Abstract
In contrast to the wealth of information concerning membrane phospholipid asymmetry in normal human erythrocytes, very little is known about membrane phospholipid organization in pathologic erythrocytes. Since the spectrin-actin lattice, which has been suggested to play an important role in stabilizing membrane phospholipid asymmetry, is abnormal in sickled erythrocytes, we determined the effects of sickling on membrane phospholipid organization. We used two enzymatic probes: been venom phospholipase A2 and Staphylococcus aureus sphingomyelinase C, which do not penetrate the membrane and react only with phospholipids located in the outer leaflet of the bilayer. Our results suggest that the distribution of glycerophospholipids within the membrane of sickled cells is different from that in nonsickled cells. Compared with the normal erythrocyte, the outer membrane leaflet of the deoxygenated, reversibly sickled cells (RSC) and irreversibly sickled cells (ISC) was enriched in phosphatidyl ethanolamine in addition to containing phosphatidyl serine. These changes were compensated for by a decrease in phosphatidyl choline in that layer. The distribution of sphingomyelin over the two halves of the bilayer was unaffected by sickling. In contrast to ICS, where the organization of phospholipids was abnormal under both oxy and deoxy conditions, reoxygenation of RSC almost completely restored the organization of membrane phospholipids to normal. These results indicate that the process of sickling induces an abnormality in the organization of membrane phospholipids to normal. These results indicate that the process of sickling induces an abnormality in the organization of membrane lipids in RSC which become permanent in ISC.
Full text
PDF

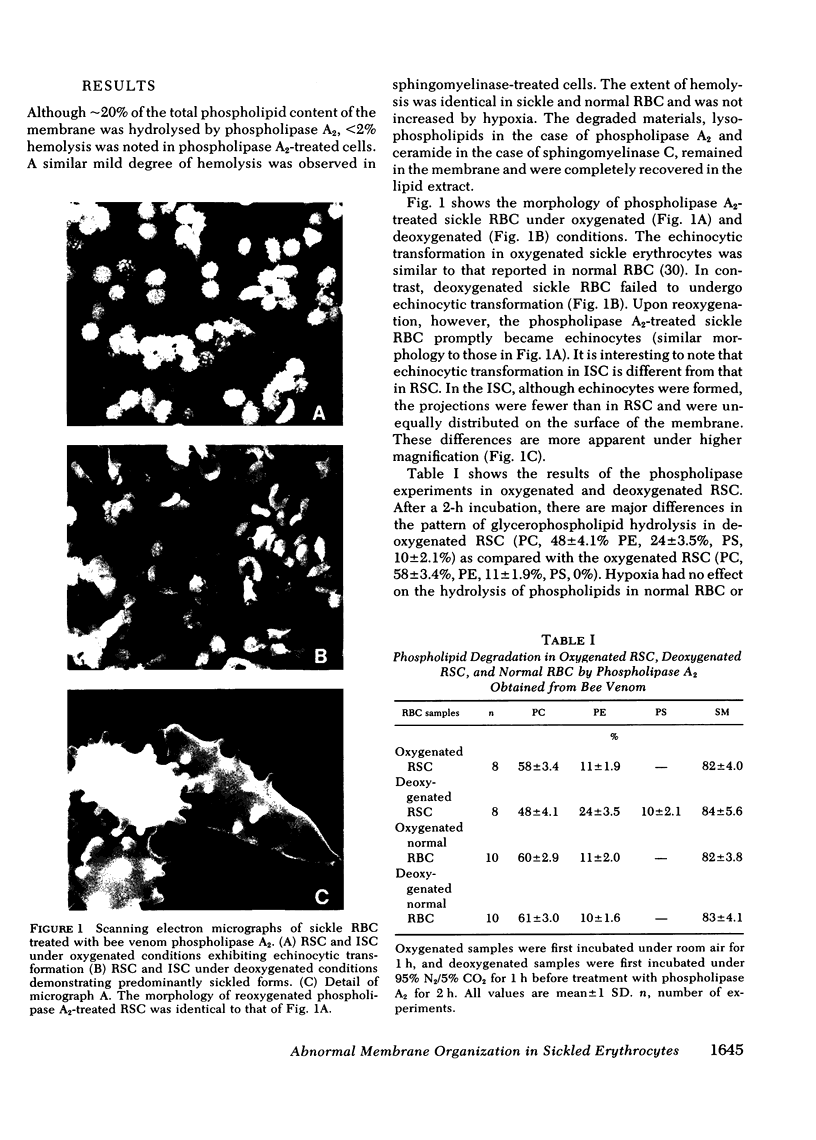
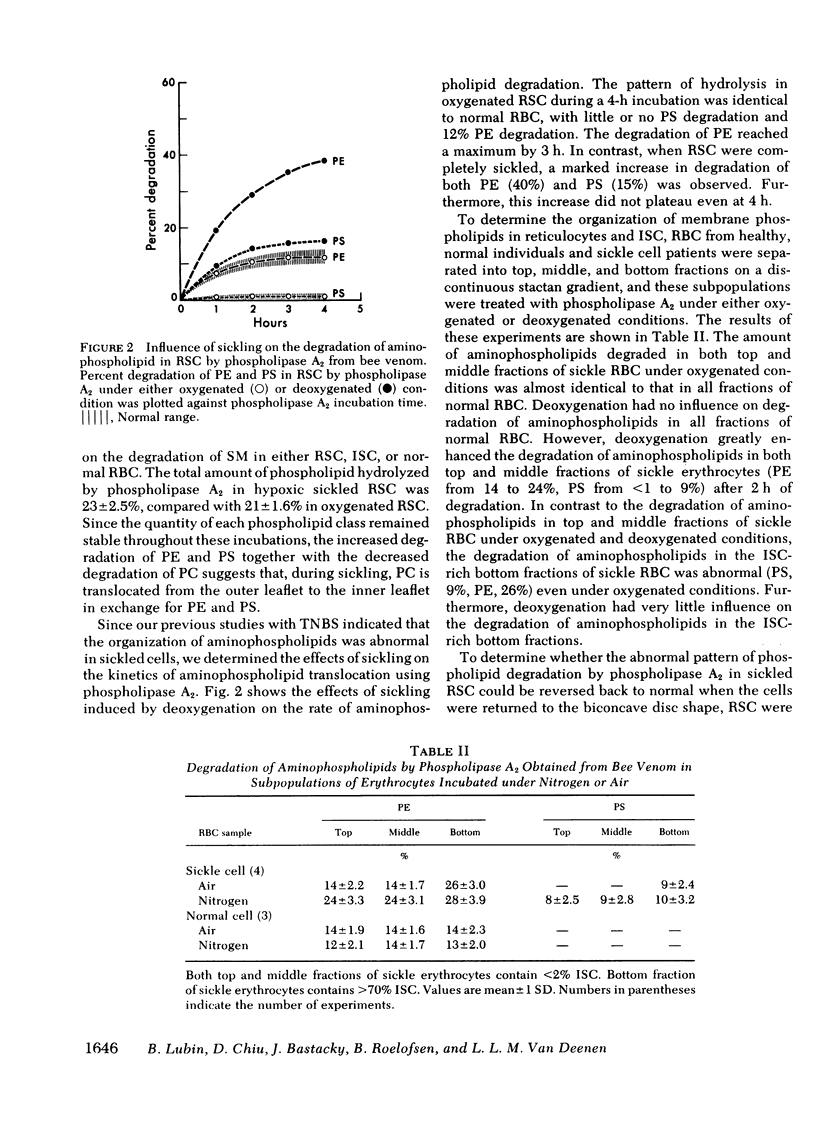



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura T., Minakata K., Adachi K., Russell M. O., Schwartz E. Denatured hemoglobin in sickle erythrocytes. J Clin Invest. 1977 Apr;59(4):633–640. doi: 10.1172/JCI108681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Chiu D., Lubin B., Shohet S. B. Erythrocyte membrane lipid reorganization during the sickling process. Br J Haematol. 1979 Feb;41(2):223–234. doi: 10.1111/j.1365-2141.1979.tb05851.x. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Unger R. C., Shohet S. B. Monovalent cation composition and ATP and lipid content of irreversibly sickled cells. Blood. 1978 Jun;51(6):1169–1178. [PubMed] [Google Scholar]
- Colley C. M., Zwaal R. F., Roelofsen B., van Deenen L. L. Lytic and non-lytic degradation of phospholipids in mammalian erythrocytes by pure phospholipases. Biochim Biophys Acta. 1973 Apr 25;307(1):74–82. doi: 10.1016/0005-2736(73)90026-6. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Fujii T., Tamura A. Asymmetric manipulation of the membrane lipid bilayer of intact human erythrocytes with phospholipase A, C, or D induces a change in cell shape. J Biochem. 1979 Nov;86(5):1345–1352. doi: 10.1093/oxfordjournals.jbchem.a132651. [DOI] [PubMed] [Google Scholar]
