Abstract
Mammographic density is a strong risk factor for breast cancer but its underlying biology in healthy women is not well-defined. Using a novel collection of core biopsies from mammographically dense versus non-dense regions of the breasts of healthy women, we examined histologic and molecular differences between these two tissue types. Eligible participants were 40+ years, had a screening mammogram and no prior breast cancer or current endocrine therapy. Mammograms were used to identify dense and non-dense regions and ultrasound-guided core biopsies were performed to obtain tissue from these regions. Quantitative assessment of epithelium, stroma, and fat was performed on dense and non-dense cores. Molecular markers including Ki-67, estrogen receptor (ER) and progesterone receptor (PR) were also assessed for participants who had >0% epithelial area in both dense and non-dense tissue. Signed rank test was used to assess within woman differences in epithelium, stroma and fat between dense and non-dense tissue. Differences in molecular markers (Ki-67, ER, and PR) were analyzed using generalized linear models, adjusting for total epithelial area. Fifty-nine women, mean age 51 years (range: 40–82), were eligible for analyses. Dense tissue was comprised of greater mean areas of epithelium and stroma (1.1 and 9.2 mm2 more, respectively) but less fat (6.0 mm2 less) than non-dense tissue. There were no statistically significant differences in relative expression of Ki-67 (P = 0.82), ER (P = 0.09), or PR (P = 0.96) between dense and non-dense tissue. Consistent with prior reports, we found that mammographically dense areas of the breast differ histologically from non-dense areas, reflected in greater proportions of epithelium and stroma and lesser proportions of fat in the dense compared to non-dense breast tissue. Studies of both epithelial and stromal components are important in understanding the association between mammographic density and breast cancer risk.
Keywords: Mammographic density, Histology, Stroma, Breast cancer
Introduction
Mammographic density is a strong and consistent risk factor for breast cancer [1, 2]. Women with dense tissue occupying over 60–75% of the breast are at 3–6 times greater risk of breast cancer than women with little or no density [3, 4]. However, what breast density represents at the tissue level is still not well-defined.
Previous studies have assessed the association between mammographic density and histologic features on mastectomy, breast biopsy, autopsy, or nipple aspirate specimens. Taken together, these studies consistently show positive associations of mammographic density with increased area of epithelial and stromal tissues, in particular, areas of collagen, as well as nuclear area of both epithelial and nonepithelial cells [5-11]. However, the majority of these studies were performed on tissue obtained in women with underlying breast symptoms, and the tissue was not specifically procured from radiologically dense areas of the breast. Examining the histology of tissue specifically obtained from dense and non-dense areas of the breast would complement these previous findings and extend our understanding of the biology of breast density.
The positive associations of mammographic density with stromal and epithelial proliferation [5, 9, 10, 12-14] have resulted in investigations of cell proliferation markers including Ki-67, MIB1, and DNA S phase percentage, and mammographic density, with inconsistent associations [7,8, 15-17]. Mammographic density has also been established as a hormonally responsive trait, with studies showing decrease in mammographic density with tamoxifen, menopause and parity and increased mammographic density with use of postmenopausal hormone therapy [18-21]. Consequently, hormone receptors for estrogen and progesterone have been examined among biopsy samples from symptomatic women or non-cancerous tissue from post-mastectomy specimens and showed no association with overall mammographic density [15, 16]. As of now, no studies have examined markers of proliferation or hormone receptors in tissue specifically derived from dense and non-dense regions of the breast.
In this report, we present results from a study that uniquely sampled tissue from mammographically dense and non-dense areas within the breasts of healthy women in order to examine histological and molecular correlates of mammographically dense and non-dense breast tissue.
Methods
Study population
Asymptomatic healthy volunteers were recruited through advertisements between 2006 and 2008 at the Mayo Clinic, Rochester, Minnesota. This study was approved by the Mayo Clinic Rochester Institutional Review Board. Eligible women were aged 40 years or older with no personal history of breast cancer and a normal screening mammogram within 6 months of biopsy. Patients were ineligible if they were currently using postmenopausal hormones, oral contraceptives, other reproductive endocrine therapy, or anticoagulants. Other exclusions included history of bleeding complications or allergy to local anesthetic agents. All participants completed a study-specific questionnaire assessing breast cancer risk factors including age at menarche, age at first parity, family history of breast cancer in a first degree relative, use of oral contraceptives or postmenopausal hormone therapy, number of breast biopsies, and prior history of atypical hyperplasia.
