Abstract
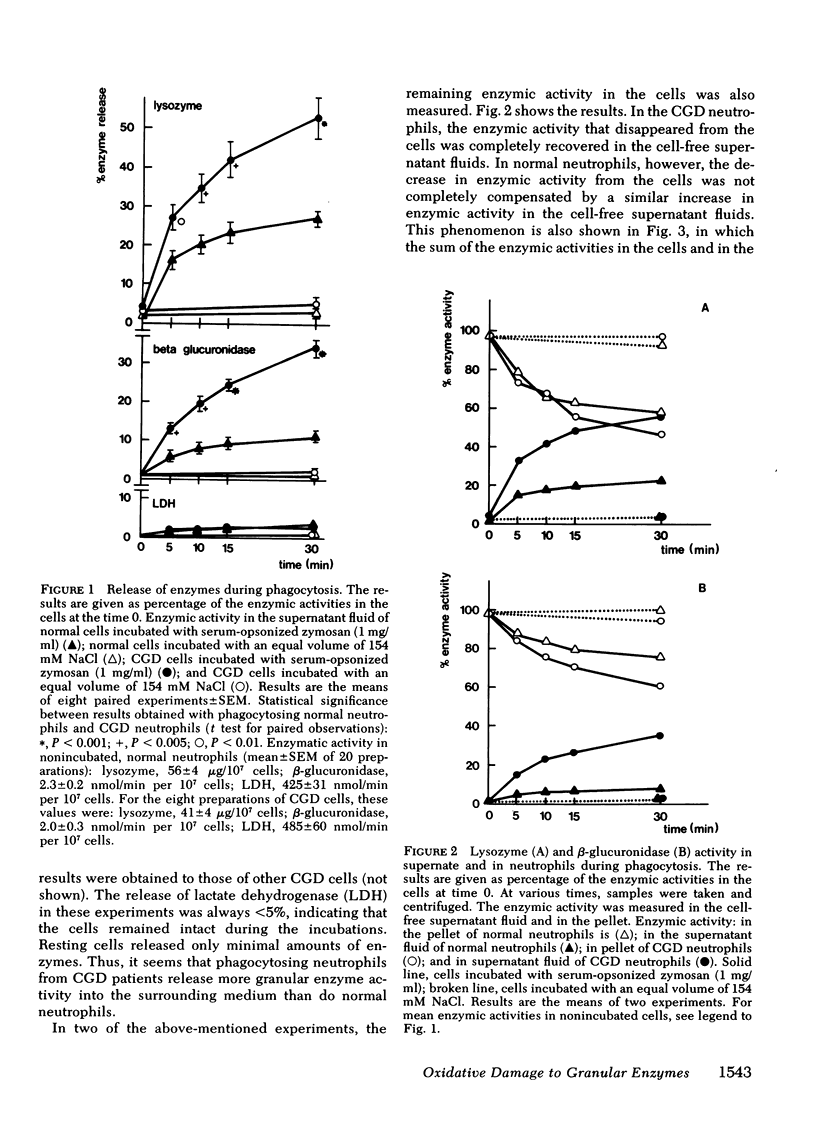
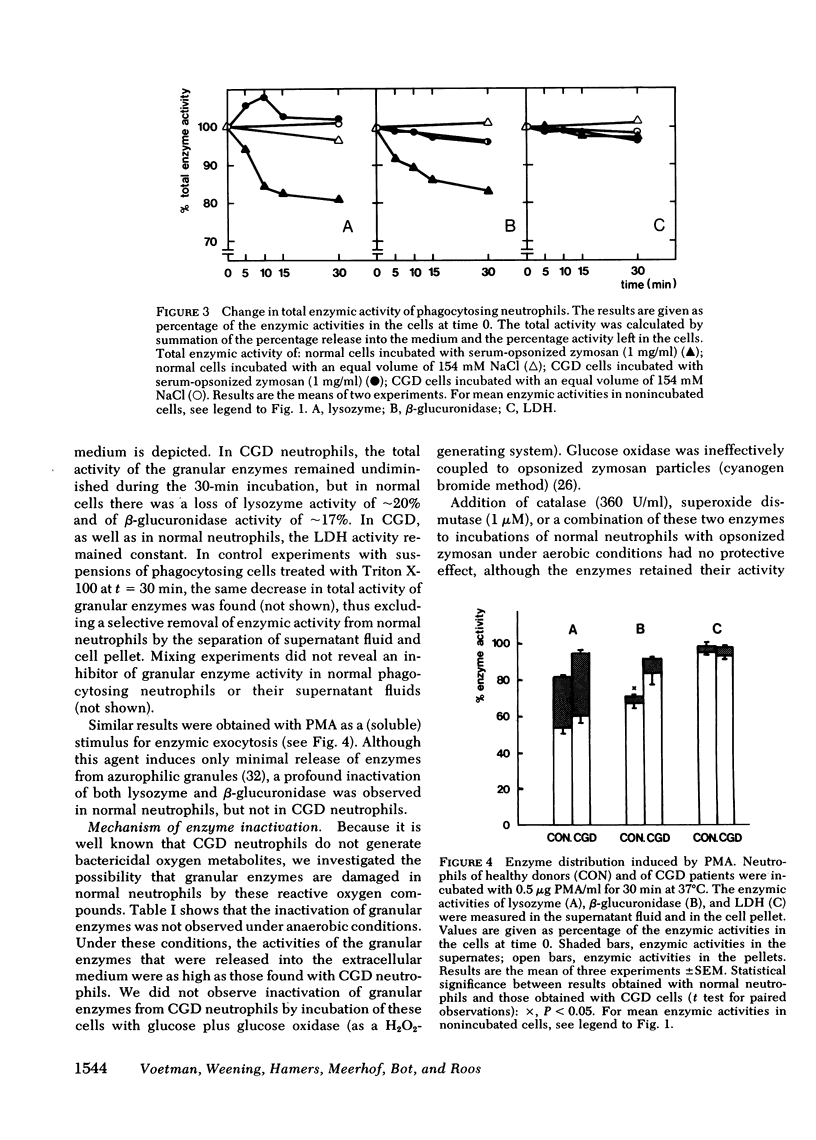
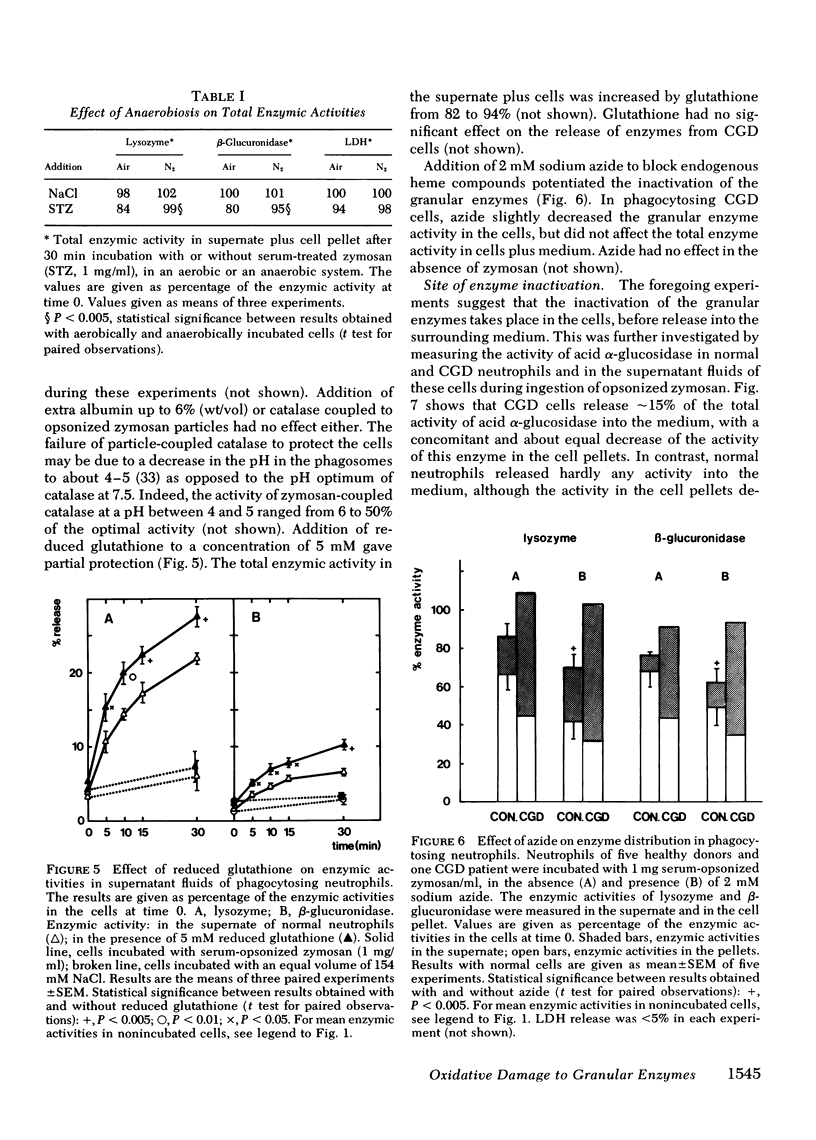
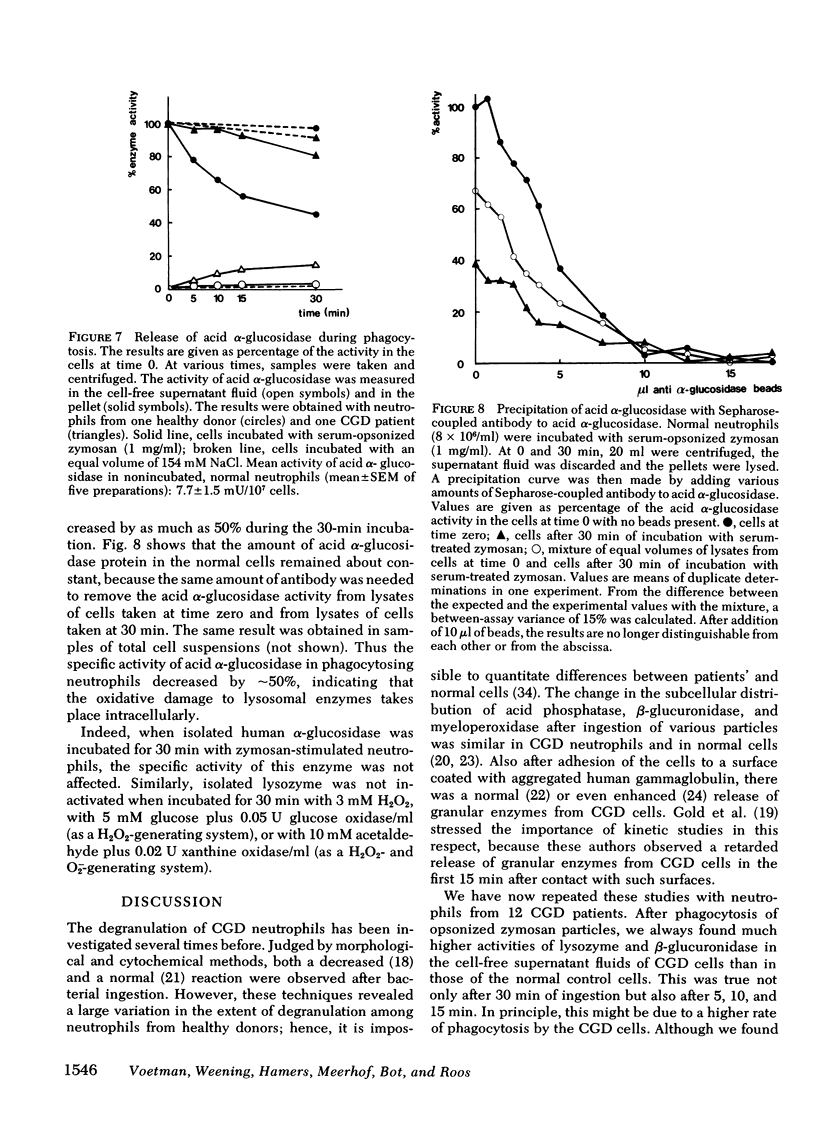
During phagocytosis, neutrophils generate reactive oxygen metabolites and release lysosomal enzymes into the extracellular medium. We have investigated the possibility that these enzyme are inactivated by the oxygen compounds. Phagocytosing neutrophils from 12 patients with chronic granulomatous disease, which do not generate these oxygen metabolites, released two to three times more activity of lysozyme and beta-glucuronidase than did normal neutrophils. This difference proved to be due to a decrease of approximately 20% of the total activity of these enzymes in normal neutrophils, but not in neutrophils of patients with chronic granulomatous disease. This inactivation of enzymes took place during phagocytosis of opsonized zymosan particles as well as during stimulation of normal cells with phorbol myristate acetate. The inactivation was not due to formation of inhibitors. The lysosomal enzymes were not activated when the neutrophils were stimulated under anaerobic conditions. Addition of catalase, superoxide dismutase, or albumin gave no protection against the oxidative damage; reduced glutathione gave partial protection. The oxidative inactivation was more pronounced in the presence of azide. Measurement of the activity and the amount of protein of acid alpha-glucosidase in the cells showed that the specific activity of this enzyme decreased by approximately 50% during 30 min of