Abstract
To examine the effect of discriminative fear conditioning on the shape of the generalization gradient, two groups of participants first learned to discriminate between two color stimuli, one paired with an electrical shock (CS+) and the other explicitly unpaired (CS−). The CS+ was held constant as an intermediate (ambiguous) value along the blue-green color dimension while the CS− varied between groups as opposite endpoints along the blue-green color dimension. Post-discrimination testing, using spectral wavelengths above and below the CS+, revealed opposing asymmetric gradients of conditioned skin conductance responses across training groups that skewed in a direction opposite the CS−. Moreover, perceptual ratings for the color of the CS+ were affected by discriminative conditioning, with the color value of the blue or green CS− inducing a shift in the frequency for ratings of the ambiguous CS+ as either “green” or “blue,” respectively. These results extend findings on gradient shifts in the animal literature, and suggest that post-discrimination testing provides a more comprehensive estimate of the effects of discriminative fear conditioning than testing responses solely to the conditioned stimuli.
Keywords: anxiety, associative learning, generalization, peak shift, skin conductance responses
The problem of stimulus generalization has concerned researchers for nearly a century (Hull, 1943; Pavlov, 1927; Watson & Rayner, 1920). In the mid-twentieth century, two landmark publications demonstrated the utility of quantifying gradients of stimulus generalization to determine the features that control learning during appetitive instrumental conditioning. Guttman and Kalish (1956) showed that pigeons trained to key-peck to a specific color (conditioned stimulus, CS) reinforced by food (unconditioned stimulus, US) will generalize pecking responses in a graded decremented manner to unreinforced values along the color spectrum. Critically, gradients centered on the CS crossed discriminable color boundaries (e.g. between blue and green), thus addressing an important theoretical issue by showing that generalization was not merely a failure to discriminate color values (Hull, 1947; Lashley & Wade, 1946). A stimulus generalization experiment by Hanson (1959), also utilizing a color dimension, showed that discrimination training between a reinforced (CS+) and unreinforced (CS−) stimulus induced a post-discrimination shift in responses peaking at an unreinforced value beyond the CS+ in a direction opposite the CS− (i.e. a peak shift), an effect predicted earlier by Spence’s (1937) gradient-interaction theory.
The response bias characteristic of discrimination learning has become an important construct in theories of animal learning, behavior, and evolution (Jansson & Enquist, 2003; Lynn, Cnaani, & Papaj, 2005; Purtle, 1973; ten Cate & Rowe, 2007). For example, these effects may explain sexual preferences for exaggerated features (ten Cate & Rowe, 2007; ten Cate, Verzijden, & Etman, 2006) and heightened avoidance of potential prey with colors that are more extreme than previously experienced distasteful prey (Gamberale-Stille & Tullberg, 1999). In humans, there is some evidence that intradimensional discrimination training affects stimulus generalization of voluntary responses (Doll & Thomas, 1967; Galizio & Baron, 1979), but effects on gradients of autonomic fear responses is scant (see Dunsmoor, Mitroff, & LaBar, 2009).
Here, we examined the effect of discriminative fear conditioning on post-discrimination gradients of conditioned fear responses as a function of the relative properties between a reinforced and unreinforced stimulus. During conditioning, two groups of participants learned to discriminate between a color associated with an aversive electrical shock US and another color associated with the absence of the US. The color of the CS+ was held constant between groups as a value near the point of subjective equality between blue and green. The CS−, on the other hand, varied across groups -- for half the participants the CS− was from the blue end of the color spectrum, whereas for the other half of participants the CS− was from the green end of the color spectrum. A generalization test followed conditioning by presenting spectral values above and below the CS+. Skin conductance responses (SCR) were used as the dependent measure of fear conditioning and generalization. In addition, subjective color perception was assessed by 2-alternative forced choice (2-AFC) ratings throughout the experimental session.
