Abstract
Emphysema is caused by the cigarette smoke (CS)–induced destruction of alveolar wall septa, and CS is the main risk factor for chronic obstructive pulmonary disease (COPD). To study the mechanisms of response to this insult, we focused on oxidant-induced lung injury and the potential role of nuclear erythroid 2–related factor–2 (Nrf2), which is a key regulator of the antioxidant defense system. We studied the protective role of N-acetylcysteine (NAC) against the injury of alveolar type II (ATII) cells induced by CS in vivo and in vitro. ATII cells were isolated and purified using magnetic MicroBeads (Miltenyi Biotec, Auburn, CA) from Nrf2−/− mice and wild-type mice. We analyzed pulmonary injury, inflammation, glutathione (GSH) concentrations, the expression of glutathione cysteine ligase catalytic subunit mRNA, glutathione cysteine ligase modifier subunit mRNA, and glutathione reductase mRNA, and Nrf2, heme oxygenase–1, and nicotinamide adenine dinucleotide phosphate–reduced:quinone oxireductase levels by Western blotting, TUNEL assay, and immunocytofluorescence for 4-hydroxynonenal as a marker of oxidative stress. We found that CS induced greater injury in ATII cells obtained from Nrf2−/− mice than from wild-type mice. Furthermore, NAC attenuated the injuries by CS in ATII cells obtained from wild-type mice both in vivo and in vitro. Moreover, NAC decreased the injury of ATII cells obtained from Nrf2−/− mice. Our results suggest that Nrf2–GSH signaling is important for the protective activity of NAC. In addition, in ATII cells deficient in Nrf2, this compound can provide partial protection through its reactive oxygen species–scavenging activities. Targeting the antioxidant system regulated by Nrf2 may provide an effective strategy against lung injury in COPD.
Keywords: murine alveolar type II cells, lung, Nrf2, NAC, cigarette smoke
Clinical Relevance
Cigarette smoke is the main contributing factor in emphysema, which is caused by destruction of the alveolar wall. Our data demonstrate that the nuclear erythroid 2–related factor–2 is a critical factor in the protective activity of N-acetylcysteine to attenuate oxidative stress and the injury of primary alveolar type II cells.
Chronic obstructive pulmonary disease (COPD) is currently the third-leading cause of death in the United States (1, 2), and mortality rates continue to rise. Emphysema is caused by the destruction of the alveolar wall septa, which is brought about by cigarette smoke (CS) and its associated lung inflammation (3, 4). CS is a complex mixture of different chemicals, including free radicals and other oxidants. Cell death (both apoptosis and necrosis) by CS has been shown to produce oxidant injury both in vitro and in vivo (5–7). However, the exact mechanism underlying CS-induced alveolar epithelial cell death in COPD remains unclear.
The nuclear erythroid 2–related factor–2 (Nrf2) transcription factor is a key determinant of COPD susceptibility (8–11), and one of the critical regulators of cellular redox status (12, 13). Nrf2 is required for the constitutive and inducible expression of the glutathione cysteine ligase catalytic subunit (GCLC) and the glutathione cysteine ligase modifier subunit (GCLM), which are required for glutathione (GSH) synthesis (14). Nrf2 also regulates the expression of other cellular antioxidant genes, such as heme oxygenase–1 (HO-1) and nicotinamide adenine dinucleotide phosphate–reduced:quinone oxireductase (NQO1). We have described the protective role of Nrf2 against human alveolar type II (ATII) cell injury induced by ozone (15), influenza A virus (16), and cigarette smoke (17). Reduced Nrf2 activity in patients with advanced COPD lends support to the importance of an antioxidant-coordinated response in the pathogenesis of CS-induced alveolar destruction (8, 10, 11). Furthermore, Nrf2−/− mice were reported to be highly susceptible to CS-induced lung injury (18, 19).
Very few treatment strategies limit the progression of COPD. N-acetylcysteine (NAC), a thiol compound, is a potential drug in the treatment of COPD (20–24), and it acts as an antioxidant to prevent lung injury. NAC is a precursor of GSH (25), which is one of the most important mechanisms for protecting cells and tissue from reactive oxygen species (ROS). GSH and NAC act as free oxygen radical scavengers (26). However, the mechanism by which NAC protects against lung injury remains to be fully elucidated. NAC was used here to study the attenuation of murine ATII cell injury, characterized by increased lipid peroxidation, inflammation, apoptosis, cytotoxicity, and the GSH depletion caused by CS in Nrf2−/− and Nrf2+/+ mice. GCLC, GCLM, and glutathione reductase (GR) are also critical for GSH biosynthesis and the conversion of NAC to GSH (12).
We chose to study cells in the gas-exchange portion of the human lung, where emphysema develops, and to use murine primary cells rather than cell lines (27). Our approach was novel in that we studied the effect of CS in murine primary ATII cells obtained from Nrf2−/− mice both in vivo and in vitro. Furthermore, we determined the protective role of NAC in ATII cells obtained from both genotypes. This allowed us to better understand the role of the Nrf2 pathway in alveolar cell injury by CS. According to our hypothesis, CS induces greater oxidative injury in ATII cells isolated from Nrf2−/− mice than from Nrf2+/+ mice, both in vitro and in vivo. NAC should protect ATII cells obtained from Nrf2+/+ mice against injury induced by CS in a GSH-dependent manner. We expect that this compound will provide at least partial protection in cells isolated from Nrf2−/− mice in an Nrf2-independent manner by decreasing oxidative stress.
Materials and Methods
Animals
Nrf2+/+ and Nrf2−/− mice (C57BL/6) were maintained at National Jewish Health (Denver, CO). Nrf2−/− mice were developed by Dr. M. Yamamoto (28). Experimental procedures were approved by the Institutional Animal Care and Use Committee at National Jewish Health. At 4 weeks of age, mice were fed the antioxidant-free AIN-76A diet for 10 days (29) before in vitro or in vivo experiments.
Exposure to CS In Vivo
We exposed 5- to 6-week-old mice to CS from Kentucky reference cigarette 3R4F for 5 hours/day for 3 days, using a Teague TE-10 smoking system (Teague Enterprises, Woodland, CA). The total suspended particulate (TSP) was 60 mg/m3, and CO concentrations were less than 300 ppm. Mice received 0.5 g/kg NAC (30) in PBS by oral gavage every 24 hours, starting 1 day before exposure to CS, and for 3 days during exposure to CS.