phagocytosis. This indicates that the inactivation of the lysosomal enzymes takes place in the phagolysosomes, before the enzymes have leaked into the extracellular medium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen V., Koch C., Vejlsgaard R., Wilken-Jensen K. Fatal granulomatous disease. Acta Paediatr Scand. 1968 Mar;57(2):110–114. doi: 10.1111/j.1651-2227.1968.tb04661.x. [DOI] [PubMed] [Google Scholar]
- BRIDGES R. A., BERENDES H., GOOD R. A. A fatal granulomatous disease of childhood; the clinical, pathological, and laboratory features of a new syndrome. AMA J Dis Child. 1959 Apr;97(4):387–408. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Allen J. M., Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977 Aug;50(2):327–335. [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976 Aug;48(2):309–313. [PubMed] [Google Scholar]
- Bainton D. F. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J Cell Biol. 1973 Aug;58(2):249–264. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Baehner R. L. Potentiation of polymorphonuclear leukocyte motile functions by 2,3-dihydroxybenzoic acid. J Lab Clin Med. 1978 Nov;92(5):730–736. [PubMed] [Google Scholar]
- Breton-Gorius J., Coquin Y., Guichard J. Activités peroxydasiques de certaines granulations des neutrophiles dans deux cas de déficit congénital en myéloperoxydase. C R Acad Sci Hebd Seances Acad Sci D. 1975 Apr 14;280(14):1753–1756. [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill B. R., Oliver J. M., Pearson C. B., Leinbach E. D., Berlin R. D. Microtubule dynamics and glutathione metabolism in phagocytizing human polymorphonuclear leukocytes. J Cell Biol. 1978 Feb;76(2):439–447. doi: 10.1083/jcb.76.