Abstract
Cannabinoid receptor type 2 (CB2R) plays a critical role in nociception. In contrast to cannabinoid receptor type 1 ligands, CB2R agonists do not produce undesirable central nervous system effects and thus promise to treat neuropathic pain that is often resistant to medical therapy. In the study presented here, we evaluated the bilateral distribution of the CB2R protein and messenger RNA (mRNA) in rat dorsal root ganglia (DRG) after unilateral peripheral nerve injury using immunohistochemistry, western blot, and in situ hybridization analysis. Unilateral chronic constriction injury (CCI) of the sciatic nerve induced neuropathic pain behavior and bilateral elevation of both CB2R protein and mRNA in lumbar L4–L5 as well as cervical C7–C8 DRG when compared with naive animals. CB2R protein and mRNA were increased not only in DRG neurons but also in satellite glial cells. The fact that changes appear bilaterally and (albeit at a lower level) even in the remote cervical DRG can be related to propagation of neuroinflammation alongside the neuraxis and to the neuroprotective effects of CB2R.
Keywords: unilateral nerve injury, remote neuroinflammation, satellite glial cells
Cannabinoids are involved in the pathogenesis of neuroinflammatory and neurodegenerative diseases, which are mostly accompanied by pain. Cannabinoid modulation of nociceptive processing has been proven to occur through central (Hohman et al. 1995; Martin et al. 1996; Tsou et al. 1996) and peripheral (Calignano et al. 1998; Richardson et al. 1998) mechanisms. Effects of cannabinoids are mediated by two main receptor subtypes: cannabinoid receptor type 1 (CB1R) and cannabinoid receptor type 2 (CB2R). Although activation of CB1R induces antinociception, this is accompanied by many neurological effects limiting its therapeutic application (Saňudo-Peňa et al. 2000; Scottet al. 2004). By contrast, CB2R-selective agonists can be used for the treatment of pain without such centrally CB1R-mediated adverse effects as sedation, loss of motor coordination, and hypothermia (Malan et al. 2001).
Diseases or trauma affecting peripheral nerves often result in neuropathic pain. Patients with neuropathic pain suffer from spontaneous pain, allodynia (pain response to normally innocuous stimuli), and hyperalgesia (aggravated pain evoked by noxious stimuli). The pharmacotherapy of patients with neuropathic pain is unsatisfactory, and the pathobiological mechanisms of the transition from acute nociception to a state of neuropathic pain are not completely understood. The pathogenesis of neuropathic pain is not simply confined to changes in the activities of neuronal systems but also involves interactions between neurons and inflammatory immune and immune-like glial cells (Austin and Moalem-Taylor 2010). One of the strongest pain management strategies may be to administer CB2R-selective agonists that inhibit tactile and thermal hypersensitivity in neuropathic pain models and avoid unwanted central nervous system (CNS) effects (Ibrahim et al. 2003; Hsieh et al. 2011).
The dorsal root ganglia (DRG), which contain bodies of the primary sensory neurons enveloped by satellite glial cells (SGCs), are critical for neuropathic pain induction associated with cellular and molecular changes following peripheral nerve injury (Kirita et al. 2007). Upregulation of CB2R messenger RNA (mRNA) (Beltramo et al. 2006) and protein (Wotherspoon et al. 2005) has been identified in rat and mouse primary afferent neurons following nerve transection or spinal nerve ligature. These may represent sites for antinociceptive activity of CB2R-selective cannabinoids, which may be of great importance for developing new analgesics (Hanuš et al. 1999; Malan et al. 2001).
Nevertheless, the precise cellular distribution and extent of CB2R mRNA and protein expression in DRG after experimental neuropathic pain induction remain unknown. Therefore, we used a rat neuropathic pain model of chronic constriction injury of the sciatic nerve to study CB2R protein and mRNA changes in DRG associated (L4–L5) and not associated (C7–C8) with the injured nerve.
Materials and Methods
Animals and Surgery
The experiments were performed on 84 adult male rats (Wistar, 200–250 g; Animal Breeding Facility of Masaryk University, Czech Republic). The animals were kept at 22C and maintained on a 12-hr light/dark cycle under specific pathogen-free conditions in the animal housing area of Masaryk University. Sterilized food and water were available ad libitum. All experimental procedures were carried out aseptically and according to protocols approved by the Ethical Committee of Masaryk University, Brno and the Departmental Committee of the Ministry of Education, Youth and Sports, Czech Republic.
Twelve naive animals were used as a control group. Other rats were anesthetized with a mixture of 5% ketamine (100 mg/kg) and 2% xylazine (10 mg/kg) administered intraperitoneally (IP). In the first group of rats (n=48), chronic constriction injury (CCI) of the left sciatic nerve was performed by three ligatures (3-0; Ethicon, Somerville, NJ) that reduced the nerve diameter by approximately one-third. The retracted muscles and skin incision were closed with 3-0 silk sutures and the animals were allowed to survive for 1 (n=12), 3 (n=12), 7 (n=12), and 14 (n=12) days. A sham operation was carried out with animals in the second group (n=24) where the left sciatic nerves were exposed only without any lesion. The sham-operated animals were allowed to survive for 3 (n=12) and 14 (n=12) days.
Behavioral Tests
Neuropathic pain behavior was evaluated by testing of mechanical allodynia (a reduction in pain threshold to mechanical stimulation) using the dynamic plantar esthesiometer (Ugo Basile; Comerio, Italy). All behavioral tests were conducted in a blind manner. The animals were placed in cages with a metal mesh platform and were allowed to habituate to the testing chamber for 10 min before the first measurement. The mean of six consecutive readings on forelimbs and hindlimbs of both sides in each animal was used for analysis. The behavioral assessments were performed on the day before operation and at 1, 3, 7, and 14 days after operation. Limb movements that were considered a part of an animal’s normal movement (walking) were not included in the assessment.
Pain behavior was also assessed by testing of paw withdrawal latency after thermal stimulation (Hargreaves et al. 1988). Rats were allowed to acclimate within the Plexiglas enclosures for 10 min before measurement. A radiant heat source connected to an automatic timer was focused onto the plantar surface of the forepaws and hindpaws, respectively (Plantar Test; Ugo Basile). Activation of the heat source started a timer that stopped when withdrawal of the paw was detected with a photo detector. Stimulus intensity was kept constant throughout the entire experiment, and it was adjusted to give approximately a 10-sec withdrawal latency in the normal or sham-operated paw. A significant reduction in paw withdrawal latency compared with normal baseline was interpreted as thermal hyperalgesia. The withdrawal latencies of both forepaws and hindpaws were tested bilaterally once every 10 min until three consistent thresholds were obtained. The mean value of three consistent thresholds was used as the thermal threshold. A cutoff time of 20 sec was used to prevent potential tissue damage.
Tissue Sampling and Immunohistochemical Staining
Experimental animals surviving 1 day (n=4), 3 days (n=4), 7 days (n=4), and 14 days (n=4); sham-operated animals surviving 3 days (n=4) and 14 days (n=4); and naive animals (n=4) were sacrificed by an overdose of anesthetics and perfused transcardially with phosphate-buffered saline (PBS) followed by Zamboni’s fixative solution (Zamboni and De Martino 1967). The L4–L5 DRG as well as C7–C8 DRG of both sides were detected within their intervertebral foramina after total laminectomy, removed, and immersed in Zamboni’s fixative solution overnight. Longitudinal cryostat sections (12 µm) were cut through ipsilateral and contralateral DRG (Leica 1800 cryostat; Leica Microsystems, Wetzlar, Germany) while being simultaneously blocked in Tissue-Tek OCT compound (Miles; Elkhart, IN), collected on gelatin-coated microscopic slides, then air-dried.
Immunostaining for CB2R
Sections were washed with PBS containing 0.3% bovine serum albumin (BSA) and 0.1% Tween-20 (PBS-BSA-TW20); treated with 3% normal donkey serum, streptavidin, and biotin (each for 15 min); and then incubated with goat polyclonal anti-CB2R antibody (1:100; sc-10076; Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 16 hr. After washing in PBS-BSA-TW20, the sections were incubated with secondary donkey anti–goat 1:400 antibody conjugated with biotin (Santa Cruz Biotechnology) and then FITC-conjugated streptavidin (Boehringer; Mannheim, Germany), both for 90 min. The cell nuclei were stained using Hoechst 33342 (Sigma; St. Louis, MO), and sections were mounted in a Vectashield aqueous mounting medium (Vector Laboratories; Burlingame, CA).
Control sections were treated by a complete immunohistochemical staining procedure without incubation with primary antibody or by using blocking peptide (sc-10076P; Santa Cruz Biotechnology) for saturation of primary antibody prior to staining using normal procedures.
Double Immunostaining
To identify CB2R in SGCs, some of the sections were treated with 3% normal donkey serum and incubated with mouse monoclonal anti–glutamine synthetase (GS) antibody (1:500; Chemicon, a part of Millipore, Billerica, MA) at room temperature for 240 min. The immunoreaction was visualized by treatment with FITC-conjugated and affinity-purified donkey anti–mouse secondary antibody (1:100) for 90 min at room temperature. Sections were washed in PBS and then immunostained for CB2R as described above, but streptavidin was conjugated with TRITC.
To detect CB2R in monocyte/macrophage lineages, a part of the sections was treated with 3% normal donkey serum and incubated with mouse monoclonal anti–ED-1 antibody (1:100; Serotec, Kidlington, UK) at room temperature for 240 min and FITC-conjugated and affinity-purified donkey anti–mouse secondary antibody (1:100) for 90 min at room temperature. After washing in PBS, sections were immunostained for CB2R as described above, but streptavidin was conjugated with TRITC.
All immunostained sections were observed and analyzed using a Leica DMLB epifluorescence microscope equipped with a Leica DFC-480 camera.
