Abstract
Cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO) utilize arachidonic acid for the synthesis of eicosanoids that have been implicated in carcinogenesis and cardiovascular disease. The ability of celecoxib, a selective COX-2 inhibitor, to redirect arachidonic acid into the 5-LO pathway can potentially reduce its efficacy as a chemopreventive agent and increase the risk of cardiovascular complications. Levels of urinary prostaglandin E metabolite (PGE-M) and leukotriene E4 (LTE4), biomarkers of the COX and 5-LO pathways, are elevated in smokers. Here we investigated the effects of zileuton, a 5-LO inhibitor, vs. zileuton and celecoxib for 6 ± 1 days on urinary PGE-M and LTE4 levels in smokers. Treatment with zileuton led to an 18% decrease in PGE-M levels (P=0.03); the combination of zileuton and celecoxib led to a 62% reduction in PGE-M levels (P<0.001). Levels of LTE4 decreased by 61% in subjects treated with zileuton alone (P<0.001) and were unaffected by the addition of celecoxib. Although zileuton use was associated with a small overall decrease in PGE-M levels, increased PGE-M levels were found in a subset (19/52) of subjects. Notably, the addition of celecoxib to the 5-LO inhibitor protected against the increase in urinary PGE-M levels (P=0.03). In conclusion, zileuton was an effective inhibitor of 5-LO activity resulting in marked suppression of urinary LTE4 levels and possible redirection of arachidonic acid into the COX-2 pathway in a subset of subjects. Combining celecoxib and zileuton was associated with inhibition of both the COX-2 and 5-LO pathways manifested as reduced levels of urinary PGE-M and LTE4.
Keywords: smoking, inflammation, cyclooxygenase, lipoxygenase, zileuton, celecoxib
Introduction
Cigarette smoking continues to be the leading cause of preventable death in the United States. The risk of lung cancer is increased in smokers with chronic lung injury (1). While primary prevention efforts through tobacco cessation are likely to be the most effective strategy for reducing disease, successful quit rates are at best around 30% (2). Given the recognized link between smoking, inflammation and cancer, identifying inflammatory pathways that are pharmacologically modifiable represents a bona fide approach to attempting to reduce the risk of smoking-related cancer.
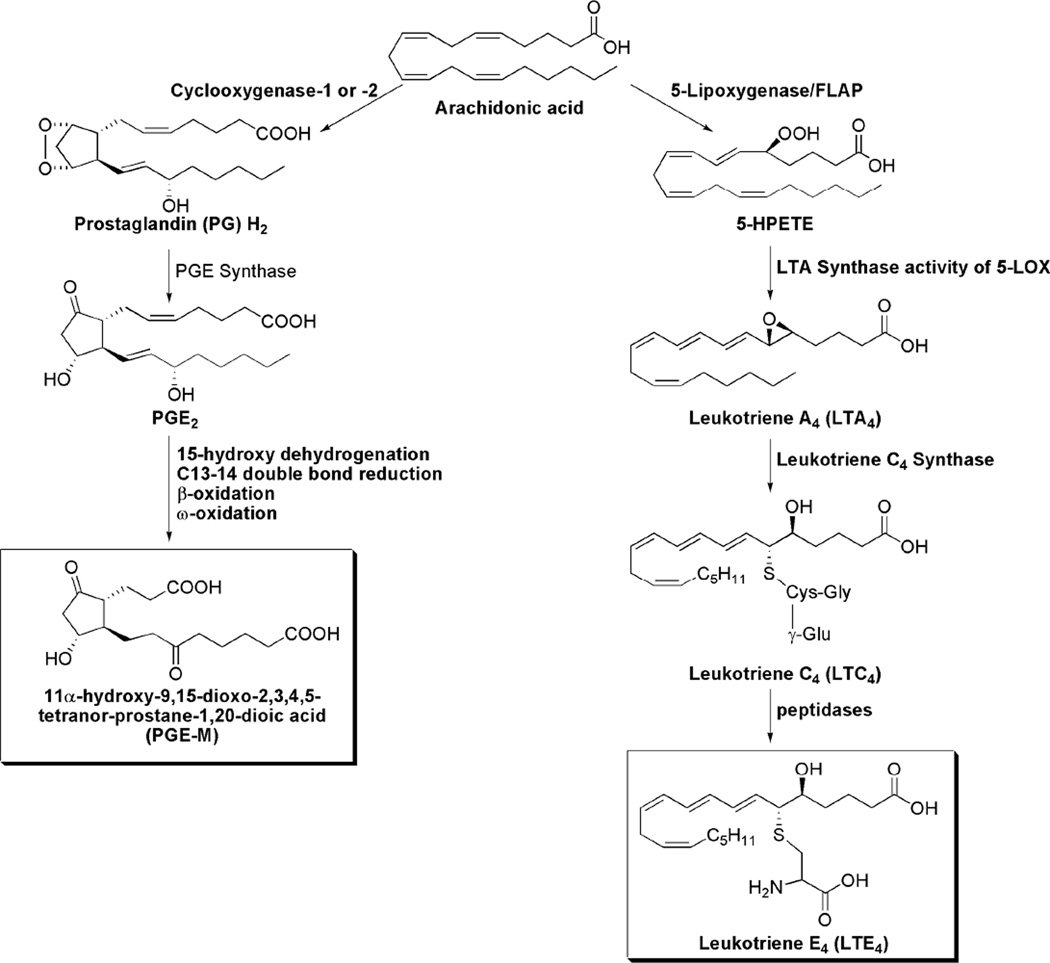
Multiple lines of evidence suggest an important role for aberrant arachidonic acid metabolism in both inflammation and carcinogenesis. The current report is focused on two proinflammatory pathways involving the enzymes cyclooxygenase (COX) and 5-lipoxygenase (5-LO). COX-1, a constitutively expressed enzyme (3), and COX-2, an enzyme rapidly induced by inflammatory stimuli, tobacco carcinogens and growth factors, catalyze the conversion of arachidonic acid to PGH2 (4–7). PGH2 is converted by microsomal prostaglandin E synthase to PGE2 (8), which can regulate cell proliferation, apoptosis, angiogenesis, and immune surveillance (9). Catabolism of PGE2 is initiated by 15-hydroxyprostaglandin dehydrogenase and results in a stable end metabolite, 11-α-hydroxy-9, 15-dioxo-2, 3, 4, 5-tetranor-prostane-1, 20-dioic acid (PGE-M) that is excreted in the urine (Fig. 1) and used as an index of systemic PGE2 production (10–12).
Figure 1. Synthesis of PGE-M and LTE4 via COX and 5-LO pathways.
