Abstract
Rationale: In San Francisco, 70% of the tuberculosis cases occur among foreign-born persons, mainly from China, the Philippines, and Mexico. We postulate that there are differences in the characteristics and risk factors for tuberculosis among these populations.
Objectives: To determine the clinical, epidemiological and microbiological characteristics of tuberculosis caused by recent infection and rapid evolution in the major groups of foreign-born and the U.S.-born populations.
Methods: We analyzed data from a 20-year prospective community-based study of the molecular epidemiology of tuberculosis in San Francisco. We included all culture-positive tuberculosis cases in the City during the study period.
Measurements and Main Results: We calculated and compared incidence rates, clinical and microbiological characteristics, and risk factors for being a secondary case between the various foreign-born and U.S.-born tuberculosis populations. Between 1991 and 2010, there were 4,058 new cases of tuberculosis, of which 1,226 (30%) were U.S.-born and 2,832 (70%) were foreign-born. A total of 3,278 (81%) were culture positive, of which 2,419 (74%) had complete data for analysis. The incidence rate, including the incidence rate of tuberculosis due to recent infection and rapid evolution, decreased significantly in the U.S.-born and the major foreign-born populations. The clinical and microbiological characteristics and the risk factors for tuberculosis due to recent infection differed among the groups.
Conclusions: There are differences in the characteristics and the risk factors for tuberculosis due to recent transmission among the major foreign-born and U.S.-born populations in San Francisco. These differences should be considered for the design of targeted tuberculosis control interventions.
Keywords: tuberculosis, foreign-born populations, epidemiology, Mycobacterium tuberculosis
At a Glance Commentary
Scientific Knowledge on the Subject
-
•
In the United States, tuberculosis occurs mainly among foreign-born persons.
-
•
The clinical characteristics and risk factors for tuberculosis (TB), including risk factors for tuberculosis due to recent infection and rapid evolution to active disease, are different in U.S.-born versus foreign-born populations.
-
•
In San Francisco, tuberculosis in the foreign-born population occurs mainly among patients born in China, the Philippines, and Mexico, each of which have their own cultural and social attributes that may lead to different risk factors for TB.
What This Study Adds to the Field
-
•
The clinical and bacterial characteristics and risk factors associated with tuberculosis due to recent infection and rapid progression to active disease differ among patients born in the United States, China, the Philippines, and Mexico.
-
•
Descriptive epidemiological analyses grouping all foreign-born cases together obscure important differences in presentation and risk factors for tuberculosis due to recent transmission.
-
•
Differences in the microbiological characteristics among various lineages of M. tuberculosis may account for some of the clinical and epidemiological differences noted among foreign-born groups.
-
•
This study suggests that to be effective it is important to consider tailored public health interventions to address various foreign-born groups.
The overall rate of tuberculosis (TB) in the United States decreased from 9.7 cases per 100,000 population in 1993 to 3.4 cases per 100,000 in 2011 (1). During these two decades, the proportion of cases occurring in foreign-born persons has increased from 29.5% of the 25,107 TB cases reported in 1993 to 62.2% of the 10,521 TB cases reported in 2011. In 2011, the case rate in the foreign-born population was 17.3 cases per 100,000 compared with 1.5 cases per 100,000 among U.S.-born persons (1). In San Francisco, we previously reported a significant decline of TB cases in United States and foreign born-populations (including TB cases due to recent transmission) from 1991 to 1999 but a slower decline since 2000, despite application of control measures (2).
In the 1990s TB among foreign-born persons was attributed mainly to reactivation of prior infection acquired in the country of origin (3). In contrast, TB among U.S.-born persons was attributed to both reactivation of a latent infection and to recent infection evolving rapidly to active TB (4–6). The risk factors for TB due to recent infection in U.S.-born patients were younger age, homelessness, and drug and alcohol use, whereas foreign-born patients with TB were older and without risk factors (2, 5).
There are a number of reasons to postulate that there are differences in the epidemiology of TB among foreign-born persons from different countries. These include variations in (1) the prevalence of TB in the country of origin (7), (2) the manner in which foreign-born persons enter the United States (with or without predeparture screening for TB) (8), (3) prevention efforts targeted toward immigrant groups (9), (4) host susceptibility (10), (5) differences in pathogenicity among the various lineages of Mycobacterium tuberculosis (M.tb) (11–13), and (6) other unidentified factors.
In San Francisco, 72% of the TB cases reported between 1992 and 2011 occurred in foreign-born persons, mainly from China, the Philippines, and Mexico, each of which have their own demographic, cultural, and social characteristics. The strains of M.tb causing most of the TB in these populations are from different lineages: Chinese patients have TB mainly by M.tb lineage 2 strains (also known as East Asian lineage); Filipino patients by lineage 1 (Indo-Oceanic lineage); and Mexicans, and U.S.-born Hispanic, white, and African American patients by lineage 4 (Euro-American lineage) (12).
In this article, we used conventional and molecular epidemiological methods and phylogeographic lineage analysis to explore the hypothesis that in San Francisco the clinical, microbiological, and epidemiological characteristics of TB differ among populations by country of origin and that these differences should be considered in the design of population-specific strategies to control TB. Some of the results of this study have been previously reported in the form of an abstract (14).
Methods
The study was approved by the University of California, San Francisco (San Francisco, CA) Human Research Protection Program. This is the fourth of a series of progressively larger prospective community-based studies conducted in San Francisco (2–4). There is partial overlap in the study population, but there is no overlap in the questions addressed.
The incidence rate of TB among U.S.-born patients was calculated using the national census population data for San Francisco (15, 16). The incidence rate of TB among patients born in the Philippines, China, and Mexico was calculated on the basis of estimates of those populations in San Francisco available only since 2000 (15–17).
Genotyping of M.tb was performed using restriction fragment length polymorphisms with the insertion sequence (IS) 6110 (18) and the polymorphic guanine-cytosine–rich sequence (PGRS) (19). The phylogeographic lineage of the M.tb isolates was determined using regions of difference (RD) and single-nucleotide polymorphisms (12).
Definitions
Clusters were defined as two or more persons having M.tb isolates with the same IS6110 (and PGRS if fewer than six IS6110 bands)-based genotype reported within 12 months of one another. The first case in the cluster was defined as the index case and subsequent cases as secondary cases. All other cases were defined as unique cases. Index cases from 2010 were excluded from this definition because a 12-month window (into 2011) could not be constructed for these cases.
Lineages of M.tb (12): Lineage 4 was defined by the presence of the codon cgg in katG463. The other lineages were defined by the absence of RD: lineage 1 by RD239, lineage 2 by RD105, lineage 3 by RD750, lineage 5 by RD711, and lineage 6 by RD702. Lineages 3, 5, and 6 were rare in our population and were not included in the analysis.
Phylogeographic associations were defined as sympatric when there was concordance between the patient’s race/ethnicity and the lineage of M.tb: Chinese patients with lineage 2 M.tb, Filipino patients with lineage 1 M.tb, and Mexican or U.S.-born Hispanic, white, or African American patients with lineage 4 M.tb. All other combinations were defined as allopatric (12).
