Abstract
Respiratory muscle remodeling occurs in human sleep apnea—a common respiratory disorder characterized by chronic intermittent hypoxia (CIH) due to recurrent apnea during sleep. We sought to determine if CIH causes remodeling in rat sternohyoid (upper airway dilator) and diaphragm muscles. Adult male Wistar rats were exposed to CIH (n=8), consisting of 90 sec of hypoxia (5% at the nadir; SaO2 ~80%)/90 sec of normoxia, 8 hr per day, for 7 consecutive days. Sham animals (n=8) were exposed to alternating air/air cycles in parallel. The effect of CIH on myosin heavy-chain (MHC) isoform (1, 2a, 2x, 2b) distribution, sarcoplasmic reticulum calcium ATPase (SERCA) isoform distribution, succinate dehydrogenase activity, glycerol phosphate dehydrogenase activity, and Na+/K+ ATPase pump content was determined. Sternohyoid muscle structure was unaffected by CIH treatment. CIH did not alter oxidative/glycolytic capacity or the Na+/K+-ATPase pump content of the diaphragm. CIH significantly increased the areal density of MHC 2b fibers in the rat diaphragm, and this was associated with a shift in SERCA proteins from SERCA2 to SERCA1. We conclude that CIH causes a slow-to-fast fiber transition in the rat diaphragm after just 7 days of treatment. Respiratory muscle functional remodeling may drive aberrant functional plasticity such as decreased muscle endurance, which is a feature of human sleep apnea.
Keywords: respiratory muscles, obstructive sleep apnea, chronic intermittent hypoxia, myosin heavy chain isoform distribution, diaphragm dysfunction
Obstructive sleep apnea (OSA) is the most common form of sleep-disordered breathing, affecting approximately 2% to 4% of the adult population (Young et al. 1997). OSA is characterized by recurrent collapse of the upper airway during sleep, leading to repeated bouts of airway occlusion and cessation of breathing (White 1995). These apneic patients have a wide spectrum of disorders, including cardiovascular, metabolic, and neurocognitive dysfunctions (Bradley 1992; Peppard et al. 2000; Chervin et al. 2002; Coughlin et al. 2004; McNicholas and Ryan 2006; Verstraeten 2007; Levy et al. 2009; Butt et al. 2010; Dempsey et al. 2010; Drager et al. 2010). Chronic intermittent hypoxia (CIH) is a central feature of OSA due to recurrent hypoxia/reoxygenation cycles that occur throughout the night. Evidence suggests that CIH may be the major culprit in the development of key morbidities associated with OSA (Brooks et al. 1997; Fletcher et al. 1992; Fletcher 2000; Gozal et al. 2003; Row et al. 2003; Bradford 2004; Veasey et al. 2004).
Skeletal muscle is recognized as a highly adaptable and malleable tissue that can change its phenotype in response to many environmental and physiological challenges (Fitts et al. 2001; Oliven et al. 2001; Sieck and Regnier 2001; Baldwin and Haddad 2002; Hawley 2002; Rhee et al. 2004; D’Antona et al. 2006; Degens and Alway 2006; Matsakas and Patel 2009). Like other skeletal muscles, respiratory muscles are highly specialized for their specific functional tasks but also show a large capacity for remodeling (van Lunteren et al. 1995; Cantillon and Bradford 1998; O’Halloran et al. 2002; Polla et al. 2004; Bradford et al. 2005; Rowley et al. 2005). OSA patients (Griggs et al. 1989; Series et al. 1995; Series et al. 1996; Carrera et al. 1999; Chien et al. 2010) and animal models of CIH (McGuire et al. 2002a; McGuire et al. 2002b; McGuire et al. 2003; Liu SS et al. 2005; Pae et al. 2005; Farkas et al. 2007; Dunleavy et al. 2008; Liu YH et al. 2009; Skelly et al. 2012) show signs of respiratory muscle dysfunction.
Skeletal muscles involved in respiration can be broadly categorized as thoracic pump muscles and striated muscles that modulate upper airway caliber. The diaphragm, innervated by the phrenic nerves, is the main inspiratory pump muscle and acts in concert with the pharyngeal respiratory muscles to maintain airway patency. More than 20 pharyngeal muscles are involved in respiratory and non-respiratory (swallowing, speech, mastication) functions (Horner 2009). Pharyngeal dilator muscles are a subset of these upper airway muscles and are critical for maintaining airway patency (Roberts et al. 1984; Horner and Guz 1991; van Lunteren and Dick 1992). The sternohyoid is an important upper airway dilator muscle. The sternohyoids are paired muscles running longitudinally from the sternum to the hyoid bone. During inspiration, the sternohyoid muscles display phasic activity (Roberts et al. 1984) and facilitate airway patency by ventral movement of the hyoid bone (Van de Graaff et al. 1984).
Upper airway (UA) muscle dysfunction may increase the vulnerability of the UA to collapse in OSA patients, thus causing further hypoxic insult, leading to increased muscle fatigue and a greater susceptibility to collapse. In addition, diaphragm muscle dysfunction may also be implicated in the pathophysiology of OSA. Patients with OSA are subjected to repetitive collapse of the UA and subsequent asphyxia, which leads to greater respiratory effort and thus increases the risk of inspiratory muscle fatigue. Reduced muscle endurance is often associated with a transition from a slow oxidative fatigue-resistant fiber to fast glycolytic fatigable muscle (McGuire et al. 2002a; Pae et al. 2005; Ray et al. 2007; Liu YH et al. 2009). Therefore, we sought to examine the effect of CIH treatment on the activity of two key metabolic enzymes (succinate dehydrogenase [SDH] and glycerol phosphate dehydrogenase [GPDH]) and myosin heavy-chain (MHC) isoform composition in a rat model of sleep-disordered breathing.
Calcium homeostasis and membrane excitability are important modulators of muscle function. Sarcoplasmic reticulum calcium ATPase (SERCA) pumps sequester calcium into the sarcoplasmic reticulum, thus facilitating muscle relaxation. There is evidence that altered SERCA function is associated with skeletal muscle fatigue (Aubier and Viires 1998; Tupling 2004; Allen et al. 2008). In addition, the Na+/K+ ATPase pump plays a major role in maintaining membrane excitability during contractility, and pump function correlates with muscle endurance (Leppik et al. 2004; Petersen et al. 2005; McKenna et al. 2006; Murphy and Clausen 2007). Moreover, a recent study demonstrated that pump content was altered in chronic hypoxic rat diaphragms (McMorrow et al. 2011). Therefore, we examined the effect of CIH on the relative distribution of SERCA1 and SERCA2 isoforms and Na+/K+ ATPase pump content in rat respiratory muscles.
Materials and Methods
In Vivo Protocol
All appropriate measures were undertaken to minimize any pain or discomfort caused to the animals during these experiments in accordance with institutional guidelines and national legislation. Adult male Wistar rats (Harlan, Oxfordshire, UK) were placed in environmental chambers with free access to food and water. Animals (n=8) were exposed to alternating cycles of 90 sec of normoxia/90 sec of hypoxia (5% oxygen at the nadir; SaO2 ~80%) for 8 hr per day (during the light cycle) for 1 week. Each hypoxic/reoxygenation cycle lasted 180 sec (i.e., 20 cycles per hour). Sham animals (n=8) were placed in environmental chambers and exposed to alternating cycles of normoxia/normoxia under identical experimental conditions in studies conducted in parallel.
