Abstract
Dynein, the retrograde motor protein, is essential for the transport of cargo along axons and proximal dendrites in neurons. The dynein heavy chain mutation, Loa, has been reported to cause degeneration of spinal motor neurons, as well as defects of spinal sensory proprioceptive neurons, but cranial nerve nuclei have received little attention. Here, we examined the number and morphology of neurons in cranial nerve nuclei of young, adult and aged heterozygous Loa mice, with focus on the trigeminal, facial, and trochlear motor nuclei, as well as the proprioceptive mesencephalic trigeminal nucleus. Using stereological counting techniques, we report a slowly progressive and significant reduction, to 75% of wildtype controls, in the number of large trigeminal motoneurons, while normal numbers were found for sensory mesencephalic trigeminal, and facial and trochlear motoneurons. The morphology of many surviving large trigeminal motoneurons was substantially altered, in particular the size and length of perpendicularly extending primary dendrites, but not those of facial or trochlear motoneurons. At the ultrastructural level, proximal dendrites of large trigeminal motoneurons, but not other neurons, were significantly depleted in organelle content such as polyribosomes and showed abnormal (vesiculated) mitochondria. These data indicate primary defects in trigeminal alpha motoneurons more than gamma motoneurons. Our findings expand the Loa heterozygote phenotype in two important ways: we reveal dendritic in addition to axonal defects or abnormalities, and we identify the Loa mutation as a mouse model for mixed motor-sensory loss when the entire neuraxis is considered, rather than a model primarily for sensory loss.
Keywords: cranial motor neuron, mesencephalic trigeminal nucleus, stereology, dynein mutation, mitochondria, ultrastructure
INTRODUCTION
The motor protein dynein transports cargoes along microtubules in axons (Goldstein and Yang, 2000; Vallee et al., 2004; Ström et al., 2008) and dendrites (Zheng et al., 2008; Kapitein et al., 2010). Mutation of dynein’s heavy chain in mice (“Legs at odd angles,” official nomenclature Dync1h1Loa, here referred to as the Loa mouse) was initially reported to affect the motor system, apparently due to a retrograde axonal transport defect (Hafezparast et al., 2003). Subsequent work implicated deficits in proprioceptive sensory neurons in addition to motor neuron deficiencies (Chen et al., 2007), or possibly primary effects on the sensory (proprioceptive muscle spindle) system (Ilieva et al., 2008). In particular, gamma (γ motoneuron axons rather than alpha (α motoneuron axons were reported reduced in the lumbar spinal cord of heterozygous Loa mice (Ilieva et al., 2008), while cervical spinal nerves may be much less compromised or may not be affected at all in this mutant (Hafezparast et al., 2003; Chen et al., 2007; Ilieva et al., 2008).
Neurodegenerative effects of dynein mutations may be due to reduced trafficking of trophic factors (Levy and Holzbaur, 2006; Chen et al., 2007; Ilieva et al., 2008; Perlson et al., 2010). While no retrograde axonal transport defects were originally reported in heterozygous Loa mice (Hafezparast et al., 2003; Kieran et al., 2005) (homozygous animals have defects in axonal transport but do not survive beyond birth), recent investigations have shown defects in retrograde axonal trophic factor transport in adult Loa/+ mice (Perlson et al., 2009). Survival functions and trophic responses differ considerably between different motor neuronal populations in mouse models of disease and other animal models (Henderson et al., 1998; Nimchinsky et al., 2000; Haenggeli and Kato, 2002; Oppenheim and von Bartheld, 2008; Gould and Enomoto, 2009). It is possible that the significance of dynein-mediated transport of trophic factors is specific for neuronal populations and/or differs between neurons along the neuraxis (Banks and Fisher, 2008; Gould and Enomoto, 2009).
Given the rather limited and controversial information about the Loa phenotype among neuronal populations, we conducted a comprehensive survey to determine whether and to what extent the dynein mutation of the Loa type alters cranial nerve nuclei in the early postnatal, adult and aged heterozygous Loa mouse. We analyzed surviving neurons in several cranial nuclei, including two motor nuclei consisting entirely of α-motoneurons (facial and trochlear, Sturrock, 1988, 1991), one mixed α/γ-motor neuron nucleus (trigeminal motor nucleus, Howard et al., 1980; Rokx et al., 1987; Maeda et al., 1993), and one purely sensory nucleus, the mesencephalic trigeminal nucleus (Mes V, Lazarov, 2007).
Based on morphometry and calibrated stereological techniques, as well as ultrastructural analysis of organelle accumulation and distribution, we report that α rather than γ trigeminal motoneurons are affected by the heterozygote dynein mutation (Loa/+). Surprisingly, survival of proprioceptive neurons in the Mes V nucleus of the trigeminal nerve was not significantly affected, unlike their counterparts in the lumbar spinal cord that lose ~40% of proprioceptive neurons (Chen et al., 2007) and ~75% of proprioceptive axons (Ilieva et al., 2008). For the first time, we provide in-vivo evidence of dendritic abnormalities in addition to previously described axonal deficits in Loa/+ mice. Taken together, the Loa/+ mutation seems to affect specific, but different subsets of neuronal populations in spinal and cranial neurons, while leaving the Mes V trigeminal proprioceptive neurons intact.
MATERIALS AND METHODS
A total of 23 heterozygote Loa/+ mice (congenic on a C57BL/6J background), 27 age-matched wildtype (wt) littermates, and two C67BL/6 mice were available for this study. Data were derived primarily from three 10-day old Loa/+ pups, three 3-month old Loa/+ adults, and four 19-month old (aged) Loa/+ mice; as well as three 10-day old wt littermate pups, three 3-month old wt littermates, four 19-month old wt littermates, and two 19-month old C57BL/6 mice. Both wt and Loa/+ mice included similar ratios of males and females. In most cases, complete serial sections through the brain area of interest could be obtained from both sides. The quantitative data reported in this study are based on the histological analysis of a total of 114 cranial nerve nuclei. Mice were housed at 21°C in 55% relative humidity with a 12-hour light and 12-hour dark cycle and food ad libitum. The animal studies reported in this paper were carried out with full approval from UCL Institute of Neurology’s local ethical review panel under the guidance issued by the Medical Research Council in Responsibility in the Use of Animals for Medical Research (1993) and under license from the UK Home Office, and, for the tissue processing and analysis at the University of Nevada, Reno, from UNR’s institutional animal care and use committee.
Genotyping
Genotyping was performed on DNA prepared from ear biopsies. The Loa mutation results in the formation of a new Rsa1 site, thus mice were genotyped by PCR with the forward primer Loa-F: ATTGAGGAGGTGAACCTGGCC and the reverse primer Loa-R: CAGTCATCGAAGATCTCCTGGG which flank the mutation and generated a 548 bp product. Restriction enzyme digestion with Rsa1 of the wildtype locus produced two fragments of 322 and 226 bp, while the Loa locus produced three fragments of 226, 185 and 137 bp.
Tissue Processing
At 10 days, 3 months or 19 months of age, the mice were anesthetized with phenobarbital, and perfused intracardially with saline, immediately followed by 4% paraformaldehyde (PFA). Brains were postfixed overnight in PFA and transferred to 70% ethanol for storage. Fixed brains were dehydrated in three changes of graded ethanols, cleared in two changes of methylsalicylate, and embedded in paraffin (Paraplast Plus). Paraffin blocks were cut in the transverse plane at 25 μm or, when thick sections were difficult to obtain, occasionally at 15 μm. Every section was collected on silane-coated slides and stained with 0.03% thionin.
Cell counting
The calibrated optical disector technique was used to obtain estimates of the number of neurons in each motor nucleus (Hatton and von Bartheld, 1999). Stereology of cranial motor nuclei was carried out during direct observation through the microscope, using an Optiphot microscope (Nikon, Tokyo, Japan) with a 100× immersion oil objective (NA=1.25), and a 10×10 square reticule in the eyepiece. The Optiphot microscope was equipped with a drawing tube (Nikon 1.25) and a microcator (MFC-1 focus controller and DRV-1 OPTI drive). The localization of each motor neuron nucleus within the z-axis and within the view field was determined by using the microcator with a nominal resolution of 0.1 μm, producing a practical (visual) resolution of about 0.4 μm. Serial tissue sections were collected through the entire brain nucleus of interest, and a systematic random series of sections was selected for counting. To be counted, a particle had to meet the following inclusion criteria, according to an unbiased counting rule (“unbiased counting box,” Schmitz and Hof, 2005): A neuronal nucleus with a diameter larger than 7 μm fell within the counting frame and did not intersect the exclusion line. The size of glial nuclei was < 7 μm. When two different types of neurons in the trigeminal motor nucleus were counted, they were distinguished based on two criteria, differences in size as well as differences in cytoplasm-nucleus ratios, as described below. There was shrinkage in trigeminal α-motoneurons between adult and aged mice, in both the wt and the mutant, but the differences in neuronal size specifically for the trigeminal α-motoneurons were much larger between the wt and mutant mice than those due to age-related shrinkage. The same investigator (L.M.W.) analyzed all serial sections to eliminate inter-observer variability. Sections were coded to assure that the observer was blind to the condition and to prevent observer bias. Parameters measured for statistical analyses included the mean, SEM, and differences between groups by Student’s t-test, and measurement of statistical significance with p values of < 0.05, using Sigma Stat software. An unbiased counting frame size of 80 × 80 μm was used for sampling with a 100× oil immersion objective. The reference volume was determined by point-counting, using sampling boxes of 40 × 40 μm with a 20× objective, and the average section thickness was applied to the volume calculation for the z-axis. The number of samples per nucleus varied with the nucleus’ volume, from 15–20 for the trochlear, 25–30 for the trigeminal, and 50–58 for the facial nucleus. The number of neurons per sample box (either 151,040 μm3 or 81,920 μm3) ranged from 1.9–2.9 (averages). Thus, at least 4% of all neurons were sampled.
