Abstract
Elevated concentrations of CO2 (hypercapnia) lead to alveolar epithelial dysfunction by promoting Na,K-ATPase endocytosis. In the present report, we investigated whether the CO2/HCO3− activated soluble adenylyl cyclase (sAC) regulates this process. We found that hypercapnia increased the production of cyclic adenosine monophosphate (cAMP) and stimulated protein kinase A (PKA) activity via sAC, which was necessary for Na,K-ATPase endocytosis. During hypercapnia, cAMP was mainly produced in specific microdomains in the proximity of the plasma membrane, leading to PKA Type Iα activation. In alveolar epithelial cells exposed to high CO2 concentrations, PKA Type Iα regulated the time-dependent phosphorylation of the actin cytoskeleton component α-adducin at serine 726. Cells expressing small hairpin RNA for PKAc, dominant-negative PKA Type Iα, small interfering RNA for α-adducin, and α-adducin with serine 726 mutated to alanine prevented Na,K-ATPase endocytosis. In conclusion, we provide evidence for a new mechanism by which hypercapnia via sAC, cAMP, PKA Type Iα, and α-adducin regulates Na,K-ATPase endocytosis in alveolar epithelial cells.
Keywords: hypercapnia; soluble adenylyl cyclase; PKA Type Iα; α-adducin; Na,K-ATPase
The Na,K-ATPase located at the basolateral membrane of alveolar epithelial cells drives the vectorial sodium transport necessary to keep the lungs free of edema (1, 2). In many animal models and in patients with acute lung injury/acute respiratory distress syndrome (ALI/ARDS), alveolar fluid reabsorption is impaired, partly because of a decreased abundance of Na,K-ATPase at the plasma membrane (3–5). We recently reported that rat lungs exposed to high CO2 concentrations (hypercapnia) have impaired alveolar fluid reabsorption because of Na,K-ATPase endocytosis, independent of intracellular and extracellular pH (6). The signaling pathways leading to hypercapnia-induced Na,K-ATPase endocytosis in alveolar epithelial cells are still being explored, with roles for protein kinase C (PKC)–ζ and AMP-activated protein kinase (AMPK) reported (6, 7).
Recently, a soluble adenylyl cyclase (sAC) responsive to CO2/HCO3− has been proposed as a metabolic sensor that generates cyclic adenosine monophosphate (cAMP) in cellular microdomains in response to changes in CO2/HCO3− concentrations (8–10). sAC does not contain transmembrane domains, and unlike transmembrane adenylyl cyclases (tmACs), sAC is insensitive to regulation by forskolin or heterotrimeric G proteins (8). cAMP mediates its cellular effects via at least three distinct classes of direct effectors: cAMP-dependent protein kinase A (PKA), guanine nucleotide exchange factors for the small G protein Rap (Epacs), and cAMP-gated ion channels (11). PKA exists as an inactive holoenzyme composed of two catalytic and two regulatory subunits (12). Four regulatory subunits lead to four types of PKA isozymes, namely, PKA Types Iα, Iβ, IIα, and IIβ (13).
Adducins comprise a protein family encoded by three closely related genes (α, β, and γ) that regulate the assembly of the spectrin–actin subcortical membrane network, and that have been described as a substrate for PKA (14, 15). α-adducin has been reported to modulate Na,K-ATPase endocytosis (16, 17). However, whether adducin phosphorylation is required for Na,K-ATPase endocytosis remains unknown.
Here we set out to determine whether alveolar epithelial cells exposed to high concentrations of CO2 exhibit increased sAC activity, and whether the cAMP produced by sAC promotes Na,K-ATPase endocytosis. We found that hypercapnia increased the production of cAMP via sAC in specific microdomains, leading to the activation of PKA Type Iα. PKA Type Iα in turn leads to the phosphorylation of α-adducin at serine (Ser) 726, a necessary step in hypercapnia-induced Na,K-ATPase endocytosis. A portion of these studies was previously reported in abstract form (18).
Materials and Methods
Reagents, antibodies, cell culture, and the isolation of primary cells are described in the online supplement.
CO2 Exposure
Exposures and solutions were performed as described elsewhere (7). A more detailed protocol can be found in the online supplement.
cAMP Assay
Cells were exposed for 1 minute to Pco2 of approximately 40 mm Hg, pH 7.4, or to Pco2 of approximately 120 mm Hg, pH 7.4, in the absence or presence of inhibitors, or small interfering (si)RNA and cellular cAMP concentration was measured using a cAMP immunoassay kit (Enzo Life Sciences, Farmingdale, NY).
Fluorescence Resonance Energy Transfer Imaging
Fluorescence resonance energy transfer imaging (FRET) imaging experiments, based on the technique of Nikolaev and colleagues (19), were performed 24 hours after A549 cells were transfected with the Epac-1 sensor for cAMP (a gift of Dr. G. Hamilton, University of Glasgow, Glasgow, Scotland, UK). The sensor is a fusion protein generated by positioning cyan fluorescent protein and yellow fluorescent protein moieties to encompass the cAMP-binding domain of Epac-1 directly. Changes in FRET were measured as changes in the background-subtracted 545/480-nm fluorescence emission intensity on excitation at 430 nm and expressed as R/R0, where R is the ratio at time t and R0 is the ratio at time = 0 seconds. Data are represented as the background-subtracted 480/545-nm fluorescence emission intensity, as described by Gesellchen and colleagues (20), with an increase in ratio values corresponding to an increase in cAMP. A more detailed protocol can be found in the online supplement.
Transfection
Cells were grown on 35-mm plates at a density of 0.35–0.6 × 105 cells/plate. siRNAs were transfected using Lipofectamine RNAimax (Life Technologies, Carlsbad, CA), and plasmids were transfected using Lipofectamine 2000 (Life Technologies). Cells were used 24 to 72 hours after transfection. A list of the siRNAs and plasmids used can be found in the online supplement.
Biotinylation of Cell-Surface Proteins
Biotinylation, streptavidin pull-down, and Western blotting were performed as previously described (7). A list of antibodies is provided in the online supplement.
PKA Assay
PKA activity was measured using a nonradioactive assay kit (Enzo Life Sciences). A detailed protocol can be found in the online supplement.
Small Hairpin RNA against the Catalytic Subunit of PKA
Small hairpin RNA (shRNA) against the catalytic subunit of PKA (PKAc) was generated using the m-cherry version of the pG-SUPER vector (21) (a gift from Dr. Shin Kojima, Northwestern University). The targeting sequence entailed PKAcα (NM_207518, 5′-GAACACACCCTGAATGAAA-3′).
Immunocytochemistry
The cellular distribution of Na,K-ATPase, the regulatory subunits of PKA, and α-adducin was determined using a Zeiss LSM 510 laser-scanning confocal microscope (objective plan apochromat, ×63/1.4 oil; Carl Zeiss Microscopy, Jena, Germany). A detailed protocol can be found in the online supplement.