Arachidonic acid can also be converted by 5-LO and its molecular partner, 5-LO-activating protein (FLAP), to leukotriene A4 (LTA4) (13). LTA4 is then conjugated to reduced glutathione by leukotriene C4 synthase, forming LTC4. The final biologically active metabolites of the 5-LOX pathway include LTB4 and the cysteinyl-LTs (cys-LTs), LTC4, LTD4 and LTE4. The cys-LTs are potent inflammatory mediators that increase vascular permeability and stimulate smooth muscle contraction (14, 15). The cys-LTs, LTC4 and LTD4 are metabolized to the end product LTE4, which is excreted in the urine without further modification (16, 17).
Selective inhibitors of both COX-2 and 5-LO possess chemopreventive activity in preclinical studies (9, 18, 19). Drug induced shunting of arachidonic acid from the COX pathway into the 5-LO pathway or vice-versa has been postulated to decrease the efficacy of these agents (20, 21). This possibility provided the rationale for combining agents that target each of the pathways to reduce shunting of arachidonic acid with the goal of improving drug efficacy. In fact, several preclinical studies have suggested that the combination of celecoxib, a selective COX-2 inhibitor, and zileuton, a 5-LO inhibitor, is more effective than either agent alone in preventing tumor formation or inhibiting tumor growth (22–24). Translating these preclinical findings into the clinic represents a significant need. Mao et al. (25) showed that treatment of active smokers with celecoxib led to increased levels of LTB4 in bronchoalveolar fluid, which suggested a drug induced shunt of arachidonic acid into the 5-LO pathway. We previously reported that celecoxib suppressed COX-2 activity leading to reduced levels of urinary PGE-M in smokers; the reduction in urinary PGE-M was associated with increased levels of urinary LTE4 in a subset of subjects (26).
The COX-2 and 5-LO pathways have been suggested to be important in the pathogenesis of cardiovascular disease in addition to carcinogenesis (27–33). The ability of celecoxib to redirect arachidonic acid into the 5-LO pathway has been suggested to help explain the link between use of COX-2 inhibitors and cardiovascular complications (26, 34). Although both the COX-2 and 5-LO pathways are activated in smokers (26), the effects of celecoxib when combined with zileuton in human smokers are unknown. In the current study, we had two main objectives: 1) to determine whether zileuton redirects arachidonic acid into the COX pathway in association with suppression of urinary LTE4 and 2) to investigate whether combining celecoxib and zileuton suppresses shunting of arachidonic acid and thereby reduces both urinary PGE-M and LTE4 levels. Our findings suggest that a COX-2 inhibitor can be combined with a 5-LO inhibitor to suppress the synthesis of both PGE-M and LTE4 and thereby prevent the redirection of arachidonic acid into a competing proinflammatory pathway.
Materials and Methods
Study design
This was a randomized biomarker trial which evaluated the effects of 1200 mg twice daily oral zileuton (Zyflo CR, Cornerstone Therapeutics) or this dose of zileuton in combination with 200 mg twice daily oral celecoxib (Celebrex, Pfizer) for 6 ± 1 days in healthy current smokers. The protocol was approved by the Weill Cornell Medical College Institutional Review Board and Clinical and Translational Science Center and conducted in accordance with an assurance filed with and approved by the Department of Health and Human Services. All subjects provided written informed consent for participation.
Participant selection
Eligible subjects were required to be 18 years of age or older, healthy (ECOG performance status of 0 or 1) current smokers with ≥ 10 pack years of smoking. The exclusion criteria included active cancer, history of cancer, chronic inflammatory conditions, ongoing or active infection, or use of certain medications (e.g., celecoxib, zileuton, corticosteroids, nonsteroidal anti-inflammatory drugs, drugs known to interact with celecoxib or zileuton, or other investigational drugs) within 30 days of consent, contraindication to celecoxib or zileuton (pregnancy, peptic ulcer disease, sulfa allergy, prior cardiovascular events), kidney or liver disease.
Study schema, treatment and study assessments
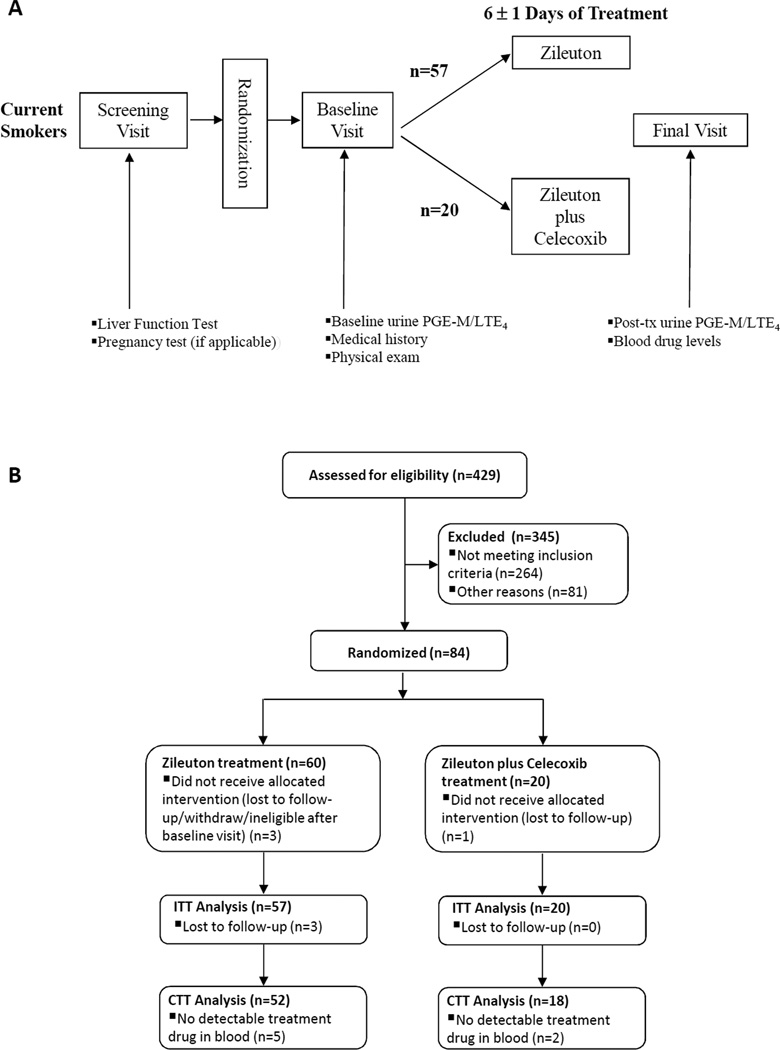
After signing informed consent, participants underwent a baseline evaluation that included smoking and concomitant medication assessment, and liver functions tests and a pregnancy test, if applicable. Upon confirmation of eligibility, participants were randomized and presented for the baseline full medical assessment including physical examination. A single void urine specimen was collected (Fig. 2A). The trial consisted of two arms (administration of zileuton 1200 mg twice daily; combined administration of zileuton 1200 mg twice daily plus celecoxib 200 mg twice daily) with subjects randomized at a 3:1 ratio to receive either zileuton alone or the combination of zileuton and celecoxib, respectively. These doses of zileuton and celecoxib are the maximum recommended for the treatment of asthma and arthritis, respectively. After the initial urine sample was collected, subjects were randomized to one of the two treatment arms and received study agents for 6 ± 1 day. This length of treatment was chosen to be certain steady-state levels of drug were achieved. At day 6 (± 1), urine and blood were collected. Toxicity was monitored according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Urine was aliquoted into 2 mL cryovials and stored at −80°C. Plasma was prepared from blood and stored at −80°C.
