR spectral data and chemical properties, it was assigned the structure of 5,12-dihydro-cycloocta[1,2-b;5,6-b′]diindole-6,13-dicarboxylic acid dimethyl ester, named caulerpine or caulerpin.
3.2. Solutions and Drugs
Caulerpine was dissolved in Cremophor® and diluted in distilled water. Carbamoylcholine chloride (carbachol), histamine dihydrochloride, serotonin hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were dissolved and diluted in distilled water. The physiological solution was a freshly modified Krebs solution (pH 7.4) with the following composition (mM): NaCl (117.0), KCl (4.7), MgSO4 (1.3), NaH2PO4 (1.2), CaCl2 (2.5), glucose (11.0) and NaHCO3 (25.0). A high K+ isosmotic solution (pH 7.4) with the following composition was also used: NaCl (51.7), KCl (70.0), MgSO4 (1.3), NaH2PO4 (1.2), glucose (11.0) and NaHCO3 (25.0). These salts were obtained from Vetec (Rio de Janeiro, RJ, Brazil) and Fmaia (Cotia, SP, Brazil).
3.3. Animals
Adult guinea pigs (Cavia porcellus) of both sexes from the Professor Thomas George Bioterium of CBiotec/UFPB, weighing 368 ± 8 g, were used. The animals had free access to food and water, were kept in rooms at 21 ± 1 °C with a 12-h light–dark cycle and fasted for 18 h before the experiments. Measures to reduce pain, stress and any suffering were taken in accordance with the ethical guidelines for animal use. All experimental procedures were previously approved and performed in accordance with the Research Ethics Committee of the Federal University of Alagoas (UFAL) guidelines (protocol CEUA 039/2012).
3.4. Measurement of Contraction of Guinea Pig Ileum
Animals were euthanized by cervical dislocation and exsanguination. The distal ileum was immediately removed, cleaned of adhering fat and connective tissue, immersed in modified Krebs solution at room temperature and continuously gassed with carbogen (95% O2 and 5% CO2). Segments of the ileum oriented along the longitudinal axis (2–3 cm in length) were suspended in a 5 mL organ bath, which contained modified Krebs solution, maintained under resting load of 1.0 g at 37 °C. The tissues were allowed to stabilize for 30 min. Isotonic contractions were recorded using isotonic levers coupled to kymographs and smoked drums (DTF, Brazil) (Experiments 3.5.1 and 3.5.2). An isometric transducer (FORT-10) coupled to an amplifier (TMB4M), both from World Precision Instruments (EUA), connected to an analog/digital converter board (Bio Data, Brazil) installed in a computer with BioMed© software version RV2 were used to record isometric contractions (Experiments 3.5.3 and 3.5.4).
3.5. Pharmacological Experiments
3.5.1. Effect of the Caulerpine on CCh-, Histamine- and Serotonin-Induced Phasic Contractions
After a stabilization period, two phasic contractions were obtained for 10−6 M CCh, 10−6 M histamine or 10−5 M serotonin with intervals of 15 min between them. Caulerpine was then added, and after an incubation period of 15 min, a third concentration-response curve was obtained in the presence of various concentrations of caulerpine in different preparations. The procedure was repeated in the absence and in the presence of various concentrations of this alkaloid. The molar concentration of a substance that inhibits the response to an agonist by 50% (IC50) [50] was obtained by non-linear regression from the individual inhibition values for caulerpine.
3.5.2. Effect of Caulerpine on Serotonin-Induced Cumulative Contractions
After a stabilization period, two similar cumulative concentration-response curves for serotonin were induced and caulerpine was incubated, in different preparations, in the absence of serotonin for 15 min in different concentrations as independent experiments. Afterwards, a new serotonin cumulative curve was obtained in the presence of caulerpine (3 × 10−5, 10−4, 3 × 10−4 and 10−3 M). The average amplitude of concentration-response curves for serotonin was considered to be 100% (control) and all contractions were assessed referring to it. Each preparation was exposed to only one caulerpine concentration. The antagonism exerted by caulerpine was evaluated based on the analysis of the Schild plot and their potencies with pD′2 values, which is defined as the negative logarithm to base 10 of molar concentration values of an antagonist that reduces the response to an agonist to 50% of its maximum effect (Emax), assessed through concentration-response curves in both absence (control) and presence of caulerpine [51].
