Abstract
The search for innovative therapeutic agents in non-small cell lung cancer (NSCLC) has witnessed a swift evolution. The number of targeted drugs that can improve patient outcomes with an acceptable safety profile is steadily increasing. In this review, we highlight current drugs that have already been approved or are under evaluation for the treatment of patients with NSCLC, either in monotherapy or combined therapy for both the first- and second-line settings. Experience with drugs targeting the vascular endothelial growth factor and its receptor, as well as the epidermal growth factor receptor is summarized. Moreover, we provide an overview of more novel targets in NSCLC and initial experience with the respective therapeutic agents.
Keywords: monoclonal antibody, non-small cell lung cancer, therapy, tyrosine kinase inhibitor
Introduction
Lung cancer is the second most common cancer and is the leading cause of cancer mortality worldwide for both men and women [Siegel et al. 2012]. Most patients will present with incurable disease and face a 5-year relative survival rate of up to 17% with current standard therapies [Askoxylakis et al. 2010]. More than 80% of lung cancers are non-small cell lung carcinoma (NSCLC), which includes adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and NSCLC that cannot be further classified. Treatment of lung cancer depends on the cell type (NSCLC versus small cell), tumor stage, and the patient’s overall condition. Patients with early disease (stage I, II, or III-a) NSCLC are generally treated with curative intent, using surgery, chemotherapy, radiation therapy, or a combined modality approach. However, patients deemed suitable for curative treatment will still maintain a high rate of relapse. Patients with advanced disease are often treated with systemic chemotherapy but response and survival rates continue to be modest [Vilmar and Sorensen, 2011]. In the last few decades, our understanding of the molecular biology of tumors has increased tremendously. This has allowed researchers to design and develop selective agents to specifically target the oncogenic pathways that drive tumor cell growth, proliferation, angiogenesis, and invasion. In this review (last literature search performed on 1 April 2013), we discuss new targeted agents that have emerged to treat these tumors, and the clinical development of novel agents with the potential to improve survival rates of patients with NSCLC.
Drugs blocking ligands and receptors
Vascular endothelial growth factor
The vascular endothelial growth factor (VEGF) production is initiated by features characteristic of tumors such as tumor hypoxia, necrosis, and oncogene expression. VEGF has a central role in tumor angiogenesis, influencing other pro-angiogenic factors. It is overexpressed in a variety of tumors including NSCLC and may be associated with reduced survival [Otrock et al. 2011].
Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody to VEGF. It has a prolonged half-life, allowing administration every 2–3 weeks, and demonstrates a high affinity to the VEGF-A isoform. Preclinical studies have shown synergistic activity of bevacizumab in combination with various chemotherapeutic agents [Browder et al. 2000]. Clinical trials have demonstrated the benefit of using bevacizumab in combination with chemotherapy in the first-line setting in patients with advanced NSCLC. The phase II AVF0757g trial randomized patients to bevacizumab, 7.5 mg/kg or 15 mg/kg once every 3 weeks, in combination with carboplatin–paclitaxel compared with carboplatin–paclitaxel alone [Johnson et al. 2004]. The greatest benefit was observed in the 15 mg/kg cohort in combination with carboplatin–paclitaxel with a significant improvement in response rates (31.5% versus 18.8%) and median time to disease progression (7.4 versus 4.2 months) compared with the control arm [Johnson et al. 2004]. Moderate improvement in overall survival was also observed (17.7 versus 14.9 months) [Johnson et al. 2004]. Bleeding events noted with bevacizumab in this study were mainly minor epistaxis; however, six patients had major bleeding events, four of them fatal, most frequently in patients with squamous carcinoma tumors located close to major blood vessels [Johnson et al. 2004].
This led to the design of the pivotal ECOG 4599 phase III study of bevacizumab, 15 mg/kg, in combination with carboplatin–paclitaxel as first-line therapy, in patients with nonsquamous cell advanced NSCLC without brain metastasis [Sandler et al. 2006]. Chemotherapy was administered every 3 weeks for 6 cycles, and bevacizumab was administered every 3 weeks until disease progression was evident or toxic effects were intolerable [Sandler et al. 2006]. There was a significant improvement in response rate (35% versus 15%, p < 0.001), median overall survival (12.3 versus 10.3 months, p = 0.003), and progression-free survival (6.2 versus 4.5 months, p < 0.001) in patients who received bevacizumab. The safety profile of bevacizumab in combination with carboplatin–paclitaxel was acceptable with the most common adverse events observed being hypertension, proteinuria, and minor bleeding. Only 3% of patients experienced grade 3–4 bleeding events. Interestingly, 24% of the patients in the bevacizumab–carboplatin–paclitaxel group had neutropenia compared with 16% in the carboplatin–paclitaxel alone group, suggesting that bevacizumab improved penetration to both the tumor and the bone marrow [Sandler et al. 2006]. The AVAil phase III trial evaluated the addition of bevacizumab to cisplatin–gemcitabine as first-line therapy in patients with advanced NSCLC. The addition of bevacizumab (7.5 or 15 mg/kg) also significantly improved progression-free survival and objective response rate [Reck et al. 2009, 2010b]. This raised the question of which is the ideal platinum-based regimen for use in combination with bevacizumab, although overall survival benefit was only noted with the carboplatin–paclitaxel and not the cisplatin–gemcitabine combination. Irrespectively, a recent meta-analysis of randomized, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced NSCLC confirmed the benefit of bevacizumab on prolonged overall and progression-free survival without unexpected toxicity [Soria et al. 2013]. Benefit of this regimen in elderly patients (>65 years), however, is controversial [Laskin et al. 2012; Spigel et al. 2012; Zhu et al. 2012]. Pretreatment measurement of circulating VEGF-A level is of prognostic value in patients receiving bevacizumab [Hegde et al. 2013].
A phase II study of first-line bevacizumab plus pemetrexed–carboplatin followed by maintenance bevacizumab in responding or stable patients showed that the regimen is well tolerated and displayed remarkable activity [Stevenson et al. 2012]. The clinical benefit and safety of continued bevacizumab treatment in patients with advanced NSCLC whose disease has progressed after first-line treatment with bevacizumab plus a platinum-based doublet is currently under evaluation [Gridelli et al. 2011; Takeda et al. 2012].
Aflibercept
The VEGF Trap AVE0005 (aflibercept) is a specific antagonist that binds and inactivates circulating VEGF. The VEGF Trap is a fusion protein consisting of human VEGF receptor extracellular domains fused to the Fc portion of human IgGl. It has a higher binding affinity to VEGF-A but also binds VEGF-B plus the related factors PlGF1 and PlGF2. The VEGF Trap may inhibit tumor growth by reducing the density of tumor vasculature and reducing the escape of tissue matrix components from leaky tumor vessels [Chu, 2009]. Aflibercept was recently studied in a phase II clinical trial, with a dose schedule of 4.0 mg/kg given every 2 weeks to patients with platinum- and erlotinib-resistant, locally advanced, or metastatic adenocarcinoma of the lung. The results of this multicenter, open-label, single-arm, study showed an acceptable safety profile in 89 patients that were evaluable after a mean of 4 cycles, with common toxicities including dyspnea, hypertension, and proteinuria seen in 21%, 23%, and 10% of patients, respectively [Leighl et al. 2010]. Its activity as a single agent, however, was minor, with an overall response rate of 2%, median progression-free survival of 2.7 months, and overall survival of 6.2 months. A more recent phase III trial showed no benefit from the addition of aflibercept to docetaxel in patients with platinum-resistant advanced or metastatic NSCLC [Ramlau et al. 2012].
Epidermal growth factor receptor
Epidermal growth factor receptor (EGFR), also known as HER1, is frequently dysregulated in carcinomas. Its activation initiates a signal transduction cascade that promotes tumor-cell proliferation and survival. The overexpression/dysregulation of EGFR is associated with poor prognosis and can transform cells in a ligand-dependent manner. EGFR blockade inhibits tumorigenicity [Okamoto et al. 2010].
Cetuximab
Cetuximab, a monoclonal antibody that binds EGFR, is used in colorectal cancer as well as squamous cell cancer of the head and neck. A phase II trial explored the role of EGFR amplification, assessed by fluorescent in-situ hybridization (FISH), in locally advanced or metastatic NSCLC patients treated with concurrent or sequential carboplatin, paclitaxel, and cetuximab [Hirsch et al. 2008]. The study showed that in those patients with FISH-positive tumors, there was an increase in both progression-free survival (6 versus 3 months, p = 0.0008) and overall survival (15 versus 7 months, p = 0.04). Considerable clinical efficacy with an acceptable toxicity were also demonstrated when a regimen including docetaxel instead of paclitaxel was used [Fischer et al. 2012]. In a randomized phase III trial (FLEX), cetuximab was also studied in the first-line setting in combination with cisplatin–vinorelbine, compared with cisplatin–vinorelbine alone, in patients with NSCLC that expressed EGFR by immunohistochemistry (IHC) [Pirker et al. 2009]. The chemotherapy was given in combination with cetuximab for up to six cycles, and in responding patients (partial response or stable disease); the cetuximab was continued until progression. Patients receiving cetuximab had an increased response rate (36% versus 29%), and improved median survival (11.3 versus 10.1 months, p = 0.044). Important in this study is the fact that the advantage in overall survival was observed not only in nonsquamous NSCLC (12.0 versus 10.3 months) but also in squamous cell NSCLC (10.2 versus 8.9 months) [Pirker et al. 2009]. More recent data on the benefit of cetuximab added to chemoradiation in unresectable advanced NSCLC are conflicting [Blumenschein et al. 2011; Govindan et al. 2011; Jensen et al. 2011]. A recent meta-analysis, however, concluded that cetuximab-based chemotherapy produces significant clinical benefit with acceptable toxicity as a first-line strategy in patients with advanced or metastatic NSCLC, although highlighting the need to identify markers predictive of cetuximab benefit [Ibrahim et al. 2011]. Irrespectively, it is now established that evaluation of EGFR expression predicts response for NSCLC patients receiving cetuximab plus chemotherapy [O’Callaghan et al. 2010; Pirker et al. 2012]. Moreover, a recent subanalysis of the FLEX trial showed that first-cycle rash was associated with a better outcome in patients with advanced NSCLC who received cisplatin–vinorelbine plus cetuximab as a first-line treatment, suggesting it could be a surrogate clinical marker used to tailor cetuximab treatment for advanced NSCLC [Gatzemeier et al. 2011]. Trials evaluating the combination of cetuximab and bevacizumab in chemo-naive patients with NSCLC are emerging [Bonomi et al. 2013] and further data are warranted.
Nimotuzumab
Nimotuzumab is a novel anti-EGFR monoclonal antibody that was recently evaluated in patients with advanced NSCLC. Phase I trials established attractive safety of the drug and further studies are awaited [Bebb et al. 2011; Kim et al. 2013].
Tyrosine kinase inhibitors
EGFR
Activating mutations in the gene encoding EGFR can confer sensitivity to EGFR tyrosine kinase inhibitors in patients with advanced NSCLC. Mutations associated with enhanced sensitivity to EGFR tyrosine kinase inhibitors are found in exons 18–21 of the tyrosine kinase domain of EGFR. Two types of mutations, short in-frame deletions in exon 19, clustered around the amino-acid residues 747–750 as well as a specific exon 21 point mutation (L858R), have been reported to comprise up to 90% of all activating EGFR mutations. Other activating mutations include point mutations in exon 18 (including mutations in codon 719) and point mutations and in-frame insertions in exon 20 (including T790M). Testing for mutations in EGFR has been an important step in clinical trials evaluating the role of EGFR tyrosine kinase inhibitors, as noted in subsequent sections. Various methods have been investigated as potential alternatives to the historical standard for EGFR mutation testing, direct DNA sequencing. Many of these are targeted methods that specifically detect the most common EGFR mutations. The development of targeted mutation testing methods and commercially available test kits has enabled sensitive, rapid, and robust analysis of clinical samples. The use of screening methods, subsequent to sample microdissection, has also ensured that identification of more rare, uncommon mutations is now feasible. Cytology samples including fine needle aspirate and pleural effusion can be used successfully to determine EGFR mutation status provided that sensitive testing methods are employed [Ellison et al. 2013]. Although patients with EGFR-mutation-positive tumors may have the greatest degree of benefit [Paz-Ares et al. 2010], the data are not clear that EGFR-mutation-negative patients (wild type) will not benefit at all [Laurie and Goss, 2013].