Statistical Analysis
Patient characteristics and risk factors for being a secondary case were explored in the various populations using chi-squared tests and logistic regression, adjusting for factors known to be associated with transmission (sputum smear examination and presence of cavitary disease). We performed a stratified analysis to identify differences in cases diagnosed in 1991–1999 and in 2000–2010. To compare the secondary case rates between phylogeographic M.tb lineages in the various populations (12), we converted the lineage-specific secondary case rates into secondary case rate ratios by dividing the secondary case rate of the lineage of interest by the secondary case rate of the other two lineages combined. To calculate the population-specific secondary case rate, we used as the denominator the sum of all index cases plus unique cases with complete genotyping data, as any patient has the potential to cause secondary cases. Trends over time were assessed using the Poisson test for trend.
Results
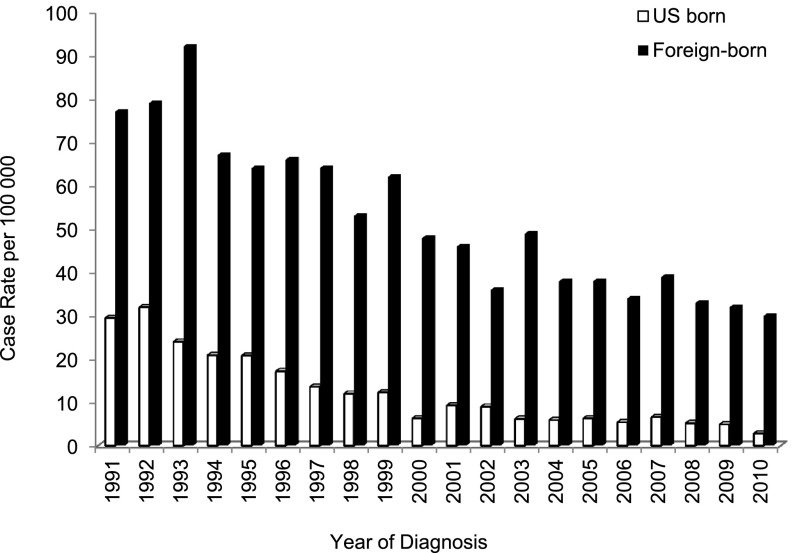
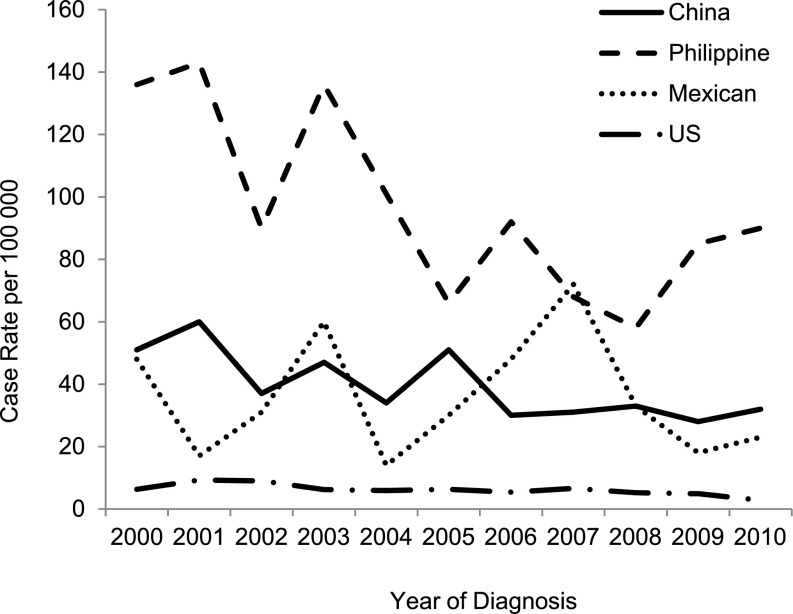
Between January 1991 and December 2010, there were 4,058 cases of TB reported to the San Francisco Department of Public Health; 81% (n = 3,288) had pulmonary disease, and 70% (n = 2,832) were foreign-born (Figure 1). The incidence rate of reported TB cases in San Francisco from 1991 to 2010 is shown in Figure 2. As reported previously (2), there was a sharp decline in the TB rate from 1991 to 1999 in both the U.S.-born population, from 29.5 to 12.3 per 100,000 inhabitants (Poisson for trend: P < 0.001; coefficient, –0.15), and in the foreign-born population, from 77 to 62 per 100,000 inhabitants (P < 0.001; coefficient, –0.06). The decline from 2000 to 2010 was smaller but still significant for both populations: from 6.3 to 2.8 per 100,000 inhabitants (P < 0.001; coefficient, –0.07) in the U.S.-born, and 48 to 30 per 100,000 inhabitants (P < 0.001; coefficient, –0.05) in the foreign-born (Figure 2). The overall reduction was greater (90.5%) in the U.S.-born population compared with the foreign-born population (62.3%; P < 0.001). This resulted in an increase of the proportion of TB cases in foreign-born persons compared with U.S.-born persons, from 58% in 1991 to 85% in 2010. Between 2000 and 2010, the incidence rate of TB decreased 33.8% in patients born in the Philippines (P = 0.001; coefficient, –0.07), and 37.2% in patients born in China (P < 0.001; coefficient, –0.06); the incidence rate in patients born in Mexico decreased by 52% overall, but rates in the intervening years were too volatile to define a consistent downward trend (P = 0.66; coefficient, –0.01) (Figure 3). The incidence rate of TB for these populations in previous years was not calculable because population sizes were not available.
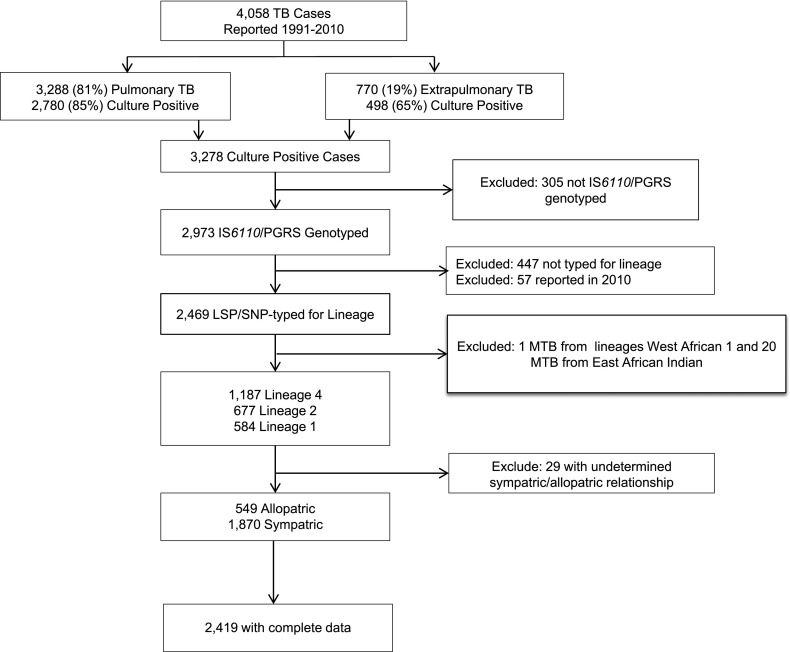
Figure 1.
