Abstract
Critically ill patients are routinely exposed to high concentrations of supplemental oxygen for prolonged periods of time, which can be life-saving in the short term, but such exposure also causes severe lung injury and increases mortality. To address this therapeutic dilemma, we studied the mechanisms of the tissue-damaging effects of oxygen in mice. We show that pulmonary invariant natural killer T (iNKT) cells are unexpectedly crucial in the development of acute oxygen-induced lung injury. iNKT cells express high concentrations of the ectonucleotidase CD39, which regulates their state of activation. Both iNKT cell–deficient (Jα18−/−) and CD39-null mice tolerate hyperoxia, compared with wild-type control mice that exhibit severe lung injury. An adoptive transfer of wild-type iNKT cells into Jα18−/− mice results in hyperoxic lung injury, whereas the transfer of CD39-null iNKT cells does not. Pulmonary iNKT cell activation and proliferation are modulated by ATP-dependent purinergic signaling responses. Hyperoxic lung injury can be induced by selective P2X7-receptor blockade in CD39-null mice. Our data indicate that iNKT cells are involved in the pathogenesis of hyperoxic lung injury, and that tissue protection can be mediated through ATP-induced P2X7 receptor signaling, resulting in iNKT cell death. In conclusion, our data suggest that iNKT cells and purinergic signaling should be evaluated as potential novel therapeutic targets to prevent hyperoxic lung injury.
Keywords: lung injury, immunology, ATP, P2X7, oxygen
Clinical Relevance
The use of high concentrations of inspiratory oxygen is widespread in patient care. The toxic effects of hyperoxia are well known to clinicians, but the etiology of hyperoxic lung injury remains poorly understood, and no treatment options are available. Hyperoxia-induced lung injury is shown to be dependent upon invariant natural killer T (iNKT) cell function, and is further mediated by purinergic signaling mechanisms that are regulated by the ectonucleotidase CD39. The targeting of iNKT cells or the specific blockade of CD39 in iNKT cells may produce a therapeutic impact in hyperoxic lung injury.
High concentrations of inspired oxygen are routinely administered to critically ill patients with cardiopulmonary disease and during major surgical interventions to improve oxygen delivery to crucial organs (1). Hyperoxia-induced lung injury is caused by prolonged exposure to high oxygen concentrations (> 50%), and is typically characterized by alveolar epithelial and endothelial damage. These effects result in capillary leak syndrome, followed by inflammatory cell recruitment (2–5).
Recently, hyperoxia and its potential for organ injury have received more attention in the clinical literature (6). Little is currently known about pulmonary immune defense mechanisms to hyperoxia in vivo. In addition, no therapeutic strategies are available to control pathophysiological processes in hyperoxic lung injury.
Invariant natural killer T (iNKT) cells bridge innate and adaptive immune responses. These cells are characterized by their ability to use invariant T-cell receptors to recognize glycolipid antigens presented by CD1d, leading to a rapid cytokine effector response (7, 8). Although the lung itself does not carry an exceptionally large population of iNKT cells, this does appear to be a site where iNKT cells can exert profound effects (9).
CD39, also known as ectonucleoside triphosphate diphosphohydrolase–1, hydrolyzes ATP and ADP to adenosine monophosphate (AMP), catalyzed by CD73, also known as 5′-nucleotidase, to form adenosine (10, 11). CD39 is highly expressed in iNKT cells (12).
CD39 deficiency has mainly been attributed to deleterious outcomes in various models of end-organ injury thought to be caused by unopposed ATP toxicity. CD39 deficiency has been described as a deleterious factor in such models as liver ischemia and reperfusion injury, liver regeneration and transplantation, and graft survival in xenograft heart transplantation (13–17). However, altered levels of CD39 expression in iNKT cells also affect the outcomes of immune responses, and so far, decreased concentrations of CD39 have been shown to be protective in a murine model of concanavalin A (Con-A) hepatitis (12).
In this study, we hypothesized that iNKT cells might induce hyperoxia-induced lung injury, and that these effects may be influenced by CD39 and purinergic signaling.
Materials and Methods
Reagents and Antibodies
Invariant NKT–reactive α-glactoceramide (α-GalCer)–loaded anti-CD1 d tetramer was provided by the National Institutes of Health Tetramer Facility (Atlanta, GA). Several antibodies were purchased from eBioscience (San Diego, CA), including PECy7-conjugated anti-NK1.1, FITC-conjugated anti-CD3, Pacific blue–conjugated anti-CD11b, phycoerythrin (PE)-conjugated anti–GR-1, and FITC-conjugated anti-F4/80. GalCer was purchased from Toronto Research Chemicals, Inc. (Toronto, ON, Canada).
Flow Cytometry
Mononuclear cells were extracted from lung tissue and stained with αGal-Cer–loaded anti-CD1 d tetramer, PECy7-conjugated anti-NK1.1, and FITC-conjugated anti-CD3. Natural killer T (iNKT) cells were defined as either CD1 d tetramer+/NK1.1+ or CD3+/NK1.1+ cells on flow cytometry (12). Polymorphonuclear leukocytes (PML) were extracted and stained with PE-conjugated anti–GR-1 or FITC-conjugated anti-F4/80. PML fractions were characterized as GR-1+/F4/80− cells (18).
Animals and Animal Model
Animals were housed in accordance with guidelines from the American Association for Laboratory Animal Care. Wild-type C57/BL6 mice (Taconic, Germantown, NJ) and CD39-null mice backcrossed six times onto C57/BL6 were studied. Littermate mice were used for control groups. Separate sets of animals were studied for different experimental outcome groups.
Eight- to 10-week-old wild-type and mutant mice were placed in cages in a customized, airtight Plexiglas chamber, and oxygen exposure was performed for 72 hours (19, 20).
Adoptive Transfer of iNKT Cells
Splenic iNKT cells were identified using αGal-Cer–loaded anti-CD1 d tetramer FACS. We injected 105 iNKT cells into the recipient mice (12).