Following exposures, the animals were anaesthetized with 5% isoflurane and killed humanely by spinal transection. Respiratory muscles (sternohyoid and diaphragm) and limb muscles (extensor digitorum longus [EDL] and soleus) were excised and snap frozen in isopentane cooled in liquid nitrogen. Samples were stored at –80C for histochemistry (measurement of SDH and GPDH activities), immunofluorescence (MHC fiber typing, SERCA), and Na+/K+-ATPase pump content analysis.
SDH and GPDH Protocol
The 10-µm serial transverse sections were cryosectioned (Model CM30505; Leica Microsystems, Nussloch, Germany) at –22C and mounted on polylysine-coated glass slides (VWR International, Dublin, Ireland). Muscle sections were stained histochemically for the mitochondrial enzyme SDH at 37C for 9 min. SDH activity was determined using an incubation solution containing sodium succinate and nitro blue tetrazolium chloride (NBT) in phosphate buffer brought to pH 7.4 using drops of NaOH (1 M). Different muscle sections were stained for the mitochondrial enzyme GPDH. Muscle sections were incubated for 20 min at 37C in a solution (pH 7.4) containing DL-3-glycerophosphate (5 mM), 0.01% menadione, NBT (0.5 mM), and phosphate buffer (0.2 mM). Following the respective incubation periods, slides were removed and the solution was drained and fresh ice-cold deionized water was added to stop the histochemical reaction. Control (blank) reactions were run in parallel, where the substrates succinate or glycerophosphate were omitted. All slides were then dehydrated in a graded series of acetone and methanol rinses. Slides were then passed through xylene and cover-slipped.
Immunofluorescence
MHC Isoform Composition
Serial transverse muscles sections (10 µm) were cryosectioned and mounted on polylysine-coated glass slides. Slides were washed in PBS (phosphate-buffered solution, 0.01 M) and then placed in a 1% solution of bovine serum albumin (BSA) for 15 min. Samples were then rinsed with PBS and subsequently washed in PBS and goat serum (5%) for another 15 min. Slides were rinsed once more in PBS. Primary monoclonal myosin antibodies, developed by S. Schiaffino (Venetian Institute of Molecular Medicine, University of Padova), were obtained from the Developmental Studies Hybridoma Bank (DSHB) at the University of Iowa, and a rabbit anti–laminin antibody was purchased from Sigma-Aldrich (St. Louis, MO; L9393). We used two approaches to tag the principal MHC isoforms and laminin (a basement membrane protein). A double-labeled approach was applied to target all fibers except for MHC 2x (mouse IgG1 primary antibody, clone number BF35; 1:500), whereas rabbit anti–laminin antibody (1:500) was used to tag for laminin. On separate sections, a triple-labeled approach was used to tag for MHC 1, 2a, and 2b isoforms. The cocktail of antibodies used for this approach consisted of mouse anti–myosin antibodies: type 1 IgG2b (clone number BAF8, 1:100), type 2a IgG1 (clone number Sc71, 1:100), and type 2b IgM (clone number BFF3, 1:25). Muscle sections were incubated with the primary antibodies in a humidity chamber overnight at 4C. Upon removal, slides were washed (3 × 5 min) in PBS before application of secondary antibodies. Dylight594-conjugated goat anti–mouse IgG1 (1:500; Jackson ImmunoResearch Europe Ltd, Suffolk, UK) and FITC-conjugated anti–rabbit secondary antibody (1:250; Sigma-Aldrich) were applied to the double-labeled muscle sections. Secondary antibodies applied to the triple-labeled sections included an Alexa Fluor 350–conjugated goat anti–mouse IgG2b (1:500; Invitrogen, Biosciences Ltd, Dublin, Ireland), a Dylight594-conjugated goat anti–mouse IgG1 (1:500; Jackson ImmunoResearch Europe Ltd), and an Alexa Fluor 488–conjugated goat anti–mouse IgM (1:250; Invitrogen). Negative control experiments were run in parallel where the primary antibody was omitted and the secondary antibody was applied to ensure that tagging of antibodies was specific. Images were merged using Adobe Photoshop (Adobe; San Jose, CA).
SERCA Isoform Distribution
Diaphragm muscle sections (10 µm) from sham and CIH-treated animals were incubated in 1% BSA in PBS for 1 hr and then washed with PBS. Primary mouse IgG SERCA2 antibody (1:500, cat. MA3-919; Affinity BioReagents, Golden, CO) and primary mouse IgG SERCA1 antibody (1:500, cat. MA3-911; Affinity BioReagents) were applied to separate muscle sections. Various sections were also co-tagged with rabbit anti–laminin antibody (1:500). The slides were placed in a humidity chamber overnight at 4C. Muscle sections were washed (3 × 5 min) in PBS and secondary antibodies were applied. SERCA1 and SERCA2 labeling was visualized with a Dylight594-conjugated goat anti–mouse IgG1 (1:500; Jackson ImmunoResearch Europe Ltd), whereas a FITC-conjugated anti–rabbit secondary antibody (1:250; Sigma-Aldrich) highlighted laminin. Negative control experiments, where the primary antibody was omitted, were also completed in parallel. Images were merged using Adobe Photoshop.
Na+/K+-ATPase Pump Content
Muscle content of the Na+/K+ pump α2 isoform was determined using the vanadate-facilitated [3H]ouabain binding method. Four biopsy specimens weighing 5 mg were prepared from each of the frozen muscles. Samples were prewashed in 2 ml of Tris-vanadate buffer (250 mM sucrose, 10 m Tris-HCl, 3 mm MgSO4, and 1 mm NaVO4; pH 7.25–7.30) for 2 × 10 min at 37C. [3H]ouabain (10–6m, 2 µCi ml–1) was then added to buffer and the samples were incubated in this solution for 120 min at 37C. Muscle samples were then washed at 0C in 2 ml buffer (4 × 30 min) to remove any unbound [3H]ouabain. Following a wash, the samples were blotted dry and weighed. Samples were then soaked overnight in 0.5 ml 5% trichloroacetic acid (TCA) containing 0.1 mM ouabain. After overnight soaking, 2.5 ml of scintillation cocktail (Opti-Fluor, Packard; PerkinElmer, Boston, MA) was added to 0.5 ml of the TCA extract for counting in the β-counter.
Data Analysis
Enzymatic Activity
SDH and GPDH enzymatic activities were quantified using the same method. Images were captured at a magnification of ×100 on the same day as staining. All images were captured under the same preset lighting conditions using a BX51 Olympus microscope (Olympus Life Science Microscopesl Munchen, Germany) and Olympus DP71 camera. Three to four sections were analyzed from each animal. Optical density of muscle sections was calculated using Scion Image software (Scion Corporation; Walkersville, MD). Images were converted to grayscale, and the optical density was calculated according to the equation y = log10 [255/(255 – X)] for a 256-grayscale image, where X equals the gray value of the image. The optical density was taken as an index of oxidative capacity. Average optical density was calculated from multiple sections for each animal normalized to total cross-sectional area and expressed as mean ± SEM. Statistical analysis was carried out using a Student’s unpaired t-test with p<0.05 taken as significant.
MHC and SERCA Isoform Composition
Muscle sections were captured at a magnification of ×100 using a BX51 Olympus microscope and Olympus DP71 camera. Cell A (Olympus) software was used to digitally analyze images, allowing the estimation of areal density and cross-sectional area (CSA) for each MHC fiber type. CSA measurements were made by fiber “circling” based on MHC labeling. A square test frame (640,000 µm2) with two inclusion boundaries and two exclusion boundaries was employed to calculate these parameters in a particular randomly chosen field. Areal density for a given fiber type was calculated by determining the sum of the CSAs for that fiber type divided by the area of the square test frame and multiplied by 100. Relative area of fibers containing SERCA1 and SERCA2 was calculated in the same fashion. Values were obtained from multiple muscle sections and averaged for each animal before computing group means. Data were expressed as mean ± SEM. A Student’s unpaired t-test or Mann-Whitney test (for non-parametric data sets) was employed to statistically compare sham versus CIH, with p<0.05 taken as significant.