3.5.3. Effect of the Caulerpine on KCl- or CCh-Induced Tonic Contractions
After a stabilization period, an isometric contraction was elicited by 40 mM KCl or 10−5 M CCh. Contractile agents remained in contact with the preparation until a contraction plateau was reached (approximately 10 min), and the tissue was then washed. After 30 min, the process was repeated and caulerpine was added cumulatively (10−7 up to 10−3 M) at the plateau phase. Relaxation was expressed as the reverse percentage of initial contraction elicited by the contractile agents. The molar concentration of a substance that produces 50% of its maximal effect (EC50) was obtained graphically from the concentration-response curves [50].
3.5.4. Effect of Caulerpine on CaCl2-Induced Contractions in Depolarizing Medium Nominally without Ca2+
After the stabilization period, the modified Krebs solution was replaced by a depolarizing (with 70 mM KCl in equimolar exchange for NaCl) and nominally without Ca2+ solution for 45 min. Two similar CaCl2 cumulative concentration-response curves were obtained and caulerpine was incubated for 15 min in the absence of CaCl2. A third CaCl2 cumulative curve was obtained in the presence of caulerpine. The maximal contraction obtained with the first CaCl2 concentration-response curve was considered to be 100% (control), and all contractions were assessed in reference to it. Each preparation was exposed to a single caulerpine concentration.
3.6. Statistical Analysis
Data are expressed as means and S.E.M. EC50 and IC50 values were determined by nonlinear regression [50]. Differences between means were statistically compared using a t-test or one-way ANOVA followed by Bonferroni’s test when appropriate. The significance level considered in all tests was p < 0.05. Schild plots were analyzed by linear regression. Antagonism was considered to be noncompetitive when the slope of the Schild plot was significantly different from unity and depression of the maximum response was observed. All values were obtained using Graph-Pad Prism® 5.01 software (GraphPad Software Inc., SanDiego, CA, USA).
4. Conclusions
In conclusion, we demonstrated that caulerpine has a non-selective spasmolytic effect on guinea pig ileum. At the functional level, this effect is due in part to the inhibition of Ca2+ influx through CaV. However, other mechanisms cannot be excluded.
Acknowledgments
The authors are grateful to CAPES, CNPq, PRONEX/FAPESQ-PB, FAPEAL, INCT-INOFAR/CNPq (573.564/2008-6), INCTAmbTropic and the Ministry of Environment (License MMA/CGEN 18/2007) for the joint funding of this research project. The authors thank their colleagues working at the Federal University of Alagoas and Federal University of Paraíba for their constructive criticism and assistance in carrying out this project. A. Leyva helped with English editing of the manuscript.