We hypothesized that post-discrimination SCR gradients would be asymmetric and skew in a direction opposite the CS−, in line with a rich body of literature on gradient shifts in the animal literature, mostly in the domain of instrumental conditioning (Honig & Urcuioli, 1981; Purtle, 1973). As a secondary hypothesis, we also predicted that discrimination learning would alter perceptual color judgments for the ambiguous CS+, in line with prior human research on the effects of fear conditioning on perceptual decisions and discrimination thresholds (Li, Howard, Parrish, & Gottfried, 2008; Lim & Pessoa, 2008; Resnik, Sobel, & Paz, 2011) and stimulus generalization experiments of human perceptual learning (Doll & Thomas, 1967).
Method
Participants
Fifty-eight healthy volunteers from the Duke community (30 women, age range: 18–22, median age = 19 years) with normal color vision provided written informed consent in accordance with the Duke University Institutional Review Board guidelines. Eight subjects were excluded from the final analysis due to a lack of measurable SCRs. Participants were randomly assigned to Group 1 (n = 25) or Group 2 (n = 25), which differed only in regard to the unpaired control stimulus presented during the fear conditioning phase. The ethnic composition consisted of 8% Black, 24% Asian, and 68% White.
Stimulus set
Circles of different colors ranging from blue to green along the color spectrum were presented on a white rectangular background with a black border in the center of a 17-inch flat panel monitor in a closed testing room with ambient overhead lighting. The circles were 230 pixels in diameter and presented at a viewing distance of approximately 60 cm for a subtended visual angle of 4.63° × 7.24°. Color stimuli were determined by converting spectral wavelength (nanometers) into Red, Green, and Blue (RGB) values.
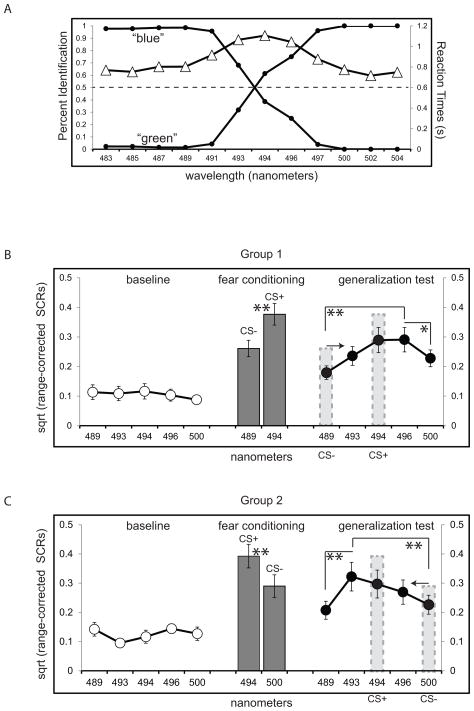
Stimuli were determined through pilot testing. A separate group of volunteers (n = 27) participated in a 2-AFC task wherein 12 different circles spanning the blue-to-green portion of the color spectrum were rated as either “blue” or “green.” Pilot subjects did not participate in the fear conditioning experiment. Based on pilot ratings (Figure 1a), we chose five incremental values that spanned between blue and green: 489, 493, 494, 496, and 500 nm. The value used for the CS+ (494 nm) was picked as it was nearest the point of subjective equality between blue and green. Two stimuli adjacent to the CS+ value (493 nm and 496 nm), and two stimuli that were consistently rated as “blue” (489 nm) and “green” (500 nm), were used as unreinforced stimuli.
Figure 1.
Pilot behavioral data and skin conductance responses (SCRs). (A) Perceptual identification (black circles) indicated that the 494 nm stimulus was nearest the point of subjective equality (dashed line) between green and blue. Reaction times (white triangles) peaked around the intermediate spectral values. SCRs broken down by experimental phase for Group 1 (B) and Group 2 (B) showed low and undifferentiated SCRs across the color dimension prior to conditioning (white circles) and heightened SCRS for the CS+ versus CS− (gray bars) during fear conditioning. Generalization gradients in Group 1 (B) and Group 2 (B) revealed peak SCRs at an unreinforced value opposite the CS−. Error bars represent standard error of the mean. * p < .05; ** p < .015 (Bonferroni-corrected for multiple comparisons). For both (B) and (C), the transparent gray bars indicate which values served as the CS+ and the CS− and the arrow indicates the direction of the gradient shift.