Na+/K+-ATPase Pump Content
The content of [3H]ouabain binding sites was calculated on the basis of both the sample wet and dry weights and the specific activity of the incubation medium. The final [3H]ouabain binding site content was then calculated by subtracting the nonspecific [3H]ouabain uptake measured using vanadate buffer containing an excess of unlabeled ouabain. Data were expressed as mean ± SEM and were statistically analyzed to compare sham versus CIH groups using a Student’s unpaired t-test with p<0.05 taken as significant.
Results
Effect of CIH on SDH and GPDH Enzyme Activities in the Sternohyoid Muscle
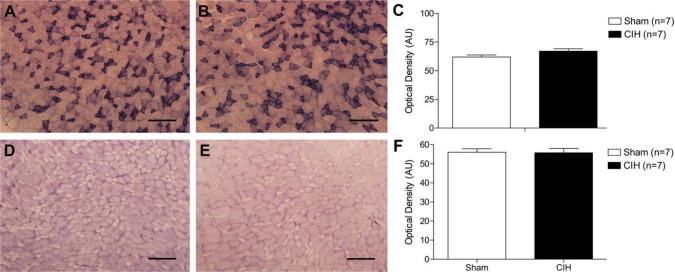
Representative images of sternohyoid muscle stained for SDH (an index of enzymatic oxidative capacity) are shown in Fig. 1A (sham) and Fig. 1B (CIH). CIH treatment did not alter SDH activity in the sternohyoid (Fig. 1C). Representative images of sternohyoid muscle stained for GPDH (an index of enzymatic glycolytic capacity) are shown in Fig. 1D (sham) and Fig. 1E (CIH). CIH treatment had no effect on sternohyoid GPDH activity (Fig. 1F).
Figure 1.
Sternohyoid enzymatic activity. Representative images of sternohyoid muscle stained for succinate dehydrogenase (SDH)—an index of enzymatic oxidative capacity in sternohyoid muscle from a sham (A) and chronic intermittent hypoxia (CIH)-treated (B) animal. (C) Group data (mean ± SEM) showing that CIH had no effect on sternohyoid SDH activity. Representative images of glycerol phosphate dehydrogenase (GPDH) staining (a marker of glycolytic enzymatic activity) in sternohyoid muscle from a sham (D) and CIH-treated (E) animal. (F) Group data (mean ± SEM) showing that CIH had no effect on sternohyoid GPDH activity. Bars = 200 µm.
Effect of CIH on SDH and GPDH Enzyme Activities in the Diaphragm Muscle
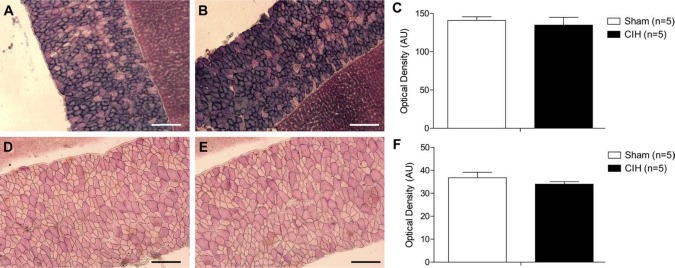
Representative images of diaphragm muscles stained for SDH (Figs. 2A, B) and GPDH (Figs. 2D, E) are shown. Similar to the results observed for the sternohyoid muscle, diaphragm SDH (Fig. 2C) and GPDH (Fig. 2E) enzymatic activities were unchanged following CIH treatment.
Figure 2.
Diaphragm enzymatic activity. Representative images of diaphragm muscle (and an adjacent piece of liver used to assist transverse cutting of the tissue) stained for succinate dehydrogenase (SDH)—an index of enzymatic oxidative capacity in a sham (A) and chronic intermittent hypoxia (CIH)–treated (B) animal. (C) Group data (mean ± SEM) showing that CIH had no effect on diaphragm SDH activity. Representative images of glycerol phosphate dehydrogenase (GPDH) staining (a marker of glycolytic enzymatic activity) in diaphragm muscle (and an adjacent piece of liver used to assist transverse cutting of the tissue) from a sham (D) and CIH-treated (E) animal. (F) Group data (mean ± SEM) showing that CIH had no effect on diaphragm GPDH activity. Bars = 200 µm.
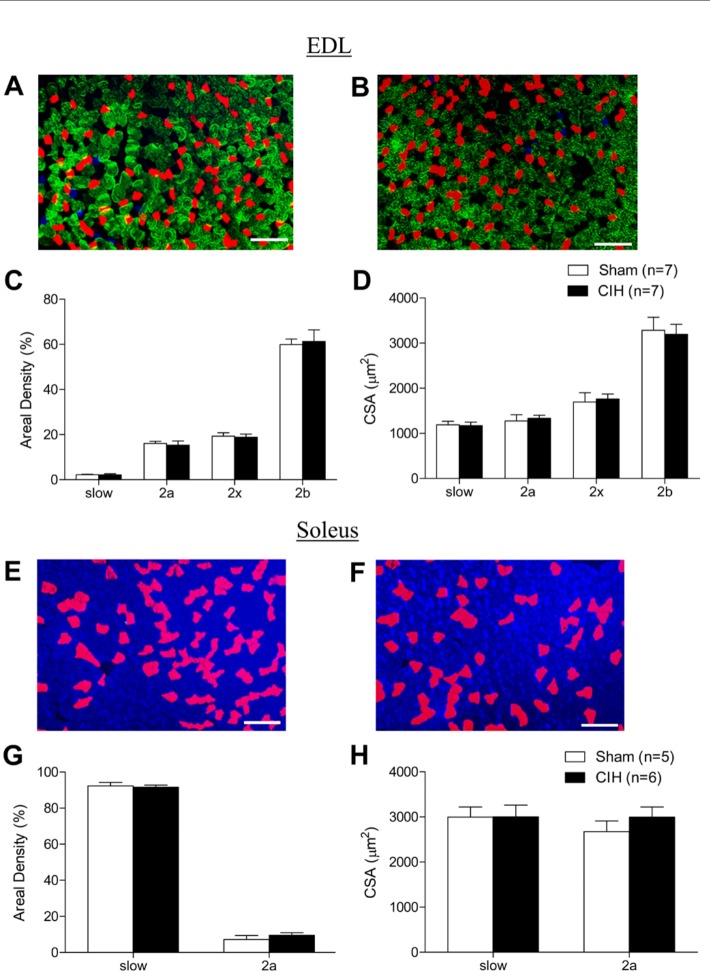
MHC Isoform Composition and Fiber CSA in Respiratory Muscles
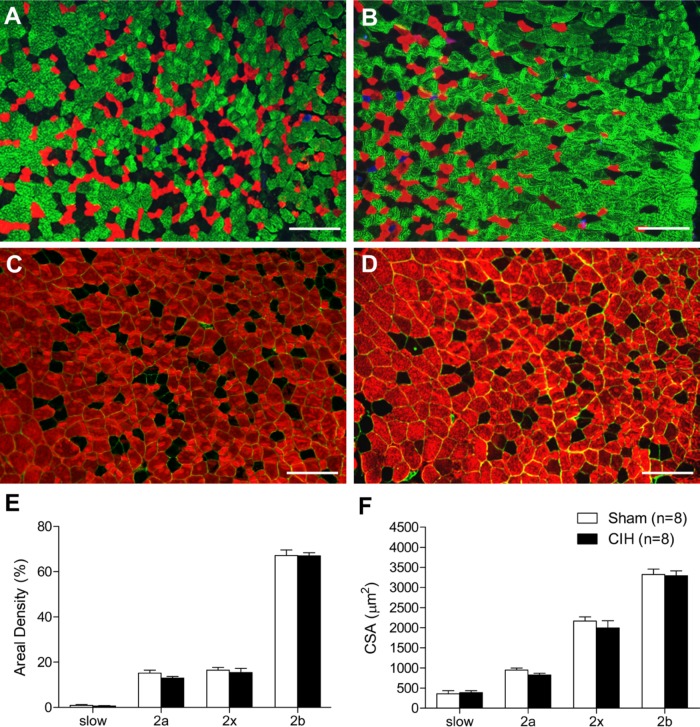
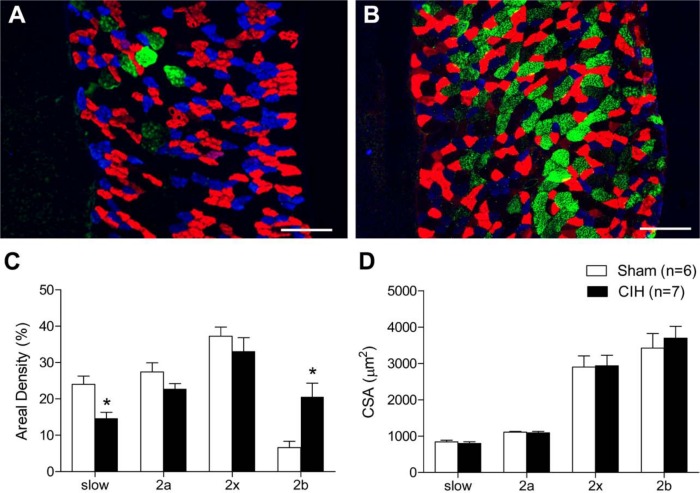
Representative immunofluorescence images of sternohyoid muscle are shown in Fig. 3A and C (sham) and Fig. 3B and D (CIH). CIH had no effect on sternohyoid MHC 1, 2a, 2x, or 2b areal density (Fig. 3E). Sternohyoid fiber CSA (Fig. 3F) and fiber type proportion (data not shown) were unaffected by CIH treatment. Representative immunofluorescence images of diaphragm muscle are shown in Fig. 4A and B. CIH significantly decreased diaphragm MHC 1 areal density (Fig. 4C; p=0.006; Student’s unpaired t-test) and significantly increased diaphragm MHC 2b areal density (Fig. 4C; p=0.008). MHC 2a and 2x areal densities were not statistically different in sham and CIH-treated animals (Fig. 4C). MHC 1 fiber proportion was significantly decreased (41.4 ± 2.8% vs 31.9 ± 2.1%; p=0.02, % of total fibers, sham vs CIH) and MHC 2b was significantly increased (2.8 ± 0.7x% vs 10.3 ± 2.0%; p=0.007) in CIH-treated diaphragm compared with sham animals. Fiber CSA was unaffected by CIH treatment (Fig. 4D). Frequency distributions of fiber CSAs for sternohyoid and diaphragm muscles were unaffected by CIH (data not shown).