Figure 2. Study procedures and accrual.
A, Study schema. B, Flow diagram of subjects who were accrued to the study. ITT= Intent to treat; CTT= Compliant to treatment
Study end points
Urine was analyzed for PGE-M and LTE4. Post-treatment plasma specimens were analyzed for zileuton and celecoxib levels as a measure of drug compliance. All measurements were carried out in a blinded manner.
Urinary PGE-M
PGE-M and d4-PGEM were custom synthesized by Dr. Doug Taber and colleagues (35). C-18 Sep-Pak extraction cartridges were purchased from Waters Corporation. Methyloxime HCl was purchased from Sigma Aldrich. All other organic reagents were of high-performance LC quality and purchased from EM Sciences.
Sample preparation
Urine samples were removed from −80°C storage and allowed to thaw at room temperature. 1mL of sample was then acidified to pH 3 with 1 mol/L HCl, and endogenous PGE-M was then converted to the O-methyloxime derivative by treatment with 0.5 mL of 16% (w/v) methyloxime HCl in 1.5 mol/L sodium acetate buffer (pH 5). Following 1 hour incubation, the methoximated PGE-M was diluted with 10 mL water adjusted to pH 3, and the aqueous sample was applied to a C-18 Sep-Pak that had been preconditioned with 5 mL methanol and 5 mL water (pH 3). The Sep-Pak was washed with 20 mL water (pH 3) and 10 mL heptane. PGE-M was then eluted from the Sep-Pak with 5 mL ethyl acetate, and any residual aqueous material was removed from the eluate by aspiration. The [2H6]-O-methyloxime PGE-M internal standard (6.2 ng in 10 µL ethanol) was then added, and the eluate was evaporated under a continuous stream of nitrogen at 37°C. The dried residue was resuspended in 50 µL (20µL injections) 95:4.9:0.1 (v/v/v) 5 mmol/L ammonium acetate: acetonitrile: acetic acid and was filtered through a 0.2-micron Spin-X filter (Corning).
LC-MS-MS
Samples were analyzed using LC-MS-MS with slight modifications to the method previously described by Murphey and colleagues (12). LC was performed on a 2.0 × 50 mm, 1.7µm particle Acquity CSH Phenyl-Hexyl column (Waters Corporation). Mobile phase A was 95:4.9:0.1 (v/v/v) 5 mmol/L ammonium acetate:acetonitrile:acetic acid, and mobile phase B was 10.0:89.9:0.1 (v/v/v) 5 mmol/L ammonium acetate:acetonitrile:acetic acid. Gradient elution was performed with 5% B for 1 minute, a linear increase to 11% B until 1.1 minute, a linear increase to 17% B until 30 minutes, a step to 60% B at 31 minutes, hold for 3 minutes at 60% B, a step to 100% B at 34.1 minutes, hold for 3 minutes at 100% B, and reequilibration from 37.1 minutes to 40 minutes with 5% B. The flow rate was set to 200µL/minute and the column was maintained at room temperature.
The analytes were detected on a Thermo Scientific Quantum Vantage triple quadrupole mass spectrometer fitted with an electrospray ion source operating in the negative ion mode using selected reaction monitoring (SRM). The following SRM reactions were monitored: PGE-M (m/z 385 ➔ 336) and d6-PGE-M (m/z 391 ➔ 339) using a collision energy of 20eV under 1.5mT argon gas. Endogenous levels were calculated by comparing the ratio of the peak heights of endogenous PGE-M with that of the deuterated internal standard.
Urinary LTE4
20,20,20-2H3-LTE4 was purchased from Enzo Life Sciences. Empore SD C-18 extraction cartridges (standard density, 6mL capacity, 3M, St. Paul, MN) were obtained from VWR International and Thermo Fisher Scientific. All organic reagents were of high-performance LC quality and purchased from EM Sciences.
Sample preparation
Urine samples were removed from −80°C storage and allowed to thaw at room temperature. 3mL of sample was then acidifed to pH 3 with 1mol/L HCl. To the acidified urine was added the internal standard, [2H3]-LTE4. The sample was next applied to an Empore C-18 solid phase extraction column that had been pre-washed with methanol (6mL) and 0.001N HCl (6mL). The column was subsequently washed with 0.001N HCl (6mL), methanol/10mmol/L ammonium acetate buffer (pH 5.6) (1/9, v/v) (6mL), and ethyl acetate/heptane (1/1, v/v) (6mL). The analyte was eluted with methanol (1mL). The eluate was evaporated under a continuous stream of dry nitrogen, then dissolved in 100µL methanol and filtered using a 0.2-µm Spin-X filter (Corning). The sample was again dried under a stream of nitrogen and then dissolved in 32µL methanol (15µL injections) for analysis by UPLC/ESI-MS/MS.
LC-MS-MS
UPLC was performed on a 2.1 × 50-mm, 1.7-µm particle Acquity UPLC BEH C-18 column (Waters Corporation) attached to an Acquity UPLC (Waters Corporation) (36). Mobile phase A was 0.1% formic acid in water; mobile phase B was 0.1% formic acid in acetonitrile. Gradient elution was performed with 5% B for 1 minute, a linear increase to 53% B until 9.5 minutes, a linear increase to 76% B until 11 minutes, a step to 100% B until 11.1 minutes, hold for 1 minute at 100% B, and reequilibration from 12.1 minutes to 14 minutes with 5% B. The flow rate was set to 600 µL/minute and the column temperature was maintained at 30°C.