Identification of mutations that can predict response to targeted therapeutics in patients with NSCLC has been the focus of the Lung Cancer Mutation Consortium who analyzed approximately 800 lung adenocarcinoma tumor samples and identified mutations in 54% of the samples; the most common molecular changes identified were KRAS mutations (22%), EGFR mutations (17%), and ALK rearrangements (7%, to be covered in subsequent section) [Kris et al. 2011]. Owing to how common KRAS mutations are in NSCLC and the fact the they are associated with a poor prognosis in NSCLC, the clinical utility of KRAS mutational analysis has also been considered for predicting benefit from EGFR tyrosine kinase inhibitors in clinical trials and will eventually become an important biomarker in the treatment of patients with advanced NSCLC outside the context of clinical trials. Of note, an association between KRAS mutational status and benefit of anti-EGFR monoclonal antibodies (covered in previous sections) has not been demonstrated in NSCLC. As the most common oncogenic event in NSCLC, KRAS mutation also represents an elusive clinical target for ongoing drug development (KRAS downstream effector pathways) [Roberts and Stinchcombe, 2013].
Gefitinib
Gefitinib is an orally active EGFR tyrosine kinase inhibitor that was shown to enhance antitumor efficacy of cytotoxics. Gefitinib was studied in several phase III trials following the two phase II trials that suggested favorable response rates and limited toxicity in patients with advanced NSCLC after failure of treatment [Fukuoka et al. 2003; Kris et al. 2003]. The ISEL (Iressa Survival Evaluation in Lung Cancer) study randomized 1692 patients with advanced NSCLC after failure of treatment into either gefitinib treatment or placebo. The results of this study showed no benefit in median overall survival [Thatcher et al. 2005]. Another study, the INTEREST trial, randomized 1433 patients with advanced NSCLC who had progression with previous platinum-based treatment to either gefitinib or docetaxel [Kim et al. 2008]. Again, there was no benefit in overall survival between those treated with gefitinib versus docetaxel. However, a recent phase III trial (INFORM) showed that maintenance treatment with gefitinib significantly prolonged progression-free survival compared with placebo in patients achieved disease control after first-line chemotherapy [Zhang et al. 2012].
The IPASS study was a phase III trial that randomized 1217 previously untreated patients who were never smokers with NSCLC (adenocarcinoma) and showed superiority of gefitinib over carboplatin–paclitaxel. Those treated with gefitinib had increased progression free survival [Mok et al. 2009]. Interestingly, in the subset analysis, those who were positive for the EGFR mutation had increased progression-free survival with gefitinib, while those who were EGFR-mutation-negative experienced increased progression-free survival on carboplatin–paclitaxel [Fukuoka et al. 2011]. Same results of progression-free survival benefit were reproduced in the NEJ002 phase III trial which recruited EGFR-mutation-positive patients [Maemondo et al. 2010]. However, an updated overall survival results from this trial failed to show benefit of gefitinib in this setting [Inoue et al. 2013]. Another phase III trial randomized 177 patients with untreated advanced NSCLC that possess an EGFR-mutation to receive cisplatin-docetaxel or gefitinib [Mitsudomi et al. 2010]. Those treated with gefitinib had significantly prolonged progression-free survival (9.2 versus 6.3 months, p = 0.0001), which was the primary endpoint of the study, supporting its use in this population of NSCLC patients expressing an EGFR-mutation. A recent meta-analysis concluded that first-line treatment with gefitinib conferred prolonged progression-free survival than treatment with systemic chemotherapy in the molecularly or histologically defined population of patients with NSCLC [Wang et al. 2012].
Two phase III trials (INTACT-1 and INTACT-2) randomized chemotherapy-naive patients with advanced NSCLC into either chemotherapy alone (one employed cisplatin–gemcitabine and the other employed carboplatin–paclitaxel) or chemotherapy with gefitinib [Giaccone et al. 2004; Herbst et al. 2004]. Neither of these studies showed an improvement in overall survival with the addition of gefitinib to chemotherapy. Sequential gefitinib following platinum-doublet chemotherapy in this setting also failed to show benefits [Niho et al. 2012; Takeda et al. 2010].
Erlotinib
Erlotinib is an orally available small molecule tyrosine kinase inhibitor of EGFR. It has a good safety profile, with common toxicities including rash and diarrhea.
The TOPICAL phase III trial compared erlotinib to placebo as first-line therapy in patients with advanced NSCLC unsuitable for chemotherapy. Although median overall survival did not differ between groups, patients with first-cycle rash had better overall survival [hazard ratio (HR) 0.76, p = 0.0058], compared with placebo [Lee et al. 2012]. The RADIANT (Randomized Double-blind Trial In Adjuvant NSCLC with Tarceva) study, a phase III clinical trial comparing 150 mg of daily erlotinib versus placebo, has completed enrolling 945 subjects who are EGFR-mutation-positive NSCLC patients treated with surgery with or without adjuvant chemotherapy [ClinicalTrials.gov identifier: NCT00373425]. The primary endpoint for this study is disease-free survival. Recent data also show that neoadjuvant erlotinib in early-stage NSCLC patients has low toxicity and sufficient activity to deserve further testing [Schaake et al. 2012].
More importantly, results from two phase III, multicenter, open-label, randomized trials of erlotinib monotherapy versus chemotherapy as first-line treatment in patients with advanced NSCLC and EGFR-mutation positivity are now available [ClinicalTrials.gov identifiers: NCT00874419 (OPTIMAL) and NCT00446225 (EURTAC)]. In the EURTAC study patients were randomized to receive either erlotinib as monotherapy or a combination of platinum-based doublet chemotherapy every 3 weeks for 4 cycles [Rosell et al. 2012]. This study is the first prospective study of an EGFR tyrosine kinase inhibitor versus chemotherapy for first-line treatment of NSCLC in Caucasian patients with an EGFR-mutation. A total of 174 patients received erlotinib 150 mg orally daily as continuous treatment or any of the following combinations of chemotherapy: cisplatin–docetaxel, cisplatin–gemcitabine, carboplatin–docetaxel, carboplatin–gemcitabine. Erlotinib conferred a significant progression-free survival (primary endpoint) benefit over standard chemotherapy with 63% reduction in risk of progression (HR 0.37). Overall survival data are immature with, as expected, a high level of known crossover. As expected, no new safety findings were found and tolerability of erlotinib was consistent with previous studies. The OPTIMAL study is also the first study as upfront therapy comparing erlotinib alone to chemotherapy in Asian patients with NSCLC and an EGFR mutation [Zhou et al. 2011]. A total of 165 patients were randomized in this phase III trials to receive either erlotinib 150 mg daily or carboplatin–gemcitabine every 3 weeks for up to 4 cycles. Erlotinib was highly potent as first-line therapy with a median progression-free survival (primary endpoint) almost tripled (13.7 months versus 4.6 months) and with an 84% reduction in the risk of progression or death with erlotinib (HR 0.16). A significant quality of life benefit with erlotinib over first-line chemotherapy was also observed (over 70% of patients in erlotinib arm had clinically relevant improvement in quality of life). Also, erlotinib had less severe toxicity than chemotherapy, except for rash (mostly mild or moderate in severity). Recently, and based on the data from these two studies erlotinib was approved by EMEA as first-line treatment for NSCLC patients with EFGR mutation. Lower rates of central nervous system involvement were also noted in EGFR-mutation-positive advanced NSCLC patients receiving erlotinib (or gefitinib) compared with chemotherapy [Heon et al. 2012].
The TALENT (Tarceva Lung Cancer Investigation Trial) study randomized previously untreated patients with advanced NSCLC to receive cisplatin plus gemcitabine, with or without erlotinib [Gatzemeier et al. 2007]. There were no statistically significant differences between the two groups, and subgroup analysis of smokers versus nonsmokers involved a very small group. The TRIBUTE (Tarceva Responses in Conjunction with Paclitaxel and Carboplatin) study randomized patients with stage III-b/IV NSCLC into two arms: carboplatin plus paclitaxel, with or without erlotinib [Herbst et al. 2005]. Patients treated with erlotinib had similar median overall survival and time to progression compared with placebo. However, those who reported to have never smoked had an improved overall survival on erlotinib versus placebo and EGFR mutations seemed to be a positive prognostic indicator of response to erlotinib. Similarly, two randomized phase II trials comparing first-line erlotinib with or with carboplatin–paclitaxel in advanced NSCLC patients showed similar outcomes in both groups. EGFR mutations identified patients most likely to benefit [Hirsch et al. 2011; Janne et al. 2012].
The TORCH (Tarceva or Chemotherapy) study is a phase III multicenter randomized control trial that investigated which sequential approach in NSCLC is most appropriate and cost-effective. The study was stopped at interim analysis because of inferiority of the experimental arm. First-line erlotinib followed at progression by cisplatin–gemcitabine was significantly inferior in terms of overall survival compared with the standard sequence of first-line chemotherapy followed by erlotinib [Gridelli et al. 2012]. Functional domains and global quality of life did not differ between both arms, although some symptoms were better controlled with chemotherapy [Di Maio et al. 2012]. Similar data were retrieved from elderly patients [LeCaer et al. 2011].
The BR.21 study was a randomized, placebo controlled trial on 731 patients with stage III/IV NSCLC after failure of first- or second-line platinum-based chemotherapy [Shepherd et al. 2005]. Two thirds of the patients received erlotinib (150 mg daily) and the remaining one third received placebo. The erlotinib arm showed significantly improved progression-free survival, overall survival, and quality of life (longer time to clinically significant deterioration) as compared with those receiving placebo. In a retrospective analysis of these patients, it was found that those with EFGR-mutation-positive tumors (by FISH) had improved overall survival, yet this was not seen in patients that were negative for the EFGR mutation [Tsao et al. 2005]. Erlotinib was thus approved for the treatment of recurrent NSCLC at a daily dose of 150 mg. More recent data from the TITAN phase III trial which was halted prematurely because of slow recruitment showed similar efficacy between erlotinib and docetaxel or pemetrexed in second-line treatment of patients with advanced NSCLC with poor prognosis [Ciuleanu et al. 2012].
