Abstract
Non-small cell lung cancer (NSCLC) is the most common cause of cancer deaths worldwide. The majority of patents presenting with NSCLC have advanced disease, which precludes curative treatment. Early detection and treatment might result in the identification of more patients with early central lung cancer and improve survival. In addition, the study of early lung cancer improves understanding of lung carcinogenesis and might also reveal new treatment targets for advanced lung cancer. Bronchoscopic investigation of the central airways can reveal both early central lung cancer in situ (stage 0) and other preinvasive lesions such as dysplasia. In the current review we discuss the detection of early squamous lung cancer, the natural history of preinvasive lesions and whether biomarkers can be used to predict progression to cancer. Finally we will review the staging and management of preinvasive lung cancer lesions and the different therapeutic modalities that are available.
Keywords: biomarkers, diagnosis, non-small cell lung cancer, treatment
Introduction
Non-small cell lung cancer (NSCLC) is the most common cause of cancer deaths worldwide [Jemal et al. 2009; Parkin et al. 2005]. The reason for the unsatisfactory overall 5-year survival rate of approximately 15% mainly lies in the often advanced stage of the disease at the time of diagnosis and the inability to cure metastatic disease. Even for early stage lung cancer (stage I and II), which is usually treated with curative intent, 5-year survival is only about 50–60% [Groome et al. 2007]. This is caused by both subsequent lung cancer primaries and metastatic disease. In contrast, the prognosis of early central squamous lung cancer in-situ (stage 0) is excellent [Cortese et al. 1983; Fujimura et al. 2000; Kennedy et al. 2007; Nakamura et al. 2001; Woolner et al. 1984]. Early detection strategies might result in identification of such microinvasive and preinvasive lung cancers that are still eligible for curative treatment. This underscores the importance of studying pre-/microinvasive lung cancer. In addition, such studies improve our understanding of lung carcinogenesis, might identify valuable prognostic and predictive markers and might reveal new molecular targets for chemoprevention and the treatment of advanced lung cancer.
Screening trials for early detection of lung cancer in high-risk subjects are ongoing and likely identify large numbers of patients with small endobronchial lesions. Radical treatments, such as surgical resection or radiotherapy, might not be in the best interest of these patients. First, early squamous lung cancer is often centrally located, which necessitates bronchotomy, sleeve lobectomy or pneumonectomy. Second, patients often present with synchronous or metachronous lesions that would require multiple resections. Third, patients with the highest risk of lung cancer often harbor significant comorbidities, such as chronic obstructive pulmonary disease (COPD) and cardiovascular disease. An alternative curative approach for these patients may be minimal invasive tissue sparing bronchoscopic treatment modalities such as electrocautery, argon plasma coagulation (APC), cryotherapy and photodynamic therapy (PDT). In this review we first discuss the detection of early squamous lung cancer. Subsequently we describe the natural history of these lesions and whether biomarkers can be used to predict progression to cancer. Finally we review the staging and management of preinvasive lung cancer lesions and the therapeutic modalities that can be applied.
Detection of early squamous lung cancer
The classic screening method for centrally located early lung cancer is sputum cytology. However, this method is limited by low sensitivity which is due to sampling error, technical difficulties in the preparation of samples and significant variations in intra- and interobserver agreement [Holiday et al. 1995; Motherby et al. 1999]. The introduction of white light flexible fiberoptic bronchoscopy (WLB) has enabled visual inspection of the central airways [Chhajed et al. 2005; Shibuya et al. 2002; Wagnieres et al. 2003]. However, regardless of the technological improvements that have led to the modern day videobronchoscopy systems, the sensitivity of WLB for detecting early stage lung cancer remains low [Chhajed et al. 2005; Venmans et al. 1998]. Autofluorescence bronchoscopy (AFB) has dramatically increased the detection of preinvasive endobronchial lesions [Chiyo et al. 2005; Ernst et al. 2005; Fielding, 2004; Goujon et al. 2003; Haussinger et al. 2005; Hirsch et al. 2001; Horvath et al. 1999; Hung et al. 1991; Ikeda et al. 2006; Kennedy et al. 2005; Kusunoki et al. 2000; Lam et al. 1994, 1998, 2000; Qu et al. 1994, 1995; Sato et al. 2001; Shibuya et al. 2001; Vermylen et al. 1999; Wagnieres et al. 2003]. Autofluorescence imaging utilizes the spectral differences in fluorescence and absorption properties of normal and dysplastic bronchial epithelium. While the reported sensitivity of WLB is 9–58%, AFB performs better with a sensitivity of 44–82% [Ernst et al. 2005; Haussinger et al. 2005; Hirsch et al. 2001; Lam et al. 1998]. A drawback of the increased sensitivity of AFB is a reduced specificity of 46–75% compared with a specificity of 62–95% for WLB [Ernst et al. 2005; Haussinger et al. 2005; Hirsch et al. 2001; Lam et al. 1998]. The detection of false-positive lesions leads to an increase in unnecessary biopsies which impacts on the cost effectiveness of this technique. However, there are some recent data showing that areas with abnormal autofluorescence but benign histopathology contain increased chromosomal aberrations and that the presence of multiple areas of abnormal autofluorescence may be an indicator of increased lung cancer risk [Helfritzsch et al. 2002; Pasic et al. 2003]. Recently, the use of a quantitative score during autofluorescence examination has been shown to improve specificity [Lee et al. 2007].
An alternative approach to AFB is high-magnification bronchoscopy (Exera, Olympus Optical Corp., Tokyo, Japan), which combines both fiberoptic and videobronchoscope technologies to produce 100–110× magnification of the bronchial wall compared with standard videobronchoscopes [Shibuya et al. 2003]. This technique enables the visualization of microvascular networks in the bronchial mucosa. Increased vessel density in the bronchial submucosa is often present in squamous dysplasia and may play an early role in cancer pathogenesis [Keith et al. 2000]. Narrow band imaging (NBI, Olympus Optical Corp.) is a novel system that also utilizes the changes seen in the microvascular network. This technique uses a narrow band filter rather than the conventional broad RGB (red/green/blue) filter used in standard videobronchoscopes. The conventional RGB filter uses 400–500 nm (blue), 500–600 nm (green) and 600–700 nm (red). NBI uses three narrow bands, 400–430 nm (blue – covers hemoglobin absorption at 410 nm), 420–470 nm (blue) and 560–590 nm (green). Blue light has a short wavelength, reaches into the bronchial submucosa and is absorbed by hemoglobin. This technique enables the detection of increased vessel growth and complex networks of tortuous vessels, dotted vessels and spiral or screw type tumor vessels of the bronchial mucosa, which might reflect the onset of angiogenesis in the process of carcinogenesis [Shibuya et al. 2010]. On evaluation of airway lesions that were abnormal under autofluorescence imaging, this technique provides more accurate images of microvessels compared with high-magnification videobronchoscopy using broadband RGB technology [Shibuya et al. 2003]. NBI in comparison to standard white light videobronchoscopy seemed to improve the detection of dysplasia/malignancy when used as an adjunct to white light in this small study [Vincent et al. 2007]. Only one prospective study compared the diagnostic yield of WLB, NBI and AFB in early squamous lung cancer [Herth et al. 2009]. The authors concluded that NBI might increase the specificity of bronchoscopic detection. NBI and AFB might be complementary techniques in the future.