References
- 1.Harvey A.L. Natural products in drug discovery. Drug Discov. Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2012;29:144–222. doi: 10.1039/c2np00090c. [DOI] [PubMed] [Google Scholar]
- 4.Molinski T.F., Dalisay D.S., Lievens S.L., Saludes J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009;81:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 5.Lira N.S., Montes R.C., Tavares J.F., da Silva M.S., da Cunha E.V., de Athayde-Filho P.F., Rodrigues L.C., da Silva Dias C., Barbosa-Filho J.M. Brominated compounds from marine sponges of the genus Aplysina and a compilation of their 13C NMR spectral data. Mar. Drugs. 2011;9:2316–2368. doi: 10.3390/md9112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Essack M., Bajic V.B., Archer J.A. Conotoxins that confer therapeutic possibilities. Mar. Drugs. 2012;10:1244–1265. doi: 10.3390/md10061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Almeida C.L., Falcão H.S., Lima G.R., Montenegro C.A., Lira N.S., de Athayde-Filho P.F., Rodrigues L.C., de Souza M.F., Barbosa-Filho J.M., Batista L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011;12:4550–4573. doi: 10.3390/ijms12074550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimenez P.C., Wilke D.V., Ferreira E.G., Takeara R., de Moraes M.O., da Silveira E.R., da Cruz Lotufo T.M., Lopes N.P., Costa-Lotufo L.V. Structure elucidation and anticancer activity of 7-oxostaurosporine derivatives from the Brazilian endemic tunicate Eudistoma vannamei. Mar. Drugs. 2012;10:1092–1102. doi: 10.3390/md10051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer A.M., Glaser K.B., Cuevas C., Jacobs R.S., Kem W., Little R.D., McIntosh J.M., Newman D.J., Potts B.C., Shuster D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Hu G.P., Yuan J., Sun L., She Z.G., Wu J.H., Lan X.J., Zhu X., Lin Y.C., Chen S.P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs. 2011;9:514–525. doi: 10.3390/md9040514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gul W., Hamann M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005;78:442–453. doi: 10.1016/j.lfs.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Güven K.C., Percot A., Sezik E. Alkaloids in marine algae. Mar. Drugs. 2010;8:269–284. doi: 10.3390/md8020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinehart K.L., Kobayashi J., Harbour G.C., Hughes R.G., Mizsak S.A., Scahill T.A. Eudistomins C, E, K and L, potent antiviral compounds containing a novel oxathiazepine ring from Caribbean tunicate Eudistoma olivaceum. J. Am. Chem. Soc. 1984;106:1524–1526. doi: 10.1021/ja00317a079. [DOI] [Google Scholar]
- 14.Kohmoto S., Kashman Y., McConnell O.J., Rinehart K.L., Wright A., Koehn F. Dragmacidin, a new cytotoxic bis (indole) alkaloid from a deep water marine sponge, Dragmacidon sp. J. Org. Chem. 1988;53:3116–3118. doi: 10.1021/jo00248a040. [DOI] [Google Scholar]
- 15.Bobzin S.C., Faulkner D.J. Aromatic alkaloids from the marine sponge Chelonaplysilla sp. J. Org. Chem. 1991;56:4403–4407. doi: 10.1021/jo00014a015. [DOI] [Google Scholar]
- 16.Souza E.T., Lira D.P., Queiroz A.C., Silva D.J.C., Aquino A.B., Mella E.A.C., Lorenzo V.L., Miranda G.E.C., Araújo-Júnior J.X., Chaves M.C.O., et al. The antinociceptive and anti-inflammatory activities of caulerpine, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs. 2009;7:689–704. doi: 10.3390/md7040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi J., Nakamura H., Ohizumi Y., Hirata Y. Eudistomidin-A, a novel calmodulin antagonist from the Okinawan tunicate Eudistoma glaucus. Tetrahedron Lett. 1986;27:1191–1194. doi: 10.1016/S0040-4039(00)84213-6. [DOI] [Google Scholar]
- 18.Christophersen C. Secondary metabolites from marine bryozoans. A review. Acta Chem. Scand. B. 1985;39:517–529. doi: 10.3891/acta.chem.scand.39b-0517. [DOI] [PubMed] [Google Scholar]
