Abstract
Rationale: Helper CD4+ T cell subsets, including IL-9– and IL-10–producing T helper cell type 9 (Th9) cells, exist under certain inflammatory conditions. Cyclooxygenase (COX)-1 and COX-2 play important roles in allergic lung inflammation and asthma. It is unknown whether COX-derived eicosanoids regulate Th9 cells during allergic lung inflammation.
Objectives: To determine the role of COX metabolites in regulating Th9 cell differentiation and function during allergic lung inflammation.
Methods: COX-1−/−, COX-2−/−, and wild-type (WT) mice were studied in an in vivo model of ovalbumin-induced allergic inflammation and an in vitro model of Th9 differentiation using flow cytometry, cytokine assays, confocal microscopy, real-time PCR, and immunoblotting. In addition, the role of specific eicosanoids and their receptors was examined using synthetic prostaglandins (PGs), selective inhibitors, and siRNA knockdown.
Measurements and Main Results: Experimental endpoints were not different between COX-1−/− and WT mice; however, the percentage of IL-9+ CD4+ T cells was increased in lung, bronchoalveolar lavage fluid, lymph nodes, and blood of allergic COX-2−/− mice relative to WT. Bronchoalveolar lavage fluid IL-9 and IL-10, serum IL-9, and lung IL-17RB levels were significantly increased in allergic COX-2−/− mice or in WT mice treated with COX-2 inhibitors. IL-9, IL-10, and IL-17RB expression in vivo was inhibited by PGD2 and PGE2, which also reduced Th9 cell differentiation of murine and human naive CD4+ T cells in vitro. Inhibition of protein kinase A significantly increased Th9 cell differentiation of naive CD4+ T cells isolated from WT mice in vitro.
Conclusions: COX-2–derived PGD2 and PGE2 regulate Th9 cell differentiation by suppressing IL-17RB expression via a protein kinase A–dependent mechanism.
Keywords: T helper cell type 9 cells, cyclooxygenase 2, asthma, prostaglandins, IL-17RB
At a Glance Commentary
Scientific Knowledge on the Subject
Cyclooxygenase (COX) enzymes are known to be regulators of T helper cell type 1 (Th1), Th2, and Th17 cells in allergic lung disease; however, it is not known whether COX-1– or COX-2–derived eicosanoids regulate Th9 cell function, or the mechanisms involved.
What This Study Adds to the Field
This study identifies COX-2 as a key negative regulator of Th9 cell differentiation and function in allergic lung inflammation via an autocrine loop that involves prostaglandin (PG) D2 and PGE2 suppression of IL-17RB through protein kinase A signaling.
Asthma is one of the most common diseases in the world, and substantially impacts public health, especially in developed nations. Asthma is characterized by reversible airflow obstruction, bronchial hyperresponsiveness, and airway inflammation. CD4+ T helper cells play a pivotal role in the pathogenesis of asthma (1–4). CD4+ T helper cell subsets, which include T helper cell type 1 (Th1), Th2, and Th17 cells, are key components of the adaptive immune response in rodents and humans (1, 5–7). Allergic responses in the lung are traditionally thought of as being Th2 mediated; however, recent studies have shown that IL-9 contributes to allergic responses by promoting mast cell expansion and production of IL-13, which stimulates the release of mucus and contributes to airway hyperresponsiveness (1, 5, 8, 9). Serum IL-9 levels, which correlate with symptom severity in patients with allergic rhinitis, depend on exposure to a causal allergen (10–14). Similarly, during the effector phase of food allergy, IL-9 promotes mastocytosis, which increases intestinal permeability (15).
Recently, a distinct CD4+ T helper cell subtype that produces IL-9 was described and termed Th9 (16–19). The most definitive work that supports the existence and functional relevance of Th9 cells in vivo comes from a study of mice with T cell–specific deletion of the transcription factor, PU.1 (20). These mice have wild-type (WT) levels of Th2 cells, but do not develop IL-9–dependent allergic lung inflammation, and have low levels of IL-9 in bronchoalveolar lavage fluid (BALF). At least in this model, PU.1 appears to be crucial for Th9 cell differentiation. IFN-regulatory factor (IRF) 4 has also been shown to be important for development and function of the Th9 cell subset (21). Generation of Th9 cells is dependent on transforming growth factor (TGF)-β and IL-4, and the addition of IL-25 further increases the production of IL-9 (16). Mice deficient in IL-25 or IL-17RB, the cell surface receptor for IL-25, have reduced airway inflammation and produce Th9 cells with decreased IL-9 expression in a model of allergic asthma (22–26). Other cytokines have additive effects in promoting Th9 cell generation in the presence of TGF-β and IL-4 in vitro (22); however, many of these reports are conflicting, and the precise molecular mechanisms involved in the regulation of Th9 cell development remain enigmatic.
Cyclooxygenase (COX)-1 and COX-2 are responsible for conversion of arachidonic acid to bioactive eicosanoids, including prostacyclin, thromboxane, and various prostaglandins (PGs), which signal through specific G protein–coupled receptors (GPCRs) (27). COX-1 is constitutively expressed in most tissues, whereas COX-2 is inducible and primarily responsible for the formation of PGs in immune cells such as macrophages. In response to infection and injury, COX-2 plays an important role in inflammation, tissue damage, and tumorigenesis (28–31). Importantly, expression of COX-2 is up-regulated during allergic lung inflammation in animal models and in humans with asthma (32, 33). COX-2 is involved in the regulation of Th1 and Th2 balance, and our recent data indicates that COX-2 acts as a key regulator of Th17 cell differentiation and function (34, 35). It is unknown whether COX-2 is also involved in Th9 differentiation or function.
In this study, we investigated the roles of both COX isoforms in Th9 cell differentiation and function during allergic lung inflammation. Using COX-1– and COX-2–deficient mice and siRNA knockdown strategies, we examined the signaling pathways that control Th9 differentiation. Our results indicate that COX-2 negatively regulates Th9 differentiation from naive CD4+ T cells during allergic lung inflammation via a mechanism that involves PGD2 and PGE2 signaling through their cognate receptors and down-regulation of IL-17RB. We further show that human Th9 cell differentiation is also regulated by COX-2–derived eicosanoids. Defining the role of COX-2–derived PGs in Th9 cell differentiation may lead to development of new therapeutic approaches for asthma and other allergic diseases.
Methods
Reagents and Animal Handling
A full listing of antibodies, eicosanoids, chemicals, and inhibitors used can be found in the Methods section of the online supplement. All animal experiments were performed according to National Institutes of Health guidelines and were approved by the National Institute of Environmental Health Sciences Animal Care and Use Committee. The 6- to 10-week-old male COX-1−/−, COX-2−/−, and WT littermate control mice on a hybrid C57BL/6J x 129P2/OlaHsd genetic background were purchased from Taconic (Germantown, NY). Male EP2−/− (6–10 wk old) mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Ovalbumin-induced Allergic Airway Inflammation Model
Mice were immunized with ovalbumin (OVA) or vehicle (adjuvant) by intraperitoneal injection on Days 0 and 1; 14–21 days later, mice were exposed to 1% OVA in sterile saline (or to saline vehicle only) via inhalation for 30 minutes per day for 4 consecutive days. PGD2 and/or PGE2 were delivered 1 week before OVA exposure via subcutaneously implanted osmotic minipumps (model 1004; Alzet, Cupertino, CA). Mice were killed for tissue collection 48 hours after the last OVA exposure to collect BALF, blood, and tissues. Staining of lung tissue, analysis of cytokines, and determination of eicosanoid levels were performed.
Lung and spleen CD4+ T cells were isolated with MACS CD4+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Naive CD4+ T cells were isolated using the CD4+ CD62L+ isolation kit (cat no. 130-093-227; Becton Dickinson, Franklin Lakes, NJ) to obtain cells of greater than 95% purity. Cell sorting was performed to obtain a population of naive CD4+ T cells of greater than 99% purity. Cells were cultured in the presence of anti-CD3, anti-CD28, anti-IFNγ, IL-4, and TGF-β to induce Th9 differentiation. A description of lung staining, analysis of cytokines, and determination of eicosanoid levels are contained in the online supplement.