ATII Cell Isolation, Purification, and Culture
ATII cells were isolated and purified as previously described (31). We used CD45 MicroBeads for negative depletion (Miltenyi Biotec, Inc., Auburn, CA), followed by epithelial cell adhesion molecule (e-Bioscience, San Diego, CA)–positive selection. The purity of these cells, as measured by flow cytometry using cytokeratin and surfactant protein A (SP-A), was greater than 90% (data not shown) (31). ATII cells were cultured as we previously described (31). Briefly, they were plated for 1 day in Dulbecco’s Modified Eagle’s medium (DMEM) with 5% rat serum and 20 mM HEPES (pH 7.4) on millicell inserts coated with a mixture of 20% Engelbreth–Holm–Swarm tumor matrix (BD Biosciences, San Jose, CA) and 80% rat-tail collagen in DMEM, and then cultured for 3 days with 1% CS-stripped FBS along with 10 ng/ml keratinocyte growth factor (R&D Systems, Inc., Minneapolis, MN).
ATII Cell Exposure to Cigarette Smoke Extract In Vitro
The cigarette smoke extract (CSE) was prepared as we previously described (17). ATII cells were treated with 0.5 μM NAC for 24 hours, followed by 0.75% CSE, 1.5% CSE, or 3% CSE for 24 hours. In some experiments, we used an opposite treatment. NAC and CSE concentrations were selected on the basis of viability assay (data not shown).
TUNEL Assay
TUNEL assay was used (17) with anti–proSP-C (Millipore, Billerica, MA) to identify apoptotic ATII cells (32). The percentage of TUNEL-positive apoptotic ATII cells was calculated (33).
Glutathione Measurement
Lung tissue was homogenized, and total GSH was analyzed spectrophotometrically (16). The increase in absorbance was measured at 412 nm. Values were normalized to protein content.
Western Blotting
The expression of protein was normalized to murine glyceraldehyde 3–phosphate dehydrogenase (GAPDH; Abcam, Cambridge, MA) (15). Antibodies included HO-1, purchased from Enzo Life Sciences, Farmingdale, NY, and Nrf2, NQO1, and matrix metalloproteinase–13 (MMP-13) from Santa Cruz Biotechnology (Santa Cruz, CA). Blots were developed using a chemiluminescence method, and images were quantitated using NIH Image 1.62 software (National Institutes of Health, Bethesda, MD).
Real-Time PCR
Total RNA was isolated using an RNeasy Mini kit (Qiagen, Frederick, MD). TaqMan PCR was performed on a C1000 Thermal Cycler (Bio-Rad, Hercules, CA). Gene expression levels were calculated as a ratio to the expression of the constitutive probe, GAPDH (relative expression). Data were analyzed using the delta delta threshold cycle (ΔΔCt) method. Assay On-Demand probes were purchased from Life Technologies (Grand Island, NY) (Table 1).
TABLE 1.
ASSAY-ON-DEMAND GENE EXPRESSION PROBES USED FOR REAL-TIME PCR
| Gene Name | Abbreviation | Assay Identification |
|---|---|---|
| Glutamate cysteine ligase, catalytic subunit | GCLC | Mm00802655_m1 |
| Glutamate cysteine ligase, modifier subunit | GCLM | Mm00514996_m1 |
| Glutathione reductase 1 | GR | Mm00833903_m1 |
| Glyceraldehyde 3–phosphate dehydrogenase | GAPDH | Mm03302249_g1 |
Statistical Analysis
One-way ANOVA by GraphPad Prism 4 (La Jolla, CA) was used. The Dunnett test was applied (P < 0.05). Data are presented as the means ± SEMs from three independent experiments.
Results
Nrf2 Protects against Inflammation Induced by CS In Vivo
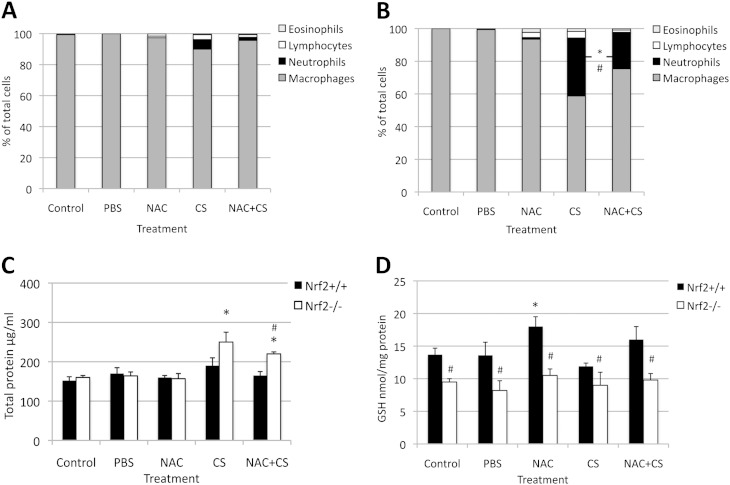
To check whether Nrf2 protects lung tissue against injury induced by CS in vivo, we compared inflammatory responses in Nrf2−/− and Nrf2+/+ mice. Exposure to CS for 3 days induced higher alveolar septa destruction and inflammation in Nrf2−/− mice than in Nrf2+/+ mice (Figures E1I and E1II in the online supplement). Moreover, we observed a higher percentage of neutrophils (Figures 1A and 1B) and a higher total protein concentration (Figure 1C) in BAL obtained from Nrf2−/− mice than from Nrf2+/+ mice after exposure to CS. Treatment with 0.5 g/kg NAC decreased inflammation in Nrf2−/− mice in comparison with CS alone. We found significantly lower concentrations of GSH in lung tissue obtained from Nrf2−/− compared with Nrf2+/+ mice (Figure 1D). NAC increased GSH concentrations only in lung tissue obtained from Nrf2+/+ mice. These results suggest a protective role of Nrf2 and NAC against lung injury induced by CS in vivo.
Figure 1.
Pulmonary injury, inflammation, and glutathione concentrations in the lungs of Nrf2−/− and Nrf2+/+ mice after treatment with N-acetylcysteine (NAC) and exposure to cigarette smoke (CS) in vivo. A higher percentage of inflammatory cells in bronchoalveolar lavage (BAL) fluid from Nrf2−/− (B) than from Nrf2+/+ mice (A) is evident after exposure to CS for 3 days (5 hours/day), as described in Materials and Methods. The oral administration of 0.5 g/kg NAC for 4 days provided partial protection. (C) Higher total protein concentrations in the BAL fluid of Nrf2−/− than of Nrf2+/+ mice after exposure to total suspended particulate (TSP) CS were evident. NAC protected lungs against injury by CS. (D) NAC increased glutathione (GSH) concentrations in the lungs of Nrf2+/+ mice. *Statistically significant increase in comparison with control mice. #Statistically significant decrease in comparison with exposure to CS alone in B and C, or in comparison with Nrf2+/+ mice in D (P < 0.05). Nrf2, nuclear erythroid 2–related factor–2.
