Abstract
In pulmonary arterial hypertension (PAH), there is overexpression of the chemokine, C-C chemokine ligand type 2 (CCL2), and infiltration of myeloid cells into the pulmonary vasculature. Inhibition of CCL2 in animals decreases PAH, suggesting that the CCL2 receptor (CCR2) plays a role in PAH development. To test this hypothesis, we exposed wild-type (WT) and CCR2-deficient (Ccr2−/−) mice to chronic hypobaric hypoxia to induce PAH. After hypoxic stress, Ccr2−/− mice displayed a more severe PAH phenotype, as demonstrated by increased right ventricular (RV) systolic pressures, RV hypertrophy, and tachycardia relative to WT mice. However, these mice also exhibited increased RV systolic pressures and increased pulmonary artery muscularization under normoxic conditions. Moreover, Ccr2−/− mice displayed decreased pulmonary vascular branching at 3 weeks of age and increased vascular muscularization at birth, suggesting that an abnormality in pulmonary vascular development leads to spontaneous PAH in these animals. No significant differences in cytokine responses were observed between WT and Ccr2−/− mice during either normoxia or hypoxia. However, Ccr2−/− mice displayed increased Notch-3 signaling and dysregulated Notch ligand expression, suggesting a possible cause for their abnormal pulmonary vascular development. Our findings imply that CCR2 does not directly contribute to the development of PAH, but does play a previously unrecognized role in pulmonary vasculature development and remodeling wherein the absence of CCR2 results in spontaneous PAH, most likely via dysregulation of Notch signaling. Our results demonstrate that CCR2 has impacts beyond leukocyte recruitment, and is required for the proper expression of Notch signaling molecules.
Keywords: pulmonary arterial hypertension, C-C chemokine type 2 receptor, Notch-3, Jagged-1, Delta-like-4
Clinical Relevance
This article describes novel interactions between the chemokine and Notch signaling pathways and effects of C-C chemokine ligand type 2 receptor (CCR2) deficiency on lung vascular development. This is likely to have implications for the numerous investigators using CCR2-deficient mice in various studies, and raises important issues about the use of CCR2 antagonists in humans.
Pulmonary arterial hypertension (PAH) is defined by elevated pulmonary arterial pressure and subsequent right ventricular (RV) hypertrophy and failure caused by progressive remodeling of small pulmonary arterioles (1). This remodeling process is induced by environmental triggers, such as hypoxia, by some types of inflammation, and by genetic factors. A search for genetic disease determinants has identified bone morphogenic protein receptor 2, which belongs to the transforming growth factor-β (TGF-β)receptor super family, but bone morphogenic protein receptor 2 (BMPR2) is only associated with a small percentage of PAH, and exhibits low penetrance (2, 3). Major determinants of disease severity in PAH remain to be identified, and there are likely multiple disease-modifying genes to be uncovered (2).
It is currently thought that pulmonary vascular remodeling involves endothelial dysfunction, vascular smooth muscle cell (VSMC) proliferation, and immune cell recruitment as pathologic consequences of dysregulation of specific cellular and molecular signaling pathways (1, 4, 5). In addition to the transforming growth factor-β pathway, Notch signaling contributes to pulmonary vasculature remodeling. In mammals, four Notch receptors (Notch 1–4) have been identified. Notch-3–mediated signaling regulates VSMC differentiation and proliferation, and is required for development of hypoxia-induced PAH in animals (6, 7). In addition, Notch-3 expression levels in patients are correlated with disease severity (7). Of the four mammalian Notch ligands expressed on endothelial cells and VSMCs (Jagged-1, Jagged-2, Delta-like [DLL]-1, and DLL-4), Jagged-1 interacts with Notch-3 on developing VSMCs (8). However, the contribution of individual Notch ligands to pulmonary vascular remodeling and PAH is not known.
Another important pathological characteristic of pulmonary vascular remodeling is inflammation. In both patients with and animal models of PAH, there are prominent inflammatory cell infiltrates around the pulmonary vasculature, consisting predominately of cells of myeloid cell lineage monocytes, dendritic cells (DCs), and macrophages (5, 9, 10). In animal models of PAH, depletion of myeloid cells attenuates the disease (11, 12). Myeloid cells in the lung arise from circulating monocytes, and, in mice, there are two subsets of monocytes: inflammatory (CD11b+Ly6C+CCR2hi) and constitutive (CD11b+Ly6C−CX3CR1hi) monocytes (13). Constitutive monocytes enter tissues continuously to form resident myeloid populations, and are thought to have a role in surveillance. Inflammatory monocytes exit the bone marrow and enter tissues under inflammatory conditions via the activity of CCR2 (14). Each monocyte population gives rise to distinct lung myeloid populations, the accumulation of which can be prevented by deleting their required chemokine receptor. Constitutive monocytes give rise to alveolar macrophages and interstitial macrophages, whereas inflammatory monocytes give rise to inflammatory DCs and exudative macrophages (15). Because inflammatory monocyte recruitment is CCR2 dependent, its accumulation is abrogated by CCR2 deletion.
The early recruitment of alternatively activated (M2) macrophages promotes PAH progression, and these macrophages are thought to be polarized and activated by T helper (Th) type 2 cytokines (i.e., IL-4 or IL-13, IL-6) and the chemokine, CCL2 (16, 17). Interestingly, endothelial cells isolated from patients with idiopathic PAH (IPAH) demonstrate increased CCL2 expression (18). Blockade of CCL2 expression attenuates the disease severity (19). Taken together, these data suggest that CCL2 may regulate pulmonary hypertension (PH) progression through macrophage accumulation or polarization. However, the role for CCR2, the receptor for CCL2, has not been investigated. Because of the capacity of CCR2 to recruit inflammatory monocytes and, possibly, when activated by CCL2, to polarize M2 macrophages, we hypothesized that CCR2 deletion would mitigate PAH due to absence of inflammatory monocyte and M2 macrophage accumulation.
Using CCR2-deficient (Ccr2−/−) and CCL2-deficient (Ccl2−/−) mice, we found that, contrary to our hypothesis, CCR2 deficiency promotes spontaneous PAH at a young age and, in the setting of hypoxia-induced stress, increases the severity of PAH. Hypoxia-induced immune responses in Ccr2−/− mice are similar to those in wild-type (WT) mice. The increase in PAH severity appears to be due to an effect on pulmonary vasculature development. In addition, we found that CCR2 deficiency has profound effects on Notch signaling pathways, which we argue affects pulmonary vascular development and contributes to spontaneous PAH in Ccr2−/− mice.