Since the volume of the Mes V nucleus is practically impossible to measure due to the widely scattered distribution of its neurons (Sturrock, 1987; see Fig. 1C), profile counting was used to estimate the neuron number for this nucleus (von Bartheld, 2002). Every section through the trochlear and the Mes V nucleus, every third section through the trigeminal motor nucleus, and every fifth section through the facial nucleus was selected for counting of neurons, with a random start site. For the estimates obtained by profile counting, cell nuclei were counted only when >50% of the nucleus appeared within the section. Thus, counting of split nuclei was minimized and Abercrombie correction was not deemed necessary. It is easier to recognize nuclear borders of cranial nerve nuclei in the mouse pups based on distribution of large neurons than small neurons. Therefore, we estimated neuron numbers only for the larger (α) neurons in 10-day old mice, unlike the adult and aged mice, where numbers of small and large neurons in the nucleus were estimated separately.
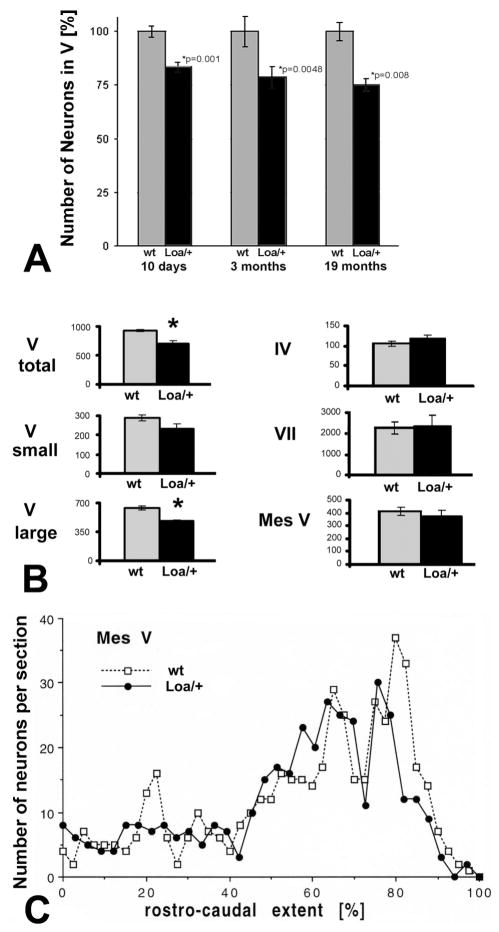
Fig. 1.
Fig. 1A–C. Estimates of neuronal numbers and their distribution in cranial nerve nuclei of wildtype (wt) and Loa/+ mice. A. Numerical estimates of α-motoneurons in the trigeminal motor nucleus (V) of wt and Loa/+ mice from 10 day-old mouse pups, 3-month old adults, and 19-month old aged mice, normalized to wt = 100% for each age group. Error bars = SEM. Note the deficit already at 10 days and slowly progressing course with aging. B. Quantification of neuron numbers in cranial nerve nuclei of aged wt and Loa/+ mice. Note significant differences (*, p<0.01) in numbers exclusively for trigeminal motoneurons (V, total and large), but not for small V neurons (p=0.12). Error bars = SEM. C. Representative plots of the number of neurons in the mesencephalic nucleus of the trigeminal nerve (Mes V) per tissue section in aged (19-month old) mice. Note that there are no major or consistent differences in either number or rostro-caudal distribution between wt and Loa/+ mice.
Section Thickness and Z-axis Analysis
Section thickness was verified by z-axis analysis. The final mean section thickness (after dehydration and coverslipping) for nominally cut 25 μm sections was 23.6 ± 0.7 μm (SEM) (range: 19 to 25.8) and 12.8 ± 0.6 μm (SEM) with a range of 10–15.6 μm (for 15 μm-thick sections). A z-axis distribution analysis was performed on several random sections to determine z-axis deformation and to verify complete stain penetration (Gardella et al., 2003). The position of nuclei in the z-axis of tissue sections was determined as described previously (Baryshnikova et al., 2006). For counting particles, centers of nuclei (rather than tops or bottoms of particles) were scored, to avoid any over- or undercount in the bins adjacent to the surface at the top or bottom of the tissue section. Z-axis deformation was as expected for conventionally collected paraffin sections (Baryshnikova et al., 2006).
Neuron Count Calibration by Serial Section 3D Reconstruction
To verify and calibrate our optical disector-derived neuronal estimates, one trochlear nucleus of an aged wt animal was reconstructed in 3D from serial sections as described (Hatton and von Bartheld, 1999; von Bartheld, 2002). Serial sections were collected from the midbrain beginning at a level rostral to the oculomotor motor nuclei. Completeness of serial sections through the entire motor nuclei was verified by comparison with a mouse brain atlas (Paxinos and Franklin, 2001).
Morphometry
The size (area, diameter and ratio of largest over smallest diameter) of cranial motor and Mes V neurons was quantified using Simple PCI Software (Thousand Oaks, CA) or NIS elements imaging software (Nikon, Tokyo, Japan). Sections from mouse pups were not quantified for morphometry, since nuclear borders were difficult to determine for the smaller cells at this age. For an example of the distinction between cell body and proximal dendrites and for the definition of diameters, see Fig. 2A. A total of ten mice were used for the morphometric analysis, with at least two mice and four nuclei for each data point. Neurons were randomly selected from the right and left side. Results for neuron area, diameter and ratio of diameters are presented as mean ± SEM. To determine whether differences between wt and Loa/+ mice were statistically significant, unpaired 2-tailed Student’s t-test was used and p values calculated (SigmaStat, Jandel Corp., San Rafael, CA). The significance level was set at p<0.05. For the trochlear nucleus, we analyzed 236 adult neurons, 38–96 neurons for each mouse, and 347 aged neurons, 52–64 neurons for each mouse. For the facial nucleus, we analyzed 110 aged neurons, 17–20 for each mouse. For the trigeminal motor nucleus we analyzed 80 postnatal α-motoneurons, 20 for each mouse, 148 adult α-motoneurons, 33–55 neurons for each mouse, and 337 aged α-neurons, 51–60 neurons for each mouse. Consistent with previous reports (Limwongse and deSantis, 1977; Howard et al., 1980; Rokx et al., 1987; Maeda et al., 1993), we distinguished trigeminal α neurons from γ or interneurons by their size. In our sections from adult and aged mice, the large neurons typically ranged in diameter from 18–37 μm vs. 13–16 μm for the small neurons, and they had a visibly higher ratio of cytoplasm to nucleus (small neurons have much less cytoplasm relatively to the size of their nucleus, Rokx et al., 1987). For the Mes V nucleus, 66 aged neurons were examined, 9–12 neurons for each mouse. For a detailed morphometric analysis of proximal trigeminal motoneuron dendrites, we measured the length of primary dendrites within the plane of tissue section. While measurement of dendritic parameters is limited within 25 μm thick Nissl-stained paraffin sectons (and impossible for distal dendrites), properties of proximal dendrites could nevertheless be reliably quantified. The number of neurons with dendrites extending 25–50 μm, 50–75 μm, or were longer than 75 μm within the section plane was recorded as a percentage of total samples (307 aged neurons from 10 different trigeminal nuclei were analyzed). This was compared between wt and Loa/+ mice and statistical significance measured by Student’s t-test. A similar analysis was performed for neurons in the facial and trochlear motor nucleus for comparison. Images were captured on a Coolpix 4300 digital camera mounted on a Nikon E600 Eclipse microscope, and processed in Adobe Photoshop exclusively for optimization of brightness and contrast.
Fig. 2.
Fig. 2A–E. Numerical estimates and morphometry of cranial nerve nuclei in wildtype (wt) and Loa/+ heterozygote mice. A. Example of a photomicrograph of a large (alpha) trigeminal motoneuron (wt) illustrates how morphometric data were obtained. The borders of the cell body (distinct from and excluding primary dendrites, stippled) are indicated, as well as the smallest and largest cell body diameter. One-sided arrow points to a glial cell for size comparison. Scale bar = 10 μm. B–C. Morphology of cranial motor neurons (MIV, trochlear; MVII, facial; MV, trigeminal, distinct for large, >18 μm neurons and small, <18 μm neurons) and sensory mesencephalic trigeminal neurons (Mes V) in the 19-month-old (wt) mouse (B) and the Loa/+ mouse (C). Scale bar (shown in bottom of panels B, same for all micrographs) = 30 μm. D–E. Quantification of area, diameter and the ratio of largest over smallest diameter of neurons (diameters as defined in A) in 3-month old mice (D) and in aged (19 month old mice, E), expressed as mean ±SEM. Statistical significance is indicated by asterisks, with *, p=0.02; **, p=0.01; ***, p=0.001. Only large trigeminal and trochlear motoneurons, but no other neurons examined showed significant differences between wt and Loa/+.
Electron Microscopy
Two 3-month old mice were anesthetized with phenobarbital, and perfused intracardially with saline, immediately followed by 4% paraformaldehyde (PFA). Brains were postfixed overnight in 2% PFA and 1.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). We dissected the right and left trigeminal nuclei and adjacent structures, and processed each sample for electron microscopy. Areas of interest were carefully trimmed from the brainstem. Postfixed brain samples were dehydrated in three changes of graded ethanols, two changes of propylene oxide, and embedded in Spurr’s resin, oriented and polymerized at 60°C overnight. Semithin cross-sections (1 μm) were prepared and stained with 1% toluidine blue to facilitate orientation and monitoring of tissue quality, followed by preparation of ultrathin sections (70–80 nm) for electron microscopy. Thin sections were stained with 2.5% aqueous lead citrate and uranyl acetate. Sections were examined at magnifications of ×1,950, ×10,500 and ×25,000. Sections were digitized in a Philips CM 10 transmission electron microscope equipped with a Gatan 792 BioScan digital imaging system.