The analytes were detected on a Thermo Scientific Quantum Vantage triple quadrupole mass spectrometer fitted with an electrospray ion source operating in the negative ion mode using SRM. The following SRM reactions were monitored: LTE4 (m/z 438 ➔ 333) and d3-LTE4 (m/z 441 ➔ 336) using a collision energy of 20eV under 1.5mT argon gas. Endogenous levels were calculated by comparing the ratio of the peak heights of endogenous LTE4 with that of the deuterated internal standard.
Urine Creatinine
Creatinine (Cr) in the urine was measured by a chemical assay based on Jaffe’s reaction according to the manufacturer’s instructions (Enzo Life Sciences) for normalization of PGE-M and LTE4. Levels of both PGE-M and LTE4 are expressed per mg Cr.
Drug levels
Celecoxib was provided by the National Cancer Institute and celecoxib-d4 was obtained from TRC. Zileuton was obtained from Sigma-Aldrich and zileuton-d4 was obtained from Santa Cruz Biotechnology. Blank human plasma was obtained from Innovative Research. Solvents were HPLC grade and purchased from Fisher Scientific.
Sample preparation
Plasma samples were removed from −80°C storage, allowed to thaw on ice and vortexed. 100 µL of thawed sample were added to 250 µL acetonitrile containing both deuterated internal standards and immediately vortexed. The samples were then centrifuged at 5000 r.c.f. for 2 minutes. The supernatants were transferred to autosampler vials, diluted 1:1 (v:v) with H2O and analyzed via LC-MS.
LC-MS-MS
Analytes were chromatographed on a Supelco C18 column (50 × 2 cm, with a C18 guard cartridge) using the following gradient: 50%B to 90%B over 4.0 minutes followed by a 0.4 minutes hold at 90%B. The column was re-equilibrated at 50%B for at least 3 minutes prior to each injection. The flow rate was 0.375 mL/minute. Component A was 10 mM ammonium acetate (aqueous) and B was 1:1 methanol: acetonitrile with 10% A.
The analytes were detected on a Thermo Quantum triple quadrupole mass spectrometer via SRM. The mass spectrometer was equipped with an atmospheric pressure chemical ionization (APCI) source and operated in the positive ion mode. The following SRM reactions were employed: celecoxib (m/z 380∧316); celecoxib-d4 (m/z 384∧320); zileuton (m/z 237∧161); zileuton-d4 (m/z 241∧165) (the Q1 masses represent [M+H]+ complexes). Unknown samples were quantitated against a standard curve prepared in blank human plasma and extracted and analyzed concurrently with the unknown samples. The standard curves were constructed by plotting the analyte response (ratio of the analyte peak area to its corresponding deuterated internal standard peak area) against the concentration of the standards.
Statistical analysis
Demographic and smoking characteristics of the subjects were compared between treatment arms using methods appropriate for the type of data. For age, the two-sample Student t-test was used to compare the means. For smoking intensity measured by pack year, the non-parametric Wilcoxon rank sum test was used. For the categorical variables, Fisher's exact test was used to compare the differences in proportions.
PGE-M and LTE4 values were analyzed primarily using nonparametric tests and reported in terms of median (range). Differences in baseline levels of PGE-M and LTE4 between two treatment arms were examined using the Wilcoxon rank sum test. Pre/post change in PGE-M and LTE4 levels following a given treatment for subjects in a specific treatment arm was evaluated using Wilcoxon signed rank test. Magnitude of change between two treatment arms was compared using Wilcoxon rank sum test.
Consistent results were obtained for log-transformed data when corresponding parametric methods were used. Further analyses adjusting for age, gender, and race were carried out using multiple regression for log-transformed PGE-M and LTE4 data. Age, gender, and race had no effect on any of the reported results.
Results
The trial opened in April 2010 with the last participant completing treatment in September of 2011. The screening and accrual to this study has been described previously (37) (Fig. 2B). A total of 84 subjects were enrolled in the trial and randomized at a 3:1 ratio to receive either zileuton alone or the combination of zileuton and celecoxib. Four subjects withdrew for different reasons before beginning study medication. 80 subjects were started on the study medication(s). 77 subjects completed the entire study. The 3 subjects who failed to complete the study withdrew for personal reasons. The study medications were undetectable in the plasma of 7 of 77 subjects (9%) who completed the trial. This indicated noncompliance with the study medication and led to exclusion of these 7 subjects in the analysis of treatment effect. Hence, data from 70 subjects who completed the treatment phase of the study and had measureable levels of drug in plasma were included in the treatment effect analyses. The urine of two subjects in the zileuton + celecoxib arm contained interfering substances possessing the same m/z as urinary PGE-M precluding analysis; these two study subjects were excluded in the analysis related to PGE-M. A description of the characteristics of the 70 subjects who were compliant to treatment is shown in Table 1. Subjects in the two arms of the study were well matched by age, gender and pack years of smoking.
Table 1.
Patient Characteristics
| Compliant-to-treatment sample |
||||
|---|---|---|---|---|
| All (n=70) |
Zileuton (n=52) |
Zileuton + Celecoxib (n=18) |
p-value | |
| Age | ||||
| Mean ± s.d. | 43.6 ± 9.1 | 43.8 ± 9.6 | 43.1 ± 7.6 | 0.76 |
| Gender | 0.58 | |||
| Female | 27 (39%) | 19 (37%) | 8 (44%) | |
| Male | 43 (61%) | 33 (63%) | 10 (56%) | |
| Race, n (%) | 0.07 | |||
| Asian | 2 (3%) | 0 (0%) | 2 (11%) | |
| Black | 35 (50%) | 28 (54%) | 7 (39%) | |
| White | 33 (47%) | 24 (46%) | 9 (50%) | |
| Smoking (Pack Year) | ||||
| Median (range) | 19.5 (10, 68) | 19.5 (10, 68) | 19.5 (10, 40) | 0.59 |
| Baseline PGE-M (ng/mg Cr) | ||||
| Median (range) | 12.6 (1.4, 50.4) | 12.8 (1.4, 50.4) | 10.2 (2.3, 35.1) | 0.61 |
| Baseline LTE4 (pg/mg Cr) | ||||
| Median (range) | 102.5 (4, 268) | 107 (4, 268) | 86.5 (37, 250) | 0.42 |
Effect of celecoxib and zileuton vs. zileuton alone on urinary PGE-M
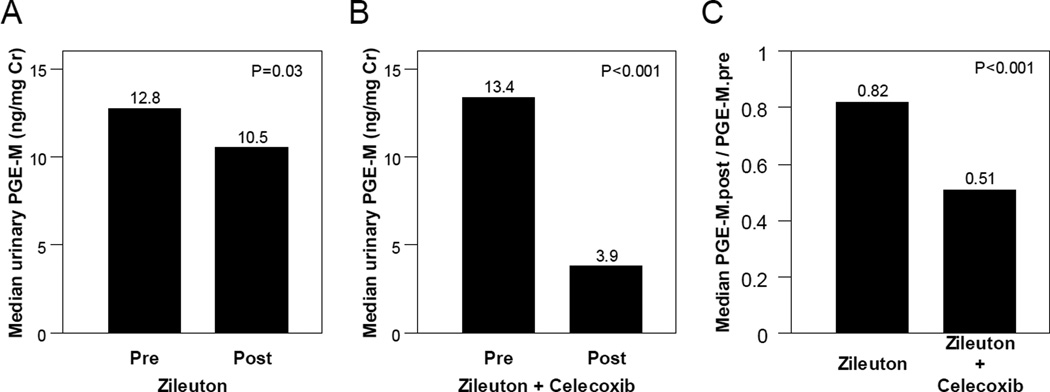
Treatment with zileuton led to a small (18%) but statistically significant decrease in median levels of PGE-M (P=0.03; Fig. 3A). The combination of celecoxib and zileuton led to a 62% reduction in median levels of PGE-M (P<0.001; Fig. 3B). As shown in Fig. 3C, the magnitude of the decrease in PGE-M was greater in subjects who received celecoxib combined with zileuton vs. zileuton alone (P<0.001).
