Abstract
Background & Aims
Neutralizing auto-antibodies (Ab) against granulocyte-macrophage colony-stimulating factor (GM-CSF Ab) have been associated with stricturing ileal Crohn’s disease (CD) in a largely pediatric patient cohort (total 394, adult CD 57). The aim of this study was to examine this association in two independent predominantly adult inflammatory bowel disease patient cohorts.
Methods
Serum samples from 745 subjects from the NIDDK IBD Genetics Consortium and 737 patients from Australia were analyzed for GM-CSF Ab and genetic markers. We conducted multiple regression analysis with backwards elimination to assess the contribution of GM-CSF Ab levels, established CD risk alleles and smoking on ileal disease location in the 477 combined CD subjects from both cohorts. We also determined associations of GM-CSF Ab levels with complications requiring surgical intervention in combined CD subjects in both cohorts.
Results
Serum samples from CD patients expressed significantly higher concentrations of GM-CSF Ab when compared to Ulcerative Colitis or controls in each cohort. Non-smokers with ileal CD expressed significantly higher GM-CSF Ab concentrations in the Australian cohort (p= 0.002). Elevated GM-CSF Ab, ileal disease location and disease duration greater than 3 years were independently associated with stricturing/penetrating behavior and intestinal resection for CD.
Conclusions
The expression of high GM-CSF Ab is a risk marker for aggressive CD behavior and complications including surgery. Modifying factors include environmental exposure to smoking and genetic risk markers.
Keywords: Inflammatory bowel disease; granulocyte-macrophage colony-stimulating factor antibody; Crohn’s Disease, smoking
Background and Significance
The onset of Inflammatory Bowel Disease (IBD) requires multiple factors. Key among them is the genetic composition of the host, environmental interactions, and a dysregulated innate and adaptive mucosal immune response 1, 2. Several genome wide association studies have identified polymorphisms within genomic regions that correspond to immune pathways. These pathways are involved in activating inflammation in response to antigen exposure 3–5.
Bacterial recognition and autophagy are two important processes that drive the appropriate response to antigens at the intestinal level. Functional mutations in NOD2, an intracellular pattern recognition receptor in Paneth cells, are a common and well replicated CD risk factor 6, 7. In addition, defects in ATG16L1 and IRGM, two well described autophagy genes, also increase risk for IBD susceptibility 8, 9.
Endogenous auto-antibodies to cytokines which may have an activating or neutralizing effect are proposed as additional modulators of mucosal inflammation. Cytokine auto-antibodies may create a relative immunodeficient state in IBD predisposing patients to chronic inflammation. In one study neutralizing antibodies to transforming growth factor (TGF)-β were measured in sera from patients with Ulcerative Colitis (UC) (n=136) and were found to be elevated (P < 0·01) when compared with levels in unaffected individuals. In addition neutralizing antibodies to IL-10 (P < 0·05) were elevated in a subset of patients with Crohn’s disease (CD)10. In a subsequent study, neutralizing autoantibodies (Ab) against granulocyte macrophage colony-stimulating factor (GM-CSF Ab) were elevated in adult and pediatric CD patients and particularly in those patients with ileal disease involvement and stricturing behavior (P < .001). In addition CD patients with increased GMCSF Ab had reduced neutrophil phagocytic capacity11. An important parallel finding in this study was that NOD2 deficient mice treated with neutralizing antibodies to GM-CSF and subsequently exposed to NSAIDS, developed a transmural ileitis. This study and others demonstrate that deficiency of the important hematopoietic growth factor GM-CSF can contribute to a relative immunodeficiency 12. Idiopathic pulmonary alveolar proteinosis (I-PAP) for example, is a rare lung disorder of impaired macrophage function and due to elevated GM-CSF autoantibodies that neutralize the bioactivity of the growth factor GM-CSF 13, 14.
We therefore asked if elevated GMCSF Ab would be associated with a stricturing ileal phenotype in an adult CD population.
Materials and Methods
Study Subjects
Our study recruited subjects from two study cohorts, the NIDDK IBD Genetics consortium and the Brisbane node of the ANZ IBD Consortium, Australia. The NIDDK IBD cohort included 350 unaffected controls, 139 UC and 253 CD subjects. The Brisbane cohort included 257 unaffected controls, 255 UC and 224 CD subjects. All research protocols for human subjects’ research were reviewed and approved by individual Institutional Review Boards (IRB) at five institutions involved in the NIDDK IBD genetics consortium and Brisbane, Australia. All IBD cases were previously diagnosed using standard criteria. IBD phenotypes including CD and UC sub-phenotypes were classified according to the Montreal Classification15. A standardized clinical questionnaire was completed for each patient at the time of enrollment and entered into a database program. The questionnaire included date of birth, sex, age at diagnosis, ethnicity, disease location, disease behavior, family history, extraintestinal manifestations, surgeries, smoking status at diagnosis, and therapeutic management at the time of study enrollment. This information was stripped of identifying information and entered in the databases linked to the serum samples.
Genotyping Methods
A blood sample was obtained at the time of study enrollment for DNA and serum isolation. DNA was isolated using standard protocols at each center. Seven single nucleotide polymorphisms (SNPs) were genotyped. SNP Genotyping was completed by various methods. These included the Illumina GoldenGate Genotyping Assay which is a flexible, pre-optimized assay that uses a discriminatory DNA polymerase and ligase to interrogate multiple loci simultaneously 16. Some samples were genotyped using Sequenom, a technology that uses primer extension chemistry and mass spectrometric analysis 17. Some SNPs were determined using TaqMan MGB technology from Applied Biosystems and following the manufacturer’s recommendations.
GM-CSF Ab Enzyme-Linked Immunosorbent Assay
Serum samples were stored at −80 degrees Celsius within 4 hours of isolation. Samples were sent for batch analysis of GM-CSF Ab to the Trapnell laboratory in Cincinnati. GM-CSF Ab to glycosylayed GM-CSF were quantified in human serum by enzyme-linked immunosorbent assay (ELISA)18. In our prior report11 we found that antibodies to both glycosylated and non-glycosylated GM-CSF were elevated in adult and pediatric CD compared to UC and controls. However, we identified a strong association with stricturing/penetrating behavior only for antibodies to glycosylated GM-CSF, and so the current study sought to replicate this association.