Optical coherence tomography (OCT) is a powerful optical imaging technique that offers high resolution visualization of structures at and below the tissue surface. It is a method similar to ultrasound, but applies near infrared light instead of sound waves to the endobronchial tissue via a small probe that reaches the lesion via the working channel of a bronchoscope. Because the velocity of light is far greater than that of sound, the light that is reflected back from the structures within the tissue cannot be detected electronically. Instead it is detected with a technique known as low-coherence interferometry. An advantage of this technique is that light waves, unlike sound waves, do not need a liquid based coupling medium which makes it ideal for use in the airways. OCT allows imaging of cellular and extracellular structures from analysis of the backscattered light with a spatial resolution of around 3–15 µm and a depth penetration of around 2 mm to provide near-histological images in the bronchial wall [Fujimoto et al. 1995; Huang et al. 1991; Lam et al. 2008; Li et al. 2006; Tearney et al. 1997; Tsuboi et al. 2005; Whiteman et al. 2006; Yang and Vitkin, 2006]. Early studies showed that dysplasia can be distinguished from metaplasia, hyperplasia or normal tissue and that carcinoma in situ (CIS) can be distinguished from invasive cancer [Lam et al. 2008; Tsuboi et al. 2005]. Severity of the histopathology grade was associated with a progressive increase in the epithelial thickness. The nuclei of the cells also became darker and showed less light scattering. The basement membrane became disrupted or disappeared with invasive carcinoma [Lam et al. 2008]. To further advance this technology, systems with higher resolution and Doppler capability that can measure both tissue microstructures in greater detail and microvascular blood flow may be useful [Yang and Vitkin, 2006]. Doppler OCT systems already exist that can detect very slow blood flow (<20 µm/s in blood vessels as small as about 15 µm diameter). In summary, OCT technology might become a useful modality for structural and functional assessment of suspicious lesions, staging (invasion of basement membrane) and provide feedback during endobronchial therapy. The use of ligand-conjugated microparticles in combination with OCT might enable in vivo identification of molecular treatment targets [Jefferson et al. 2011]. However, these promising techniques require further technical improvements and assessment of their clinical value.
Natural history of early squamous lung cancer
The World Health Organization (WHO) has classified preinvasive squamous lesions into nine categories, ranging from normal (A) to invasive cancer (I) (Table 1) [WHO, 1999]. Both the invasive potential of these lesions and the need for curative treatment are controversial [Kennedy et al. 2007; Vonk Noordegraaf et al. 2003]. The natural history of premalignant lesions is difficult to study for several reasons. First, these lesions are often asymptomatic and discovered by chance. Second, longitudinal studies are required with repeated biopsies from the same site(s). Third, there is considerable interobserver variability in the assessment of these lesions by pathologists [Nicholson et al. 2001; Venmans et al. 2000b]. Finally, by taking biopsies of small mucosal lesions one can affect their natural course because these lesions can be very small in size [Auerbach et al. 1957].
Table 1.
Classification of endobronchial (pre)malignant lesions according to the World Health Organization [Holiday et al. 1995].
| Histologic grade | |
|---|---|
| A | Normal |
| B | Inflammation/bronchitis |
| C | Hyperplasia |
| D | Squamous metaplasia |
| E | Mild dysplasia |
| F | Moderate dysplasia |
| G | Severe dysplasia |
| H | Carcinoma in situ |
| I | Invasive carcinoma |
Several institutions have performed longitudinal studies with serial AFB and biopsies (Table 2) [Bota et al. 2001; Breuer et al. 2005; George et al. 2007; Hoshino et al. 2004; Moro-Sibilot et al. 2004; Pasic et al. 2004; Venmans et al. 2000a; Salaun et al. 2008, 2009]. There are several difficulties when comparing these studies. The initial histology of the patient varies among the different studies. The follow-up time of some studies is relatively short (±2 years), considering that the progression of precancerous lesions to invasive cancer can take a long time [Ishizumi et al. 2010; Satoh et al. 1997]. Furthermore and importantly, ‘progression’ is not uniformly defined. Whereas most authors define progression as the progression of the initial precancerous lesion to invasive cancer [George et al. 2007; Hoshino et al. 2004; Moro-Sibilot et al. 2004; Salaun et al. 2008, 2009; Venmans et al. 2000a], other authors also use CIS or even severe dysplasia as the end point of progression [Bota et al. 2001; Breuer et al. 2005; Pasic et al. 2004]. For future publications it is important to use a uniform definition. Because high-grade lesions often do not progress to invasive cancer (Table 2), including severe dysplasia or CIS in the definition of progression might overestimate the carcinogenic potential of certain preinvasive lesions. We therefore propose that progression is defined as the site-specific progression of a lesion of any histological grade (WHO grade A-H) to invasive lung cancer. Another reason why studies are difficult to compare is that different follow-up and treatment regimens were employed. Finally, the patient cohorts from these studies were predominantly established in expert referral centers, which evidently results in referral bias. Nonetheless, these institutions have greatly contributed to the understanding of lung carcinogenesis. For example, these studies show that high-grade lesions (WHO G, H) are more likely to progress to invasive cancer than low-grade lesions (WHO A–F). This finding in itself supports the theory of slow stepwise carcinogenesis. However, even low-grade lesions (hyperplasia, metaplasia) can sometimes progress rapidly to lung cancer, thereby skipping several histological grades [Bota et al. 2001; Breuer et al. 2005; Ishizumi et al. 2010]. In addition, these studies demonstrate that the majority of precancerous lesions, even high-grade dysplasia, regress spontaneously instead of progressing step by step towards invasive cancer. In the study by Breuer and colleagues [Breuer et al. 2005], for example, 32% of severe dysplasia lesions progressed to CIS or invasive cancer and in the study by George and colleagues [George et al. 2007] only 17% of high-grade lesions (severe dysplasia and CIS) progressed to invasive cancer. Taken together the course of precancerous lesions, with the exception of CIS, is highly unpredictable. As a result, many patients without clinically relevant disease are repeatedly exposed to bronchoscopic examinations while patients with true precancers might be undertreated or treated with delay.
Table 2.
Longitudinal studies investigating the natural history of endobronchial squamous preinvasive lesions.
| Reference | N (lesions/patients) | Follow-up time (months)* | Initial histologic grade$ | Definition of progression$ | Treatment of high-grade lesions | Progression according to definition$ | Progression to invasive cancer$ |
|---|---|---|---|---|---|---|---|
| Breuer et al. [2005] | 134/52 | Not stated | D–G | H and I | Yes | D: 4/45 (9%) | Not stated |
| E, F: 6/64 (9%) | |||||||
| G: 8/25 (32%) | |||||||
| Venmans et al. [2000] | 9/9 | Not stated | H | I | Yes | 56% | 56% |
| Moro Sibilot et al. [2004] | 31/27 | 24 (13–41) | G and H | I | Partly | Gtreated: 1/2 (50%) | Gtreated: 1/2 (50%) |
| Guntreated: 0/1 (0%) | Guntreated: 0/1 (0%) | ||||||
| Htreated: 9/21 (43%) | Htreated: 9/21 (43%) | ||||||
| Huntreated: 2/7 (29%) | Huntreated: 2/7 (29%) | ||||||
| Bota et al.[2001] | 416/104 | 21% > 24 | A–H | G–I | Yes | A: 0/36 (0%) | A: 0/36 (0%) |
| D: 3/152 (2%) | D: 1/152 (1.5%) | ||||||
| E, F: 6/169 (3.5%) | E, F: 0/169 (0%) | ||||||
| G: 10/27 (37%) | G: 0/27 (0%) | ||||||
| H: 27/31 (87%) | H: 0/31 (0%) | ||||||
| Salaun et al. [2008,2009] | 54/37‡ | 68 (19–117) | G and H | I | Yes | G: 0/23 (0%) | G: 0/23 (0%) |
| H: 7/31 (23%) | H: 7/31 (23%) | ||||||
| Hoshino et al. [2004] | 99/55 | Mean 7, range 5–17 | E–G | I | No | E: 0/32 (0%) | E: 0/32 (0%) |
| F: 1/56 (2%) | G: 2/11 (2%) | ||||||
| F: 1/56 (2%) | G: 2/11 (2%) | ||||||
| Pasic et al. [2004] | 135/52 | 21 (1–72) | D–G | H and I | Yes | 27/135 (20%) | Not stated |
| George et al. [2007] | 53/22 | E–H | I | no | E, F: 0/17 (0%) | E, F: 0/17 (0%) | |
| G, H: 6/36 (17%) | G, H: 6/36 (17%) |
Median (interquartile range) unless stated otherwise.
Histologic grade according to the World Health Organization classification: A = normal, B = inflammation/bronchitis, C = hyperplasia, D = squamous metaplasia, E = mild dysplasia, F = moderate dysplasia, G = severe dysplasia, H = carcinoma in situ (CIS), I = invasive carcinoma.
