Abstract
Objective
Elevated plasma aldosterone concentrations in patients have been linked to a spectrum of cardiovascular diseases. Mineralocorticoid receptor antagonists provide additional benefits in heart failure patients. However, whether aldosterone and the mineralocorticoid receptor are involved in aortic aneurysm is unknown.
Approach and Results
We report that administration of deoxycorticosterone acetate (DOCA) and salt or aldosterone and salt, but not DOCA or salt alone, to C57BL/6 male mice induced abdominal and thoracic aortic aneurysm formation and rupture in an age-dependent manner. DOCA and salt or aldosterone and salt induced aortic aneurysm mimicked human aortic aneurysm with respect to elastin degradation, inflammatory cell infiltration, smooth muscle cell degeneration and apoptosis, and oxidative stress. Aortic aneurysm formation did not correlate with the increase in blood pressure induced by DOCA and salt. Systemic administration of the angiotensin converting enzyme inhibitor, enalapril, or angiotensin type 1 receptor antagonist, losartan, did not affect DOCA and salt induced aortic aneurysm. In contrast, the mineralocorticoid receptor antagonists, spironolactone or eplerenone, significantly attenuated DOCA and salt or aldosterone and salt induced aortic aneurysm.
Conclusions
The current study describes a novel aortic aneurysm animal model induced by mineralocorticoid receptor agonist and high salt, and reveals a previously unrecognized but potentially significant role of aldosterone in the pathogenesis of aortic aneurysm. These findings imply that mineralocorticoid receptor antagonists may be effective in the treatment of some aortic aneurysms.
Keywords: aneurysm, receptors, DOCA, salt, aldosterone
Introduction
Aortic aneurysms can be divided into thoracic aortic aneurysms (TAAs) and abdominal aortic aneurysms (AAAs) according to their location. TAAs occur in all age people without sexual dimorphism and are highly associated with hereditary conditions.1 In contrast, AAAs are typically associated with aging, male gender, atherosclerosis, and smoking but have weak genetic association.2 AAAs are the most common form of aortic aneurysms, affects 4–8% of men over the age of 60, and accounting for about 2% of all deaths in Western countries.1, 2 Currently, open surgery repair and endovascular repair are the only widely used therapies for treatment of aortic aneurysm,2 and no drug has been approved for treatment of this devastating disease.2
The mineralocorticoid receptor, also known as the aldosterone receptor or nuclear receptor subfamily 3, group C, member 2, (NR3C2), is a protein that in humans is encoded by the NR3C2 gene (Gene ID: 4306).3, 4 Multiple steroid hormones including aldosterone and its precursor deoxycorticosterone acetate (DOCA) can bind to and activate NR3C2, but aldosterone is considered the primary physiological ligand in humans.3, 4 The mineralocorticoid receptor was initially identified in epithelial cells of the kidney and has been well recognized for its pivotal role in regulation of salt excretion, plasma volume, and blood pressure.3, 4 Subsequent studies show that the mineralocorticoid receptor is also expressed in nonepithelial cells and tissues (e.g. heart and aorta),3, 4 which raise the possibility that the mineralocorticoid receptor may also exert functions beyond the kidney. Indeed, a growing body of evidence suggests that the mineralocorticoid receptor plays a critical role in cardiac fibrosis, heart failure, and myocardial infarction.4–6 In particular, evidence from two large clinical trials, the Randomized ALdactone Evaluation Study (RALES)7 and EPlerenone neuroHormonal Efficacy and SUrvival Study (EPHESUS),8 demonstrated that mortality, risk of hospitalization, and onset of cardiovascular events in patients with heart failure were decreased significantly after administration of a mineralocorticoid receptor antagonist (spironolactone or eplerenone) in addition to existing therapies including angiotensin-converting-enzyme (ACE) inhibitors and angiotensin II (Ang II) receptor blockers (ARBs).
In contrast to overwhelming evidence for a significant role of the mineralocorticoid receptor in heart diseases, little is known about whether mineralocorticoid receptor activation by aldosterone plays a role in the pathogenesis of AAAs or TAAs. In a clinical case report, aortic dissection was found in patients with primary aldosteronism (also known as hyperaldosteronism).9 In addition, an analysis of drug modulation of AAA growth through 25 years of surveillance in 1,269 patients demonstrated a strong association between mineralocorticoid receptor blockers and slowed AAA progression.10 However, a cause and effect relationship between the mineralocorticoid receptor and aortic aneurysms has not been tested.
In an independent study using 10- to 12-month-old male C57BL/6 mice to investigate DOCA and salt-induced hypertension,11 we unexpectedly observed that a number of mice died from aortic aneurysm rupture. This raises the possibility that activation of the mineralocorticoid receptor by DOCA and salt may be involved in aortic aneurysm formation. A series of experiments were therefore designed to test this possibility and mechanisms thereof and the results are reported here.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Both DOCA and high Salt Are Required to Induce Aortic Aneurysm
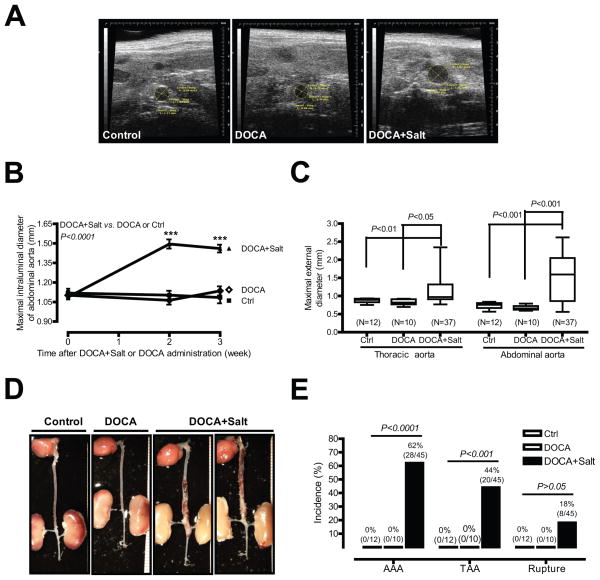
To determine whether DOCA and high salt are able to induce aortic aneurysm, ten-month-old male mice were randomly divided into 3 groups that received 1) no treatment (control, N=12); 2) DOCA alone (N=10); or 3) DOCA and salt (N=45). The representative ultrasound images (Figure 1A) and quantitative data (Figure 1B) illustrate that mice administered DOCA and salt exhibited a significant increase in aortic dilation [1.09 mm (o week, N=45) vs. 1.50 mm (2 week, N=44) or 1.46 mm (3 week, N=38); P<0.001]. In contrast, no aortic dilation was found in control mice or in mice administered DOCA alone. In addition to lumen dilation, a significant increase in external diameters of abdominal aortas was observed in mice administered DOCA and salt compared to control or mice administered DOCA alone [1.48 mm (DOCA and salt, N=37) vs. 0.67 mm (DOCA, N=10) or 0.74 mm (control, N=12); P<0.001; Figure 1C]. A similar increase in external diameter was also observed in thoracic aortas from DOCA and salt, but not control or DOCA only mice [1.16 mm (DOCA and salt, N=37) vs. 0.83 mm (DOCA, N=10) or 0.88 mm (control, N=12); P<0.05; Figure 1C].