Figure 3.
Sternohyoid myosin heavy-chain (MHC) areal density and fiber cross-sectional area (CSA). Representative images of sternohyoid muscle in a sham (A) and chronic intermittent hypoxia (CIH)–treated (B) animal triple-labeled with monoclonal antibodies showing MHC 1 (blue), MHC 2a (red), and MHC 2b (green); MHC 2x fibers are untagged. Representative images of sternohyoid muscle in a sham (C) and CIH-treated (D) animal double-labeled with monoclonal antibodies tagging the basement membrane protein laminin (green) and all MHC isoforms (red) except for MHC 2x (untagged). (E) Group data (mean ± SEM) showing that CIH had no significant effect on the areal density of sternohyoid muscle MHC 1 (slow), MHC 2a, MHC 2x, or MHC 2b fibers. (F) Group data (mean ± SEM) showing that CIH had no significant effect on sternohyoid fiber CSAs. Bars = 200 µm.
Figure 4.
Diaphragm myosin heavy-chain (MHC) areal density and fiber cross-sectional area (CSA). Representative images of diaphragm muscle from a sham (A) and chronic intermittent hypoxia (CIH)–treated animal (B) labeled with monoclonal antibodies showing MHC 1 (blue), MHC 2a (red), and MHC 2b (green); MHC 2x fibers are untagged. Note the decrease in MHC 1 (blue) and large increase in MHC 2b (green) fibers in the CIH-treated animal (B) compared with the sham animal (A). (C) Group data (mean ± SEM) showing that CIH significantly decreased MHC 1 (slow) areal density (*p=0.006, Student’s unpaired t-test) and increased MHC 2b areal density (*p=0.008) in the diaphragm. MHC 2a and MHC 2x areal densities were not statistically different in sham and CIH-treated animals (C). (D) Group data (mean ± SEM) showing that CIH had no significant effect on diaphragm fiber CSAs. Bars = 200 µm.
MHC Isoform Composition and Fiber CSA in Non-Respiratory Muscles
Figure 5A (sham) and 5B (CIH) show representative images of immunolabeled EDL muscle. EDL MHC 1, 2a, 2x, and 2b areal densities were not altered by CIH treatment (Fig. 5C). The CSAs of EDL muscle fibers were not different in sham and CIH muscle (Fig. 5D). Figure 5E (sham) and 5F (CIH) show representative images of soleus muscle. MHC 1 (blue) and 2a (red) are the only two fiber types expressed in rat soleus muscle. CIH did not affect the areal density (Fig. 5G), numerical density (data not shown), or CSA (Fig. 5H) of either fiber type.
Figure 5.
Limb myosin heavy-chain (MHC) areal density and fiber cross-sectional area (CSA). Representative images of extensor digitorum longus (EDL) muscle from a sham (A) and chronic intermittent hypoxia (CIH)–treated (B) animal labeled with monoclonal antibodies showing MHC 1 (blue), MHC 2a (red), and MHC 2b (green); MHC 2x fibers are untagged. (C) Group data (mean ± SEM) showing that CIH had no significant effect on the areal density of MHC 1 (slow), MHC 2a, MHC 2x, or MHC 2b EDL muscle fibers. (D) Group data (mean ± SEM) showing that CIH had no significant effect on EDL fiber CSAs. Representative images of soleus muscle from a sham (E) and CIH-treated (F) animal labeled with monoclonal antibodies showing MHC 1 (blue) and MHC 2a (red). (G) Group data (mean ± SEM) showing that CIH had no significant effect on the areal density of MHC 1 (slow) or MHC 2a soleus muscle fibers. (H) Group data (mean ± SEM) showing that CIH had no significant effect on soleus fiber CSAs. Bars = 200 µm.
Effect of CIH on Fibers Expressing SERCA1 and SERCA2 in the Diaphragm
CIH caused a significant increase in fibers expressing SERCA1 in the diaphragm. This is illustrated in the representative images of diaphragm labeled with SERCA1 (red) in a sham (Fig. 6A) and CIH-treated (Fig. 6B) animal. Figure 6C shows the group data illustrating the significant increase in fibers expressing SERCA1 in CIH muscle compared with control (Fig. 6C; p=0.004). CIH treatment also caused a significant decrease in fibers expressing SERCA2 in the diaphragm. This is illustrated in the representative images of diaphragm from a sham (Fig. 6D) and CIH-treated (Fig. 6E) animal labeled with SERCA2 (red). It is clear from these images and the group data that CIH significantly decreased fibers expressing SERCA2 in the diaphragm (Fig. 6F; p=0.05).
