Abstract
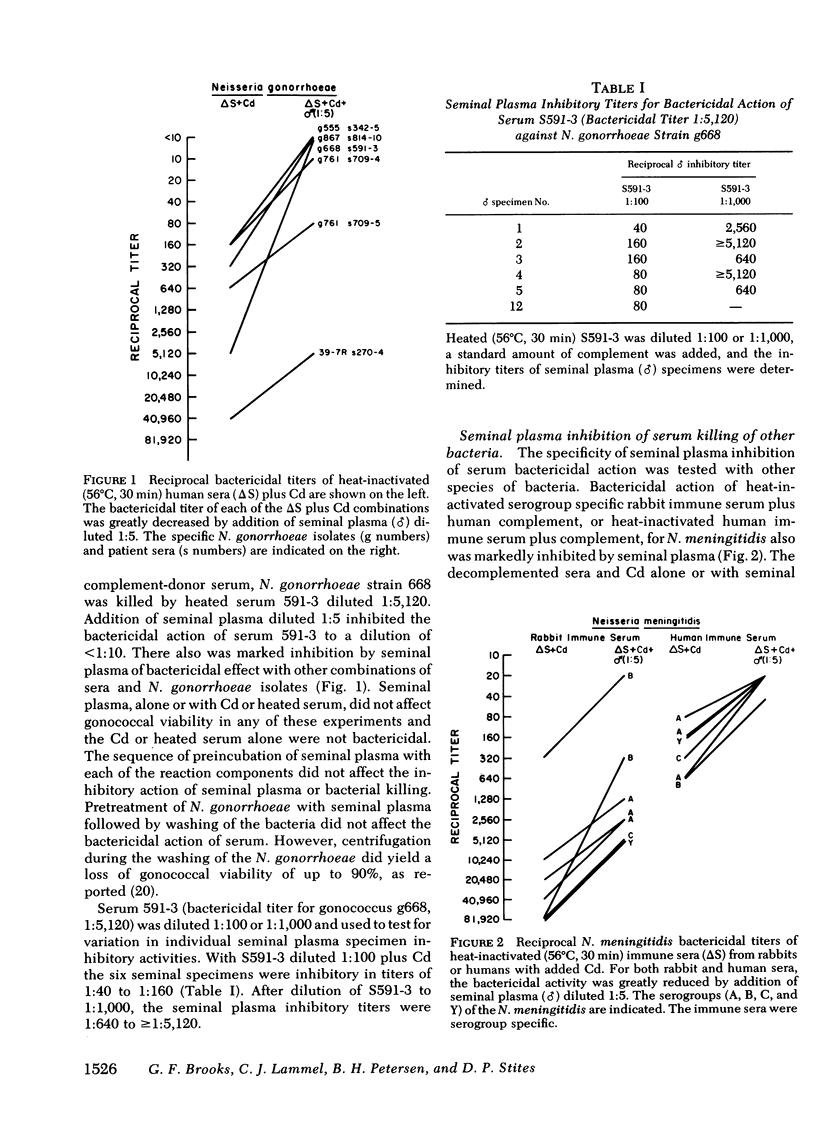
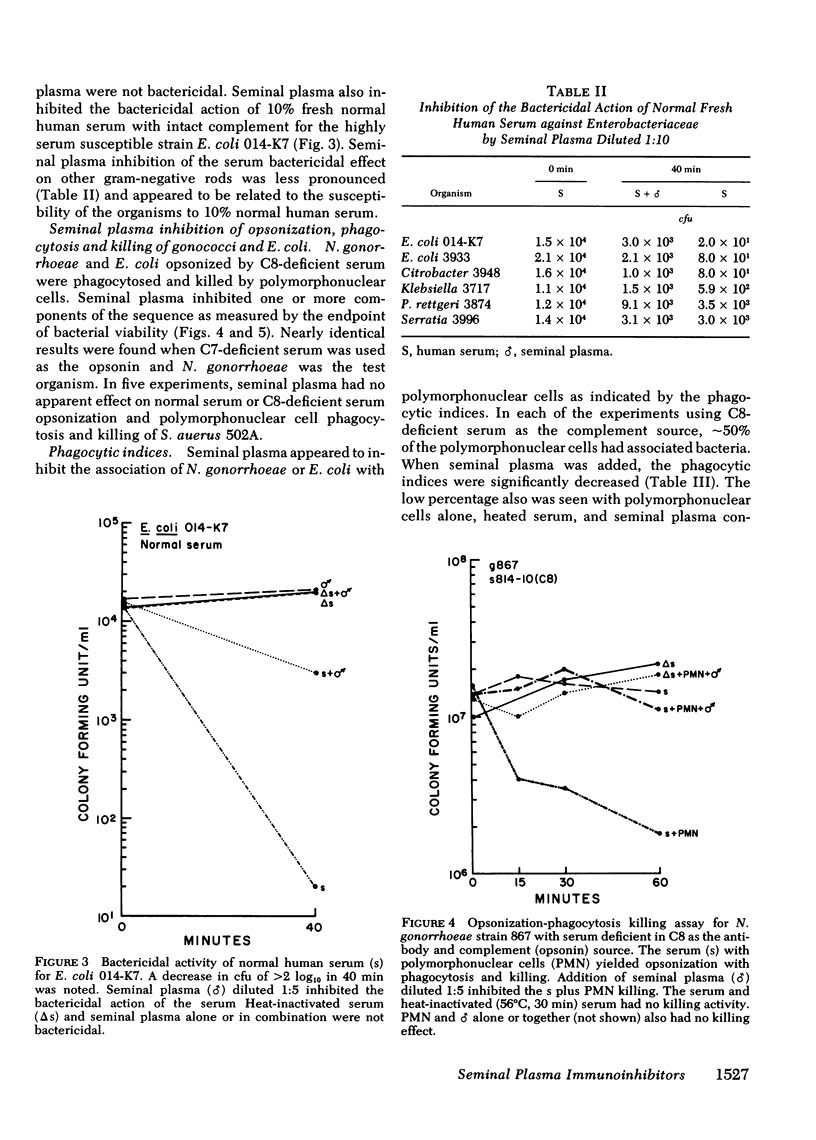
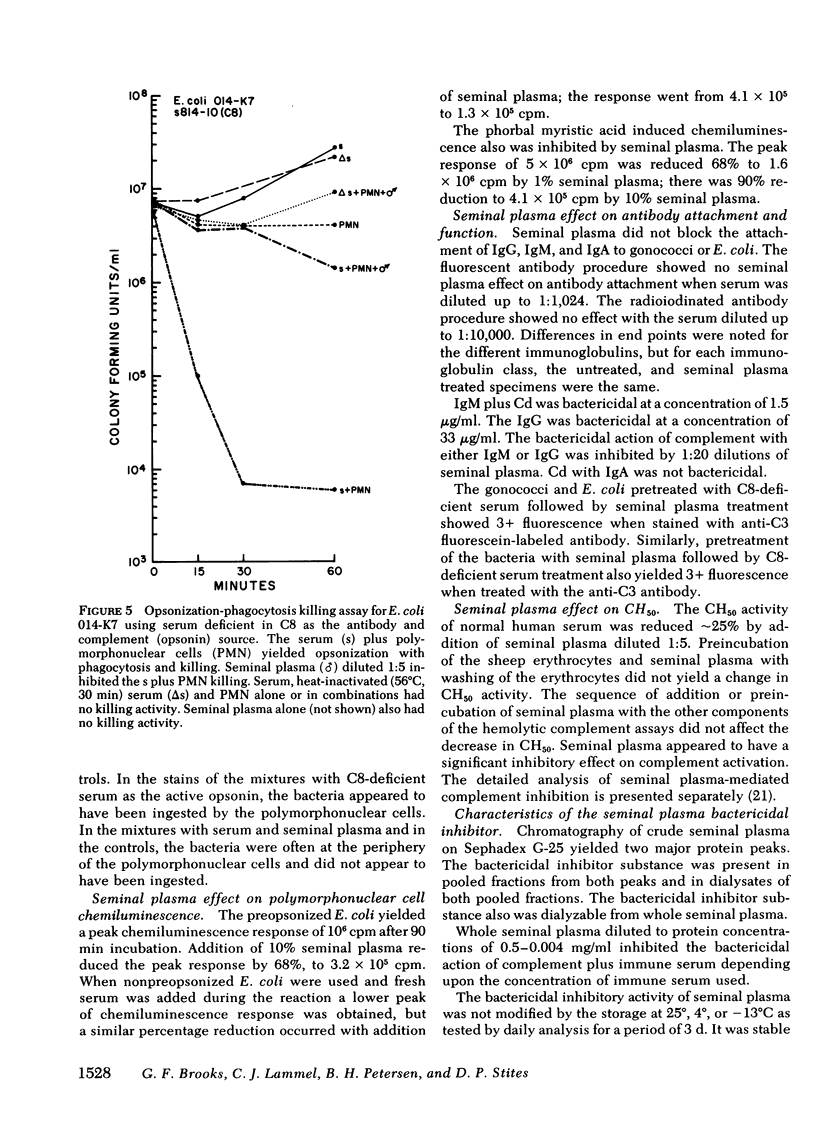
Seminal plasma diluted 1:5-1:1,000 gave marked inhibition of serum antibody complement-mediated bactericidal and opsonic effects against Neisseria gonorrhoeae and other gram-negative organisms. Serum that was bactericidal at a dilution of 1:5,120 was not bactericidal at a dilution of 1:10 when seminal plasma was added. Bactericidal action of immune human or rabbit sera, or purified immunoglobulin (Ig)G or IgM plus complement for six strains of N. gonorrhoeae, serogroups A, B, C, and Y of Neisseria meningitidis, Escherichia coli and other gram-negative rods was inhibited by seminal plasma. Using C8- or C7-deficient sera as antibody and complement sources, opsonization, phagocytosis, and killing of N. gonorrhoeae and E. coli 014-K7 were inhibited by seminal plasma. Opsonization, phagocytosis, and killing of Staphylococcus aureus 502A was not inhibited. For the gram-negative organisms, the early phase of the opsonization process, probably complement activation, appeared to be inhibited rather than the ingestion or polymorphonuclear leukocyte killing steps; addition of seminal plasma yielded a significant reduction in the percentage of polymorphonuclear cells with associated bacteria. Seminal plasma did not prevent attachment of IgG, IgM, or IgA antibodies to gonococci. It reduced serum hemolytic whole complement activity by 25%. The seminal plasma inhibitor was of low molecular weight and was stable at 56 degrees C for 30 min, but inhibitory activity was lost after heating to 100 degrees C for 10 min. It is likely that the inhibitory factor(s) is a low-molecular weight protease or protease inhibitor. Seminal plasma probably has an important role in inhibition of complement and antibody