Abstract
α-Synuclein (α-Syn) is a presynaptic protein implicated in Parkinson’s disease (PD). Mice overexpressing human wildtype (WT) α-Syn under the Thy1 promoter show high levels of α-Syn in cortical and subcortical regions, exhibit progressive sensorimotor anomalies, as well as non-motor abnormalities and are considered models of pre-manifest PD as there is little evidence of early loss of dopaminergic (DA) neurons. We used whole-cell patch clamp recordings from visually identified striatal medium-sized spiny neurons (MSSNs) in slices from α-Syn and WT littermate control mice at 35, 90 and 300 days of age to examine corticostriatal synaptic function. MSSNs displayed significant decreases in the frequency of spontaneous excitatory postsynaptic currents (EPSCs) in α-Syn mice at all ages. This difference persisted in the presence of tetrodotoxin indicating it was independent of action potentials. Stimulation thresholds for evoking EPSCs were significantly higher and responses were smaller in α-Syn mice. These data suggest a decrease in neurotransmitter release at the corticostriatal synapse. At 90 days the frequency of spontaneous GABAA receptor-mediated synaptic currents was decreased in MSSNs but increased in cortical pyramidal neurons. These observations indicate that high levels of expression of α-Syn alter corticostriatal synaptic function early and they provide evidence for early synaptic dysfunction in a pre-manifest model of PD. Of importance, these changes are opposite to those found in DA-depletion models, suggesting that before degeneration of DA neurons in the substantia nigra synaptic adaptations occur at the corticostriatal synapse that may initiate subtle preclinical manifestations.
Keywords: Glutamate receptors, Postsynaptic currents, Parkinson’s disease
Introduction
Human α-Synuclein (α-Syn) is highly expressed in presynaptic terminals (Iwai et al. 1995) and also is present in Lewy bodies, a hallmark of idiopathic and familial Parkinson’s disease (PD) (Sidhu et al. 2004). Accumulating evidence indicates that α-Syn plays a role in synaptic transmission (Sidhu et al. 2004), synaptic plasticity (George et al. 1995; Liu et al. 2004) and neuroprotection (Wersinger et al. 2003). α-Syn is distributed throughout the brain including the corticostriatal, nigrostriatal and striatonigral pathways (Totterdell and Meredith 2005). Findings indicate that presynaptic mobilization of reserve pool neurotransmitter vesicles, including dopamine (DA) and glutamate, is impaired in α-Syn knock-out (KO) mice and mice expressing α-Syn with A30P mutations (Gureviciene et al. 2007; Yavich et al. 2004), which would alter neurotransmitter release.
Although rare cases of familial PD have been linked to point mutations in α-Syn (Polymeropoulos et al. 1997), most neurodegenerative disorders exhibiting Lewy bodies are associated with abnormal accumulation of wild type (WT), not mutant, α-Syn (Spillantini et al. 1997; Trojanowski and Lee 1998). Recently, a number of different lines of mice overexpressing WT α-Syn have been generated (Fernagut et al. 2007; Fleming et al. 2005; Springer and Kahle 2006). Among them, mice overexpressing WT α-Syn under the Thy1 promoter have been extensively characterized. The Thy1 promoter confers widespread, high levels of WT human α-Syn overexpression in cortical and subcortical neurons, including the substantia nigra pars compacta (Rockenstein et al. 2002). These mice show progressive sensorimotor alterations that are similar to deficits observed in mouse models displaying nigrostriatal degeneration (Fleming et al. 2004) as well as early non-motor olfactory deficits and autonomic changes characteristic of pre-manifest PD (Fleming et al. 2008; Wang et al. 2008). α-Syn mice have a full complement of striatal DA terminals at the times when they show deficits in these tests (Fernagut et al. 2007), raising the question of which mechanisms lead to behavioral abnormalities and indicating that these mice are a model of pre-manifest PD (Chesselet 2008).
Most electrophysiological research on PD concentrated on using toxin-based models to destroy nigrostriatal DA neurons and then analyzed changes in striatal neural activity at selected postlesion time points (Dauer and Przedborski 2003). One general result of these studies has been to demonstrate a postlesion increase in excitatory corticostriatal synaptic inputs of striatal medium-sized spiny neurons (MSSNs) (Calabresi et al. 1993; Cepeda et al. 1989; Galarraga et al. 1987). Mouse models of basal ganglia disorders, including PD and Huntington’s disease, show alterations in synaptic transmission along the corticostriatal pathway with development of the phenotype (Cepeda et al. 2007; Goldberg et al. 2003; Klapstein et al. 2001). In contrast to effects from toxin-based lesions, in the parkin KO mouse model of PD, we showed decreases in MSSN excitability to corticostriatal synaptic activation (Goldberg et al. 2003). Since toxin-based studies cannot easily evaluate changes in putative pre-manifest phases, there may be unique physiological alterations in synaptic communication in MSSNs that precede the occurrence of marked DA cell degeneration. These early phases are characterized by progressive α-Syn pathology including abnormal accumulation in many brain regions (Halliday et al. 2006). Although overt neurological effects are not evident, this phase also is characterized by significant non-motor impairments similar to those observed in α-Syn mice (Fleming et al. 2008; Wang et al. 2008). The goal of the present study was to examine neuronal dysfunction in MSSNs at different time points in the progression of the phenotype in α-Syn mice. We assessed spontaneous and evoked excitatory postsynaptic currents (EPSCs) and spontaneous inhibitory currents (IPSCs) in MSSNs in α-Syn mice at three ages 35, 90 and 300 days. At 90 days and older, transgenic mice display sensorimotor and a wide range of non-motor deficits (Fleming et al. 2004; Fleming et al. 2008).
Methods
All experimental procedures were carried out in accordance with the USPHS Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at UCLA. Transgenic α-Syn mice were obtained from Eliezer Masliah at the University of California San Diego (Rockenstein et al. 2002) and a colony was maintained on the mixed C57BL/6-DBA/2 background in our mouse breeding facilities at UCLA. Genotypes were verified with polymerase chain reaction amplification of tail DNA.
Experiments were conducted on α-Syn and age-matched littermate controls of both genders. A total of 45 α-Syn mice with average ages of 37±0.6 (n=12), 94±1.6 (n=26) and 306±4 (n=7) days and 49 WT mice with average ages of 36±0.6 (n=14), 93±1.4 (n=27) and 306±4 (n=8) days were used. An additional group of animals at 90 days (n=8, 4 WT and 4 α-Syn) was used for experiments in isolated cells. There were no differences in electrophysiological results between genders in each age group and data were subsequently pooled.
Brain slice preparation
Mice were deeply anesthetized with halothane and after sacrifice the brains were dissected and immediately placed in oxygenated ice-cold, low-Ca2+ artificial cerebrospinal fluid (ACSF) containing (in mM) NaCl, 130; NaH2PO4, 1.25; NaHCO3, 26; MgCl2, 5; CaCl2, 1 and glucose, 10. The hemispheres were separated and 350 µm coronal slices were cut and transferred to an incubating chamber containing ACSF (with 2 mM CaCl2 and 2 mM MgCl2) oxygenated with 95% O2-5% CO2 (pH 7.2–7.4, 290–310 mOsm, 25±2°C). After 1 h slices were placed on the stage of an upright fixed-stage microscope (Olympus BX51; Center Valley, PA), submerged in continuously flowing ACSF (4 ml/min).
Electrophysiological recordings
Whole-cell patch clamp recordings in voltage clamp mode were obtained using a MultiClamp 700A amplifier (Axon Instruments, Inc.; Foster City, CA). MSSNs in the dorsolateral striatum were visualized with the aid of infrared videomicroscopy (Cepeda et al. 1998) and identified by somatic size and basic membrane properties (input resistance, membrane capacitance and time constant). Series resistance was <25 MΩ, compensated 70–80% and checked periodically. If the series resistance changed >10% at the end of the experiment data from the cell were discarded. In most experiments the patch pipette (3–5 MΩ) contained the following solution (in mM): Cs-methanesulfonate 130, CsCl 10, NaCl 4, MgCl2 1, MgATP 5, EGTA 5, HEPES 10, GTP 0.5, phosphocreatine 10, leupeptin 0.1 (pH 7.25–7.3, osmolarity 280–290 mOsm). The use of Cs+ as the main charge carrier also blocked several K+ conductances and facilitated holding the membrane at depolarized potentials when necessary. Lidocaine N-ethyl bromide (QX-314, 4 mM) was added to the internal solution to block Na+ channels.
Passive membrane properties of MSSNs were determined by applying a depolarizing step voltage command (10 mV) and using the membrane test function integrated in the pClamp8 software (Axon Instruments, Foster City, CA). This function reports membrane capacitance (in pF), input resistance (in MΩ) and time constant (in msec).