Measurement of Lung Injury
Pulmonary vascular permeability was quantified by an intravenous administration of Evans blue (21). Data are expressed as light adsorption in arbitrary units per gram of tissue weight (22).
Histology and Immunohistochemistry
Lungs were harvested and stained as described elsewhere (10, 23). A lung injury score was established, based on four histological criteria (alveolar edema, interstitial edema, leukocyte [PML] infiltration, and hemorrhage), according to severity (0, not present; 4, severe and present throughout) (24).
Cytokine Measurement by ELISA
Pulmonary iNKT cells were cultured (104 cells per well, in duplicate) and exposed to either room air or 95% O2/5% CO2 for 72 hours. Markers of iNKT cell activation (IFN-γ and IL-17) were measured in cell supernatants, using commercially available ELISA kits (eBioscience).
In Vivo Bromodeoxyuridine Proliferation Assay
After 60 hours of oxygen exposure, animals were injected with 200 μl bromodeoxyuridine (BrdU) intraperitoneally, and the oxygen exposure continued. Pulmonary mononuclear cells were isolated and stained as already described (Abcam, Cambridge, MA).
In Vivo Inhibition of Purinergic Receptors
Oxidized ATP (oATP; Sigma, St. Louis, MO) was used to block P2X7 signaling (25).
Purification of Pulmonary and Splenic Mononuclear Cells
Organs were harvested, and Ficoll gradient isolations of mononuclear cells were performed (26).
iNKT Cell Cultures and Cell Activation
Lungs were harvested, and iNKT cells were extracted with CD1 d tetramer sorting by FACS (26). iNKT cells were cultured (27) and exposed to room air (21% oxygen) or 95% oxygen/5% CO2 for 72 hours.
High-Performance Liquid Chromatography
Blood was collected from the inferior vena cava, and extracellular nucleotides were analyzed by high-performance liquid chromatography (28).
Expression of P2X7 Receptors in iNKT Cells (Reverse-Transcription Polymerase Chain Reaction)
RNA from iNKT cells was reversed-transcribed to complementary DNA, using a Reverse Transcription Kit (Applied Biosystems, Foster City, CA) (23). The P2X7 primer sequence reads as TCACTGGAGGAACTGGAAGT (forward) and TTGCATGGATTGGGGAGCTT (reverse).
Statistical Analyses
Results are expressed as the median range and as the mean ± SEM. For statistical analyses, the Student t test was used. Significance was defined as P < 0.05 (29).
Results
iNKT Cell–Deficient and CD39-Null Mice Are Protected from Hyperoxia-Induced Lung Injury
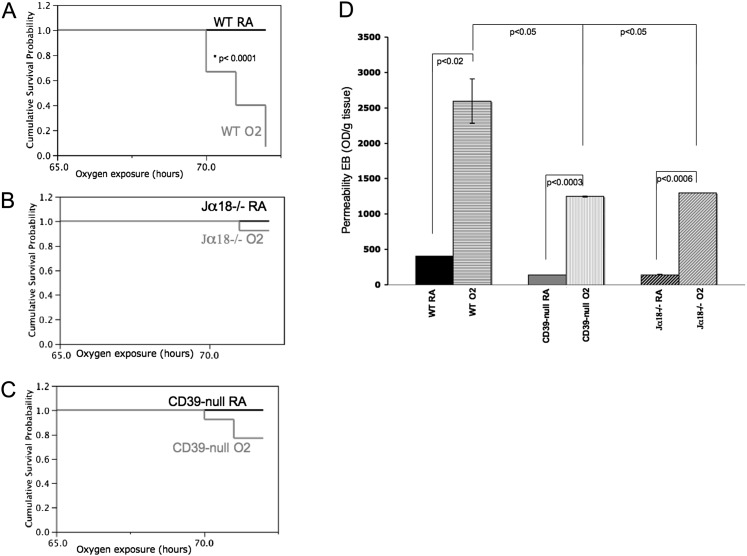
Wild-type animals showed severe systemic signs of illness such as lethargy, hypothermia, and ruffling of the fur after 72 hours of 100% oxygen exposure, and were killed (Figure 1A). Lungs from these wild-type mice with hyperoxia-induced lung injury showed large areas of hemorrhage, pronounced interstitial edema, and complete destruction of their bronchial epithelia (Figure 2D and Figure E3 in the online supplement). In contrast, iNKT cell–deficient mice (Jα18−/−) remained healthy, with excellent survival (Figure 1B) and minimal lung injury after hyperoxia (Figure 2G and Figure E3).
Figure 1.
Parameters of hyperoxia-induced lung injury. Kaplan-Meier survival curves for (A) wild-type (WT) (n = 15), (B) invariant natural killer T (iNKT) cell–deficient (Jα18−/−) (n = 13), and (C) CD39-null (n = 13) animals after 72 hours in 100% oxygen demonstrate a clear survival benefit of Jα18−/− and CD39-null mice, compared with wild-type animals. (D) Vascular permeability was assessed using an Evan’s blue extraction assay. Wild-type animals show significantly higher vascular leakage than do Jα18−/− and CD39-null animals after 100% oxygen for 72 hours (n = 3 per group). Error bars represent the SEM. EB, Evans Blue; OD, optical density; RA, room air.
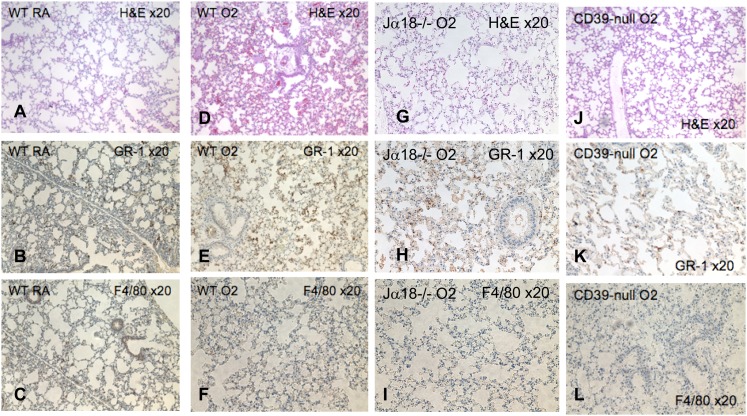
Figure 2.