With regards to the use of erlotinib as maintenance therapy, the SATURN (Sequential Tarceva in Unresectable Lung Cancer) study randomized 889 patients in remission after 4 cycles of chemotherapy to either erlotinib therapy (150 mg daily) or placebo with the primary endpoint of progression free survival including EFGR-mutation-positive patients [Cappuzzo et al. 2010]. All patients experienced improved progression-free survival on erlotinib, and those with the EGFR mutation had a marked improvement [Brugger et al. 2011]. Median overall survival was also improved with patients in the erlotinib arm. In addition, erlotinib significantly extended the time to pain and analgesic use. Erlotinib was also tolerated well with similar quality of life scores in both groups [Cappuzzo et al. 2010]. It should be noted, however, that in a meta-analysis (BioLOGUE) of data from BR.21 and SATURN, no biomarker was felt sufficiently robust to select for the use of erlotinib in the maintenance or refractory setting [Soulieres et al. 2011]. A recent study also showed that gemcitabine continuation maintenance or erlotinib switch maintenance significantly reduces disease progression in patients with advanced NSCLC treated with cisplatin–gemcitabine as first-line chemotherapy [Perol et al. 2012]. The ATLAS trial was a randomized, double-bind, placebo-controlled study of 743 patients with advanced NSCLC who were in remission after receiving 4 cycles of chemotherapy [Miller et al. 2009]. The two arms were bevacizumab alone versus bevacizumab and erlotinib with the primary endpoint of progression-free survival. The trial was cut short during the interim analysis since there was an observed statistically significant improvement with the combination therapy over the use of bevacizumab alone. The data from these two trials suggest that erlotinib is indicated for use in maintenance therapy for NSCLC. Pooled analysis of the three trials confirmed that the addition of maintenance erlotinib significantly improves progression-free and overall survival in patients with advanced NSCLC who had not progressed after four cycles of first-line chemotherapy. The benefit did not seem to be limited to a particular subgroup, although it is more pronounced in never-smoking women patients with nonsquamous histology and a performance status of zero [Petrelli et al. 2011].
Several trials evaluated dual targeted therapy for both EGFR and VEGF in patients with NSCLC, primarily using erlotinib and bevacizumab. In a phase II trial, survival in an unselected population of patients with advanced NSCLC treated with first-line bevacizumab and erlotinib approximated that expected with conventional chemotherapy [Akerley et al. 2013]. Another trial evaluated the same regimen and showed a nonprogression rate of up to 75%, although overall survival was disappointing [Dingemans et al. 2011]. Another two phase II trials evaluated the same regimen followed by platinum-based chemotherapy on progression and demonstrated acceptable toxicity and activity [Cohen et al. 2012; Zappa et al. 2012]. VeriStrat® analyses (a serum proteomic test) distinguished patients who were likely or unlikely to benefit from this combination in all such trials [Akerley et al. 2013; Gautschi et al. 2013]. Similar data were not as promising in elderly patients [Riggs et al. 2012]. Moreover, when both agents were incorporated with chemotherapy (carboplatin–paclitaxel) and used all together for first-line therapy in advanced NSCLC patients, lack of efficacy and substantial risk of esophageal toxicity were noted [Socinski et al. 2012]. A recent meta-analysis showed that combining targeted therapy with erlotinib seems superior over erlotinib monotherapy as second-line treatment for advanced NSCLC. Subgroup analysis based on phases of trials, EGFR status, and KRAS status also showed that there was a tendency to improve progression-free and overall survival in combining targeted therapy, except that progression-free survival for patients with EGFR-mutation or wild-type KRAS favored erlotinib monotherapy. In addition, greater incidence of grade 3 or 4 rash, fatigue, and hypertension were observed in combining targeted therapy [Qi et al. 2013].
Afatinib
Afatinib (BIBW 2992) is a next-generation tyrosine kinase inhibitor that irreversibly inhibits both ErbB:EGFR (HER 1) as well as HER2 [Yap et al. 2010]. The drug has been recently evaluated in NSCLC patients who have failed erlotinib or gefitinib therapy. Initial data from an ongoing phase III trial in NSCLC patients (LUX-Lung 5) suggested the drug extended progression-free survival threefold compared with placebo, but did not extend overall survival [Metro and Crino, 2011]. The phase IIb/III trial (LUX-Lung 1) came to the same conclusion [Miller et al. 2012].
Dacomatinib
Dacomatinib (PF-00299804) is an irreversible pan-inhibitor of ErbB (EGFR/HER 1, HER 2, and HER 4). In a recent randomized phase II study dacomitinib demonstrated significantly improved progression-free survival versus erlotinib, with acceptable toxicity in advanced NSCLC. Progression-free survival benefit was observed in most clinical and molecular subsets, notably KRAS wild-type/EGFR any status, KRAS wild-type/EGFR wild-type, and EGFR mutants [Ramalingam et al. 2012].
Vascular endothelial growth factor receptor
Sorafenib
Sorafenib targets Raf-1, VEGFR-2 and 3, PDGFR-α, c-kit, and Flt-3. It has received regulatory approval for the treatment of relapsed or refractory renal cell and hepatocellular carcinomas. A phase II study of sorafenib was conducted in patients who had previously received only one chemotherapy regimen for advanced NSCLC [Liu et al. 2006]. Patients received 400 mg sorafenib twice daily continuously on a 28-day cycle. Results demonstrated that sorafenib was well tolerated with the expected skin toxicities, hypertension, anemia, hyponatremia, and nausea, which are clinically manageable. Positive responses were observed in four out of five evaluable patients with relapsed NSCLC (two with partial responses and two with stable disease). A more recent phase II trial showed that treatment with sorafenib has relevant clinical activity in NSCLC patients that progressed after at least one platinum-containing regimen. Response was associated with harboring KRAS mutations [Dingemans et al. 2013]. However, the phase III trial (NEXUS) did not meet its primary endpoint of improved overall survival when sorafenib (400 mg twice daily) was added to first-line gemcitabine–cisplatin in patients with advanced nonsquamous NSCLC [Paz-Ares et al. 2012]. Similarly, a phase II study evaluating maintenance sorafenib therapy in patients receiving first-line paclitaxel–carboplatin did not meet its primary survival endpoint [Gervais et al. 2011].
Sunitinib
Sunitinib is another tyrosine kinase inhibitor that targets VEGFR-1,-2, α3, PDGFR-α, -β, c-kit, and Flt3 [Mendel et al. 2003]. Sunitinib is approved as a first-line therapy in metastatic renal cell carcinoma and in second-line therapy for the treatment of gastrointestinal stromal tumors [Demetri et al. 2006; Faivre et al. 2006; Motzer et al. 2008]. Preclinical studies have demonstrated antitumor activity in a wide range of tumor models, including NSCLC. Sunitinib was studied in a phase I trial in combination with cisplatin–gemcitabine. The maximum tolerated dose was 37.5 mg. A total of 4 out of 18 evaluable patients achieved a partial tumor response; however, frequent dose delays due to myelosuppression occurred [Reck et al. 2010a]. Similarly, trials in patients with advanced solid malignancies (with an expanded cohort in NSCLC) showed that sunitinib at 37.5 mg was not tolerated when combined with standard pemetrexed and cisplatin doses, although it was well tolerated when combined with pemetrexed alone [Camidge et al. 2013; Chow et al. 2012]. A phase II study assessing the safety and efficacy of sunitinib in addition to carboplatin–paclitaxel in nonsquamous NSCLC found that this combination was also not well tolerated [Socinski et al. 2010]. Collectively, these studies indicated that the combination of sunitinib and platinum-based chemotherapy is not a favorable option as it is associated with high toxicity. In a phase II trial of sunitinib monotherapy in 47 patients with previously treated advanced NSCLC, the response rate was 2% (seen in only one patient) and was generally well tolerated [Novello et al. 2009]. A more recent trial in previously treated patients with advanced NSCLC showed that sunitinib plus erlotinib did not improve overall survival compared with erlotinib alone, but the combination was associated with a statistically significantly longer progression-free survival and greater overall response rate [Scagliotti et al. 2012a]. Sunitinib is currently being studied in a phase III trial as maintenance therapy for patients with stage III/IV NSCLC that have previously been treated with combination chemotherapy [ClinicalTrials.gov identifier: NCT00693992].
Cediranib
Cediranib is an orally available and potent small-molecule tyrosine kinase inhibitor that targets VEGFR-2. Two phase I dose-escalating studies of cediranib in combination with cisplatin–gemcitabine or carboplatin–paclitaxel were conducted in patients with advanced NSCLC. The cediranib–carboplatin–paclitaxel combination reported responses in 9 out of 20 patients (45%) [Goss et al. 2009; Laurie et al. 2008]. A phase II/III randomized control double-blinded trial studied the safety and efficacy of cediranib (30 mg daily) with carboplatin–paclitaxel in 251 patients with advanced NSCLC [Goss et al. 2010]. The study results showed that there was an improved response rate and progression free survival with the addition of cediranib to carboplatin–paclitaxel; however, increased toxicity, including hypertension, gastrointestinal toxicity, hypothyroidism, and hand-foot syndrome, was observed at the dose of 30 mg daily. Similar data were reported when the chemotherapy regimen included carboplatin-gemcitabine [Dy et al. 2013]. A randomized, double-blind, placebo-controlled trial is currently underway to assess the efficacy and safety of cediranib at a dose 20 mg daily [ClinicalTrials.gov identifier: NCT00795340].
Vandetanib
Vandetanib is a tyrosine kinase inhibitor of both VEGFR and EGFR. It was mainly evaluated as a second-line treatment for advanced NSCLC. A recent meta-analysis of four randomized controlled trials concluded that therapy with vandetanib offered a clinically meaningful and statistically significant improvement in progression-free survival and overall response in patients with advanced NSCLC but did not benefit overall survival. Therapy with vandetanib regimens might be suggested as second-line treatment for advanced NSCLC based on a similar toxicity profile compared with standard second-line therapy [Qi et al. 2011].
Figure 1 summarizes the drugs and their targets.
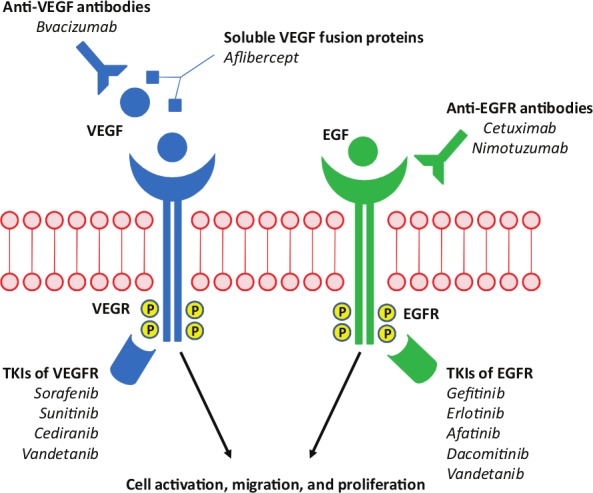
Drug targets in non-small cell lung carcinoma.
VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; EGF, epidermal growth factor; EFGR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Other agents and future perspective
Targeting HER2
Human epidermal growth factor receptor 2 (HER2, or ErBB2) is overexpressed in 13–20% of NSCLC cases (with 3+ expression in only 2–6%), typically in the adenocarcinoma subtype, and more frequently in women and never-smokers [Oxnard et al. 2013]. Although the study of trastuzumab in HER2-overexpressing NSCLC did not identify significant clinical activity [Clamon et al. 2005], the role of some of the aforementioned irreversible tyrosine kinase inhibitors targeting both EGFR and HER2 (afatinib and dacomitinib) has received recent attention. An early report from a phase II trial of afatinib alone in molecularly selected lung adenocarcinoma [ClinicalTrials.gov identifier: NCT00730925] described partial responses in two of five patients with tumors harboring HER2 mutations; although efficacy may have been limited by toxicity, which required several patients to come off study before progression [De Greve et al. 2012]. However, a prospective trial of dacomitinib has now reported only 3 responses out of 18 HER2-mutant lung cancers, suggesting that dacomitinib alone may be inadequate to fully inhibit this particular oncogene in patients [Oxnard et al. 2013].
Targeting BRAF
BRAF is a serine/threonine kinase downstream from KRAS in the mitogen-activated protein kinase (MAPK) signaling cascade. The high incidence of oncogenic BRAF mutations in melanoma led investigators to search for BRAF mutations in NSCLC. Upon sequencing of exons 11 and 15 of BRAF missense mutations were identified in 1.6–4.9% of NSCLC cases. Approximately half of the mutations identified were V600E, with one group finding that this particular mutation was more common in never-smokers [Oxnard et al. 2013]. A phase II trial of the BRAF inhibitor GSK2118436 is currently under way in BRAF V600E–mutant NSCLC [ClinicalTrials.gov identifier: NCT01336634] to prospectively study this approach in lung cancer. Because cancers with non-V600E BRAF mutations are unlikely to be inhibited by V600E-specific inhibitors, inhibitors of downstream targets like MEK are being explored [selumetinib (AZD6244)] in this population [ClinicalTrials.gov identifier: NCT00888134].
