Abstract
Nitrogen dioxide (NO2) is an environmental pollutant and endogenously generated oxidant associated with the development, severity, and exacerbation of asthma. NO2 exposure is capable of allergically sensitizing mice to the innocuous inhaled antigen ovalbumin (OVA), promoting neutrophil and eosinophil recruitment, and a mixed Th2/Th17 response upon antigen challenge that is reminiscent of severe asthma. However, the identity of IL-17A–producing cells and the mechanisms governing their ontogeny in NO2-promoted allergic airway disease remain unstudied. We measured the kinetics of lung inflammation after antigen challenge in NO2-promoted allergic airway disease, including inflammatory cells in bronchoalveolar lavage and antigen-specific IL-17A production from the lung. We determined that IL-17A+ cells were predominately CD4+T cell receptor (TCR)β+ Th17 cells, and that a functional IL-1 receptor was required for Th17, but not Th2, cytokine production after in vitro antigen restimulation of lung cells. The absence of natural killer T cells, γδ T cells, or the inflammasome scaffold nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain (Nlrp)3 did not affect the development of NO2-promoted allergic inflammation or IL-17A production. Similarly, neutrophil depletion or the neutralization of IL-1α during sensitization exerted no effect on these parameters. However, the absence of caspase-1 significantly reduced IL-17A production from lung cells without affecting Th2 cytokines or lung inflammation. Finally, the intranasal administration of IL-1β and the inhalation of antigen promoted allergic sensitization that was reflected by neutrophilic airway inflammation and IL-17A production from CD4+TCRβ+ Th17 cells subsequent to antigen challenge. These data implicate a role for caspase-1 and IL-1β in the IL-1 receptor–dependent Th17 response manifest in NO2-promoted allergic airway disease.
Keywords: asthma, Th17, IL-1R, IL-17, nitrogen dioxide
Clinical Relevance
Exposure to nitrogen dioxide (NO2), an environmental pollutant and endogenously generated reactant, is capable of allergically sensitizing mice to an inhaled antigen, promoting an antigen-induced airway disease reminiscent of severe asthma subsequent to antigen challenge. Generation of the antigen-specific Th17 response requires IL-1 receptor (R) signaling and caspase-1, but not Nlrp3, IL-1α, neutrophils, natural killer T cells, or γδ T cells. Furthermore, inhalational exposure to IL-1β and antigen elicits neutrophilic airway inflammation and IL-17A production from pulmonary TCRβ+CD4+ T cells after a subsequent antigen challenge. These experiments implicate the IL-1R as a pharmacologic target for selectively interfering with the Th17 response in allergic airway disease, an approach that may enable glucocorticoids to attenuate the Th2 response effectively.
Severe, glucocorticoid-resistant asthma comprises 5 to 7% of the population with asthma, but represents 40 to 50% of asthma healthcare costs (1). Whereas the hallmarks of mild/moderate allergic asthma include eosinophils and Th2 cytokine elevation, additional biomarkers of severe asthma include neutrophils and IL-1β–producing and IL-17–producing cells in the airway (1–3). IL-17A+ and IL-17F+ lung cells and CD4+ Th17 blood cells in patients with asthma correlate with disease severity (4, 5). Furthermore, heterogeneity exists within the severe asthmatic population, and not all patients with severe asthma exhibit this phenotype (1). Understanding the functional relevance and the sources of these biomarkers would assist in the better characterization of severe asthma subtypes and the development of more efficacious treatments for severe asthma.
Nitrogen dioxide (NO2) is a toxic byproduct of combustion, an environmental pollutant, and an endogenously generated mediator of inflammation (6, 7). Exposure to NO2 positively correlates with asthma severity, the risk of hospitalization, disease exacerbation, the risk of death, and the development of asthma in previously healthy children (8–10). We have reported that NO2 exposure allergically sensitizes mice to ovalbumin (OVA; we define sensitization as the act or process of inducing an acquired allergy), inducing methacholine airway hyperresponsiveness (AHR), antigen-specific IgG1 and IgE, inflammatory leukocyte recruitment to the airway, and the production of Th2 cytokines and IL-17 during in vitro restimulation of CD4+ T cells (11, 12).
Th17 cells comprise a distinct subset of T cell receptor (TCR)αβ+CD4+ T cells that are characterized by the production of IL-17A, IL-17F, and IL-22 and the transcription factor retinoic acid receptor-related orphan receptor (ROR)γt. IL-17A can also be produced by natural killer (NK) cells, NK T cells, γδ T cells, and granulocytes (13). IL-17A may contribute to the pathogenesis of asthma by stimulating fibroblasts and epithelial cells to produce cytokines, promoting glucocorticoid insensitivity, inducing smooth muscle hypercontractility, and enhancing neutrophil recruitment to the airway (3, 14–17). Mice genetically deficient in the IL-17 receptor (R) fail to develop allergic airway disease (18, 19). Adoptive transfer of in vitro polarized MHC class II-restricted OVA-specific TCR transgenic mice (OTII) Th17 cells, followed by antigen challenge, is sufficient to promote IL-17R–dependent AHR and neutrophil recruitment to the airway (20). Whereas Th17-dependent allergic airway disease is glucocorticoid-resistant, Th2-mediated pulmonary inflammation is glucocorticoid-sensitive (20). Finally, the administration of IL-17A is sufficient to exacerbate pulmonary inflammation in a Th2-mediated alum/OVA model of asthma (19). As such, the Th17 pathway is an attractive target for pharmacologic interventions in severe asthma.
The Type 1 IL-1R is a heterodimeric complex comprised of the IL-1RI (Il1ra) protein and the IL-1R accessory protein (21). The requirement for IL-1R signaling in the generation of Th17 responses has been demonstrated through in vitro and in vivo models (22–25). Endogenous agonists of IL-1R signaling include IL-1α and IL-1β, both of which initiate the recruitment of the IL-1R accessory protein and the downstream adaptor myeloid differentiation factor 88 (MyD88), kinase phosphorylation, the activation of NF-κB, and finally, the increased expression of many proinflammatory genes (21). Whereas the functional outcomes of IL-1R signaling by IL-1α and IL-1β are similar, these cytokines are differentially regulated at the level of both expression and activation. Under basal conditions, IL-1α remains intracellular, but upon cell death, extracellular IL-1α functions as an alarmin, promoting sterile inflammation (26). The release of IL-1α from house dust mite–stimulated airway epithelia promotes Th2 polarization and allergic airway disease (27). In contrast, IL-1β is inducibly synthesized as pro–IL-1β, which requires cleavage by proteases for activation. Although several proteases can cleave pro–IL-1β, the caspase-1 inflammasome is conventionally considered the critical activator of IL-1β (28).