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 (SPSS Inc., IBM Corporation, Somers NY, USA), Minitab Statistical Software, Release 16 (State College, Pennsylvania), GraphPad Prism version 5.00 (San Diego California, USA), and SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Demographic and baseline disease variables were summarized by cohort. Simple analyses were conducted by the Pearson chi-square test to compare discrete variables, and by 2-sample t test, 1-way ANOVA or the nonparametric alternative, the Kruskal–Wallis test, to compare continuous variables. All statistical tests were two-sided, and results were interpreted at the significance level of 0.05; variables with P-values<0.05 were reported as statistically significant. Odds ratio estimates were computed with 95% confidence intervals. Multiple linear regression and logistic regression analyses were conducted to determine relationships of serum GM-CSF Ab levels or its elevation (≥ 5mcg/mL) respectively with disease location, behavior, and surgery, controlling for the effects of other factors. Factors controlled for included age of diagnosis (by Montreal classification), gender, disease duration, and NOD2 SNP carriage. We evaluated the sensitivity of the regression results against the definition of GM-CSF Ab elevation by using different cutoff values (2, 3 or 4 mcg/mL). Consistent results were obtained (see Tables, Supplemental Digital Content 1–4, http://links.lww.com/IBD/A148, http://links.lww.com/IBD/A149, http://links.lww.com/IBD/A150, and http://links.lww.com/IBD/A151) and validated our main results. The multiple regression analyses were conducted in the combined cohorts (NIDDK and Brisbane). We included cohort effect in the regression analyses in order to account for any non-random discrepancies between the two cohorts unaccounted for by the aforementioned factors. We also included interaction effects by the cohort and smoking with the other factors included in the analyses to entertain the possibility that the cohort and smoking factor may modify the effects of the other factors such that the elevation of serum GM-CSF Ab level may only be significantly associated with ileal disease location among current smokers or ex-smokers. Non-significant interactions indicate no significant modifications by the cohort or smoking factor and allow interpreting the effects of the factors (main effects) as they appear. Conversely significant interactions indicate significant modifications and require the effects of the factors to be interpreted separately by the levels of the cohort or smoking factor. We simplified statistical models by removing non-significant interaction effects until all remaining interaction effects were significant via backward variable selection procedure. We reported the resulting parsimonious model results here, interpreting only significant interaction and main effects in the forms of odds ratio estimates. . We therefore reported OR estimates in the tables for the regression models after accounting for significant interactions between the individual factors considered. In such a case in which there were significant interactions between the individual factors, the OR estimates for the individual effects were not provided because the significant interactions mean that the individual effects are modified by these interactions. In cases where significant interactions were not identified, then the OR estimates for the individual factors were reported.
Results
Clinical and Demographic Characteristics
Descriptive characteristics of the two IBD cohorts are listed in Table 1. Clinical and demographics features of the two cohorts were similar with the following exceptions. The proportion of unaffected males in the Brisbane cohort was significantly higher (p=0.002). Unaffected and UC cases from NIDDK were younger (p<0.001, and p=0.006, respectively). The proportions of unaffected and CD cases of European ancestry from Brisbane were higher (p<0.001 and p<0.001, respectively). Smokers and ex-smokers from Brisbane were significantly higher (p=0.001, p=0.01, and p=0.001 for unaffected, UC and CD subsets, respectively). The proportion of unaffected controls with a history of appendectomy were greater in the Brisbane cohort (p<0.001).
Table 1.
Demographic Characteristics and Serum GM-CSF Ab Concentrations of the Cohorts.
| Diagnosis | Unaffected | UC | CD | |||
|---|---|---|---|---|---|---|
| Cohort | NIDDK | Brisbane | NIDDK | Brisbane | NIDDK | Brisbane |
| Count | 350 | 257 | 139 | 255 | 253 | 224 |
| Male/female (% male ) | 116/233 (33.2) | 118/139 (45.9)**1 | 68/71 (48.9) | 116/139 (45.5) | 121/132 (47.8) | 103/121 (46.0) |
| Age, mean ± S.d. (range) | 33.1±12.9 (16–76) | 58.3 ± 13.5** (22–83) | 43.6 ± 16.6 (1–85) | 48.6 ± 16.1** (18–86) | 41.2 ± 15.1 (3–86) | 42.9 ± 13.8 (16–81) |
| Race | ||||||
| European Ancestry ( % ) | 284 (81.1) | 248 (96.5)** | 126 (90.6) | 218 (85.5) | 218 (86.2) | 222 (99.1)** |
| Other | 65 | 9 | 13 | 16 | 35 | 2 |
| Smokers (at diagnosis) | ||||||
| Yes( % ) | 30(8.7) | 12(6.6)** | 14(10.1) | 36(15.8)* | 46(18.3) | 108(48.2)** |
| Ex-smoker( % ) | 27(7.8) | 103(56.6) | 23(16.5) | 59(25.9) | 24(9.6) | 19(8.5) |
| No( % ) | 289(83.5) | 67(36.8) | 102(73.4) | 133(58.3) | 181(72.1) | 97(43.3) |
| Appendectom y | ||||||
| Yes( % ) | 18(5.2) | 67(26.5)** | 6(4.6) | 20(9.14) | 25(10.6) | 33(15.5) |
| No( % ) | 330(94.8) | 186(73.5) | 124(95.4) | 199(90.9) | 210(89.4) | 180(84.5) |
| GM-CSF Ab (mcg/mL) | ||||||
| Median (IQR) | 0.77 (.41, 1.5) | 0.62 (0.33, 1.5)* | 1.16 (0.46, 3.8) | 0.65 (0.31, 1.5)** | 4.24 (1.4, 10.6) | 2.59 (0.81, 7.6)** |
p-value <0.05 denotes significantly different proportions of affection subsets between NIDDK and Brisbane cohorts; specific values are cited in Results section
The phenotypic characteristics of the two IBD cohorts are listed in Table 2. The NIDDK cohort had a higher proportion of younger patients (p=0.02). Brisbane UC patients had a higher proportion of cases with disease duration longer than 3 years (p=0.01). Isolated Ileal disease was higher among Brisbane CD cases (p<0.001). The proportions of patients who had intestinal resection for surgery was higher in Brisbane (p=0.008 and p=0.01 for UC and CD subsets, respectively). Isolated proctitis in UC was greater in the NIDDK cohort (p=0.001). Exposure to biologic medication was significantly higher in the NIDDK UC cohort (p=0.026).