Materials and Methods
Animals
Mouse experiments were preapproved by the Institutional Animal Care and Use Committee of Duke University School of Medicine. Male Ccr2−/− and C57BL/6 (WT) mice between 6 and 7 weeks of age were placed either in room air or at 18,000 ft altitude in an environmentally controlled hypobaric chamber. The duration of treatments are specified in each experiment.
Cardiac Function Evaluation
Animals were anesthetized with ketamine (100 mg/kg) and xylazine (2.5 mg/kg), intubated using a 20-gauge catheter, and ventilated (Volume = 0.3 ml; Rate = 110/min). After bilateral vagatomy, the right jugular vein was cannulated with a transducer (Millar Instruments Inc., Houston, TX) and PE-10 tubing (BD Biosciences, Franklin Lakes, NJ). The transducer was advanced to the RV, and heart rate and RV hemodynamic values were recorded with PowerLab (ADInstruments, Colorado Springs, CO). Hemoglobin concentrations were measured using a 682 Co-oximeter (Instrumentation Laboratory, Bedford, MA). Subsequently, RV and left ventricle (LV) plus septum weights were quantified.
Flow Cytometry
Single-cell suspensions were prepared from whole lung. Lung tissues were minced and treated with 1 mg/ml collagenase A and 0.6 mg/ml DNase I (Roche USA, Indianapolis, IN) in Hanks’ balanced salt solution. This mixture was incubated at 37°C for 35 minutes and strained through a 70-μm cell strainer. Red blood cells were removed using red blood cell lysis buffer. The resulting single-cell suspensions were enumerated and analyzed using flow cytometry. Approximately 5 × 105 lung cells were stained with CD11c, CD11b, F4/80, CD31, Ly6G, CD45, and IA/IE (eBiosciences, San Diego, CA), and Gr-1 (BD Bioscience, San Jose, CA). Cell viability was assessed using the Aqua Live/Dead Fixable Dead Cell staining kit (Invitrogen, Grand Island, NY). To distinguish between macrophages and DCs, at least one channel was dedicated to autofluorescence monitoring.
Quantitative Real-Time RT-PCR
Quantitative analysis of mRNA expression was performed using real-time RT-PCR. Total RNA was isolated from whole lung cells using TRIzol Reagent (Invitrogen), and DNA was removed with a DNase-free kit (Invitrogen). Subsequently, cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Amplification was performed using IQ SYBR Green Supermix reagent (Bio-Rad) on a OneStepPlus Real-time PCR system (Applied Biosystems, Carlsbad, CA). The specificity of real-time PCR was confirmed via melting-curve analysis. All analyzed targets were normalized to glyceraldehyde 3-phosphate dehydrogenase (GADPH) level in each sample. The primers used are listed in the online supplement.
Immunohistochemistry
Animals were anesthetized and perfused via the RV with PBS, followed by 4% paraformaldehyde. Lungs were inflated with 4% paraformaldehyde and fixed overnight at 4°C. Sections were deparaffinized, hydrated, permeabilized, and stained sequentially for von Willebrand factor (vWF) (Clone A0082; Dako, Carpinteria, CA) and anti–α-smooth muscle actin (α-SMA) conjugated to alkaline phosphatase (Clone 1A4; Sigma-Aldrich, St. Louis, MO). The number of vWF-positive and α-SMA–positive vessels were counted.
Vascular casting
Casts of the pulmonary vasculature were generated by injecting 3 ml of Microfil (Flow Tech Inc., Carver, MA) into the pulmonary artery. Lungs were cured at 4°C overnight. Tissues were cleared with SCALEA2 solution (Olympus, Center Valley, PA) for 1–2 weeks.
Statistical Analysis
All data are expressed as means (±SEM). Group comparisons were made using Student’s t test or one-way ANOVA.
Results
Inflammatory Responses to Hypoxia in WT and Ccr2−/− Mice
To determine whether CCR2 plays a role in PAH, we exposed WT and Ccr2−/− mice to either normoxia or hypobaric hypoxia for 4 weeks. In initial studies under normoxic conditions, we found no significant differences in the body weight, hemoglobin, heart rate, or left ventricular plus septum weight/body weight ([LV+S]/body weight) between WT and Ccr2−/− mice (Table 1). After 4 weeks of hypoxia, neither WT nor Ccr2−/− mice developed LV hypertrophy, and WT and Ccr2−/− mice exhibited a similar degree of weight loss and increase in hemoglobin levels (Table 1). Overall, WT and Ccr2−/− mice displayed a similar LV mass, body weight, and hemoglobin response under both normoxic and hypoxic conditions.
TABLE 1.
CHARACTERISTICS OF NORMOXIC AND HYPOXIC WILD-TYPE AND C-C CHEMOKINE LIGAND TYPE 2 RECEPTOR–DEFICIENT MICE
| Normoxia | Hypoxia | |
|---|---|---|
| Body weight, g | ||
| WT | 24.29 ± 0.25 | 22.36 ± 0.40* |
| CCR2 | 24.70 ± 0.46 | 22.00 ± 0.47* |
| Hemoglobin, g/dl | ||
| WT | 12.90 ± 0.20 | 19.00 ± 0.31* |
| CCR2 | 13.00 ± 0.25 | 19.60 ± 0.53* |
| Heart rate, bpm | ||
| WT | 381.40 ± 9.00 | 403.00 ± 12.00* |
| CCR2 | 416.70 ± 22.31 | 480.70 ± 30.38*† |
| LV + S/weight, mg/g | ||
| WT | 3.59 ± 0.07 | 3.43 ± 0.07 |
| CCR2 | 3.49 ± 0.09 | 3.59 ± 0.20 |
Definition of abbreviations: bpm, beats per minute; LV, left ventricular; S, septum; WT, wild type.
Normoxic WT mice (n = 24) and CCR2-deficient (Ccr2−/−) mice (n = 17) have similar baseline body weight, hemoglobin, heart rate, and [LV + S]/body weight. After 4 weeks of continuous hypoxia, WT mice (n = 27) and Ccr2−/− mice (n = 22) had responded similarly in body weight, hemoglobin, and [LV + S]/body weight. After hypoxic exposure, Ccr2−/− animals had significant increases in resting heart rate.
P < 0.001 (comparison between normoxia and hypoxia).
P < 0.001 (comparison between WT and Ccr2−/−).
