Abstract
Osteoporotic fractures are one of the major causes of increased morbidity and mortality in postmenopausal women and the overall aging population. One of the major issues in the management of postmenopausal osteoporosis is to find a safe and effective treatment in the long term (>3 years) to achieve and maintain a reduction in the risk of fracture. Strontium ranelate (PROTELOS®) is a relatively novel drug, currently approved in Europe for the treatment of postmenopausal osteoporosis. Strontium ranelate is the first agent of a new therapeutic class in osteoporosis, capable of both promoting bone formation and, to a lesser extent, inhibiting bone resorption. This uncoupling in bone turnover results in a net gain in bone mineral density (BMD), bone quality improvement and reduction in risk of vertebral and nonvertebral fractures, as initially demonstrated in the preplanned long-term registrative trials SOTI (Spinal Osteoporosis Therapeutic Intervention) and TROPOS (Treatment of Peripheral Osteoporosis) at 5 years. Recently, open-label extensions of the SOTI and TROPOS trials up to 8 and, recently, 10 years have confirmed the sustained efficacy of strontium ranelate in increasing BMD, the long-term safety profile and the high compliance to treatment, independently from baseline BMD or other risk factors for osteoporotic fractures. Recent economic impact analyses have proved that long-term treatment with strontium ranelate is highly cost effective, especially in women older than 70 years of age. Histomorphometric analyses in animals and humans participating in the phase III trials have proved that the quality of mineralization is preserved in the long term and bone microarchitecture is ameliorated, with increased bone strength. Thus, strontium ranelate has been confirmed to be an effective compound for the long-term, chronic treatment of postmenopausal osteoporosis.
Keywords: anabolic, antiresorptive, bone formation, bone mineral density, bone resorption, mineralization, safety, tolerability
Introduction
Postmenopausal osteoporosis is a chronic condition characterized by decreased bone mass, deterioration of bone microarchitecture compromising bone strength thus predisposing women to fragility fractures, a major cause of morbidity and mortality [Raisz, 2005]. The most prevalent type of fracture in postmenopausal osteoporosis is vertebral fracture, while nonvertebral fractures, including hip fractures, occurring at the level of cortical bone, account for 80% of all fractures. Trabecular bone loss has been classically linked to the development of osteoporosis. However, cortical trabecularization (i.e. cortical porosity) is now considered one of the major mechanisms through which bone strength is reduced, because of the greater impact on bone strength [Zebaze et al. 2010]. During the very first years after menopause bone loss is mainly due to an acceleration of bone turnover with prevailing of bone resorption over bone formation [Khosla et al. 2012]. The decrease in bone mineral density (BMD) is associated with a progressively increased fracture risk. This very short phase is followed by a sustained and prolonged period of defective bone formation, which is the main reason for the uncoupling of bone turnover during menopause and aging. However, the process of resorption is necessary for microfracture repair. Thus, an ideal treatment for this chronic and progressive condition should mainly provide a long-lasting enhancement of bone formation with relative inhibition of bone resorption, while being safe in the long term [Seeman, 2003]. In addition, mineralization, which is the completion process of each remodeling cycle, should be maintained in the physiological range to preserve optimal biomechanical properties. Current available and worldwide approved treatments for osteoporosis are antiresorptive medications, which include bisphosphonates, selective estrogen-receptor modulators and calcitonin, and bone-forming agents, such as teriparatide (PTH1-34) and parathyroid hormone (PTH1-84). Strontium ranelate (PROTELOS®, Les Laboratoires Servier, Neuilly-Sur-Seine, France) is a bone-forming agent with antiresorptive capacity, currently approved in Europe for the treatment of postmenopausal osteoporosis. This review discusses the long-term, sustained antifracture efficacy and safety profile of strontium ranelate, which has been proved in the results of the preplanned phase III registrative trials at 5 years and recently confirmed in open-label extension studies of the initial trials, starting from the basic in vitro and in vivo pharmacologic properties of this compound.
Rational for long-term treatment with strontium ranelate: in vitro and in vivo analyses
Bone turnover is a physiological process in which osteoclast-mediated bone resorption is constantly coupled to osteoblast-mediated bone formation to provide minerals to extracellular fluids, adapt to stress and strain, and constantly repair microfractures.
Since osteoclasts secrete factors that stimulate osteoblasts, drugs inhibiting bone resorption will constantly lead to a proportional inhibition of bone formation. Conversely, since osteoblasts are necessary for osteoclast activation, agents that stimulate only bone formation will subsequently elicit bone resorption. The net recovery in BMD during therapy with anabolic agents is obtained mainly during the so-called ‘anabolic window’, a period during which bone formation is uncoupled from resorption.
Mineralization is the completion stage of every cycle of bone turnover at the level of a single basic multicellular unit, after old bone is removed by osteoclasts and new bone is formed by osteoblasts. During primary mineralization the bone matrix is rapidly mineralized, while secondary mineralization is a slow process characterized by a gradual maturation of the mineral component. It is believed that the heterogeneity index of mineralization, and not the degree of mineralization itself, influences bone strength [Boivin et al. 2009]. Long-term inhibition of bone remodeling preserves structure but affects bone composition by increasing the mean degree of mineralization but decreasing the heterogeneity index. This is likely able to compromise bone quality and micromechanical properties in the long term.
The rational for the long-term treatment of osteoporosis with strontium ranelate {5-[bis(carboxymethyl)amino]-2-carboxy-4-cyano-3-thiophen-acetic acid distrontium salt} comes from in vitro and in vivo studies, and in particular from histomorphometric analyses showing that bone quality, the major determinant of bone strength, is enhanced and preserved.
Determinants at the cellular and molecular level: in vitro evidence
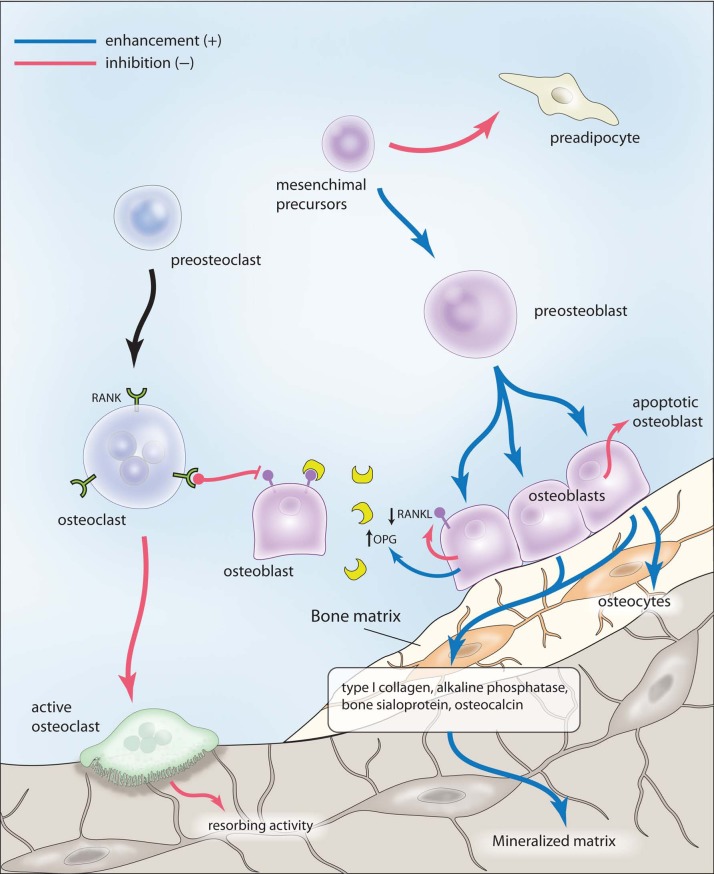
Preliminary in vitro studies showed that strontium ranelate mediates an uncoupling in bone turnover since it enhances osteoblastogenesis and osteoblast activity while decreasing osteoclast differentiation and function [Bonnelye et al. 2008; Marie et al. 2011] (Figure 1).