Figure 3. Combining celecoxib and zileuton is more effective than zileuton alone in reducing levels of urinary PGE-M.
A, Treatment with zileuton alone led to a small yet statistically significant decrease in urinary PGE-M levels [median (range)] (ng/mg creatinine) [pre PGE-M = 12.8 (1.4, 50.4) vs. post PGE-M =10.5 (2.0, 34.6); P=0.03, Wilcoxon signed rank test] among study participants compliant to treatment (n=52). B, Treatment with zileuton and celecoxib led to significant decrease in urinary PGE-M levels [pre PGE-M=13.4 (2.3, 35.1) vs. 3.9 (1.2, 10.1); P<0.001, Wilcoxon signed rank test] among study participants compliant to treatment and with evaluable urine samples at both time points (n=16). C, Significantly greater decrease in urinary PGE-M levels in terms of post/pre fold change [median (range)] was observed in subjects treated with zileuton and celecoxib combined therapy compared to subjects treated with zileuton alone [0.51 (0.11,1.04) vs. 0.82 (0.31,6.82); P<0.001, Wilcoxon rank-sum test].
Effect of celecoxib and zileuton vs. zileuton alone on urinary LTE4
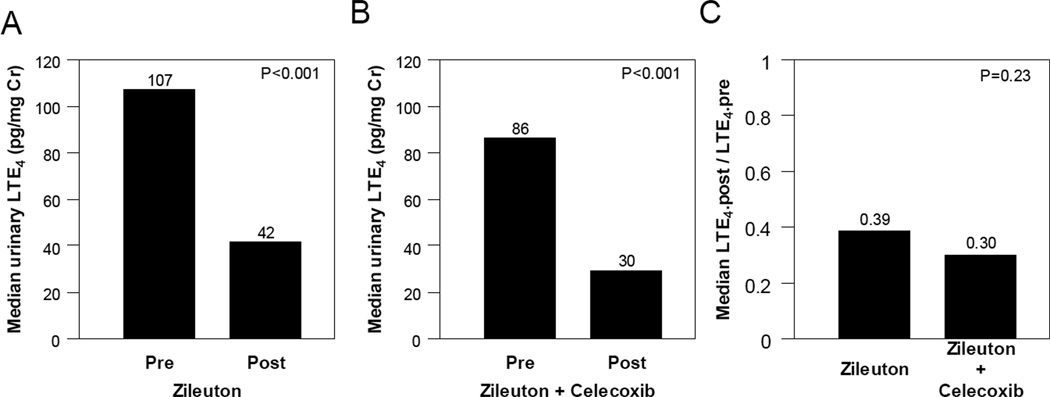
Median levels of LTE4 decreased by 61% in participants treated with zileuton alone (P<0.001; Fig. 4A). Treatment with the combination of zileuton and celecoxib led to approximately a 65% reduction in median levels of urinary LTE4 (P<0.001; Fig. 4B). The combination of zileuton and celecoxib was no more effective than zileuton alone in suppressing levels of urinary LTE4 (Fig. 4C).
Figure 4. Adding celecoxib to zileuton does not enhance the suppression of urinary LTE4.
A, Treatment with zileuton alone led to significant decrease in urinary LTE4 levels [median (range)] (ng/mg creatinine) [pre LTE4=107 (4, 268) vs. post LTE4=42 (4, 292); P<0.001, Wilcoxon signed rank test] among study participants compliant to treatment (n=52). B, Treatment with zileuton and celecoxib led to significant decrease in urinary LTE4 levels [pre LTE4=86 (37, 250) vs. post LTE4=30 (10, 84); P<0.001, Wilcoxon signed rank test] among study participants compliant to treatment (n=18). C, Similar magnitude of decrease in urinary LTE4 levels in terms of post/pre fold change [median (range)] was observed in subjects treated with zileuton and celecoxib combined therapy compared to subjects treated with zileuton alone [0.30 (0.12,1.01) vs. 0.39 (0.05,4.00); P=0.23 , Wilcoxon rank-sum test].