Western Blot Analysis
DRG from the experimental rats surviving 1 day (n=6), 3 days (n=6), 7 days (n=6), and 14 days (n=6); sham-operated animals surviving 3 days (n=6) and 14 days (n=6); and naive animals (n=6) were harvested and snap frozen in liquid nitrogen. Frozen tissue samples were homogenized in PBS containing 0.1% Triton X-100 (PBST) and protease inhibitors (Roche, Basel, Switzerland), then centrifuged at 10,000 × g for 5 min at 4C. The protein concentration of the supernatant was determined by Nanodrop ND-1000 (Thermo Scientific; Wilmington, DE). Proteins were denatured by boiling in sample buffer for 5 min, separated by SDS-PAGE (50 V, 15 min; 100 V, 10 min; and 150 V, 60 min), and then transferred to nitrocellulose membranes by electroblotting at 150 mA for 90 min on a Bio-Rad (Hercules, CA) Mini-PROTEAN device (for details, see Brázda et al. 2006). Blots were blocked for 1 hr (1% BSA in PBST), incubated overnight with goat anti–CB2R polyclonal antibody (1:250; sc-10076, Santa Cruz Biotechnology), washed, and then treated with peroxidase-conjugated donkey anti–goat IgG (1:500; Santa Cruz Biotechnology) for 1 hr. Equal loading of proteins was confirmed by a-tubulin staining. All incubations were performed at room temperature. Protein bands were visualized using the ECL detection kit (Amersham, a part of GE Healthcare Life Sciences; Piscataway, NJ) on the LAS-3000 chemiluminometer reader (Fujifilm; Tokyo, Japan), analyzed using densitometry ImageJ software (National Institutes of Health; Bethesda, MD), and normalized to data of naive lumbar and cervical DRG. According to a molecular marker (prestained SDS-PAGE Standards, Broad Range [Bio-Rad, cat. 161-0318]), the molecular weight of the protein displayed by CB2R antibodies by western blot analysis was approximately 40 kDa.
In Situ Hybridization
Before DRG harvesting for in situ hybridization, the experimental rats surviving 1 day (n=2), 3 days (n=2), 7 days (n=2), and 14 days (n=2); sham-operated animals surviving 3 days (n=2) and 14 days (n=2); and naive animals (n=2) were perfused transcardially with 500 ml PBS followed by 500 ml of 4% paraformaldehyde, both containing 0.1% diethylpyrocarbonate (DEPC). The DRG samples were washed in 20% phosphate-buffered sucrose for 12 hr and blocked in Tissue-Tek OCT compound (Miles). Serial longitudinal cryostat sections (12 µm) were cut (Leica 1800 cryostat) and mounted on chrome-alum covered slides.
In situ hybridization to localize the CB2R gene transcript was performed according to the protocol of Harnicarova et al. (2006). We used three 50-mer oligoprobes that had been synthesized for the target CB2R gene transcript. Digoxigenin (DIG)-dT was used for probe labeling. The sequences of oligoprobes were as follows: (1) 5′-CTCCGGCCAA GCTACCGATG AACAGGTACG AGGGCTTCCT GCGGAGCCGC-3′, 5′-GACAACAAGT CCACCCCATG AGCGGTAGGT AGGAGATCAA CGCCGAGAGG-3′, and 5′-GCCTGTCAGG CAGTGCTGGG CAGCAGAGCG GATCTCTCCA CTCCGCAGGG-3′ (VBC-Biotech, Vienna, Austria). All solutions used in this procedure were prepared in double-distilled water treated with DEPC. The sections were treated as described previously (Dubový et al. 2010a). DIG was detected using the DIG Colorimetric Nucleid Acid Detection Kit (Roche). The sections were mounted in a Vectashield aqueous mounting medium (Vector Laboratories) and analyzed using a Leica DMLB microscope equipped with a Leica DFC-480 camera (Leica Microsystems). The control sections were incubated while omitting the DIG-oligonucleotide probes and displayed no color staining.
Double CB2R Immunostaining and CB2R mRNA In Situ Hybridization
To analyze CB2R mRNA signals together with protein localization, we used simultaneous fluorescence in situ hybridization and immunostaining of CB2R protein. Digoxigenin from in situ hybridization was detected by incubation with a TRITC-conjugated anti-DIG monoclonal antibody, dilution 1:1000 (product code ab420; Abcam, Cambridge, MA), for 180 min. The sections were then washed with PBS containing 0.1% Tween-20 (PBS-TW20) and 0.3% BSA for 30 min, treated with 3% normal donkey serum in PBS-TW20 for 30 min, and incubated with CB2R antibody, dilution 1:50 (Santa Cruz Biotechnology), in a humid chamber at room temperature (21–23C) overnight. The CB2R immunoreaction was visualized by treatment with biotin-conjugated and affinity-purified donkey anti–goat antibody, dilution 1:400 (S.C. 2042; Santa Cruz Biotechnology), for 90 min at room temperature. The sections were then washed with PBS and incubated with FITC–streptavidin, dilution 1:100 (SA-5001; Vector Laboratories), for 90 min at room temperature. Sections of naive DRG and those removed from CCI-operated rats for both survival periods as well as from sham-operated rats were incubated simultaneously under identical conditions. Immunostained sections were rinsed, stained with Hoechst 33342 to detect positions of the cell nuclei, and then mounted in a Vectashield aqueous mounting medium (Vector Laboratories). The control sections were incubated with omission of the primary antibody and displayed no immunostaining. The immunostained sections were analyzed using a Leica SP5 confocal microscope (Leica Microsystems).
Image Analysis
The sections of DRG from naive and operated rats were processed simultaneously for CB2R immunostaining as well as in situ hybridization of CB2R mRNA. Neuronal diameter, immunofluorescence intensity (brightness), and density of mRNA staining were measured using a LUCIA-G image analysis system (Laboratory Imaging Ltd.; Prague, Czech Republic) according to our protocol (Dubový et al. 2002). Briefly, stained structures were detected for measurement after subtraction of background by an interactive thresholding technique (HSI, hue, saturation, and intensity) performed with the LUCIA software and transformed to binary mode. The binary map was monitored at every step of thresholding, manually edited if needed, and used to overlay the original image. At least 50 neuronal profiles with visible nucleoli from 10 sections for each DRG group from naive, CCI-operated, and sham-operated rats were measured for their diameters, CB2R immunofluorescence brightness, and CB2R mRNA staining intensity. The intensity of CB2R immunofluorescence was measured as brightness, whereas that of CB2R mRNA staining was measured as optical density. Analysis of DRG images from naive and operated rats was performed by an investigator blinded to the treatment groups.
The neuronal bodies were categorized by diameter calculated from areas measured as small (<25 µm), medium (25–40 µm), or large (>40 µm).
Statistical Analysis
Behavioral data were evaluated using Kruskal-Wallis one-way analysis of variance with Bonferroni post hoc testing, and p values less than 0.05 were considered significant. Data for CB2R immunofluorescence brightness and density of CB2R mRNA staining were tested for normal distribution. The values of behavioral tests are reported as mean ± SE.
Statistical differences between data of naive, CCI-operated, and sham-operated rats were tested using a Mann-Whitney U test (STATISTICA 5.5; StatSoft, Tulsa, OK) with 0.05 indicating significant differences. All values are reported as mean ± SD.
Results
Behavioral Tests
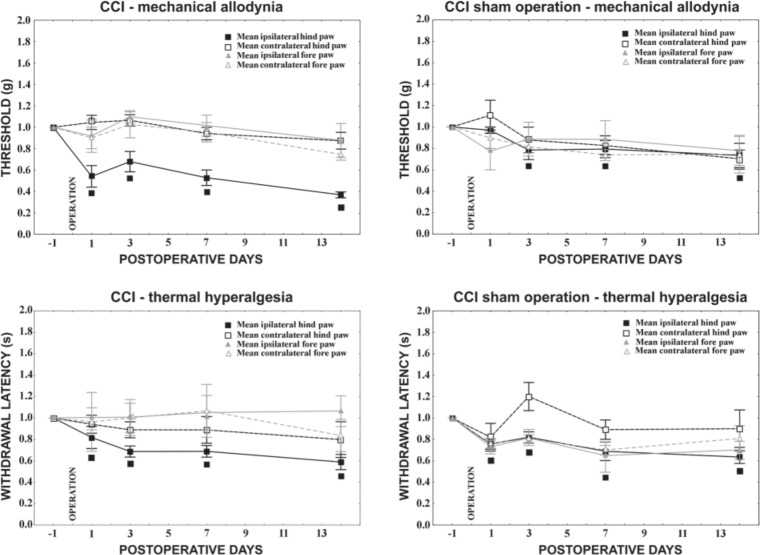
Starting the first day from the unilateral CCI, animals developed clear behaviors indicative of neuropathic pain such as mechanical allodynia and thermal hyperalgesia. Ipsilateral to nerve ligatures, the affected hindpaw skin became allodynic as demonstrated by earlier withdrawal of the injured paw from the pushing probe. This was assessed as decreased pain threshold when compared with the measurement taken on the day before the operation (Fig. 1). Contralateral hindpaws as well as forepaws did not exhibit statistically significant changes to withdrawal thresholds. Similar to the test for mechanical allodynia, there was a significant decline in withdrawal latency for the ipsilateral hindpaw in comparison with that of naive animals, whereas no signs of thermal hyperalgesia were found for either the contralateral hindpaw or the two forepaws.
Figure 1.
Behavioral tests in chronic constriction injury (CCI) and sham-operated rats. CCI operation evoked mechanical allodynia as well as thermal hyperalgesia in the ipsilateral hindpaw starting the first day from surgery. Sham-operated rats also exhibited both mechanical allodynia and thermal hyperalgesia in ipsilateral hindpaws, albeit less so than did the CCI animals. No signs of neuropathy were observed in forepaws either after mechanical or thermal stimuli. Data are expressed as mean ± standard error of withdrawal thresholds in grams and withdrawal latency in seconds for mechanical allodynia and thermal hyperalgesia, respectively. The threshold index and index of withdrawal latency indicate the ratio of after-surgery measurement to measurement before operation. ■ Indicates statistically significant difference of ipsilateral hindpaw values after CCI when compared with the measurement in naive animals (p<0.05).
