Abstract
The maintenance DNA methyltransferase (DNMT) 1 and the de novo methyltransferases DNMT3A and DNMT3B are all essential for mammalian development. DNA methylation, catalyzed by the DNMTs, plays an important role in maintaining genome stability. Aberrant expression of DNMTs and disruption of DNA methylation patterns are closely associated with many forms of cancer, although the exact mechanisms underlying this link remain elusive. DNA damage repair systems have evolved to act as a genome-wide surveillance mechanism to maintain chromosome integrity by recognizing & repairing both exogenous and endogenous DNA insults. Impairment of these systems gives rise to mutations and directly contributes to tumorigenesis. Evidence is mounting for a direct link between DNMTs, DNA methylation, and DNA damage repair systems, which provide new insight into the development of cancer. Like tumor suppressor genes (TSGs), an array of DNA repair genes frequently sustain promoter hypermethylation in a variety of tumors. In addition, DNMT1, but not the DNMT3’s, appear to function coordinately with DNA damage repair pathways to protect cells from sustaining mutagenic events, which is very likely through a DNA methylation-independent mechanism. This chapter is focused on reviewing the links between DNA methylation and the DNA damage response.
Keywords: DNA methyltransferase, DNA methylation, genome stability, DNA damage repair
Introduction
DNA methyltransferases (DNMTs), responsible for the transfer of a methyl group from the universal methyl donor, S-adenosyl-L-methionine (SAM), to the 5-position of cytosine residues in DNA, are essential for mammalian development1. There are four members of the DNMT family, including DNMT1, DNMT3A, DNMT3B and DNMT3L. DNMT3L, unlike the other DNMTs, does not possess any inherent enzymatic activity2. The other three family members are active on DNA. DNMT1 encodes the maintenance methyltransferase and DNMT3A/DNMT3B encode the de novo methyltransferases3–4, required to establish and maintain genomic methylation. While this maintenance vs de novo division has been convenient, there is clear evidence for functional overlap between the maintenance and the de novo methyltransferases5–6. Gene knockout analysis in mice has shown that Dnmt1 and Dnmt3a/Dnmt3b genes are all essential for viability. Dnmt1-inactivation leads to very early lethality at embryonic day (E) 9.5, shortly after gastrulation 7–9, whereas Dnmt3b knockout induces embryo death at E14.5–18.5, due to multiple developmental defects including growth impairment and rostral neural tube defects3, 8–9. Dnmt3a−/− mice become runted and die at about 4 weeks of age, although they appear to be relatively normal at birth3.
DNMTs play an important role in genomic integrity, disruption of which may result in chromosome instability and tumor progression. It is well established that DNMTs are required for transcriptional silencing of a number of sequence classes, including imprinted genes, genes on the inactive X chromosome and transposable elements1, 10, and silencing of these sequences is essential for maintaining chromosome stability. Much compelling evidence has come from targeted deletion experiments showing that all three DNA methyltransferases are involved in stabilization of the genome, particularly repetitive sequences3, 11–12. For example, either single knockout of Dnmt1 or double knockout of Dnmt3a and Dnmt3b, enhances telomere recombination11. DNMT3B is specifically required for stabilization of pericentromeric satellite repeats. DNMT3B deficiency results in expansion and rearrangements of pericentromeric repeats3, 12. ICF (Immunodeficiency, Centromere instability and Facial anomalies) syndrome is the only human genetic disorder known to involve biallelic mutations in DNMT3B. It is characterized by chromosomal instability arising due to destabilization of pericentromeric repeats, particular those at juxtacentromeric regions of chromosomes 1, 9 and 163, 12. Of note, cells null for DNMT1 or with hypomorphic mutations in DNMT1 that partially reduce its levels to 30% of WT DNMT1, display significantly greater microsatellite instability13–17, a greater frequency of chromosomal translocations18 and much higher sensitivity to genotoxic agents17, which may promote the development of cancer.
The DNA damage response (DDR) is a genome-wide surveillance system that protects cells from potentially mutagenic DNA insults derived from either endogenous or exogenous sources. The DDR usually functions through the coordinated actions of DNA repair and checkpoint systems to promote DNA damage repair before replication or to activate cell death pathways if excessive damage exists19. Like the cellular DNA methylation machinery, an intact DDR is crucial for preventing cancer. Evidence is mounting to support a link between the DNA methylation and DNA damage repair systems, as first suggested by promoter hypermethylation & silencing of DNA repair genes in multiple types of cancer20. More importantly, DNMT1 may be directly involved in DNA damage repair in a DNA methylation-independent manner14, 17, 21–23. Strong support for this latter notion comes from recent observations that DNMT1 is rapidly and transiently recruited to regions of DNA double strand breaks (DSBs) via its interaction with proliferating cell nuclear antigen (PCNA)21, 24, as well the PCNA-like DNA damage sliding clamp component RAD9 (of the 9-1-1 complex)21. In this chapter, we examine and outline the links between DNMTs and DNA repair systems and discuss the possible mechanisms of how they are orchestrated, with a focus on cancer.
1. Epigenetic Silencing of DNA Repair Pathways through Aberrant Promoter Hypermethylation
DNA repair systems have evolved to maintain genomic integrity by countering threats posed by DNA lesions19. Deficiency in the DNA repair pathways may leave these lesions unrepaired or cause them to be repaired incorrectly, eventually leading to genome instability or mutations that contribute directly to a large array of human diseases including cancer. Carcinogenesis is believed to originate from and be driven by the acquisition of abnormal genetic and/or epigenetic changes. Aberrant DNA hypermethylation, when it occurs at promoter CpG islands (CGIs), leads to potent and heritable transcriptional silencing that inactivates key cellular pathways much like genetic changes (e.g. mutation/deletion) do. In addition to genetic mutations, promoter hypermethylation in DNA repair genes is closely linked to a variety of human tumor types including colorectal, breast, lung cancers and glioma20 (Table 1), suggesting that epigenetic silencing of DNA repair pathways is an important contributor to the development of cancer.
Table 1.
Genes in DNA Damage Repair Pathways that are Hypermethylated
| Repair Pathway | Methylated Gene | Cancer Type | Samples Studied | Samples Methylated | Methylation Frequency (%) | References |
|---|---|---|---|---|---|---|
| MMR | MLH1 | sporadic CRC (MSI+) | 110 | 67 | 61 | 41–44¤ |
| sporadic CRC (MSI−) | 128 | 38 | 30 | 42–43¤ | ||
| sporadic early-onset CRC | 110 | 55 | 50 | 45 | ||
| NSCLC | 77 | 43 | 56 | 32 | ||
| acute myeloid leukemia | 177 | 11 | 6 | 34–36¤ | ||
| ovarian cancer | 672 | 72 | 11 | 33 | ||
| oral squamous cell carcinoma | 99 | 8 | 8 | 29 | ||
| HNPCC | 179 | 2 | 1 | 39–40¤ | ||
| gastric cancer | 306 | 58 | 19 | 30–31¤ | ||
| HNSCC | 49 | 14 | 29 | 37 | ||
| MSH2 | NSCLC | 14 | 4 | 29 | 32 | |
| gastric cancer | 200 | 27 | 14 | 30 | ||
| ovarian cancer | 56 | 29 | 52 | 46 | ||
| sporadic CRC | 36 | 1 | 3 | 47 | ||
| HNPCC | 46 | 11 | 24 | 48 | ||
| MSH3 | gastric cancer | 200 | 25 | 13 | 30 | |
| MSH6 | breast cancer | 33 | 92–95*/20© | 50 | ||
| BER | TDG | multiple myeloma | KAS-6/1 cell line | 52 | ||
| MBD4 | CRC | 339 | 24*/14© | 53 | ||
| OGG1 | thyroid cancer | 38 | 2 | 5 | 54 | |
| NER | XPC | bladder cancer | 37 | 12 | 32 | 56 |
| ERCC1 | glioma | 32 | unknown | 57 | ||
| XRCC1 | gastric cancer | 25 | unknown | 60 | ||
| RAD23B | multiple myeloma | KAS-6/1 cell line | 61 | |||
| HR | BRCA1 | NSCLC | 98 | 29 | 30 | 69 |
| sporadic ovarian cancer | 81 | 12 | 15 | 66 | ||
| sporadic breast cancer | 190 | 24 | 13 | 64,70¤ | ||
| hereditary breast cancer | 162 | 18 | 11 | 70 | ||
| early onset gastric cancer | 104 | 0.6 | 1 | 67 | ||
| bladder cancer | 96 | 0.71 | 1 | 68 | ||
| BRCA2 | breast cancer | 33 | 59–64*/10© | 50 | ||
| NSCLC | 98 | 41 | 42 | 69 | ||
| FANCC | acute lymphoblastic leukaemia | 97 | 3 | 3 | 74 | |
| acute myeloid leukaemia | 143 | 1 | 1 | 74 | ||
| FANCF | HNSCC | 89 | 13 | 15 | 75 | |
| NSCLC | 158 | 22 | 14 | 75 | ||
| cervical cancer | 91 | 27 | 30 | 76 | ||
| ovarian cancer | 53 | 7 | 13 | 77 | ||
| FANCL | acute lymphoblastic leukaemia | 97 | 1 | 1 | 74 | |
| NHEJ | XRCC5 | NSCLC | 98 | 19 | 19 | 69 |
| ATM/ATR | ATM | HNSCC | 100 | 25 | 25 | 101 |
| breast cancer | 23 | 18 | 78 | 100 | ||
| CRC | HCT116 cell line | 99 | ||||
| CHK2 | NSCLC | 139 | 39 | 28 | 106 | |
| glioma | 5 | 5 | 100 | 107 | ||
| Others | MGMT | oral squamous cell carcinoma | 99 | 40 | 40 | 29 |
| gastric cancer | 200 | 50 | 25 | 30 | ||
| CRC | 36 | 14 | 39 | 80 | ||
| HNSCC | 21 | 6 | 29 | 80 | ||
| NSCLC | 34 | 10 | 29 | 80 | ||
| lymphomas | 61 | 15 | 25 | 80 | ||
| glioma | 140 | 54 | 39 | 80 | ||
| WRN | gastric cancer | 38 | 10 | 26 | 91 | |
| CRC | 182 | 69 | 38 | 91 | ||
| NSCLC | 56 | 21 | 38 | 91 | ||
| prostate cancer | 20 | 4 | 20 | 91 | ||
| breast cancer | 58 | 10 | 17 | 91 | ||
| thyroid cancer | 32 | 4 | 13 | 91 | ||
| non-Hodgkin lymphoma | 118 | 28 | 24 | 91 | ||
| acute lymphoblastic leukemia | 21 | 2 | 10 | 91 | ||
| acute myeloblastic leukemia | 36 | 3 | 8 | 91 | ||
| chondrosarcomas | 15 | 5 | 33 | 91 | ||
| osteosarcomas | 27 | 3 | 11 | 91 |
Mean methylation level (%) in cancer vs © mean methylation level (%) in control
Indicates references from which methylation data derived from similar samples was pooled for this summary
CRC-colorectal cancer; NSCLC-non-small cell lung cancer; HNPCC-hereditary non-polyposis colorectal cancer; HNSCC-head and neck squamous cell carcinoma
1.1 Epigenetic Inactivation of the DNA Mismatch Repair (MMR) Pathway
MMR is a genome-surveillance system to maintain genomic integrity through recognizing and correcting mismatched nucleotides arising during DNA replication, homologous recombination (HR), or other forms of DNA damage. Impairment of this system gives rise to microsatellite instability (MSI)25–26, which has now been recognized as a hallmark of MMR gene-deficient cancers. Microsatellite loci, widely dispersed in the genome, are repetitive sequences consisting of short runs of nucleotides, typically one to four bases in length. Repetitive regions may give rise to the formation of secondary structures, which are subject to expansion or contraction. The secondary structures, if incorrectly resolved, lead to slippage of DNA polymerases along repetitive sequences during replication. Microsatellites are particularly susceptible to length change mutations during replication and transcription, resulting in frameshift mutations if they are located within a gene25–26. MMR deals with these changes to maintain microsatellite stability. MMR comprises the MutS complex and the MutL complex. MutS recognizes the mismatched base, while MutL recruits repair enzymes to damage sites via its binding with MutS27. There are two main MutS complexes in humans, MutSα and MutSβ. MutSα, consisting of the MutS homologue 2 (MSH2) protein bound to MSH6, recognizes single-base mismatches or small insertion/deletion loops (indels), whereas MutSβ, consisting of MSH2 and MSH3, repairs only indels28. The main complex for MutL in humans is MutLα, consisting of a heterodimer of MLH1 and PMS226. Mutations in or epigenetic silencing of MMR genes like MLH1 and MSH2 is closely associated with a variety of human cancers such as hereditary non-polyposis colon cancer (HNPCC), sporadic colon cancer, and ovarian cancer29.