Targeting insulin-like growth factor 1 receptor
The insulin-like growth factor 1 receptor (IGF-1R) belongs to a family of receptors that can interact with its ligands, insulin, IGF-1, and IGF-2, to activate cellular proliferation, differentiation, and antiapoptotic proteins [Sachdev and Yee, 2007]. The circulating concentrations of IGF-1R ligands are tightly regulated by a range of high-affinity IGF binding proteins 1–6 (IGFBP1–6). There is evidence to suggest that the IGF pathway is active across a range of malignancies, including NSCLC [Yu et al. 1999].
Figitumumab (CP-751,871) is a selective fully human IgG2 monoclonal antibody against the IGF-1R pathway. A recent randomized phase II trial of CP-751871 in combination with carboplatin–paclitaxel in patients with advanced NSCLC, in first-line setting, found an overall response rate of 54% for the IGF-1R inhibitor–carboplatin–paclitaxel combination compared with 41% for carboplatin–paclitaxel alone [Rodon et al. 2008]. Furthermore, a subgroup analysis found that the overall response rates of patients with squamous histology were as high as 78%. The most common grade 3–4 adverse events observed with the combination therapy included neutropenia, hyperglycemia, and fatigue [Rodon et al. 2008]. Cixutumumab (IMC-A12) is another IGF-1R inhibitor which showed unfavorable efficacy and tolerability when combined with erlotinib in patients with advanced NSCLC [Weickhardt et al. 2012]. Similarly, the combination of R1507, another IGF-1R inhibitor, with erlotinib did not provide progression-free or overall survival advantage over erlotinib alone in an unselected group of patients with advanced NSCLC [Ramalingam et al. 2011]. Clinical trials of other IGF-1R inhibitors in patients with advanced NSCLC in chemotherapy naive and chemotherapy refractory disease are ongoing.
Targeting the PI3K-AKT pathway using mTOR inhibitors
Growth factors, such EGF or IGFs, primarily regulate mTOR activity through signaling via the PI3K-AKT pathway [Cantley, 2002]. In various cancers, growth factor receptors such as IGF-1R, EGFR, HER2/neu, and PDGFR may be overexpressed, amplified, or mutated and drive the PI3K-AKT-mTOR pathway [Gadgeel and Wozniak, 2013; Hennessy et al. 2005]. These data suggest that targeting of this pathway with specific inhibitors could be used to treat these particular cancers. Preclinical studies have demonstrated that the inhibition of the PI3K-AKT-mTOR pathway restores gefitinib sensitivity and shows a synergistic effect in EGFR resistant cancer cell lines [Dong et al. 2012]. A phase I study of the combination of everolimus (RAD001), an mTOR inhibitor, and gefitinib in advanced NSCLC confirmed tolerability and showed encouraging responses [Milton et al. 2007]. However, a phase II trial of this combination showed a 13% partial response rate and did not meet the prespecified response threshold to pursue further study of the combination [Price et al. 2010]. Combination therapy with everolimus and erlotinib provided acceptable tolerability and disease control [Papadimitrakopoulou et al. 2012]. A randomized phase II study evaluating this combination in comparison with erlotinib alone is complete and is being analyzed. Promising anticancer activity was also noted in a phase I studies of everolimus in combination with docetaxel or pemetrexed for recurrent/refractory NSCLC [Ramalingam et al. 2010; Vansteenkiste et al. 2011].
Targeting histone deacetylases
Histone deacetylases (HDACs) play an important role in the epigenetic regulation of gene expression by catalyzing the removal of acetyl groups from histones. This deacetylation results in chromatin condensation and the transcriptional repression of tumor suppressor genes [Bolden et al. 2006]. HDACs also target nonhistone acetylated proteins, such as p53, Rb, E2F1, HSP90, and tubulin [Carew et al. 2008]. Acetylation status of both tubulin and tubulin-associated proteins can modulate microtubule dynamics suggesting that HDAC inhibitors could enhance taxane-mediated disruption of microtubules and induce apoptosis [Carew et al. 2008]. As inhibition of HDACs could reverse the epigenetic silencing observed in many cancers, several HDAC inhibitors with various chemical structures have been developed for cancer therapy. These include the hydroxamic acids (vorinostat and panobinostat), short-chain fatty acids (valproic acid and pivanex), benzamides (MS-275 and CI-994), and cyclic tetrapeptides (desipeptide), among others. Treatment with these inhibitors stimulated cellular differentiation and cell-cycle arrest typically at G1/S, with an associated increase in p21 due to p53-independent induction of CDKN1A [Zhang et al. 2003]. Preclinical findings have led to phase I/II clinical trials of HDAC inhibitors either alone or in combination with standard chemotherapeutic or biological agents for NSCLC [Capelletto and Novello, 2012]. HDAC inhibitors were also shown to overcome resistance to EGFR tyrosine kinase inhibitors linked to epigenetic changes and epithelial-mesenchymal transition state. However, initial clinical trials did not yield promising results [Witta et al. 2012].
Targeting c-MET
c-MET is a proto-oncogene that encodes a protein known as hepatocyte growth factor receptor (HGFR) which possesses tyrosine kinase activity. c-MET receptor activation is associated with poor prognosis and EGFR tyrosine kinase inhibitor resistance in NSCLC. A recent randomized phase II study of erlotinib plus tivantinib (ARQ 197), a c-MET inhibitor, versus erlotinib plus placebo in previously treated NSCLC showed that the combination is well tolerated. Although the study did not meet its primary endpoint, evidence of activity was demonstrated, especially among patients with KRAS mutations [Sequist et al. 2011]. The phase III trial of this regimen (MARQUEE) [Scagliotti et al. 2012b] has been halted following a planned interim analysis because it was not expected to meet its primary endpoint. Onartuzumab (MetMAb) is a humanized monovalent monoclonal antibody that binds to MET with high specificity. In a phase II study, patients with MET-positive tumors (≥50% of tumor cells stain 2+ or 3+ intensity by IHC) who received onartuzumab and erlotinib had nearly twofold reduction in the risk of disease progression and threefold reduction in the risk of death compared with erlotinib alone [Spigel et al. 2011]. A randomized, phase III, multicenter, double-blind study (MetLUNG) is currently recruiting adults with MET-positive advanced NSCLC who failed at least one but no more than two prior lines of platinum-based chemotherapy, to receive erlotinib + placebo or onartuzumab [ClinicalTrials.gov identifier: NCT01456325].
Targeting the proteasome
The proteasome is a multicatalytic intracellular complex responsible for the degradation of misfolded or damaged proteins that are targeted by ubiquitination. Key proteins degraded by the proteasome include those involved in cell cycle progression, proliferation, and apoptosis, such as p53, p21, p27, inhibitor of NFκB, Bcl-2, Bcl-xL, and Bax. Inhibition of the proteasome disrupts protein homeostasis and attenuates multiple signaling pathways that promote tumor transformation. This has led to the development of proteasome inhibitors, such as bortezomib, which has been extensively studied and has received regulatory approval for treatment of multiple myeloma and mantle cell lymphoma [Davies et al. 2007; Gettinger, 2008].
Single-agent bortezomib in patients with NSCLC has displayed modest results [Besse et al. 2012; Li et al. 2010]. However, phase I combination studies of bortezomib with cisplatin–gemcitabine in patients with NSCLC reported that, among 21 evaluable patients, 10 patients (48%) achieved a partial response, whereas stable disease was observed in 6 patients [Davies et al. 2008]. These results led to the SWOG 0339 phase II trial of this combination for first-line treatment of advanced NSCLC. Of the 114 patients enrolled in this study, 20% had objective responses and 45% had stable disease [Davies et al. 2009]. The combination of bortezomib with carboplatin and bevacizumab in the first-line setting was also very well tolerated with interesting clinical activity [Piperdi et al. 2012]. The encouraging results observed with bortezomib and in combination with chemotherapy may represent an exciting opportunity for further clinical trials, particularly in chemo-naïve patients. Of note, a randomized phase II trial of docetaxel plus cetuximab or docetaxel plus bortezomib as first-line therapy in patients with advanced NSCLC and a performance status of 2 showed that neither combination met the prespecified progression-free survival endpoint [Lilenbaum et al. 2009]. In previously treated patients, the addition of bortezomib to pemetrexed was well tolerated but offered no statistically significant response or survival advantage versus pemetrexed alone, while bortezomib alone showed no clinically significant activity [Scagliotti et al. 2010]. A more recent trial demonstrated that bortezomib plus docetaxel given sequentially or concurrently has similar response rate and progression-free survival with the median survival in the sequential arm exceeding published survival estimates for either agent alone or in combination [Lara et al. 2011b]. The addition of bortezomib to erlotinib in previously treated patients also showed insufficient activity [Lynch et al. 2009].
Targeting the apoptotic pathway
Apoptosis, a programmed form of cell death, is a highly regulated process [Samali et al. 1996]. A diverse array of cellular stresses trigger apoptosis by activating one or more signal transduction pathways which converge to activate a conserved family of aspartic acid-specific cysteine proteases called caspases [Alnemri et al. 1996]. The extrinsic, or death receptor-mediated, apoptotic pathway is activated following binding of death ligands to specific cognate death receptors expressed on the cell surface. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis by binding to its transmembrane, death domain-containing receptors, TRAIL receptor 1 (death receptor 4; DR4), and TRAIL receptor 2 (death receptor 5; DR5) [Mahalingam et al. 2009]. Preclinical studies have shown antitumor activity with TRAIL across a wide variety of cancer models, including NSCLC, when used alone and in combination with other anticancer agents [Mahalingam et al. 2009]. TRAIL and its variants, including death receptor selective monoclonal antibodies, are in early clinical trials and are being evaluated in advanced NSCLC. A phase I study of conatumumab (AMG 655), a monoclonal agonistic antibody targeting the DR5 death receptor, found that the agent was well tolerated up to 20 mg/kg and showed activity in NSCLC [Doi et al. 2011; Herbst et al. 2010]. The combination of dulanermin (recombinant human Apo2L/TRAIL) with paclitaxel–carboplatin–bevacizumab has also been studied in a phase Ib trial, in chemo-naïve patients with NSCLC. No grade 3/4 adverse events related to TRAIL were reported and 14 of the 24 patients (58%) responded to the combination [Soria et al. 2010]. In the phase II trial, the addition of dulanermin to paclitaxel–carboplatin–bevacizumab and paclitaxel–carboplatin–bevacizumab did not improve outcomes in unselected patients with previously untreated advanced or recurrent NSCLC [Soria et al. 2011].