Ascertainment of dense and non-dense breast core biopsies
Mammography and ultrasound were both used to localize regions of dense and non-dense tissue. Using mammogram films (two views of each breast) taken within 6 months of the study biopsy, the radiologist (KRB) identified areas of high and low density in the upper outer quadrant of the right breast. If the patient had a previous benign surgical biopsy in the right breast, the upper outer quadrant of the left breast was selected for biopsy. Mammographically identified dense and non-dense areas were then localized by use of 3-D ultrasound. Patients were excluded if the radiologist could not localize areas of both high- and low-density in the same breast.
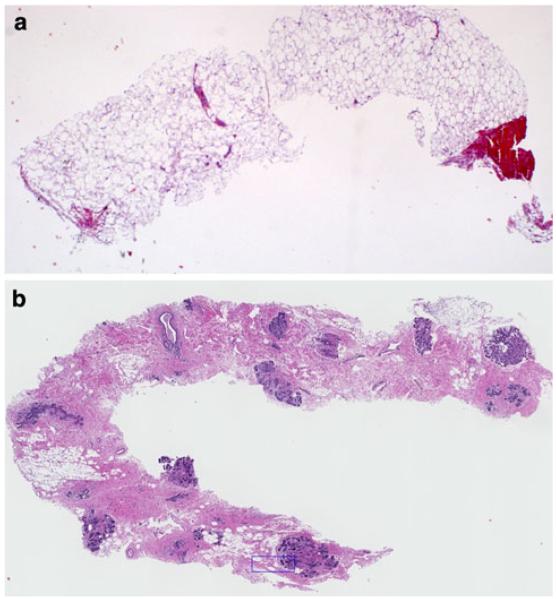
Sonographically, the dense tissue selected for biopsy was either homogenously hyperechoic (relative to subcutaneous breast fat) or a heterogenous mixture of hyperechoic tissue and hypoechoic tissue. Sonographically, the non-dense tissue selected for biopsy was isoechoic to subcutaneous breast fat. Following injection of local anesthetic, using a 14-gauge needle, an ultrasound-guided core-needle biopsy was performed in the dense and non-dense regions. Four tissue cores were taken from both dense and non-dense regions (Fig. 1). Three tissue cores were fixed in 10% normal buffered formalin and then paraffin embedded into one tissue block. The fourth tissue core was frozen for future studies. Thirty serial sections (5 μ) were cut from each paraffin-embedded tissue block. Sections 1, 15, and 30 from both the dense and non-dense tissue blocks were used for conventional staining with hematoxylin and eosin (H&E) and histologically examined to insure that the tissue cores contained benign tissue.
Fig. 1.
Core-needle breast biopsies taken from non-dense (a) and dense (b) areas of the healthy breast
Histological and molecular marker assessment
Quantitative assessment of the areas of epithelium, stroma, and fat was performed on blinded sections (section 15) from the dense and non-dense areas using image analysis. Each H&E stained slide was scanned using the Hamamatsu NanoZoomer Digital Pathology (NDP) (Bacus Laboratories, Inc.) to generate high resolution images at 20 times magnification.
The total area of tissue on each section was manually outlined to include the combined area of epithelium, stroma, and fat. Next, the individual areas of epithelium, stroma, and fat (in mm2) were each outlined separately [22]. The area measurement tool within the Bacus Laboratories Webslide Browser [23] program was used for all area measurements.
For cores with adequate epithelium, extent of lobular involution was also assessed on the H&E stained slides from dense and non-dense areas by a pathologist (DVW) experienced with the assessment of involution [24]. Involution was categorized as no involution (0% lobules involuted), partial involution (1–74% lobules involuted) or complete involution (≥75% lobules involuted) [24].
One section (slide 16, 17, and 18, respectively) from each of the dense and non-dense tissue blocks was stained for Ki-67, estrogen receptor (ER), and progesterone receptor (PR) using standard antibodies (MIB-1, Dako for Ki67; Clone ID5, Dako for ER; PgR363, Dako for PR). Computer assisted analysis was perform by using the IHCScore software provided by Bacus Laboratories, Inc. This software allows for individual analysis of regions of interest for measurements of (1) total area, (2) absolute stained (IHC) area, and (3) percentage stained (IHC) area (referenced to the total area). Only women with >0% (some) epithelial cells on both dense and non-dense sections were eligible for the comparisons of hormone receptors (estrogen and progesterone) and cell proliferation marker, Ki-67.
