Abstract
Background
It is unknown whether the spleen size correlates with disease severity and outcomes in patients with idiopathic and heritable pulmonary arterial hypertension (PAH).
Objectives
To determine the prevalence of splenomegaly in PAH and assess whether it correlates with severity of disease and outcomes.
Methods
We identified subjects with either heritable or idiopathic PAH who had Doppler echocardiography, right heart catheterization (RHC) and computed tomography (CT) of the chest and/or abdomen that included the spleen.
Results
We included 62 subjects with mean age (±SD) of 49 (±15) years, 82% women. Spleen dimensions were 10 (± 3) cm, 6 (± 2) cm, 9 (± 2) cm for the for the cranio-caudal length, thickness and width measurements, respectively. The median (interquartile (IQR)) spleen volume was 344 (225–533) cm3. Splenomegaly was observed in 52 to 63 % of the patients, depending on the formula used. The spleen volume was not associated with clinical, echocardiographic or hemodynamic variables. Spleen volume was not associated with adjusted mortality.
We studied the characteristics of the spleen during autopsy in nine patients with idiopathic PAH who died of right heart failure. The mean (IQR) spleen weight was 220 (151–325) g. We observed early congestion in all but two patients who had chronic congestion.
Conclusions
Splenomegaly of predominantly mild degree is common in idiopathic and heritable PAH. However, spleen size was not associated with clinical, echocardiographic, hemodynamic and survival data in these patients.
Keywords: Pulmonary arterial hypertension, Splenomegaly, Spleen, Outcome Assessment
Introduction
Pulmonary arterial hypertension (PAH) is a disease characterized by a restrictive flow through the pulmonary circulation that can lead to right heart failure and death.1 It involves a variety of diseases included in group I of the 4th World Symposium on pulmonary hypertension (PH) updated in 20082. Pulmonary arterial hypertension is hemodynamically characterized by a mean pulmonary artery pressure of ≥ 25 mm Hg with pulmonary vascular resistance (PVR) ≥ 3 Wood Units and pulmonary arterial occlusion pressure (PAOP) ≤ 15 mm Hg1. When the right ventricular (RV) compensatory response to the increased PVR is overwhelmed, right heart failure ensues. Clinical manifestations of right heart failure include symptoms and signs resulting from venous congestion and low cardiac output3. Characteristic physical signs related to venous congestion include hepatomegaly, ascites and peripheral edema4–5. Splenomegaly has been associated with right heart failure6, but it is usually not described as part of the signs observed in patients with advanced PAH7–8.
It is unknown whether patients with idiopathic or heritable PAH have abnormal spleen volume and whether its volume correlates with clinical, echocardiographic and hemodynamic parameters or outcomes. We hypothesized that patients with idiopathic and heritable PAH have splenomegaly which may correlate with severity of disease and outcomes. We evaluated whether assessing the spleen volume in the CT of the chest could provide valuable prognostic information in these patients.
Methods
a) Study design and inclusion criteria
This study was approved by the Cleveland Clinic Institutional Review Board (protocol number: 10-1127). Informed consent was waived. We identified subjects using the Cleveland Clinic Pulmonary Hypertension Registry. We selected patients with PAOP ≤ 15 mm Hg, PVR ≥ 3 Wood Units, forced expiratory volume in one second over forced vital capacity (FEV1/FVC) ≥ 0.6, and total lung capacity ≥ 60%. Of those, we only included patients with either idiopathic or heritable PAH (n=140)2 who had a CT of the chest (including the spleen) or abdomen between 1994 and 2010.
Each patient underwent a careful selection process to exclude other etiologies of pulmonary hypertension. We excluded all patients in whom at least two PH physicians were not in complete agreement about the diagnosis of idiopathic or heritable PAH. Except for one patient who had splenectomy and was excluded, no other patient had any medical condition linked to changes in the spleen volume.
b) Measurements and calculations
We selected the CTs of the chest and abdomen that incorporated the spleen in its entirety and were performed closest in time to any right heart catheterization (RHC) performed at our institution. In the case of several studies we selected the first one. Studies were obtained with commercially available single and four slice CT scanners using 5mm slice thickness at 4 mm intervals before 2003. Since 2004, studies were obtained initially with 16- and subsequently with 64-detector CT scanners using a 5-mm, 3mm and 1mm section thicknesses at 2.5, 1.5 and 1 mm intervals, respectively. Two radiologists (RY, AG) reviewed all the CTs performed in these patients.
