Abstract
Central nervous system inflammatory demyelinating disease can affect patients across the life span. Consensus definitions and criteria of all of the different acquired demyelinating diseases that fall on this spectrum have magnetic resonance imaging criteria. The advances of both neuroimaging techniques and important discoveries in immunology have produced an improved understanding of these conditions and classification. Neuroimaging plays a central role in the accurate diagnosis, prognosis, disease monitoring and research efforts that are being undertaken in this disease. This review focuses on the imaging spectrum of acquired demyelinating disease.
Keywords: demyelinating disease, multiple sclerosis, MRI, neuroimaging
Background
Magnetic resonance imaging (MRI) has become a critically important tool in diagnosis and differentiation of different demyelinating disorders. Prognosis, disease monitoring and treatment changes are often based on the combination of clinical symptoms and neuroimaging findings. MRI is the most commonly used imaging modality as it offers high-resolution images in a noninvasive and safe method, without exposing patients to ionizing radiation. The multitude of available pulse sequences provides unparalleled structural and even functional information that allows investigation of different pathological tissue properties. Since the introduction of MRI, this technique has truly altered the approach to demyelinating diseases, including that of multiple sclerosis (MS) [Noseworthy et al. 2000]. The latter is the most common demyelinating disease in adults and leads to significant morbidity and disability within the population. Neuroimaging has played an increasing role in the diagnosis of MS since its initial incorporation in the 2001 McDonald’s criteria [McDonald et al. 2001] and subsequent revisions [Polman et al. 2005, 2011]. In predicting conversion risk from the presentation with clinically isolated (demyelinating) syndrome (CIS), MRI measures have been shown to play a large role [Barkhof et al. 1997; Brex et al. 2002; Morrissey et al. 1993; O’Riordan et al. 1998]. Also, there has been increasing recognition of MRI patterns typical for MS, meeting imaging criteria in the absence of any clinically related symptoms, also known as radiologically isolated syndrome (RIS), which over time has the potential to evolve into clinically definite MS [Lebrun et al. 2009; Okuda et al. 2009; Siva et al. 2009]. In other demyelinating or neuro-inflammatory diseases MRI has been incorporated as well into diagnostic criteria, including neuromyelitis optica (NMO) [Wingerchuk et al. 1999, 2006], transverse myelitis [Transverse Myelitis Consortium Working Group, 2002], pediatric MS [Callen et al. 2009b] and acute disseminated encephalomyelitis (ADEM) [Tenembaum et al. 2007].
However, there is a clear discrepancy between the clinical course of the disease and the ‘severity’ of the appearance of the disease on MRI, also known as the clinicoradiological paradox [Barkhof, 2002]. This has become apparent even in recent subanalyses of data from several clinical studies; the exclusive use of MRI derived surrogate markers of disease activity in clinical trials in its current state was disputed based on its lack of correlation with clinical outcomes [Daumer et al. 2009]. Advances in the MRI acquisitions are revealing aspects of MS beyond visibility on conventional imaging techniques. The expectation is that once validated, such new sequences gradually will be incorporated into future clinical trials.
In this review we discuss routinely available MRI sequences, their current role in diagnosis, management and disease monitoring of MS and other acquired inflammatory demyelinating diseases, including NMO and ADEM. In addition, we will briefly review some advanced MRI sequences, currently mostly utilized in research studies. We will first review typical imaging features of CIS and MS. Subsequently we briefly review some of the other acquired demyelinating diseases.
Imaging appearance of typical lesions in MS
Although MS is a demyelinating disease that predominantly affects the white matter, anywhere within the central nervous system (CNS) different pathologies can be detected.
White matter lesions
T2 weighted imaging
The hallmark lesions of demyelinating disease are within the white matter of the brain. The classical sequences that visualize MS plaques in vivo are T2 weighted imaging (T2WI) acquisition techniques, where lesions are sensitively detected with a hyperintense signal change (Figures 1C and 2A). However, the specificity of detecting the underlying pathology is poor. These lesions typically remain hyperintense over time [Paty and Li, 1993]. It can be difficult to make a clear distinction on T2WI alone between demyelination and other pathological features associated with demyelinating disease (e.g. remyelination, inflammation, edema, Wallerian degeneration, axonal loss) or with other diseases (e.g. cell infiltration, neoplasm, infection, etc.). All of these can have similar hyperintense characteristics on T2WI. The most common T2WI sequences used in clinical practice are fast spin echo (FSE)-based techniques and fluid attenuation inversion recovery (FLAIR). In the latter acquisition technique, an additional inversion recovery pulse is used to suppress signal arising from cerebrospinal fluid (CSF). Owing to the increased tissue contrast in this sequence, it has improved detection of cerebral hemispheric lesions, especially increased sensitivity to the detection of juxtacortical lesions [Filippi et al. 1996] (Figure 1B and 2B). The improved tissue contrast of FLAIR images makes it overall easier to spot lesions at the first glance, probably one of the reasons why this sequence is often preferred over standard T2 weighted sequences by practicing neurologists. Often forgotten, they come with a major shortcoming, which is the relative decreased sensitivity to detect posterior fossa lesions [Gawne-Cain et al. 1997; Stevenson et al. 1997] (Figure 1C and D). Such infratentorial lesions are common in MS and CIS and one of the main reasons to continue to include standard T2WI in the evaluation of possible demyelinating disease. The location of T2 weighted hyperintense lesions is of great importance from the diagnostic perspective in MS. It can help differentiate MS from its numerous mimics and is one of the main reasons why lesion location is prominently featured in the diagnostic criteria of MS [McDonald et al. 2001; Polman et al. 2005, 2011]. These criteria will be reviewed in further detail later. The most frequently seen lesions are located periventricularly, which have a unique feature in MS where seemingly arising from the ventricle with the main axis almost perpendicular to the main axis of the lateral ventricle [Gean-Marton et al. 1991; Offenbacher et al. 1993], also known as Dawson’s fingers.
Figure 1.
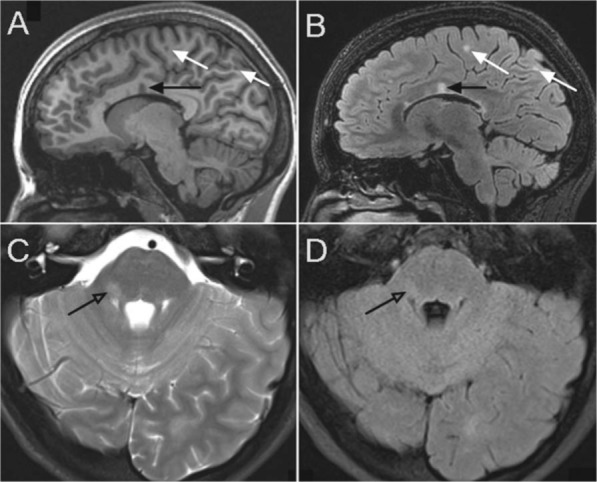
Lesions in subject with relapsing remitting MS. Top images are sagittal images with corpus callosum lesions (black arrows) and juxtacortical lesions on the T1 weighted image (A) and T2 FLAIR weighted image (B). Bottom images clearly demonstrate the improved sensitivity of posterior fossa lesion detection (open arrow) on standard T2 weighted images (C) over T2 FLAIR (D).
Figure 2.
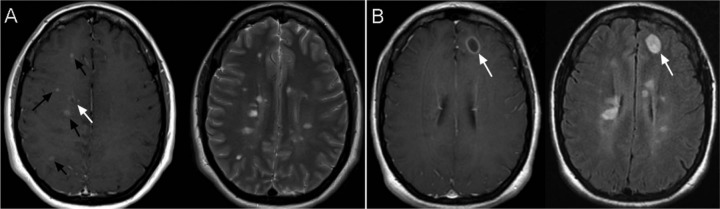
Variety of contrast enhancing lesions in a patient with RRMS during a clinical relapse. (A) shows numerous small, nodular contrast enhancing lesions (black arrow) and a small ring enhancing lesion (white arrow) in a T1 weighted post contrast image (left image), correlating to the hyperintensities on T2 weighted images (right image). Images in (B) show a larger ring enhancing lesion in the right frontal lobe on the T1 weighted post gadolinium contrast images (left image) with multiple older lesions appearing on FLAIR imaging (right image).
There are multiple factors that contribute to what has been called the clinicoradiological or ‘MRI paradox’: there is a limited weak correlation between standard T2 lesion load and clinical disability. In early relapsing–remitting (RRMS) this correlation is slightly stronger [Molyneux et al. 1998; O’Riordan et al. 1998; Sailer et al. 1999; Zivadinov and Leist, 2005] and when the disability scale is extended to include higher Expanded Disability Status Scale (EDSS) scores, this correlation does not seem to plateau [Caramanos et al. 2012]. Part of this is discrepancy is the location and biological features of lesion recovery that do not have great imaging differentiating factors as remyelinated lesions can have an indistinct appearance on T2WI. In advanced MS the multifocal T2 lesions can ‘merge’ and become confluent and at the same time brain atrophy has been shown to progress [Pirko et al. 2007]. Owing to the combination of these, the lesion load may actually decrease in advanced cases, another potential reason of the paradox.
T1 weighted imaging
T1 weighted scans can be quite unimpressive compared with T2WI in many patients. On first observation, these images may almost appear entirely normal in MS in such patients. However, in some patients many of the typical T2 hyperintense signal change has a T1 hypointense imaging characteristic [Uhlenbrock and Sehlen, 1989], the so-called ‘T1 black holes’. This phenomenon can be seen in two instances with largely independent mechanisms.
Acute T1 black holes are seen in an early transient stage in lesion formation [Bagnato et al. 2003; Levesque et al. 2005]. Cellular infiltration in emerging lesions and associated ‘focal edema’ are thought to be the pathological correlate. These often resolve [Brex et al. 2001; Losseff et al. 2001a].
However, approximately 30% of T1 ‘black holes’ will persist, becoming a lifelong present feature [Ciccarelli et al. 1999; van Waesberghe et al. 1999] (Figure 1A). When persistent, these are thought to represent more severe tissue loss with areas of axonal injury [Bitsch et al. 2001; van Walderveen et al. 1998], the degree of hypointensity correlated best with axonal density [Bitsch et al. 2001]. The most hypointense black holes can have little to no remaining neuronal tissue [van Waesberghe et al. 1999]. However, in most cases the signal intensity is higher than CSF in these areas, indicating persistence of (some) CNS tissue.
On advanced MRI, T1 black holes have low magnetization transfer ratios (MTRs) associated with them (even lower than in T1 isointense lesions) [Hiehle et al. 1995]. On MRS, the hallmark of chronic black holes is severely decreased N-acetlyaspartate (NAA) [Brex et al. 2000]. These are additional indicators of more profound tissue damage in these lesions. This seems to correlate better with clinical disability, as in contrast to T2WI lesion load, T1 black hole load correlates better with chronic disability [Truyen et al. 1996; van Walderveen et al. 1995, 2001; Zivadinov and Leist, 2005].
Gadolinium contrast enhancement
Gadolinium enhancement (Figure 1D) is considered the hallmark of ‘active lesions’ as it represents the presence of inflammatory infiltrates through leakage of the blood–brain barrier (BBB). Where most lesions noted on noncontrast T2WI and T1 weighted imaging are chronically present, gadolinium contrast-enhanced lesions (CELs) provide a ‘time-stamp’ for recent or ongoing inflammatory activity. Gadolinium enhancement is typically observable in the first 4–6 weeks of lesion formation [Cotton et al. 2003; Miller et al. 1988]. Very rarely, it is detectable beyond 2–3 months, which should raise the concern for alternative diagnoses (neoplasm, [neuro]sarcoidosis, etc.). Chelated gadolinium is the most commonly used contrast material and is detected as a T1 hyperintense signal change. Spontaneous T1 hyperintensity on conventional spin-echo-based T1 weighted studies have been reported [Janardhan et al. 2007; Zhou et al. 2010], therefore correlation between pre- and post-contrast administrated T1 weighted images should be made. There are alternative iron oxide nanoparticle-based contrast agents that can give hypointense signal changes on T2* weighted imaging, but most of these are experimental and with limited use in human studies [Dousset et al. 2006; Pirko et al. 2004, 2005; Vellinga et al. 2008].