NAC Reduces Apoptosis Induced by CS in ATII Cells In Vivo
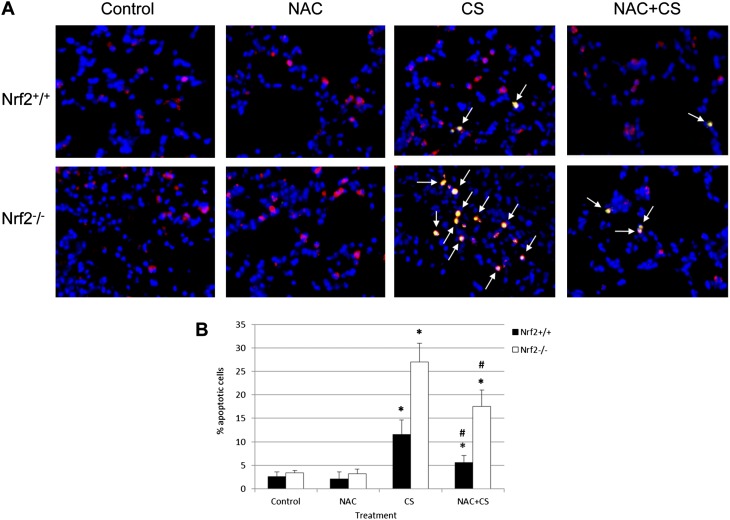
We wanted to compare the susceptibility of ATII cells in Nrf2−/− and Nrf2+/+ mice to injury induced by CS and the protection conferred by NAC. We found a statistically significantly higher percentage of TUNEL-positive ATII cells in lungs from Nrf2−/− compared with Nrf2+/+ mice after exposure to CS (Figure 2). NAC provided protection against injury in ATII cells obtained from both genotypes. We also compared oxidative stress by staining for 4-hydroxynonenal (4-HNE), which is a product of lipid peroxidation. We observed that NAC decreased oxidative stress in lung tissue and ATII cells obtained from both Nrf2+/+ and Nrf2−/− mice exposed to CS in vivo and in vitro (Figures E2 and E3). This indicates the protective role of NAC as a scavenger of ROS induced by CS. However, this compound protected ATII cells obtained from Nrf2+/+ more effectively than from Nrf2−/− mice. This suggests the protective role of Nrf2–GSH signaling, which is in concordance with the higher GSH concentrations in Nrf2+/+ mice.
Figure 2.
CS induces greater apoptosis in alveolar type II (ATII) cells obtained from Nrf2−/− mice than from Nrf2+/+ mice, and NAC protects cells against injury in vivo. (A) Mice were treated with NAC for 4 days and exposed to CS for 3 days. ATII cells in lung sections were identified by staining with proSP-C (red). Apoptotic cells were detected by TUNEL assay (green). Apoptotic ATII cells (arrows indicate overlapping red and green) are shown, at magnification ×400. (B) Quantitation of apoptotic pro–surfactant protein C (proSP–C) positive ATII cells, as detected by TUNEL assay. *Statistically significant increase in comparison with control (P < 0.05). #Statistically significant decrease in comparison with CS alone.
NAC Protects ATII Cells against Injury Induced by CS In Vivo
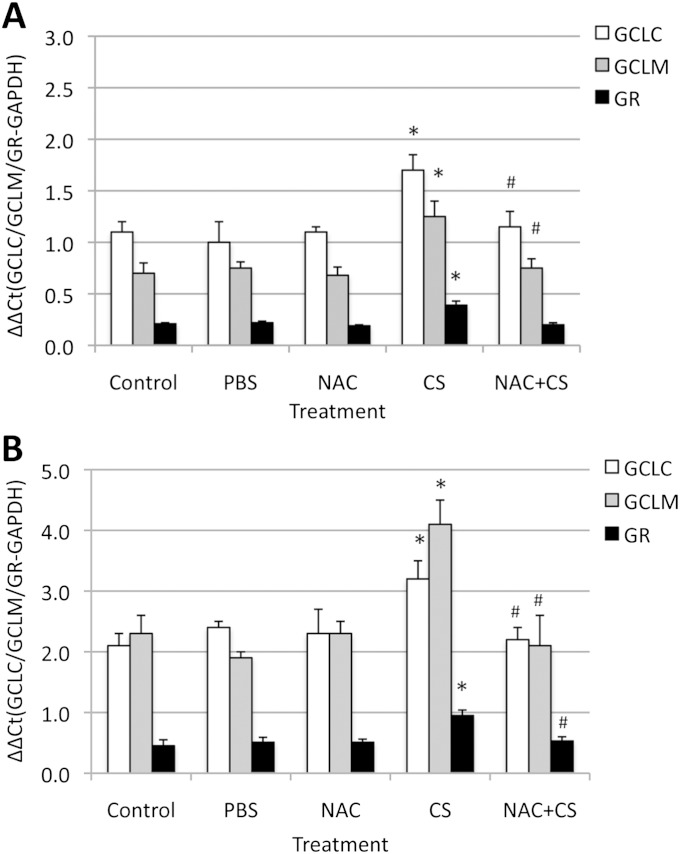
To further determine the role of the GSH pathway in protection against CS, we measured the expression of GCLC, GCLM, and GR. We found that CS increased the mRNA concentrations of these genes in ATII cells and lung tissue obtained from Nrf2+/+ mice (Figure 3), but did not change their expression in Nrf2−/− mice (data not shown). We also observed that NAC decreased GCLC and GCLM concentrations induced by CS only in Nrf2+/+ mice. Our results indicate that the Nrf2-dependent activation of antioxidant genes is involved in the protection of lung tissue and ATII cells against injury induced by CS. These results are in agreement with our observations that NAC increased GSH concentrations in lung tissue obtained from Nrf2+/+ mice, but not from Nrf2−/− mice (Figure 1D). This indicates that NAC can protect against injuries induced by CS in a GSH-dependent manner in Nrf2+/+ mice and in a GSH-independent manner in both genotypes as a scavenger of ROS. This compound can more effectively protect ATII cells and lung tissue obtained from Nrf2+/+ mice than from Nrf2−/− mice against injury induced by CS.
Figure 3.