We measured the splenic length, width and thickness in all patients. The length was obtained by multiplying the number of sections where the spleen was visualized by the thickness of the cuts. We determined splenic width as the largest diameter of the spleen on any transaxial slice and we measured maximum splenic thickness on any section, as the distance between the inner and outer borders of the spleen on a plane perpendicular to the splenic width (Figure 1). We calculated the spleen volume using the formula described by Prassopoulos et al.9 [(0.58 × splenic length × splenic width × splenic thickness) + 30] and the one used to calculate the volume of a prolate ellipsoid (0.524 × splenic length × splenic width × splenic thickness).10 Splenic index is the multiplication of the 3 splenic measurements without the incorporation of a correction factor.
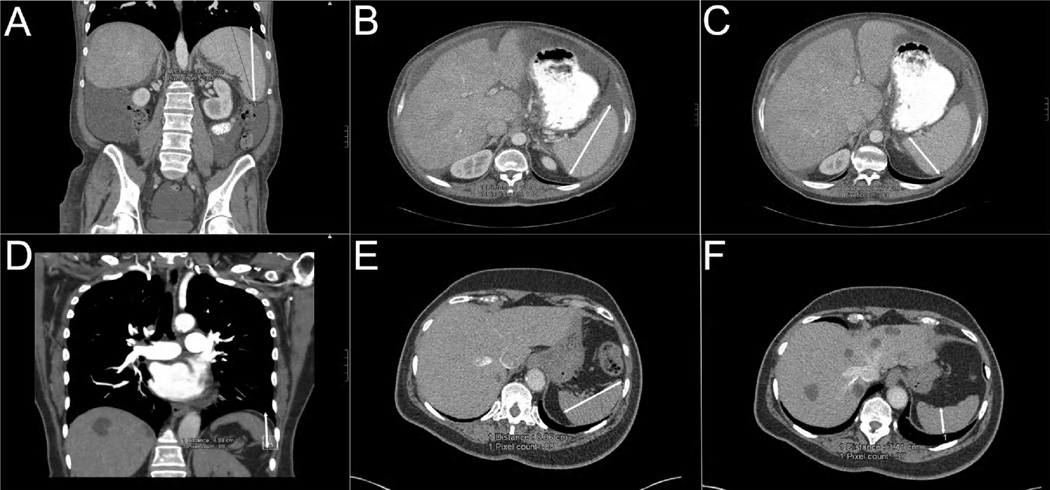
Figure 1. Splenomegaly and hyposplenia in patients with idiopathic and heritable PAH.
Panel A, B and C: splenic length, width and thickness, respectively, in a patient with splenomegaly. Panel D, E, F: splenic length, width and thickness, respectively, in a subject with hyposplenia. White double-headed arrows mark the splenic measurements.
Splenomegaly was considered if the calculated splenic volume exceeded the 95% limit for spleen size by height and weight, as described by Sprogøe-Jakobsen et al.11 Similarly we defined hyposplenia when the calculated splenic volume was less than the 5% limit for spleen size by height and weight11. Normal spleen size was considered present when the splenic volume was contained in the 5–95% limit interval.
Right heart catheterization data was obtained from studies done as part of clinical evaluation. The procedures were performed in the standard manner in the cardiac catheterization laboratory using a 7 F pulmonary artery catheter. Pressure measurements were obtained from paper recordings at end-expiration. Cardiac output (CO) was obtained by the thermodilution method and transpulmonary gradient (mean PAP – pulmonary artery occlusion pressure) and pulmonary vascular resistance (transpulmonary gradient / CO) were calculated.
In addition, we reviewed the transthoracic Doppler echocardiogram performed the closest to the CT-RHC pair included in the analysis. Experienced operators, blinded to the results of the CT chest / abdomen, reviewed the echocardiograms and assessed right ventricular size and function subjectively. Six-minute walk test was performed following American Thoracic Society (ATS) guidelines12. Predicted six-minute walk distance was estimated using formulae suggested by Enright et al13. Plasma brain natriuretic peptide was measured at the time of right heart catheterization in 40 patients.
In order to support our findings, we also reviewed the reports of all autopsies performed at the Cleveland Clinic from 1990 to 2010 that included the phrases “primary pulmonary arterial hypertension”, “idiopathic pulmonary arterial hypertension” and “plexiform lesions” in any form within the text. Only the patients with idiopathic or heritable PAH who died of right heart failure were included to ensure the absence of any other condition that could result in alteration of the spleen weight. We recorded the age at the time of death, gender, height, weight, heart weight, right and left ventricular heart thickness, presence and amount of pericardial / pleural effusions and ascites, and the liver and spleen weights and characteristics.