Sham operation resulted also in significantly increased sensitivity to both mechanical and thermal stimuli in the ipsilateral hindpaws when compared with naive rats.
CB2R Immunohistochemical Staining
Sections of naive L4–L5 and C7–C8 DRG revealed very weak immunofluorescence staining for CB2R protein in both neurons and SGCs (Figs. 2A and 3A). No immunoreactivity was observed in the control sections treated by the complete immunostaining procedure with omission or saturation of the primary antibody (Figs. 2B and 3C). The same immunostaining pattern for CB2R was observed in DRG removed from rats surviving 1 or 3 days and 7 or 14 days from the CCI of the sciatic nerve. Therefore, the results are interpreted together for the early (1–3 days) and later (7–14 days) periods of survival. In addition, no basic differences were found between ipsilateral and contralateral cervical DRG removed from experimental rats, and for that reason, results will be predominantly described and illustrated without side differentiation, unless where obvious differences were present.
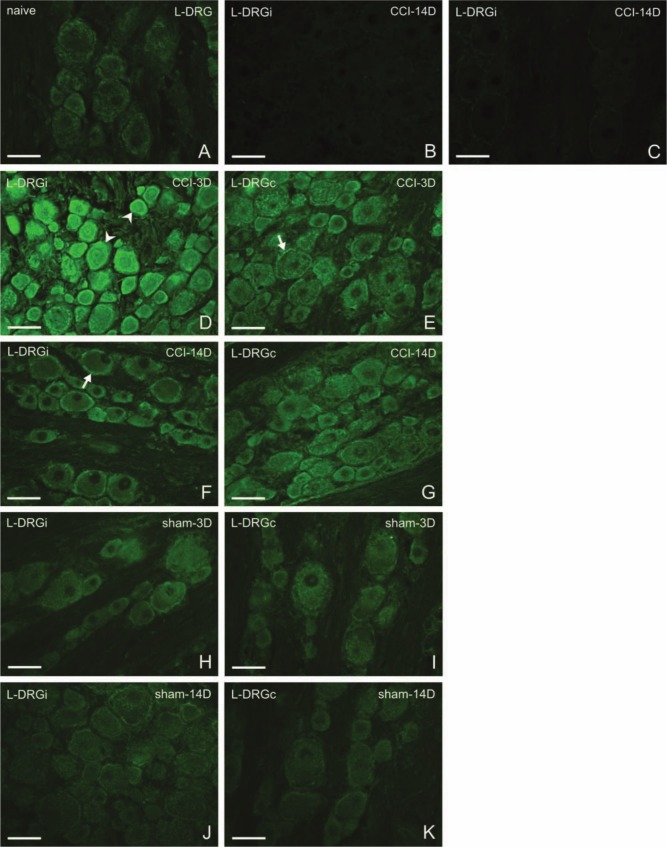
Figure 2.
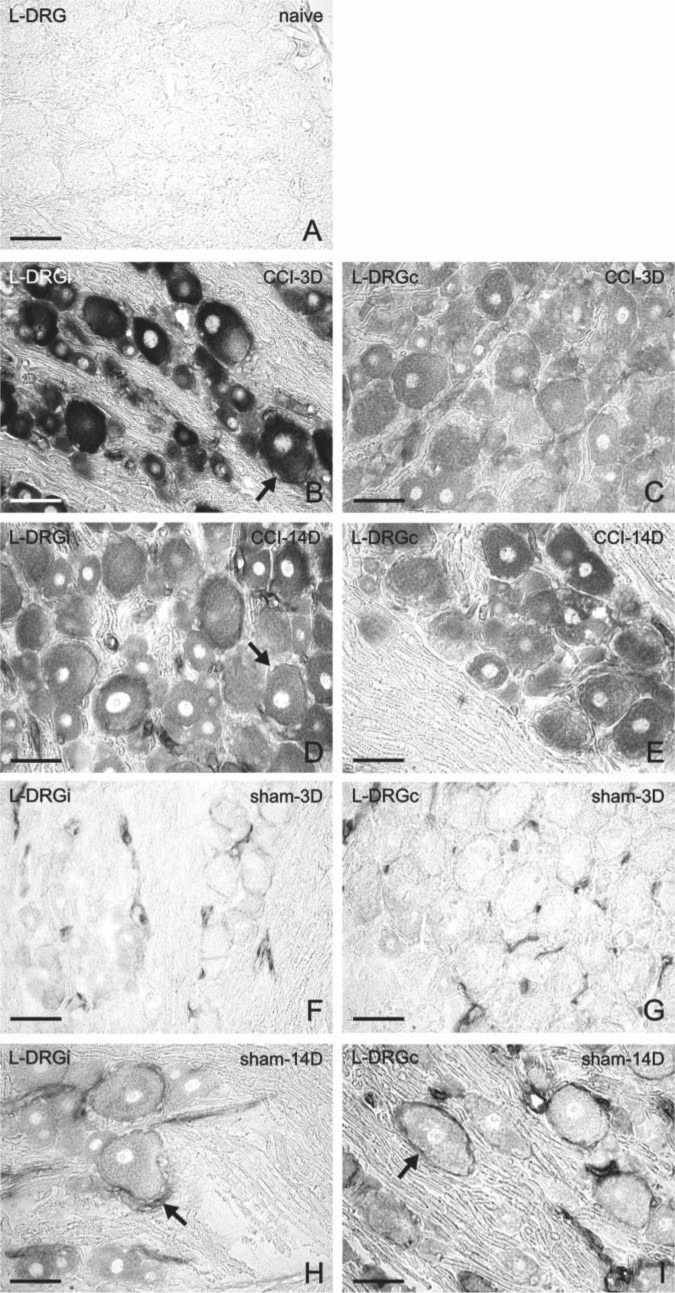
Representative sections of L4–L5 dorsal root ganglia (DRG) immunostained for CB2R from naive rats (A), from ipsilateral (D, F) and contralateral (E, G) DRG of chronic constriction injury (CCI) rats, and ipsilateral (H, J) and contralateral (I, K) DRG of sham-operated rats (sham). Control incubations with antibody after its absorption by protein (B) and with omission of primary antibody (C) displayed no immunostaining. Three days after CCI (D, E), both neurons (arrowheads) and satellite glial cells (SGCs; arrows) exhibited immunofluorescence for CB2R. Immunostaining was more pronounced ipsilaterally (D), and small- and medium-sized neurons displayed accentuated staining. Fourteen days from surgery (F, G), the immunostaining was much less. Sham-operated animals exhibited also higher immunostaining in contrast to that of naive rats, both ipsilaterally and contralaterally. Scale bars = 50 µm.
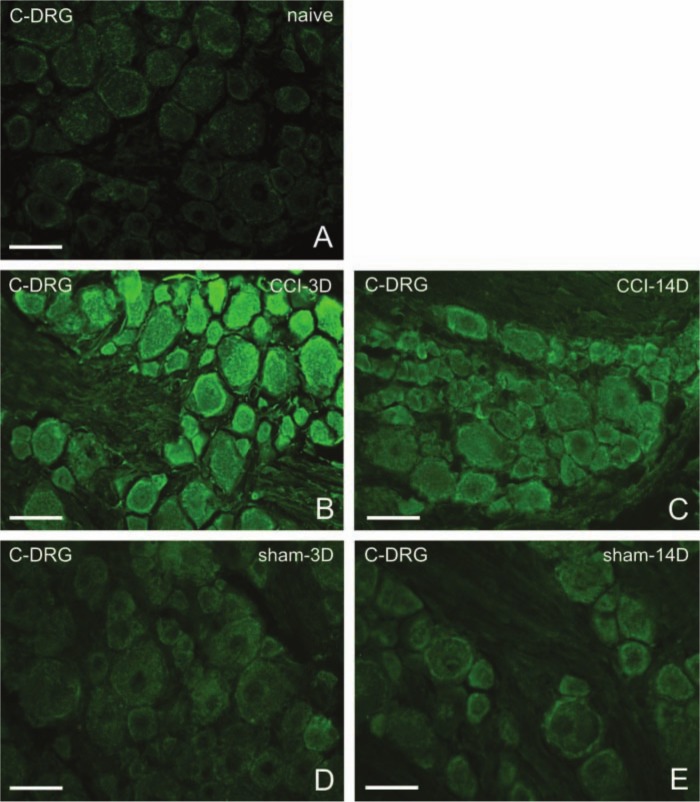
Figure 3.