In Vitro Treatment and Analyses
After Th9 differentiation from naive CD4+ T cells with TGF-β and IL-4, cells were fixed and stained for IL-9 and IL-10 cytokines and/or the prostanoid receptor subtypes EP1–EP4, DP1, DP2, FP, and IP. For some experiments, CD4+ T cells were transfected with siRNAs targeted to the EP1, EP3, EP4, DP1, DP2, or IL-17RB receptors using mouse T cell Nucleofector solution (Amaxa, Cologne, Germany). Total RNA was isolated using the RNeasy mini kit (Qiagen, Germantown, MD) and cDNA was synthesized with the High Capacity cDNA Archive Kit (Applied Biosystems, Carlsbad, CA). Luciferase reporter constructs were transfected into Jurkat T cells. At 24 hours after transfection, cells were treated with 1 μM PGE2 or vehicle for 4 hours. Luciferase activity was detected using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Human CD4+ T cells were isolated from blood collected under a protocol that was approved by the National Institute of Environmental Health Sciences Institutional Review Board and stained for Th9 and Th2 markers. Additional details, primers, and TaqMan primer/probe sets are listed in the Methods section of the online supplement.
Statistical Analysis
Data are presented as means (±SEM). Statistical comparisons among treatment groups were performed by randomized-design two-way ANOVA, followed by the Newman-Keuls post hoc test for more than two groups, or by unpaired Student’s t test for two groups using Prism software (GraphPad Inc., La Jolla, CA), as appropriate. Statistical significance was defined as a P value of less than 0.05.
Results
COX-2−/− Mice Have Enhanced Lung Th9 Cell Responses to Allergen Exposure
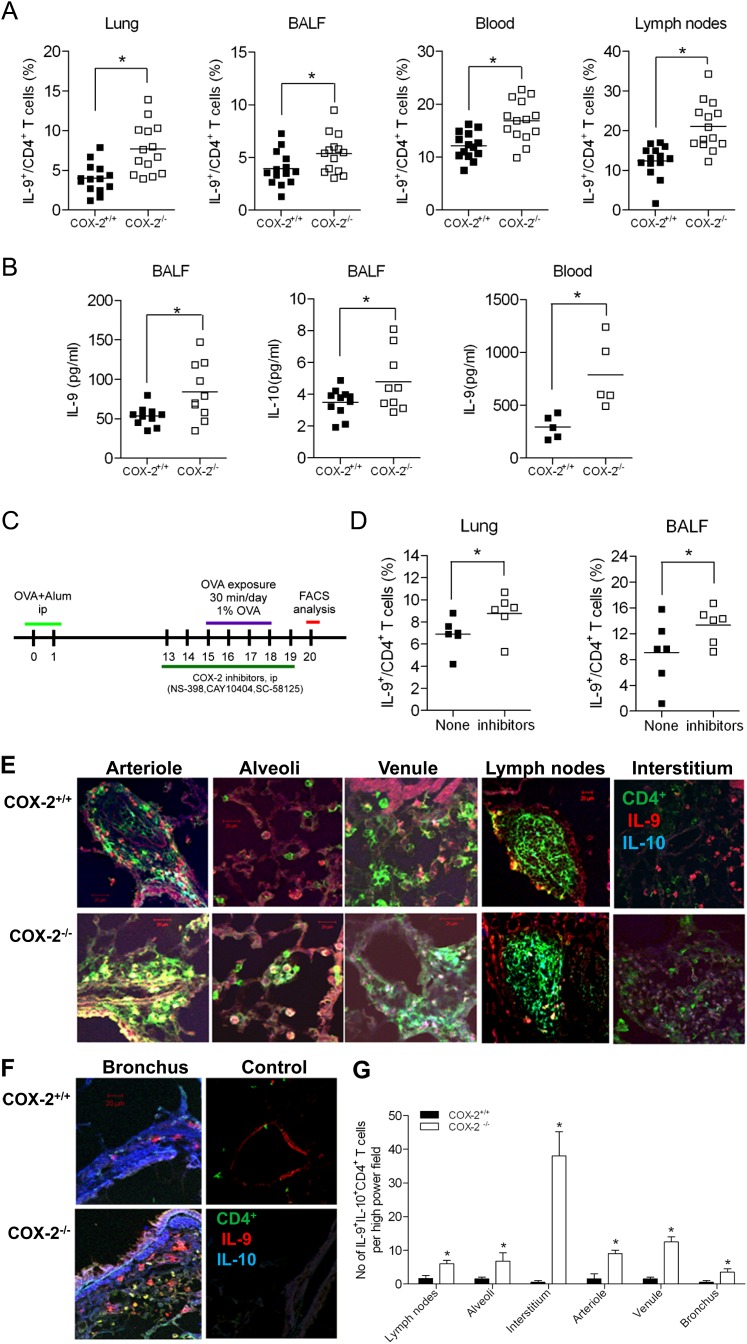
To investigate the role of COX isoforms in regulating Th9 cell differentiation during allergic lung inflammation, we exposed COX-1−/−, COX-2−/−, and WT control mice to the allergen OVA. After OVA sensitization/exposure, the percentage of Th9 cells (IL-9+ CD4+) was significantly increased in lung (7.7 ± 0.8 versus 4.0 ± 0.5%), BALF (5.4 ± 0.5 versus 3.9 ± 0.4%), lymph nodes (21.1 ± 5.8 versus 12.4 ± 3.9%), and blood (16.7 ± 1.0 versus 12.1 ± 0.6%) of COX-2−/− mice compared with WT mice (P < 0.05 for all). The total number of Th9 cells in lung, BALF, lymph nodes, and blood was also dramatically increased in COX-2−/− mice (see Figure E1B in the online supplement; P < 0.05 for all), but not COX-1−/− mice (Figure E1C), relative to WT controls. Consistent with these findings, BALF IL-9 (84.1 ± 11.4 versus 53.5 ± 4.0 pg/ml), IL-10 (4.8 ± 0.6 versus 3.5 ± 0.3 pg/ml), and serum IL-9 (787 ± 144 versus 295 ± 49 pg/ml) levels were increased in COX-2−/− mice relative to WT control animals (Figure 1B, P < 0.05 for all). Interestingly, levels of IL-10 were not significantly increased in the serum of COX-2−/− mice. A similar increase in Th9 differentiation was observed after treatment with selective COX-2 inhibitors in vivo (Figures 1C and 1D), which further confirms that COX-2 plays an essential role in regulating Th9 cells during allergic lung inflammation.
Figure 1.
Increased T helper cell type 9 (Th9) cells in lung, bronchoalveolar lavage fluid (BALF), lymph nodes, and blood of cyclooxygenase (COX)-2−/− mice after ovalbumin (OVA) sensitization/exposure in vivo. (A) COX-2+/+ and COX-2−/− mice (n = 14 each) were sensitized with OVA in adjuvant. At 15–18 days later, mice were exposed to inhaled OVA for 4 consecutive days. The percentages of IL-9+ CD4+ T cells in lung, BALF, lymph nodes, and blood from COX-2+/+ and COX-2−/− mice were analyzed by flow cytometry 48 hours after the last OVA exposure. (B) IL-9 and IL-10 concentrations in BALF and blood were measured a BioPlex assay 48 hours after the last OVA exposure. (C) Schematic of COX-2 inhibitor experiment. Wild-type (WT) mice were treated with the selective COX-2 inhibitors, NS-398, CAY10404, and SC-58125, from Day 13 to Day 19 after OVA sensitization. (D) The percentages of IL-9+ CD4+ cells were dramatically increased in the lungs and BALF of COX-2 inhibitor–treated mice (n = 5–6). (A, B, and D) Lines indicate the mean, and each symbol (COX-2+/+, filled squares; COX-2−/−, open squares) represents an individual mouse. (E and F) Th9 cells in mouse lung tissue sections were visualized by immunofluorescent staining using anti–IL-9, anti–IL-10 (labeled with Alexa Fluor 376), and anti-CD4 antibodies (labeled with Alexa Fluor 488). The control image is from a mouse that did not receive OVA. All images are shown at original magnification = ×60; numerical aperture = 1.4; scale bars = 25 μm. Results are representative of at least five independent experiments. (G) Quantitation of the number of IL-9+ IL-10+ CD4+ T cells in various lung compartments (n = 5–10). *P < 0.05 versus COX-2+/+.
To determine the localization of Th9 cells during allergic lung inflammation, adjacent lung tissue sections were stained with antibodies against CD4, IL-9, and IL-10. Th9 cells (IL-9+ IL-10+ CD4+) were more abundant in arterioles, alveoli, venules, lymph nodes, interstitium, and bronchi of allergic COX-2−/− mice compared with allergic WT mice (Figures 1E–1G). Th9 cells were not observed in the lungs of mice that were not sensitized/exposed to OVA (Figure 1F). Together, these data indicate that Th9 cell numbers are significantly increased in the inflammatory loci of allergic COX-2−/− mice relative to WT mice, and that Th9 cells localize to sites of allergic lung inflammation.