Figure 1.
Proformative and antiresorptive effects of strontium ranelate. See text for details. OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor κB ligand. Illustration courtesy of Alessandro Baliani © (2013).
Osteoblasts are considered the major direct target of this compound [Brennan et al. 2009]. Strontium induces preosteobast proliferation and enhances osteoblast activity, as demonstrated by the increase in the expression of several early and late osteoblastic markers, such as type I collagen, alkaline phosphatase, bone sialoprotein, osteocalcin and, ultimately, bone matrix mineralization and nodule formation in bone marrow stromal cell cultures and immature osteoblasts [Canalis et al. 1996; Barbara et al. 2004; Choudhary et al. 2007; Caversazio, 2008, Brennan et al. 2009]. Moreover, strontium inhibits osteoblast apoptosis and induces terminal differentiation of osteoblasts into osteocytes, as demonstrated by the increase in sclerostin expression [Atkins et al. 2009]. This latter effect could play a pivotal role in the uncoupling of bone turnover induced by strontium ranelate, since osteocytes can influence both osteoblasts and osteoclast function by producing paracrine signals to these cells. Recent studies have shown that strontium is also able to inhibit adipogenesis while enhancing osteogenesis both in bone marrow stromal cells and in multipotent C3H10T1/2 cells by selective peroxisome proliferator-activated receptor γ2 repression [Fournier et al. 2012; Li et al. 2012]. Considering that an increase in adiposity in bone marrow stroma sustained by an increase in adipogenesis may occur in patients with osteoporosis [Meunier et al. 1971], this novel mechanism could explain at least in part the long-term efficacy of strontium ranelate in preventing fractures.
Strontium ranelate is able to decrease osteoclast differentiation and activity in vitro, as demonstrated by the reduction in the expression of markers of osteoclast function and the disruption of osteoclast cytoskeleton, essential for resorbing activity [Takahashi et al. 2003]. The principal mechanism by which strontium inhibits osteoclast activity in vitro is by enhancing the secretion of osteoprotegerin (OPG) and by reducing the expression of the receptor activator of nuclear factor κB ligand (RANKL) in osteoblasts [Atkins et al. 2009; Brennann et al. 2009]. Indeed, the OPG/RANKL system plays a key role in osteoclast differentiation. The increase in OPG is considered to be the main mediator of the strontium ranelate uncoupling effect on bone metabolism, as demonstrated by in vitro and subsequently in vivo studies [Peng et al. 2011a]. Moreover, the effect of strontium ranelate on bone formation and resorption is blunted in mice devoid of OPG [Peng et al. 2011b]. Thus, enhancement of OPG expression and inhibition of RANKL production by osteoblasts at various differentiation stages impairs osteoclast differentiation and function and would explain, at least in part, the uncoupling of bone turnover generated by strontium through a rebalance of this system. According to these in vitro findings, it has recently been reported that OPG is increased in postmenopausal women receiving strontium ranelate, as early as 3 months after treatment initiation and up to 3 years [Reginster et al. 2012].
Bone cells (osteoblasts, osteoclasts and osteocytes) harbor the calcium-sensing receptor (CaSR), a G-protein coupled receptor that is activated by extracellular divalent cations such as calcium and, with lower affinity, strontium. CaSR has been shown to mediate, at least in part, strontium-induced osteoblast proliferation [Chattopadhyay et al. 2007; Caudrillier et al. 2010], and osteoclast apoptosis [Hurtel-Lemaire et al. 2009]. CaSR signaling has been shown to play a key role in mediating the modulation of the OPG/RANKL system by strontium. However, since osteoblasts devoid of CaSR can still respond to strontium in terms of proliferation and apoptosis, other cation-sensing molecules and different mechanisms can be involved in mediating the effects of strontium on bone cells [Fromigue et al. 2009]. In this respect, it has been recently shown that the strontium-mediated activation of calcineurin/nuclear factor of activated Tc pathway induces osteoblastogenesis via stimulation of both canonical and noncanonical Wnt signaling pathways [Fromigue et al. 2010].
Determinants at the tissue level: in vivo evidence
Studies in rats, nonhuman primates, ovariectomized rodents and animal models of immobility-induced osteoporosis showed that strontium ranelate is able to increase bone mass, mineral content and strength while improving skeletal microarchitecture in vivo. Preliminary histomorphometric studies demonstrated that bone formation and mineral apposition rate are enhanced, while bone resorption indices are blunted by strontium ranelate over the first 6 months of treatment. This confirms in vivo the dissociating effect of strontium ranelate on bone turnover observed in vitro, indicating that this agent harbors the potential of a novel drug for the treatment of osteoporosis. In mice, this is reflected in an increase in vertebral bone mass. Studies in rats have demonstrated that in addition to the increase in bone mass, there is an increase in cortical thickness and an improvement in trabecular and cortical microarchitecture, leading to an increased bone quality and strength [Ammann et al. 2004, 2007]. In ovariectomized rats, an established animal model for postmenopausal osteoporosis, strontium ranelate was able to prevent bone loss by decreasing bone resorption while maintaining bone formation not only in the short term [Marie et al. 1993], but also in the long term [Bain et al. 2009]. In this latter study, the ovariectomy-induced decline in bone mass, volume and strength was prevented by achieving a plasma concentration of strontium ranelate similar to that obtained in women with osteoporosis treated with the therapeutic daily dose of 2 g.
In mice overexpressing Runx2, leading to an increased RANKL expression and accelerated bone loss and spontaneous fractures, strontium ranelate has been proved to be effective in increasing bone volume, cortical thickness and trabecular number, decreasing trabecular separation, with an amelioration of bone microarchitecture leading to improved bone strength and reduced vertebral fracture risk [Geoffroy et al. 2011]. This is the first study demonstrating in an experimental animal model the antifracture efficacy of strontium ranelate in the relative long term (9 weeks).