In an alum-independent murine model of allergic asthma, the inflammasome scaffold nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain (Nlrp)3 is required for IL-1β production, and IL-1β and the IL-1R are critical for airway inflammation (29). Clinical data demonstrating elevated concentrations of IL-1β in status asthmaticus and neutrophilic asthma further support the contributions of IL-1R to asthma severity (2, 30, 31). Although data are limited regarding the role of Nlrp3 and caspase-1 in human asthma (32), gene analysis studies have linked nucleotide-binding oligomerization (NOD)-like receptors, including Nlrp3, to asthma and asthma subtypes (33, 34). Given IL-1R’s regulation of Th17 development, these data suggest a role for the Nlrp3–IL-1R axis in Th17-dependent allergic airway disease, and identify IL-1R as a potential pharmacologic target in severe asthma (35).
We previously reported that CD11c+ antigen-presenting cells from the lungs of NO2-exposed mice secrete elevated concentrations of IL-1α and IL-1β during ex vitro stimulation (12). Furthermore, the presence of CD11c+ cells during NO2-promoted allergic sensitization was required for antigen-specific Th2 cytokine and IL-17 production from CD4+ T cells after antigen challenges (12). The objective of the experiments reported here involved identifying IL-17–producing cells in the lungs and investigating the relationship of IL-1, IL-1R, and Th17 during NO2-promoted allergic airway disease. Our results demonstrate the sufficiency of IL-1β and the requirement for caspase-1, but not Nlrp3 or IL-1α, in the generation of IL-1R–dependent Th17 responses in NO2-promoted allergic airway disease.
Materials and Methods
Mice
C57BL/6 and IL-1Rα−/− (Jackson Laboratories, Bar Harbor, ME), CD1d−/−, TCRδ−/−, Nlrp3−/−, and caspase-1−/− mice (C57BL/6 background; bred at the University of Vermont) were housed as described elsewhere (28). Our experiments were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
Allergic Sensitization and Challenge
A schematic summarizing treatments and sensitization schemes is presented in the online supplement (Figure E1). For NO2-promoted allergic sensitization, a single 1-hour exposure to 15 ppm NO2 on Day 1 was followed by 30 minutes of nebulized 1% OVA and Fraction V (Sigma-Aldrich, St. Louis, MO) in saline on Days 1, 2, and 3. Additional details on NO2 dosing can be found in the online supplement’s Materials and Methods. Alum/OVA-sensitized mice were injected intraperitoneally on Days 1 and 7 with 20 μg OVA in Imject Alum (Thermo Scientific, Rockford, IL). IL-1β–sensitized mice were anesthetized using isoflurane and instilled intranasally with 1 μg IL-1β (R&D Systems, Minneapolis, MN) in 50 μl 0.1% BSA and saline on Day 1, and were OVA-nebulized on Days 1, 2, and 3. All mice were OVA-challenged on Days 14, 15, and 16, as described elsewhere (28). Analysis was performed at 48 hours on Day 18, unless otherwise indicated. During analysis, bronchoalveolar lavage (BAL) was processed as described (28).
Antibody Treatments
For IL-1α neutralization, 40 μg of neutralizing anti–IL-1α antibody (ACF-161; BioXCell, West Lebanon, NH) or IgG isotype control antibody (HTK888; BioXCell) were administered intraperitoneally in 200 μl saline on Days 0–4.
Preparation of Tissue for Single-Cell Suspensions
Spleen and mediastinal lymph nodes (MLNs) were processed as described elsewhere (12). For single-cell suspensions, lungs were enzymatically digested and mechanically disrupted, as described in the online supplement.
In Vitro Antigen Restimulation and Cytokine Quantitation
In CD4+ complete medium containing 5% FBS (Cell Generation, Fort Collins, CO), pen/strep, L-glutamine, folic acid, glucose, and 2-mercaptoethanol in RPMI-1640, 1 × 106 cells/ml were activated with 400 μg/ml OVA for 96 hours. Supernatants were analyzed by ELISA (R&D Systems) or a Luminex-based multiplex assay (Millipore, Billerica, MA).
Intracellular Staining and Flow Cytometric Analysis
We stimulated 1 × 106 cells/ml lung cells with 2.5 μg/ml phorbol-myristate-acetate (PMA; Invivogen, San Diego, CA), 0.25 μg/ml ionomycin (Sigma-Aldrich), and 1 μl/ml GolgiPlug (BD Pharmingen, San Jose, CA) for 3–4 hours at 37°C. Live/dead (Invitrogen, Grand Island, NY) staining was followed by fragment crystallizable (Fc) receptor blocking (anti-CD16/CD32; BD Pharmingen) and surface staining with anti–CD4–FITC (BD Pharmingen), anti–TCRγδ–PE, anti–TCRβ–PECy7, and anti–CD8–PerCPCy5.5 (BioLegend, San Diego, CA) in FACS buffer (Dulbecco's PBS with 5% FBS and 0.05% sodium azide; MP Biomedicals, Santa Ana, CA). Cells were fixed overnight in 1% paraformaldehyde at 4°C, permeabilized in 0.2% saponin in FACS buffer, and stained with anti–IL-17A–APC (BioLegend). Analysis was performed using a Becton Dickinson LSR II FACS and BD FACSDIVA (Becton Dickinson, Franklin Lakes, NJ) and FlowJo (Tree Star, Ashland, OR).
Statistical Analysis
Data were analyzed according to a two-tailed unpaired Student t test, one-way ANOVA, or two-way ANOVA, using GraphPad Prism version 4 for Macintosh (GraphPad Software, La Jolla, CA). Statistically significant (P < 0.05) results according to ANOVA were further analyzed by Bonferroni or Newman-Keuls post hoc tests.
Results
Kinetics of Inflammation and IL-17A Production after Antigen Challenge in NO2-Promoted Allergic Airway Disease
Given the correlation of neutrophillic influx and IL-17 in severe asthma, we first sought to characterize the kinetics of leukocyte influx into the BAL fluid and IL-17A production from antigen-restimulated lung, draining lymph node, and spleen cells in NO2-promoted allergic airway disease. After a single antigen challenge on Day 14, we performed analyses at 2, 4, 24, and 48 hours. After challenges with antigen for 3 consecutive days, on Days 14, 15, and 16, we performed analyses at 24, 48, and 72 hours after the final antigen challenge (Figure 1A). We contrasted NO2-sensitized and OVA-challenged mice with noninflamed mice and mice that were sensitized with alum/OVA and OVA-challenged on Days 14, 15, and 16, and analyzed on Day 18. After NO2-promoted allergic sensitization and challenge, neutrophils and eosinophils were significantly elevated, whereas neither naive nor alum/OVA mice demonstrated neutrophils in the BAL (Figure 1B). In the NO2 model, BAL neutrophils peaked at 24 hours, whereas at 48 hours, an eosinophilic response was observed in all sensitized mice that had received three antigen challenges (Figure 1C). Alum/OVA-sensitized as well as NO2-sensitized mice that had been challenged three times exhibited a robust eosinophilic response. Eosinophils in the airway remained elevated at 72 hours after three challenges (not different from 48 hours according to Bonferroni post hoc analysis). Forty-eight hours after a single antigen challenge, eosinophils were also elevated, although this increase did not reach statistical significance. These data indicate that neutrophils infiltrate the lavageable airspace shortly after either a brief, 1-day challenge or an extended 3-day challenge during NO2-promoted allergic sensitization. The airway recruitment of eosinophils occurs later, and increases in magnitude after three antigen challenges.