Assessment of reproducibility was performed for the image analysis of morphometry (areas and proportions of epithelium, stroma, and fat) and antibody expression (percentage stained area for Ki-67, ER, and PR). A total of five pairs of sections were selected randomly from the entire set of 59 pairs and evaluated in a blind fashion. Both the intraclass correlation coefficient [25] and concordance correlation coefficient [26-28] were used to assess agreement of the histologic and molecular marker reads. Both the intraclass and concordance correlations have been shown equivalent to extensions of the weighted kappa statistic, and therefore appropriately measure agreement. The correlation coefficients exceeded 0.98 for all markers examined, providing evidence for strong agreement of our marker assessment.
Classification of benign breast disease
The H&E stained slide (section 15) of breast tissue from dense and non-dense areas was assessed for the type of benign breast tissue. Tissue was classified by the breast pathologist (CR and DVW) into the following categories: non-proliferative disease, proliferative disease without atypia, and atypical hyperplasia using standard criteria described by Dupont et al. [29, 30].
Participant acceptance of procedure
Participants were also asked to complete a questionnaire after the procedure to determine if they had experienced pain (numeric rating scale ranging from 0 to 10) [31-33], anxiety related to the procedure (numeric rating scale from 0 to 10) [34], and if they would recommend this procedure to other women (yes; maybe; no).
Statistical analyses
Characteristics of study participants were summarized with means and standard deviations, or counts and percentages, as appropriate. The Gail model risk score was calculated for all women using the Gail model 2 risk calculator. This score is based on risk factor information including age, race, history of breast cancer, age at menarche, age at first parity, family history of breast cancer in a first degree relative, number of previous breast biopsies, and atypical hyperplasia on prior breast biopsy. A woman is considered at elevated risk of breast cancer if her risk score is ≥1.66 [35, 36].
The area of epithelium, stroma and fat was quantified both as absolute area (mm2) and as the ratio of the area of the cell type by the total area of tissue on the slide (proportion). The percent of the assessed area staining positive for each of the molecular markers (Ki67, ER, and PR) was measured from each tissue sample from women whose dense and non-dense tissues had any (>0%) epithelial cells. Comparisons between the area and percent of dense and non-dense histologic tissue types within a woman were made using signed rank tests. Generalized linear models were applied to further compare the histologic and molecular correlates of dense and non-dense samples after adjusting for subject-level covariates, including amount of epithelial area, while controlling for the repeated measurements within subjects with generalized estimating equations approaches [37, 38]. Where the data were not normally distributed, a link function was applied within the analyses (i.e., logit or logarithmic). All analyses were performed within the SAS statistical computing platform (Cary, NC) and all tests of significance were two-sided.
Results
A total of 238 women inquired about study participation; of these, 146 (61.3%) were ineligible, 23 (9.7%) expressed interest but did not participate, 3 (1.3%) were scheduled for the biopsy but did not show for their appointment and 66 (27.7%) completed the study. Since our comparisons in the current study required tissue from both dense and non-dense regions in individual participants, seven participants were excluded who only had cores available from a non-dense (n = 6) or dense (n = 1) region of their breasts. Thus, 59 women were included in the current analyses.
Participant characteristics are described in Table 1. All were Caucasian and the mean age of the participants was 51 years (range 40–82 years). In addition, 37.3% of the women were premenopausal. The mean 5-year Gail model risk score of the participants was 1.3% (SD = 0.9%). Only a small proportion of participants had breasts that were almost all fat (3.4% had BI-RADS = 1) or extremely dense (3.4% had BI-RADS = 4), since a requirement that participants had representative areas of high and low densities was part of the eligibility criteria.
Table 1.