In rodents as well as in humans only a small fraction of strontium incorporates into the bone matrix and associates with hydroxyapatite crystals in the place of calcium, thus not directly influencing mineralization [Dahl et al. 2001]. Maintenance of the degree of bone mineralization and bone minerals at the crystal level was confirmed in monkeys after long-term administration of strontium ranelate, confirming the absence of deleterious effects of prolonged therapy with this agent on the quality of mineralization [Farlay et al. 2005]. It has recently been demonstrated in human bone biopsies from postmenopausal women with osteoporosis treated with strontium ranelate for up to 3 years that strontium accumulated only in newly formed bone during treatment, reaching a plateau in global strontium bone content after 3 years, with preservation of mineralization and focal strontium bone content [Li et al. 2010; Boivin et al. 2010].
This issue has been further analyzed in a recent study on bone biopsies from postmenopausal women treated with strontium ranelate for up to 5 years [Doublier et al. 2011]. This analysis shows that even after long-term treatment with strontium ranelate and independently of the distribution of the mineral at different bone sites, the quality of bone mineralization and the heterogeneity index, which reflects secondary mineralization, are maintained. Preservation of bone matrix quality in terms of mineralization has the great potential of maintaining bone strength over the long term, as discussed above.
It is also likely that the properties of the bone matrix are improved by treatment with strontium ranelate as demonstrated by nanoindentation analyses in ovariectomized rats [Bain et al. 2009; Ammann et al. 2007]. However, further studies are necessary on this subject since a small study on bone biopsies from postmenopausal women has not shown changes in nanoindentation parameters in a small group treated with strontium ranelate [Roschger et al. 2010].
Nonetheless, the consequences of the dissociating effect of strontium ranelate on bone turnover and modifications of bone matrix quality can at least in part explain the improved skeletal microarchitecture in patients treated with strontium ranelate, which can contribute to increased bone strength and resistance to fractures. Histomorphometry studies on unpaired human bone biopsies in women with osteoporosis participating in the Spinal Osteoporosis Therapeutic Intervention (SOTI) and Treatment of Peripheral Osteoporosis (TROPOS) trials, performed in the long term (after 1–5 years of treatment; see below for study details in terms of antifracture efficacy), have demonstrated an increased mineral apposition rate and osteoblast surface. A three-dimensional analysis by means of microcomputed tomography documented significant improvements in microarchitecture, such as increased cortical thickness and trabecular number and decreased trabecular separation compared with the placebo group, without changes in cortical porosity [Arlot et al. 2008]. These changes can reflect the enhanced bone formation and decreased bone resorption observed in in vitro and in vivo preclinical experiments. The modifications of bone microarchitecture seem to be particularly evident in the long term, since short-term studies on human bone biopsies obtained after 6 months of treatment with strontium ranelate do not display any change in histomorphometric indices [Recker et al. 2009]. However, a randomized study comparing the effects on bone microstructure (distal tibia) of therapy with strontium ranelate and alendronate for 1 year (versus double placebo) in women with osteoporosis by means of high-resolution peripheral quantitative computed tomography have confirmed increased cortical thickness and increased bone volume/total volume fraction (BV/TV) at 1 year in patients receiving strontium ranelate, while no modifications were observed in the bones of the alendronate-treated or placebo-treated subjects [Rizzoli et al. 2010]. This could explain the early protection against fracture obtained with strontium ranelate (within 1 year of treatment). In the analysis on distal tibia and radius at 2 years, cortical thickness and density and overall cancellous BV/TV increased by 6.3%, 2.4% and 2.5% respectively with strontium ranelate [Rizzoli et al. 2012]. Moreover, the quantification of bone strength and biomechanical properties of cortical and trabecular bone by means of finite element analysis shows that the estimated failure load increased, while cortical and trabecular stress decreased with strontium ranelate [Rizzoli et al. 2012].
Regarding secondary osteoporosis, strontium ranelate (900 mg/kg) has proven to be more effective than alendronate (1 mg/kg) in preventing glucocorticoid-induced osteopenia in rats, according to BMD and histomorphometric analysis [Sun et al. 2010]. Indeed, in a 2-year observational study strontium ranelate was more effective than risedronate in increasing BMD and decreasing back pain in patients with glucocorticoid-induced osteoporosis, although the number of patients with new fractures did not differ between the two treatment groups [Ringe et al. 2009]. However, to date, this drug is not indicated in secondary osteoporosis since the phase III trials have not included such patients.
Additional evidence of improved bone strength during strontium ranelate treatment comes from animal and human studies on implant osseointegration and fracture healing (Maimoun et al. 2010). Indeed, in rats receiving strontium ranelate for 8 weeks, the resistance to pullout bone implants was increased. In ovariectomized rats, 4–8 weeks of treatment with strontium ranelate enhanced screw fixation in osteoporotic bone and improved fracture healing, by amelioration of microstructure, callus volume and biomechanical properties [Li et al. 2010], suggesting potential novel clinical applications for this drug in vivo [Goldhahn et al. 2012].
Long-term efficacy: intervention trials
The final endpoint of an antiosteoporosis therapy is to reduce fracture risk, by improving BMD, bone quality and strength.
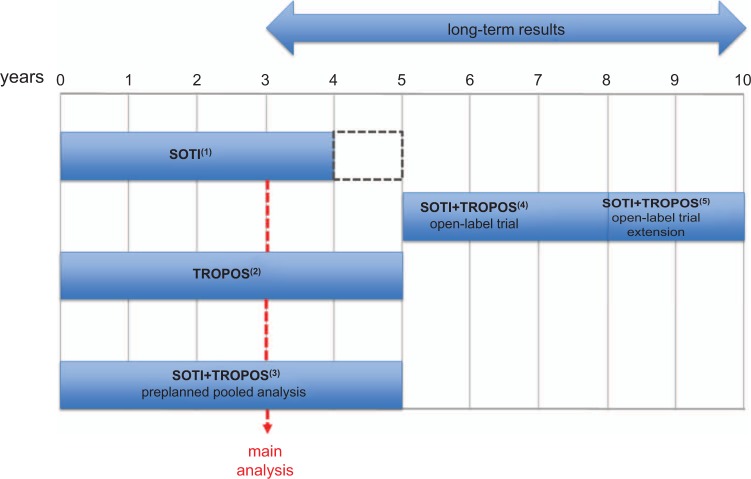
Phase III randomized controlled trials (RCTs) for the clinical development of strontium ranelate in women with postmenopausal osteoporosis have been specifically designed to test the antifracture efficacy of this drug in the long term (5 years) with the main statistical analysis after 3 years of treatment (Figure 2).
Figure 2.
Randomized controlled trials and open-label extension studies designed to assess the antifracture efficacy of strontium ranelate 2 g/day in the long term. The dotted rectangle in the Spinal Osteoporosis Therapeutic Intervention (SOTI) study indicates the interruption of the study against placebo and the subsequent new randomization (see text). TROPOS, Treatment of Peripheral Osteoporosis.
1Meunier et al. [2004, 2009], Marquis et al. [2008].
2Reginster et al. [2005, 2008].