COX-2 Expression Is Regulated by IL-4 and TGF-β In Vitro
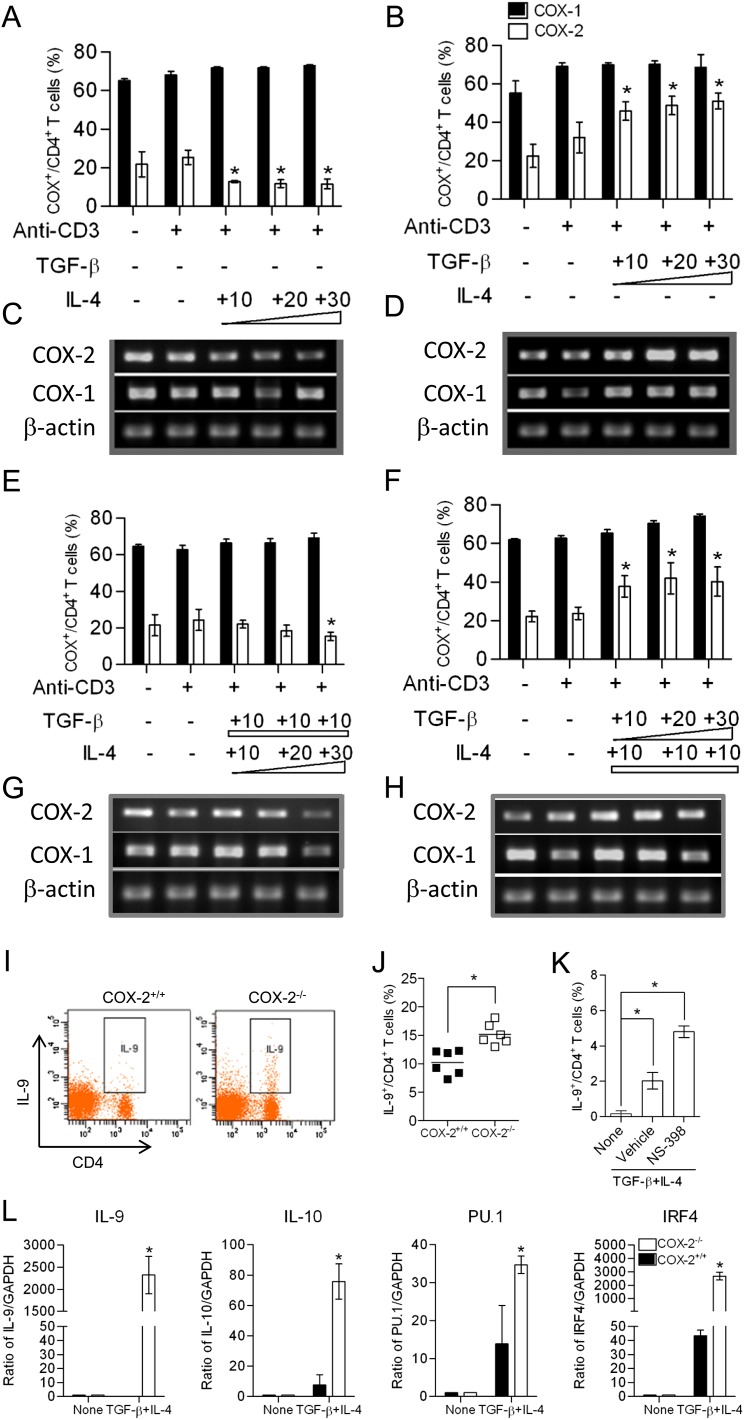
To examine COX expression during conditions that support Th9 cell differentiation in vitro, CD4+ CD62L+ naive T cells were isolated from WT mice and incubated with 3 μg/ml anti-CD3, 1 μg/ml anti-CD28, and various amounts of TGF-β and/or IL-4. IL-4 treatment inhibited COX-2 expression (Figures 2A and 2C), whereas TGF-β treatment increased COX-2 expression in a concentration-dependent manner (Figures 2B and 2D). These results are consistent with previous studies in dendritic cells (36). The combination of IL-4 (10 ng/ml) and TGF-β (10 ng/ml), which induced Th9 cell differentiation of naive T cells in vitro, did not significantly alter expression of COX-1, and had variable effects on COX-2 expression (Figures 2E–2H). These data suggest that the coordinate regulation of COX-2 by TGF-β and IL-4 might play an important role in Th9 cell differentiation.
Figure 2.
Increased T helper cell type 9 (Th9) differentiation in cyclooxygenase (COX)-2−/− naive T cells in vitro. (A, B, E, and F) Naive CD4+ T cells were isolated from wild-type (WT) mice and differentiated in vitro with anti-CD3, anti-CD28, IL-4 (10–30 ng/ml), and transforming growth factor (TGF)-β1 (10–30 ng/ml) for 3–5 days. CD4+ COX-1+ and CD4+ COX-2+ T cells were analyzed by flow cytometry. (C, D, G, and H) COX-1 and COX-2 mRNA levels were detected by RT-PCR in the experiments in (C and D) and (G and H). Results are representative of three independent experiments. (I and J) Naive CD4+ T cells were sorted from WT and COX-2−/− mice, differentiated in the presence of anti-CD3, CD28, TGF-β, and IL-4 for 3–5 days in vitro, and the percentages of IL-9+/CD4+ T cells were analyzed by flow cytometry. Th9 cell differentiation was significantly increased in COX-2−/− cells relative to WT (n = 6 each). (K) Incubation of WT naive CD4+ T cells with NS-398 also increased Th9 cell differentiation in vitro. (L) mRNA levels of IL-9, IL-10, PU.1, and IFN-regulatory factor (IRF) 4 were detected by real-time RT-PCR in naive CD4+ T cells and in vitro–differentiated Th9 cells (n = 6). *P < 0.05 versus WT.
COX-2−/− Naive CD4+ T Cells Exhibit Increased Th9 Differentiation In Vitro
To examine the role of COX-2 in Th9 cell differentiation in vitro, we treated WT and COX-2−/− naive CD4+ T cells isolated from mouse spleens with anti-CD3, anti-CD28, and the Th9-inducing cytokines, TGF-β and IL-4, and quantified the number of IL-9+ CD4+ T cells by flow cytometry (Figure 2I). Interestingly, we observed that 15.2 (±1.7)% of COX-2−/− naive CD4+ T cells differentiated into Th9 cells, compared with only 10.2 (±1.9)% of WT naive CD4+ T cells (Figure 2J). Consistently, the selective COX-2 inhibitor, NS-398, also enhanced Th9 cell differentiation of WT naive CD4+ T cells in vitro (Figure 2K).
Th9 cell lineage markers (IL-9, IL-10, PU.1, and IRF4) were significantly increased after treatment of COX-2−/− naive CD4+ T cells with TGF-β and IL-4 relative to WT cells (Figure 2L). In contrast, Th2 lineage markers (IL-4 and GATA3) were similar in WT and COX-2−/− naive CD4+ T cells after treatment with TGF-β and IL-4 (Figure E2). Together, these results indicate that COX-2 is a negative regulator of Th9 cell differentiation, and confirm that the increased IL-9 production after treatment with TGF-β and IL-4 was mainly from Th9 cells, rather than Th2 cells.