San Francisco study population, 1991–2010. LSP = large sequence polymorphism; MTB = Mycobacterium tuberculosis; PGRS = polymorphic guanine-cytosine–rich sequence; SNP = single-nucleotide polymorphism; TB = tuberculosis.
Figure 2.
Incidence rate of reported tuberculosis (TB) cases per 100,000 inhabitants by year among U.S.-born and foreign-born populations, San Francisco, 1991–2010 (n = 4,058). The U.S.-born case rate decreased from 29.5 in 1991 to 2.8 in 2010 (P < 0.001; coefficient, –0.12). The foreign-born case rate decreased from 77 in 1991 to 30 in 2010 (P < 0.001; coefficient, –0.06).
Figure 3.
Incidence rate of tuberculosis (TB) cases by year among three foreign-born populations and the U.S.-born population, San Francisco, 2000–2010. The incidence rate of TB decreased 33.8% in patients born in the Philippines (P = 0.001; coefficient, –0.07), 37.2% in patients born in China (P < 0.001; coefficient, –0.06), and 52% in patients born in Mexico (P = 0.66; coefficient, –0.01).
Eighty-one percent of all reported cases had a positive culture for M.tb. Of the 3,278 culture-positive cases, 74% (n = 2,419) were genotyped using IS6110 (and PGRS where indicated); were determined by RD/single-nucleotide polymorphisms to be in lineage 1, 2, or 4; and had reported a race/ethnicity known to be sympatric or allopatric with their TB lineage (Figure 1); of these, 731 (30%) were U.S.-born and 1,688 (70%) were foreign-born. The distribution of the place of birth was similar between those included and excluded from the final analysis. Patients with incomplete genotyping data differed from those with complete data in that they were less likely to have smear-positive sputum, cavitary lesions on chest radiographs, or isoniazid (INH)-resistant M.tb, and less likely to be coinfected with HIV or homeless.
Patient and Bacterial Characteristics Were Different in the Various Patient Groups
When compared with U.S.-born patients, China- and Philippine-born patients were older, whereas Mexican patients were younger (Table 1). The frequency of extrapulmonary disease only was similar among the various populations, but the frequency of combined extrapulmonary and pulmonary disease was more frequent among Mexicans and U.S-born patients. Also, when compared with U.S.-born patients, China-born patients had a lower proportion of cavitary lesions on chest radiographs, and patients born in the Philippines and Mexico had a higher proportion of cavitary TB. The frequency of smear-positive TB, coinfection with HIV, as well as the association with homelessness and alcohol abuse were different among the populations. In patients from the Philippines the proportion of INH-resistant (15.4%) and multidrug-resistant (MDR) TB (2.3%) was higher. The relevant differences in the characteristics of the cases diagnosed in 1991–1999 and those diagnosed in 2000–2011 were (1) a decrease in U.S.-born patients with TB coinfected with HIV from 40% in 1991–1999 to 29% in 2000–2011, and (2) an increase in proportions of homeless patients from 37 to 45%, respectively.
TABLE 1.
CLINICAL AND DEMOGRAPHIC CHARACTERISTICS OF PATIENTS WITH ACTIVE TUBERCULOSIS BY PLACE OF BIRTH (n = 4,058, 1991–2010)
| China [n (%)] | The Philippines [n (%)] | Mexico [n (%)] | United States (Referent) [n (%)] | Others [n (%)] | |
|---|---|---|---|---|---|
| n |
941 |
677 |
197 |
1,226 |
1,017 |
| Age, yr |
|
|
|
|
|
| Median (IQR) |
66 (50–77) |
55 (38–70) |
33 (26–42) |
41.5 (33–54) |
40 (29–58) |
| Mean ± SD |
62.1 ± 19.1 |
53.8 ± 19.9 |
35.9 ± 16.0 |
43.2 ± 18.4 |
44.1 ± 19.6 |
| P value |
<0.001 |
<0.001 |
<0.001 |
1.00 |
0.26 |
| Sex, no. (%) |
|
|
|
|
|
| Male |
582 (61.9) |
460 (68.0) |
158 (80.2) |
918 (74.9) |
555 (54.6) |
| Female |
359 (38.2) |
217 (32.1) |
39 (19.8) |
308 (25.1) |
462 (45.4) |
| P value |
<0.001 |
0.001 |
0.11 |
1.00 |
<0.001 |
| Site of disease |
|
|
|
|
|
| Pulmonary only |
706 (75.0) |
520 (76.8) |
127 (64.5) |
845 (68.9) |
653 (64.2) |
| Pulmonary and extrapulmonary |
68 (7.2) |
40 (5.9) |
36 (18.3) |
192 (15.7) |
101 (9.9) |
| Extrapulmonary only |
167 (17.8) |
117 (17.3) |
34 (17.3) |
189 (15.4) |
263 (25.9) |
| P value* |
0.15 |
0.29 |
0.51 |
1.00 |
<0.001 |
| Sputum smear positive (n = 3,046) |
222 (31.1) |
183 (33.8) |
58 (38.4) |
410 (44.0) |
260 (36.7) |
| P value |
<0.001 |
<0.001 |
0.20 |
1.00 |
0.003 |
| Chest X-ray (n = 3,265) |
|
|
|
|
|
| Cavities |
82 (10.7) |
102 (18.3) |
32 (19.6) |
141 (13.8) |
129 (17.2) |
| Abnormal (NC) |
680 (88.4) |
445 (79.8) |
127 (77.9) |
855 (83.4) |
611 (81.5) |
| Normal |
7 (0.9) |
11 (2.0) |
4 (2.5) |
29 (2.8) |
10 (1.3) |
| P value† |
0.050 |
0.02 |
0.049 |
1.00 |
0.046 |
| HIV result |
|
|
|
|
|
| Positive |
3 (0.3) |
29 (4.3) |
41 (20.8) |
454 (37.0) |
78 (7.7) |
| Negative |
271 (28.8) |
280 (41.4) |
102 (51.8) |
394 (32.1) |
428 (42.1) |
| Unknown |
667 (70.9) |
368 (54.4) |
54 (27.4) |
378 (30.8) |
511 (50.3) |
| P value‡ |
<0.001 |
<0.001 |
<0.001 |
1.00 |
<0.001 |
| Homeless (n = 3,361) |
2 (0.2) |
14 (2.4) |
52 (30.8) |
373 (40.2) |
54 (6.3) |
| P value |
<0.001 |
<0.001 |
0.02 |
1.00 |
<0.001 |
| Alcohol abuse (n = 3,026) |
21 (2.9) |
25 (4.9) |
57 (36.3) |
276 (32.8) |
84 (10.8) |
| P value |
<0.001 |
<0.001 |
0.39 |
1.00 |
<0.001 |
| Any INH resistant (n = 3,372) |
80 (9.8) |
86 (15.4) |
9 (6.0) |
52 (5.1) |
94 (11.5) |
| P value |
<0.001 |
<0.001 |
0.64 |
1.00 |
<0.001 |
| Any RIF resistant (n = 3,372) |
13 (1.6) |
15 (2.7) |
5 (3.3) |
28 (2.7) |
23 (2.8) |
| P value |
0.11 |
0.96 |
0.68 |
1.00 |
0.92 |
| MDR (n = 3,372) |
12 (1.5) |
13 (2.3) |
2 (1.3) |
8 (0.8) |
14 (1.7) |
| P value | 0.16 | 0.01 | 0.50 | 1.00 | 0.08 |
Definition of abbreviations: HIV = human immunodeficiency virus; INH = isoniazid; IQR = interquartile range; MDR = multidrug resistant; NC = noncavitary; RIF = rifampin.