Important factors in the appearance of CELs (sensitivity) are the dose and delay between administration and scanning time in addition to imaging parameters (e.g. slice thickness, field strength, use of ‘sensitizing’ magnetization transfer pulse [Silver et al. 1999]). Typical recommended doses are 0.1 mmol/kg, and a delay of minimal 5 minutes. Between administration and obtaining the T1 weighted post-contrast images, often another noncontrast sensitive sequence is obtained. With the increasing use of gadolinium a linkage between nephrogenic systemic fibrosis and administration of gadolinium was made, most strongly correlated with poor kidney function, the reason to screen for this prior to administration of gadolinium [Sadowski et al. 2007]. In cases of limited estimated glomerular filtration rate (eGFR), caution should be applied in the use of gadolinium-based contrast agents [Colletti, 2008; Haemel et al. 2011].
It deserves mention that, similar to other lesion-based clinicoradiological discordances, in many cases the presence of enhancement will not result in an obvious new clinical relapse. As a matter of fact, presence of gadolinium enhancement is 5–10 times more common than clinical observation alone [Thompson et al. 1992]. This increased sensitivity to recent disease activity is the main reason that gadolinium-enhanced lesion-based quantitative metrics have been used as surrogate markers of outcome measures in clinical trials of MS therapies. While efficiently capturing recent inflammatory activity, these lesions also do not correlate well with disability on longitudinal studies. The mean number of CELs in the first 6 months after the diagnosis of MS showed only a weak correlation with disability 1 and 2 years later [Kappos et al. 1999]. In biopsy-proven demyelinating disease, also no such association was found between ring CELs and disability [Lucchinetti et al. 2008]. CELs are not single predictors of subsequent relapses and many factors, including overall clinical course, imaging appearance and temporal changes in addition to most importantly patient preference is what should guide decision making regarding treatment change. This is one of the most important features for the practicing neurologist, treatment changes are made based on disease activity overall and not the appearance of imaging features alone at this point.
In progressive forms of MS, contrast enhancement can be present, albeit with much lower incidence. In secondary progressive MS (SPMS), CELs are rarely seen and often are not observed at all [Kidd et al. 1996; Tubridy et al. 1998]. In primary progressive MS (PPMS), CEL have been reported in as low as approximately 5% of cases [Thompson et al. 1991]. This is thought to occur more frequently in the earlier stages of the disease, in the first 5 years of PPMS as high as 40% of patients may have associated cerebral CELs [Ingle et al. 2005].
The physical appearance of CELs can take many different forms. The differential diagnosis of ring enhanced lesions is wide and includes gliomas, metastases, abscesses, etc. Demyelinating CELs can appear as homogenously enhanced lesions, heterogeneous/nodular pattern or with (open) ring enhanced patterns [Grossman et al. 1986, 1988; Miller et al. 1988; Thompson et al. 1992; Thorpe et al. 1996] (Figure 2). In the case of the very specific open ring enhancement pattern, the opening is typically outward, towards the cortex [Masdeu et al. 1996, 2000]. Ring enhanced lesions are often larger and can have specific imaging features, including shorter duration of enhancement [Minneboo et al. 2005], low signal intensity on apparent diffusion coefficient (ADC) [Leist et al. 2001] and MTR [Morgen et al. 2001]. There are some reports on the evolution into T1 black holes and the association with the development of brain atrophy [Bagnato et al. 2003].
Tumefactive demyelinating white matter lesions
A small subset of patients will initially present with unusually large or tumefactive solitary or multiple demyelinating lesion. These typically have contrast enhancement patterns as described above and the differentiating between the potential diagnoses is even more pressing as entirely different treatment pathways and prognosis follow. Other similar appearing lesions primarily include primary brain neoplasms (e.g. high-grade gliomas), metastatic malignancies and abscess. Biopsies are often obtained to complete the evaluation of these atypical lesions as differentiation on imaging presentation alone often is not sufficient. Efforts to differentiate using magnetic resonance spectroscopy (MRS) has not been proven helpful thus far. MRS of tumefactive demyelinating lesions may show elevated choline and decreased NAA peak in addition to lactate and lipid peaks, similar findings have been seen in neoplastic lesions [Saindane et al. 2002]. Changes in MRS patterns over time may be suggestive of demyelinating etiology [Butteriss et al. 2003]. Some had reviewed specific diagnostic algorithms [Al-Okaili et al. 2007] to incorporate perfusion MRI, diffusion weighted imaging and conventional MRI. Other subtle imaging findings can include the presence of a similar T2 hypointense rim and a pattern of open ring enhancement favoring demyelination. The hypointense borders are thought to correlate with the location of macrophages in these lesions, but are not specific. However, a recent correlation study between histopathology and MRI findings of tumefactive demyelinating disease found that distinguishing appearance of tumefactive lesions include appearance on the ADC maps of diffusion weighted imaging [Abou Zeid et al. 2012]. The behavior of peripheral ADC patterns at the lesion edge was found to have significant peripheral diffusion restriction in demyelination, compared with abscesses and tumors (p = 0.006); whereas central restriction was only seen in abscesses.
Spinal cord lesions
Spinal cord lesions (Figure 3) are prominently present in MS and are commonly not fully investigated. As reviewed later, the significance of spinal cord involvement is also underlined in the newly revised diagnostic criteria of MS [McDonald et al. 2001; Polman et al. 2005]. The best way to detect spinal cord lesions are T2 weighted short tau inversion recovery (STIR) and FSE sequences. On sagittal scans, smaller MS lesions can be missed and opposed to NMO, these rarely extend longitudinally beyond one spinal cord segment. When imaging quality and patient factors permit (e.g. movement, obesity), axial cord images commonly show smaller lesions with involvement of small sections anywhere within the spinal cord, commonly affecting the posterior or lateral aspects. This typically is around a quarter to half of the axial cord slice surface area, with both white and gray matter involvement. STIR imaging is often used to increase the sensitivity of spinal cord lesion detection [Bot et al. 2000]. These images also provide fat suppression, which may be useful in optic nerve imaging as well [Campi et al. 2000; Moseley et al. 1998]. Similar to T1 black holes that are most commonly found in the cerebral white matter, T1 hypointense signal changes can be found in the spinal cord [Losseff et al. 2001b]. In progressive forms of MS, where the clinical picture is predominated by myelopathic symptoms, subtle cord atrophy and increasing cord lesion load may be the only MRI manifestations.
Figure 3.
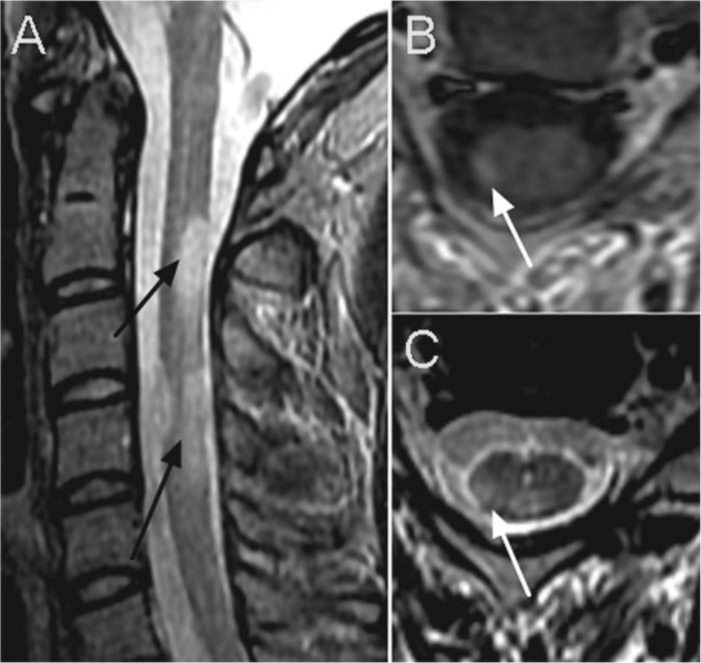
Typical spinal cord lesions in a patient with RRMS. On the sagittal STIR sequence (A) multiple ovoid lesions (black arrows) are seen extending across one vertebral segment. The images on the right show axial cuts of the same individual, showing the typical pattern of ovoid lesion in the right dorsolateral aspect of the cervical spinal cord (white arrow). (B) post gadolinium contrast T1 weighted image, (C) T2 weighted image.
Optic nerve lesions
The optic nerve is frequently involved in demyelinating disease and optic neuritis (ON) can be seen as CIS or as part of MS, ADEM or NMO. The diagnosis of ON is clinical with most frequently gradual worsening of visual acuity, color perception and associated eye pain. Imaging findings can be supportive, best demonstrated with dedicated orbital/optic nerve imaging (Figure 4). The most significant contribution of imaging at initial presentation with isolated ON is evaluation with brain MRI. The overall conversion risk to MS in the optic neuritis treatment trial (ONTT) was approximately 50% in 15 years after initial presentation with ON [Brodsky et al. 2008]. When brain MRI findings were incorporated, risk stratification revealed that approximately 75% of patients converted when brain lesions were present, where only 25% of patients with a normal MRI developed MS in that time frame. Advanced imaging methods such as diffusion tensor imaging have been used to study ON as well and only have been described to possibly predict outcome [Naismith et al. 2009, 2010], without any clear diagnostic advantage.
Figure 4.
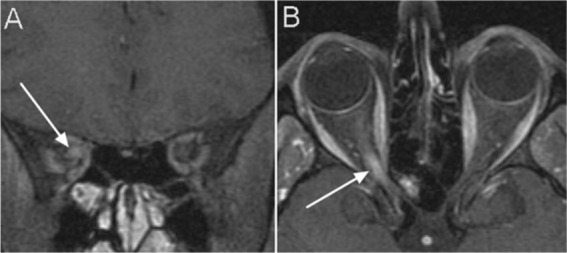
Post gadolinium fat saturated T1 weighted images showing contrast enhancement and thickening of the optic nerve (white arrows) on coronal (A) and axial image (B).
Subcortical gray matter lesions
On standard T2 weighted images, hypointensities have been observed and described in the thalamus and deep gray nuclei [Bakshi et al. 2001, 2002; Bermel et al. 2005; Grimaud et al. 1995; Tjoa et al. 2005]. The presence of such lesions seems to correlate with specific imaging outcomes such as brain atrophy, and with clinical outcome measures, including an increased likelihood for cognitive dysfunction, fatigue and disability. In addition, quantitative volumetric MRI metrics in these regions seem to correlate with similar clinical outcomes [Batista et al. 2012; Benedict et al. 2009]. Although not yet conclusively proven, pathological iron deposition seems to play a role in this, further supported by imaging features using susceptibility weighted imaging [Haacke et al. 2007; Hagemeier et al. 2012].