Cloning and Site-Directed Mutagenesis
The PKA regulatory subunit Iα (RIα) was amplified from A549 cells and cloned into the vector p3XFLAG-CMV (Sigma-Aldrich, St. Louis, MO). The glycine-to-aspartic acid mutation (G325D) was generated using a QuikChange Mutagenesis Kit from Agilent Technologies (Santa Clara, CA). A more detailed protocol is included in the online supplement.
Statistical Analysis
Data are represented as mean ± SEM. Multiple comparisons were performed using one-way ANOVA, followed by a Dunnett multiple-comparisons test when the F statistic indicated significance. Results were considered significant when P < 0.05.
Results
High CO2 Exposure Increases the Production of cAMP via sAC in Alveolar Epithelial Cells, Which Is Necessary for Na,K-ATPase Endocytosis
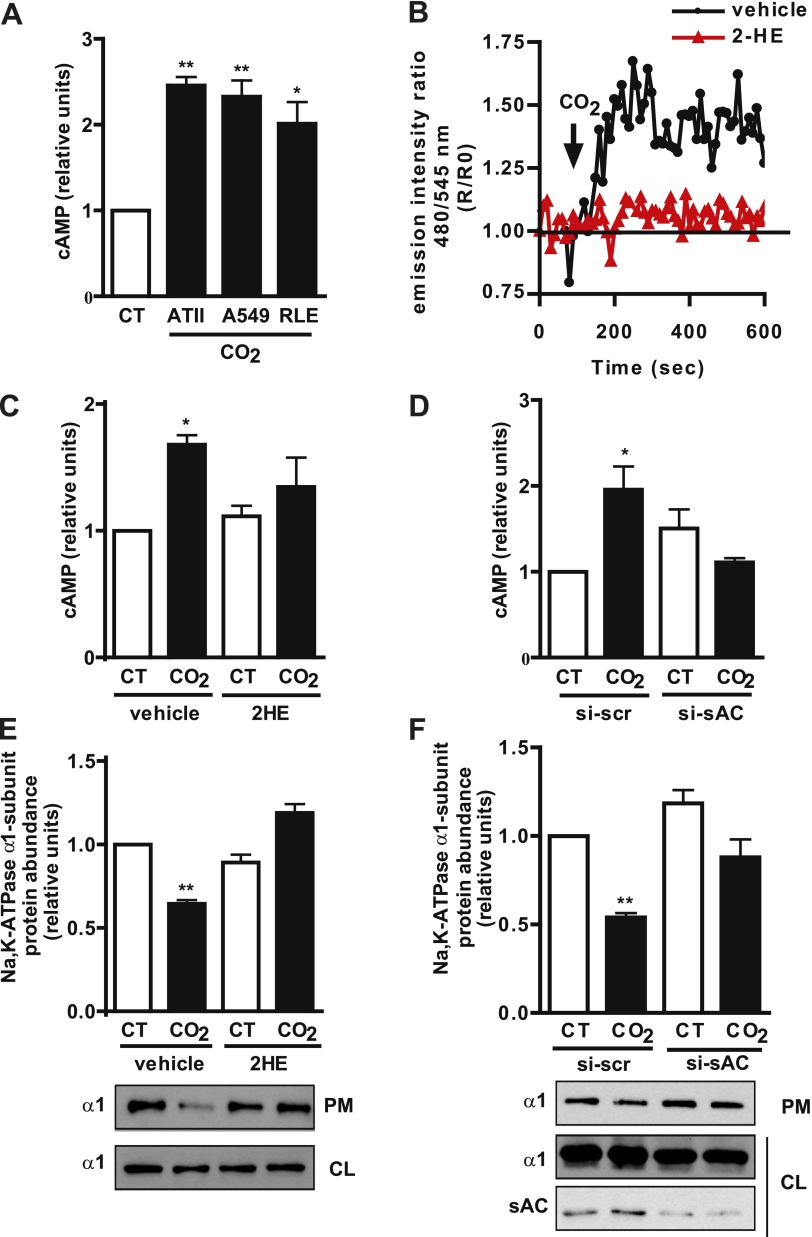
To determine whether hypercapnia induces an increase in cAMP production, we exposed rat alveolar type II (ATII), human A549, and rat RLE-6TN cells for 1 minute to 120 mm Hg Pco2, and measured the cellular concentration of cAMP by immunoassay. As shown in Figure 1A, cAMP concentrations increased in the different cell lines in a fashion similar to that in primary cells. We confirmed the increased in cAMP by FRET, using the Epac-1 sensor. Figure 1B shows a FRET tracing that indicates the increased production of cAMP after the exposure of transfected A549 cells to 120 mm Hg Pco2. We determined that sAC was the enzyme involved in the hypercapnia-induced increase in cAMP, because preincubation with the specific sAC inhibitor 2-hydroxyestradiol (5 μM) (2HE) (22) (Figures 1B and 1C) and sAC knockdown by siRNA (Figure 1D) prevented it. Finally, to study whether the sAC–cAMP pathway mediated hypercapnia-induced Na,K-ATPase endocytosis, we used a biotinylation assay to determine the amount of Na,K-ATPase α1 subunit at the plasma membrane in cells exposed for 30 minutes to 120 mm Hg Pco2 in the absence or presence of 2HE or si-sAC. We found that both 2HE (Figure 1E) and si-sAC (Figure 1F) prevented the hypercapnia-induced Na,K-ATPase endocytosis, suggesting an important role for the cAMP generated by sAC.
Figure 1.
High CO2 exposure increases the production of cyclic adenosine monophosphate (cAMP) via soluble adenylyl cyclase (sAC) in alveolar epithelial cells, which is necessary for Na,K-ATPase endocytosis. (A) Rat alveolar type II (ATII), human A549, and rat RLE-6TN cells were exposed to 40 mm Hg Pco2 (CT) or 120 mm Hg Pco2 (CO2) for 1 minute, and cAMP production was measured by immunoassay (n = 4). (B) Graph shows a representative fluorescence resonance energy transfer imaging (FRET) tracing in A549 cells expressing the guanine nucleotide exchange factor for the small G protein Rap-1 (Epac-1) sensor and exposed to CT or CO2 in the presence or absence of 5 μM 2-hydroxyestradiol (2HE). An increase in the ratio corresponds to an increase in cAMP production. Arrow indicates when cells were exposed to high CO2 (n = 4). (C) ATII cells were preincubated for 30 minutes with vehicle or with 5 μM 2HE and were exposed to CT or CO2 for 1 minute, and cAMP production was measured by immunoassay (n = 5). (D) RLE cells were transfected with scrambled small interfering (si)RNA (si-scr) or siRNA for sAC (si-sAC) and were exposed to CT or CO2 for 1 minute, and cAMP production was measured by immunoassay (n = 3). (E) Graph represents the relative abundance of the Na,K-ATPase α1 subunit at the plasma membrane in ATII cells preincubated for 30 minutes with vehicle or 5 μM 2HE and exposed to CT or CO2 for 30 minutes. Below: A representative Western blot shows the abundance of the Na,K-ATPase α1 subunit at the plasma membrane (PM) and cell lysate (CL) (n = 5). (F) Graph represents the relative abundance of the Na,K-ATPase α1 subunit at the plasma membrane in RLE cells transfected with si-scr or si-sAC and exposed to CT or CO2 for 30 minutes. Below: A representative Western blot shows the abundance of the Na,K-ATPase α1 subunit at the plasma membrane (PM) and cell lysate (CL) and sAC knockdown (n = 3). *P < 0.05. **P < 0.01.