- 19.Lidgren G., Bohlin L. Studies of Swedish marine organisms VII. A novel biologically active indole alkaloid from the sponge Geodia baretti. Tetrahedron Lett. 1986;27:3283–3284. doi: 10.1016/S0040-4039(00)84776-0. [DOI] [Google Scholar]
- 20.Aguilar-Santos G., Doty M.S. Chemical Studies on Three Species of the Marine Algal Genus Caulerpa. In: Freudenthal H.D., editor. Drugs from the Sea. Marine Technology Society; Washington, DC, USA: 1968. pp. 173–176. [Google Scholar]
- 21.Aguilar-Santos G. Caulerpine, a new red pigment from green algae of the genus Caulerpa. J. Chem. Soc. C. 1970;6:1842–1843. doi: 10.1039/j39700000842. [DOI] [PubMed] [Google Scholar]
- 22.Anjaneyulu A.S.R., Prakash C.V.S., Mallavadhani U.V. Sterols and terpenes of the marine green algal species Caulerpa racemosa and Codium decorticatum. J. Indian Chem. Soc. 1991;68:480. [Google Scholar]
- 23.Yan S., Su J., Wang Y., Zeng L. Studies on chemical constituents of Halimeda incrassata. Trop. Ocean. 1999;18:91–94. [Google Scholar]
- 24.Govenkar M.B., Wahidulla S. Constituents of Chondria armata. Phytochemistry. 2000;54:979–981. doi: 10.1016/S0031-9422(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 25.Ayyad S.E.N., Badria F.A. Caulerpin: An antitumor indole alkaloid from Caulerpa racemosa. Alex. J. Pharm. Sci. 1994;8:217–219. [Google Scholar]
- 26.Vairappan C.S. Antibacterial activity of major secondary metabolities: Found in four species of edible green macroalgae genus Caulerpa. Asian J. Microbiol. Biotechnol. Environ. Sci. 2004;6:197–201. [Google Scholar]
- 27.Mao S.C., Guo Y.W., Shen X. Two novel aromatic valerenane-type sesquiterpenes from the Chinese green alga Caulerpa taxifolia. Bioorg. Med. Chem. Lett. 2006;16:2947–2950. doi: 10.1016/j.bmcl.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Morgan J.B., Coothankandaswamy V., Liu R., Jekabsons M.B., Mahdi F., Nagle D.G., Zhou Y.D. The Caulerpa pigment caulerpine inhibits HIF-1 activation and mitochondrial respiration. J. Nat. Prod. 2009;72:2104–2109. doi: 10.1021/np9005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macedo N.R.P.V., Ribeiro M.S., Villaça R.C., Ferreira W., Pinto A.M., Teixeira V.L., Cirne-Santos C., Paixão I.C.N.P., Giongo V. Caulerpine as a potential antiviral drug against herpes simplex virus type 1. Braz. J. Pharmacogn. 2012;22:861–867. [Google Scholar]
- 30.Cavalcante-Silva L.H.A., Barbosa Brito da Matta C., de Araújo M.V., Barbosa-Filho J.M., Pereira de Lira D., de Oliveira Santos B.V., de Miranda G.E.C., Alexandre-Moreira M.S. Antinociceptive and anti-inflammatory activities of crude methanolic extract of red alga Bryothamnion triquetrum. Mar. Drugs. 2012;10:1977–1992. doi: 10.3390/md10091977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brito da Matta C.B., de Souza É.T., de Queiroz A.C., de Lira D.P., de Araújo M.V., Cavalcante-Silva L.H.A., de Miranda G.E.C., de Araújo-Júnior J.X., Barbosa-Filho J.M., de Oliveira Santos B.V., et al. Antinociceptive and anti-inflammatory activity from algae of the genus Caulerpa. Mar. Drugs. 2011;9:307–318. doi: 10.3390/md9030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Souza E.T., Queiroz A.C., Miranda G.E.C., Lorenzo V.P., Silva E.F., Freire-Dias T.L.M., Cupertino-Silva Y.K., Melo G.M.A., Chaves M.C.O., Barbosa-Filho J.M., et al. Antinociceptive activities of crude methanolic extract and phases, n-butanolic, chloroformic and ethyl acetate from Caulerpa racemosa (Caulerpaceae) Braz. J. Pharmacogn. 2009;19:115–120. [Google Scholar]
- 33.Sorkin L.S., Wallace M.S. Acute pain mechanisms. Surg. Clin. North Am. 1999;79:213–229. doi: 10.1016/S0039-6109(05)70380-7. [DOI] [PubMed] [Google Scholar]
