Abstract
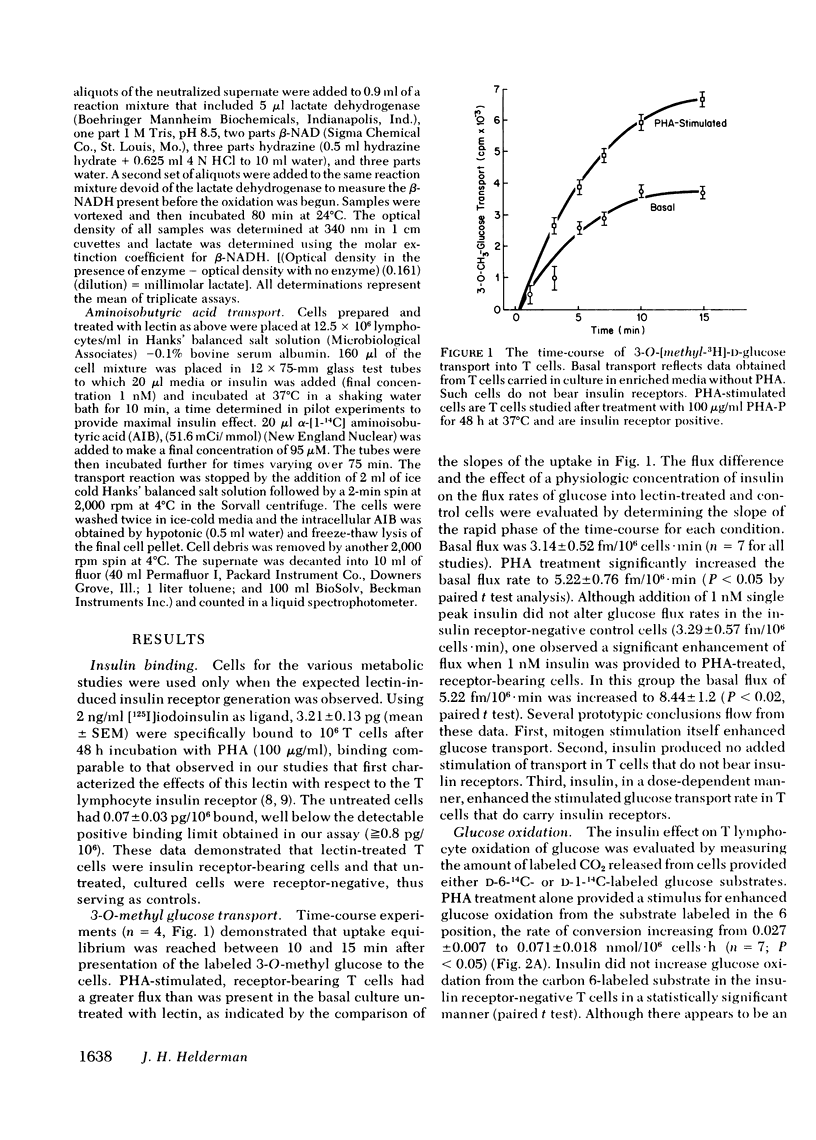
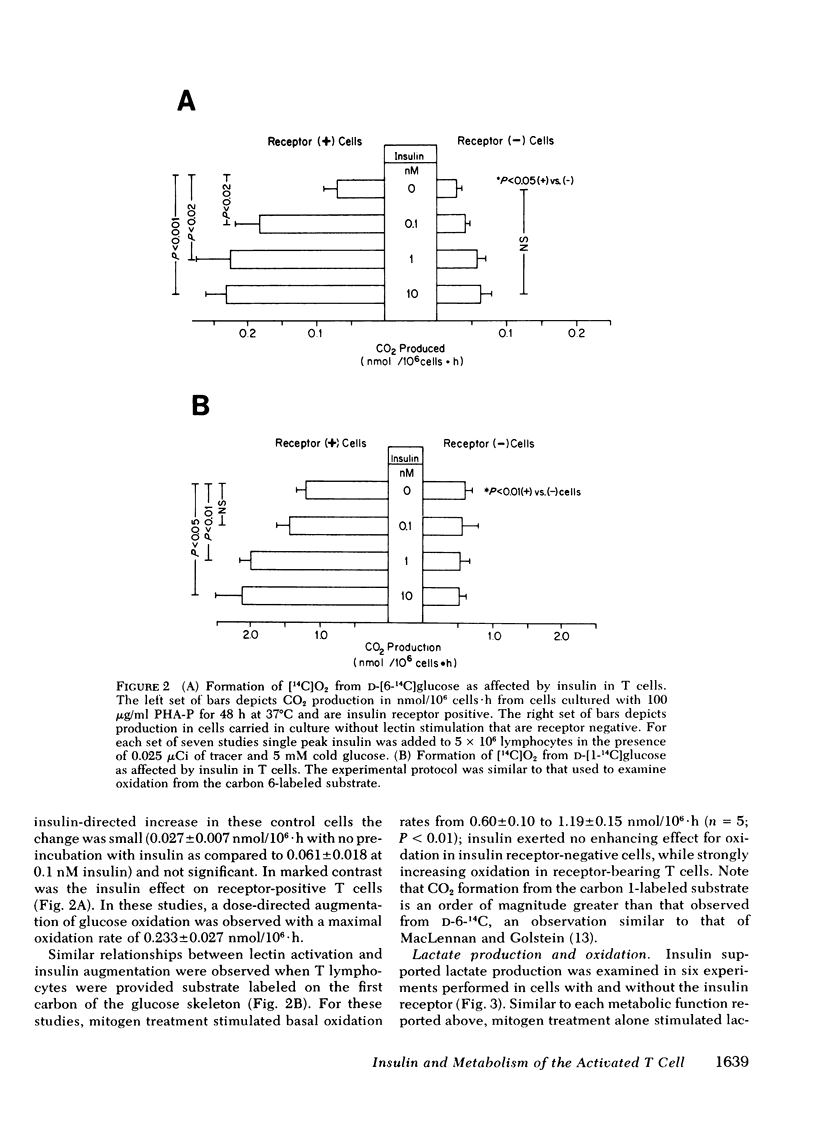
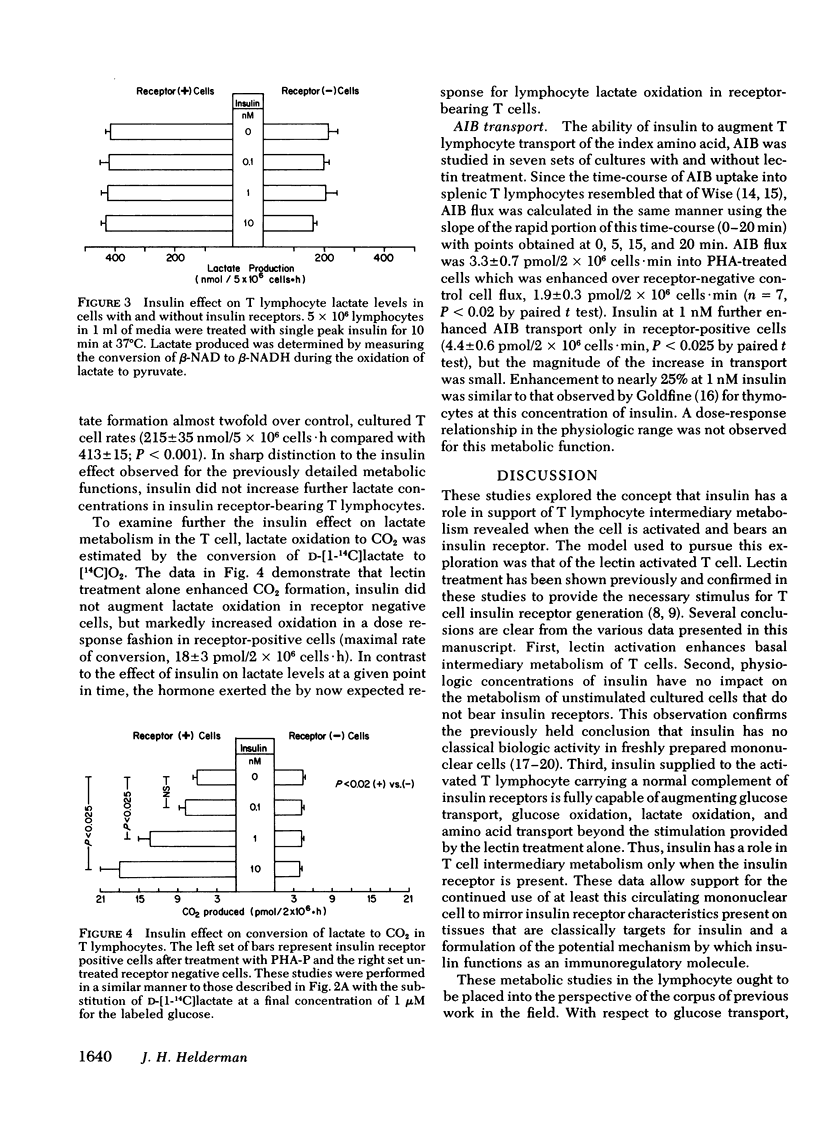
The hypothesis that a role for insulin in the metabolism of T cells would be evident after cell activation when receptors appear was tested to validate the T cell model and to analyze the mechanism by which insulin may function in immunoregulation. Measuring the flux rates of 3-O-[methyl-3H]-D-glucose and aminoisobutyric acid, alpha-[1-14C], lactate production and oxidation, and glucose oxidation from carbon 1- and carbon 6-labeled substrates, it was determined that (a) mitogens such as phytohemagglutinin enhance basal T lymphocyte intermediary metabolism, (b) physiologic concentrations of insulin have no impact on the metabolism of unstimulated, cultured, receptor-negative lymphocytes, and (c) insulin provided to receptor bearing lymphocytes augments intermediary metabolism above mitogen stimulated levels. The importance of the pentose phosphate shunt pathway for energy metabolism in the stimulated lymphocyte was confirmed. These studies demonstrate that insulin has a classical physiologic role to play in the activated lymphocyte further validating the use of this cell to examine potential receptor defects in disorders of carbohydrate metabolism. By enhancing energy metabolism of stimulated lymphocytes, insulin serves biologic economy and thus may perform its immunoregulatory role.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonioli J. A., Felber J. P., Vannotti A. Effet de l'insuline et de quelques autres facteurs sur la glycolyse des leucocytes humains mesurée in vitro. Acta Haematol. 1967;37(4):161–173. doi: 10.1159/000209066. [DOI] [PubMed] [Google Scholar]
- Archer J. A., Gorden P., Gavin J. R., 3rd, Lesniak M. A., Roth J. Insulin receptors in human circulating lymphocytes: application to the study of insulin resistance in man. J Clin Endocrinol Metab. 1973 Apr;36(4):627–633. doi: 10.1210/jcem-36-4-627. [DOI] [PubMed] [Google Scholar]