COX-2 Inhibits Th9 Cell Differentiation through PGD2 and PGE2 Signaling
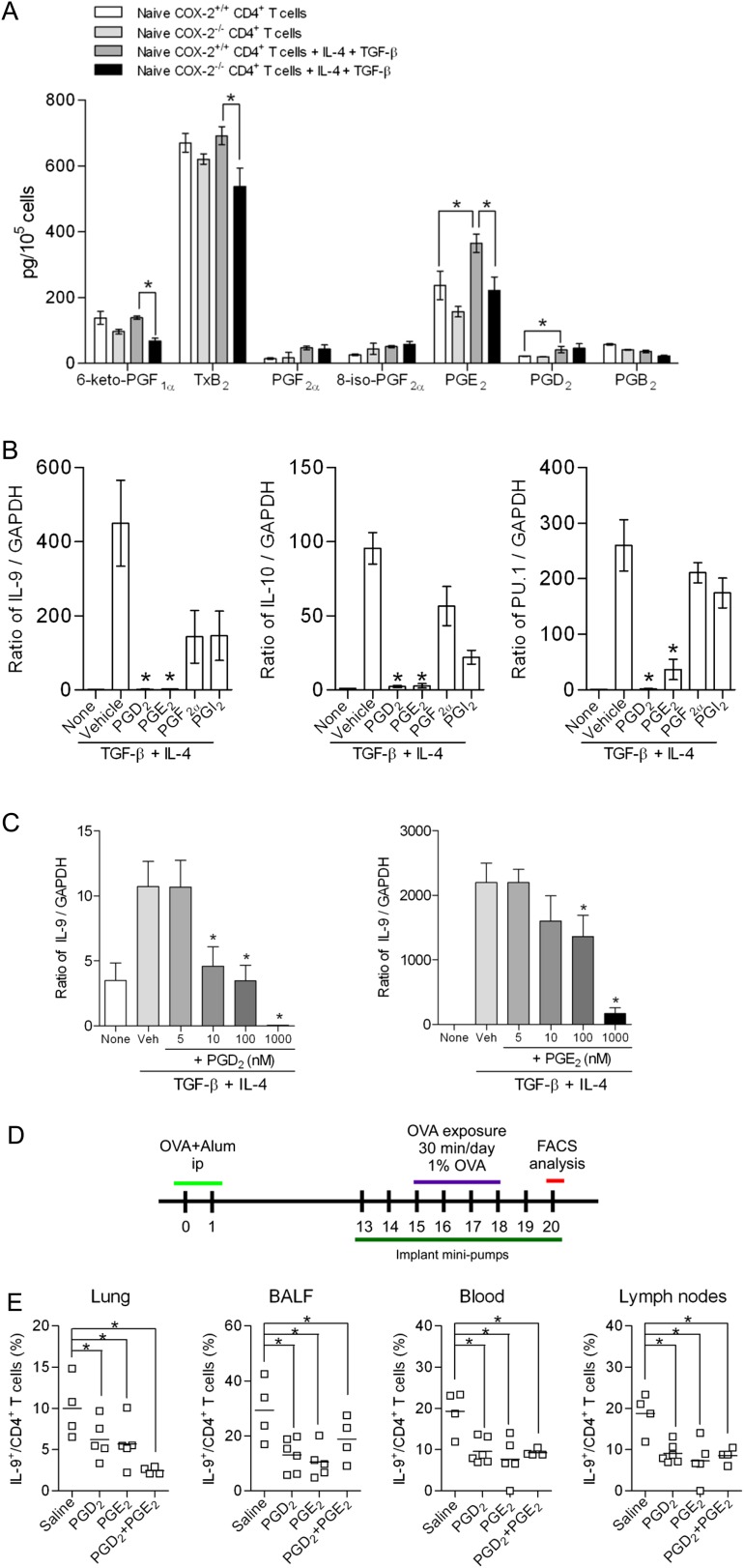
To explore the molecular mechanisms by which COX-2 regulates Th9 cell differentiation, eicosanoid levels in supernatants of naive CD4+ T cells and in vitro–differentiated Th9 cells from WT and COX-2−/− mice were measured by liquid chromatography/tandem mass spectrometry. As we have previously published (34), PG levels were low in naive CD4+ T cells and not significantly different between the two genotypes (Figure 3A). Levels of PGE2 and PGD2 increased significantly with Th9 cell differentiation in cells from WT mice. Importantly, levels of PGE2 were reduced in Th9 cells differentiated from CD4+ T cells isolated from COX-2−/− mice relative to WT mice (Figure 3A). These data suggest that PG production is increased during Th9 differentiation, and that COX-2 is critical for optimal PGE2 production in Th9 cells.
Figure 3.
Cyclooxygenase (COX)-2–derived prostaglandins (PGs) regulate T helper cell type 9 (Th9) differentiation in vitro. (A) Eicosanoid levels in supernatants of naive CD4+ T cells and differentiated Th9 cells from COX-2+/+ and COX-2−/− mice were measured by liquid chromatography–tandem mass spectrometry (n = 5 per group; *P < 0.05). (B) Naive CD4+ T cells were cultured with anti-CD3, anti-CD28, anti–INF-γ, IL-4, and transforming growth factor (TGF)-β in the presence or absence of PGD2, PGE2, PGF2α, or PGI2 (1 μM each). Th9 differentiation from naive CD4+ T cells was examined by RT-PCR. Results are representative of at least three independent experiments. *P < 0.05 versus vehicle. (C) Th9 differentiation, as above, was measured in the presence of increasing concentrations of PGD2 and PGE2, *P < 0.05 versus vehicle. (D) Schematic of in vivo minipump experiment. Alzet minipumps filled with either PGD2, PGE2, or the combination of PGD2 and PGE2 were implanted into sensitized COX-2−/− mice from Day 13 to Day 20. (E) Sensitized mice were exposed to ovalbumin (OVA) daily for 4 days. At 48 hours after the last OVA exposure, mice were killed and the percentages of IL-9+ CD4+ T cells in lung, bronchoalveolar lavage fluid (BALF), lymph nodes, and blood were determined by flow cytometry (n = 4–5; *P < 0.05 versus saline containing minipump). Lines indicate the mean, and each symbol represents an individual mouse.
We then incubated naive CD4+/CD62L+ T cells from WT mice with PGD2, PGE2, PGF2α, or PGI2 during Th9 differentiation. PGD2 and PGE2, but not PGF2α or PGI2, inhibited Th9 cell differentiation in vitro in a concentration-dependent manner (Figures 3B and 3C and Figure E3). A role for PGD2 and PGE2 in the regulation of Th9 cell numbers in vivo was further confirmed in allergic COX-2−/− mice treated with synthetic PGD2, PGE2, or a combination of the two prostanoids via osmotic minipumps. Th9 cell numbers were significantly reduced in lungs, BALF, lymph nodes, and blood of these mice compared with those treated with vehicle (Figures 3D and 3E). Taken together, these data suggest that COX-2–derived PGD2 and PGE2 inhibit Th9 cell differentiation in vitro and in the allergic lung in vivo.
PGD2 and PGE2 Regulate Th9 Cell Differentiation through Down-regulation of IL-17RB
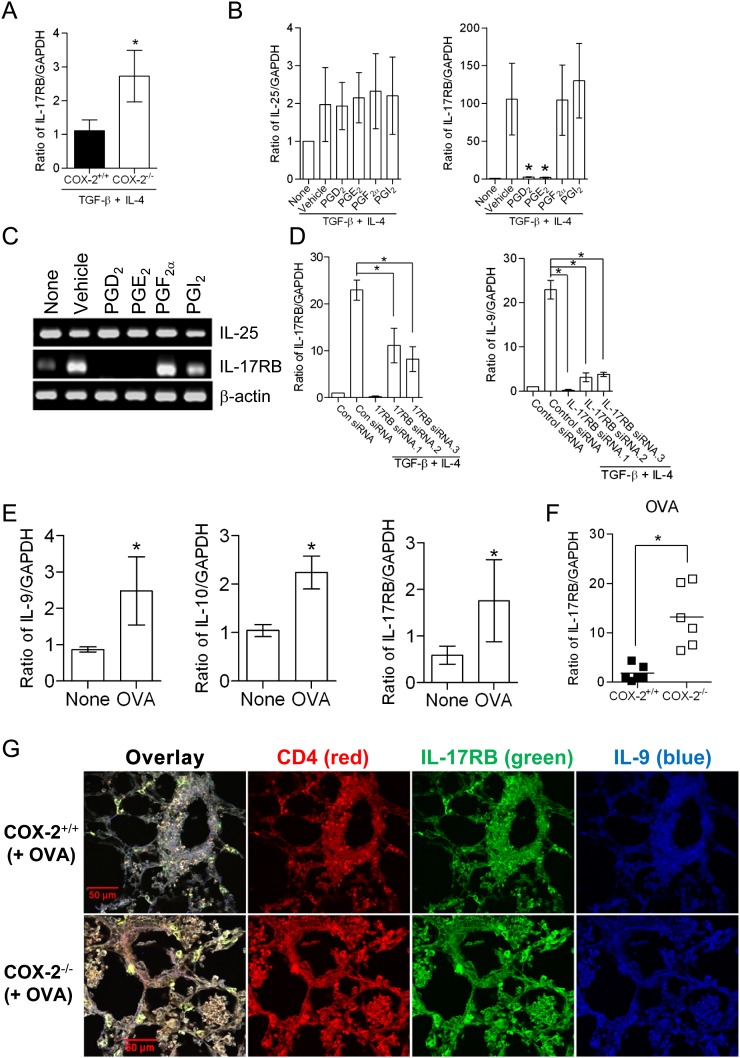
Recent studies have identified a role for IL-25 in the regulation of Th9 cells (22). IL-25/IL-17RB signaling promotes IL-9 expression through a mechanism that is independent of endogenous IL-4 production from differentiating T cells, but dependent upon endogenous TGF-β (22). To identify the signaling mechanisms involved in the regulation of Th9 cell differentiation by COX-2–derived PGs, we examined expression of IL-17RB and IL-25 during Th9 cell differentiation from WT and COX-2−/− naive CD4+ T cells, and also studied the effects of PGs on these endpoints. IL-17RB expression was significantly increased in Th9 cells differentiated from COX-2−/− naive CD4+ T cells compared with WT cells (Figure 4A). Treatment of naive CD4+ T cells from COX-2−/− mice with either PGD2 or PGE2, but not PGF2α or PGI2, markedly inhibited IL-17RB expression, but had no significant effect on IL-25 levels (Figures 4B and 4C). siRNA knockdown of IL-17RB during Th9 cell differentiation prevented the increase in IL-9 in COX-2−/− naive CD4+ T cells (Figure 4D). In addition, CD4+ T cells isolated from WT mice exhibited increased expression of IL-17RB, IL-9, and IL-10 after OVA exposure (Figure 4E). In CD4+ T cells isolated from lungs of allergic COX-2−/− mice, IL-17RB expression was significantly increased compared with WT mice (Figure 4F). IL-17RB+ IL-9+ CD4+ T cells were also increased at inflammatory loci in COX-2−/− lungs relative to WT lungs after OVA exposure (Figure 4G).