Stimulus presentation and button press recordings were managed with Presentation software (Neurobehavioral Systems). The aversive US consisted of a 6-ms electrical shock delivered to the right wrist that was calibrated for each subject prior to the start of the experiment using an ascending staircase procedure to reach a level deemed “highly annoying but not painful” by the participant [see Dunsmoor et al. (2009) for similar procedures].
Task and procedure
The experiment consisted of four phases in the following order: habituation, baseline, fear conditioning, and the generalization test. Each trial was 4 s and followed by a waiting period consisting of a fixation on a blank background. The habituation phase contained 1 trial of each stimulus (data not reported). The baseline phase included 6 trials of each stimulus (30 total) with a waiting period between trials of 4, 5, or 6 s (average = 5 s). This phase was identical for Groups 1 and 2 and allowed for baseline measures of arousal, reaction time (RT), and subjective color ratings prior to fear conditioning. A 5–6 m break followed the baseline phase, during which time participants passively viewed a silent movie of a train traveling through British Columbia (Highball Productions).
Fear conditioning included 10 trials of the CS+ and 10 trials of the CS−. Trials were separated by a waiting period of 8, 9, or 10 s (average = 9 s). The intermediate color stimulus (494 nm) served as the CS+ and was paired with the US on 6 out of 10 trials (pseudorandomly determined). The CS− served as an explicitly unpaired control and was never paired with the US. Critically, the CS− was different between groups: for Group 1, the CS− was a stimulus from the blue end of the dimension (489 nm), whereas Group 2 received a CS− that was from the green end of the dimension (500 nm). Fear conditioning was followed by a 5–6 m break that included the silent train video.
The generalization test contained a total of 9 trials of each stimulus (45 total) with a waiting period between trials of 7, 8, or 9 s (average = 8 s). The CS+ was intermittently reinforced by the US on 6 out of 9 trials (pseudorandomly determined) in order to reduce the effects of habituation and avoid extinction over the course of a lengthy generalization test (‘steady-state’ generalization test) (Honig & Urcuioli, 1981). This phase was identical across Groups 1 and 2.
At the conclusion of the study, participants identified which stimulus had been paired with the shock. Each stimulus was arranged in a random and counterbalanced (across subjects) order on the monitor (i.e. not in sequence from blue-to-green) to ensure that participants could not simply indicate the intermediate value as the CS+. One subject from Group 1 could not decide on a stimulus and is excluded from the retrospective CS+ identification analysis.
On each trial, participants rated whether the circle was blue or green as quickly and accurately as possible upon CS presentation by pressing one of two buttons. The scale was presented below the circle, and the button press corresponding to blue or green was counterbalanced between subjects. The order of stimulus presentation was counterbalanced and pseudorandomized such that no more than two presentations of the same stimulus occurred in a row. Participants were not informed of the CS-US contingencies.
Skin conductance responses (SCRs)
Psychophysiological recordings and shock administration were controlled with the MP-150 BIOPAC system (BIOPAC systems, Goleta, CA). SCRs were collected from the middle phalanx of the second and third digits of the left hand and scored using our previous criteria (Dunsmoor et al., 2009). In short, an SCR was considered related to stimulus presentation if the trough-to-peak response began 1–4 seconds after stimulus onset, lasted between 0.5 and 5.0 s, and was greater than 0.02 microsiemens (μS). A response that did not meet these criteria was scored as a zero. Raw SCR scores were normalized by range correction using each participant’s maximum SCR (Lykken & Venables, 1971), which was elicited by the shock US. Range-corrected values were square-root transformed to normalize the distribution.
Statistical analysis
SCR and RT data from fear conditioning were analyzed by two-sample t tests. SCR and RT data from the baseline and generalization test phases were analyzed by repeated measures ANOVA (using the 5 stimuli as factors) with polynomial trend analyses, considered significant at p < .05. Additional analyses on the generalization data were conducted to examine gradient shifts. For this analysis, paired sample t tests were used to compare responses to the stimulus with the greatest mean response against each other color stimulus, using a Bonferroni-correction for multiple comparisons of p = .0125.