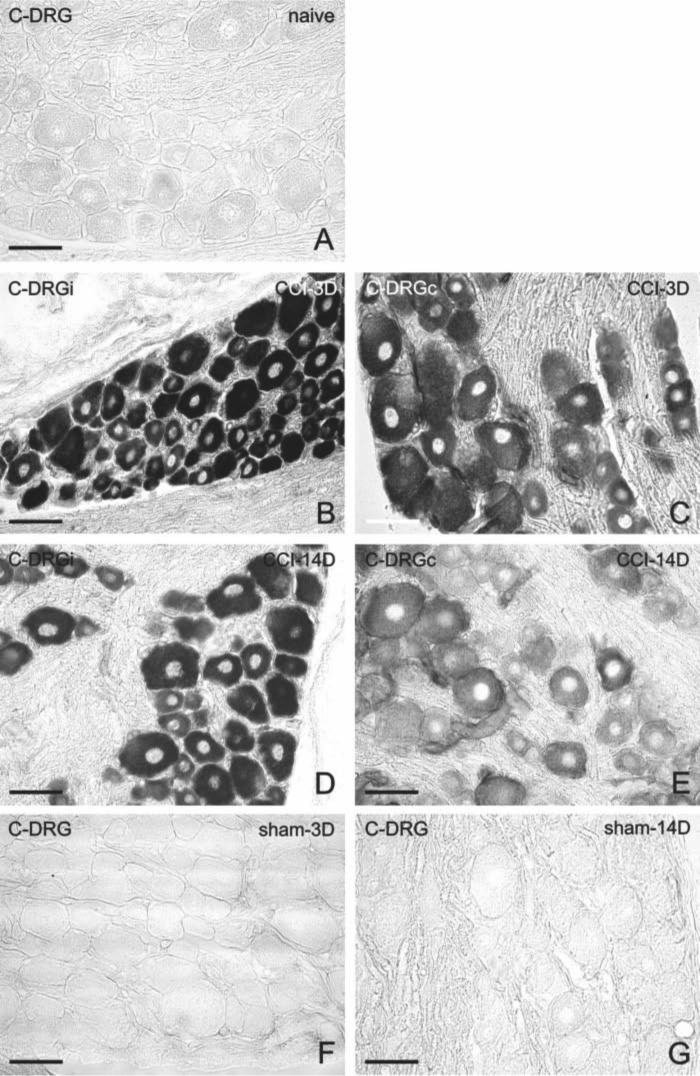
Representative sections of C7–C8 dorsal root ganglia (DRG) immunostained for CB2R from naive rats (A), from DRG of chronic constriction injury (CCI) rats (B, C), or sham-operated rats (D, E). Immunostaining for CB2R is intensified 3 days after CCI (B) but decreases 14 days after CCI (C). Three days (D) as well as 14 days (E) after sham operation, the immunostaining is also greater than that of naive animals. Scale bars = 50 µm.
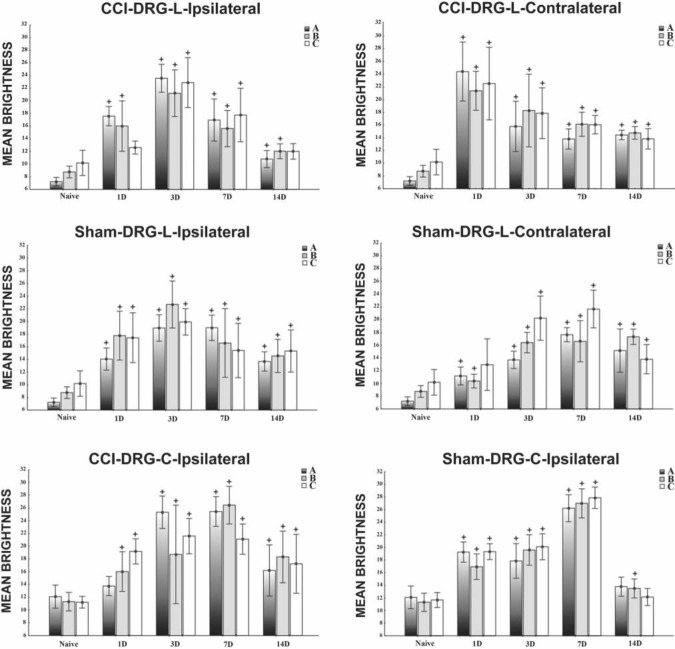
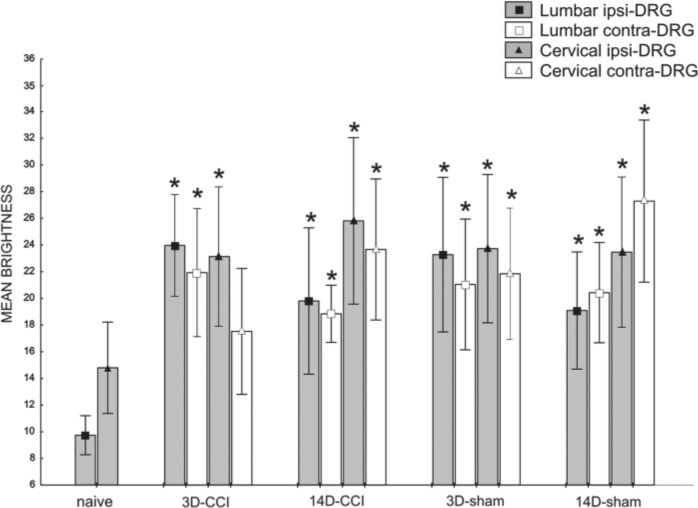
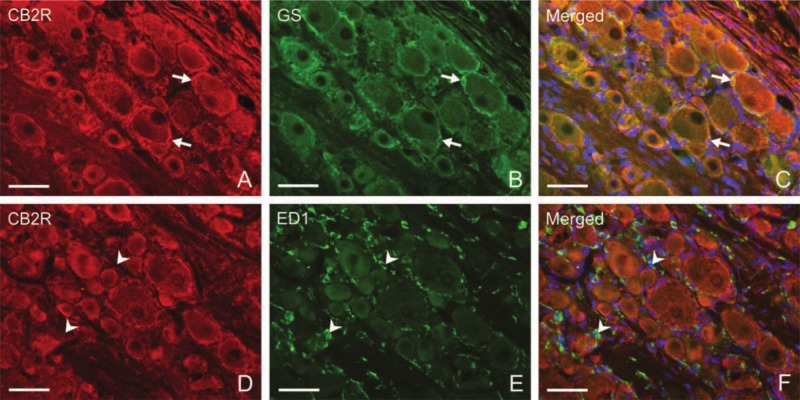
Unilateral CCI of the sciatic nerve induced more intense immunostaining for CB2R in lumbar as well as cervical DRG of both sides (Figs. 2D–G, 3B, and 3C). Immunostaining was more pronounced in ipsilateral lumbar DRG three days after CCI (Fig. 2D). Neuronal bodies of all sizes were more intensely stained after CCI. The most notable increase of immunofluorescence was measured in the largest neurons (type A), in which values more than 10 times higher were found on the third day after operation in ipsilateral lumbar DRG and three times higher values in cervical DRG in comparison with those of naive DRG (Fig. 5). Decreased CB2R immunostaining was observed in DRG after CCI for the later versus early period of survival (Figs. 2D, 2F, 3B, 3C, and 5). Sham-operated rats displayed also significantly higher immunofluorescence in both lumbar and cervical DRG neurons when compared with naive DRG, although this was lower than in CCI-operated animals (Figs. 2H–K, 3D, 3E, and 5). The SGCs immunolabeled for CB2R were observed particularly around large neuronal bodies, which displayed weak immunostaining in comparison with small- and medium-sized neuronal somata (Fig. 2E, F). Measurement of immunofluorescence brightness revealed significantly higher CB2R staining in SGCs of both lumbar and cervical DRG after CCI as well as sham operation when compared with naive DRG. In contrast to neurons, there was no decrease in intensity in the later periods of survival (Fig. 6). Double immunostaining for CB2R and GS proved CB2R protein expression also in SGCs (Fig. 4A–C). By contrast, the ED-1+ macrophages invading the neuronal area did not display immunoreaction for the CB2R protein (Fig. 4D–F).
Figure 5.
Measurement of CB2R immunofluorescence brightness of lumbar and cervical dorsal root ganglia (DRG) neurons after chronic constriction injury (CCI) and sham operation compared with brightness in naive animals. All values are reported as mean brightness ± SD. A indicates large (>40 µm), B intermediate (25–40 µm), and C small neurons (<25 µm). +Mean brightness is significantly higher in both lumbar and cervical DRG neurons after both CCI and sham operation when compared with the corresponding type of neurons in naive rats (p<0.05).
Figure 6.
Measurement of CB2R immunofluorescence brightness of lumbar and cervical satellite glial cells (SGCs) after chronic constriction injury (CCI) and sham operation compared with brightness in naive animals. All values are reported as mean brightness ± SD. *Mean brightness is significantly higher in both lumbar and cervical dorsal root ganglia (DRG) SGCs after both CCI (with the exception of cervical contralateral after 3 days) and sham operation when compared with the SGCs in naive DRG (p<0.05). Ipsilateral, ipsi; contralateral, contra.
Figure 4.
Representative sections illustrating double immunostaining for either CB2R and glutamine synthetase (GS) (A–C) or CB2R and ED1 (D–F) in L4–L5 dorsal root ganglia (DRG) of the ipsilateral side harvested from rats 7 days after unilateral chronic constriction injury (CCI) of the sciatic nerve. Strong immunostaining for CB2R induced in satellite glial cells (SGCs) (A, arrows) surrounding large neuronal bodies displays co-localization with GS immunostaining (C, arrows). ED-1+ macrophages (E, arrowheads) display no co-localization with CB2R immunostaining (F, arrowheads). Nuclei of the cells were stained by Hoechst 33342. Scale bars = 50 µm.
Western Blot Analysis
A certain level of CB2R protein was detected in lumbar and cervical DRG obtained from naive rats by western blot analysis. Western blot analyses from rat DRG revealed only one (major) CB2R band of approximately 40 kDa corresponding with the CB2R molecular weight. Densitometry of western blots of total L4–L5 DRG fractions showed a significant bilateral elevation of CB2R protein at 1, 3, 7, and 14 days after CCI operation (Fig. 7A). The amounts of CB2R protein remained elevated during the entire experimental period up to 14 days after operation in all DRG samples analyzed. The highest elevation of CB2R protein was detected 1 day after CCI. This level decreased slightly at day 3 and continued to decrease 7 and 14 days after CCI. At 1 day after CCI, the level of CB2R protein increased more than twice in the ipsilateral L4–L5 DRG versus the naive control (Fig. 7A). At days 3, 7, and 14, the elevation was lower compared with day 1 in the ipsilateral DRG, but it was still evident.
Figure 7.