26 out of 37 patients were from the cohort described by Bota and colleagues [Bota et al. 2001].
Prognostic biomarkers
Identification of molecular, immunohistochemical, proteomic or genetic markers that predict progression to invasive cancer might streamline the management of patients with precancerous lesions and further improve our understanding of lung carcinogenesis. Jeanmart and colleagues studied the value of immunohistochemistry in the development of lung cancer from precancerous lesions [Jeanmart et al. 2003]. Immunohistochemical analysis of P53, cyclin D1, cyclin E, Bax and Bcl2 was performed. Aberrant expression of two or more of these proteins was associated with the combined endpoint of CIS and invasive lung cancer, although no isolated parameter could predict the development of invasive lung cancer. Proteomic analysis of preinvasive lesions might help to identify patients harboring lung cancer or patients whose condition progresses to lung cancer [Rahman et al. 2011], but this has not been prospectively validated in a cohort at risk. Several research groups have tried to identify genetic markers of progression to lung cancer. Salaun and colleagues investigated the molecular profile of 23 severe dysplasia and 31 CIS lesions from 37 patients and showed that baseline pathology and 3p loss of heterozygosity were associated with progression to cancer [Salaun et al. 2008]. McCaughan and colleagues analyzed the structure of chromosome 3 in 10 high-grade and 7 low-grade preinvasive lesions from 15 patients [McCaughan et al. 2010]. No low-grade lesions progressed to cancer, whereas eight high-grade lesions progressed. All high-grade lesions exhibited 3q amplification and three patients showed incremental amplification of the SOX2 gene. In a recent study by van Boerdonk and colleagues six subjects with squamous metaplastic (SqM) lesions at baseline that had progressed to lung cancer over time were compared with 23 patients with nonprogressive SqM lesions at baseline [van Boerdonk et al. 2011]. Immunohistochemistry at baseline (p53, p63, Ki-67) was not predictive of lung cancer, whereas array comparative genomic hybridization analysis identified specific DNA copy number alterations at baseline, especially in the regions 3p26.3–p11.1, 3q26.2–q29 and 6p25.3–24.3, which predicted cancer with 97% accuracy when combined. Taken together, immunohistochemistry has no additional prognostic value to histologic grading. Genetic markers, on the contrary, hold promise as prognostic markers, although their value in the management of preinvasive mucosal lesions remains to be established in future prospective studies.
Staging of early central lung cancer
Once early lung cancer lesions are detected by one of the above-mentioned methods, clinicians have to decide whether additional workup is necessary, whether treatment is indicated and with what modality. When a high-grade lesion is diagnosed, the first step is to assess whether there is extraluminal growth. The presence or absence of extraluminal growth has direct implications for selection of the optimal treatment modality, because the depth of the effect of the available modalities varies greatly. In addition, clinicians should be aware that a patient with an endobronchial high-grade lesion might harbor invasive lung cancer nearby. To avoid tunnel vision, some form of imaging prior to treatment is crucial. Sutedja and colleagues showed that high-resolution computed tomography (HRCT, slice thickness ≤1 mm) proved peribronchial tumor extension or lymph node enlargement in 35% of cases referred for endobronchial treatment, and have implemented this in a treatment algorithm [Sutedja et al. 1996, 2001]. In this algorithm high-grade dysplastic, CIS and cancerous lesions with a visible distal margin and occult on HRCT were treated with an endobronchial approach. Also fludeoxyglucose F18 positron emission tomography (FDG-PET) scanning in addition to CT has been evaluated in this setting. A pilot study with 18 patients harboring 20 lesions suggested that FDG-PET scan might be useful [Pasic et al. 2005]. However, more data are needed to determine the full potential of FDG-PET in these patients. Also endobronchial ultrasound (EBUS) can be applied in the staging of central airway lesions. Lymph nodes adjacent to the airways can be sampled with EBUS fine needle aspiration, which has become an established staging technique for lung cancer. In addition, EBUS can be used to study the depth of invasion in the layers of the bronchial wall. Kurimoto and colleagues compared the depth of invasion on ultrasonographic images with histopathologic findings in 24 cases [Kurimoto et al. 1999]. The estimation of depth of invasion by ultrasound was correct in 23 cases (96%). In a larger study 105 cases were assessed for tumor invasion and the authors found a sensitivity and specificity of 89% and 100% respectively for EBUS and a far inferior sensitivity and specificity of 75% and 28% for CT scan [Herth et al. 2003]. As mentioned above, OCT can also be used to determine the depth of tumor invasion.
Bronchoscopic treatment of early squamous lung cancer
Although endoscopic minimally invasive techniques are becoming increasingly sophisticated, surgery is still the gold standard for the treatment of CIS and results in over 80–90% 5-year survival rate [Cortese et al. 1983; Fujimura et al. 2000; Kennedy et al. 2007; Nakamura et al. 2001; Woolner et al. 1984]. A drawback of surgery is that, in many cases, significant normal lung parenchyma also has to be removed. Up to 30% of patients with early proximal lung cancer require bilobectomy or pneumonectomy, and the remaining 70% require lobectomy [Nakamura et al. 2001]. In addition, these patients often harbor synchronous lesions or develop subsequent primaries (field cancerization). Moreover, significant comorbidities such as COPD often limit the amount of lung parenchyma that can be removed. Minimally invasive techniques have the additional advantages of less morbidity and lower cost in comparison to surgery, while the reported outcomes are similar [van Boxem et al. 2001; Furuse et al. 1993; Sutedja et al. 2001; Venmans et al. 2000; Vonk Noordegraaf et al. 2003]. The cost of treatment and follow up of bronchoscopically treated small stage IA cancers in patients with inoperable disease was 30% of the cost of surgical resection in matched patients with operable disease in one cost-effectiveness analysis [Pasic et al. 2004]. As discussed above, treatment success with bronchoscopic techniques strongly depends on accurate staging. Lymph node metastasis, although rare in early squamous lung cancer, has to be excluded. Tumor invasion of the airway wall can be assessed with EBUS or OCT and extraluminal tumor growth can be assessed with EBUS or HRCT (slice thickness ≤1 mm).
Treatment modalities
Commonly used bronchoscopic treatment modalities include electrocautery, APC, cryotherapy, laser, PDT and intraluminal irradiation therapy or brachytherapy. The concepts between these different techniques vary substantially and therefore the effects that these modalities exert on tissue vary equally.
Endobronchial electrocautery is the application of heat, produced by electrical current that is transferred to the target tissue with the use of a specifically designed probe or a snare (Figure 1) [Barlow, 1982]. With a monopolar probe superficial coagulative necrosis can be achieved with relative sparing of the cartilage. Electrocautery can be applied with both rigid and flexible instruments and under local or general anesthesia, which depends on the experience of the bronchoscopist, risk assessment and the availability of instruments. The effects on the tissue depend on the tissue itself (how it conducts the electrical current) and the energy level that is used. A high energy level will result in carbonization, whereas a low energy level will result in blanching of the mucosa. Electrocautery is the most used technique and is cheap compared with other techniques such as laser.
Figure 1.
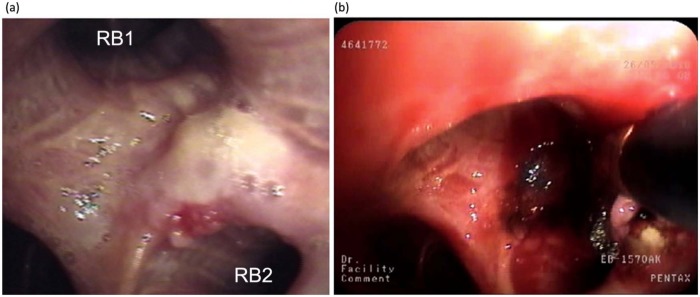
Carcinoma in situ on the carina between RB1 and RB2 before (a) and during (b) electrocauterization. Note the charring of the mucosa during treatment (the electrocautery probe is in place).