functions in the genital tract. It may enhance pathogenesis of agents of sexually transmitted diseases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boxer L. A., Watanabe A. M., Rister M., Besch H. R., Jr, Allen J., Baehner R. L. Correction of leukocyte function in Chediak-Higashi syndrome by ascorbate. N Engl J Med. 1976 Nov 4;295(19):1041–1045. doi: 10.1056/NEJM197611042951904. [DOI] [PubMed] [Google Scholar]
- Brooks G. F., Ingwer I. Studies on the relationships between serum bactericidal activity and uncomplicated genital infections due to Neisseria gonorrhoeae. J Infect Dis. 1978 Sep;138(3):333–339. doi: 10.1093/infdis/138.3.333. [DOI] [PubMed] [Google Scholar]
- Brooks G. F., Israel K. S., Petersen B. H. Bactericidal and opsonic activity against Neisseria gonorrhoeae in sera from patients with disseminated gonococcal infection. J Infect Dis. 1976 Nov;134(5):450–462. doi: 10.1093/infdis/134.5.450. [DOI] [PubMed] [Google Scholar]
- Chipperfield E. J., Evans B. A. Effect of local infection and oral contraception on immunoglobulin levels in cervical mucus. Infect Immun. 1975 Feb;11(2):215–221. doi: 10.1128/iai.11.2.215-221.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Rice P. A., McCormick W. M. Bactericidal antibody in genital infection due to Neisseria gonorrhoeae. J Infect Dis. 1977 Feb;135(2):243–251. doi: 10.1093/infdis/135.2.243. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Kraut E. H., Metz E. N., Sagone A. L., Jr In vitro effects of ascorbate on white cell metabolism and the chemiluminescence response. J Reticuloendothel Soc. 1980 Apr;27(4):359–366. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai a Fat R. F., Suurmond D., van Furth R. In vitro synthesis of immunoglobulins, secretory component and complement in normal and pathological skin and the adjacent mucous membranes. Clin Exp Immunol. 1973 Jul;14(3):377–395. [PMC free article] [PubMed] [Google Scholar]