Figure 1.
Kinetics of airway inflammation and IL-17A production after antigen challenge in nitrogen dioxide (NO2)–promoted allergic airway disease. For antigen sensitization, wild-type (WT) C57BL/6 mice were exposed on Day 1 to 15 ppm NO2, followed by the inhalation of nebulized 1% ovalbumin (OVA) for 30 minutes. The mice were then exposed on Days 1, 2, and 3 to 1% OVA for 30 minutes. Single-challenged mice were antigen-challenged on Day 14 only with a 30-minute exposure to nebulized 1% OVA and analyzed at 2, 4, 24, or 48 hours after antigen challenge. Mice challenged three times were antigen-challenged on Days 14, 15, and 16 with a 30-minute exposure to nebulized 1% OVA, and were analyzed at 24, 48, or 72 hours after the final antigen challenge. Control mice were either noninflamed or allergically sensitized with OVA in alum (Al/O). Al/O mice were antigen-challenged on Days 14, 15, and 16 with a 30-minute exposure to nebulized 1% OVA, and were analyzed at 48 hours after the final antigen challenge. (A) This schematic is presented with a box depicting the analysis time point for most of the experiments performed. Percent neutrophils (PMNs; B) and eosinophils (Eos; C) were enumerated from BAL cytospins. IL-17A production upon 96-hour restimulation in the presence of OVA antigen was measured from lung (D), mediastinal lymph node (MLN; E), and spleen (F) single-cell suspensions by ELISA. *P < 0.05, **P < 0.01, *** P < 0.001, and ****P < 0.0001 by one-way ANOVA and the Newman-Keuls multiple-comparison test (B and C) or by Bonferroni post hoc analysis (D–F). For NO2-sensitized mice, n = 5–10/group. For naive and alum/OVA control mice, n = 3/group. ch, challenge.
IL-17A was robustly produced from antigen-restimulated lung single-cell suspensions after three antigen challenges, with the greatest production by cells enriched from the 48-hour time point (Figure 1D). In mice analyzed at 24 hours and 48 hours after a single antigen challenge, only a small amount of IL-17A was produced from lung cells. No IL-17A was observed in mice that received a single antigen challenge at earlier time points. MLN and spleen IL-17A production increased significantly over time (P = 0.02 and P = 0.035, respectively). Although the data did not reach statistical significance on post hoc analysis, time-dependent trends in IL-17A production from both the MLNs (Figure 1E) and spleen (Figure 1F) were observed. Because mice demonstrated peak IL-17A production at 48 hours after three challenges, we chose this time point for the majority of subsequent analyses.
NO2-Promoted Allergic Sensitization Induces Neutrophil and Eosinophil Recruitment, as Well as a Mixed Th2/Th17 Phenotype, in the Lung after Antigen Challenge
We next sought to further characterize the immune response in the lung at 48 hours after three antigen challenges. Because multiple low doses of OVA can induce allergic sensitization (29, 36), we compared NO2-sensitized and OVA-sensitized and challenged mice with mice that were exposed to OVA but not NO2. We found a decreased percentage of macrophages in the BAL, and an increase of neutrophils and eosinophils, in mice that were NO2-sensitized compared with mice that were exposed to air (Figures 2A–2C). In comparison with control mice, single-cell suspensions of lungs from NO2-sensitized mice produced increased IL-17A and the Th2 cytokines IL-5 and IL-13 (trend only) upon restimulation in the presence of OVA antigen (Figures 2D–2F).
Figure 2.
NO2-promoted allergic sensitization and challenge induce pulmonary inflammation, a mixed Th2/Th17 adaptive immune response, and the production of IL-17A from CD4+T cell receptor (TCR)β+ Th17 cells. Mice were exposed to OVA aerosol (air/O) only, or were subjected to NO2-promoted allergic sensitization (NO2/O), OVA-challenged on Days 14–16, and analyzed 48 hours after the final antigen challenge. Bronchoalveolar lavage (BAL) was analyzed for percent macrophages (Macs; A), neutrophils (PMNs; B), and eosinophils (Eos; C). Cytokine production by lung cells upon 96-hour restimulation in the presence of OVA antigen was measured by ELISA for IL-17A (D), IL-5 (E), and IL-13 (F). Enzymatically digested lungs were counted (G) and stimulated for 3 hours in phorbol myristate acetate (PMA) and ionomycin in the presence of GolgiPlug (BD Pharmingen, San Jose, CA), stained for surface markers, permeabilized, and then stained for intracellular IL-17A. We determined the percent (H) and total (I) IL-17A+ cells of total lung cells. To characterize the IL-17A+ population further, we determined the percentages of CD4+TCRβ+ (J) and CD8+TCRβ+ (K) cells within the IL-17A+ gate. (L) The breakdown of the IL-17A+ gate into CD4+TCRβ+, CD8+TCRβ+, or “other” (non-CD4+TCRβ+/CD8+TCRβ+) is illustrated (n = 4/group). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, according to unpaired Student t test (A–K) or two-way ANOVA (L) and Bonferroni post hoc analysis.
IL-17A+ Cells in the Lung Are CD4+TCRβ+ Th17 Cells
To determine the major cell type in the lung contributing to IL-17A production during NO2-promoted allergic airway disease, we stained stimulated lung cells for intracellular IL-17A. After gating on live cells, our analysis revealed that IL-17A+ cells exhibited side and forward scatters characteristic of lymphocytes (Figures E2A and E2B in the online supplement). Additional analyses revealed that IL-17A+ cells in the lungs of NO2-sensitized and challenged mice were CD11b− or CD11bmed, 90% of which were CD4+TCRβ+ cells in NO2-sensitized and OVA-challenged mice (data not shown).