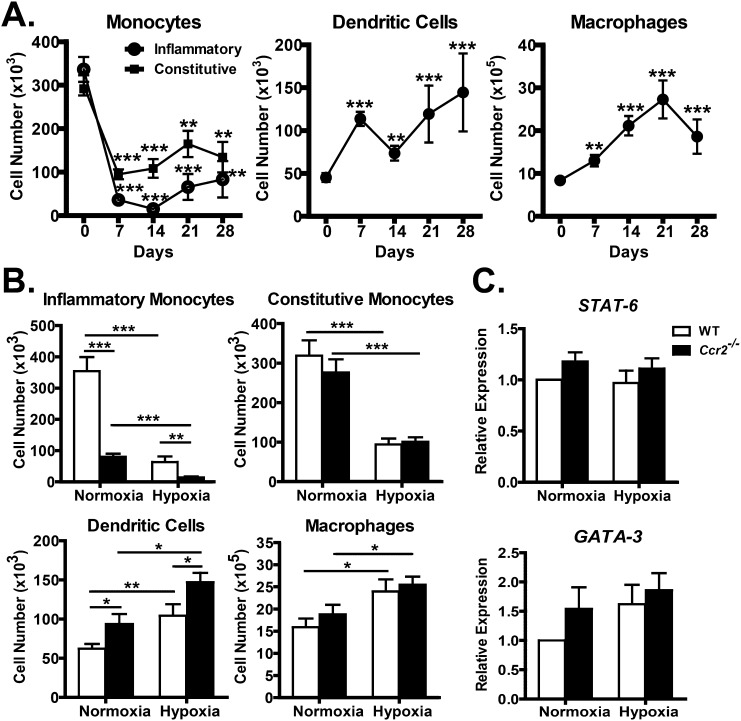
To determine the effects of hypoxia on lung myeloid cell populations, WT mice were exposed to hypobaric hypoxia for increasing periods of time (0, 7, 14, 21, and 28 d) and their total lung immune cells subjected to flow cytometric analysis using, with minor modification, an established protocol (15). As we have previously shown, both inflammatory (Ly6C+) and constitutive (Ly6C−) monocytes are present in the lung in significant and roughly equal numbers at baseline. Unexpectedly, there was a substantial decrease in both monocyte populations after just 7 days of hypoxia, with a 9-fold reduction in inflammatory monocytes and a 3-fold reduction in constitutive monocytes (Figure 1A). Despite a slight recovery in both monocyte populations, the significant reduction in lung monocyte numbers persisted throughout the hypoxia exposure (Figure 1A). Both DC and macrophage numbers increased roughly 3-fold over the course of hypoxia exposure (Figure 1A). Thus, hypoxia appears to reduce the migration of monocytes into the lung, while stimulating their differentiation into more mature cell types.
Figure 1.
Effects of C-C chemokine ligand type 2 (CCL2) receptor (CCR2) deficiency on cellular immune response. (A) Flow cytometry of monophagocytic cell responses under chronic hypoxic stress shows that, after 7 days of hypoxia, there is a significant and persistent decline in both inflammatory and constitutive monocytes. Along with decreasing monocytes, there is an accumulation of monocyte-derived dendritic cells (DCs) and macrophages. (B) Effects of CCR2 deficiency on monophagocytic cell response. CCR2-deficient (Ccr2−/−) mice have decreased inflammatory monocytes under normoxic and hypoxic conditions, and these mice also have increased accumulation of monocyte-derived DCs. Baseline difference in monocytes and DCs are preserved in hypoxic Ccr2−/− mice. (C) Quantitative RT-PCR of T helper (Th) type 2 regulatory genes. There were no significant differences in Stat-6 and GATA-3 between WT and Ccr2−/−. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine the role of CCR2 in the accumulation of lung myeloid cell populations during hypoxia, WT and Ccr2−/− mice were exposed to hypoxia for 28 days, and whole-lung digests were analyzed by flow cytometric analysis. Consistent with reports that CCR2 is critical for the trafficking of inflammatory monocytes into the lung, Ccr2−/− mice displayed a 4.5-fold reduction in lung inflammatory monocyte numbers relative to WT mice during both normoxia and hypoxia (Figure 1B). Despite the baseline differences in cell numbers, WT and Ccr2−/− mice displayed a similar 5.5-fold decrease in inflammatory monocyte numbers after 4 weeks of hypoxia (Figure 1B). Neither constitutive monocyte nor macrophage cell numbers were altered by CCR2 deficiency. However, Ccr2−/− mice displayed a ∼1.5-fold increase in DC numbers during both normoxic and hypoxic conditions (Figure 1B). Thus, although CCR2 deficiency led to a decrease in inflammatory monocytes and an increase in DCs at baseline, it had no effect on the relative changes in myeloid cell populations in hypoxia.
Due to a defect in the accumulation of inflammatory monocyte-derived DCs in lymph nodes, Ccr2−/− mice lack Th1 immune responses and display increased Th2 responses in some conditions (20, 21). To determine if CCR2 deficiency alters the profile of cytokine production in lungs, we collected bronchoalveolar lavage fluid, total lung homogenates, and total lung RNA from WT and Ccr2−/− mice for cytokine analysis after 28 days of normoxia or hypoxia. By multiplex cytokine analysis, we found no significant differences in the levels of IL-1β, IL-2, TNF-α, vascular endothelial growth factor (VEGF), or eotaxin between WT and Ccr2−/− mice, and no significant change in the cytokines with hypoxia (data not shown). Levels of IL-4, IL-5, IL-6, IL-10, IL-13, and IFN-γ were below the limit of detection. To evaluate further the possible changes in Th2 response, we measured signal transducer and activator of transcription 6 (Stat-6) and GATA binding protein 3 (GATA-3) transcript levels, but found no significant differences in their expression levels, regardless of treatment (Figure 1C). We conclude that CCR2 deficiency does not significantly increase lung inflammatory cytokine levels during hypoxia.
Ccr2−/− Mice Have Increased Severity of Hypoxia-Induced PAH
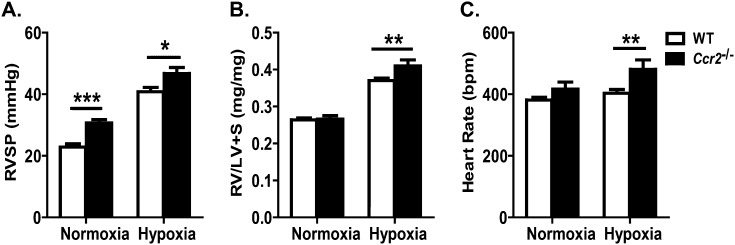
To test the hypothesis that Ccr2−/− mice would be protected from hypoxia-induced PAH, we examined several indices of pulmonary hypertension in WT and Ccr2−/− mice that underwent hypoxia for 4 weeks. In contrast to our hypothesis, Ccr2−/− mice were found to have increased indices of pulmonary hypertension relative to WT mice (Figure 2A). Ccr2−/− mice displayed a significantly higher RV systolic pressure (RVSP) than WT mice (46.71 ± 1.99 mm Hg versus 40.81 ± 1.40 mm Hg; P < 0.05), as well as more pronounced RV hypertrophy than WT mice (RV/[LV + S]: 0.414 ± 0.016 versus 0.370 ± 0.007; P < 0.01) (Figure 2B). In PAH, resting tachycardia is associated with a poor outcome (22). Upon hypoxic stress, Ccr2−/− mice became more tachycardic than WT mice (480.70 ± 30.38 bpm versus 403.00 ± 12.00 bpm; P < 0.01) (Figure 2C).