In each brainstem sample, we examined three nuclei: The trigeminal motor nucleus (MV), the reticulotegmental nucleus of the pons (RtTg), and the mesencephalic trigeminal nucleus (Mes V), from both left and right sides. A total of 45 grids were examined, 26 from wt mice and 19 from Loa/+ mice, with 1–2 sections per grid, resulting in a total of 67 sections examined. As control for changes in trigeminal motoneuron dendrites of Loa/+ mice, we examined the RtTg nucleus - whose neurons have comparable long dendrites. As control for changes in the cytoplasm of trigeminal motoneurons we examined the Mes V nucleus.
For quantification, we determined the fractional area (%) of organelles in dendrites. Using point counting (size of counting box = 0.964 μm2), we measured the fractional area of each organelle divided by the total area of the dendrite. Total area of dendrite samples examined in Loa/+ trigeminal neurons (MV) with 30 dendrites was 742 μm2, total area of MV in wt mice with 22 dendrites was 267 μm2, and that of the RtTg nucleus in the Loa/+ mouse with 19 dendrites was 332 μm2. The total area of cell bodies of MV neurons sampled in Loa/+ mice with 10 neurons was 308 μm2, and of MV neurons in wt mice with 10 neurons was 492 μm2. Means, errors of the mean, and calculation of p-values for statistical significance was done using SigmaStat (Jandel Corp., San Rafael, CA).
RESULTS
Location of nuclei
Since some cranial motor neurons in the homozygous Loa mouse exhibit migration defects during embryonic development (Hafezparast et al., 2003), we first examined and assessed the location of motor and sensory cranial nerve nuclei in the adult heterozygote Loa mouse, as compared with wt littermates, normal C57BL/6 mice, as well as the mouse brain atlas (Paxinos and Franklin, 2001). All cranial nerve nuclei examined (trochlear, oculomotor, facial, trigeminal motor, and Mes V) were found in their normal locations (data not shown). There was no evidence for any significant migration defect as described for the facial motor nucleus in the embryonic homozygous Loa mouse.
Estimates of neuron number
We obtained estimates of motoneuron numbers for selected cranial nerve nuclei in mouse pups, adult mice (3-months old) and aged mice (19-months old).
Mouse pups
The mean number of motoneurons in four trochlear nuclei from two wt mice was 126.0 ± 1.6 (SEM) on each side, compared with a mean number of 119.7 ± 2.3 (SEM) motoneurons on each side in four trochlear nuclei from two Loa/+ mice. The difference between the two groups was not statistically significant (Student’s t-test, p = 0.077). The mean number of large, αmotoneurons in six trigeminal motor nuclei from three wt-mice was 539.6 ± 13.0 (SEM) on each side, but 450.6 ± 12.4 (SEM) in six trigeminal motor nuclei from three Loa/+ mice – a reduction of 17% (Fig. 1A). This difference was statistically significant with p ≤ 0.001. We did not attempt to count the mean number of all trigeminal neurons (including small cells), because at this age the nuclear borders are somewhat arbitrary when based on the distribution of small cells.
Adult mice
The mean number of motoneurons in four trochlear nuclei from two wt mice was 100.7 ± 4.4 (SEM) on each side, and in two trochlear nuclei from one Loa/+ mice was 99.0 ± 2.0 (SEM, no significant difference, p = 0.810). The mean number of all neurons in four trigeminal motor nuclei from two wt mice was 1,032.0 ± 110.9 (SEM) on each side, but was 794.0 ± 89.3 (SEM) in four trigeminal motor nuclei from two Loa/+ mice. This difference was statistically significant (Student’s t-test, p = 0.016). The number of large, α-motoneurons in wt mice was 649.0 ± 92.4 (SEM) and in Loa/+ mice 510.2 ± 63.2 (SEM) on each side, a 22% reduction (Fig. 1A). This difference was also statistically significant with p = 0.048. The number of the small neurons in the trigeminal motor nucleus did not differ significantly between the Loa/+ and the wt mice: 383.0 ± 77.6 (SEM) in the wt, and 288.5 ± 83.6 (SEM) in the Loa/+, p = 0.149.
Aged mice
We performed a detailed analysis of cranial nerve nuclei in the aged mouse, because we expected any deficits to be most pronounced with advanced age. The mean number of motoneurons in ten trochlear nuclei from five wt mice was 105.6 ± 5.3 (SEM) on each side, and 119.7 ± 6.8 (SEM) in four trochlear nuclei from two Loa/+ mice, a difference that was not statistically significant (p = 0.161). To confirm the validity of our stereological estimates, a 3D serial section reconstruction was performed for one trochlear nucleus of a wt mouse (von Bartheld, 2002). The optical disector estimate of this particular nucleus was 109 neurons, while the serial section reconstruction rendered 108 trochlear motoneurons on that same side. Thus, our estimate was accurate within about 1%. The mean number of motoneurons in three facial nuclei from three wt mice was 2,300 ± 295.9 (SEM) on each side, and 2,385 ± 543.4 (SEM) in three facial nuclei from three Loa/+ mice, a difference that was not statistically significant (p = 0.897).
The mean number of all neurons in three trigeminal motor nuclei from two wt mice was 931.3 ± 17.0 (SEM) on each side, but 714.0 ± 42.5 (SEM) in three trigeminal motor nuclei from two Loa/+ mice. This difference between the two groups was statistically significant (p =0.009, Fig. 1B). In the trigeminal motor nucleus, two populations of neurons have been distinguished (Limwongse and deSantis, 1977; Rokx et al., 1987): large neurons (consisting of α-motoneurons) and small neurons (consisting of γ-motoneurons and interneurons). In our study, the ratio of large (α) to small neurons was about 2:1 in wt mice (number of nuclei: four; from three mice), consistent with previous reports in rats (Howard et al., 1980) and mice (Maeda et al., 1993), but was 0.76–0.91:1 in Loa/+ mice (number of nuclei = four; from two mice) which is a significant difference (p = 0.001). The number of α-motoneurons decreased by about 25% in Loa/+ mice, from a mean of 641.6 ± 27.7 (SEM) in wt mice to 481.6 ± 18.1 (SEM) on each side (Fig. 1B). This difference was statistically significant with p = 0.008. The number of the small neurons in the trigeminal motor nucleus did not differ significantly between the Loa/+ and the wt mice: 289.6 ± 13.3 (SEM) in the wt, and 232.3 ± 25.1 (SEM) in the Loa/+, p = 0.115 (Fig. 1B).
The mean number of neurons in six Mes V nuclei from three wt mice was 410.4 ± 30.6 (SEM) on each side, and 375.0 ± 46.9 (SEM) in six Mes V nuclei from three Loa/+ mice (Fig. 1B). This difference between the two groups was not statistically significant (p = 0.532). Since there was a difference in numbers between the Loa/+ and the wt of ~ 9% (albeit not statistically significant), we asked whether any losses in the Loa/+ mouse may be concentrated in particular areas of Mes V as previously shown in the Mes V nucleus of embryonic chick during the period of normal developmental cell death (von Bartheld and Bothwell, 1993). Plotting of Mes V neurons from rostral to caudal and comparison between wt and Loa/+ mice revealed no localized areas of losses or any observable differences in gradients of cell density along the neuraxis (Fig. 1C).
Morphology and morphometry at the light-microscopic level
Mouse pups
At the age of P10, nuclear borders and thus identities of small neurons in cranial nerve nuclei were difficult to recognize, but the large α-motoneurons could be easily identified. Although the area and diameters of trigeminal α-motoneurons from wt mice was slightly larger (Table 1), this difference was not statistically significant (p=0.547 and p=0.542, respectively). Likewise, the ratio of largest to smallest diameter was increased, but again without statistical significance (borderline with p=0.052). We conclude that changes in morphometry of these neurons may already be beginning but are too subtle yet for significance at this age.
TABLE 1.
Morphometry of neurons in cranial nerve nuclei in the early postnatal (pn), adult (ad) and 19 month-old aged (ag) wildtype (wt) and Loa/+ mouse.
| Area [μm2] | Diameter [μm] | Diameter Ratios | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| wt | Loa/+ | wt | Loa/+ | wt | Loa/+ | |
| MIVad | 460.0 ± 17.8 | 527.4 ± 33.4 | 26.4 ± 0.4 | 28 ± 0.8 | 1.7 ± 0.1 | 1.4 ± 0.1 |
| MIVag | 447 ± 11*** | 541 ± 14.2*** | 23.6 ± 0.2*** | 25.9 ± 0.3*** | 1.3 ±0.02 | 1.2 ±0.01 |
| MVpn | 445.2 ± 21.4 | 427.0 ± 21.1 | 23.6 ± 0.5 | 23.1 ± 0.5 | 1.4 ± 0.06 | 1.6 ± 0.07 |
| MVad α | 670.7 ± 15.1*** | 528.6 ± 16.3*** | 29.0 ± 0.3*** | 25.6 ± 0.4*** | 1.34 ± 0.03*** | 2.72 ± 0.11*** |
| MVag α | 544.8 ± 31.6* | 460.4 ± 18.6* | 28.8 ± 0.6** | 26.0 ± 0.6** | 1.7 ± 0.04*** | 3.4 ± 0.1*** |
| MVag γ | 156.8 ± 2.9 | 153.2 ± 3.8 | 14.6 ± 0.14 | 15.4 ± 0.18 | 1.38 ± 0.07 | 1.24 ± 0.06 |
| Mes Vag | 429.4 ± 12.8 | 458.4 ± 16.6 | 25.8 ± 0.4 | 26.6 ± 0.5 | 1.3 ± 0.06 | 1.2 ± 0.04 |
| MVIIag | 389.4 ± 12.84 | 395.0 ± 22.4 | 24.46 ± 0.4 | 24.12 ± 0.3 | 1.35 ± 0.09 | 1.30 ± 0.08 |
Values are mean ± SEM. P values for significant difference (values in bold) between wt and Loa/+:
p<0.05;
p<0.01,
p<0.001. Diameter Ratios: Longest diameter divided by shortest diameter.