Targeting the EML4-ALK fusion protein
A subset of patients with NSCLC exhibits an EML4-ALK fusion protein mutation, which is presumed to be an oncogenic driver. It appears to be associated with young, male, never-smokers, the adenocarcinoma histological subtype, and the lack of EFGR or KRAS mutations [Inamura et al. 2008; Koivunen et al. 2008; Martelli et al. 2009; Shaw et al. 2009; Wong et al. 2009]. Crizotinib (PF-02341066) has been studied in a phase I trial with positive response seen in more than half of patients and an estimated 6-month progression-free survival of 72%, with a tolerable gastrointestinal side-effect profile [Kwak et al. 2010; Camidge et al. 2012]. An ongoing phase III trial, PROFILE 1007 [ClinicalTrials.gov identifier: NCT00932893], is comparing crizotinib with standard second-line chemotherapy (pemetrexed or taxotere) in the treatment of ALK-positive NSCLC. In addition, a phase II trial, PROFILE 1005 [ClinicalTrials.gov identifier: NCT00932451], is studying patients meeting similar criteria who have received more than one line of prior chemotherapy. On 26 August 2011, the US Food and Drug Administration approved crizotinib (fast-track) to treat certain late-stage (locally advanced or metastatic) NSCLC that express the abnormal ALK gene. Approval required a companion molecular test for the EML4-ALK fusion. The FDA approval was based on two phase II studies involving 255 ALK-positive NSCLC patients. The drug resulted in 3 complete response (1%) and treated patients had 50% and 61% overall response rate in studies A and B. There were 45 deaths out of which 32 were due to progressive disease. The adverse events observed were: ALT elevation, neutropenia, QTs prolongation, vision disorder, nausea, diarrhea, vomiting, edema, pneumonia, and constipation. A recent retrospective analysis also showed that in patients with advanced, ALK-positive NSCLC, crizotinib therapy is associated with improved survival compared with that of crizotinib-naïve controls. ALK rearrangement is not a favorable prognostic factor in advanced NSCLC [Shaw et al. 2011]. On 15 March 2013, the highly selective inhibitor of ALK, LDK378, received Breakthrough Therapy designation by the US FDA for the treatment of patients with metastatic NSCLC who had progressed during treatment with, or were intolerant to, crizotinib. Initial results from a phase I study investigating the maximum tolerated dose, safety, pharmacokinetics and antitumor activity of LDK378 in 88 patients with ALK-positive advanced malignancies, as detected by an FDA-approved test and who had progressed during treatment with, or were intolerant to crizotinib, showed marked responses in a majority of patients with ALK-positive NSCLC. A response rate of 80% was observed in the patients who had experienced disease progression after crizotinib treatment. Two phase II clinical trials were initiated to further evaluate the compound in this patient population with plans to initiate several phase III clinical trials later this year [Shaw and Engelman, 2013].
The heat shock protein-90 (Hsp90) has also become a target for therapy. A preliminary study has shown that a novel Hsp90 inhibitor (IPI-504) has clinical activity in patients with NSCLC, particularly among patients with ALK rearrangements [Sequist et al. 2010]. A more recent phase II study evaluating this agent was terminated due to low accrual [ClinicalTrials.gov identifier: NCT01228435].
Immunotherapy
Ipilimumab, an anticytotoxic T-cell lymphocyte-4 monoclonal antibody, is commonly used in melanoma patients for evidence of survival benefit. A recent randomized phase II trial evaluated ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV NSCLC established benefit in progression-free survival and recommended additional investigation of this agent in NSCLC [Lynch et al. 2012].
Anti-PD-1 (BMS-936558, nivolumab) is a fully-human antibody that targets the inhibitory receptor expressed on activated T-cells called PD-1 or programmed death-1. In a phase I dose-ranging study the drug showed clinical activity in patients with previously-treated NSCLC, metastatic melanoma, and renal cell carcinoma [Topalian et al. 2012]. Data on a second investigational immunotherapeutic agent, the anti-PD-L1 (BMS-936559), were also published recently [Brahmer et al. 2012]. BMS-936559 targets one of the immunosuppressive ligands for PD-1, PD-L1, which is often expressed on tumor, stromal and immune cells. Antibody-mediated blockade of PD-L1 induced durable tumor regression (objective response rate of 6 to 17%) and prolonged stabilization of disease (rates of 12–41% at 24 weeks) in patients with advanced cancers, including NSCLC, melanoma, and renal-cell carcinoma. These encouraging data supported further investigation of anti-PD-1 in large-scale phase II and phase III trials [Brahmer, 2013].
Mucin 1 (MUC1) is a glycoprotein that is overexpressed and aberrantly glycosylated in many carcinomas including NSCLC. MUC1 can stimulate cell proliferation and suppress apoptosis and therefore may have a role in tumour progression. Moreover, abnormal MUC1 expression is associated with progressive disease and metastasis [Bafna et al. 2010]. BLP25 liposome vaccine or L-BLP25 (Stimuvax®) is a therapeutic cancer vaccine that targets the MUC1 antigen. A phase II study comparing L-BLP25 plus best supportive care (BSC) with BSC alone in 171 patients with stage IIIB/IV NSCLC reported median overall survival times of 17.4 and 13 months, respectively, after a median follow up of 26 months (adjusted HR 0.74, p = 0.112). The greatest difference was observed in patients with stage IIIB locoregional disease in whom the median survival time had not been reached for the L-BLP-25 arm, compared with 13.3 months for the BSC arm (adjusted HR 0.524, p = 0.069) at the time of the initial publication [Butts et al. 2005]. An updated survival analysis in patients with stage IIIB locoregional disease reported a median survival time of 30.6 months with L-BLP25 compared with 13.3 months in the control arm [Butts et al. 2011]. On the basis of these findings, the phase III trial, START was initiate investigating the efficacy and safety of L-BLP25 as maintenance therapy for unresectable stage III NSCLC [ClinicalTrials.gov identifier: NCT00409188]. However, on 19 December 2012, the manufacturer announced that the trial did not meet its primary endpoint of an improvement in overall survival.
The Melanoma AntiGEn A3 (MAGE-A3) is expressed in 35% of early-stage NSCLC cases [Sienel et al. 2004]. The MAGE-A3 vaccine showed favorable results in a phase II trial recruiting MAGE-A3-positive patients with completely resected, pathological stage IB/II NSCLC; a possible gene expression signature predictive of clinical activity of the MAGE-A3 vaccine was also identified [Decoster et al. 2012]. A double-blind phase III trial (MAGRIT) [ClinicalTrials.gov identifier: NCT00480025] is recruiting patients with completely resected stage IB/II/IIIA MAGE-A3-positive NSCLC.
Belagenpumatucel-L, the EGFR, and the TG4010 vaccines are also being evaluated in advanced stage NSCLC, either as an adjunct to chemotherapy or as maintenance after completion of chemotherapy [Decoster et al. 2012].
Tumor vascular disrupting agents
Vadimezan (ASA404) is a tumor vascular disrupting agent (VDA) that attacks the blood supply of a cancerous tumor to cause tumor regression. This agent was studied in combination with carboplatin–paxcitaxel as first-line therapy in two phase II trials for advanced NSCLC and showed survival benefit of around 5 months when compared with chemotherapy alone [McKeage et al. 2009; McKeage et al. 2008]. However, in a recent phase III trial the addition of vadimezan to carboplatin and paclitaxel, although generally well tolerated, failed to improve first-line efficacy in advanced NSCLC [Lara et al. 2011a].
Conclusion
Despite recent advances in treatment of NSCLC, clinical outcome of these patients still remains a challenge. The search for innovative therapeutic agents in NSCLC that are more effective and have fewer side effects than older chemotherapeutic drugs has spurred the development of more than 500 molecularly targeted agents and thereby has introduced the concept of individualized therapy. In the process of identifying targets for therapy, our understanding of the molecular pathways involved in malignancy has also increased, and this knowledge of mechanisms of tumor cell growth and survival has translated into clinical trials of drugs that have clearly changed the treatment landscape. With a median age at diagnosis of 70 years, many NSCLC patients previously considered ineligible for anticancer therapy because of comorbidities and frailty may now receive treatment. Yet despite these apparent advances, for most patients with NSCLC targeted therapies have not dramatically changed clinical outcome. The molecular complexity of lung cancer underlies these disappointments and stresses the need for optimizing treatment by seeking a more personalized approach to care. Therefore, clinical trials that investigate activity of novel agents, and incorporate patient selection based on clinical and molecular factors, are required.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Fadi S. Farhat, Hematology-Oncology Division (Head), Hammoud Hospital University Medical Center, Ghassan Hammoud Street, 652, Saida, Lebanon
Wissam Houhou, Hammoud Hospital University Medical Center, Saida, Lebanon.
References
- Akerley W., Boucher K., Rich N., Egbert L., Harker G., Bylund J., et al. (2013) A phase II study of bevacizumab and erlotinib as initial treatment for metastatic non-squamous, non-small cell lung cancer with serum proteomic evaluation. Lung Cancer 79: 307–311 [DOI] [PubMed] [Google Scholar]
- Alnemri E., Livingston D., Nicholson D., Salvesen G., Thornberry N., Wong W., et al. (1996) Human ICE/CED-3 protease nomenclature. Cell 87: 171. [DOI] [PubMed] [Google Scholar]
- Askoxylakis V., Thieke C., Pleger S., Most P., Tanner J., Lindel K., et al. (2010) Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer 10: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafna S., Kaur S., Batra S. (2010) Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 29: 2893–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebb G., Smith C., Rorke S., Boland W., Nicacio L., Sukhoo R., et al. (2011) Phase I clinical trial of the anti-EGFR monoclonal antibody nimotuzumab with concurrent external thoracic radiotherapy in Canadian patients diagnosed with stage IIb, III or IV non-small cell lung cancer unsuitable for radical therapy. Cancer Chemother Pharmacol 67: 837–845 [DOI] [PubMed] [Google Scholar]
- Besse B., Planchard D., Veillard A., Taillade L., Khayat D., Ducourtieux M., et al. (2012) Phase 2 study of frontline bortezomib in patients with advanced non-small cell lung cancer. Lung Cancer 76: 78–83 [DOI] [PubMed] [Google Scholar]
- Blumenschein G., Jr, Paulus R., Curran W., Robert F., Fossella F., Werner-Wasik M., et al. (2011) Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol 29: 2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden J., Peart M., Johnstone R. (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5: 769–784 [DOI] [PubMed] [Google Scholar]
- Bonomi P., Mace J., Mandanas R., Min M., Olsen M., Youssoufian H., et al. (2013) Randomized phase II study of cetuximab and bevacizumab in combination with two regimens of paclitaxel and carboplatin in chemonaive patients with stage IIIB/IV non-small-cell lung cancer. J Thorac Oncol 8: 338–345 [DOI] [PubMed] [Google Scholar]
- Brahmer J. (2013) Harnessing the immune system for the treatment of non-small-cell lung cancer. J Clin Oncol 31: 1021–1028 [DOI] [PubMed] [Google Scholar]
- Brahmer J., Tykodi S., Chow L., Hwu W., Topalian S., Hwu P., et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browder T., Butterfield C., Kraling B., Shi B., Marshall B., O’Reilly M., et al. (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60: 1878–1886 [PubMed] [Google Scholar]
- Brugger W., Triller N., Blasinska-Morawiec M., Curescu S., Sakalauskas R., Manikhas G., et al. (2011) Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol 29: 4113–4120 [DOI] [PubMed] [Google Scholar]