Cortical gray matter lesions
Whereas T2WI sequences are very sensitive in detecting demyelinated white matter lesions, they are relatively insensitive in the detection of cortical gray matter pathology. The presence of pathological abnormality within the cortex in MS has been discussed since the early descriptions by Charcot. A recent study investigating pathological samples obtained from biopsies of demyelinating white matter lesions found that in 38% of the specimens cortical demyelination was present en route to the biopsy core [Lucchinetti et al. 2011]. Lesions can be located in subpial, intracortical or leukocortical areas and even meningeal inflammation can be seen in early phases of the disease [Popescu and Lucchinetti, 2012]. Together with inflammatory demyelinating lesions in the cortex, over time there has been substantial evidence for advanced brain and cortical atrophy developing over time with MRI-based studies. Interestingly, these changes are more typically associated with clinical outcomes [Pirko et al. 2007; Zivadinov and Leist, 2005].
The detection of cortical lesions is so far the most sensitive of the T2 weighted inversion recovery techniques, including FLAIR and the recently developed double inversion recovery (DIR) acquisition techniques. DIR, which will be reviewed in further detail in the advanced imaging section, has an increased sensitivity of detecting cortical lesions compared with FLAIR, although in a recent pathology and MRI correlation study, this sensitivity was only 18% compared with the gold standard, histopathology [Seewann et al. 2012]. Part of this is that the greatest sensitivity is to detecting leukocortical or juxtacortical lesions, where the vast majority of intracortical and subpial cortical lesions remain undetected.
Imaging findings of other demyelinating diseases
The differential diagnosis of typical imaging findings of MS is large. As reviewed above, one of the most important features in detecting typical MS lesions is location. In contrast to MS lesions, nonspecific lesions due to small vessel ischemia and migraines tend to spare the U-fibers (juxtacortical lesions) and the hallmark periventricular locations of demyelinating disease. In addition, the nonspecific white matter lesions of such etiologies are typically absent in the infratentorial brain, with the exception of rare pontine lesions that can be seen in small vessel ischemic disease. Spinal cord lesions are typically not seen in small vessel ischemic disease, underlining the importance of diagnostic use of spinal cord imaging to assess demyelinating disease [Bot et al. 2002].
A workshop of the European MAGNIMS (Magnetic Resonance Network in Multiple Sclerosis) defined ‘MRI red flags’ derived from evidence-based findings and expert opinion [Charil et al. 2006]. Table 1 summarizes some of the identified MRI features that are suggestive of specific other pathologies and in the appropriate clinical setting should trigger consideration of work up targeted to other etiologies.
Table 1.
Table summarizing important entities in the differential diagnosis of MS and potential ‘red flags’ in the misdiagnosis of MS.
| Brain white matter | Disease | Cortical gray matter | Disease |
|---|---|---|---|
| Normal | NMO (absent or few lesions), ATM | Cortical/subcortical lesions crossing vascular territories | MELAS |
| Large lesions | AMS (can be confluent with perilesional edema), BCS, PACNS (with mass effect) | Infiltrating lesions that do not remain in gray or white matter boundaries | Abscess |
| Symmetrically distributed lesions | ADEM, AFL | Prevalent involvement versus white matter | Encephalitis |
| Poorly defined lesion margins | ADEM, pattern III MS | Deep gray matter | |
| Absent MRI activity at follow-up | ADEM | Bilateral lesions | ADEM (GM-WM junction), CADASIL |
| Involvement temporal pole, vertex U-fibers, external capsule, insula. | CADASIL | Lacunar infarcts | CADASIL, SVD |
| Bilateral microhemorrhagic foci | CADASIL, SVD, AHEM/AHLE, PACNS | T1-hyperintensity of the pulvinar | FD |
| Frequent sparing of CC and cerebellum | CADASIL, SVD | Multiple discrete lesions in the basal ganglia and thalamus | Susac’s syndrome |
| Predominant CC involvement | Susac’s syndrome | Large and infiltrating basal ganglia lesions | NBD |
| Enhancement of all lesions | ADEM, PACNS, sarcoidosis | Infiltrating lesions without respecting gray-matter or white-matter boundaries | Abscesses |
| Infarcts | SID, PACNS, SVD | T2-hyperintense lesions in the dentate nuclei | AFL (CTX) |
| Punctate parenchyma enhancement | PACNS, sarcoidosis, NBD | Spinal cord | |
| Predominance of lesions at the cortical/subcortical junction | SID | Large and swelling lesions | NMO, ADEM, ATM, Sjögren’s syndrome |
| Diffuse white matter involvement | NBD, encephalitis, SVD, CADASIL | Diffuse posterior column abnormalities | B12D, ACD |
| Cerebral venous sinus thrombosis | NBD | Other | |
| Infiltrating (large) brainstem lesions | NBD | No ‘occult’ changes in the NAWM | NMO, Lyme disease, SID (except in NSLE) |
| Anterior temporal and inferior frontal lobe involvement, associated with enhancement or mass effect | Encephalitis (HSE) | Pontine lacunar infarcts | CADASIL, SVD |
| Isolated lesions with ring enhancement (often complete) | Abscess | Dilation of Virchow–Robin spaces | HHC, PACNS |
| Mass effect | Abscess, metastasis, malignancy, tumefactive MS | Diffuse lactate increase on brain MRS | MELAS |
| Progressively enlarging lesions, asymmetric lesions (juxtacortical origin) | PML | Meningeal enhancement | Susac’s syndrome, PACNS, NBD, meningitis, Lyme disease, sarcoidosis |
| Large lesions, absent or rare mass effect | PML | Hydrocephalus | Sarcoidosis |
| Extensive and bilateral periventricular abnormalities in isolation | B12D, ACD | Absence of optic-nerve lesions | PML |
| Regional atrophy | HHC (hippocampus and amygdala), NBD (brainstem) |
ACD, acquired copper deficiency; ADEM, acute disseminated encephalomyelitis; AFL, adult forms of leukoencephalopathies; AMS, acute multiple sclerosis (Marburg type); ATM, acute transverse myelitis; B12D, vitamin B12 deficiency; BCS, Balo’s concentric sclerosis; CNS, central nervous system; CTX, cerebrotendinous xanthomatosis; FD, Fabry’s disease; HHC, hyper homocystinemia; HIVE, HIV encephalitis; HSE, herpes simplex encephalitis; MELAS, mitochondrial encephalopathy with lactic acidosis and stroke-like episodes; MRS, magnetic resonance spectroscopy; MS, multiple sclerosis; NAWM, normal-appearing white matter; NBD, Behçet’s disease with CNS involvement; NMO, neuromyelitis optica; NSLE, neuropsychiatric systemic lupus erythematosus; PACNS, primary angiitis of the CNS; PML, progressive multifocal leukoencephalopathy; SID, systemic immune-mediated disease; SSP, subacute sclerosing panencephalitis; SVD, small-vessel disease.
Neuromyelitis optica
The diagnostic criteria of NMO have been established after recognition that this was more than a variant of MS and now is recognized as a separate disease entity with an entirely different epidemiology, pathology and radiographic appearance. The breakthrough has been the discovery of a serum marker (NMO-IgG) against aquaporin 4 (AQP-4) [Lennon et al. 2004, 2005]. Although highly specific (>99%) [McKeon et al. 2009; Waters et al. 2012], sensitivity of the serological disease marker remains around 70% and depends on the performed laboratory method [Waters et al. 2012]. This is reflected in the revised diagnostic criteria; it remains a clinical diagnosis with ancillary support of radiographic and serological features. These criteria [Wingerchuk et al. 2006] include the presence of (1) clinical history of both ON and transverse myelitis, with (2) two out of the three supporting criteria (MRI evidence of longitudinally extensive transverse myelitis in >3 vertebral segments, brain MRI is nondiagnostic for MS at onset, or positive NMO-IgG testing).
NMO spinal cord lesions demonstrate a ‘swollen cord’ appearance with mass effect. Not uncommonly initially this would raise the question of neoplastic or ischemic disease. The lesions are longitudinally extensive (defined as stretching over >3 vertebral segments) and in most typical circumstances involve over half of the area on axial cuts (Figure 5A and B). This often distinguishes readily from MS-like lesions, which are smaller lesions as reviewed earlier. NMO lesions more often have central cord involvement and can affect both gray and white matter within the spinal cord. In addition, optic nerve lesions also have a tendency to be more extensive in length, often crossing the optic chiasm, a feature not commonly seen in MS-related ON. MS and NMO both have the presence of demyelination on histopathology. However, in NMO lesions there is generally more tissue destruction than what is seen in MS lesions, resulting in some features of the characteristic imaging appearance. Related to that is the finding that on average it seems that NMO attacks can be associated with more significant disability after a single attack. It is critical to differentiate NMO from MS as commonly used therapies for MS (interferon β) have been reported to possibly worsen the clinical course of NMO [Kim et al. 2012; Shimizu et al. 2010].
Figure 5.
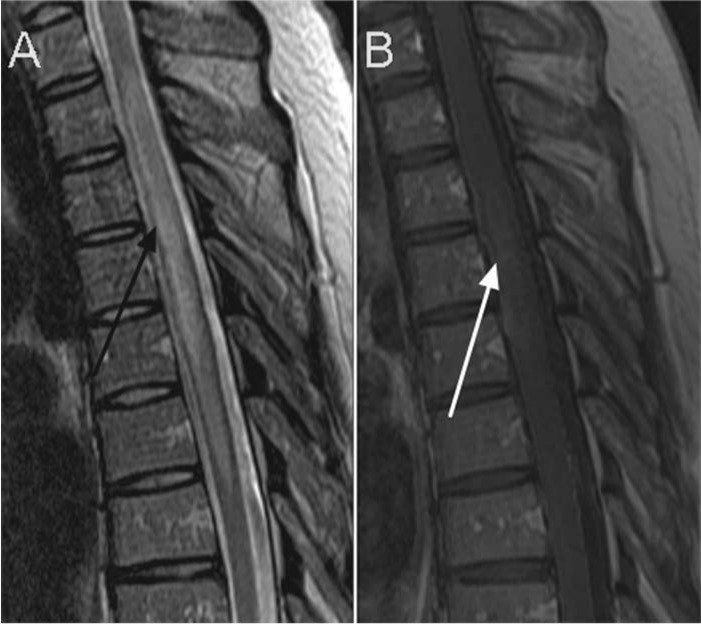
Spinal cord lesions in a patient with neuromyelitis optica (NMO). These images very nicely demonstrate the longitudinally extensive involvement of the spinal cord in this disease. In this patient this extended over a region of 5 vertebral segments as seen on the sagittal STIR image (A), with in affected area a faint, heterogeneous hyperintense signal change after gadolinium contrast administration on sagittal T1 image (B).
One of the most important revisions was to no longer exclude the presence of brain lesions as these were described to be frequently present in NMO [Pittock et al. 2006a, 2006b]. Cerebral white matter findings could even meet imaging criteria for MS, but more often are fairly nonspecific in imaging characteristics and confined to regions known to be rich in AQP-4 [Pittock et al. 2006b]. These typically include periventricular regions of the third (diencephalic) and fourth ventricle (brainstem). In comparison with MS, where abnormalities are found in the unaffected white and gray matter (normal-appearing white matter [NAWM] and normal-appearing gray matter [NAGM]), such abnormalities are not commonly found in NMO and if present seem to be in areas involved in connection to the active disease, e.g. corticospinal tract or optic radiation [Filippi et al. 1999b; Pichiecchio et al. 2012; Yu et al. 2007].