APC is a noncontact mode that utilizes a specialized flexible catheter to apply a flow of argon around a high-frequency electrode. This produces a plasma jet that transfers the energy homogeneously to the tissue. The coagulative necrosis after standard electrocautery and APC are similar [Go et al. 1991; Lanzafame et al. 1988; Rusch et al. 1990; Sessler et al. 1995]. APC is a very safe method because it causes very superficial tissue destruction, but is therefore less efficient in the case of bulky tumor.
The cyrotherapy system uses the expansion of a pressurized gas (e.g. nitrous oxide) that cools the metal tip of a flexible probe, thereby freezing the adjacent tissue (Figure 2). Cryotherapy causes cell death by repetitive fast freezing and slow thawing [Deygas et al. 2001]. Tissues with high water content are especially sensitive to cryotherapy. Poorly vascuralized tissues such as cartilage and connective tissue are cryoresistant, which is an advantage when treating endobronchial lesions. Only a few studies with cyrotherapy have been performed, mostly on patients with advanced lung cancer [Beeson, 2007; Deygas et al. 2001; Hetzel et al. 2004; Jung et al. 2008; Noppen et al. 2001; Walsh et al. 1990]. Two studies specifically investigated early central lung cancer [Deygas et al. 2001; Noppen et al. 2001]. Deygas and colleagues reported a complete response rate of 91% after 1 year [Deygas et al. 2001] and Noppen and colleagues reported favorable outcome in four patients with CIS [Noppen et al. 2001]. Obviously, more studies are required to determine its effectiveness. A new freezing technique is the spraying of liquid nitrogen through a flexible catheter. This technique enables treatment of large epithelial surfaces, which might be useful for patients with superficial spreading squamous cell carcinoma. Although there is some experience with cryospray in the treatment of Barrett’s esophagus [Dumot et al. 2009], no studies have been published on its application in bronchial lesions.
Figure 2.
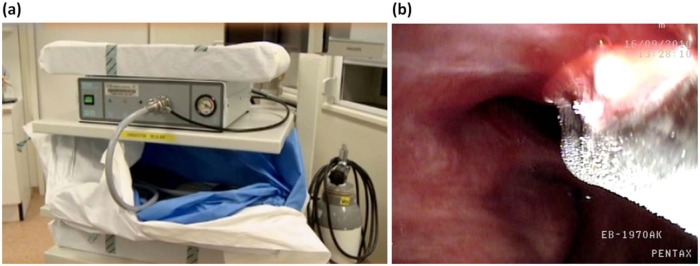
Cryotherapy unit (a) and cryotherapy for a central dysplastic lesion using a flexible probe (b). Note the clear demarcation of the frozen tissue.
Laser is also suitable for bronchoscopic treatment because of its high power and durability. Laser can be applied through a flexible catheter or rigid instruments. The effects of laser depend on the wavelength of the laser light and the settings of the machine. The most commonly used laser is the neodymium-doped yttrium aluminum garnet (Nd:YAG) laser, which emits light with a wavelength of 1064 nm. The most common indication for laser treatment is the debulking of central tumors. For the application of laser in superficial squamous lesions, excellent depth control is necessary. Two-micron thulium continuous wave laser might become an alternative for such lesions because of its shallow effects (0.5 mm) and excellent tissue vaporization.
PDT causes selective death of tumor cells by the interaction between tumor-selective photosensitizers and laser light. Interaction between the photosensitive molecules, light of a specific wavelength and tissue oxygen results in the formation of active forms of oxygen that induce cellular necrosis. Second-generation photosensitizers such as mono-L-aspartyl chlorine e6 (NPe6) have excellent antitumor activity and cause lower skin photosensitivity than older agents. The depth of the treatment effects is limited to the penetration of the laser light, which is usually around 4 mm. Careful selection of patients with imaging techniques such as EBUS is important because patients with extracartilaginous should be referred for conventional treatment modalities such as surgery or radiotherapy [Miyazu et al. 2002]. The arrival of second-generation photosensitizers and modern imaging techniques such as OCT and EBUS warrant a careful reassessment of PDT as a minimal invasive treatment for early lung cancer. A recent study by Usuda and colleagues suggests that NPe6-PDT has strong antitumor effects, also in larger and more invasive central lesions [Usuda et al. 2010].
Endoluminal brachytherapy is irradiation of tissue by placing a radioactive source at the site of the endobronchial lesion. Brachytherapy has the advantage of delivering a higher dose to the tumor while sparing normal tissues more than with external beam radiation. In the absence of lymph node metastasis, brachytherapy can be applied in case of CIS or radiographically occult squamous cell cancer with excellent results. The border between the first and second isodoses is located 1 cm from the source, which allows treatment of lesions that reach beyond the intercartilaginous space [Fisher and Huber, 2000]. No randomized controlled trials have investigated the role of brachytherapy in CIS/early stage NSCLC. Saito and colleagues applied a combination of external beam radiation (40 Gy in 20 fractions) and low-dose-rate endobronchial brachytherapy (25 Gy in five fractions) in 79 lesions of 71 patients with roentgenographically occult lung cancer [Saito et al. 2000]. The 5-year cause-specific survival was 96% and the overall survival was 72%. Bronchial obstruction was common (32%), but no severe or fatal toxicity was observed. Marsiglia and colleagues used high-dose-rate brachytherapy (30 Gy in six fractions) as the only modality in 34 patients with early stage lung cancer [Marsiglia et al. 2000]. The local control rate was 85%, the survival rate was 78% and no acute toxicity was observed, except one pneumothorax. Another series of 35 tumors in 33 patients with either CIS or small invasive carcinoma treated with 6×5 Gy showed a local control rate of 86% after 6 months and an overall 1-year survival rate of 71% [Lorchel et al. 2003]. One patient developed pneumothorax and 12 developed bronchial stenosis.
Considerations with regard to minimally invasive treatment
The choice of the modality depends on the characteristics of the individual patient as well as factors related to care providers and institutions. On a patient level the most important factors for determining the right modality are the depth of invasion of the lesion, the lymph node status and comorbidities that might preclude other treatments such as surgical resection. The treating physicians should possess the knowledge and skills required to correctly and safely apply the modality in question. Importantly, sufficient technical support should be available, especially with the use of complex modalities such as PDT, brachytherapy and laser.
There is currently no evidence from randomized controlled studies that early detection and minimally invasive treatment of central early lung cancer is beneficial. In case endobronchial high-grade preinvasive lesions are discovered, careful assessment of treatment options other than surgical resection as the primary therapeutic choice should be considered, especially for syn- or metachronous lung cancer or significant comorbidities. Although cure rates of 43–97% for endobronchial minimally invasive techniques have been reported, these studies are often small series with a short follow-up time and include patients with more advanced disease [Chhajed et al. 2005; Holiday et al. 1995; Hung et al. 1991; Jung et al. 2008; Kennedy et al. 2007; Lam et al. 1994, 1998, 2000; Motherby et al. 1999; Qu et al. 1994, 1995; Shibuya et al. 2002; Venmans et al. 1998; Wagnieres et al. 2003; Woolner et al. 1984].
Another limitation is that many studies were performed without modern imaging techniques for careful assessment of extension on and beyond the epithelial surface.
Chemoprevention
While doctors and scientists put a lot of effort into the systemic treatment of advanced lung cancer, the preceding process of lung carcinogenesis receives little attention. This is understandable because patients with advanced lung cancer are symptomatic and in urgent need of treatment. Treating lung carcinogenesis, however, might reduce the worldwide burden of lung cancer. Because squamous lung cancer is induced by carcinogens, it exhibits a marked genomic complexity and alteration of many metabolic pathways. Therefore, in the absence of specific oncogene addiction, such as in epidermal growth factor receptor and EML4-ALK associated lung adenocarcinoma, probably multiple pathways will have to be targeted to effectively impede squamous lung carcinogenesis [Hecht et al. 2009]. The first step is to perform preclinical studies and assess whether a single agent possesses chemopreventive effects. Subsequently, the agent can be tested in clinical studies with the use of surrogate endpoints, such as regression of histologic grade and biomarkers. A comprehensive review of all preclinical and clinical work is beyond the scope of this review and has been performed by others [Cohen and Khuri, 2004; Hecht et al. 2009; Hirsch and Lippman, 2005; Keith and Miller, 2005; Lee et al. 2008; Shaipanisch et al. 2006; Soria et al. 2003; Winterhalder et al. 2004; van Zandwijk, 2005]. Here we review several agents that have been tested on patients with preinvasive lesions.