These RCTs, together with open-label extension studies, have clearly demonstrated that the in vitro and in vivo effects of strontium ranelate translate into a long-term vertebral and nonvertebral antifracture efficacy and improvement in bone strength in postmenopausal osteoporosis. Open-label extensions of these trials have confirmed the increased BMD and safety of strontium ranelate up to 10 years of treatment. The limitations of these studies are comparable to those of any long-term trial. The observed reduction in fracture rates was not evaluated against a proper placebo group, which was unethical to pursue given the beneficial effects of strontium ranelate in a selected osteoporotic population at increased risk of fracture. Moreover, patients enrolled in the long-term studies were not randomized but they autonomously decided whether to continue or not with the medication. In general, patients with higher compliance and those who responded to therapy were enrolled in the open-label RCT extensions. These issues have to be considered in the interpretation of the study results, which can lead to overestimation of the beneficial long-term effect of a given drug [Cooper et al. 2012].
The SOTI trial [Meunier et al. 2004] and the TROPOS trial [Reginster et al. 2005, 2008] are the first extensive international, prospective, double-blind RCTs which have been designed to test strontium ranelate (2 g/day) efficacy against placebo (calcium + vitamin D) in the prevention of vertebral and nonvertebral fractures respectively in postmenopausal women. In the SOTI trial, 1649 postmenopausal women with at least one osteoporotic vertebral fracture (age > 50 years and had been postmenopausal for at least 5 years), all receiving calcium and vitamin D, were randomized to receive 2 g/day of strontium ranelate or placebo for 4 years, followed by a 1-year period in which subjects receiving strontium ranelate were switched to placebo or continued on the active treatment, while all patients who had been treated with placebo were switched to strontium ranelate. In the main analysis at 3 years, the SOTI trial demonstrated that strontium ranelate is able to reduce the risk of new vertebral fractures by 41% [relative risk (RR) 0.59; 95% confidence interval (CI) 0.48–0.73; p < 0.001 versus placebo-treated group]. A significant reduction in fracture risk was observed after just 1 year of treatment (49%; RR 0.51; 95% CI 0.36–0.74; p < 0.001) [Meunier et al. 2004]. Subsequent analyses of the preplanned study demonstrated that the decrease in vertebral fracture risk is maintained after 4 and 5 years of treatment [Meunier et al. 2009]. In particular, the reduction in fracture risk was 33% after 4 years (RR 0.67; 95% CI 0.55–0.81; p < 0.001 versus placebo), with a concomitant improvement in quality of life (p = 0.025), especially in terms of absence of back pain (p = 0.005). In this study, the rise in BMD continued up to 5 years in the group that continued strontium ranelate, while it decreased in the group that was switched to placebo after 4 years.
The TROPOS study is the first RCT specifically designed to assess the efficacy of strontium ranelate on nonvertebral (peripheral) fractures in the long term (5 years) [Reginster et al. 2005, 2008], with the evaluation of vertebral fracture risk reduction as a secondary endpoint. In this study 5091 postmenopausal women with osteoporosis aged over 74 years (or aged 70–74 years with one additional fracture risk factor) were enrolled. The study was completed by 2714 subjects. A preliminary analysis of the results at 3 years showed that the RR reduction for all nonvertebral fractures was reduced by 16% (RR 0.84; 95% CI 0.702–0.995; p = 0.04), while the risk for major nonvertebral fragility fractures (hip, wrist, pelvis and sacrum, ribs and sternum, clavicle and humerus) and new vertebral fractures was reduced by 19% (RR 0.81; 95% CI 0.66–0.98; p < 0.001) and by 39% (RR 0.61; 95% CI 0.51–0.73; p < 0.001) respectively in the group receiving strontium ranelate compared with placebo [Reginster et al. 2005]. In addition, therapy with strontium ranelate provided significant improvement in quality of life, analyzed as a secondary endpoint [Marquis et al. 2008]. In a post hoc analysis, the reduction in the relative risk of hip fracture, the type of fracture associated with greatest morbidity and mortality, was assessed in a group at higher risk for this type of fracture (age > 74 years, T score < –3) [Reginster et al. 2005]. In these high-risk women, strontium ranelate treatment decreased the relative risk of hip fracture by 36% (RR 0.64; 95% CI 0.412–0.997; p = 0.046). The complete analysis at 5 years confirmed the sustained antifracture efficacy of strontium ranelate, with a decrease in the risk of experiencing a nonvertebral, hip or vertebral fracture of 15% (RR 0.85; 95% CI 0.73–0.99), 43% (RR 0.57; 95% CI 0.33–0.97) or 24% (RR 0.76; 95% CI 0.65–0.88) respectively [Reginster et al. 2008].
The preplanned analysis of the pooled data from the SOTI and TROPOS studies at 3 years showed that the efficacy of strontium ranelate in reducing vertebral and nonvertebral fracture risk was independent of individual risk factors for osteoporotic fractures, such as baseline BMD, family history of osteoporosis, baseline body mass index and addiction to smoking [Roux et al. 2006]. This study also demonstrated that strontium ranelate was able to decrease the risk of first, second or third (or more) new vertebral fractures by 48%, 45% and 33% respectively. Strontium ranelate also displayed antifracture efficacy in the group with osteopenia at moderate risk of fracture [Seeman et al. 2008]. An additional preplanned analysis at 3 and 5 years of the two RCTs explored the specific antifracture efficacy of strontium ranelate in preventing either vertebral or nonvertebral fractures in the subgroup of 1488 women aged at least 80 years. In the intention-to-treat analysis the reduction in the risk of vertebral and nonvertebral fractures was reduced by 32% and 31% at 3 years [Seeman et al. 2006], and 32% and 27% at 5 years [Seeman et al. 2010] respectively, with a long-term enhancement of quality of life.
Other surrogate markers for efficacy include BMD and bone turnover markers [Cooper et al. 2012]. Despite BMD being overestimated during treatment with strontium ranelate because of the uptake of strontium in bone, there is a strong correlation between the positive change in BMD and the decrease in fracture risk. It has been demonstrated that for each 1% increase in femoral neck BMD there is a 3% reduction in the risk of a new vertebral fracture within 3 years [Bruyere et al. 2007]. In a group of older women (≥74 years, with low femoral neck BMD) for each 1% increase in femoral neck BMD there was a 7% reduction in the risk of experiencing a hip fracture within 3 years [Bruyere et al. 2007].
Regarding markers of bone remodeling, the early dissociation of markers of bone remodeling (significant increase in bone formation markers and decrease in bone resorption markers as early as 3 months after treatment initiation) correlates with the increase in vertebral and femoral neck BMD at 3 years [Bruyere et al. 2010]. Thus, the early positive balance in bone remodeling reflecting the uncoupling of the two different processes could offer at least one rational for the anabolic effect of strontium ranelate documented since preclinical studies [Marie et al. 2011].