PKA Mediates Hypercapnia-Induced Na,K-ATPase Endocytosis
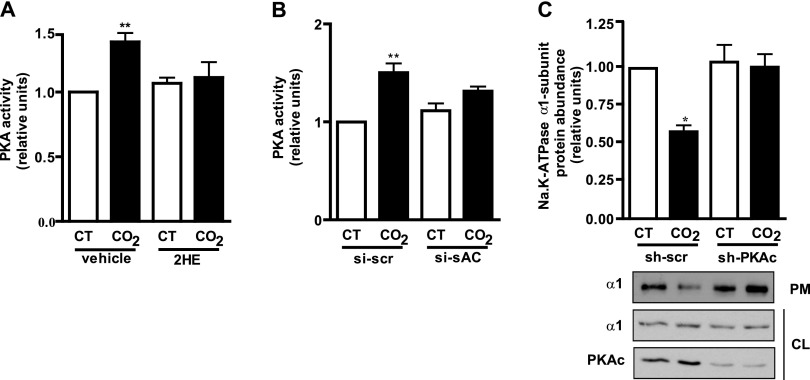
PKA, one of the main effectors of cAMP (11, 13), has been reported to regulate Na,K-ATPase traffic (23, 24). To determine whether PKA is the downstream effector of cAMP in hypercapnia, we exposed cells for 2.5 minutes to 120 mm Hg Pco2, and determined PKA activity by immunoassay. As shown in Figure 2, hypercapnia increased PKA activity in both rat primary ATII cells (Figure 2A) and RLE cells (Figure 2B). Moreover, we determined that sAC-generated cAMP mediated the increase in PKA activity, because it was prevented by preincubation with 2HE (Figure 2A) and by si-sAC (Figure 2B). To study whether PKA mediated the hypercapnia-induced Na,K-ATPase endocytosis, we used a biotinylation assay to determine the amount of Na,K-ATPase α1 subunit at the plasma membrane in cells transfected with shRNA against the catalytic subunit of PKA and exposed for 30 minutes to 120 mm Hg Pco2. We found that PKA was necessary for the hypercapnia-induced Na,K-ATPase endocytosis, because transfection with shRNA against the catalytic subunit of PKA prevented it (Figure 2C).
Figure 2.
Protein kinase A (PKA) mediates the hypercapnia-induced Na,K-ATPase endocytosis. (A) ATII cells were preincubated for 30 minutes with vehicle or 5 μM 2HE and exposed to 40 mm Hg Pco2 (CT) or 120 mm Hg Pco2 (CO2) for 2.5 minutes, and PKA activity was measured by immunoassay (n = 3). (B) RLE cells were transfected with scrambled siRNA (si-scr) or siRNA for sAC (si-sAC) and exposed to CT or CO2 for 2.5 minutes, and PKA activity was measured by immunoassay (n = 3). (C) Graph represents the relative abundance of the Na,K-ATPase α1 subunit at the plasma membrane in A549 cells transfected with small hairpin (sh)–scrambled (sh-scr) or shRNA for the catalytic subunit of PKA (sh-PKAc) and exposed to CT or CO2 for 30 minutes. Below: A representative Western blot shows the abundance of Na,K-ATPase at the plasma membrane (PM) and cell lysate (CL) and PKAc knockdown (n = 6). *P < 0.05. **P < 0.01.
Hypercapnia Increases cAMP Concentrations in Discrete Microdomains Where Na,K-ATPase and PKA Type Iα Colocalize
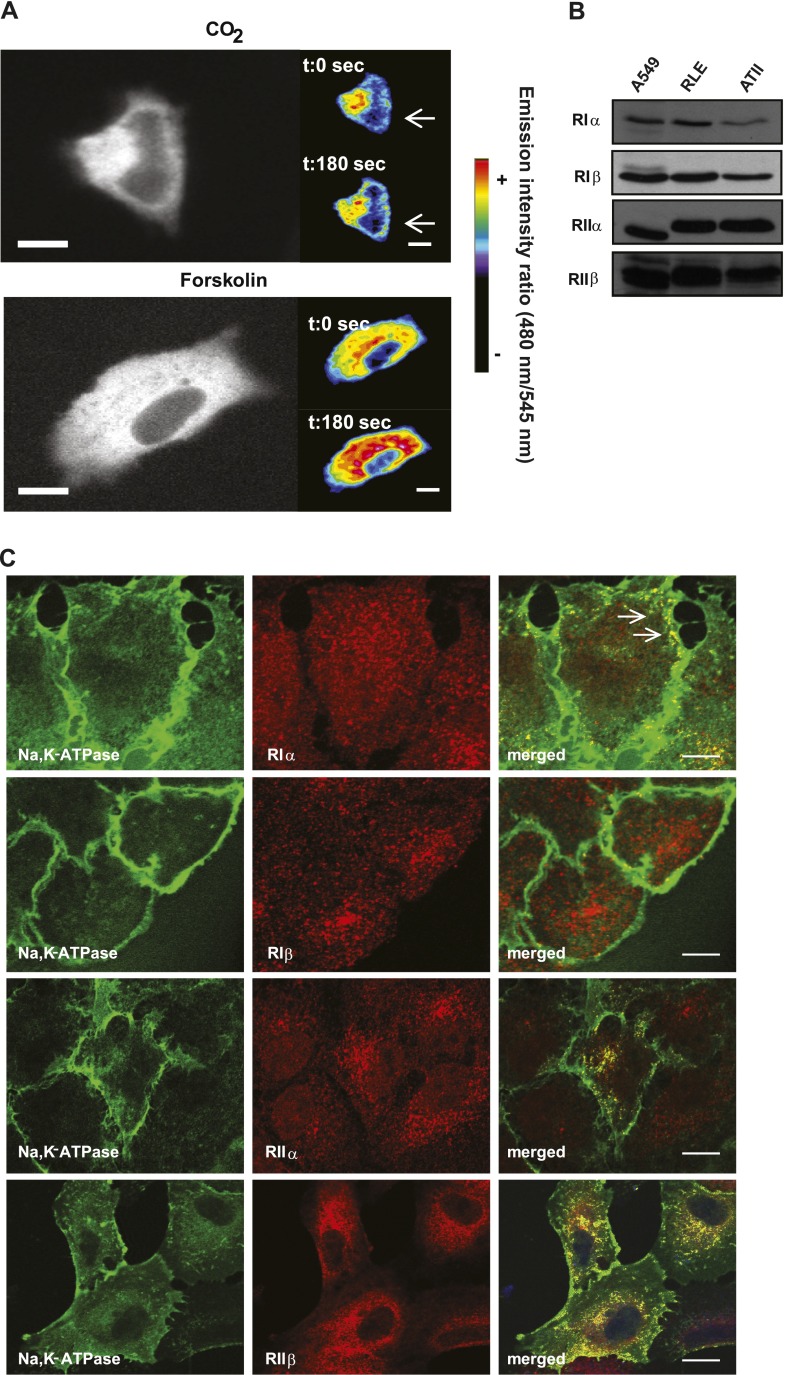
Recently, we reported that cAMP mediates the recruitment of Na,K-ATPase to the plasma membrane in alveolar epithelial cells (25). Therefore, to understand the discrepancy with our present results, we explored whether cAMP compartmentalization could explain the different effects of cAMP-PKA in these cells. The possibility of visualizing localized increases in cAMP by using pseudocolors of the images obtained by FRET has been reported (26). With this approach, we found that A549 cells transfected with the Epac-1 sensor and incubated with 120 mm Hg Pco2 have increased cAMP production in the proximity of the plasma membrane (Figure 3A, top). This cAMP distribution clearly differs from that observed after incubation with forskolin (an activator of tmAC), which occurs broadly in the cell, including the perinuclear region (Figure 3A, bottom). These results indicate that hypercapnia increased the concentration of cAMP in discrete microdomains, and specifically in the subplasma membrane of alveolar epithelial cells, suggesting the activation of a specific subset of PKA isoforms located in that region.