- Blecher M., Goldstein S. Hormone receptors: VI. On the nature of the binding of glucagon and insulin to human circulating mononuclear leukocytes. Mol Cell Endocrinol. 1977 Oct;8(4):301–315. doi: 10.1016/0303-7207(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Culvenor J. G., Weidemann M. J. Phytohaemagglutinin stimulation of rat thymus lymphocytes glycolysis. Biochim Biophys Acta. 1976 Jul 21;437(2):354–363. doi: 10.1016/0304-4165(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Jen P., Freychet P. Insulin receptors in human circulating cells and fibroblasts. Proc Natl Acad Sci U S A. 1972 Mar;69(3):747–751. doi: 10.1073/pnas.69.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Gardner J. D., Neville D. M., Jr Insulin action in isolated rat thymocytes. I. Binding of 125 I-insulin and stimulation of -aminoisobutyric acid transport. J Biol Chem. 1972 Nov 10;247(21):6919–6926. [PubMed] [Google Scholar]
- HELMREICH E., EISEN H. N. The distribution and utilization of glucose in isolated lymph node cells. J Biol Chem. 1959 Aug;234(8):1958–1965. [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Wilson E. E., Good R. A., Coffey R. G. Direct action of insulin on plasma membrane ATPase activity in human lymphocytes. Nat New Biol. 1972 Feb 9;235(58):174–177. doi: 10.1038/newbio235174a0. [DOI] [PubMed] [Google Scholar]
- Handwerger B. S., Schwartz R. H. Separation of murine lymphoid cells using nylon wool columns. Recovery of the B cell-enriched population. Transplantation. 1974 Dec;18(6):544–548. doi: 10.1097/00007890-197412000-00013. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J. Early effects of phytohaemagglutinin on glucose metabolism of normal human lymphocytes. Biochem J. 1968 Nov;110(2):373–380. doi: 10.1042/bj1100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helderman J. H., Reynolds T. C., Strom T. B. The insulin receptor as a universal marker of activated lymphocytes. Eur J Immunol. 1978 Aug;8(8):589–595. doi: 10.1002/eji.1830080810. [DOI] [PubMed] [Google Scholar]