- Lebherz T. B., Ladudovich M. Changes in endometrial ground substance. J Reprod Med. 1970 Jul;5(1):13–14. [PubMed] [Google Scholar]
- Lee T. J., Utsinger P. D., Snyderman R., Yount W. J., Sparling P. F. Familial deficiency of the seventh component of complement associated with recurrent bacteremic infections due to Neisseria. J Infect Dis. 1978 Sep;138(3):359–368. doi: 10.1093/infdis/138.3.359. [DOI] [PubMed] [Google Scholar]
- Lord E. M., Sensabaugh G. F., Stites D. P. Immunosuppressive activity of human seminal plasma. I. Inhibition of in vitro lymphocyte activation. J Immunol. 1977 May;118(5):1704–1711. [PubMed] [Google Scholar]
- Marcus Z. H., Freisheim J. H., Houk J. L., Herman J. H., Hess E. V. In vitro studies in reproductive immunology. 1. Suppression of cell-mediated immune response by human spermatozoa and fractions isolated from human seminal plasma. Clin Immunol Immunopathol. 1978 Mar;9(3):318–326. doi: 10.1016/0090-1229(78)90103-4. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- McCall C. E., DeChatelet L. R., Cooper M. R., Ashburn P. The effects of ascorbic acid on bactericidal mechanisms of neutrophils. J Infect Dis. 1971 Aug;124(2):194–198. doi: 10.1093/infdis/124.2.194. [DOI] [PubMed] [Google Scholar]
- Petersen B. H., Graham J. A., Brooks G. F. Human deficiency of the eighth component of complement. The requirement of C8 for serum Neisseria gonorrhoeae bactericidal activity. J Clin Invest. 1976 Feb;57(2):283–290. doi: 10.1172/JCI108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B. H., Lammel C. J., Stites D. P., Brooks G. F. Human seminal plasma inhibition of complement. J Lab Clin Med. 1980 Oct;96(4):582–591. [PubMed] [Google Scholar]
