Abstract
Collagen from human skin was fractionated into neutral salt-soluble, acid-soluble, pepsin-released, and insoluble fractions. No age-related changes were observed in the proportion of collagen extracted by neutral salt. A significant age-related decrease in the proportion of acid-soluble collagen was found. A highly significant (P less than 0.001) age-related decrease in the amount of collagen released by pepsin digestion was observed, with a concomitant age-related increase in the fraction of insoluble collagen. The amount of ketoamine-linked glucose bound to this insoluble collagen also increased significantly with age. Skin collagen from three juvenile onset diabetics (JOD) and one young maturity onset diabetic (MOD) appeared to have undergone accelerated aging. JOD and the young MOD had significantly less collagen released by pepsin digestion and significantly more insoluble collagen than would be predicted by their ages. The collagen released by pepsin digestion of the diabetic samples had more high molecular weight components than similar fractions obtained from age-matched nondiabetic controls. There was also more ketoamine-linked glucose bound to the insoluble collagen of JOD than to that fraction from comparably aged control subjects. The apparent acceleration of collagen aging in diabetes mellitus may play a role in complications of diabetes that occur in collagen-rich tissues.
Full text
PDF

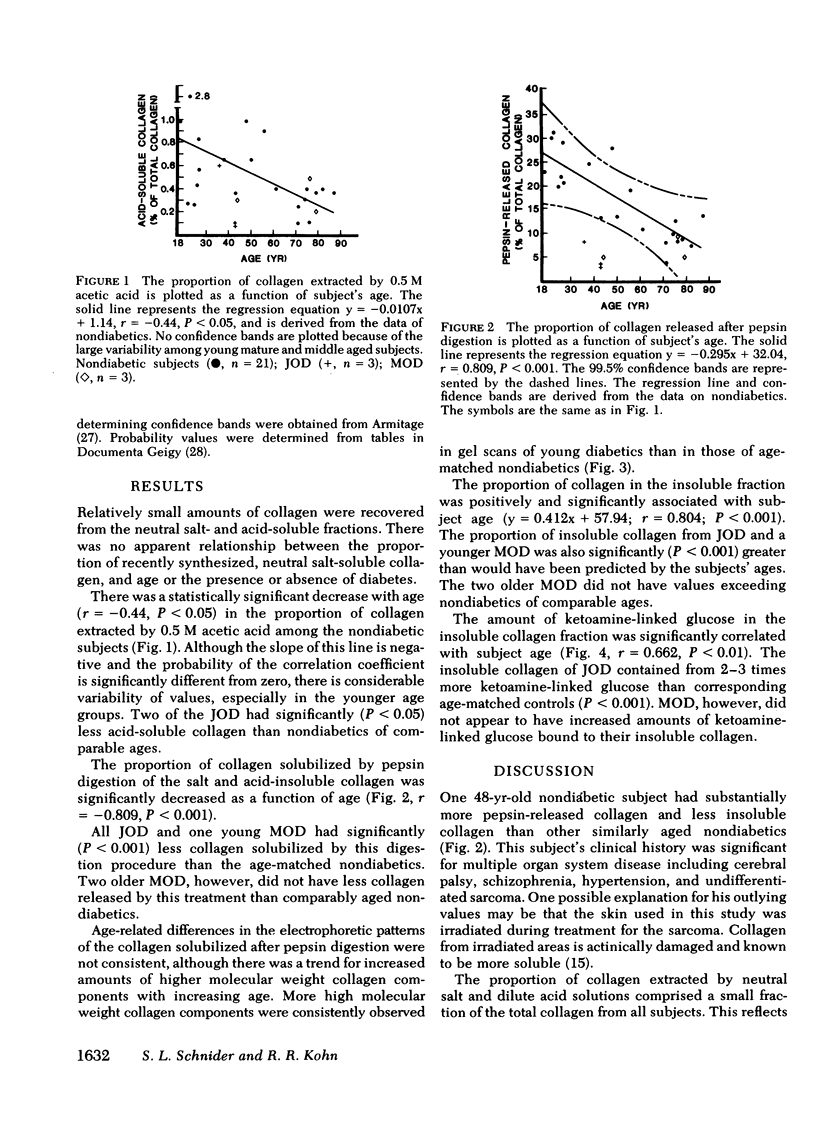
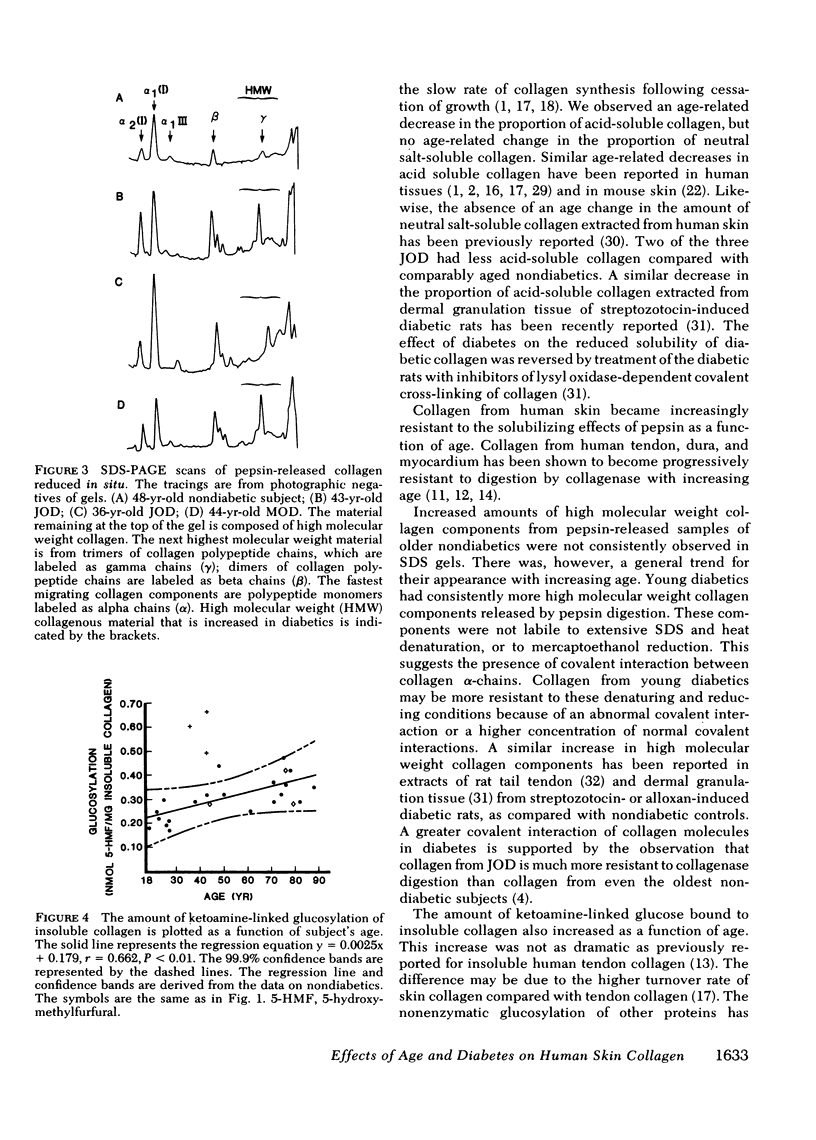


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKERMAN S. Quantitative extraction of acid-soluble human skin collagen with age. Nature. 1962 Oct 27;196:375–376. doi: 10.1038/196375a0. [DOI] [PubMed] [Google Scholar]
- Chang K., Uitto J., Rowold E. A., Grant G. A., Kilo C., Williamson J. R. Increased collagen cross-linkages in experimental diabetes: reversal by beta-aminopropionitrile and D-penicillamine. Diabetes. 1980 Oct;29(10):778–781. doi: 10.2337/diacare.20.10.778. [DOI] [PubMed] [Google Scholar]
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979 Dec;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Urdanivia E., Surma M., Wu V. Y. Increased glycosylation of glomerular basement membrane collagen in diabetes. Biochem Biophys Res Commun. 1980 Jul 31;95(2):765–769. doi: 10.1016/0006-291x(80)90852-9. [DOI] [PubMed] [Google Scholar]