Statistics
Means and standard deviations (SD) are provided for all continuous variables when normally distributed and median (interquartile range (IQR)) when not. Two-group comparisons were performed by Welch’s t-test or Mann-Whitney U-test when appropriate. Categorical data were compared using Fischer’s exact test. We used Pearson’s correlation and linear regression analysis (adjusted for age, gender, height and weight)14 to compare splenic volume with other continuous variables. Non linear relationships were assessed by fitting a LOWESS (locally weighted scatterplot smoothing) line. Survival was assessed by Kaplan-Meier methodology. Time 0 was the date at the time of the CT chest / abdomen, and the end of follow-up was December, 2010 or the time of the recipient’s death (or transplantation, when a combined endpoint was used). Survival analysis was also performed with Cox proportional hazards modeling, using spleen volume and other factors as covariates, adjusted by age and gender, height and weight. Hazard ratios (HR) and the corresponding 95% confidence interval (95% CI) are shown. At Cleveland Clinic patients are transplanted using criteria established by the International Society for Heart and Lung Transplantation15. Death of the study participants was ascertained by reviewing our records and querying the U.S. Social Security Death Index. All p values reported are two-tailed. A p value of < 0.05 was considered significant. The statistical analyses were performed using the statistical package IBM SPSS, version 20 (IBM; IBM; Armonk, New York) and R statistical software, version 2.13.0 (www.r-project.org).
Results
Patient characteristics
We included 62 unique subjects (82% women) with mean age (±SD) of 49 (± 15) years. Fifty-four had idiopathic and eight had heritable PAH. Race was Caucasian in 81 %, African-American in 15 % and others in 4 %. New York Heart Association (NYHA) functional class was II, III and IV in 36, 48 and 16 % of the patients, respectively. Mean (SD) height was 1.62 (± 8.5) m, weight 77 (± 23) kg and BMI 29 (± 8) kg/m2. Six patients (10 %) were receiving PAH-targeted therapy at the time of the CT chest / abdomen, three patients sildenafil and three bosentan. Brain natriuretic peptide was 424 (± 592) pg/mL and the six-minute walk distance was 327 (± 104) m or 58.5 (± 17) % of predicted13. Hemodynamic and echocardiographic characteristics are shown in table 1 and 2, respectively.
Table 1.
Hemodynamic parameters in all patients, with or without splenomegaly.
| All patients (n=62) Mean ± SD |
No splenomegaly (n=22) Mean ± SD |
Splenomegaly (n=39) Mean ± SD |
P (Welch’s t- test) |
|
|---|---|---|---|---|
| RA pressure (mmHg) | 12 ± 7 | 10 ± 5 | 13 ± 7 | 0.08 |
| RV systolic pressure (mmHg) | 83 ± 22 | 85 ± 20 | 82 ± 23 | 0.6 |
| RV diastolic pressure (mmHg) | 14 ± 10 | 13 ± 7 | 15 ± 11 | 0.39 |
| PA systolic pressure (mmHg) | 86 ± 21 | 87 ± 21 | 85 ± 22 | 0.75 |
| PA diastolic pressure (mmHg) | 37 ± 11 | 39 ± 12 | 37 ± 11 | 0.61 |
| PA mean pressure (mmHg) | 54 ± 14 | 55 ± 14 | 53 ± 14 | 0.66 |
| PAOP (mmHg) | 10 ± 4 | 10 ± 4 | 10 ± 4 | 0.96 |
| CO (L/min) | 4.1 ± 1.5 | 3.8 ± 1.4 | 4.2 ± 1.5 | 0.28 |
| CI (L/min/m2) | 2.2 ± 0.8 | 2 ± 0.6 | 2.3 ± 0.8 | 0.13 |
| PVR (Wood Units) | 12 ± 7 | 13 ± 7 | 12 ± 7 | 0.85 |
| TPG (mm Hg) | 43 ± 14 | 44 ± 14 | 43 ± 14 | 0.8 |
CI indicates cardiac index; CO, cardiac output; PA, pulmonary artery; PAOP, pulmonary artery occlusion pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV, right ventricular; TPG, transpulmonary gradient. Splenomegaly was defined by a volume (using Prassopoulos et al.9 formula) higher than 95% limit according to the expected spleen volume by height and weight described by Sprogøe-Jakobsen et al.11.
Table 2.
Echocardiographic parameters in all patients, with or without splenomegaly.
| All patients (n=62) n (%) |
No splenomegaly (n=22)* n (%) |
Splenomegaly (n=39) n (%) |
p (Fisher’s exact test) |
|
|---|---|---|---|---|
| RV dilation | ||||
| Absent | 7 (11) | 1 (4) | 6 (15) | |
| Mild | 4 (6) | 2 (9) | 2 (5) | 0.59 |
| Moderate | 24 (39) | 10 (46) | 14 (36) | |
| Severe | 27 (44) | 9 (41) | 17 (44) | |
| RV dysfunction | ||||
| Absent | 8 (13) | 1 (4) | 7 (18) | |
| Mild | 3 (5) | 3 (14) | 0 (0) | 0.08 |
| Moderate | 29 (47) | 11 (50) | 18 (46) | |
| Severe | 22 (35) | 7 (32) | 14 (36) | |
One patient with hyposplenia was removed from the subgroup analysis.