Figure 4.
Cyclooxygenase (COX)-2–derived prostaglandins (PGs) inhibit T helper cell type 9 (Th9) differentiation through down-regulation of IL-17RB. (A) Naive CD4+ T cells from COX-2+/+ and COX-2−/− mice were differentiated into Th9 cells and IL-17RB mRNA levels were assayed by real-time RT-PCR. (B and C) The effects of PGD2, PGE2, PGF2α, and PGI2 (1 μM each) on IL-25 and IL-17RB levels was examined by RT-PCR and real-time RT-PCR (n = 5; *P < 0.05 versus vehicle). (D) Naive CD4+ T cells from wild-type (WT) mice were transfected with IL-17RB receptor siRNAs or control siRNA. Transfected cells were then differentiated in the presence of anti-CD3, anti-CD28, anti–INF-γ, IL-4, and transforming growth factor (TGF)-β for 5 days. Levels of IL-9 and IL-17RB relative to GAPDH were determined by real-time RT-PCR (n = 3; *P < 0.05 versus control siRNA). (E) WT mice were sensitized and exposed to ovalbumin (OVA), after which lung CD4+ T cells were isolated and levels of IL-9, IL-10, and IL-17RB mRNAs were determined by real-time RT-PCR. (F) Levels of IL-17RB in allergic COX-2−/− and WT mice by real-time RT-PCR. (G) Immunofluorence staining of lungs from COX-2+/+ and COX-2−/− mice with anti–CD4+-phycoerythrin, anti–IL-17RB–fluorescein isothiocyanate and anti–IL-9–antigen-presenting cells after OVA exposure. IL-17RB-positive Th9 cells are increased in inflammatory loci of COX-2−/− lungs. Scale bars = 50 μm. GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
To determine the molecular mechanisms through which PGE2 and PGD2 signaling inhibit IL-17RB expression, we interrogated the mouse IL-17RB promoter using a series of luciferase reporter constructs (Figure E4). cAMP response element (CRE) binding protein (CREB) is a transcription factor that binds to CREs and regulates transcription of a number of downstream genes. Two consensus CRE sites were identified in the IL-17RB promoter at positions −1.771 kb and −3.894 kb relative to the transcription start site. In T cells transfected with luciferase constructs containing the proximal CRE element, PGE2 decreased luciferase activity compared with vehicle-treated cells. In contrast, the response to PGE2 was attenuated in a truncation mutant that lacked the proximal CRE element (plasmid IL17RB.1.1kb). Importantly, when the proximal CRE element was specifically mutated (plasmid IL17RB-CREB-3.3kb), PGE2 treatment resulted in increased luciferase activity. Based on these data, we conclude that PGE2 negatively regulates the IL-17RB promoter through the proximal CRE site. Consistent with its suppression of endogenous IL-17RB expression (Figure 4B), PGD2 treatment resulted in suppression of IL-17RB promoter activity (Figure E4). Importantly, deletion of the proximal CRE site of IL-17RB promoter significantly increased luciferase activity, suggesting that PGD2 may act through a CREB-dependent pathway. PGD2–DP2 signaling can activate phosphoinositide-3-kinase signaling, may activate CREB in a protein kinase (PK) A–independent manner, or may act on critical pathway components in addition to IL-17RB. Our data suggest that PGD2 suppression of Th9 differentiation is PKA independent. Thus, whereas the PKA inhibitor, H89, promoted Th9 cell differentiation in vitro, PGD2 strongly inhibited Th9 differentiation, even in the presence of H89 (Figure E4). Together, these findings indicate that PGD2 inhibits Th9 differentiation through a CREB-dependent, PKA-independent mechanism.
Regulation of Th9 Cell Differentiation by PGD2 and PGE2 Is Mediated through Prostanoid Receptors
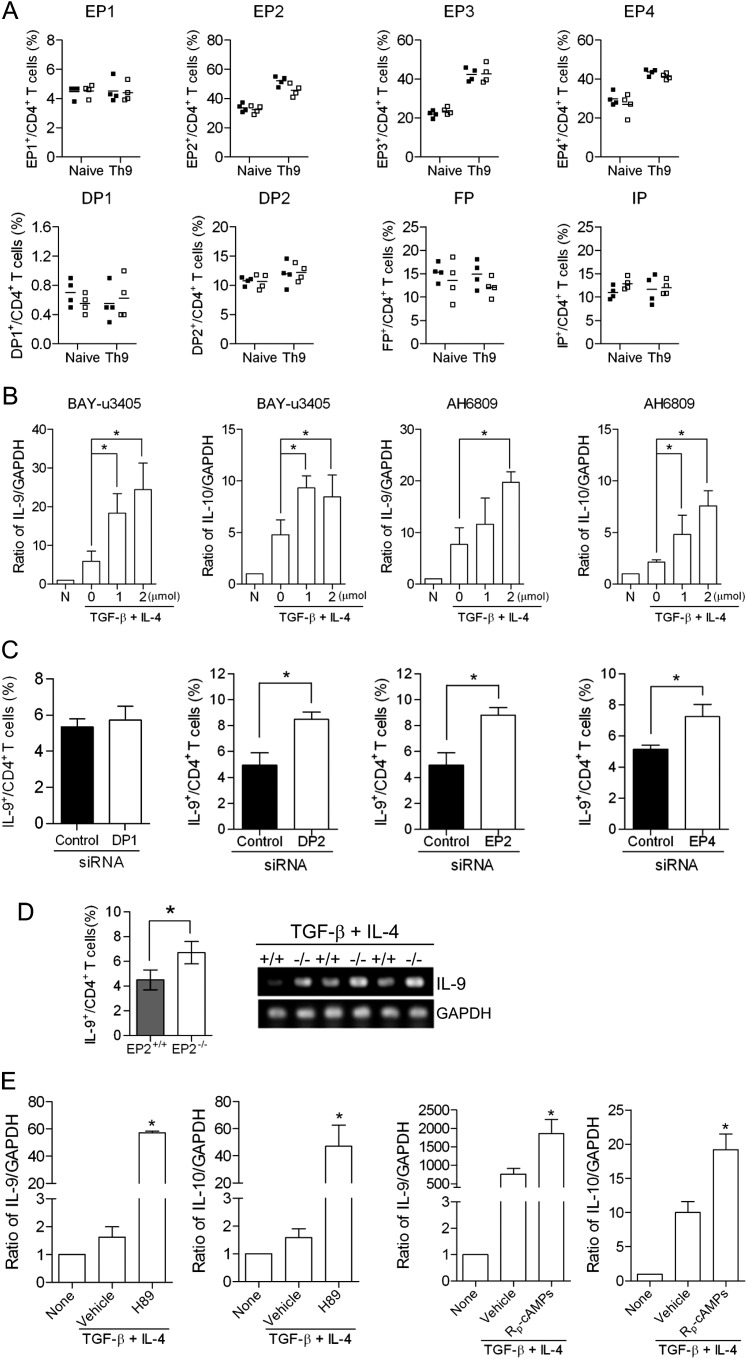
To determine whether PGs regulate Th9 cell differentiation through their cognate receptors, we first identified the receptors that were expressed in naive CD4+ T cells and in vitro–differentiated Th9 cells from WT and COX-2−/− mice. Each of the prostanoid receptors were expressed at similar levels in naive CD4+ T cells from WT and COX-2−/− mice, as assessed by flow cytometry. Interestingly, the expression of EP2, EP3, and EP4 receptors was increased in Th9 cells differentiated from both WT and COX-2−/− naive CD4+ T cells, but, again, there were no differences between the two genotypes (Figure 5A). EP2 and EP4 receptor transcripts were up-regulated in differentiated Th9 cells from both WT and COX-2−/− mice (Figure E5). DP1 receptors were detected on less than 1% of naive CD4+ T cells and Th9 cells, whereas DP2 receptors were more widely expressed (Figure 5A). DP1 receptor transcripts were down-regulated, whereas DP2 receptor transcripts were up-regulated, during Th9 differentiation in vitro (Figure E5B).