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., McCall C. E., McPhail L. C., Johnston R. B., Jr Superoxide dismutase activity in leukocytes. J Clin Invest. 1974 Apr;53(4):1197–1201. doi: 10.1172/JCI107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., White J. G., Holmes B. Specific degranulation of human polymorphonuclear leukocytes. Nature. 1974 Mar 22;248(446):347–348. doi: 10.1038/248347a0. [DOI] [PubMed] [Google Scholar]
- Gold S. B., Hanes D. M., Stites D. P., Fudenberg H. H. Abnormal kinetics of degranulation in chronic granulomatous disease. N Engl J Med. 1974 Aug 15;291(7):332–337. doi: 10.1056/NEJM197408152910704. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M. Polymorphonuclear Leukocyte lysosomes and immune tissue injury. Prog Allergy. 1976;20:301–340. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad H. L., Beatty D. W., Dowdle E. B. Chronic granulomatous disease of childhood. S Afr Med J. 1976 Dec 4;50(52):2068–2072. [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Quie P. G., Windhorst D. B., Good R. A. Fatal granulomatous disease of childhood. An inborn abnormality of phagocytic function. Lancet. 1966 Jun 4;1(7449):1225–1228. doi: 10.1016/s0140-6736(66)90238-8. [DOI] [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- Jensen M. S., Bainton D. F. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol. 1973 Feb;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauder E., Kahle L. L., Moreno H., Partin J. C. Leukocyte degranulation and vacuole formation in patients with chronic granulomatous disease of childhood. J Clin Invest. 