- Helderman J. H., Strom T. B., Dupuy-D'Angeac A. A close relationship between cytotoxic T lymphocytes generated in the mixed lymphocyte culture and insulin receptor-bearing lymphocytes: enrichment by density gradient centrifugation. Cell Immunol. 1979 Sep 1;46(2):247–258. doi: 10.1016/0008-8749(79)90414-3. [DOI] [PubMed] [Google Scholar]
- Helderman J. H., Strom T. B. Emergence of insulin receptors upon alloimmune T cells in the rat. J Clin Invest. 1977 Feb;59(2):338–344. doi: 10.1172/JCI108646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helderman J. H., Strom T. B. Role of protein and RNA synthesis in the development of insulin binding sites on activated thymus-derived lymphocytes. J Biol Chem. 1979 Aug 10;254(15):7203–7207. [PubMed] [Google Scholar]
- Helderman J. H., Strom T. B. Specific insulin binding site on T and B lymphocytes as a marker of cell activation. Nature. 1978 Jul 6;274(5666):62–63. doi: 10.1038/274062a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofert J. F., Phillips K. J. In vitro insulin-stimulated conversion of [U-14C]glucose to 14CO2 by rat thymocytes. Endocrinology. 1978 Mar;102(3):751–756. doi: 10.1210/endo-102-3-751. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Epidermal growth factor: receptors in human fibroblasts and modulation of action by cholera toxin. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2964–2968. doi: 10.1073/pnas.70.10.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of C14O2 yields from glucose-1- and -6-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1963 Feb;238:517–523. [PubMed] [Google Scholar]
- MacLennan I. C., Golstein P. Requirement for hexose, unrelated to energy provision, in T-cell-mediated cytolysis at the lethal hit stage. J Exp Med. 1978 Jun 1;147(6):1551–1567. doi: 10.1084/jem.147.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A. Glucocorticoid inhibition of glucose uptake by peripheral tissues: old and new evidence, molecular mechanisms, and physiological significance. Perspect Biol Med. 1971 Winter;14(2):265–269. doi: 10.1353/pbm.1971.0002. [DOI] [PubMed] [Google Scholar]