After NO2-promoted allergic sensitization and antigen challenge, total lung cells increased in comparison to mice exposed to OVA alone (Figure 2G). We found that the fraction (Figure 2H) and total number (Figure 2I) of IL-17A+ lung cells increased after NO2 sensitization and antigen challenge. Gating on IL-17A+ cells (Figure E2D), we found that IL-17A+CD4+TCRβ+ cells (Figure 2J), but not IL-17A+CD8+TCRβ+ cells (Figure 2K) or IL-17A+CD4−TCRβ−/CD8− TCRβ− cells (“other”; Figure 2L), increased after antigen challenge. These data identify CD4+TCRβ+ T cells, and not CD8+TCRβ+ T cells, as the major source of IL-17A in the lung in NO2-promoted allergic airway disease.
In a reciprocal flow cytometric analysis (Figure E3), the fraction of CD4+TCRβ+ cells and CD8+TCRβ+ T cells in the lung after antigen challenge was equivalent between air-exposed and NO2-sensitized mice (Figures E4A and E4B), and the fraction of IL-17A+ cells within each T-cell subset increased after antigen challenge (Figures E4C and E4D). Calculating cells counts based on total lung cells (Figure 2G), we found a significant increase in the number of IL-17A+CD4+TCRβ+ (Figure E4E) cells, but not IL-17A+CD8+TCRβ+ cells (Figure E4F), further supporting CD4+TCRβ+ Th17 cells as the major source of IL-17A in NO2-promoted allergic airway disease.
Whereas flow cytometric analyses illustrate CD4+TCRβ+ Th17 cells as the most abundant cell type contributing to IL-17A in the lung as a consequence of NO2-promoted sensitization and challenge, other T-cell subsets, notably NK T cells and γδ T cells, can contribute to IL-17A production and airway inflammation (13). Therefore, we subjected C57BL/6 wild-type (WT) mice, CD1d−/− mice that lack NK T cells, and TCRδ−/− mice that lack γδ T cells to NO2-promoted allergic sensitization and challenge. CD1d−/− mice demonstrated no difference from C57BL/6 WT mice in BAL cells or IL-17A production by lung cells after antigen-specific in vitro restimulation (Figures 3A–3C). Similarly, γδ knockout animals demonstrated no difference from WT mice in BAL cells or in IL-17A production upon the restimulation of lung cells (Figures 3D–3F). These data indicate that NK T cells and γδ T cells are not critical for allergic sensitization, cellular recruitment into the airway, and IL-17A production by lung cells 48 hours after antigen challenge in NO2-promoted allergic airway disease.
Figure 3.
Natural killer (NK) T cells and γδ cells are not required for cellular recruitment to the lavageable airspaces or for IL-17 production in NO2-promoted allergic airway disease. C57BL/6 and CD1d−/− mice were subjected to NO2-promoted allergic sensitization, challenged, and analyzed 24 hours after the final antigen challenge. BAL cell fractions of macrophages (Macs), neutrophils (PMNs), eosinophils (Eos), and lymphocytes (Lymphs) (A) and differential cell counts from BAL (B) were assessed. (C) Lung-cell production of IL-17A upon 96-hour restimulation in the presence of OVA antigen was measured by ELISA. C57BL/6 and TCRδ−/− mice were subjected to NO2-promoted allergic sensitization and challenge, and analyzed 48 hours after the final antigen challenge. BAL cell fractions (D) and differential cell counts from BAL (E) were assessed. (F) Lung-cell production of IL-17 upon 96 hours of restimulation in the presence of OVA antigen was measured by ELISA. Statistics were performed using either two-way ANOVA (for A, B, D, and E) or an unpaired Student t test (C and F). n = 3/group for CD1d−/− experiments, and n = 5/group for TCRδ−/− experiments. WT, wild-type. ND, not statistically different.
IL-1R Is Required for Th17 Cytokine Production in NO2-Promoted Allergic Airway Disease
To test the hypothesis that the IL-1R is critical for the generation of antigen-specific Th17 cells in NO2-promoted allergic airway disease, we subjected WT and IL-1R−/− mice to NO2-promoted allergic sensitization, challenge, and performed an analysis 48 hours after the antigen challenge. BAL cellularity revealed no differences in macrophage, neutrophil, or eosinophil counts (Figures 4A–4C) in IL-1R−/− mice compared with WT mice. Lung single-cell suspensions from WT and IL-1R−/− mice restimulated in vitro in the presence of antigen produced robust and equivalent concentrations of Th2 cytokines (Figure 4D), whereas lung cells from IL-1R−/− mice produced significantly less of the Th17 cytokines IL-17A, IL-17F, IL-21, IL-22, and granulocyte/macrophage colony-stimulating factor (GM-CSF) (Figure 4E) in comparison with WT mice. These results indicate that the IL-1R is required for the production of Th17 cytokines during NO2-mediated allergic airway disease.
Figure 4.
IL-1R is required for Th17 cytokine production by lung cells in NO2-promoted allergic airway disease. Wild-type (WT) C57BL/6 and IL-1R−/− mice were subjected to NO2-promoted allergic sensitization, challenged, and analyzed 48 hours after the final antigen challenge. Differential counts of BAL macrophages (A), neutrophils (B), and eosinophils (C) were performed. Lung Th2 cytokines were measured by ELISA (D), and lung Th17 cytokines were measured by Milliplex (Millipore, Billerica, MA; E) after the 96-hour in vitro restimulation of single-cell suspensions in the presence of OVA antigen. **P < 0.01 and ****P < 0.0001, according to an unpaired Student t test (n = 5–6/group). GM-CSF, granulocyte/macrophage colony-stimulating factor.
IL-1R Deficiency Principally Affects the TCRβ+CD4+ T-Cell Population of IL-17A+ Lung Cells
Because IL-17A production from both γδ T cells and CD4+ TCRαβ T cells requires IL-1R signaling and can subsequently impact disease pathogenesis (25, 37), we sought to determine the IL-17A–producing cells regulated by IL-1R signaling in our model of NO2-promoted allergic asthma. We stimulated lung single-cell suspensions from allergically inflamed WT and IL-1R−/− mice with PMA and ionomycin, and performed intracellular staining for IL-17A. Gating on live cells (as in Figure E2), we observed a decrease in the percentage of IL-17A+ cells in the lungs of IL-1R−/− mice compared with WT mice (Figure 5A). Further analysis of the IL-17A+ cell population revealed a significant decrease in the fraction of CD4+TCRβ+ cells within the IL-17A+ gate in IL-1R−/− lungs (Figures 5B and 5C). A similar trend was noted in the CD8+TCRβ+ and TCRγδ+ fractions (Figure 5B). Furthermore, the CD8+TCRβ+ and TCRγδ+ subsets comprised only approximately 8–9% and 3–4%, respectively, of the IL-17A+ lymphocyte population, representing a small fraction compared with the IL-17A+CD4+TCRβ+ T cells. In the reciprocal analysis (as in Figure E3), no differences were noted in the percentages of lung CD4+TCRβ+, CD8+TCRβ+, or TCRγδ+ cells from IL-1R−/− versus WT mice (Figures E5A–E5C). However, a significant decrease in IL-17A+ cells within the CD4+TCRβ+ and TCRγδ+ T-cell populations was observed in IL-1R−/− mice (Figures E5D–E5I). Furthermore, despite a lack of statistical difference in the total number of IL-17A+ lung cells between IL-1R−/− and WT mice, a decrease in the total number of IL-17A+CD4+TCRβ+ cells, but not IL-17A+CD8+TCRβ+ cells or IL-17A+TCRγδ+ cells, was observed in IL-1R−/− lungs compared with WT lungs (Figure 5D). These data identify Th17 cells as the major IL-17A–producing cell type regulated by IL-1R signaling in NO2-promoted allergic airway disease.