- Day J. F., Thornburg R. W., Thorpe S. R., Baynes J. W. Nonenzymatic glucosylation of rat albumin. Studies in vitro and in vivo. J Biol Chem. 1979 Oct 10;254(19):9394–9400. [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Flückiger R., Winterhalter K. H. In vitro synthesis of hemoglobin AIc. FEBS Lett. 1976 Dec 1;71(2):356–360. doi: 10.1016/0014-5793(76)80969-6. [DOI] [PubMed] [Google Scholar]
- Golub L. M., Greenwald R. A., Zebrowski E. J., Ramamurthy N. S. The effect of experimental diabetes on the molecular characteristics of soluble rat-tail tendon collagen. Biochim Biophys Acta. 1978 May 24;534(1):73–81. doi: 10.1016/0005-2795(78)90477-4. [DOI] [PubMed] [Google Scholar]
- Hamlin C. R., Kohn R. R. Evidence for progressive, age-related structural changes in post-mature human collagen. Biochim Biophys Acta. 1971 May 25;236(2):458–467. doi: 10.1016/0005-2795(71)90226-1. [DOI] [PubMed] [Google Scholar]
- Hamlin C. R., Kohn R. R., Luschin J. H. Apparent accelerated aging of human collagen in diabetes mellitus. Diabetes. 1975 Oct;24(10):902–904. doi: 10.2337/diab.24.10.902. [DOI] [PubMed] [Google Scholar]
- Hamlin C. R., Luschin J. H., Kohn R. R. Partial characterization of the age-related stabilizing factor of post-mature human collagen--I. By the use of bacterial collagenase. Exp Gerontol. 1978;13(6):403–414. doi: 10.1016/0531-5565(78)90051-7. [DOI] [PubMed] [Google Scholar]
- Hamlin C. R., Luschin J. H., Kohn R. R. Partial characterization of the age-related stabilizing factor of post-mature human collagen--II. By the use of trypsin. Exp Gerontol. 1978;13(6):415–423. doi: 10.1016/0531-5565(78)90052-9. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., McGee D. L. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979 May 11;241(19):2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I. Urinary excretion of hydroxyproline in health and disease. Int Rev Connect Tissue Res. 1970;5:93–163. doi: 10.1016/b978-0-12-363705-5.50008-7. [DOI] [PubMed] [Google Scholar]
- Kohn R. R., Hamlin C. R. Genetic effects on aging of collagen, with special reference to diabetes mellitus. Birth Defects Orig Artic Ser. 1978;14(1):387–401. [PubMed] [Google Scholar]
- Maekawa T., Rathinasamy T. K., Altman K. I., Forbes W. F. Changes in collagen with age. I. The extraction of acid soluble collagens from the skin of mice. Exp Gerontol. 1970 Jul;5(2):177–186. doi: 10.1016/0531-5565(70)90007-0. [DOI] [PubMed] [Google Scholar]
- McCormick P. J., Chandrasekhar S., Millis A. J. Direct visualization of collagens and procollagens in polyacrylamide gels. Anal Biochem. 1979 Sep 1;97(2):359–366. doi: 10.1016/0003-2697(79)90086-1. [DOI] [PubMed] [Google Scholar]