Figure 1. DOCA and salt, but not DOCA alone, induce AAA, TAA, and aortic aneurysm rupture.
(A) Representative ultrasound images of aortas from control mice or mice administrated DOCA and salt or DOCA alone. (B) Quantification of ultrasound data. (C) ex vivo aortic diameter quantification. (D) Representative photographs of aortas from three groups of mice. (E) The incidence of AAAs, TAAs, and aortic aneurysm ruptures. ***P < 0.001 vs. basal (0 week).
Human aortic aneurysm, including TAA and AAA, is defined as a permanent localized dilatation of aorta, having at least a 50% increase in diameter compared with the normal diameter of aorta.12 Based on this definition, of the 45 mice administered DOCA and salt, 28 developed AAAs (Figures 1D and 1E). Of the 28 mice that developed AAAs, 20 also developed TAAs, 8 mice died of aortic aneurysm rupture, and no TAAs were seen in mice that did not also exhibit an AAA. The incidence of AAAs, TAAs, and aortic aneurysm rupture is 62%, 44%, and 18%, respectively (Figure 1E). AAAs were only found in the suprarenal abdominal aorta and TAAs were only found in the descending thoracic aorta. On the contrary, no AAA, TAA, or aortic aneurysm rupture were observed in control or DOCA alone (Figure 1E).
Of the 8 aortic ruptures, 1 occurred at 1 week and 7 occurred at 3 weeks after DOCA and salt administration (Supplemental Figure IA). We found a significant increase in maximal intraluminal diameters of abdominal aorta in ruptured aortas compared with that in control mice at 2 weeks after DOCA and salt administration [1.80 mm (DOCA and salt, N=7) vs. 1.10 mm (Ctrl, N=12); P<0.001; Supplemental Figure IB). This result illustrates that most if not all mice that developed aortic ruptures were first seen with enlarged aortic diameters.
To investigate whether high salt alone is sufficient to induce aortic aneurysms, ten-month old male mice received drinking water containing high salt for 3 weeks. Administration of high salt alone had no effect on aortic dilation as measured by ultrasound imaging and ex vivo aortic quantification (Supplemental Figures IIA and IIB). Importantly, neither AAA, TAA nor aortic rupture were evident in mice administered high salt.
Aldosterone and Salt Induce Aortic Aneurysms
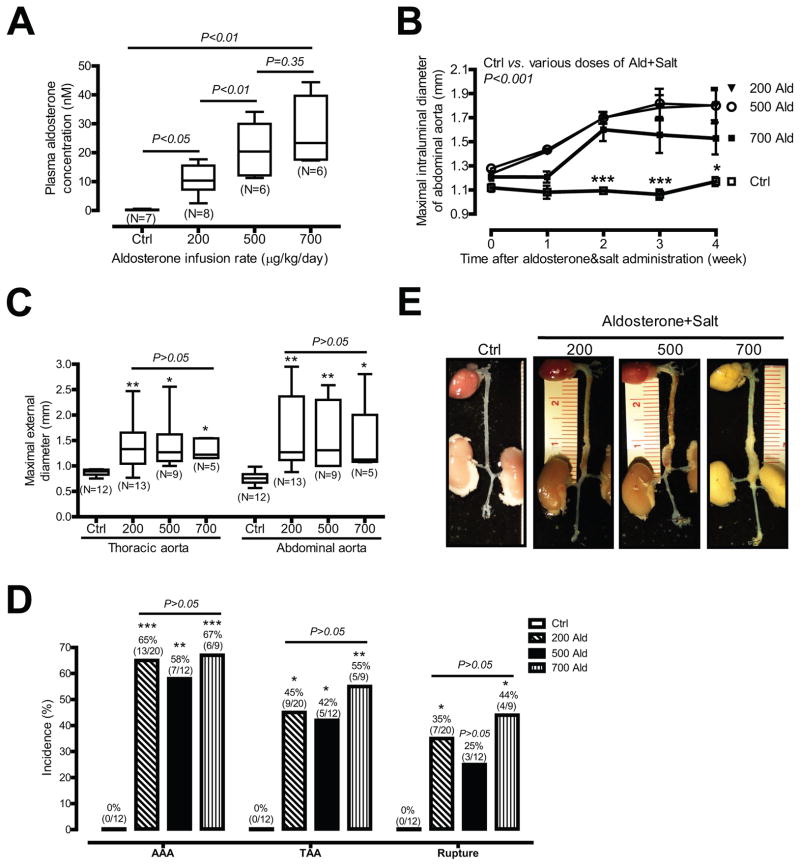
Since DOCA is a mineralocorticoid receptor ligand, we defined whether aldosterone, a physiologic ligand of the mineralocorticoid receptor, can induce aortic aneurysms in a dose-dependent manner. Ten-month old mice receiving salt were infused with 3 different doses of aldosterone (200, 500, and 700 μg/kg/day) for 4 weeks. Figure 2A shows that plasma aldosterone concentrations were significantly elevated in mice administered aldosterone and salt in a dose-dependent manner [0.27 nM (ctrl, N=7) vs. 10.58 nM (200 μg/kg/day, N=8, P<0.05), 21.21 nM (500 μg/kg/day, N=6, P<0.01) or 27.36 nM (700 μg/kg/day, N=6, P<0.01)].
Figure 2. Aldosterone and salt induce aortic aneurysms.
(A) Plasma aldosterone concentrations of control mice or mice infused with three different doses of aldosterone. (B) Quantification of ultrasound data. (C) ex vivo aortic diameter quantification. (D) The incidence of AAAs, TAAs, and aortic aneurysm ruptures. (E) Representative photographs of aortas from four groups of mice. *P <0.05; **P < 0.01; ***P < 0.001 vs. control mice (Ctrl).
All 3 doses of aldosterone and salt markedly increased maximal intraluminal diameters of suprarenal abdominal aortas 2 weeks after administration of mice with aldosterone and salt (Figure 2B and Supplemental Table II). Similar significant increases were also seen in maximal external diameters of abdominal and thoracic aortas 4 weeks after administration of mice with aldosterone and salt (Figure 2C and Supplemental Table III). Accordingly, all 3 doses of aldosterone and salt also markedly induced AAAs [65%, 58%, and 67%, respectively), TAAs [45%%, 42%, and 55%, respectively), and aortic aneurysm rupture [35%, 25%, and 44%, respectively; Figure 2D]. Interestingly, 200 μg/kg/day aldosterone infusion plus high salt appeared to be sufficient for induction of aortic aneurysm formation since effects were not dose-dependent above this aldosterone dose (Figures 2B through 2D). To rule out the possibility that the vehicle [50% dimethyl sulfoxide (DMSO)] may have had an effect on aldosterone and salt-induced aortic aneurysm formation, mice were infused with 50% DMSO plus high salt for 4 weeks. Supplemental Figures IIIA and IIIB illustrate that DMSO and salt did not cause aortic dilation as measured by ultrasound imaging and ex vivo aortic diameter quantification. In addition, neither AAAs, TAAs, nor aortic ruptures were seen in mice administered DMSO and salt.