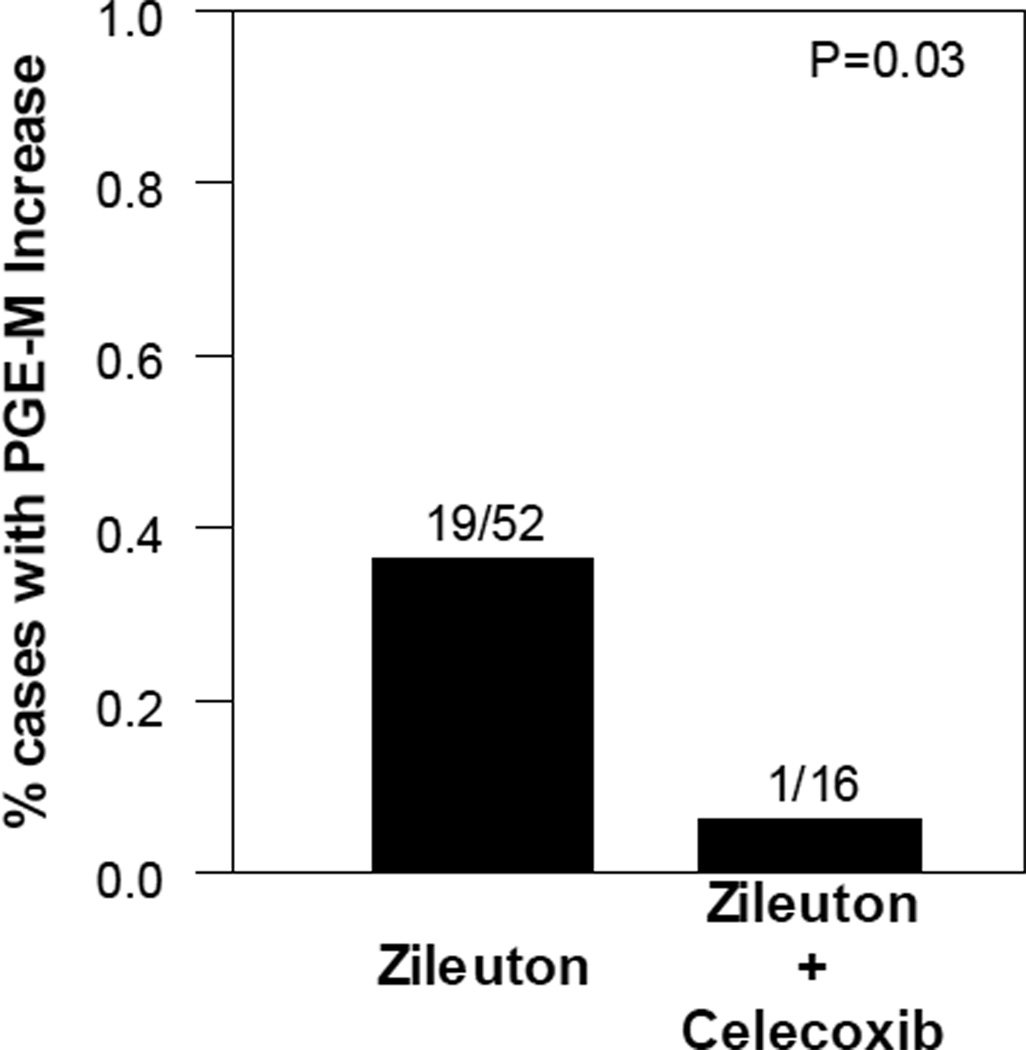
Celecoxib protects against an increase in urinary PGE-M in a subset of zileuton-treated subjects
As mentioned above, arachidonic acid is a substrate for both the COX and 5-LO pathways (Fig. 1). In theory, zileuton, a 5-LO inhibitor, might divert arachidonic acid into the COX pathway resulting in elevated levels of urinary PGE-M in a subset of subjects. Overall zileuton led to small decrease in urinary PGE-M levels (n=52; Fig. 3A). However, a subset of subjects had increased levels of PGE-M following treatment where the median (range) of the increase was 3.4 (0.13–17.1) ng/mg Cr. Additionally, the proportion of subjects with increased PGE-M levels following treatment was significantly higher among those treated with zileuton alone, 37% (19/52), than that among those treated with celecoxib in combination with zileuton, 6.2% (1/16), P=0.03 (Fig. 5). Thus, the addition of celecoxib to zileuton protected against the increase in urinary PGE-M. Treatment with celecoxib has been reported to increase levels of urinary LTE4 (26). Hence, we hypothesized that the addition of zileuton to celecoxib would protect against this effect. Levels of LTE4 were quantified at baseline and following treatment with the combination of celecoxib and zileuton. Interestingly, LTE4 levels increased in only 1 of 18 subjects following treatment with this combination regimen.
Figure 5. Celecoxib protects against an increase in urinary PGE-M in a subset of zileuton treated subjects.
Proportion of cases with a post-treatment increase in urinary PGE-M levels was significantly smaller in those treated with zileuton and celecoxib combined therapy compared to those treated with zileuton alone [6% (1/16) vs. 37% (19/52); P=0.03, Fisher’s exact test].
Safety and tolerability
Both zileuton and the combination of zileuton and celecoxib were well tolerated with no reports of serious adverse events and no statistically significant differences between the adverse event profiles of the two treatment arms. One grade 3 adverse event (sore throat), which was felt to be unlikely to be related to study drug, occurred in the zileuton arm. Thirteen adverse events that were deemed to be potentially related to drug treatment were reported in the two arms (nine in the zileuton arm and 4 in the combination arm). One grade 2 headache occurred in the zileuton arm. All other reported adverse events were grade 1 in severity and included abdominal pain, diarrhea, nausea, dizziness, fatigue, headache, increased lacrimation, and postnasal drip.
Discussion
Numerous studies have suggested a role for both COX-2 and 5-LO in inflammation and carcinogenesis (9, 14, 18–24, 38–40). Previously, we carried out a study in human subjects and demonstrated that celecoxib could redirect arachidonic acid into the 5-LO pathway resulting in enhanced urinary LTE4 levels (26). In theory, both the anti-inflammatory and chemopreventive properties of celecoxib may be compromised in cells and tissues in which arachidonic acid is shunted into the 5-LO pathway. Therefore, co-administration of an inhibitor of 5-LO, which inhibits the synthesis of leukotrienes, may increase the utility of a COX-2 inhibitor in conditions in which this type of shunt occurs.