- Pecoraro R. E., Graf R. J., Halter J. B., Beiter H., Porte D., Jr Comparison of a colorimetric assay for glycosylated hemoglobin with ion-exchange chromatography. Diabetes. 1979 Dec;28(12):1120–1125. doi: 10.2337/diab.28.12.1120. [DOI] [PubMed] [Google Scholar]
- Pillsbury H. C., 3rd, Hung W., Kyle M. C., Freis E. D. Arterial pulse waves and velocity and systolic time intervals in diabetic children. Am Heart J. 1974 Jun;87(6):783–790. doi: 10.1016/0002-8703(74)90427-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Modrak J. B., Hassing J. M., Al-Turk W. A., Stohs S. J. Glycosylated collagen. Biochem Biophys Res Commun. 1979 Nov 28;91(2):498–501. doi: 10.1016/0006-291x(79)91549-3. [DOI] [PubMed] [Google Scholar]
- Rosenbloom A. L. Nature and nurture in the expression of diabetes mellitus and its vascular manifestations. Am J Dis Child. 1977 Oct;131(10):1154–1159. doi: 10.1001/archpedi.1977.02120230100019. [DOI] [PubMed] [Google Scholar]
- Schuyler M. R., Niewoehner D. E., Inkley S. R., Kohn R. Abnormal lung elasticity in juvenile diabetes mellitus. Am Rev Respir Dis. 1976 Jan;113(1):37–41. doi: 10.1164/arrd.1976.113.1.37. [DOI] [PubMed] [Google Scholar]
- Shagan B. P. Is diabetes a model for aging? Med Clin North Am. 1976;60(6):1209–1211. doi: 10.1016/s0025-7125(16)31876-4. [DOI] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Tenni R., Tavella D., Donnelly P., Di Ferrante N., Hill L., Leach C., Hatton D. Cultured fibroblasts of juvenile diabetics have excessively soluble pericellular collagen. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1071–1075. doi: 10.1016/0006-291x(80)90395-2. [DOI] [PubMed] [Google Scholar]
- Uitto J., Ohlenschläger K., Lorenzen I. Solubility of skin collagen in normal human subjects and in patients with generalised scleroderma. Clin Chim Acta. 1971 Jan;31(1):13–18. doi: 10.1016/0009-8981(71)90356-1. [DOI] [PubMed] [Google Scholar]
- Vracko R., Thorning D., Huang T. W. Basal lamina of alveolar epithelium and capillaries: quantitative changes with aging and in diabetes mellitus. Am Rev Respir Dis. 1979 Nov;120(5):973–983. doi: 10.1164/arrd.1979.120.5.973. [DOI] [PubMed] [Google Scholar]
- Weiss J. B. Enzymic degradation of collagen. Int Rev Connect Tissue Res. 1976;7:101–157. doi: 10.1016/b978-0-12-363707-9.50009-5. [DOI] [PubMed] [Google Scholar]
- Zwolinski R. J., Hamlin C. R., Kohn R. R. Age-related alteration in human heart collagen. Proc Soc Exp Biol Med. 1976 Jul;152(3):362–365. doi: 10.3181/00379727-152-39397. [DOI] [PubMed] [Google Scholar]



