Abstract
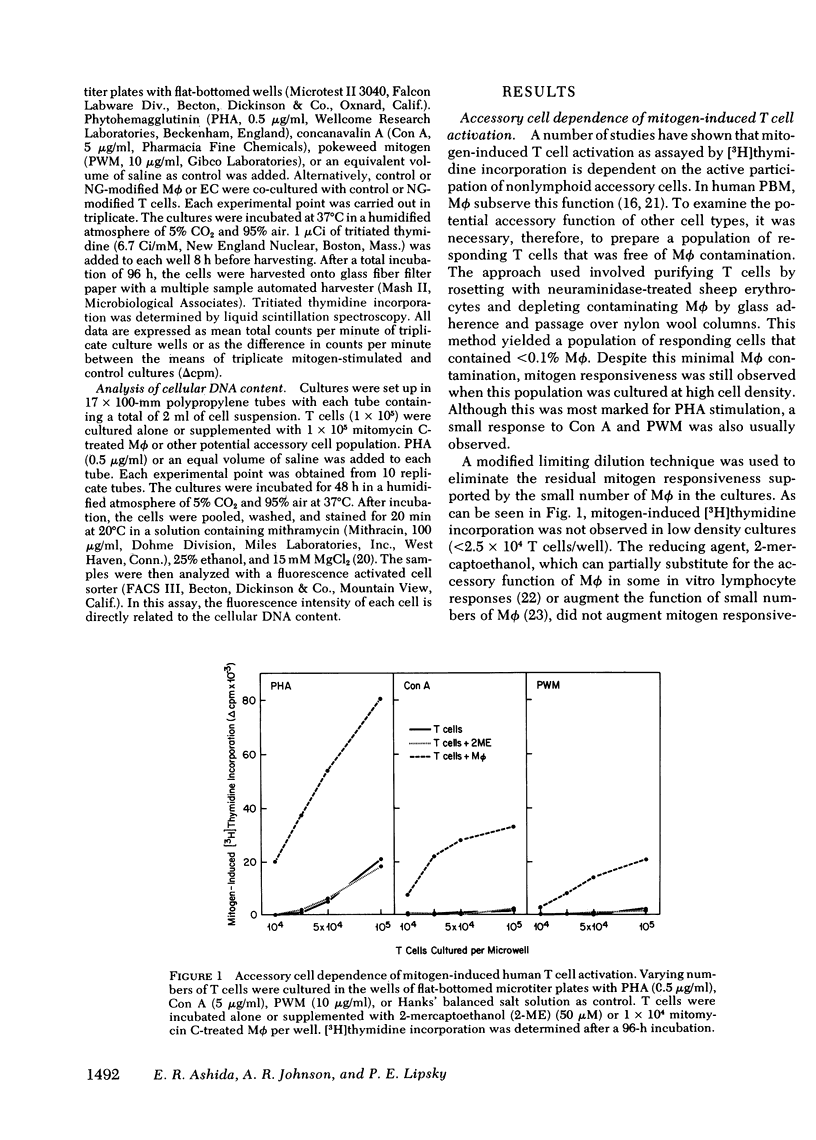
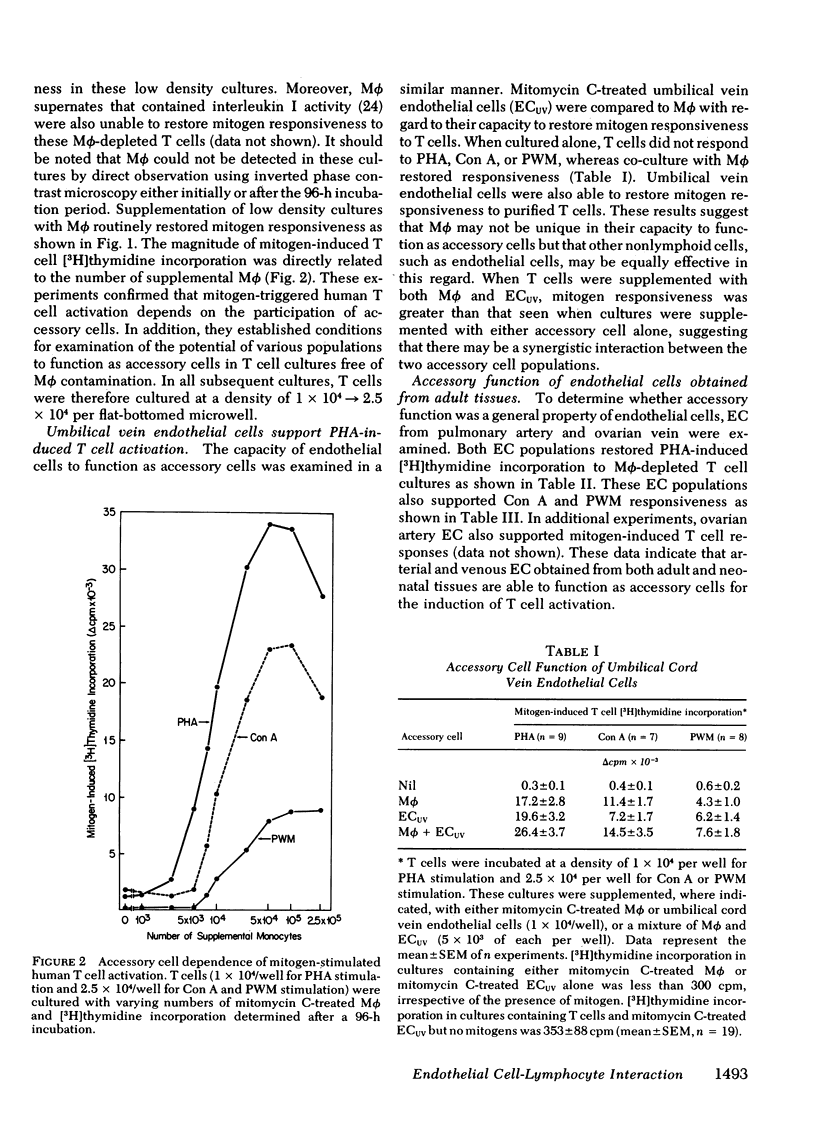
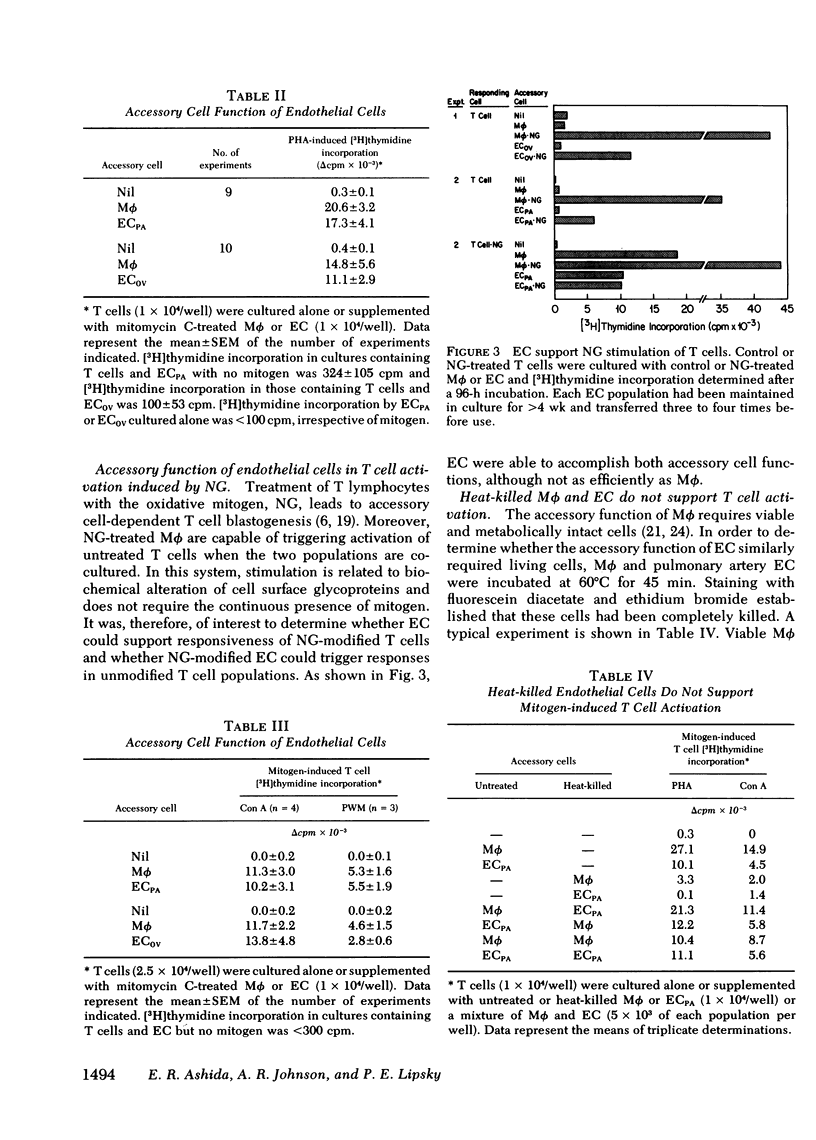
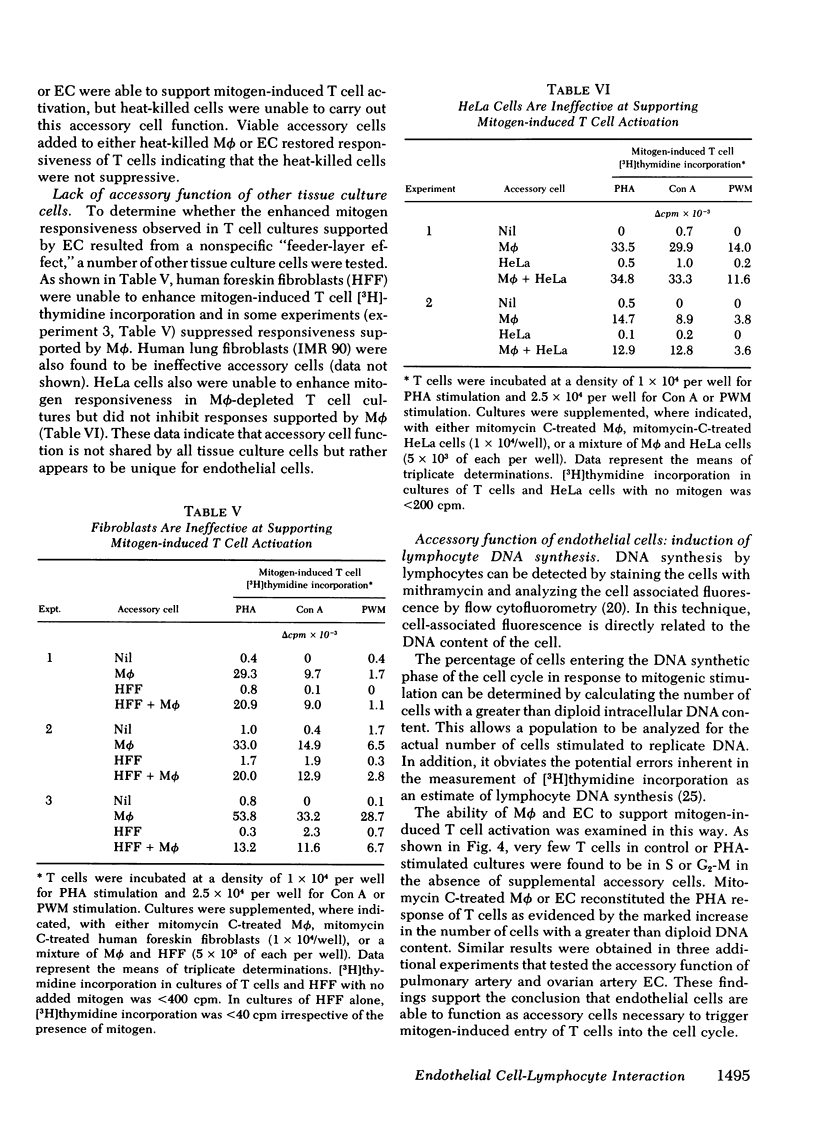
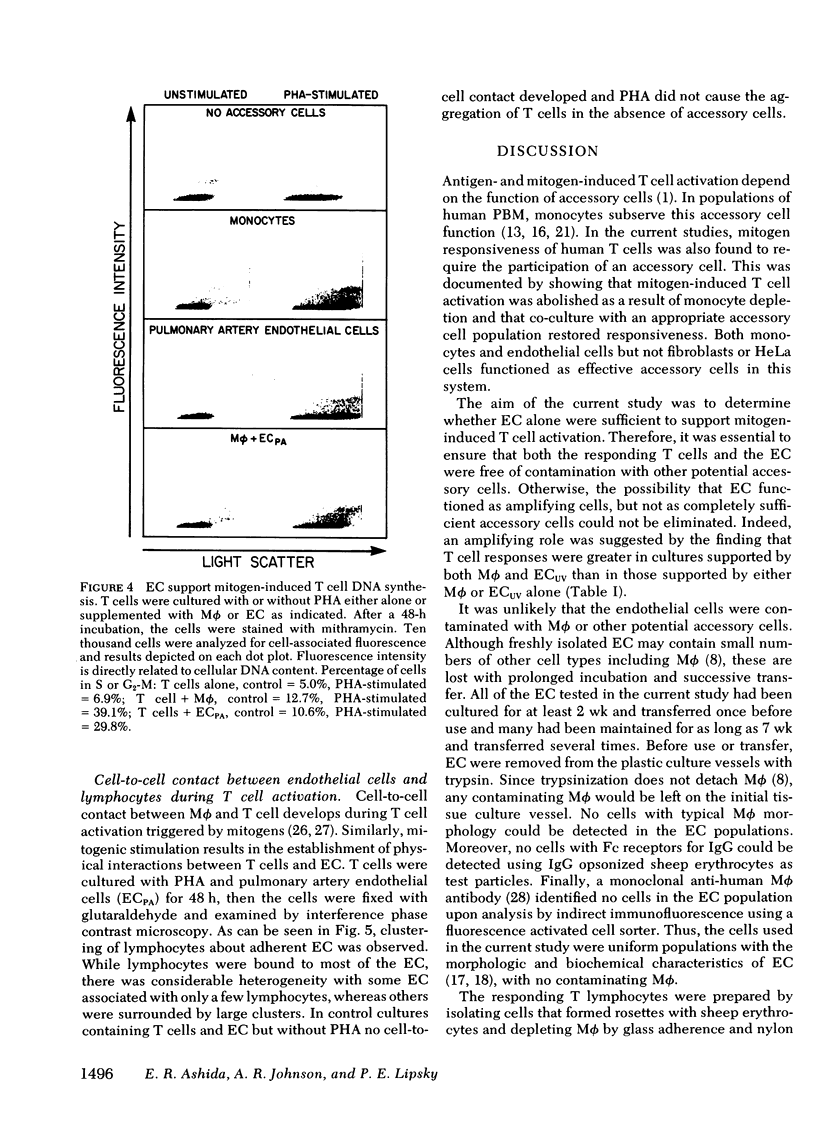
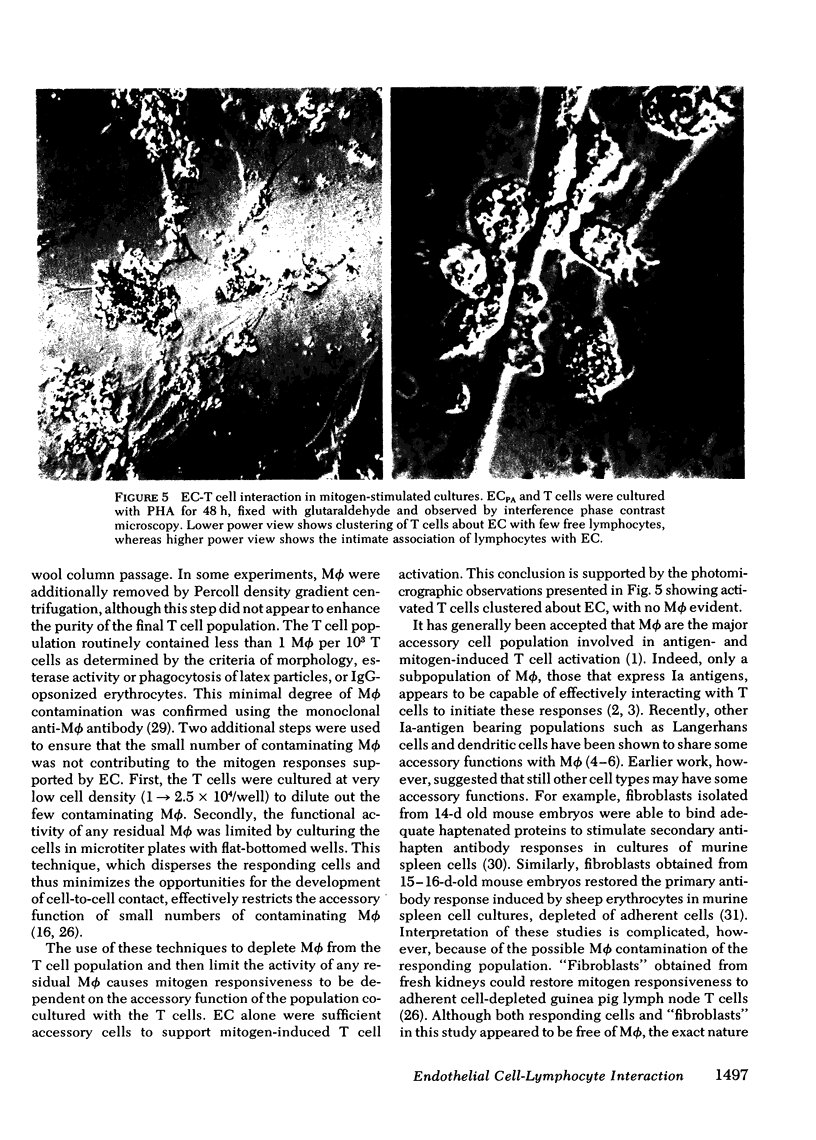
Mitogen-stimulated human T cell activation is absolutely dependent on the participation of a nonresponding accessory cell. In populations of human peripheral blood mononuclear cells, monocytes function as the requisite accessory cells. The possibility that cultured endothelial cells (EC) might also function as accessory cells was studied by examining the potential of endothelial cells to restore mitogen responsiveness to monocyte-depleted human T cells. Highly purified T cells were prepared by isolating cells rosetting with sheep erythrocytes and removing monocyte contamination by glass adherence and nylon wool column passage. When cultured at low cell density, T cells failed to respond to stimulation with various mitogenic lectins, whereas co-culture with monocytes restored responsiveness. Similarly, EC obtained from umbilical vein, pulmonary artery, and ovarian vein restored the capacity of T cells to respond to mitogens. Mitogen-stimulated T cell