Morphological features of hyperoxic lung injury. Hematoxylin-and-eosin (H&E) and immunohistochemical staining of lung tissue. (A) Hematoxylin-and-eosin staining of wild-type control lung tissue is compared with hematoxylin-and-eosin staining in (D) wild-type, (G) Jα18−/−, and (J) CD39-null lungs that were exposed to 100% oxygen for 72 hours. The oxygen-exposed wild-type lungs show severe injury with pulmonary edema and hemorrhage, whereas the Jα18−/− and CD39-null lungs express milder injury (light microscopy; magnification, ×20). Immunohistochemical staining using primary rat anti-mouse GR-1 (BD Pharmingen) and F4/80 (Abd Serotec, Raleigh, NC) antibodies, followed by biotin-labeled secondary rabbit anti-rat antibodies, reveals large populations of GR-1+ and F4/80− cells, characteristic of polymorphonuclear leukocytes (PMNs), in the (E and F) wild-type lung, which is less pronounced in the (H and I) Jα18−/− and (K and L) CD39-null lungs (light microscopy; magnification, ×20; n = 4 per group).
In parallel, CD39-null mice were significantly healthier than wild-type animals, showing better survival after 72 hours of 100% oxygen exposure (Figure 1C), with less lethargy, less ruffling of the fur, and significantly milder lung injury (Figure 2J and Figure E3).
Evan’s blue vascular permeability assays clearly show that wild-type animals exhibit significantly increased pulmonary capillary leakage after 100% oxygen exposure, compared with Jα18−/− and CD39-null animals (Figure 1D).
Wild-Type Mice Show Increased Pulmonary iNKT Cell Populations and Increased PMN/Granulocyte Infiltration after Hyperoxia
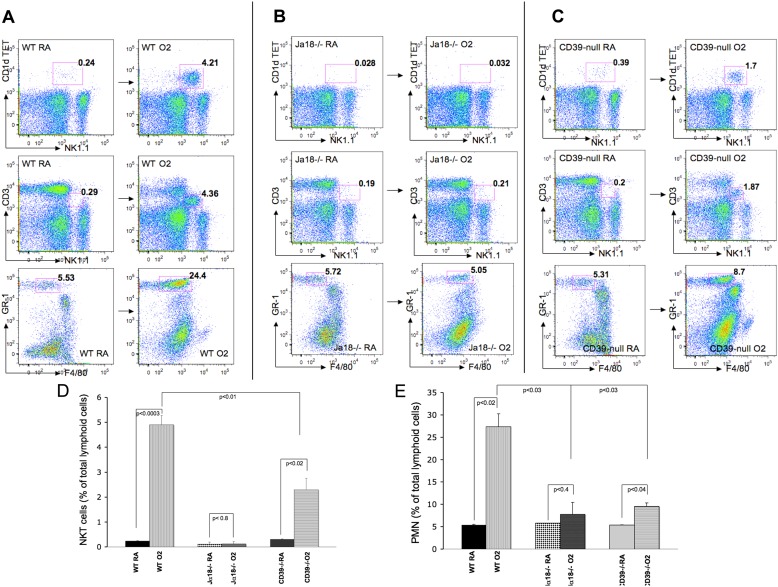
Baseline iNKT cell populations in the lungs did not differ between wild-type and CD39-null mice under normoxic conditions (< 0.5% of all mononuclear cells) (Figures 3A and 3C). NK1.1/αGalCer-loaded CD1 d tetramer double-positive cells as well as CD3/NK1.1 double intermediate positive cells were defined as iNKT cells, as previously described (12). After 72 hours of 100% exposure, wild-type animals show significant increases of iNKT cells, compared with their baseline (0.23% versus 4.7%, respectively, of all pulmonary mononuclear cells) (Figures 3A and 3D). CD39-null mice show only a small increase of pulmonary iNKT cells (0.33% versus 1.9%, respectively, of all pulmonary mononuclear cells) in response to hyperoxia (Figures 3C and 3D).
Figure 3.
Hyperoxia-associated changes of invariant natural killer T-cell (iNKT) populations in the lung. (A–C) Room-air control mice (wild-type, Jα18−/−, and CD39-null) show pulmonary iNKT cell populations at less than 0.5% of all pulmonary mononuclear cells. (A and D) Wild-type iNKT cell numbers increase significantly after 72 hours of 100% oxygen exposure, whereas (C and D) CD39-null INKT cells increase only mildly. (B) Jα18−/− animals do not express iNKT cells and consecutively do not show significant populations. (A and E) Wild-type animals show a significantly increased pulmonary GR-1+/F4/80− PMNs infiltrate after 100% oxygen, compared with (B and E) Jα18−/− and (C and E) CD39-null animals. All error bars represent the SEM (n = 5 per group).
Negligible numbers of INKT cells were identified in the Jα18−/− animals, with or without oxygen exposure (Figures 3B and 3D).
The immunohistochemical staining of hyperoxia-exposed lungs revealed increased numbers of GR-1+/F4/80− cells. GR-1+–positive staining was consistent with increased polymorphonuclear leukocytes (PMNs) in the wild-type lung (Figures 2E and 2F), compared with Jα18−/− lungs (Figures 2H and 2I) and CD39-null lungs (Figures 2K and 2L). The lack of immunostaining for F4/80 in Figures 2C, 2F, 2I, and 2L implies that macrophages are not increased in the lungs of control or oxygen-exposed animals.
Flow cytometry confirmed significantly increased populations of GR-1+/F4/80− PMN cells in 100% oxygen–exposed wild-type lungs (Figures 3A and 3E), compared with the CD39-null (Figures 3C and 3E) and Jα18−/− (Figures 3B and 3E) mice.