Inhaled corticosteroids (ICS) were studied because chronic inflammation seems to play an important role in squamous carcinogenesis and pooled data from randomized clinical trials indicated that ICS reduced cancer mortality [Sin et al. 2005]. Lam and colleagues performed a randomized phase IIb study of 6 months’ duration of inhaled budesonide (1600 µg daily) in smokers with at least one site of bronchial dysplasia [Lam et al. 2004]. After a repeat biopsy of the same lesions at the end of the intervention, no differences in histologic progression or regression were observed. Another target of interest is the phosphatidylinositol 3-kinase (PI3K) pathway. Myo-inositol, an inhibitor of the PI3K pathway, was tested in a phase I clinical trial of 36 patients with preinvasive bronchial lesions [Lam et al. 2006]. After 3 months, 91% (10/11) of dysplastic lesions had regressed to normal. This lesion-specific regression rate was considerably higher in comparison to the budesonide trial (91% versus 48%, p = 0.014) [Lam et al. 2004]. It should be noted, however, that these groups are from different trials, the numbers in the myo-inositol trial were small and that most lesions showed only mild dysplasia, which mostly regresses spontaneously (Table 2). An additional analysis of the patients from the myo-inositol trial [Lam et al. 2006] suggests that PI3K activity is reduced in histological responders to myo-inositol [Gustafson et al. 2010]. Preclinical work suggests that the chemopreventive effects of ICS are enhanced by myo-inositol [Wattenberg et al. 2000]. Sulindac, a nonsteroidal anti-inflammatory drug, was recently tested in a phase II randomized trial on current and ex smokers with at least one dysplastic bronchial lesion [Limburg et al. 2013]. Inclusion was prematurely closed after 61 patients because of slow recruitment. No differences in histological regression rates or Ki67 expression were observed. Iloprost, a synthetic analogue of prostacyclin, has shown chemopreventive effects in preclinical studies [Keith et al. 2002, 2004; Nemenoff et al. 2008]. A recent phase II placebo-controlled trial enrolled 152 subjects with sputum atypia or bronchial dysplasia [Keith et al. 2011]. Bronchoscopy was performed before and 6 months after treatment. Oral iloprost significantly improved histology (average histological grade and dysplasia index) of bronchoscopic biopsies in former smokers, but no effect was seen in current smokers.
Taken together, there are currently no established chemopreventive agents for lung cancer. However, several agents, such as myo-inositol and iloprost, seem to have anticarcinogenic effects, which warrants further research. Identification of prognostic and predictive biomarkers is essential for the development and future clinical application of these agents. The use of small histological changes in preinvasive lesions as surrogate endpoints is questionable because most preinvasive lesions regress spontaneously (Table 2). Early genetic markers of progression to invasive cancer could assist in selecting high-risk patients that could really benefit from chemoprevention.
Conclusion
At present, sufficient technology is available for the detection, staging and treatment of early squamous lung cancer. The greatest challenge for the future is to determine whether early detection and treatment of early squamous lung cancer in high-risk cohorts improves patient survival and whether such an approach is cost effective. No conclusions can be drawn about the superiority of certain treatment modalities above others because randomized trials are lacking. Biomarkers (especially genetic makers) hold promise as predictors of progression to cancer, and response to (targeted) treatments should be studied prospectively and possibly implemented in future treatment strategies.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Johannes M.A. Daniels, Department of Pulmonary Diseases, Z 4A48, VU University Medical Center, De Boelelaan 1117, 1081HV Amsterdam, The Netherlands
Thomas G. Sutedja, Department of Pulmonary Diseases, VU University Medical Center, Amsterdam, The Netherlands
References
- Auerbach O., Gere J., Forman J., Gere J., Kassouny D., Meuhsam G., et al. (1957) Changes in bronchial epithelium in relation to smoking and cancer of the lung: a report of progress. N Engl J Med 256: 97–104 [DOI] [PubMed] [Google Scholar]
- Barlow D. (1982) Endoscopic applications of electrosurgery: a review of basic principles. Gastrointest Endosc 28: 73–76 [DOI] [PubMed] [Google Scholar]
- Beeson J. (2007) Palliation of tracheobronchial carcinoma: the role of cryosurgery. J Perioper Pract 17: 332, 334,–336, 338–339 [DOI] [PubMed] [Google Scholar]
- Bota S., Auliac J., Paris C., Metayer J., Sesboue R., Nouvet G., et al. (2001) Follow-up of bronchial precancerous lesions and carcinoma in situ using fluorescence endoscopy. Am J Resp Crit Care Med 164: 1688–1693 [DOI] [PubMed] [Google Scholar]
- Breuer R., Pasic A., Smit E., van Vliet E., Vonk Noordegraaf A., Risse E., et al. (2005) The natural course of preneoplastic lesions in the bronchial epithelium. Clin Cancer Res 11: 537–543 [PubMed] [Google Scholar]
- Chhajed P., Shibuya K., Hoshino H., Chiyo M., Yasufuku K., Hiroshima K., et al. (2005) A comparison of video and autofluorescence bronchoscopy in patients at high risk of lung cancer. Eur Respir J 25: 951–955 [DOI] [PubMed] [Google Scholar]
- Chiyo M., Shibuya K., Hoshino H., Yasufuku K., Sekine Y., Iizasa T., et al. (2005) Effective detection of bronchial preinvasive lesions by a new autofluorescence imaging bronchovideoscope system. Lung Cancer 48: 307–313 [DOI] [PubMed] [Google Scholar]
- Cohen V., Khuri F. (2004). Chemoprevention of lung cancer. Curr Opin Pulm Med 10: 279–283 [DOI] [PubMed] [Google Scholar]
- Cortese D., Pairolero P., Bergstralh E., Woolner L., Uhlenhopp M., Pichler J., et al. (1983) Roentgenographically occult lung cancer. A ten-year experience. J Thorac Cardiovasc Surg 86: 373–380 [PubMed] [Google Scholar]
- Deygas N., Froudarakis M., Ozenne G., Vergnon J. (2001) Cryotherapy in early superficial bronchogenic carcinoma. Chest 120: 26–31 [DOI] [PubMed] [Google Scholar]
- Dumot J., Vargo J., Falk G., Frey L., Lopez R., Rice T. (2009) An open-label, prospective trial of cryospray ablation for Barrett’s esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc 70: 635–644 [DOI] [PubMed] [Google Scholar]
- Ernst A., Simoff M., Mathur P., Yung R., Beamis J., Jr (2005) D-light autofluorescence in the detection of premalignant airway changes: A multicenter trial. J Bronchol 12: 133–138 [Google Scholar]
- Fielding D. (2004) Practical issues in autofluorescence bronchoscopy with Storz D Light bronchoscope. Photodiagnosis Photodyn Ther 1: 247–251 [DOI] [PubMed] [Google Scholar]
- Fisher R., Huber R. (2000) Endoluminal brachytherapy in central lung cancer. In: Bolliger C. (ed.), Interventional Bronchoscopy, Vol. 30 Basel: S. Karger AG [Google Scholar]
- Fujimoto J., Brezinski M., Tearney G., Boppart S., Bouma B., Hee M., et al. (1995) Optical biopsy and imaging using optical coherence tomography. Nat Med 1: 970–972 [DOI] [PubMed] [Google Scholar]
- Fujimura S., Sagawa M., Saito Y., Takahashi H., Tanita T., Ono S., et al. (2000) A therapeutic approach to roentgenographically occult squamous cell carcinoma of the lung. Cancer 89(11 Suppl.): 2445–2448 [DOI] [PubMed] [Google Scholar]