Figure 5.
IL-1R deficiency selectively inhibits CD4+TCRβ+ T-cell IL-17A production. Wild-type (WT) C57BL/6 and IL-1R−/− mice were subjected to NO2-promoted allergic sensitization, challenged, and analyzed 48 hours after the final antigen challenge. Lung single-cell suspensions were stimulated with PMA and ionomycin before staining for IL-17A and surface markers. Cell populations were gated by cell type. The percentages of IL-17A+ lymphocytes (A) of the total events analyzed (at least 50,000) and of the IL-17A+ subsets (B) were determined. (C) A representative histogram depicts the fluorescence intensity of CD4+ cells within the IL-17A+ gate. (D) The total number of IL-17A+ cells in each identified subset was determined by applying the percentages to total lung cell counts. *P < 0.05 and ****P < 0.0001, according to two-way ANOVA and Bonferroni post hoc analysis. ++P < 0.01, according to unpaired Student t test (n = 5–6/group).
Caspase-1 Contributes to Th17 Polarization
We previously demonstrated that acute NO2 exposure induces the expression of serum amyloid A3 (Saa3) in the lung (28), and promotes the ability of CD11c+ antigen-presenting cells to secrete IL-1α and IL-1β (12). Inhalational exposure of recombinant SAA protein promotes IL-1β production and Th17 polarization that requires the Nlrp3/caspase-1 inflammasome (28). To determine the contribution of the Nlrp3/caspase-1 inflammasome in Th17 response development, we subjected Nlrp3−/− and caspase-1−/− mice to NO2-promoted allergic sensitization and antigen challenge. Whereas we observed no differences in BAL cells (Figure 6A), we observed a decrease in IL-17A production from in vitro restimulated lung cells from caspase-1−/−, but not Nlrp3−/−, mice compared with WT mice (Figure 6B). No significant differences in Th2 cytokine production were observed between Nlrp3−/−, caspase-1−/−, and WT mice (data not shown). We tested the contribution of IL-1α to antigen-specific IL-17A production in NO2-promoted allergic airway disease by neutralizing IL-1α at the time of allergic sensitization. Compared with IgG-treated mice, anti–IL-1α–treated mice exhibited no differences in BAL cells (Figure 6C) or IL-17A production during the in vitro restimulation of lung single-cell suspensions in the presence of antigen (Figure 6D). Because neutrophils are capable of expressing and activating IL-1β (38), we tested whether neutrophils at sensitization contribute to the development of Th17 responses. Our data indicate that neutrophils do not contribute to IL-1R–dependent IL-17A production after an antigen challenge (see Results in the online supplement and Figure E6). Thus, the Nlrp3-independent activation of IL-1β by caspase-1 partly contributes to antigen-specific IL-17A production and the development of Th17 cells in the lung during NO2-promoted allergic airway disease.
Figure 6.
Caspase-1 (Casp1) activity is involved in the generation of IL-17A during NO2-promoted allergic airway disease. For antigen sensitization, WT C57BL/6, nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain (Nlrp)3−/− or caspase-1−/− mice were subjected to NO2-promoted allergic sensitization, challenged, and analyzed 48 hours after the final antigen challenge. BAL cells were assessed (A), and cytokine production from lung single-cell suspensions was measured by ELISA after restimulation in the presence of OVA antigen (B). For the IL-1α neutralization experiment, mice were untreated or received isotype control antibody or anti–IL-1α–neutralizing antibody as a single intraperitoneal injection dispensed daily for 5 days during allergic sensitization, on Days 0–4. All mice were antigen-challenged on Days 14, 15, and 16, and analyzed 48 hours later on Day 18. BAL cells were assessed (C), and the lung-cell production of IL-17A upon 96-hour restimulation in the presence of OVA antigen was measured by ELISA (D). Nlrp3/caspase-1−/− BAL data are compiled from three experiments (n = 10–23/group). Nlrp3/caspase-1−/− lung restimulation data (n = 6/group) represent a single experiment. The IL-1α neutralization data (n = 5/group) represent a single experiment. *P < 0.05, according to one-way ANOVA and Bonferroni post hoc analysis.
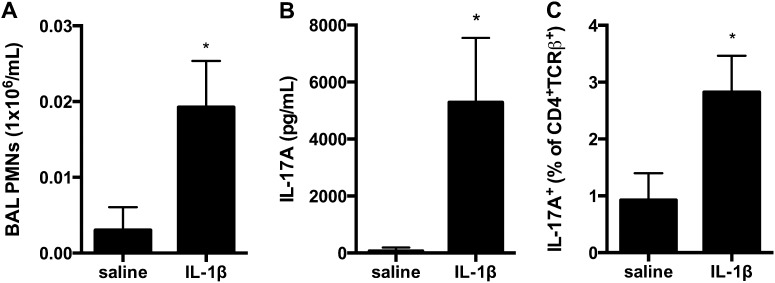
IL-1β and Antigen at Sensitization Is Sufficient to Elicit IL-17A Production from Pulmonary CD4+ T Cells upon Antigen Challenge
Because our findings implicate IL-1β production at the time of sensitization as critical for generating IL-17A production at the time of antigen challenge in NO2-promoted allergic airway disease, we tested whether the administration of IL-1β at the time of initial inhaled antigen exposure (sensitization) would be sufficient to facilitate the antigen-specific production of IL-17A as a consequence of antigen challenge. Mice received 1 μg of IL-1β or 0.1% BSA in saline intranasally on Day 1, followed by OVA nebulization on Days 1, 2, and 3 and again during challenge on Days 14, 15, and 16. Because neutrophil recruitment is a characteristic of Th17 responses in the lung, and neutrophils are recruited early into the airways (Figure 1), our analyses were performed 24 hours after the final antigen challenge. We found that mice sensitized with IL-1β recruited an increased number of neutrophils into the airway after antigen challenge, compared with mice that received vehicle control (Figure 7A). Upon antigen restimulation of lung cells, IL-1β sensitization elicited increased IL-17A production (Figure 7B). IL-17A was not detected in lung cells that were not incubated with OVA antigen during in vitro restimulation (data not shown). Furthermore, our earlier findings implicated IL-1R signaling in the generation of IL-17A production by CD4+ T cells. Therefore, we measured the percentage of IL-17A+ cells within the CD4+TCRβ+ cell population from the lung. We found that antigen sensitization with IL-1β was sufficient to amplify the percentages of IL-17A+CD4+TCRβ+ cells in the lungs of OVA-challenged mice (Figure 7C). Together, these data demonstrate that the administration of IL-1β during initial antigen exposure manifests in a sensitization that is sufficient to generate Th17 responses during subsequent antigen challenge.