- Pincus S. H., Klebanoff S. J. Quantitative leukocyte iodination. N Engl J Med. 1971 Apr 8;284(14):744–750. doi: 10.1056/NEJM197104082841402. [DOI] [PubMed] [Google Scholar]
- Prakash C., Coutinho A., Möller G. Inhibition of in vitro immune responses by a fraction from seminal plasma. Scand J Immunol. 1976;5(1-2):77–85. doi: 10.1111/j.1365-3083.1976.tb02994.x. [DOI] [PubMed] [Google Scholar]
- Price R. J., Boettcher B. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil Steril. 1979 Jul;32(1):61–66. doi: 10.1016/s0015-0282(16)44117-8. [DOI] [PubMed] [Google Scholar]
- Schumacher G. F. Biochemistry of cervical mucus. Fertil Steril. 1970 Oct;21(10):697–705. [PubMed] [Google Scholar]
- Senff L. M., Wegener W. S., Brooks G. F., Finnerty W. R., Makula R. A. Phospholipid composition and phospholipase A activity of Neisseria gonorrhoeae. J Bacteriol. 1976 Aug;127(2):874–880. doi: 10.1128/jb.127.2.874-880.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Winston D. J., Van Dyke K. In vitro evaluation of opsonic and cellular granulocyte function by luminol-dependent chemiluminescence: utility in patients with severe neutropenia and cellular deficiency states. Infect Immun. 1978 Oct;22(1):41–51. doi: 10.1128/iai.22.1.41-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stites D. P., Erickson R. P. Suppressive effect of seminal plasma on lymphocyte activation. Nature. 1975 Feb 27;253(5494):727–729. doi: 10.1038/253727a0. [DOI] [PubMed] [Google Scholar]
- TALIAFERRO L. G., TALIAFERRO W. H. X-ray effects on hemolysin formation in rabbits with the spleen shielded or irradiated. J Infect Dis. 1956 Sep-Oct;99(2):109–128. doi: 10.1093/infdis/99.2.109. [DOI] [PubMed] [Google Scholar]
- Tramont E. C. Inhibition of adherence of Neisseria gonorrhoeae by human genital secretions. J Clin Invest. 1977 Jan;59(1):117–124. doi: 10.1172/JCI108608. [DOI] [PMC free article] [PubMed] [Google Scholar]