Figure 3.
Hypercapnia increases the concentration of cAMP in discrete microdomains in which Na,K-ATPase and PKA Type Iα colocalize. (A) Images depict cAMP production by FRET, using pseudocolors. Above: Increased cAMP after exposure to 120 mm Hg Pco2 (CO2). Below: Increased cAMP after exposure to 5 μM forskolin. The color scale is shown at right. Bar, 10 μm. (B) Representative Western blots show the expression of the different regulatory subunits of PKA in A549, RLE, and ATII cells. (C) Confocal images show the colocalization (arrow, yellow) between Na,K-ATPase (green) and the different regulatory subunits of PKA (red, RIα, RIβ, RIIα, RIIβ) in A549 cells expressing the Na,K-ATPase α1-subunit tagged with GFP (GFPα1-A549 cells). Bar, 10 μm. t, time; sec, seconds.
To determine whether the specificity of the response to hypercapnia was dependent on a subset of PKA isoforms activated by cAMP, we first analyzed their expression and distribution in alveolar epithelial cells. As Figure 3B shows, we found that all four isoforms of the regulatory subunit of PKA are expressed in primary ATII, A549, and RLE cells. Moreover, confocal images showed that the Na,K-ATPase mainly colocalized with PKA Type Iα at the plasma membrane, whereas both PKA Type II isoforms mostly colocalized with Na,K-ATPase in the perinuclear region (Figure 3C). We found that PKA Type Iβ and Na,K-ATPase did not colocalize (Figure 3C).
PKA Type Iα Mediates the Hypercapnia-Induced Na,K-ATPase Endocytosis
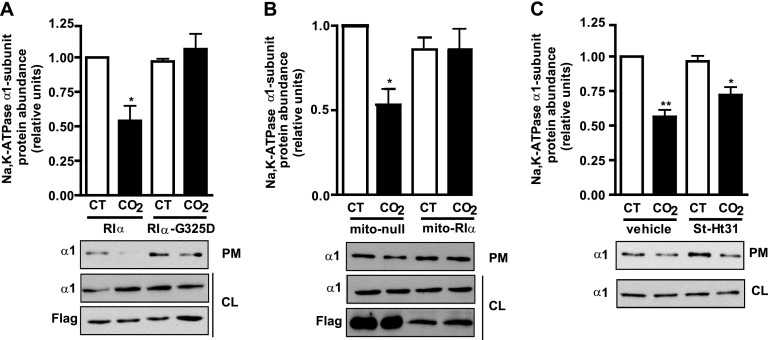
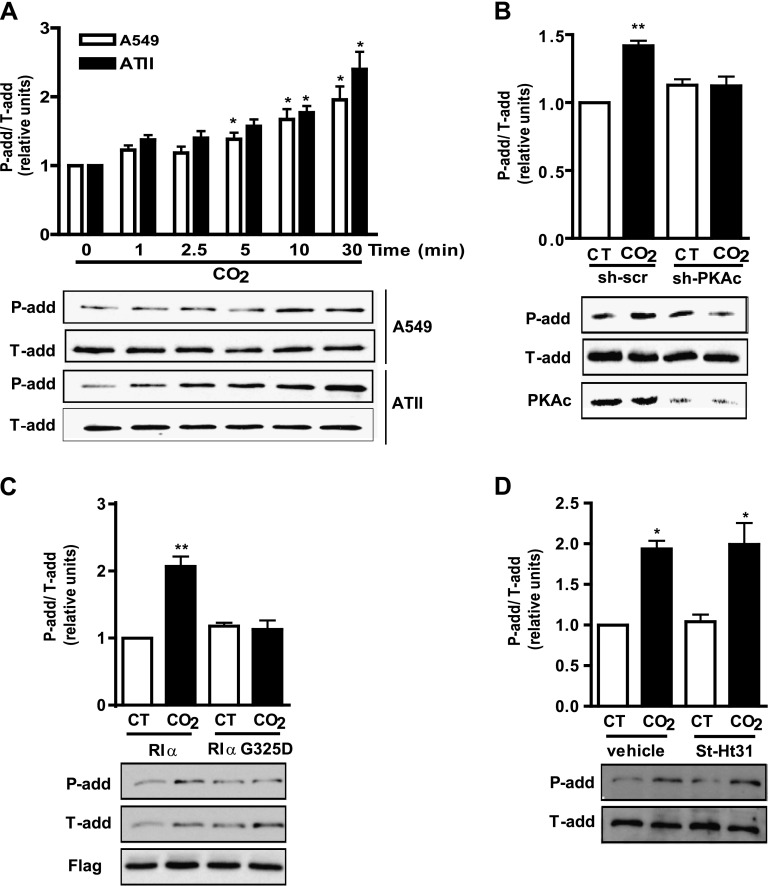
Our data suggest that hypercapnia (via sAC) increases cAMP production close to the plasma membrane, probably activating PKA Type Iα. To study whether PKA Type Iα mediates the hypercapnia-induced Na,K-ATPase endocytosis, we determined, in A549 cells transiently transfected with wild-type PKA Type Iα or a dominant negative mutant where the glycine in position 325 has been mutated to an aspartic acid (G325D) (which prevents cAMP from binding to site B) (27), the Na,K-ATPase abundance at the plasma membrane after exposure for 30 minutes to 120 mm Hg Pco2. We found that the expression of the G325D mutant blunted the hypercapnia-induced Na,K-ATPase endocytosis (Figure 4A). We confirmed the role of PKA Type Iα by overexpressing a specific isoform peptide disruptor that targets the RIα subunit to the mitochondria, acting also as a dominant negative (28) (Figure 4B). As shown in Figure 4B, cells exposed to 120 mm Hg Pco2 and expressing a null nondisruptive peptide have less Na,K-ATPase at the plasma membrane. However, hypercapnia-induced Na,K-ATPase endocytosis was prevented in cells expressing a specific RIα disruptor peptide. A possible role for PKA Type II isoforms was ruled out by determining the abundance of the Na,K-ATPase at the plasma membrane in ATII cells incubated with the St-Ht31 peptide, which disrupts the binding of the PKA regulatory subunit II to the catalytic subunit (29). As shown in Figure 4C, hypercapnia-induced Na,K-ATPase endocytosis still occurred in the presence of the St-Ht31 peptide.