After characterizing the basic membrane properties of the neuron, spontaneous EPSCs or IPSCs were recorded for 3–6 min in ACSF. For EPSCs cells were held at −70 mV to minimize the contribution of GABAA receptors and that of voltage-gated conductances. In addition, in most experiments bicuculline (BIC, 20 µM) also was added to block the contribution of spontaneous currents mediated by activation of GABAA receptors. To record spontaneous IPSCs cells were held at +20 mV to increase the driving force of GABAA receptor-mediated currents. At this holding potential glutamate currents did not contribute significantly as application of BIC (20 µM) completely abolished all synaptic activity. The membrane current was filtered at 1 kHz and digitized at 100 µs using Clampex (gap free mode) (Axon Instruments, Inc.; Foster City, CA). For some cells tetrodotoxin (TTX, 1 µM) was added to isolate miniature (m)EPSCs, events that were not dependent on presynaptic action potentials.
EPSCs were evoked by stimulating the corpus callosum and deep cortical layers with a bipolar electrode and isolating responses mediated by specific glutamate receptor subtypes pharmacologically. The distance between the stimulating and recording electrodes was 150–250 µm. Responses mediated by activation of N-methyl-D-aspartate (NMDA) receptors were isolated by bathing the slice in ACSF containing picrotoxin (PIC, 100 µM), to block activation of GABAA receptors and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX 10 µM, a non-NMDA receptor antagonist) and changing the holding potential to +40 mV. Responses mediated by activation of non-NMDA receptors were isolated by bathing the slice in PIC while holding the membrane at −70 mV. Drugs were purchased from Sigma-Aldrich, Inc. (St. Louis, MO) except for BIC, AP5 (Tocris; Ellisville, MO) and TTX (Calbiochem; San Diego, CA).
Isolated cell preparation
To more selectively evaluate postsynaptic receptor function, acutely isolated MSSNs were obtained using previously described procedures (Bargas et al. 1994; Starling et al. 2005). Briefly, mice were anesthetized with halothane, transcardially perfused with 10 ml cold sucrose solution and decapitated. Brains were dissected and sliced in oxygenated cold sucrose solution. Coronal slices (350 µm thick) were incubated in NaHCO3-buffered Earl's balanced salt solution (EBSS, pH 7.4, 300–310 mOsm) bubbled with 95% O2-5% CO2 and supplemented with (in mM) 1 pyruvic acid, 0.005 glutathione, 0.1 NG-nitro-L-arginine, and 1 kynurenic acid until isolation. After 1 h incubation, dorsal striata were dissected, placed in an oxygenated cell-stir chamber (Wheaton, Millville, NJ), and enzymatically treated for 20 min with papain (0.5 mg/ml, Calbiochem, San Diego, CA) at 35°C in a HEPES-buffered Hank's balanced salt solution (HBSS, pH 7.4, 300–310 mOsm). After enzymatic digestion, the tissue was rinsed with a low Ca2+ HEPES-buffered Na-isethionate solution containing (in mM): 140 Na+ isethionate, 2 KCl, 2 MgCl2, 0.1 CaCl2, 23 Glucose and 15 HEPES (pH 7.4, 300–310 mOsm) and mechanically isolated with a graded series of fire-polished Pasteur pipettes. The cell suspension was plated into a Petri dish containing a HEPES-buffered salt solution consisting of (in mM): 140 NaCl, 23 glucose, 15 HEPES, 2 KCl, 2 MgCl2, and 1 CaCl2 (pH 7.4, 300–310 mOsm). For whole-cell voltage clamp recordings, the internal pipette solution contained (in mM): 175 N-methyl-D-glucamine (NMDG), 40 HEPES, 2 MgCl2, 10 ethylene glycol-bis (-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), 12 phosphocreatine, 2 Na2ATP, 0.2 Na2GTP, and 0.1 leupeptin (pH 7.2–7.3, 265–270 mOsm, Sigma Chemicals). The Mg2+-free external solution consisted of (in mM): 135 NaCl, 20 CsCl, 3 BaCl2, 2 CaCl2, 10 glucose, 10 HEPES, 0.02 glycine, and 0.0003 TTX (pH 7.4, 300–310 mOsm). The presence of Cs+ and TTX in the external solution blocked some voltage-gated K+ and all Na+ currents, respectively. Electrode resistance was typically 5–6 MΩ in the bath. After seal rupture, series resistance was compensated (70–90%) and periodically monitored. Only data from cells for which access resistance values were <20 MΩ were included. Signals were detected with an Axoclamp 1D amplifier (Axon Instruments Inc.; Foster City, CA). Membrane capacitances and input resistances were measured by applying a 10 mV depolarizing step voltage command and using the membrane test function integrated in the pClamp software.
Drugs were applied using a pressure-driven perfusion system with an array of capillaries positioned ∼500 µm from the cell. Solution changes were performed by changing the position of the array with a DC drive system controlled by a SF-77B perfusion system (Warner Instruments, Hamden, CT) synchronized by pClamp. Agonists were applied for 3 sec every 30 sec. Responsiveness of cells to α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) or NMDA was examined at a holding potential of −70 mV. Responsiveness of cells to AMPA was determined in the presence of 10 µM cyclothiazide (CTZ) to reduce AMPA receptor desensitization.
Data analysis and statistics
Spontaneous synaptic events were analyzed off-line using the Mini Analysis Program (Jaejin Software, Leonia, NJ). This software was used to calculate spontaneous and mEPSC frequency, amplitude of events, and to construct amplitude-frequency and cumulative amplitude and frequency histograms. The threshold amplitude for the detection of an event was set at 6 pA for spontaneous EPSCs and 10 pA for IPSCs [2–3 times above root mean square noise level (generally ∼2 pA at −70 mV, ∼4 pA at +20 mV)]. All events were confirmed visually by an experimenter blind to genotype. Frequencies were expressed as number of events per second (Hz). EPSC kinetic analyses were performed using the Mini Analysis Program. Events with peak amplitudes between 6–50 pA were grouped, aligned by half-rise time and averaged to obtain rise and decay times and durations at half-amplitude. Events with complex peaks were eliminated from this analysis. First and second order exponential curves were fit with a maximum of 5000 iterations. For amplitude and inter-event interval cumulative probability plots, all spontaneous EPSCs contained in a 3 min continuous recording period were included. Evoked synaptic currents were analyzed off-line using Clampfit (Axon Instruments, Foster City, CA). This software was used to measure evoked EPSC amplitudes, areas, rise and decay times and half-amplitude durations.
Unless otherwise noted, values in the figures, table and text are mean±standard error of the mean (SE). Differences among group means were assessed with appropriately designed analyses of variance followed by multiple comparisons using Bonferroni t-tests. Student t-tests alone were used when only two group means were compared. Differences between means were considered statistically significant if p<0.05.
Results
Based on somatic size and typical membrane properties (Cepeda et al. 1998) all recorded cells were MSSNs. The passive membrane properties of MSSNs were similar in α-Syn and WT mice at 35 days. At 90 days, while the membrane capacitance and time constant were similar, the input resistance was slightly, but significantly increased in cells from α-Syn compared to WT (Table 1). This increase was due to a decrease in input resistance occurring in WTs between 35 and 90 days which did not occur in the α-Syn mice. At 300 days, although there were no significant differences between genotypes, the input resistance of α-Syn MSSNs remained elevated compared to that of WTs.
Table 1.