CD39 Expression Affects iNKT Cell–Derived and Not T-Cell–Derived Cytokine Secretion Release In Vivo and In Vitro after Hyperoxia
Pulmonary wild-type and CD39-null iNKT cells were cultured and exposed to either room air or 95% O2/5% CO2 for 72 hours. Concentrations of IFN-γ and IL-17 in culture supernatants were significantly higher in the wild-type iNKT cells than in the supernatants of CD39-null iNKT cells (Figures E4A and E4B).
These in vitro data were validated in vitro by flow cytometry analysis and the intracellular staining of isolated pulmonary iNKT cells after oxygen exposure. Wild-type iNKT cells expressed higher concentrations of IFN-γ and IL-17 than did CD39-null cells after 72 hours of exposure to hyperoxia (Figure E4C).
Flow cytometry analysis and the intracellular staining of isolated pulmonary T cells after oxygen exposure revealed that wild-type T cells (Figures E2a–E2c) did not show increased proliferation or IFN-γ or IL-17 release, compared with CD39-null cells (Figures E2d–E2f).
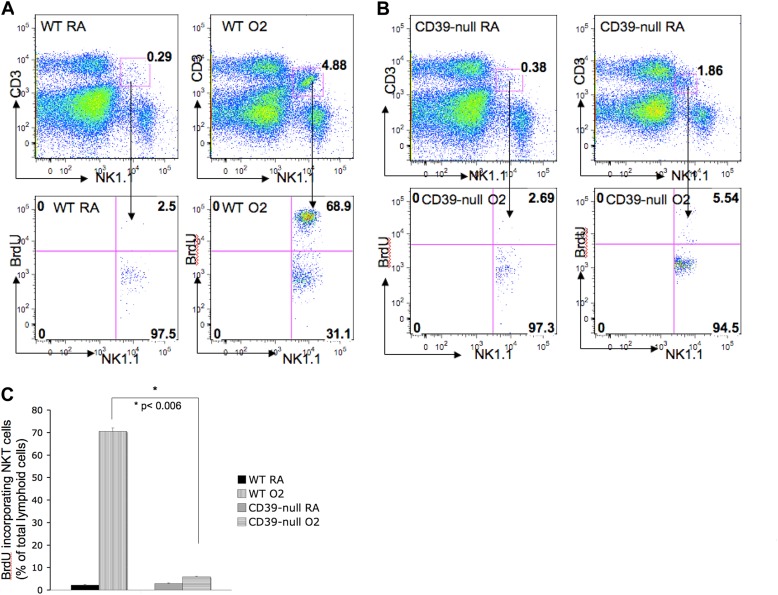
Pulmonary Wild-Type iNKT Cells Proliferate More than CD39-Null iNKT Cells after Hyperoxia
A BrdU proliferation study showed that pulmonary iNKT cells derived from wild-type animals proliferated significantly more after 72 hours of hyperoxia than did iNKT cells derived from CD39-deficient animals (P < 0.006) (Figures 4A–4C). Of the wild-type pulmonary iNKT cells, 69% ± 2.26% (SD) incorporated BrdU as a sign of cell proliferation after oxygen exposure (Figures 4A and 4C). Only 6% ± 0.49% (SD) of the CD39-null pulmonary iNKT cells incorporated BrdU after oxygen exposure (Figures 4B and 4C).
Figure 4.
CD39 deletion prevents iNKT cell proliferation after hyperoxia. (A–C) Bromodeoxyuridine (BrdU) proliferation assay for pulmonary iNKT cells in vivo after oxygen exposure. FACS analysis showed that (A and C) wild-type pulmonary iNKT cells proliferate significantly more than do (B and C) CD39-null pulmonary iNKT cells (P < 0.006) after exposure to hyperoxia. All error bars represent the SEM (n = 2 per group).
Splenic iNKT cells isolated from oxygen-exposed animals did not show a significant difference in cell proliferation, in either wild-type or CD39-null animals, as determined by BrdU incorporation (Figure E1).
Adoptive Transfers Reveal that Wild-Type iNKT Cells Result in More Lung Injury and Proliferate More than Transplanted CD39−/−iNKT Cells under Hyperoxic Conditions
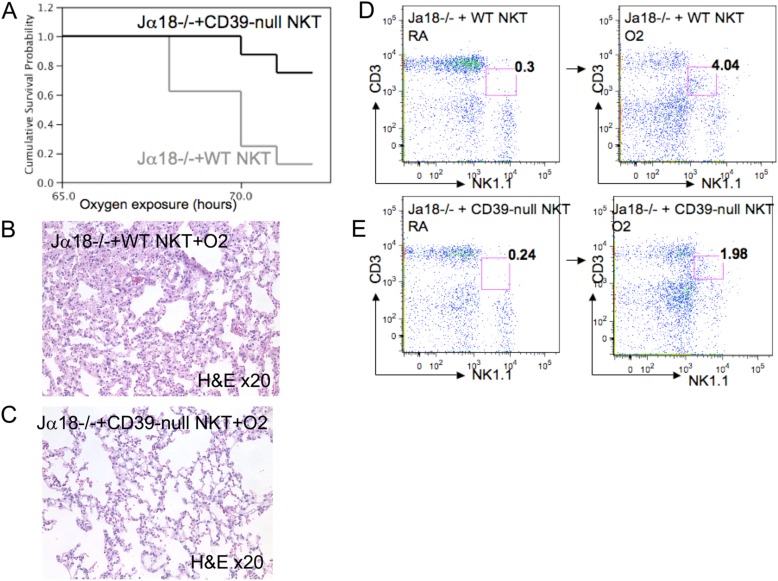
Wild-type and CD39-null cells were CD1d tetramer–purified, FACS-sorted, and injected into Jα18−/− mice. After 72 hours of oxygen exposure, the Jα18−/− mice adoptively transferred with wild-type iNKT cells exhibited significantly decreased survival (Figure 5A), more severe lung injury (Figure 5B), and a nearly 20-fold increase in their pulmonary iNKT cell populations, compared with the room-air control mice (4.3% versus 0.23%, respectively) (Figure 5D).
Figure 5.
