Abstract
Because successful allotransplantation of islets of Langerhans isolated by collagenase digestion has been difficult in many animal species, we asked whether isolated islet preparations might have tissue specific determinants conferring amplified immunogenicity in vitro. Lymphocyte proliferative responses ([3H]thymidine uptake) were studied in beagle dogs in mixed culture combinations of lymphocyte vs. lymphocyte (MLC) and lymphocyte vs. islet (MLIC). In five MLC responder and five nonresponder pairs, peripheral blood lymphocytes of dogs A and B were used as responding cells, and dog B x-irradiated lymphocytes (Bx), x-irradiated (or nonirradiated) islets (BI), or hepatic cells (BH) were used as stimulating cells in primary and secondary reactions. For the secondary reactions, A + Bx, A + BI, or B + BI were incubated for 9 d (A′B, A′BI, B′BI, respectively) before addition of new stimulating cells.
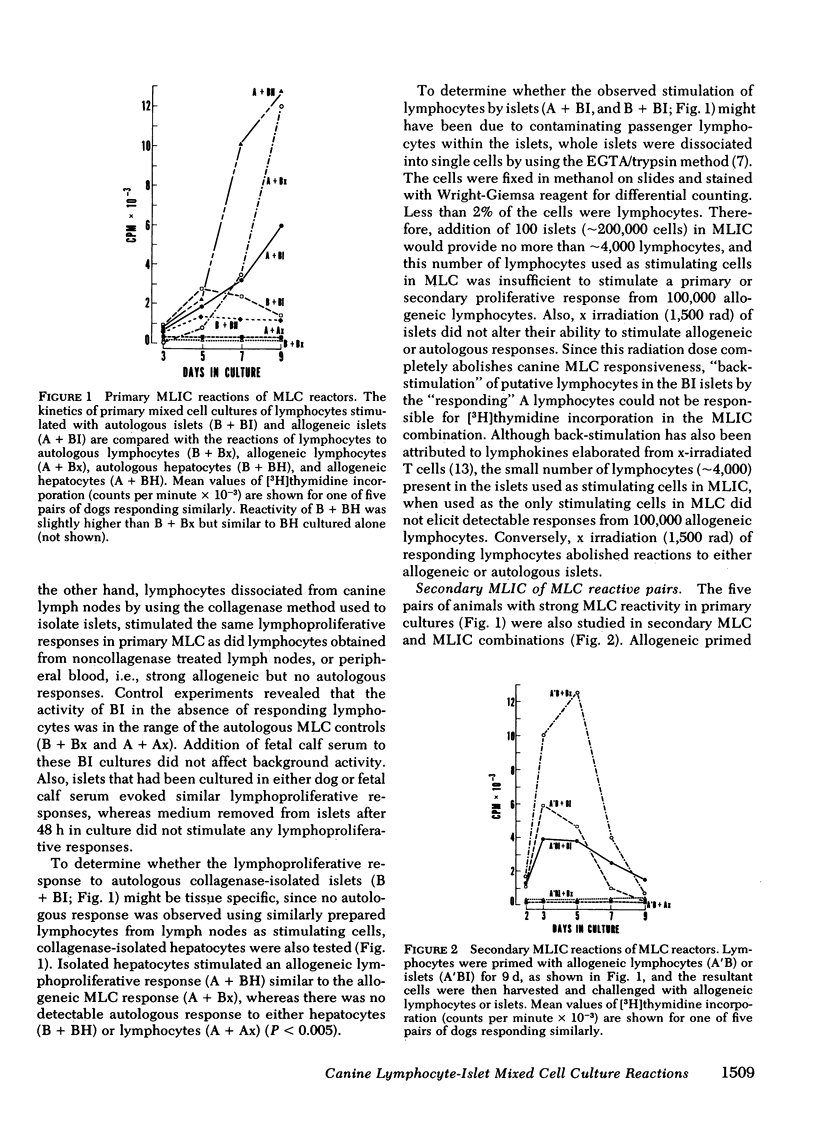
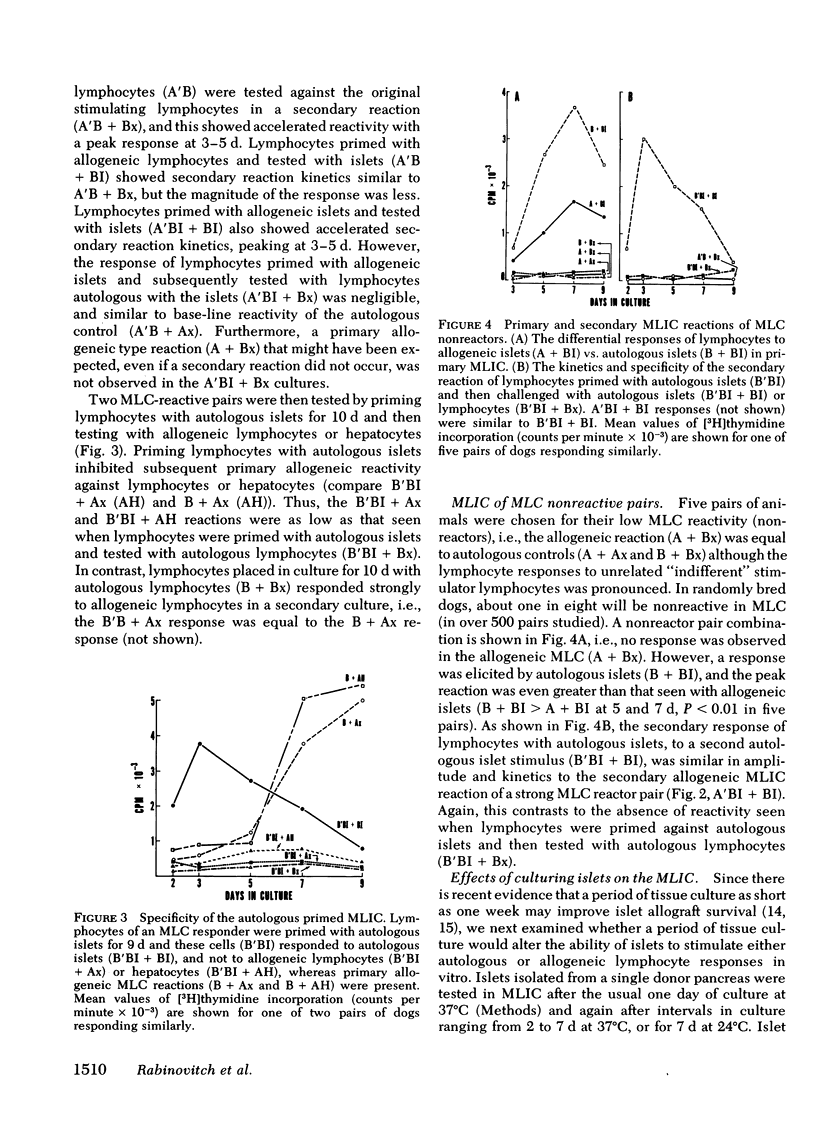
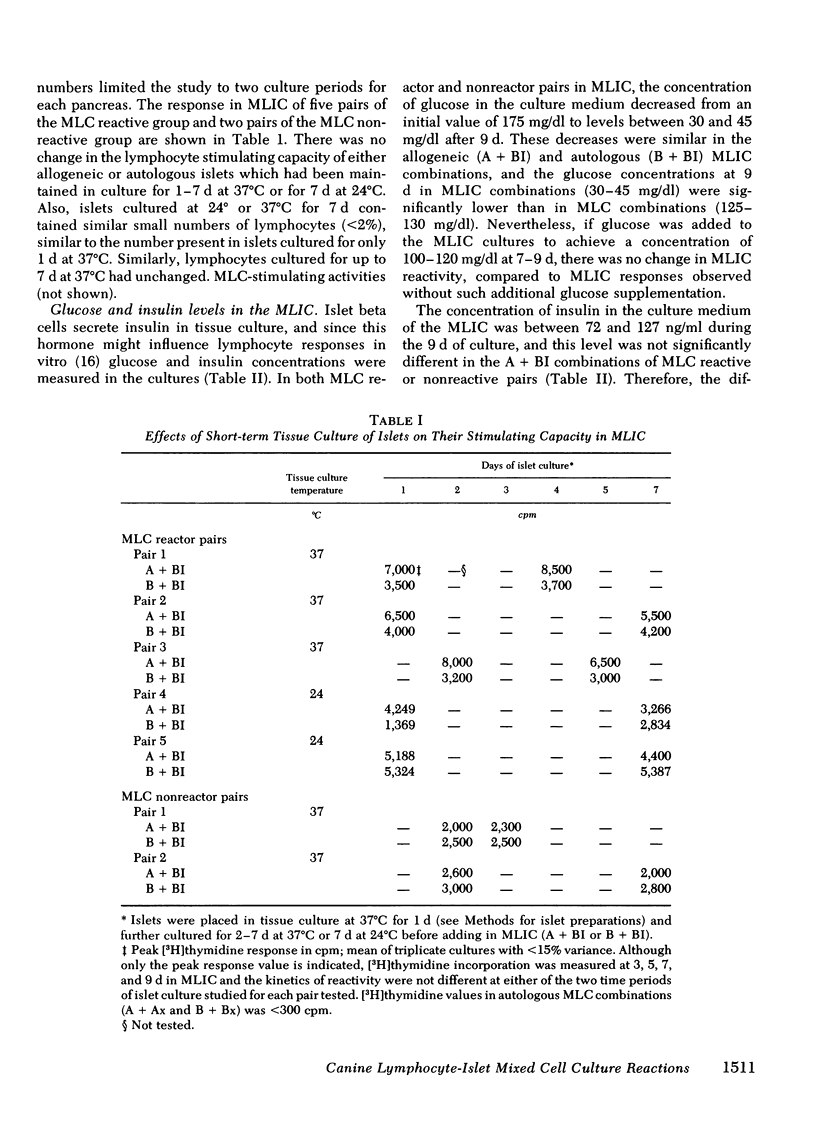
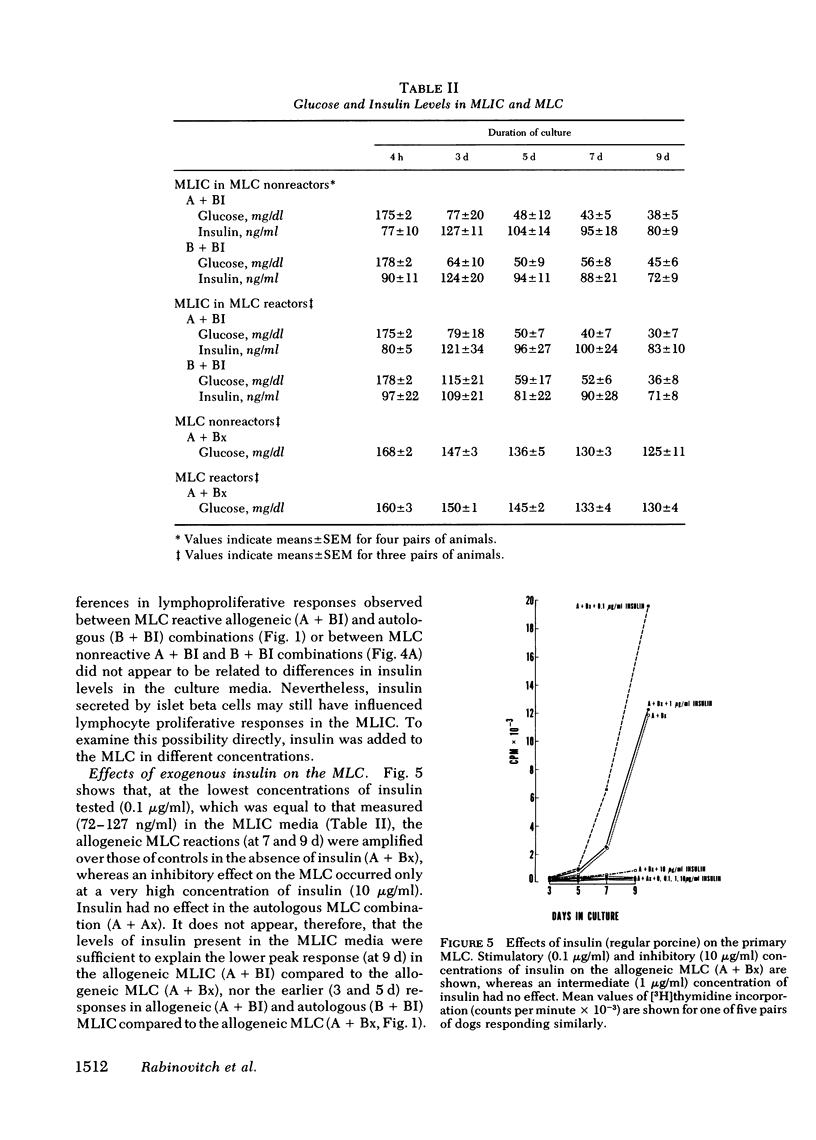
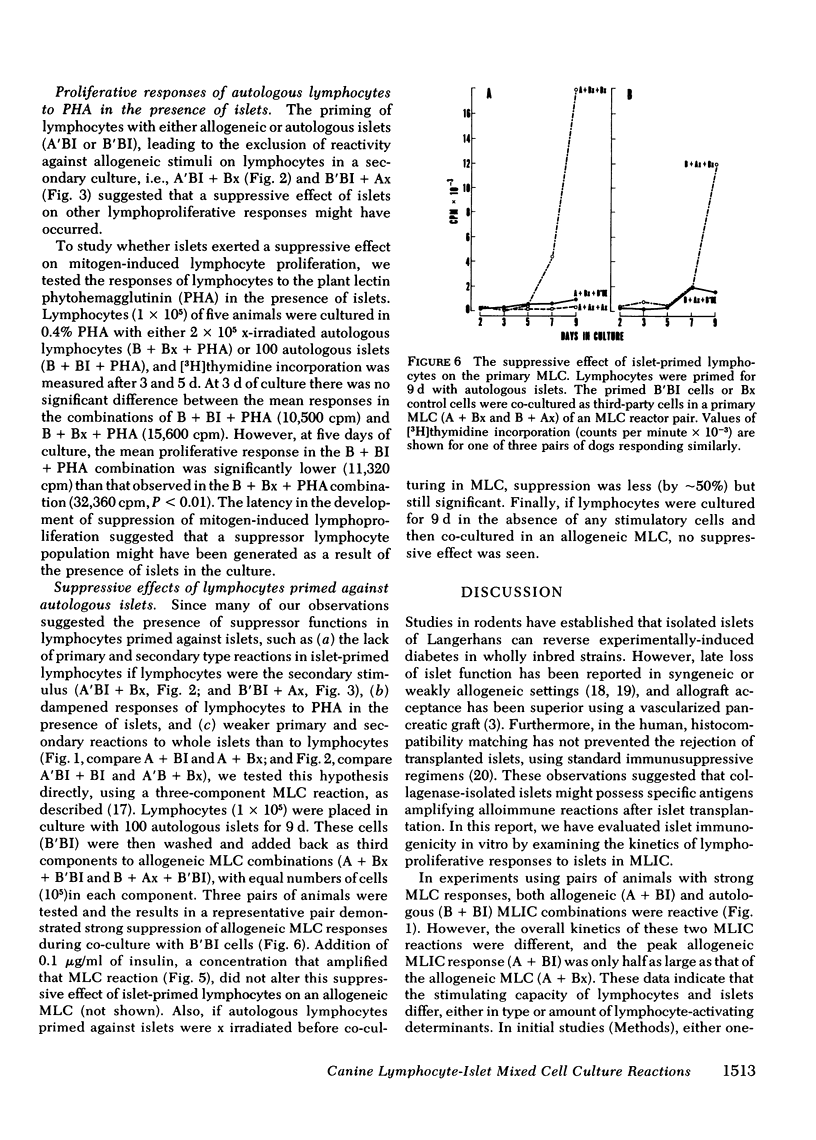
The results showed that islets were autostimulatory, eliciting a tissue-specific lymphoproliferative response in a primary MLIC. Thus, B + BI reactivity was evident at 3,5, and 7 d in primary culture, whereas collagenase-digested liver cells, or lymphocytes obtained from collagenase-digested lymph nodes did not stimulate autologous lymphocytes. A separate reactivity was observed in the allogeneic A + BI combination in MLC responder pairs, and the peak response of A + BI at 9 d was markedly greater than that of B + BI, suggesting the presence of major histocompatibility complex lymphocyte-defined