Figure 7.
IL-1β at sensitization is sufficient to elicit IL-17A production by CD4+TCRβ+ T cells at antigen challenge. For antigen sensitization, wild-type (WT) C57BL/6 mice received IL-1β or 0.1% BSA in saline (control) by intranasal instillation before OVA nebulization on Day 1. Mice were again exposed to OVA on Days 2 and 3. Mice were OVA-challenged on Days 14, 15, and 16, and analyzed on Day 17. (A) Total BAL neutrophils were enumerated. (B) Lung single-cell suspensions were restimulated in the presence of OVA antigen, and IL-17A was measured by ELISA. Lung single-cell suspensions were stimulated in PMA and ionomycin for 3 hours before staining for intracellular IL-17A. (C) The percentage of IL-17A+ cells within the CD4+TCRβ+ gate is shown. *P < 0.05, according to unpaired Student t test (n = 5/group).
Discussion
Whereas a traditional alum/OVA model for studying allergic asthma generates a robust Th2 response and eosinophil recruitment to the airway, alternative methodologies better model the heterogeneity of clinical asthma (39). Notably, the route of sensitization seems to determine the immune response generated. Inhalational antigen exposure is thought to best replicate the route of sensitization leading to allergic asthma (40). A sensitizing scheme in which LPS and antigen were administered via the airway generated more neutrophils and eosinophils in the airway, IL-17 in the BAL, and CD4+IL-17+ T cells in the lung, whereas the alum/OVA model elicited purely eosinophilic recruitment to the airway and Th2 cytokines in the BAL (19). In the experiments presented here, we used NO2, a toxic byproduct of combustion and an environmental pollutant that is correlated with asthma development (10) and exacerbation (41), to sensitize mice to the innocuous antigen OVA. We previously reported that NO2-promoted allergic airway disease generates a mixed Th2/Th17 response upon the in vitro restimulation of CD4+ splenic T cells stimulated with OVA-exposed antigen-presenting cells (APCs) from antigen-naive mice (12).
This series of studies first investigated the generation of IL-17A responses in NO2-promoted allergic airway disease. After the identification of Th17 cells as the prominent source for IL-17A in the lungs during NO2-promoted allergic airway disease, we investigated the upstream events required for the Th17 response, illustrating the critical role of IL-1R and caspase-1 in IL-17A production. Whereas the Nlrp3 inflammasome–caspase-1–Th17 link was explicitly hypothesized in a recent review (35), this is the first airway-sensitizing model of asthma that specifically examines the requirement for Nlrp3/caspase-1 and the IL-1R in the generation of Th17 responses.
In our initial studies determining the kinetics of IL-17A production from antigen-restimulated lung, MLN, and spleen cells, we found that the IL-17A response from MLN cells and splenocytes was abbreviated in comparison with that of restimulated lung cells. These results support earlier findings (42), and reinforce the notion that the lung is the critical organ from which to measure immune responses subsequent to airway challenge.
Although many cell types can produce IL-17A (43–45), we found that the major producer of IL-17A in the lung during NO2-promoted allergic airway disease was the CD4+TCRβ+ Th17 cell, a finding similar to that in another inhalational asthma model (19). Although both γδ T cells (37) and NK T cells (46) can augment the production of IL-17 by conventional T cells, we found that neither cell type affected the inflammatory cells in the BAL after antigen challenge or IL-17A production by lung cells upon in vitro restimulation with OVA antigen. NK T cells or γδ T cells may participate in eliciting immune responses at time points not measured in this study, but these data reinforce our conclusion that the critical cell type producing IL-17A in the lung as a consequence of NO2 sensitization and antigen challenge is the CD4+TCRβ+ Th17 cell.
One of the requirements in the generation of a Th17 response IL-1R signaling (24, 25). In house dust mite–promoted allergic airway disease, the IL-1R was required for cytokine production by antigen-restimulated MLN cells after antigen challenge, including IL-17A and Th2 cytokines IL-10, IL-5, and IL-13 (27). In contrast, the IL-1R was not required in an alum/OVA model (36). These studies and others (29, 47) implicate the importance of the IL-1R in the generation of both Th2 and Th17 responses in the absence of alum adjuvant. Although we found that eosinophil recruitment and Th2 cytokine production upon the in vitro restimulation of lung single-cell suspensions were independent of IL-1R signaling, IL-1R−/− mice demonstrated a compromised ability to recruit neutrophils into the airways at challenge (trend only) and a selective diminution in the generation of Th17 responses upon in vitro restimulation of lung single-cell suspensions. Our experiments demonstrate the critical role of IL-1R in promoting Th17 responses in an alum-independent model of asthma. In addition, the Th17 response does not affect eosinophil recruitment or Th2 cytokine production during restimulation (18, 48).
IL-1R signaling is implicated in the regulation of IL-17A production from multiple cell types (25, 37). Similar to observations from an LPS-mediated airway-sensitizing scheme, both TCRβ+ and TCRγδ+ T cell populations produced IL-17A in NO2-promoted allergic airway disease (19). However, gating on the IL-17A+ cell population revealed that only the CD4+TCRβ+ Th17 subset, and not the TCRγδ+ T-cell subset, was significantly diminished in lungs from IL-1R−/− mice compared with WT mice after antigen challenge. IL-1R signaling on CD4+ T cells regulates Th17 polarization and maintenance (22), and may promote IL-17 signaling by Th2 cells (42). Further investigation is required to elucidate whether IL-1R controls IL-17A production at the level of naive CD4+ T-cell polarization, mature Th2 cells, or both.