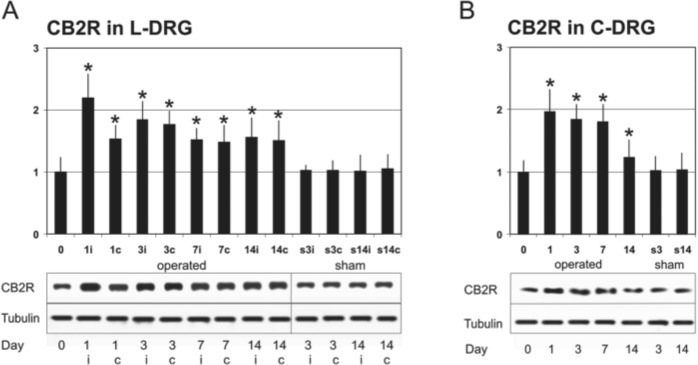
Western blot analysis. (A) Western blot analysis of CB2R total protein levels (first lane) and tubulin levels (loading control, second lane) in L4–L5 dorsal root ganglia (DRG) after chronic constriction injury (CCI) (in naive animals = 0, then 1, 3, 7, and 14 days postinjury). Densitometry of western blot analyses expressed a relative amount of CB2R protein in lumbar DRG (L-DRG). Taking the intensity of the CB2R band on the SDS-PAGE gel of the unoperated rat as 100% (lane 1), the intensity of the band increased in operated groups. The y-axis shows relative changes in the CB2R band intensity, and the x-axis indicates periods of survival; “i” indicates ipsilateral and “c” contralateral DRG. (B) Western blot analysis of CB2R total protein levels (first lane) and tubulin levels (loading control, second lane) in cervical DRG (C-DRG) following CCI in naive rats = 0, then 1, 3, 7, and 14 days postinjury. Densitometry of western blot analyses expresses the relative amount of CB2R protein in cervical DRG. No significant changes in CB2R protein levels were found after sham operation in comparison with those of naive rats. All values are reported as mean ± SD. *Significant increase of density (p<0.05).
The CB2R protein levels were very similar in both ipsilateral and contralateral DRG at days 3, 7, and 14. Moreover, elevation of CB2R protein was also observed in cervical DRG in all tested survival periods (Fig. 7B), thus indicating its upregulation in remote DRG. In comparison with naive DRG, no significant changes in the levels of CB2R protein were assessed in the lumbar and cervical DRG removed from sham-operated animals.
The data provide evidence for bilateral elevation of CB2R protein levels not only in L4–L5 but also in C7–C8 DRG following unilateral CCI of the sciatic nerve used as an experimental neuropathic pain model.
In Situ Hybridization for CB2R mRNA
Lumbar and cervical DRG sections of naive rats displayed no to very low detectable signals for CB2R mRNA in neuronal bodies and their SGCs (Figs. 8A and 9A). Similar to immunohistochemistry of CB2R protein, the pattern of CB2R mRNA staining was very much like that in DRG of rats surviving for 1 and 3 days as well as for 7 and 14 days from CCI. Therefore, representative CB2R mRNA staining is described and illustrated for early and later periods of survival, using 3 and 14 days, respectively.
Figure 8.
Detection of CB2R messenger RNA (mRNA) by in situ hybridization in sections of L4–L5 dorsal root ganglia (DRG) from naive (A), chronic constriction injury (CCI) (B–E), and sham-operated (F–I) rats. Bilateral staining for CB2R mRNA was induced in neuronal bodies of all sizes and in satellite glial cell (SGCs; arrows) by unilateral CCI of the sciatic nerve for 3 days (B, C) and 14 days (D, E). In contrast, sections of L4–L5 DRG removed from sham-operated rats after 3 days (F, G) or 14 days (H, I) displayed only moderate or no CB2R mRNA staining in neurons. Higher intensity of CB2R mRNA staining was observed in SGCs predominantly surrounding the large neurons (arrows), especially on day 14 after sham operation. Scale bars = 50 µm.
Figure 9.
Detection of CB2R messenger RNA (mRNA) by in situ hybridization in sections of C7–C8 dorsal root ganglia (DRG) from naive (A), chronic constriction injury (CCI) (B–E), and sham-operated (F, G) rats. Strong staining for CB2R mRNA was observed bilaterally in neuronal bodies 3 days after unilateral CCI of the sciatic nerve (B, C) and was reduced 14 days after CCI (D, E). No staining was displayed in the sections of cervical DRG removed 3 and 14 days after sham operation. Scale bars = 50 µm.
Unilateral CCI of the sciatic nerve induced bilateral elevation in staining for CB2R mRNA in both lumbar and cervical DRG after early and later survival periods (Figs. 8B–E and 9B–E). In comparison with naive DRG, a higher intensity of CB2R mRNA staining was observed, particularly in neuronal somata of all sizes and their SGCs. Intense staining for CB2R mRNA in neuronal bodies did not usually allow distinguishing the signal for CB2R mRNA in the SGCs. This was possible only when staining of neuronal bodies was already lower (e.g., Figs. 8C–E, 9C, and 9E). Moderate staining for CB2R mRNA was found also in neuronal bodies and their SGCs of both ipsilateral and contralateral L4–L5 DRG removed from sham-operated rats (Figs. 8F–I), albeit more distinctly in the later survival periods (Figs. 8H, I). With no or very low signals for CB2R mRNA in neuronal bodies, a distinct staining could be observed in blood vessels and SGCs. Intensity of staining for CB2R mRNA in SGCs was bilaterally higher in the later rather than in the early period of survival (Figs. 8F–I). In contrast to the lumbar DRG, no CB2R mRNA staining was observed in cervical DRG of the sham-operated rats (Figs. 9F, G).
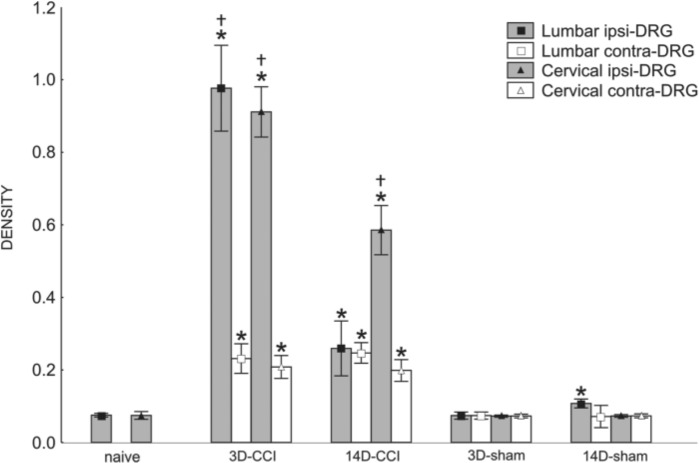
To compare signals for CB2R mRNA in DRG from naive rats with those after unilateral CCI of the sciatic nerve for early and later periods, we analyzed the density of in situ hybridization staining. Mean density of CB2R mRNA signals increased significantly at days 3 and 14 in both ipsilateral and contralateral lumbar and cervical DRG after unilateral CCI of the sciatic nerve. At 3 days after injury, the level of CB2R mRNA was increased in the lumbar and cervical ipsilateral DRG by about 10 times in comparison with the naive control (Fig. 10). We also observed a strong increase in the signal in contralateral and cervical DRG from both survival periods. The level of CB2R mRNA in sham-operated animals increased significantly only in the ipsilateral lumbar DRG when compared with the naive control (Fig. 10). We can conclude that unilateral injury leads to changes not only in CB2R protein but also in mRNA levels in both DRG associated and not associated with the injured nerve. Simultaneous immunostaining and in situ hybridization revealed that CB2R protein and CB2R mRNA are co-localized in neurons and SGCs (Fig. 11).
Figure 10.
Measurement of CB2R messenger RNA (mRNA) density of lumbar and cervical dorsal root ganglia (DRG) after chronic constriction injury (CCI) and sham operation compared with density in naive animals. Staining intensity was calibrated from 0 to 1 with background (white) level set at 0 and the value of 1 set at black depicting the densest level of DAB reaction product. Mean density of both lumbar and cervical DRG increased significantly at days 3 and 14 after unilateral CCI of the sciatic nerve. All values are reported as mean ± SD. *At both 3 and 14 days after injury, the levels of CB2R mRNA in the ipsilateral lumbar and cervical DRG were significantly higher in comparison with the naive control. We can observe also a strong increase of the signal in the contralateral DRG for both tested periods (p<0.05). †Density of ipsilateral DRG was much lower when compared with contralateral ganglia (p<0.05). The changes of the density in sham-operated animals did not significantly increase except for the ipsilateral lumbar DRG (p<0.05).
Figure 11.
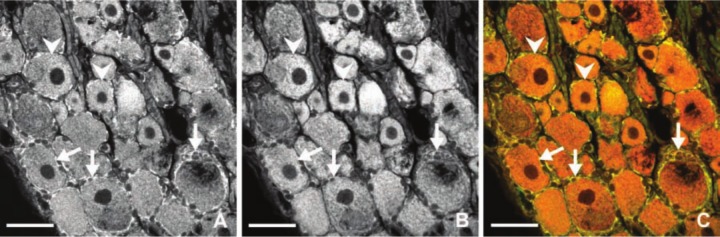
Representative sections illustrating simultaneous immunofluorescence of CB2R protein (A) and in situ hybridization fluorescence of CB2R messenger RNA (mRNA) (B) using a confocal microscope in the green and red fluorescence channel, respectively. CB2R protein was co-localized with CB2R mRNA signal (C) as yellow/orange in both neurons (arrowheads) and satellite glial cell (SGCs; arrows) when red and green channels were merged. Scale bars = 50 µm.
Discussion
Unilateral CCI of the sciatic nerve induced bilateral elevation of CB2R mRNA and protein not only in DRG of the L4–L5 segments anatomically related to the ligated sciatic nerve but also in the remote cervical ganglia. Increased staining for CB2R protein and mRNA was observed in both neuronal bodies and their SGCs. Nevertheless, clear behavioral signs of neuropathic pain developed only in hindpaws ipsilateral to CCI of the sciatic nerve.