- Gordesky S. E., Marinetti G. V., Love R. The reaction of chemical probes with the erythrocyte membrane. J Membr Biol. 1975;20(1-2):111–132. doi: 10.1007/BF01870631. [DOI] [PubMed] [Google Scholar]
- Gordesky S. E., Marinetti G. V., Segel G. B. Differences in the reactivity of phospholipids with FDNB in normal RBC, sickle cells and RBC ghosts. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1004–1009. doi: 10.1016/0006-291x(72)90932-1. [DOI] [PubMed] [Google Scholar]
- Haest C. W., Plasa G., Kamp D., Deuticke B. Spectrin as a stabilizer of the phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta. 1978 May 4;509(1):21–32. doi: 10.1016/0005-2736(78)90004-4. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Boogaerts M. A., Eaton J. W., Steinberg M. H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980 May 1;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R., Rubin R., Wise G., Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979 Oct;54(4):872–876. [PubMed] [Google Scholar]
- Hosey M. M., Tao M. Altered erythrocyte membrane phosphorylation in sickle cell disease. Nature. 1976 Sep 30;263(5576):424–425. doi: 10.1038/263424a0. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Kimelberg H. K., Papahadjopoulos D. Synergistic effects of a membrane protein (spectrin) and Ca 2+ on the Na + permeability of phospholipid vesicles. Biochim Biophys Acta. 1971 Sep 14;241(3):894–905. doi: 10.1016/0005-2736(71)90017-4. [DOI] [PubMed] [Google Scholar]
- Luer C. A., Wong K. P. Altered erythrocyte membrane proteins in sickle cell patients associated with the severity of the disease. Biochem Med. 1978 Feb;19(1):95–107. doi: 10.1016/0006-2944(78)90010-8. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Karnovsky M. J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J Clin Invest. 1976 Oct;58(4):955–963. doi: 10.1172/JCI108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., Wolfe L. C. Inherited disorders of the red cell membrane skeleton. Pediatr Clin North Am. 1980 May;27(2):463–486. doi: 10.1016/s0031-3955(16)33862-7. [DOI] [PubMed] [Google Scholar]
- Martin J. K., Luthra M. G., Wells M. A., Watts R. P., Hanahan D. J. Phospholipase A2 as a probe of phospholipid distribution in erythrocyte membranes. Factors influencing the apparent specificity of the reaction. Biochemistry. 1975 Dec 16;14(25):5400–5408. doi: 10.1021/bi00696a003. [DOI] [PubMed] [Google Scholar]