Passive Membrane Properties of MSSNs in WT and α-Syn mice
| 35 day | 90 day | 300 day | ||||
|---|---|---|---|---|---|---|
| WT | α-Syn | WT | α-Syn | WT | α-Syn | |
| Cm (pF) | 95±3 | 92±4 | 95±3 | 90±3 | 96±4 | 102±3 |
| Rm (MΩ) | 137±11 | 137±14 | 105±8 | 129±12* | 105±8 | 117±9 |
| Tau (ms) | 1.6±0.1 | 1.6±0.1 | 1.6±0.1 | 1.7±0.1 | 1.6±0.1 | 1.9±0.1 |
p<0.05
α-SYN mice exhibit a lower frequency of spontaneous EPSCs
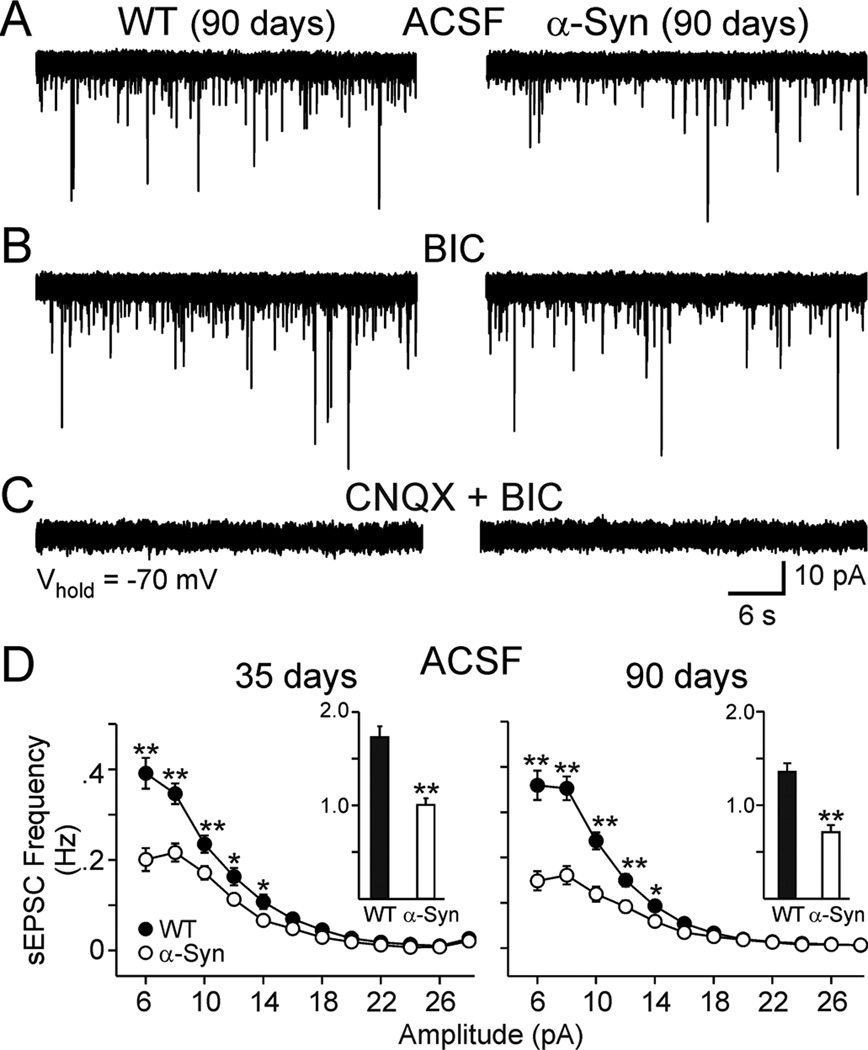
Spontaneous EPSCs were recorded in voltage clamp mode at a holding potential of −70 mV in standard ACSF (Fig. 1A). These EPSCs were blocked by CNQX (10 µM), a non-NMDA receptor antagonist, demonstrating they were predominantly mediated by AMPA-kainate receptors (Fig. 1C). The mean frequency of spontaneous EPSCs was significantly reduced in MSSNs from α-Syn [1.73±0.12 (n=26) versus 1.01±0.07 Hz (n=27) and 1.36±0.09 (n=52) versus 0.71±0.08 Hz (n=37) for WTs and α-Syn groups at 35 and 90 days respectively p<0.001] (Fig. 1D insets). There was no significant interaction between age and genotype. Amplitude-frequency distributions indicated that the primary decreases in frequency were due to a significant reduction in currents in the 6–16 pA range at both ages (p<0.05–0.001, Fig. 1D).
Figure 1.
A. Representative traces showing spontaneous synaptic currents from MSSNs from 90 day WT and α-Syn mice in ACSF (Vhold=−70 mV). B. Glutamate receptor-mediated spontaneous EPSCs were isolated by bath applying BIC (20 µM). C. Spontaneous EPSCs were blocked by CNQX (10 µM), a non-NMDA receptor antagonist, demonstrating that they were mediated by non-NMDA ionotropic glutamate receptors. D. Amplitude-frequency distributions of spontaneous EPSCs in ASCF at 35 and 90 days, respectively. Insets show the mean frequency of spontaneous EPSCs. Note the lower frequency of events 6–14 pA in the α-Syn mice compared to the WTs at both ages. Asterisks indicate the mean frequency for WT and α-Syn mice were significantly different. In this and other figures * indicates p<0.05 and ** indicates p<0.01.
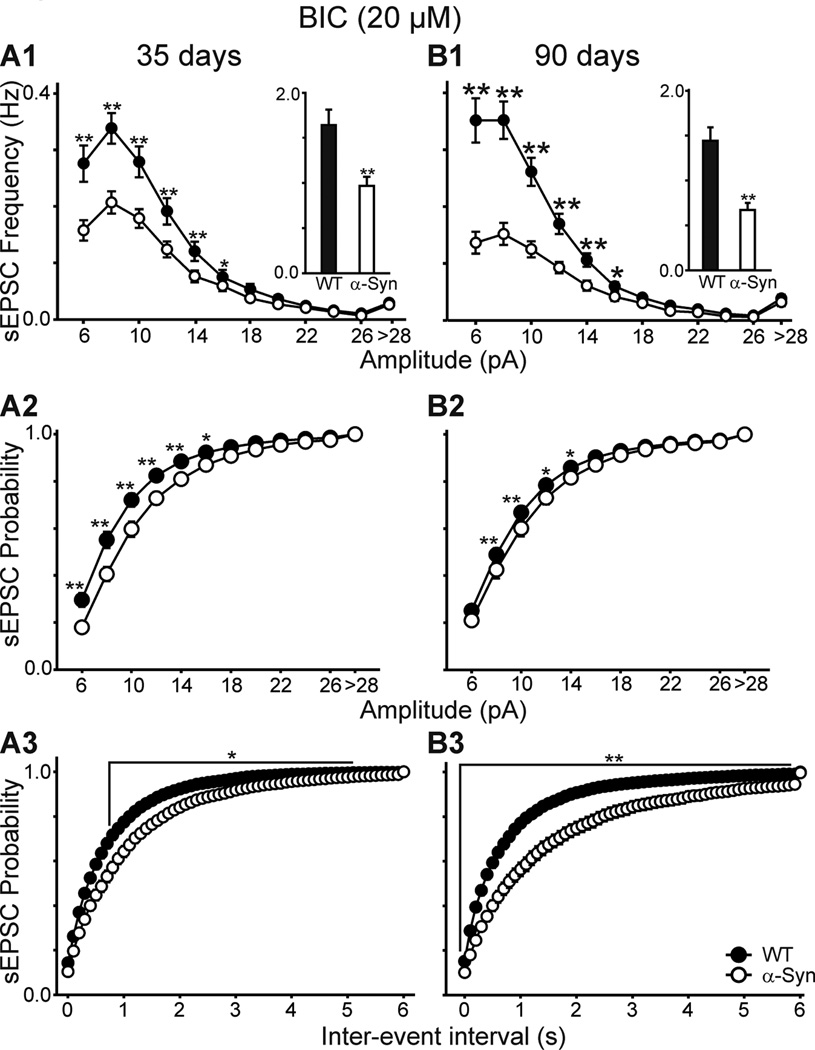
Because at a holding potential of −70 mV, GABAA receptor-mediated currents may also contribute to the total number of spontaneous synaptic currents (Cepeda et al. 2003), we examined the frequencies of spontaneous synaptic currents in the presence of BIC (20 µM) a GABAA receptor antagonist (Fig. 1B). BIC produced either no change or a small reduction (<10%) in the mean frequency of events when compared to standard ACSF in neurons from WT and α-Syn mice (Fig. 1D). In the presence of BIC, spontaneous EPSCs in MSSNs from α-Syn mice in both age groups remained significantly reduced compared to values obtained from WTs [1.58±0.11 (n=26) versus 0.99±0.04 Hz (n=27) at 35 days (p<0.001); 1.42±0.11 (n=52) versus 0.75±0.08 Hz (n=37) at 90 days (p<0.001)] (Fig. 2A1, B1 insets). Again, there was no significant interaction between age and genotype. Amplitude-frequency distributions indicated that the primary decreases in frequency were due to a significant reduction in currents in the 6–16 pA range in both age groups (p<0.05–0.001, Fig. 2A1, B1). Cumulative amplitude probability histograms also demonstrated a significant effect of genotype on currents in the 6–16 pA range (p<0.05–0.01, Fig. 2A2, B2). Cumulative inter-event interval probability histograms were constructed to more precisely analyze event frequency differences. Cells from α-Syn mice at both ages showed significantly longer inter-event intervals (p<0.05–0.001, Fig 2A3, B3). Together the findings demonstrate that overexpressing human α-Syn under the Thy1 promoter in mice results in a decrease in spontaneous EPSCs in MSSNs, and that this effect begins at approximately 1 month of age.
Figure 2.
A1, B1. Amplitude-frequency distributions of spontaneous EPSCs at 35 and 90 days, respectively. Insets are the mean frequencies of spontaneous EPSCs. A2, B2. Cumulative amplitude distributions of events at each age. A3, B3. Cumulative inter-event distributions at each age. All data obtained in the presence of BIC. In this (A3 and B3) and other figures lines over distributions indicate all differences between bins from α-Syn and WTs are statistically significantly.