Note: Sputum smear results: 242 of 3,288 pulmonary patients had missing smear status.
Note: Chest radiograph: 23 of 3,288 pulmonary patients had missing radiograph information.
P value tests extrapulmonary versus pulmonary and both combined.
P value tests cavitary versus all others combined.
P value tests HIV-positive versus all others combined.
Prevalent Strains in San Francisco Were Strongly Associated with Sympatric Patient Populations
On the basis of the reported ethnicity/race, 98.8% of the patients born in China were reported to be ethnically Chinese, 99.8% of the patients born in the Philippines were Filipino, 100% of the patients born in Mexico were Mexicans/Hispanics, and 92.8% of the patients born in the United States were either Hispanic, white, or African American.
In patients from the Philippines and Mexico more than 85% of the phylogeographic associations were sympatric. Of the China-born patients 68.2% had lineage 2 M.tb, 24.3% had lineage 4 M.tb, and the remaining 7.2% had lineage 1 M.tb (Table 2). Further analysis showed that among those with lineage 4, all but two (1.5%) were ethnically Chinese. China-born patients infected with lineage 4 M.tb had been in the United States for a shorter time than those infected with lineage 2 or 1 (median time in years between entry into United States and diagnosis: 9, 14, and 10.8 yr, respectively) and the proportion of secondary cases due to strains from lineage 4 (7.4%) was similar to the proportion of secondary cases due to strains from lineage 2 (6.5%). The frequency of HIV infection was low in patients infected with either lineage.
TABLE 2.
FREQUENCY OF Mycobacterium tuberculosis LINEAGES BY PLACE OF BIRTH FOR THE FOUR GROUPS OF INTEREST (n = 1,814)*
| Birthplace |
||||
|---|---|---|---|---|
| China [n (%)] | The Philippines [n (%)] | Mexico [n (%)] | United States [n (%)] | |
| n |
559 |
399 |
125 |
731 |
| Lineage 2 (East Asian) |
383 (68.5%) |
20 (5.0%) |
7 (5.6%) |
85 (11.6%) |
| Lineage 4 (Euro-American) |
136 (24.3%) |
24 (6.0%) |
113 (90.4%) |
595 (81.4%) |
| Lineage 1 (Indo-Oceanic) |
40 (7.2%) |
355 (89.0%) |
5 (4.0%) |
51 (7.0%) |
| Allopatric association |
178 (31.8%) |
45 (11.3%) |
12 (9.6%) |
134 (18.3%) |
| P value | <0.001 | 0.002 | 0.02 | 1.00 |
Excluding 605 patients born elsewhere.
The Incidence Rate of Secondary TB Cases Decreased, but the Reduction Was Greater among U.S.-Born Patients
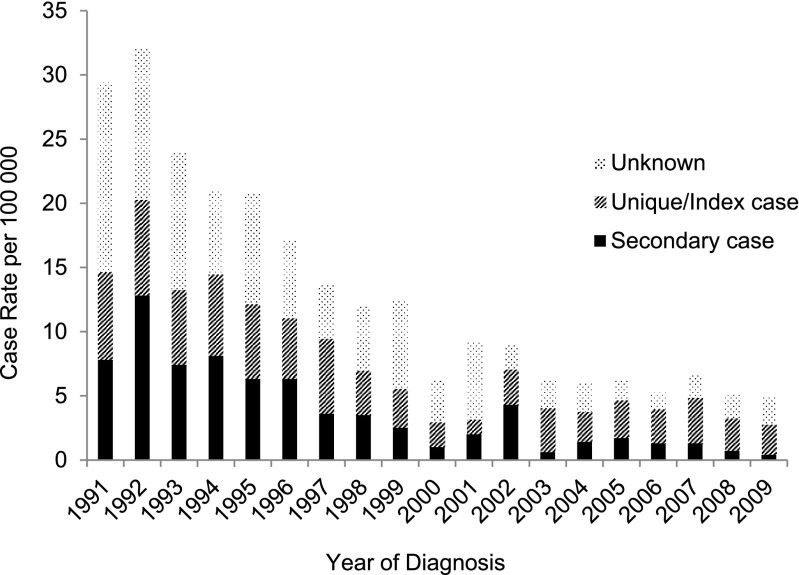
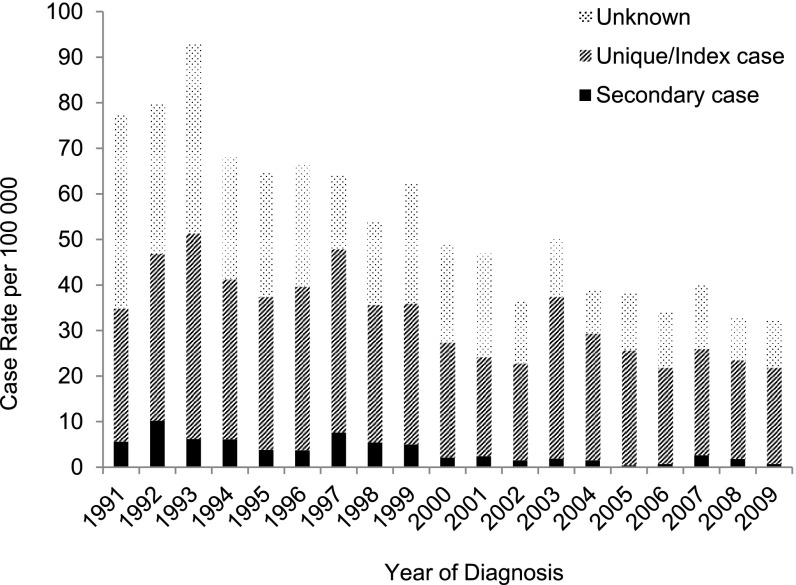
Between 1991 and 2009, the incidence rate of secondary cases decreased significantly in both U.S.-born (Figure 4) and foreign-born (Figure 5) populations (94.9 vs. 87.5%, respectively), but decreased relatively more among the U.S.-born compared with the foreign-born (P value for trend = 0.045). The stratified analysis showed a significant decrease in the incidence rate of secondary cases in the U.S.-born between 1991 and 1999 (P < 0.001; coefficient, –0.15) and between 2000 and 2009 (P < 0.001; coefficient, –0.12). Although the incidence rate of secondary cases in the foreign-born declined significantly between 1991 and 2009 (P < 0.001; coefficient, –0.12), when the study period was divided for stratified analysis, there was no significant decrease in either the earlier or later decade. There was a reduction of unique and index cases in both the foreign- and U.S.-born, but the reduction was lower in foreign-born patients (27.4 vs. 65.2%, respectively; P value for trend = 0.001) (Figures 4 and 5).