Table 2.
Phenotypic Characteristics of the IBD Patients.
| Diagnosis | UC | CD | ||
|---|---|---|---|---|
| Cohort | NIDDK | Brisbane | NIDDK | Brisbane |
| Age at diagnosis, mean ± s.d. (range) | 32.7 ± 16 (3–82) | 32.8 ± 14.6 (3–75) | 26.9 ± 13.1 (0–80) | 27.1 ± 11.1 (2–66) |
| Age at diagnosis of CD (Montreal A) | ||||
| A1 (≤16 years) | 20 (14.4) | 26 (10.2) | 48 (19) | 23 (10.3)*2 |
| A2 (17 – 40 years) | 83 (59.7) | 155 (60.4) | 170 (67.2) | 173 (77.2) |
| A3 (> 40 years) | 36 (25.9) | 73 (28.5) | 35 (13.8) | 28 (12.5) |
| Disease duration > 3 years (%) | 110 (79.1) | 226(88.3)* | 225 (88.9) | 204 (91.1) |
| Disease location, CD (Montreal L) | ||||
| L1 ileal | 56 (22.1) | 109 (48.7)** | ||
| L2 colonic | 64 (25.3) | 26 (11.6) | ||
| L3 ileocolonic | 128 (50.6) | 88 (39.3) | ||
| Perianal ± other involvement | 78 (30.8) | 63 (28.1) | ||
| Disease behavior, CD (Montreal B) | ||||
| B1 non-stricturing, non enetrating | 113 (44.7) | 83 (37.1) | ||
| B2 stricturing | 63 (24.9) | 64 (28.6) | ||
| B3 penetrating – excludes perianal | 71 (28.1) | 77 (34.4) | ||
| Surgery for IBD – Yes (%) | 22 (15.8) | 66(25.8)** | 136 (53.8) | 147 (65.6)* |
| Disease location, UC (Montreal E) | ||||
| E1 ulcerative proctitis | 21 (15.1) | 12 (4.7)** | ||
| E2 left-sided UC (distal UC) | 37 (26.6) | 91 (35.5) | ||
| E3 extensive UC (pancolitis) | 79 (56.8) | 136 (53.1) | ||
| Exposure to IBD Medication – Yes (%) | ||||
| 5-Amino Salicylic Acids3 | 127 (91.4) | 212 (82.8) | 189 (74.7) | 62 (27.7) |
| Immunomodulators4 | 64 (46.0) | 108 (42.2) | 167 (66.0) | 167 (74.6) |
| Biologics5 | 23 (16.5) | 23 (9.0) | 100 (39.5) | 74 (33.0)* |
P-value <0.05 denotes significantly different proportions of affection subsets between NIDDK and Brisbane; specific values are cited in results section
5-ASAs:Balsalazide, Mesalamine, Olsalazine, Sulfasalazine
Immunomodulators: Imuran, Methotrexate, Purinethol, Neoral
Biologics: Adalimumab, Certolizumab, Infliximab
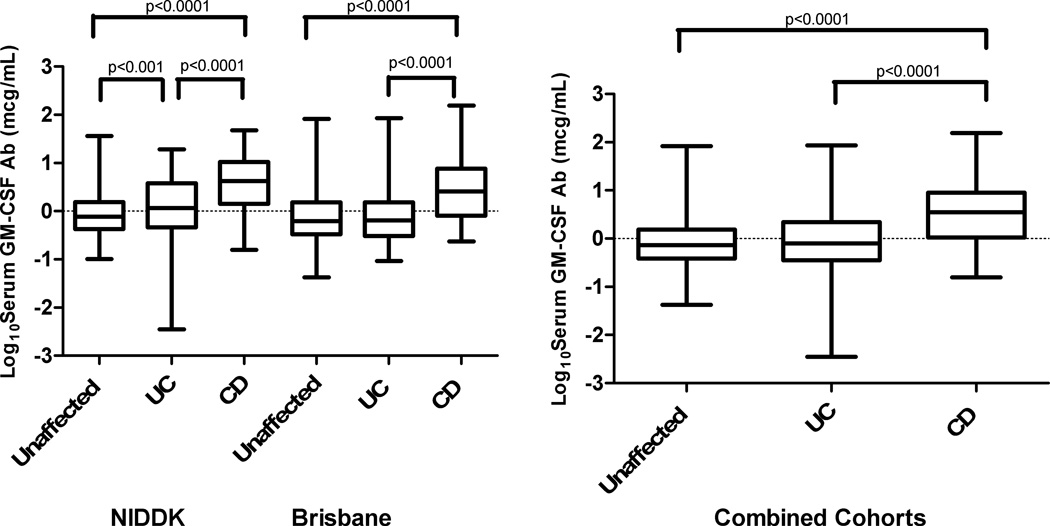
Initial studies were undertaken to define levels of GM-CSF Ab in serum from large cohorts of adult IBD and control patients. Using an assay designed to detect binding of GM-CSF Ab to glycosylated GM-CSF we found that patients with CD expressed significantly higher concentrations when compared to Ulcerative colitis or controls in each cohort, and for the combined cohorts (Figure 1). However, median GM-CSF Ab concentrations were significantly different between the two cohorts (p=0.04, p<0.001, and p=0.007 for unaffected, UC and CD, respectively). Interestingly, median GM-CSF Ab concentrations were significantly higher in non-smokers compared to smokers with CD in the Brisbane cohort (p=0.002) and a similar trend was observed for the NIDDK cohort although not statistically significant (Figure, Supplemental Digital Content 5, http://links.lww.com/IBD/A152). A similar result was observed for the combined cohort, demonstrating that smoking was associated with reduced GM-CSF Ab concentration.
Figure 1.