- Furuse K., Fukuoka M., Kato H., Horai T., Kubota K., Kodama N., et al. (1993) A prospective phase II study on photodynamic therapy for centrally located early-stage lung cancer. J Clin Oncol 11: 1852–1858 [DOI] [PubMed] [Google Scholar]
- George P., Banerjee A., Read C., O’Sullivan C., Falzon M., Pezella F., et al. (2007) Surveillance for the detection of early lung cancer in patients with bronchial dysplasia. Thorax 62: 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go P., Goodman G., Bruhn E., Hunter J. (1991) The Argon beam coagulator provides rapid hemostasis of experimental hepatic and splenic hemorrhage in anticoagulated dogs. J Trauma 31: 1294–1300 [DOI] [PubMed] [Google Scholar]
- Goujon D., Zellweger M., Radu A., Grosjean P., Weber B., van den Bergh H., et al. (2003) In vivo autofluorescence imaging of early cancers in the human tracheobronchial tree with a spectrally optimized system. Biomed Opt J 8: 17–25 [DOI] [PubMed] [Google Scholar]
- Groome P., Bolejack V., Crowley J., Kennedy C., Krasnik M., Sobin L., et al. (2007) The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007. 2: 694–705 [DOI] [PubMed] [Google Scholar]
- Gustafson A., Soldi R., Anderlind C., Scholand M., Qian J., Zhang X., et al. (2010) Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med 2: 26ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussinger K., Becker H., Stanzel F., Kreuzer A., Schmidt B., Strausz J., et al. (2005) Autofluorescence bronchoscopy with white light bronchoscopy compared with white light bronchoscopy alone for the detection of precancerous lesions: a European randomized controlled multicentre trial. Thorax 60: 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S., Kassie F., Hatsukami D. (2009) Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer 9: 476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfritzsch H., Junker K., Bartel M., Scheele J. (2002) Differentiation of positive autofluorescence bronchoscopy findings by comparative genomic hybridization. Oncol Rep 9: 697–701 [PubMed] [Google Scholar]
- Herth F., Eberhardt R., Anantham D., Gompelmann D., Zakaria M., Ernst A. (2009) Narrow-band imaging increases the specificity of bronchoscopic early lung cancer detection. J Thorac Oncol 4: 1060–1065 [DOI] [PubMed] [Google Scholar]
- Herth F., Ernst A., Schulz M., Becker H. (2003) Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest 123: 458–462 [DOI] [PubMed] [Google Scholar]
- Hetzel M., Hetzel J., Schumann C., Marx N., Babiak A. (2004) Cryorecanalization: a new approach for the immediate management of acute airway obstruction. J Thorac Cardiovasc Surg 127: 1427–1431 [DOI] [PubMed] [Google Scholar]
- Hirsch F., Lippman S. (2005) Advances in the biology of lung cancer chemoprevention. J Clin Oncol 23: 3186–3197 [DOI] [PubMed] [Google Scholar]
- Hirsch F., Prindiville S., Miller Y., Franklin W., Dempsey E., Murphy J., et al. (2001) Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst 93: 1385–1391 [DOI] [PubMed] [Google Scholar]
- Holiday D., McLarty J., Farley M., Mabry L., Cozens D., Roby T., et al. (1995) Sputum cytology within and across laboratories. A reliability study. Acta Cytol 39: 195–206 [PubMed] [Google Scholar]
- Horvath T., Horvathova M., Salajka F., Habanec B., Foretova L., Kana J., et al. (1999) Detection of bronchial neoplasia in uranium miners by autofluorescence bronchoscopy. Diagnostic and Therapeutic Endoscopy 5: 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H., Shibuya K., Chiyo M., Iyoda A., Yoshida S., Sekine Y., et al. (2004) Biological features of bronchial squamous dysplasia followed up by autofluorescence bronchoscopy. Lung Cancer 46: 187–196 [DOI] [PubMed] [Google Scholar]
- Huang D., Swanson E., Lin C., Schuman J., Stinson W., Chang W., et al. (1991) Optical coherence tomography. Science 254: 1178–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J., Lam S., leRiche J., Palcic B. (1991) Autofluorescence of normal and malignant bronchial tissue. Laser Surg Med 11: 99–105 [DOI] [PubMed] [Google Scholar]
- Ikeda N., Honda H., Hayashi A., Usuda J., Kato Y., Tsuboi M., et al. (2006) Early detection of bronchial lesions using newly developed videoendoscopy-based autofluorescence bronchoscopy. Lung Cancer 52: 21–27 [DOI] [PubMed] [Google Scholar]
- Ishizumi T., McWilliams A., MacAulay C., Gazdar A., Lam S. (2010) Natural history of bronchial preinvasive lesions. Cancer Metastasis Rev 29: 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmart M., Lantuejoul S., Fievet F., Moro D., Sturm N., Brambilla C., et al. (2003) Value of immunohistochemical markers in preinvasive bronchial lesions in risk assessment of lung cancer. Clin Cancer Res 9: 2195–2203 [PubMed] [Google Scholar]
- Jefferson A., Wijesurendra R., McAteer M., Digby J., Douglas G., Bannister T., et al. (2011) Molecular imaging with optical coherence tomography using ligand-conjugated microparticles that detect activated endothelial cells: rational design through target quantification. Atherosclerosis 219: 579–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M. (2009) Cancer statistics, 2009. CA Cancer J Clin 59: 225–249 [DOI] [PubMed] [Google Scholar]
- Jung J., Lee S., Kim D., Lee K., Lee E., Kang E., et al. (2008) Clinical benefits and complications of cryotherapy in advanced lung cancer with central airway obstruction. Tuberc Respir Dis 64: 272–277 [Google Scholar]
- Keith R., Blatchford P., Kittelson J., Minna J., Kelly K., Massion P., et al. (2011) Oral iloprost improves endobronchial dysplasia in former smokers. Cancer Prev Res 4: 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith R., Miller Y. (2005). Lung cancer: genetics of risk and advances in chemoprevention. Curr Opin Pulm Med 11: 265–271 [DOI] [PubMed] [Google Scholar]
- Keith R., Miller Y., Gemmill R., Drabkin H., Dempsey E., Kennedy T., et al. (2000) Angiogenic squamous dysplasia in bronchi of individuals at high risk for lung cancer. Clin Cancer Res 6: 1616–1625 [PubMed] [Google Scholar]
- Keith R., Miller Y., Hoshikawa Y., Moore M., Gesell T., Gao B., et al. (2002) Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res 62: 734–740 [PubMed] [Google Scholar]
- Keith R., Miller Y., Hudish T., Girod C., Sotto-Santiago S., Franklin W., et al. (2004) Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Res 64: 5897–5904 [DOI] [PubMed] [Google Scholar]
- Kennedy T., Franklin W., Prindiville S., Cook R., Dempsey E., Keith R., et al. (2005) High prevalence of occult endobronchial malignancy in high risk patients with moderate sputum atypia. Lung Cancer 49: 187–191 [DOI] [PubMed] [Google Scholar]
- Kennedy T., McWilliams A., Edell E., Sutedja T., Downie G., Yung R., et al. (2007) American College of Chest Physicians. Bronchial intraepithelial neoplasia/early central airways lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132(3 Suppl.): 221S–233S [DOI] [PubMed] [Google Scholar]
- Kurimoto N., Murayama M., Yoshioka S., Nishisaka T., Inai K., Dohi K. (1999) Assessment of usefulness of endobronchial ultrasonography in determination of depth of tracheobronchial tumor invasion. Chest 11: 1500–1506 [DOI] [PubMed] [Google Scholar]
- Kusunoki Y., Imamura F., Uda H., Mano M., Horai T. (2000) Early detection of lung cancer with laser-induced fluorescence endoscopy and spectrofluorometry. Chest 118: 1776–1782 [DOI] [PubMed] [Google Scholar]
- Lam S., Kennedy T., Unger M., Miller Y., Gelmont D., Rusch V., et al. (1998) Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest 113: 696–702 [DOI] [PubMed] [Google Scholar]