- Butts C., Maksymiuk A., Goss G., Soulieres D., Marshall E., Cormier Y., et al. (2011) Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 137: 1337–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts C., Murray N., Maksymiuk A., Goss G., Marshall E., Soulieres D., et al. (2005) Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 23: 6674–6681 [DOI] [PubMed] [Google Scholar]
- Camidge D., Bang Y., Kwak E., Iafrate A., Varella-Garcia M., Fox S., et al. (2012) Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 13: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge D., Blais N., Jonker D., Soulieres D., Doebele R., Ruiz-Garcia A., et al. (2013) Sunitinib combined with pemetrexed and cisplatin: results of a phase I dose-escalation and pharmacokinetic study in patients with advanced solid malignancies, with an expanded cohort in non-small cell lung cancer and mesothelioma. Cancer Chemother Pharmacol 71: 307–319 [DOI] [PubMed] [Google Scholar]
- Cantley L. (2002) The phosphoinositide 3-kinase pathway. Science 296: 1655–1657 [DOI] [PubMed] [Google Scholar]
- Capelletto E., Novello S. (2012) Emerging new agents for the management of patients with non-small cell lung cancer. Drugs 72(Suppl. 1): 37–52 [DOI] [PubMed] [Google Scholar]
- Cappuzzo F., Ciuleanu T., Stelmakh L., Cicenas S., Szczesna A., Juhasz E., et al. (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11: 521–529 [DOI] [PubMed] [Google Scholar]
- Carew J., Giles F., Nawrocki S. (2008) Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Cancer Lett 269: 7–17 [DOI] [PubMed] [Google Scholar]
- Chow L., Blais N., Jonker D., Laurie S., Diab S., Canil C., et al. (2012) A phase I dose-escalation and pharmacokinetic study of sunitinib in combination with pemetrexed in patients with advanced solid malignancies, with an expanded cohort in non-small cell lung cancer. Cancer Chemother Pharmacol 69: 709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Q. (2009) Aflibercept (AVE0005): an alternative strategy for inhibiting tumour angiogenesis by vascular endothelial growth factors. Expert Opin Biol Ther 9: 263–271 [DOI] [PubMed] [Google Scholar]
- Ciuleanu T., Stelmakh L., Cicenas S., Miliauskas S., Grigorescu A., Hillenbach C., et al. (2012) Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 13: 300–308 [DOI] [PubMed] [Google Scholar]
- Clamon G., Herndon J., Kern J., Govindan R., Garst J., Watson D., et al. (2005) Lack of trastuzumab activity in nonsmall cell lung carcinoma with overexpression of erb-B2: 39810: a phase II trial of Cancer and Leukemia Group B. Cancer 103: 1670–1675 [DOI] [PubMed] [Google Scholar]
- Cohen E., Subramanian J., Gao F., Szeto L., Kozloff M., Faoro L., et al. (2012) Targeted and cytotoxic therapy in coordinated sequence (TACTICS): erlotinib, bevacizumab, and standard chemotherapy for non-small-cell lung cancer, a phase II trial. Clin Lung Cancer 13: 123–128 [DOI] [PubMed] [Google Scholar]
- Davies A., Chansky K., Lara P., Jr, Gumerlock P., Crowley J., Albain K., et al. (2009) Bortezomib plus gemcitabine/carboplatin as first-line treatment of advanced non-small cell lung cancer: a phase II Southwest Oncology Group Study (S0339). J Thorac Oncol 4: 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Lara P., Jr, Mack P., Gandara D. (2007) Incorporating bortezomib into the treatment of lung cancer. Clin Cancer Res 13: s4647–s4651 [DOI] [PubMed] [Google Scholar]
- Davies A., Ruel C., Lara P., Lau D., Gumerlock P., Bold R., et al. (2008) The proteasome inhibitor bortezomib in combination with gemcitabine and carboplatin in advanced non-small cell lung cancer: a California Cancer Consortium Phase I study. J Thorac Oncol 3: 68–74 [DOI] [PubMed] [Google Scholar]
- De Greve J., Teugels E., Geers C., Decoster L., Galdermans D., De Mey J., et al. (2012) Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 76: 123–127 [DOI] [PubMed] [Google Scholar]
- Decoster L., Wauters I., Vansteenkiste J. (2012) Vaccination therapy for non-small-cell lung cancer: review of agents in phase III development. Ann Oncol 23: 1387–1393 [DOI] [PubMed] [Google Scholar]
- Demetri G., van Oosterom A., Garrett C., Blackstein M., Shah M., Verweij J., et al. (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368: 1329–1338 [DOI] [PubMed] [Google Scholar]
- Di Maio M., Leighl N., Gallo C., Feld R., Ciardiello F., Butts C., et al. (2012) Quality of life analysis of TORCH, a randomized trial testing first-line erlotinib followed by second-line cisplatin/gemcitabine chemotherapy in advanced non-small-cell lung cancer. J Thorac Oncol 7: 1830–1844 [DOI] [PubMed] [Google Scholar]
- Dingemans A., de Langen A., van den Boogaart V., Marcus J., Backes W., Scholtens H., et al. (2011) First-line erlotinib and bevacizumab in patients with locally advanced and/or metastatic non-small-cell lung cancer: a phase II study including molecular imaging. Ann Oncol 22: 559–566 [DOI] [PubMed] [Google Scholar]
- Dingemans A., Mellema W., Groen H., van Wijk A., Burgers S., Kunst P., et al. (2013) A phase II study of sorafenib in patients with platinum-pretreated, advanced (Stage IIIb or IV) non-small cell lung cancer with a KRAS mutation. Clin Cancer Res 19: 743–751 [DOI] [PubMed] [Google Scholar]
- Doi T., Murakami H., Ohtsu A., Fuse N., Yoshino T., Yamamoto N., et al. (2011) Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 68: 733–741 [DOI] [PubMed] [Google Scholar]
- Dong S., Zhang X., Cheng H., Zhu J., Chen Z., Zhang Y., et al. (2012) Everolimus synergizes with gefitinib in non-small-cell lung cancer cell lines resistant to epidermal growth factor receptor tyrosine kinase inhibitors. Cancer Chemother Pharmacol 70: 707–716 [DOI] [PubMed] [Google Scholar]
- Dy G., Mandrekar S., Nelson G., Meyers J., Adjei A., Ross H., et al. (2013) A Randomized Phase II Study of Gemcitabine and Carboplatin with or without Cediranib as First-Line Therapy in Advanced Non-Small-Cell Lung Cancer: North Central Cancer Treatment Group Study N0528. J Thorac Oncol 8: 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison G., Zhu G., Moulis A., Dearden S., Speake G., McCormack R. (2013) EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol 66: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre S., Delbaldo C., Vera K., Robert C., Lozahic S., Lassau N., et al. (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24: 25–35 [DOI] [PubMed] [Google Scholar]
- Fischer J., Griesinger F., Fink T., Salm T., Marseille A., Wolf M. (2012) Docetaxel-carboplatin chemotherapy combined with cetuximab in patients with locally advanced or metastatic non small-cell lung cancer (NSCLC) - results of the nonrandomised phase II study TaxErb. Lung Cancer 75: 348–352 [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Wu Y., Thongprasert S., Sunpaweravong P., Leong S., Sriuranpong V., et al. (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29: 2866–2874 [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Yano S., Giaccone G., Tamura T., Nakagawa K., Douillard J., et al. (2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 21: 2237–2246 [DOI] [PubMed] [Google Scholar]
- Gadgeel S., Wozniak A. (2013) Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer. Clin Lung Cancer, in press. [DOI] [PubMed] [Google Scholar]
- Gatzemeier U., Pluzanska A., Szczesna A., Kaukel E., Roubec J., De Rosa F., et al. (2007) Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 25: 1545–1552 [DOI] [PubMed] [Google Scholar]
- Gatzemeier U., von Pawel J., Vynnychenko I., Zatloukal P., de Marinis F., Eberhardt W., et al. (2011) First-cycle rash and survival in patients with advanced non-small-cell lung cancer receiving cetuximab in combination with first-line chemotherapy: a subgroup analysis of data from the FLEX phase 3 study. Lancet Oncol 12: 30–37 [DOI] [PubMed] [Google Scholar]
- Gautschi O., Dingemans A., Crowe S., Peters S., Roder H., Grigorieva J., et al. (2013) VeriStrat((R)) has a prognostic value for patients with advanced non-small cell lung cancer treated with erlotinib and bevacizumab in the first line: Pooled analysis of SAKK19/05 and NTR528. Lung Cancer 79: 59–64 [DOI] [PubMed] [Google Scholar]
- Gervais R., Hainsworth J., Blais N., Besse B., Laskin J., Hamm J., et al. (2011) Phase II study of sunitinib as maintenance therapy in patients with locally advanced or metastatic non-small cell lung cancer. Lung Cancer 74: 474–480 [DOI] [PubMed] [Google Scholar]
- Gettinger S. (2008) Targeted therapy in advanced non-small-cell lung cancer. Sem Resp Crit Care Med 29: 291–301 [DOI] [PubMed] [Google Scholar]
- Giaccone G., Herbst R., Manegold C., Scagliotti G., Rosell R., Miller V., et al. (2004) Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial - INTACT 1. J Clin Oncol 22: 777–784 [DOI] [PubMed] [Google Scholar]
- Goss G., Arnold A., Shepherd F., Dediu M., Ciuleanu T., Fenton D., et al. (2010) Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol 28: 49–55 [DOI] [PubMed] [Google Scholar]
- Goss G., Shepherd F., Laurie S., Gauthier I., Leighl N., Chen E., et al. (2009) A phase I and pharmacokinetic study of daily oral cediranib, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer: a study of the National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer 45: 782–788 [DOI] [PubMed] [Google Scholar]
- Govindan R., Bogart J., Stinchcombe T., Wang X., Hodgson L., Kratzke R., et al. (2011) Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol 29: 3120–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridelli C., Bennouna J., de Castro J., Dingemans A., Griesinger F., Grossi F., et al. (2011) Randomized phase IIIb trial evaluating the continuation of bevacizumab beyond disease progression in patients with advanced non-squamous non-small-cell lung cancer after first-line treatment with bevacizumab plus platinum-based chemotherapy: treatment rationale and protocol dynamics of the AvaALL (MO22097) trial. Clin Lung Cancer 12: 407–411 [DOI] [PubMed] [Google Scholar]
- Gridelli C., Ciardiello F., Gallo C., Feld R., Butts C., Gebbia V., et al. (2012) First-line erlotinib followed by second-line cisplatin–gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol 30: 3002–3011 [DOI] [PubMed] [Google Scholar]
- Hegde P., Jubb A., Chen D., Li N., Meng Y., Bernaards C., et al. (2013) Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res 19: 929–937 [DOI] [PubMed] [Google Scholar]
- Hennessy B., Smith D., Ram P., Lu Y., Mills G. (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4: 988–1004 [DOI] [PubMed] [Google Scholar]
- Heon S., Yeap B., Lindeman N., Joshi V., Butaney M., Britt G., et al. (2012) The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res 18: 4406–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R., Giaccone G., Schiller J., Natale R., Miller V., Manegold C., et al. (2004) Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial - INTACT 2. J Clin Oncol 22: 785–794 [DOI] [PubMed] [Google Scholar]
- Herbst R., Kurzrock R., Hong D., Valdivieso M., Hsu C., Goyal L., et al. (2010) A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res 16: 5883–5891 [DOI] [PubMed] [Google Scholar]
- Herbst R., Prager D., Hermann R., Fehrenbacher L., Johnson B., Sandler A., et al. (2005) TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23: 5892–5899 [DOI] [PubMed] [Google Scholar]
- Hirsch F., Herbst R., Olsen C., Chansky K., Crowley J., Kelly K., et al. (2008) Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol 26: 3351–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch F., Kabbinavar F., Eisen T., Martins R., Schnell F., Dziadziuszko R., et al. (2011) A randomized, phase II, biomarker-selected study comparing erlotinib to erlotinib intercalated with chemotherapy in first-line therapy for advanced non-small-cell lung cancer. J Clin Oncol 29: 3567–3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim E., Abouelkhair K., Al-Masri O., Chaudry N., Kazkaz G. (2011) Cetuximab-based therapy is effective in chemotherapy-naive patients with advanced and metastatic non-small-cell lung cancer: a meta-analysis of randomized controlled trials. Lung 189: 193–198 [DOI] [PubMed] [Google Scholar]