Figure 4.
Incidence rate of secondary cases, index cases/unique cases, and unknown (not determined) genotyping cases of tuberculosis among U.S.-born patients in San Francisco from 1991 through 2009 (n = 2,419), assessed using a 1-year window period (U.S.-born secondary cases decreasing: P < 0.001; coefficient, –0.16; U.S.-born index/unique cases decreasing: P < 0.001; coefficient, –0.11).
Figure 5.
Incidence rate of secondary cases, index cases/unique cases, and unknown (not determined) genotyping cases of tuberculosis among foreign-born patients in San Francisco from 1991 through 2009 (n = 2,419), assessed using a 1-year window period (foreign-born secondary cases decreasing: P < 0.001; coefficient, –0.12; foreign-born index/unique cases decreasing: P < 0.001; coefficient, –0.05).
Risk Factors for TB Due to Recent Infection Were Different in the Various Populations
Stratified analysis of secondary case rates of M.tb lineages showed that in each of the populations, the secondary case rate ratios were significantly higher among sympatric compared with allopatric associations. This association persisted after adjusted analysis considering other known risk factors for transmission and rapid progression to disease, such as age, HIV status, and direct smear examination results (Table 3). Besides the sympatric association, the risk factors associated with recent infection and rapid evolution to active TB in the adjusted analysis differed among the populations: persons born in the Philippines with known risk factors for infection and for developing TB were no more likely to be secondary cases than persons without risk factors; persons born in China who were 50 years of age or older and persons from Mexico who were younger than 50 years and male were more likely to be secondary cases. U.S.-born patients had several risk factors for TB due to recent infection, including age younger than 50 years, coinfection with HIV, homelessness, alcohol abuse, injection drug use, and INH-resistant TB. The stratified analysis showed that HIV was a significant risk factor before and after 2000 in spite of the decrease of HIV in the U.S. population in San Francisco. Similarly, homelessness and alcohol abuse were significant in both periods (data not shown).
TABLE 3.
RISK FACTORS FOR TUBERCULOSIS DUE TO RECENT INFECTION AND RAPID EVOLUTION TO DISEASE (SECONDARY CASES) BY PLACE OF BIRTH, USING A RATE RATIO DENOMINATOR COMPOSED OF ALL UNIQUE AND INDEX CASES: UNADJUSTED AND ADJUSTED ANALYSIS
| Place of Birth | Patient Characteristic | Secondary Cases* [n (%)] | Unique or Index Cases (n) | Unadjusted OR (95% CI), P Value | Adjusted† OR (95% CI), P Value |
|---|---|---|---|---|---|
| China |
|
40 cases |
1,879 cases |
|
|
| |
Ethnicity/lineage relationship |
|
|
|
|
| |
Sympatric |
25 (4.0) |
597 |
3.58 (1.87–6.84), <0.001 |
3.28 (1.67–6.41), <0.001 |
| |
Allopatric |
15 (1.2) |
1,282 |
|
|
| |
Age, yr |
|
|
|
|
| |
<50 |
10 (1.1) |
892 |
0.37 (0.18–0.76), 0.01 |
0.48 (0.23–1.00), 0.049 |
| |
≥50 |
30 (3.0) |
987 |
|
|
| The Philippines |
|
42 cases |
1,879 cases |
|
|
| |
Ethnicity/lineage relationship |
|
|
|
|
| |
Sympatric |
30 (5.6) |
503 |
6.84 (3.47–13.5), <0.001 |
6.75 (3.43–13.3), <0.001 |
| |
Allopatric |
12 (0.9) |
1,376 |
|
|
| Mexico |
|
31 cases |
1,879 cases |
|
|
| |
Ethnicity/lineage relationship |
|
|
|
|
| |
Sympatric |
24 (3.0) |
779 |
4.84 (2.08–11.3), <0.001 |
2.97 (1.21–7.30), 0.02 |
| |
Allopatric |
7 (0.6) |
1,100 |
|
|
| |
Age, yr |
|
|
|
|
| |
<50 |
26 (2.8) |
892 |
5.75 (2.20–15.0), <0.001 |
3.91 (1.43–10.7), 0.01 |
| |
≥50 |
5 (0.5) |
987 |
|
|
| |
Sex |
|
|
|
|
| |
Male |
28 (2.2) |
1,231 |
4.91 (1.49–16.2), 0.01 |
3.92 (1.14–13.4), 0.03 |
| |
Female |
3 (0.5) |
648 |
|
|
| |
Smear status |
|
|
|
|
| |
Positive |
16 (2.5) |
613 |
2.20 (1.08–4.49), 0.03 |
1.55 (0.70–3.44), 0.28 |
| |
Negative/unknown |
15 (1.2) |
1,266 |
|
|
| |
HIV status |
|
|
|
|
| |
Positive |
8 (3.6) |
216 |
2.68 (1.18–6.06), 0.02 |
1.03 (0.42–2.53), 0.94 |
| |
Negative/unknown |
23 (1.4) |
1,663 |
|
|
| |
Housing status |
|
|
|
|
| |
Homeless |
9 (4.6) |
188 |
3.68 (1.67–8.11), 0.001 |
1.30 (0.52–3.26), 0.57 |
| |
Housed/unknown |
22 (1.3) |
1,691 |
|
|
| |
Alcohol abuse |
|
|
|
|
| |
Yes |
10 (4.7) |
204 |
3.91 (1.82–8.42), <0.001 |
1.46 (0.60–3.59), 0.41 |
| |
No/unknown |
21 (1.2) |
1,675 |
|
|
| |
Chest X-ray |
|
|
|
|
| |
Cavities |
9 (3.3) |
266 |
2.46 (1.12–5.40), 0.03 |
1.73 (0.70–4.28), 0.24 |
| |
No cavities |
22 (1.4) |
1,598 |
|
|
| United States |
|
355 cases |
1,879 cases |
|
|
| |
Ethnicity/lineage relationship |
|
|
|
|
| |
Sympatric |
313 (28.7) |
779 |
10.5 (7.53–14.7), <0.001 |
5.94 (4.14–8.52), <0.001 |
| |
Allopatric |
42 (3.7) |
1,100 |
|
|
| |
Age, yr |
|
|
|
|
| |
<50 |
273 (23.4) |
892 |
3.68 (2.83–4.79), <0.001 |
1.45 (1.06–1.99), 0.02 |
| |
≥50 |
82 (7.7) |
987 |
|
|
| |
Sex |
|
|
|
|
| |
Male |
285 (18.8) |
1,231 |
2.14 (1.62–2.83), <0.001 |
1.17 (0.84–1.64), 0.35 |
| |
Female |
70 (9.8) |
648 |
|
|
| |
Site of disease |
|
|
|
|
| |
Pulmonary |
322 (17.2) |
1,552 |
2.06 (1.41–3.00), <0.001 |
1.36 (0.87–2.14), 0.18 |
| |
Extrapulmonary |
33 (9.2) |
327 |
|
|
| |
Smear status |
|
|
|
|
| |
Positive |
147 (19.3) |
613 |
1.46 (1.16–1.84), 0.001 |
1.21 (0.90–1.64), 0.21 |
| |
Negative/unknown |
208 (14.1) |
1,266 |
|
|
| |
HIV status |
|
|
|
|
| |
Positive |
169 (43.9) |
216 |
7.00 (5.44–9.00), <0.001 |
2.68 (1.94–3.70), <0.001 |
| |
Negative/unknown |
186 (10.1) |
1,663 |
|
|
| |
Housing status |
|
|
|
|
| |
Homeless |
132 (41.3) |
188 |
5.32 (4.09–6.92), <0.001 |
2.10 (1.49–2.97), <0.001 |
| |
Housed/unknown |
223 (11.7) |
1,691 |
|
|
| |
Alcohol abuse |
|
|
|
|
| |
Yes |
102 (33.3) |
204 |
3.31 (2.52–4.34), <0.001 |
1.92 (1.34–2.75), <0.001 |
| |
No/unknown |
253 (13.1) |
1,675 |
|
|
| |
Injection drug use |
|
|
|
|
| |
Yes |
68 (56.7) |
52 |
11.5 (7.73–17.0), <0.001 |
2.56 (1.60–4.09), <0.001 |
| |
No |