Figure 6.
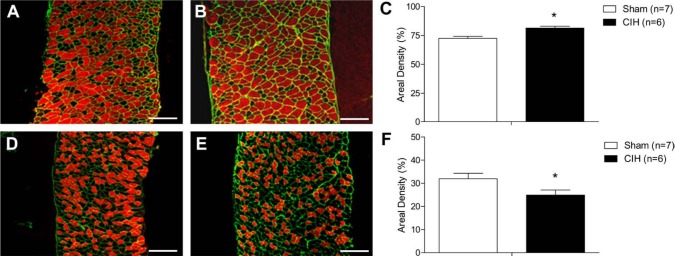
Diaphragm sarcoplasmic reticulum calcium ATPase (SERCA) isoform distribution. Representative images of diaphragm muscle from a sham (A) and chronic intermittent hypoxia (CIH)–treated (B) animal positively labeled using antibodies for SERCA1 isoform (red) and laminin (green). Note the increase in SERCA1 isoform distribution in the CIH-treated animal (B) compared with the sham animal (A). (C) Group data (mean ± SEM) show that CIH significantly increased SERCA1 isoform distribution compared with sham (*p=0.004, Student’s unpaired t-test). Representative images of diaphragm muscle from a sham (D) and CIH-treated (E) animal positively labeled using antibodies for SERCA2 isoform (red) and laminin (green). Note the decrease in SERCA2 isoform distribution in the CIH-treated animal (E) compared with the sham animal (D). (F) Group data (mean ± SEM) shows that SERCA2 isoform distribution was significantly decreased in CIH-treated animals compared with sham animals (*p=0.05, Student’s unpaired t-test). Bars = 200 µm.
Na+/K+-ATPase Pump Content in Skeletal Muscle following CIH
CIH treatment had no effect on diaphragm (Fig. 7A), EDL (Fig. 7B), or soleus (Fig. 7C) Na+/K+-ATPase pump content.
Figure 7.
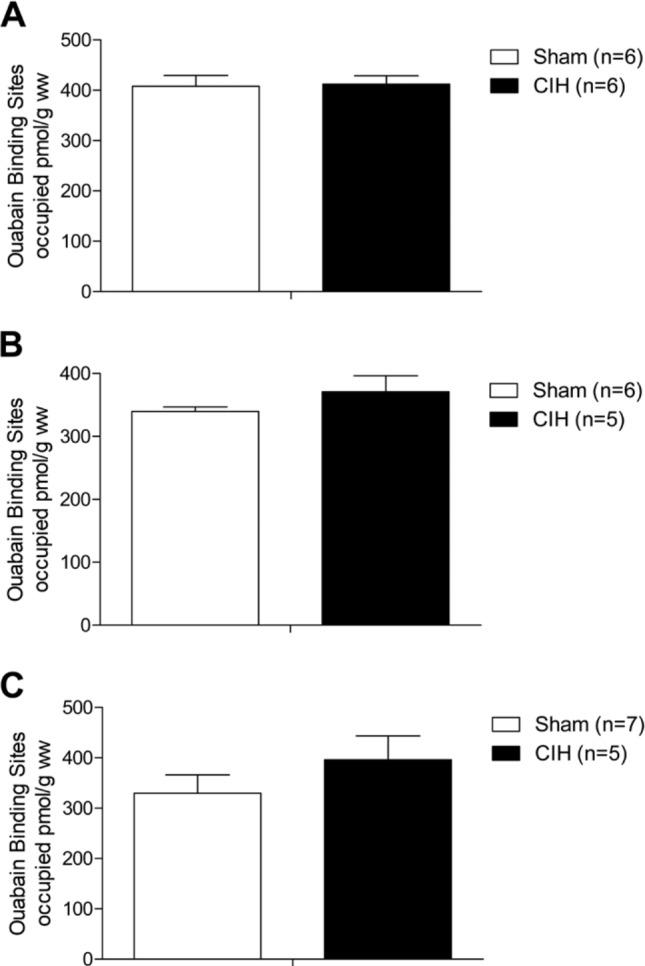
Na+/K+-ATPase pump content in skeletal muscle. Group data (mean ± SEM) show that chronic intermittent hypoxia (CIH) had no significant effect on diaphragm (A), soleus (B), or extensor digitorum longus (C) Na+/K+-ATPase pump content.
Discussion
The main findings of this study are as follows: 1) CIH had no effect on muscle SDH and GPDH enzymatic activities; 2) CIH causes a significant shift in diaphragm fiber phenotype from slow to fast, concomitant with a shift in SERCA proteins from SERCA2 to SERCA1; and 3) Na+/K+-ATPase pump content was unaffected by CIH treatment.
The upper airway dilator muscles play a pivotal role in maintaining airway patency. Injury or fatigue to this group of muscles predisposes the airway to collapse and exacerbates OSA. Growing evidence suggests that upper airway (UA) muscle dysfunction is implicated in the pathophysiology of OSA syndrome. Both OSA patients (Series et al. 1995; Series et al. 1996; Carrera et al. 1999) and CIH animal models (McGuire et al. 2002a; McGuire et al. 2002b; McGuire et al. 2003; Liu SS et al. 2005; Pae et al. 2005; Dunleavy et al. 2008; Liu YH et al. 2009; Skelly et al. 2012) show signs of UA muscle dysfunction. We reasoned that UA muscle dysfunction may be related to CIH-induced phenotypic changes within the muscle. However, we found no significant alteration in sternohyoid muscle fiber distribution. A previous study showed that a longer bout (5 weeks) of CIH was associated with a shift in UA (geniohyoid) muscle phenotype toward a faster, more fatigable muscle, with an increase in MHC 2b fibers and a reduction in MHC 2a fibers (McGuire et al. 2002a). Therefore, it seems likely that the pattern, duration, and intensity of the hypoxic stimulus, among other factors, determine the phenotypic response of airway dilator muscles. We acknowledge that the animal model used in these studies is not a model of OSA per se but rather a model of CIH—the central feature of the disorder. These animals do not have a compromised airway as observed in many OSA patients, and the rats do not experience obstructive airway events that themselves are likely to cause muscle remodeling in OSA. UA muscle remodeling is known to occur in the English bulldog (Petrof et al. 1994), a natural animal model of OSA, and in OSA patients (Series et al. 1995). We acknowledge that OSA is a complicated disorder and that it is likely that UA muscle dysfunction in OSA is multifactorial. However, it is now increasingly recognized that the recurrent hypoxia/reoxygenation cycling that is representative of OSA due to recurrent apnea is a major culprit in the development of key morbidities associated with sleep apnea (Brooks et al. 1997; Fletcher 2000; Gozal et al. 2003; Row et al. 2003; Bradford 2004; Veasey et al. 2004), and this hypothesis also extends to respiratory muscle function (McGuire et al. 2002b; Dunleavy et al. 2008; Liu YH et al. 2009; Skelly et al. 2012).