activation required viable endothelial cells. Moreover, effective endothelial T cell cooperation appeared to involve the establishment of cell-to-cell contact between EC and responding T cells. Accessory cell function was not a nonspecific property of all tissue culture cells as evidenced by the finding that human foreskin fibroblasts, lung fibroblasts, and HeLa cells were unable to restore responsiveness to monocyte-depleted T cells. These observations indicate that endothelial cells can support the induction of mitogen-induced T cell activation and suggest that cells lining blood vessels may play an active role in the initiation of immune responses in vivo.
Full text
PDF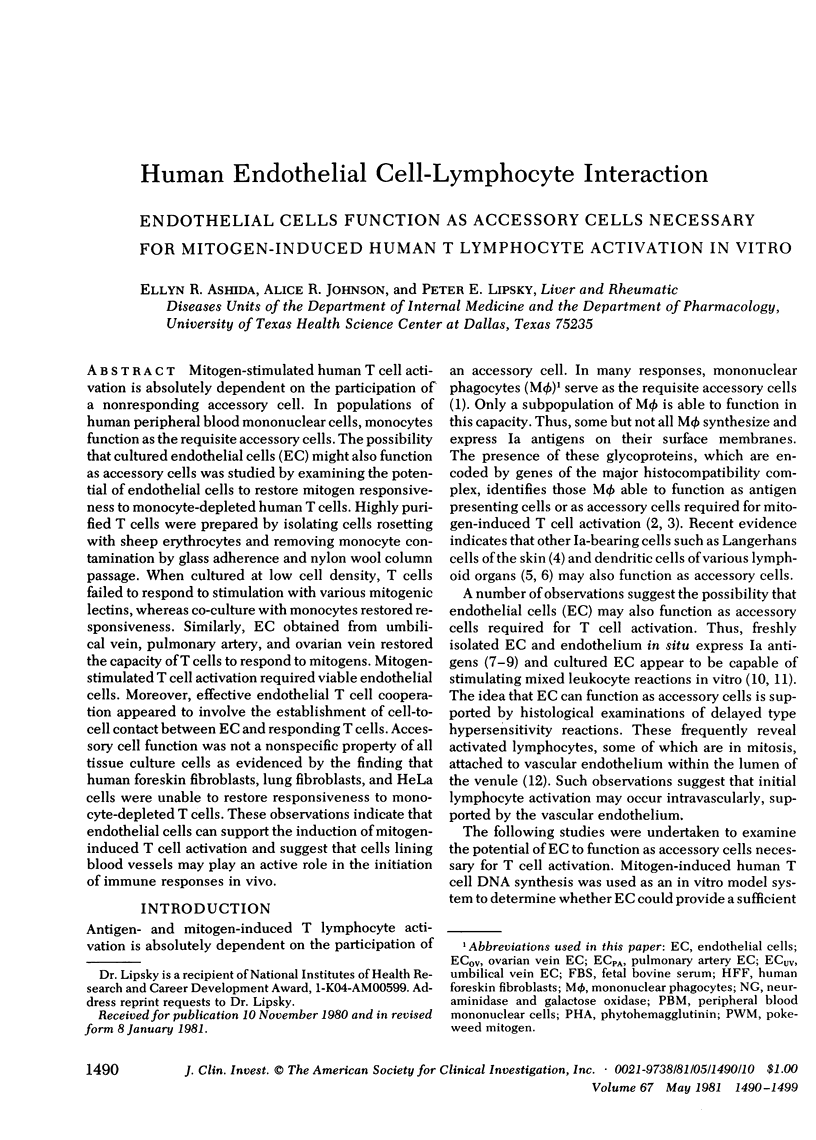



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmann G. B., Sachs D. H., Hodes R. J. Requirement for an Ia-bearing accessory cell in Con A-induced T cell proliferation. J Immunol. 1978 Nov;121(5):1981–1989. [PubMed] [Google Scholar]
- Buckley P. J., Wedner H. J. Measurement of the DNA synthetic capacity of activated lymphocytes: nucleotide triphosphate incorporation by permeabilized cells. J Immunol. 1978 Jun;120(6):1930–1940. [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. J Exp Med. 1972 Sep 1;136(3):604–617. doi: 10.1084/jem.136.