- Inamura K., Takeuchi K., Togashi Y., Nomura K., Ninomiya H., Okui M., et al. (2008) EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 3: 13–17 [DOI] [PubMed] [Google Scholar]
- Inoue A., Kobayashi K., Maemondo M., Sugawara S., Oizumi S., Isobe H., et al. (2013) Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin–paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 24: 54–59 [DOI] [PubMed] [Google Scholar]
- Janne P., Wang X., Socinski M., Crawford J., Stinchcombe T., Gu L., et al. (2012) Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol 30: 2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A., Munter M., Bischoff H., Haselmann R., Haberkorn U., Huber P., et al. (2011) Combined treatment of nonsmall cell lung cancer NSCLC stage III with intensity-modulated RT radiotherapy and cetuximab: the NEAR trial. Cancer 117: 2986–2994 [DOI] [PubMed] [Google Scholar]
- Johnson D., Fehrenbacher L., Novotny W., Herbst R., Nemunaitis J., Jablons D., et al. (2004) Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22: 2184–2191 [DOI] [PubMed] [Google Scholar]
- Kim E., Hirsh V., Mok T., Socinski M., Gervais R., Wu Y., et al. (2008) Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 372: 1809–1818 [DOI] [PubMed] [Google Scholar]
- Kim S., Shim H., Cho J., Jeong J., Kim S., Hong Y., et al. (2013) A phase I trial of gefitinib and nimotuzumab in patients with advanced non-small cell lung cancer (NSCLC). Lung Cancer 79: 270–275 [DOI] [PubMed] [Google Scholar]
- Koivunen J., Mermel C., Zejnullahu K., Murphy C., Lifshits E., Holmes A., et al. (2008) EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 14: 4275–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris M., Johnson B., Kwiatkowski D., Iafrate A., Wistuba I., Aronson S., et al. (2011) Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI’s Lung Cancer Mutation Consortium (LCMC) [abstract]. J Clin Oncol 29(15 Suppl.): CRA7506. [Google Scholar]
- Kris M., Natale R., Herbst R., Lynch T., Jr, Prager D., Belani C., et al. (2003) Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 290: 2149–2158 [DOI] [PubMed] [Google Scholar]
- Kwak E., Bang Y., Camidge D., Shaw A., Solomon B., Maki R., et al. (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363: 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara P., Jr, Douillard J., Nakagawa K., von Pawel J., McKeage M., Albert I., et al. (2011a) Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol 29: 2965–2971 [DOI] [PubMed] [Google Scholar]
- Lara P., Jr, Longmate J., Reckamp K., Gitlitz B., Argiris A., Ramalingam S., et al. (2011b) Randomized phase II trial of concurrent versus sequential bortezomib plus docetaxel in advanced non-small-cell lung cancer: a California cancer consortium trial. Clin Lung Cancer 12: 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin J., Crino L., Felip E., Franke F., Gorbunova V., Groen H., et al. (2012) Safety and efficacy of first-line bevacizumab plus chemotherapy in elderly patients with advanced or recurrent nonsquamous non-small cell lung cancer: safety of avastin in lung trial (MO19390). J Thorac Oncol 7: 203–211 [DOI] [PubMed] [Google Scholar]
- Laurie S., Gauthier I., Arnold A., Shepherd F., Ellis P., Chen E., et al. (2008) Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: the National Cancer Institute of Canada clinical trials group. J Clin Oncol 26: 1871–1878 [DOI] [PubMed] [Google Scholar]
- Laurie S., Goss G. (2013) Role of epidermal growth factor receptor inhibitors in epidermal growth factor receptor wild-type non-small-cell lung cancer. J Clin Oncol 31: 1061–1069 [DOI] [PubMed] [Google Scholar]
- LeCaer H., Barlesi F., Corre R., Jullian H., Bota S., Falchero L., et al. (2011) A multicentre phase II randomised trial of weekly docetaxel/gemcitabine followed by erlotinib on progression, vs the reverse sequence, in elderly patients with advanced non small-cell lung cancer selected with a comprehensive geriatric assessment (the GFPC 0504 study). Br J Cancer 105: 1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Khan I., Upadhyay S., Lewanski C., Falk S., Skailes G., et al. (2012) First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 13: 1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighl N., Raez L., Besse B., Rosen P., Barlesi F., Massarelli E., et al. (2010) A multicenter, phase 2 study of vascular endothelial growth factor trap (Aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J Thorac Oncol 5: 1054–1059 [DOI] [PubMed] [Google Scholar]
- Li T., Ho L., Piperdi B., Elrafei T., Camacho F., Rigas J., et al. (2010) Phase II study of the proteasome inhibitor bortezomib (PS-341, Velcade) in chemotherapy-naive patients with advanced stage non-small cell lung cancer (NSCLC). Lung Cancer 68: 89–93 [DOI] [PubMed] [Google Scholar]
- Lilenbaum R., Wang X., Gu L., Kirshner J., Lerro K., Vokes E. (2009) Randomized phase II trial of docetaxel plus cetuximab or docetaxel plus bortezomib in patients with advanced non-small-cell lung cancer and a performance status of 2: CALGB 30402. J Clin Oncol 27: 4487–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Barrett T., Choyke P., Maynard K., Wright J., Kummar S., et al. (2006) A phase II study of BAY 43–9006 (Sorafenib) in patients with relapsed non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol 24(Suppl. 18): 17119 [Google Scholar]
- Lynch T., Bondarenko I., Luft A., Serwatowski P., Barlesi F., Chacko R., et al. (2012) Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 30: 2046–2054 [DOI] [PubMed] [Google Scholar]
- Lynch T., Fenton D., Hirsh V., Bodkin D., Middleman E., Chiappori A., et al. (2009) A randomized phase 2 study of erlotinib alone and in combination with bortezomib in previously treated advanced non-small cell lung cancer. J Thorac Oncol 4: 1002–1009 [DOI] [PubMed] [Google Scholar]
- Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., et al. (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388 [DOI] [PubMed] [Google Scholar]
- Mahalingam D., Szegezdi E., Keane M., de Jong S., Samali A. (2009) TRAIL receptor signalling and modulation: Are we on the right TRAIL? Cancer Treat Rev 35: 280–288 [DOI] [PubMed] [Google Scholar]
- Martelli M., Sozzi G., Hernandez L., Pettirossi V., Navarro A., Conte D., et al. (2009) EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol 174: 661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeage M., Reck M., Jameson M., Rosenthal M., Gibbs D., Mainwaring P., et al. (2009) Phase II study of ASA404 (vadimezan, 5,6-dimethylxanthenone-4-acetic acid/DMXAA) 1800mg/m(2) combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Lung Cancer 65: 192–197 [DOI] [PubMed] [Google Scholar]
- McKeage M., Von Pawel J., Reck M., Jameson M., Rosenthal M., Sullivan R., et al. (2008) Randomised phase II study of ASA404 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Br J Cancer 99: 2006–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel D., Laird A., Xin X., Louie S., Christensen J., Li G., et al. (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9: 327–337 [PubMed] [Google Scholar]
- Metro G., Crino L. (2011) The LUX-Lung clinical trial program of afatinib for non-small-cell lung cancer. Expert Rev Anticancer Ther 11: 673–682 [DOI] [PubMed] [Google Scholar]
- Miller V., Hirsh V., Cadranel J., Chen Y., Park K., Kim S., et al. (2012) Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 13: 528–538 [DOI] [PubMed] [Google Scholar]
- Miller V., O’Connor P., Soh C., Kabbinavar F. (2009) A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol 27: LBA8002. [DOI] [PubMed] [Google Scholar]
- Milton D., Riely G., Azzoli C., Gomez J., Heelan R., Kris M., et al. (2007) Phase 1 trial of everolimus and gefitinib in patients with advanced nonsmall-cell lung cancer. Cancer 110: 599–605 [DOI] [PubMed] [Google Scholar]
- Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121–128 [DOI] [PubMed] [Google Scholar]
- Mok T., Wu Y., Thongprasert S., Yang C., Chu D., Saijo N., et al. (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947–957 [DOI] [PubMed] [Google Scholar]
- Motzer R., Bukowski R., Figlin R., Hutson T., Michaelson M., Kim S., et al. (2008) Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer 113: 1552–1558 [DOI] [PubMed] [Google Scholar]
- Niho S., Ohe Y., Ishikura S., Atagi S., Yokoyama A., Ichinose Y., et al. (2012) Induction chemotherapy followed by gefitinib and concurrent thoracic radiotherapy for unresectable locally advanced adenocarcinoma of the lung: a multicenter feasibility study (JCOG 0402). Ann Oncol 23: 2253–2258 [DOI] [PubMed] [Google Scholar]
- Novello S., Scagliotti G., Rosell R., Socinski M., Brahmer J., Atkins J., et al. (2009) Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer 101: 1543–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan D., O’Donnell D., O’Connell F., O’Byrne K. (2010) The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol 5: 2024–2036 [DOI] [PubMed] [Google Scholar]
- Okamoto I., Mitsudomi T., Nakagawa K., Fukuoka M. (2010) The emerging role of epidermal growth factor receptor (EGFR) inhibitors in first-line treatment for patients with advanced non-small cell lung cancer positive for EGFR mutations. Ther Adv Med Oncol 2: 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otrock Z., Hatoum H., Musallam K., Awada A., Shamseddine A. (2011) Is VEGF a predictive biomarker to anti-angiogenic therapy? Crit Rev Oncol Hematol 79: 103–111 [DOI] [PubMed] [Google Scholar]
- Oxnard G., Binder A., Janne P. (2013) New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol 31: 1097–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitrakopoulou V., Soria J., Jappe A., Jehl V., Klimovsky J., Johnson B. (2012) Everolimus and erlotinib as second- or third-line therapy in patients with advanced non-small-cell lung cancer. J Thorac Oncol 7: 1594–1601 [DOI] [PubMed] [Google Scholar]
- Paz-Ares L., Biesma B., Heigener D., von Pawel J., Eisen T., Bennouna J., et al. (2012) Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol 30: 3084–3092 [DOI] [PubMed] [Google Scholar]
- Paz-Ares L., Soulieres D., Melezinek I., Moecks J., Keil L., Mok T., et al. (2010) Clinical outcomes in non-small-cell lung cancer patients with EGFR mutations: pooled analysis. J Cell Mol Med 14: 51–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perol M., Chouaid C., Perol D., Barlesi F., Gervais R., Westeel V., et al. (2012) Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin–gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 30: 3516–3524 [DOI] [PubMed] [Google Scholar]
- Petrelli F., Borgonovo K., Cabiddu M., Barni S. (2011) Erlotinib as maintenance therapy in patients with advanced non-small cell lung cancer: a pooled analysis of three randomized trials. Anticancer Drugs 22: 1010–1019 [DOI] [PubMed] [Google Scholar]
- Piperdi B., Walsh W., Bradley K., Zhou Z., Bathini V., Hanrahan-Boshes M., et al. (2012) Phase-I/II study of bortezomib in combination with carboplatin and bevacizumab as first-line therapy in patients with advanced non-small-cell lung cancer. J Thorac Oncol 7: 1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker R., Pereira J.R., Szczesna A., von Pawel J., Krzakowski M., Ramlau R., et al. (2009) Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 373: 1525–1531 [DOI] [PubMed] [Google Scholar]
- Pirker R., Pereira J., von Pawel J., Krzakowski M., Ramlau R., Park K., et al. (2012) EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 13: 33–42 [DOI] [PubMed] [Google Scholar]
- Price K., Azzoli C., Krug L., Pietanza M., Rizvi N., Pao W., et al. (2010) Phase II trial of gefitinib and everolimus in advanced non-small cell lung cancer. J Thorac Oncol 5: 1623–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Tang L., He A., Shen Z., Yao Y. (2011) The role of vandetanib in the second-line treatment for advanced non-small-cell-lung cancer: a meta-analysis of four randomized controlled trials. Lung 189: 437–443 [DOI] [PubMed] [Google Scholar]