Open-label extension studies of the SOTI and TROPOS trials, specifically designed to directly assess the long-term change in BMD, tolerability and safety and, indirectly, the antifracture efficacy, were carried out in a group of 879 subjects for up to 8 years [Reginster et al. 2009] and extended in a group of 233 subjects for up to 10 years [Reginster et al. 2012]. The great limitation of these studies, as stated above, is the absence of a placebo group, which does not let us draw any definitive conclusion on the antifracture efficacy of strontium ranelate in this prolonged time period. Nevertheless, the characteristics of the final population enrolled in the 8- and 10-year study were representative of the whole SOTI and TROPOS population (mean age 72 ± 5.5 years, mean lumbar spine and femoral neck BMD T score of −3.3 ± 1.38 and −2.95 ± 0.57, respectively). Postmenopausal women who had participated in the phase III RCTs for 5 years in the treatment arm were invited to be enrolled in a 3-year open-label extension to continue to receive strontium ranelate 2 g/day [Reginster et al. 2009]. The initial 3-year extension was then increased to 10 years in a subset of patients [Reginster et al. 2012]. These studies clearly demonstrated that the rise in lumbar spine BMD was significant and sustained over the 10 years of treatment (up to 34.5 ± 20.2% versus baseline), while neck and total hip BMD reached a plateau after 7 years of treatment (10.7 ± 12.% and 11.7 ± 13.6% respectively). These studies also provide indirect evidence of maintained antifracture efficacy since the cumulative incidence of new vertebral and nonvertebral fractures in the 5-year extension (20.6% and 13.7% respectively) did not significantly differ from the cumulative incidences relative to the first 5 years of the original RCTs (18.5% and 12.9% respectively). To obtain an estimate of the antifracture efficacy, the authors selected a control group from the initial placebo group of the TROPOS study by means of the 10-year probability of major osteoporotic fracture derived from the FRAX algorithm (developed by World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK). In the group receiving strontium ranelate for 10 years a RR reduction of 35% and 38% for vertebral and nonvertebral fractures was then calculated [Reginster et al. 2012].
Recently, the results of a 2-year double-blind, placebo-controlled study to test the efficacy and safety of strontium ranelate in 243 men (intention-to-treat population, 161 patients received strontium ranelate) with osteoporosis have been reported (MALEO, i.e. MALE Osteoporosis, study) [Kaufman et al. 2013]. Statistically significant increases in BMD were observed as early as 6 months following initiation of strontium ranelate treatment versus placebo. The increase in BMD in men treated with strontium ranelate was similar to that observed in women in the same time period. In addition, bone resorption markers were significantly reduced in the first 3 months of treatment. The incidence of vertebral and nonvertebral fractures was lower in the treated men (5.8% and 3.5% versus 7.8% and 4.6% in the strontium and placebo groups respectively). Quality of life, as reflected by some of the QUALIOST (Quality of Life Questionnaire in Osteoporosis) scores [Marquis et al. 2001], improved in the strontium ranelate treated group versus placebo. The treatment was well tolerated. This study demonstrates that strontium ranelate offers a valid therapeutic option in men with osteoporosis.
A recent analysis on the economic impact of this therapy has assessed the cost effectiveness of strontium ranelate compared with no treatment over the long term in the target populations for routine use of the product [Hiligsmann et al. 2010a]. This study has used data from the TROPOS trial over 5 years of treatment in women aged 70 or older with a T score of −2.5 or lower or prevalent vertebral fractures (i.e. criteria for reimbursability in Belgium and other European countries) by means of a validated Markov simulation model with a Belgian healthcare cost perspective. Strontium ranelate was cost saving at the age of 80 years in both groups. Indeed, the cost per quality-adjusted life year gained of strontium ranelate compared with no treatment, when assuming adherence similar to bisphosphonate therapy, was inferior to the cutoffs established in other countries (i.e. the UK, Sweden) at all ages, in both groups. In particular, the cost-saving benefit of strontium ranelate was proved to be very sensitive to the risk of hip fracture during treatment.
A recent meta-analysis of cost-effectiveness studies in different settings (Belgium, the UK and Sweden) confirmed that treatment with strontium ranelate is cost saving in women with osteoporosis aged 80 years and older [Hiligsmann et al. 2010b]. Moreover it showed that strontium ranelate is cost effective compared with no treatment in women aged 70 years and older and in younger women with clinical risk factors for fragility fractures.
Other widely used osteoporotic therapies, such as bisphosphonates, have been proven to be cost effective compared with no treatment in the long term, but their long-term efficacy in terms of cost-saving benefit can be compromised by a poor adherence to treatment. Unfortunately, no head-to-head clinical trials are available. However, when strontium ranelate was indirectly compared with a branded risedronate it is cost effective in women older than 75 years. In another study strontium ranelate was proven to be less cost effective than a generic alendronate, but the authors have assumed a low antifracture efficacy of strontium ranelate at the hip [Hiligsmann et al. 2010b].
Further analyses are necessary to establish the cost effectiveness, adherence and persistence of strontium ranelate in other countries and in different settings other than clinical trials.
Long-term tolerability and safety issues
Strontium ranelate has a good tolerability and safety profile in long-term studies (RCTs at 5 years and open-label extension studies) and in the general population after 7 years of postapproval surveillance [Reginster et al. 2012; Rizzoli et al. 2011; Cooper et al. 2012]. The high tolerability leads to increased compliance, as demonstrated in a post hoc analysis of pooled data from the SOTI and TROPOS trials at 5 years [Rabenda and Reginster, 2010]. The medication possession ratio was high (82.6% in the active treatment group versus 86.8% in the placebo group) and influenced the risk of hip and nonvertebral fractures, since women with higher compliance displayed a greater reduction in this risk.
In the SOTI and TROPOS trials, adverse events, serious adverse events and withdrawals relative to adverse events were comparable in the active treatment group versus placebo and their incidence did not differ in women older than 80 years [Meunier et al. 2004, 2009; Reginster et al. 2005, 2008]. Nausea in the SOTI trial and nausea, diarrhea, headache, skin alterations (dermatitis) in the TROPOS trial were associated with strontium ranelate therapy, but only at the beginning of treatment and not after 3 months.
Uncommon side effects associated with a certain therapy are more likely to emerge in larger populations than in those included in the initial registrative trials. Thus, information on the long-term safety comes from postmarketing analyses, pharmacovigilance, case reports and after prolonged treatment, such as data from open-label studies [Cooper et al. 2012].
The rate of venous thromboembolism was not increased in the group of women receiving strontium ranelate versus placebo in the SOTI and TROPOS trials. However, when the data were pooled in a postmarketing analysis by the European Medicine Agency (EMA), the annual incidence of venous thromboembolism was 0.9% in the active treatment group versus 0.6% in the placebo group [EMA, 2009]. However, these rates of thromboembolism are similar in an age-matched general population and postmenopausal women per se are at increased risk of venous thromboembolism. A retrospective analysis using data from the British General Practice Research Database investigated this issue further by examining the rates of venous thromboembolism in women with osteoporosis treated with strontium ranelate (2408), those treated with alendronate (20,084), and those who were untreated (11,546), and women without osteoporosis (115,009) [Breart et al. 2010]. There was an increase in the rate of venous thromboembolism in the untreated women with osteoporosis versus women without osteoporosis, but no difference was observed between treated and untreated women with osteoporosis or between different treatments. These observations were confirmed in two observational studies [Perrio et al. 2008; Breart et al. 2009]. However, in a recent recommendation by the EMA, since venous thromboembolism is more frequent in people with a positive history of thromboembolism, or temporarily or permanently immobilized, it is advised to avoid administration of strontium ranelate under these conditions. In addition, strontium ranelate should be used with caution in patients at risk of venous thromboembolism and discontinued as soon as possible in the event of an illness or a condition leading to immobilization and adequate preventive measures taken. Therapy should not be restarted until the initiating condition has resolved and the patient is fully mobile. Treatment is not contraindicated in patients aged 80 years and over in which the efficacy of strontium ranelate is well documented, but when treating patients over 80 years at risk of venous thromboembolism, the need for continued treatment should be re-evaluated [EMA, 2012].