MLH1 plays a central role in coordinating various steps in MMR via interacting with other MMR proteins and modulating their activities. Hypermethylation of the MLH1 promoter is observed in a variety of cancers including oral squamous cell carcinoma30, gastric cancer31–32, non-small cell lung cancer (NSCLC)33, ovarian cancer34, acute myeloid leukemia35–37, head and neck squamous cell carcinoma (HNSCC)38, HNPCC39–41, and particularly in colorectal cancer (CRC)42–45 (Table 1). The reduced MLH1 protein expression is correlated with high-level methylation detected in human CRC samples, whereas samples with low-level methylation display expression levels similar to those observed in methylation-negative samples46, strongly suggesting that the MLH1 gene is inactivated via promoter hypermethylation in a dose-dependent manner. Nonetheless, it is not clear whether a moderate degree of methylation affects MLH1 gene expression or not. On the basis of observations made in germline cells, it has long been believed that MLH1 promoter methylation involves only one allele of maternal origin. However, more recent findings demonstrate that there is biallelic involvement of MLH1 promoter hypermethylation in many cancers46. The causal link between MSI and epigenetic inactivation of MLH1 is further highlighted by the observation that 90% of MSI+ HNPCC have MLH1 hypermethylation, while 95% of MSI- samples do not20.
MSH2 is also hypermethylated in multiple tumor types, including gastric cancer31, NSCLC33, ovarian cancer47, sporadic CRC48, and HNPCC49 (Table 1). Interestingly, promoter methylation of MSH2 in HNPCC occurs primarily in patients with germline mutations in MSH2 rather than in germline mutation–negative cases49. Seventy percent of patients with MSH2 methylation also present germline mutations in this gene, clearly indicating that methylation is the second inactivating hit in these tumors49. DNA hypermethylation can be caused by transcription across a CpG island within a promoter region. Recent studies have revealed that deletions of the last exons of the EpCAM gene, located immediately upstream of MSH2, give rise to somatic hypermethylation of the MSH2 promoter50. Deletions at the most 3′-end of the EpCAM gene result in loss of its polyadenylation signal, which abolishes transcription termination. Transcription of EpCAM then continues downstream into the MSH2 promoter and induces promoter hypermethylation of MSH2. DNA methylation triggered by transcriptional read-through of a neighboring gene, in either sense or antisense, may represent a general mutational mechanism that promotes aberrant epigenetic changes. Like MLH2, other MutS homologues, including MSH3 and MSH6, are also inactivated by hypermethylation in tumors such as breast51 and gastric cancers31 (Table 1).
1.2 Epigenetic Inactivation of the Base Excision Repair (BER) and Nucleotide Excision Repair (NER) Pathway
The specific pairing of DNA bases in the genome is constantly challenged by endogenous metabolic byproducts and environmental insults. BER is responsible for the removal of damaged DNA bases and their backbones to prevent mutations that could give rise to cancer19, 52. In BER, abnormal DNA bases are recognized and removed by specific glycosylases, followed by recruitment of other enzymes including nuclease, polymerase and ligase proteins, to complete the repair process via excising the remaining sugar fragments and reinstalling an intact correctly based-paired nucleotide19.
Either thymine DNA glycosylase (TDG) or methyl-CpG binding domain 4 (MBD4), mediate a specific BER pathway for the correction of G/T mismatches arising due to 5-methylcytosine deamination leading to C to T transitions. DNA hypermethylation-mediated silencing of TDG and MBD4 may contribute to the frequent genomic instability that occurs in cancer cells53 (Table 1). TDG promoter hypermethylation negatively correlates with its expression. TDG down-regulation leads to less efficient DNA repair activity in response to hydrogen peroxide-induced DNA damage. Ectopic expression of TDG, however, functionally compensates for lower repair activities of damaged DNA in the KAS-6/1 myeloma cell line with extensive endogenous TDG gene hypermethylation53. MBD4, like TDG, is also subject to promoter hypermethylation and gene silencing in tumors like sporadic CRC and ovarian cancer54. Another DNA glycosylase, OGG1, which mediates removal of 8-oxoguanine induced by oxidative damage, is also subject to inactivation via promoter methylation in cancer cells55 (Table 1).
Of all the repair systems, NER recognizes the most varied types of DNA lesions, contending with the diverse class of helix-distorting damage that interferes with base pairing and obstructs replication and transcription. In NER, there exist two sub-pathways that differ in the mechanism of lesion recognition: global genome NER (GG-NER) that surveys the entire genome for distortions, and transcription-coupled repair (TCR), which targets damage that blocks elongating RNA polymerases19, 56. NER, therefore, plays a particularly important role in preventing mutations. Thus far, three syndromes, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy (TTD), are closely associated with NER defects56. Of these, patients with xeroderma pigmentosum, attributable to mutations in one of seven xeroderma pigmentosum (XP) group genes (XPA–XPG), show a dramatically increased incidence of UV light-induced skin cancer19, 56.
It was reported recently that the XPC promoter is epigenetically inactivated in bladder cancer57 (Table 1). XPC promoter methylation is significantly elevated in cancerous bladder compared to normal tissue, leading to reduced mRNA levels in the tumor57. Epigenetic defects in the XPC gene may also influence malignant behavior and prognosis. ERCC1 is a crucial protein in the NER pathway primarily involved in the repair of platinum-DNA adducts. Aberrant CpG island methylation in the ERCC1 promoter region has been observed in human glioma cell lines and primary tumors, which is associated with cisplatin chemosensitivity58. In a rat lung cancer model, however, ERCC1 methylation is detected in only a very small proportion of samples59. Deficiency in XRCC1, a scaffolding protein for BER and single-strand break repair (SSBR), is associated with enhanced risk of lung cancer60. XRCC1 is subject to aberrant promoter methylation in human gastric cancer tissues61. In lung cancer, infiltrating carcinomas exhibit statistically higher levels of methylation at the XRCC1 promoter compared to normal, hyperplastic, and squamous metaplastic tissues59. RAD23B, a key component for damage recognition in NER, is also hypermethylated in multiple myeloma62.
1.3 Epigenetic Inactivation of HR and Non-Homologous End-Joining (NHEJ) DNA Repair Pathway Components
HR not only provides an important mechanism to repair several types of DNA lesions that pose a threat to genome integrity, including DNA DSBs, DNA damage encountered during DNA replication and DNA interstrand cross links (ICLs), but is also required to restart stalled replication forks during the late S and G2 phases of the cell cycle63–64. HR promotes precise repair of DNA damage using the intact sister chromatid as a template. Deficiency of HR leads to more error-prone repair, which is associated with mutagenesis and predisposition to cancer63.
The BRCA1 and BRCA2 genes are both essential for HR-mediated DNA repair. BRCA1 appears to act as a signal integrator that links DNA damage sensors with response mechanisms. BRCA2, however, is more directly involved in homology-directed DSB repair, as it mediates formation of a RAD51-DNA nucleoprotein filament that catalyzes strand invasion during HR. BRCA1 and BRCA2 are frequently mutated in hereditary breast and ovarian cancers, but seldom in sporadic cases of these tumor types. Epigenetic inactivation of BRCA1 via promoter hypermethylation, however, plays an important role in tumorigenesis in a wide array of cancers including breast65–66, ovarian67, gastric68, bladder69 and non-small cell lung cancers70, both hereditary71 and sporadic forms20, 39 (Table 1). It is believed that epigenetic silencing of BRCA1 creates a new mutator pathway that generates mutations and gross chromosomal rearrangements via p53 signaling. This idea is supported by several observations including one demonstrating that p53 inactivation rescues the impact of BRCA1 deficiency on cell survival20, 72. Although much less frequently than BRCA1, BRCA2 also acquires promoter region hypermethylation that is closely associated with its reduced expression in breast cancer51 and NSCLC70 (Table 1).
The primary function of the Fanconi anemia (FA) pathway is to repair inter-strand DNA cross-links, which promotes HR via coordinating other DNA damage-responsive events to stabilize stalled replication forks, to convey signals to DNA checkpoint pathways, and to facilitate recovery of replication forks73. FA is a genomic instability syndrome characterized by bone marrow failure, developmental abnormalities, and increased cancer incidence, which is caused by mutations in one of thirteen distinct genes (FANCA, FANCB, FANCC, FANCD1, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ, FANCL, FANCM, and FANCN)73. Eight of them (FANCA, B, C, E, F, G, L and M) form the FA core complex. This group of genes contain a high GC content and CpG islands at their promoter regions, making them potential targets for aberrant hypermethylation-mediated silencing74. This idea has received support from observations that FANCC, FANCF and FANCL acquire promoter methylation during human carcinogenesis39, 75. Of these, FANCF displays hypermethylation the most frequently, occurring in 14% to 28% of different cancers including NSCLC76, HNSCC76, cervical77, and ovarian 39, 78 (Table 1).