Somewhat surprisingly, very few studies have examined the effect of CIH on diaphragm structure despite its tremendous importance in the maintenance of respiratory homeostasis. Evidence suggestive of diaphragm fatigue in OSA and animal models of OSA is somewhat controversial. Griggs et al. (1989) showed that patients with sleep apnea have impaired inspiratory muscle contractility (Griggs et al. 1989). This was supported by Chien and colleagues (2010), who observed significantly lower inspiratory muscle functional performance and increased muscle fatigue in OSA patients compared with control subjects. However, other studies have failed to find evidence of diaphragm fatigue (Cibella et al. 1997; Montserrat et al. 1997) or reduced diaphragm force (Shepherd et al. 2006) in patients with OSA. McGuire et al. (2003) demonstrated an increase in diaphragm muscle fatigue in a rodent model exposed to chronic intermittent asphyxia (McGuire et al. 2003). In addition, dystrophic mice exposed to episodic hypoxia had a 30% reduction in diaphragm strength (Farkas et al. 2007). In contrast, other studies report no effect of CIH on diaphragm endurance or structural properties (Clanton et al. 2001; Pae et al. 2005).
The results obtained in this study show that CIH (1 week; 5% at the nadir) causes a shift in the structural phenotype of the diaphragm toward fast, glycolytic fibers, suggesting that diaphragm endurance could be reduced in patients with OSA. Of interest, we did not observe a structural alteration in either EDL or soleus muscle following CIH treatment, which suggests that increased ventilation in CIH-treated animals may have caused this structural alteration in the diaphragm. It is plausible to suggest that the shift toward a faster phenotype may not be due to CIH per se but instead due to a “training effect”. However, if a training effect were responsible, one would also expect an alteration in sternohyoid muscle structure given that these accessory muscles of breathing are also recruited during CIH-induced hyperventilation. However, sternohyoid structure was unchanged. Nonetheless, independent of the mechanism causing structural remodeling, our observations suggest that transitions in the MHC complement may underpin the development of diaphragm dysfunction in response to CIH.
Alternatively, respiratory muscle function could be preserved or perhaps even improved with this phenotypic alteration (an increase in fast fibers would be expected to improve force-generating capacity). This “adaptation” may not necessarily be beneficial for respiration. If the force generated by the inspiratory muscles is increased compared with the force generated by the pharyngeal dilator muscles, respiratory homeostasis could be adversely affected with the increased “negative” intrathoracic pressure, leaving the upper airway susceptible to collapse. Therefore, a putative CIH-induced alteration in diaphragm performance may have serious consequences for patients with OSA.
CIH had no effect on MHC fiber mean CSA. We acknowledge the potential for error in the accuracy of CSA measurements using the fiber “circling” approach, which is dependent on the angle of the transverse section. Feret’s minimum diameter is now recognized as a superior method for the determination of fiber remodeling (TREAT-NMD network), and this would have been a superior approach. As such, absolute measurements of CSA reported herein should be viewed with caution, but we suggest that our data illustrate that CIH does not affect muscle CSA, assuming that oblique sectioning was not unique to one group or one particular muscle. Of interest, we found that CIH—although it caused an MHC isoform shift from MHC 1 to 2—had no effect on SDH or GPDH activity. Otis et al. (2004) also found that a transition in the MHC isoform from a slow to fast phenotype does not necessarily coincide with a shift from an oxidative to a glycolytic phenotype following spinal cord transection. In fact, in that study, oxidative capacity was upregulated, emphasizing a lack of correlation between MHC isoform composition and muscle metabolism in some scenarios. Other studies have shown that the metabolic properties of a muscle are not always directly related to the fatigability of the muscle (Sieck et al. 1989; Ohira et al. 1992; Watchko and Sieck 1993; Caiozzo et al. 1994; Castro et al. 1999). Therefore, it is plausible to suggest that muscle endurance may be altered independent of changes in muscle enzymatic capacity.
SERCA is the second largest energy-consuming protein in skeletal muscle (de Meis 2002). There are two SERCA isoforms found in skeletal muscle fibers: SERCA1 is expressed in fast type 2 muscle fibers, whereas SERCA2 is expressed in slow type 1 muscle fibers. Although the expression of the fast and slow isoforms of MHC and SERCA is highly coordinated (Hamalainen and Pette 1997), it is plausible that both isoforms of these two functionally distinct proteins could coexist in hybrid fibers. We examined the relative area of fibers expressing SERCA1 and SERCA2 proteins in the diaphragm and found that CIH treatment increased fibers expressing SERCA1 and decreased fibers expressing SERCA2. This correlates with our findings showing that MHC isoform expression is significantly shifted from MHC 1 (slow) to MHC 2 (fast) in the same muscles. Diaphragm fatigue is associated with an increase in SERCA1 and/or a decrease in SERCA2 isoform function (Aubier and Viires 1998). In addition, improved soleus muscle endurance is accompanied by an increase in SERCA2 and a decrease in SERCA1 isoform expression (Johansson et al. 2003). Therefore, it is plausible to suggest that a SERCA2-to-SERCA1 transition may cause the diaphragm to develop a greater fatigability.
The Na+/K+ pump is important in maintaining Na+ and K+ concentrations in skeletal muscle in addition to preserving membrane excitability and force production. Reduced Na+/K+-ATPase pump activity led to a decline in muscle contractile function (Leppik et al. 2004; Petersen et al. 2005; McKenna et al. 2006; Murphy and Clausen 2007), whereas increased Na+/K+-ATPase pump content with exercise improved plasma and skeletal muscle K+ regulation after sprint training (McKenna et al. 1993). Furthermore, an increase in diaphragm muscle endurance and Na+/K+ pump content was observed in the diaphragm from chronic hypoxic animals (McMorrow et al. 2011), suggesting that pump content is affected by hypoxia and is implicated in muscle fatigue. In the present study, we found that CIH had no effect on the Na+/K+-ATPase pump in the soleus, EDL, or diaphragm muscles. It must be noted, however, that pump content and not pump activity were measured in this experiment, and therefore it is possible that pump activity may have been reduced independent of expression. This scenario was observed after prolonged exercise in humans (Leppik et al. 2004; Aughey et al. 2005) and following electrical stimulation in rat muscle (McKenna et al. 2003).
Our results demonstrate that CIH causes a slow-to-fast fiber transition in rat diaphragm after just 7 days of treatment. This effect is restricted to the diaphragm and not uniform for other respiratory and non-respiratory skeletal muscles (sternohyoid, EDL, and soleus). The observed shift in diaphragm MHC isoform composition is associated with a shift in SERCA isoform composition (SERCA2 to SERCA1) but occurs independent of an alteration in diaphragm enzymatic capacity. A shift in MHC isoform composition toward a fast fatigable fiber complement may be a contributing factor in the development of diaphragm fatigue. Our results may have implications for respiratory muscle function in disorders characterized by CIH, such as human OSA.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Health Research Board Ireland and the School of Medicine and Medical Science, University College Dublin, Ireland.