- Lam S., leRiche J., McWIlliams A., Macaulay C., Dyachkova Y., Szabo E., et al. (2004) A randomized phase IIb trial of pulmicort turbuhaler (budenoside) in people with dysplasia of the bronchial epithelium. Clin Cancer Res 10: 6502–6511 [DOI] [PubMed] [Google Scholar]
- Lam S., MacAulay C., leRiche J., Ikeda N., Palcic B. (1994) Early localization of bronchogenic carcinoma. Diagn Ther Endosc 1: 75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S., MacAulay C., leRiche J., Palcic B. (2000) Detection and localization of early lung cancer by fluorescence bronchoscopy. Cancer 89(11 Suppl.): 2468–2473 [DOI] [PubMed] [Google Scholar]
- Lam S., McWilliams A., LeRiche J., MacAulay C., Wattenberg L., Szabo E. (2006) A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev 15: 1526–1531 [DOI] [PubMed] [Google Scholar]
- Lam S., Standish B., Baldwin C., McWilliams A., LeRiche J., Gazdar A., et al. (2008) In-vivo optical coherence tomography imaging of pre-invasive bronchial lesions. Clin Cancer Res 14: 2006–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzafame R., Qiu K., Rogers D., Naim J., Caldwell F., Perry F., et al. (1988) Comparison of local tumor recurrence following excision with the CO2 laser, Nd:YAG laser and argon beam coagulator. Lasers Surg Med 8: 515–520 [DOI] [PubMed] [Google Scholar]
- Lee J., Yanagawa J., Peebles K., Mao J., Dubinett S. (2008). Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit Rev Oncol Hematol 66: 208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., McWilliams A., Lam S., Sutedja T. (2007) Quantitative image analysis for intra-epithelial neoplasia. J Thorac Oncol 2(Suppl. 4): S812 [Google Scholar]
- Li H., Standish B., Mariampillai A., Munce N., Mao Y., Chiu S., et al. (2006) Feasibility of interstitial Doppler optical coherence tomography for in vivo detection of microvascular changes during photodynamic therapy. Lasers Surg Med 38: 754–761 [DOI] [PubMed] [Google Scholar]
- Limburg P., Mandrekar S., Aubry M., Ziegler K., Zhang J., Yi J., et al. (2013) Randomized phase II trial of silundac for lung cancer chemoprevention. Lung Cancer 79: 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorchel F., Spaeth D., Scheid P., Aletti P., Thariat J., Peiffert D. (2003) High dose rate brachytherapy: a potentially curative treatment for small invasive T1N0 endobronchial carcinoma and carcinoma in situ. Rev Mal Respir 20: 515–520 [PubMed] [Google Scholar]
- Marsiglia H., Baldeyrou P., Lartigau E., Briot E., Haie-Meder C., Le Chevalier T., et al. (2000) High-dose-rate brachytherapy as sole modality for early-stage endobronchial carcinoma. Int J Radiat Oncol Biol Phys 47: 665–672 [DOI] [PubMed] [Google Scholar]
- McCaughan F., Pole J., Bankier A., Konfortov B., Carroll B., Falzon M., et al. (2010) Progressive 3q amplification consistently targets SOX2 in preinvasive squamous lung cancer. Am J Respir Crit Care Med 182: 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazu Y., Miyazawa T., Kurimoto N., Iwamoto Y., Kanoh K., Kohno N. (2002) Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am J Respir Crit Care Med 165: 832–837 [DOI] [PubMed] [Google Scholar]
- Moro-Sibilot D., Fievet F., Jeanmart M., Lantuejoul S., Arbib F., Laverribre M., et al. (2004) Clinical prognostic indicators of high-grade pre-invasive bronchial lesions. Eur Respir J 24: 24–29 [DOI] [PubMed] [Google Scholar]
- Motherby H., Nicklaus S., Berg A., Ohler S., Ross B., Sarbia M., et al. (1999) Semiautomated monolayer preparation of bronchial secretions using AutoCyte PREP. Acta Cytol 43: 47–57 [DOI] [PubMed] [Google Scholar]
- Nakamura H., Kawasaki N., Hagiwara M., Ogata A., Saito M., Konaka C., et al. (2001) Early hilar lung cancer-risk for multiple lung cancers and clinical outcome. Lung Cancer 33: 51–57 [DOI] [PubMed] [Google Scholar]
- Nemenoff R., Meyer A., Hudish T., Mozer A., Snee A., Narumiya S., et al. (2008) Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator–activated receptor gamma. Cancer Prev Res 1: 349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A., Perry L., Cury O., Jackson P., McCormick C., Corrin B., et al. (2001) Reproducibility of the WHO/IASLC grading system for pre-invasive squamous lesions of the bronchus: a study of inter-observer and intraobserver variation. Histopathology 38: 202–208 [DOI] [PubMed] [Google Scholar]
- Noppen M., Meysman M., Van Herreweghe R., Lamote J., D’Haese J., Vincken W. (2001) Bronchoscopic cryotherapy: preliminary experience. Acta Clin Belg 56: 73–77 [DOI] [PubMed] [Google Scholar]
- Parkin D., Bray F., Ferlay J., Pisani P. (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108 [DOI] [PubMed] [Google Scholar]
- Pasic A., Brokx H., Vonk Noordegraaf A., Paul R., Postmus P., Sutedja T. (2004) Cost-effectiveness of early intervention: comparison between intraluminal bronchoscopic treatment and surgical resection for T1N0 lung cancer patients. Respiration 71: 391–396 [DOI] [PubMed] [Google Scholar]
- Pasic A., Comans E., Herder G., Risse E., Hoekstra O., Postmus P., et al. (2005) Detection and staging of pre-invasive lesions and occult lung cancer in the central airways with 18fluorodeoxyglucose positron emission tomography: a pilot study. Clin Cancer Res 11: 6186–6189 [DOI] [PubMed] [Google Scholar]
- Pasic A., van Vliet E., Breur R., Risse E., Snijders P., Postmus P., et al. (2004) Smoking behavior does not influence the natural course of pre-invasive lesions in bronchial mucosa. Lung Cancer 45: 153–154 [DOI] [PubMed] [Google Scholar]
- Pasic A., Vonk-Noordegraaf A., Risse E., Postmus P., Sutedja T. (2003) Multiple suspicious lesions detected by autofluorescence bronchoscopy predict malignant development in the bronchial mucosa in high risk patients. Lung Cancer 41: 295–301 [DOI] [PubMed] [Google Scholar]
- Qu J., MacAulay C., Lam S., Palcic B. (1994) Optical properties of normal and carcinoma bronchial tissue. Appl Optics 33: 7397–7405 [DOI] [PubMed] [Google Scholar]
- Qu J., MacAulay C., Lam S., Palcic B. (1995) Laser-induced fluorescence spectroscopy at endoscopy: tissue optics, Monte Carlo modeling, and in vivo measurements. Opt Eng 34: 3334–3343 [Google Scholar]
- Rahman S., Gonzalez A., Li M., Seeley E., Zimmerman L., Zhang X., et al. (2011) Lung cancer diagnosis from proteomic analysis of preinvasive lesions. Cancer Res 71: 3009–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch V., Schmidt R., Shoij Y., Fujimura Y. (1990) Use of the argon beam electrocoagulator for performing pulmonary wedge resections. Ann Thorac Surg 49: 287–291 [DOI] [PubMed] [Google Scholar]
- Saito M., Yokoyama A., Kurita Y., Uematsu T., Tsukada H., Yamanoi T. (2000) Treatment of roentgenographically occult endobronchial carcinoma with external beam radiotherapy and intraluminal low-dose-rate brachytherapy: second report. Int J Radiat Oncol Biol Phys 47: 673–680 [DOI] [PubMed] [Google Scholar]
- Salaun M., Bota S., Thiberville L. (2009) Long-term follow up of severe dysplasia and carcinoma in-situ of the bronchus. J Thorac Oncol 4: 1187–1188 [DOI] [PubMed] [Google Scholar]
- Salaun M., Sesboue R., Moreno-Swire S., Metayer J., Bota S., Bourguignon J., et al. (2008) Molecular predictive factors for progression of high-grade preinvasive bronchial lesions. Am J Resp Crit Care Med 177: 880–886 [DOI] [PubMed] [Google Scholar]