Unlike HR, which performs error-free repair, NHEJ simply restores DNA integrity by joining the two DNA ends. This type of repair is error-prone and frequently results in the loss or addition of several nucleotides at the break site. Despite its mutagenic consequences, NHEJ is the major DSB repair pathway in mammalian cells. Defects in NHEJ lead to chromosomal translocations and genomic instability. In NHEJ, DSBs are detected by the KU70/KU80 heterodimer; the KU complex then activates the protein kinase DNA-PKcs (DNA-dependent protein kinase catalytic subunit), leading to recruitment and activation of end-processing enzymes, polymerases and finally ligation of the breaks by the XRCC4/DNA ligase IV complex. In the NHEJ pathway, only the XRCC5 gene, encoding the KU80 protein, has been reported to be inactivated via epigenetic mechanisms70 (Table 1). Low expression of XRCC5 in squamous cell carcinoma and NSCLC is significantly associated with promoter region hypermethylation. Treatment of NSCLC cells with the DNA demethylating agent 5-aza-2′-deoxycytidine (5-aza-CdR), however, does not result in increased KU80 expression70. Thus, the underlying mechanisms promoting and maintaining XRCC5 silencing await further investigation, particularly in more samples and more types of cancer.
1.4 Epigenetic Silencing of O6-methylguanine-DNA Methyltransferase (MGMT)
O6-methylguanine, which arises due to alkylation reactions, pairs with thymine rather than cytosine, resulting in G:C to A:T mutations during DNA replication. MGMT, also known as O6-alkylguanine-DNA alkyltransferase (AGT), repairs DNA damage by transferring the methyl groups on the O6 position of guanine to an active site cysteine residue to protect cells from sustaining mutagenic events, which has been demonstrated by gain- or loss-of function experiments in vitro and in vivo79. The MGMT protein is unique among DNA-repair components because it acts alone to remove DNA adducts. Although MGMT is ubiquitously expressed in normal human tissues, mean enzymatic activity in malignant tissues is usually higher than in their normal counterparts. However, there is a variety of tumors such as glioma, CRC, NSCLC and HNSCC that lack MGMT expression20, 39 (Table 1). It has been well documented that MGMT deficiency often arises due to abnormal promoter methylation20, 39, 80. For example, 29% of NSCLCs and 38% of CRCs display aberrant MGMT methylation, in which the presence of hypermethylation is highly associated with loss of MGMT protein81. MGMT is the most frequently methylated gene in central nervous system tumors. Epigenetic silencing of MGMT via promoter hypermethylation occurs in approximately 40% of primary glioblastomas and over 70% of secondary glioblastomas. It is also detected in 50% of the diffuse and anaplastic astrocytomas and approximately two thirds of oligodendroglial and mixed tumors82. These results, together with a causal relationship between DNA methylation of the MGMT CpG island and decreased transcription of the gene in cell culture-based studies, demonstrates that DNA methylation is an important mechanism for silencing the MGMT gene in human cancers.
Epigenetic silencing of MGMT may initiate an important mutator signaling cascade in human cancers since MGMT loss causes G:C to A:T transitions, which lead to downstream gene mutations. This proposal is strongly supported by an analysis of point mutations in KRAS and p53. KRAS, the most commonly altered oncogene in cancer, is an early key player in multiple signal pathways. Loss of MGMT is associated with increased KRAS mutations possessing G:C to A:T transitions in colon83 and gastric cancer84. p53 is the most frequently mutated TSG (tumor suppressor gene) in human cancer, and the majority of known p53 mutations are G:C to A:T transitions66, 85. Epigenetic inactivation of MGMT may lead to G:C to A:T transition mutations in p53, which has been observed in several types of cancer including colorectal66, liver86, lung87, esophageal squamous cell carcinomas88 and glioma89. Interestingly, MGMT promoter methylation is associated with improved disease chemo-sensitivity and prolonged survival time in patients treated with alkylating agent-based therapies90. However, it is unclear whether the improved survival is specifically due to loss of MGMT expression or accompanying drug sensitivity.
1.5 Epigenetic Silencing of WRN
Werner syndrome (WS) is a rare autosomal recessive disease, characterized by premature onset of aging, genomic instability, and increased cancer incidence. WS is caused by null mutations at the WRN locus at 8p11.2–p12, which codes for a DNA helicase belonging to the RecQ family. Deficiency in WRN function causes defects in DNA replication and recombination, as well as DNA repair.
WRN is a 180-kd nuclear protein that has a unique interaction with its DNA substrates through its C terminal RQC domain during base-separation91. In addition to two C-terminal ATPase domains encoding for helicase activity, the WRN protein contains an N-terminal domain coding for exonuclease activity. Its helicase and exonuclease activities function in a coordinated manner, suggestive of roles in DNA repair, recombination, and replication. Recently, the WRN protein was also shown to be involved in telomere maintenance based on the discovery that its deficiency leads to accelerated telomere shortening in WS cells92. These multiple roles of the WRN protein highlight its importance in aging and cancer.
The evidence suggesting that WRN acts as a tumor suppressor gene are derived primarily from WS, which is characterized by the early onset development of a variety of cancers due to germline WRN mutation; somatic mutations in the WRN gene have not been reported. Epigenetic inactivation of WRN provides additional support for its TSG role in sporadic cancer. The WRN promoter undergoes hypermethylation in a wide array of tumors including colorectal, gastric, prostate, non-small cell lung and breast cancers93–94 (Table 1). Epigenetic silencing of WRN via methylation not only leads to the loss of protein and enzyme activity, but also to chromosomal instability. Furthermore, the above phenotype is reversed by DNA-demethylating agents. Most importantly, restoration of WRN expression induces its tumor-suppressor effects, such as inhibition of colony formation and tumor growth93. Taken together, aberrant epigenetic silencing of WRN, a candidate TSG, may play an important role in human cancers. Interestingly, WRN was recently shown to be associated with promoter methylation of the OCT4 gene95, which encodes a crucial transcription factor for the maintenance of cell pluripotency. During differentiation of human pluripotent NCCIT embryonic carcinoma cells, WRN localizes to the OCT4 promoter region with de novo DNA methyltransferase DNMT3B and promotes differentiation-dependent OCT4 silencing and promoter methylation95. Deficiency in WRN blocks DNMT3B recruitment to the promoter and leads to decreased promoter methylation of OCT495. Therefore, WRN may also contribute to the control of stem cell differentiation via epigenetic silencing of the key pluripotency transcription factor OCT4.
1.6 Epigenetic Inactivation of ATM/ATR Signaling
DNA damage signaling requires the coordinated action of a large array of molecules that can be categorized as DNA damage sensors, transducers, mediators, and effectors according to their functions. Upon damage of DNA, the MRE11–RAD50–NBS1 (MRN) sensor complex recognizes DSBs and the replication protein A (RPA) complex processes accumulated single stranded DNA (ssDNA). The transducer ATM and ATR kinases are recruited to and activated by DSBs and RPA-coated ssDNA, respectively. With the help of mediators (including 53BP1, MDC1, BRCA1, MCPH1 and PTIP in ATM signaling, and TopBP1 and Claspin in ATR signaling), ATM and ATR activate the effector kinase CHK2 and CHK1, respectively, which then spread the signal throughout the nucleus96–98. CHK1 and CHK2 decrease cyclin-dependent kinase (CDK) activity, which slows down or arrests cell-cycle progression. Meanwhile, ATM/ATR signaling promotes DNA repair through various mechanisms. Through ATM/ATR signaling, DNA repair and cell cycle progression are closely coordinated. The coordinated action of DNA repair and cell cycle controls either promotes the resumption of normal cell functioning before replication or triggers apoptosis/cell death when normal cell functioning cannot be restored; both mechanisms act as barriers to tumorigenesis19.
Ataxia-telangiectasia (AT) is a rare autosomal recessive disorder, characterized by progressive cerebellar ataxia, oculocutaneous telangiectasia, susceptibility to bronchopulmonary disease, and lymphoid tumors. AT is caused by deficiency in the ATM (Ataxia-telangiectasia mutated) gene, localized on chromosome 11q22–23. ATM is a Ser/Thr protein kinase of the phosphoinositide 3-kinase (PI3K)-related protein kinase (PIKK) family, which also includes ATR, DNA-PKcs and SMG1. ATM may have as many as 700 substrates99–100, highlighting its multiple functions in various biological processes including cancer. Loss of heterozygosity in ATM results in reduced protein expression, however this mechanism explains only a small proportion of cancers where ATM down-regulation is observed. In sporadic cancer, which accounts for 90–95% of tumors, the probability of ATM gene mutations are low, whereas altered expression of ATM is frequently observed. It is therefore likely that the epigenetic modifications have an impact on ATM expression in these cases (Table 1). Initial proof for this idea came from studies using the human colon cancer cell line HCT116101. In this cell line, ATM displays aberrant promoter methylation, which inversely correlates with its low expression and low radio-sensitivity. The significance of this finding is underscored by further observations that treatment of HCT116 cells with 5-azacytidine (a DNA demethylating agent) restores expression of ATM and radio-sensitivity101. ATM is also epigenetically silenced in primary cancers. For example, 78% of surgically removed breast tumors102 and 25% of HNSCC103 display aberrant methylation in the ATM promoter region accompanied by reduced ATM.
CHK2, the mammalian homolog of the yeast Rad53 and Cds1, is located at chromosome 22q12.1, spans approximately 50 kilobases, and consists of 14 exons104. CHK2, activated by ATM, responds primarily to DSBs. Its fundamental role is to coordinate cell-cycle progression with DNA repair and cell survival or death. Germ line mutations in the CHK2 gene predispose to Li-Fraumeni syndrome (LFS), characterized by multiple tumors at early age with a predominance of breast cancer and sarcomas105. Somatic mutations in CHK2 exist also, although they occur in only a small subset of sporadic human malignancies, including carcinomas of the breast, lung, colon, and ovary, osteosarcomas, and lymphomas106. The finding of both germ line and somatic mutations suggests that CHK2 acts as a TSG. This is further supported by the observation that down-regulation of CHK2 is associated with promoter methylation in sporadic cancers including lung cancer, glioma and Hodgkin’s lymphoma107–109. For example, DNA hypermethylation of the distal CHK2 CGIs occurs in 28.1% of NSCLCs and 40.0% of squamous cell carcinomas, which inversely correlates with CHK2 mRNA levels. It should be noted, however, that observations in breast, colon, and ovarian cancers, do not support a causative link between DNA methylation and gene expression of CHK2110–111.