Results
SCRs
As expected, baseline SCRs were low, with no main effect of stimulus in Group 1 [F (4, 96) = .67, p > .6] or Group 2 [F (4, 96) = 2.12, p = .08]. During fear conditioning, both groups demonstrated successful acquisition of conditioned fear to the CS+: Group 1 exhibited greater SCRs to the intermediate 494 nm CS+ (mean ± standard error: .38 ± .04) than to the 489 nm (blue) CS− (.26 ± .03), t (24) = 4.00, p = .001, d = .65. Group 2 exhibited greater SCRs to the 494 nm CS+ (.39 ± .04) than to the 500 nm (green) CS− (.29 ± .04), t (24) = 3.29, p = .003, d = .51.
Post-discrimination SCR gradients emerged in both groups that were asymmetric and skewed away from the CS−. In Group 1 (Fig 1b), SCRs exhibited a main effect of stimulus (F (4, 96) = 4.05, p = .005, η2 = .14), with significant linear (p = .01, η2 = .24) and quadratic (p = .01, η2 = .18) trends. Paired t tests revealed enhanced SCRs to the stimulus with the greatest mean response (496 nm) versus the 489 nm CS− (p = .002, d = .67). SCRs between the 496 nm stimulus were not enhanced relative to the 493 nm (p > .1) or CS+ (p > .5), and comparison to the 500 nm stimulus at the extreme opposite end of the continuum from the CS− did not survive correction for multiple comparisons (p = .032).
Group 2 (Fig 1c) also exhibited a main effect of SCRs during the generalization test (F (4, 96) = 4.45, p = .002, η2 = .16), with significant quadratic (p = .02, η2 = .20) and cubic (p = .03, η2 = .17) trends. Paired t tests revealed enhanced SCRs to the stimulus with the greatest mean response (in this case, 493 nm) versus the 500 nm CS− (p = .007, d = .47) and the 489 nm stimulus at the extreme opposite end of the continuum from the CS−, p = .008, d = .57. Comparison between the 493 nm stimulus and the 494 nm CS+ (p = .34), and between the 493 nm and the 496 nm (p = .05) stimulus, were not significant.
A planned comparison, 2-way mixed ANOVA [Group (1, 2) X color value (496 nm, 493 nm)] was conducted to examine the interaction between training group and responses to the unreinforced values adjacent to the CS+ along the color dimension. This analysis showed a significant group X color value interaction, F (1, 48) = 6.58, p = .013, η2 = .12. This interaction provides evidence for opposing patterns of SCRs as a function of discrimination training group, with enhanced SCRs to the stimulus opposite the CS− relative to the stimulus nearer the CS−.
Perceptual classification
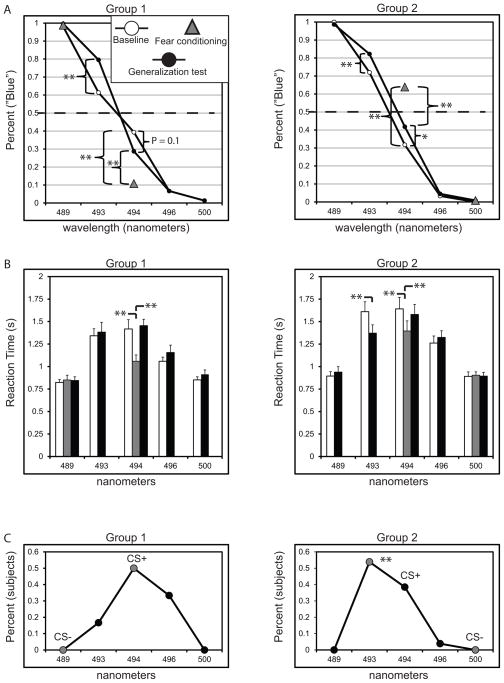
For clarity, the blue end of the continuum is used as the point of reference. Participants in Groups 1 and 2 did not alter their ratings for the CS− from baseline to fear conditioning as “blue” or “green,” respectively. However, in Group 1 ratings for the 494 nm CS+ as “blue” fell by 28.53 ± 6.46% from baseline, a significant change, p <.001, d = 1.04. In Group 2, ratings for the 494 nm CS+ “blue” increased by 32.16 ± 5.45% from baseline, p <.001, d = .89. A between-groups comparison of ratings for the CS+ was also significant, t (24) = 5.96, p < .001, d = 1.79. Thus, ratings for the 494 nm CS+ changed from baseline to fear conditioning but in opposite directions across groups.