Adoptive transfer of iNKT cells and hyperoxic lung injury. (A) Kaplan-Meier survival curves show a significant survival advantage after the transfer of CD39-null iNKT cells into Jα18−/− animals, compared with the transfer of wild-type INKT cells into Jα18−/− animals (P = 0.006; n = 5 per group). Hematoxylin-and-eosin staining (light microscopy; magnification, ×20) reveals severe lung injury in the (B) wild-type iNKT cells transferred into Jα18−/− animals, whereas lung injury in the (C) CD39 iNKT cells transferred into Jα18−/− animals is only mild. (D) Flow cytometry (FACS) analysis showed a strong increase in the pulmonary iNKT cell populations in the wild-type iNKT cells transferred into Jα18−/− animals (> 4% of total pulmonary mononuclear cells), compared with (E) CD39-null iNKT cells transferred into Jα18−/− animals (1.98% of total mononuclear cells). All error bars represent the SEM (n = 5 per group).
In contrast, Jα18−/− mice with CD39-null iNKT cells showed significantly improved survival (Figure 5A) and mild lung injury (Figure 5C) with an eightfold increase in their iNKT cell populations, compared with the room-air control mice (1.98% versus 0.23%, respectively) (Figure 5E).
CD39 Deletion in iNKT Cells Is Protective to Hyperoxia-Induced Lung Injury by Enhancing iNKT Cell Apoptosis
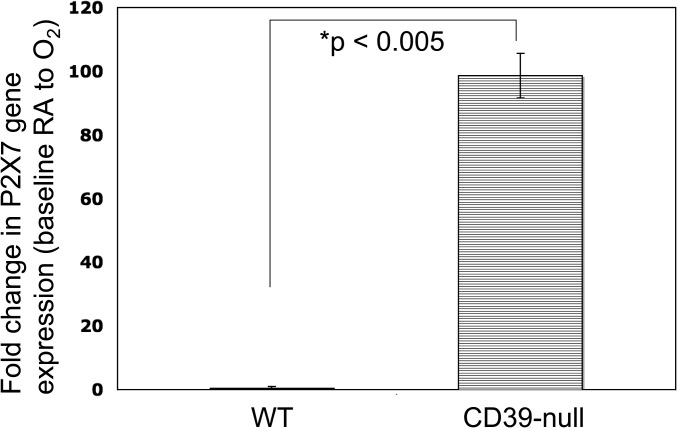
RT-PCR analysis of isolated and oxygen-exposed iNKT cells revealed a substantial up-regulation of the P2X7 receptor in CD39-null cells (Figure 6), compared with wild-type cells.
Figure 6.
Altered patterns of iNKT cell expression of P2X7 receptors after hyperoxia. Real-time PCR analyses of cultured iNKT cells were done after 72 hours in room air (RA) or 95% O2/5% CO2 (elevated O2) exposure. Expression patterns of P2X7 at the transcriptional level are presented as “fold change” from room air to that seen in elevated oxygen levels (RA/O2) in both wild-type and CD39-null iNKT cells. Wild-type iNKT cells exhibit minimal changes, whereas CD39-null iNKT cells show dramatic increases in P2X7 receptor levels after hyperoxia.
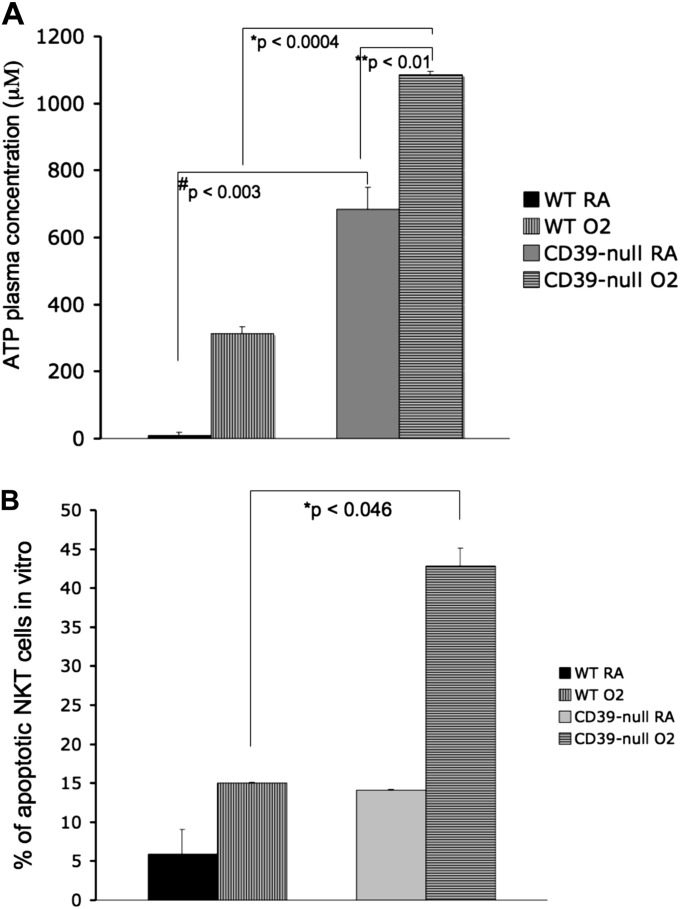
We measured systemic ATP plasma concentrations in CD39-null and wild-type mice under room air and after 72 hours of hyperoxia. As expected, CD39-null mice had higher plasma ATP concentrations in room air than did wild-type mice (Figure 7A). After oxygen exposure, CD39-null mice significantly increased systemic ATP concentrations compared with wild-type mice (Figure 7A).
Figure 7.
Increased plasma ATP concentrations and iNKT apoptosis in CD39-null mice. (A) High-performance liquid chromatography analysis of plasma shows that ATP plasma concentrations were significantly increased in the CD39-null animals after oxygen exposure, compared with wild-type animals (P < 0.0004). All error bars represent the SEM (n = 3 per group). (B) In vitro apoptosis assay. iNKT cells from wild-type and CD39-null animals were cultured (104 cells per well) in room air (RA) or 95% O2/5% CO2 for 72 hours. Cells were then stained with PECy7-conjugated anti-NK1.1, FITC-conjugated anti-CD3, and APC-conjugated anti–annexin V and propidium iodide (PI). Apoptotic cells were defined as annexin V+/PI− and annexin V+/PI+. CD39-null iNKT cells showed significantly higher levels of iNKT cell apoptosis under oxygen than did the wild-type iNKT cells (P < 0.046). All error bars represent the SEM (n = 2 per group).