Figure 5.
Regulation of T helper cell type 9 (Th9) cell differentiation is mediated through prostanoid receptors. (A) The percentage of prostanoid receptor (EP1, EP2, EP3, EP4, DP1, DP2, FP, and IP)–positive naive CD4+ T cells and in vitro differentiated Th9 cells from cyclooxygenase (COX)-2+/+ and COX-2−/− mice was determined by flow cytometry. Lines indicate the mean, and each symbol (COX-2+/+, filled squares; COX-2−/−, open squares) represents an individual mouse. (B) The effects of the TP/DP2 receptor antagonist, BAY-u3405, or the EP1–3/DP1 receptor antagonist, AH6809, on Th9 differentiation of naive CD4+ T cells were investigated by real-time RT-PCR (n = 3; *P < 0.05 versus vehicle). (C) Naive CD4+ T cells from wild-type (WT) mice were transfected with DP1, DP2, EP2, or EP4 receptor siRNAs, or control siRNA. Transfected cells were then differentiated in the presence of anti-CD3, anti-CD28, anti–INF-γ, transforming growth factor (TGF)-β, and IL-4 for 5 days and the percentage of Th9 cells was analyzed by flow cytometry (n = 3; *P < 0.05 versus control siRNA). (D) Th9 cell differentiation of naive CD4+ T cells isolated from EP2 receptor knockout mice and WT controls were investigated by flow cytometry and RT-PCR (n = 5; *P < 0.05 versus WT). (E) Two different protein kinase A (PKA) inhibitors (H89 and Rp-cAMPs) significantly increase Th9 cell differentiation, as measured by IL-9 and IL-10 expression in vitro (n = 3; *P < 0.05 versus vehicle).
To elucidate further the role of specific prostanoid receptors in Th9 cell differentiation, we examined the effects of DP and EP receptor agonists/antagonists and siRNA knockdown in vitro. Th9 cell differentiation from naive CD4+ T cells was significantly increased in the presence of the TP/DP2 receptor antagonist, BAY-u3405, or the EP1–3/DP1 receptor antagonist, AH6809 (Figure 5B). The DP2-selective agonist, 15(R)-PGD2, strongly suppressed Th9 differentiation, whereas the DP1-selective agonist, BW246C, had no significant effect (Figure E5C). Likewise, DP1 receptor siRNA treatment did not alter Th9 cell differentiation, whereas knockdown of the DP2, EP2, or EP4 receptors significantly increased Th9 cell differentiation in vitro (Figure 5C and Figure E6). Consistent with these data, we observed an increase in Th9 cell differentiation from naive CD4+ T cells isolated from EP2−/− mice compared with cells isolated from WT mice (Figure 5D).
Activation of EP2 and EP4 receptors increases cAMP (Figure E7) leading to activation of cAMP-dependent PKA which activates CREB. We found that inhibition of PKA by H89 or Rp-cAMPs significantly increased Th9 cell differentiation of naive CD4+ T cells isolated from WT mice in vitro, as measured by IL-9 and IL-10 mRNA levels (Figure 5E). Taken together, these results indicate that COX-2–derived PGE2 activates EP2 and/or EP4 receptors to regulate Th9 cell differentiation via a mechanism that involves activation of PKA.
Human Th9 Cell Differentiation Is Also Regulated by COX-2–derived Prostanoids
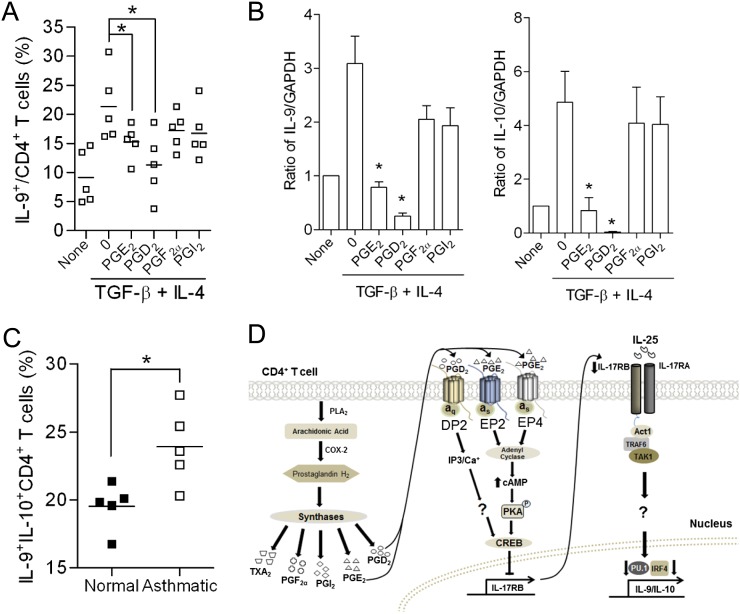
Studies have shown that IL-9 is involved in the pathogenesis of human asthma (8, 10, 22, 37–40). Indeed, monoclonal antibodies to IL-9 are currently being evaluated in asthma clinical trials (41). To test whether COX-2–derived PGs could regulate human Th9 cell differentiation, we isolated naive CD4+ T cells from peripheral blood of healthy volunteers and differentiated them to Th9 cells ex vivo with anti-CD3, anti-CD28, IL-4, and TGF-β in the presence or absence of PGs. PGD2 and PGE2, but not PGF2α or PGI2, significantly reduced the percentage of Th9 cells and decreased levels of IL-9 and IL-10 mRNAs (Figures 6A and 6B). Interestingly, subjects with mild to moderate asthma had significantly more circulating Th9 cells compared with age- and sex-matched control subjects without asthma (Figure 6C and Table E1). Thus, human Th9 cell differentiation is also regulated by COX-2–derived eicosanoids.
Figure 6.
Human T helper cell type 9 (Th9) cell differentiation is regulated by cyclooxygenase (COX)-2–derived prostanoids. (A) Naive CD4+ T cells were isolated from peripheral blood of normal human volunteers and differentiated in vitro in the presence of anti-CD3, anti-CD28, anti–INF-γ, IL-4, and transforming growth factor (TGF)-β, in the presence or absence of prostaglandin (PG) E2, PGD2, PGF2α, and PGI2 (1 μM each). The percentage of Th9 cells was measured by flow cytometry (n = 5; *P < 0.05). (B) IL-9 and IL-10 mRNA levels in Th9 cells differentiated from human naive CD4+ T cells were measured by real-time RT-PCR (n = 5; *P < 0.05 versus vehicle). (C) Percentage of Th9 cells isolated from peripheral blood of individuals with and without asthma was analyzed by flow cytometry (n = 5; *P < 0.05). (D) Proposed mechanisms for regulation of Th9 cell differentiation by COX-2–derived PGs. Binding of PGD2 and PGE2 to DP2, EP2, and EP4 prostanoid receptor subtypes on CD4+ T cells leads to decreased IL-17RB expression. This results in less up-regulation of PU.1 and IFN-regulatory factor (IRF) 4 transcription factors, which leads to less induction of IL-9 expression and reduced Th9 cell differentiation. Act1 = NF-κB activator 1; CREB = cAMP response element binding protein; IP3 = inositol triphosphate; TAK1 = transforming growth factor-β–activated kinase 1; TRAF6 = TNF receptor–associated factor 6; TXA2 = thromboxane A2.
Discussion
Th9 cells are a unique subset of effector T cells distinct from Th1, Th2, and Th17 cell subsets (1). Th9 cells secrete IL-9 and IL-10, which have been reported to play important roles in the pathogenesis of asthma in humans (39, 40). COX-derived eicosanoids are known to regulate inflammatory responses, and we recently reported that COX-2 regulates Th17 cell differentiation and function (34, 42). In the current study, we investigated the roles of COX-1 and COX-2 in regulating Th9 cell differentiation and function in allergic lung inflammation. Our main finding is that COX-2, but not COX-1, is a critical negative regulator of Th9 cell differentiation and IL-9 production during allergic lung inflammation. COX-2–derived PGD2 and PGE2 act through DP2 and EP2/4 receptors to down-regulate IL-17RB expression in CD4+ T cells and suppress Th9 cell differentiation. Our studies reveal key mechanistic pathways involved in regulation of COX-2, activation of PG signaling, and modulation of transcription factors that coordinate Th9 cell differentiation of naive CD4+ T cells.