In Group 1, ratings for the 494 nm CS+ as “blue” decreased from baseline to the generalization test by 10.61 ± 7.63%, but this change was not significant (p = .18). In contrast, ratings for the CS+ as “blue” increased by 10.04 ± 4.59% in Group 2 from pre-to-post fear conditioning, and this change was significant (p = .038, d = .29). Pre-to-post fear conditioning ratings for the 493 nm stimulus as “blue” increased significantly in both Group 1 and 2 (ps <.01).
RT
In Group 1, baseline RTs showed a main effect of stimulus, F (4, 96) = 38.02, p < .001, η2 = .61, with linear (p = .004, η2 = .29), quadratic (p < .001, η2 = .73), and cubic (p < .001, η2 = .48) trends. Baseline RTs in Group 2 also exhibited a main effect of stimulus F (4, 96) = 34.06, p < .001, η2 = .58, with linear (p = .005, η2 = .28), quadratic (p < .001, η2 = .72), and cubic (p = .001, η2 = .36) trends. Response latency peaked at the intermediate 494 nm stimulus in both groups, suggesting that this stimulus was the most difficult to classify.
During fear conditioning, RTs to identify the 494 nm CS+ were slower relative to the 489 nm CS− in Group 1 (p < .001, d = .67) and the 500 nm CS− in Group 2 (p < .001, d =1.02).
The generalization test was similarly marked by a main effect of stimulus in both groups (ps < .001), with the peak RT for the intermediate 494 nm CS+. For Group 1, ANOVA using stimulus and phase (baseline, generalization test) as factors did not show an effect of phase (p = .27) nor a stimulus X phase interaction (p = .9), demonstrating that the pattern of RTs was largely unchanged from pre- to post-conditioning. Similarly, Group 2 did not exhibit an effect of phase (p = .28); however, a stimulus X phase interaction was significant (p = .003, η2 = .15). Post-hoc analysis revealed that pre- to post-conditioning changes in RT were driven by faster RTs to classify the 493 nm stimulus adjacent to the CS+ but opposite the 500 nm CS−, p = .002, d = .47.
Retrospective CS+ identification
After the conclusion of the generalization test, 12 out of 24 participants in Group 1were able to correctly identify the 494 nm CS+, while 8 participants (33%) mistakenly identified the greener 496 nm value as the CS+. In contrast, the CS+ was mistakenly identified as the bluer 493 nm stimulus by 14 out 25 of participants in Group 2, and a chi-square test revealed that stimulus color significantly affected CS+ identification ratings in this group, χ2 (2) = 10.64, p = .005.
Discussion
Opposing fear generalization gradients were observed across two training groups as a function of intradimensional discrimination learning. Physiological data were accompanied by a shift in perceptual classification for the color of the CS+. These results provide new evidence that discriminative fear conditioning affects autonomic indices of fear generalization as well as perceptual classification of color categories.