Acute disseminated encephalomyelitis
ADEM is an immune-mediated inflammatory demyelinating disorder that often is preceded by infection or vaccination and usually behaves as a monophasic disease [Leake et al. 2004; Torisu et al. 2010]. The diagnosis of ADEM has not been as rigidly reviewed and revised as those of MS and NMO. Part of this is the low incidence of the disease and its variable clinical presentation and course. While ADEM can occur at any age it is most commonly encountered in the pediatric age group. An international pediatric study group proposed clinical criteria [Krupp et al. 2007]. Part of one of the main distinguishing clinical features of ADEM that was included most commonly in clinical criteria [Krupp et al. 2007] is the presence of encephalopathy. In addition to a variable degree of encephalopathy, both clinical and imaging findings need to be consistent with polyfocal demyelinating disease. Around the same time another study group that focused more on presentation at older age (>15 years) proposed similar diagnostic criteria that could differentiate from MS: clinical symptoms atypical for MS, absence of oligoclonal bands (OCBs), and gray matter involvement [de Seze et al. 2007].
Neuroimaging of ADEM has multiple characteristic features. Brain parenchymal abnormalities are typically large, multiple, bilateral but asymmetric, poorly demarcated, areas of increased signal on T2-weighted and FLAIR sequences which can affect both white and gray matter (Figure 6). Gray matter involvement can be neocortical or deep gray matter (thalamus, basal ganglia). Brainstem and cerebellum are commonly involved [Callen et al. 2009a; Ketelslegers et al. 2010; Tenembaum et al. 2007]. Associated edema, swelling and localized mass effect can be seen in larger lesions. Enhancement is not commonly encountered (10–30%), can be marginal and/or nodular in distribution. Four imaging patterns have been proposed based on evaluation of pediatric patients with ADEM [Tenembaum et al. 2002,2007] These categories included ADEM with (1) multifocal numerous small (<5 mm) lesions, (2) large, confluent white matter lesions with frequent edema and mass effect, (3) bithalamic involvement, and (4) acute hemorrhagic encephalomyelitis (AHEM) [Tenembaum et al. 2002]. The latter is with T2* hypointensity within areas of T2 hyperintense signal change. In pediatric patients, spinal cord involvement has been reported to potentially include long segments (>3 vertebral segments) [Tenembaum et al. 2007; Yiu et al. 2009] that are NMO-IgG negative [Banwell et al. 2008].
Figure 6.
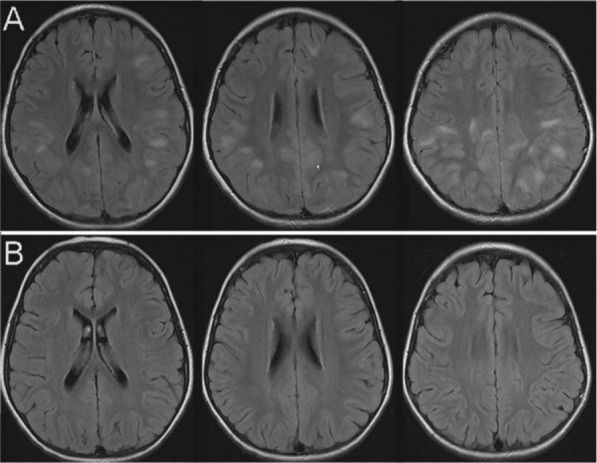
Images of acute disseminated encephalomyelitis (ADEM) in a pediatric patient. The T2 FLAIR axial images were acquired during the symptomatic phase of the disease (A) with typical poorly demarcated lesions with white matter and grey matter involvement. There was no contrast enhancement of any of these lesions (not shown). On follow up imaging three months later (B) no residual signal change is noted.
Follow-up imaging often documents resolution of the signal change abnormalities and the development of additional lesions are not compatible with a monophasic course of ADEM. Complete resolution of lesions can occur in up to half of patients with ADEM, however residual hyperintensities on T2WI likely represent gliosis and demyelination [Dale et al. 2000].
Pathologically, ADEM is different from MS and shows a striking perivenular inflammatory demyelination [Wingerchuk and Lucchinetti, 2007; Young et al. 2010]. The risk of development of MS has varied significantly in the last few decades, partly due to variable inclusion criteria that were applied. Initially, this risk was estimated to be as high as 20–30% [Mikaeloff et al. 2004, 2007; Tenembaum et al. 2002, 2007]. Using MRI to refine diagnostic criteria better to distinguish between pediatric MS and ADEM suggested the presence of two out of the following three criteria to distinguish MS from ADEM: (1) absence of a diffuse bilateral lesion distribution pattern; (2) presence of T1 hypointense lesions; (3) presence of two or more periventricular lesions. On imaging appearance alone, this was found to be 81% sensitive and 95% specific[Callen et al. 2009a], later confirmed by others [Ketelslegers et al. 2010]. Using these and the more stringent clinical criteria, a recent prospective population based study in Canada revealed conversion rates of MS in ADEM in pediatric patients as low as 3.3% [Banwell et al. 2011].
The role of MRI in diagnostic criteria of MS
Relapsing–remitting multiple sclerosis
Since the first international consensus criteria for MS that incorporated MRI criteria (also known as McDonald criteria) [McDonald et al. 2001], two subsequent revisions [Polman et al. 2005, 2011] have reflected the increased role of MRI in the diagnostic process. These criteria are based on the ‘dissemination in space’ and ‘dissemination of time’ in MS. Although MS-related pathology and MRI change can be seen anywhere in the CNS, the most commonly encountered location remains the periventricular white matter, where ovoid lesions are often observed (Dawson’s fingers). In addition to periventricular (1) lesions, juxtacortical (2), infratentorial (3) and spinal cord lesions (4) are specifically included in the 2010 dissemination in space criteria. With these current criteria, the presence of lesions in any of two of these four locations meets the ‘dissemination in space’ criteria.
The criterion for dissemination in time was simplified in most recent 2010 revision of the McDonald criteria. Where previously either new enhanced or nonenhanced new lesions needed to develop between two defined time points, now the diagnosis can be made within one single scan. Simply, the presence of asymptomatic CELs and nonenhanced T2 hyperintense lesions fulfill the prior requirement of lesions developing over a minimum of 3 months. Thus, one single MRI is sufficient to establish dissemination in time. The importance of imaging findings in the different stages of lesion formation come from prior studies showing that these findings hold up in early stages of MS and conversion of CIS [Montalban et al. 2010; Rovira et al. 2009]. The only caveat to such measures of increasing sensitivity (capturing disease earlier) is that it can be at the cost of specificity. Special care should be maintained in interpreting scans: the prior mentioned persisting gadolinium-enhanced ‘lesions’ can include other inflammatory pathologies (e.g. sarcoidosis) or occasionally observed structural anomalies (e.g. venous angiomas) could erroneously conclude that dissemination in time could be present or only related to MS. MS remains a clinical diagnosis with data derived from clinical history, neurological examination supplemented by ancillary support from both laboratory and MRI. It can and will not fully be replaced by any one of these components alone.
Primary progressive multiple sclerosis
PPMS typically presents as a slowly progressive myelopathy. As reviewed earlier, MRI features of PPMS include a cord predominant lesion pattern with minimal to no CELs in comparison with RRMS. The current (in 2010 revised) MS criteria were further simplified and require 1 year of slow clinical progression, and two out of the following three findings: (1) limited evidence of DIS in the brain (with one or more periventricular, juxtacortical and infratentorial lesion); (2) evidence of DIS in the cord with two or more characteristic T2 weighted lesions; (3) positive CSF (OCB elevation and/or IgG Index elevation).
Clinically isolated syndrome
The initial presentations of MS include well-characterized subacute onset demyelinating syndromes that can include either ON, transverse myelitis, or isolated brainstem/cerebellar syndromes, but the differential diagnosis can be broad. CIS is when there is a single clinical event that is demyelinating in nature, where the clinical and MRI criteria for dissemination in time are not met. The clinical presentation of CIS immediately raises the question whether this represents a first episode of MS and what to expect in terms of prognosis and risk of relapse. The question of evaluation of the risk of conversion to MS has been studied extensively and these studies have contributed to the revision of some of the MRI criteria to allow earlier diagnosis of MS. The most sensitive measure has been the presence of typical MS lesions on MRI at time of presentation. Most of this knowledge comes through the series of longitudinal studies from the National Hospital in London, UK and were confirmed in (usually smaller) studies [Brex et al. 2002; Chard et al. 2003; Fisniku et al. 2008; Morrissey et al. 1993; O’Riordan et al. 1998]. This has established that at different time points in follow up, the conversion risk of developing MS was significantly higher when other CNS lesions were present. It has become evident that most of the conversion takes place in the first few years after the initial demyelinating event. These time points were 1 year (30% versus 0%), 5 year (65% versus 3%), 10 year (83% versus 11%), 14 year (88% versus 19%) and 20 years (82% versus 21%). Part of some of this fluctuation is the availability of follow up [Fisniku et al. 2008]. Those that converted with an unremarkable MRI at presentation typically had a milder course of the disease. The follow up at the last time point (20-year follow up) [Fisniku et al. 2008] addressed the disability rate and in those that had converted to MS, there was a significant correlation between T2 lesion volume and EDSS, most evident in the first 5 years. This study established that change in lesion volume at earlier time points are correlated with disability at 20 years. There statistical significant difference in the rate of lesion growth over the duration of the study was 0.80 cm3/year in those patients who continued in a relapsing–remitting course, and 2.89 cm3/year in those who developed SPMS.
Radiologically isolated syndrome
RIS is an entity when the typical MRI findings of MS can be seen in entirely asymptomatic patients. These MRIs are often obtained in the work up of unrelated diseases, e.g. headache, head injury, etc. When upon further historical or and/or laboratory investigations, no other evidence can be found for demyelinating disease or other explanatory diagnoses, this finding is commonly labeled as the RIS. This is somewhat of a misnomer as patients by definition do not have any ‘syndrome’, there is no constellation of symptoms that would fit with CIS or MS. Thorough evaluation investigating other etiologies is often warranted. There is evidence that a subgroup of these patients will develop clinically definite MS, over as long as 10 years [Lebrun et al. 2009; Okuda et al. 2009; Siva et al. 2009]. In one of these series (n = 44), radiological evidence for new lesion formation was seen in 59%, only 22.8% had clinical symptoms and ‘converted’ to CIS or MS [Okuda et al. 2009]. The presence on initial imaging of CELs [Okuda et al. 2009] or asymptomatic spinal cord lesions [Okuda et al. 2011] have been reported to be predictors of clinical conversion to either CIS or MS. Extensive cognitive testing demonstrated similar cognitive abnormalities in the patients with RIS as compared with patients with MS [Lebrun et al. 2010]. Cases of RIS need radiological and clinical follow up and treatment decisions should be dependent on radiological or clinical conversion. Further efforts to improve the understanding of this entity are ongoing.
Advances in MRI in demyelinating disease
Both MRI acquisition and post-processing of MRI data have gone through tremendous improvements in the recent years. It is beyond the scope of this paper to review all research MRI sequences and processing techniques in a comprehensive matter, as currently many of these are not yet available for routine clinical practice. Despite the advances that have been made, not many have made it into clinical practice. The validation of such improved acquisitions or analysis tools in the solution to the discrepancy between clinical outcome and imaging findings will greatly advance diagnostic and therapeutic decision points. Some of the main hurdles to implementation in clinical practice are that many of these methods require pulse sequences and post-processing software that is often not installed in the standard package of MRIs. In addition, the post-processing steps are often time and labor intensive, requiring significant computational power and interpretation and can be clouded by fairly easily introduced artifacts. We will briefly highlight some of the potential advances in evaluation with the most commonly used techniques.