Figure 4.
PKA Type Iα mediates the hypercapnia-induced Na,K-ATPase endocytosis in alveolar epithelial cells. (A) Graph represents the relative abundance of the Na,K-ATPase α1 subunit at the plasma membrane in A549 cells transfected with plasmids expressing Flag-RIα or the mutant Flag-RIα–G325D and exposed to 40 mm Hg Pco2 (CT) or 120 mm Hg Pco2 (CO2) for 30 minutes. Below: A representative Western blot shows the abundance of NA,K-ATPase at the plasma membrane (PM) and cell lysate (CL) and the expression levels of Flag-RIα and Flag-RIα–G325D (n = 4). (B) Graph represents the relative abundance of the Na,K-ATPase α1 subunit at the plasma membrane in A549 cells transfected with plasmids Flag-mito–null (mito-null) or Flag-mito–RIα (mito-RIα) and exposed to CT or CO2 for 30 minutes. Below: A representative Western blot shows the abundance of Na,K-ATPase at the plasma membrane (PM) and cell lysate (CL) and the expression levels of Flag-mito–null and Flag-mito-RIα (n = 3). (C) Graph represents the relative abundance of the Na,K-ATPase α1 subunit at the plasma membrane in ATII cells preincubated for 30 minutes with vehicle or 10 μM St-Ht31 before exposure to CT or CO2 for 30 minutes. Below: A representative Western blot shows the abundance of Na,K-ATPase at the plasma membrane (PM) and cell lysate (CL) (n = 6). *P < 0.05. **P < 0.01.
α-Adducin Is Phosphorylated at Ser726 in Alveolar Epithelial Cells Exposed to High CO2 Concentrations
The reorganization of the actin cytoskeleton and its component α-adducin has been shown to play an important role in the endocytosis of Na,K-ATPase (17, 30). α-adducin has a myristoylated alanine-rich C kinase substrate–related domain that could constitute a substrate for PKA (31). To determine whether α-adducin is phosphorylated under hypercapnic conditions, we performed a time-course experiment where we exposed ATII and A549 cells up to 30 minutes to 120 mm Hg Pco2, and determined α-adducin phosphorylation by using a specific phospho-antibody for Ser726. As Figure 5A shows, we found that α-adducin was phosphorylated in a time-dependent manner at Ser726 in as few as 5 minutes of CO2 exposure. To explore whether α-adducin phosphorylation at Ser726 was mediated by PKA, and particularly by PKA Type Iα, we studied its phosphorylation in A549 cells transiently transfected with sh-PKAc or with the dominant-negative G325D. Figures 5B and 5C show that both constructs prevented α-adducin phosphorylation at Ser726 in cells exposed to 120 mm Hg Pco2 for 10 minutes. This phosphorylation was not prevented in ATII cells incubated with the St-Ht31 peptide (Figure 5D), suggesting that PKA Type Iα regulates the phosphorylation of α-adducin at Ser726 in cells exposed to high concentrations of CO2.
Figure 5.
α-adducin becomes phosphorylated at serine (Ser) 726 in alveolar epithelial cells exposed to high CO2 via PKA Type Iα. (A) ATII and A549 cells were exposed to 40 mm Hg Pco2 (CT) or 120 mm Hg Pco2 (CO2) for different times, and α-adducin phosphorylation at Ser726 was studied with a phospho-specific antibody. Above: Graph depicts phosphorylated (P-add) versus total (T-add) α-adducin. Below: A representative Western blot shows the phosphorylation of α-adducin at Ser726 (n = 6). (B) Graph represents the relative abundance of phosphorylated versus total α-adducin in A549 cells transfected with sh-scramble (sh-scr) or shRNA for the catalytic subunit of PKA (sh-PKAc) and exposed to CT or CO2 for 10 minutes. Below: A representative Western blot shows the phosphorylation of α-adducin at Ser726 and PKAc knockdown (n = 6). (C) Graph represents the relative abundance of phosphorylated versus total α-adducin in A549 cells transfected with plasmids expressing Flag-RIα or the mutant Flag-RIα–G325D and exposed to CT or CO2 for 10 minutes. Below: A representative Western blot shows the phosphorylation of α-adducin at Ser726 and the expression levels of Flag-RIα and Flag-RIα–G325D (n = 3). (D) Graph represents the relative abundance of phosphorylated versus total α-adducin in ATII cells preincubated for 30 minutes with vehicle or 10 μM St-Ht31 before exposure to CT or CO2 for 10 minutes. Below: A representative Western blot shows the phosphorylation of α-adducin at Ser726 (n = 3). *P < 0.05. **P < 0.01.
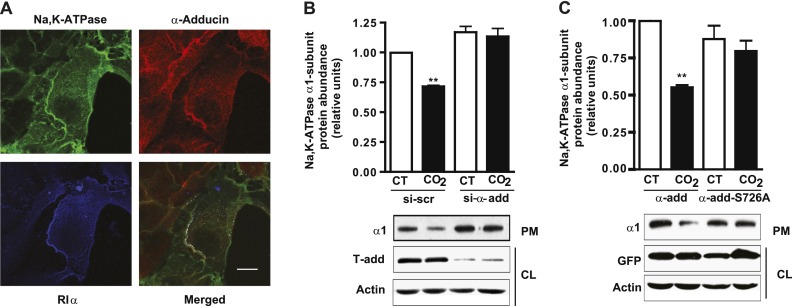
Phosphorylation of α-Adducin at Ser726 Is Necessary for Hypercapnia-Induced Na,K-ATPase Endocytosis
To determine whether the phosphorylation of α-adducin plays a role in the hypercapnia-induced Na,K-ATPase endocytosis, we performed triple-localization experiments with Na,K-ATPase, PKA Type Iα, and α-adducin. As Figure 6A shows, we observed that the three proteins colocalized at the plasma membrane. Finally, to confirm the importance of α-adducin and its phosphorylation in the hypercapnia-induced Na,K-ATPase endocytosis, we determined the abundance of Na,K-ATPase at the plasma membrane in A549 cells exposed to 120 mm Hg Pco2, where α-adducin has been knocked down by siRNA, and in A549 cells transfected with a plasmid expressing α-adducin with the Ser726 mutated to an alanine. As shown in Figures 6B and 6C, hypercapnia-induced Na,K-ATPase endocytosis was prevented in both instances.
Figure 6.