Adult mice
Neurons in the facial and Mes V nuclei were normal in appearance and indistinguishable from their wt counterparts by visual inspection. Some neurons in the trochlear nucleus (MIV) were larger in size in the Loa/+ mouse, showing two types, while only one type was apparent in the wt (Fig. 2B, C). This difference between genotypes was quantified by measuring area, diameter, and the ratio of largest vs. smallest diameter of the cell body (Fig. 2A). The results for the MIV revealed a significant difference between wt and Loa/+, as shown in Table 1. In the trigeminal motor nucleus (MV), it was conspicuous that many large motoneurons (identified by the criteria of greater than 17 μm for the diameter and a high cytoplasm/nucleus ratio: 4.0 ±0.26 for large; 1.5 ±0.16 for small neurons) of the Loa/+ mice were abnormally shaped with elongated primary dendrites, giving the neurons a spindle- or triangular shaped appearance (Fig. 2C) rather than a spherical or ellipsoid shape as in the wt (Fig. 2A, B). This difference was consistently seen in all four adult trigeminal motor nuclei from Loa/+ mice that were examined, but was not apparent in the same brains in other motor nuclei (trochlear, oculomotor or facial, see Fig. 2B, C). To quantify these apparent differences, the area of randomly selected motor neuron profiles containing the nucleus was measured, as well as the largest and smallest diameter and the ratio between the two values. As shown in Fig. 2D and Table 1, the large trigeminal motor neurons (criteria as defined above) in the Loa/+ mouse differed significantly by these measures from those in the wt littermates. There was a significant (about 20%) decrease in the mean area of the α-trigeminal motoneurons in Loa/+ mice, and the diameter was likewise decreased by about 15%, both statistically significant reductions (Table 1, Fig. 2D). The ratio of largest over smallest diameter was dramatically increased, from 1.3 to 2.7, a highly significant difference (p=0.001). Similar data were obtained for the aged Loa/+ mouse (see below and Table 1 for details). In addition to the large trigeminal motoneurons, the mean area and diameter of trochlear motoneurons were also significantly different in the Loa/+ mouse, although the ratio of largest over smallest diameter did not differ significantly.
Aged mice
To determine whether the abnormal morphological phenotype progressed further with aging, we examined the morphology of neurons in cranial nerve nuclei in aged (19 month old) mice. In the MV nucleus, the large neurons were also abnormally shaped in the aged Loa/+ mouse, as seen in the adult. This aspect was quantified by measuring area, diameter and ratio of largest over smallest diameter. Quantification of the morphology showed that the shape-defect of cell bodies was restricted to the large MV neurons (Table 1).
Similar to the changes in the adult MV, the primary dendrites of large MV neurons were elongated, giving the neurons a spindle- or triangular shaped appearance (Fig. 3D–F), rather than a spherical or ellipsoid shape as in the wt (Fig. 3C). At higher magnification with a 100× oil immersion lens, bundles of filaments were conspicuous in the Loa/+ dendrites of trigeminal neurons (Fig. 3D, inset) and could often be followed for 40–50 μm in the transverse plane in the Loa/+, but not in the wt mice. This was consistently seen in all eight of the aged Loa/+ mice that were examined, but was not apparent in the same brains in other motor nuclei (trochlear, oculomotor or facial, data not shown).
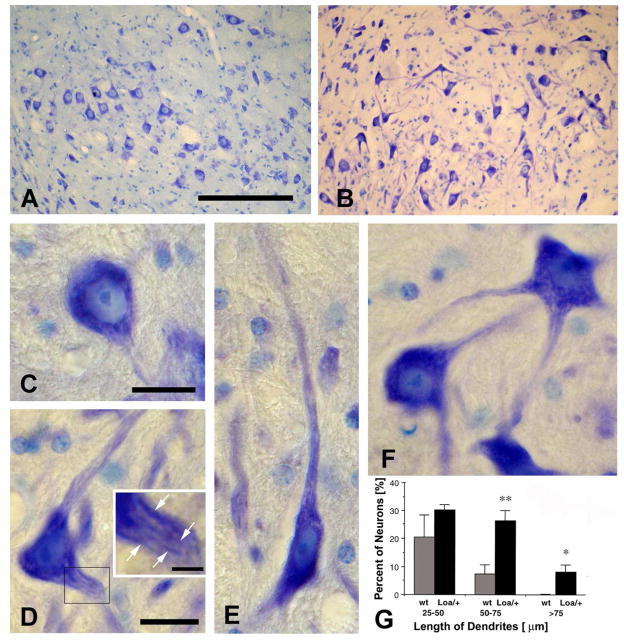
Fig. 3.
Fig. 3A–G. Micrographs illustrate morphological differences between large trigeminal motoneurons in the aged Loa/+ mouse (B, D–F) compared with wildtype (wt) littermates (A, C). Note the enlarged, but often relatively pale primary dendrites, packed with neurofibrils (arrows in panel D, inset) extending longitudinally within the primary dendritic processes (D–F). Quantitative analysis of dendritic length within the section plane revealed that primary dendrites were significantly enlarged in the Loa/+ mouse (G). Error bars = SEM. * denotes p = 0.011; ** denotes p = 0.005. Scale bars = 150 μm (A, B), 20 μm (C–F), and 5 μm (D, inset).
Quantification of the frequency of trigeminal motoneurons with long dendrites in the plane of sectioning showed substantial differences between the wt and the Loa/+ mouse (Fig. 3G). In the wt mouse, 20.5% (± 7.8% SEM) of trigeminal motoneurons had dendrites 25–50 μm long within the plane of sectioning, while the Loa/+ mouse had 30.3% (± 1.9% SEM) such neurons (not a significant difference, p = 0.259). However, in the wt mouse, 7.3% (± 3.3% SEM) of trigeminal motoneurons had visible dendrites 50–75 μm long, while the Loa/+ mouse had 26.2% (± 3.6% SEM) such neurons (a significant difference, p = 0.005). In the wt mouse, none of the trigeminal motoneurons had visible dendrites 75 μm or longer, while the Loa/+ mouse had 8.2% (± 2.5% SEM) such neurons (a significant difference, p = 0.011). Thus, super-sized dendrites in the plane of sectioning were significantly more frequent in the Loa/+ mouse than the wt littermates, while trochlear motoneurons showed no such difference between the wt and Loa/+, and facial motoneurons also showed no differences in these parameters (data not shown).
In the trochlear nucleus, there was a similar difference in size in the aged Loa/+ mouse as in the adult, that was not apparent in the wt, but the difference was not statistically significant (although it approached significance with p=0.08 for area). The mean area and diameter of facial motoneurons did not differ significantly between aged Loa/+ and wt mice, and the ratio of largest over smallest diameter was not different (Table 1). However, similar to the adult, there was a significant (about 10–15%) decrease in the mean area and diameter of the α-trigeminal motoneurons in Loa/+ mice, while the ratio of largest over smallest diameter was dramatically increased, from 1.7 to 3.4, a highly significant difference (Fig. 2E). On the other hand, neither the area, nor the diameter of small trigeminal neurons was significantly reduced in the Loa/+ mouse, and there was no significant difference in the ratio of largest over smallest diameter. Likewise, the Mes V nucleus was not different in area, diameter, or largest-to-smallest diameter ratio between the Loa/+ and the wt mice (Table 1).
The volume of three cranial motor nuclei was measured, based on point-counting and mean section thickness. The mean volume of the trochlear and facial nucleus was not reduced in the Loa/+ mouse. The mean volume of the trigeminal motor nucleus was reduced by 30%, a statistically significant reduction.
Taken together, these data indicate that qualitative and quantitative differences in dendrite morphology between the wt and the Loa/+ mice were restricted to the large trigeminal motoneurons, and the only other difference in morphometric parameters for cell bodies was for cell bodies in the trochlear, but not in the facial motoneurons or the Mes V sensory neurons (Fig. 2C–E). Thus, among the cranial nerve nuclei examined, the Loa/+ mouse harbors a distinct and selective alteration in the morphology of large trigeminal and trochlear motor neurons that progresses very little from 3 to 19 months of age.
Electron microscopy
Since adult Loa/+ mice showed the most significant alterations in the morphometry of large trigeminal motoneurons (Table 1), we next sought to confirm and further examine these changes at the ultrastructural level, using neurons in an adjacent pontine nucleus (RtTg) as well as Mes V neurons as within-animal control, and age-matched littermates as genotype controls. Upon qualitative observation of the proximal dendrites of trigeminal motor neurons in wt and Loa/+ mice at the ultrastructural level, the dendrites appeared conspicuously “empty” in the adult Loa/+ mouse compared with wt littermates. Such differences were not seen in the dendrites of large neurons in the adjacent pontine nucleus, the RtTg. To verify these subjective observations, the organelle content in dendrites and cell bodies of trigeminal neurons was quantified and compared between wt and Loa/+, and between motor trigeminal and RtTg pontine neurons and, for the cell body, also for Mes V neurons. Significant differences were observed selectively in dendrites of large (α) MV neurons in Loa/+ mice (Table 2), but not in the dendrites of smaller trigeminal neurons or of pontine tegmental neurons (Table 2). When parameters for the cell body were quantified, there were no consistent or significant changes apparent (data not shown). In proximal dendrites of trigeminal motoneurons, however, major changes were obvious in the distribution of polyribosomes and in the structure of mitochondria in the Loa/+ mice. While ribosomes were present in MV dendrites of both wt and Loa/+ mice, polyribosomes were virtually absent in MV dendrites of Loa/+ mice. On the other hand, the quantity of vesiculated mitochondria was dramatically increased in MV dendrites of Loa/+ mice (Table 2). Nearly all mitochondria in dendrites of Loa/+ mice were abnormal, and more than 70% of these mitochondria contained vesicular structures within the cristae that are similar to mitochondria described as typical for pre-apoptotic cellular stages (Sun et al., 2007). We did not find any signs of increased autophagic vesicles, neither in the soma nor dendrites; in proximal dendrites, our data rather show an absence of lysosomes, a significant, 10-fold reduction of multivesicular bodies, and an almost twofold reduction of late endosomes in Loa/+ mice (Table 2). The endoplasmic reticulum was reduced fourfold in dendrites of Loa/+ mice. Overall, our electron microscopy study indicated a change in metabolism of Loa/+ mice with reduction of traffic and/or synthesis of molecules and organelles in dendrites selectively of large trigeminal motoneurons.