The present study used procedures previously shown to induce peak-shift effects of instrumental responding in pigeons (Hanson, 1959) and stimulus generalization of voluntary behaviors in humans along color dimensions (Doll & Thomas, 1967) to examine patterns of fear generalization in humans. Holding the color of the CS+ constant across training groups, we found that autonomic responding was asymmetric and skewed in a direction away from the CS−. SCR results provide clear evidence that discrimination learning induced a post-discrimination shift in autonomic responses towards an unreinforced value that is more unlike the CS−. Although a statistically significant increase from the CS+ value to the adjacent value was not observed, there was a numeric increase in SCR responding away from the CS+ value, consistent with the original behavioral descriptions of the peak-shift effect (Hanson, 1959) and more recent studies in humans (Kahnt, Park, Burke, & Tobler, 2012). Stronger statistical evidence for a peak-shift effect would likely be obtained if a steady-state generalization procedure were not employed so that extinction of CS+ responses would occur, leading to a more pronounced peak shift away from the CS+ value during the generalization test.
We also observed a change in perceptual classification for the CS+ seemingly affected by the color of the CS−. That is, a blue CS− led to increased ratings for the ambiguous CS+ as green, whereas a green CS− led to increased ratings for the CS+ as blue. This finding is in line with recent research showing that fear conditioning alters the ability to discriminate low-level sensory stimuli like auditory tones (Resnik et al., 2011) and odors (Li et al., 2008), and can change perceptual thresholds for classifying emotional faces as “fearful” (Lim & Pessoa, 2008). In the present study, changes in perceptual ratings for the CS+, which was near the point of subjective equality, appeared to be driven by comparison to the CS−, which was clearly blue or clearly green. In Group 1, the blue CS− increased the endorsement for the CS+ as “green” from baseline to conditioning, but this change did not extend to the generalization test in which subjects once again saw several different spectral values in addition to the CS+ and CS−. In contrast, Group 2, for whom the CS− was green, increased the endorsement for the CS+ as “blue” from baseline to conditioning, and this change extended to the generalization test. This group difference may be owed to the fact that the intermediate value was classified as “green” with slightly more frequency than “blue” at baseline, and thus the CS+ and CS− may have been perceptually more similar in Group 2 than Group 1. Hanson (1959) showed that the difference between the CS+ and CS− is inversely related to the magnitude of the peak shift. In this regard, discrimination learning between a somewhat green (494 nm) CS+ and clearly green (500 nm) CS− may also explain why Group 2 mistakenly identified the bluer 493 nm stimulus as the CS+, and pre-to-post conditioning RTs for the 493 nm significantly changed in Group 2 but not Group 1.
Gradient shifts have been described as an emergent property of discrimination training that leads to biases for extreme stimulus properties (ten Cate & Rowe, 2007). The preponderance of research on this phenomenon has occurred in the non-human animal literature and has incorporated instrumental conditioning techniques (Purtle, 1973). Most human fear conditioning experiments utilize discriminative techniques as a control for non-associative effects (e.g. sensitization), but rarely consider the effects of discrimination learning on post-discrimination fear responses.
Our findings suggest that post-discrimination testing with values that approximate the CS+ and CS− may provide a more comprehensive estimate of fear conditioning. In particular, testing for peak shift effects may be worthwhile in patients with clinical anxiety disorder, including posttraumatic stress disorder, obsessive compulsive disorder, and specific phobias. Prior research has shown overgeneralization of learned fear to be a marker of clinical anxiety (Lissek, 2012), but selective generalization to cues that are more salient or extreme than the CS+ has yet to be tested across anxiety disorders.
Figure 2.
Subjective ratings and reaction times. (A) Endorsements for the CS+ (494 nm) as “blue” decreased in Group 1 from baseline (white circles) to fear conditioning (gray triangle). Endorsements for the CS+ (494 nm) as “blue” increased in Group 2 from baseline (white circles) to fear conditioning (gray triangle) and from baseline to the generalization test (black circle). (B) Reaction times peaked at the CS+ in both groups. Gray bars reflect RTs to the CS− and CS+ during fear conditioning. (C) When asked to identify which stimulus was the CS+ at the end of the experiment, 56% of participants in Group 2 mistakenly identified a stimulus that was more blue than the actual CS+. Error bars represent standard error of the mean. * p < .05; ** p < .015 (Bonferroni-corrected for multiple comparisons).
Acknowledgments
This work was supported by NSF grant 0745919 and NIH grants R01 DA027802 and F31 MH090682.