Figure 2.
CCR2 deficiency leads to increased severity of hypoxia-induced pulmonary arterial hypertension (PAH). (A) Measurements of right ventricular (RV) systolic pressure (RVSP) in WT and Ccr2−/− mice. After 4 weeks of exposure to hypoxia, Ccr2−/− mice (n = 22) have increased RVSP compared with WT (n = 27). (B) Measurements of RV/[LV + S] ratio in WT and Ccr2−/− mice. With hypoxic stress, Ccr2−/− mice exhibit increased RV hypertrophy. (C) Measurement of heart rate (bpm, beats per minute). Compared with hypoxic WT mice, hypoxic Ccr2−/− mice have more tachycardia. *P < 0.05; **P < 0.01; ***P < 0.001.
These findings suggest that Ccr2−/− mice have a heightened hypoxic response; however, some of the abnormalities seen in hypoxic Ccr2−/− mice were also present under normoxic conditions. Ccr2−/− mice displayed an elevated RVSP compared with that of WT mice after 4 weeks of normoxia (30.67 ± 1.09 mm Hg versus 22.82 ± 1.06 mm Hg; P < 0.001) (Figure 2A). In addition, although not reaching statistical significance, RV:body weight ratios in Ccr2−/− mice tended to be higher than in WT mice (1.018 ± 0.031 versus 0.961 ± 0.032; data not shown). These findings suggest that pulmonary hypertension develops spontaneously in Ccr2−/− mice.
Ccr2−/− Mice Display Increased Muscularization of Pulmonary Arterioles
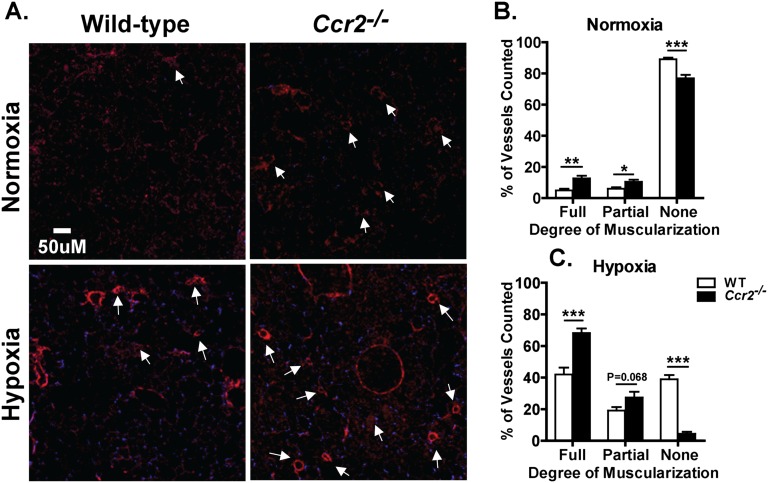
In PAH, increased RVSP and RV hypertrophy arise as a consequence of changes in the anatomy and vascular resistance of pulmonary arterioles, and are measures of the process that leads to pulmonary hypertension. A diagnostic hallmark of PAH is the increased muscularization of small pulmonary arteries. To determine if the pulmonary hypertension and RV hypertrophy seen in Ccr2−/− mice is actually due to abnormalities in the pulmonary vasculature, we exposed WT and Ccr2−/− mice to hypoxia or normoxia for 4 weeks and examined the muscularization of small pulmonary arteries. To identify vascular endothelium and smooth muscle cells, lung sections were stained for vWF and α-SMA. Compared with WT mice, Ccr2−/− mice had increased α-SMA staining regardless of experimental conditions. In Ccr2−/− mice, the number of muscularized small arteries was increased at baseline, and this number increased to a much greater extent than in WT mice after hypoxia (Figure 3A). To quantify these differences, the number of fully muscularized intra-acinar arteries (decorated with a full ring of α-SMA), partially muscularized intra-acinar arteries (partially decorated with α-SMA), and nonmuscularized (vWF staining only) were enumerated and expressed as percentage of the total number of vessels examined. In hypoxic Ccr2−/− mice, 68.23 (±3.65)% of vessels were fully muscularized compared with 41.98 (±4.34)% in WT mice (P < 0.001; Figure 3C). There was also a non–statistically significant trend toward an increased percentage of partially muscularized vessels in Ccr2−/− mice (27.39 ± 3.65% versus 19.13 ± 2.22%; P = 0.068). Overall, hypoxic Ccr2−/− mice had a substantially lower percentage of nonmuscularized vessels than WT mice (4.38 ± 1.32% versus 38.89 ± 2.76%; P < 0.001; Figure 3C). In addition, during normoxia, Ccr2−/− mice already displayed an increased percentage of fully muscularized vessels (12.6 ± 1.69% versus 4.90 ± 1.08%; P < 0.01) and partially muscularized vessels (10.51 ± 1.33% versus 6.03 ± 0.87%; P < 0.05) compared with WT mice (Figure 3B). Increased arteriolar muscularization in normoxic Ccr2−/− mice further supports an abnormality in pulmonary vascular development in these animals.
Figure 3.
CCR2 deficiency affects muscularization of pulmonary arteries. (A) Fluorescent α-smooth muscle actin (α-SMA) staining of normoxic and hypoxic lungs of WT and Ccr2−/− mice. Ccr2−/− mice show increased muscularization of vessels (white arrows). Original magnification, 50×. (B) Quantification of muscularized vessels in WT and Ccr2−/− mice. Under normoxic conditions, Ccr2−/− mice have a higher proportion of vessel muscularization; with hypoxia, Ccr2−/− mice have a significantly increased percentage of muscularized pulmonary artery compared with WT mice (n = 5 to 7 mice per group). *P < 0.05; **P < 0.01; ***P < 0.001.