In the current study, we compared the effects of zileuton vs. the combination of zileuton and celecoxib in smokers. As predicted, zileuton was an effective inhibitor of 5-LO and markedly suppressed urinary LTE4 levels (Fig. 4A). However, the effects of zileuton on urinary PGE-M were more complex. Overall, zileuton led to a small but statistically significant decrease in urinary PGE-M levels (Fig. 3A). This finding fits with prior evidence that zileuton suppressed PGE2 levels in bronchoalveolar fluid following exposure to an allergen (41). Recently, zileuton was reported to inhibit the translocation of phospholipase A2 to cellular membranes resulting in decreased arachidonic acid release, which can explain the small decrease in urinary PGE-M that we observed (42). Alternatively, if zileuton had an anti-inflammatory effect, the small decrease in PGE-M might be explained by a decrease in the number of inflammatory cells in the lungs of smokers. Originally, we had hypothesized that zileuton might shunt arachidonic acid into the COX pathway resulting in increased levels of urinary PGE-M in a subset of patients. Although treatment with zileuton led to an overall reduction in urinary PGE-M levels, use of zileuton was associated with increased urinary PGE-M levels in 19 of 52 subjects, thereby suggesting that a shunt may occur in a minority of individuals (Fig. 5). Future studies are warranted to elucidate the mechanistic basis for this heterogeneous effect. Neither imaging nor pulmonary function tests were carried out in this study. Hence, one possible contributor to the observed heterogeneity could be variability in the severity of underlying lung disease. Additional possibilities include genetic differences in key enzymes involved in arachidonic acid metabolism (e.g., SNPs) as well as effects of bioactive food components on these pathways. For instance, Norris and Dennis recently reported the effects of omega-3 fatty acids on inhibition of cyclooxygenase and shunting of arachidonic acid to lipoxygenase pathways (43). This raises the possibility that various dietary constituents affecting arachidonic acid metabolism could be partially responsible for the heterogeneity seen in this study. Regardless of the underlying mechanism, the addition of celecoxib to zileuton protected against the increase in urinary PGE-M found in individuals who received zileuton alone (Fig. 5). Consistent with its known mechanism of action, the selective COX-2 inhibitor celecoxib when combined with zileuton suppressed urinary PGE-M levels (Fig. 3C) without modulating LTE4 levels (Fig. 4C). Taken together, it appears that combining a COX-2 inhibitor with a 5-LO inhibitor prevents the shunting of arachidonic acid that can be observed in a subset of individuals when either a COX-2 inhibitor or 5-LO inhibitor is used alone. Selected cytochrome P450s can also metabolize arachidonic acid to eicosanoids (44). Based on the current results, it will be of considerable interest to determine whether inhibition of the COX-2 and 5-LO pathways alters P450-mediated arachidonic acid metabolism. In theory, targeting two pathways that utilize arachidonic acid as a substrate could result in increased levels of free arachidonic acid. Increased levels of free arachidonic acid can stimulate apoptosis in model systems but whether this occurs in humans is uncertain (45).
Use of selective COX-2 inhibitors has been associated with an increased risk of cardiovascular complications (27, 28). A number of different mechanisms may be important for understanding this risk. One possibility is that selective COX-2 inhibitors block the production of cardioprotective prostacyclin without inhibiting COX-1-dependent platelet thromboxane A2 synthesis, supporting a prothrombotic mechanism (29, 46). Drug induced redirection of arachidonic acid into the 5-LO pathway could also be important for understanding the cardiovascular toxicity associated with selective COX-2 inhibitors. Both COX-2 and 5-LO are expressed in atherosclerotic plaques (47, 48). 5-LO has been linked to atherosclerosis in some mouse models (30). Recently, disruption of the 5-LO pathway was found to attenuate the proatherogenic effects of COX-2 deletion in hyperlipidemic mice (49). This finding lends further support for the idea that the cardiovascular risk associated with use of COX-2 inhibitors might be reduced by co-administering an agent that targets the 5-LO pathway. Another recent preclinical study found that treatment with a selective 5-LO inhibitor protected against the reduction of renal blood flow that was induced by COX-2 inhibition (50). In the current study, we provide clear evidence in human smokers that celecoxib and zileuton can be combined to reduce both COX-2 and 5-LO activity.
In summary, preclinical evidence suggests that targeting the 5-LO pathway may both augment the antitumor activity of COX-2 inhibition and potentially reduce the cardiovascular toxicity associated with COX-2 inhibition. The results of the current study highlight the feasibility of using a 5-LO inhibitor in combination with a selective COX-2 inhibitor to inhibit the activities of both proinflammatory pathways. Future studies will be required to determine whether inhibitors of the 5-LO pathway can either increase the chemopreventive activity or decrease the cardiovascular risk associated with use of COX-2 inhibitors. This strategy could be relevant in both former and current smokers (40).
Acknowledgements
We would like to thank Judy Smith, Lauren Tyrell and Lana Vornik for help in the conduct of the trial.
Grant Support
This work was supported by NIH grants UL1-RR024996, T32 CA09685 and NIH contract N01-CN-35159
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare
References
- 1.Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messer K, Trinidad DR, Al-Delaimy WK, Pierce JP. Smoking cessation rates in the United States: a comparison of young adult and older smokers. Am J Public Health. 2008;98:317–322. doi: 10.2105/AJPH.2007.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith WL, DeWitt DL. Biochemistry of prostaglandin endoperoxide H synthase-1 and synthase-2 and their differential susceptibility to nonsteroidal anti-inflammatory drugs. Semin Nephrol. 1995;15:179–194. [PubMed] [Google Scholar]
- 4.DuBois RN, Awad J, Morrow J, Roberts LJ, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-α and phorbol ester. J Clin Invest. 1994;93:493–498. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W, Reinmuth N, Stoeltzing O, Parikh AA, Tellez C, Williams S, et al. Cyclooxygenase-2 is up-regulated by interleukin-1β in human colorectal cancer cells via multiple signaling pathways. Cancer Res. 2003;63:3632–3636. [PubMed] [Google Scholar]
- 6.Moraitis D, Du B, De Lorenzo MS, Boyle JO, Weksler BB, Cohen EG, et al. Levels of cyclooxygense-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor and its ligands. Cancer Res. 2005;65:664–670. [PubMed] [Google Scholar]
- 7.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 8.Jakobsson P-J, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal glutathione-dependent, inducible enzyme constituting a potential novel drug target. Proc Natl Acad Sci USA. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 10.Hamberg M, Samuelsson B. On the metabolism of prostaglandins E1 and E2 in man. J Biol Chem. 1971;246:6713–6721. [PubMed] [Google Scholar]