Aortic aneurysms induced by DOCA and salt versus those induced by aldosterone and salt had similar features, but there were some differences. First, in contrast to the massive and diffusive aortic aneurysms induced by DOCA and salt (Figure 1D), aortic aneurysms induced by aldosterone and salt were more discretely localized to the suprarenal abdominal aorta (Figure 2E). Second, aortic aneurysm ruptures occurred more frequently in mice administered aldosterone and salt compared to those administered DOCA and salt [35%, 25%, and 44% respectively with the three doses of aldosterone (Figure 2D) vs. 18% in DOCA and salt (Figure 1E)].
Vascular Pathology of DOCA and Salt or Aldosterone and Salt Induced Aortic Aneurysms
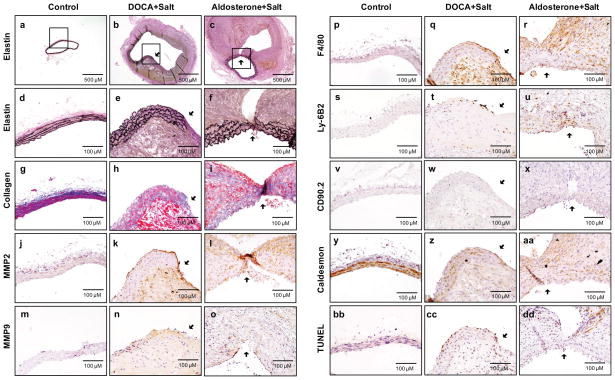
Human aortic aneurysms are characterized by elastin and collagen degradation, matrix metalloproteinase (MMP) upregulation, inflammatory cell infiltration, vascular smooth muscle cell degeneration, and oxidative stress.13 To investigate whether DOCA and salt or aldosterone and salt induced aortic aneurysms have these pathologic features, sequential 5 μm cross sections were collected throughout the entire aorta (Supplemental Figure IV). Of the 12 aortas sectioned, elastin degradation was only observed in AAAs induced by DOCA and salt or by aldosterone and salt (Supplemental Figures V and VI). Of these 12 aortas sectioned, 9 were administrated DOCA and salt and 3 were administrated aldosterone and salt. Interestingly, 4 of 9 (44.4%) and 1 of 3 (33.3%) of aortic dissections were seen in administration of DOCA and salt or aldosterone and salt, respectively (Supplemental Figure VIIA).
Compared to sections from control mice, elastin staining in aortas from mice administered DOCA and salt or aldosterone and salt demonstrated pronounced elastin degradation and extensive vascular remodeling (Figures 3a through 3f). Quantitative analysis showed that elastin breaks were significantly increased in aortas administrated DOCA and salt or aldosterone and salt compared to controls (Supplemental Figure VIIB). In addition to elastin, collagen content was also markedly suppressed in the media and adventitia of aortas from mice administrated DOCA and salt or aldosterone and salt relative to that of control mice (Figures 3g through 3i).
Figure 3. DOCA and salt or aldosterone and salt increase elastin and collagen degradation, MMP2 and MMP9 protein expression, vascular macrophage and neutrophil accumulation, smooth muscle degeneration, and apoptosis in abdominal aortas.
Abdominal aortas were isolated from control mice (a, d, g, j, m, p, s, v, y, and bb) and mice administered DOCA and salt for 3 weeks (b, e, h, k, n, q, t, w, z, and cc) or aldosterone and salt for 4 weeks (c, f, i, l, o, r, u, x, aa, and dd). Paraffin-embedded cross-sections of abdominal aortas were stained with Elastic-Van Gieson staining for elastin (a–f), Masson trichrome for collagen (g–i), MMP2 Ab (j–l), MMP9 Ab (m–o), F4/80 Ab for macrophages (p–r), Ly6B.2 Ab for neutrophils (s–u), CD90.2 Ab for T cells (v–x) caldesmon (smooth) Ab for vascular smooth muscle cells (VSMCs) (y–aa), and TUNEL for apoptosis (bb–dd). Among representative images, a–c are low magnification whereas the rest are higher magnification. Arrows indicates elastin degradation sites.
MMP2 and MMP9 are enzymatic proteins that play a critical role in extracellular matrix degradation and vascular remodeling.13 MMP2 immunostaining was evident in the endothelial/medial layer of aortas from mice administered DOCA and salt or aldosterone and salt, and was apparent at elastin degradation sites of aortas compared to the control mouse aorta in which MMP2 protein was barely detectable (Figures 3j through 3i). The similar immunostaining pattern was also seen for MMP9 although it was less evident than MMP2 immunostaining (Figure 3m through 3o). Moreover, in situ zymography illustrated that MMP activity was markedly elevated in the medial and adventitial layers of aortas from mice administrated DOCA and salt or aldosterone and salt compared to that in control mice (Supplemental Figure VIII).
To investigate inflammatory cell infiltration, aorta cross sections were immunostained with an F4/80, Ly-6B.2, and CD90.2 antibodies, which recognizes monocytes/macrophages, neutrophils, and T cells, respectively. Monocyte/macrophage immunostaining was minimal in control aortic sections, but it was readily seen in the endothelial/medial layer of aortic sections from mice administered DOCA and salt or aldosterone and salt (Figures 3p through 3r). The similar immunostaining pattern was also seen for neutrophils although it was less evident than monocyte/macrophage immunostaining (Figure 3s through 3u). Interestingly, no obvious T Cells were observed in the endothelial/medial layer of aortic sections (Figure 3v through 3x) although they were found in adventitial layers of aortas from mice administrated DOCA and salt or aldosterone and salt (Supplemental Figure IX).
To further investigate the role of vascular inflammation in DOCA and salt induced aortic aneurysm, we determined mRNA expression of several inflammatory genes, including vascular cell adhesion molecule 1 (Vcam-1), chemokine (C-C motif) ligand 2 (Ccl2, also known as MCP-1), and tumor necrosis factor (Tnf, also known as TNFα) in both abdominal and thoracic aortas from mice administrated DOCA and salt or control mice. We found that Vcam-1, Ccl2, and Tnf were all markedly upregulated in thoracic aortas from mice administrated DOCA and salt compared to control mice (Supplemental Figures XA, XB, and XC). Interestingly, Vcam-1 and Ccl2, but not Tnf, were also significantly upregulated by DOCA and salt in abdominal aorta from mice administrated DOCA and salt compared to control mice (Supplemental Figures XD, XE, and XF).