Th9-inducing cytokines, TGF-β and IL-4, had opposing effects on COX-2 expression in CD4+ T cells. COX-2 expression was promoted by TGF-β and inhibited by IL-4 during Th9 differentiation. This is consistent with published data that IL-4 suppressed COX-2 expression in dendritic cells (36). IL-4 addition enhanced Th9 cell differentiation and IL-9 production, which may be due, at least in part, to suppression of COX-2–derived PGs and downstream signaling. Thus, an important function of IL-4 may be to attenuate COX-2 expression to allow for efficient Th9 differentiation.
The role of COX-2–derived PGs in lung immunity and asthma is complex. PGD2 production is induced by allergens and is released by mast cells during asthma attacks (43). PGD2 causes several proinflammatory effects in the lung, including bronchoconstriction and eosinophilia (44). In contrast, PGE2 has protective effects in models of allergic inflammation by suppressing bronchoconstriction, eosinophilia, and T cell proliferation (45). PGE2 may also act through down-regulation of PGD2 synthesis to achieve these effects (45). We previously showed that COX-2−/− mice have increased antigen-induced lung inflammation, which suggested a loss of PGs that have a protective role in the lung (35). Consistent with those findings, we found that levels of PGE2 were significantly reduced in differentiating T cells from COX-2−/− mice. Loss of COX-2–derived PGs led to increased Th9 cell differentiation and IL-9 secretion, which may then contribute to the lung inflammation and eosinophilia observed in COX-2−/− mice (35, 40).
We identified the specific DP and EP receptors and signaling pathways involved in inhibition of Th9 cell differentiation by COX-2–derived eicosanoids. PGD2 is known to signal through two GPCRs, DP1 and DP2 (also known as CRTH2 or GPR44) (43, 46, 47). The DP1 receptor is coupled to a Gs protein and mediates the activity of mast cell–derived PGD2 in asthma and other allergic diseases (48). The DP2 receptor couples to Gi protein and mediates chemotaxis of Th2 lymphocytes and eosinophils (43). PGE2 signals through four GPCRs, EP1–EP4. Activation of EP2 and EP4 receptors is linked to Gs proteins, which stimulate cAMP production (45). PGD2 and PGE2 receptors are either constitutively expressed in CD4+ T cells or are induced by TGF-β and IL-4 (34). Knockdown of DP2, EP2, and EP4 receptor expression in naive CD4+ T cells by siRNA significantly increased Th9 cell differentiation. The role of EP2 was further confirmed using EP2-deficient mice. Although previous studies with knockout mice or receptor antagonists revealed that these receptors can influence asthma and allergy phenotypes (43, 49), our data provide additional mechanistic insights into how these receptors influence the immune response to allergens. PGE2 receptor activation suppressed Th9 cell differentiation through activation of cAMP/PKA–dependent signaling, as both Rp-cAMPs and H-89 significantly increased IL-9 and IL-10 production. Taken together, our results show that the increased Th9 cell differentiation observed in COX-2−/− CD4+ T cells is due to decreased PGE2 and/or PGD2 signaling through their respective cognate receptors.
Our results define the precise molecular mechanisms underlying the regulation of Th9 cell differentiation by COX-2–derived PGs. During Th9-inducing conditions, IL-25 activates IL-17RA/IL-17RB receptors on naive CD4+ T cells (22). IL-25 signaling then up-regulates PU.1 and IRF4 to induce production of IL-9 and IL-10, which are the hallmarks of Th9 cell differentiation (20, 25). Treatment with OVA allergen in vivo or TGF-β/IL-4 in vitro significantly induced IL-17RB expression to facilitate Th9 cell differentiation. COX-2–derived PGs blocked this signaling at the level of IL-17RB transcription. Indeed, PGD2 or PGE2 treatment abolished IL-17RB induction, diminished PU.1 and IRF4 expression, and attenuated Th9 cell differentiation. Furthermore, PGE2 negatively regulated IL-17RB transcription via a proximal CRE site in the IL-17RB promoter. In contrast, PGD2 inhibited IL-17RB transcription and Th9 cell differentiation through a separate CREB-dependent, PKA-independent pathway.
We confirmed that COX-2–derived PGs also regulate Th9 cell differentiation of human T cells. Similar to observations in mice, both PGD2 and PGE2 diminished Th9 cell differentiation of human peripheral blood naive CD4+ T cells. In addition, subjects with asthma were found to have increased numbers of circulating Th9 cells. These results suggest the possibility that differential COX-2 activity, either through genetic variation or the use of COX-2 inhibitors, may be involved in the progression of allergy and asthma in humans through aberrant regulation of Th9 cell differentiation.
In conclusion, we have provided in vitro and in vivo evidence that COX-2–derived PGs act as critical regulators of Th9 cell differentiation and function (Figure 6D). COX-2 is up-regulated by TGF-β, which, together with IL-4, induces Th9 differentiation. PGD2 and PGE2 act through their cognate receptors to down-regulate IL-17RB expression and IL-9/IL-10 induction. Our results demonstrate an important role of PGs in coordinating both IL-17RB and IL-9 expression in CD4+ T cells. Further research on the role of COX-2–derived PGs in the regulation of Th9 cell subsets and their function in immune-mediated diseases will be required to better understand the complex effector mechanisms involved.
Footnotes
Supported by National Institutes of Health/National Institute of Environmental Health Sciences, Division of Intramural Research grants Z01 ES025043 and Z01 ES050167.
Author Contributions: The work presented here was performed in collaboration with all authors. H.L.: conception, design, performance and interpretation of experiments, and writing the manuscript. M.L.E.: Acquisition and analysis of liquid chromatography/mass spectrometry data, and revision of manuscript. J.A.B.: Acquisition and analysis of in vivo, Western blot, and ELISA experiments. J.P.G.: Acquisition and analysis of RT-PCR data. L.M.D.: Acquisition and analysis of prostanoid mini-pump data. A.G.: Acquisition and analysis of liquid chromatography/mass spectrometry data, and revision of manuscript. J.C.: Acquisition and analysis of RT-PCR data, and revision of manuscript. R.T.D.: Acquisition and analysis of prostanoid mini-pump data, and revision of manuscript. P.M.W.: Acquisition and analysis of lung immunostaining data. C.D.B.: Acquisition and analysis of FACS data. S.G.: Acquisition and analysis of human experimental data. A.M.J.: Hypothesis, conception, and design of the study. D.C.Z.: Hypothesis, conception, and design of the study, interpretation of data, and revision of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201211-2073OC on March 1, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Durrant DM, Metzger DW. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunol Invest. 2010;39:526–549. doi: 10.3109/08820131003615498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T cells. Eur J Immunol. 2009;39:1301–1312. doi: 10.1002/eji.200838969. [DOI] [PubMed] [Google Scholar]
- 3.Corren J. Cytokine inhibition in severe asthma: current knowledge and future directions. Curr Opin Pulm Med. 2011;17:29–33. doi: 10.1097/MCP.0b013e3283413105. [DOI] [PubMed] [Google Scholar]
- 4.Wan YY. Multi-tasking of helper T cells. Immunology. 2010;130:166–171. doi: 10.1111/j.1365-2567.2010.03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrigan CJ, Wang W, Meng Q, Fang C, Wu H, Reay V, Lv Z, Fan Y, An Y, Wang YH, et al. T-helper cell type 2 (Th2) memory T cell–potentiating cytokine IL-25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci USA. 2011;108:1579–1584. doi: 10.1073/pnas.1014241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jager A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173–184. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jutel M, Akdis CA. T-cell subset regulation in atopy. Curr Allergy Asthma Rep. 2011;11:139–145. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011;183:865–875. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma CS, Tangye SG, Deenick EK. Human Th9 cells: inflammatory cytokines modulate IL-9 production through the induction of IL-21. Immunol Cell Biol. 2010;88:621–623. doi: 10.1038/icb.2010.73. [DOI] [PubMed] [Google Scholar]