References
- Allen DG, Lamb GD, Westerblad H. 2008. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 88:287–332 [DOI] [PubMed] [Google Scholar]
- Aubier M, Viires N. 1998. Calcium ATPase and respiratory muscle function. Eur Respir J. 11:758–766 [PubMed] [Google Scholar]
- Aughey RJ, Gore CJ, Hahn AG, Garnham AP, Clark SA, Petersen AC, Roberts AD, McKenna MJ. 2005. Chronic intermittent hypoxia and incremental cycling exercise independently depress muscle in vitro maximal Na+-K+-ATPase activity in well-trained athletes. J Appl Physiol. 98:186–192 [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Haddad F. 2002. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil. 81:S40–S51 [DOI] [PubMed] [Google Scholar]
- Bradford A. 2004. Effects of chronic intermittent asphyxia on haematocrit, pulmonary arterial pressure and skeletal muscle structure in rats. Exp Physiol. 89:44–52 [DOI] [PubMed] [Google Scholar]
- Bradford A, McGuire M, O’Halloran KD. 2005. Does episodic hypoxia affect upper airway dilator muscle function? Implications for the pathophysiology of obstructive sleep apnoea. Respir Physiol Neurobiol. 147:223–234 [DOI] [PubMed] [Google Scholar]
- Bradley TD. 1992. Right and left ventricular functional impairment and sleep apnea. Clin Chest Med. 13:459–479 [PubMed] [Google Scholar]
- Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. 1997. Obstructive sleep apnea as a cause of systemic hypertension: evidence from a canine model. J Clin Invest. 99:106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt M, Dwivedi G, Khair O, Lip GY. 2010. Obstructive sleep apnea and cardiovascular disease. Int J Cardiol. 139:7–16 [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Baker MJ, Herrick RE, Tao M, Baldwin KM. 1994. Effect of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of a slow muscle. J Appl Physiol. 76:1764–1773 [DOI] [PubMed] [Google Scholar]
- Cantillon D, Bradford A. 1998. Effect of gender on rat upper airway muscle contractile properties. Respir Physiol. 113:147–156 [DOI] [PubMed] [Google Scholar]
- Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Agusti AG. 1999. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med. 159:1960–1966 [DOI] [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Jr, Staron RS, Campos GE, Dudley GA. 1999. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 86:350–358 [DOI] [PubMed] [Google Scholar]
- Chervin RD, Archbold KH, Dillon JE, Panahi P, Pituch KJ, Dahl RE, Guilleminault C. 2002. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics. 109:449–456 [DOI] [PubMed] [Google Scholar]
- Chien MY, Wu YT, Lee PL, Chang YJ, Yang PC. 2010. Inspiratory muscle dysfunction in patients with severe obstructive sleep apnoea. Eur Respir J. 35:373–380 [DOI] [PubMed] [Google Scholar]
- Cibella F, Cuttitta G, Romano S, Bellia V, Bonsignore G. 1997. Evaluation of diaphragmatic fatigue in obstructive sleep apnoeas during non-REM sleep. Thorax. 52:731–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton TL, Wright VP, Reiser PJ, Klawitter PF, Prabhakar NR. 2001. Selected contribution: improved anoxic tolerance in rat diaphragm following intermittent hypoxia. J Appl Physiol. 90:2508–2513 [DOI] [PubMed] [Google Scholar]
- Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. 2004. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 25:735–741 [DOI] [PubMed] [Google Scholar]
- D’Antona G, Lanfranconi F, Pellegrino MA, Brocca L, Adami R, Rossi R, Moro G, Miotti D, Canepari M, Bottinelli R. 2006. Skeletal muscle hypertrophy and structure and function of skeletal muscle fibres in male body builders. J Physiol. 570:611–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meis L. 2002. Ca2+-ATPases (SERCA): energy transduction and heat production in transport ATPases. J Membr Biol. 188:1–9 [DOI] [PubMed] [Google Scholar]
- Degens H, Alway SE. 2006. Control of muscle size during disuse, disease, and aging. Int J Sports Med. 27:94–99 [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. 2010. Pathophysiology of sleep apnea. Physiol Rev. 90:47–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager LF, Jun JC, Polotsky VY. 2010. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 24:843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy M, Bradford A, O’Halloran KD. 2008. Oxidative stress impairs upper airway muscle endurance in an animal model of sleep-disordered breathing. Adv Exp Med Biol. 605:458–462 [DOI] [PubMed] [Google Scholar]
- Farkas GA, McCormick KM, Gosselin LE. 2007. Episodic hypoxia exacerbates respiratory muscle dysfunction in DMD(mdx) mice. Muscle Nerve. 36:708–710 [DOI] [PubMed] [Google Scholar]
- Fitts RH, Riley DR, Widrick JJ. 2001. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol. 204:3201–3208 [DOI] [PubMed] [Google Scholar]
- Fletcher EC. 2000. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol. 119:189–197 [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Qian W, Miller CC, III, Unger T. 1992. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 19:555–561 [DOI] [PubMed] [Google Scholar]
- Gozal D, Row BW, Gozal E, Kheirandish L, Neville JJ, Brittian KR, Sachleben LR, Jr, Guo SZ. 2003. Temporal aspects of spatial task performance during intermittent hypoxia in the rat: evidence for neurogenesis. Eur J Neurosci. 18:2335–2342 [DOI] [PubMed] [Google Scholar]
- Griggs GA, Findley LJ, Suratt PM, Esau SA, Wilhoit SC, Rochester DF. 1989. Prolonged relaxation rate of inspiratory muscles in patients with sleep apnea. Am Rev Respir Dis. 140:706–710 [DOI] [PubMed] [Google Scholar]
- Hamalainen N, Pette D. 1997. Coordinated fast-to-slow transitions of myosin and SERCA isoforms in chronically stimulated muscles of euthyroid and hyperthyroid rabbits. J Muscle Res Cell Motil. 18:545–554 [DOI] [PubMed] [Google Scholar]
- Hawley JA. 2002. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin Exp Pharmacol Physiol. 29:218–222 [DOI] [PubMed] [Google Scholar]
- Horner RL. 2009. Emerging principles and neural substrates underlying tonic sleep-state–dependent influences on respiratory motor activity. Philos Trans R Soc Lond B Biol Sci. 364:2553–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Guz A. 1991. Some factors affecting the maintenance of upper airway patency in man. Respir Med. 85(Suppl A):27–30 [DOI] [PubMed] [Google Scholar]
- Johansson C, Lunde PK, Gothe S, Lannergren J, Westerblad H. 2003. Isometric force and endurance in skeletal muscle of mice devoid of all known thyroid hormone receptors. J Physiol. 547:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, McKenna MJ. 2004. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+-K+-ATPase activity, sarcoplasmic reticulum Ca2+ release, and Ca2+ uptake. J Appl Physiol. 97:1414–1423 [DOI] [PubMed] [Google Scholar]
- Levy P, Bonsignore MR, Eckel J. 2009. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 34:243–260 [DOI] [PubMed] [Google Scholar]
- Liu SS, Liu HG, Xiong SD, Niu RJ, Xu YJ, Zhang ZX. 2005. Effects of Shen-Mai injection on sternohyoid contractile properties in chronic intermittent hypoxia rat [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi. 28:611–614 [PubMed] [Google Scholar]
- Liu YH, Huang Y, Shao X. 2009. Effects of estrogen on genioglossal muscle contractile properties and fiber-type distribution in chronic intermittent hypoxia rats. Eur J Oral Sci. 117:685–690 [DOI] [PubMed] [Google Scholar]
- Matsakas A, Patel K. 2009. Skeletal muscle fibre plasticity in response to selected environmental and physiological stimuli. Histol Histopathol. 24:611–629 [DOI] [PubMed] [Google Scholar]