Neonatal Ccr2−/− Mice Exhibit Abnormal Vascular Content and Muscularization
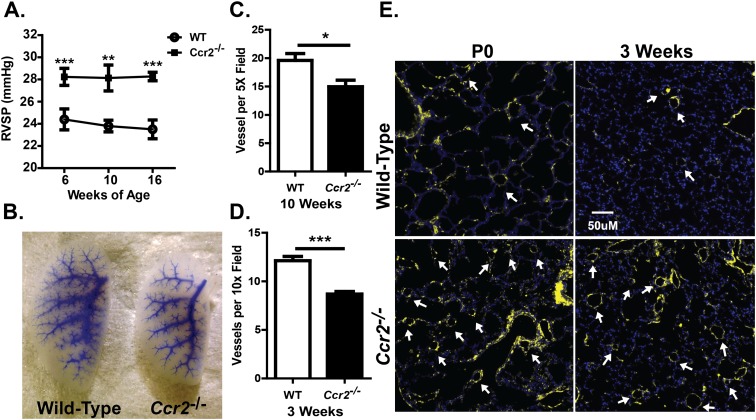
Under normoxic condition, adult Ccr2−/− mice exhibited elevated RVSPs and pulmonary arteriolar muscularization, suggesting the spontaneous development of PAH. To determine if pulmonary hypertension is an innate characteristic of Ccr2−/− mice or develops in response to some exogenous stimulus, we tracked the RVSPs of WT and Ccr2−/− mice beginning at 6 weeks of age, the earliest time that such a measurement is feasible due to animal size. As shown in Figure 4A, Ccr2−/− mice displayed a significantly elevated RVSP at all times examined. RVSP values were elevated at 6 weeks in these mice, but did not increase further over the next 10 weeks, suggesting that pulmonary hypertension develops spontaneously in Ccr2−/− mice.
Figure 4.
CCR2 deficiency leads to spontaneous PAH and abnormal vascular development. (A) Measurement of RVSP in WT and Ccr2−/− mice at 6, 10, and 16 weeks of age. Under normoxic conditions, Ccr2−/− mice have persistently elevated RVSP compared with WT mice at 6, 10, and 16 weeks of age (n = 9–18 for each group). (B) Microfil pulmonary vascular casting of WT and Ccr2−/− mice at 3 weeks of age. Entire left lobe of the lung is shown in the picture. There is significant reduction of small arteriolar branching in lungs of Ccr2−/− mice (representative of four animals). (C–D) Pulmonary arteriolar counts in Ccr2−/− and WT mice. Ccr2−/− mice have ∼25–30% reductions in pulmonary arteriolar count at 10 weeks (C) and 3 weeks (D) of age (n = 6 per group). (E) Fluorescent α-SMA staining of lungs of untreated WT and Ccr2−/− mice at Postnatal Day 0 (P0) and 3 weeks of age. Ccr2−/− mice have increased arteriolar muscularization as early as time of birth (representative of five animals). *P < 0.05; **P < 0.01; ***P < 0.001.
To characterize the pulmonary vasculature, lung sections from 3- and 10-week-old mice were stained for vWF, and the number of arterioles enumerated. At 10 weeks of age, Ccr2−/− mice displayed a decreased number of vessels per 5× field compared with WT mice (14.97 ± 1.15 versus 19.60 ± 1.22; P < 0.05; Figure 4C). This abnormality was also observed at 3 weeks of age, with Ccr2−/− mice having ∼30% fewer pulmonary arterioles per 10× field than WT mice (8.69 ± 0.26 versus 12.13 ± 0.43; P < 0.001; Figure 4D). Higher magnification was used to accommodate the small size of the lung in young animals. A general decrease in vascular branching and complexity was observed in pulmonary vascular casts of Ccr2−/− compared with WT mice (Figure 4B). To determine if abnormal pulmonary arteriolar muscularization arises during development in Ccr2−/− mice, lung sections from postnatal Day-0 (P0) and 3-week-old mice were stained for α-SMA expression. At both P0 and 3 weeks of age, Ccr2−/− mice displayed increased muscularization of vessels relative to WT mice (Figure 4E), to an extent similar to that seen at 10 weeks of age.
Ccr2−/− Mice Have Increased Notch-3 Expression and Signaling
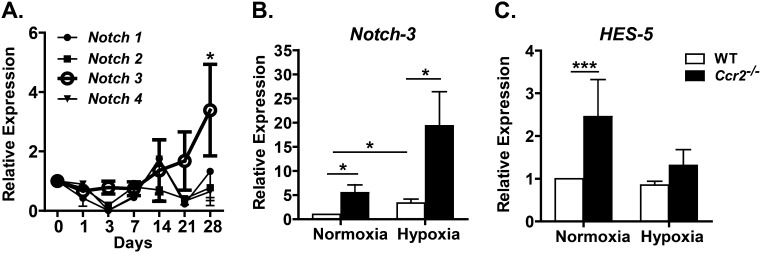
The unexpected finding that Ccr2−/− mice displayed increased pulmonary vascular density, pulmonary arteriolar hypertrophy, and pulmonary hypertension raises the question of mechanism. Because Ccr2−/− mice showed no evidence of increased inflammation or abnormal cytokine production, we sought to determine if these mice have abnormalities in the Notch signaling pathway, which is known to participate in vascular development and the development of pulmonary hypertension in mice. To examine Notch expression in hypoxia-induced PH, we determined transcript levels of the four Notch receptors in the lungs of WT mice exposed to hypoxia for various times (0, 1, 3, 7, 14, 21, and 28 days) using quantitative RT-PCR. Of the four receptors, only Notch-3 expression changed significantly during hypoxia, demonstrating a 3-fold increase in transcript levels after 28 days (Figure 5).
Figure 5.
CCR2 deficiency affects hypoxia-induced Notch signaling. (A) Time course of pulmonary Notch receptor expression in hypoxia by quantitative RT-PCR. Of the four Notch receptors, only Notch-3 shows significant increase in expression under chronic hypoxic stress. (B) Notch-3 expression in WT and Ccr2−/− mice. At baseline, Ccr2−/− mice have a 5-fold higher Notch-3 expression, and a 6.5-fold increase with hypoxic stress compared with WT. (C) Hairy and enhancer of Split-5 (HES-5) expression in WT and Ccr2−/− mice. At baseline, Ccr2−/− mice exhibited a 2.5-fold increase in HES-5 expression (there were approximately n = 6 per group). *P < 0.05; ***P < 0.001.
To determine if Notch-3 expression is abnormal in Ccr2−/− mice, lung Notch-3 transcript levels were compared in WT and Ccr2−/− mice during normoxia and after 4 weeks of hypoxia. Remarkably, relative to WT mice, Ccr2−/− mice displayed a roughly 5-fold increase in Notch-3 expression at baseline and a roughly 6.5-fold increase in expression after hypoxia (Figure 5). Regardless of conditions, there were no significant differences in Notch-1, Notch-2, or Notch-4 expression between WT and Ccr2−/− mice (data not shown). To examine Notch-3 activity, we measured expression of Hairy and enhancer of Split-5, a transcriptional target of Notch-3 that is associated with an undifferentiated VSMC phenotype and increased VSMC proliferation. Importantly, normoxic Ccr2−/− mice exhibited a ∼2.5-fold increase in Hairy and enhancer of Split-5 (Hes-5) expression (Figure 5), implying baseline Notch-3 activation in these animals.