To investigate possible vascular smooth muscle apoptosis, aortic sections were stained with an antibody that specifically recognizes smooth muscle-specific caldesmon. In control aortas, caldesmon protein was highly expressed in the media of the vessel. In contrast, in aortas from mice administrated DOCA and salt or aldosterone and salt, caldesmon protein expression was markedly suppressed (Figures 3y through 3aa). Aortic sections were also stained with a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit that recognizes apoptotic cells. In contrast to caldesmon staining, TUNEL-positive cells were absent in control aortas but could be found in aortas from mice administered DOCA and salt or aldosterone and salt (Figures 3bb through 3dd). Importantly, TUNEL staining was present in the same areas as elastin degradation and the loss of immunostaining of caldesmon in sections examined. Quantitative analysis also showed that TUNEL positive cells in the tunica media of aortic section were significantly increased in mice administrated DOCA and salt or aldosterone and salt compared to that in control mice (Supplemental Figure XI).
To investigate a potential role for oxidative stress, cross-sections of abdominal aortas from mice administrated DOCA and salt were stained with dihydroethidium (DHE). In contrast to control aortas, in which only auto-florescence of elastin was seen, additional nuclear staining derived from DHE was observed in abdominal aortas from mice administrated DOCA and salt (Supplemental Figure XIIA). To investigate the mechanism that may be responsible for the DOCA and salt-induced increase in oxidative stress, we determined the time course of DOCA and salt-induced Ncf1 (also known as p47phox) mRNA expression in thoracic and abdominal aortas. We selected Ncf1 because NCF1 has been shown to be upregulated in human aortic aneurysms14 and has been demonstrated to be critical in Ang II-induced aortic aneurysms.15 Supplemental Figures XIIB and XIIC illustrate that Ncf1 mRNA was upregulated in a time-dependent manner in both thoracic and abdominal aortas in response to DOCA and salt. Importantly, Ncf1 mRNA upregulation by DOCA and salt preceded aortic aneurysm formation (9 days vs. 3 weeks). In addition, a similar increase in Cyba (also known as p22phox; Gene ID: 13057) mRNA was also observed (data not shown).
DOCA and Salt-Induced Aortic Aneurysm Is Age-Dependent
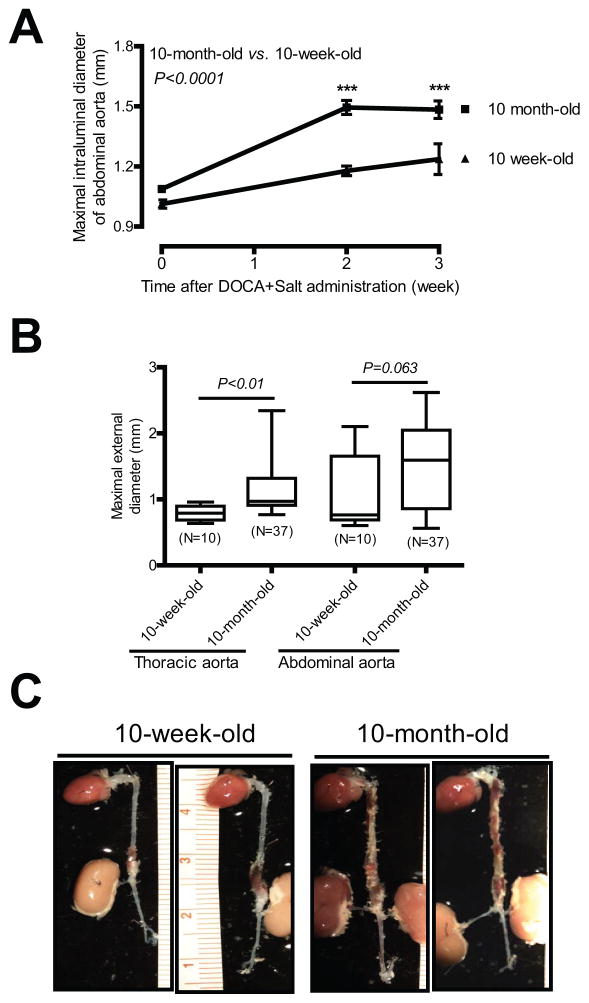
In humans, the prevalence of AAAs increases with age.2 Therefore, we compared aortic aneurysm formation in response to DOCA and salt administration in 10-week-old vs. 10-month-old mice. DOCA and salt administration induced a significant increase in abdominal aortic dilation in mice of both ages (Figure 4A). However, luminal dilation was more pronounced in 10-month-old mice compared to 10-week-old mice [e.g. 2 week after DOCA and salt, 1.50 mm (10 month-old, N=44) vs. 1.18 mm (10 week-old, N=12); P<0.001]. Similarly, maximal external diameters of the thoracic aorta were also greater in 10-month-old mice administered DOCA and salt than that in 10-week-old mice [1.16 mm (10 month-old, N=37) vs. 0.80 mm (10 week-old, N=10); P<0.01; Figure 4B]. A trend towards increased maximal external diameters of abdominal aortas was also seen in 10-month-old mice compared to 10-week-old mice [1.48 mm (10 month-old, N=37) vs. 1.06 mm (10 week-old, N=10); P=0.06; Figure 4B]. A trend towards increased incidences of AAAs [42% (5 of 12, 10-week-old) vs. 64% (29 of 42, 10-month-old); P=0.10] and TAAs [17% (2 of 12, 10-week-old) vs. 44% (20 of 45, 10-month-old); P=0.20] were observed in 10-month-old mice compared to 10-week-old mice. In contrast, there was no difference of aortic aneurysm ruptures between two groups of mice [17% (2 of 12, 10-week-old) vs. 18% (8 of 45, 10-month-old); P=0.33]. Moreover, DOCA and salt-induced aortic aneurysms in 10-month-old mice were more severe and diffuse and often extended from the suprarenal abdominal aorta to the descending thoracic aorta (Figure 4C and Figure 1D). In contrast, 10-week old mice administered DOCA and salt had aortic aneurysms that primarily localized to the suprarenal abdominal aorta (Figure 4C).
Figure 4. DOCA and salt induces more severe aortic aneurysm in 10-month-old mice than in 10-week-old mice.
(A) Quantification of ultrasound data of 10- week-old and 10-month-old mice administrated DOCA and salt. (B) ex vivo aortic diameter quantification. (C) Representative photographs ***P < 0.001 vs. 10-week-old mice.