In vitro analysis of cultured wild-type iNKT cells and CD39-null iNKT cells revealed a significantly higher apoptosis rate in the oxygen-treated CD39-null cells (Figure 7B).
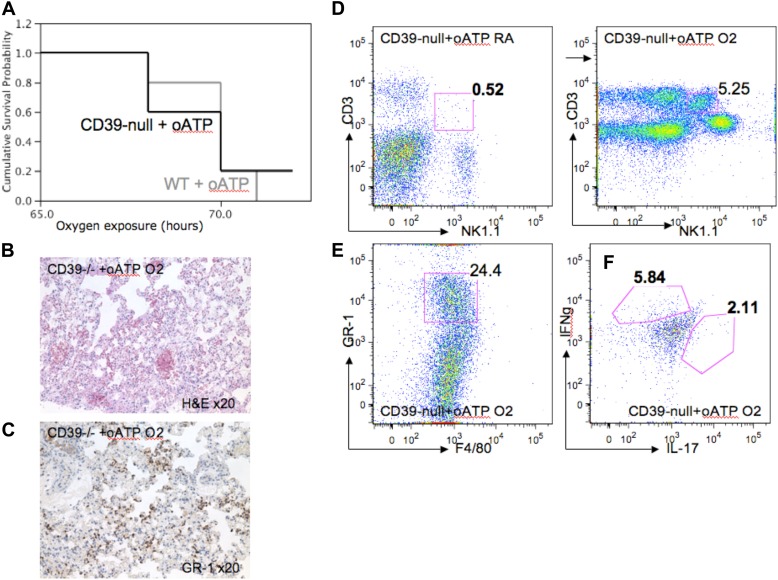
To test the impact of P2X7 receptor signaling in our model, we injected wild-type and CD39-null mice with oxidized ATP (oATP), a specific P2X7-receptor antagonist, and exposed the mice to hyperoxia. CD39-null mice treated with oATP showed significantly decreased survival rates (Figure 8A), compared with the untreated CD39-null mice (Figure 1C). The oATP-treated CD39-null mice also expressed serious signs of systemic illness, with severe lung injury (Figure 8B).
Figure 8.
P2X7 receptor antagonism induces hyperoxic lung injury in CD39-null mice. The P2X7 antagonist oxidized ATP (oATP) was administered to wild-type and CD39-null animals. Mice were then exposed to either room air or 100% oxygen for 72 hours (n = 3 per group). (A) Kaplan-Meier survival curves show that survival in CD39-null animals treated with oATP decreases dramatically, and approaches survival rates of the wild-type mice after oATP treatment. (B) Hematoxylin-and-eosin staining of oxygen-exposed lungs reveals severe lung injury in the oATP-treated CD39-null mice, with prominent (C) GR-1+ PMN lung infiltrates. Pulmonary mononuclear cells were extracted and stained with PECy7-conjugated anti-NK1.1, FITC-conjugated anti-CD3, PE-conjugated anti–GR-1, and FITC-conjugated anti-F4/80. (D) Flow cytometry analysis (FACS) confirmed significant increases in the pulmonary iNKT cell population in oATP-treated animals (5.2% of total pulmonary mononuclear cells), compared with the untreated CD39-null mice (Figure 3C) (1.75% of total mononuclear cells). All error bars represent the SEM (n = 3 per group). (E) Pulmonary polymorphonuclear cell (PMN) infiltrates were identified in the oATP-treated CD39-null animals. (F) Intracellular staining using flow cytometry (FACS) from pulmonary-derived iNKT cells. Mononuclear cells were extracted from oxygen-exposed lungs and stained with PECy7-conjugated anti-NK1.1, FITC-conjugated anti-CD3, PE-conjugated anti–IL-17, and Pacific blue–conjugated anti–IFN-γ. NK1.1+/CD3+ cells were assessed for IL-17 and IFN-γ expression. (F) oATP-treated CD39-null mice produced higher concentrations of IL-17 and IFN-γ, compared with (Figure 4D) untreated CD39-null cells after 100% oxygen exposure.
Flow cytometry analysis and immunohistochemistry staining revealed a significantly increased pulmonary number of neutrophil granulocytes (PMNs; GR-1+ and F4/80−) in the oATP-treated CD39-null animals (Figures 8C and 8E), compared with untreated CD39-null mice (Figures 3C and 3E).
Interestingly, oATP-treated CD39-null animals demonstrated significantly increased pulmonary iNKT cell numbers (Figure 8D) and an augmented release of IFN-γ and IL-17 (Figure 8F), compared with untreated CD39-null mice (Figures 3C and 3D).
Discussion
In this study, we demonstrate that iNKT cells are crucial for the development of hyperoxia-induced lung injury. We show that hyperoxia provokes severe lung injury in wild-type mice, whereas iNKT cell–deficient (Jα18−/−) and CD39-null mice are largely protected from hyperoxic injury. Wild-type mice exhibit lethal inflammatory responses to hyperoxia, with high numbers of infiltrating pulmonary iNKT cells and polymorphonuclear leukocytes. CD39-null mice express milder inflammatory responses, with lower pulmonary iNKT and polymorphonuclear leukocyte cell numbers.
Importantly, lung injury in iNKT cell–deficient (Jα18−/−) mice can be caused by transferring wild-type iNKT cells into iNKT cell–deficient mice. However, transplanting CD39−/− INKT cells into Jα18−/− mice does not result in increased lung injury after hyperoxia. Pulmonary injury with increases in inflammatory INKT cells and polymorphonuclear leukocytes can also be induced by the administration of a P2X7 antagonist (oATP) to CD39-null mice.