Exposure to CS in vivo induced the expression of antioxidant genes in lung tissue (A) and ATII cells (B) obtained from Nrf2+/+ mice. Mice were treated with 0.5 g/kg NAC, followed by exposure to CS for 3 days (5 hours/day) in vivo, as described in Materials and Methods. Glutathione cysteine ligase catalytic subunit (GCLC), glutathione cysteine ligase modifier subunit (GCLM), and glutathione reductase (GR) mRNA levels were measured by RT-PCR. *Statistically significant increase in comparison with control mice (P < 0.05). #Statistically significant decrease in comparison with CS alone. GADPH, glyceraldehyde 3–phosphate dehydrogenase.
CS Induces the Response of Nrf2-Modulated Genes in ATII Cells In Vivo
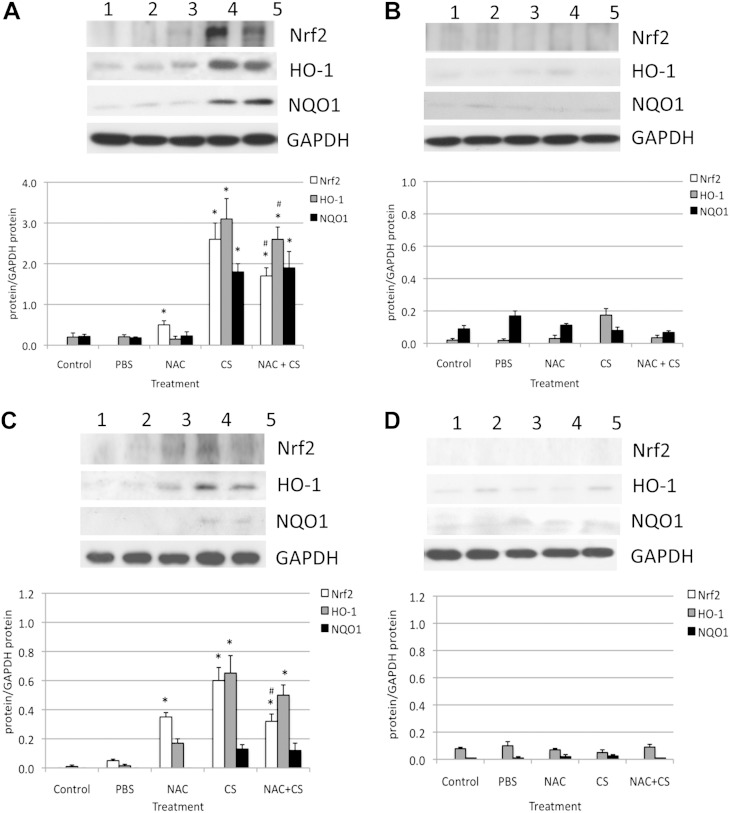
Our results indicated the involvement of the GSH pathway and Nrf2 target genes (GCLC and GCLM) in the protection of ATII cells and lung tissue against injury induced by CS in vivo. Therefore, we wanted to focus on the response of antioxidant and detoxification genes regulated by Nrf2. We analyzed protein expression in lung tissue and ATII cells obtained from Nrf2+/+ and Nrf2−/− mice after exposure to CS in vivo, with and without the administration of NAC. We found that CS significantly increased the expression of Nrf2, HO-1, and NQO1 in lung tissue and ATII cells isolated from Nrf2+/+ mice (Figure 4). Higher Nrf2 levels were detected after the administration of 0.5 g/kg NAC, in comparison with control mice. Furthermore, cotreatment with NAC and CS significantly decreased the expression of Nrf2, NQO1, and HO-1 in comparison with CS alone. However, we observed a higher expression of these proteins in lung tissue than in ATII cells. This suggests the lower antioxidant capacity of ATII cells and their higher susceptibility to injury. We did not detect Nrf2, HO-1, and NQO1 expression in lung tissue (Figure 4IB) or in ATII cells (Figure 4IIB) obtained from Nrf2−/− mice. Our results indicate the protective role of NAC and the Nrf2 pathway against ATII cell and lung tissue injury by CS in vivo.
Figure 4.
Exposure to CS in vivo induced antioxidant defense system in lung tissue (top panels) and in ATII cells (bottom panels) obtained from Nrf2+/+ mice (by immunoblotting). (A and C) Nrf2+/+ mice. (B and D) Nrf2−/− mice. Mice were treated with 0.5 g/kg NAC for 4 days and exposed to CS for 5 hours/day for 3 days. Lane 1, control; lane 2, PBS; lane 3, 0.5 g/kg NAC; lane 4, CS; lane 5, 0.5 g/kg NAC with CS. The relative expression of these proteins is also shown. *Statistically significant difference in comparison with control mice (P < 0.05). #Statistically significant decrease in comparison with CS alone. HO-1, heme oxygenase–1; NQO1, nicotinamide adenine dinucleotide phosphate–reduced:quinone oxireductase.
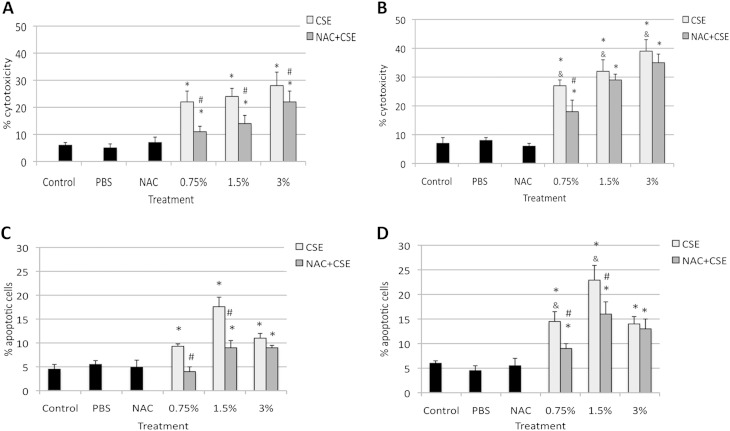
CSE Induces Apoptosis and Cytotoxicity in Murine ATII Cells In Vitro
Because we observed ATII cell and lung injury induced by CS in vivo, we also wanted to focus on the response of murine ATII cells to CSE in vitro. We isolated these cells from Nrf2+/+ mice and Nrf2−/− mice, and exposed them to 0.75%, 1.5%, and 3% CSE with and without 0.5 μM NAC (Figure 5). We observed significantly higher cytotoxicity in ATII cells isolated from Nrf2−/− than from Nrf2+/+ mice (Figures 5A and 5B) after exposure to all applied concentrations of CSE, as detected by Hoechst 33342 and propidium iodide double staining. The treatment of these ATII cells with 0.5 μM NAC significantly decreased injuries in ATII cells obtained from Nrf2+/+ mice in comparison with CSE alone for all concentrations of CSE evaluated. However, only cotreatment with the lowest concentration of CSE (0.75%) and NAC significantly decreased the percentages of propidium iodide–positive ATII cells from Nrf2−/− mice in comparison with CSE alone. These results indicate (1) the cytotoxicity of CSE in murine ATII cells, (2) that these cells isolated from Nrf2−/− mice are more susceptible to injury induced by CSE in comparison with Nrf2+/+ mice, and (3) that NAC more efficiently protects ATII cells obtained from Nrf2+/+ than from Nrf2−/− mice against injury. These in vitro findings confirm the results obtained in vivo.