NO2 exposure rapidly leads to NF-κB activation in airway epithelia, and later, the up-regulation of Saa3 and the production of SAA3 from airway epithelia (6, 11, 28). SAA is a candidate downstream mediator of NO2-promoted allergic sensitization (6, 28, 49). Similar to NO2, apoSAA can promote allergic sensitization and induce an IL-1R–dependent Th17 and an IL-1R–independent Th2 adaptive immune response after antigen challenge (42). After exposure to apoSAA, Nlrp3/caspase-1 inflammasome components are required for IL-1β production and pulmonary inflammation (28). In NO2-promoted allergic airway disease, a deficiency of Nlrp3 did not result in decreased IL-17A production upon in vitro restimulation. We observed no change in Th2 cytokines (data not shown), which contradicts the requirement of Nlrp3 for Th2 cytokine production reported by others (29). In line with our finding that airway inflammation is independent of the Nlrp3 inflammasome, a recent study questioned the role of the Nlrp3 inflammasome in multiple in vivo models of asthma (50). Results from our expriments showed that in contrast to Nlrp3-deficient mice, lungs from caspase-1–deficient mice exhibited decreased IL-17A production from lung single-cell suspensions restimulated in the presence of OVA antigen, suggesting that caspase-1–derived IL-1β does contribute to the generation of Th17 responses.
Caspase-1–dependent IL-1β activation only partly explains the generation of Th17 in NO2-promoted allergic airway disease, because the diminution of Th17 responses in caspase-1−/− mice was blunted in comparison with that in IL-1R−/− mice. These data implicate either IL-1α (12, 51) or an alternative source of IL-1β activation, such as neutrophil-derived proteases, in promoting the IL-1R–dependent Th17 response (38). Because a proteolytic activation step is required for the generation of active IL-1β, but not IL-1α, NO2 could regulate IL-1β activity separately from the regulation of IL-1α (21). We found that neither the neutralization of IL-1α nor neutrophil depletion at the time of NO2 exposure and antigen sensitization resulted in changes in IL-17A production at the time of antigen challenge. We may not have achieved sufficient concentrations of neutralizing antibody to neutralize local IL-1α signaling effectively (52), and intratracheal administration may confer a different result in our model (27, 53, 54). In addition, we cannot exclude the involvement of alternative proteases capable of cleaving IL-1β, such as mast cell–derived chymase (38). Nonetheless, we conclude that IL-1β signals through IL-1R to promote Th17 responses in NO2-promoted allergic airway disease. The requirement for caspase-1 in generating a Th17 response introduces the possibility for a role of active IL-18 (55). However, given the critical nature of IL-1R in the generation of Th17 responses, IL-18 seems unlikely to be vital for the generation of Th17 in our model.
We demonstrate that the administration of IL-1β along with antigen is sufficient to generate Th17 responses at the time of antigen challenge. Earlier studies also demonstrated that IL-1β instillation generates IL-17 production (56), and is sufficient to allergically sensitize mice via the airway (27). However, according to Willart and colleagues, IL-1β was sufficient to induce Th2 cytokine production in MLNs upon antigen restimulation, but not IL-17 (27). In comparison, we restimulated lung cells with OVA antigen and found IL-17A production to be elevated from mice sensitized with IL-1β. Furthermore, we did not note significant differences in Th2 cytokine production, although a trend toward elevated IL-13 was present (data not shown). These differences may be attributed to the IL-1β dose or route of administration. In a study of pulmonary fibrosis, the amount of IL-1β we used during sensitization (1 μg) was sufficient for IL-17 production upon the restimulation of MLNs with anti-CD3. Over a protracted time course, this dose caused pathologic changes in the lung characteristic of fibrotic disease, thus presenting a caveat when testing the role of IL-1β in promoting allergic airway disease (56). Nonetheless, our data show that IL-1β is sufficient to generate antigen-specific IL-17A production in the lung, similar to observations in NO2-promoted allergic airway disease.
Our experiments provide insight into the development and upstream requirements for the in vivo generation of an antigen-specific Th17 response in an airway-sensitizing model of asthma. Here, we have determined that the antigen-specific Th17 response in NO2-promoted allergic airway disease requires IL-1R signaling and caspase-1, but is independent of γδ T cells, NK T cells, and Nlrp3. Given that IL-1β and IL-17 are present in some patients with severe asthma, and given the availability of IL-1–blocking pharmaceutical agents, it will be interesting to determine whether Th17 responses in human asthma require similar IL-1–associated components.
Acknowledgments
Acknowledgments
The authors thank Kate Foley for technical assistance during these experiments, and Dr. Steven Lidofsky for his support of the MD/PhD program at the University of Vermont.
Footnotes
This work was supported by grants R01 HL089177, R01 HL107291, P20 RR15557, P20 RR021905, and T32 HL076122 from the National Institutes of Health, by a grant from Hoffman La-Roche, and by a Clinical Innovator Award from the Flight Attendant Medical Research Institute.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0423OC on January 31, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brasier AR, Victor S, Ju H, Busse WW, Curran-Everett D, Bleecker E, Castro M, Chung KF, Gaston B, Israel E, et al. Predicting intermediate phenotypes in asthma using bronchoalveolar lavage–derived cytokines. Clin Transl Sci. 2010;3:147–157. doi: 10.1111/j.1752-8062.2010.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–1036. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151:297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 6.Poynter ME. Airway epithelial regulation of allergic sensitization in asthma. Pulm Pharmacol Ther. 2012;25:438–446. doi: 10.1016/j.pupt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesterberg TW, Bunn WB, McClellan RO, Hamade AK, Long CM, Valberg PA. Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: evidence for NO2 no-effect levels. Crit Rev Toxicol. 2009;39:743–781. doi: 10.3109/10408440903294945. [DOI] [PubMed] [Google Scholar]
- 8.Delamater PL, Finley AO, Banerjee S. An analysis of asthma hospitalizations, air pollution, and weather conditions in Los Angeles County, California. Sci Total Environ. 2012;425:110–118. doi: 10.1016/j.scitotenv.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunyer J, Basagana X, Belmonte J, Anto JM. Effect of nitrogen dioxide and ozone on the risk of dying in patients with severe asthma. Thorax. 2002;57:687–693. doi: 10.1136/thorax.57.8.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takenoue Y, Kaneko T, Miyamae T, Mori M, Yokota S. The influence of outdoor NO(2) exposure on asthma in childhood: a meta-analysis. Pediatr Int. 2012;54:762–769. doi: 10.1111/j.1442-200X.2012.03674.x. [DOI] [PubMed] [Google Scholar]