Reduced frequency of spontaneous EPSCs in α-Syn mice is independent of action potentials
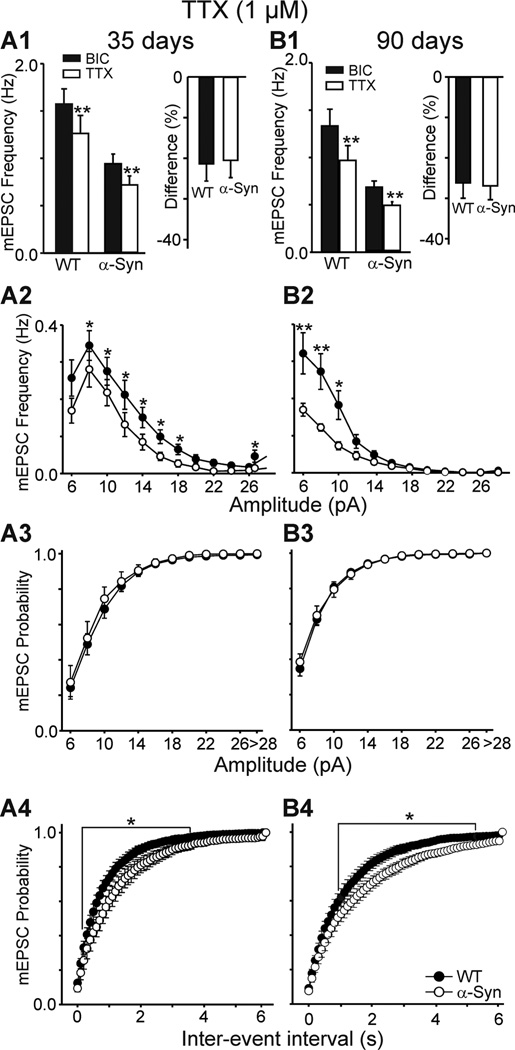
One possible explanation for reduced spontaneous EPSC frequency in α-Syn MSSNs is reduced glutamate release from corticostriatal terminals. To examine this possibility we applied TTX (1 µM) in the presence of BIC to assess EPSCs independent of action potential generation (mEPSCs). Although TTX significantly decreased the frequency of spontaneous EPSCs in all MSSNs from WT and α-Syn mice at both ages (Fig. 3A1, B1), percentage changes were similar at each age (Fig. 3A1, B1 insets). The difference in frequency of mEPSCs between cells from WT and α-Syn mice persisted in the presence of TTX (Fig. 3A1, B1) in both age groups [1.26±0.19 (n=11) versus 0.71±0.09 Hz (n=7) for WTs and α-Syn at 35 days, respectively; p<0.05 and 0.81±0.15 (n=13) versus 0.48±0.04 Hz (n=14) for WTs and α-Syn at 90 days, respectively; p<0.05]. Between 75–80% of spontaneous EPSCs in WT and α-Syn MSSNs were TTX-resistant. The amplitude-frequency distributions indicated that the reduction was mainly in the events of 8–16 pA range in cells from 35-day mice (p<0.05, Fig. 3A2), while greater decreases of small amplitude events (6–10 pA) were observed in cells from 90-day α-Syn mice (p<0.01, Fig. 3B2). This suggests progressive corticostriatal alterations with age. Cumulative amplitude probability distributions of mEPSCs were similar in MSSNs from WT and α-Syn mice at both ages (Fig. 3A3, B3). In contrast, cumulative inter-event interval probability distributions were significantly different in cells from α-Syn compared to WT mice at 35 days and 90 days (Fig. 3A4, B4).
Figure 3.
A1, B1. Bar graphs show the mean frequencies of mEPSCs and the mean percentage decreases in spontaneous EPSCs in cells at 35 and 90 days in the presence of TTX. A2, B2. Amplitude-frequency distributions of mEPSCs in TTX. Note the lower frequency of small amplitude events in α-Syn mice persisted in the presence of TTX. A3, B3. Cumulative amplitude probability distributions in TTX at each age. A4, B4. Cumulative inter-event interval probability distributions in TTX at each age.
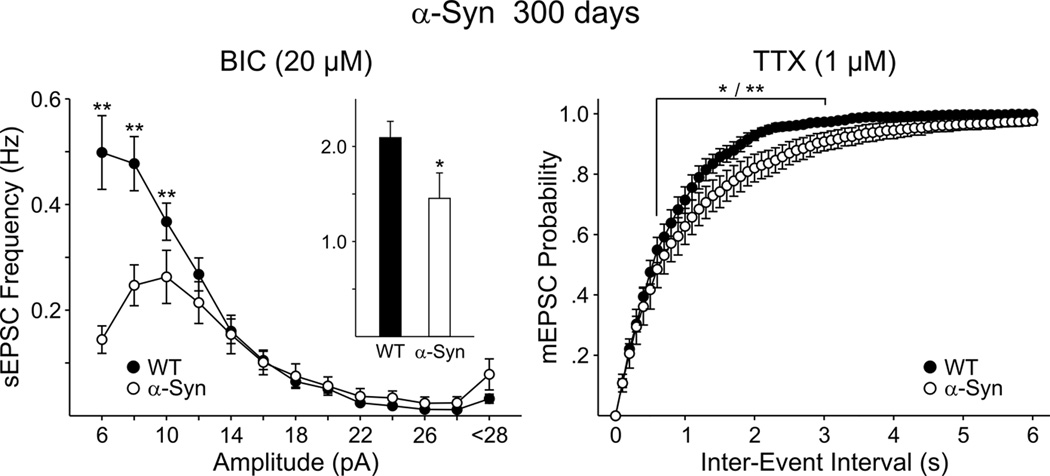
This decrease in spontaneous EPSCs was maintained as the α-Syn mice aged. At 300 days both in ACSF (data not shown) and in BIC, there was a significant decrease in mean frequency of spontaneous EPSCs which was due primarily to decreases of events in the 6–12 pA range (Fig. 4, left graph). Similar to effects at 35 and 90 days, at 300 days the difference in frequency and probability of release also was maintained in TTX (Fig. 4, right graph). Together, these observations suggest that the decreased frequency of EPSCs in MSSNs from α-Syn mice is mainly due to reduced neurotransmitter release, independent of action potential generation, and persists in older animals.
Figure 4.
Left graph shows amplitude-frequency distributions of spontaneous EPSCs at 300 days for MSSNs from α-Syn and WT mice. Insets are the mean frequencies of spontaneous EPSCs. Right graph shows cumulative inter-event interval probability distributions in TTX.
Average spontaneous EPSC amplitude and kinetic properties
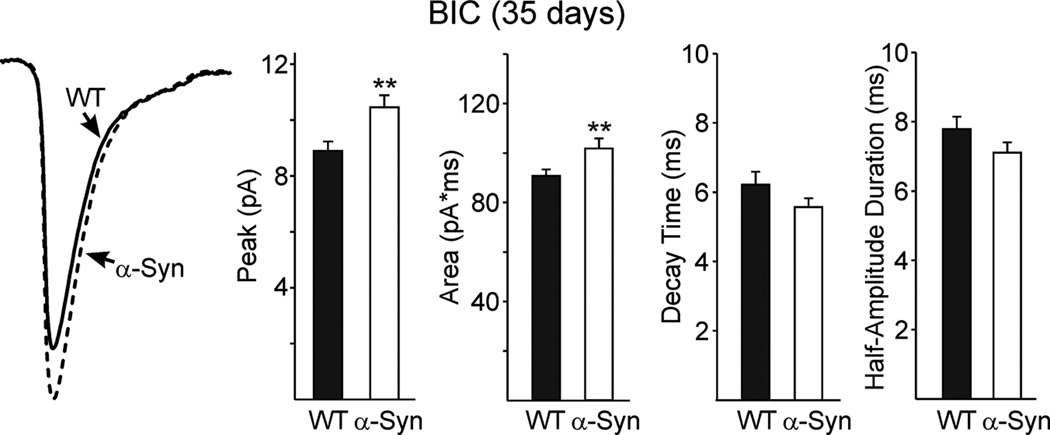
There were differences in mean event amplitudes and areas at 35 but not at 90 days (Fig. 5). In the presence of BIC, at 35 days the amplitude of EPSCs was significantly larger in cells from α-Syn mice than those from WTs [10.5±0.4 (n=26) versus 8.9±0.3pA (n=39), p<0.01]. Mean EPSC area was significantly increased in cells from α-Syn mice compared to those from WTs [101.9±4.0 (n=26) versus 90.7±2.7 pA*ms (n=39), p<0.01]. There were no significant differences in rise (data not shown) and decay times or half-amplitude durations between genotypes. The increases in amplitude and area of EPSCs in α-Syn mice at 35 days were dependent on action potential firing as TTX (1 µM) application abolished the differences (data not shown).
Figure 5.
Left traces are means of spontaneous EPSCs with amplitudes 6–50 pA from 35 day WT and α-Syn MSSNs in the presence of BIC. Bar graphs show the peak amplitude and area were significantly increased whereas the decay time and duration at half-amplitude were similar in cells from α-Syn mice compared to those of WTs.
Spontaneous EPSC frequency is unchanged in cortical pyramidal neurons
Since the primary sources of glutamatergic activity within the striatum are the cortex and thalamus (Fonnum et al. 1981a; Fonnum et al. 1981b; Smith and Bolam 1990), we next determined whether the reduction in frequency of events in MSSNs also occurred in cortical pyramidal neurons. Pyramidal neurons in layers II/III of the somatosensory cortex were patched and voltage clamp recordings made at a holding potential of −70 mV. The frequency of spontaneous EPSCs recorded in ACSF was similar in cells from 90 day α-Syn and WT mice [2.84±0.33 (n=15) versus 2.38±0.37 Hz (n=13), respectively]. Addition of BIC reduced the frequency similarly in both groups (2.10±0.47 versus 2.09±0.29 Hz in α-Syn and WT groups, respectively). The kinetic properties also were similar between the two groups of mice. The results suggest that there are no obvious changes in spontaneous excitatory inputs to cortical pyramidal neurons in α-Syn compared to WT mice at 90 days.