2. DNMT1 and MMR
The function of the MMR pathway is to correct base substitution mismatches and insertion-deletion mismatches generated in newly replicated DNA112. Deficiencies in or inactivation of this pathway have profound biological consequences. Loss of MMR activity is attributed to the initiation and promotion of multistage carcinogenesis113. A growing number of reports have demonstrated that loss of DNMT1 function has a significant impact on MSI - a hallmark of MMR efficiency, suggesting it has a role in the MMR pathway (Fig. 1). Using genetic screens in Blm deficient embryonic stem (ES) cells, Dnmt1 was identified as an MMR modifier gene. Dnmt1 deficiency in murine ES cells results in a 4-fold increase in the MSI rate13. Further support for this finding comes from several other laboratories14–17, 114. DNMT1 deficiency enhances microsatellite mutations for both integrated reporter genes13–14, 16–17 and endogenous repeats15. This finding holds true for both ES cells and somatic cells. In a murine ES cell line with homologous deletion of Dnmt1, the stability of five endogenous microsatellite repeats (two mononucleotide and three dinucleotide), exhibiting instabilities in mismatch repair-deficient cells were analyzed. A significantly higher frequency of instability was detected at three of the five markers in Dnmt1−/− ES cells compared to the wild-type ES cells15. The slippage rate of a stable reporter gene was also monitored. Dnmt1 deficiency led to a 7-fold higher rate of microsatellite slippage in Dnmt1−/− ES cells compared to wild type cells14. Notably, no DNA methylation in the region flanking the reporter gene was discovered, regardless of Dnmt1 status, suggesting that the effect of Dnmt1 on MMR was not at the level of DNA methylation14. Enhanced MSI is associated with higher levels of histone H3 acetylation and lower MeCP2 binding at regions near the assayed microsatellite, suggesting that Dnmt1 loss decreases MMR efficiency by modifying chromatin structure. CAG repeat expansions are closely associated with human age-related diseases including twelve neurodegenerative disorders. Repeat instability induced by CAG repeat expansion requires the mismatch repair components16, 115. DNMT1 deficiency induces destabilization and intergenerational expansion of CAG triplet repeats16. Double knockdown of MLH1 and DNMT1, however, additively increases the frequency of CAG contraction114. Specific targeting of DNMT1 in hTERT-immortalized normal human fibroblasts by siRNA induces both resistance to MSI and the drug 6-thioguanine (which induces cytotoxic DNA damage due to its misincorporation opposite thymine116) at a CA17 reporter gene; two hallmarks of MMR deficiency. Mutation rates correspond well with DNMT1 levels, ranging from 4.1-fold in cells with 31% of the normal DNMT1 protein level, to 10-fold in cells with 12% of the normal DNMT1 protein level17. This suggests that DNMT1 regulates microsatellite stability in a dose-dependent manner. The exact underling mechanism of how DNMT1 is involved in MSI appears complex and remains elusive. Microsatellite methylation probably provides a mechanism for length stabilization by subsequent transcriptional repression of genes containing or proximal to microsatellites with methylated CpG repeats. However, increased mutations usually occur at microsatellite repeats that do not contain any CpG sites in the repeat itself 13, 15–16, 114 or nearby14, indicating that DNA methylation changes around microsatellite repeats, at least in some cases, are not the primary cause of the instability. Alternatively, DNMT1 might influence transcriptional repression and MSI through chromatin remodeling14.
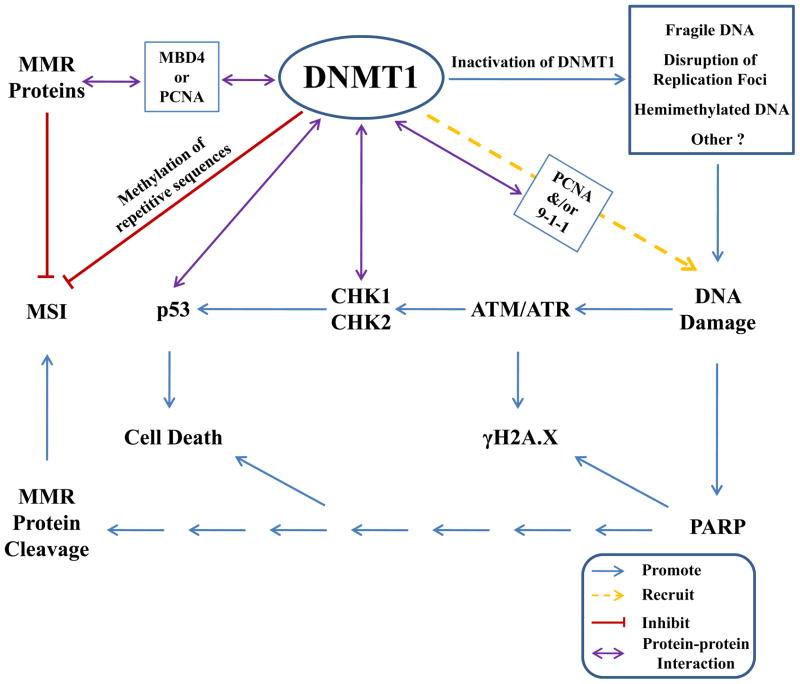
Fig. 1. Impact of DNMT1 on MMR and DDR.
DNMT1 may promote stabilization of microsatellites via methylation of CpG repeats and it also interacts with DNA repair proteins via third-party mediators (e.g. MBD4 and PCNA). Moreover, deficiency in DNMT1 leads to activation of PARP signaling, eventually resulting in MMR protein cleavage. DNMT1 is also closely associated with DDR. Inactivation of DNMT1 may induce several changes to DNA and/or chromatin including increased DNA fragility, disruption of replication foci, and accumulation of hemimethylated DNA, which may be recognized as “damage” and activate the DDR. Strong support for a direct link between DNMT1 and DDR comes from the identification of several protein-protein interactions involving DNMT1 and DDR proteins. DNMT1 is recruited to sites of DNA damage via its interaction with PCNA and 9-1-1. DNMT1 is also capable of binding CHK1 and p53, which promote cell cycle arrest and apoptosis, respectively.
The impact of DNMT1 on the MMR pathway is further highlighted by the observation that DNMT1 and the MMR proteins probably interact with each other through a third-party mediator (Fig. 1). The methyl CpG-binding protein MBD4/MED1 may provide a functional link between MMR and DNMT1 through protein-protein interaction. MBD4, which possesses glycosylase repair activity for G:T mismatches, is involved in NER as well as MMR. MBD4 binds MLH1 via its C-terminal glycosylase domain117–118. Deletion of Mbd4 in MEFs induced destabilization of MMR proteins and conferred resistance to antitumor drugs including 5-FU and platinum119. MBD4 and TDG have functional overlap and they interact with the de novo methyltransferases DNMT3A and DNMT3B120–121. MBD4 also interacts with maintenance methyltransferase DNMT1 via its N-terminal MBD domain118. Based on a combination of immunoprecipitation and GST-pull down experiments in mouse, rat and Xenopus, a minimal domain of approximately 70 amino acids in the N-terminal targeting sequence region of DNMT1 was shown to be required for MBD4 to bind to DNMT1118, which overlaps with a region in rat DNMT1 that interacts with MECP2122. Through interacting directly with both DNMT1 and MLH1, MBD4 recruits MLH1 to heterochromatic sites that are coincident with DNMT1 localization118. Similarly, MBD4/MLH1 accumulates at DNA damage sites where DNMT1 is recruited after laser microirridation118. Loss of DNMT1 induces p53-dependent apoptosis, which can be rescued by inactivation of p53123. The MBD4/MLH1 complex also mediates the apoptotic response to DNMT1 depletion118. Colocalization of these proteins at damaged regions implies that they function coordinately in the cellular decision to repair the lesion or activate apoptosis. Like MBD4, PCNA may act as a mediator between MMR and DNMT1 because of its direct interaction with both systems. PCNA interacts with multiple components of the MMR pathway including MSH6, MSH3, and MLH1. Disruption of this interaction confers a mismatch repair defect in vivo and in vitro124–126. Both MSH6 and MSH3 co-localize with PCNA at replication foci during S-phase127. MLH1 is recruited to damage sites where PCNA and DNMT1 also accumulate, although with slower kinetics than DNMT1118, 128. The recruitment of DNMT1 to both the replication fork and DNA damage sites is through a direct interaction with PCNA and possibly CHK1 and the 9-1-1 complex as well21, 24. However, there is no report showing that PCNA, MLH1 and DNMT1 colocalize together, implying that PCNA might interact with each protein at a different time. Nonetheless, the functional mechanisms of whether and how these factors are orchestrated in response to DNA damage remains to be further investigated.
Most recently, DNMT1 deficiency has been shown to induce the depletion of multiple repair factors at the protein level (Fig. 1)17, highlighting its importance not only in MMR efficiency, but also in DDR signaling. In normal human fibroblasts and CRC cell lines, DNMT1 knockdown leads to a matching decrease in MLH1 at the protein, but not the mRNA level17. Loss of MLH1, however, does not lead to expression changes in DNMT117. Promoter hypermethylation of MLH1, although frequently observed in sporadic colon cancers39, does not appear to be the cause leading to gene inactivation in the context of DNMT1 deficiency. MLH1 hypermethylation in DNMT1-deficient cells was further ruled out using a bisulfite pyrosequencing assay17. Further observations suggest that DNMT1 deficiency affects the steady-state levels of a number of repair proteins, including MSH2, MSH6, and PMS2, as well as MBD417. Loss of multiple MMR components in DNMT1 hypomorphic cells indicates that DNMT1 might play an indirect role in the stabilization or proteolytic cleavage of these proteins, rather than directly interacting with each of them. It is documented that DNMT1 deficiency activates the DDR, which leads to cell cycle arrest21, 123 and the triggering of cell-death pathways123 that may result in cleavage of proteins including MLH1129, which might account for MMR protein depletion after DNMT1 knockdown. Loss of DNMT1 activates ATM/ATR, which normally phosphorylate H2A.X leading to focal accumulation of γH2A.X, a hallmark of DDR21. If excessive damage exists, p53-dependent123 and other cell-death pathways are activated to maintain genomic integrity. Elevated γH2A.X levels in DNMT1 hypomorphic cells can be partially reduced through inhibition of ATM/ATR singaling17. However, the PARP (PAR polymerase) inhibitor DPQ also reduces the level of γH2A.X, to an extent exceeding that observed with the ATM/ATR inhibitor caffeine. In keeping with these observations, the viability of DNMT1-depleted cells treated with DPQ is enhanced to a greater extent than treatment of cells with agents that inhibit caspases or p5317. These findings, together with the observation that PARylation increases after DNMT1 loss, clearly demonstrate that PARP is involved in the DDR and cell death process in cells deficient in DNMT1 (Fig. 1). PARP catalyzes the polymerization of ADP-ribose (PAR) units on target proteins using NAD+ (nicotinamide adenine dinucleotide) molecules as a donor130. NAD+ depletion, induced by severe DNA damage, gives rise to mitochondrial membrane depolarization and AIF (apoptosis initiation factor) translocation. It eventually results in an activation of caspases that lead to protein cleavage and cell death. DNA repair protein MLH1129, along with BLM1131 and ATM132, are preferred targets of caspases. Treatment with the PARP inhibitor DPQ, as expected, leads to an increase in full-length MLH1 protein levels in DNMT1-depleted cells17. Taken together, DDR signaling, particularly the cell death pathway mediated by PARP, may play a substantial role in regulating cleavage of MMR repair proteins in cells deficient for DNMT1 (Fig. 1).