Characteristics of the study population (N = 59)
| Characteristic | Variable | N (%) |
|---|---|---|
| Age at biopsy (years) | ||
| 40-49 | 34 (57.6%) | |
| 50-59 | 13 (22.0%) | |
| 60-69 | 7 (11.9%) | |
| 70-79 | 4 (6.8%) | |
| 80-89 | 1 (1.7%) | |
| Categories of BMI (kg/m2) | ||
| 18-24 | 25 (42.4%) | |
| 25-30 | 23 (39.0%) | |
| 31-36 | 10 (17.0%) | |
| ≥37 | 1 (1.7%) | |
| Age at menarche (years) | ||
| ≤11 | 8 (14.8%) | |
| 12 | 12 (22.2%) | |
| 13 | 20 (37.0%) | |
| ≥14 | 14 (25.9%) | |
| Age at first parity (years)a | ||
| Mean (SD) | 25.9 ± 7.1 | |
| Parity (births) | ||
| Nulliparous | 10 (17.5%) | |
| 1 | 6 (10.5%) | |
| 2 | 21 (36.8%) | |
| ≥3 | 20 (35.1%) | |
| Involution status (%) |
||
| Unable to assess | 6 (10.2%) | |
| 0% | 0 (0%) | |
| 1-74% | 24 (40.7%) | |
| ≥75% | 29 (49.2%) | |
| BI-RADS density category | ||
| Fatty replaced | 2 (3.4%) | |
| Scattered density | 24 (40.7%) | |
| Heterogeneously dense | 31 (52.5%) | |
| Extremely dense | 2 (3.4%) | |
| Oral contraceptive use | ||
| Former | 46 (78.0%) | |
| Never | 11 (18.6%) | |
| Missingb | 2 (3.4%) | |
| Menopausal status | ||
| Premenopausal | 22 (37.3%) | |
| Postmenopausal | 37 (62.7%) | |
| Postmenopausal hormone use | ||
| Former | 13 (22.0%) | |
| Never | 45 (76.3%) | |
| Missingb | 1 (1.7%) |
Parous women only
Patients not currently on treatment but unknown if former or never user
Table 2 describes the differences in area (mm2) and proportion (ratio of the area of the cell type by the total area of tissue on the slide) measures of each cell type-epithelium, stroma or fat, between dense and non-dense breast tissue. Dense tissue was comprised of greater areas of epithelium and stroma (1.1 and 9.2 mm2 more, respectively) but less fat (6.0 mm2 less) than non-dense tissue. The differences in proportions were 4.8% greater epithelium, 46.1% greater stroma but 50.9% less fat in dense than non-dense tissue (all P < 0.0001). These differences in histology did not differ by Gail model risk score. There was also a statistically significant difference between dense and non-dense tissue with regard to the type of benign breast tissue, with greater proportions of proliferative disease without atypia among the dense sections (88.1% of dense sections were non-proliferative versus 100% in non-dense tissue; 11.9% proliferative disease without atypia in dense versus 0% in non-dense; atypical hyperplasia 0% in both dense and non-dense tissue) (P = 0.01).
Table 2.
Histology of dense and non-dense areas of the breast of healthy women (N = 59 women)
| Breast tissue type | Dense tissue | Dense tissue | Non-dense tissue |
Non-dense tissue |
Difference between dense and non-dense |
Difference between dense and non-dense |
|---|---|---|---|---|---|---|
| Mean area (mm2) |
Proportion (%)a |
Mean area (mm2) |
Proportion (%)a |
Mean area (mm2) | Proportion (%)a | |
| Epithelium | 1.21 (1.43) | 5.6 (5.0) | 0.14 (0.36) | 0.7 (1.8) | 1.07 (1.33)b | 4.8 (4.9)b |
| Stroma | 12.07 (6.17) | 64.7 (17.7) | 2.92 (3.57) | 18.6 (14.5) | 9.15 (5.90)b | 46.1 (20.6)b |
| Fat | 5.51 (4.27) | 29.7 (18.5) | 11.50 (6.0) | 80.6 (15.8) | −5.99 (7.09)b | −50.9 (21.5)b |
| Epithelium and stroma combined | 13.28 (7.10) | 70.3 (18.5) | 3.06 (3.86) | 19.4 (15.8) | 10.22 (6.65)b | 50.9 (21.5)b |
Numbers in parentheses represent standard deviation
Proportion refers to the ratio of the area of the specific tissue type to the total area of breast tissue on the slide
Pvalues for within woman difference between dense and non-dense <0.0001
Involution status was compared between dense and nondense tissue, using 23 pairs for whom involution could be evaluated in the core biopsy (Table 3). There was suggestion of a greater proportion of complete involution in the non-dense areas (73.9%) compared to dense areas (52.2%) but the findings were non-significant. Similarly, there was little difference in mean involution score between these two tissue types (Table 3).
Table 3.
Comparison of involution status in dense and non-dense tissue
| Characteristic | N a | Dense | Non-dense | Adjusted difference between dense and non-dense tissuec |
P value |
|---|---|---|---|---|---|
| Involution statusb | 23 | 1.52 (0.51) | 1.74 (0.45) | 0.08 (0.12) | 0.52 |
| Proportion with complete involution |
23 | 52.2% | 73.9% | Odds ratio = 0.35d 95% Confidence interval: 0.07, 1.7 |
0.20 |
SD standard deviation
Only women with >0% epithelial cells and involution assessed on each section were eligible for this analysis
Mean value (SD). Involution defined as: 0 no involution (0% involuted TDLUs); 1 partial involution (1-74% involuted TDLUs); 2 complete involution (≥75% involuted TDLUs). Mean involution status is average of 0, 1, and 2
Adjusted for epithelial area
Odds of complete involution among dense tissue compared to odds of complete involution among non-dense tissue
Preliminary analyses of Ki-67, ER, and PR were performed on the 24 women who had >0% epithelial cells present on both dense and non-dense tissue sections. There were no statistically significant differences in relative expression of Ki-67 (P = 0.82), ER (P = 0.09), or PR (P = 0.96) between dense and non-dense tissue (Table 4). However, the difference in relative expression of ER was 2.7-fold greater in dense compared to non-dense tissue.