Indications for computed tomography
CT chest / abdomen was performed with a median (IQR) difference from the RHC of 0 (0–6) months. A total of 53 (86 %) CTs were of the chest and the rest of the abdomen (9 patients, 14 %). Computed tomographies were done for further evaluation of lung parenchyma (47 %), dyspnea (19 %), abdominal distension (9 %), pre- lung transplant evaluation (7 %), lung nodule assessment (7 %), and other reasons (11 %) such as research, chest pain, and follow up of ovarian carcinoma.
Computed tomography measurements
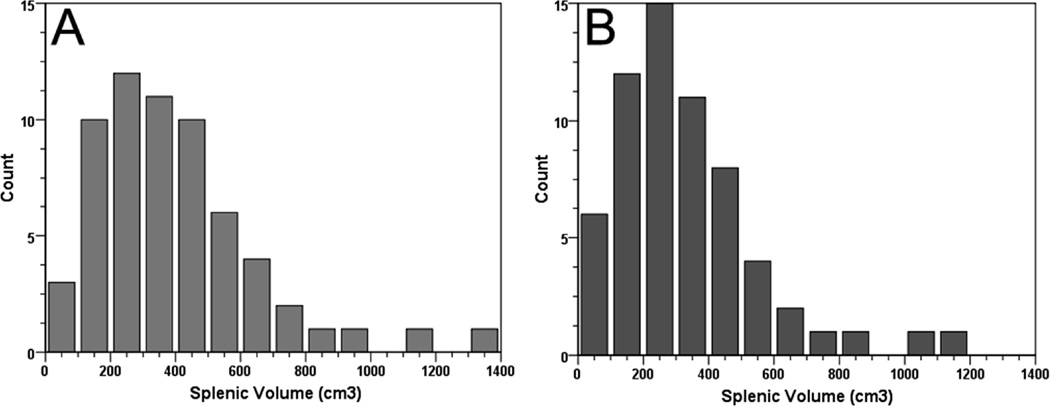
The aorta and pulmonary artery diameters were 3 (± 0.5) and 3.4 (± 0.5) cm, respectively; with a ratio of aorta / pulmonary artery diameter of 1.2 (± 0.2). Spleen dimensions were 10 (± 3) cm, 6 (± 2) cm, 9 (± 2) cm for the cranio-caudal, thickness and width measurements, respectively. The median (IQR) splenic index was 541 (337–868) cm3. Sixty percent of patients had a splenic index above the upper limit of the normal range (480 cm3).16–17 The median (IQR) splenic volumes measured by Prassopoulos et al.9 and prolate ellipsoid volume10 formulae were 344 (225–533) and 283 (176–455) cm3, respectively (Figure 2).
Figure 2. Histogram of splenic volumes in idiopathic and heritable PAH.
Panel A: Histogram of splenic volumes using the Prassopoulos et al.9 formula. Panel B: Histogram of splenic volumes utilizing the formula for calculation volume of prolate ellipsoid10.
The splenic volume measured by Prassopoulos et al.9 formula was 339 (222–291) cm3 and 378 (279–553) cm3 in patients that underwent CT of the chest and abdomen, respectively (p = 0.39). The spleen size was directly associated with weight (R=0.43, p=0.001) but not with age and height. The splenic size was higher in males (461 (339–673) cm3) than females (333 (218–491) cm3) (p=0.021 with Mann-Whitney test). In multivariate analysis that included weight and gender, only weight was significantly associated with spleen size (p=0.027).
Using the table for spleen volume suggested by Sprogøe-Jakobsen et al11, splenomegaly was observed in 63 % of the patients using the formula to calculate volume of Prassopoulos et al.9 and 51.6 % of the individuals by applying the formula to calculate the volume of a prolate ellipsoid10. Only one patient (1.6%) had hyposplenia (below 5% limit for spleen size CI) by the formula described by Prassopoulos et al.9 and two (3.2%) by the formula used to determine the volume of a prolate ellipsoid10 (Table 3).
Table 3.
| Formulae for splenic volume |
Prassopoulos et al.9 n (%) |
Prolate ellipsoid volume10 n (%) |
|---|---|---|
| Splenomegaly* | 39 (63 %) | 32 (52 %) |
| Normal spleen size* | 22 (35 %) | 28 (45 %) |
| Hyposplenia* | 1 (2 %) | 2 (3 %) |
See methods for definition of these terms.