Volumetric studies of the brain and spinal cord have been important in detecting ongoing and early presence of atrophy in MS. Atrophic changes are most pronounced in progressive stages of advanced MS, but are detectable even in the earliest stages of the disease [Sastre-Garriga et al. 2004, 2005; Tiberio et al. 2005] (Figure 7B). In addition, atrophy keeps progressing even when new lesion formation or gadolinium enhancement are no longer detectable in progressive MS, and is overall at least partially independent of lesion load in most studies [Anderson et al. 2007; Fox et al. 2000; Jasperse et al. 2007; Losseff et al. 1996; Miller et al. 2002]. Studies have shown that this is strongly correlated with clinical disability [Bermel and Bakshi, 2006; De Stefano et al. 2007; Kutzelnigg and Lassmann, 2005; Simon, 2006; Zivadinov and Bakshi, 2004]. Improvements in automated methods to generate volumetric assessments have resulted in different software applications to study this in an organized method. In clinical practice the application is still somewhat limited despite the ready availability of this software, there have been no great studies where this is implemented in outcome measures of clinical therapeutic intervention trials. MS-related atrophy includes both gray and white matter loss, with the potential predominance of gray matter atrophy emerging in more recent studies [Pirko et al. 2007; Zivadinov and Cox, 2007].
Figure 7.
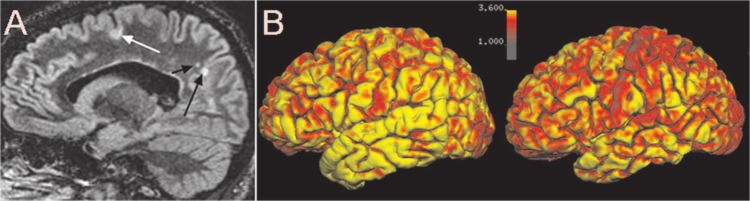
(A) Sample image of double inversion recovery imaging (DIR) demonstratting the presence of leukocortical lesion (white arrow) and typical white matter lesions (black arrows). Image (B) shows a sample of cortical thickness measurements using Freesurfer (software available on http://surfer.nmr.mgh.harvard.edu) showing a sample of a healthy control (left image) and a patient with RRMS. The color bar representing the cortical thickness goes from thin (red) to thicker cortex (yellow) measurements.
High field strength MRI has become increasingly available. These have become more widely available. Many institutions have 3 T scanners, with up to 7 T or higher becoming available in a smaller number of centers. Current FDA approval for clinical use goes up to 4 T, making the others currently only useful for research purposes with local IRB approval. The energy absorption in tissue is higher and sequences need to be specifically designed to allow the desired acquisition techniques without crossing the specific absorption rate (SAR). These sequences have been generally without described adverse events. Higher field strength results in higher signal-to-noise ratio, enabling higher-resolution acquisitions in shorter time. Along with that comes the potential of improved contrast-to-noise ratio. In MS, research studies applying higher field strength MRIs have resulted in some interesting studies, although validation of many will have to come with time. There has been the potential for improved lesion detection in white and cortical gray matter [de Graaf et al. 2012; Mistry et al. 2011; Nielsen et al. 2012], deep gray matter pathology [Lebel et al. 2012] and unusual imaging features of white matter lesions with persistent ring phase white matter lesions [Bian et al. 2012] all potentially provide additional insight in the pathology of MS, where clinical application may be still far out.
Magnetization transfer imaging (MTI) was first performed in MS patients in 1992 [Dousset et al. 1992]. An additional saturation pulse is directed at macromolecules such as myelin and subsequent transfer of magnetization to the mobile proton pool is measured. It allows the influence of these macromolecules on the local environment to be studied, thus providing a sensitive marker for tissue integrity. In lesions, the MTR is decreased proportional to the degree of tissue abnormality. In NAWM and NAGM it has been shown that the MT ratio is altered, reflecting nonlesional pathology [Chen et al. 2005; Filippi et al. 1999a; Laule et al. 2003; Rovaris et al. 2003; Santos et al. 2002]. Changes in NAWM and NAGM are often detectable very early in MS, and correlate at least moderately with future disability according to most [Rovaris et al. 2003; Santos et al. 2002; Traboulsee et al. 2002] but not all studies [Rocca et al. 2008]. MTR signal intensity may normalize as part of normal lesion evolution [Filippi and Rocca, 2004; Inglese et al. 2005] or may remain decreased [Dousset et al. 1998; Inglese et al. 2005; Oreja-Guevara et al. 2006]. The potential normalization is thought to reflect underlying tissue repair (remyelination) [Barkhof et al. 2003] and could be used potentially in studies investigating therapeutics addressing remyelination and regeneration.
Diffusion tensor imaging has found similar changes within the NAWM and NAGM. By measuring the relative displacement of free water it provides information on the directional migration of water along and perpendicular to nerve fibers. This provides a marker of white matter integrity as this is altered in injured nerve fibers. This is often measured in fractional anisotropy (FA) and mean diffusivity measures along the nerve fibers (axial diffusivity), perpendicular to the nerve fibers (radial diffusivity) or mean diffusivity measures. This method is similar to measuring changes of restricted diffusion commonly used in other pathologies (such as ischemic strokes), but in diffusion tensor imaging more gradient directions are applied, which allows for more accurate vector measurement, which is used to calculate diffusivity measures and FA. The latter is a mathematical value that describes whether the overall direction of the diffusion is linear. When the direction of movement is uniformly in the same direction (highly organized tissue in one direction) the FA is larger. When movement in all directions is equal, e.g. free water), the FA approaches zero. Therefore, white matter has higher FA values than gray matter and is highest in areas of organized parallel fiber bundles. In MS, FA values have been reported to be lower. This goes for lesional areas, NAWM and NAGM [Ciccarelli et al. 2001, 2003, 2005; Griffin et al. 2001; Werring et al. 1999]. These subtle changes can be present in the earliest stages of MS, even in pediatric patients [Tillema et al. 2012; Vishwas et al. 2009].
Double inversion recovery imaging was introduced relatively recently. The acquisition is similar to FLAIR with an additional inversion recovery pulse compared with FLAIR. These are calibrated to suppress signal arising from both CSF and white matter. The sequence has an inherent low SNR, but excellent CNR, leaving better visibility of white matter pathology and cortical lesions (Figure 7A). It has mostly been described in studies improving the detection of cortical lesions, but there is great interrater variability when different acquisition parameters are used [Geurts et al. 2011]. In a recent study comparing DIR with the gold standard, histopathology, while it did have improved detection compared with FLAIR, but still only had a sensitivity of 18% [Geurts et al. 2005; Seewann et al. 2012].
Conclusions and future directions
MRI has become the single most important paraclinical biomarker of demyelinating diseases and these are routinely used in the diagnostic process, prognosis, disease and treatment monitoring of inflammatory and demyelinating diseases. A growing body of imaging research, using advanced acquisition and post-processing techniques, has revolutionized the understanding of these diseases. Despite these enormous advances, the relative lack of specificity of standard MRI techniques and lack of clear correlation between these measures and clinical outcome stress the need for further research in this field. No paraclinical diagnostic modality can replace solid clinical judgment using all available sources, but MRI has been and will continue to represent an important supplement to the decision-making process. Validation of newer imaging acquisitions will continually result in gradual changes in the implementation of MRI in clinical practice.
Footnotes
Funding: Part of this research received funding from the NIH (R01 NS 58698), from Mayo Clinic Intramural Funds and by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS).
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Jan-Mendelt Tillema, Department of Neurology, Mayo Clinic, Rochester, MN, USA.
Istvan Pirko, Department of Neurology, Mayo Clinic, 200 First Street SW, Rochester,MN 55905, USA.
References
- Abou Zeid N., Pirko I., Erickson B., Weigand S., Thomsen K., Scheithauer B., et al. (2012) Diffusion-weighted imaging characteristics of biopsy-proven demyelinating brain lesions. Neurology 78: 1655–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Okaili R., Krejza J., Woo J., Wolf R., O’Rourke D., Judy K., et al. (2007) Intraaxial brain masses: MR imaging-based diagnostic strategy - initial experience. Radiology 243: 539–550 [DOI] [PubMed] [Google Scholar]
- Anderson V., Fernando K., Davies G., Rashid W., Frost C., Fox N., et al. (2007) Cerebral atrophy measurement in clinically isolated syndromes and relapsing remitting multiple sclerosis: a comparison of registration-based methods. J Neuroimaging 17: 61–68 [DOI] [PubMed] [Google Scholar]
- Bagnato F., Jeffries N., Richert N., Stone R., Ohayon J., McFarland H., et al. (2003) Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain 126:1782–1789 [DOI] [PubMed] [Google Scholar]
- Bakshi R., Benedict R., Bermel R., Caruthers S., Puli S., Tjoa C., et al. (2002) T2 hypointensity in the deep gray matter of patients with multiple sclerosis: a quantitative magnetic resonance imaging study. Arch Neurol 59: 62–68 [DOI] [PubMed] [Google Scholar]
- Bakshi R., Dmochowski J., Shaikh Z., Jacobs L. (2001) Gray matter T2 hypointensity is related to plaques and atrophy in the brains of multiple sclerosis patients. J Neurol Sci 185: 19–26 [DOI] [PubMed] [Google Scholar]
- Banwell B., Bar-Or A., Arnold D., Sadovnick D., Narayanan S., McGowan M., et al. (2011) Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol 10: 436–445 [DOI] [PubMed] [Google Scholar]
- Banwell B., Tenembaum S., Lennon V., Ursell E., Kennedy J., Bar-Or A., et al. (2008) Neuromyelitis optica-IgG in childhood inflammatory demyelinating CNS disorders. Neurology 70: 344–352 [DOI] [PubMed] [Google Scholar]
- Barkhof F. (2002) The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 15: 239–245 [DOI] [PubMed] [Google Scholar]
- Barkhof F., Bruck W., De Groot C., Bergers E., Hulshof S., Geurts J., et al. (2003) Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch Neurol 60: 1073–1081 [DOI] [PubMed] [Google Scholar]
- Barkhof F., Filippi M., Miller D., Scheltens P., Campi A., Polman C., et al. (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120: 2059–2069 [DOI] [PubMed] [Google Scholar]
- Batista S., Zivadinov R., Hoogs M., Bergsland N., Heininen-Brown M., Dwyer M., et al. (2012) Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis.J Neurol 259: 139–146 [DOI] [PubMed] [Google Scholar]
- Benedict R., Ramasamy D., Munschauer F., Weinstock-Guttman B., Zivadinov R. (2009) Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy.J Neurol Neurosurg Psychiatry 80: 201–206 [DOI] [PubMed] [Google Scholar]