Table 4.
Comparison of molecular markers in dense and non-dense breast tissue
| Biomarker | N a | Within-woman percent area Staining positive (mm2) |
Adjustedb relative differencec between dense and non-dense tissue (95% CI) |
|
|---|---|---|---|---|
| Mean (SD) | Dense Non-dense | |||
| Ki-67 | 24 | 0.73 (1.01) | 1.03 (1.62) | 0.91 (0.42, 1.99) |
| ER | 23 | 6.82 (9.96) | 2.68 (6.23) | 2.73 (0.85, 8.79) |
| PR | 24 | 11.90 (13.73) | 11.14 (19.28) | 0.98 (0.47, 2.07) |
Only women with >0% epithelial cells on each section were eligible tor this analysis
Adjusted for epithelial area
c Relative difference reflects the ratio of the cells staining positive for the marker, with the measurement in dense tissue serving as the numerator and the measurement of the non-dense tissue serving as the denominator
Participants reported little pain (mean value (SD) = 0.6 (1.0); range 0–4) or anxiety (anxiety: mean value (SD) = 1.6 (1.8); range 0–7, both on a scale of 1–10) related to the biopsy procedure. Most participants indicated that they would recommend this procedure to other women (no = 0%; maybe = 7%; yes = 93%).
Discussion
This report describes histological and molecular characteristics of breast tissue specifically sampled from dense and non-dense regions of the breasts of healthy women. We found that mammographically dense areas of the breast differ histologically from non-dense areas, reflected in greater proportions of epithelium and stroma, lesser proportions of fat, and higher proportion of proliferative disease without atypia in the dense compared to non-dense breast tissue. However, we found no significant differences in expression of Ki-67, ER, or PR, or involution status, in dense versus non-dense tissue.
Our findings are consistent with those from prior studies assessing the association between histological and mammographic features of the breast [5, 6, 10, 14, 16, 39]. Bartow et al. [10] studied mastectomy specimens and found that mammographic density was more likely to be associated with epithelial hyperplasia. Fisher et al. [14] also assessed mastectomy specimens and reported that dense areas as depicted on mammograms of the mastectomy specimens were more likely to have fibrous tissue than radiolucent areas. Studies of tissue obtained at clinical breast biopsies also reported epithelial hyperplasia associated with mammographic density in some studies [13, 39-41] but no associations in other studies [42, 43]. In the most comprehensive study to date, Li et al. [6] examined breast tissue at the time of forensic autopsy, and correlated estimates of mammographic density on tissue sections with histologic features using quantitative microscopy. They reported that percent mammographic density on the section was associated with epithelial and non-epithelial nuclear area, collagen, and area of glandular structures [6]. Lin et al. [11] used image-guided technique to sample high and low mammographic density tissues from mastectomy specimens and showed that high mammographic density areas of the breast had higher stromal and lower fat composition compared to low mammographic density regions of the same breast. We extend these prior findings to breast tissue from targeted dense and non-dense regions from healthy, living women. Our histology findings are consistent with most prior reports showing that mammographically dense areas reflect increased proportion of stroma and epithelium and less fat compared to non-dense areas. Taken together, these studies suggest need for further investigation of stromal and epithelial contributions to the mammographic density and breast cancer association.
Benign breast conditions such as atypical hyperplasia and proliferative disease without atypia are associated with increased breast cancer risk and have been considered to be histological precursors of breast cancer [29, 30]. Several studies have examined the association between benign breast conditions on breast core-biopsies or mastectomy specimens, and mammographic density [5, 13, 14, 39, 40,42-44]. Our findings of higher proportion of proliferative disease without atypia in dense compared to non-dense tissue are consistent with those that found associations between proliferative changes in the epithelium and mammographic density [5, 13, 14, 39, 40, 44]. In one of the largest case–control studies to date (Canadian National Breast Screening Study), women with greater than 75% density had increased risk of ductal cancer in situ or atypical hyperplasia (Odds Ratio 9.7; 95% Confidence Interval: 1.7, 53.9), as well as increased risk of proliferative disease without atypia (Odds Ratio 12.2; 95% Confidence Interval: 2.9, 50.1) compared to women with non-dense breasts [39]. Our data would suggest that epithelium in dense tissue is more susceptible to cancer than epithelium in non-dense tissue. Moreover, all dense tissue may not reflect similar features, as dense tissue from only a small proportion of women (12%) was seen at greater risk of breast cancer, as defined by proliferative disease.