When using a spleen volume to define splenomegaly of 314.5 cm3, the formula by Prasspoulous et al9 revealed that 36 patients (58 %) had splenomegaly. Using the upper 95 % limit (334 cm3) from the normal spleen volume proposed by Henderson et al.18, we observed that 34 (55%) patients had splenomegaly. If only craniocaudal splenic length is used, splenomegaly (craniocaudal length ≥ 10 cm)19 was observed in 22 patients (36.1 %).
Correlation of computed tomography findings
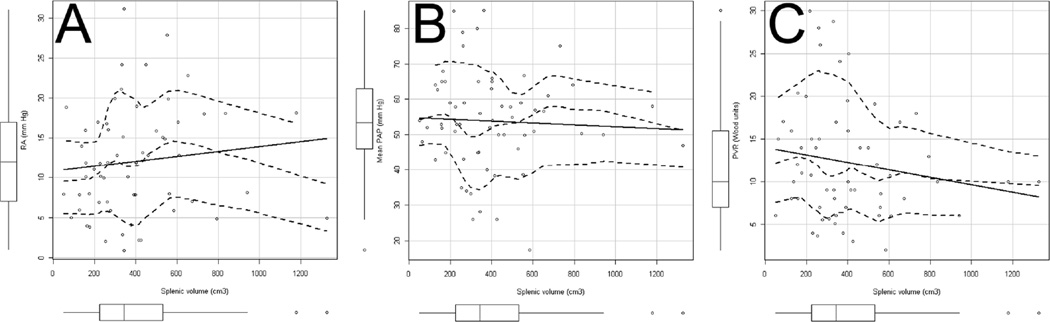
We did not observed a significant association between spleen volume obtained by the formula described by Prassopoulos et al.9 and either pulmonary artery diameter (R=0.24, p=0.09) or aortic / pulmonary ratio (p=0.97). Similarly, no significant associations were observed between spleen volume and NYHA functional class, laboratory data (brain natriuretic peptide serum levels, hemoglobin, white blood cells, platelets, sodium, creatinine, BUN, albumin, bilirubin, alkaline phosphatase and alanine aminotransferase), pulse oximeter oxygen saturation (SpO2) at rest on RA, six-minute walk distance (in meters or % of predicted), echocardiographic (left ventricular ejection fraction, tricuspid jet velocity, degree of RV dilation or dysfunction) or hemodynamic parameters (Figure 3). Lack of association was also observed for all these variables when splenic volume was adjusted for age, height, weight and gender. Aspartate aminotransferase was significantly associated with splenic volume (R=−0.33, p=0.026), although this association disappeared when adjusting for other factors (p=0.09).
Figure 3. Scatter plots with linear regression and LOWESS line.
All panels have boxplots corresponding to the variable shown in the × and y axis. Panel A, B and C show scatter plots of right atrial (RA) pressure, mean pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR) versus splenic volume. All scatter plots have linear regression (solid) and LOWESS nonparametric regression line (middle broken line) with 95-percent confidence envelopes around the fit (external broken lines). All relationships were non-significant (p > 0.05).