177 (10.2) |
1,551 |
Referent |
Referent |
| |
Unknown |
110 (28.5) |
276 |
3.49 (2.67–4.58), <0.001 |
3.39 (2.43–4.73), <0.001 |
| |
Chest X-ray |
|
|
|
|
| |
Cavities |
38 (12.5) |
266 |
0.73 (0.51–1.04), 0.08 |
0.80 (0.52–1.24), 0.32 |
| |
No cavities |
314 (16.4) |
1,598 |
|
|
| |
Initial drug resistance |
|
|
|
|
| |
INH resistant |
12 (5.1) |
225 |
0.26 (0.14–0.47), <0.001 |
0.33 (0.17–0.62), <0.001 |
| INH susceptible | 342 (17.2) | 1,650 |
Definition of abbreviations: CI = confidence interval; HIV = human immunodeficiency virus; INH = isoniazid; OR = odds ratio.
Matched by restriction fragment length polymorphism (RFLP) typing within 1 year.
Adjusted for positive sputum smear and cavitary disease (known risk factors for transmission), and all factors with P < 0.20 in bivariate (unadjusted) analysis as shown.
Discussion
In the United States, analysis of TB in foreign-born populations has largely been limited to the analysis of TB case rates according to the countries of origin, age, time of diagnosis of TB relative to entry into the United States and the frequency of drug resistance (20, 21). In this study we have shown that there are clinical and epidemiological differences among persons with TB from Mexico, China, the Philippines, and the United States.
The incidence rate of TB in San Francisco showed a significant decrease in all populations during the study period. This reduction reflects in part on the success of TB prevention and control measures such as contact investigation and treatment of contacts with latent TB infection (LTBI), targeted testing and treatment of foreign-born individuals, intensified screening and treatment of visa applicants in their countries of origin (8, 22), and dedicated programs to intensify prevention efforts among the Chinese and homeless populations (J. A. Grinsdale, personal communication).
The decline in the incidence rates of TB may also be associated with the decrease of TB in the countries of origin: from 2000 to 2010, the rate of TB in the Philippines decreased from 329 to 275 cases per 100,000 individuals (23, 24), in Mexico from 32 to 16 cases per 100,000 individuals (25, 26), and in China from 109 to 78 cases per 100,000 (23, 24).
The clinical characteristics of patients with TB differed among the groups. TB among the Chinese was a disease of older people with an abnormal chest radiograph without cavities, and a negative sputum smear examination. The differences in clinical and demographic features may be influenced by preimmigration overseas screening, in which active TB is excluded before an immigrant visa is issued, as well as activities of the San Francisco TB control section targeted to the Chinese population. Our findings also suggest that additional research is needed to identify foreign-born persons at higher risk of TB, but who do not meet the current national targeted testing and LTBI treatment criteria (27). Some of the characteristics of TB among Mexico-born patients were similar to the U.S.-born patients: higher rates occurred among young people, the HIV-infected, the homeless, and alcohol users. Patients born in Mexico had the highest proportion of cavitary TB, suggesting that these patients may have had a longer duration of disease. Studies have demonstrated that the Hispanic population living in the United States does not seek medical attention in the early stages of any disease because of language barriers, lack of access to health care, or fear of deportation (28–30). The overall profile of this group speaks to the need for culturally appropriate strategies of aggressive outreach such as awareness campaigns for HIV and TB screening.
In each of the populations included in the study, most of the TB cases had a sympatric association with the M.tb lineage, although 25% of Chinese patients had an allopatric association (TB due to lineage 4 M.tb). However, recent population-based studies in several regions of China showed that lineage 4 caused TB in 12 to 30% of the cases of TB (31, 32). Therefore, the distribution observed in San Francisco may simply reflect the current M.tb populations circulating in China.
There has been a decline in the incidence rate of secondary cases; however, the decline in incidence in the past 20 years has been greater for the U.S.-born population. As previously demonstrated, sympatric associations were more conducive to the occurrence of active TB than allopatric associations in all four populations, probably because mycobacterial lineages are adapted to specific human populations (12). However, social and environmental factors could also have an impact on these associations. The other risk factors for TB due to recent infection were different among the major foreign-born and U.S. populations. Younger age has been traditionally associated with TB due to recent infection and older age with TB due to reactivation of latent infection (33). Surprisingly, we found the opposite among China-born patients; older patients had TB due to recent infection with rapid progression to disease, which may be due to transmission within households. Higher secondary cases from elderly China-born patients should signal the need for intensified contact investigation and LTBI treatment in this group.
Patients infected with an INH-susceptible M.tb strain were associated with secondary case generation only within the U.S.-born group. Stated differently, we found that patients with TB caused by INH-resistant strains were less likely to cause secondary cases in the U.S.-born population. Several studies have shown that INH-resistant mutations in katG other than S315T are less likely to cause secondary cases (34–37). Although analysis of the resistance-conferring mutations was not within the scope of this project, it is possible that patients from the United States with INH-resistant TB have M.tb with mutations other than katG S315T.