- Bermel R., Bakshi R. (2006) The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 5: 158–170 [DOI] [PubMed] [Google Scholar]
- Bermel R., Puli S., Rudick R., Weinstock-Guttman B., Fisher E., Munschauer F., III., et al. (2005) Prediction of longitudinal brain atrophy in multiple sclerosis by gray matter magnetic resonance imaging T2 hypointensity. Arch Neurol 62: 1371–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian W., Harter K., Hammond-Rosenbluth K., Lupo J., Xu D., Kelley D., et al. (2012) A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult Scler, in press. [DOI] [PubMed] [Google Scholar]
- Bitsch A., Kuhlmann T., Stadelmann C., Lassmann H., Lucchinetti C., Bruck W. (2001) A longitudinal MRI study of histopathologically defined hypointense multiple sclerosis lesions. Ann Neurol 49: 793–796 [DOI] [PubMed] [Google Scholar]
- Bot J., Barkhof F., Lycklama a Nijeholt G., Bergers E., Polman C., Ader H., et al. (2000) Comparison of a conventional cardiac-triggered dual spin-echo and a fast STIR sequence in detection of spinal cord lesions in multiple sclerosis. Eur Radiol 10: 753–758 [DOI] [PubMed] [Google Scholar]
- Bot J., Barkhof F., Lycklama a, Nijeholt G., van Schaardenburg D., Voskuyl A., Ader H., et al. (2002) Differentiation of multiple sclerosis from other inflammatory disorders and cerebrovascular disease: value of spinal MR imaging. Radiology 223: 46–56 [DOI] [PubMed] [Google Scholar]
- Brex P., Ciccarelli O., O’Riordan J., Sailer M., Thompson A., Miller D. (2002) A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 346: 158–164 [DOI] [PubMed] [Google Scholar]
- Brex P., Molyneux P., Smiddy P., Barkhof F., Filippi M., Yousry T., et al. (2001) The effect of IFNbeta-1b on the evolution of enhancing lesions in secondary progressive MS. Neurology 57: 2185–2190 [DOI] [PubMed] [Google Scholar]
- Brex P., Parker G., Leary S., Molyneux P., Barker G., Davie C., et al. (2000) Lesion heterogeneity in multiple sclerosis: a study of the relations between appearances on T1 weighted images, T1 relaxation times, and metabolite concentrations.J Neurol Neurosurg Psychiatry 68: 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky M., Nazarian S., Orengo-Nania S., Hutton G., Buckley E., Massey E., et al. for the Optic Neuritis Study Group (2008) Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol 65: 727–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butteriss D., Ismail A., Ellison D., Birchall D. (2003) Use of serial proton magnetic resonance spectroscopy to differentiate low grade glioma from tumefactive plaque in a patient with multiple sclerosis. Br J Radiol 76: 662–665 [DOI] [PubMed] [Google Scholar]
- Callen D., Shroff M., Branson H., Li D., Lotze T., Stephens D., et al. (2009a) Role of MRI in the differentiation of ADEM from MS in children. Neurology 72: 968–973 [DOI] [PubMed] [Google Scholar]
- Callen D., Shroff M., Branson H., Lotze T., Li D., Stephens D., et al. (2009b) MRI in the diagnosis of pediatric multiple sclerosis. Neurology 72: 961–967 [DOI] [PubMed] [Google Scholar]
- Campi A., Pontesilli S., Gerevini S., Scotti G.(2000) Comparison of MRI pulse sequences for investigation of lesions of the cervical spinal cord. Neuroradiology 42: 669–675 [DOI] [PubMed] [Google Scholar]
- Caramanos Z., Francis S., Narayanan S., Lapierre Y., Arnold D. (2012) Large, nonplateauing relationship between clinical disability and cerebral white matter lesion load in patients with multiple sclerosis. Arch Neurol 69: 89–95 [DOI] [PubMed] [Google Scholar]
- Chard D., Brex P., Ciccarelli O., Griffin C., Parker G., Dalton C., et al. (2003) The longitudinal relation between brain lesion load and atrophy in multiple sclerosis: a 14 year follow up study. J Neurol Neurosurg Psychiatry 74: 1551–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charil A., Yousry T., Rovaris M., Barkhof F., De Stefano N., Fazekas F., et al. (2006) MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol 5: 841–852 [DOI] [PubMed] [Google Scholar]
- Chen J., Collins D., Freedman M., Atkins H., Arnold D. (2005) Local magnetization transfer ratio signal inhomogeneity is related to subsequent change in MTR in lesions and normal-appearing white-matter of multiple sclerosis patients. Neuroimage 25: 1272–1278 [DOI] [PubMed] [Google Scholar]
- Ciccarelli O., Giugni E., Paolillo A., Mainero C., Gasperini C., Bastianello S., et al. (1999) Magnetic resonance outcome of new enhancing lesions in patients with relapsing-remitting multiple sclerosis. Eur J Neurol 6: 455–459 [DOI] [PubMed] [Google Scholar]
- Ciccarelli O., Parker G., Toosy A., Wheeler-Kingshott C., Barker G., Boulby P., et al. (2003) From diffusion tractography to quantitative white matter tract measures: a reproducibility study. Neuroimage 18: 348–359 [DOI] [PubMed] [Google Scholar]
- Ciccarelli O., Toosy A., Hickman S., Parker G., Wheeler-Kingshott C., Miller D., et al. (2005) Optic radiation changes after optic neuritis detected by tractography-based group mapping. Hum Brain Mapp 25: 308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O., Werring D., Wheeler-Kingshott C., Barker G., Parker G., Thompson A., et al. (2001) Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 56: 926–933 [DOI] [PubMed] [Google Scholar]
- Colletti P. (2008) Nephrogenic systemic fibrosis and gadolinium: a perfect storm. AJR Am J Roentgenol 191: 1150–1153 [DOI] [PubMed] [Google Scholar]
- Cotton F., Weiner H., Jolesz F., Guttmann C.(2003) MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology 60: 640–646 [DOI] [PubMed] [Google Scholar]
- Dale R., de Sousa C., Chong W., Cox T., Harding B., Neville B. (2000) Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain 123: 2407–2422 [DOI] [PubMed] [Google Scholar]
- Daumer M., Neuhaus A., Morrissey S., Hintzen R., Ebers G. (2009) MRI as an outcome in multiple sclerosis clinical trials. Neurology 72: 705–711 [DOI] [PubMed] [Google Scholar]
- de Graaf W., Zwanenburg J., Visser F., Wattjes M., Pouwels P., Geurts J., et al. (2012) Lesion detection at seven Tesla in multiple sclerosis using magnetisation prepared 3D-FLAIR and 3D-DIR. Eur Radiol 22: 221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Seze J., Debouverie M., Zephir H., Lebrun C., Blanc F., Bourg V., et al. (2007) Acute fulminant demyelinating disease: a descriptive study of 60 patients. Arch Neurol 64: 1426–1432 [DOI] [PubMed] [Google Scholar]
- De Stefano N., Battaglini M., Smith S. (2007) Measuring brain atrophy in multiple sclerosis. J Neuroimaging 17(Suppl. 1): 10S–15S [DOI] [PubMed] [Google Scholar]
- Dousset V., Brochet B., Deloire M., Lagoarde L., Barroso B., Caille J., et al. (2006) MR imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium. AJNR Am J Neuroradiol 27: 1000–1005 [PMC free article] [PubMed] [Google Scholar]
- Dousset V., Gayou A., Brochet B., Caille J. (1998) Early structural changes in acute MS lesions assessed by serial magnetization transfer studies. Neurology 51: 1150–1155 [DOI] [PubMed] [Google Scholar]
- Dousset V., Grossman R., Ramer K., Schnall M., Young L., Gonzalez-Scarano F., et al. (1992) Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 182: 483–491 [DOI] [PubMed] [Google Scholar]
- Filippi M., Iannucci G., Tortorella C., Minicucci L., Horsfield M., Colombo B., et al. (1999a) Comparison of MS clinical phenotypes using conventional and magnetization transfer MRI. Neurology 52: 588–594 [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M. (2004) Magnetization transfer magnetic resonance imaging in the assessment of neurological diseases. J Neuroimaging 14: 303–313 [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M., Moiola L., Martinelli V., Ghezzi A., Capra R., et al. (1999b) MRI and magnetization transfer imaging changes in the brain and cervical cord of patients with Devic’s neuromyelitis optica. Neurology 53: 1705–1710 [DOI] [PubMed] [Google Scholar]
- Filippi M., Yousry T., Baratti C., Horsfield M., Mammi S., Becker C., et al. (1996) Quantitative assessment of MRI lesion load in multiple sclerosis. A comparison of conventional spin-echo with fast fluid-attenuated inversion recovery. Brain 119: 1349–1355 [DOI] [PubMed] [Google Scholar]
- Fisniku L., Brex P., Altmann D., Miszkiel K., Benton C., Lanyon R., et al. (2008) Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 131: 808–817 [DOI] [PubMed] [Google Scholar]
- Fox N., Jenkins R., Leary S., Stevenson V., Losseff N., Crum W., et al. (2000) Progressive cerebral atrophy in MS: a serial study using registered, volumetric MRI. Neurology 54: 807–812 [DOI] [PubMed] [Google Scholar]
- Gawne-Cain M., O’Riordan J., Thompson A., Moseley I., Miller D. (1997) Multiple sclerosis lesion detection in the brain: a comparison of fast fluid-attenuated inversion recovery and conventional T2-weighted dual spin echo. Neurology 49: 364–370 [DOI] [PubMed] [Google Scholar]
- Gean-Marton A., Vezina L., Marton K., Stimac G., Peyster R., Taveras J., et al. (1991) Abnormal corpus callosum: a sensitive and specific indicator of multiple sclerosis. Radiology 180: 215–221 [DOI] [PubMed] [Google Scholar]
- Geurts J., Pouwels P., Uitdehaag B., Polman C., Barkhof F., Castelijns J. (2005) Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 236: 254–260 [DOI] [PubMed] [Google Scholar]
- Geurts J., Roosendaal S., Calabrese M., Ciccarelli O., Agosta F., Chard D., et al. (2011) Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology 76: 418–424 [DOI] [PubMed] [Google Scholar]
- Griffin C., Chard D., Ciccarelli O., Kapoor B., Barker G., Thompson A., et al. (2001) Diffusion tensor imaging in early relapsing-remitting multiple sclerosis. Mult Scler 7: 290–297 [DOI] [PubMed] [Google Scholar]
- Grimaud J., Millar J., Thorpe J., Moseley I., McDonald W., Miller D. (1995) Signal intensity on MRI of basal ganglia in multiple sclerosis. J Neurol Neurosurg Psychiatry 59: 306–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman R., Braffman B., Brorson J., Goldberg H., Silberberg D., Gonzalez-Scarano F. (1988) Multiple sclerosis: serial study of gadolinium-enhanced MR imaging. Radiology 169: 117–122 [DOI] [PubMed] [Google Scholar]
- Grossman R., Gonzalez-Scarano F., Atlas S., Galetta S., Silberberg D. (1986) Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology 161: 721–725 [DOI] [PubMed] [Google Scholar]
- Haacke E., Ayaz M., Khan A., Manova E., Krishnamurthy B., Gollapalli L., et al. (2007) Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. J Magn Reson Imaging 26: 256–264 [DOI] [PubMed] [Google Scholar]
- Haemel A., Sadowski E., Shafer M., Djamali A. (2011) Update on nephrogenic systemic fibrosis: are we making progress? Int J Dermatol 50: 659–666 [DOI] [PubMed] [Google Scholar]
- Hagemeier J., Weinstock-Guttman B., Bergsland N., Brown M., Carl E., Kennedy C., et al. (2012) Iron deposition on SWI-filtered phase in the subcortical deep gray matter of patients with clinically isolated syndrome may precede structure-specific atrophy. AJNR Am J Neuroradiol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiehle J., Jr, Grossman R., Ramer K., Gonzalez-Scarano F., Cohen J. (1995) Magnetization transfer effects in MR-detected multiple sclerosis lesions: comparison with gadolinium-enhanced spin-echo images and nonenhanced T1-weighted images. AJNR Am J Neuroradiol 16: 69–77 [PMC free article] [PubMed] [Google Scholar]