Serum GM-CSF Ab Levels in Unaffected, UC, and CD Patients. The Log10 transformation of serum GM-CSF Ab (µg/ml) are shown for the NIDDK cohort, the Brisbane cohort, and the combined cohort. The middle line represents the median, and the lower edge and the upper edge of the box represent the 25% and 75% quartiles. The bottom and top lines represent the minimum and maximum values, respectively. For the Brisbane cohort there were 257 Unaffected, 255 UC and 224 CD subjects. For the NIDDK cohort there were 350 Unaffected, 139 UC and 253 CD subjects.
Kruskal-Wallis with Dunn’s post-test revealed significant differences between Unaffected and CD subjects, and UC and CD subjects in both cohorts analyzed separately and when combined. Pairwise comparison of each disease phenotype between the two cohorts revealed that the median log transformed serum GM-CSF Ab level in the NIDDK UC group was higher than the Brisbane UC group.
We then asked whether GM-CSF Ab level would vary with IBD risk allele carriage. Serum GM-CSF Ab expression level was elevated in CD patients independent of SNP carriage for the genetic markers NOD2, ATG16L1, IRGM, JAK2 or STAT3 (Tables, Supplemental Digital Content 6, http://links.lww.com/IBD/A153). This was true whether the cohorts were analyzed individually, or combined. Associations between smoking and elevated GM-CSF Ab, and between elevated GM-CSF Ab and ileal CD location, stricturing/penetrating disease behavior, and surgery were examined using multiple linear and logistic regression as described in the statistical analysis section after accounting for the effects of age of diagnosis, gender, disease duration, NOD2 risk allele carriage, smoking status, and perianal disease location.
First, we examined the effect of smoking upon serum GM-CSF Ab concentration (Table 3). Significant two way and three way interactions of smoking status with ileal location and cohort indicate a modified effect of smoking status, different by ileal location and between the cohorts. Current or past smoking was significantly associated with the lower odds of GM-CSF Ab elevation in the NIDDK cohort with ileal location (OR=0.32 (0.17–0.61)). A similar trend was observed within the Brisbane cohort with ileal location but did not reach significance. No significant effect was observed within the smaller subset of CD patients with colon-only location. These results conversely demonstrated that non-smoking status at diagnosis was associated with elevated serum GM-CSF Ab amongst CD patients with ileal location, with a significant effect observed within the NIDDK cohort.
Table 3.
Multiple Logistic Regression Results for Elevated Serum GM-CSF Ab Concentration (≥ 5mcg/mL)
| Factors | P-values | ||
|---|---|---|---|
| Age at diagnosis (>16yrs) | 0.6758 | ||
| Gender | 0.8317 | ||
| NOD2 risk allele carriage | 0.7367 | ||
| Cohort | 0.7530 | ||
| Smoking | 0.0686 | ||
| Ileal Location | 0.0086 | ||
| Perianal | 0.3149 | ||
| Disease duration (>3yrs) | 0.5496 | ||
| Cohort*smoking | 0.0322 | ||
| smoking*Ileal Location | 0.0015 | ||
| Cohort*smoking*Ileal Location | 0.0038 | ||
| Factors | By Ileal location | By Cohort | OR (95%CI) |
| Smoking Yes vs. No | Yes | Brisbane | 0.70 (0.35–1.41) |
| NIDDK | 0.32 (0.17–0.61) | ||
| No | Brisbane | 0.63 (0.21–1.89) | |
| NIDDK | 4.35 (0.83–20.4) | ||
A multiple logistic regression model was fitted with elevated GM-CSF-Ab as the response variable. Main effects for the variables age at diagnosis, gender, NOD2 allele, Cohort, Smoking, Ileal location, Perianal location and disease duration were included in the model automatically, while interaction terms between these main effects were included only if significant after backward elimination. Table 3 displays the p-values for all fitted terms in the final model, as well as the estimated odds ratios (OR) for significant association of Elevated GM-CSF-Ab with smoking. The odds ratio estimates were broken down by Ileal location and cohort as the significant association was defined by interactions of smoking with Ileal location and cohort factors.
Second, we tested the relationship between elevated serum GM-CSF Ab concentration and ileal disease location (Table 4). Significant two way and three way interactions with smoking status at diagnosis and cohort suggest a modified effect of elevated GM-CSF Ab level, different by smoking status at diagnosis and between the cohorts. Elevated GM-CSF Ab level was associated with the higher odds of ileal location, and was only significant for non-smokers. Among current or ex-smokers at the time of diagnosis, no significant association was found and the direction of the association was not consistent between the cohorts. This analysis held true for all the GM-CSF Ab cutoff values tested (Table, Supplemental Digital Content 2, http://links.lww.com/IBD/A149).
Table 4.
Multiple Logistic Regression Results for Ileal Disease Location.
| Fctors | P-values | ||
|---|---|---|---|
| Age at diagnosis (>16yrs) | 0.6809 | ||
| Gender | 0.3071 | ||
| NOD2 risk allele carriage | 0.1408 | ||
| Cohort | 0.2269 | ||
| Smoking | 0.0044 | ||
| Elevated GM-CSF Ab | 0.0108 | ||
| Perianal location | 0.0029 | ||
| Smoking* Elevated GM-CSF Ab | 0.0023 | ||
| Cohort*Smoking | 0.0010 | ||
| Cohort*Smoking* Elevated GM-CSF Ab | 0.0048 | ||
| Factors | By Smoking | By Cohort | OR (95%CI) |
|
GM-CSF Ab
Elevation Yes vs. No |
Yes | Brisbane | 0.19 (0.04–0.81) |
| NIDDK | 2.67 (0.88–8.06) | ||
| No | Brisbane | 2.27 (1.21–4.26) | |
| NIDDK | 2.25 (1.21–4.25) | ||
A multiple logistic regression model was fitted with Ileal CD location as the response variable. Main effects for the variables age at diagnosis, gender, NOD2 allele, Cohort, Smoking, elevated GM-CSF Ab (≥ 5mcg/mL), and Perianal location were included in the model automatically, while interaction terms between these main effects were included only if significant after backward elimination. Table 4 displays the p-values for all fitted terms in the final model, as well as the estimated odds ratios for association of Ileal CD location with Elevated GM-CSF-Ab. The odds ratio estimates were broken down by smoking and cohort as the significant association was defined by interactions of elevated GM-CSF-Ab with smoking and cohort.