TABLE 2.
Fractional area [%] of organelles in dendrites including afferent synapses of wildtype (wt) and Loa/+ mice.
| Organelle | wt MV | Loa/+ MV | Loa/+ RtTg | |||
|---|---|---|---|---|---|---|
| Mean | ±SEM | Mean | ±SEM | Mean | ±SEM | |
| Total Cytosol | 55.9 | 59.1 | 43.0 | |||
| Cytosol without polyribosomes | 25.1 | ±6.3 | 59.1** | ±3.3 | 25.3 | ±4.4 |
| Cytosol with polyribosomes | 30.8 | ±0.3 | 0.0* | ±0.0 | 17.7 | ±0.4 |
| Endoplasmic reticulum | 8.9 | ±3.2 | 2.15* | ±0.2 | 11.9 | ±0.4 |
| Total Mitochondria | 10.3 | ±0.4 | 12.5 | ±1.5 | 8.5 | ±0.2 |
| Normal Mitochondria | 9.2 | ±1.5 | 3.75* | ±0.3 | 7.5 | ±0.1 |
| “Aged” Mitochondria | 1.1 | ±1.1 | 8.75** | ±1.2 | 1.0 | ±0.1 |
| Early endosomes | 2.8 | ±0.4 | 4.65 | ±1.2 | 4.6 | ±1.8 |
| Late endosomes | 3.4 | ±1.0 | 1.8* | ±0.5 | 3.2 | ±0.2 |
| Multivesicular bodies | 2.0 | ±1.2 | 0.25* | ±0.2 | 1.6 | ±0.5 |
| Lysosomes | 1.6 | ±1.6 | 0.0* | ±0.0 | 2.6 | ±1.7 |
| Tubules | 1.7 | ±0.6 | 1.9 | ±0.5 | 1.8 | ±0.7 |
| Plasma membrane | 7.6 | ±1.2 | 8.8 | ±0.1 | 8.8 | ±3.3 |
| Synapses | 5.8 | ±0.5 | 8.3 | ±2.2 | 13.1 | ±1.2 |
| Unidentified | 0.0 | ±0.0 | 0.55 | ±0.1 | 0.9 | ±0.8 |
| 100.0 | 100.0 | 100.0 | ||||
MV, Trigeminal Motor neurons; RtTg, reticulotegmental nucleus.
Major differences in bold:
decrease;
increase.
DISCUSSION
Our study is the first that examines effects of the dynein mutation on cranial nerve nuclei in heterozygote Loa mice and it is the first that examines such neuronal changes at the ultrastructural level. When compared with the phenotype of this mutation at the spinal cord level, some cranial nerve neurons show major differences that allow new insights into the extent and consequences of the dynein heavy chain mutation (Loa/+). We show that besides neuron number, dendritic morphology and organelle density are affected in cranial nerve nuclei, but both defects appear to be surprisingly selective for one particular cranial nerve neuronal population, the large trigeminal motor neurons.
Loa phenotype: the relevance of cranial nerve nuclei
Initial work on effects of the heterozygote dynein heavy chain mutation (Loa/+) was performed mostly on the spinal level and implicated the motor system (α-motoneurons) in the loss of hindlimb muscle strength (Rogers et al., 2001; Hafezparast et al., 2003). However, subsequent studies found additional defects in sensory (proprioceptive) spinal neurons and also in axons that appeared to originate from spinal γ-motoneurons rather than α-motoneurons (Chen et al., 2007; Ilieva et al., 2008; Dupuis et al., 2009). These findings pointed to an early postnatal and primary defect of the muscle spindle system with sensory neuropathy and loss of γ-motoneuron axons, rather than a progressive degeneration of α-motor neurons that innervate the extrafusal limb muscles.
We show that the α-motoneurons in the trigeminal motor nucleus of Loa/+ mice have significantly reduced numbers, as well as severe morphological abnormalities, while the γ-motoneurons appear less affected. This was an unexpected finding, since a recent study concluded that only γ-motoneurons, but not α-motoneurons were compromised in the Loa/+ spinal cord (Ilieva et al., 2008). We show that survival of cranial sensory proprioceptive neurons (Mes V) is not significantly reduced in the Loa/+ mouse, neither in the adult nor the aging animal. This was another surprise, since the spinal counterparts of the Mes V nucleus, the proprioceptive component in the lumbar spinal cord, lose ~40% of proprioceptive neurons (Chen et al., 2007) and ~75% of proprioceptive axons already during the first six postnatal weeks (Ilieva et al., 2008). Thus, the phenotype of the Loa/+ mouse differs considerably between the cranial and the spinal levels of the neuraxis (Table 3). Previous studies that focused on the spinal cord revealed a significant caudal-to-rostral gradient in the susceptibility of sensory or motor neurons to the Loa/+ mutation, with the lumbar neurons being significantly more affected than the cervical neurons (Chen et al., 2007; Ilieva et al., 2008). We show that the numerical deficit in the trigeminal motor nucleus is already apparent at an early postnatal age and progresses only minimally with age (Fig. 1A). Thus, our study adds important novel insights into the consequences of the dynein mutation in the brainstem, complementing previous work in the spinal cord and the striatum (Braunstein et al., 2010).
TABLE 3.
Numerical deficits in cranial and spinal nerves and/or their nuclei in Loa/+ mice compared with wild type mice, expressed as % loss in the heterozygote mutant.
| Motor | Sensory | References | |
|---|---|---|---|
| Cranial | |||
| V total | −23.1 to −23.4% | Mes V −8.7% (N.S.) | Current Study |
| V alpha | −22 to −25% | Current Study | |
| V gamma | −19.8 to −24.7% (N.S.) | Current Study | |
| IV | −1.7 to +11.8% (N.S.) | Current Study | |
| VII | +3.6% (N.S.) | Current Study | |
| Spinal | |||
| Lumbar | |||
| total | DRG −42% (neurons) | Chen et al., 2007 | |
| −10% (axons) | −60% (axons) | Ilieva et al., 2008 | |
| alpha | −50–80% (neurons) | Hafezparast et al., 2003 | |
| ±0% (axons) | DRG, −75% (axons) | Ilieva et al., 2008 | |
| gamma | −45% (axons) | Ilieva et al., 2008 | |
| grip-strength | −52% | Chen et al., 2007 | |
| −65% | Ilieva et al., 2008 | ||
| Cervical | |||
| grip-strength | −5% (N.S.) | DRG −10% (neurons) (N.S.) | Chen et al., 2007 |
| −30% | Ilieva et al., 2008 | ||
Data based on present study (cranial level) and previous reports (References); DRG, dorsal root ganglia; Mes V, mesencephalic nucleus of the trigeminal nerve; N.S., not significant.
Technical considerations of neuronal number estimates
Reports of precise numbers and ranges of numerical estimates for neuronal populations vary greatly between studies, making it difficult to compare absolute numbers between studies and mouse strains. Reasons include not only different techniques or variations of techniques and tissue processing, but also particle identification and inclusion criteria, recognition of precise borders of nuclei, as well as sexual dimorphism and biological variation between individual mice and mouse strains (Maeda et al., 1993; von Bartheld, 2002; Schmitz and Hof, 2005; Baquet et al., 2009). In our study, we have taken into account commonly recommended safeguards to minimize biases in estimates of neuronal numbers, such as z-axis distortion, observer bias, particle recognition and inclusion criteria, stain penetration, and tissue quality. Importantly, we calibrated our counts by comparison with a serial section 3D reconstruction, considered the “gold standard” in cell counting (von Bartheld, 2002; Baquet et al., 2009). Each of our number estimates for neurons in cranial nuclei for the wildtype (wt) mouse is within the range of numbers previously reported (Table 4). In rodents, the trigeminal motor nucleus consists of three neuronal types: α-motoneurons, γ-motoneurons, and interneurons (Card et al. 1986; Rokx et al., 1987; Ringstedt et al., 1998). The ratio of large to small neurons is typically reported as 2:1 (Howard et al., 1980; Maeda et al., 1993). This heterogeneous composition likely explains at least some of the divergent accounts of the number of neurons in the trigeminal motor nucleus (Table 4).
TABLE 4.