The mechanism by which CD39 deletion in mice elicits protection during hyperoxia appears to be linked to low levels of pulmonary iNKT cell proliferation and increased INKT cell apoptosis in response to hyperoxia. This response is mediated by increased concentrations of systemic ATP, which triggers increased levels of P2X7-receptor signaling in iNKT cells, leading to their depletion.
The deleterious effects of iNKT cells were recently described in different disease models, such as airway hyperreactivity/bronchial asthma (9, 26) and acute concanavalin A–induced hepatitis (12). The impact of iNKT cells in the lung has been studied in depth by Akbari and colleagues, who proposed the crucial coregulatory role of iNKT cells in Th2-mediated asthma (9, 26). Although controversy persists in regard to whether iNKT cells comprise the major T cells responsible for the pathogenesis of asthma, iNKT cells clearly play an important regulatory role in lung pathology, as discussed by Thomas and colleagues (30), Bratke and colleagues (31), and Pham-Thi and colleagues (32). In our model of hyperoxic lung injury, we further confirm the important immunoregulatory role of iNKT cells. We also infer that iNKT cells mediate hyperoxic lung injury without substantial T-cell involvement. Although iNKT cells constitute less than 1% of all lymphoid cells in the lung, they definitely play an important immunoregulatory role. The ability of iNKT cells to proliferate rapidly and to produce large amounts of cytokines such as IFN-γ and IL-17 appears to be key in the development of acute lung injury in this murine hyperoxia model.
We also showed that wild-type iNKT cells, and not T cells, produce large quantities of IFN-γ and IL-17 after 72 hours of hyperoxia. Both IFN-γ and IL-17 are potent chemoattractants for polymorphonuclear leukocytes, and were found in large quantities in our hyperoxia-exposed wild-type lungs (8, 33, 34). iNKT cells seem to be directly stimulated and activated by hyperoxia via unclear mechanisms, whereas T cells do not show signs of activation after hyperoxia. Whether the direct endothelial and epithelial damage induced by hyperoxia plays an additional role in iNKT cell activation and lymphocyte migration needs to be determined more conclusively.
The underlying mechanisms linking inflammatory responses and hyperoxic lung injury remain unclear. Previous studies demonstrated a link between lung injury and purinergic signaling mechanisms influencing immune responses. The role of extracellular adenosine in acute lung injury has gained some attention, with studies showing protective properties in a model of LPS-induced pulmonary inflammation (35) as well as ventilator-induced lung injury (36). We and others have proposed a protective pathway that mediates tissue protection during hypoxia through extracellular adenosine and the A2A-dependent inhibition of the immune response (37–40). More specifically, peripheral T-cell activation, proliferation, and cytokine production can be inhibited via A2A receptor activation in a cyclic AMP–dependent manner.
Chen and colleagues (28) recently linked extracellular ATP concentrations to polymorphonuclear leukocyte migration. ATP at lower concentrations (< 100 μM) promoted PMN migration, whereas ATP concentrations at more than 100 μM inhibited PMN migration. In addition to P2X7 receptor–mediated iNKT cell apoptosis, this inhibition of PMN migration could be partly responsible for the lower PMN cell numbers and the milder lung injury evident in our CD39-null animals, which show high plasma concentrations of ATP (> 1,000 μM) after hyperoxia.
Kawamura and colleagues (41) and Aswad and Dennert (42) recently showed that the high expression of P2X7 receptors in T cells after stimulation by high concentrations of ATP leads to the formation of pores permeable to small molecules, ultimately leading to cell death. The results of those studies are in keeping with our findings of P2X7 receptor–mediated iNKT cell death. This effect was associated with P2X7 receptor up-regulation and increased plasma ATP concentrations in CD39-null mice after hyperoxia.
A growing body of literature has emerged about purinergic signaling and iNKT cell–mediated liver injury. Lappas and colleagues showed that A2A-dependent iNKT cell inhibition results in improved liver ischemia–reperfusion injury (8). We noted in a model of Con-A hepatitis that iNKT cells in CD39-null mice became apoptotic, and that the consequent lack of iNKT cells increased tissue protection (12). The purinergic receptor that induced iNKT cell apoptosis in CD39-null animals was also found to be P2X7. Our data suggest a similar pathway leading to decreased pulmonary iNKT cell proliferation in CD39-null animals, as mediated through ATP-dependent P2X7-receptor signaling.
The etiology of hyperoxia-induced lung injury seems to be multifactorial and complex, involving at least two different immune cells and two different purinergic receptors as crucial players. Kolliputi and colleagues recently linked hyperoxic lung injury to immunoregulation by macrophages, as mediated by purinergic signaling mechanisms (43). Again, P2X7 receptor stimulation by ATP resulted in a putative activation of the inflammasome complex in alveolar macrophages, ultimately inducing lung injury.
Hyperoxic lung injury is a well-described and clinically important syndrome that affects morbidity and mortality in critically ill patients. We have identified new pathways linking INKT cell functions and ATP signaling via P2X7 receptors in mediating hyperoxia-induced lung injury. Blocking iNKT cell activation or the transient depletion of these cells may become a therapeutic option that limits hyperoxic lung injury in critically ill patients.
Footnotes
Author Contributions: M.N.-M. and S.C.R. designed the research. M.N.-M., M.S., D.H., Y.W., E.C., and G.D. performed the research. M.N.-M., S.C.R., W.J., M.E., L.O., and M.S. analyzed the data. M.N.-M. and S.C.R. wrote the manuscript.