HIV has traditionally been associated with recent TB infection and rapid evolution to disease in U.S.-born persons (2, 4–6). The stratified analysis showed that HIV is still a factor for recent infection and rapid evolution to disease in the period of 2000 to 2010 in spite of the broad use of antiretroviral treatment and the decrease in HIV-TB cases.
Policy Implications
The rate of TB decline in San Francisco has slowed in the last decade, and has not occurred uniformly among the various populations. This study suggests that to eliminate TB, population-specific strategies are required. The smallest decline occurred among the Mexican-born patients, who had the highest frequency of coinfection with HIV among the foreign-born groups and had the highest proportion of cavitary TB, perhaps due to delay in seeking medical attention. For this group, an aggressive outreach program with public awareness campaigns and bidirectional screening of persons living with HIV for TB and persons with TB for HIV should be implemented. Among Filipino patients, the high rate of INH resistance (15%) suggests the need for implementing rifamycin-based preventive treatment, such as the 12-dose, 3-month short-course rifapentine-based regimen (38). In the U.S.-born patients, the efforts started in the 1990s to address both TB and HIV among the homeless in San Francisco should be continued and expanded to the Mexican-born population, as these are still important risk factors for TB in San Francisco. Consideration should be given to target testing for LTBI regardless of age and time living in the United States in the Chinese population to identify those who would benefit from preventive therapy, especially among those with other risk factors for TB infection and progression (i.e., tobacco and diabetes). However, neither the risk benefit nor the cost benefit of such a strategy is known.
At this point, knowledge of M.tb lineage does not have a direct impact on TB control in San Francisco. However, we and others have demonstrated that the various lineages and sublineages of M.tb have distinct clinical and epidemiological phenotypes, including their pathogenicity (11, 39–42). If bacterial factors associated with pathogenicity are found, it may be possible in the future to determine which patients are more likely to transmit and cause secondary cases among their contacts, enabling implementation of specific strategies directed toward these contacts.
There are some limitations in our study. First, the denominator of each foreign-born population used to estimate the incidence rate was drawn from various sources and available only since 2000 and may not account for unregistered immigrants or for changes in the immigration/emigration patterns over time. Second, the proportion of foreign-born populations that were prescreened for tuberculosis in their home country before entering permanently to the United States may be different. Third, we included only 2,419 (74%) of 3,278 culture-positive cases. However, we believe that the differences observed between those included and those excluded from the study will not impact our conclusions.
Conclusions
We have presented evidence that the clinical, epidemiological, and microbiological characteristics of TB differ among patients born in the United States, China, the Philippines, and Mexico. The causes of these differences are unclear but are likely multifactorial and may include social, environmental, and comorbid conditions, and the presence or absence of effective public health interventions as well as preimmigration screening in their countries of origin. These differences can be used to inform TB control policies and measures and point to more targeted interventions of the future.
Footnotes
Supported by the National Institutes of Health (NIH AI034238) and King Chulalongkorn Memorial Hospital, Thai Red Cross Society. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions: G.S., L.G.J., D.O., P.C.H., and M.K.-M. contributed to the conception and design of the study; G.S., L.G.J., J.A.G., and L.M.K. were responsible for acquisition of the data. G.S., L.G.J., and D.O. performed the data analysis, and G.S., L.G.J., J.A.G., D.O., L.M.K., P.C.H., and M.K.-M. interpreted the data. G.S. and M.K.-M. drafted the manuscript and G.S., L.G.J., J.A.G., D.O., L.M.K., P.C.H., and M.K.-M. contributed, revising it critically for important intellectual content, and approved the final version.
Originally Published in Press as DOI: 10.1164/rccm.201212-2239OC on March 8, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers of Disease Control and Prevention. Trends in tuberculosis—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:181–185. [PubMed] [Google Scholar]
- 2.Cattamanchi A, Hopewell PC, Gonzalez LC, Osmond DH, Masae Kawamura L, Daley CL, Jasmer RM. A 13-year molecular epidemiological analysis of tuberculosis in San Francisco. Int J Tuberc Lung Dis. 2006;10:297–304. [PubMed] [Google Scholar]
- 3.Chin DP, DeRiemer K, Small PM, de Leon AP, Steinhart R, Schecter GF, Daley CL, Moss AR, Paz EA, Jasmer RM, et al. Differences in contributing factors to tuberculosis incidence in US-born and foreign-born persons. Am J Respir Crit Care Med. 1998;158:1797–1803. doi: 10.1164/ajrccm.158.6.9804029. [DOI] [PubMed] [Google Scholar]
- 4.Jasmer RM, Hahn JA, Small PM, Daley CL, Behr MA, Moss AR, Creasman JM, Schecter GF, Paz EA, Hopewell PC. A molecular epidemiologic analysis of tuberculosis trends in San Francisco, 1991–1997. Ann Intern Med. 1999;130:971–978. doi: 10.7326/0003-4819-130-12-199906150-00004. [DOI] [PubMed] [Google Scholar]
- 5.El Sahly HM, Adams GJ, Soini H, Teeter L, Musser JM, Graviss EA. Epidemiologic differences between United States- and foreign-born tuberculosis patients in Houston, Texas. J Infect Dis. 2001;183:461–468. doi: 10.1086/318079. [DOI] [PubMed] [Google Scholar]
- 6.Geng E, Kreiswirth B, Driver C, Li J, Burzynski J, DellaLatta P, LaPaz A, Schluger NW. Changes in the transmission of tuberculosis in New York City from 1990 to 1999. N Engl J Med. 2002;346:1453–1458. doi: 10.1056/NEJMoa012972. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Global tuberculosis control. Report No. WHO/HTM/TB/2012.6. Geneva, Switzerland: World Health Organization; 2012.