- 11.Seyberth HW, Sweetman BJ, Frolich JC, Oates JA. Quantifications of the major urinary metabolite of the E prostaglandins by mass spectrometry: evaluation of the method's application to clinical studies. Prostaglandins. 1976;11:381–397. doi: 10.1016/0090-6980(76)90160-x. [DOI] [PubMed] [Google Scholar]
- 12.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, Johnson DH, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Peters-Golden M, Henderson WR. Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 14.De Caterina R, Zampolli A. From asthma to atherosclerosis—5-lipoxygenase, leukotrienes, and inflammation. N Engl J Med. 2004;350:4–7. doi: 10.1056/NEJMp038190. [DOI] [PubMed] [Google Scholar]
- 15.Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med Res Rev. 2007;27:469–527. doi: 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- 16.Westcott JY, Voelkel NF, Jones K, Wenzel SE. Inactivation of leukotriene C4 in the airways and subsequent urinary leukotriene E4 excretion in normal and asthmatic subjects. Am Rev Respir Dis. 1993;148:1244–1251. doi: 10.1164/ajrccm/148.5.1244. [DOI] [PubMed] [Google Scholar]
- 17.Kumlin M. Measurement of leukotrienes in humans. Am J Respir Crit Care Med. 2000;161:S102–S106. doi: 10.1164/ajrccm.161.supplement_1.ltta-20. [DOI] [PubMed] [Google Scholar]
- 18.Howe LR, Subbaramaiah K, Patel J, Masferrer JL, Deora A, Hudis C, et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62:5405–5407. [PubMed] [Google Scholar]
- 19.Rioux N, Castonguay A. Inhibitors of lipoxygenase: a new class of cancer chemopreventive agents. Carcinogenesis. 1998;19:1393–1400. doi: 10.1093/carcin/19.8.1393. [DOI] [PubMed] [Google Scholar]
- 20.Ye YN, Wu WKK, Shin VY, Bruce IC, Wong BCY, Cho CH. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 21.Cianchi F, Cortesini C, Magnelli L, Fanti E, Papucci L, Schiavone N, et al. Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol Cancer Ther. 2006;5:2716–2726. doi: 10.1158/1535-7163.MCT-06-0318. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Wang S, Wu N, Sood S, Wang P, Jin Z, et al. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. Clin Cancer Res. 2004;10:6703–6709. doi: 10.1158/1078-0432.CCR-04-0838. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Sood S, Wang S, Fang M, Wang P, Sun Z, et al. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clin Cancer Res. 2005;11:2089–2096. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- 24.Fegn L, Wang Z. Topical chemoprevention of skin cancer in mice, using combined inhibitors of 5-lipoxygenase and cyclo-oxygenase-2. J Laryngol Otol. 2009;123:880–884. doi: 10.1017/S0022215109004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao JT, Tsu IH, Dubinett SM, Adams B, Sarafian T, Baratelli F, et al. Modulation of pulmonary leukotriene B4 production by cyclooxygenase-2 inhibitors and lipopolysaccharide. Clin Cancer Res. 2004;10:6872–6878. doi: 10.1158/1078-0432.CCR-04-0945. [DOI] [PubMed] [Google Scholar]
- 26.Duffield-Lillico AJ, Boyle JO, Zhou XK, Ghosh A, Butala GS, Subbaramaiah K, et al. Levels of prostaglandin E metabolite and leukotriene E4 are increased in the urine of smokers: evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev Res. 2009;2:322–329. doi: 10.1158/1940-6207.CAPR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 28.Solomon SC, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, et al. Cardiovascular risk of celecoxib in 6 randomized placebo controlled trials: the cross trial safety analysis. Circulation. 2008;117:2104–2113. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grosser T, Fries S, Fitzgerald GA. Biologic basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehrabian M, Allayee H, Wong J, Shi W, Wang XP, Shaposhnik Z, et al. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–126. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 31.Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 32.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 33.Hakonarson H, Thorvaldsson S, Helgadottir A, Gudbjartsson D, Zink F, Andresdottir M, et al. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction. JAMA. 2005;293:2245–2256. doi: 10.1001/jama.293.18.2245. [DOI] [PubMed] [Google Scholar]
- 34.Marnett LJ. Mechanisms of cyclooxygenase-2 inhibition and cardiovascular side effects-the plot thickens. Cancer Prev Res. 2009;2:288–290. doi: 10.1158/1940-6207.CAPR-09-0033. [DOI] [PubMed] [Google Scholar]
- 35.Taber DF, Teng D. Total synthesis of the ethyl ester of the major urinary metabolite of prostaglandin E(2) J Org Chem. 2002;67:1607–1612. doi: 10.1021/jo011017i. [DOI] [PubMed] [Google Scholar]
- 36.Sterz K, Scherer G, Ecker JA. Combination therapies that inhibit cyclooxygenase-2 and leukotriene synthesis prevent disease in murine collagen induced arthritis. Lipid Res. 2012;53:1026–1036. doi: 10.1007/s00011-009-8149-3. [DOI] [PubMed] [Google Scholar]
- 37.Mohebati A, Knutson A, Zhou XK, Smith JJ, Brown PH, Dannenberg AJ, et al. A web-based screening and accrual strategy for a cancer prevention clinical trial in healthy smokers. Contemp Clin Trials. 2012;33:942–948. doi: 10.1016/j.cct.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson GD, Keys KL, DeCiechi PA, Masferrer JL. Combination therapies that inhibit cyclooxygenase-2 and leukotriene synthesis prevent disease in murine collagen induced arthritis. Inflamm Res. 2009;58:109–117. doi: 10.1007/s00011-009-8149-3. [DOI] [PubMed] [Google Scholar]
- 39.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 40.Mao JT, Roth MD, Fishbein MC, Aberle DR, Zhang ZF, Rao JY, et al. Lung cancer chemoprevention with celecoxib in former smokers. Cancer Prev Res. 2011;4:984–993. doi: 10.1158/1940-6207.CAPR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kane GC, Tollino M, Pollice M, Kim CJ, Cohn J, Murray JJ, et al. Insights into IgE-mediated lung inflammation derived from a study employed a 5-lipoxygenase inhibitor. Prostaglandins. 1995;50:1–18. doi: 10.1016/0090-6980(95)00088-r. [DOI] [PubMed] [Google Scholar]
- 42.Rossi A, Pergola C, Koeberle A, Hoffmann M, Dehm F, Bramanti P, et al. The 5-lipoxygenase inhibitor, zileuton, suppresses prostaglandin biosynthesis by inhibition of arachidonic acid release in macrophages. Br J Pharmacol. 2010;161:555–570. doi: 10.1111/j.1476-5381.2010.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci USA. 2012;109:8517–8522. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theken KN, Schuck RN, Edin ML, Tran B, Ellis K, Bass A, et al. Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis. 2012;222:530–536. doi: 10.1016/j.atherosclerosis.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA. 2000;97:11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 47.Schonbeck U, Sukhova GK, Graber P, Coulter S, Libby P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am J Pathol. 1999;155:1281–1291. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, et al. Expanding expression of the 5-lipoxygenase pathway with the arterial wall during human atherogenesis. Proc Natl Acad Sci USA. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Z, Crichton I, Tang SY, Hui Y, Ricciotti E, Levin MD, et al. Disruption of the 5-lipoxygenase pathway attenuates atherogenesis consequent to COX-2 deletion in mice. Proc Natl Acad Sci USA. 2012;109:6727–6732. doi: 10.1073/pnas.1115313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salazar F, Salazar FJ, Saez F, Reverte V, Zweifel B, Dufield D, et al. Leukotrienes, but not angiotensin II, are involved in the renal effects elicited by the prolonged COX-2 inhibition when sodium intake is low. Cardiovasc Pharmacol. 2013;61:329–336. doi: 10.1097/FJC.0b013e31828399ae. [DOI] [PubMed] [Google Scholar]