Ccr2−/− Mice Have Dysregulated of Notch Ligand Expression
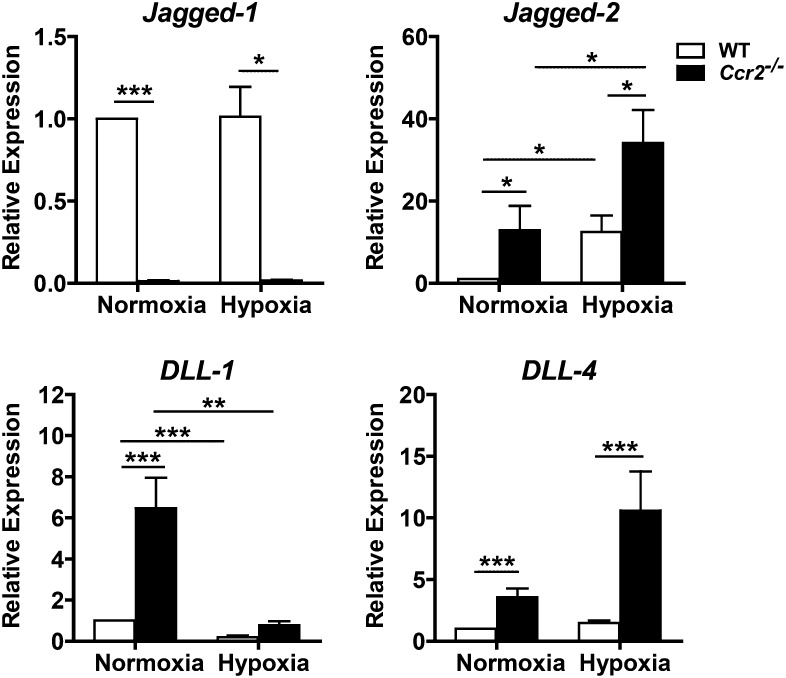
To determine the cause of the increased Notch-3 activity in Ccr2−/− mice, we examined the transcript expression for the Notch ligands, Jagged-1, Jagged-2, DLL-1, and DLL-4, in these animals during normoxia and after 4 weeks of hypoxia. We found a complex dysregulation of Notch ligand transcript expression in Ccr2−/− mice. Ccr2−/− mice displayed markedly reduced and barely detectable levels of Jagged-1 mRNA both at baseline and under hypoxic conditions (Figure 6). At the same time, these mice demonstrated significant increases in Jagged-2 (∼12.5-fold), DLL-1 (∼6.5-fold), and DLL-4 (∼3.5-fold) expression during normoxia (Figure 6). After hypoxia, Ccr2−/− mice displayed increased Jagged-2 (∼2.7-fold) and DLL-4 (∼7-fold) expression relative to WT mice (Figure 6). Although the full implications of this abnormal Notch ligand expression are unclear, CCR2-deficiency has a profound impact on the Notch signaling pathway.
Figure 6.
Effects of CCR2 deficiency on Notch ligand expression. Quantitative RT-PCR of normoxic and hypoxic lungs from Ccr2−/− mice show significantly decreased Jagged-1 expression; these mice also had significantly increased baseline expression of Jagged-2, Delta-like (DLL)-1, and DLL-4. Expression of Jagged-2 and DLL-4 were further enhanced with hypoxic exposure (n = 5–7 animals per group). *P < 0.05; **P < 0.01; ***P < 0.001.
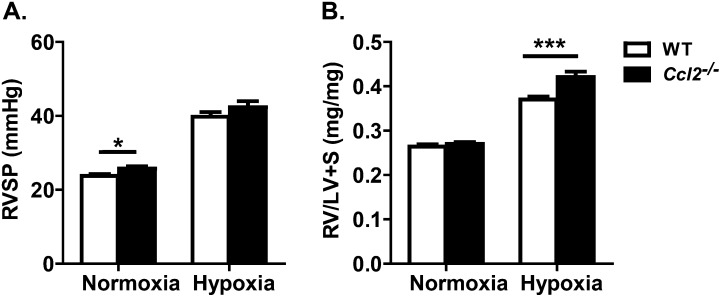
Ccl2−/− Mice Have Increased Baseline RVSP and Hypoxia-Induced PAH
Because Ccr2−/− mice are predisposed to develop pulmonary hypertension, we sought to determine if this abnormal phenotype is directly related to CCR2 signaling. We reasoned that, if this phenotype is related to CCR2 signaling, it should be present in mice that lack one or more CCR2 ligands. Because CCL2 is the major CCR2 ligand in the lung, and Ccl2−/− mice typically exhibit phenotypes that are similar to, albeit less severe than, those of Ccr2−/− mice, we chose to examine the development of pulmonary hypertension in Ccl2−/− mice. We exposed WT and Ccl2−/− mice to either normoxia or hypoxia for 4 weeks and examined RV pressures and weights, as described previously here. Compared with normoxic WT mice, Ccl2−/− mice displayed a mildly elevated RVSP at baseline (25.7 ± 0.59 mm Hg versus 23.8 ± 0.53 mm Hg; P < 0.05; Figure 7A). Although not reaching statistical significance, RVSP tended to increase more in Ccl2−/− mice than in WT mice during hypoxia (42.3 ± 1.67 mm Hg versus 39.8 ± 1.21 mm Hg). Ccl2−/− mice displayed significantly more RV hypertrophy than WT mice (RV/[LV + S]: 0.421 ± 0.012 versus 0.370 ± 0.007; P < 0.001; Figure 7B). These findings demonstrate that both Ccr2−/− and Ccl2−/− mice have a propensity to develop pulmonary hypertension, suggesting that this phenotype is directly related to CCR2 signaling, rather than, for example, an abnormality in the CCR2 locus associated with the deletion of this gene.
Figure 7.
CCL2 deficiency affects development of hypoxia-induced PAH. After 4 weeks of hypoxia exposure, the RVs of WT and CCL2-deficient (Ccl2−/−) animals were analyzed. (A) Ccl2−/− animals show a mild baseline increase in RVSP (n = 23–26 animals per group). With hypoxic exposure, Ccl2−/− mice (n = 13) trended toward a higher RVSP compared with WT mice (n = 28). (B) RV/[LV + S] ratios of normoxic and hypoxic animals are analyzed. Compared with WT (n = 29), Ccl2−/− mice (n = 21) have worsening RV hypertrophy. *P < 0.05; ***P < 0.001.