Survival analysis
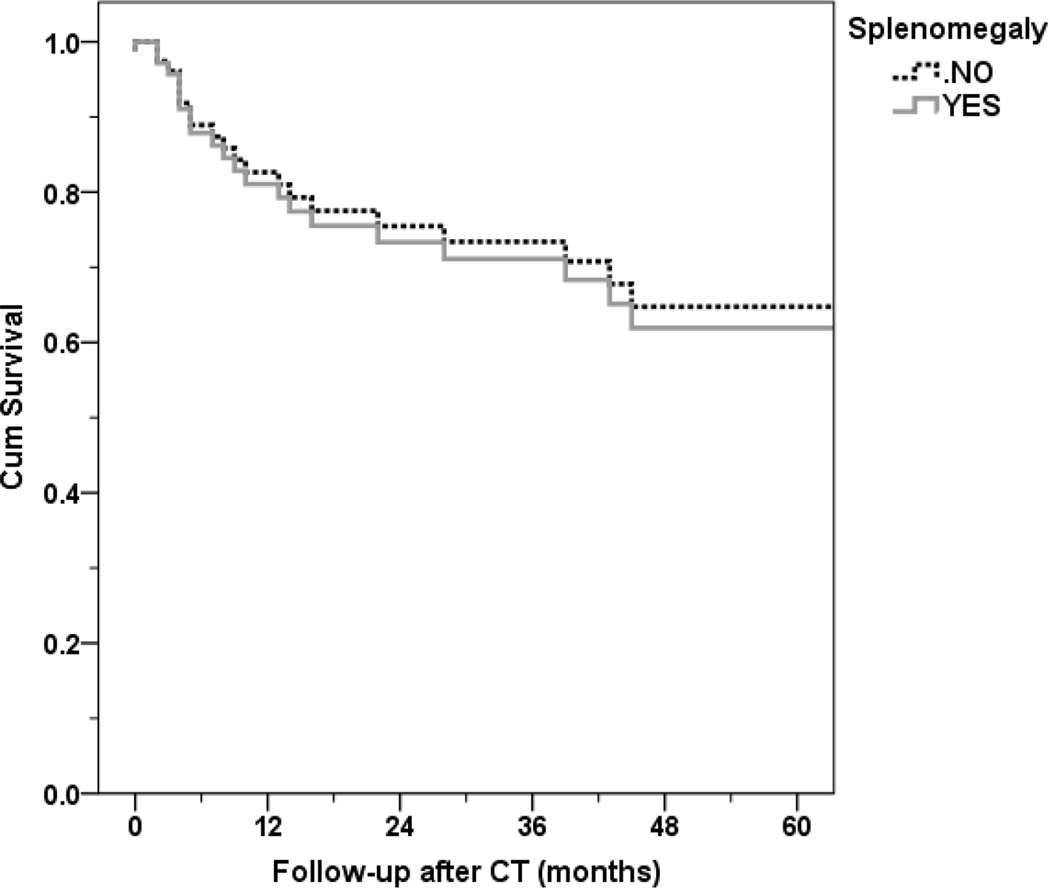
Survival at one, two and three years was 89.7 %, 73.1 % and 64.3 %, respectively. When survival was adjusted for age, gender, height and weight, we did not find a significant effect of splenic volume as a continuous variable (hazard ratio (HR): 1 (95% CI: 0.997–1.002), p = 0.74) (Figure 4). Similarly, no survival difference was noted when the binary variable splenomegaly (defined by Sprogøe-Jakobsen et al11 table) was included in the model, adjusted for age, gender, weight, height and (HR: 0.91 (95% CI: 0.36–2.3), p=0.84).
Figure 4. Cox survival analysis in patients with or without splenomegaly.
Splenomegaly defined by Sprogøe-Jakobsen et al11 table. No statistical survival difference was observed between the groups of patient with or without splenomegaly, adjusted for age, gender, height and weight (p=0.84).
Autopsy findings from patients with idiopathic PAH
We identified the autopsies of 9 patients with idiopathic PAH patients who died of right heart failure. None of these 9 patients had a CT of the chest or abdomen close to the RHC to be included in the main analysis. The mean (± SD) age of these patients was 44 (± 20) years and 67 % of them were females. Mean (± SD) height and weight were 161 (± 9) cm and 69 (± 11) kg, respectively. On the autopsies, the heart, left and right lung weights were 490 g (IQR: 360–605), 650 g (IQR: 400–770) and 570 g (IQR: 338–725), respectively. Pericardial and pleural effusions of at least mild degree were noted in three and six cases, respectively and ascites in six. The liver weight was 1650 g (IQR: 1475–1950) and the spleen weight was 220 g (IQR: 151–325). Marked RV hypertrophy (0.8 cm (IQR: 0.6–1.1) and dilation was noted in all cases, as well as plexogenic pulmonary hypertensive arteriopathy on microscopic evaluation and some degree of hepatic congestion. Splenic congestion was seen in all cases, two spleens revealed fibrosis of the capsule (Figure 5).
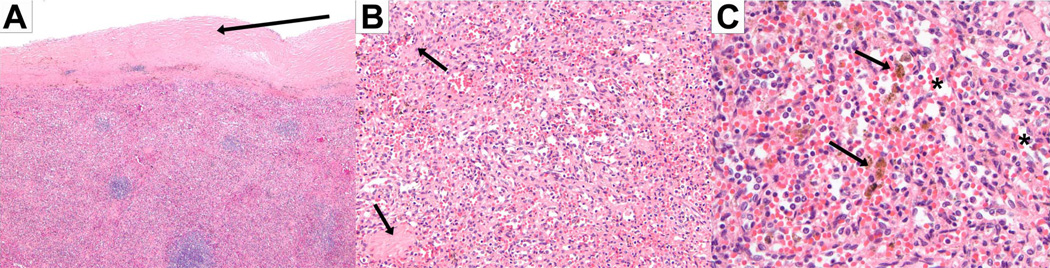
Figure 5. Pathology examination of spleen in idiopathic and heritable PAH.