CB2R in DRG Neurons and Neuropathic Pain
Peripheral neuropathic pain develops as a result of lesions or disease affecting a peripheral nerve, DRG, or spinal nerve roots (Woolf and Mannion 1999). Injured and neighboring non-injured primary sensory neurons can develop a change in their excitability sufficient to generate pacemaker-like potentials, which evoke ectopic action potential discharges and sensory inflow independent of any peripheral stimulus (Devor and Seltzer 1999; Liu CN et al. 2002). Ectopic input is most prominent in A-fibers, but it also occurs to a more limited extent in cells with unmyelinated axons (Devor and Seltzer 1999).
We observed a robust bilateral elevation of CB2R protein and mRNA in DRG neurons of all sizes following unilateral CCI of the sciatic nerve. A presence of CB2R in the primary sensory neurons of DRG has been demonstrated using various methods but most usually after in vitro cultivation (Ross et al. 2001; Wotherspoon et al. 2005; Anand et al. 2008). In addition, CB2R immunoreactivity has been identified in large dermal myelinated nerve fibers and small subepidermal nerve fibers of healthy human skin (Ständer et al. 2005). Increased CB2R protein has been detected by western blot analysis in the ipsilateral DRG of neuropathic rats when compared with naive and sham-operated animals (Walczak et al. 2005). A controversy has emerged, however, as to what is the true location of CB2R in the nervous system due to the inducibility of CB2R, the lack of sufficiently specific antibodies, and imperfect pharmacology. Therefore, proper controls must be borne in mind when interpreting the data presented from any study (Atwood and Mackie 2010). In this study, the specificity of immunohistochemical staining was controlled by saturation of the antibody with the immunizing peptide and omission of the primary antibody from the staining procedure. The antibody specificity was confirmed by our western blot analyses of rat DRG that revealed a CB2R band of approximately 40 kDa, thus corresponding with the CB2R molecular weight; this is considered a satisfactory control of antibody specificity (Burry 2000). Changes in CB2R protein in DRG neurons were verified by western blotting, in situ hybridization of CB2R mRNA, and simultaneous immunostaining for CB2R protein and in situ hybridization fluorescence for CB2R mRNA.
Cannabinoid agonists are potential analgesics for the management of pain (Pertwee 2001). Analgesic effects of CB2R agonists have been demonstrated in experimental models of neuropathic pain (Whiteside et al. 2007; Brownjohn and Ashton 2009; Wilkerson et al. 2012). In contrast to CB1R ligands, CB2R agonists are devoid of psychoactive effects and therefore are of particular therapeutic interest, especially for reducing neuropathic pain. The presence of CB2R in primary sensory neurons suggests that CB2R agonists can modulate pain transmission through a direct neuronal action. The exact mechanism of CB2R-mediated analgesic effects remains to be determined, however. It has been reported that CB2R possesses constitutive activity when overexpressed in neurons (Bouaboula et al. 1999). In neurons (e.g., if it is induced), it will modulate synaptic transmission. Overexpression of CB2R leads to a smaller excitatory postsynaptic current (EPSC). CB2R agonists probably exert their inhibitory effect on neurotransmission by inhibition of neuronal voltage-gated calcium channels (Atwood et al. 2012). It also has been shown that this is likely due to ongoing synthesis of an endocannabinoid (most likely 2-AG) that can activate CB2R (Atwood et al. 2012). This is supported by reports that mice with the gene deleted for the enzyme DAG Lipase α that is responsible for the production of 2-AG exhibit abolition of retrograde signaling to inhibit neurotransmitter release in their brains (Gao et al. 2010; Tanimura et al. 2010). Upregulated CB2R may contribute to antinociception through inhibition of pronociceptive neuropeptides such as galanin, which are upregulated after nerve damage (Wotherspoon et al. 2005). Another neuropeptide released by primary afferent fibers and involved in pain transmission is CGRP (Brooks et al. 2004). A simple way to stimulate CGRP release in these neurons is by activation of TRPV1. Administration of such selective CB2R agonists as (+)-AM1241 and L768242 has been shown to inhibit capsaicin-induced CGRP release. This effect was competitively antagonized by CB2R antagonist/inverse agonist SR 144528, confirming that the effect was CB2R mediated (Beltramo et al. 2006). Anand et al. (2008) demonstrated co-localization of CB2R with TRPV1 in small- to medium-diameter DRG neurons and determined that CB2R-mediated cAMP depletion attenuated TRPV1 activation. Increased cAMP levels produced by inflammatory mediators such as prostaglandins are known to activate PKA in nociceptive afferents, resulting in hyperalgesia, and the direct activation of PKA with cAMP analogues is known to cause behavioral hypersensitivity (Taiwo et al. 1989; Taiwo and Levine 1991). Thus, CB2R agonists appear to block TRPV1 activation by depleting cAMP.
Expression of CB2R in Satellite Glial Cells of DRG
SGCs enveloping bodies of the primary sensory neurons in DRG support normal sensory transmission and nociception by maintaining metabolic and ionic homeostasis. SGCs identified by the GS antibody are similar in many respects to other glial cells, but they may differ in certain specific details (Hanani 2005).
In models of neuropathy and peripheral inflammation, SGCs proliferate and become activated (Lu and Richardson 1991; Liu F-Y et al. 2012), thus resulting in increased expression of various molecules such as proinflammatory cytokines (Dubový et al. 2006; Takeda M et al. 2007; Dubový et al. 2010a) and neurotrophins (Hanani 2005; Ohara et al. 2009; Takeda, Muramatsu, et al. 2009) and changes in functional gap junctions (Huang et al. 2005; Jasmin et al. 2010). Mechanisms by which peripheral nerve injury causes activation of SGCs may involve ATP, and ATP released from damaged neurons is believed to be one of the critical mediators involved in activating SGCs through stimulation of purinergic receptors (reviewed by Ohara et al. 2009). Recent studies have demonstrated that SGCs were able to modulate neuronal excitability leading to neuropathic pain (Hanani 2005; Takeda, Takahashi, and Matsumoto 2009).
We found co-localization of CB2R and GS as SGC markers, whereas the ED-1+ macrophages invading the neuronal area did not display immunoreaction for CB2R protein. ED1+ cells are regarded as activated, invading monocyte/macrophage cells, whereas ED2 antibody is used for detecting resident macrophage populations (Dijkstra and Damoiseaux 1993). The naive DRG contains resident macrophages that display immunostaining with ED2 antibody and also weak ED1 positivity. In addition to ED2+ cells, however, a robust amount of ED1+ macrophages has been shown to invade the DRG in CCI-operated rats (Hu and McLachlan 2003; Dubový et al. 2007; Hu et al. 2007). Therefore, the ED1+ macrophages in the DRG of our CCI-operated rats were recruited macrophages that had no immunostaining for CB2R. Moreover, co-localization of CB2R and ED2 immunostaining also was not observed (data not shown).
The SGCs displayed immunofluorescence for CB2R protein and signals for CB2R mRNA, particularly around large neuronal bodies. Our results provide unequivocal evidence that activated SGCs are able to synthesize CB2R. We presume that modulation of CB2R in SGCs may affect the activity of DRG neurons and provide another target for therapeutic intervention by external ligands. This corresponds with the presumption that cannabinoid activation of CB2R in activated microglial cells induced by neuropathic pain states may reduce secretion of neuroexcitatory substances in the spinal cord (Zhang et al. 2003). Our evidence that DRG neurons and their SGCs are able to synthesize CB2R in a neuropathic pain model is in accordance with the conclusion that DRG constitute a crucial site for CB2R-mediated analgesia (Mitrirattanakul et al. 2006; Hsieh et al. 2011).
Bilateral CB2R Expression in DRGs Associated and Not Associated with Injured Nerves
We found upregulation of CB2R protein and mRNA in the DRG not only ipsilateral but also contralateral to CCI of the sciatic nerve. This phenomenon was demonstrated by immunohistochemistry and western blot analysis for CB2R protein and by in situ hybridization for CB2R mRNA. Our results showing bilateral upregulation of CB2R in lumbar DRG are supported by quantitative RT-PCR in a rat spinal nerve ligation neuropathic pain model (Hsieh et al. 2011). Various experimental models have shown that a unilateral nerve injury evokes bilateral changes (Pitcher et al. 1999; Woolf and Mannion 1999; Yamaguchi et al. 1999; Kleischnitz et al. 2005; Brázda et al. 2009; Dubový et al. 2010b), but the underlying mechanisms are largely unknown. One of the possible mechanisms for contralateral signaling is transmission via commissural interneurons (Koltzenburg et al. 1999).
In our experiments, however, unilateral CCI of the sciatic nerve induced elevation of CB2R mRNA and protein in the DRG of L4–L5 spinal cord segments and also C7–C8 segments not associated with the injured nerve. Bilateral upregulation of CB2R protein and mRNA not only in L4–L5 but also in C7–C8 DRG suggests that systemic signaling (e.g., by circulating signal molecules in the bloodstream) cannot be excluded (Klusáková and Dubový 2009; Dubový 2011). Wallerian degeneration distal to nerve injury results in interruption of the blood-nerve barrier (Mellick and Cavanagh 1968), allowing diffusion of signaling molecules produced by the Schwann and immune cells (Üçeyler and Sommer 2006) into the blood flow. An absence of a blood-nerve barrier in the intact DRG (Jacobs et al. 1976) supports a possibility for circulating signaling molecules to be diffused into the microenvironment of DRG not associated with the injured peripheral nerve. Several candidate molecules have been suggested as signaling from the damaged nerve. For example, multiple components of complement activation are regulated in DRG following nerve injury (Levin et al. 2008), but further experiments are needed to examine their potential involvement in signaling and changes to CB2R levels in heteronymous DRG.