1968 Aug;47(8):1753–1762. doi: 10.1172/JCI105865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Hook E. W. Leukocyte function in chronic granulomatous disease of childhood. Studies on a seventeen year old boy. Am J Med. 1969 Sep;47(3):473–486. doi: 10.1016/0002-9343(69)90231-9. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Baehner R. L., Weaver D. K. Failure of nitro blue tetrazolium reduction in the phagocytic vacuoles of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Oct;48(10):1895–1904. doi: 10.1172/JCI106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Herron M., Simmons R. L., Quie P. G. Chemotactic deactivation of human neutrophils: possible relationship to stimulation of oxidative metabolism. Infect Immun. 1979 Feb;23(2):282–286. doi: 10.1128/iai.23.2.282-286.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARRY R. M., Jr, CHANDAN R. C., SHAHANI K. M. A RAPID AND SENSITIVE ASSAY OF MURAMIDASE. Proc Soc Exp Biol Med. 1965 Jun;119:384–386. doi: 10.3181/00379727-119-30188. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Weening R. S., Voetman A. A., van Schaik M. L., Bot A. A., Meerhof L. J., Loos J. A. Protection of phagocytic leukocytes by endogenous glutathione: studies in a family with glutathione reductase deficiency. Blood. 1979 May;53(5):851–866. [PubMed] [Google Scholar]