Estimates of numbers of neurons in adult wildtype mouse cranial nerve nuclei.
| Nucleus | Our study adult–aged | Previous ranges | References |
|---|---|---|---|
| MIV | 101–106 | 77–197 | Sturrock, 1991; Parsons et al., 2003 |
| MVII | n.d.–2,300 | 2,010–6,060 | Sturrock, 1988; Ernfors et al., 1994; Nimchinsky et al., 2000; Parsons et al., 2003 |
| MV all | 1,032–1,514 | n.d. | |
| MV large | 649–1,001 | 482–1,052 | Hinrichsen and Larramendi, 1969; Sturrock, 1987; Liebl et al., 1997; Ringstedt et al., 1998; Haenggeli and Kato, 2002; Parsons et al., 2003 |
| MV small | 383–513 | n.d. | |
| Mes V | 410 | 410–1,020 | Hinrichsen and Larramendi, 1969; Sturrock, 1987; Ernfors et al., 1994; Fan et al., 2000; Matsuo et al., 2000 |
Abbreviations: MIV, trochlear nucleus; MVII, facial motor nucleus; MV, trigeminal nucleus; Mes V, mesencephalic nucleus of the trigeminal nerve; n.d., not determined. Numbers are per side.
Can a neurotrophin-specific transport deficit explain the Loa/+ phenotype?
Morphological abnormalities in the Loa mouse are believed to result most likely from dynein-mediated defects in trafficking of neurotrophic factors such as neurotrophin-3 (Levy and Holzbaur, 2006; Ilieva et al., 2008). The extent of deficits reported for the spinal muscle spindle system of Loa/+ mice showed striking similarities with those previously reported for mice with a knock-out of neurotrophin 3 (NT-3) or its receptor, trkC (for reviews, see Liebl et al., 1997; Chen et al., 2003). Indeed, NT-3 is essential for the development and postnatal maturation of the muscle spindle system in the limbs and trunk, including the normal contingent of proprioceptive sensory neurons in the lumbar dorsal root ganglia, and γ-motoneurons in the anterior horn of the lumbar spinal cord (Ernfors et al., 1994; Kucera et al., 1995a,b; Liebl et al., 1997; Fan et al., 2000; Chen et al., 2003). Furthermore, trk receptors can bind directly to dynein (Yano et al., 2001; Ha et al., 2008). Therefore, impairment of specific neurotrophin transport along axons of the muscle-spindle innervating neurons seemed a plausible mechanistic explanation for some or all of the motor-sensory deficits observed at the spinal level of Loa/+ mice (Ilieva et al., 2008).
While muscle spindles innervated by neurons in the lumbar spinal cord require NT-3 signaling, those in the muscles of mastication of the jaw do not (Kucera et al., 1998; Ringstedt et al., 1998; Matsuo et al., 2000). Consistent with NT-3’s non-essential role in the formation or maintenance of muscle spindles in muscles of mastication (Kucera et al., 1998; Ringstedt et al., 1998; Chen et al., 2003), Loa/+ mice showed no significant reductions in the number (and no morphological abnormalities in the soma) of Mes V neurons which comprise up to 90% of the trigeminal proprioceptive sensory neurons (Lazarov, 2007). Since the number of Mes V neurons is reduced to half in NT-3 knockouts (Ernfors et al., 1994; Fan et al., 2000), but Mes V numbers were near normal in the Loa/+, we conclude that the NT-3 supply cannot be significantly impaired in the Loa/+ Mes V neurons. In addition to NT-3, GDNF has been implicated in both survival (Mikaels et al., 2000; Oppenheim et al., 2000; Gould et al., 2008) and dendritic morphogenesis (Vrieseling and Arber, 2006) of distinct pools of motoneurons. Therefore, trafficking of GDNF may be involved in the Loa/+ phenotype we describe.
Muscle spindles are believed to have evolved phylogenetically in jaw muscles prior to limb muscles, and may therefore utilize a different set of neurotrophic factors and receptors than those in limb muscles (von Bartheld and Fritzsch, 2006). Recent work indicates that although retrograde axonal transport of neurotrophic factors is reduced, the Loa/+ mouse has a relatively mild and very slowly progressive phenotype of neurodegeneration (Perlson et al., 2009), consistent with our finding of relatively modest, early loss of neuron numbers and only in a select cranial nerve nucleus. Interestingly, defects of striatal neurons in a related dynein mutation (Cra1/+) do not appear to be caused primarily by a neurotrophin trafficking problem (Braunstein et al., 2010).
Dendritic changes of trigeminal motoneurons at the light-microscopic level
Initial work in vertebrates has focused primarily on axonal phenotypes of dynein mutations (Lamonte et al., 2002; Hafezparast et al., 2003; Levy and Holzbaur, 2006; Chen et al., 2007; Ilieva et al., 2008; Dupuis et al., 2009; Perlson et al., 2009). Recent work has revealed essential functions of dynein in dendritic organelle traffic and dendritic morphogenesis in vitro (Zheng et al., 2008; Kapitein et al., 2010; Braunstein et al., 2010); this often overlooked aspect of dynein function in dendrites may be particularly relevant to explain the abnormalities we describe in trigeminal motoneurons.
Large (α) trigeminal motoneurons in rodents are known to have on average four primary dendrites that extend predominantly within a plane perpendicular to the long axis of the brainstem (Card et al., 1986), and these dendrites extend and ramify extensively throughout the brain (Mong et al., 1988). We have documented significant morphological alterations in the dendrites of large trigeminal motor neurons in the Loa/+ mouse. Many of the Loa mutant trigeminal motoneurons assume a shape that is reminiscent of those in vertebrates with more primitive or pedomorphic characters such as lampreys (Koyama et al., 1987) and lungfishes (von Bartheld, 1992) – species that lack the full repertoire of mammalian neurotrophins and their receptors (Hallböök et al., 2006). These changes include a reduction in the cell body size and increases in the length and diameter of their primary dendrites with an altered appearance of neurofibrils (Fig. 3D) that consist of aggregates of neurofilaments and microtubules. Aberrant accumulation of neurofilaments is a common pathological hallmark in motoneuron degeneration (Bruijn et al., 2004). Our findings of abnormal dendrites in large trigeminal motoneurons are consistent with the report that altered neurofilament expression has the most pronounced effect on the primary dendrites of large spinal cord motoneurons (Zhang et al., 2002). Furthermore, in a dynein mutation (Cra1) that is similar to the Loa mutation, striatal neurons show atrophy and highly abnormal dendritic arborization (Braunstein et al., 2010), indicating that dynein may have essential functions in dendrite development.
Dendritic changes of trigeminal motoneurons at the ultrastructural level
We further explored cytoskeletal and other organelle perturbations as a possible consequence of the Loa mutation by ultrastructural analysis to better understand the nature of the dendritic phenotype in the Loa/+ mouse. At the ultrastructural level, we found multiple signs of “cellular stress” in large trigeminal neurons, including dramatically reduced numbers of polyribosomes and the endoplasmic reticulum, structurally abnormal mitochondria, and reduced numbers of other small organelles (lysosomes, multivesicular bodies, and late endosomes, Table 2). This is consistent with defects in trafficking of organelles, as would be expected for a dynein motor mutation that leads to reduced cellular functions dependent on trafficking of organelles along microtubules (Deng et al., 2010; Ori-McKenney et al., 2010; Eschbach and Dupuis, 2011). The findings of reduced polyribosomes and endoplasmic reticulum are consistent with the notion that dynein plays a critical role in the translation of mRNAs (Carson and Barbarese, 2005; Tsai et al., 2009).
Another organelle that was conspicuously altered in the trigeminal Loa/+ dendrites was the mitochondrion (Table 2), with accumulation of vesiculated mitochondria. Vesiculated mitochondria have been described in dendrites of axotomized neurons (Ling et al., 1986) and in pre-apoptotic stages (Sun et al., 2007), but not in dendrites subjected to glutamate excitotoxicity (Greenwood and Connolly, 2007). Increased numbers of vesiculated mitochondria in proximal dendrites may indicate delays in removal of peripheral mitochondria en route for degradation. It is possible that changes in the structure of mitochondria of Loa/+ mice impair energy homeostasis as well as dynein-mediated movements along the microtubules (Ström et al., 2008; Magrané and Manfredi, 2009). The mitochondria of Loa/+ mice have increased respiratory capacity and lower membrane potential (El-Kadi et al., 2010), and the large majority of such mitochondria are transported retrogradely towards the cell body (Miller and Sheetz, 2004). The distribution and size homeostasis of mitochondria is also disrupted in the superoxidase dismutase 1 (SOD1) mouse (Vande Velde et al., 2011). Based on biochemical evidence, it was shown that mitochondria are altered by the Loa/+ mutation, either due to changes in mitochondrial respiration or to changes in mitochondrial transport, resulting in an amelioration of the phenotype in the SOD1 mutant (El Kadi et al., 2010).
Cell-type specificity is characteristic of many neurodegenerative diseases (Perlson et al., 2010). It is interesting that the histological and ultrastructural abnormalities in the Loa/+ mice appear to be restricted to one type of motor neurons, the large trigeminal motoneurons. One may speculate that this is due to particularly long primary dendrites of this neuronal population with extensive coverage of afferent nerve terminals (Card et al., 1986; Mong et al., 1988). There may also be a threshold for impairment in trafficking capacity that differs between neuronal populations. Neurons with long neurites – such as sensory and motor neurons – may be particularly susceptible to trafficking dysfunction (Ori-McKenney et al., 2010). Loa/+ mice express similar amounts of dynein in the brain and spinal cord as do wildtype controls (Hafezparast et al., 2003), as would be expected for a point-mutation. To our knowledge, there is no information about differential expression of dynein in spinal vs. bulbar motoneurons, in either wt or mutant mice. However, it is known that the Loa mutation impacts on the assembly of the dynein complex, leading to the formation of lighter complexes (Deng et al., 2010). Regardless of the precise mechanism, the numerical (survival) and/or morphological / morphometric deficits in select neuronal populations of the Loa/+ mouse appear to be “instant” (early onset and detectable early postnatal) rather than adult onset progressive (degenerative, with autophagic markers), as was initially assumed when the Loa/+ phenotype was first described. In this respect, the Loa/+ dynein mutation appears to have a different phenotype and underlying mechanisms than other mouse models of neurodegeneration such as mice with mutations in SOD1 (Perlson et al., 2009).