- Olefsky J., Reaven G. M. The human lymphocyte: a model for the study of insulin-receptor interaction. J Clin Endocrinol Metab. 1974 Apr;38(4):554–560. doi: 10.1210/jcem-38-4-554. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Hausen P. Effect of phytohemagglutinin on lymphocyte membrane transport. 2. Stimulation of "facilitated diffusion" of 3-O-methyl-glucose. Eur J Biochem. 1971 Apr 30;19(4):509–513. doi: 10.1111/j.1432-1033.1971.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Reeves J. P. 3-O-methylglucose transport by rat thymocyte subpopulations. J Cell Physiol. 1977 Aug;92(2):309–318. doi: 10.1002/jcp.1040920220. [DOI] [PubMed] [Google Scholar]
- Reeves J. P. Stimulation of 3-O-methylglucose transport by anaerobiosis in rat thymocytes. J Biol Chem. 1975 Dec 25;250(24):9413–9420. [PubMed] [Google Scholar]
- Soll A. H., Goldfine I. D., Roth J., Kahn C. R. Thymic lymphocytes in obese (ob-ob) mice. A mirror of the insulin receptor defect in liver and fat. J Biol Chem. 1974 Jul 10;249(13):4127–4131. [PubMed] [Google Scholar]
- Soulillou J. P., Carpenter C. B., d'Apice A. J., Strom T. B. The role of nonclassical Fc receptor-associated, Ag-B antigens (Ia) in rat allograft enhancement. J Exp Med. 1976 Feb 1;143(2):405–421. doi: 10.1084/jem.143.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise W. C. Amino acid transport in thymic- and spleen-derived lymphocytes. J Cell Physiol. 1978 Nov;97(2):161–167. doi: 10.1002/jcp.1040970205. [DOI] [PubMed] [Google Scholar]
- Wise W. C. Maturation of membrane function: transport of amino acid by rat erythroid cells. J Cell Physiol. 1975 Dec;87(2):199–201. doi: 10.1002/jcp.1040870208. [DOI] [PubMed] [Google Scholar]
- Yasmeen D., Laird A. J., Hume D. A., Weidemann M. J. Activation of 3-O-methyl-glucose transport in rat thymus lymphocytes by concanavalin A. Temperature and calcium ion dependence and sensitivity to puromycin but to cycloheximide. Biochim Biophys Acta. 1977 Nov 7;500(1):89–102. doi: 10.1016/0304-4165(77)90049-6. [DOI] [PubMed] [Google Scholar]