- Roos D., Weening R. S., Wyss S. R., Aebi H. E. Protection of human neutrophils by endogenous catalase: studies with cells from catalase-deficient individuals. J Clin Invest. 1980 Jun;65(6):1515–1522. doi: 10.1172/JCI109817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram A. W., Brouwer-Kelder B., Donker-Koopman W. E., Loonen C., Hamers M. N., Tager J. M. Use of immobilized antibodies in investigating acid alpha-glucosidase in urine in relation to Pompe's disease. Biochim Biophys Acta. 1979 Apr 12;567(2):370–383. doi: 10.1016/0005-2744(79)90123-2. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Peters T. J. Analytical subcellular fractionation of human granulocytes with special reference to the localization of enzymes involved in microbicidal mechanisms. Clin Sci Mol Med. 1977 Apr;52(4):429–442. doi: 10.1042/cs0520429. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Evaluation of opsonic and leukocyte function with a spectrophotometric test in patients with infection and with phagocytic disorders. Blood. 1973 Jul;42(1):121–130. [PubMed] [Google Scholar]
- Stossel T. P., Root R. K., Vaughan M. Phagocytosis in chronic granulomatous disease and the Chediak-Higashi syndrome. N Engl J Med. 1972 Jan 20;286(3):120–123. doi: 10.1056/NEJM197201202860302. [DOI] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Henson P., Holmes B., Good R. A. An in vitro study of exocytosis of neutrophil granule enzymes in chronic granulomatous disease neutrophils. J Immunol. 1974 Apr;112(4):1383–1386. [PubMed] [Google Scholar]
- Weening R. S., Roos D., Loos J. A. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974 Apr;83(4):570–577. [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]