Mineralocorticoid Receptor Antagonism, but not AT1 Receptor Antagonism, Attenuates DOCA and Salt Induced Aortic Aneurysms
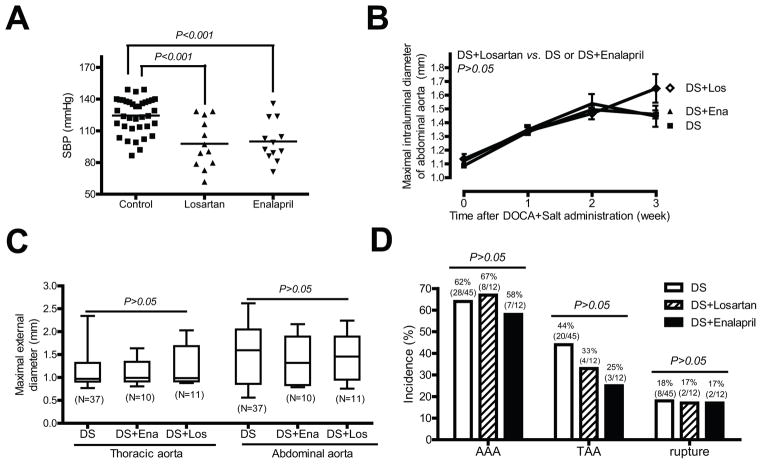
Although plasma renin and Ang II concentrations are suppressed in animals administered DOCA and salt,11 results suggest that a local vascular Ang II may be increased.16 Moreover, infusion of Ang II is an established model of abdominal and ascending aortic aneurysms,17, 18 and Ang II is a primary stimulus for aldosterone release.4 Therefore, we administered either an ACE inhibitor (enalapril) or an ARB (losartan) to define the role of Ang II in DOCA and salt induced aneurysms. Administration of losartan or enalapril resulted in a significant reduction in basal BP (Figure 5A), demonstrating effective blockage of the renin-angiotensin system. However, neither enalapril nor losartan significantly reduced maximal intraluminal diameters of suprarenal abdominal aortas (Figure 5B), maximal external diameters of thoracic and abdominal aorta (Figure 5C), or the incidence of AAAs, TAAs, and aortic aneurysm ruptures induced by DOCA and salt administration (Figure 5D).
Figure 5. Enalapril or losartan has no effect on DOCA and salt induced aortic aneurysms.
(A) Effects of losartan or enalapril on basal SBP. (B) Quantification of ultrasound data of aortas from mice administrated DOCA and salt (DS) or mice administrated DOCA and salt plus losartan (Los) or enalapril (Ena). (C) ex vivo aortic diameter quantification. (D) The incidence of AAAs, TAAs, and aortic aneurysm ruptures.
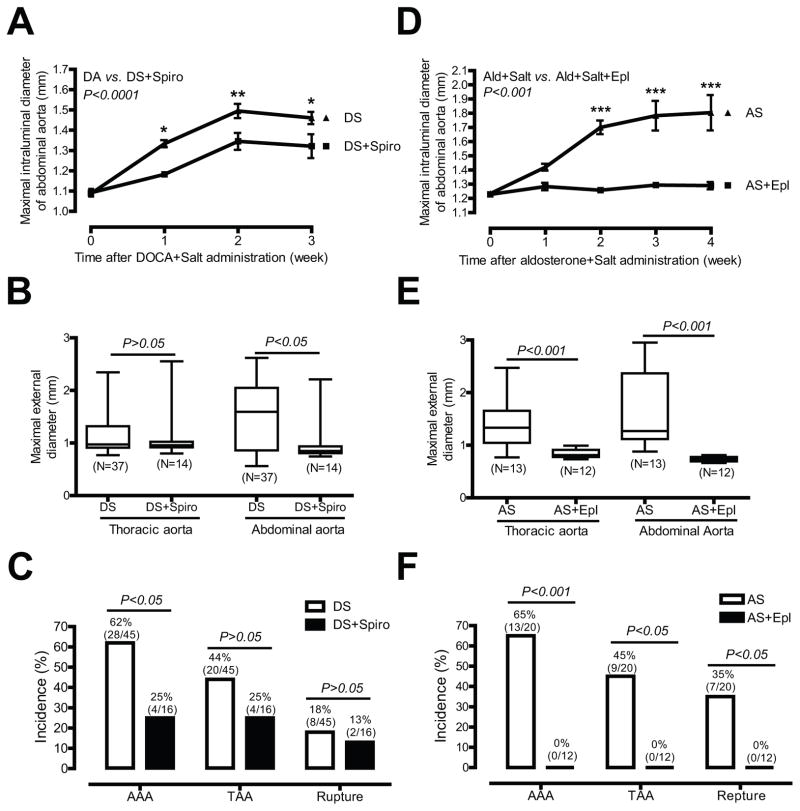
To define the role of mineralocorticoid receptor in DOCA and salt induced aortic aneurysm, we determined whether a mineralocorticoid receptor antagonist spironolactone affects DOCA and salt induced aortic aneurysm. Administration of spironolactone resulted in a significant reduction in maximal intraluminal diameter of suprarenal abdominal aortas [e.g. 2 weeks after treatment, 1.35 mm (DOCA and salt plus spironolactone, N=16) vs. 1.50 mm (DOCA and salt, N=44); P<0.01); Figure 6A], maximal external diameters of abdominal aortas [1.04 mm (DOCA and salt plus spironolactone, N=14) vs. 1.48 mm (DOCA and salt, N=37); P<0.05; Figure 6B], and the incidence of AAAs [25% (4/16, DOCA and salt plus spironolactone) vs. 62% (28 of 45, DOCA and salt); P<0.05; Figure 6C]. In addition, a trend towards reduced incidence of TAAs [25% (4/16, DOCA and salt plus spironolactone) vs. 44% (20 of 45, DOCA and salt)] and aortic aneurysm ruptures [13% (2/16, DOCA and salt plus spironolactone) vs. 18% (8 of 45, DOCA and salt)] were also seen in DOCA and salt mice administered spironolactone (Figure 6C).
Figure 6. Spironolactone or eplerenone significantly inhibits DOCA and salt or aldosterone and salt induced aortic aneurysms.
(A) Quantification of ultrasound data of aortas from mice administrated DOCA and salt (DS) or mice administrated DS and spironolactone (Spiro). (B) ex vivo aortic diameter quantification. (C) The incidence of AAAs, TAAs, and aortic aneurysm ruptures. (D) Quantification of ultrasound data of aortas from mice administrated aldosterone and salt (AS) or mice administrated AS and Eplerenone (Epl). (E) ex vivo aortic diameter quantification. (F) The incidence of AAAs, TAAs, and aortic aneurysm ruptures.
It is well known that spironolactone cross-reacts with sex-steroid receptors.6 It was also noted that spironolactone only partially blunted aortic aneurysm formation (Figure 6A through 6C). To further define the role of mineralocorticoid receptor in aortic aneurysm, we determined the effect of eplerenone, a more specific mineralocorticoid receptor antagonist, on aldosterone and salt induced aortic aneurysm. We used a low dose aldosterone (200 μg/kg/day), but not DOCA (50 mg, 21 day release) in attempting to increase the ratio of mineralocorticoid receptor antagonist to mineralocorticoid receptor agonist to achieve a better inhibition. Eplerenone treatment completely abolished aldosterone and salt induced increases in maximal intraluminal diameters of abdominal aortas [e.g. 2 week after treatment, 1.26 mm (aldosterone and salt plus eplerenone, N=12) vs. 1.70 mm (aldosterone and salt, N=16); P<0.001); Figure 6D] and maximal external diameters of abdominal and thoracic aortas [1.08 mm (aldosterone and salt plus eplerenone, N=12) vs. 1.40 mm (aldosterone and salt, N=13); P<0.001; Figure 6E] and thoracic aortas [0.84 mm (aldosterone and salt plus eplerenone, N=12) vs. 1.34 mm (aldosterone and salt, N=13); P<0.001; Figure 6E]. Moreover, none of the 12 mice treated with eplerenone developed any aortic aneurysm in any part of aorta (Figure 6F). In contrast, control mice administrated aldosterone and salt had significant abdominal and thoracic aortic dilations (Figure 6D and 6E) and dramatic incidences of AAAs, TAAs, and aortic aneurysm ruptures [65%, 45%, and 35%, respectively; Figure 6F).