The phosphorylation of α-adducin (α-add) at Ser726 is necessary for the hypercapnia-induced Na,K-ATPase endocytosis in alveolar epithelial cells. (A) Confocal images show triple localization (white) among Na,K-ATPase (green), α-adducin (red), and PKA-RIα (blue) in A549-GFPα1 cells. Bar, 10 μm. (B) Graph represents the relative abundance of the Na,K-ATPase α1 subunit at the plasma membrane in A459 cells transfected with scrambled siRNA (si-scr) or siRNA for α-adducin (si-α-add) and exposed to 40 mm Hg Pco2 (CT) or 120 mm Hg Pco2 (CO2) for 30 minutes. Below: A representative Western blot shows the abundance of Na,K-ATPase at the plasma membrane (PM) and α-adducin knockdown (n = 5). (C) Graph represents the relative abundance of the Na,K-ATPase α1 subunit at the plasma membrane in A459 cells transfected with plasmids GFP-α-add or GFP-α-add-S726A and exposed to CT or CO2 for 30 minutes. Below: A representative Western blot shows the abundance of Na,K-ATPase at the plasma membrane (PM) and the expression levels of GFP-α-add or GFP-α-add-S726A (n = 9). **P < 0.01.
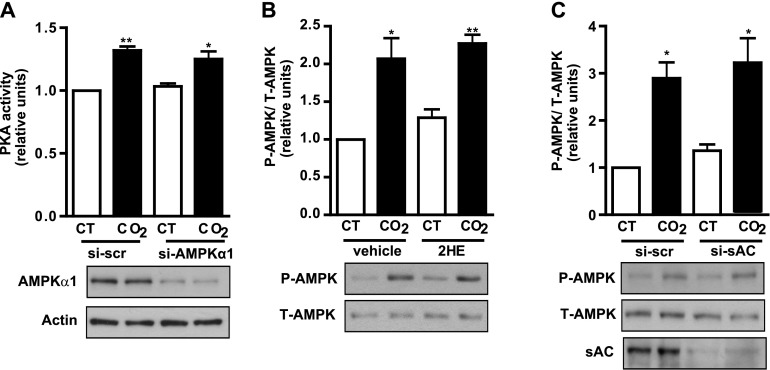
Both sAC and AMPK Are Necessary for the Hypercapnia-Induced Na,K-ATPase Endocytosis
We previously showed that AMPK mediates the high CO2-induced Na,K-ATPase endocytosis in alveolar epithelial cells (7). In the present report, we show that the activation of sAC is also necessary for Na,K-ATPase endocytosis to occur. To determine whether both pathways are interrelated or independent of one another, we studied PKA activation in the presence of siRNA for AMPK, and AMPK activation in the presence of 2HE or siRNA for sAC. As Figure 7A shows, RLE cells exposed to 120 mm Hg Pco2 exhibit increased PKA activity, even when AMPK has been silenced by siRNA. On the other hand, AMPK phosphorylation still occurred in cells exposed to 120 mm Hg Pco2 and incubated with the pharmacological sAC inhibitor 2HE (Figure 7B) or with siRNA for sAC (Figure 7C). Taken together, these results suggest that both pathways are independent of one another, but necessary for the hypercapnia-induced Na,K-ATPase endocytosis.
Figure 7.
sAC and AMP-activated protein kinase (AMPK) are necessary for hypercapnia-induced Na,K-ATPase endocytosis. (A) RLE cells transfected with scrambled siRNA (si-scr) or with siRNA for AMPKα1 (si-AMPKα1) were exposed to 40 mm Hg Pco2 (CT) or 120 mm Hg Pco2 (CO2) for 2.5 minutes, and PKA activity was measured by immunoassay. Below: A representative Western blot shows AMPKα1 knockdown (n = 3). (B) RLE cells were incubated for 30 minutes with vehicle or 5 μM 2HE and exposed to 40 mm Hg Pco2 (CT) or 120 mm Hg Pco2 (CO2) for 10 minutes, and AMPK phosphorylation was studied with a phospho-specific antibody. Upper panel depicts a graph of phosphorylated (P) versus total (T) AMPK. Below: A representative Western blot shows AMPK phosphorylation (n = 6). (C) Graph represents the relative abundance of phosphorylated versus total AMPK in RLE cells transfected with scrambled siRNA (si-scr) or siRNA for sAC (si-sAC) and exposed to CT or CO2 for 10 minutes. Below: A representative Western blot shows AMPK phosphorylation and sAC knockdown (n = 3). *P < 0.05. **P < 0.01.
Discussion
Elevated concentrations of CO2 in blood and tissues (hypercapnia) can occur in patients with inadequate alveolar gas exchange, leading to increased morbidity (32–34). Recently, hypercapnia was suggested to exert deleterious effects on innate immunity in Drosophila, C. elegans, and lung tissue (35–37), and also to impair alveolar epithelial function (6, 7, 38). One of the major effects of hypercapnia on the alveolar epithelium involves impaired Na,K-ATPase function because of the endocytosis of Na,K-ATPase (6, 7). In the present report, we provide evidence that hypercapnia-induced Na,K-ATPase endocytosis is dependent on the cAMP generated by sAC in a discrete subcellular microdomain that activates a specific subset of PKA isoforms, namely, PKA Type Iα. PKA Type Iα, in turn, leads to the phosphorylation of a critical component of the actin cytoskeleton, α-adducin, resulting in Na,K-ATPase endocytosis.
sAC has been recently identified as a source of cAMP in mammals (9), generating cAMP when activated by CO2/HCO3−. We found that alveolar epithelial cells exposed to high CO2 concentrations have an increase in cAMP within 1 minute because of the activation of sAC. The activation of sAC has been suggested to be regulated by bicarbonate rather than by carbon dioxide itself (39). However, in phylogenetically related prokaryotic adenylyl cyclases, CO2 was demonstrated to be the ligand (40).
The cAMP generated by sAC regulates ion transport in different cell types by modulating the activity of different channels and ATPases such as V-ATPase, cystic fibrosis transmembrane conductance regulator (CFTR), and Na,K-ATPase (22, 41, 42). We found that sAC activation was necessary for the hypercapnia-induced Na,K-ATPase endocytosis, in contrast with reports on kidney cells where sAC regulated sodium reabsorption by enhancing Na,K-ATPase activity (42). This discrepancy is in keeping with the well-known differences in the regulation of Na,K-ATPase between lung and kidney tissue, namely, β-adrenergic and dopaminergic agonists induce Na,K-ATPase endocytosis in the kidney, while inducing its recruitment to the plasma membrane in the alveolar epithelium (23, 24, 43).
We found that the site of generation of cAMP was quite different in cells exposed to high concentrations of CO2, in comparison to cells incubated with forskolin, suggesting that sAC generates cAMP in a compartmentalized fashion close to the plasma membrane, whereas the cAMP generated by tmACs has a more generalized and perinuclear location, probably reflecting a greater increase in the cAMP induced by forskolin than by CO2. This agrees with the notion that sAC is restricted to specific locations in the cell, generating cAMP microdomains (10). cAMP exerts opposing effects in the cell dependent on the site of production, namely, it strengthens the cortical actin rim when synthesized at the plasma membrane (44), whereas it can induce gap formation when synthesized in the cytoplasm (45). These opposing effects, depending on the site of production, could explain why the cAMP produced by tmACs regulates alveolar Na,K-ATPase by promoting its recruitment to the plasma membrane (24, 25), whereas when cAMP is produced by sAC, it leads to Na,K-ATPase endocytosis.