- Mombers C., van Dijck P. W., van Deenen L. L., de Gier J., Verkleij A. J. The interaction of spectrin - actin and synthetic phospholipids. Biochim Biophys Acta. 1977 Oct 17;470(2):152–160. doi: 10.1016/0005-2736(77)90096-7. [DOI] [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Riggs M. G., Ingram V. M. Differences in erythrocyte membrane proteins and glycoproteins in sickle cell disease. Biochem Biophys Res Commun. 1977 Jan 10;74(1):191–198. doi: 10.1016/0006-291x(77)91393-6. [DOI] [PubMed] [Google Scholar]
- Roelofsen B., Trip M. S., Verheij H. M., Zevenbergen J. L. The action of cobra venom phospholipase A2 isoenzymes towards intact human erythrocytes. Biochim Biophys Acta. 1980 Aug 14;600(3):1012–1017. doi: 10.1016/0005-2736(80)90504-0. [DOI] [PubMed] [Google Scholar]
- Rubin R. W., Milikowski C., Wise G. E. Organizational differences in the membrane proteins of normal and irreversibly sickled erythrocytes. Biochim Biophys Acta. 1980;595(1):1–8. doi: 10.1016/0005-2736(80)90241-2. [DOI] [PubMed] [Google Scholar]
- Shohet S. B. Reconstitution of spectrin-deficient, spherocytic mouse erythrocyte membranes. J Clin Invest. 1979 Aug;64(2):483–494. doi: 10.1172/JCI109486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. D., Coleman R., Finean J. B., Michell R. H. The use of phospholipase c to detect structural changes in the membranes of human erythrocytes aged by storage. Biochim Biophys Acta. 1978 Sep 22;512(2):341–349. doi: 10.1016/0005-2736(78)90258-4. [DOI] [PubMed] [Google Scholar]
- Sweet C., Zull J. E. Interaction of the erythrocyte--membrane protein, spectrin, with model membrane systems. Biochem Biophys Res Commun. 1970 Oct 9;41(1):135–141. doi: 10.1016/0006-291x(70)90479-1. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., CARLSEN E., DUNHAM E. T. The effects of sickling on ion transport. I. Effect of sickling on potassium transport. J Gen Physiol. 1955 Sep 20;39(1):31–53. doi: 10.1085/jgp.39.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilschut J., Papahadjopoulos D. Ca2+-induced fusion of phospholipid vesicles monitored by mixing of aqueous contents. Nature. 1979 Oct 25;281(5733):690–692. doi: 10.1038/281690a0. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Comfurius P., van Deenen L. L. Membrane asymmetry and blood coagulation. Nature. 1977 Jul 28;268(5618):358–360. doi: 10.1038/268358a0. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Comfurius P., van Deenen L. L. Organization of phospholipids in human red cell membranes as detected by the action of various purified phospholipases. Biochim Biophys Acta. 1975 Sep 16;406(1):83–96. doi: 10.1016/0005-2736(75)90044-9. [DOI] [PubMed] [Google Scholar]
- van Meer G., Poorthuis B. J., Wirtz K. W., Op den Kamp J. A., van Deenen L. L. Transbilayer distribution and mobility of phosphatidylcholine in intact erythrocyte membranes. A study with phosphatidylcholine exchange protein. Eur J Biochem. 1980 Jan;103(2):283–288. doi: 10.1111/j.1432-1033.1980.tb04313.x. [DOI] [PubMed] [Google Scholar]