Third, we tested the relationship between elevated serum GM-CSF Ab concentration and stricturing (B2) or penetrating (B3) disease behavior (Table 5). Absence of significant interaction suggests a consistent effect of GM-CSF Ab elevation between the cohorts and by smoking status. Elevated GM-CSF Ab level was significantly associated with higher odds of stricturing or penetrating disease behavior uniformly in both cohorts regardless of their smoking status. A significant interaction of ileal location with the cohort indicates that ileal location was associated differently between the cohorts. Ileal location was significantly associated with higher odds of the more aggressive CD phenotypes in both the cohorts with the odds much higher in the NIDDK cohort. As expected, disease duration greater than 3 years was independently significantly associated with higher odds (p=0.015) for stricturing/penetrating disease behavior.
Table 5.
Multiple Logistic Regression Results for Stricturing/Penetrating Behavior.
| Factors | P-values | |
|---|---|---|
| Age at diagnosis (>16yrs) | 0.6526 | |
| Gender | 0.1788 | |
| NOD2 risk allele carriage | 0.7334 | |
| Cohort | 0.0205 | |
| Smoking | 0.8690 | |
| Elevated GM-CSF Ab | 0.0001 | |
| Ileal Location | 0.0481 | |
| Perianal location | 0.2250 | |
| Disease Duration (>3yrs) | 0.0146 | |
| Cohort* Ileal Location | 0.0722 | |
| Factors | By Cohort | OR (95%CI) |
| Ileal Location Yes vs. No | Brisbane | 2.48(1.01–6.10) |
| NIDDK | 7.09(3.47–14.49) | |
| Disease Duration >3yrs vs. ≤3 | 2.46 (1.20–5.07) | |
| Elevated GM-CSF Ab Yes vs. No | 2.37 (1.53–3.67) | |
A multiple logistic regression model was fitted with Stricturing/Penetrating behavior as the response variable. Main effects for the variables age at diagnosis, gender, NOD2 allele, Cohort, Smoking, elevated GM-CSF Ab (≥ 5mcg/mL), Perianal location and disease duration were included in the model automatically, while interaction terms between these main effects were included only if significant after backward elimination. Table 5 displays the p-values for all fitted terms in the final model, as well as the estimated odds ratios for association of Stricturing/Penetrating CD behavior with Ileal CD location, disease duration longer than 3 years, and Elevated GM-CSF-Ab. The Ileal location odds ratio estimates were broken down by cohort as the significant association was defined by interaction of Ileal location with cohort. OR estimates are reported for the individual factors disease duration and elevated GM-CSF Ab as significant interactions for these factors were not identified.
Finally, we tested the relationship between elevated serum GM-CSF Ab concentration and a surgical event (intestinal resection) (Table 6). Absence of significant interaction suggests a consistent effect of GM-CSF Ab concentration; elevated GM-CSF Ab level was significantly associated with the higher odd of surgery uniformly in both the cohorts regardless of their smoking status (OR=1.80, (1.17–2.76)). Ileal location was also uniformly significantly associated with the higher odds for surgery (OR=2.30, (1.35, 3.91)). Disease duration greater than 3 years was also independently significantly associated with surgery (p=0.0005). We observed an interaction between NOD2 risk allele carriage, smoking status at diagnosis, and cohort. In this regard, smokers within the NIDDK cohort who carried a NOD2 risk allele exhibited a greater risk for surgery. A similar trend was observed within the Brisbane cohort which did not reach significance. Collectively, these results demonstrated that elevated GM-CSF Ab was associated with increased risk for surgery in CD, after controlling for NOD2 risk allele carriage, ileal disease location, smoking status, and duration of disease.
Table 6.
Multiple Logistic Regression Results for Surgery.
| Factors | P-values | ||
|---|---|---|---|
| Age at diagnosis (>16yrs) | 0.5205 | ||
| Gender | 0.8879 | ||
| NOD2 risk allele carriage | 0.2884 | ||
| Cohort | <.0001 | ||
| Smoking | 0.2016 | ||
| Elevated GM-CSF Ab | 0.0078 | ||
| Ileal Location | 0.0022 | ||
| Perianal location | 0.2866 | ||
| Disease Duration (>3yrs) | 0.0005 | ||
| Cohort*NOD2 | 0.0346 | ||
| NOD2*smoking | 0.0356 | ||
| Cohort*smoking | 0.0330 | ||
| Cohort*Perianal | 0.0020 | ||
| Factors | By Smoking | By Cohort | OR (95%CI) |
| NOD2 Mutation Yes vs. No | Yes | Brisbane | 1.98(0.86–4.57) |
| NIDDK | 5.92(1.90–18.18) | ||
| No | Brisbane | 0.62(0.26–1.49) | |
| NIDDK | 1.86(0.96–3.58) | ||
| Ileal Location Yes vs. No | 2.30 (1.35–3.91) | ||
| Disease Duration >3yrs vs. ≤3 | 3.75 (1.79–7.88) | ||
| Elevated GM-CSF Ab Yes vs No | 1.80 (1.17–2.76) | ||
A multiple logistic regression model was fitted with Surgery as the response variable. Main effects for the variables age at diagnosis, gender, NOD2 allele, Cohort, Smoking, elevated GM-CSF Ab (≥ 5mcg/mL), Ileal CD location, Perianal location and disease duration were included in the model automatically, while interaction terms between these main effects were included only if significant after backward elimination. Table 6 displays the p-values for all fitted terms in the final model, as well as the estimated odds ratios for association of surgery with NOD2 risk allele carriage, Ileal CD location, disease duration longer than 3 years, and Elevated GM-CSF-Ab. The NOD2 risk allele odds ratio estimates were broken down by smoking and cohort as the significant association was defined by interactions of NOD2 risk allele carriage with smoking and cohort. OR estimates are reported for the individual factors ileal location, disease duration, and elevated GM-CSF Ab as significant interactions for these factors were not identified.