GABAergic activity progressively decreases in striatum but increases in cerebral cortex of α-Syn mice
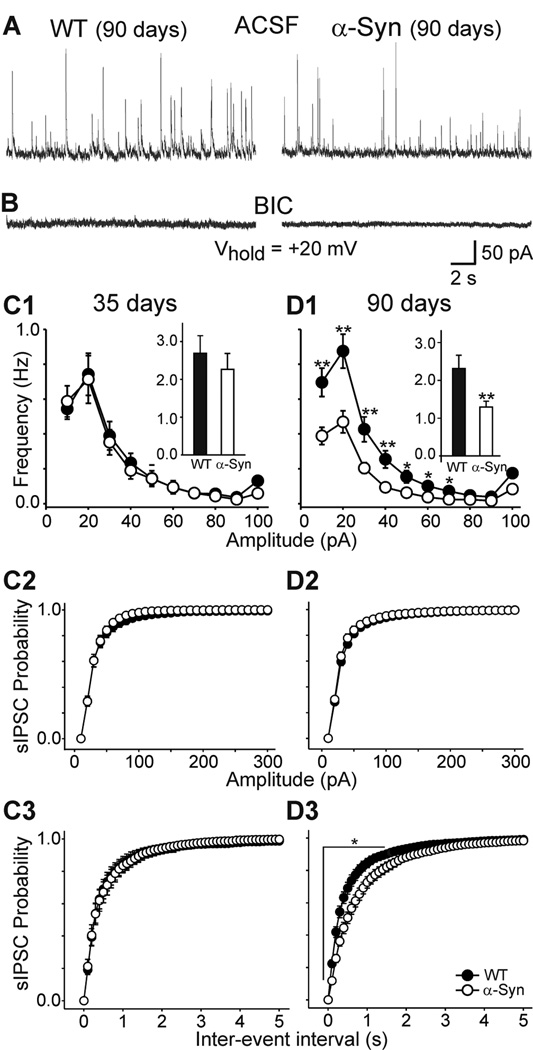
GABA is the principal inhibitory neurotransmitter in the striatum and the neurotransmitter of MSSNs mediating inhibition in their targets as well as collateral inhibition in the striatum. In addition, a variety of striatal inhibitory interneurons use GABA as a neurotransmitter (Czubayko and Plenz 2002; Koos and Tepper 1999; Oertel and Mugnaini 1984; Venance et al. 2004). Therefore, we examined spontaneous currents mediated by activation of GABAA receptors in neurons from α-Syn and WT mice. In ACSF cells were held at +20 mV to increase the driving force of GABAA synaptic currents (in our experimental conditions the equilibrium potential for GABAA currents is around −60 mV). At this holding potential, spontaneous IPSCs were outward (Fig. 6A) and glutamate receptor-mediated currents did not contribute significantly as application of BIC (20 µM) completely abolished events (Fig. 6B). The mean frequency of spontaneous IPSCs did not differ between cells from α-Syn and WT mice at 35 days [2.3±0.4 (n=11) versus 2.7± 0.5 Hz (n=16) for α-Syn and WT, respectively] (Fig. 6C1). However, at 90 days the mean frequency was significantly reduced in MSSNs from α-Syn mice compared to those from WTs [2.3±0.3 (n=38) versus 1.3± 0.2 Hz (n=24); p<0.01] (Fig. 6D1). Amplitude-frequency distributions showed that the significant frequency reduction occurred mainly in the 10–70 pA amplitude currents (p<0.05–0.01). Cumulative amplitude probability distributions of IPSCs were similar in MSSNs from WT and α-Syn mice at both ages (Fig. 6C2, D2). Cumulative inter-event interval probability distributions were not significantly different at 35 days but at 90 days inter-event distributions were significantly different (p<0.05–0.001, Fig. 6C3, D3), indicating proportionately more longer inter-event intervals.
Figure 6.
A. Spontaneous IPSCs mediated by activation of MSSN GABAA receptors. Vhold=+20 mV in ACSF. B. BIC application eliminated the events, demonstrating they were mediated by GABAA receptors. C1. Amplitude-frequency distributions of spontaneous IPSCs were similar in the cells from 35 day WT and α-Syn mice. Insets are the mean frequencies. D1. Amplitude-frequency distributions of spontaneous EPSCs in ASCF at 90 days. Insets show the mean frequencies of spontaneous EPSCs. Note the lower frequencies of events 10–70 pA in the α-Syn mice compared to the WTs. C2, D2. Cumulative amplitude probability distributions were similar between the two genotypes at both ages. C3, D3. Cumulative inter-event interval probability distributions were similar at 35 days but were significantly different at 90 days.
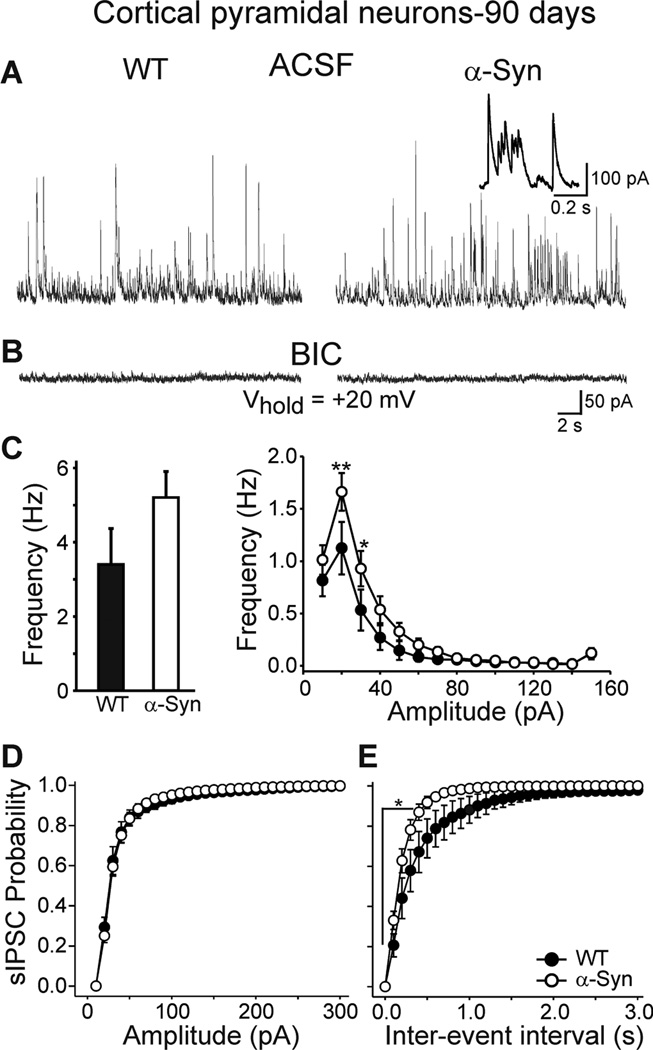
In contrast to MSSNs, the mean frequency of spontaneous IPSCs in cortical pyramidal neurons increased in α-Syn mice at 90 days (Fig. 7A). Although the overall increase was not statistically significant [5.2±0.7 (n=8) versus 3.4±0.9 Hz (n=7), p=0.122], cumulative amplitude and inter-event interval probability distributions displayed significant differences between α-Syn and WT mice in several amplitude and inter-event interval bins [20–30 pA (p<0.05) for amplitude and 0.1–1.0 sec (p<0.05) for inter-event intervals, Fig. 7C right, 7E]. In addition there were complex bursts that only occurred in cortical cells from α-Syn mice (Fig. 7A right inset). The observation that GABAergic synaptic activity is increased in cortical pyramidal neurons indicates that the output of cortical pyramidal neurons could be reduced.
Figure 7.
A. Spontaneous IPSCs mediated by activation of GABAA receptors in cortical pyramidal neurons were recorded by holding the membrane at +20 mV in standard ACSF. Inset shows bursting from the same cell at a faster time scale. B. BIC application eliminated the events, demonstrating they were mediated by GABAA receptors. C. Left bar graph shows that mean frequency of spontaneous IPSCs was higher in cells from α-Syn mice compared to WTs, but the difference was not statistically significant. Right graph shows amplitude-frequency distributions of spontaneous IPSCs. Significant differences between α-Syn and WT mice occurred in events of 20–30 pA. D. Cumulative amplitudes probability distributions were similar. E. Cumulative inter-event interval probability distributions were significantly different for short duration events.