Fig. 4.
Fig. 4A–F. Comparison of the ultrastructure of primary dendrites in 3-month old wildtype (wt) and heterozygote mice (Loa/+). A. A representative primary dendrite from a large wt alpha trigeminal motoneuron contains numerous organelles. B. A representative primary dendrite from a Loa/+ alpha trigeminal motoneuron is nearly devoid of organelles, with the exception of mitochondria. Also note the lack of polyribosomes. C. A representative primary dendrite from a Loa/+ primary dendrite of a reticulo-tegmental neuron adjacent to the trigeminal motor nucleus in the pons. Note that this neuronal cell type contains normal organelle density. D. Typical mitochondrion in a primary dendrite from a wt trigeminal motoneuron. Note the normal cristae in the interior. E. Mitochondrion from a Loa/+ trigeminal dendrite showing major parts with vesicular material. Polyribosomes are indicated with arrows. F. Another mitochondrion from a Loa/+ primary trigeminal dendrite shows pronounced vesicles, a sign of dysfunctional or “pre-apoptotic” mitochondria. Error bars = 1 μm (for panels A–C), and 0.5 μm (for panels D–F).
Acknowledgments
The authors thank Dr. Bernadett Kalmar (Institute of Neurology, University College London, UK) for assistance with the initial tissue processing, and Drs. Amy Altick, Linda Greensmith, Majid Hazfezparast, and Giampetro Schiavo for critical reading of earlier versions of the manuscript. Our work was supported by NIH grant EY012841 (CSvB) and a grant from the Sanford Center for Aging (University of Nevada, Reno, CSvB), as well as Center of Biomedical Research Excellence grants from the NIH (RR015581, RR024210 and GM103554), INBRE grant (RR016464 and GM103440), grants from the UK Medical Research Council and Brain Research Trust (JCS, EMCF), and the ENDOCYTE Research and Training Network funded by the European Union (AK, EMCF).
Grant Support: NIH grants EY012841, RR15581, RR016464 and RR024210, a grant from the Sanford Center for Aging (University of Nevada, Reno), grants from the UK Medical Research Council and Brain Research Trust, and the ENDOCYTE Research and Training Network (EU).
ABBREVIATIONS
- GDNF
glial cell line-derived neurotrophic factor
- Loa
“Legs at odd angles” dynein heavy chain mutation
- Mes V
mesencephalic nucleus of the trigeminal nerve
- MIV
trochlear nucleus
- MV
trigeminal motor nucleus
- MVII
facial motor nucleus
- NT-3
neurotrophin 3
- PFA
paraformaldehyde
- RtTg
reticulotegmental nucleus of the pons
- SEM
standard error of the mean
- wt
wildtype
LITERATURE CITED
- Banks GT, Fisher EM. Cytoplasmic dynein could be key to understanding neurodegeneration. Genome Biol. 2008;9:214. doi: 10.1186/gb-2008-9-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquet ZC, Williams D, Brody J, Smeyne RJ. A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience. 2009;161:1082–1090. doi: 10.1016/j.neuroscience.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryshnikova LM, von Bohlen und Halbach O, Kaplan S, von Bartheld CS. Two distinct events, section compression and loss of particles (“lost caps”), contribute to z-axis distortion and bias in optical disector counting. Microsc Res Tech. 2006;69:738–756. doi: 10.1002/jemt.20345. [DOI] [PubMed] [Google Scholar]
- Braunstein KE, Eschbach J, Ròna-Vörös K, Soylu R, Mikrouli E, Larmet Y, René F, Gonzalez De Aguilar JL, Loeffler JP, Müller HP, Bucher S, Kaulisch T, Niessen HG, Tillmanns J, Fischer K, Schwalenstöcker B, Kassubek J, Pichler B, Stiller D, Petersen A, Ludolph AC, Dupuis L. A point mutation in the dynein heavy chain gene leads to striatal atrophy and compromises neurite outgrowth of striatal neurons. Hum Mol Genet. 2010;19:4385–4398. doi: 10.1093/hmg/ddq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Card JP, Riley JN, Moore RY. The motor trigeminal nucleus of the rat: analysis of neuronal structure and the synaptic organization of noradrenergic afferents. J Comp Neurol. 1986;250:469–484. doi: 10.1002/cne.902500406. [DOI] [PubMed] [Google Scholar]
- Carson JH, Barbarese E. Systems analysis of RNA trafficking in neural cells. Biol Cell. 2005;97:51–62. doi: 10.1042/BC20040083. [DOI] [PubMed] [Google Scholar]
- Chen HH, Hippenmeyer S, Arber S, Frank E. Development of the monosynaptic stretch reflex circuit. Curr Opin Neurobiol. 2003;13:96–102. doi: 10.1016/s0959-4388(03)00006-0. [DOI] [PubMed] [Google Scholar]
- Chen XJ, Levedakou EN, Millen KJ, Wollmann RL, Soliven B, Popko B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic dynein heavy chain 1 gene. J Neurosci. 2007;27:14515–14524. doi: 10.1523/JNEUROSCI.4338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Garrett C, Dombert B, Soura V, Banks G, Fisher EM, van der Brug MP, Hafezparast M. Neurodegenerative mutation in cytoplasmic dynein alters its organization and dynein-dynactin and dynein-kinesin interactions. J Biol Chem. 2010;285:39922–39934. doi: 10.1074/jbc.M110.178087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, Fergani A, Braunstein KE, Eschbach J, Holl N, Rene F, Gonzalez De Aguilar JL, Zoerner B, Schwalenstocker B, Ludolph AC, Loeffler JP. Mice with a mutation in the dynein heavy chain 1 gene display sensory neuropathy but lack motor neuron disease. Exp Neurol. 2009;215:146–152. doi: 10.1016/j.expneurol.2008.09.019. [DOI] [PubMed] [Google Scholar]
- El-Kadi AM, Bros-Facer V, Deng W, Philpott A, Stoddart E, Banks G, Jackson GS, Fisher EM, Duchen MR, Greensmith L, Moore AL, Hafezparast M. The legs at odd angles (Loa) mutation in cytoplasmic dynein ameliorates mitochondrial function in SOD1G93A mouse model for motor neuron disease. J Biol Chem. 2010;285:18627–18639. doi: 10.1074/jbc.M110.129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Eschbach J, Dupuis L. Cytoplasmic dynein in neurodegeneration. Pharmacol Ther. 2011;130:348–363. doi: 10.1016/j.pharmthera.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Fan G, Copray S, Huang EJ, Jones K, Yan Q, Walro J, Jaenisch R, Kucera J. Formation of a full complement of cranial proprioceptors requires multiple neurotrophins. Dev Dyn. 2000;218:359–370. doi: 10.1002/(SICI)1097-0177(200006)218:2<359::AID-DVDY9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Gardella D, Hatton WJ, Rind HB, Rosen GD, von Bartheld CS. Differential tissue shrinkage and compression in the z-axis: implications for optical dissector counting in vibratome-, plastic- and cryosections. J Neurosci Methods. 2003;124:45–59. doi: 10.1016/s0165-0270(02)00363-1. [DOI] [PubMed] [Google Scholar]
- Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- Gould TW, Yonemura S, Oppenheim RW, Ohmori S, Enomoto H. The neurotrophic effects of glial cell line-derived neurotrophic factor on spinal motoneurons are restricted to fusimotor subtypes. J Neurosci. 2008;28:2131–2146. doi: 10.1523/JNEUROSCI.5185-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TW, Enomoto H. Neurotrophic modulation of motor neuron development. Neuroscientist. 2009;15:105–116. doi: 10.1177/1073858408324787. [DOI] [PubMed] [Google Scholar]
- Greenwood SM, Connolly CN. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology. 2007;53:891–898. doi: 10.1016/j.neuropharm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Ha J, Lo KW, Myers KR, Carr TM, Humsi MK, Rasoul BA, Segal RA, Pfister KK. A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J Cell Biol. 2008;181:1027–1039. doi: 10.1083/jcb.200803150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenggeli C, Kato AC. Differential vulnerability of cranial motoneurons in mouse models with motor neuron degeneration. Neurosci Lett. 2002;335:39–43. doi: 10.1016/s0304-3940(02)01140-0. [DOI] [PubMed] [Google Scholar]
- Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, Morgan PJ, Oozageer R, Priestley JV, Averill S, King VR, Ball S, Peters J, Toda T, Yamamoto A, Hiraoka Y, Augustin M, Korthaus D, Wattler S, Wabnitz P, Dickneite C, Lampel S, Boehme F, Peraus G, Popp A, Rudelius M, Schlegel J, Fuchs H, Hrabe de Angelis M, Schiavo G, Shima DT, Russ AP, Stumm G, Martin JE, Fisher EM. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- Hallböök F, Wilson K, Thorndyke M, Olinski RP. Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain Behav Evol. 2006;68:133–144. doi: 10.1159/000094083. [DOI] [PubMed] [Google Scholar]
- Hatton WJ, von Bartheld CS. Analysis of cell death in the trochlear nucleus of the chick embryo: calibration of the optical disector counting method reveals systematic bias. J Comp Neurol. 1999;409:169–186. [PubMed] [Google Scholar]
- Henderson CE, Yamamoto Y, Livet J, Arce V, Garces A, deLapeyrière O. Role of neurotrophic factors in motoneuron development. J Physiol Paris. 1998;92:279–281. doi: 10.1016/s0928-4257(98)80033-8. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF, Larramendi LM. Features of trigeminal mesencephalic nucleus structure and organization. I. Light microscopy. Am J Anat. 1969;126:497–505. doi: 10.1002/aja.1001260408. [DOI] [PubMed] [Google Scholar]
- Howard V, Scales L, Lynch R. The numerical densities of alpha and gamma-motoneurons in the trigeminal motor nucleus of the rat - a method of determining the separate numerical densities of 2 mixed populations of anatomically similar cells. Mikroskopie. 1980;37(Suppl):229–236. [PubMed] [Google Scholar]