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman H. A., Tobey R. A. Cell-cycle analysis in 20 minutes. Science. 1974 Jun 21;184(4143):1297–1298. doi: 10.1126/science.184.4143.1297. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Lipsky P. E., Rosenthal A. S. Phytohemagglutinin-induced proliferation of guinea pig thymus-derived lymphocytes. II. Accessory cell function. J Immunol. 1976 Mar;116(3):876–880. [PubMed] [Google Scholar]
- Greineder D. K., Rosenthal A. S. The requirement for macrophage-lymphocyte interaction in T lymphocyte proliferation induced by generation of aldehydes on cell membranes. J Immunol. 1975 Oct;115(4):932–938. [PubMed] [Google Scholar]
- Habu S., Raff M. C. Accessory cell dependence of lectin-induced proliferation of mouse T lymphocytes. Eur J Immunol. 1977 Jul;7(7):451–457. doi: 10.1002/eji.1830070710. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Bergh O. J., Thorsby E. Antigen-presenting properties of human vascular endothelial cells. J Exp Med. 1980 Aug 1;152(2 Pt 2):249s–255s. [PubMed] [Google Scholar]
- Hirschberg H., Evensen S. A., Henriksen T., Thorsby E. The human mixed lymphocyte-endothelium culture interaction. Transplantation. 1975 Jun;19(6):495–504. doi: 10.1097/00007890-197506000-00008. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Moen T., Throsby E. Specific destruction of human endothelial cell monolayers by anti-DRw antisera. Transplantation. 1979 Aug;28(2):116–120. doi: 10.1097/00007890-197908000-00009. [DOI] [PubMed] [Google Scholar]
- Häyry P., von Willebrand E., Andersson L. C. Expression of HLA-ABC and -DR locus antigens on human kidney, endothelial, tubular and glomerular cells. Scand J Immunol. 1980;11(3):303–310. doi: 10.1111/j.1365-3083.1980.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Metabolism of vasoactive peptides by human endothelial cells in culture. Angiotensin I converting enzyme (kininase II) and angiotensinase. J Clin Invest. 1977 Apr;59(4):684–695. doi: 10.1172/JCI108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R. Human pulmonary endothelial cells in culture. Activities of cells from arteries and cells from veins. J Clin Invest. 1980 Apr;65(4):841–850. doi: 10.1172/JCI109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Unanue E. R. Critical role of determinant presentation in the induction of specific responses in immunocompetent lymphocytes. J Exp Med. 1973 Apr 1;137(4):967–990. doi: 10.1084/jem.137.