We found PKA to be necessary for the hypercapnia-induced Na,K-ATPase endocytosis, in contrast with the well-established role of PKA in the recruitment of Na,K-ATPase to the plasma membrane in these cells (25). Ample evidence shows that the different isoforms of PKA are not functionally redundant, and are located in distinct intracellular signaling compartments (46, 47). Our confocal experiments indicate that the Na,K-ATPase located at the plasma membrane of alveolar epithelial cells mainly colocalizes with PKA Type Iα, and we propose that this specific PKA is activated during hypercapnia by sAC-generated cAMP, leading to Na,K-ATPase endocytosis. We hypothesize that the differences in the Na,K-ATPase traffic we observed in alveolar epithelial cells (endocytosis or recruitment to the plasma membrane) depend on the PKA isoform activated, because β-adrenergic agonists preferentially stimulate PKA Type II isoforms (46).
Reorganization of the actin cytoskeleton is crucial in endocytosis processes (48). Adducins have been reported not only to exert a modulatory effect on actin cytoskeleton dynamics, but also to participate in the clathrin-dependent endocytosis of Na,K-ATPase in kidney cells (16, 17). We demonstrated that α-adducin is necessary for the endocytosis of Na,K-ATPase, and moreover, that it needs to be phosphorylated. The phosphorylation of proteins belonging to the spectrin–actin cytoskeleton was shown to modulate their interaction and function (49). α-adducin has a myristoylated alanine-rich C kinase substrate–related domain (a phosphorylation site for PKA and PKC) (14, 31) that regulates adducin activity when phosphorylated. In particular, the phosphorylation of α-adducin at Ser726 promotes its dissociation from the actin–spectrin complex (50).
We previously showed that AMPK and PKC-ζ are involved in the hypercapnia-induced Na,K-ATPase endocytosis, probably by regulating the signaling cascade leading to the phosphorylation of the Na,K-ATPase α1-subunit at Ser18, a necessary step toward triggering its endocytosis (6, 7). Here we report that hypercapnia leads to Na,K-ATPase endocytosis via remodeling of the actin cytoskeleton by the phosphorylation of α-adducin.
In conclusion, we provide evidence that alveolar epithelial cells exposed to high CO2 concentrations lead to an increased production of cAMP via sAC in discrete cellular microdomains, resulting in the activation of PKA Type Iα, which in turn mediates α-adducin phosphorylation at Ser726 and Na,K-ATPase endocytosis. We describe a novel signaling pathway regulating Na,K-ATPase trafficking, and demonstrate the importance of the type of adenylyl cyclase involved in cAMP generation, cAMP compartmentalization, and PKA isoform specificity, to determine endocytosis versus recruitment to the plasma membrane of the Na,K-ATPase in alveolar epithelial cells.
Acknowledgments
Acknowledgments
The authors appreciate discussions with, and the help of, Dr. Laura Dada and Lynn Welch.
Footnotes
This work was supported in part by National Institutes of Health grant HL85534 (J.I.S.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0373OC on January 24, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sznajder JI. Alveolar edema must be cleared for the acute respiratory distress syndrome patient to survive. Am J Respir Crit Care Med. 2001;163:1293–1294. doi: 10.1164/ajrccm.163.6.ed1801d. [DOI] [PubMed] [Google Scholar]
- 2.Lecuona E, Trejo H, Sznajder J. Regulation of Na,K-ATPase during acute lung injury. J Bioenerg Biomembr. 2007;39:391–395. doi: 10.1007/s10863-007-9102-1. [DOI] [PubMed] [Google Scholar]
- 3.Lecuona E, Saldias F, Comellas A, Ridge K, Guerrero C, Sznajder JI. Ventilator-associated lung injury decreases lung ability to clear edema in rats. Am J Respir Crit Care Med. 1999;159:603–609. doi: 10.1164/ajrccm.159.2.9805050. [DOI] [PubMed] [Google Scholar]
- 4.Litvan J, Briva A, Wilson MS, Budinger GRS, Sznajder JI, Ridge KM. Beta-adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na,K-ATPase and alveolar fluid reabsorption. J Biol Chem. 2006;281:19892–19898. doi: 10.1074/jbc.M602064200. [DOI] [PubMed] [Google Scholar]
- 5.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briva A, Vadász I, Lecuona E, Welch LC, Chen J, Dada LA, Trejo HE, Dumasius V, Azzam ZS, Myrianthefs PM, et al. High CO2 levels impair alveolar epithelial function independently of pH. PLoS ONE. 2007;2:e1238. doi: 10.1371/journal.pone.0001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vadász I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Tóth PT, Lecuona E, Witters LA, Schumacker PT, et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest. 2008;118:752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zippin JH, Levin LR, Buck J. CO2/HCO3-responsive soluble adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol Metab. 2001;12:366–370. doi: 10.1016/s1043-2760(01)00454-4. [DOI] [PubMed] [Google Scholar]
- 10.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 11.Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- 12.Kopperud R, Krakstad C, Selheim F, Døskeland SO. cAMP effector mechanisms: novel twists for an “old” signaling system. FEBS Lett. 2003;546:121–126. doi: 10.1016/s0014-5793(03)00563-5. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SS, Kim C, Cheng CY, Brown SHJ, Wu J, Kannan N. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. BBA Proteins Proteomics. 2008;1784:16–26. doi: 10.1016/j.bbapap.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin–actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142:485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci. 2000;57:884–895. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Efendiev R, Krmar RT, Ogimoto G, Zwiller J, Tripodi G, Katz AI, Bianchi G, Pedemonte CH, Bertorello AM. Hypertension-linked mutation in the adducin alpha–subunit leads to higher AP2-mu2 phosphorylation and impaired Na+,K+-ATPase trafficking in response to GPCR signals and intracellular sodium. Circ Res. 2004;95:1100–1108. doi: 10.1161/01.RES.0000149570.20845.89. [DOI] [PubMed] [Google Scholar]
- 17.Torielli L, Tivodar S, Montella RC, Iacone R, Padoani G, Tarsini P, Russo O, Sarnataro D, Strazzullo P, Ferrari P, et al. Alpha-adducin mutations increase Na/K pump activity in renal cells by affecting constitutive endocytosis: implications for tubular Na reabsorption. Am J Physiol Renal Physiol. 2008;295:F478–F487. doi: 10.1152/ajprenal.90226.2008. [DOI] [PubMed] [Google Scholar]
- 18.Lecuona E, Sun H, Trejo H, Chen J, Gelfand V, Sznajder J. Diverging effects of cAMP/PKA in the traffic and function of the Na,K-ATPase in alveolar epithelial cells. Am J Respir Crit Care Med. 2011;183:A4233. [Google Scholar]