DRESS syndrome (Drug Rash with Eosinophilia and Systemic Symptoms) is a rare hypersensitivity reaction. The highest risk usually occurs around 3–6 weeks after treatment initiation. The mortality rate can reach 8–10%. DRESS syndrome usually requires intensive care unit admission, with prompt drug withdrawal and proper management, sometimes including the administration of systemic glucocorticoids, which can improve the prognosis [Musette et al. 2011]. This syndrome is not unique to strontium ranelate since it has also been observed during other treatments (e.g. sulfonamides, aromatic antiepileptic agents, nonsteroidal anti-inflammatory drugs, neuroleptics, etc). This reaction is characterized by the development of a severe generalized eruption, facial edema, lymph node enlargement, high body temperature (>38.5ºC) and multisystemic involvement (hepatitis, interstitial nephritis, interstitial pneumonia and hematological abnormalities). The macular then papular confluent exanthema begins on the upper trunk and face and descends to the lower extremities. Following the EMA recommendation, treatment with strontium ranelate should be discontinued in the case of rash, and should not be restarted [EMA, 2009, Musette et al. 2010].
Conclusion
Efficacy and safety have to be taken into account in the long-term treatment of any chronic condition such as osteoporosis. Long-term therapy with strontium ranelate has proven to be effective in reducing fracture risk, it is well tolerated and associated with a low incidence of side effects, making it a first-line treatment for postmenopausal women at high risk of fractures. Strontium ranelate is currently licensed in Europe for the treatment of postmenopausal osteoporosis and male osteoporosis.
More studies are needed to explore the mechanisms by which strontium ranelate promotes bone formation and inhibits bone resorption and how these effects reflect on amelioration of the biomechanical properties of skeletal tissue.
Footnotes
Funding: This work was supported through a grant from F.I.R.M.O. Fondazione Raffaella Becagli (to MLB).
Conflict of interest statement: MLB has received speaking honoraria and research funding from Amgen, Eli Lilly, MSD, Novartis, Roche and Servier. LC and FDA have no conflicts of interest to disclose.
Contributor Information
Luisella Cianferotti, Unit of Bone and Mineral Metabolism, Department of Surgery and Translational Medicine, University of Florence, Medical School, Florence, Italy.
Federica D’Asta, Unit of Bone and Mineral Metabolism, Department of Surgery and Translational Medicine, University of Florence, Medical School, Florence, Italy.
Maria Luisa Brandi, Department of Surgery and Translational Medicine, University of Florence, Viale Pieraccini 6, 50139 Florence, Italy.
References
- Ammann P., Badoud I., Barraud S., Dayer R., Rizzoli R. (2007) Strontium ranelate treatment improves trabecular and cortical intrinsic bone tissue quality, a determinant of bone strength. J Bone Miner Res 22: 1419–1425 [DOI] [PubMed] [Google Scholar]
- Ammann P., Shen V., Robin B., Mauras Y., Bonjour J., Rizzoli R. (2004) Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J Bone Miner Res 19: 2012–2020 [DOI] [PubMed] [Google Scholar]
- Arlot M., Jiang Y., Genant H., Zhao J., Burt-Pichat B., Roux J., et al. (2008) Histomorphometric and microCT analysis of bone biopsies from postmenopausal osteoporotic women treated with strontium ranelate. J Bone Miner Res 23: 215–222 [DOI] [PubMed] [Google Scholar]
- Atkins G., Welldon K., Halbout P., Findlay D. (2009) Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos Int 20: 653–664 [DOI] [PubMed] [Google Scholar]
- Bain S., Jerome C., Shen V., Dupin-Roger I., Ammann P. (2009) Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporos Int 20: 1417–1428 [DOI] [PubMed] [Google Scholar]
- Barbara A., Delannoy P., Denis B., Marie P. (2004) Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism 53: 532–537 [DOI] [PubMed] [Google Scholar]
- Boivin G., Farlay D., Bala Y., Doublier A., Meunier P., Delmas P. (2009) Influence of remodeling on the mineralization of bone tissue. Osteoporos Int 20: 1023–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G., Farlay D., Khebbab M., Jaurand X., Delmas P., Meunier P. (2010) In osteoporotic women treated with strontium ranelate, strontium is located in bone formed during treatment with a maintained degree of mineralization. Osteoporos Int 21: 667–677 [DOI] [PubMed] [Google Scholar]
- Bonnelye E., Chabadel A., Saltel F., Jurdic P. (2008) Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 42: 129–138 [DOI] [PubMed] [Google Scholar]
- Breart G., Audran M., Brandi M., Bröll J., Hamdy A., Jakob F., et al. (2009) Good safety and persistence of strontium ranelate in a prospective observational cohort study. Osteoporos Int 20: S94 [Google Scholar]
- Breart G., Cooper C., Meyer O., Speirs C., Deltour N., Reginster J. (2010) Osteoporosis and venous thromboembolism: a retrospective cohort study in the UK General Practice Research Database. Osteoporos Int 21: 1181–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T., Rybchyn M., Green W., Atwa S., Conigrave A., Mason R. (2009) Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br J Pharmacol 157: 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyere O., Collette J., Rizzoli R., Decock C., Ortolani S., Cormier C., et al. (2010) Relationship between 3-month changes in biochemical markers of bone remodelling and changes in bone mineral density and fracture incidence in patients treated with strontium ranelate for 3 years. Osteoporos Int 21: 1031–1036 [DOI] [PubMed] [Google Scholar]
- Bruyere O., Roux C., Badurski J., Isaia G., De Vernejoul M., Cannata J., et al. (2007) Relationship between change in femoral neck bone mineral density and hip fracture incidence during treatment with strontium ranelate. Curr Med Res Opin 23: 3041–3045 [DOI] [PubMed] [Google Scholar]
- Canalis E., Hott M., Deloffre P., Tsouderos Y., Marie P. (1996) The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 18: 517–523 [DOI] [PubMed] [Google Scholar]
- Caudrillier A., Hurtel-Lemaire A., Wattel A., Cournarie F., Godin C., Petit L., et al. (2010) Strontium ranelate decreases RANKL-induced osteoclastic differentiation in vitro: involvement of the calcium sensing receptor. Mol Pharmacol 78: 569–576 [DOI] [PubMed] [Google Scholar]