Discussion
The pathogenesis of PAH is complex and involves genetic and environmental factors that stimulate small pulmonary artery remodeling (1, 5). The remodeling process involves not only all components of the vessel wall, but is also modulated by immune response. Accordingly, CCR2, an essential chemokine for inflammatory monocytes, serves to recruit precursors for inflammatory DC and exudative macrophages into the lung. On this basis, we hypothesized that CCR2 promotes inflammation and PAH, and that deletion of CCR2 would protect animals from PAH. Our findings argue against this hypothesis, because Ccr2−/− mice had more severe PAH than WT mice, and hypoxic Ccr2−/− mice had increased RVSP, increased RV hypertrophy, and increased pulmonary vascular muscularization compared with hypoxic WT mice. We also demonstrate that Ccr2−/− mice have decreased vascular density, and develop increased pulmonary vascular muscularization and increased RV pressure, even in the absence of hypoxia. The spontaneous development of PAH is seen in mice as young as 6 weeks of age, abnormal vascular content at 3 weeks of age, and increased vascular muscularization at birth, suggesting that embryonic CCR2 deficiency leads to an abnormality in the development or physiology of the pulmonary vasculature. Therefore, we cannot totally exclude a contribution of CCR2 to the hypoxic induction of PAH. In addition, because inflammation is not a prominent feature of hypoxia-induced PH in mice, CCR2 deficiency in this model may not accurately predict the effects of CCR2 inhibition in human PAH. A definitive answer to the question of whether or not CCR2 contributes to PAH in adults will likely require the use of CCR2-inducible knockout mice or CCR2 antagonists. In addition, our studies did not address the role of noninflammatory myeloid cell types, such as constitutive monocytes, on the development of PAH.
The spontaneous PAH and increased severity of hypoxia-induced PAH in Ccr2−/− and Ccl2−/− animals are unexpected. Previous studies have shown that inhibition of CCL2 ameliorates monocrotaline-induced PH (19). Although this may be explained by the difference in experimental models, it more likely reflects an unexpected role of CCR2-mediated signaling in pulmonary vasculature development. At this point, it is not clear if the spontaneous development of pulmonary hypertension in Ccr2−/− mice is due to the decreased pulmonary vascular density in these animals and a presumed increase in pulmonary vascular resistance, or if the increased muscularization of pulmonary arterioles is due to increased Notch-3 signaling. It appears that the defect in vascular development seen in Ccr2−/− mice is limited to the lungs. Skeletal muscle vascular density in these mice has been reported to be normal (23), and, to our knowledge, no developmental defect or morphologic abnormality has previously been described in these animals.
In an attempt to identify the mechanism by which CCR2 deficiency leads to pulmonary hypertension, we examined the Notch signaling pathway and found increased Notch-3 expression and activation, a dramatic decrease in Jagged-1 transcript levels, and significant increases in Jagged-2, DLL-1, and DLL-4 transcripts. Notch-3 activation has been demonstrated to stimulate VSMC proliferation and pulmonary arteriolar muscularization in animal models of PAH, and Notch-3 displays increased activity in humans with pulmonary hypertension (7, 8). In early vascular development, sprouting, and tumor angiogenesis, four of the five Notch ligands are expressed in the endothelial cells (Jagged-1, Jagged-2, DLL-1, and DLL-4); VSMCs also express Jagged-1 (24). Endothelial Jagged-1 and/or DLL-1 are thought to induce Jagged-1 expression and activate Notch-3 in VSMCs and pericytes; these interactions regulate VSMC proliferation and maturation (8). During sprouting angiogenesis, Jagged-1 antagonizes DLL-4 on endothelial cells to promote vascular branching (25), and DLL-4 and DLL-1 regulate arterial specification and identity maintenance (26). The role of Jagged-2 on vascular development and angiogenesis is unclear. In Ccr2−/− mice, a decrease in vascular branching–promoting transcript (Jagged-1) and increase in branching-inhibiting transcript (DLL-4) is associated with decreased pulmonary arteriolar branching. Thus, although we have not demonstrated a causal link between altered Notch signaling, increased pulmonary vascular density, increased vascular muscularization, and the spontaneous development of pulmonary hypertension, the phenotypes we observe in Ccr2−/− mice are fully consistent with the pattern of Notch signaling abnormalities that we find in these animals.
At present, the reason that Notch signaling is increased in Ccr2−/− mice is not clear. To our knowledge, no abnormality in Notch signaling or Notch ligand expression has been described in Ccr2−/− mice. Two papers have described alterations in Notch receptor expression in response to CCL2. In one, Notch-4 was 1 of 31 angiogenesis-related genes up-regulated in human umbilical vein endothelial cells by CCL2 treatment (27). In the second, CCL2 induced Notch-1 expression in breast cancer cells (28). It is known that β-arrestin, a mediator of G protein-coupled receptor (GPCR) desensitization, through interactions with Deltex, can regulate ubiquination of Notch receptors (29); however, this mechanism does not seem to apply here. It is possible that CCR2 may regulate Notch expression indirectly through an autocrine or paracrine pathway. For example, during muscle regeneration, CCR2 deficiency leads to delayed VEGF production (30), and VEGF is known to regulate Notch receptor and ligand expressions (31). Delayed VEGF responses may potentially impact Notch expression; however, the lack of significant differences in VEGF or other cytokine levels in our system argues for a novel regulation of Notch expression. Because CCR2 is expressed by VSMCs, it may modulate Notch expression directly through intracellular cross-talk. CCR2 signaling does activate several mitogen-activated protein kinases (MAPK): extracellular signal–regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK (32), and differential activation of various MAPK pathways can modulate both Notch receptor and ligand expression (33, 34). For example, inhibition of p38 MAPK has been shown to decrease and inhibition of p42/44 MAP to increase Notch-4 expression (34). Thus, CCR2, via MAPK activation, might directly modulate Notch-3 and Notch ligand expression. Clearly, additional studies will be required to define the mechanisms by which CCR2 influences Notch signaling.
The predisposition to PAH in the setting of CCR2 deletion has implications in understanding disease pathogenesis and treatment. Polymorphisms of CCR2 have been linked to several diseases (35–37); thus, the examination of genetic polymorphisms of CCR2 in patients with IPAH may provide useful insights. From a therapeutic standpoint, there is interest in using CCR2 antagonists as therapeutic anti-inflammatory agents, but the complex role of CCR2 in pulmonary vasculature development and remodeling shown here indicates a need for further understanding of the CCL2–CCR2 functional axis.
In conclusion, using Ccr2−/− mice in altitude-induced PH, we define a role for CCR2 in pulmonary vascular development and in regulating PAH pathogenesis. CCR2 has a clear impact on VSMC development and/or maturation and regulation of Notch signaling pathway; thus, CCR2 deletion leads to spontaneous PAH at a young age, and hypoxia leads to significantly more severe PAH. Our findings show that the functions of CCR2 reach far beyond the recruitment inflammatory cells. Based on its effects on pulmonary vascular development, CCR2 may be a candidate susceptibility locus for IPAH.