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnick J. T., Ostberg L., Stegagno M., Kimura A. K., Orn A., Sjöberg O. A rapid method for the separation of functional lymphoid cell populations of human and animal origin on PVP-silica (Percoll) density gradients. Scand J Immunol. 1979;10(6):563–573. doi: 10.1111/j.1365-3083.1979.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Ellner J. J., Rosenthal A. L. Phytohemagglutinin-induced proliferation of guinea pig thymus-derived lymphocytes. I. Accessory cell dependence. J Immunol. 1976 Mar;116(3):868–875. [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. Inhibition of antigen- and mitogen-induced human lymphocyte proliferation by gold compounds. J Clin Invest. 1977 Mar;59(3):455–466. doi: 10.1172/JCI108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel A. L., Mehta S., Ford R. J. T-lymphocyte/monocyte interaction in response to phytohemagglutinin. Cell Immunol. 1979 Dec;48(2):383–397. doi: 10.1016/0008-8749(79)90133-3. [DOI] [PubMed] [Google Scholar]
- Moen T., Moen M., Thorsby E. HLA-D region products are expressed in endothelial cells. Tissue Antigens. 1980 Feb;15(2):112–122. doi: 10.1111/j.1399-0039.1980.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Moraes J. R., Stastny P. A new antigen system expressed in human endothelial cells. J Clin Invest. 1977 Aug;60(2):449–454. doi: 10.1172/JCI108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Lemke H., Opitz H. G. The role of adherent cells in the immune response. Fibroblasts and products released by fibroblasts and peritoneal cells can substitute for adherent cells. Scand J Immunol. 1976;5(3):269–280. doi: 10.1111/j.1365-3083.1976.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Parker J. W., Frelinger J. A., O'Brien R. L. Characterization of responding cells in oxidative mitogen stimulation. II. Identification of an Ia-bearing adherent accessory cell. J Immunol. 1980 Jun;124(6):2700–2707. [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl G., Katz S. I., Shevach E. M., Rosenthal A. S., Green I. Analogous functions of macrophages and Langerhans cells in the initiation in the immune response. J Invest Dermatol. 1978 Jul;71(1):59–64. doi: 10.1111/1523-1747.ep12544055. [DOI] [PubMed] [Google Scholar]