Evoked EPSCs exhibit smaller amplitudes in α-Syn mice
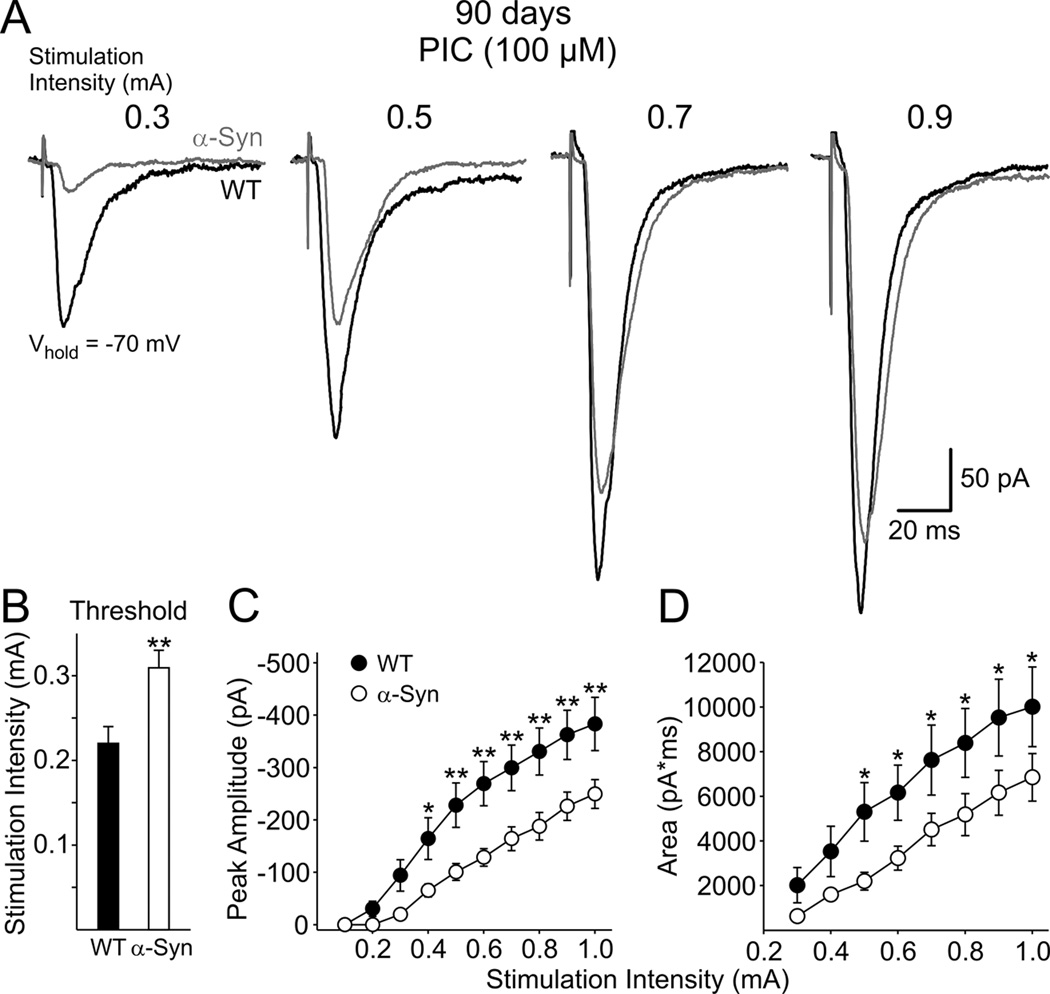
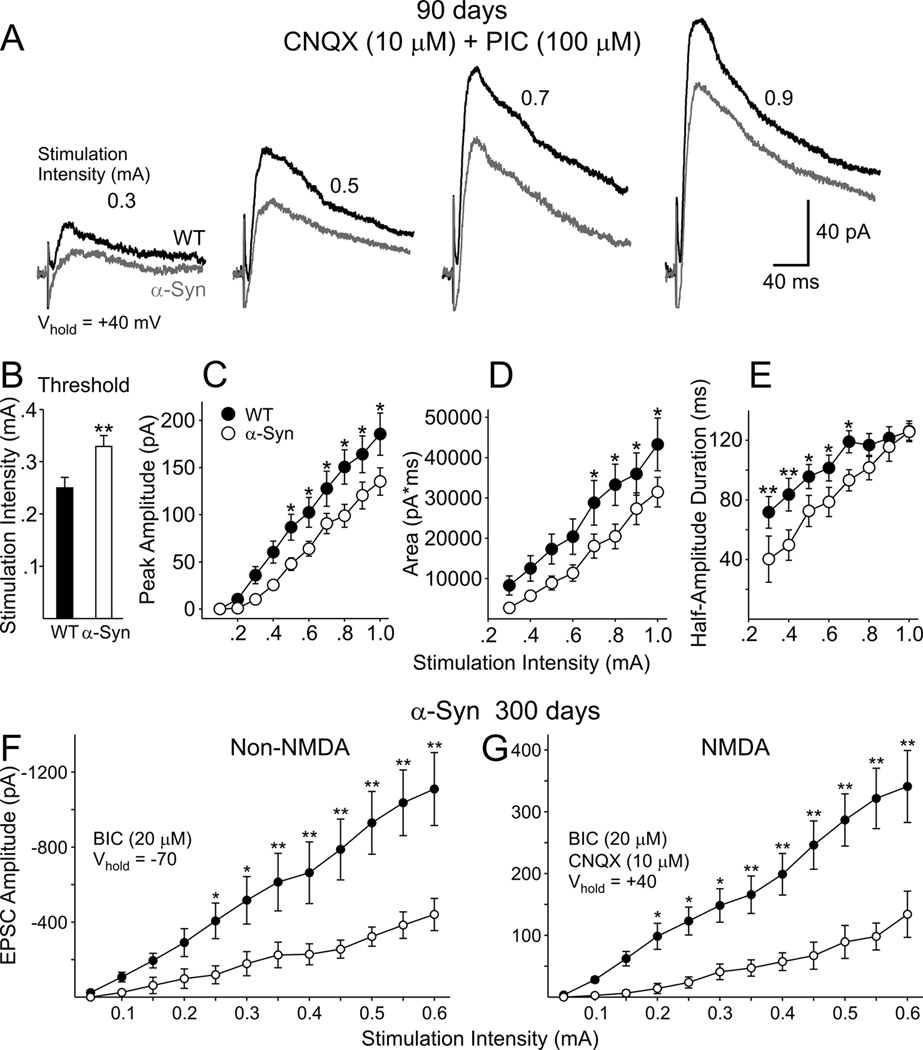
EPSCs in MSSNs were evoked by stimulating the corpus callosum and deep cortical layers in 90 day mice. MSSNs were held at −70 mV and responses were evoked at stimulation intensities of 0.1–1.0 mA in the presence of PIC (100 µM) to block currents mediated by GABAA receptors (Fig. 8A). The majority of the current is mediated by non-NMDA glutamate receptors and is almost completely blocked by bath application of CNQX (10 µM) (data not shown). The stimulation threshold for evoking EPSCs was significantly higher in cells from α-Syn than WT mice [0.31±0.02 (n=19) versus 0.22±0.02 mA (n=16); p<0.001] (Fig. 8B). Mean peak response amplitudes and response areas were smaller in MSSNs from α-Syn than those from WTs (ANOVA main effects, p=0.004 for response amplitudes and p=0.025 for response areas). Bonferroni post hoc analysis demonstrated that, at stimulation intensities of 0.4–1.0 mA, mean amplitudes were significantly smaller in cells from α-Syn than those from WT mice (p<0.05–0.01) (Fig. 8C). Mean response areas in MSSNs of α-Syn mice were significantly smaller at stimulation intensities of 0.5–1.0 mA (Fig. 8D, p<0.05). There were no significant differences in rise and decay times and half-amplitude durations between groups suggesting the smaller areas in α-Syn mice were primarily the result of smaller response amplitudes. Taken together, the results suggest a reduction in transmitter release and or a potential loss of corticostriatal synaptic connections in α-Syn mice.
Figure 8.
A. Representative non-NMDA receptor-mediated EPSCs evoked by an increasing series of stimulation intensities and recorded in MSSNs from α-Syn and WT mice at 90 days in ACSF in the presence of PIC (100 µM). B. Mean stimulation threshold was significantly higher in α-Syn mice than in WTs. C. Mean peak EPSC amplitudes were significantly reduced in α-Syn mice compared to those of WTs. D. Mean EPSC areas were significantly reduced in α-Syn mice compared to those of WTs.
NMDA receptor mediated currents were isolated by bath applying CNQX and PIC, and stepping to a holding potential of +40 mV to remove the Mg2+ block of the NMDA receptor (Fig. 9A). Under these conditions the majority of the evoked current is NMDA receptor-mediated, as AP5 (an NMDA receptor antagonist) abolished the response (data not shown). The stimulation threshold for evoking response was significantly higher in α-Syn than in WT mice [0.33±0.02 (n=19) versus 0.25±0.02 mA (n=15) in α-Syn and in WTs, respectively; p<0.02] (Fig. 9B). Mean peak response amplitudes and response areas were smaller in MSSNs from α-Syn than those of WTs (ANOVA main effects, p=0.016). Bonferroni post hoc analysis demonstrated that, at stimulation intensities of 0.5–1.0 mA, mean NMDA receptor-mediated current amplitudes were significantly smaller in α-Syn than in WT mice (Fig. 9C; p<0.05–0.01). At stimulation intensities of 0.7–1.0 mA, mean areas of NMDA receptor-mediated responses also were significantly smaller in α-Syn than in WT mice (Fig. 9D; p<0.05). At the lower stimulation intensities (0.3–0.7 mA), the half-amplitude duration was significantly shorter in α-Syn than in WT mice (Fig 9E; p<0.05–0.01). There were no significant differences in rise and decay times between responses evoked in α-Syn and WT animals. At 300 days there also were significant decreases in evoked non-NMDA and NMDA receptor-mediated currents (Fig 9F, G) again indicating that the response decreases were maintained as the mice aged. Taken together the results provide additional evidence that MSSNs from α-Syn mice have reduced excitatory synaptic inputs.