- 8.Centers of Disease Control and PreventionTechnical instructions for tuberculosis screening and treatment. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009 [accessed 2012 Aug 12]Available from: http://www.cdc.gov/immigrantrefugeehealth/pdf/tuberculosis-ti-2009.pdf
- 9.CDC's Division of Global Migration and Quarantine. Notice to readers: revised technical instructions for tuberculosis screening and treatment for panel physicians. MMWR Morb Mortal Wkly Rep. 2008;57:292–293. [Google Scholar]
- 10.Qu HQ, Fisher-Hoch SP, McCormick JB. Knowledge gaining by human genetic studies on tuberculosis susceptibility. J Hum Genet. 2011;56:177–182. doi: 10.1038/jhg.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong BC, Hill PC, Aiken A, Awine T, Antonio M, Adetifa IM, Jackson-Sillah DJ, Fox A, Deriemer K, Gagneux S, et al. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in the Gambia. J Infect Dis. 2008;198:1037–1043. doi: 10.1086/591504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, et al. Variable host–pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103:2869–2873. doi: 10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato-Maeda M, Shanley CA, Ackart D, Jarlsberg LG, Shang S, Obregon-Henao A, Harton M, Basaraba RJ, Henao-Tamayo M, Barrozo JC, et al. Beijing sublineages of Mycobacterium tuberculosis differ in pathogenicity in the guinea pig. Clin Vaccine Immunol. 2012;19:1227–1237. doi: 10.1128/CVI.00250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suwanpimolkul G, Jarlsberg L, Osmond D, Grinsdale J, Kawamura M, Hopewell PC, Kato-Maeda M. Molecular epidemiology of tuberculosis in foreign-born persons in San Francisco [abstract] Am J Respir Crit Care Med. 2012;185:A3250. doi: 10.1164/rccm.201212-2239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Census BureauSan Francisco California Population and Demographics Resources. 2000Available from: http://sanfrancisco.areaconnect.com/statistics.htm
- 16.US Census BureauState & County QuickFacts. San Francisco County, California. 2010Available from: http://quickfacts.census.gov/qfd/states/06/06075.html
- 17.Grantmakers Concerned with Immigrants and Refugees. San Francisco, California [accessed 2012 May 10]. Available from: http://www.gcir.org/node/2873
- 18.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee JT, Tanaka MM, Behr MA, Agasino CB, Paz EA, Hopewell PC, Small PM. Use of multiple markers in population-based molecular epidemiologic studies of tuberculosis. Int J Tuberc Lung Dis. 2000;4:1111–1119. [PubMed] [Google Scholar]
- 20.Cain KP, Haley CA, Armstrong LR, Garman KN, Wells CD, Iademarco MF, Castro KG, Laserson KF. Tuberculosis among foreign-born persons in the United States: achieving tuberculosis elimination. Am J Respir Crit Care Med. 2007;175:75–79. doi: 10.1164/rccm.200608-1178OC. [DOI] [PubMed] [Google Scholar]
- 21.Cain KP, Benoit SR, Winston CA, Mac Kenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA. 2008;300:405–412. doi: 10.1001/jama.300.4.405. [DOI] [PubMed] [Google Scholar]
- 22.Linas BP, Wong AY, Freedberg KA, Horsburgh CR., Jr. Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med. 2011;184:590–601. doi: 10.1164/rccm.201101-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. Global tuberculosis control. Geneva, Switzerland: World Health Organization; 2003. Report No. WHO/CDS/TB/2003.316.
- 24. World Health Organization. Global tuberculosis control. Geneva, Switzerland: World Health Organization; 2011. Report No. WHO/HTM/TB/2011.16.
- 25. World Health Organization. Global tuberculosis control. Geneva, Switzerland: World Health Organization; 2010. Report No. WHO/HTM/TB/2010.7.
- 26.Trading Economicscidence of tuberculosis (per 100,000 people) in Mexico. 2010Available from: http://www.tradingeconomics.com/mexico/incidence-of-tuberculosis-per-100,000-people-wb-data.html
- 27.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49:1–51. [PubMed] [Google Scholar]
- 28.Wells CD, Ocana M, Moser K, Bergmire-Sweat D, Mohle-Boetani JC, Binkin NJ. A study of tuberculosis among foreign-born hispanic persons in the US States bordering Mexico. Am J Respir Crit Care Med. 1999;159:834–837. doi: 10.1164/ajrccm.159.3.9712122. [DOI] [PubMed] [Google Scholar]
- 29.Lurie N, Dubowitz T. Health disparities and access to health. JAMA. 2007;297:1118–1121. doi: 10.1001/jama.297.10.1118. [DOI] [PubMed] [Google Scholar]
- 30.Dennis AM, Napravnik S, Sena AC, Eron JJ. Late entry to HIV care among Latinos compared with non-Latinos in a southeastern US cohort. Clin Infect Dis. 2011;53:480–487. doi: 10.1093/cid/cir434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Xu P, Shen X, Qi L, DeRiemer K, Mei J, Gao Q. Non-Beijing strains of Mycobacterium tuberculosis in China. J Clin Microbiol. 2011;49:392–395. doi: 10.1128/JCM.00754-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang Y, Zhou Y, Zhao B, Liu G, Jiang G, Xia H, Song Y, Shang Y, Wang S, Zhao YL. Spoligotyping and drug resistance analysis of Mycobacterium tuberculosis strains from national survey in China. PLoS One. 2012;7:e32976. doi: 10.1371/journal.pone.0032976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stead WW. The pathogenesis of pulmonary tuberculosis among older persons. Am Rev Respir Dis. 1965;91:811–822. doi: 10.1164/arrd.1965.91.6.811. [DOI] [PubMed] [Google Scholar]
- 34.Gagneux S, Burgos MV, DeRiemer K, Encisco A, Munoz S, Hopewell PC, Small PM, Pym AS. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoS Pathog. 2006;2:e61. doi: 10.1371/journal.ppat.0020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metcalfe JZ, Kim EY, Lin SY, Cattamanchi A, Oh P, Flood J, Hopewell PC, Kato-Maeda M. Determinants of multidrug-resistant tuberculosis clusters, California, USA, 2004–2007. Emerg Infect Dis. 2010;16:1403–1409. doi: 10.3201/eid1609.100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Doorn HR, de Haas PE, Kremer K, Vandenbroucke-Grauls CM, Borgdorff MW, van Soolingen D. Public health impact of isoniazid-resistant Mycobacterium tuberculosis strains with a mutation at amino-acid position 315 of katG: a decade of experience in the Netherlands. Clin Microbiol Infect. 2006;12:769–775. doi: 10.1111/j.1469-0691.2006.01495.x. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Hoffner S, Jiang W, Wang W, Xu B. Extensive transmission of isoniazid resistant M. tuberculosis and its association with increased multidrug-resistant TB in two rural counties of eastern China: a molecular epidemiological study. BMC Infect Dis. 2010;10:43. doi: 10.1186/1471-2334-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, McIntyre JA, Gray GE, Chaisson RE. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365:11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed MB, Gagneux S, Deriemer K, Small PM, Barry CE., III The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J Bacteriol. 2007;189:2583–2589. doi: 10.1128/JB.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thwaites G, Caws M, Chau TT, D'Sa A, Lan NT, Huyen MN, Gagneux S, Anh PT, Tho DQ, Torok E, et al. Relationship between Mycobacterium tuberculosis genotype and the clinical phenotype of pulmonary and meningeal tuberculosis. J Clin Microbiol. 2008;46:1363–1368. doi: 10.1128/JCM.02180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Click ES, Moonan PK, Winston CA, Cowan LS, Oeltmann JE. Relationship between Mycobacterium tuberculosis phylogenetic lineage and clinical site of tuberculosis. Clin Infect Dis. 2012;54:211–219. doi: 10.1093/cid/cir788. [DOI] [PubMed] [Google Scholar]
- 42.Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE., III A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature. 2004;431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]