Discussion
There has been tremendous progress in the discovery of genetic markers that predict the risk of developing Inflammatory Bowel Disease. The 71 confirmed Crohn’s susceptibility loci explain just 23.1% of the heritability 19. Many risk polymorphisms implicate defects in innate immunity in CD 19, 20. Other factors that may contribute to CD expression could result from the abnormal immune response in genetically predisposed individuals. GM-CSF is produced by several laminia propria immune cells21 and has a role in promoting intestinal epithelial barrier integrity, stimulating crypt cell proliferation in acute injury, and decreasing inflammation 22–24. Recombinant GM-CSF has also been examined in clinical trials with both favorable and negative efficacy results suggesting an effect in a subset of patients 25–30. Elevated GM-CSF antibodies have been shown to impair neutrophil cell function in patients with CD and to exacerbate NSAID-induced transmural ileitis in mouse models11.
In this cross-sectional study we examined the correlation of GM-CSF neutralizing Ab with CD location and behavior. Similar to the study by Han et al11, we confirmed that GMCSF Ab expression is elevated in CD when compared to UC or unaffected individuals. Serum GM-CSF Ab expression level was elevated in CD patients independent of SNP carriage for the genetic markers NOD2, ATG16L1, IRGM, JAK2 or STAT3. Studies regarding a potential association between NOD2 genotype and antimocrobial serologies have yielded conflicting results, although several have suggested that CD patients with NOD2 risk allele carriage are more likely to be positive for ASCA. The lack of association between the NOD2 genotype and serum GM-CSF antibody concentration in the current study is consistent with our prior report in 354 primarily pediatric-onset IBD patients, and so is unlikely to be due to a methodological issue 11. Future studies will utilize genome-wide approaches and larger cohorts to test for genetic variants associated with GM-CSF Ab concentration.
We also examined for the first time, the effect of smoking on GM-CSF Ab expression. Our primary finding was that smokers with ileal CD in both the Brisbane and NIDDK CD cohorts had significantly lower GM-CSF Ab concentrations. This result was surprising, as smoking is associated with a higher prevalence of complicated ileal CD31 which is less responsive to anti-TNF therapy. In our prior report11 we showed that patients with elevated GM-CSF antibodies are more likely to have elevated titers of ASCA. Therefore, similar immune pathways may drive production of GM-CSF antibodies and ASCA. A prior study32 reported that the frequency of ASCA seropositivity was significantly lower in smokers with adult-onset CD compared to non-smokers with adult-onset CD. The authors concluded that smoking may regulate immune responses to intestinal antigens. A more recent study confirmed a trend towards a lower rate of ASCA seropositivity in adult-onset CD smokers compared to non-smokers, although this did not reach significance33. Smoking has immune-suppressive effects which could result in reduced production of ASCA and GM-CSF auto-antibodies. These include suppression of dendritic cell maturation and antigen presentation, inhibition of T cell antibody-forming responses, and reduction in circulating levels of immune-globulins. Nicotine and more recently carbon monoxide have been implicated in mediating these effects 34. However, a large cohort study of adults with pulmonary alveolar proteinosis did not identify an association between smoking status and serum GM-CSF Ab concentration35. Conversely, smoking may augment auto-antibody production in rheumatoid arthritis and lupus 34. Collectively, these data suggest that smoking may exert a fundamentally different effect with regard to development of sero-reactivity to intestinal versus extra-intestinal antigens. This mechanism will be addressed in future studies
Smoking, elevated GM-CSF Ab, and perianal disease were significantly associated with ileal disease location in the combined cohort analysis. Smokers had significantly higher odds (by 6.76 times) of having ileal disease location compared to non-smokers in the Brisbane cohort when GM-CSF Ab was less than 5mcg/mL. Consistent with prior studies, perianal location was associated with significantly lower odds (by 0.461 times) of having ileal disease location. After controlling for these factors, elevated GM-CSF Ab (≥5mcg/mL) was associated with significantly higher odds (by 2.27 times) of having ileal disease location among non-smokers both in the Brisbane and the NIDDK cohort.
Ileal CD, elevated GM-CSF Ab, and disease duration longer than 3 years were risk factors for stricturing or penetrating disease behavior and for surgery. Interestingly, smoking and NOD2 risk genotypes, factors that are associated with ileal disease location were not independent risk factors for stricturing or penetrating behavior in our combined cohort analysis except as effect modifiers for surgery. Prior studies looking at the effect of smoking in CD report a higher prevalence of ileal disease and a lower prevalence of colonic involvement36, 37. Smoking is also linked to a greater likelihood of more complicated CD behavior including more frequency of stricturing or penetrating disease behavior, more frequent disease flares, and a higher risk of surgery38–42. In our study, non-smokers with ileal CD and high GM-CSF Ab concentration had the greatest frequency of stricturing/penetrating CD behavior. Thus, smoking appeared to offer some protection against high GM-CSF Ab production and a resultant lower frequency of stricturing or penetrating disease behavior among patients with ileal disease.
The processes by which deficiency of GM-CSF culminates in a more severe CD phenotype are currently being explored. In a recent study, NOD2 deficient and WT mice demonstrated an increase in CCL25, a chemokine involved in immune cell homing to the small intestine, and CCR9 it’s cognate receptor after neutralization of GM-CSF. This correlated with an increased fraction of CCR9+ lymphocytes and a transmural ulcerating ileal disease 11, 43. Samson et al43 also observed an increase in ileal CCR9+ lamina propria mononuclear cells (LPMC) obtained from ileal biopsies and in CCR9+ lymphocytes from peripheral blood in pediatric CD patients expressing high neutralizing antibodies to GM-CSF. Therapeutic blockade of CCR9 can ameliorate early murine ileitis44 and a phase II study of a CCR9 antagonist has demonstrated efficacy in patients with moderate to severe CD43, 45.
In our study elevated GM-CSF Ab was associated with higher incidence of surgery for IBD. One limitation was that since this was a cross-sectional study, we could not determine if elevated GM-CSF Ab expression results in an earlier time to surgery. Another limitation of our study was that serum samples and phenotype data from patients were obtained only at the time of enrollment. As such, we could not assess the direct impact of GM-CSF antibody titers on disease activity. Longitudinal assessment and disease activity measurements using standardized instruments such as the CDAI, may be more useful in correlating with mucosal inflammation.