- 34.Berridge M.J. Smooth muscle cell calcium activation mechanisms. J. Physiol. 2008;586:5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva B.A., de Araújo A.P., Mukherjee R., Chiappeta A.D. Bisnordihydrotoxiferine and vellosimine from Strychnos divaricans root: Spasmolytic properties of bisnordihydrotoxiferine. Phytother. Res. 1993;7:419–424. doi: 10.1002/ptr.2650070607. [DOI] [Google Scholar]
- 36.Diniz M.F.F.M., Silva B.A., Mukherjee R. Spasmolytic actions of the new indole alkaloid trinervine from Strychnos trinervis root. Phytomedicine. 1994;1:205–207. doi: 10.1016/S0944-7113(11)80066-8. [DOI] [PubMed] [Google Scholar]
- 37.Gutierrez S.J., Claudino F.S., Silva B.A., Câmara C.A., Almeida R.N., Souza M.F.V., Silva M.S., da-Cunha E.V., Barbosa-Filho J.M. Nb-benzoyltryptamine derivatives with relaxant activity in guinea-pig ileum. Farmaco. 2005;60:475–477. doi: 10.1016/j.farmac.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Ash A.S., Schild H.O. Receptors mediating some actions of histamine. Br. J. Pharmacol. Chemother. 1966;27:427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehlert F.J., Sawyer G.W., Esqueda E.E. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal smooth muscle. Life Sci. 1999;64:387–394. doi: 10.1016/S0024-3205(98)00584-0. [DOI] [PubMed] [Google Scholar]
- 40.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 41.Engel G., Hoyer D., Kalkman H.O., Wick M.B. Identification of 5HT2-receptors on longitudinal muscle of the guinea pig ileum. J. Recept. Res. 1984;4:113–126. doi: 10.3109/10799898409042543. [DOI] [PubMed] [Google Scholar]
- 42.Fox A., Morton I.K. An examination of the 5-HT3 receptor mediating contraction and evoked [3H]-acetylcholine release in the guinea-pig ileum. Br. J. Pharmacol. 1990;101:553–558. doi: 10.1111/j.1476-5381.1990.tb14119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoyer D., Hannon J.P., Martin G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/S0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 44.Bolton T.B. Mechanism of action of transmitters and other substances on smooth-muscle. Physiol. Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Latif A.A. Calcium-mobilizing receptors, polyphosphoinositides, generation of second messengers and contraction in the mammalian iris smooth muscle: Historical perspectives and current status. Life Sci. 1989;45:757–786. doi: 10.1016/0024-3205(89)90170-7. [DOI] [PubMed] [Google Scholar]
- 46.Honda K., Takano Y., Kamiya H. Involvement of protein kinase C in muscarinic agonist-induced contractions of guinea pig ileal longitudinal muscle. Gen. Pharmacol. 1996;27:957–961. doi: 10.1016/0306-3623(95)02124-8. [DOI] [PubMed] [Google Scholar]
- 47.Nouailhetas V.L., Shimuta S.I., Paiva A.C., Paiva T.B. Calcium and sodium dependence of the biphasic response of the guinea-pig ileum to agonists. Eur. J. Pharmacol. 1985;116:41–47. doi: 10.1016/0014-2999(85)90183-9. [DOI] [PubMed] [Google Scholar]
- 48.Triggle C.R., Swamy V.C., Triggle D.J. Calcium antagonists and contractile responses in rat vas deferens and guinea pig ileal smooth muscle. Can. J. Physiol. Pharmacol. 1979;57:804–818. doi: 10.1139/y79-124. [DOI] [PubMed] [Google Scholar]
- 49.Rembold C.M. Electromechanical and Pharmacomechanical Coupling. In: Bárány M., editor. Biochemistry of Smooth Contraction. Academic Press; San Diego, CA, USA: 1996. pp. 227–239. [Google Scholar]
- 50.Neubig R.R., Spedding M., Kenakin T., Christopoulos A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol. Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- 51.Dunne A. Comparison of individual and cumulative dose-response curves [proceedings] Br. J. Pharmacol. 1979;67:491–492. [PMC free article] [PubMed] [Google Scholar]