Upregulation of CB2R in Sham-Operated Rats
Sham surgery is sufficient to induce changes in mechanically evoked responses in the hindpaws. However, the magnitude is less and the duration not so long-lasting as are those observed in rats with CCI of the sciatic nerve (Pitcher et al. 1999). A surgical procedure without major nerve damage induces transsynaptic degeneration in laminae I–III of the spinal dorsal horn (Nachemson and Bennett 1993). In addition, a skin incision alone, without injury to the main nerve trunk, is able to initiate SGC proliferation in DRG (Elson et al. 2004) and induce axonal growth and regeneration genes (Hill et al. 2010). Our data, in line with these results, show increased sensitivity to both mechanical and thermal stimuli in sham-operated rats, but this is less than that of CCI animals. Although western blot analysis provided no evidence for increased CB2R protein in the DRG of sham-operated rats, a moderate intensity of CB2R mRNA and protein staining was observed in both neurons and SGCs predominantly surrounding the large neurons, especially on day 14 after sham operation. In line with our results, Sagar et al. (2005) have provided ex vivo evidence that activation of CB2R in the DRG attenuates evoked responses from neuropathic as well as sham-operated rats. In addition, it has been observed that thermal hyperalgesia and mechanical allodynia induced by unilateral sciatic nerve injury were enhanced in CB2–/– mice on the contralateral side (Racz et al. 2008).
Upregulation of CB2R mRNA and Protein in DRG and Neuroinflammation
There is now accumulating evidence that inflammatory processes at the site of nerve injury, in DRGs and in the spinal cord projection area contribute significantly to neuropathic pain induction and maintenance (Moalem and Tracey 2006). Nerve injury stimulates immune cell invasion and changes in immune mediators in the distal nerve stump (Ruohonen et al. 2005; Rotshenker 2011), followed by the activation of SGCs in the DRG (Dubový et al. 2006; Dublin and Hanani 2007; Dubový et al. 2010a), spinal cord microglia and astrocytes (Scholz and Woolf 2007). The immune response is orchestrated by inflammatory mediators, including cytokines, chemokines, ATP, neuropeptides, prostaglandins, and endocannabinoids (DeLeo and Yezierski 2001; Clark et al. 2007).
The endocannabinoid system has physiological and/or pathophysiological roles in the modulation of immune reactions (Klein 2005) and pain (Pertwee 2001). Effects of endogenous ligands are mediated by cannabinoid receptors CB1 and CB2, the upregulation of which reflects a possible enhanced action of endocannabinoid ligands. It has been demonstrated that immune mediators such as cytokines or interferon-γ (IFN-γ) increase the expression of CB2R in animal models of neuropathic pain. The primary sensory neurons and their SGCs increase synthesis of immune mediators such as cytokines and IFN-γ (Brázda et al. 2006; Dubový et al. 2006; Austin and Moalem-Taylor 2010; Dubový et al. 2010a; Jančálek et al. 2010), which are able to upregulate the expression of CB2R (Jean-Gilles et al. 2010) in the DRG of animal models of neuropathic pain. The crucial role of CB2R in neuropathic pain is mediated through an immune mechanism linked to modified IFN-γ activity. Our previous findings revealed that a bilateral neuroinflammatory reaction in DRG is propagated alongside the neuraxis from the spinal segments in relation to segments not related to the injured nerve (Jančálek et al. 2010, 2011). The results showing upregulation of CB2R mRNA and protein in DRG presented here correspond with the propagation of a neuroinflammatory reaction indicated by the expression of cytokines and chemokines to remote DRG far from the injured nerve. This indicates that CB2R mRNA and protein are upregulated not only in relation to neuropathic pain induction but also to other general reactions of the nervous system to injury.
Neuroinflammation may induce or aggravate neuron damage through increased release of neurotoxic mediators, such as proinflammatory cytokines (tumor necrosis factor–α, interleukin-1β, interleukin-6), eicosanoids, nitric oxide, and reactive oxygen species. Alternatively, it could increase neuronal vulnerability to these cytotoxic stimuli (Fernández-Ruiz et al. 2005). Upregulation of anti-inflammatory cytokines (Jančálek et al. 2010) and CB2R reflecting a possible enhanced efficacy of endocannabinoids in remote DRG may protect the primary sensory neurons from detrimental effects of neuroinflammation spreading from spinal segments associated with the injured nerve. This is supported by a neuroprotective effect of CB2R upregulation by blocking cyt-c–associated apoptosis through Akt/PI3K signaling linked with a reduction in remote neuron death (Viscomi et al. 2009, 2010).
Acknowledgments
We thank Ms. Dana Kutějová, Ms. Marta Lněníčková, Ms. Jitka Mikulášková, Ms. Stana Bartová, Ms. Eva B. Jagelská, and Mr. Lumír Trenčanský for their skilful technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the projects “CEITEC—Central European Institute of Technology” (CZ.1.05/1.1.00/02.0068) from the European Regional Development Fund, SYLICA/286154 (FP7-REGPOT-2011-1).
References
- Anand U, Otto WR, Sanchez-Herrera D, Facer P, Yiangou Y, Korchev Y, Birch R, Benham C, Bountra C, Chessell IP, et al. 2008. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 138:667–680 [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. 2010. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 160:467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Straiker A, Mackie K. 2012. CB2 cannabinoid receptors inhibit synaptic transmission when expressed in cultured autaptic neurons. Neuropharmacology. 63:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PJ, Moalem-Taylor G. 2010. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 229:26–50 [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. 2006. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. J Neurosci. 23:1530–1538 [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. 1999. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Mol Pharmacol. 55:473–480 [PubMed] [Google Scholar]
- Brázda V, Klusáková I, Svíženská I, Veselková Z, Dubový P. 2009. Bilateral changes in IL-6 protein, but not in its receptor gp130, in rat dorsal root ganglia following sciatic nerve ligature. Cell Mol Neurobiol. 29:1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brázda V, Muller P, Brozkova K, Vojtesek B. 2006. Restoring wild-type conformation and DNA-binding activity of mutant p53 is insufficient for restoration of transcriptional activity. Biochem Biophys Res Commun. 351:499–506 [DOI] [PubMed] [Google Scholar]
- Brooks JW, Thompson SW, Rice AS, Malcanqio M. 2004. (S)-AMPA inhibits electrically evoked calcitonin gene–related peptide (CGRP) release from the rat dorsal horn: reversal by cannabinoid receptor antagonist SR141716A. Neurosci Lett. 372:85–88 [DOI] [PubMed] [Google Scholar]
- Brownjohn PW, Ashton JC. 2009. Novel targets in pain research: the case for CB2 receptors as a biorational pain target. Curr Anaesth Crit Care. 20:198–203 [Google Scholar]
- Burry RW. 2000. Specificity controls for immunocytochemical methods. J Histochem Cytochem. 48:163–165 [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. 1998. Control of pain initiation by endogenous cannabinoids. Nature. 394:277–281 [DOI] [PubMed] [Google Scholar]
- Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. 2007. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 11:223–230 [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. 2001. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 90:1–; 6. [DOI] [PubMed] [Google Scholar]
- Devor M, Seltzer Z. 1999. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, editors. Textbook of pain, 4th ed. Edinburgh, UK: Churchill Livingstone; p. 129–164 [Google Scholar]
- Dijkstra CD, Damoiseaux J. 1993. Macrophage heterogeneity established by immunocytochemistry. Prog Histochem Cytochem. 27:1–65 [DOI] [PubMed] [Google Scholar]
- Dublin P, Hanani M. 2007. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 21:592–598 [DOI] [PubMed] [Google Scholar]
- Dubový P. 2011. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann Anat. 193:267–275 [DOI] [PubMed] [Google Scholar]
- Dubový P, Jančálek R, Klusáková I, Svíženská I, Pejchalova K. 2006. Intra- and extraneuronal changes of immunofluorescence staining for TNF-alpha and TNFR1 in the dorsal root ganglia of rat peripheral neuropathic pain models. Cell Mol Neurobiol. 26:1205–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubový P, Klusáková I, Svíženská I. 2002. A quantitative immunohistochemical study of the endoneurium in the rat dorsal and ventral spinal roots. Histochem Cell Biol. 117:473–480 [DOI] [PubMed] [Google Scholar]
- Dubový P, Klusáková I, Svíženská I, Brázda V. 2010a. Satellite glial cells express IL-6 and corresponding signal-transducing receptors in the dorsal root ganglia of rat neuropathic pain model. Neuron Glia Biol. 6:73–83 [DOI] [PubMed] [Google Scholar]
- Dubový P, Klusáková I, Svíženská I, Brázda V. 2010b. Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem Cell Biol. 133:323–337 [DOI] [PubMed] [Google Scholar]
- Dubový P, Tuckova L, Jančálek R, Svíženská I, Klusáková I. 2007. Increased invasion of ED-1 positive macrophages in both ipsi- and contralateral dorsal root ganglia following unilateral nerve injuries. Neurosci Lett. 427:88–93 [DOI] [PubMed] [Google Scholar]
- Elson K, Simmons A, Speck P. 2004. Satellite cell proliferation in murine sensory ganglia in response to scarification of the skin. Glia. 45:105–109 [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, González S, Romero J, Ramos JA. 2005. Cannabinoids in neurodegeneration and neuroprotection. In: Mechoulam R, editor. Cannabinoids as therapeutics. Basel, Switzerland: Birkhäuser Verlag; p. 79–109. 10.1007/3-7643-7358-X_5 [DOI] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Mechoulam R, Shen R, Zhang MY, Strassle BW, Lu P, et al. 2010. Loss of retrograde endocannabinoids signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 30:2017–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanani M. 2005. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 48:457–476 [DOI] [PubMed] [Google Scholar]
- Hanuš L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R, Fride E. 1999. HU-308 a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci U S A. 96:14228–14233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 32:77–88 [DOI] [PubMed] [Google Scholar]
- Harnicarova A, Kozubek S, Pachernik J, Krejci J, Bartova E. 2006. Distinct nuclear arrangement of active and inactive c-myc genes in control and differentiated colon carcinoma cells. Exp Cell Res. 312:4019–4035 [DOI] [PubMed] [Google Scholar]