Figure 5.
NAC protects ATII cells against injury induced by cigarette smoke extract (CSE) in vitro, as detected by Hoechst 33342 and propidium iodide double staining (top panels) and TUNEL assay (bottom panels). ATII cells were isolated from Nrf2+/+ (A and C) and Nrf2−/− (B and D) mice, cultured as described in Materials and Methods, and treated with 0.5 μM NAC for 24 hours, followed by 0.75%, 1.5%, and 3% CSE for 24 hours. *Statistically significant increase in comparison with negative control samples (P < 0.05). #Statistically significant decrease in comparison with CSE alone. &Statistically significant increase in comparison with cells isolated from Nrf2+/+ mice and treated with CSE (A and C).
We also observed a significant induction of apoptosis in ATII cells obtained from both genotypes upon exposure to 0.75%, 1.5%, and 3% CSE as detected by TUNEL assay (Figures 5C and 5D). Furthermore, treatment with 0.75% or 1.5% CSE and 0.5 μM NAC significantly decreased the percentage of apoptotic cells isolated from Nrf2+/+ mice, compared with CSE alone. However, the percentage of apoptotic ATII cells isolated from Nrf2−/− mice was significantly higher after treatment with 0.75% and 1.5% CSE, in comparison with cells obtained from Nrf2+/+ mice. Treatment with 0.75% and 1.5% CSE and NAC significantly decreased apoptosis in ATII cells from Nrf2−/− mice. However, NAC did not decrease apoptosis after treatment with the highest concentration of CSE. This can be explained by the very high cytotoxicity of 3% CSE in ATII cells. We also found a statistically significant decrease in apoptosis among ATII cells treated with CSE followed by 0.5 μM NAC (Figure E4). For this experiment, we selected 1.5% CSE, which induced the highest percentage of apoptotic ATII cells as measured by TUNEL assay (Figures 5C and 5D). Our results indicate (1) that CSE induces apoptosis in ATII cells; (2) a confirmation of our observations in vivo that ATII cells isolated from Nrf2−/− mice are more sensitive to injury than cells obtained from Nrf2+/+ mice; and (3) that pretreatment or posttreatment with NAC can protect ATII cells against injury in both genotypes as a ROS scavenger, and by increasing antioxidant capacity.
NAC Protects Murine ATII Cells against Injury by CSE In Vitro
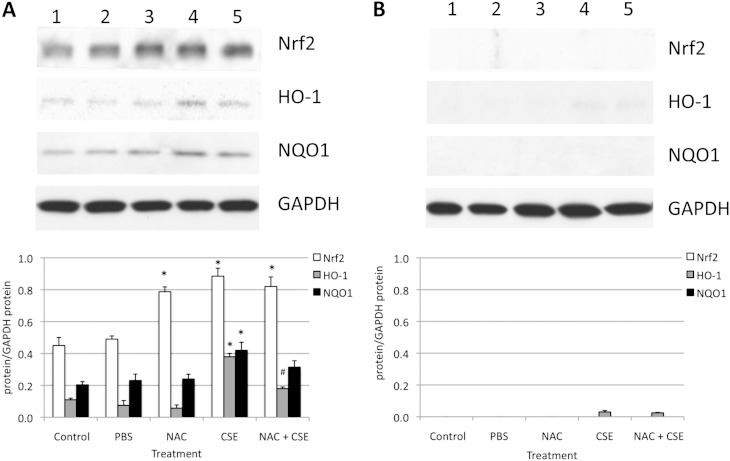
Because we observed the Nrf2-dependent response in ATII cells and lung tissue after exposure to CS in vivo, we also wanted to investigate Nrf2 induction in ATII cells exposed to CSE in vitro. We found that 3% CSE significantly induced the expression of Nrf2, HO-1, and NQO1 in ATII cells obtained from Nrf2+/+ mice (Figure 6A). Moreover, cotreatment with NAC and CSE significantly decreased HO-1 expression, which is regulated by Nrf2, in comparison with CSE alone. We did not detect the expression of Nrf2, HO-1, and NQO1 in ATII cells obtained from Nrf2−/− mice (Figure 6B). These results suggest an Nrf2-dependent response of ATII cells to CSE. NAC is a precursor to GSH biosynthesis, and Nrf2-regulated GSH signaling can protect ATII cells against injury in vitro.
Figure 6.
Protein expression in ATII cells isolated from Nrf2+/+ mice (A) and Nrf2−/− mice (B) after treatment with 0.5 μM NAC for 24 hours, followed by 3% CSE for 24 hours in vitro (by immunoblotting). Lane 1, control; lane 2, PBS; lane 3, 0.5 μM NAC; lane 4, 3% CSE; lane 5, 0.5 μM NAC and 3% CSE. The relative expression of these proteins is also shown. *Statistically significant increase in comparison with control samples (P < 0.05). #Statistically significant decrease in comparison with CSE alone.
We also checked the effect of 3% CSE, followed by treatment with 0.5 μM NAC, on MMP-13 expression in ATII cells in vitro. We found that treatment with NAC decreased MMP-13 expression in ATII cells obtained from Nrf2−/− mice and exposed to CSE (Figure E5). We did not observe a higher expression of MMP-13 in ATII cells from Nrf2+/+ mice, probably because of the lower sensitivity of this genotype to exposure to 60 mg/m3 TSP. Our results indicate the important protective role of pretreatment and posttreatment with NAC against the ATII cell injury induced by CSE.