locus determinants in the islet preparations, in addition to islet-specific determinants. A secondary reaction was observed if lymphocytes were primed with islets and challenged with islets (A′BI + BI or B′BI + BI), but not if they were challenged with lymphocytes (A′BI + Bx, B′BI + Bx) or hepatic cells (A′BI + BH, B′BI + AH). Furthermore, priming of lymphocytes with autologous islets (B′BI) led to exclusion of any reactivity against allogeneic lymphocytes, i.e., B′BI suppressed A + Bx, and B′BI also markedly suppressed phytohemagglutinin-stimulated lymphoproliferative responses. Experiments were performed that excluded the possibility that the insulin levels present in the MLIC, the presence of passenger lymphocytes in the islets, or the maintenance of islets in tissue culture for 1-7 d affected the observations. These results provide evidence for the existence of alloantigens as well as tissue-specific antigens on collagenase-isolated islets of Langerhans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker C. F., Naji A., Silvers W. K. Immunologic problems in islet transplantation. Diabetes. 1980;29 (Suppl 1):86–92. doi: 10.2337/diab.29.1.s86. [DOI] [PubMed] [Google Scholar]
- Bergholtz B., Albrechtsen D., Thorsby E. Stimulation of T lymphocytes by autologous non-T lymphoid cells. Participation of HLA-D? Tissue Antigens. 1977 Jul;10(1):27–38. doi: 10.1111/j.1399-0039.1977.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Billingham R. E. The passenger cell concept in transplantation immunology. Cell Immunol. 1971 Feb;2(1):1–12. doi: 10.1016/0008-8749(71)90022-0. [DOI] [PubMed] [Google Scholar]
- Frangipane L. G., Barker C. F., Silvers W. K. Importance of weak histocompatibility factors in survival of pancreatic islet transplants. Surg Forum. 1977;28:294–296. [PubMed] [Google Scholar]