- Caverzasio J. (2008) Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 42: 1131–1136 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N., Quinn S., Kifor O., Ye C., Brown E. (2007) The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem Pharmacol 74: 438–447 [DOI] [PubMed] [Google Scholar]
- Choudhary S., Halbout P., Alander C., Raisz L., Pilbeam C. (2007) Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: involvement of prostaglandins. J Bone Miner Res 22: 1002–1010 [DOI] [PubMed] [Google Scholar]
- Cooper C., Reginster J., Cortet B., Diaz-Curiel M., Lorenc R., Kanis J., et al. (2012) Long-term treatment of osteoporosis in postmenopausal women: a review from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Curr Med Res Opin 28: 475–491 [DOI] [PubMed] [Google Scholar]
- Dahl S., Allain P., Marie P., Mauras Y., Boivin G., Ammann P., et al. (2001) Incorporation and distribution of strontium in bone. Bone 28: 446–453 [DOI] [PubMed] [Google Scholar]
- Doublier A., Farlay D., Khebbab M., Jaurand X., Meunier P., Boivin G. (2011) Distribution of strontium and mineralization in iliac bone biopsies from osteoporotic women treated long-term with strontium ranelate. Eur J Endocrinol 165: 469–476 [DOI] [PubMed] [Google Scholar]
- EMA (2009) Strontium ranelate. Summary of product characteristics. European Medicines Agency; Available at: http://www.emea.europa.eu (accessed 3 March 2013). [Google Scholar]
- EMA (2012) Questions and answers on the review of Protelos and Osseor (strontium ranelate). European Medicines Agency; Available at: http://www.emea.europa.eu (accessed 3 March 2013). [Google Scholar]
- Farlay D., Boivin G., Panczer G., Lalande A., Meunier P. (2005) Long-term strontium ranelate administration in monkeys preserves characteristics of bone mineral crystals and degree of mineralization of bone. J Bone Miner Res 20: 1569–1578 [DOI] [PubMed] [Google Scholar]
- Fournier C., Perrier A., Thomas M., Laroche N., Dumas V., Rattner A., et al. (2012) Reduction by strontium of the bone marrow adiposity in mice and repression of the adipogenic commitment of multipotent C3H10T1/2 cells. Bone 50: 499–509 [DOI] [PubMed] [Google Scholar]
- Fromigue O., Hay E., Barbara A., Marie P. (2010) Essential role of nuclear factor of activated T cells (NFAT)-mediated WNT signalling in osteoblast differentiation induced by strontium ranelate. J Biol Chem 285: 25251–25258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromigue O., Hay E., Barbara A., Petrel C., Traiffort E., Ruat M., et al. (2009) Calcium sensing receptor-dependent and -independent activation of osteoblast replication and survival by strontium ranelate. J Cell Mol Med 13: 2189–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy V., Chappard D., Marty C., Libouban H., Ostertag A., Lalande A., et al. (2011) Strontium ranelate decreases the incidence of new caudal vertebral fractures in a growing mouse model with spontaneous fractures by improving bone microarchitecture. Osteoporos Int 22: 289–297 [DOI] [PubMed] [Google Scholar]
- Goldhahn J., Féron J., Kanis J., Papapoulos S., Reginster J., Rizzoli R., et al. (2012) Implications for fracture healing of current and new osteoporosis treatments: an ESCEO consensus paper. Calcif Tissue Int 90: 343–353 [DOI] [PubMed] [Google Scholar]
- Hiligsmann M., Bruyère O., Reginster J. (2010a) Cost-utility of long-term strontium ranelate treatment for postmenopausal osteoporotic women. Osteoporos Int 21: 157–165 [DOI] [PubMed] [Google Scholar]
- Hiligsmann M., Vanoverberghe M., Neuprez A., Bruyère O., Reginster J. (2010b) Cost-effectiveness of strontium ranelate for the prevention and treatment of osteoporosis. Expert Rev Pharmacoecon Outcomes Res 10:359–366 [DOI] [PubMed] [Google Scholar]
- Hurtel-Lemaire A., Mentaverri R., Caudrillier A., Cournarie F., Wattel A., Kamel S., et al. (2009) The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. New insights into the associated signaling pathways. J Biol Chem 284: 575–584 [DOI] [PubMed] [Google Scholar]
- Kaufman J., Audran M., Bianchi G., Braga V., Diaz-Curiel M., Francis R., et al. (2013) Efficacy and safety of strontium ranelate in the treatment of osteoporosis in men. J Clin Endocrinol Metab 98: 592–601 [DOI] [PubMed] [Google Scholar]
- Khosla S., Oursler M., Monroe D. (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23: 576–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Paris O., Siegel S., Roschger P., Paschalis E., Klaushofer K., et al. (2010) Strontium is incorporated into mineral crystals only in newly formed bone during strontium ranelate treatment. J Bone Miner Res 25: 968–975 [DOI] [PubMed] [Google Scholar]
- Li Y., Feng G., Gao Y., Luo E., Liu X., Hu J. (2009) Strontium ranelate treatment enhances hydroxyapatite-coated titanium screws fixation in osteoporotic rats. J Orthop Res 28: 578–582 [DOI] [PubMed] [Google Scholar]
- Li Y., Li J., Zhu S., Luo E., Feng G., Chen Q., et al. (2012) Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem Biophys Res Commun 418: 725–730 [DOI] [PubMed] [Google Scholar]
- Li Y., Luo E., Feng G., Zhu S., Li J., Hu J. (2010) Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos Int 21: 1889–1897 [DOI] [PubMed] [Google Scholar]
- Maimoun L., Brennan T., Badoud I., Dubois-Ferriere V., Rizzoli R., Ammann P. (2010) Strontium ranelate improves implant osseointegration. Bone 46: 1436–1441 [DOI] [PubMed] [Google Scholar]
- Marie P., Felsenberg D., Brandi M. (2011) How strontium ranelate, via opposite effects on bone resorption and formation, prevents osteoporosis. Osteoporos Int 22: 1659–1667 [DOI] [PubMed] [Google Scholar]
- Marie P., Hott M., Modrowski D., De Pollak C., Guillemain J., Deloffre P., et al. (1993) An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J Bone Miner Res 8: 607–615 [DOI] [PubMed] [Google Scholar]
- Marquis P., Cialdella P., De La Loge C. (2001) Development and validation of a specific quality of life module in post-menopausal women with osteoporosis: the QUALIOST. Qual Life Res 10: 555–566 [DOI] [PubMed] [Google Scholar]
- Marquis P., Roux C., de la Loge C., Diaz-Curiel M., Cormier C., Isaia G., et al. (2008) Strontium ranelate prevents quality of life impairment in post-menopausal women with established vertebral osteoporosis. Osteoporos Int 19: 503–510 [DOI] [PubMed] [Google Scholar]
- Meunier P., Aaron J., Edouard C., Vignon G. (1971) Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies, Clin Orthop Relat Res 80: 147–154 [DOI] [PubMed] [Google Scholar]