- Qi W., Wang Q., Jiang Y., Sun Y., Tang L., He A., et al. (2013) Overall survival benefits for combining targeted therapy as second-line treatment for advanced non-small-cell-lung cancer: a meta-analysis of published data. PLoS One 8(2): e55637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam S., Blackhall F., Krzakowski M., Barrios C., Park K., Bover I., et al. (2012) Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 30: 3337–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam S., Harvey R., Saba N., Owonikoko T., Kauh J., Shin D., et al. (2010) Phase 1 and pharmacokinetic study of everolimus, a mammalian target of rapamycin inhibitor, in combination with docetaxel for recurrent/refractory nonsmall cell lung cancer. Cancer 116: 3903–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam S., Spigel D., Chen D., Steins M., Engelman J., Schneider C., et al. (2011) Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J Clin Oncol 29: 4574–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlau R., Gorbunova V., Ciuleanu T., Novello S., Ozguroglu M., Goksel T., et al. (2012) Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 30: 3640–3647 [DOI] [PubMed] [Google Scholar]
- Reck M., Frickhofen N., Cedres S., Gatzemeier U., Heigener D., Fuhr H., et al. (2010a) Sunitinib in combination with gemcitabine plus cisplatin for advanced non-small cell lung cancer: a phase I dose-escalation study. Lung Cancer 70: 180–187 [DOI] [PubMed] [Google Scholar]
- Reck M., von Pawel J., Zatloukal P., Ramlau R., Gorbounova V., Hirsh V., et al. (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27: 1227–1234 [DOI] [PubMed] [Google Scholar]
- Reck M., von Pawel J., Zatloukal P., Ramlau R., Gorbounova V., Hirsh V., et al. (2010b) Overall survival with cisplatin–gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 21: 1804–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs H., Jalal S., Baghdadi T., Bhatia S., McClean J., Johnson C., et al. (2012) Erlotinib and bevacizumab in newly diagnosed performance status 2 or elderly patients with nonsquamous non-small-cell lung cancer, a phase II study of the Hoosier Oncology Group: LUN04-77. Clin Lung Cancer, in press. [DOI] [PubMed] [Google Scholar]
- Roberts P., Stinchcombe T. (2013) KRAS mutation: should we test for it, and does it matter? J Clin Oncol 31: 1112–1121 [DOI] [PubMed] [Google Scholar]
- Rodon J., DeSantos V., Ferry R., Jr, Kurzrock R. (2008) Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther 7: 2575–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239–246 [DOI] [PubMed] [Google Scholar]
- Sachdev D., Yee D. (2007) Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther 6: 1–12 [DOI] [PubMed] [Google Scholar]
- Samali A., Gorman A., Cotter T. (1996) Apoptosis - the story so far. Experientia 52: 933–941 [DOI] [PubMed] [Google Scholar]
- Sandler A., Gray R., Perry M., Brahmer J., Schiller J., Dowlati A., et al. (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550 [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Germonpre P., Bosquee L., Vansteenkiste J., Gervais R., Planchard D., et al. (2010) A randomized phase II study of bortezomib and pemetrexed, in combination or alone, in patients with previously treated advanced non-small-cell lung cancer. Lung Cancer 68: 420–426 [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Krzakowski M., Szczesna A., Strausz J., Makhson A., Reck M., et al. (2012a) Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol 30: 2070–2078 [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Novello S., Schiller J., Hirsh V., Sequist L., Soria J., et al. (2012b) Rationale and design of MARQUEE: a phase III, randomized, double-blind study of tivantinib plus erlotinib versus placebo plus erlotinib in previously treated patients with locally advanced or metastatic, nonsquamous, non-small-cell lung cancer. Clin Lung Cancer 13: 391–395 [DOI] [PubMed] [Google Scholar]
- Schaake E., Kappers I., Codrington H., Valdes Olmos R., Teertstra H., van Pel R., et al. (2012) Tumor response and toxicity of neoadjuvant erlotinib in patients with early-stage non-small-cell lung cancer. J Clin Oncol 30: 2731–2738 [DOI] [PubMed] [Google Scholar]
- Sequist L., Gettinger S., Senzer N., Martins R., Janne P., Lilenbaum R., et al. (2010) Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol 28: 4953–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist L., von Pawel J., Garmey E., Akerley W., Brugger W., Ferrari D., et al. (2011) Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 29: 3307–3315 [DOI] [PubMed] [Google Scholar]
- Shaw A., Engelman J. (2013) ALK in lung cancer: past, present, and future. J Clin Oncol 31: 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A., Yeap B., Mino-Kenudson M., Digumarthy S., Costa D., Heist R., et al. (2009) Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27: 4247–4253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A., Yeap B., Solomon B., Riely G., Gainor J., Engelman J., et al. (2011) Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 12: 1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd F., Rodrigues Pereira J., Ciuleanu T., Tan E., Hirsh V., Thongprasert S., et al. (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353: 123–132 [DOI] [PubMed] [Google Scholar]
- Siegel R., Naishadham D., Jemal A. (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10–29 [DOI] [PubMed] [Google Scholar]
- Sienel W., Varwerk C., Linder A., Kaiser D., Teschner M., Delire M., et al. (2004) Melanoma associated antigen (MAGE)-A3 expression in Stages I and II non-small cell lung cancer: results of a multi-center study. Eur J Cardiothorac Surg 25: 131–134 [DOI] [PubMed] [Google Scholar]
- Socinski M., Scappaticci F., Samant M., Kolb M., Kozloff M. (2010) Safety and efficacy of combining sunitinib with bevacizumab + paclitaxel/carboplatin in non-small cell lung cancer. J Thorac Oncol 5: 354–360 [DOI] [PubMed] [Google Scholar]
- Socinski M., Stinchcombe T., Moore D., Gettinger S., Decker R., Petty W., et al. (2012) Incorporating bevacizumab and erlotinib in the combined-modality treatment of stage III non-small-cell lung cancer: results of a phase I/II trial. J Clin Oncol 30: 3953–3959 [DOI] [PubMed] [Google Scholar]
- Soria J., Mark Z., Zatloukal P., Szima B., Albert I., Juhasz E., et al. (2011) Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol 29: 4442–4451 [DOI] [PubMed] [Google Scholar]
- Soria J., Mauguen A., Reck M., Sandler A., Saijo N., Johnson D., et al. (2013) Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 24: 20–30 [DOI] [PubMed] [Google Scholar]
- Soria J., Smit E., Khayat D., Besse B., Yang X., Hsu C., et al. (2010) Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol 28: 1527–1533 [DOI] [PubMed] [Google Scholar]
- Soulieres D., Wolf J., Shepherd F., Cappuzzo F., Bunn P., Herbst R., et al. (2011) Meta-analysis of the predictive and prognostic value of erlotinib-related biomarkers in phase III, placebo-controlled trials in non-small cell lung cancer (NSCLC): Recommendations of the BioLOGUE advisors [abstract]. J Clin Oncol 29(15 Suppl.): 7533 [Google Scholar]
- Spigel D., Ervin T., Ramlau R., Daniel D., Goldschmidt J., Blumenschein G., et al. (2011) Final efficacy results from OAM4558g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC [abstract]. J Clin Oncol 20(15 Suppl.): 7505 [Google Scholar]
- Spigel D., Hainsworth J., Shipley D., Ervin T., Kohler P., Lubiner E., et al. (2012) A randomized phase II trial of pemetrexed/gemcitabine/bevacizumab or pemetrexed/carboplatin/bevacizumab in the first-line treatment of elderly patients with advanced non-small cell lung cancer. J Thorac Oncol 7: 196–202 [DOI] [PubMed] [Google Scholar]
- Stevenson J., Langer C., Somer R., Evans T., Rajagopalan K., Krieger K., et al. (2012) Phase 2 trial of maintenance bevacizumab alone after bevacizumab plus pemetrexed and carboplatin in advanced, nonsquamous nonsmall cell lung cancer. Cancer 118: 5580–5587 [DOI] [PubMed] [Google Scholar]
- Takeda K., Hida T., Sato T., Ando M., Seto T., Satouchi M., et al. (2010) Randomized phase III trial of platinum-doublet chemotherapy followed by gefitinib compared with continued platinum-doublet chemotherapy in Japanese patients with advanced non-small-cell lung cancer: results of a west Japan thoracic oncology group trial (WJTOG0203). J Clin Oncol 28: 753–760 [DOI] [PubMed] [Google Scholar]
- Takeda M., Okamoto I., Yamanaka T., Nakagawa K., Nakanishi Y. (2012) Impact of treatment with bevacizumab beyond disease progression: a randomized phase II study of docetaxel with or without bevacizumab after platinum-based chemotherapy plus bevacizumab in patients with advanced nonsquamous non-small cell lung cancer (WJOG 5910L). BMC Cancer 12: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher N., Chang A., Parikh P., Rodrigues Pereira J., Ciuleanu T., von Pawel J., et al. (2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366: 1527–1537 [DOI] [PubMed] [Google Scholar]
- Topalian S., Hodi F., Brahmer J., Gettinger S., Smith D., McDermott D., et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao M., Sakurada A., Cutz J., Zhu C., Kamel-Reid S., Squire J., et al. (2005) Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med 353: 133–144 [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J., Solomon B., Boyer M., Wolf J., Miller N., Di Scala L., et al. (2011) Everolimus in combination with pemetrexed in patients with advanced non-small cell lung cancer previously treated with chemotherapy: a phase I study using a novel, adaptive Bayesian dose-escalation model. J Thorac Oncol 6: 2120–2129 [DOI] [PubMed] [Google Scholar]
- Vilmar A., Sorensen J. (2011) Customising chemotherapy in advanced nonsmall cell lung cancer: daily practice and perspectives. Eur Respir Rev 20: 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Wang L., Li B., Sheng Z. (2012) Gefitinib compared with systemic chemotherapy as first-line treatment for chemotherapy-naive patients with advanced non-small cell lung cancer: a meta-analysis of randomised controlled trials. Clin Oncol (R Coll Radiol) 24: 396–401 [DOI] [PubMed] [Google Scholar]
- Weickhardt A., Doebele R., Oton A., Lettieri J., Maxson D., Reynolds M., et al. (2012) A phase I/II study of erlotinib in combination with the anti-insulin-like growth factor-1 receptor monoclonal antibody IMC-A12 (cixutumumab) in patients with advanced non-small cell lung cancer. J Thorac Oncol 7: 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witta S., Jotte R., Konduri K., Neubauer M., Spira A., Ruxer R., et al. (2012) Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol 30: 2248–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D., Leung E., So K., Tam I., Sihoe A., Cheng L., et al. (2009) The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 115: 1723–1733 [DOI] [PubMed] [Google Scholar]
- Yap T., Vidal L., Adam J., Stephens P., Spicer J., Shaw H., et al. (2010) Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol 28: 3965–3972 [DOI] [PubMed] [Google Scholar]
- Yu H., Spitz M., Mistry J., Gu J., Hong W., Wu X. (1999) Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst 91: 151–156 [DOI] [PubMed] [Google Scholar]
- Zappa F., Droege C., Betticher D., von Moos R., Bubendorf L., Ochsenbein A., et al. (2012) Bevacizumab and erlotinib (BE) first-line therapy in advanced non-squamous non-small-cell lung cancer (NSCLC) (stage IIIB/IV) followed by platinum-based chemotherapy (CT) at disease progression: a multicenter phase II trial (SAKK 19/05). Lung Cancer 78: 239–244 [DOI] [PubMed] [Google Scholar]
- Zhang L., Ma S., Song X., Han B., Cheng Y., Huang C., et al. (2012) Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 13: 466–475 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li N., Caron C., Matthias G., Hess D., Khochbin S., et al. (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22: 1168–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Wu Y., Chen G., Feng J., Liu X., Wang C., et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735–742 [DOI] [PubMed] [Google Scholar]
- Zhu J., Sharma D., Gray S., Chen A., Weeks J., Schrag D. (2012) Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA 307: 1593–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]