Figure 9.
A. Representative NMDA receptor-mediated EPSCs evoked by an increasing series of stimulation intensities and recorded from MSSNs from α-Syn and WT mice at 90 days in ACSF in the presence of CNQX (10 µM) and PIC (100 µM). B. Mean stimulation threshold was significantly higher in α-Syn mice than in WTs. C. Mean peak EPSC amplitudes were significantly reduced in α-Syn mice compared to those of WTs. D. Mean EPSC areas were significantly reduced in α-Syn mice compared to those of WTs. E. Mean EPSC half-amplitude durations were significantly shorter in α-Syn mice than in WTs. F. Graph shows mean peak non-NMDA receptor-mediated EPSC amplitudes evoked by an increasing series of stimulation intensities and recorded in MSSNs from α-Syn and WT mice at 300 days in ACSF in the presence of BIC (20 µM). Mean peak EPSC amplitudes were significantly reduced in α-Syn mice compared to those of WTs at stimulation intensities of 0.25 to 0.6 mA. G. Graph shows mean peak NMDA receptor-mediated EPSCs evoked by an increasing series of stimulation intensities and recorded from MSSNs from α-Syn and WT mice at 300 days in ACSF in the presence of CNQX (10 µM) and BIC (20 µM). Mean peak EPSC amplitudes were significantly reduced in α-Syn mice compared to those of WTs at stimulation intensities of 0.2 to 0.6 mA.
NMDA/non-NMDA peak current ratios also were calculated by dividing the peak amplitude of the non-NMDA response by the peak amplitude of the NMDA response at each stimulation intensity. There were no differences in these ratios between cells from α-Syn and WT mice. In addition, paired-pulse ratios were evaluated at 50 and 100 msec interstimulus intervals using an intensity of about twice threshold to evoke responses. These ratios were slightly greater in the cells from α-Syn compared to WT mice at the 50 msec interval [1.11±0.08 (n=21) versus 1.05±0.08 (n=21) for α-Syn and WTs, respectively], but very similar at the 100 msec interval [1.01±0.08 (n=21) versus 1.02±0.07 (n=21) for α-Syn and WTs, respectively]. There was considerable variation in this ratio among cells in each group and the group differences were not statistically significant.
No changes in AMPA or NMDA receptor-mediated currents occur in isolated MSSNs from α-Syn mice
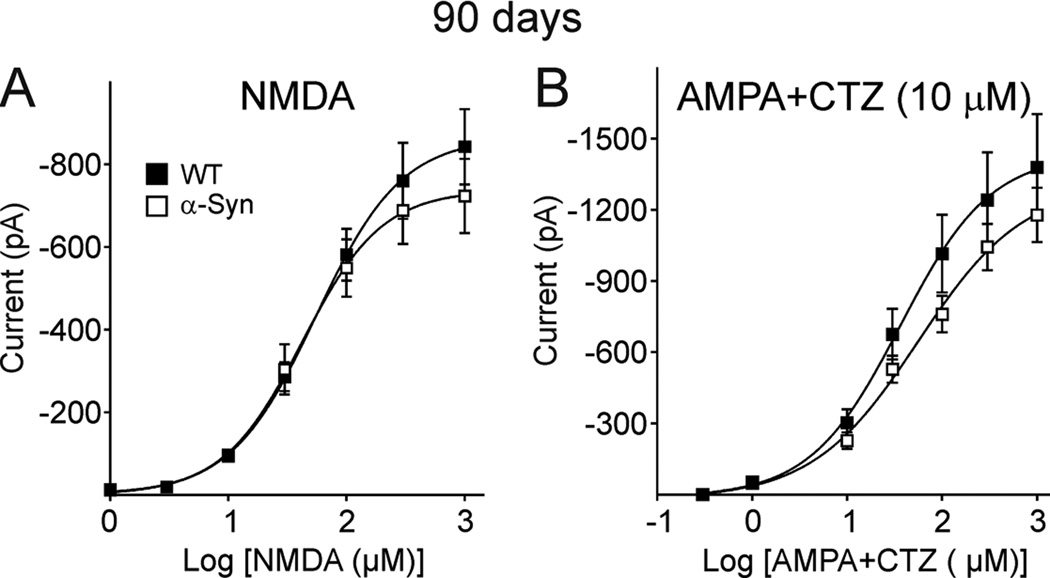
We used an acutely isolated cell preparation to examine postsynaptic receptor function in MSSNs from 90 day mice. Currents mediated by activation of NMDA and AMPA receptors were induced by applying a series of increasing concentrations of the respective agonist (10–1000 µM) and the membrane was held at −70 mV. No statistically significant differences in concentration-response functions occurred (Fig. 10) again suggesting that the decreases in frequency of spontaneous and evoked EPSCs were due to presynaptic alterations.
Figure 10.
A. Graphs show mean NMDA concentration-response function obtained from acutely dissociated MSSNs from 90 day α-Syn and WT mice in Mg2+-free ACSF in the presence of TTX (0.3 µM). B. Graphs show mean AMPA-CTZ concentration-response function obtained from acutely dissociated MSSNs from 90 day α-Syn and WT mice in Mg2+-free ACSF in the presence of TTX (0.3 µM).
Discussion
The present study demonstrated marked decreases in both spontaneous and evoked excitatory synaptic inputs to MSSNs in α-Syn overexpressing mice at all ages examined. At 90 days decreases in GABAergic synaptic activity also occurred. Changes in excitatory synaptic inputs were action potential-independent as they persisted after blockade of sodium channels with TTX. Although synaptic currents mediated by activation of both non-NMDA (primarily AMPA receptors) and NMDA receptors were decreased, postsynaptic glutamate receptor activation was similar in isolated MSSNs from α-Syn and WT mice suggesting reduced transmitter release and/or reduced cortical inputs.
Several transgenic mouse lines overexpressing α-Syn have been described (Chesselet 2008; Fernagut and Chesselet 2004). Mice overexpressing human α-Syn under the Thy1 promoter develop progressive sensorimotor deficits starting as early as two months of age (Fleming et al. 2005). They show increased expression of α-Syn in cortical and subcortical regions of the brain, including the substantia nigra pars compacta, but not in the spinal cord, neuromuscular junction, or in glial cells (Rockenstein et al. 2002), suggesting that sensorimotor deficits can be partially attributed to alterations in the brain. These mice also display early non-motor olfactory deficits and autonomic changes characteristic of pre-manifest PD (Fleming et al. 2008; Wang et al. 2008) and proteinase K-resistant α-Syn inclusions in many brain regions (Fernagut et al. 2007).
Spontaneous excitatory synaptic activity was significantly reduced in MSSNs from α-Syn mice as early as 35 days of age and persisted in older mice. These effects are specific to synaptic inputs to MSSNs since excitatory synaptic inputs to cortical neurons appeared unaffected at 90 days. The reduction of striatal synaptic activity was independent of presynaptic action potentials and the likely explanation is that presynaptic glutamate release is reduced in the striatum of these α-Syn mice due to overexpression of α-Syn in corticostriatal terminals. It is conceivable that α-Syn negatively interacts with signaling pathways that underlie neurotransmitter release. Supporting this finding, previous studies of evoked DA release in nigrostriatal terminals from α-Syn KO mice, as well as in neuronal cultures from transgenic mice overexpressing α-Syn, suggest that human α-Syn negatively regulates reserve pools of synaptic vesicles and “primed” synaptic vesicles required for DA release (Abeliovich et al. 2000; Larsen et al. 2006; Yavich et al. 2004). The reduced excitatory synaptic activity is not likely due to a decrease in inputs to cortical pyramidal neurons as at 90 days pyramidal neurons exhibited normal synaptic inputs in α-Syn mice. However, the observation that spontaneous GABA synaptic inputs were increased in cortical pyramidal neurons at 90 days indicates that cortical neurons may be under greater inhibitory control and their output may be altered. One explanation for the progressive decrease in spontaneous IPSCs in the striatum is the reduced cortical input to both inhibitory interneurons and MSSNs. Such reduced input would be reflected by reduced GABA-mediated output of both MSSNs and striatal interneurons synapsing on MSSNs (Koos et al. 2004).
These data are consistent with previous studies demonstrating alterations in synaptic transmission in other brain areas by alterations in α-Syn expression. Observations from striatal (Abeliovich et al. 2000) and hippocampal (Cabin et al. 2002) slices have revealed altered kinetics of neurotransmitter release in α-Syn KO mice. Similarly, neurons in hippocampal slices from mice with altered α-Syn (α-Syn KO, α-Syn A30P mutation, and human WT α-Syn overexpression) exhibit impairment in presynaptic mobilization of glutamate from the reserve pool (Gureviciene et al. 2007).