References
- Doll TJ, Thomas DR. Effects of discrimination training on stimulus generalization for human subjects. Journal of Experimental Psychology. 1967;75(4):508. doi: 10.1037/h0025116. [DOI] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory. 2009;16(7):460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, Baron A. Human postdiscrimination gradients: The effects of three-stimulus discrimination training. Animal Learning & Behavior. 1979;7(1):53–56. doi: 10.3758/bf03209657. [DOI] [Google Scholar]
- Gamberale-Stille G, Tullberg BS. Experienced chicks show biased avoidance of stronger signals: an experiment with natural colour variation in live aposematic prey. Evolutionary Ecology. 1999;13(6):579–589. doi: 10.1023/a:1006741626575. [DOI] [Google Scholar]
- Guttman N, Kalish HI. Discriminability and stimulus-generalization. Journal of Experimental Psychology. 1956;51(1):79–88. doi: 10.1037/h0046219. [DOI] [PubMed] [Google Scholar]
- Hanson HM. Effects of discrimination-training on stimulus-generalization. Journal of Experimental Psychology. 1959;58(5):321–334. doi: 10.1037/h0042606. [DOI] [PubMed] [Google Scholar]
- Honig WK, Urcuioli PJ. The legacy of Guttman and Kalish (1956) - 25 years of research on stimulus-generalization. Journal of the Experimental Analysis of Behavior. 1981;36(3):405–445. doi: 10.1901/jeab.1981.36-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CL. Principles of Behavior. New York: Appleton-Century-Crofts; 1943. [Google Scholar]
- Hull CL. The problem of primary stimulus generalization. Psychological Review. 1947;54(3):120–134. doi: 10.1037/h0061159. [DOI] [PubMed] [Google Scholar]
- Jansson L, Enquist M. Receiver bias for colourful signals. Animal Behaviour. 2003;66:965–971. doi: 10.1006/anbe.2003.2249. [DOI] [Google Scholar]
- Kahnt, Thorsten, Park, Soyoung Q, Burke Christopher J, Tobler Philippe N. How glitter relates to gold: similarity-dependent reward prediction errors in the human striatum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(46):16521–16529. doi: 10.1523/jneurosci.2383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley KS, Wade M. The Pavlovian theory of generalization. Psychological Review. 1946;53(2):72–87. doi: 10.1037/h0059999. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319(5871):1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SL, Pessoa L. Affective learning increases sensitivity to graded emotional faces. Emotion. 2008;8(1):96–103. doi: 10.1037/1528-3542.8.1.96. [DOI] [PubMed] [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depress Anxiety. 2012;29(4):257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: a proposal for standardization. Psychophysiology. 1971;8(5):656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Lynn SK, Cnaani J, Papaj DR. Peak shift discrimination learning as a mechanism of signal evolution. Evolution. 2005;59(6):1300–1305. [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Purtle RB. Peak shift: a review. Psychological Bulletin. 1973;80(5):408–421. doi: 10.1037/h0035233. [DOI] [Google Scholar]
- Resnik J, Sobel N, Paz R. Auditory aversive learning increases discrimination thresholds. Nat Neurosci. 2011;14(6):791–796. doi: 10.1038/nn.2802. [DOI] [PubMed] [Google Scholar]
- Spence KW. The differential response in animals to stimuli varying within a single dimension. Psychological Review. 1937;44:430–444. [Google Scholar]
- ten Cate C, Rowe C. Biases in signal evolution: learning makes a difference. Trends in Ecology & Evolution. 2007;22(7):380–387. doi: 10.1016/j.tree.2007.03.006. [DOI] [PubMed] [Google Scholar]
- ten Cate C, Verzijden MN, Etman E. Sexual imprinting can induce sexual preferences for exaggerated parental traits. Current Biology. 2006;16(11):1128–1132. doi: 10.1016/j.cub.2006.03.068. [DOI] [PubMed] [Google Scholar]
- Watson JB, Rayner R. Conditioned emotional reactions. Journal of Experimental Psychology. 1920;3:1–14. [Google Scholar]