Discussion
Constituents of CS play a significant role in the injury of the alveolar epithelium and the pathogenesis of COPD (5, 17). This study sought to understand the protective role of NAC against murine ATII cell injury. Nrf2 is expressed predominantly in the epithelium, and plays an essential protective role in the lungs through the activation of cytoprotective genes (34). To our knowledge, no previous findings have been reported on the role of NAC in ATII cells obtained from Nrf2−/− mice after exposure to CS. This was addressed by histological and BAL analysis and by evaluations of oxidative stress, apoptosis, and gene and protein expression both in vivo and in vitro. We confirmed our hypothesis that NAC protects ATII cells against CS in an Nrf2-dependent manner through GSH-dependent redox homeostasis. This compound also suppressed oxidative stress in an Nrf2-independent manner in Nrf2−/− mice. Our findings demonstrated for the first time the important role of Nrf2–GSH signaling in murine ATII cells supplemented with NAC and exposed to CS both in vivo and in vitro. Furthermore, ATII cells isolated from Nrf2−/− mice are more sensitive to injuries induced by CS than are ATII cells isolated from Nrf2+/+ mice because of the decreased concentrations of GSH and the decreased detoxification process.
Our in vivo experiments revealed greater inflammation, oxidative stress, pulmonary injury, apoptosis in ATII cells, and necrosis of alveolar septa (observed as rupture and loss) in Nrf2−/− mice than in wild-type mice after exposure to CS. The high infiltration of inflammatory cells and total protein concentrations in the BAL fluid of Nrf2−/− mice may indicate that ATII cell and lung injury in vivo is related more to necrosis than to apoptosis. These results are in agreement with previous observations that the disruption of the Nrf2 gene in mice leads to earlier and more extensive CS-induced lung injury (19). This indicates the significant role of the Nrf2 pathway in the prevention of lung injury. We also observed that GCLC, GCLM, and GR mRNA genes were increased in ATII cells obtained from Nrf2+/+ but not from Nrf2−/− mice after exposure to CS, which indicates an activation of the Nrf2 pathway. Yageta and colleagues (35) and Sussan and colleagues (8) reported GCLC, GCLM, and HO-1 mRNA up-regulation in the lungs of wild-type mice exposed to CS in vivo. However, in both of those studies, the effect of CS on alveolar epithelial cells was not examined. Because Nrf2 is expressed mainly in the epithelium (34), we wanted to study the ATII cell response. We also found that CSE in vitro induced greater apoptosis and cytotoxicity in ATII cells isolated from Nrf2−/− mice. The lower lung injury in Nrf2+/+ in comparison with Nrf2−/− mice is also a result of their resistance to CS and of the decreased concentration of CS in vivo. However, in our study, we wanted to determine the extent of ATII cell injury in Nrf2−/− mice, and 60 TSP was the highest nonlethal concentration of cigarette smoke exposure that we could use in these mice. We used the same dose to compare, for the first time, ATII cell injury in both genotypes in vivo. However, C57BL/6 wild-type mice can be exposed to as much as 101 mg/m3 TSP (36).
Furthermore, we found that NAC provides protection against injuries induced by CS in ATII cells and in lung tissue obtained from Nrf2−/− mice, both in vivo and in vitro. This indicates the protective role of NAC as a scavenger of ROS in an Nrf2-independent manner. NAC antioxidant and anti-inflammatory properties were previously described (25). NAC acts as a direct scavenger of free radicals such as OH•, H2O2, and O2−• (37), and therefore can protect ATII cells isolated from Nrf2−/− mice against the ROS generated by cigarette smoke. Furthermore, CS causes sustained lung inflammation (38). NAC also has anti-inflammatory properties because it restores cellular redox status and can modulate the activity of redox-sensitive cell-signaling and transcription pathways, thereby regulating a variety of proinflammatory genes (37). Our results are in agreement with those of Reddy and colleagues (39), who found that supplementation with NAC eliminated the high concentrations of ROS induced by H2O2 in ATII cells obtained from Nrf2−/− mice. Furthermore, NAC partly reduced H2O2–induced cell death in these cells.
We emphasize that NAC provided greater protection against injuries induced by CS in ATII cells and lung tissue obtained from Nrf2+/+ mice than from Nrf2−/− mice, both in vivo and in vitro. NAC, a cysteine-donating reducing compound, acts as a cellular precursor of GSH (40), and genes involved in GSH biosynthesis are regulated by Nrf2 (41). This confirms the requirement of the Nrf2 pathway to restore GSH by NAC and protect against injury by CSE. We found that NAC up-regulated GSH concentrations in Nrf2+/+ mice, but not in Nrf2−/− mice. This is in agreement with previous observations in rat lungs that the availability of cysteine in NAC for GSH provides an important mechanism for elevations in intracellular GSH and Nrf2 (25). The lack of an effect of NAC on GSH concentrations in Nrf2−/− mice may be attributed to the diminished concentrations of GCLC and GCLM enzymes, which are critical for GSH synthesis and the conversion of NAC to GSH (12). Supplementation with NAC reportedly did not restore GSH in ATII cells obtained from Nrf2−/− mice (39). Our findings indicate the activation of the GSH pathway by NAC in an Nrf2-dependent manner. Valenca and colleagues (42) reported that C57BL/6 mice exposed to CS and treated with NAC showed a significantly decreased number of leukocytes and macrophages in BAL, in comparison with CS alone. Cotreatment with CS and NAC for 60 days decreased oxidative stress (as measured by 4-HNE levels) in the lungs of C57BL/6 mice, in comparison with CS alone (42).
We observed that NAC administration abolished the GCLC, GCLM, GR, and HO-1 mRNA up-regulation induced by CS in Nrf2+/+ mice. These findings suggest that ROS were generated as a consequence of the initial depletion of GSH by CS, or as an adaptive response (43) that induced Nrf2 activation and triggered redox-sensitive signaling. NAC was found to decrease the GCLC levels in PC12 cells after treatment with carbon monoxide, which generated ROS (44). Exposure to CS for 1 day also induced GR concentrations in the lungs of Nrf2+/+ mice, but not in Nrf2−/− (ICR) mice (14). NAC was reported to alleviate phosgene-induced pulmonary edema in rats significantly by up-regulating the Nrf2/GR/GSH pathway (25).
The matrix metalloproteinases (MMPs) comprise a diverse group of zinc-containing endopeptidases that are able to degrade most extracellular matrix components (45). We examined MMP-13 expression because it is probably the closest murine MMP in terms of function to human MMP-1 (mice lack MMP-1), which has been shown to increase in COPD. We found that NAC decreased the apoptosis and MMP-13 expression induced by CSE in ATII cells. The increased expression of MMP-13 was shown to be related to the COPD smoker, and elevated levels of this protein were found in ATII cells obtained from patients with COPD (46). Our results also indicate that both pretreatment and posttreatment with NAC provide protection against injuries induced by CSE in ATII cells.