We previously demonstrated that involution assessed from a breast biopsy was inversely associated with mammographic density of the whole breast [45]. Further, Lin et al. [11] showed that areas of high mammographic density in mastectomy specimens had higher glandular counts and greater proportion of smaller and less complex glands or type I lobules compared to areas of low mammographic density. Based on these reports, we had expected to see a difference in involution corresponding to tissue from targeted dense and non dense regions, but we instead found no evidence of association. However, our evaluation of involution in the dense versus non-dense tissue was limited in power, with only 23 pairs for analysis. Also, as noted previously [45], the inverse association between mammographic density and involution is complex; the current definition for lobular involution does not differentiate the replacement of involuted lobules by stroma and/or fat [24] and mammographic density likely has differential associations with involution depending on the replacement by stroma and/or fat [45]. Our findings of a lack of difference in involution by dense and non-dense tissue are consistent, though, with a previous study by our group that found involution was similar across the breast [46]. Continued research with larger number of women will be needed to study these issues further.
The lack of association between ER and PR in dense versus non-dense regions of the breast is consistent with the few studies that examined these markers with mammographic density [15, 16]. Verheus et al. [15] studied hormonal and proliferation markers in benign breast tissue cores obtained from breast cancer tumor blocks in 159 women. They showed no significant association between mammographic density and expression of steroid receptors or proliferation markers. Similar to our findings for Ki-67, mammographic density has not been shown to correlate with proliferation markers in the majority of studies to date. Five studies have examined mammographic density and markers of proliferation in benign tissue or nipple aspirates in breast cancer cases, mastectomy specimens [16], high risk women [8, 17], and reduction mammoplasty [7]. The only positive association was reported by Harvey et al. [16] who studied non-cancerous tissue from mastectomy specimens of 56 women; half of them were on postmenopausal hormone therapy. They found increased density was significantly associated with Ki-67 activity in the ducts and lobule type 1 for both pre- and post-menopausal women. Even with our limited power, the estimates of relative difference for Ki-67 and PR between the two tissue types were close to unity, consistent with no difference in these markers. Interestingly, there was a 2.7-fold difference between ER expression in dense versus non-dense tissue. Owing to the small sample size, we cannot rule out a true difference. Thus, examining ER expression in these two tissue types is an area for future investigation.
The patients’ response to the biopsy study demonstrated the feasibility of performing tissue-based studies of healthy women to study breast disease. This is important, as the use of clinical information, combined with radiologic information (such as mammographic density) and tissue markers from breast biopsy tissue or possibly even random breast biopsies may be able to stratify women based on breast cancer risk.
We acknowledge that this study has limitations in terms of the generalizability of the findings as the study population was a group of healthy women volunteers from the upper Midwest of the United States. This study will need to be validated in larger and diverse populations. We used both mammography and ultrasound to localize dense and non-dense regions, but recognize the utility of using a modality such as magnetic resonance imaging for better precision of localizing these regions. Also, our findings with estrogen and progesterone receptor as well as involution are limited by power and must be viewed as exploratory, since only 24 women had sufficient epithelial tissue on their dense and non-dense sections and involution status was only available on 23 of these. However, this is the first study of its kind, wherein tissue was specifically obtained from dense and non-dense areas of the healthy breast to better characterize mammographic density. Our findings confirm that mammographically dense tissue is comprised of greater epithelium and stroma and less fat compared to non-dense tissue. Hence, study of both epithelium and stroma are likely to be important in understanding mammographic density and its association with breast cancer.
Acknowledgments
We are very grateful to the volunteers who participated in this study. We acknowledge the efforts of the study coordinators (Jenny Mentlick, Shannin Renn), members of the Mayo BAP and TACMA labs (especially Karla Kopp and Lorna Lubinski), the Breast Imaging Unit, Department of Radiology, and the Clinical Research Unit. This study was supported by the National Institute of Health (NIH) [Grant numbers K12 RR24151; Mayo Clinic Breast SPORE; NCI P50 CA116201; NCI R01 CA128931; NCI R01 CA140286] and Mayo Clinic Cancer Center.
Footnotes
Conflict of interest None declared.
Contributor Information
Karthik Ghosh, Department of Medicine, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA.
Kathleen R. Brandt, Department of Radiology, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA
Carol Reynolds, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA.
Christopher G. Scott, Department of Health Sciences Research, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA
V. S. Pankratz, Department of Health Sciences Research, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA
Darren L. Riehle, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA
Wilma L. Lingle, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA
Tonye Odogwu, Department of Health Sciences Research, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA.