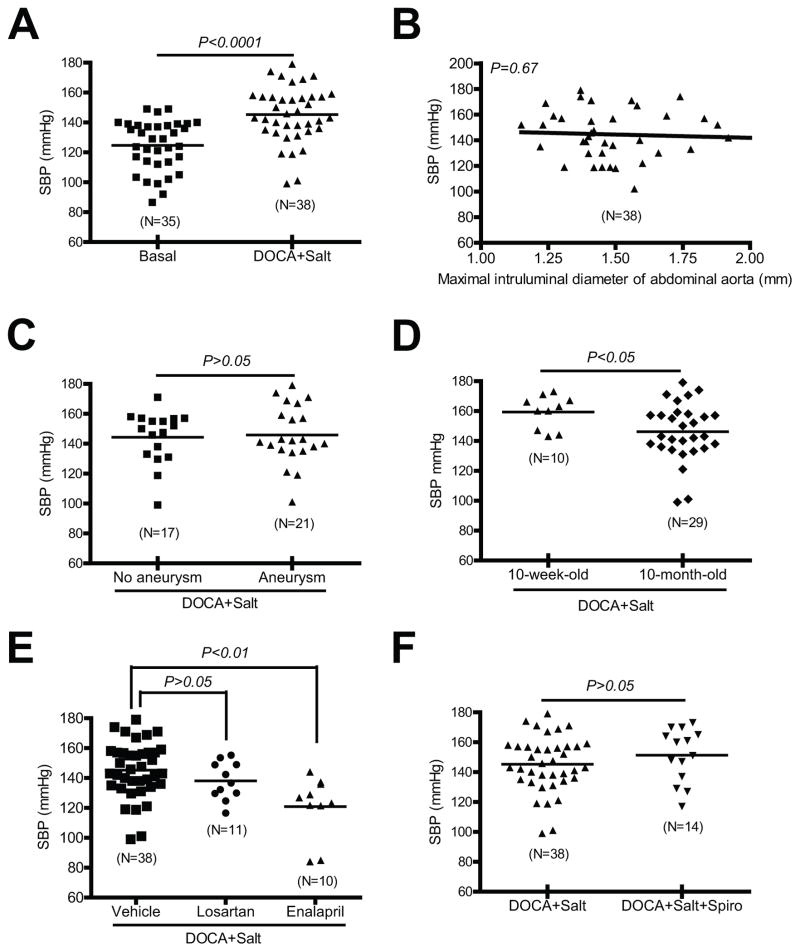
DOCA and Salt-Induced Hypertension does not Correlate with Aortic Aneurysms
To investigate whether DOCA and salt-induced hypertension contributes to aortic aneurysm formation, we correlated DOCA and salt induced hypertension (Figure 7A) to several quantified parameters of aortic aneurysm formation. In DOCA and salt mice, there was no significant correlation between systolic blood pressure (SBP) and maximal intraluminal diameters of suprarenal abdominal aortas (Figure 7B), and there was also no difference in SBP between the mice with and those without aortic aneurysms (Figure 7C). Moreover, DOCA and salt-induced aortic aneurysms were more severe in 10-month-old compared to 10-week-old mice (Figure 4), but DOCA and salt-induced hypertension was significantly less in 10-month-old mice compared to 10-week-old mice (Figure 7D).
Figure 7. DOCA and salt induced blood pressure increase does not correlate with presence of aortic aneurysm.
(A) DOCA and salt induced hypertension. (B) There was no correlation between SBP and maximal intraluminal diameter of suprarenal abdominal aortas. (C) There was no difference in SBP between mice with AAA and those without aortic aneurysms. (D) DOCA and salt induced SBP in 10-month-old mice was lower than that in 10-week-old mice. (E) Enalapril decreased DOCA and salt induced SBP. (F) There was no difference in SBP between mice administrated DOCA and salt and those administrated DOCA and salt plus spironolactone (Spiro).
There was also no correlation between the effectiveness of pharmacological inhibition of DOCA and salt-induced hypertension and aortic aneurysms. Administration of enalapril significantly decreased DOCA and salt-induced increases in SBP (Figure 7E) without affecting DOCA and salt-induced aortic aneurysms (Figures 5B–D). In contrast, administration of spironolactone had no effect on DOCA and salt-induced hypertension (Figure 7F), but attenuated DOCA and salt-induced aortic aneurysms (Figures 6A–C).
Discussion
The current study describes a novel mouse model of aortic aneurysms induced by administration of mineralocorticoid receptor agonist and high salt, and reveals a previously unrecognized but potentially significant role of aldosterone in the pathogenesis of aortic aneurysms. The major findings are: 1) DOCA and salt or aldosterone and salt were able to induce AAAs and TAAs in 10-month-old male C57BL/6 mice. The pathology of aortic aneurysms induced by DOCA and salt or aldosterone and salt resembled several features of human aneurysms including elastin degradation, inflammatory cell infiltration, smooth muscle cell degeneration and apoptosis, and oxidative stress; 2) the incidence of aortic aneurysms and the severity of the vascular pathologies were age-dependent; 3) aortic aneurysm formation did not correlate with blood pressure increases; and 4) mineralocorticoid receptor antagonism, but not blockade of Ang II synthesis or the AT1 receptor, reduced DOCA and salt induced aortic aneurysms.
In humans, elevated plasma aldosterone levels are associated with essential hypertension, primary aldosteronism, and congestive heart failure,4, 5, 19 suggesting that aldosterone is emerging as a major independent cardiovascular risk factor.4–6 The normal plasma aldosterone concentration range in humans (0.139 to 0.416 nM) can increase up to 58 to 173 fold (24 nM) in patients with congestive heart failure.5, 20 In the current study, infusions of 200, 500 or 700 μg/kg/day, respectively, resulted in plasma aldosterone concentrations (10.58, 21.21, and 27.36 nM, respectively) that are comparable to those observed under pathological conditions. Whether aortic aneurysms are present in congestive heart failure patients with high plasma aldosterone concentrations is not known. However, aortic dissection has been demonstrated in patients with primary aldosteronism.9 In addition, patients with glucocorticoid-remediable aldosteronism, an inherited form of primary hyperaldosteronism, often die at a young age due to rupture of cerebral aneurysms.21
Little is known regarding the level of NR3C2 mRNA and/or protein in human aortic aneurysm. Lenk et al. performed a genome-wide microarray-based expression study and reported that NR3C2 mRNA level was significantly down-regulated in human AAA tissue samples compared to age-, sex-, ethnicity- and location-matched controls of the infrarenal abdominal aorta.22 While it remains elusive that this significant down-regulation of NR3C2 mRNA (P=0.0004) alters the level of NR3C2 protein and function, an analysis of drug modulation of AAA growth through 25 years of surveillance in 1,269 patients demonstrated that there is a strong association between mineralocorticoid receptor blockers and slowed AAA progression,10 implicating mineralocorticoid receptor in human AAAs.