- Ilieva HS, Yamanaka K, Malkmus S, Kakinohana O, Yaksh T, Marsala M, Cleveland DW. Mutant dynein (Loa) triggers proprioceptive axon loss that extends survival only in the SOD1 ALS model with highest motor neuron death. Proc Natl Acad Sci USA. 2008;105:12599–12604. doi: 10.1073/pnas.0805422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran D, Hafezparast M, Bohnert S, Dick JR, Martin J, Schiavo G, Fisher EM, Greensmith L. A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J Cell Biol. 2005;169:561–567. doi: 10.1083/jcb.200501085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Koyama H, Kishida R, Goris RC, Kusunoki T. Organization of sensory and motor nuclei of the trigeminal nerve in lampreys. J Comp Neurol. 1987;264:437–448. doi: 10.1002/cne.902640402. [DOI] [PubMed] [Google Scholar]
- Kucera J, Fan G, Jaenisch R, Linnarsson S, Ernfors P. Dependence of developing group Ia afferents on neurotrophin-3. J Comp Neurol. 1995a;363:307–320. doi: 10.1002/cne.903630211. [DOI] [PubMed] [Google Scholar]
- Kucera J, Ernfors P, Walro J, Jaenisch R. Reduction in the number of spinal motor neurons in neurotrophin-3-deficient mice. Neuroscience. 1995b;69:321–330. doi: 10.1016/0306-4522(95)00221-4. [DOI] [PubMed] [Google Scholar]
- Kucera J, Fan G, Walro J, Copray S, Tessarollo L, Jaenisch R. Neurotrophin-3 and trkC in muscle are non-essential for the development of mouse muscle spindles. Neuroreport. 1998;9:905–909. doi: 10.1097/00001756-199803300-00026. [DOI] [PubMed] [Google Scholar]
- LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascaño J, Tokito M, Van Winkle T, Howland DS, Holzbaur EL. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- Lazarov NE. Neurobiology of orofacial proprioception. Brain Res Rev. 2007;56:362–383. doi: 10.1016/j.brainresrev.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Levy JR, Holzbaur EL. Cytoplasmic dynein/dynactin function and dysfunction in motor neurons. Int J Dev Neurosci. 2006;24:103–111. doi: 10.1016/j.ijdevneu.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limwongse V, DeSantis M. Cell body locations and axonal pathways of neurons innervating muscles of mastication in the rat. Am J Anat. 1977;149:477–488. doi: 10.1002/aja.1001490405. [DOI] [PubMed] [Google Scholar]
- Ling EA, Wong WC, Yick TY, Leong SK. Ultrastructural changes in the dorsal motor nucleus of monkey following bilateral cervical vagotomy. J Neurocytol. 1986;15:1–15. doi: 10.1007/BF02057900. [DOI] [PubMed] [Google Scholar]
- Maeda N, Sugiyama H, Suemune S, Wakisaka H, Niida S, Ogata K, Miyata K. Sexual dimorphism in the trigeminal motor neurons innervating the mouse masseter muscle. Brain Res. 1993;627:177–180. doi: 10.1016/0006-8993(93)90763-d. [DOI] [PubMed] [Google Scholar]
- Magrané J, Manfredi G. Mitochondrial function, morphology, and axonal transport in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1615–1626. doi: 10.1089/ars.2009.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo S, Ichikawa H, Silos-Santiago I, Arends JJ, Henderson TA, Kiyomiya K, Kurebe M, Jacquin MF. Proprioceptive afferents survive in the masseter muscle of trkC knockout mice. Neuroscience. 2000;95:209–216. doi: 10.1016/s0306-4522(99)00424-8. [DOI] [PubMed] [Google Scholar]
- Mikaels A, Livet J, Westphal H, De Lapeyrière O, Ernfors P. A dynamic regulation of GDNF-family receptors correlates with a specific trophic dependency of cranial motor neuron subpopulations during development. Eur J Neurosci. 2000;12:446–456. doi: 10.1046/j.1460-9568.2000.00924.x. [DOI] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- Mong FS, Chen YC, Lu CH. Dendritic ramifications of trigeminal motor neurons innervating jaw-closing muscles of rats. J Neurol Sci. 1988;86:251–264. doi: 10.1016/0022-510x(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Young WG, Yeung G, Shah RA, Gordon JW, Bloom FE, Morrison JH, Hof PR. Differential vulnerability of oculomotor, facial, and hypoglossal nuclei in G86R superoxide dismutase transgenic mice. J Comp Neurol. 2000;416:112–125. doi: 10.1002/(sici)1096-9861(20000103)416:1<112::aid-cne9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Parsadanian AS, Prevette D, Snider WD, Shen L. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci. 2000;20:5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW, von Bartheld CS. Programmed cell death and neurotrophic factors. In: Squire L, editor. Fundamental Neuroscience. 3. San Diego: Elsevier; 2008. pp. 437–467. [Google Scholar]
- Ori-McKenney KM, Xu J, Gross SP, Vallee RB. A cytoplasmic dynein tail mutation impairs motor processivity. Nat Cell Biol. 2010;12:1228–1234. doi: 10.1038/ncb2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SA, Banks GB, Rowland JA, Coschigano KT, Kopchick JJ, Waters MJ, Noakes PG. Genetic disruption of the growth hormone receptor does not influence motoneuron survival in the developing mouse. Int J Dev Biol. 2003;47:41–49. doi: 10.1387/15. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. San Diego: Acad Press; 2001. [Google Scholar]
- Perlson E, Jeong GB, Ross JL, Dixit R, Wallace KE, Kalb RG, Holzbaur EL. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J Neurosci. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Maday S, Fu MM, Moughamian AJ, Holzbaur EL. Retrograde axonal transport: pathways to cell death? Trends Neurosci. 2010;33:335–344. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstedt T, Copray S, Walro J, Kucera J. Development of fusimotor innervation correlates with group Ia afferents but is independent of neurotrophin-3. Dev Brain Res. 1998;111:295–300. doi: 10.1016/s0165-3806(98)00146-1. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Peters J, Martin JE, Ball S, Nicholson SJ, Witherden AS, Hafezparast M, Latcham J, Robinson TL, Quilter CA, Fisher EM. SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci Lett. 2001;306:89–92. doi: 10.1016/s0304-3940(01)01885-7. [DOI] [PubMed] [Google Scholar]
- Rokx JT, Liem RS, van Willigen JD. Identification of alpha and gamma trigeminal motoneurons by the vibratome paraplast technique for HRP histochemistry. Acta Anat (Basel) 1987;129:333–336. doi: 10.1159/000146425. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Ström AL, Gal J, Shi P, Kasarskis EJ, Hayward LJ, Zhu H. Retrograde axonal transport and motor neuron disease. J Neurochem. 2008;106:495–505. doi: 10.1111/j.1471-4159.2008.05393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR. Changes in the number of neurons in the mesencephalic and motor nuclei of the trigeminal nerve in the ageing mouse brain. J Anat. 1987;151:15–25. [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR. Loss of neurons from the motor nucleus of the facial nerve in the ageing mouse brain. J Anat. 1988;160:189–94. [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR. Stability of motor neuron number in the oculomotor and trochlear nuclei of the ageing mouse brain. J Anat. 1991;174:125–129. [PMC free article] [PubMed] [Google Scholar]
- Sun MG, Williams J, Munoz-Pinedo C, Perkins GA, Brown JM, Ellisman MH, Green DR, Frey TG. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nat Cell Biol. 2007;9:1057–1065. doi: 10.1038/ncb1630. [DOI] [PubMed] [Google Scholar]
- Tsai NP, Tsui YC, Wei LN. Dynein motor contributes to stress granule dynamics in primary neurons. Neuroscience. 2009;159:647–656. doi: 10.1016/j.neuroscience.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- Vande Velde C, McDonald KK, Boukhedimi Y, McAlonis-Downes M, Lobsiger CS, Bel Hadj S, Zandona A, Julien JP, Shah SB, Cleveland DW. Misfolded SOD1 associated with motor neuron mitochondria alters mitochondrial shape and distribution prior to clinical onset. PLoS One. 2011;6(7):e22031. doi: 10.1371/journal.pone.0022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS. Oculomotor and sensory mesencephalic trigeminal neurons in lungfishes: phylogenetic implications. Brain Behav Evol. 1992;39:247–263. doi: 10.1159/000114122. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Counting particles in tissue sections: choices of methods and importance of calibration to minimize biases. Histol Histopathol. 2002;17:639–648. doi: 10.14670/HH-17.639. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Bothwell M. Development of the mesencephalic nucleus of the trigeminal nerve in chick embryos: target innervation, neurotrophin receptors, and cell death. J Comp Neurol. 1993;328:185–202. doi: 10.1002/cne.903280203. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Fritzsch B. Comparative analysis of neurotrophin receptors and ligands in vertebrate neurons: tools for evolutionary stability or changes in neural circuits? Brain Behav Evol. 2006;68:157–172. doi: 10.1159/000094085. [DOI] [PubMed] [Google Scholar]
- Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Yano H, Lee FS, Kong H, Chuang J, Arevalo J, Perez P, Sung C, Chao MV. Association of Trk neurotrophin receptors with components of the cytoplasmic dynein motor. J Neurosci. 2001;21RC125:1–7. doi: 10.1523/JNEUROSCI.21-03-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Casey DM, Julien JP, Xu Z. Normal dendritic arborization in spinal motoneurons requires neurofilament subunit L. J Comp Neurol. 2002;450:144–152. doi: 10.1002/cne.10306. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol. 2008;10:1172–1180. doi: 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]