We also observed higher expressions of Nrf2, HO-1, and NQO1 in lung tissue than in ATII cells obtained from Nrf2+/+ mice and exposed to CS in vivo. This observation suggests a greater susceptibility of ATII cells to injury induced by CS. Furthermore, we found that treatment with NAC and CS significantly decreased the expression of HO-1, which is regulated by Nrf2, in ATII cells and in lung tissue obtained from Nrf2+/+ mice in vitro and in vivo, in comparison with CS alone. This confirms our previous observations that NAC can effectively protect ATII cells and lung tissue against injury by CS through both ROS-scavenging properties and, in an Nrf2-dependent manner, by increasing GSH concentrations. However, higher doses of NAC may be required to restore balance fully in ATII cells isolated from Nrf2+/+ mice. The effects of NAC in vivo and in vitro were suggested to be highly dose-dependent (37). We also reported that HO-1 was up-regulated in human alveolar type I–like cells exposed to CSE in vitro, and this expression was attenuated by NAC (17).
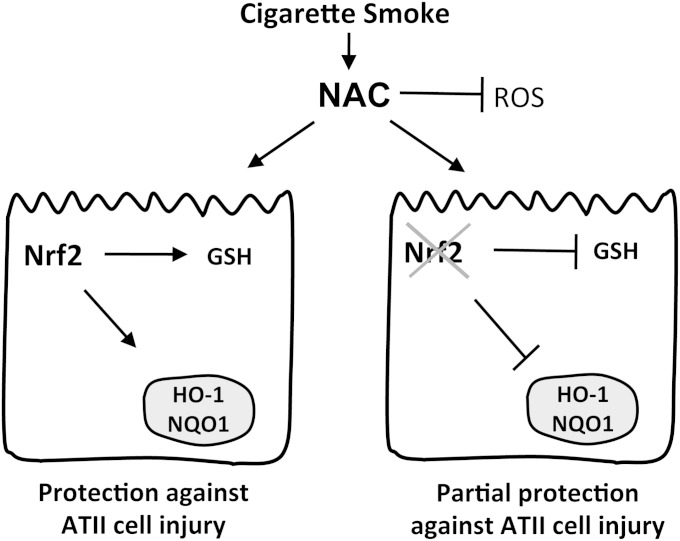
In conclusion, the decrease in the antioxidant defense system regulated by Nrf2 reduces the ability of murine ATII cells to protect against the oxidative stress induced by CS. Our studies demonstrated, for the first time, the protective role of NAC in murine ATII cells isolated from Nrf2−/− mice and exposed to CS both in vivo and in vitro. NAC supplementation can partly rescue the ATII cells obtained from Nrf2−/− mice in an Nrf2-independent manner (Figure 7). Because of the very limited therapy against COPD, NAC may be a promising medication because of its multiple molecular modes of action, once doses have been optimized. Targeting the antioxidant system regulated by Nrf2 may provide an effective strategy against lung injury in COPD.
Figure 7.
Model of ATII cell response in Nrf2+/+ and Nrf2−/− mice to treatment with NAC and exposure to CS. NAC protects ATII cells in an Nrf2-dependent manner by increasing GSH concentrations and the expression of antioxidant genes (e.g., HO-1 and NQO1). NAC also protects cells against injury as a reactive oxygen species (ROS) scavenger in an Nrf2-independent manner. The lack of Nrf2 in ATII cells obtained from Nrf2−/− mice induces a dysregulated antioxidant response and lung injury, which can be partly prevented by the reduction of oxidative stress by NAC.
Acknowledgments
Acknowledgments
The authors thank Erika Ross and Karen E. Edeen for assistance with murine alveolar type II cell isolations, and Joshua Chandler for helpful discussions.
Footnotes
This work was supported by a Young Clinical Scientist Award from the Flight Attendant Medical Research Institute (B.K.). S.R.K. was supported by the Intramural Program of the National Institutes of Health.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0295OC on March 14, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 inflammasome–mediated production of IL-1beta modulates mucosal immunity in the lung against Gram-negative bacterial infection. J Immunol. 2012;188:5623–5635. doi: 10.4049/jimmunol.1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkissoon R, Lommatzsch S, Carolan B, Make B. Chronic obstructive pulmonary disease: a concise review. Med Clin North Am. 2011;95:1125–1141. doi: 10.1016/j.mcna.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Hogg JC, Senior RM. Chronic obstructive pulmonary disease—part 2: pathology and biochemistry of emphysema. Thorax. 2002;57:830–834. doi: 10.1136/thorax.57.9.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest. 2008;118:394–402. doi: 10.1172/JCI31811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, et al. Mitochondrial localization and function of heme oxygenase–1 in cigarette smoke–induced cell death. Am J Respir Cell Mol Biol. 2007;36:409–417. doi: 10.1165/rcmb.2006-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004;125:626–632. doi: 10.1378/chest.125.2.626. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 8.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke–induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutten A, Goven D, Boczkowski J, Bonay M. Oxidative stress targets in pulmonary emphysema: focus on the Nrf2 pathway. Expert Opin Ther Targets. 2010;14:329–346. doi: 10.1517/14728221003629750. [DOI] [PubMed] [Google Scholar]
- 10.Goven D, Boutten A, Lecon-Malas V, Marchal-Somme J, Amara N, Crestani B, Fournier M, Leseche G, Soler P, Boczkowski J, et al. Altered Nrf2/KEAP1–BACH1 equilibrium in pulmonary emphysema. Thorax. 2008;63:916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, et al. Decline in Nrf2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Reddy NM, Kleeberger SR, Bream JH, Fallon PG, Kensler TW, Yamamoto M, Reddy SP. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signaling. Oncogene. 2008;27:5821–5832. doi: 10.1038/onc.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 14.Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med. 2009;46:443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosmider B, Loader JE, Murphy RC, Mason RJ. Apoptosis induced by ozone and oxysterols in human alveolar epithelial cells. Free Radic Biol Med. 2010;48:1513–1524. doi: 10.1016/j.freeradbiomed.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, Mason RJ. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res. 2012;13:13–43. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS ONE. 2011;6:e26059. doi: 10.1371/journal.pone.0026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, et al. Nrf2-deficient mice are highly susceptible to cigarette smoke–induced emphysema. Genes Cells. 2005;10:1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 19.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke–induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai S, Chen P, Zhang C, Chen JB, Wu J. Oral N-acetylcysteine attenuates pulmonary emphysema and alveolar septal cell apoptosis in smoking-induced COPD in rats. Respirology. 2009;14:354–359. doi: 10.1111/j.1440-1843.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 21.Dekhuijzen PN, van Beurden WJ. The role for N-acetylcysteine in the management of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:99–106. doi: 10.2147/copd.2006.1.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadowska AM, Luyten C, Vints AM, Verbraecken J, Van Ranst D, De Backer WA. Systemic antioxidant defences during acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2006;11:741–747. doi: 10.1111/j.1440-1843.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 23.Stav D, Raz M. Effect of N-acetylcysteine on air trapping in COPD: a randomized placebo-controlled study. Chest. 2009;136:381–386. doi: 10.1378/chest.09-0421. [DOI] [PubMed] [Google Scholar]