3. DNMT1 and the DNA Damage Response
Reduction of DNMT1 levels activates a DNA damage response usually initiated by the most lethal form of DNA damage-DSBs (Fig. 1). DNMT1 deficiency also inhibits DNA replication22–23, 133. It was reported that DNMT1 knockdown triggers an intra-S-phase arrest of DNA replication, independent of DNA demethylation22. Similar to the observations for DNA damage checkpoints134, the intra-S-phase arrest is transient, disappearing after 10 days of treatment with DNMT1 siRNA. The S-phase cells induced by DNMT1 knockdown exist in two distinct populations: 70% incorporate BrdUr, while 30% do not, consistent with the presence of an intra-S-phase checkpoint triggering cell cycle arrest134. Cells are arrested at different positions throughout S-phase, suggesting that this response is not specific to distinct classes of origins of DNA replication. 5-aza-CdR, a nucleoside analogue, is a well-characterized and widely-used inhibitor of DNA methylation, which inhibits DNA methylation by trapping DNMT1 at the replication fork after being incorporated into DNA. 5-aza-CdR does not inhibit the de novo synthesis of DNMT1 protein or its presence in the nucleus. S-phase cells treated with 5-aza-CdR, which causes genome-wide demethylation, do not exhibit two distinct population distributions as observed in cells deficient in DNMT1. These results suggest that the intra-S-phase arrest is not correlated with the degree of DNA methylation, consistent with observations that DNA replication arrest following DNMT1 inhibition is probably due to a reduction in the physical presence of DNMT1 at the replication fork, rather than DNA demethylation133. As discussed above, the cell cycle distribution in DNMT1 knockdown cells resembles the transient intra-S-phase arrest in DNA replication that is evoked by genotoxic insults135–137. In addition, DNMT1 inhibition also leads to the induction of a set of genes that are implicated in the genotoxic stress response including p21133, p53123, and the growth arrest DNA damage inducible 45β gene (GADD45β)22. These results imply that DNMT1 is linked to DNA damage repair machineries to maintain chromosome integrity via blocking DNA replication, a notion further strengthened by observations that DNMT1 knockdown activates the checkpoint pathways in a ATR-dependent manner23. Upon DNMT1 depletion, CHK1 and CHK2, key proteins in ATM/ATR signaling, are phosphorylated, which in turn induce phosphorylation and degradation of cell division control protein 25 A (CDC25A) as well as CDC25B23. As a consequence, the capacity for loading CDC45, an essential factor for DNA replication138, onto replication forks is decreased, resulting in replication arrest. DNMT1 knockdown also induces the formation of histone γH2A.X foci, a hallmark of the DNA DSB response. The response elicited by DNMT1 knockdown is blocked by siRNA mediated-depletion of ATR, suggestive of its ATR-dependency. Further support for the importance of ATR came from the finding that the cellular response to DNMT1 depletion is markedly attenuated in cells derived from a patient with Seckel syndrome, a disorder due to ATR deficiency23. However, it is not clear whether ATM, another key transducer like ATR in the checkpoint pathway, is involved in the process or not. DNA demethylating agents do not trigger the stress response like genetic DNMT1 depletion does23. Moreover, this response is abolished by ectopic expression of either wild-type DNMT1 or a mutant form of DNMT1 lacking the catalytic domain23, suggesting that loss of catalytic activity of DNMT1 is not driving this response. Also of importance, DNMT1 knockdown leads to very limited genomic demethylation22–23, consistent with observations made in cells containing hypomorphic mutations in DNMT1139–140. One explanation for this limited demethylation is that de novo DNMTs compensate for the reduction of DNMT1 activity139. Another possibility is that DNMT1 loss triggers a checkpoint pathway (Fig. 1) to block DNA replication, preventing loss of DNA methylation in an attempt to maintain genome stability. Double knockdown of DNMT1 and ATR does indeed induce global DNA demethylation, whereas single knockdowns of either DNMT1 or ATR do not, implying that the arrest of DNA replication activated by ATR signaling following DNMT1 depletion prevents loss of DNA methylation and that blocking this response results in global loss of DNA methylation23. Taken together, it appears that reduction of DNMT1 levels activates ATR signaling to block DNA replication in a DNA methylation-independent manner (Fig. 1). How this response to DNMT1 reduction is initiated, however, is still uncertain. It is possible that removal of DNMT1 from replication forks disrupts fork progression and eventually results in DSBs that elicit checkpoint signaling (Fig. 1). Alternatively, the presence of low levels of hemimethylated DNA due to the absence of DNMT1 may trigger this response (Fig. 1).
Complete inactivation of DNMT1 via genetic mechanisms also activates the DNA damage response and causes genomic demethylation. The degree of demethylation, however, varies greatly depending on cellular context, ranging from 20% loss in human cancer cells141 to 90% loss of genomic methylation in murine ES cells7–8. As the principal enzyme responsible for maintaining DNA methylation, DNMT1 is essential for embryonic development and cell survival. Disruption of Dnmt1 in mice results in loss of 90% of genomic methylation and embryonic lethality 7–8. Murine ES cells deficient for Dnmt1 die when introduced to differentiate7, mouse fibroblasts die within 2–4 cell divisions after conditional deletion in Dnmt1123, and the human colon cancer cell line HCT116 undergoes marked apoptosis and cell death within one cell division if DNMT1 is completely inactivated by cre-mediated conditional knockout141–142. Notably, complete inactivation of DNMT1 triggers the DNA damage response before cells die141. Deletion of DNMT1 activates p53123, 141, a target of ATM whose phosphorylation correlates with accumulation of p53 in response to DNA damage143. Disruption of both alleles of DNMT1 leads to activation of the G2/M checkpoint and G2 arrest, as verified by the presence of phosphorylated ATM and γH2A.X at discrete nuclear DNA damage foci141. Further support for checkpoint activation comes from the finding that treatment of cells with an ATM/ATR inhibitor, caffeine, facilitates mitotic entry and cell death in DNMT1 null cells141. Most of these cells, however, eventually escape G2 arrest and re-enter interphase with their unrepaired DNA, resulting in severe chromosomal and mitotic abnormalities (mitotic catastrophe)141. Thus far, the mechanisms by which DNMT1 inactivation leads to activation of the DNA damage repair remains elusive. In the complete absence of DNMT1, DNA may become more fragile owing to reduced methylation and/or defective chromatin structure in critical regions of the genome, leading to activation of DNA damage signaling (Fig. 1)142. Alternatively, the accumulation of hemimethylated DNA in DNMT1 mutant cells may be recognized as damage and trigger the damage response (Fig. 1). Both of these possibilities are consistent with the observation that agents that affect overall chromatin structure without damaging DNA also activate ATM144. Nonetheless, it cannot be excluded that oncogene activation or gene mutations initiate the DNA damage response, as Dnmt1-deficient ES cells exhibit significantly increased mutation rates, particularly in the form of deletions and mutations145.
Recruitment of DNMT1 to sites of DNA damage has been observed by our laboratory21, 146 and others24, providing compelling evidence to support the notion that DNMT1 is directly involved in DNA damage repair (Fig. 1). Immediately after laser microirradiation-induced DSBs, an accumulation of DNMT1 and PCNA occurs at the damage sites in S and non-S phase cells, colocalizing with γH2A.X - a marker of DSBs. Recruitment of DNMT1 to damage sites is dependent on its interaction with PCNA through its PCNA binding domain (PBD)21, 24, but is independent of its catalytic activity21. In addition to PCNA, DNMT1 also interacts with other components of the DNA damage machinery including CHK121, 146 and the 9-1-1 complex21. PCNA, along with CHK1 and 9-1-1, is essential for DNMT1’s recruitment to DNA damage sites. After recruitment to damaged regions, DNMT1 modulates the rate of ATR signaling and is essential for suppressing abnormal activation of the DNA damage response in the absence of exogenous damage21. Taken together, these data have revealed a direct link between DNMT1 and the DNA damage repair process.
PCNA mediates recruitment of DNMT1, not only to DNA replication sites, but also to DNA damage sites. The DNMT1-PCNA interaction implies that the role of DNMT might be to restore epigenetic information after damage repair. However, recent studies demonstrate that this interaction is not essential for maintaining DNA methylation5, 147. Furthermore, the observation21 that DNMT1 is very rapidly recruited and retained only transiently, likely before re-synthesis is completed, suggest that genomic methylation is not the main function of DNMT1 at these sites, at least in the early part of the DNA damage response. The recruitment kinetics of WT DNMT1 and DNMT1 with a point mutation in the catalytic domain are almost identical21. CHK1/CHK2 activation and γH2A.X foci formation induced by DNMT1 deficiency are rescued by expression of a catalytically inactive form of DNMT123. Therefore, although the possibility that DNMT1 participates in the restoration of DNA methylation patterns during damage repair cannot be excluded, it seems more likely that DNMT1 functions in sensing and/or mobilizing the response to certain forms of DNA damage (Fig. 1).
In summary, both DNMTs and DNA damage repair systems have evolved to maintain genomic integrity and disruption of these pathways contributes to the development of cancer19. Therefore, we have examined and outlined the interaction of DNMTs and DNA methylation with DNA damage repair systems, and have discussed possible mechanisms for how the two systems may function coordinately to deal with DNA damage. Promoter methylation, catalyzed by DNMTs, plays an established role in silencing key genes in multiple DNA damage repair pathways; inactivation of these pathways may predispose to a large array of tumors20. These findings are consistent with observations that TSGs are frequently silenced via epigenetic mechanisms in cancer cells. Unexpectedly perhaps, more recent observations strongly suggest that DNMTs, particular DNMT1, are directly involved in DNA damage repair systems via what is likely to be a DNA-methylation-independent mechanism17, 21–23, 141. The exact nature of the links between the DNMTs, DNA methylation and DNA damage repair systems are likely complex and remain to be further investigated. A more thorough understanding of these links will not only help dissect the mechanisms of tumor development, but also identify new anti-tumor targets and therapeutic strategies.
Acknowledgments
Work in the Robertson laboratory is supported by NIH grants R01CA116028, R01CA114229, and the Georgia Cancer Coalition (KDR). KDR is a Georgia Cancer Coalition Distinguished Cancer Scholar.