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965 Oct;14(10):619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Harrison M. R., Paul W. E. Stimulus-response in the mixed lymphocyte reaction. J Exp Med. 1973 Dec 1;138(6):1602–1607. doi: 10.1084/jem.138.6.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzman R. J., Segall M., Bach M. L., Bach F. H. Histocompatibility matching. VI. Miniaturization of the mixed leukocyte culture test: a preliminary report. Transplantation. 1971 Mar;11(3):268–273. doi: 10.1097/00007890-197103000-00005. [DOI] [PubMed] [Google Scholar]
- Helderman J. H., Strom T. B. Emergence of insulin receptors upon alloimmune T cells in the rat. J Clin Invest. 1977 Feb;59(2):338–344. doi: 10.1172/JCI108646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Huang S. W., Maclaren N. K. Insulin-dependent diabetes: a disease of autoaggression. Science. 1976 Apr 2;192(4234):64–66. doi: 10.1126/science.769160. [DOI] [PubMed] [Google Scholar]
- Krug U., Krug F., Cuatrecasas P. Emergence of insulin receptors on human lymphocytes during in vitro transformation. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2604–2608. doi: 10.1073/pnas.69.9.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. Lymphocyte transformation induced by autologous cells. IV. Human T-lymphocyte proliferation induced by autologous or allogeneic non-T lymphocytes. J Exp Med. 1976 May 1;143(5):1042–1054. doi: 10.1084/jem.143.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Davie J. M., Finke E. H. Prolongation of islet allograft survival following in vitro culture (24 degrees C) and a single injection of ALS. Science. 