- Sato M., Sakurada A., Sagawa M., Minowa M., Takahashi H., Oyaizu T., et al. (2001) Diagnostic results before and after introduction of autofluorescence bronchoscopy in patients suspected of having lung cancer detected by sputum cytology in lung cancer mass screening. Lung Cancer 32: 247–253 [DOI] [PubMed] [Google Scholar]
- Satoh Y., Ishikawa Y., Nakagawa K., Hirano T., Tsuchiya E. (1997) A follow-up study of progression from dysplasia to squamous cell carcinoma with immunohistochemical examination of p53 protein overexpression in the bronchi of ex-chromate workers. Br J Cancer 75: 678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessler M., Becker H., Flesch I. (1995) Therapeutic effect of argon plasma coagulation on small malignant gastrointestinal tumors. J Cancer Res Clin Oncol 121: 235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaipanisch T., McWilliams A., Lam S. (2006) Early detection and chemoprevention of lung cancer. Respirology 11: 366–372 [DOI] [PubMed] [Google Scholar]
- Shibuya K., Fujisawa T., Hoshino H., Baba M., Saitoh Y., Iizasa T., et al. (2001) Fluorescence bronchoscopy in the detection of preinvasive bronchial lesions in patients with sputum cytology suspicious or positive for malignancy. Lung Cancer 32: 19–25 [DOI] [PubMed] [Google Scholar]
- Shibuya K., Hoshino H., Chiyo M., Iyoda A., Yoshida S., Sekine Y., et al. (2003) High magnification bronchovideoscopy combined with narrow band imaging could detect capillary loops of angiogenic squamous dysplasia in heavy smokers at high risk for lung cancer. Thorax 58: 989–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K., Hoshino H., Chiyo M., Yasufuku K., Lizasa T., Saitoh Y., et al. (2002) Subepithelial vascular patterns in bronchial dysplasias using a high magnification bronchovideoscope. Thorax 57: 902–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K., Nakajima T., Fujiwara T., Chiyo M., Hoshino H., Moriya Y., et al. (2010) Narrow band imaging with high-resolution bronchovideoscopy: a new approach for visualizing angiogenesis in squamous cell lung carcinoma of the lung. Lung Cancer 69: 194–202 [DOI] [PubMed] [Google Scholar]
- Sin D., Wu L., Anderson J., Anthonisen N., Buist A., Burge P., et al. (2005) Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax 60: 992–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria J., Izzo J., Mao L., Hong W., Papadimitrakopoulou V. (2003) Chemoprevention of lung cancer. Lancet Oncol 4: 659–669 [DOI] [PubMed] [Google Scholar]
- Sutedja T., Codrington H., Risse E., Breuer R., van Mourik J., Golding R., et al. (2001) Autofluorescence bronchoscopy improves staging of radiographically occult lung cancer and has an impact on therapeutic strategy. Chest 120: 1327–1332 [DOI] [PubMed] [Google Scholar]
- Sutedja T., Golding R., Postmus P. (1996) High resolution computed tomography in patients referred for intraluminal bronchoscopic therapy with curative intent. Eur Respir J 9: 1020–1023 [DOI] [PubMed] [Google Scholar]
- Tearney G., Brezinski M., Bouma B., Boppart S., Pitris C., Southern J., et al. (1997) In vivo endoscopic optical biopsy with optical coherence tomography. Science 276: 2037–2039 [DOI] [PubMed] [Google Scholar]
- Tsuboi M., Hayashi A., Ikeda N., Honda H., Kato Y., Ichinose S., et al. (2005) Optical coherence tomography in the diagnosis of bronchial lesions. Lung Cancer 49: 387–394 [DOI] [PubMed] [Google Scholar]
- Usuda J., Ichinose S., Ishizumi T., Hayashi H., Ohtani K., Maehara S., et al. (2010) Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clin Cancer Res 16: 2198–2204 [DOI] [PubMed] [Google Scholar]
- van Boerdonk R., Sutedja T., Snijders P., Reinen E., Wilting S., van de Wiel M., et al. (2011) DNA copy number alterations in endobronchial squamous metaplastic lesions predict lung cancer. Am J Respir Crit Care Med 184: 948–956 [DOI] [PubMed] [Google Scholar]
- van Boxem A., Westerga J., Venmans B., Postmus P., Sutedja T. (2001) Photodynamic therapy, Nd-YAG laser and electrocautery for treating early-stage intraluminal cancer: which to choose? Lung Cancer 31: 31–36 [DOI] [PubMed] [Google Scholar]
- Van Zandwijk N. (2005) Chemoprevention in lung carcinogenesis – an overview. Eur J Cancer 41: 1990–2000 [DOI] [PubMed] [Google Scholar]
- Venmans B., van Boxem A., Smit E., Postmus P., Sutedja T. (2000a) Outcome of bronchial carcinoma in-situ. Chest 117: 1572–1576 [DOI] [PubMed] [Google Scholar]
- Venmans B., van der Linden H., Elbers H., van Boxem T., Smit E., Postmus P., et al. (2000b) Observer variability in histologic reporting of bronchial biopsy specimens. J Bronchol 7: 210–214 [Google Scholar]
- Venmans B., van der Linden H., van Boxem T., Postmus P., Smit E., Sutedja T. (1998) Early detection of preinvasive lesions in high-risk patients. A comparison of conventional flexible and fluorescence bronchoscopy. J Bronchol 5: 280–283 [Google Scholar]
- Vermylen P., Pierard P., Roufosse C., Bosschaerts T., Verhest A., Sculier J., et al. (1999) Detection of bronchial preneoplastic lesions and early lung cancer with fluorescence bronchoscopy: a study about its ambulatory feasibility under local anesthesia. Lung Cancer 25: 161–168 [DOI] [PubMed] [Google Scholar]
- Vincent B., Fraig M., Silvestri G. (2007) A pilot study of narrow-band imaging compared to white light bronchoscopy for evaluation of normal airways and premalignant and malignant airways disease. Chest 131: 1794–1788 [DOI] [PubMed] [Google Scholar]
- Vonk Noordegraaf A., Postmus P., Sutedja T. (2003) Bronchoscopic treatment of patients with intraluminal microinvasive radiographically occult lung cancer not eligible for surgical resection: a follow-up study. Lung Cancer 39: 49–53 [DOI] [PubMed] [Google Scholar]
- Wagnieres G., McWilliams A., Lam S. (2003) Lung cancer imaging with fluorescence endoscopy. In: Mycek M., Pogue B. (eds), Handbook of Biomedical Fluorescence. New York: Marcel Dekker [Google Scholar]
- Walsh D., Maiwand M., Nath A., Lockwood P., Lloyd M., Saab M. (1990) Bronchoscopic cryotherapy for advanced bronchial carcinoma. Thorax 45: 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg L., Wiedmann T., Estensen R., Zimmerman C., Galbraith A., Steele V., et al. (2000) Chemoprevention of pulmonary carcinogenesis by brief exposures to aerosolized bidesonide or beclomethasone dipropionate and by the combination of aerosolized budesonide and dietary myo-inositol. Carcinogenesis 21: 179–182 [DOI] [PubMed] [Google Scholar]
- Whiteman S., Yang Y., van Pittius D., Stephens M., Parmer J., Spiteri M. (2006) Optical coherence tomography: Real-time imaging of bronchial airways microstructure and detection of inflammatory/neoplastic morphological changes. Clin Cancer Res 12: 813–818 [DOI] [PubMed] [Google Scholar]
- WHO (1999) Histological Typing of Lung and Pleural Tumors. 3rd ed. Berlin: Springer-Verlag [Google Scholar]
- Winterhalder R., Hirsch F., Kotantoulas G., Franklin W., Bunn P., Jr (2004) Chemoprevention of lung cancer – from biology to clinical reality. Ann Oncol 15: 185–196 [DOI] [PubMed] [Google Scholar]
- Woolner L., Fontana R., Cortese D., Sanderson D., Bernatz P., Payne W., et al. (1984) Roentgenographically occult lung cancer: pathologic findings and frequency of multicentricity during a 10-year period. Mayo Clin Proc 59: 453–466 [DOI] [PubMed] [Google Scholar]
- Yang V., Vitkin I. (2006) Principles of Doppler optical coherence tomography. In: Regar E., van Leeuwen T., Serruys P. (eds), Handbook of Optical Coherence Tomography in Cardiology. Oxford: Taylor and Francis Medical [Google Scholar]