Derek C. Radisky, Department of Laboratory Medicine and Pathology, Mayo Clinic, Jacksonville, FL, USA
Daniel W. Visscher, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA
James N. Ingle, Department of Oncology, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA
Lynn C. Hartmann, Department of Oncology, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA
Celine M. Vachon, Department of Health Sciences Research, Mayo Clinic College of Medicine, Charlton 6-239, 200 First Street SW, Rochester, MN 55905, USA
References
- 1.Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101:384–398. doi: 10.1093/jnci/djp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Sellers TA. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:43–49. doi: 10.1158/1055-9965.EPI-06-0738. [DOI] [PubMed] [Google Scholar]
- 3.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102:1224–1237. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;6:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Jensen HM, Cooke G, Han HL, Lockwood GA, Miller AB. Mammographic densities and the prevalence and incidence of histological types of benign breast disease. Reference Pathologists of the Canadian National Breast Screening Study. Eur J Cancer Prev. 2000;1:15–24. doi: 10.1097/00008469-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 7.Hawes D, Downey S, Pearce CL, Bartow S, Wan P, Pike MC, Wu AH. Dense breast stromal tissue shows greatly increased concentration of breast epithelium but no increase in its proliferative activity. Breast Cancer Res. 2006;8:R24. doi: 10.1186/bcr1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stomper PC, Penetrante RB, Edge SB, Arredondo MA, Blumenson LE, Stewart CC. Cellular proliferative activity of mammographic normal dense and fatty tissue determined by DNA S phase percentage. Breast Cancer Res Treat. 1996;37:229–236. doi: 10.1007/BF01806504. [DOI] [PubMed] [Google Scholar]
- 9.Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–R135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartow SA, Pathak DR, Mettler FA, Key CR, Pike MC. Breast mammographic pattern: a concatenation of confounding and breast cancer risk factors. Am J Epidemiol. 1995;142:813–819. doi: 10.1093/oxfordjournals.aje.a117720. [DOI] [PubMed] [Google Scholar]
- 11.Lin SJ, Cawson J, Hill P, Haviv I, Jenkins M, Hopper JL, Southey MC, Campbell IG, Thompson EW. Image-guided sampling reveals increased stroma and lower glandular complexity in mammographically dense breast tissue. Breast Cancer Res Treat. 2011;128:505–516. doi: 10.1007/s10549-011-1346-0. [DOI] [PubMed] [Google Scholar]
- 12.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian national breast screening study. J Natl Cancer Inst. 1995;87:670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 13.Bright RA, Morrison AS, Brisson J, Burstein NA, Sadowsky NS, Kopans DB, Meyer JE. Relationship between mammographic and histologic features of breast tissue in women with benign biopsies. Cancer. 1988;61:266–271. doi: 10.1002/1097-0142(19880115)61:2<266::aid-cncr2820610212>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Fisher ER, Palekar A, Kim WS, Redmond C. The histopathology of mammographic patterns. Am J Clin Pathol. 1978;69:421–426. doi: 10.1093/ajcp/69.4.421. [DOI] [PubMed] [Google Scholar]
- 15.Verheus M, Maskarinec G, Erber E, Steude JS, Killeen J, Hernandez BY, Cline JM. Mammographic density and epithelial histopathologic markers. BMC Cancer. 2009;9:182. doi: 10.1186/1471-2407-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey JA, Santen RJ, Petroni GR, Bovbjerg VE, Smolkin ME, Sheriff FS, Russo J. Histologic changes in the breast with menopausal hormone therapy use: correlation with breast density, estrogen receptor, progesterone receptor, and proliferation indices. Menopause. 2008;15:67–73. doi: 10.1097/gme.0b013e318054e29a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan QJ, Kimler BF, O’Dea AP, Zalles CM, Sharma P, Fabian CJ. Mammographic density does not correlate with Ki-67 expression or cytomorphology in benign breast cells obtained by random periareolar fine needle aspiration from women at high risk for breast cancer. Breast Cancer Res. 2007;9:R35. doi: 10.1186/bcr1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerlikowske K, Cook AJ, Buist DS, Cummings SR, Vachon C, Vacek P, Miglioretti DL. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830–3837. doi: 10.1200/JCO.2009.26.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vachon CM, Sellers TA, Vierkant RA, Wu FF, Brandt KR. Case-control study of increased mammographic breast density response to hormone replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11:1382–1388. [PubMed] [Google Scholar]