1979 Apr 20;204(4390):312–313. doi: 10.1126/science.107588. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Davie J. M., Finke E. H. Prolongation of islet xenograft survival without continuous immunosuppression. Science. 1980 Jul 11;209(4453):283–285. doi: 10.1126/science.6770465. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lendrum R., Walker G., Gamble D. R. Islet-cell antibodies in juvenile diabetes mellitus of recent onset. Lancet. 1975 Apr 19;1(7912):880–882. doi: 10.1016/s0140-6736(75)91683-9. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Lazarow A., Hegre O. D. Pancreatic islet transplantation in the rat. Diabetes. 1973 Jun;22(6):413–428. doi: 10.2337/diab.22.6.413. [DOI] [PubMed] [Google Scholar]
- Meda P., Hooghe-Peters E. L., Orci L. Monolayer cultures of adult pancreatic islet cells on osmotically disrupted fibroblasts. Diabetes. 1980 Jun;29(6):497–500. doi: 10.2337/diab.29.6.497. [DOI] [PubMed] [Google Scholar]
- Miller J., Clark C., Sigmon E., Azar M. Development of suppressor cells as a second generation mixed lymphocyte culture phenomenon in MLS and non-H-2-disparate inbred mice. Transplantation. 1978 Nov;26(5):308–314. doi: 10.1097/00007890-197811000-00009. [DOI] [PubMed] [Google Scholar]
- Miller J., Lifton J., Hattler B. G., Jr Alteration of the antigenicity of human lymphocytes by plant lectins and short-term tissue culture. Cell Immunol. 1975 Oct;19(2):306–317. doi: 10.1016/0008-8749(75)90212-9. [DOI] [PubMed] [Google Scholar]
- Miller J., Lifton J., Wilcox C., DeWolf W. C. The monitoring of canine LD responsiveness using two systems: (1) blocking antibody in second-set renal allografts and (2) modulation of cellular response stimuli. Transplant Proc. 1978 Jun;10(2):395–402. [PubMed] [Google Scholar]