- 11.Bevelander M, Mayette J, Whittaker LA, Paveglio SA, Jones CC, Robbins J, Hemenway D, Akira S, Uematsu S, Poynter ME. Nitrogen dioxide promotes allergic sensitization to inhaled antigen. J Immunol. 2007;179:3680–3688. doi: 10.4049/jimmunol.179.6.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgkins SR, Ather JL, Paveglio SA, Allard JL, LeClair LA, Suratt BT, Boyson JE, Poynter ME. NO2 inhalation induces maturation of pulmonary CD11c+ cells that promote antigen-specific CD4+ T cell polarization. Respir Res. 2010;11:102. doi: 10.1186/1465-9921-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011;134:8–16. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vazquez-Tello A, Semlali A, Chakir J, Martin JG, Leung DY, Eidelman DH, Hamid Q. Induction of glucocorticoid receptor–beta expression in epithelial cells of asthmatic airways by T-helper Type 17 cytokines. Clin Exp Allergy. 2010;40:1312–1322. doi: 10.1111/j.1365-2222.2010.03544.x. [DOI] [PubMed] [Google Scholar]
- 15.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zijlstra GJ, Ten Hacken NH, Hoffmann RF, van Oosterhout AJ, Heijink IH. Interleukin-17A induces glucocorticoid insensitivity in human bronchial epithelial cells. Eur Respir J. 2012;39:439–445. doi: 10.1183/09031936.00017911. [DOI] [PubMed] [Google Scholar]
- 17.Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1beta–mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1023–L1029. doi: 10.1152/ajplung.00306.2006. [DOI] [PubMed] [Google Scholar]
- 18.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, et al. Th17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state Th17 cells in the intestine. J Exp Med. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)–1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 27.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ather JL, Ckless K, Martin R, Foley KL, Suratt BT, Boyson JE, Fitzgerald KA, Flavell RA, Eisenbarth SC, Poynter ME. Serum amyloid A activates the Nlrp3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187:64–73. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besnard AG, Guillou N, Tschopp J, Erard F, Couillin I, Iwakura Y, Quesniaux V, Ryffel B, Togbe D. Nlrp3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy. 2011;66:1047–1057. doi: 10.1111/j.1398-9995.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- 30.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–967. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 31.Tillie-Leblond I, Pugin J, Marquette CH, Lamblin C, Saulnier F, Brichet A, Wallaert B, Tonnel AB, Gosset P. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999;159:487–494. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 32.Birrell MA, Eltom S. The role of the Nlrp3 inflammasome in the pathogenesis of airway disease. Pharmacol Ther. 2011;130:364–370. doi: 10.1016/j.pharmthera.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Melen E, Kho AT, Sharma S, Gaedigk R, Leeder JS, Mariani TJ, Carey VJ, Weiss ST, Tantisira KG. Expression analysis of asthma candidate genes during human and murine lung development. Respir Res. 2011;12:86. doi: 10.1186/1465-9921-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hitomi Y, Ebisawa M, Tomikawa M, Imai T, Komata T, Hirota T, Harada M, Sakashita M, Suzuki Y, Shimojo N, et al. Associations of functional Nlrp3 polymorphisms with susceptibility to food-induced anaphylaxis and aspirin-induced asthma. J Allergy Clin Immunol. 2009;124:779–785. doi: 10.1016/j.jaci.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 35.Besnard AG, Togbe D, Couillin I, Tan Z, Zheng SG, Erard F, Le Bert M, Quesniaux V, Ryffel B. Inflammasome–IL-1–Th17 response in allergic lung inflammation. J Mol Cell Biol. 2012;4:3–10. doi: 10.1093/jmcb/mjr042. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz N, Kurrer M, Kopf M. The IL-1 receptor 1 is critical for Th2 cell type airway immune responses in a mild but not in a more severe asthma model. Eur J Immunol. 2003;33:991–1000. doi: 10.1002/eji.200323801. [DOI] [PubMed] [Google Scholar]
- 37.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1–independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dykewicz MS. Occupational asthma: current concepts in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2009;123:519–528. doi: 10.1016/j.jaci.2009.01.061. quiz 529–530. [DOI] [PubMed] [Google Scholar]
- 41.Castellsague J, Sunyer J, Saez M, Anto JM. Short-term association between air pollution and emergency room visits for asthma in Barcelona. Thorax. 1995;50:1051–1056. doi: 10.1136/thx.50.10.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu YJ. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17–producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hunig T, Mittrucker HW, Brustle A, Kamradt T, et al. Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 45.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, Feng ZH. IL-17–producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 46.Wingender G, Rogers P, Batzer G, Lee MS, Bai D, Pei B, Khurana A, Kronenberg M, Horner AA. Invariant NKT cells are required for airway inflammation induced by environmental antigens. J Exp Med. 2011;208:1151–1162. doi: 10.1084/jem.20102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakae S, Komiyama Y, Yokoyama H, Nambu A, Umeda M, Iwase M, Homma I, Sudo K, Horai R, Asano M, et al. IL-1 is required for allergen-specific Th2 cell activation and the development of airway hypersensitivity response. Int Immunol. 2003;15:483–490. doi: 10.1093/intimm/dxg054. [DOI] [PubMed] [Google Scholar]
- 48.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, et al. IL-23 and Th17 cells enhance Th2-cell–mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178:1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 49.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor–kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol. 2002;160:1325–1334. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen IC, Jania CM, Wilson JE, Tekeppe EM, Hua X, Brickey WJ, Kwan M, Koller BH, Tilley SL, Ting JP. Analysis of Nlrp3 in the development of allergic airway disease in mice. J Immunol. 2012;188:2884–2893. doi: 10.4049/jimmunol.1102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Switalla S, Knebel J, Ritter D, Krug N, Braun A, Sewald K. Effects of acute in vitro exposure of murine precision-cut lung slices to gaseous nitrogen dioxide and ozone in an air–liquid interface (ALI) culture. Toxicol Lett. 2010;196:117–124. doi: 10.1016/j.toxlet.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface–bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corsini E, Bruccoleri A, Marinovich M, Galli CL. Endogenous interleukin-1 alpha associated with skin irritation induced by tributyltin. Toxicol Appl Pharmacol. 1996;138:268–274. doi: 10.1006/taap.1996.0125. [DOI] [PubMed] [Google Scholar]
- 54.Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Vanden Berghe T, Vandenabeele P, Lambrecht BN, Joos GF, Brusselle GG. Role of IL-1alpha and the Nlrp3/caspase-1/IL-1beta axis in cigarette smoke–induced pulmonary inflammation and COPD. Eur Respir J. 2011;38:1019–1028. doi: 10.1183/09031936.00158110. [DOI] [PubMed] [Google Scholar]
- 55.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1–processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 56.Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L, Charron S, Lagente V, Eberl G, et al. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS ONE. 2011;6:e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]