In agreement with our previous study in parkin KO mice, there is decreased excitation of MSSNs (Goldberg et al. 2003). However, the site of the deficit in the two different genetic models appears different. In the parkin KO mice the data suggested a postsynaptic site of the effect. In the α-Syn mice the lack of postsynaptic changes in glutamate receptor-mediated currents and differences in spontaneous mEPSCs suggest a presynaptic site. In recent experiments using striatal field potentials in α-Syn overexpressing mice we demonstrated increased paired-pulse ratios to activation of corticostriatal afferents as well as altered synaptic plasticity and provided additional evidence for a presynaptic effect (Watson et al. 2009).
In the present experiments, although we interpret the decreases in synaptic responses as presynaptic alteration, we only observed a slight, non-significant increase in the paired-pulse ratios in MSSNs from α-Syn mice. If changes are presynaptic one expectation is that paired-pulse ratios increase (Zucker and Regehr 2002). There was high variability of paired-pulse ratios among neurons. Such variability may have been due to sampling different populations of MSSNs. For example, recent studies in striatal D1 and D2 receptor-containing MSSNs indicate that paired-pulse ratios are not always a reliable predictor of pre- or postsynaptic effects (Cepeda et al. 2008) and at least in dorsal striatum, an increase in paired-pulse ratios does not necessarily reflect reduced probability of release. Additional studies segregating the populations of MSSNs in α-Syn overexpressing mice will be required to provide a more definitive answer.
The present findings as well as our studies in parkin KO mice contrast with the outcomes of electrophysiological studies in DA-depletion models of PD. Electrolytic or neurotoxic lesions of DA neurons have demonstrated increased glutamate synaptic activity and excitability in MSSNs after damage to the substantia nigra (Calabresi et al. 1993; Cepeda et al. 1989; Galarraga et al. 1987), indicating that DA exerts a tonic inhibition of glutamate release (Bamford et al. 2004; Cepeda et al. 2001). In α-Syn mice, at the ages examined, there is little evidence of DA neuron loss (Fernagut et al. 2007). In addition, striatal DA levels in α-Syn overexpressing and parkin KO mice are not reduced and actually appear to be increased (Goldberg et al. 2003; Maidment et al. 2006), raising the possibility of altered modulation of cortical input. There are other potential reasons why the outcomes in toxin-based and genetic models differ. Although toxin models reproduce DA cell degeneration and loss, the mechanisms producing cellular degeneration are not the same as in idiopathic or genetic PD (Chesselet 2008; Dauer and Przedborski 2003). Furthermore, toxin models do not reproduce the pathology and cell loss observed in other brain regions in PD (Halliday et al. 2006). Although genetic models also have limitations, their major strength is that animals can be studied before the development of an overt phenotype and the contributions of multiple brain regions can be examined (Meredith et al. 2008). Thus, our studies in α-Syn mice are evaluating early effects on synaptic communication that cannot be observed in the toxin-based models.
In conclusion, we have demonstrated marked glutamatergic synaptic deficits at the corticostriatal synapse of α-Syn mice in the absence of loss of DA terminals. These changes appear specific to striatal MSSNs as they were not observed in cortical pyramidal neurons. The α-Syn mice probably represent a very early stage of PD and can provide important information on mechanisms that initiate later pathophysiological process.
Acknowledgments
We thank Donna Crandall for help with the illustrations and M-F. Chesselet and J. Watson for critical evaluation of earlier versions of this manuscript. This work was supported by UPHS grants P50NS38367, U54ES12078, NS33538 and P30-HD04612.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Bargas J, Howe A, Eberwine J, Cao Y, Surmeier DJ. Cellular and molecular characterization of Ca2+ currents in acutely isolated, adult rat neostriatal neurons. J Neurosci. 1994;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACalabresi P, Mercuri NB, Sancesario G, Bernardi G. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson's disease. Brain. 1993;116(Pt 2):433–452. [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol. 2001;85:659–670. doi: 10.1152/jn.2001.85.2.659. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Calvert CR, Hernandez-Echeagaray E, Nguyen OK, Jocoy E, Christian LJ, Ariano MA, Levine MS. Transient and progressive electrophysiological alterations in the corticostriatal pathway in a mouse model of Huntington's disease. J Neurosci. 2003;23:961–969. doi: 10.1523/JNEUROSCI.23-03-00961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Walsh JP, Hull CD, Howard SG, Buchwald NA, Levine MS. Dye-coupling in the neostriatum of the rat: I. Modulation by dopamine-depleting lesions. Synapse. 1989;4:229–237. doi: 10.1002/syn.890040308. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Wu N, Andre VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington's disease. Prog Neurobiol. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson's disease? Exp Neurol. 2008;209:22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubayko U, Plenz D. Fast synaptic transmission between striatal spiny projection neurons. Proc Natl Acad Sci U S A. 2002;99:15764–15769. doi: 10.1073/pnas.242428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Chesselet MF. Alpha-synuclein and transgenic mouse models. Neurobiol Dis. 2004;17:123–130. doi: 10.1016/j.nbd.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse. 2007;61:991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF. Olfactory deficits in mice overexpressing human wildtype alpha-synuclein. Eur J Neurosci. 2008;28:247–256. doi: 10.1111/j.1460-9568.2008.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Soreide A, Kvale I, Walker J, Walaas I. Glutamate in cortical fibers. Adv Biochem Psychopharmacol. 1981a;27:29–41. [PubMed] [Google Scholar]
- Fonnum F, Storm-Mathisen J, Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neuroscience. 1981b;6:863–873. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Galarraga E, Bargas J, Martinez-Fong D, Aceves J. Spontaneous synaptic potentials in dopamine-denervated neostriatal neurons. Neurosci Lett. 1987;81:351–355. doi: 10.1016/0304-3940(87)90409-5. [DOI] [PubMed] [Google Scholar]
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Gureviciene I, Gurevicius K, Tanila H. Role of alpha-synuclein in synaptic glutamate release. Neurobiol Dis. 2007;28:83–89. doi: 10.1016/j.nbd.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Del Tredici K, Braak H. Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson's disease. J Neural Transm. 2006;(Suppl):99–103. doi: 10.1007/978-3-211-45295-0_16. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Klapstein GJ, Fisher RS, Zanjani H, Cepeda C, Jokel ES, Chesselet MF, Levine MS. Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington's disease transgenic mice. J Neurophysiol. 2001;86:2667–2677. doi: 10.1152/jn.2001.86.6.2667. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, Narasanna A, Kolodilov N, Dauer W, Hawkins RD, Arancio O. Alpha-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004;23:4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidment NT, Lam HA, Ackerson LC, Rockenstein E, Masliah E. Dysregulation of dopamine transmission in mice overexpressing human wildtype alpha-synuclein. Society for Neuroscience. 2006 Abstracts Program No. 378.374. [Google Scholar]
- Meredith GE, Sonsalla PK, Chesselet MF. Animal models of Parkinson's disease progression. Acta Neuropathol. 2008;115:385–398. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel WH, Mugnaini E. Immunocytochemical studies of GABAergic neurons in rat basal ganglia and their relations to other neuronal systems. Neurosci Lett. 1984;47:233–238. doi: 10.1016/0304-3940(84)90519-6. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004;18:637–647. doi: 10.1096/fj.03-1112rev. [DOI] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Springer W, Kahle PJ. Mechanisms and models of alpha-synuclein-related neurodegeneration. Curr Neurol Neurosci Rep. 2006;6:432–436. doi: 10.1007/s11910-996-0025-8. [DOI] [PubMed] [Google Scholar]
- Starling AJ, Andre VM, Cepeda C, de Lima M, Chandler SH, evine MS. Alterations in N-methyl-D-aspartate receptor sensitivity and magnesium blockade occur early in development in the R6/2 mouse model of Huntington's disease. J Neurosci Res. 2005;82:377–386. doi: 10.1002/jnr.20651. [DOI] [PubMed] [Google Scholar]
- Totterdell S, Meredith GE. Localization of alpha-synuclein to identified fibers and synapses in the normal mouse brain. Neuroscience. 2005;135:907–913. doi: 10.1016/j.neuroscience.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. Aggregation of neurofilament and alpha-synuclein proteins in Lewy bodies: implications for the pathogenesis of Parkinson disease and Lewy body dementia. Arch Neurol. 1998;55:151–152. doi: 10.1001/archneur.55.2.151. [DOI] [PubMed] [Google Scholar]
- Venance L, Glowinski J, Giaume C. Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J Physiol. 2004;559:215–230. doi: 10.1113/jphysiol.2004.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fleming SM, Chesselet MF, Tache Y. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport. 2008;19:873–876. doi: 10.1097/WNR.0b013e3282ffda5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JB, Hatami A, David H, Masliah E, Roberts K, Evans CE, Levine MS. Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein. Neuroscience. 2009;159:501–513. doi: 10.1016/j.neuroscience.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger C, Prou D, Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003;17:2151–2153. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]