Our study confirms that the expression of elevated GM-CSF Ab in Crohn’s disease is correlated with aggressive disease behavior. Smoking appeared to be a negative modifier of GM-CSF Ab concentration in this study. Longitudinal studies including dosage of nicotine exposure, along with other factors such as exercise and dietary factors are indicated to better define the role of smoking in GM-CSF Ab production. The studies primary finding was that CD patients with elevated GM-CSF auto-antibodies were more likely to experience stricturing/penetrating behavior. Longitudinal studies are needed to examine the natural history of GM-CSF Ab expression and its effect on the bioavailability of the GM-CSF cytokine. It would also be beneficial to identify the antigenic trigger for the expression of autoantibodies. This will require an examination of the composition of the intestinal microbiota and other mediators of the intestinal barrier integrity. The results of this kind of study will help to identify the subset of patients expressing high GMCSF Ab who may benefit from more aggressive therapy to induce disease remission and mucosal healing.
Supplementary Material
Acknowledgments
The authors thank Dr Ellen Li and Dr Catherine Messina (Stony Brook University Medical Center) for critical reading of the manuscript and for technical assistance.
Grant Support: This work was supported in part by the National Institutes of Health grants U01DK 062422-08 S1, R01DK078683-03, NIDDK U01 DK062429 (J.H.C.), U01 DK062422 (J.H.C.), and R01HL085453
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Judy H. Cho is a medical consultant for Pfizer. All remaining authors have nothing to disclose
- conception and design of the study;
- generation, collection, assembly, analysis and/or interpretation of data;
- drafting or revision of the manuscript;
- approval of the final version of the manuscript;
Contributor Information
Mi-Ok Kim, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States of America.
John P. Ferguson, Department of Medicine, Section of Digestive Diseases, Yale University School of Medicine, New Haven, CT, 06520 United States of America.
Yashoda Sharma, Department of Medicine, Section of Digestive Diseases, Yale University School of Medicine, New Haven, CT, 06520 United States of America.
Wei Zhang, Department of Medicine, Section of Digestive Diseases, Yale University School of Medicine, New Haven, CT, 06520 United States of America.
Sok Meng E. Ng, Department of Medicine, Section of Digestive Diseases, Yale University School of Medicine, New Haven, CT, 06520 United States of America
Erin Bonkowski, Gastroenterology, Hepatology, and Nutrition, Cincinnati Children's Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH, 45267 United States of America.
Kaida Ning, Department of Medicine, Section of Digestive Diseases, Yale University School of Medicine, New Haven, CT, 06520 United States of America.
Lisa A. Simms, Inflammatory Bowel Diseases, Genetic Epidemiology, Queensland Institute of Medical Research and Department of Gastroenterology, Royal Brisbane and Women’s Hospital, Brisbane, Qld 4029 Australia.
Anthony R. Croft, Department of Gastroenterology, Royal Brisbane and Women’s Hospital, Brisbane, Australia.
Joanne M. Stempak, IBD Research Unit, Zane Cohen Centre, Mount Sinai Hospital, Toronto, ON, M5T 3L9 Canada.
Nicole Walker, Department of Gastroenterology, Royal Brisbane and Women’s Hospital, Brisbane, Australia.
Ning Huang, Inflammatory Bowel Diseases, Genetic Epidemiology, Queensland Institute of Medical Research, Brisbane, Australia.
Yang Xiao, Department of Mathematical Sciences, University of Cincinnati, Cincinnati, OH, United States of America.
Mark S. Silverberg, IBD Research Unit, Zane Cohen Centre, Mount Sinai Hospital, Toronto, ON, M5T 3L9 Canada.
Bruce C. Trapnell, Pulmonary Biology, Cincinnati Children's Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH, United States of America.
Judy H. Cho, Department of Medicine, Section of Digestive Diseases and Department of Genetics, Yale University School of Medicine, New Haven, CT, United States of America.
Graham L. Radford-Smith, Inflammatory Bowel Diseases, Genetic Epidemiology, Queensland Institute of Medical Research and Department of Gastroenterology, Royal Brisbane and Women’s Hospital, Brisbane, Qld 4029 Australia.
Lee A. Denson, Gastroenterology, Hepatology, and Nutrition, Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH, 45267 United States of America.
References
- 1.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 2.Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol. 2010;45:9–16. doi: 10.1007/s00535-009-0138-3. [DOI] [PubMed] [Google Scholar]
- 3.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franke A, Balschun T, Karlsen TH, et al. Replication of signals from recent studies of Crohn's disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet. 2008;40:713–715. doi: 10.1038/ng.148. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg MS, Cho JH, Rioux JD, et al. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41:216–220. doi: 10.1038/ng.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 7.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 8.Parkes M, Barrett JC, Prescott NJ, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rioux JD, Xavier RJ, Taylor KD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebert EC, Panja A, Das KM, et al. Patients with inflammatory bowel disease may have a transforming growth factor-beta-, interleukin (IL)-2- or IL-10-deficient state induced by intrinsic neutralizing antibodies. Clin Exp Immunol. 2009;155:65–71. doi: 10.1111/j.1365-2249.2008.03802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han X, Uchida K, Jurickova I, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn's disease. Gastroenterology. 2009;136:1261–1271. doi: 10.1053/j.gastro.2008.12.046. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Liu CH, Roberts AI, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura T, Tanaka N, Watanabe J, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoch OD, Schanz U, Koller M, et al. BAL findings in a patient with pulmonary alveolar proteinosis successfully treated with GM-CSF. Thorax. 2002;57:277–280. doi: 10.1136/thorax.57.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan JB, Chee MS, Gunderson KL. Highly parallel genomic assays. Nat Rev Genet. 2006;7:632–644. doi: 10.1038/nrg1901. [DOI] [PubMed] [Google Scholar]