- Hill CE, Harrison BJ, Rau KK, Hougland MT, Bunge MB, Mendell LM, Petruska JC. 2010. Skin incision induces expression of axonal regeneration-related genes in adult rat spinal sensory neurons. J Pain. 11:1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Martin WJ, Tsou K, Walker JM. 1995. Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212-2. Life Sci. 56:2111–2118 [DOI] [PubMed] [Google Scholar]
- Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, Wensink EJ, Zhan CC, Carroll WA, Dart MJ, et al. 2011. Central and peripheral sites of action for CB2 receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 162:428–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Bembrick AL, Keay KA, McLachlan EM. 2007. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun. 21:599–616 [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM. 2003. Distinct functional types of macrophage in dorsal root ganglia and spinal nerves proximal to sciatic and spinal nerve transections in the rat. Exp Neurol. 184:590–605 [DOI] [PubMed] [Google Scholar]
- Huang TY, Cherkas PS, Rosenthal DW, Hanani M. 2005. Dye coupling among satellite glial cells in mammalian dorsal root ganglia. Brain Res. 1036:42–49 [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, et al. 2003. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 100:10529–10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JM, Macfarlane RM, Cavanagh JB. 1976. Vascular leakage in the dorsal root ganglia of the rat, studied with horseradish peroxidase. J Neurol Sci. 29:95–107 [DOI] [PubMed] [Google Scholar]
- Jančálek R, Dubový P, Svíženská I, Klusáková I. 2010. Bilateral changes of TNFalpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J Neuroinflamm. 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jančálek R, Svíženská HI, Klusáková I, Dubový P. 2011. Bilateral changes of IL-10 protein in lumbar and cervical dorsal root ganglia following proximal and distal chronic constriction injury of peripheral nerve. Neurosci Lett. 501:86–91 [DOI] [PubMed] [Google Scholar]
- Jasmin L, Vit J-P, Bhargava A, Ohara PT. 2010. Can satellite glial cells be therapeutic target for pain control? Neuron Glia Biol. 6:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Gilles L, Gran B, Constantinescu CS. 2010. Interaction between cytokines, cannabinoids and the nervous system. Immunobiology. 215:606–610 [DOI] [PubMed] [Google Scholar]
- Kirita T, Takebayashi T, Mizuno S, Takeuchi H, Kobayashi T, Fukao M, Yamashita T, Tohse N. 2007. Electrophysiologic changes in dorsal root ganglion neurons and behavioral changes in a lumbar radiculopathy model. Spine. 32:E65–E72 [DOI] [PubMed] [Google Scholar]
- Klein TW. 2005. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 5:400–411 [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Brinkhoff J, Sommer C, Stoll G. 2005. Contralateral cytokine gene induction after peripheral nerve lesions: dependence on the mode of injury and NMDA receptor signaling. Mol Brain Res. 136:23–28 [DOI] [PubMed] [Google Scholar]
- Klusáková I, Dubový P. 2009. Experimental models of peripheral neuropathic pain based on traumatic nerve injuries—an anatomical perspective. Ann Anat. 191:248–259 [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Wall PD, McMahon SB. 1999. Does the right side know what the left is doing? Trends Neurosci. 22:122–127 [DOI] [PubMed] [Google Scholar]
- Levin ME, Jin JG, Ji RR, Tong J, Pomonis JD, Lavery DJ, Miller SW, Chiang LW. 2008. Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain. Pain. 137:182–201 [DOI] [PubMed] [Google Scholar]
- Liu CN, Devor M, Waxman SG, Kocsis JD. 2002. Subthreshold oscillations induced by spinal nerve injury in dissociated muscle and cutaneous afferents of mouse DRG. J Neurophysiol. 87:2009–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F-Y, Sun Y-N, Wang F-T, Li Q, Su L, Zhao Z-F, Meng X-L, Zhao H, Wu X, Sun Q, et al. 2012. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res. 1427:65–77 [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. 1991. Inflammation near the nerve cell body enhances axonal regeneration. J. Neurosci. 11:972–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. 2001. CB2 cannabinoid receptor–mediated peripheral antinociception. Pain. 93:239–245 [DOI] [PubMed] [Google Scholar]
- Martin WJ, Hohmann AG, Walker JM. 1996. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: correlation between electrophysiological and antinociceptive effects. J Neurosci. 16:6601–6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellick RS, Cavanagh JB. 1968. Changes in blood vessel permeability during degeneration and regeneration in peripheral nerves. Brain. 91:141–160 [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, Ramakul N, Guerrero AV, Matsuka Y, Ono T, Iwase H, Mackie K, Faull KF, Spigelman I. 2006. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain. 126:102–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem G, Tracey DJ. 2006. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 51:240–264 [DOI] [PubMed] [Google Scholar]
- Nachemson AK, Bennett GJ. 1993. Does pain damage spinal cord neurons? Transsynaptic degeneration in rat following a surgical incision. Neurosci Lett. 162:78–80 [DOI] [PubMed] [Google Scholar]
- Ohara PT, Vit JP, Bhargava A, Romero M, Sundberg C, Charles AC, Jasmin L. 2009. Gliopathic pain: when satellite glial cells go bad. Neuroscientist. 15:450–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. 2001. Cannabinoid receptors and pain. Prog Neurobiol. 63:569–611 [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Ritchie J, Henry LJ. 1999. Nerve constriction in the rat: model of neuropathic, surgical and central pain. Pain. 83:37–46 [DOI] [PubMed] [Google Scholar]
- Racz I, Nadal X, Alferink J, Baños JE, Rehnelt J, Martín M, Pintado B, Gutierrez-Adan A, Sanguino E, Manzanares J, et al. 2008. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J Neurosci. 28:12125–12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. 1998. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 75:111–119 [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, MacEwan DJ, Scott RH. 2001. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 40:221–232 [DOI] [PubMed] [Google Scholar]
- Rotshenker S. 2011. Wallerian degeneration: the innate immune response to traumatic nerve injury. J Neuroinflammation. 8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruohonen S, Khademi M, Jagodic M, Taskinen H-S, Olsson T, Roytta M. 2005. Cytokine responses during chronic denervation. J Neuroinflamm. 2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Kelly S, Millns PJ, O’Shaughnessey CT, Kendall DA, Chapman V. 2005. Inhibitory effects of CB1 receptor and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur J Neurosci. 22:371–379 [DOI] [PubMed] [Google Scholar]
- Saňudo-Peňa MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. 2000. Activational role of cannabinoids on movement. Eur J Pharmacol. 391:269–274 [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. 2007. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 10:1361–1368 [DOI] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. 2004. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 109:124–131 [DOI] [PubMed] [Google Scholar]
- Ständer S, Schmelz M, Metze D, Luger T, Rukwied R. 2005. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 38:177–188 [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. 1989. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 32:577–580 [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. 1991. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 44:131–135 [DOI] [PubMed] [Google Scholar]
- Takeda K, Muramatsu M, Chikuma T, Kato T. 2009. Effect of memantine on the levels of neuropeptides and microglial cells in the brain regions of rats with neuropathic pain. J Mol Neurosci. 39:380–390 [DOI] [PubMed] [Google Scholar]
- Takeda M, Takahashi M, Matsumoto S. 2009. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 33:784–792 [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S. 2007. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain 129:155–166 [DOI] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, et al. 2010. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 65:320–327 [DOI] [PubMed] [Google Scholar]
- Tsou K, Lowitz KA, Hohmann AG, Martin WJ, Hathaway CB, Bereiter DA, Walker JM. 1996. Suppression of noxious stimulus-evoked expression of FOS protein-like immunoreactivity in rat spinal cord by a selective cannabinoid agonist. Neuroscience. 70:791–798 [DOI] [PubMed] [Google Scholar]
- Üçeyler N, Sommer C. 2006. Wallerian degeneration and neuropathic pain. Drug Discov Today Dis Mech. 3:351–356 [Google Scholar]
- Viscomi MT, Oddi S, Latini L, Bisicchia E, Maccarrone M, Molinari M. 2010. The endocannabinoid system: a new entry in remote cell death mechanisms. Exp Neurol. 224:56–65 [DOI] [PubMed] [Google Scholar]
- Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, Molinari M, Maccarrone M. 2009. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3 K/Akt pathway. J Neurosci. 29:4564–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak J-S, Pichette V, Leblond F, Desbiens K, Beaulieu P. 2005. Behavioural, pharmacological and molecular characterization of the saphenous nerve partial ligation: a new model of neuropathic pain. Neuroscience. 132:1093–1102 [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Lee GP, Valenzano KJ. 2007. The role of the cannabinoid CB2 receptor in pain transmission and therapeutic potential of small molecule CB2 receptor agonists. Curr Med Chem. 14:917–936 [DOI] [PubMed] [Google Scholar]
- Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Kuhn MN, Zvonok AM, Thakur GA, Makriyannis A, Milligan ED. 2012. Immunofluorescent spectral analysis reveals the intrathecal cannabinoid agonist, AM1241, produces spinal anti-inflammatory cytokine responses in neuropathic rats exhibiting relief from allodynia. Brain Behav. 2:155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. 1999. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 353:1959–1964 [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. 2005. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 135:235–245 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Ochi M, Mori R, Ryoke K, Yamamoto S, Iwata A, Uchio Y. 1999. Unilateral sciatic nerve injury stimulates contralateral nerve regeneration. Neuroreport. 10:1359–1362 [DOI] [PubMed] [Google Scholar]
- Zamboni L, De Martino C. 1967. Buffered picric acid–formaldehyde—a new rapid fixative for electron microscopy. J Cell Biol. 35:A148 [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnel D. 2003. Induction of CB2 receptor expression in the rat spinal cord neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 17:2750–2754 [DOI] [PubMed] [Google Scholar]