- Ugolini V., Nunez G., Smith R. G., Stastny P., Capra J. D. Initial characterization of monoclonal antibodies against human monocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6764–6768. doi: 10.1073/pnas.77.11.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer A. J., Flad H. D. Discontinuous density gradient separation of human mononuclear leucocytes using Percoll as gradient medium. J Immunol Methods. 1979;30(1):1–10. doi: 10.1016/0022-1759(79)90268-0. [DOI] [PubMed] [Google Scholar]
- Yamashita U., Shevach E. M. The expression of Ia antigens on immunocompetent cells in the guinea pig. II. Ia antigens on macrophages. J Immunol. 1977 Nov;119(5):1584–1588. [PubMed] [Google Scholar]
- de Bono D. Endothelial-lymphocyte interactions in vitro. I. Adherence of nonallergised lymphocytes. Cell Immunol. 1976 Sep;26(1):78–88. doi: 10.1016/0008-8749(76)90349-x. [DOI] [PubMed] [Google Scholar]
- de Bono D. Endothelium-lymphocyte interactions in vitro. II. Adherence of allergized lymphocytes. Cell Immunol. 1979 Apr;44(1):64–70. doi: 10.1016/0008-8749(79)90028-5. [DOI] [PubMed] [Google Scholar]
- de Vries J. E., Caviles A. P., Jr, Bont W. S., Mendelsohn J. The role of monocytes in human lymphocyte activation by mitogens. J Immunol. 1979 Mar;122(3):1099–1107. [PubMed] [Google Scholar]