Given that DOCA and salt or aldosterone and salt-induced hypertension have been used extensively as experimental models of low-renin hypertension,11 it is surprising that aortic aneurysms have not been reported in previous studies. While exact reasons for this discrepancy are unclear, our results suggest that the age of mice may be critical to aneurysm formation. Specifically, 10-month old mice exhibited more severe aneurysms at a higher incidence compared to 10 week old, an age (or even younger) commonly used in previous studies studying DOCA and salt or aldosterone and salt hypertension.16, 23 Moreover, previous studies demonstrated that mineralocorticoid receptor expression in aorta increases with age,24 as does the functional response to mineralocorticoid receptor stimulation (e.g. mice with smooth muscle -specific deficiency of the mineralocorticoid receptor decreased blood pressure as they aged25). Since age is a risk factor for human AAAs,2 the ability to induce AAAs by DOCA and salt or aldosterone and salt with increasing age may have contributed to an inability to detect aneurysms in young mice.
An important finding of the present study is that administration of two distinct mineralocorticoid receptor antagonists, spironolactone and eplerenone, significantly attenuated DOCA and salt or aldosterone and salt induced aortic aneurysm. This suggests that the mineralocorticoid receptor mediates DOCA and salt or aldosterone and salt induced aortic aneurysm. Compared with an inability of spironolactone to completely abolish DOCA and salt induced aortic aneurysms, the inhibitory effect of eplerenone on aldosterone and salt induced aortic aneurysm was much more dramatic. The difference between spironolactone and eplerenone in their ability to inhibit aortic aneurysm is likely attributed to the doses of the mineralocorticoid receptor agonists vs. antagonists employed, since the molecular ratio of aldosterone to eplerenone and DOCA to spironolactone employed was 1:2 and 1:900, respectively. Spironolactone, but not eplerenone, exerts anti-androgen and pro-estrogen effects,6 which may have contributed to the spironolactone’s ability to inhibit DOCA and salt induced aneurysms, since it has been shown that androgen increases Ang II-induced AAAs26 while exogenous estrogen decreases AAAs.27
Although the molecular mechanism that links activation of the mineralocorticoid receptor and aortic aneurysm formation remains elusive, our results suggest that elastin degradation and collagen suppression are likely involved in DOCA and salt- or aldosterone and salt induced aortic aneurysm. These findings seem to contradict the result by Bunda et al. who found that aldosterone induced elastin and collagen production when they studied cultured cardiac fibroblasts.28 However, these findings do not necessarily contradict each other since elastin and collagen degradation may be dominant over their production in aortic aneurysm due to smooth muscle cell degeneration and activation of MMPs by vascular cells and inflammatory cells. Obviously, these two factors are absent in cultured cardiac fibroblasts.28
Intriguingly, the current study also suggests that ACE and AT1 receptor unlikely play a major role in DOCA and salt aortic aneurysm model. These results, however, do not rule out that stimulation of aldosterone synthesis by Ang II still play an important role in pathogenesis of aortic aneurysms under pathological conditions (e.g. in patients with elevated RAS and sodium intake). Our results also suggest that hypertension is not a contributory mechanism to aldosterone and salt or DOCA and salt-induced aortic aneurysms. These results are consistent with previous studies that Ang II infusion promotes AAAs was independent of increased blood pressure.29 More important, we demonstrated that DOCA and salt-induced vascular oxidative stress preceded aortic aneurysm formation and specifically we showed that Ncf1 mRNA expression was upregulated by DOCA-salt in thoracic and abdominal aortas in a time-dependent manner. These results fit well with previous studies that NCF1 protein expression was increased in human AAA segments compared with non-AAA segments,14 and genetic deletion of Ncf1 attenuated Ang II-induced AAA formation in mice,15 supporting a key role of NAD(P)H oxidase in vascular oxidative stress and the pathogenesis of AAAs.30
Several aortic aneurysm animal models have been developed.1 Among them, the Ang II-infusion-induced aortic aneurysm mouse model is mostly used.15, 18, 23, 26, 29, 31 Similar to the Ang II-infusion-induced aortic aneurysm mouse model, DOCA and salt or aldosterone and salt induced mouse aortic aneurysms were only found in suprarenal aortas but not infrarenal aorta. While the exact reasons why mice developed no aortic aneurysms in the infrarenal aorta that are different from human remains elusive, Rush et al. performed a whole genome expression analysis in the Ang II induced aortic aneurysm.32 Interestingly, they found that 304 transcripts were differentially expressed between suprarenal and infrarenal aortas. It is noted that a number of genes that may be relevant to the predilection of the suprarenal aorta to aortic aneurysm formation were down-regulated.32 These differentially expressed genes may potentially account for why mice developed aortic aneurysm in suprarenal aorta but not infrarenal aorta.
Compared with the Ang II-infusion model, the DOCA or aldosterone plus high salt model described here had several unique features, including using aged wild-type mice, requiring high salt, and exhibiting more severe elastin degradation and aortic aneurysm formation. Ang II is the primary stimulator of adrenal aldosterone synthesis,4 but Ang II-stimulated aldosterone synthesis is involved in aortic aneurysm formation only in the presence of high salt. It has been shown that spironolactone had no significant effect on Ang II-induced aortic aneurysm formation in the absence of high salt.23 It has also been shown that in hypertensive angiotensinogen and renin transgenic mice that overproduce Ang II, aortic aneurysms did not occur unless transgenic mice were fed a high-salt diet.33 Consistent with these findings, we demonstrated that high salt was required for DOCA-induced aortic aneurysm formation. According to the National Health and Nutrition Examination Survey (NHANES), 34 sodium intake has increased among all age groups since 1970, and only 32% of females and 9% of males meet the recommendation of sodium intake (2,400 mg per day). Under the circumstances that the majority of Americans are under the risk of high salt intake, the finding that activation of mineralocorticoid receptor by aldosterone plus high salt induced aortic aneurysm may have a potentially important impact on the current understanding regarding the pathogenesis of aortic aneurysm.
Based on the current study and literatures on the function of mineralocorticoid receptor,3–6 it is tentative to propose a potential mechanism that may underlie DOCA and salt or aldosterone and salt induced aortic aneurysms. Activation of mineralocorticoid receptor in circulating inflammatory cells and vascular cells by DOCA or aldosterone in the presence of high salt promotes monocyte/macrophage, neutrophil, and T cell infiltration into aorta to produce MMPs and oxidative stress, leading to a loss of structural integrity (e.g. elastin degradation and vascular smooth cell degeneration) and aortic aneurysm formation and rupture. It should be pointed out that this is an oversimplified mechanism that needs to be further studied.