- Ingle G., Sastre-Garriga J., Miller D., Thompson A. (2005) Is inflammation important in early PPMS? a longitudinal MRI study. J Neurol Neurosurg Psychiatry 76: 1255–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M., Grossman R., Filippi M. (2005) Magnetic resonance imaging monitoring of multiple sclerosis lesion evolution. J Neuroimaging 15(4 Suppl.): 22S–29S [DOI] [PubMed] [Google Scholar]
- Janardhan V., Suri S., Bakshi R. (2007) Multiple sclerosis: hyperintense lesions in the brain on nonenhanced T1-weighted MR images evidenced as areas of T1 shortening. Radiology 244: 823–831 [DOI] [PubMed] [Google Scholar]
- Jasperse B., Valsasina P., Neacsu V., Knol D., De Stefano N., Enzinger C., et al. (2007) Intercenter agreement of brain atrophy measurement in multiple sclerosis patients using manually-edited SIENA and SIENAX. J Magn Reson Imaging 26: 881–885 [DOI] [PubMed] [Google Scholar]
- Kappos L., Moeri D., Radue E., Schoetzau A., Schweikert K., Barkhof F., et al. (1999) Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet 353: 964–969 [DOI] [PubMed] [Google Scholar]
- Ketelslegers I., Neuteboom R., Boon M., Catsman-Berrevoets C., Hintzen R. (2010) A comparison of MRI criteria for diagnosing pediatric ADEM and MS. Neurology 74: 1412–1415 [DOI] [PubMed] [Google Scholar]
- Kidd D., Thorpe J., Kendall B., Barker G., Miller D., McDonald W., et al. (1996) MRI dynamics of brain and spinal cord in progressive multiple sclerosis.J Neurol Neurosurg Psychiatry 60: 15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim W., Li X., Jung I., Kim H. (2012) Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler, in press. [DOI] [PubMed] [Google Scholar]
- Krupp L., Banwell B., Tenembaum S. (2007) Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 68(16 Suppl. 2): S7–S12 [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A., Lassmann H. (2005) Cortical lesions and brain atrophy in MS. J Neurol Sci 233: 55–59 [DOI] [PubMed] [Google Scholar]
- Laule C., Vavasour I., Whittall K., Oger J., Paty D., Li D., et al. (2003) Evolution of focal and diffuse magnetisation transfer abnormalities in multiple sclerosis. J Neurol 250: 924–931 [DOI] [PubMed] [Google Scholar]
- Leake J., Albani S., Kao A., Senac M., Billman G., Nespeca M., et al. (2004) Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infectious Disease J 23: 756–764 [DOI] [PubMed] [Google Scholar]
- Lebel R., Eissa A., Seres P., Blevins G., Wilman A. (2012) Quantitative high-field imaging of sub-cortical gray matter in multiple sclerosis. Mult Scler 18: 433–441 [DOI] [PubMed] [Google Scholar]
- Lebrun C., Bensa C., Debouverie M., Wiertlevski S., Brassat D., de Seze J., et al. (2009) Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: follow-up of 70 patients. Arch Neurol 66: 841–846 [DOI] [PubMed] [Google Scholar]
- Lebrun C., Blanc F., Brassat D., Zephir H., de Seze J. CFSEP (2010) Cognitive function in radiologically isolated syndrome. Mult Scler 16: 919–925 [DOI] [PubMed] [Google Scholar]
- Leist T., Gobbini M., Frank J., McFarland H.(2001) Enhancing magnetic resonance imaging lesions and cerebral atrophy in patients with relapsing multiple sclerosis. Arch Neurol 58: 57–60 [DOI] [PubMed] [Google Scholar]
- Lennon V., Kryzer T., Pittock S., Verkman A., Hinson S. (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202: 473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V., Wingerchuk D., Kryzer T., Pittock S., Lucchinetti C., Fujihara K., et al. (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364: 2106–2112 [DOI] [PubMed] [Google Scholar]
- Levesque I., Sled J., Narayanan S., Santos A., Brass S., Francis S., et al. (2005) The role of edema and demyelination in chronic T1 black holes: a quantitative magnetization transfer study. J Magn Reson Imaging 21: 103–110 [DOI] [PubMed] [Google Scholar]
- Losseff N., Miller D., Kidd D., Thompson A.(2001a) The predictive value of gadolinium enhancement for long term disability in relapsing–remitting multiple sclerosis - preliminary results. Mult Scler 7: 23–25 [DOI] [PubMed] [Google Scholar]
- Losseff N., Wang L., Lai H., Yoo D., Gawne-Cain M., McDonald W., et al. (1996) Progressive cerebral atrophy in multiple sclerosis. A serial MRI study. Brain 119: 2009–2019 [DOI] [PubMed] [Google Scholar]
- Losseff N., Wang L., Miller D., Thompson A.(2001b) T1 hypointensity of the spinal cord in multiple sclerosis. J Neurol 248: 517–521 [DOI] [PubMed] [Google Scholar]
- Lucchinetti C., Gavrilova R., Metz I., Parisi J., Scheithauer B., Weigand S., et al. (2008) Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 131: 1759–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C., Popescu B., Bunyan R., Moll N., Roemer S., Lassmann H., et al. (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365: 2188–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masdeu J., Moreira J., Trasi S., Visintainer P., Cavaliere R., Grundman M. (1996) The open ring. A new imaging sign in demyelinating disease.J Neuroimaging 6: 104–107 [DOI] [PubMed] [Google Scholar]
- Masdeu J., Quinto C., Olivera C., Tenner M., Leslie D., Visintainer P. (2000) Open-ring imaging sign: highly specific for atypical brain demyelination. Neurology 54: 1427–1433 [DOI] [PubMed] [Google Scholar]
- McDonald W., Compston A., Edan G., Goodkin D., Hartung H., Lublin F., et al. (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 50: 121–127 [DOI] [PubMed] [Google Scholar]
- McKeon A., Fryer J., Apiwattanakul M., Lennon V., Hinson S., Kryzer T., et al. (2009) Diagnosis of neuromyelitis spectrum disorders: comparative sensitivities and specificities of immunohistochemical and immunoprecipitation assays. Arch Neurol 66: 1134–1138 [DOI] [PubMed] [Google Scholar]
- Mikaeloff Y., Adamsbaum C., Husson B., Vallee L., Ponsot G., Confavreux C., et al. (2004) MRI prognostic factors for relapse after acute CNS inflammatory demyelination in childhood. Brain 127: 1942–1947 [DOI] [PubMed] [Google Scholar]
- Mikaeloff Y., Caridade G., Husson B., Suissa S., Tardieu M. (2007) Acute disseminated encephalomyelitis cohort study: prognostic factors for relapse. Eur J Paediatric Neurol 11(2): 90–95 [DOI] [PubMed] [Google Scholar]
- Miller D., Barkhof F., Frank J., Parker G., Thompson A. (2002) Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125: 1676–1695 [DOI] [PubMed] [Google Scholar]
- Miller D., Rudge P., Johnson G., Kendall B., Macmanus D., Moseley I., et al. (1988) Serial gadolinium enhanced magnetic resonance imaging in multiple sclerosis. Brain 111: 927–939 [DOI] [PubMed] [Google Scholar]
- Minneboo A., Uitdehaag B., Ader H., Barkhof F., Polman C., Castelijns J. (2005) Patterns of enhancing lesion evolution in multiple sclerosis are uniform within patients. Neurology 65: 56–61 [DOI] [PubMed] [Google Scholar]
- Mistry N., Tallantyre E., Dixon J., Galazis N., Jaspan T., Morgan P., et al. (2011) Focal multiple sclerosis lesions abound in ‘normal appearing white matter’. Mult Scler 17: 1313–1323 [DOI] [PubMed] [Google Scholar]
- Molyneux P., Filippi M., Barkhof F., Gasperini C., Yousry T., Truyen L., et al. (1998) Correlations between monthly enhanced MRI lesion rate and changes in T2 lesion volume in multiple sclerosis. Ann Neurol 43: 332–339 [DOI] [PubMed] [Google Scholar]
- Montalban X., Tintore M., Swanton J., Barkhof F., Fazekas F., Filippi M., et al. (2010) MRI criteria for MS in patients with clinically isolated syndromes. Neurology 74: 427–434 [DOI] [PubMed] [Google Scholar]
- Morgen K., Jeffries N., Stone R., Martin R., Richert N., Frank J., et al. (2001) Ring-enchancement in multiple sclerosis: marker of disease severity. Mult Scler 7: 167–171 [DOI] [PubMed] [Google Scholar]
- Morrissey S., Miller D., Kendall B., Kingsley D., Kelly M., Francis D., et al. (1993) The significance of brain magnetic resonance imaging abnormalities at presentation with clinically isolated syndromes suggestive of multiple sclerosis. A 5-year follow-up study. Brain 116: 135–146 [DOI] [PubMed] [Google Scholar]
- Moseley I., Miller D., Gass A. (1998) The contribution of magnetic resonance imaging to the assessment of optic nerve and spinal cord involvement in multiple sclerosis. J Neurol Neurosurg Psychiatry 64(Suppl. 1): S15–S20 [PubMed] [Google Scholar]
- Naismith R., Xu J., Tutlam N., Snyder A., Benzinger T., Shimony J., et al. (2009) Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology 72: 589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith R., Xu J., Tutlam N., Trinkaus K., Cross A., Song S. (2010) Radial diffusivity in remote optic neuritis discriminates visual outcomes. Neurology 74: 1702–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A., Kinkel R., Tinelli E., Benner T., Cohen-Adad J., Mainero C. (2012) Focal cortical lesion detection in multiple sclerosis: 3 Tesla DIR versus 7 Tesla FLASH-T2. J Magnet Resonance Imaging 35: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy J., Lucchinetti C., Rodriguez M., Weinshenker B. (2000) Multiple sclerosis. N Engl J Med 343: 938–952 [DOI] [PubMed] [Google Scholar]
- O’Riordan J., Thompson A., Kingsley D., MacManus D., Kendall B., Rudge P., et al. (1998) The prognostic value of brain MRI in clinically isolated syndromes of the CNS. A 10-year follow-up. Brain 121: 495–503 [DOI] [PubMed] [Google Scholar]
- Offenbacher H., Fazekas F., Schmidt R., Freidl W., Flooh E., Payer F., et al. (1993) Assessment of MRI criteria for a diagnosis of MS. Neurology 43: 905–909 [DOI] [PubMed] [Google Scholar]
- Okuda D., Mowry E., Beheshtian A., Waubant E., Baranzini S., Goodin D., et al. (2009) Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 72: 800–805 [DOI] [PubMed] [Google Scholar]
- Okuda D., Mowry E., Cree B., Crabtree E., Goodin D., Waubant E., et al. (2011) Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 76: 686–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreja-Guevara C., Charil A., Caputo D., Cavarretta R., Sormani M., Filippi M. (2006) Magnetization transfer magnetic resonance imaging and clinical changes in patients with relapsing-remitting multiple sclerosis. Arch Neurol 63: 736–740 [DOI] [PubMed] [Google Scholar]
- Paty D., Li D. (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 43: 662–667 [DOI] [PubMed] [Google Scholar]
- Pichiecchio A., Tavazzi E., Poloni G., Ponzio M., Palesi F., Pasin M., et al. (2012) Advanced magnetic resonance imaging of neuromyelitis optica: a multiparametric approach. Mult Scler 18: 817–824 [DOI] [PubMed] [Google Scholar]
- Pirko I., Fricke S., Johnson A., Rodriguez M., Macura S. (2005) Magnetic resonance imaging, microscopy, and spectroscopy of the central nervous system in experimental animals. NeuroRx 2: 250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirko I., Johnson A., Ciric B., Gamez J., Macura S., Pease L., et al. (2004) In vivo magnetic resonance imaging of immune cells in the central nervous system with superparamagnetic antibodies. Faseb J 18: 179–182 [DOI] [PubMed] [Google Scholar]
- Pirko I., Lucchinetti C., Sriram S., Bakshi R. (2007) Gray matter involvement in multiple sclerosis. Neurology 68: 634–642 [DOI] [PubMed] [Google Scholar]