- 17.Jurinke C, Oeth P, van den Boom D. MALDI-TOF mass spectrometry: a versatile tool for high-performance DNA analysis. Mol Biotechnol. 2004;26:147–164. doi: 10.1385/MB:26:2:147. [DOI] [PubMed] [Google Scholar]
- 18.Uchida K, Nakata K, Trapnell BC, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–1098. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- 19.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks DJ, Rahman FZ, Sewell GW, et al. Crohn's disease: an immune deficiency state. Clin Rev Allergy Immunol. 2010;38:20–31. doi: 10.1007/s12016-009-8133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung HC, Eckmann L, Yang SK, et al. A Distinct Array of Proinflammatory Cytokines Is Expressed in Human Colon Epithelial-Cells in Response to Bacterial Invasion. Journal of Clinical Investigation. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sennikov SV, Temchura VV, Kozlov VA, et al. The influence of conditioned medium from mouse intestinal epithelial cells on the proliferative activity of crypt cells: role of granulocyte-macrophage colony-stimulating factor. J Gastroenterol. 2002;37:1048–1051. doi: 10.1007/s005350200176. [DOI] [PubMed] [Google Scholar]
- 23.Sainathan SK, Hanna EM, Gong Q, et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2008;14:88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernasconi E, Favre L, Maillard MH, et al. Granulocyte-Macrophage Colony-Stimulating Factor Elicits Bone Marrow-Derived Cells that Promote Efficient Colonic Mucosal Healing. Inflamm Bowel Dis. 2010;16:428–441. doi: 10.1002/ibd.21072. [DOI] [PubMed] [Google Scholar]
- 25.Korzenik JR, Dieckgraefe BK, Valentine JF, et al. Sargramostim for active Crohn's disease. N Engl J Med. 2005;352:2193–2201. doi: 10.1056/NEJMoa041109. [DOI] [PubMed] [Google Scholar]
- 26.Takazoe M, Matsui T, Motoya S, et al. Sargramostim in patients with Crohn's disease: results of a phase 1–2 study. J Gastroenterol. 2009;44:535–543. doi: 10.1007/s00535-009-0029-7. [DOI] [PubMed] [Google Scholar]
- 27.Valentine JF, Fedorak RN, Feagan B, et al. Steroid-sparing properties of sargramostim in patients with corticosteroid-dependent Crohn's disease: a randomised, double-blind, placebo-controlled, phase 2 study. Gut. 2009;58:1354–1362. doi: 10.1136/gut.2008.165738. [DOI] [PubMed] [Google Scholar]
- 28.Kelsen JR, Rosh J, Heyman M, et al. Phase I trial of sargramostim in pediatric Crohn's disease. Inflamm Bowel Dis. 2010;16:1203–1208. doi: 10.1002/ibd.21204. [DOI] [PubMed] [Google Scholar]
- 29.Magno P, Jimenez CE, Ortiz Z, et al. Recombinant Human Granulocyte-Macrophage Colony Stimulating Factor (sargramostim) as an Alternative Therapy for Fistulizing Crohn's Disease. Puerto Rico Health Sciences Journal. 2010;29:60–65. [PubMed] [Google Scholar]
- 30.Wong AC, Lee SD. Long-term therapy with sargramostim in a patient with Crohn's disease. Inflamm Bowel Dis. 2011;17:1447–1448. doi: 10.1002/ibd.21542. [DOI] [PubMed] [Google Scholar]
- 31.Lawrance IC, Batman B, Gearry RB, et al. Smoking Cessation Following the Diagnosis of Crohn's Disease Still Reduces the Rates of Surgery and Complicated Disease. Gastroenterology. 2011;140:S429–S429. [Google Scholar]
- 32.Van Kemseke C, Belaiche J, Steeman C, et al. Negative association between smoking and anti-saccharomyces cerevisiae antibodies in Crohn's disease. Acta Gastro-Enterologica Belgica. 2003;66:1–6. [PubMed] [Google Scholar]
- 33.Vander Cruyssen B, Peeters H, Hoffman IEA, et al. CARD15 polymorphisms are associated with anti-Saccharomyces cerevisiae antibodies in caucasian Crohn's disease patients. Clinical and Experimental Immunology. 2005;140:354–359. doi: 10.1111/j.1365-2249.2005.02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. Journal of Autoimmunity. 2010;34:J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008;177:752–762. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindberg E, Jarnerot G, Huitfeldt B. Smoking in Crohn's disease: effect on localisation and clinical course. Gut. 1992;33:779–782. doi: 10.1136/gut.33.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahid SS, Minor KS, Stevens PL, et al. The role of smoking in crohn's disease as defined by clinical variables. Digestive Diseases and Sciences. 2007;52:2897–2903. doi: 10.1007/s10620-006-9624-0. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland LR, Ramcharan S, Bryant H, et al. Effect of cigarette smoking on recurrence of Crohn's disease. Gastroenterology. 1990;98:1123–1128. doi: 10.1016/0016-5085(90)90324-t. [DOI] [PubMed] [Google Scholar]
- 39.Cosnes J, Carbonnel F, Carrat F, et al. Effects of current and former cigarette smoking on the clinical course of Crohn's disease. Aliment Pharmacol Ther. 1999;13:1403–1411. doi: 10.1046/j.1365-2036.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 40.Louis E, Michel V, Hugot JP, et al. Early development of stricturing or penetrating pattern in Crohn's disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut. 2003;52:552–557. doi: 10.1136/gut.52.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picco MF, Bayless TM. Tobacco consumption and disease duration are associated with fistulizing and stricturing behaviors in the first 8 years of Crohn's disease. Am J Gastroenterol. 2003;98:363–368. doi: 10.1111/j.1572-0241.2003.07240.x. [DOI] [PubMed] [Google Scholar]
- 42.Aldhous MC, Drummond HE, Anderson N, et al. Does cigarette smoking influence the phenotype of Crohn's disease? Analysis using the Montreal classification. American Journal of Gastroenterology. 2007;102:577–588. doi: 10.1111/j.1572-0241.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- 43.Samson CM, Jurickova I, Molden E, et al. Granulocyte-macrophage colony stimulating factor blockade promotes ccr9(+) lymphocyte expansion in Nod2 deficient mice. Inflamm Bowel Dis. 2011 doi: 10.1002/ibd.21672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivera-Nieves J, Ho J, Bamias G, et al. Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine ileitis. Gastroenterology. 2006;131:1518–1529. doi: 10.1053/j.gastro.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 45.Walters MJ, Wang Y, Lai N, et al. Characterization of CCX282-B, an orally bioavailable antagonist of the CCR9 chemokine receptor, for treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2010;335:61–69. doi: 10.1124/jpet.110.169714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.