References
- 1.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005 Aug;6(8):597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 2.Kareta MS, Botello ZM, Ennis JJ, Chou C, Chedin F. Reconstitution and mechanism of the stimulation of de novo methylation by human DNMT3L. J Biol Chem. 2006 Sep 8;281(36):25893–25902. doi: 10.1074/jbc.M603140200. [DOI] [PubMed] [Google Scholar]
- 3.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999 Oct 29;99(3):247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 4.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998 Jul;19(3):219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 5.Egger G, Jeong S, Escobar SG, et al. Identification of DNMT1 (DNA methyltransferase 1) hypomorphs in somatic knockouts suggests an essential role for DNMT1 in cell survival. Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):14080–14085. doi: 10.1073/pnas.0604602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riggs AD, Xiong Z. Methylation and epigenetic fidelity. Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):4–5. doi: 10.1073/pnas.0307781100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 8.Lei H, Oh SP, Okano M, et al. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development. 1996 Oct;122(10):3195–3205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- 9.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002 Sep;3(9):662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 10.Yoder JA, Walsh CP, Bestor TH. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997 Aug;13(8):335–340. doi: 10.1016/s0168-9525(97)01181-5. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalo S, Jaco I, Fraga MF, et al. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol. 2006 Apr;8(4):416–424. doi: 10.1038/ncb1386. [DOI] [PubMed] [Google Scholar]
- 12.Xu GL, Bestor TH, Bourc’his D, et al. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999 Nov 11;402(6758):187–191. doi: 10.1038/46052. [DOI] [PubMed] [Google Scholar]
- 13.Guo G, Wang W, Bradley A. Mismatch repair genes identified using genetic screens in Blm-deficient embryonic stem cells. Nature. 2004 Jun 24;429(6994):891–895. doi: 10.1038/nature02653. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Trinh BN, Long TI, Oghamian S, Laird PW. Dnmt1 deficiency leads to enhanced microsatellite instability in mouse embryonic stem cells. Nucleic Acids Res. 2004;32(19):5742–5749. doi: 10.1093/nar/gkh912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang KY, James Shen CK. DNA methyltransferase Dnmt1 and mismatch repair. Oncogene. 2004 Oct 14;23(47):7898–7902. doi: 10.1038/sj.onc.1208111. [DOI] [PubMed] [Google Scholar]
- 16.Dion V, Lin Y, Hubert L, Jr, Waterland RA, Wilson JH. Dnmt1 deficiency promotes CAG repeat expansion in the mouse germline. Hum Mol Genet. 2008 May 1;17(9):1306–1317. doi: 10.1093/hmg/ddn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loughery JE, Dunne PD, O’Neill KM, Meehan RR, McDaid JR, Walsh CP. DNMT1 deficiency triggers mismatch repair defects in human cells through depletion of repair protein levels in a process involving the DNA damage response. Hum Mol Genet. 2011 Aug 15;20(16):3241–3255. doi: 10.1093/hmg/ddr236. [DOI] [PubMed] [Google Scholar]
- 18.Karpf AR, Matsui S. Genetic disruption of cytosine DNA methyltransferase enzymes induces chromosomal instability in human cancer cells. Cancer Res. 2005 Oct 1;65(19):8635–8639. doi: 10.1158/0008-5472.CAN-05-1961. [DOI] [PubMed] [Google Scholar]
- 19.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009 Oct 22;461(7267):1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacinto FV, Esteller M. Mutator pathways unleashed by epigenetic silencing in human cancer. Mutagenesis. 2007 Jul;22(4):247–253. doi: 10.1093/mutage/gem009. [DOI] [PubMed] [Google Scholar]
- 21.Ha K, Lee GE, Palii SS, et al. Rapid and transient recruitment of DNMT1 to DNA double-strand breaks is mediated by its interaction with multiple components of the DNA damage response machinery. Hum Mol Genet. 2011 Jan 1;20(1):126–140. doi: 10.1093/hmg/ddq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milutinovic S, Zhuang Q, Niveleau A, Szyf M. Epigenomic stress response. Knockdown of DNA methyltransferase 1 triggers an intra-S-phase arrest of DNA replication and induction of stress response genes. J Biol Chem. 2003 Apr 25;278(17):14985–14995. doi: 10.1074/jbc.M213219200. [DOI] [PubMed] [Google Scholar]
- 23.Unterberger A, Andrews SD, Weaver IC, Szyf M. DNA methyltransferase 1 knockdown activates a replication stress checkpoint. Mol Cell Biol. 2006 Oct;26(20):7575–7586. doi: 10.1128/MCB.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortusewicz O, Schermelleh L, Walter J, Cardoso MC, Leonhardt H. Recruitment of DNA methyltransferase I to DNA repair sites. Proc Natl Acad Sci U S A. 2005 Jun 21;102(25):8905–8909. doi: 10.1073/pnas.0501034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laghi L, Bianchi P, Malesci A. Differences and evolution of the methods for the assessment of microsatellite instability. Oncogene. 2008 Oct 23;27(49):6313–6321. doi: 10.1038/onc.2008.217. [DOI] [PubMed] [Google Scholar]
- 26.Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 27.Raschle M, Dufner P, Marra G, Jiricny J. Mutations within the hMLH1 and hPMS2 subunits of the human MutLalpha mismatch repair factor affect its ATPase activity, but not its ability to interact with hMutSalpha. J Biol Chem. 2002 Jun 14;277(24):21810–21820. doi: 10.1074/jbc.M108787200. [DOI] [PubMed] [Google Scholar]
- 28.Kantelinen J, Kansikas M, Korhonen MK, et al. MutSbeta exceeds MutSalpha in dinucleotide loop repair. Br J Cancer. 2010 Mar 16;102(6):1068–1073. doi: 10.1038/sj.bjc.6605531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thibodeau SN, French AJ, Cunningham JM, et al. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998 Apr 15;58(8):1713–1718. [PubMed] [Google Scholar]
- 30.Viswanathan M, Tsuchida N, Shanmugam G. Promoter hypermethylation profile of tumor-associated genes p16, p15, hMLH1, MGMT and E-cadherin in oral squamous cell carcinoma. Int J Cancer. 2003 May 20;105(1):41–46. doi: 10.1002/ijc.11028. [DOI] [PubMed] [Google Scholar]
- 31.Kim HG, Lee S, Kim DY, et al. Aberrant methylation of DNA mismatch repair genes in elderly patients with sporadic gastric carcinoma: A comparison with younger patients. J Surg Oncol. 2010 Jan 1;101(1):28–35. doi: 10.1002/jso.21432. [DOI] [PubMed] [Google Scholar]
- 32.Brucher BL, Geddert H, Langner C, et al. Hypermethylation of hMLH1, HPP1, p14(ARF), p16(INK4A) and APC in primary adenocarcinomas of the small bowel. Int J Cancer. 2006 Sep 15;119(6):1298–1302. doi: 10.1002/ijc.21990. [DOI] [PubMed] [Google Scholar]
- 33.Wang YC, Lu YP, Tseng RC, et al. Inactivation of hMLH1 and hMSH2 by promoter methylation in primary non-small cell lung tumors and matched sputum samples. J Clin Invest. 2003 Mar;111(6):887–895. doi: 10.1172/JCI15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy MA, Wentzensen N. Frequency of mismatch repair deficiency in ovarian cancer: A systematic review. Int J Cancer. 2010 Dec 7; doi: 10.1002/ijc.25835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seedhouse CH, Das-Gupta EP, Russell NH. Methylation of the hMLH1 promoter and its association with microsatellite instability in acute myeloid leukemia. Leukemia. 2003 Jan;17(1):83–88. doi: 10.1038/sj.leu.2402747. [DOI] [PubMed] [Google Scholar]
- 36.Lenz G, Hutter G, Hiddemann W, Dreyling M. Promoter methylation and expression of DNA repair genes hMLH1 and MGMT in acute myeloid leukemia. Ann Hematol. 2004 Oct;83(10):628–633. doi: 10.1007/s00277-004-0925-0. [DOI] [PubMed] [Google Scholar]
- 37.Nomdedeu JF, Perea G, Estivill C, et al. Microsatellite instability is not an uncommon finding in adult de novo acute myeloid leukemia. Ann Hematol. 2005 Jun;84(6):368–375. doi: 10.1007/s00277-005-1035-3. [DOI] [PubMed] [Google Scholar]
- 38.Tawfik HM, El-Maqsoud NM, Hak BH, El-Sherbiny YM. Head and neck squamous cell carcinoma: mismatch repair immunohistochemistry and promoter hypermethylation of hMLH1 gene. Am J Otolaryngol. 2011 Feb 23; doi: 10.1016/j.amjoto.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Lahtz C, Pfeifer GP. Epigenetic changes of DNA repair genes in cancer. J Mol Cell Biol. 2011 Feb;3(1):51–58. doi: 10.1093/jmcb/mjq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valle L, Carbonell P, Fernandez V, et al. MLH1 germline epimutations in selected patients with early-onset non-polyposis colorectal cancer. Clin Genet. 2007 Mar;71(3):232–237. doi: 10.1111/j.1399-0004.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 41.Gazzoli I, Loda M, Garber J, Syngal S, Kolodner RD. A hereditary nonpolyposis colorectal carcinoma case associated with hypermethylation of the MLH1 gene in normal tissue and loss of heterozygosity of the unmethylated allele in the resulting microsatellite instability-high tumor. Cancer Res. 2002 Jul 15;62(14):3925–3928. [PubMed] [Google Scholar]
- 42.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagawa H, Nuovo GJ, Zervos EE, et al. Age-related hypermethylation of the 5′ region of MLH1 in normal colonic mucosa is associated with microsatellite-unstable colorectal cancer development. Cancer Res. 2001 Oct 1;61(19):6991–6995. [PubMed] [Google Scholar]
- 44.Kuismanen SA, Holmberg MT, Salovaara R, et al. Epigenetic phenotypes distinguish microsatellite-stable and -unstable colorectal cancers. Proc Natl Acad Sci U S A. 1999 Oct 26;96(22):12661–12666. doi: 10.1073/pnas.96.22.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wheeler JM, Beck NE, Kim HC, Tomlinson IP, Mortensen NJ, Bodmer WF. Mechanisms of inactivation of mismatch repair genes in human colorectal cancer cell lines: the predominant role of hMLH1. Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):10296–10301. doi: 10.1073/pnas.96.18.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auclair J, Vaissiere T, Desseigne F, et al. Intensity-dependent constitutional MLH1 promoter methylation leads to early onset of colorectal cancer by affecting both alleles. Genes Chromosomes Cancer. 2011 Mar;50(3):178–185. doi: 10.1002/gcc.20842. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Zhang S, Cui J, Zhang A, Shen L, Yu H. Expression and promoter methylation status of mismatch repair gene hMLH1 and hMSH2 in epithelial ovarian cancer. Aust N Z J Obstet Gynaecol. 2008 Oct;48(5):505–509. doi: 10.1111/j.1479-828X.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 48.Vlaykova T, Mitkova A, Stancheva G, et al. Microsatellite instability and promoter hypermethylation of MLH1 and MSH2 in patients with sporadic colorectal cancer. J BUON. 2011 Apr-Jun;16(2):265–273. [PubMed] [Google Scholar]