In conclusion, results from this study demonstrate that mineralocorticoid receptor agonism via DOCA or aldosterone, coupled with increased salt consumption, results in severe aneurysms in the thoracic and abdominal aortas of mice, and age, but not blood pressure, is a contributory mechanism to mineralocorticoid receptor and salt-induced aneurysms. In addition, mineralocorticoid receptor antagonism, but not Ang II synthesis or AT1 receptor antagonism, attenuates mineralocorticoid receptor and salt-induced aortic aneurysms. Given that there are no effective drug therapies for aortic aneurysms, our results may lead to mineralocorticoid receptor antagonism as a novel therapy to potentially prevent and/or attenuate aortic aneurysm formation.
Supplementary Material
SIGNIFICANCE.
Aortic aneurysm is a devastating disease but currently no drug has been approved for its treatment. This study describes a novel mouse model of aortic aneurysms induced by administration of mineralocorticoid receptor agonist and high salt, which mimics human aortic aneurysm in many aspects. This study reveals a previously unrecognized but potentially significant role of aldosterone/mineralocorticoid receptor and high salt-intake in the pathogenesis of aortic aneurysms. The results identify the high salt intake as a potential new risk factor for aortic aneurysm formation. Moreover, the results implicate that spironolactone and eplerenone, two FDA approved and widely used mineralocorticoid receptor antagonists, may be effective in the prevention and attenuation of some aortic aneurysm formation.
Acknowledgments
We thank Dr. Katz Wendy for her excellent technical assistance in preparing the histology samples.
Funding sources
This work was supported by National Institutes of Health grants HL088389 and HL088389-02S1 (to Z.G.), HL082791 (to M.G.), and HL106843 (to M.G. and Z.G.), funds from the Commonwealth of Kentucky Diabetes Research Trust Fund (to Z. G.), a Postdoctoral Fellowship from the American Heart Association (to S.L.), and a grant from the National Institute of General Medical Sciences (8 P20 GM103527-05) of the National Institutes of Health.
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–316. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: Pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 3.Young MJ, Rickard AJ. Mechanisms of mineralocorticoid salt-induced hypertension and cardiac fibrosis. Mol Cell Endocrinol. 2012;350:248–255. doi: 10.1016/j.mce.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Funder JW, Reincke M. Aldosterone: A cardiovascular risk factor? Biochim Biophys Acta. 2010;1802:1188–1192. doi: 10.1016/j.bbadis.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 6.Maron BA, Leopold JA. Aldosterone receptor antagonists: Effective but often forgotten. Circulation. 2010;121:934–939. doi: 10.1161/CIRCULATIONAHA.109.895235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed SH, Husain NM, Khawaja SN, Massey CV, Pettyjohn FS. Is primary hyperaldosteronism a risk factor for aortic dissection? Cardiology. 2007;108:48–50. doi: 10.1159/000095787. [DOI] [PubMed] [Google Scholar]
- 10.Thompson A, Cooper JA, Fabricius M, Humphries SE, Ashton HA, Hafez H. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J Vasc Surg. 2010;52:55–61. e52. doi: 10.1016/j.jvs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Schenk J, McNeill JH. The pathogenesis of doca-salt hypertension. J Pharmacol Toxicol Methods. 1992;27:161–170. doi: 10.1016/1056-8719(92)90036-z. [DOI] [PubMed] [Google Scholar]
- 12.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–e129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Curci JA. Digging in the “soil” of the aorta to understand the growth of abdominal aortic aneurysms. Vascular. 2009;17 (Suppl 1):S21–29. doi: 10.2310/6670.2008.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller FJ, Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: A potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol. 2002;22:560–565. doi: 10.1161/01.atv.0000013778.72404.30. [DOI] [PubMed] [Google Scholar]
- 15.Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr, Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss D, Taylor WR. Deoxycorticosterone acetate salt hypertension in apolipoprotein E−/− mice results in accelerated atherosclerosis: The role of angiotensin II. Hypertension. 2008;51:218–224. doi: 10.1161/HYPERTENSIONAHA.107.095885. [DOI] [PubMed] [Google Scholar]
- 17.Daugherty A, Manning MW, Cassis LA. Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor−/− mice. Circ Res. 2011;108:574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousseau MF, Gurne O, Duprez D, Van Mieghem W, Robert A, Ahn S, Galanti L, Ketelslegers JM. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: Results from the rales neurohormonal substudy. J Am Coll Cardiol. 2002;40:1596–1601. doi: 10.1016/s0735-1097(02)02382-3. [DOI] [PubMed] [Google Scholar]
- 20.He BJ, Joiner ML, Singh MV, Luczak ED, et al. Oxidation of camkii determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon GT, Dluhy RG. Glucocorticoid-remediable aldosteronism. Cardiol Rev. 2004;12:44–48. doi: 10.1097/01.crd.0000096417.42861.ce. [DOI] [PubMed] [Google Scholar]
- 22.Lenk GM, Tromp G, Weinsheimer S, Gatalica Z, Berguer R, Kuivaniemi H. Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMC Genomics. 2007;8:237. doi: 10.1186/1471-2164-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassis LA, Helton MJ, Howatt DA, King VL, Daugherty A. Aldosterone does not mediate angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Br J Pharmacol. 2005;144:443–448. doi: 10.1038/sj.bjp.0706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krug AW, Allenhofer L, Monticone R, Spinetti G, Gekle M, Wang M, Lakatta EG. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 55:1476–1483. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18(9):1429–33. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, Cassis LA. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circ Res. 2012;110:e73–85. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novotny NM. Estrogen in abdominal aortic aneurysms: Potential for therapy. J Surg Res. 2009;155:181–182. doi: 10.1016/j.jss.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Bunda S, Liu P, Wang Y, Liu K, Hinek A. Aldosterone induces elastin production in cardiac fibroblasts through activation of insulin-like growth factor-I receptors in a mineralocorticoid receptor-independent manner. Am J Pathol. 2007;171:809–819. doi: 10.2353/ajpath.2007.070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. Ang II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:461–469. doi: 10.1161/01.ATV.0000257552.94483.14. [DOI] [PubMed] [Google Scholar]
- 31.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rush C, Nyara M, Moxon JV, Trollope A, Cullen B, Golledge J. Whole genome expression analysis within the angiotensin II-apolipoprotein E deficient mouse model of abdominal aortic aneurysm. BMC Genomics. 2009;10:298. doi: 10.1186/1471-2164-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishijo N, Sugiyama F, Kimoto K, Taniguchi K, Murakami K, Suzuki S, Fukamizu A, Yagami K. Salt-sensitive aortic aneurysm and rupture in hypertensive transgenic mice that overproduce angiotensin II. Lab Invest. 1998;78:1059–1066. [PubMed] [Google Scholar]
- 34.Briefel RR, Johnson CL. Secular trends in dietary intake in the united states. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.