Chronic congestive splenomegaly showing thickened and fibrous capsule with hemosiderin deposition (a, hematoxylin and eosin 4×), fibrotic and cellular red pulp (b, hematoxylin and eosin 20×), dilated sinusoids (c, hematoxylin and eosin 20×) and hemosiderin deposition from episodes of old hemorrhage (4, hematoxylin and eosin 40×).
Discussion
This study demonstrates for the first time that splenomegaly, predominantly of mild degree, is common in patients with advanced idiopathic or heritable PAH. The presence of chronic congestion as the sole macro and microscopic finding in the enlarged spleens on autopsies suggests that chronic passive congestion is likely the cause of the splenomegaly. However, the spleen volume estimated by CT showed no association with clinical, echocardiographic or hemodynamic parameters or survival.
To the best of our knowledge there is no available information on the spleen size in patients with idiopathic and heritable PAH. Hepatomegaly and ascities, but not splenomegaly are frequently included as part of signs indicative of the presence of right heart failure in these group of patients.4,20 However, one of the common causes of splenomegaly is congestion due to heart failure. We hypothesized that as patients develop right heart failure as a result of longstanding PAH, some degree of splenomegaly would become evident.
We found that splenomegaly was common (52 – 63 %) in patients with advanced idiopathic and heritable PAH, irrespective of the definition used. We measured the spleen volume by using two multidimensional indices9–10, and compared the volumes obtained with the expected for height and weight as proposed by Sprogøe-Jakobsen et al11. We defined splenomegaly when splenic volumes were above the 95% CI for height and weight. This last approach was important as in our cohort, splenic volume correlated with body weight in multivariate analysis. Interestingly, we observed that the overall increase in spleen size was modest and this is in correlation with what is seen in congestive heart failure from other etiologies.6
Hyposplenia was rare, since only one or two patients, depending on the formula used, had this condition (Figure 1). Hoeper et al21 described a higher prevalence of asplenia in patients with idiopathic PAH when compared with individuals with other lung diseases who received lung transplant (11.5 % versus 0 %). Fahy et al.22 reported hyposplenia in a patient with idiopathic PAH potentially explained by splenic ischemia due to reduced cardiac output, hypoxia and polycythemia. In our study, we did not find an association between splenic size and either cardiac output or hemoglobin concentration in blood. In our cohort, the patient with hyposplenia has a cardiac index of 3.4 (L/min/m2), PVR of 6 Wood Units, hemoglobin of 13.7 g/dL and SpO2 at rest on RA of 98 %.
We were not able to detect an association (linear or non-linear) between splenic volume, either as a continuous or dichotomized variable, and NYHA functional class, laboratory data (including complete blood counts and comprehensive metabolic panel), six-minute walk distance, echocardiographic or hemodynamic parameters. Similarly, splenic volume at the time of the initial CT was not associated with survival. These findings do not support the routine measurement of spleen volume during CT performed to further patients with idiopathic or heritable PAH.
As part of the present study, we collected information from autopsies performed in patients with idiopathic or heritable PAH who died of right heart failure. We observed that the spleen weight was 220 (IQR: 151–325) g, and 55 % and 33 % of the patients had a splenic weight of ≥ 200 and ≥ 250 g, respectively (proposed cut-off points for splenomegaly).6,16,23–24 There is significant variability in the normal spleen weight or volume11; hence we used different methods to define splenomegaly. The weight of the spleen differs in-vivo from autopsy specimens due to blood loss during the process of extraction of the organ to be weighed.16–17 We also noted these differences in our study given that the in-vivo splenic weight was estimated (splenic volume × spleen specific gravity of 1.05 g / mL) at 361 (IQR: 236–560) g.
There are limitations to our study. We did not determine the splenic volume by summation-of-volumes technique (gold standard), although spleen length and the multidimensional indices that include length, width and thickness have shown excellent correlation with the gold standard technique.19,25–26 Thus we decided to use these simpler indexes that reliably estimate splenic size instead of other labor intensive and clinically impractical methodologies.19,25 We included patients (14%) in whom the splenic volume was determined in a CT of the abdomen, performed for abdominal distension. This could have potentially selected a subgroup of patients with larger spleen; however, the spleen size was similar between patients who underwent CT of the abdomen or chest and we took precautions not to include patients with any other pathology known to affect the volume of the spleen.
Conclusions
Splenomegaly of mild degree is common in patients with advanced idiopathic and heritable PAH. We did not find supportive data to routinely assess splenic volume in idiopathic or heritable PAH patients.