- 49.Nagasaka T, Rhees J, Kloor M, et al. Somatic hypermethylation of MSH2 is a frequent event in Lynch Syndrome colorectal cancers. Cancer Res. 2010 Apr 15;70(8):3098–3108. doi: 10.1158/0008-5472.CAN-09-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat Genet. 2009 Jan;41(1):112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 51.Moelans CB, Verschuur-Maes AH, van Diest PJ. Frequent promoter hypermethylation of BRCA2, CDH13, MSH6, PAX5, PAX6 and WT1 in ductal carcinoma in situ and invasive breast cancer. J Pathol. 2011 Oct;225(2):222–231. doi: 10.1002/path.2930. [DOI] [PubMed] [Google Scholar]
- 52.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007 Jun 21;447(7147):941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng B, Hurt EM, Hodge DR, Thomas SB, Farrar WL. DNA hypermethylation and partial gene silencing of human thymine- DNA glycosylase in multiple myeloma cell lines. Epigenetics. 2006 Jul-Sep;1(3):138–145. doi: 10.4161/epi.1.3.2938. [DOI] [PubMed] [Google Scholar]
- 54.Howard JH, Frolov A, Tzeng CW, et al. Epigenetic downregulation of the DNA repair gene MED1/MBD4 in colorectal and ovarian cancer. Cancer Biol Ther. 2009 Jan;8(1):94–100. doi: 10.4161/cbt.8.1.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan H, Ji M, Hou P, et al. Hypermethylation of the DNA mismatch repair gene hMLH1 and its association with lymph node metastasis and T1799A BRAF mutation in patients with papillary thyroid cancer. Cancer. 2008 Jul 15;113(2):247–255. doi: 10.1002/cncr.23548. [DOI] [PubMed] [Google Scholar]
- 56.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001 May 17;411(6835):366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Xu Z, Li J, et al. XPC epigenetic silence coupled with p53 alteration has a significant impact on bladder cancer outcome. J Urol. 2010 Jul;184(1):336–343. doi: 10.1016/j.juro.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 58.Chen HY, Shao CJ, Chen FR, Kwan AL, Chen ZP. Role of ERCC1 promoter hypermethylation in drug resistance to cisplatin in human gliomas. Int J Cancer. 2010 Apr 15;126(8):1944–1954. doi: 10.1002/ijc.24772. [DOI] [PubMed] [Google Scholar]
- 59.Liu WB, Ao L, Cui ZH, et al. Molecular analysis of DNA repair gene methylation and protein expression during chemical-induced rat lung carcinogenesis. Biochem Biophys Res Commun. 2011 May 20;408(4):595–601. doi: 10.1016/j.bbrc.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 60.Jiang J, Liang X, Zhou X, et al. DNA repair gene X-ray repair cross complementing group 1 Arg194Trp polymorphism on the risk of lung cancer: a meta-analysis on 22 studies. J Thorac Oncol. 2010 Nov;5(11):1741–1747. doi: 10.1097/JTO.0b013e3181f0c409. [DOI] [PubMed] [Google Scholar]
- 61.Wang P, Tang JT, Peng YS, Chen XY, Zhang YJ, Fang JY. XRCC1 downregulated through promoter hypermethylation is involved in human gastric carcinogenesis. J Dig Dis. 2010 Dec;11(6):343–351. doi: 10.1111/j.1751-2980.2010.00459.x. [DOI] [PubMed] [Google Scholar]
- 62.Peng B, Hodge DR, Thomas SB, et al. Epigenetic silencing of the human nucleotide excision repair gene, hHR23B, in interleukin-6-responsive multiple myeloma KAS-6/1 cells. J Biol Chem. 2005 Feb 11;280(6):4182–4187. doi: 10.1074/jbc.M412566200. [DOI] [PubMed] [Google Scholar]
- 63.Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat Rev Mol Cell Biol. 2010 Mar;11(3):196–207. doi: 10.1038/nrm2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazon G, Mimitou EP, Symington LS. SnapShot: Homologous recombination in DNA double-strand break repair. Cell. 2010 Aug 20;142(4):646, 646 e641. doi: 10.1016/j.cell.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Esteller M, Silva JM, Dominguez G, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000 Apr 5;92(7):564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 66.Esteller M, Risques RA, Toyota M, et al. Promoter hypermethylation of the DNA repair gene O(6)-methylguanine-DNA methyltransferase is associated with the presence of G:C to A:T transition mutations in p53 in human colorectal tumorigenesis. Cancer Res. 2001 Jun 15;61(12):4689–4692. [PubMed] [Google Scholar]
- 67.Baldwin RL, Nemeth E, Tran H, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000 Oct 1;60(19):5329–5333. [PubMed] [Google Scholar]
- 68.Bernal C, Vargas M, Ossandon F, et al. DNA methylation profile in diffuse type gastric cancer: evidence for hypermethylation of the BRCA1 promoter region in early-onset gastric carcinogenesis. Biol Res. 2008;41(3):303–315. [PubMed] [Google Scholar]
- 69.Cabello MJ, Grau L, Franco N, et al. Multiplexed methylation profiles of tumor suppressor genes in bladder cancer. J Mol Diagn. 2011 Jan;13(1):29–40. doi: 10.1016/j.jmoldx.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MN, Tseng RC, Hsu HS, et al. Epigenetic inactivation of the chromosomal stability control genes BRCA1, BRCA2, and XRCC5 in non-small cell lung cancer. Clin Cancer Res. 2007 Feb 1;13(3):832–838. doi: 10.1158/1078-0432.CCR-05-2694. [DOI] [PubMed] [Google Scholar]
- 71.Esteller M, Fraga MF, Guo M, et al. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum Mol Genet. 2001 Dec 15;10(26):3001–3007. doi: 10.1093/hmg/10.26.3001. [DOI] [PubMed] [Google Scholar]
- 72.Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 1997 May 15;11(10):1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 73.Kee Y, D’Andrea AD. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 2010 Aug 15;24(16):1680–1694. doi: 10.1101/gad.1955310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meier D, Schindler D. Fanconi anemia core complex gene promoters harbor conserved transcription regulatory elements. PLoS One. 2011;6(8):e22911. doi: 10.1371/journal.pone.0022911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hess CJ, Ameziane N, Schuurhuis GJ, et al. Hypermethylation of the FANCC and FANCL promoter regions in sporadic acute leukaemia. Cell Oncol. 2008;30(4):299–306. doi: 10.3233/CLO-2008-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marsit CJ, Liu M, Nelson HH, Posner M, Suzuki M, Kelsey KT. Inactivation of the Fanconi anemia/BRCA pathway in lung and oral cancers: implications for treatment and survival. Oncogene. 2004 Jan 29;23(4):1000–1004. doi: 10.1038/sj.onc.1207256. [DOI] [PubMed] [Google Scholar]
- 77.Narayan G, Arias-Pulido H, Nandula SV, et al. Promoter hypermethylation of FANCF: disruption of Fanconi Anemia-BRCA pathway in cervical cancer. Cancer Res. 2004 May 1;64(9):2994–2997. doi: 10.1158/0008-5472.can-04-0245. [DOI] [PubMed] [Google Scholar]
- 78.Lim SL, Smith P, Syed N, et al. Promoter hypermethylation of FANCF and outcome in advanced ovarian cancer. Br J Cancer. 2008 Apr 22;98(8):1452–1456. doi: 10.1038/sj.bjc.6604325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pegg AE, Dolan ME, Moschel RC. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol. 1995;51:167–223. doi: 10.1016/s0079-6603(08)60879-x. [DOI] [PubMed] [Google Scholar]
- 80.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004 Apr;4(4):296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 81.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999 Feb 15;59(4):793–797. [PubMed] [Google Scholar]
- 82.Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010 Jan;6(1):39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 83.Whitehall VL, Walsh MD, Young J, Leggett BA, Jass JR. Methylation of O-6-methylguanine DNA methyltransferase characterizes a subset of colorectal cancer with low-level DNA microsatellite instability. Cancer Res. 2001 Feb 1;61(3):827–830. [PubMed] [Google Scholar]
- 84.Park TJ, Han SU, Cho YK, Paik WK, Kim YB, Lim IK. Methylation of O(6)-methylguanine-DNA methyltransferase gene is associated significantly with K-ras mutation, lymph node invasion, tumor staging, and disease free survival in patients with gastric carcinoma. Cancer. 2001 Dec 1;92(11):2760–2768. doi: 10.1002/1097-0142(20011201)92:11<2760::aid-cncr10123>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 85.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- 86.Zhang YJ, Chen Y, Ahsan H, et al. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation and its relationship to aflatoxin B1-DNA adducts and p53 mutation in hepatocellular carcinoma. Int J Cancer. 2003 Feb 10;103(4):440–444. doi: 10.1002/ijc.10852. [DOI] [PubMed] [Google Scholar]
- 87.Wolf P, Hu YC, Doffek K, Sidransky D, Ahrendt SA. O(6)-Methylguanine-DNA methyltransferase promoter hypermethylation shifts the p53 mutational spectrum in non-small cell lung cancer. Cancer Res. 2001 Nov 15;61(22):8113–8117. [PubMed] [Google Scholar]
- 88.Zhang L, Lu W, Miao X, Xing D, Tan W, Lin D. Inactivation of DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation and its relation to p53 mutations in esophageal squamous cell carcinoma. Carcinogenesis. 2003 Jun;24(6):1039–1044. doi: 10.1093/carcin/bgg062. [DOI] [PubMed] [Google Scholar]
- 89.Nakamura M, Watanabe T, Yonekawa Y, Kleihues P, Ohgaki H. Promoter methylation of the DNA repair gene MGMT in astrocytomas is frequently associated with G:C --> A:T mutations of the TP53 tumor suppressor gene. Carcinogenesis. 2001 Oct;22(10):1715–1719. doi: 10.1093/carcin/22.10.1715. [DOI] [PubMed] [Google Scholar]
- 90.Sabharwal A, Middleton MR. Exploiting the role of O6-methylguanine-DNA-methyltransferase (MGMT) in cancer therapy. Curr Opin Pharmacol. 2006 Aug;6(4):355–363. doi: 10.1016/j.coph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 91.Kitano K, Kim SY, Hakoshima T. Structural basis for DNA strand separation by the unconventional winged-helix domain of RecQ helicase WRN. Structure. 2010 Feb 10;18(2):177–187. doi: 10.1016/j.str.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 92.Opresko PL. Telomere ResQue and preservation--roles for the Werner syndrome protein and other RecQ helicases. Mech Ageing Dev. 2008 Jan-Feb;129(1–2):79–90. doi: 10.1016/j.mad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 93.Agrelo R, Cheng WH, Setien F, et al. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proc Natl Acad Sci U S A. 2006 Jun 6;103(23):8822–8827. doi: 10.1073/pnas.0600645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawasaki T, Ohnishi M, Suemoto Y, et al. WRN promoter methylation possibly connects mucinous differentiation, microsatellite instability and CpG island methylator phenotype in colorectal cancer. Mod Pathol. 2008 Feb;21(2):150–158. doi: 10.1038/modpathol.3800996. [DOI] [PubMed] [Google Scholar]
- 95.Smith JA, Ndoye AM, Geary K, Lisanti MP, Igoucheva O, Daniel R. A role for the Werner syndrome protein in epigenetic inactivation of the pluripotency factor Oct4. Aging Cell. 2010 Aug;9(4):580–591. doi: 10.1111/j.1474-9726.2010.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011 Mar 1;25(5):409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harrison JC, Haber JE. Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet. 2006;40:209–235. doi: 10.1146/annurev.genet.40.051206.105231. [DOI] [PubMed] [Google Scholar]