- 10.Ciprandi G, De Amici M, Castellazzi AM, Tosca MA, Marseglia G. Serum IL-9 levels depend on allergen exposure: Preliminary study. Int Arch Allergy Immunol. 2011;154:246–248. doi: 10.1159/000321111. [DOI] [PubMed] [Google Scholar]
- 11.Fujisawa T, Katsumata H, Kato Y. House dust mite extract induces interleukin-9 expression in human eosinophils. Allergol Int. 2008;57:141–146. doi: 10.2332/allergolint.O-07-498. [DOI] [PubMed] [Google Scholar]
- 12.Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54:577–589. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J, Wong B, Cundall M, Goncharova S, Conway M, Dalrymple A, Coyle AJ, Waserman S, Jordana M. Immunoreactivity profile of peripheral blood mononuclear cells from patients with ragweed-induced allergic rhinitis. Clin Exp Allergy. 2007;37:901–908. doi: 10.1111/j.1365-2222.2007.02723.x. [DOI] [PubMed] [Google Scholar]
- 14.Thunberg S, Gafvelin G, Nord M, Gronneberg R, Grunewald J, Eklund A, van Hage M. Allergen provocation increases Th2-cytokines and Foxp3 expression in the asthmatic lung. Allergy. 2010;65:311–318. doi: 10.1111/j.1398-9995.2009.02218.x. [DOI] [PubMed] [Google Scholar]
- 15.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, et al. IL-9– and mast cell–mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, Orban T, Elyaman W, Khoury SJ, Kuchroo VK, Baecher-Allan C, et al. TGF-beta induces IL-9 production from human Th17 cells. J Immunol. 2010;185:46–54. doi: 10.4049/jimmunol.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta–induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak EC, Noelle RJ. Interleukin-9 as a T helper type 17 cytokine. Immunology. 2010;131:169–173. doi: 10.1111/j.1365-2567.2010.03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh CK, Raible D, Geba GP, Molfino NA. Biology of the interleukin-9 pathway and its therapeutic potential for the treatment of asthma. Inflamm Allergy Drug Targets. 2011;10:180–186. doi: 10.2174/187152811795564073. [DOI] [PubMed] [Google Scholar]
- 20.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, et al. The transcription factor PU.1 is required for the development of IL-9–producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Mobini R, Fang Y, Barrenas F, Zhang H, Xiang Z, Benson M. Allergen challenge of peripheral blood mononuclear cells from patients with seasonal allergic rhinitis increases IL-17RB, which regulates basophil apoptosis and degranulation. Clin Exp Allergy. 2010;40:1194–1202. doi: 10.1111/j.1365-2222.2010.03542.x. [DOI] [PubMed] [Google Scholar]
- 25.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25–induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 26.Stock P, Lombardi V, Kohlrautz V, Akbari O. Induction of airway hyperreactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J Immunol. 2009;182:5116–5122. doi: 10.4049/jimmunol.0804213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandler DB, Fulmer JD. Prostaglandin synthesis and release by subpopulations of rat alveolar macrophages. J Immunol. 1987;139:893–898. [PubMed] [Google Scholar]
- 28.Machado ER, Carlos D, Lourenco EV, Souza GE, Sorgi CA, Silva EV, Ueta MT, Ramos SG, Aronoff DM, Faccioli LH. Cyclooxygenase-derived mediators regulate the immunological control of Strongyloides venezuelensis infection. FEMS Immunol Med Microbiol. 2010;59:18–32. doi: 10.1111/j.1574-695X.2010.00656.x. [DOI] [PubMed] [Google Scholar]
- 29.Diaconu CC, Neagu AI, Lungu R, Tardei G, Alexiu I, Bleotu C, Economescu MC, Bumbacea RS, Pele I, Bumbacea D. Plasticity of regulatory T cells under cytokine pressure. Roum Arch Microbiol Immunol. 2010;69:190–196. [PubMed] [Google Scholar]
- 30.Hou Z, Falcone DJ, Subbaramaiah K, Dannenberg AJ. Macrophages induce cox-2 expression in breast cancer cells: role of IL-1beta autoamplification. Carcinogenesis. 2011;32:695–702. doi: 10.1093/carcin/bgr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toller IM, Hitzler I, Sayi A, Mueller A. Prostaglandin E2 prevents helicobacter-induced gastric preneoplasia and facilitates persistent infection in a mouse model. Gastroenterology. 2010;138:1455–1467. doi: 10.1053/j.gastro.2009.12.006. , 1467.e1–4. [DOI] [PubMed] [Google Scholar]
- 32.Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, Tiano HF, Lee CA, Langenbach R, Roggli VL, et al. Allergic lung responses are increased in prostaglandin H synthase–deficient mice. J Clin Invest. 1999;104:721–732. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sousa A, Pfister R, Christie PE, Lane SJ, Nasser SM, Schmitz-Schumann M, Lee TH. Enhanced expression of cyclo-oxygenase isoenzyme 2 (COX-2) in asthmatic airways and its cellular distribution in aspirin-sensitive asthma. Thorax. 1997;52:940–945. doi: 10.1136/thx.52.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Bradbury JA, Dackor RT, Edin ML, Graves JP, DeGraff LM, Wang PM, Bortner CD, Maruoka S, Lih FB, et al. Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation. Am J Respir Crit Care Med. 2011;184:37–49. doi: 10.1164/rccm.201010-1637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carey MA, Germolec DR, Bradbury JA, Gooch RA, Moorman MP, Flake GP, Langenbach R, Zeldin DC. Accentuated T helper type 2 airway response after allergen challenge in cyclooxygenase-1−/− but not cyclooxygenase-2−/− mice. Am J Respir Crit Care Med. 2003;167:1509–1515. doi: 10.1164/rccm.200211-1383OC. [DOI] [PubMed] [Google Scholar]
- 36.Teloni R, Giannoni F, Rossi P, Nisini R, Gagliardi MC. Interleukin-4 inhibits cyclo-oxygenase-2 expression and prostaglandin E production by human mature dendritic cells. Immunology. 2007;120:83–89. doi: 10.1111/j.1365-2567.2006.02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baraldo S, Faffe DS, Moore PE, Whitehead T, McKenna M, Silverman ES, Panettieri RA, Jr, Shore SA. Interleukin-9 influences chemokine release in airway smooth muscle: role of Erk. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1093–L1102. doi: 10.1152/ajplung.00300.2002. [DOI] [PubMed] [Google Scholar]
- 38.Nouri-Aria KT, Pilette C, Jacobson MR, Watanabe H, Durham SR. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. J Allergy Clin Immunol. 2005;116:73–79. doi: 10.1016/j.jaci.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127:450–458. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xing J, Wu Y, Ni B. Th9: a new player in asthma pathogenesis? J Asthma. 2011;48:115–125. doi: 10.3109/02770903.2011.554944. [DOI] [PubMed] [Google Scholar]
- 41.Parker JM, Oh CK, LaForce C, Miller SD, Pearlman DS, Le C, Robbie GJ, White WI, White B, Molfino NA. Safety profile and clinical activity of multiple subcutaneous doses of MEDI-528, a humanized anti–interleukin-9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulm Med. 2011;11:14. doi: 10.1186/1471-2466-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocca B, Spain LM, Pure E, Langenbach R, Patrono C, FitzGerald GA. Distinct roles of prostaglandin H synthases 1 and 2 in T-cell development. J Clin Invest. 1999;103:1469–1477. doi: 10.1172/JCI6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arima M, Fukuda T. Prostaglandin D2 receptors DP and CRTH2 in the pathogenesis of asthma. Curr Mol Med. 2008;8:365–375. doi: 10.2174/156652408785160970. [DOI] [PubMed] [Google Scholar]
- 44.Arima M, Fukuda T. Prostaglandin D and T(h)2 inflammation in the pathogenesis of bronchial asthma. Korean J Intern Med. 2011;26:8–18. doi: 10.3904/kjim.2011.26.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2006;290:L797–L805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettipher R, Hansel TT, Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat Rev Drug Discov. 2007;6:313–325. doi: 10.1038/nrd2266. [DOI] [PubMed] [Google Scholar]
- 47.Gallant MA, Slipetz D, Hamelin E, Rochdi MD, Talbot S, de Brum-Fernandes AJ, Parent JL. Differential regulation of the signaling and trafficking of the two prostaglandin D2 receptors, prostanoid DP receptor and CRTH2. Eur J Pharmacol. 2007;557:115–123. doi: 10.1016/j.ejphar.2006.11.058. [DOI] [PubMed] [Google Scholar]
- 48.Jabbour HN, Sales KJ. Prostaglandin receptor signalling and function in human endometrial pathology. Trends Endocrinol Metab. 2004;15:398–404. doi: 10.1016/j.tem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med. 2008;8:335–349. doi: 10.2174/156652408785160989. [DOI] [PubMed] [Google Scholar]