Acknowledgments
Acknowledgments
The authors thank Craig Marshall (Duke University Health Systems) for technical help and Dr. Howard Rockman (Duke University School of Medicine) for expertise in cardiac evaluation.
Footnotes
This work was supported by National Institute of Health grants 5T32-HL007538 and R01HL085473.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0182OC on March 14, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 2.Southgate L, Machado RD. Molecular genetics of pulmonary hypertension [last update December 2011]. Available from: http://www.els.net/WileyCDA/ElsArticle/refId-a0022444.html.
- 3.Newman JH, Phillips JA, III, Loyd JE. Narrative review: the enigma of pulmonary arterial hypertension: new insights from genetic studies. Ann Intern Med. 2008;148:278–283. doi: 10.7326/0003-4819-148-4-200802190-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hall S, Brogan P, Haworth SG, Klein N. Contribution of inflammation to the pathology of idiopathic pulmonary arterial hypertension in children. Thorax. 2009;64:778–783. doi: 10.1136/thx.2008.106435. [DOI] [PubMed] [Google Scholar]
- 5.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1 Suppl):S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med. 2009;15:1289–1297. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Kennard S, Lilly B. Notch3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed Jagged1. Circ Res. 2009;104:466–475. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambrecht BN, van den Toorn LM. The pressure mounts on lung dendritic cells. Eur Respir J. 2007;29:435–437. doi: 10.1183/09031936.00002507. [DOI] [PubMed] [Google Scholar]
- 10.Mathew R. Inflammation and pulmonary hypertension. Cardiol Rev. 2010;18:67–72. doi: 10.1097/CRD.0b013e3181cd612f. [DOI] [PubMed] [Google Scholar]
- 11.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Stenmark KR. Circulating mononuclear cells with a dual, macrophage-fibroblast phenotype contribute robustly to hypoxia-induced pulmonary adventitial remodeling. Chest. 2005;128(6 Suppl):583S–584S. doi: 10.1378/chest.128.6_suppl.583S. [DOI] [PubMed] [Google Scholar]
- 12.Thenappan T, Goel A, Marsboom G, Fang YH, Toth PT, Zhang HJ, Kajimoto H, Hong Z, Paul J, Wietholt C, et al. A central role for CD68(+) macrophages in hepatopulmonary syndrome: reversal by macrophage depletion. Am J Respir Crit Care Med. 2011;183:1080–1091. doi: 10.1164/rccm.201008-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Manivannan A, Dawson R, Crane IJ, Mack M, Sharp P, Liversidge J. Differentiation to the CCR2+ inflammatory phenotype in vivo is a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators. J Immunol. 2005;175:6915–6923. doi: 10.4049/jimmunol.175.10.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 16.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123:1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez O, Marcos E, Perros F, Fadel E, Tu L, Humbert M, Dartevelle P, Simonneau G, Adnot S, Eddahibi S. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;176:1041–1047. doi: 10.1164/rccm.200610-1559OC. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda Y, Yonemitsu Y, Kataoka C, Kitamoto S, Yamaoka T, Nishida K, Takeshita A, Egashira K, Sueishi K. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2002;283:H2021–H2028. doi: 10.1152/ajpheart.00919.2001. [DOI] [PubMed] [Google Scholar]
- 20.Szymczak WA, Deepe GS., Jr The CCL7–CCL2–CCR2 axis regulates IL-4 production in lungs and fungal immunity. J Immunol. 2009;183:1964–1974. doi: 10.4049/jimmunol.0901316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassoun PM. Drumming up prognostic significance in a heartbeat in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:354–355. doi: 10.1164/rccm.201112-2178ED. [DOI] [PubMed] [Google Scholar]
- 23.Tang G, Charo DN, Wang R, Charo IF, Messina L. CCR2−/− knockout mice revascularize normally in response to severe hindlimb ischemia. J Vasc Surg. 2004;40:786–795. doi: 10.1016/j.jvs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Kume T. Novel insights into the differential functions of Notch ligands in vascular formation. J Angiogenes Res. 2009;1:8. doi: 10.1186/2040-2384-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The Notch ligands DLL4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23:543–553. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- 27.Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1–induced protein (MCPIP) J Biol Chem. 2008;283:14542–14551. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, Luu T, Li AX, Wu X, Ye W, et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012;72:2768–2779. doi: 10.1158/0008-5472.CAN-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shenoy SK, Lefkowitz RJ. Receptor regulation: beta-arrestin moves up a Notch. Nat Cell Biol. 2005;7:1159–1161. doi: 10.1038/ncb1205-1059. [DOI] [PubMed] [Google Scholar]
- 30.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2007;293:R651–R661. doi: 10.1152/ajpregu.00069.2007. [DOI] [PubMed] [Google Scholar]
- 31.Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. Bioessays. 2008;30:303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- 32.Werle M, Schmal U, Hanna K, Kreuzer J. MCP-1 induces activation of MAP-kinases ERK, JNK and p38 MAPK in human endothelial cells. Cardiovasc Res. 2002;56:284–292. doi: 10.1016/s0008-6363(02)00600-4. [DOI] [PubMed] [Google Scholar]
- 33.Cho J, Gruol DL. The chemokine CCL2 activates p38 mitogen-activated protein kinase pathway in cultured rat hippocampal cells. J Neuroimmunol. 2008;199:94–103. doi: 10.1016/j.jneuroim.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiec-Wilk B, Grzybowska-Galuszka J, Polus A, Pryjma J, Knapp A, Kristiansen K. The MAPK-dependent regulation of the Jagged/Notch gene expression by VEGF, BFGF or PPAR gamma mediated angiogenesis in HUVEC. J Physiol Pharmacol. 2010;61:217–225. [PubMed] [Google Scholar]
- 35.Katrancioglu N, Manduz S, Karahan O, Yilmaz MB, Sezgin I, Bagci G, Berkan O. The role of the CCR2 gene polymorphism in abdominal aortic aneurysms. Angiology. 2011;62:140–143. doi: 10.1177/0003319710385335. [DOI] [PubMed] [Google Scholar]
- 36.Raoul W, Auvynet C, Camelo S, Guillonneau X, Feumi C, Combadiere C, Sennlaub F. CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J Neuroinflammation. 2010;7:87. doi: 10.1186/1742-2094-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh CB, Tsai HT, Chen YC, Kuo WH, Chen TY, Hsieh YH, Chou MC, Yang SF. Genetic polymorphism of CCR2-64I increased the susceptibility of hepatocellular carcinoma. J Surg Oncol. 2010;102:264–270. doi: 10.1002/jso.21623. [DOI] [PubMed] [Google Scholar]