- Meunier P., Roux C., Ortolani S., Diaz-Curiel M., Compston J., Marquis P., et al. (2009) Effects of long-term strontium ranelate treatment on vertebral fracture risk in postmenopausal women with osteoporosis. Osteoporos Int 20: 1663–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier P., Roux C., Seeman E., Ortolani S., Badurski J., Spector T., et al. (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350: 459–468 [DOI] [PubMed] [Google Scholar]
- Musette P., Brandi M., Cacoub P., Kaufman J., Rizzoli R., Reginster J. (2010) Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int 21: 723–732 [DOI] [PubMed] [Google Scholar]
- Musette P., Kaufman J., Rizzoli R., Cacoub P., Brandi M., Reginster J. (2011) Cutaneous side effects of antiosteoporosis treatments. Ther Adv Musculoskelet Dis 3: 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Liu X., Huang S., Li Z., Pan H., Zhen W., et al. (2011a) The cross-talk between osteoclasts and osteoblasts in response to strontium treatment: involvement of osteoprotegerin. Bone 49: 1290–1298 [DOI] [PubMed] [Google Scholar]
- Peng S., Liu X., Zhou G., Li Z., Luk K., Guo X., et al. (2011b) Osteoprotegerin deficiency attenuates strontium-mediated inhibition of osteoclastogenesis and bone resorption. J Bone Miner Res 26: 1272–1282 [DOI] [PubMed] [Google Scholar]
- Perrio M., Wilton L., Shakir S. (2008) Analysis of venous thromboembolism in the strontium ranelate prescription-event monitoring (PEM) cohort: interim results. Drug Saf 31: 1. [DOI] [PubMed] [Google Scholar]
- Rabenda V., Reginster J. (2010) Positive impact of compliance to strontium ranelate on the risk of nonvertebral osteoporotic fractures. Osteoporos Int 21: 1993–2002 [DOI] [PubMed] [Google Scholar]
- Raisz L. (2005) Pathogenesis of osteoporosis: concepts, conflicts and prospects. J Clin Invest 115: 3318–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recker R., Marin F., Ish-Shalom S., Moricke R., Hawkins F., Kapetanos G., et al. (2009) Comparative effects of teriparatide and strontium ranelate on bone biopsies and biochemical markers of bone turnover in postmenopausal women with osteoporosis. J Bone Miner Res 24: 1358–1368 [DOI] [PubMed] [Google Scholar]
- Reginster J., Bruyere O., Collette J. (2012) Strontium ranelate treatment increases osteoprotegerin serum levels in postmenopausal osteoporotic women. Bone 50: 1201–1202; author reply 1203–1204. [DOI] [PubMed] [Google Scholar]
- Reginster J., Bruyère O., Sawicki A., Roces-Varela A., Fardellone P., Roberts A., et al. (2009) Long-term treatment of postmenopausal osteoporosis with strontium ranelate: results at 8 years. Bone 45: 1059–1064 [DOI] [PubMed] [Google Scholar]
- Reginster J., Felsenberg D., Boonen S., Diez-Perez A., Rizzoli R., Brandi M., et al. (2008) Effects of long-term strontium ranelate treatment on the risk of nonvertebral and vertebral fractures in postmenopausal osteoporosis: results of a five-year, randomized, placebo-controlled trial. Arthritis Rheum 58: 1687–1695 [DOI] [PubMed] [Google Scholar]
- Reginster J., Kaufman J., Goemaere S., Devogelaer J., Benhamou C., Felsenberg D., et al. (2012) Maintenance of antifracture efficacy over 10 years with strontium ranelate in postmenopausal osteoporosis. Osteoporos Int 23: 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster J., Seeman E., De Vernejoul M., Adami S., Compston J., Phenekos C., et al. (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90: 2816–2822 [DOI] [PubMed] [Google Scholar]
- Ringe J., Dorst A., Farahmand P. (2009) Treatment of glucocorticoid-induced osteoporosis with strontium ranelate: a 2-year observational, controlled study versus risedronate (abstract). Osteoporos Int 20(Suppl. 1): S72 [Google Scholar]
- Rizzoli R., Chapurlat R., Laroche J., Krieg M., Thomas T., Frieling I., et al. (2012) Effects of strontium ranelate and alendronate on bone microstructure in women with osteoporosis. Results of a 2-year study. Osteoporos Int 23: 305–315 [DOI] [PubMed] [Google Scholar]
- Rizzoli R., Laroche M., Krieg M., Frieling I., Thomas T., Delmas P., et al. (2010) Strontium ranelate and alendronate have differing effects on distal tibia bone microstructure in women with osteoporosis. Rheumatol Int 30: 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli R., Reginster J., Boonen S., Bréart G., Diez-Perez A., Felsenberg D., et al. (2011) Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int 89: 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschger P., Manjubala I., Zoeger N., Meirer F., Simon R., Li C., et al. (2010) Bone material quality in transiliac bone biopsies of postmenopausal osteoporotic women after 3 years of strontium ranelate treatment. J Bone Miner Res 25: 891–900 [DOI] [PubMed] [Google Scholar]
- Roux C., Reginster J., Fechtenbaum J., Kolta S., Sawicki A., Tulassay Z., et al. (2006) Vertebral fracture risk reduction with strontium ranelate in women with postmenopausal osteoporosis is independent of baseline risk factors. J Bone Miner Res 21: 536–542 [DOI] [PubMed] [Google Scholar]
- Seeman E. (2003) Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporos Int 14(Suppl. 3): S2–S8 [DOI] [PubMed] [Google Scholar]
- Seeman E., Boonen S., Borgström F., Vellas B., Aquino J., Semler J., et al. (2010) Five years treatment with strontium ranelate reduces vertebral and nonvertebral fractures and increases the number and quality of remaining life-years in women over 80 years of age. Bone 46: 1038–1042 [DOI] [PubMed] [Google Scholar]
- Seeman E., Devogelaer J., Lorenc R., Spector T., Brixen K., Balogh A., et al. (2008) Strontium ranelate reduces the risk of vertebral fractures in patients with osteopenia. J Bone Miner Res 23: 433–438 [DOI] [PubMed] [Google Scholar]
- Seeman E., Vellas B., Benhamou C., Aquino J., Semler J., Kaufman J., et al. (2006) Strontium ranelate reduces the risk of vertebral and nonvertebral fractures in women eighty years of age and older. J Bone Miner Res 21: 1113–1120 [DOI] [PubMed] [Google Scholar]
- Sun P., Cai D., Li Q., Chen H., Deng W., He L., et al. (2010) Effects of alendronate and strontium ranelate on cancellous and cortical bone mass in glucocorticoid-treated adult rats. Calcif Tissue Int 86: 495–501 [DOI] [PubMed] [Google Scholar]
- Takahashi N., Sasaki T., Tsouderos Y., Suda T. (2003) S12911–2 inhibits osteoclastic bone resorption in vitro. J Bone Miner Res 18: 1082–1087 [DOI] [PubMed] [Google Scholar]
- Zebaze R., Ghasem-Zadeh A., Bohte A., Iuliano-Burns S., Mirams M., Price R., et al. (2010) Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 375: 1729–1736 [DOI] [PubMed] [Google Scholar]