- 19.Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 20.Gesellchen F, Stangherlin A, Surdo N, Terrin A, Zoccarato A, Zaccolo M. Measuring spatiotemporal dynamics of cyclic AMP signaling in real-time using FRET-based biosensors. Methods Mol Biol. 2011;746:297–316. doi: 10.1007/978-1-61779-126-0_16. [DOI] [PubMed] [Google Scholar]
- 21.Kojima S, Vignjevic D, Borisy G. Improved silencing vector co-expressing GFP and small hairpin RNA. Biotechniques. 2004;36:74–79. doi: 10.2144/04361ST02. [DOI] [PubMed] [Google Scholar]
- 22.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng XJ, Fisone G, Aizman O, Aizman R, Levenson R, Greengard P, Aperia A. PKA-mediated phosphorylation and inhibition of Na+-K+-ATPase in response to β-adrenergic hormone. Am J Physiol. 1997;273:C893–C901. doi: 10.1152/ajpcell.1997.273.3.C893. [DOI] [PubMed] [Google Scholar]
- 24.Bertorello AM, Ridge KM, Chibalin AV, Katz AI, Sznajder JI. Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am J Physiol. 1999;276:L20–L27. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- 25.Lecuona E, Minin A, Trejo HE, Chen J, Comellas AP, Sun H, Grillo D, Nekrasova OE, Welch LC, Szleifer I, et al. Myosin-VA restrains the trafficking of Na+/K+-ATPase–containing vesicles in alveolar epithelial cells. J Cell Sci. 2009;122:3915–3922. doi: 10.1242/jcs.046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 27.Willis BS, Niswender CM, Su T, Amieux PS, McKnight GS. Cell-type specific expression of a dominant negative PKA mutation in mice. PLoS ONE. 2011;6:e18772. doi: 10.1371/journal.pone.0018772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns-Hamuro LL, Ma Y, Kammerer S, Reineke U, Self C, Cook C, Olson GL, Cantor CR, Braun A, Taylor SS. Designing isoform-specific peptide disruptors of protein kinase A localization. Proc Natl Acad Sci USA. 2003;100:4072–4077. doi: 10.1073/pnas.2628038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A–anchoring inhibitor peptides arrest mammalian sperm motility. J Biol Chem. 1997;272:4747–4752. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]
- 30.Dada LA, Novoa E, Lecuona E, Sun H, Sznajder JI. Role of the small GTPase RhoA in the hypoxia-induced decrease of plasma membrane Na,K-ATPase in A549 cells. J Cell Sci. 2007;120:2214–2222. doi: 10.1242/jcs.003038. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka Y, Hughes CA, Bennett V. Adducin regulation: definition of the calmodulin-binding domain and sites of phosphorylation by protein kinases A and C. J Biol Chem. 1996;271:25157–25166. doi: 10.1074/jbc.271.41.25157. [DOI] [PubMed] [Google Scholar]
- 32.Mutlu GM, Factor P, Schwartz DE, Sznajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia. Crit Care Med. 2002;30:477–480. doi: 10.1097/00003246-200202000-00034. [DOI] [PubMed] [Google Scholar]
- 33.Varughese M, Patole S, Shama A, Whitehall J. Permissive hypercapnia in neonates: the case of the good, the bad, and the ugly. Pediatr Pulmonol. 2002;33:56–64. doi: 10.1002/ppul.10032. [DOI] [PubMed] [Google Scholar]
- 34.Belkin RA, Henig NR, Singer LG, Chaparro C, Rubenstein RC, Xie SX, Yee JY, Kotloff RM, Lipson DA, Bunin GR. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med. 2006;173:659–666. doi: 10.1164/rccm.200410-1369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helenius IT, Krupinski T, Turnbull DW, Gruenbaum Y, Silverman N, Johnson EA, Sporn PH, Sznajder JI, Beitel GJ. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc Natl Acad Sci USA. 2009;106:18710–18715. doi: 10.1073/pnas.0905925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cummins EP, Oliver KM, Lenihan CR, Fitzpatrick SF, Bruning U, Scholz CC, Slattery C, Leonard MO, McLoughlin P, Taylor CT. NF-κB links CO2 sensing to innate immunity and inflammation in mammalian cells. J Immunol. 2010;185:4439–4445. doi: 10.4049/jimmunol.1000701. [DOI] [PubMed] [Google Scholar]
- 37.Wang N, Gates KL, Trejo H, Favoreto S, Schleimer RP, Sznajder JI, Beitel GJ, Sporn PHS. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 2010;24:2178–2190. doi: 10.1096/fj.09-136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Toole D, Hassett P, Contreras M, Higgins BD, McKeown STW, McAuley DF, O’Brien T, Laffey JG. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-κB dependent mechanism. Thorax. 2009;64:976–982. doi: 10.1136/thx.2008.110304. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 40.Hammer A, Hodgson DR, Cann MJ. Regulation of prokaryotic adenylyl cyclases by CO2. Biochem J. 2006;396:215–218. doi: 10.1042/BJ20060372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Lam CS, Wu F, Wang W, Duan Y, Huang P. Regulation of CFTR channels by HCO3-sensitive soluble adenylyl cyclase in human airway epithelial cells. Am J Physiol Cell Physiol. 2005;289:C1145–C1151. doi: 10.1152/ajpcell.00627.2004. [DOI] [PubMed] [Google Scholar]
- 42.Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NM. Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem. 2009;284:5774–5783. doi: 10.1074/jbc.M805501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertorello A, Sznajder JI. The dopamine paradox in lung and kidney epithelia: sharing the same target but operating different signaling networks. Am J Respir Cell Mol Biol. 2005;33:432–437. doi: 10.1165/rcmb.2005-0297TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson C, Lum H, Schaphorst K, Verin A, Garcia J. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium. 2000;7:287–308. doi: 10.3109/10623320009072215. [DOI] [PubMed] [Google Scholar]
- 45.Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase–dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol. 2009;297:L73–L83. doi: 10.1152/ajplung.90577.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein kinase A Type I and Type II define distinct intracellular signaling compartments. Circ Res. 2008;103:836–844. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 47.Day ME, Gaietta GM, Sastri M, Koller A, Mackey MR, Scott JD, Perkins GA, Ellisman MH, Taylor SS. Isoform-specific targeting of PKA to multivesicular bodies. J Cell Biol. 2011;193:347–363. doi: 10.1083/jcb.201010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qualmann B, Kessels M. Endocytosis and the cytoskeleton. Int Rev Cytol. 2002;220:93–144. doi: 10.1016/s0074-7696(02)20004-2. [DOI] [PubMed] [Google Scholar]
- 49.Woroniecki R, Ferdinand JR, Morrow JS, Devarajan P. Dissociation of spectrin–ankyrin complex as a basis for loss of Na-K-ATPase polarity after ischemia. Am J Physiol Renal Physiol. 2003;284:F358–F364. doi: 10.1152/ajprenal.00100.2002. [DOI] [PubMed] [Google Scholar]
- 50.Barkalow KL, Italiano JE, Chou DE, Matsuoka Y, Bennett V, Hartwig JH. Alpha-adducin dissociates from F-actin and spectrin during platelet activation. J Cell Biol. 2003;161:557–570. doi: 10.1083/jcb.200211122. [DOI] [PMC free article] [PubMed] [Google Scholar]