- Najarian J. S., Sutherland D. E., Matas A. J., Steffes M. W., Simmons R. L., Goetz F. C. Human islet transplantation: a preliminary report. Transplant Proc. 1977 Mar;9(1):233–236. [PubMed] [Google Scholar]
- Naji A., Barker C. F. Differential susceptibility of endocrine tissues to humoral immunity. Surg Forum. 1978;29:349–351. [PubMed] [Google Scholar]
- Nash J. R., Peters M., Bell P. R. Comparative survival of pancreatic islets, heart, kidney, and skin allografts in rats, with and without enhancement. Transplantation. 1977 Jul;24(1):70–73. doi: 10.1097/00007890-197707000-00010. [DOI] [PubMed] [Google Scholar]
- Opelz G., Kiuchi M., Takasugi M., Terasaki P. I. Autologous stimulation of human lymphocyte subpopulation. J Exp Med. 1975 Nov 1;142(5):1327–1333. doi: 10.1084/jem.142.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. A., Gantan Z., Siegel S., Howell E., Belzer F. O., Kountz S. L. Reactions of kidney cells with cytotoxic antisera: possible evidence of kidney-specific antigens. Tissue Antigens. 1975 Apr;5(2):89–98. [PubMed] [Google Scholar]
- Ptak W., Rewicka M., Kollat M. Development of specific suppressor cells in hypoinsulinaemic mice. Nature. 1980 Jan 10;283(5743):199–200. doi: 10.1038/283199a0. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Gold G., Reaven G. M. Effect of age on glucose-stimulated insulin release by the beta-cell of the rat. J Clin Invest. 1979 Aug;64(2):591–599. doi: 10.1172/JCI109498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse H. G., Oxender D. L., Pek S., Ar D. Complement-mediated cytotoxic effects on pancreatic islets with sera from diabetic patients. Diabetes. 1980 Apr;29(4):317–322. doi: 10.2337/diab.29.4.317. [DOI] [PubMed] [Google Scholar]