- 24.Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, van Schayck CP, Olivieri D, Del Donno M, De Backer W, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost–Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 25.Ji L, Liu R, Zhang XD, Chen HL, Bai H, Wang X, Zhao HL, Liang X, Hai CX. N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal Toxicol. 2010;22:535–542. doi: 10.3109/08958370903525183. [DOI] [PubMed] [Google Scholar]
- 26.Nagata K, Iwasaki Y, Yamada T, Yuba T, Kono K, Hosogi S, Ohsugi S, Kuwahara H, Marunaka Y. Overexpression of manganese superoxide dismutase by N-acetylcysteine in hyperoxic lung injury. Respir Med. 2007;101:800–807. doi: 10.1016/j.rmed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro SD. Smoke gets in your cells. Am J Respir Cell Mol Biol. 2004;31:481–482. doi: 10.1165/rcmb.F285. [DOI] [PubMed] [Google Scholar]
- 28.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small MAF heterodimer mediates the induction of Phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 29.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the KEAP1–Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 30.Balansky RM, D'Agostini F, De Flora S. Induction, persistence and modulation of cytogenetic alterations in cells of smoke-exposed mice. Carcinogenesis. 1999;20:1491–1497. doi: 10.1093/carcin/20.8.1491. [DOI] [PubMed] [Google Scholar]
- 31.Messier EM, Mason RJ, Kosmider B. Efficient and rapid isolation and purification of mouse alveolar Type II epithelial cells. Exp Lung Res. 2012;38:363–373. doi: 10.3109/01902148.2012.713077. [DOI] [PubMed] [Google Scholar]
- 32.Le A, Damico R, Damarla M, Boueiz A, Pae HH, Skirball J, Hasan E, Peng X, Chesley A, Crow MT, et al. Alveolar cell apoptosis is dependent on p38 MAP kinase–mediated activation of xanthine oxidoreductase in ventilator-induced lung injury. J Appl Physiol. 2008;105:1282–1290. doi: 10.1152/japplphysiol.90689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginzberg HH, Shannon PT, Suzuki T, Hong O, Vachon E, Moraes T, Abreu MT, Cherepanov V, Wang X, Chow CW, et al. Leukocyte elastase induces epithelial apoptosis: role of mitochondrial permeability changes and Akt. Am J Physiol Gastrointest Liver Physiol. 2004;287:G286–G298. doi: 10.1152/ajpgi.00350.2003. [DOI] [PubMed] [Google Scholar]
- 34.Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. Nrf2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 2011;17:363–371. doi: 10.1016/j.molmed.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Yageta Y, Ishii Y, Morishima Y, Masuko H, Ano S, Yamadori T, Itoh K, Takeuchi K, Yamamoto M, Hizawa N. Role of Nrf2 in host defense against influenza virus in cigarette smoke–exposed mice. J Virol. 2011;85:4679–4690. doi: 10.1128/JVI.02456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer VL, Kassmeier MD, Willcockson J, Akhter MP, Cullen DM, Swanson PC. N-acetylcysteine increases the frequency of bone marrow pro-B/pre-B cells, but does not reverse cigarette smoking–induced loss of this subset. PLoS ONE. 2011;6:e24804. doi: 10.1371/journal.pone.0024804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadowska AM, manuel YKB, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther. 2007;20:9–22. doi: 10.1016/j.pupt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Sundar IK, Chung S, Hwang JW, Lapek JD, Jr, Bulger M, Friedman AE, Yao H, Davie JR, Rahman I. Mitogen- and stress-activated kinase 1 (MSK1) regulates cigarette smoke–induced histone modifications on NF-kappaB–dependent genes. PLoS ONE. 2012;7:e31378. doi: 10.1371/journal.pone.0031378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy NM, Kleeberger SR, Cho HY, Yamamoto M, Kensler TW, Biswal S, Reddy SP. Deficiency in Nrf2–GSH signaling impairs Type II cell growth and enhances sensitivity to oxidants. Am J Respir Cell Mol Biol. 2007;37:3–8. doi: 10.1165/rcmb.2007-0004RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman I. Antioxidant therapeutic advances in COPD. Ther Adv Respir Dis. 2008;2:351–374. doi: 10.1177/1753465808098224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N, et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–3432. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- 42.Valenca SS, Rueff-Barroso CR, Pimenta WA, Melo AC, Nesi RT, Silva MA, Porto LC. l-NAME and l-arginine differentially ameliorate cigarette smoke–induced emphysema in mice. Pulm Pharmacol Ther. 2011;24:587–594. doi: 10.1016/j.pupt.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Gould NS, Min E, Gauthier S, Martin RJ, Day BJ. Lung glutathione adaptive responses to cigarette smoke exposure. Respir Res. 2011;12:133. doi: 10.1186/1465-9921-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li MH, Jang JH, Na HK, Cha YN, Surh YJ. Carbon monoxide produced by heme oxygenase–1 in response to nitrosative stress induces expression of glutamate–cysteine ligase in PC12 cells via activation of phosphatidylinositol 3–kinase and Nrf2 signaling. J Biol Chem. 2007;282:28577–28586. doi: 10.1074/jbc.M701916200. [DOI] [PubMed] [Google Scholar]
- 45.Wright JL, Tai H, Wang R, Wang X, Churg A. Cigarette smoke upregulates pulmonary vascular matrix metalloproteinases via TNF-alpha signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L125–L133. doi: 10.1152/ajplung.00539.2005. [DOI] [PubMed] [Google Scholar]
- 46.Lee EJ, In KH, Kim JH, Lee SY, Shin C, Shim JJ, Kang KH, Yoo SH, Kim CH, Kim HK, et al. Proteomic analysis in lung tissue of smokers and COPD patients. Chest. 2009;135:344–352. doi: 10.1378/chest.08-1583. [DOI] [PubMed] [Google Scholar]