- 98.Lazzaro F, Giannattasio M, Puddu F, et al. Checkpoint mechanisms at the intersection between DNA damage and repair. DNA Repair (Amst) 2009 Sep 2;8(9):1055–1067. doi: 10.1016/j.dnarep.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 99.Linding R, Jensen LJ, Ostheimer GJ, et al. Systematic discovery of in vivo phosphorylation networks. Cell. 2007 Jun 29;129(7):1415–1426. doi: 10.1016/j.cell.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsuoka S, Ballif BA, Smogorzewska A, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007 May 25;316(5828):1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 101.Kim WJ, Vo QN, Shrivastav M, Lataxes TA, Brown KD. Aberrant methylation of the ATM promoter correlates with increased radiosensitivity in a human colorectal tumor cell line. Oncogene. 2002 May 30;21(24):3864–3871. doi: 10.1038/sj.onc.1205485. [DOI] [PubMed] [Google Scholar]
- 102.Vo QN, Kim WJ, Cvitanovic L, Boudreau DA, Ginzinger DG, Brown KD. The ATM gene is a target for epigenetic silencing in locally advanced breast cancer. Oncogene. 2004 Dec 16;23(58):9432–9437. doi: 10.1038/sj.onc.1208092. [DOI] [PubMed] [Google Scholar]
- 103.Ai L, Vo QN, Zuo C, et al. Ataxia-telangiectasia-mutated (ATM) gene in head and neck squamous cell carcinoma: promoter hypermethylation with clinical correlation in 100 cases. Cancer Epidemiol Biomarkers Prev. 2004 Jan;13(1):150–156. doi: 10.1158/1055-9965.epi-082-3. [DOI] [PubMed] [Google Scholar]
- 104.Bartek J, Falck J, Lukas J. CHK2 kinase--a busy messenger. Nat Rev Mol Cell Biol. 2001 Dec;2(12):877–886. doi: 10.1038/35103059. [DOI] [PubMed] [Google Scholar]
- 105.Bell DW, Varley JM, Szydlo TE, et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science. 1999 Dec 24;286(5449):2528–2531. doi: 10.1126/science.286.5449.2528. [DOI] [PubMed] [Google Scholar]
- 106.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003 May;3(5):421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 107.Kato N, Fujimoto H, Yoda A, et al. Regulation of Chk2 gene expression in lymphoid malignancies: involvement of epigenetic mechanisms in Hodgkin’s lymphoma cell lines. Cell Death Differ. 2004 Dec;11 (Suppl 2):S153–161. doi: 10.1038/sj.cdd.4401461. [DOI] [PubMed] [Google Scholar]
- 108.Kim DS, Kim MJ, Lee JY, et al. Epigenetic inactivation of checkpoint kinase 2 gene in non-small cell lung cancer and its relationship with clinicopathological features. Lung Cancer. 2009 Aug;65(2):247–250. doi: 10.1016/j.lungcan.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 109.Wang H, Wang S, Shen L, et al. Chk2 down-regulation by promoter hypermethylation in human bulk gliomas. Life Sci. 2010 Jan 30;86(5–6):185–191. doi: 10.1016/j.lfs.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 110.Sullivan A, Yuille M, Repellin C, et al. Concomitant inactivation of p53 and Chk2 in breast cancer. Oncogene. 2002 Feb 21;21(9):1316–1324. doi: 10.1038/sj.onc.1205207. [DOI] [PubMed] [Google Scholar]
- 111.Williams LH, Choong D, Johnson SA, Campbell IG. Genetic and epigenetic analysis of CHEK2 in sporadic breast, colon, and ovarian cancers. Clin Cancer Res. 2006 Dec 1;12(23):6967–6972. doi: 10.1158/1078-0432.CCR-06-1770. [DOI] [PubMed] [Google Scholar]
- 112.Jascur T, Boland CR. Structure and function of the components of the human DNA mismatch repair system. Int J Cancer. 2006 Nov 1;119(9):2030–2035. doi: 10.1002/ijc.22023. [DOI] [PubMed] [Google Scholar]
- 113.Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):776–781. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin Y, Wilson JH. Diverse effects of individual mismatch repair components on transcription-induced CAG repeat instability in human cells. DNA Repair (Amst) 2009 Aug 6;8(8):878–885. doi: 10.1016/j.dnarep.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin Y, Dion V, Wilson JH. Transcription promotes contraction of CAG repeat tracts in human cells. Nat Struct Mol Biol. 2006 Feb;13(2):179–180. doi: 10.1038/nsmb1042. [DOI] [PubMed] [Google Scholar]
- 116.Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull. 2006:79–80. 153–170. doi: 10.1093/bmb/ldl020. [DOI] [PubMed] [Google Scholar]
- 117.Bellacosa A, Cicchillitti L, Schepis F, et al. MED1, a novel human methyl-CpG-binding endonuclease, interacts with DNA mismatch repair protein MLH1. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3969–3974. doi: 10.1073/pnas.96.7.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ruzov A, Shorning B, Mortusewicz O, Dunican DS, Leonhardt H, Meehan RR. MBD4 and MLH1 are required for apoptotic induction in xDNMT1-depleted embryos. Development. 2009 Jul;136(13):2277–2286. doi: 10.1242/dev.032227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cortellino S, Turner D, Masciullo V, et al. The base excision repair enzyme MED1 mediates DNA damage response to antitumor drugs and is associated with mismatch repair system integrity. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15071–15076. doi: 10.1073/pnas.2334585100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boland MJ, Christman JK. Characterization of Dnmt3b:thymine-DNA glycosylase interaction and stimulation of thymine glycosylase-mediated repair by DNA methyltransferase(s) and RNA. J Mol Biol. 2008 Jun 6;379(3):492–504. doi: 10.1016/j.jmb.2008.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li YQ, Zhou PZ, Zheng XD, Walsh CP, Xu GL. Association of Dnmt3a and thymine DNA glycosylase links DNA methylation with base-excision repair. Nucleic Acids Res. 2007;35(2):390–400. doi: 10.1093/nar/gkl1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kimura H, Shiota K. Methyl-CpG-binding protein, MeCP2, is a target molecule for maintenance DNA methyltransferase, Dnmt1. J Biol Chem. 2003 Feb 14;278(7):4806–4812. doi: 10.1074/jbc.M209923200. [DOI] [PubMed] [Google Scholar]
- 123.Jackson-Grusby L, Beard C, Possemato R, et al. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet. 2001 Jan;27(1):31–39. doi: 10.1038/83730. [DOI] [PubMed] [Google Scholar]
- 124.Flores-Rozas H, Clark D, Kolodner RD. Proliferating cell nuclear antigen and Msh2p-Msh6p interact to form an active mispair recognition complex. Nat Genet. 2000 Nov;26(3):375–378. doi: 10.1038/81708. [DOI] [PubMed] [Google Scholar]
- 125.Iyer RR, Pohlhaus TJ, Chen S, et al. The MutSalpha-proliferating cell nuclear antigen interaction in human DNA mismatch repair. J Biol Chem. 2008 May 9;283(19):13310–13319. doi: 10.1074/jbc.M800606200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Plotz G, Welsch C, Giron-Monzon L, et al. Mutations in the MutSalpha interaction interface of MLH1 can abolish DNA mismatch repair. Nucleic Acids Res. 2006;34(22):6574–6586. doi: 10.1093/nar/gkl944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kleczkowska HE, Marra G, Lettieri T, Jiricny J. hMSH3 and hMSH6 interact with PCNA and colocalize with it to replication foci. Genes Dev. 2001 Mar 15;15(6):724–736. doi: 10.1101/gad.191201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Umar A, Buermeyer AB, Simon JA, et al. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell. 1996 Oct 4;87(1):65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- 129.Chen F, Arseven OK, Cryns VL. Proteolysis of the mismatch repair protein MLH1 by caspase-3 promotes DNA damage-induced apoptosis. J Biol Chem. 2004 Jun 25;279(26):27542–27548. doi: 10.1074/jbc.M400971200. [DOI] [PubMed] [Google Scholar]
- 130.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005 Sep 1;19(17):1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 131.Bischof O, Galande S, Farzaneh F, Kohwi-Shigematsu T, Campisi J. Selective cleavage of BLM, the bloom syndrome protein, during apoptotic cell death. J Biol Chem. 2001 Apr 13;276(15):12068–12075. doi: 10.1074/jbc.M006462200. [DOI] [PubMed] [Google Scholar]
- 132.Wang J, Pabla N, Wang CY, Wang W, Schoenlein PV, Dong Z. Caspase-mediated cleavage of ATM during cisplatin-induced tubular cell apoptosis: inactivation of its kinase activity toward p53. Am J Physiol Renal Physiol. 2006 Dec;291(6):F1300–1307. doi: 10.1152/ajprenal.00509.2005. [DOI] [PubMed] [Google Scholar]
- 133.Knox JD, Araujo FD, Bigey P, et al. Inhibition of DNA methyltransferase inhibits DNA replication. J Biol Chem. 2000 Jun 16;275(24):17986–17990. doi: 10.1074/jbc.C900894199. [DOI] [PubMed] [Google Scholar]
- 134.Bartek J, Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol. 2001 Dec;13(6):738–747. doi: 10.1016/s0955-0674(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 135.Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000 Dec;1(3):179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 136.Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001 Apr 12;410(6830):842–847. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- 137.Maser RS, Mirzoeva OK, Wells J, et al. Mre11 complex and DNA replication: linkage to E2F and sites of DNA synthesis. Mol Cell Biol. 2001 Sep;21(17):6006–6016. doi: 10.1128/MCB.21.17.6006-6016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hardy CF. Identification of Cdc45p, an essential factor required for DNA replication. Gene. 1997 Mar 18;187(2):239–246. doi: 10.1016/s0378-1119(96)00761-5. [DOI] [PubMed] [Google Scholar]
- 139.Rhee I, Bachman KE, Park BH, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002 Apr 4;416(6880):552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 140.Ting AH, Jair KW, Suzuki H, Yen RW, Baylin SB, Schuebel KE. CpG island hypermethylation is maintained in human colorectal cancer cells after RNAi-mediated depletion of DNMT1. Nat Genet. 2004 Jun;36(6):582–584. doi: 10.1038/ng1365. [DOI] [PubMed] [Google Scholar]
- 141.Chen T, Hevi S, Gay F, et al. Complete inactivation of DNMT1 leads to mitotic catastrophe in human cancer cells. Nat Genet. 2007 Mar;39(3):391–396. doi: 10.1038/ng1982. [DOI] [PubMed] [Google Scholar]
- 142.Brown KD, Robertson KD. DNMT1 knockout delivers a strong blow to genome stability and cell viability. Nat Genet. 2007 Mar;39(3):289–290. doi: 10.1038/ng0307-289. [DOI] [PubMed] [Google Scholar]
- 143.Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998 Sep 11;281(5383):1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 144.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003 Jan 30;421(6922):499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 145.Chen RZ, Pettersson U, Beard C, Jackson-Grusby L, Jaenisch R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998 Sep 3;395(6697):89–93. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- 146.Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. DNA methylation inhibitor 5-Aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. 2008 Jan;28(2):752–771. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spada F, Haemmer A, Kuch D, et al. DNMT1 but not its interaction with the replication machinery is required for maintenance of DNA methylation in human cells. J Cell Biol. 2007 Feb 26;176(5):565–571. doi: 10.1083/jcb.200610062. [DOI] [PMC free article] [PubMed] [Google Scholar]