- Sakane T., Green I. Specificity and suppressor function of human T cells responsive to autologous non-T cells. J Immunol. 1979 Aug;123(2):584–589. [PubMed] [Google Scholar]
- Scheid M., Boyse E. A., Carswell E. A., Old L. J. Serologically demonstrable alloantigens of mouse epidermal cells. J Exp Med. 1972 Apr 1;135(4):938–955. doi: 10.1084/jem.135.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B. Stimulation of autologous and allogeneic human T cells by B cells occurs through separate B-cell antigen systems. Cell Immunol. 1978 Mar 15;36(2):203–209. doi: 10.1016/0008-8749(78)90264-2. [DOI] [PubMed] [Google Scholar]
- Steinmuller D. Immunization with skin isografts taken from tolerant mice. Science. 1967 Oct 6;158(3797):127–129. doi: 10.1126/science.158.3797.127. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Bear R. A., Carpenter C. B. Insulin-induced augmentation of lymphocyte-mediated cytotoxicity. Science. 1975 Mar 28;187(4182):1206–1208. doi: 10.1126/science.163492. [DOI] [PubMed] [Google Scholar]
- Tomonari K. Cytotoxic T cells generated in the autologous mixed lymphocyte reaction. I. Primary autologous mixed lymphocyte reaction. J Immunol. 1980 Mar;124(3):1111–1121. [PubMed] [Google Scholar]
- Weksler M. E., Kozak R. Lymphocyte transformation induced by autologous cells. V. Generation of immunologic memory and specificity during the autologous mixed lymphocyte reaction. J Exp Med. 1977 Dec 1;146(6):1833–1838. doi: 10.1084/jem.146.6.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F. Differentiation antigens of the lymphocyte cell surface. Biochem Soc Trans. 1976;4(1):4–8. doi: 10.1042/bst0040004. [DOI] [PubMed] [Google Scholar]