- Pittock S., Lennon V., Krecke K., Wingerchuk D., Lucchinetti C., Weinshenker B. (2006a) Brain abnormalities in neuromyelitis optica. Arch Neurol 63: 390–396 [DOI] [PubMed] [Google Scholar]
- Pittock S., Weinshenker B., Lucchinetti C., Wingerchuk D., Corboy J., Lennon V. (2006b) Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 63: 964–968 [DOI] [PubMed] [Google Scholar]
- Polman C., Reingold S., Banwell B., Clanet M., Cohen J., Filippi M., et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69: 292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C., Reingold S., Edan G., Filippi M., Hartung H., Kappos L., et al. (2005a) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58: 840–846 [DOI] [PubMed] [Google Scholar]
- Popescu B., Lucchinetti C. (2012) Meningeal and cortical grey matter pathology in multiple sclerosis. BMC Neurol 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M., Agosta F., Sormani M., Fernando K., Tintore M., Korteweg T., et al. (2008) A three-year, multi-parametric MRI study in patients at presentation with CIS. J Neurol, in press. [DOI] [PubMed] [Google Scholar]
- Rovaris M., Agosta F., Sormani M., Inglese M., Martinelli V., Comi G., et al. (2003) Conventional and magnetization transfer MRI predictors of clinical multiple sclerosis evolution: a medium-term follow-up study. Brain 126: 2323–2332 [DOI] [PubMed] [Google Scholar]
- Rovira A., Swanton J., Tintore M., Huerga E., Barkhof F., Filippi M., et al. (2009) A single, early magnetic resonance imaging study in the diagnosis of multiple sclerosis. Arch Neurol 66: 587–592 [DOI] [PubMed] [Google Scholar]
- Sadowski E., Bennett L., Chan M., Wentland A., Garrett A., Garrett R., et al. (2007) Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 243: 148–157 [DOI] [PubMed] [Google Scholar]
- Sailer M., O’Riordan J., Thompson A., Kingsley D., MacManus D., McDonald W., et al. (1999) Quantitative MRI in patients with clinically isolated syndromes suggestive of demyelination. Neurology 52: 599–606 [DOI] [PubMed] [Google Scholar]
- Saindane A., Cha S., Law M., Xue X., Knopp E., Zagzag D. (2002) Proton MR spectroscopy of tumefactive demyelinating lesions. AJNR Am J Neuroradiol 23: 1378–1386 [PMC free article] [PubMed] [Google Scholar]
- Santos A., Narayanan S., de Stefano N., Tartaglia M., Francis S., Arnaoutelis R., et al. (2002) Magnetization transfer can predict clinical evolution in patients with multiple sclerosis. J Neurol 249: 662–668 [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J., Ingle G., Chard D., Cercignani M., Ramio-Torrenta L., Miller D., et al. (2005) Grey and white matter volume changes in early primary progressive multiple sclerosis: a longitudinal study. Brain 128: 1454–1460 [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J., Ingle G., Chard D., Ramio-Torrenta L., Miller D., Thompson A. (2004) Grey and white matter atrophy in early clinical stages of primary progressive multiple sclerosis. Neuroimage 22: 353–359 [DOI] [PubMed] [Google Scholar]
- Seewann A., Kooi E., Roosendaal S., Pouwels P., Wattjes M., van der Valk P., et al. (2012) Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology 78: 302–308 [DOI] [PubMed] [Google Scholar]
- Shimizu J., Hatanaka Y., Hasegawa M., Iwata A., Sugimoto I., Date H., et al. (2010) IFNbeta-1b May severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology 75:1423–1427 [DOI] [PubMed] [Google Scholar]
- Silver N., Lai M., Symms M., Barker G., McDonald I., Miller D. (1999) Serial gadolinium-enhanced and magnetization transfer imaging to investigate the relationship between the duration of blood-brain barrier disruption and extent of demyelination in new multiple sclerosis lesions.J Neurol 246: 728–730 [DOI] [PubMed] [Google Scholar]
- Simon J. (2006) Brain atrophy in multiple sclerosis: what we know and would like to know. Mult Scler 12: 679–687 [DOI] [PubMed] [Google Scholar]
- Siva A., Saip S., Altintas A., Jacob A., Keegan B., Kantarci O. (2009) Multiple sclerosis risk in radiologically uncovered asymptomatic possible inflammatory-demyelinating disease. Mult Scler 15: 918–927 [DOI] [PubMed] [Google Scholar]
- Stevenson V., Gawne-Cain M., Barker G., Thompson A., Miller D. (1997) Imaging of the spinal cord and brain in multiple sclerosis: a comparative study between fast FLAIR and fast spin echo. J Neurol 244: 119–124 [DOI] [PubMed] [Google Scholar]
- Tenembaum S., Chamoles N., Fejerman N. (2002) Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology 59: 1224–1231 [DOI] [PubMed] [Google Scholar]
- Tenembaum S., Chitnis T., Ness J., Hahn J.(2007) Acute disseminated encephalomyelitis. Neurology 68(16 Suppl. 2): S23–S36 [DOI] [PubMed] [Google Scholar]
- Thompson A., Kermode A., Wicks D., MacManus D., Kendall B., Kingsley D., et al. (1991) Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol 29: 53–62 [DOI] [PubMed] [Google Scholar]
- Thompson A., Miller D., Youl B., MacManus D., Moore S., Kingsley D., et al. (1992) Serial gadolinium-enhanced MRI in relapsing/remitting multiple sclerosis of varying disease duration. Neurology 42: 60–63 [DOI] [PubMed] [Google Scholar]
- Thorpe J., Kidd D., Moseley I., Kenndall B., Thompson A., MacManus D., et al. (1996) Serial gadolinium-enhanced MRI of the brain and spinal cord in early relapsing-remitting multiple sclerosis. Neurology 46: 373–378 [DOI] [PubMed] [Google Scholar]
- Tiberio M., Chard D., Altmann D., Davies G., Griffin C., Rashid W., et al. (2005) Gray and white matter volume changes in early RRMS: a 2-year longitudinal study. Neurology 64: 1001–1007 [DOI] [PubMed] [Google Scholar]
- Tillema J., Leach J., Pirko I. (2012) Non-lesional white matter changes in pediatric multiple sclerosis and monophasic demyelinating disorders. Mult Scler, in press. [DOI] [PubMed] [Google Scholar]
- Tjoa C., Benedict R., Weinstock-Guttman B., Fabiano A., Bakshi R. (2005) MRI T2 hypointensity of the dentate nucleus is related to ambulatory impairment in multiple sclerosis. J Neurol Sci 234: 17–24 [DOI] [PubMed] [Google Scholar]
- Torisu H., Kira R., Ishizaki Y., Sanefuji M., Yamaguchi Y., Yasumoto S., et al. (2010) Clinical study of childhood acute disseminated encephalomyelitis, multiple sclerosis, and acute transverse myelitis in Fukuoka Prefecture, Japan. Brain Developm 32: 454–462 [DOI] [PubMed] [Google Scholar]
- Traboulsee A., Dehmeshki J., Brex P., Dalton C., Chard D., Barker G., et al. (2002) Normal-appearing brain tissue MTR histograms in clinically isolated syndromes suggestive of MS. Neurology 59: 126–128 [DOI] [PubMed] [Google Scholar]
- Transverse Myelitis Consortium Working Group (2002) Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 59: 499–505 [DOI] [PubMed] [Google Scholar]
- Truyen L., van Waesberghe J., van Walderveen M., van Oosten B., Polman C., Hommes O., et al. (1996) Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology 47: 1469–1476 [DOI] [PubMed] [Google Scholar]
- Tubridy N., Coles A., Molyneux P., Compston D., Barkhof F., Thompson A., et al. (1998) Secondary progressive multiple sclerosis: the relationship between short-term MRI activity and clinical features. Brain 121: 225–231 [DOI] [PubMed] [Google Scholar]
- Uhlenbrock D., Sehlen S. (1989) The value of T1-weighted images in the differentiation between MS, white matter lesions, and subcortical arteriosclerotic encephalopathy (SAE). Neuroradiology 31: 203–212 [DOI] [PubMed] [Google Scholar]
- van Waesberghe J., Kamphorst W., De Groot C., van Walderveen M., Castelijns J., Ravid R., et al. (1999) Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 46: 747–754 [DOI] [PubMed] [Google Scholar]
- van Walderveen M., Barkhof F., Hommes O., Polman C., Tobi H., Frequin S., et al. (1995) Correlating MRI and clinical disease activity in multiple sclerosis: relevance of hypointense lesions on short-TR/short-TE (T1-weighted) spin-echo images. Neurology 45: 1684–1690 [DOI] [PubMed] [Google Scholar]
- van Walderveen M., Kamphorst W., Scheltens P., van Waesberghe J., Ravid R., Valk J., et al. (1998) Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology 50: 1282–1288 [DOI] [PubMed] [Google Scholar]
- van Walderveen M., Lycklama A., Ader H., Jongen P., Polman C., Castelijns J., et al. (2001) Hypointense lesions on T1-weighted spin-echo magnetic resonance imaging: relation to clinical characteristics in subgroups of patients with multiple sclerosis. Arch Neurol 58: 76–81 [DOI] [PubMed] [Google Scholar]
- Vellinga M., Oude Engberink R., Seewann A., Pouwels P., Wattjes M., van der Pol S., et al. (2008) Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement. Brain 131: 800–807 [DOI] [PubMed] [Google Scholar]
- Vishwas M., Chitnis T., Pienaar R., Healy B., Grant P. (2009) Tract-based analysis of callosal, projection, and association pathways in pediatric patients with multiple sclerosis: a preliminary study. AJNR Am J Neuroradiol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters P., McKeon A., Leite M., Rajasekharan S., Lennon V., Villalobos A., et al. (2012) Serologic diagnosis of NMO: A multicenter comparison of aquaporin-4-IgG assays. Neurology 78: 665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werring D., Clark C., Barker G., Thompson A., Miller D. (1999) Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 52: 1626–1632 [DOI] [PubMed] [Google Scholar]
- Wingerchuk D., Hogancamp W., O’Brien P, Weinshenker B. (1999) The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 53: 1107–1114 [DOI] [PubMed] [Google Scholar]
- Wingerchuk D., Lennon V., Pittock S., Lucchinetti C., Weinshenker B. (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66: 1485–1489 [DOI] [PubMed] [Google Scholar]
- Wingerchuk D., Lucchinetti C. (2007) Comparative immunopathogenesis of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. Curr Opin Neurol 20: 343–350 [DOI] [PubMed] [Google Scholar]
- Yiu E., Kornberg A., Ryan M., Coleman L., Mackay M. (2009) Acute transverse myelitis and acute disseminated encephalomyelitis in childhood: spectrum or separate entities? J Child Neurol 24: 287–296 [DOI] [PubMed] [Google Scholar]
- Young N., Weinshenker B., Parisi J., Scheithauer B., Giannini C., Roemer S., et al. (2010) Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain 133: 333–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Zhu C., Li K., Xuan Y., Qin W., Sun H., et al. (2007) Relapsing neuromyelitis optica and relapsing-remitting multiple sclerosis: differentiation at diffusion-tensor MR imaging of corpus callosum. Radiology 244: 249–256 [DOI] [PubMed] [Google Scholar]
- Zhou F., Shiroishi M., Gong H., Zee C. (2010) Multiple sclerosis: hyperintense lesions in the brain on T1-weighted MR images assessed by diffusion tensor imaging. J Magnet Resonance Imaging 31: 789–795 [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Bakshi R. (2004) Role of MRI in multiple sclerosis II: brain and spinal cord atrophy. Front Biosci 9: 647–664 [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Cox J. (2007) Neuroimaging in multiple sclerosis. Int Rev Neurobiol 79: 449–474 [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Leist T. (2005) Clinical-magnetic resonance imaging correlations in multiple sclerosis. J Neuroimaging 15(4 Suppl.): 10S–21S [DOI] [PubMed] [Google Scholar]