- 20.Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–37. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 21.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 22.Russo J. Molecular basis of breast cancer prevention and treatment. In: Russo J, Russo IH, editors. The breast as a developing organ. Springer-Verlag; Berlin: 2004. pp. 11–46. Chapter 2. [Google Scholar]
- 23.Garrity MM, Burgart LJ, Riehle DL, Hill EM, Sebo TJ, Witzig T. Identifying and quantifying apoptosis: navigating technical pitfalls. Mod Pathol. 2003;16:389–394. doi: 10.1097/01.MP.0000062657.30170.92. [DOI] [PubMed] [Google Scholar]
- 24.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, et al. Age-related lobular involution and reduced risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 25.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 26.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 27.Lin L. A note on the concordance correlation coefficient. Biometrics. 2000;56:324–325. [Google Scholar]
- 28.King TS, Chinchilli VM. A generalized concordance correlation coefficient for continuous and categorical data. Stat Med. 2001;20:2131–2147. doi: 10.1002/sim.845. [DOI] [PubMed] [Google Scholar]
- 29.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 31.Moe RH, Uhlig T, Kjeken I, Hagen KB, Kvien TK, Grotle M. Multidisciplinary and multifaceted outpatient management of patients with osteoarthritis: protocol for a randomised, controlled trial. BMC Musculoskelet Disord. 2010;11:253. doi: 10.1186/1471-2474-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chanques G, Viel E, Constantin JM, Jung B, de Lattre S, Carr J, Cisse M, Lefrant JY, Jaber S. The measurement of pain in intensive care unit: comparison of 5 self-report intensity scales. Pain. 2010;151:711–721. doi: 10.1016/j.pain.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 33.Gagliese L, Weizblit N, Ellis W, Chan VW. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117:412–420. doi: 10.1016/j.pain.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Rosen S, Svensson M, Nilsson U. Calm or not calm: the question of anxiety in the perianesthesia patient. J Perianesth Nurs. 2008;23:237–246. doi: 10.1016/j.jopan.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, et al. Tamoxifen for prevention of breast cancer: report of the National surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 36.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 37.McCullagh P, Nelder JA. Generalized linear models. 2nd edn. Chapman and Hall/CPC; New York: 1989. [Google Scholar]
- 38.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 39.Boyd NF, Jensen HM, Cooke G, Han HL. Relationship between mammographic and histological risk factors for breast cancer. J Natl Cancer Inst. 1992;84:1170–1179. doi: 10.1093/jnci/84.15.1170. [DOI] [PubMed] [Google Scholar]
- 40.Bland KI, Kuhns JG, Buchanan JB, Dwyer PA, Heuser LF, O’Connor CA, Gray LA, Sr, Polk HC., Jr A clinicopathologic correlation of mammographic parenchymal patterns and associated risk factors for human mammary carcinoma. Ann Surg. 1982;195:582–594. doi: 10.1097/00000658-198205000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urbanski S, Jensen HM, Cooke G, McFarlane D, Shannon P, Kruikov V, Boyd NF. The association of histological and radiological indicators of breast cancer risk. Br J Cancer. 1988;58:474–479. doi: 10.1038/bjc.1988.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moskowitz M, Gartside P, McLaughlin C. Mammographic patterns as markers for high-risk benign breast disease and incident cancers. Radiology. 1980;134:293–295. doi: 10.1148/radiology.134.2.7352202. [DOI] [PubMed] [Google Scholar]
- 43.Arthur JE, Ellis IO, Flowers C, Roebuck E, Elston CW, Blamey RW. The relationship of “high risk” mammographic patterns to histological risk factors for development of cancer in the human breast. Br J Radiol. 1990;63:845–849. doi: 10.1259/0007-1285-63-755-845. [DOI] [PubMed] [Google Scholar]
- 44.Bartow SA, Pathak DR, Mettler FA. Radiographic microcalcification and parenchymal patterns as indicators of histologic “high-risk” benign breast disease. Cancer. 1990;66:1721–1725. doi: 10.1002/1097-0142(19901015)66:8<1721::aid-cncr2820660812>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh K, Hartmann LC, Reynolds C, Visscher DW, Brandt KR, Vierkant RA, Scott CG, Radisky DC, Sellers TA, Pankratz VS, et al. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol. 2010;28:2207–2212. doi: 10.1200/JCO.2009.23.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vierkant RA, Hartmann LC, Pankratz VS, Anderson SS, Radisky D, Frost MH, Vachon CM, Ghosh K, Distad TJ, Degnim AC, et al. Lobular involution: localized phenomenon or field effect? Breast Cancer Res Treat. 2009;117:193–196. doi: 10.1007/s10549-008-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]