Acknowledgments
Funding sources: This publication was made possible by CTSA KL2 [Grant # RR024990] (A.R.T.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Disclosures: The authors have no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article. Dr G.A.H. has received a board fee from Lung Rx United Therapeutics and his institution has received grants from Gilead Sciences Research Scholars Program and from Bayer HealthCare Pharmaceuticals.
Authorship: All authors should have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Mahmud M, Champion HC. Right ventricular failure complicating heart failure: pathophysiology, significance, and management strategies. Curr Cardiol Rep. 2007;9:200–208. doi: 10.1007/BF02938351. [DOI] [PubMed] [Google Scholar]
- 4.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 5.Dresdale DT, Schultz M, Michtom RJ. Primary pulmonary hypertension. I. Clinical and hemodynamic study. Am J Med. 1951;11:686–705. doi: 10.1016/0002-9343(51)90020-4. [DOI] [PubMed] [Google Scholar]
- 6.Pozo AL, Godfrey EM, Bowles KM. Splenomegaly: investigation, diagnosis and management. Blood Rev. 2009;23:105–111. doi: 10.1016/j.blre.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 8.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Prassopoulos P, Daskalogiannaki M, Raissaki M, et al. Determination of normal splenic volume on computed tomography in relation to age, gender and body habitus. Eur Radiol. 1997;7:246–248. doi: 10.1007/s003300050145. [DOI] [PubMed] [Google Scholar]
- 10.Yetter EM, Acosta KB, Olson MC, et al. Estimating splenic volume: sonographic measurements correlated with helical CT determination. AJR Am J Roentgenol. 2003;181:1615–1620. doi: 10.2214/ajr.181.6.1811615. [DOI] [PubMed] [Google Scholar]
- 11.Sprogoe-Jakobsen S, Sprogoe-Jakobsen U. The weight of the normal spleen. Forensic Sci Int. 1997;88:215–223. doi: 10.1016/s0379-0738(97)00103-5. [DOI] [PubMed] [Google Scholar]
- 12.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 13.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 14.DeLand FH. Normal spleen size. Radiology. 1970;97:589–592. doi: 10.1148/97.3.589. [DOI] [PubMed] [Google Scholar]
- 15.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Cools L, Osteaux M, Divano L, et al. Prediction of splenic volume by a simple CT measurement: a statistical study. J Comput Assist Tomogr. 1983;7:426–430. doi: 10.1097/00004728-198306000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Strijk SP, Wagener DJ, Bogman MJ, et al. The spleen in Hodgkin disease: diagnostic value of CT. Radiology. 1985;154:753–757. doi: 10.1148/radiology.154.3.3969481. [DOI] [PubMed] [Google Scholar]
- 18.Henderson JM, Heymsfield SB, Horowitz J, et al. Measurement of liver and spleen volume by computed tomography. Assessment of reproducibility and changes found following a selective distal splenorenal shunt. Radiology. 1981;141:525–527. doi: 10.1148/radiology.141.2.6974875. [DOI] [PubMed] [Google Scholar]
- 19.Bezerra AS, D'Ippolito G, Faintuch S, et al. Determination of splenomegaly by CT: is there a place for a single measurement? AJR Am J Roentgenol. 2005;184:1510–1513. doi: 10.2214/ajr.184.5.01841510. [DOI] [PubMed] [Google Scholar]
- 20.Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Hoeper MM, Niedermeyer J, Hoffmeyer F, et al. Pulmonary hypertension after splenectomy? Ann Intern Med. 1999;130:506–509. doi: 10.7326/0003-4819-130-6-199903160-00014. [DOI] [PubMed] [Google Scholar]
- 22.Fahy G, Robinson K, Deb B, et al. Primary pulmonary hypertension and functional hyposplenism. Postgrad Med J. 1992;68:383–385. doi: 10.1136/pgmj.68.799.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers J, Segal RJ. Weight of the spleen. I. Range of normal in a nonhospital population. Arch Pathol. 1974;98:33–35. [PubMed] [Google Scholar]
- 24.Sternberg S. Histology for pathologists. Second ed. PA: Lippincott-Raven; 1997. [Google Scholar]
- 25.Rezai P, Tochetto SM, Galizia MS, et al. Splenic volume model constructed from standardized one-dimensional MDCT measurements. AJR Am J Roentgenol. 2011;196:367–372. doi: 10.2214/AJR.10.4453. [DOI] [PubMed] [Google Scholar]
- 26.Lamb PM, Lund A, Kanagasabay RR, et al. Spleen size: how well do linear ultrasound measurements correlate with three-dimensional CT volume assessments? Br J Radiol. 2002;75:573–577. doi: 10.1259/bjr.75.895.750573. [DOI] [PubMed] [Google Scholar]