M.N.-M. was supported by National Institutes of Health grant T32 GM007592-32.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0180OC on January 24, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, et al. Hyperoxia causes angiopoietin 2–mediated acute lung injury and necrotic cell death. Nat Med. 2006;12:1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baleeiro CE, Wilcoxen SE, Morris SB, Standiford TJ, Paine R., III Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol. 2003;171:955–963. doi: 10.4049/jimmunol.171.2.955. [DOI] [PubMed] [Google Scholar]
- 3.Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980;122:123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- 4.Davis WB, Rennard SI, Bitterman PB, Crystal RG. Pulmonary oxygen toxicity: early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med. 1983;309:878–883. doi: 10.1056/NEJM198310133091502. [DOI] [PubMed] [Google Scholar]
- 5.Kapanci Y, Weibel ER, Kaplan HP, Robinson FR. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys: II. Ultrastructural and morphometric studies. Lab Invest. 1969;20:101–118. [PubMed] [Google Scholar]
- 6.Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS. Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomized clinical trial. Anesth Analg. 2012;115:849–854. doi: 10.1213/ANE.0b013e3182652a51. [DOI] [PubMed] [Google Scholar]
- 7.Nowak M, Lynch L, Yue S, Ohta A, Sitkovsky M, Balk SP, Exley MA. The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur J Immunol. 2010;40:682–687. doi: 10.1002/eji.200939897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1 d–dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell–receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 10.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, II, Imai M, Edelberg JM, Rayburn H, Lech M, et al. Targeted disruption of CD39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 11.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, Haschemi A, Yegutkin GG, Candinas D, Exley M, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A–induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai M, Takigami K, Guckelberger O, Lin Y, Sevigny J, Kaczmarek E, Goepfert C, Enjyoji K, Bach FH, Rosenberg RD, et al. CD39/vascular ATP diphosphohydrolase modulates xenograft survival. Transplant Proc. 2000;32:969. doi: 10.1016/s0041-1345(00)01065-4. [DOI] [PubMed] [Google Scholar]
- 14.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E, Candinas D, Erb L, Robson SC. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135:1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Imai M, Nowak-Machen M, Guckelberger O, Enjyoji K, Wu Y, Khalpey Z, Berberat P, Munasinghe J, Robson SC. Liver damage and systemic inflammatory responses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia. Purinergic Signal. 2011;7:427–434. doi: 10.1007/s11302-011-9239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 19.Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest. 1998;101:1970–1982. doi: 10.1172/JCI1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B, Chen Q, Zhu Z, Zhong M, Nakayama K, et al. Bcl-2–related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest. 2005;115:1039–1048. doi: 10.1172/JCI23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barone GW, Farley PC, Conerly JM, Flanagan TL, Kron IL. Morphological and functional techniques for assessing endothelial integrity: the use of Evans blue dye, silver stains, and endothelial derived relaxing factor. J Card Surg. 1989;4:140–148. doi: 10.1111/j.1540-8191.1989.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 22.Guckelberger O, Sun XF, Sevigny J, Imai M, Kaczmarek E, Enjyoji K, Kruskal JB, Robson SC. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase–1 in murine intestinal ischemia–reperfusion injury. Thromb Haemost. 2004;91:576–586. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- 23.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, Rellstab A, Nowak M, Enjyoji K, Li X, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinclair SE, Kregenow DA, Lamm WJ, Starr IR, Chi EY, Hlastala MP. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2002;166:403–408. doi: 10.1164/rccm.200112-117OC. [DOI] [PubMed] [Google Scholar]
- 25.Moayeri M, Wickliffe KE, Wiggins JF, Leppla SH. Oxidized ATP protection against anthrax lethal toxin. Infect Immun. 2006;74:3707–3714. doi: 10.1128/IAI.00051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 27.Molling JW, Moreno M, van der Vliet HJ, von Blomberg BM, van den Eertwegh AJ, Scheper RJ, Bontkes HJ. Generation and sustained expansion of mouse spleen invariant NKT cell lines with preserved cytokine releasing capacity. J Immunol Methods. 2007;322:70–81. doi: 10.1016/j.jim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 29.Schmelzle M, Eisenberger CF, Am Esch JS, Jr, Matthaei H, Krausch M, Knoefel WT. Non-colorectal, non-neuroendocrine, and non-sarcoma metastases of the liver: resection as a promising tool in the palliative management. Langenbecks Arch Surg. 2010;395:227–234. doi: 10.1007/s00423-009-0580-y. [DOI] [PubMed] [Google Scholar]
- 30.Thomas SY, Chyung YH, Luster AD. Natural killer T cells are not the predominant T cell in asthma and likely modulate, not cause, asthma. J Allergy Clin Immunol. 2010;125:980–984. doi: 10.1016/j.jaci.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bratke K, Julius P, Virchow JC. Invariant natural killer T cells in obstructive pulmonary diseases. N Engl J Med. 2007;357:194. author reply 194–195. [PubMed] [Google Scholar]
- 32.Pham-Thi N, de Blic J, Leite-de-Moraes MC. Invariant natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:2613–2616. author reply 2613–2616. [PubMed] [Google Scholar]
- 33.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammaT and rapidly produce IL-17 upon receptor ligation in an IL-6–independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakamaki F, Ishizaka A, Urano T, Sayama K, Nakamura H, Terashima T, Waki Y, Soejima K, Tasaka S, Sawafuji M, et al. Attenuation by intravenous 2-chloroadenosine of acute lung injury induced by live Escherichia coli or latex particles added to endotoxin in the neutropenic state. J Lab Clin Med. 2003;142:128–135. doi: 10.1016/S0022-2143(03)00105-7. [DOI] [PubMed] [Google Scholar]
- 36.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 37.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohta A, Sitkovsky M. Role of G-protein–coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 39.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 40.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawamura H, Aswad F, Minagawa M, Malone K, Kaslow H, Koch-Nolte F, Schott WH, Leiter EH, Dennert G. P2X7 receptor–dependent and –independent T cell death is induced by nicotinamide adenine dinucleotide. J Immunol. 2005;174:1971–1979. doi: 10.4049/jimmunol.174.4.1971. [DOI] [PubMed] [Google Scholar]
- 42.Aswad F, Dennert G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell Immunol. 2006;243:58–65. doi: 10.1016/j.cellimm.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolliputi N, Shaik RS, Waxman AB. The inflammasome mediates hyperoxia-induced alveolar cell permeability. J Immunol. 2010;184:5819–5826. doi: 10.4049/jimmunol.0902766. [DOI] [PMC free article] [PubMed] [Google Scholar]