- McGuire M, MacDermott M, Bradford A. 2002a. The effects of chronic episodic hypercapnic hypoxia on rat upper airway muscle contractile properties and fiber-type distribution. Chest. 122:1400–1406 [DOI] [PubMed] [Google Scholar]
- McGuire M, MacDermott M, Bradford A. 2002b. Effects of chronic episodic hypoxia on rat upper airway muscle contractile properties and fiber-type distribution. Chest. 122:1012–1017 [DOI] [PubMed] [Google Scholar]
- McGuire M, MacDermott M, Bradford A. 2003. Effects of chronic intermittent asphyxia on rat diaphragm and limb muscle contractility. Chest. 123:875–881 [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Gissel H, Clausen T. 2003. Effects of electrical stimulation and insulin on Na+-K+-ATPase ([3H]ouabain binding) in rat skeletal muscle. J Physiol. 547:567–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. 2006. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 576:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MJ, Schmidt TA, Hargreaves M, Cameron L, Skinner SL, Kjeldsen K. 1993. Sprint training increases human skeletal muscle Na(+)-K(+)-ATPase concentration and improves K+ regulation. J Appl Physiol. 75:173–180 [DOI] [PubMed] [Google Scholar]
- McMorrow C, Fredsted A, Carberry J, O’Connell RA, Bradford A, Jones JF, O’Halloran KD. 2011. Chronic hypoxia increases rat diaphragm muscle endurance and sodium-potassium ATPase pump content. Eur Respir J. 37:1474–1481 [DOI] [PubMed] [Google Scholar]
- McNicholas WT, Ryan S. 2006. Obstructive sleep apnoea syndrome: translating science to clinical practice. Respirology. 11:136–144 [DOI] [PubMed] [Google Scholar]
- Montserrat JM, Kosmas EN, Cosio MG, Kimoff RJ. 1997. Lack of evidence for diaphragmatic fatigue over the course of the night in obstructive sleep apnoea. Eur Respir J. 10:133–138 [DOI] [PubMed] [Google Scholar]
- Murphy KT, Clausen T. 2007. The importance of limitations in aerobic metabolism, glycolysis, and membrane excitability for the development of high-frequency fatigue in isolated rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 292:R2001–R2011 [DOI] [PubMed] [Google Scholar]
- O’Halloran KD, McGuire M, O’Hare T, Bradford A. 2002. Chronic intermittent asphyxia impairs rat upper airway muscle responses to acute hypoxia and asphyxia. Chest. 122:269–275 [DOI] [PubMed] [Google Scholar]
- Ohira Y, Jiang B, Roy RR, Oganov V, Ilyina-Kakueva E, Marini JF, Edgerton VR. 1992. Rat soleus muscle fiber responses to 14 days of spaceflight and hindlimb suspension. J Appl Physiol. 73:51S–57S [DOI] [PubMed] [Google Scholar]
- Oliven A, Carmi N, Coleman R, Odeh M, Silbermann M. 2001. Age-related changes in upper airway muscles morphological and oxidative properties. Exp Gerontol. 36:1673–1686 [DOI] [PubMed] [Google Scholar]
- Otis JS, Roy RR, Edgerton VR, Talmadge RJ. 2004. Adaptations in metabolic capacity of rat soleus after paralysis. J Appl Physiol. 96:584–596 [DOI] [PubMed] [Google Scholar]
- Pae EK, Wu J, Nguyen D, Monti R, Harper RM. 2005. Geniohyoid muscle properties and myosin heavy chain composition are altered after short-term intermittent hypoxic exposure. J Appl Physiol. 98:889–894 [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. 2000. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 342:1378–1384 [DOI] [PubMed] [Google Scholar]
- Petersen AC, Murphy KT, Snow RJ, Leppik JA, Aughey RJ, Garnham AP, Cameron-Smith D, McKenna MJ. 2005. Depressed Na+-K+-ATPase activity in skeletal muscle at fatigue is correlated with increased Na+-K+-ATPase mRNA expression following intense exercise. Am J Physiol Regul Integr Comp Physiol. 289:R266–R274 [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Pack AI, Kelly AM, Eby J, Hendricks JC. 1994. Pharyngeal myopathy of loaded upper airway in dogs with sleep apnea. J Appl Physiol. 76:1746–1752 [DOI] [PubMed] [Google Scholar]
- Polla B, D’Antona G, Bottinelli R, Reggiani C. 2004. Respiratory muscle fibres: specialisation and plasticity. Thorax. 59:808–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray AD, Magalang UJ, Michlin CP, Ogasa T, Krasney JA, Gosselin LE, Farkas GA. 2007. Intermittent hypoxia reduces upper airway stability in lean but not obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 293:R372–R378 [DOI] [PubMed] [Google Scholar]
- Rhee HS, Lucas CA, Hoh JF. 2004. Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. J Histochem Cytochem. 52:581–590 [DOI] [PubMed] [Google Scholar]
- Roberts JL, Reed WR, Thach BT. 1984. Pharyngeal airway-stabilizing function of sternohyoid and sternothyroid muscles in the rabbit. J Appl Physiol. 57:1790–1795 [DOI] [PubMed] [Google Scholar]
- Row BW, Liu R, Xu W, Kheirandish L, Gozal D. 2003. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 167:1548–1553 [DOI] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Sieck GC. 2005. Respiratory muscle plasticity. Respir Physiol Neurobiol. 147:235–251 [DOI] [PubMed] [Google Scholar]
- Series F, Cote C, Simoneau JA, Gelinas Y, St Pierre S, Leclerc J, Ferland R, Marc I. 1995. Physiologic, metabolic, and muscle fiber type characteristics of musculus uvulae in sleep apnea hypopnea syndrome and in snorers. J Clin Invest. 95:20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Series F, Cote C, Simoneau JA, St Pierre S, Marc I. 1996. Upper airway collapsibility, and contractile and metabolic characteristics of musculus uvulae. FASEB J. 10:897–904 [DOI] [PubMed] [Google Scholar]
- Shepherd KL, Jensen CM, Maddison KJ, Hillman DR, Eastwood PR. 2006. Relationship between upper airway and inspiratory pump muscle force in obstructive sleep apnea. Chest. 130:1757–1764 [DOI] [PubMed] [Google Scholar]
- Sieck GC, Lewis MI, Blanco CE. 1989. Effects of undernutrition on diaphragm fiber size, SDH activity, and fatigue resistance. J Appl Physiol. 66:2196–2205 [DOI] [PubMed] [Google Scholar]
- Sieck GC, Regnier M. 2001. Invited review: plasticity and energetic demands of contraction in skeletal and cardiac muscle. J Appl Physiol. 90:1158–1164 [DOI] [PubMed] [Google Scholar]
- Skelly JR, Edge D, Shortt CM, Jones JF, Bradford A, O’Halloran KD. 2012. Tempol ameliorates pharyngeal dilator muscle dysfunction in a rodent model of chronic intermittent hypoxia. Am J Respir Cell Mol Biol. 46:139–148 [DOI] [PubMed] [Google Scholar]
- Tupling AR. 2004. The sarcoplasmic reticulum in muscle fatigue and disease: role of the sarco(endo)plasmic reticulum Ca2+-ATPase. Can J Appl Physiol. 29:308–329 [DOI] [PubMed] [Google Scholar]
- Van de Graaff WB, Gottfried SB, Mitra J, Van Lunteren E, Cherniack NS, Strohl KP. 1984. Respiratory function of hyoid muscles and hyoid arch. J Appl Physiol. 57:197–204 [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Dick TE. 1992. Intrinsic properties of pharyngeal and diaphragmatic respiratory motoneurons and muscles.J Appl Physiol. 73:787–800 [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Vafaie H, Salomone RJ. 1995. Comparative effects of aging on pharyngeal and diaphragm muscles. Respir Physiol. 99:113–125 [DOI] [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. 2004. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 27:194–201 [DOI] [PubMed] [Google Scholar]
- Verstraeten E. 2007. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 7:161–166 [DOI] [PubMed] [Google Scholar]
- Watchko JF, Sieck GC. 1993. Respiratory muscle fatigue resistance relates to myosin phenotype and SDH activity during development. J Appl Physiol. 75:1341–1347 [DOI] [PubMed] [Google Scholar]
- White DP. 1995. Sleep-related breathing disorder, 2: pathophysiology of obstructive sleep apnoea. Thorax. 50:797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Evans L, Finn L, Palta M. 1997. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 20:705–706 [DOI] [PubMed] [Google Scholar]