Much of the research focus on the sensory and cognitive aspects of language centers on cortical activities. The cerebral cortex, however, is inextricably linked to the collection of forebrain nuclei known as the thalamus. Thalamocortical pathways dictate the sensory and higher-order representations in cortex while corticothalamic pathways generate dynamic, context-dependent changes in thalamic responsiveness to form an iterative signalling loop. This review will describe the functional organization of the auditory thalamus as it relates to the representation of sound features that are relevant for speech perception.
The thalamus is a collection of nuclei whose main and best-studied projections are to the cerebral cortex, comprising projections to all areas of cortex. The main auditory-responsive portion of the thalamus is called the medial geniculate body (MGB), and it is the information bottleneck for neural representations of sounds being sent to auditory cortex.
Whereas early views of the thalamus were that it served as a simple ‘relay’ or ‘gateway’ to the cortex, numerous studies have demonstrated that thalamic neurons transform their inputs en route to their cortical or subcortical targets (Hubel and Wiesel 1961; Sherman and Guillery 2002). As we will show, the MGB actively and dynamically shapes the auditory representations that reach the cerebral cortex. Rather than acting as a simple conduit for incoming auditory representations, the MGB acts like a funhouse mirror in the sense that it can filter and distort incoming inputs to enhance representation and perception of acoustic features for use by the auditory cortex (AC).
Acoustic characteristics of the speech signal relevant for auditory processing and receptive language
Human speech and animal vocalizations have complex sound characteristics that must be represented accurately by neural populations in order to recognize, understand, and converse with individuals in a variety of sound backgrounds and in the presence of multiple speakers. For speech, this includes not only the ability to identify the words that are spoken, but also the identity and gender of the speaker as well as the emotional content of their speech. Unlike the tones often used to probe auditory function in the lab, the speech spectrum is distributed over multiple octaves ranging from approximately 0.1 to 10 kHz. Although there are spectral peaks in the speech spectrum, such as in formants, there is also significant energy in non-peak frequencies.
One way that the speech signal can be parsed by auditory neuroscientists for understanding neural representations is to decompose the acoustic signal into components defined by different frequency ranges (Rosen 1992). With appropriate spectral resolution in quiet conditions, most speech fluctuations important for intelligibility occur at <10 Hz (Elliott and Theunissen 2009). Under a variety of conditions of spectral degradation, envelope modulations of up to 50 Hz are able to produce an adequate level of speech intelligibility (Shannon 1995, Elliott and Theunissen 2009). Thus, these modulation frequencies in the 1-10 Hz and 10-50 Hz range, collectively called the sound envelope, are important for speech intelligibility. However, for gender, emotion, and speaker identification, higher frequencies are needed. Higher frequency modulations in the range of 50-500 Hz are referred to as stimulus periodicity or temporal fine structure (Rosen 1992). This range also overlaps with the fundamental frequencies of speaker’s voices. Higher frequencies in speech signal contribute to speaker identity and emotional content and speech perception in noise (Rosen 1992). What separates these three frequency regions (<50 Hz, 50-500 Hz, and >500 Hz) from a neural standpoint are the auditory nuclei that are able to encode the modulations in a stimulus-synchronized manner. All stations in the ascending auditory pathway, including the auditory cortex, are able to encode stimulus envelope. Temporal fine structure cues (>50 Hz) are generally not represented by stimulus-synchronized responses in the auditory cortex, but often are in the auditory thalamus and inferior colliculus (Rouiller et al. 1981, Bartlett and Wang 2011, Krishna and Semple 2000). Finally, while auditory nerve and some cochlear nucleus fibers can synchronize to stimulus modulations or carriers up to 5 kHz, these high frequency fluctuations are encoded as changes in firing rates at higher levels of the auditory system (Joris et al. 2004).
In addition to temporal processing, the ability to parse the acoustic speech signal into different frequency bands is critical for speech comprehension. This is a job that is performed well by a properly functioning cochlea and then subsequently sharpened in the auditory thalamus and cortex of primates (Bitterman et al. 2008, Bartlett et al. 2011). Whereas adequate speech comprehension can occur with as few as 4 logarithmically spaced frequency channels (Shannon 1995)), comprehension improves in normal hearing listeners with up to 20 channels (Friesen et al. 2001, Baskent and Shannon 2006). Furthermore, gender identification and music recognition/appreciation increase with increasing spectral resolution of the auditory signal (Shannon 2005, Elliott and Theunissen 2009). Complicating the matter even further is that speech may be understood over a wide range of intensities, from whispers to shouts (40-60 dB dynamic range).
Therefore, for the difficult perceptual tasks of segregating and representing speech signals for comprehension, speaker identification, and emotional content in a variety of backgrounds and over a wide range of sound levels, the auditory system must be able to:
Represent and segregate carrier frequencies with high resolution
Represent temporal modulations up to 500 Hz, especially those ≤ 50 Hz.
Maintain neural representations over a large range of sound levels.
The MGB is heavily involved in shaping these representations in primates and other mammals. The following section will demonstrate the organization of the MGB, how the MGB transforms neural representations of auditory inputs, how representations are shaped by contextual and non-sensory factors, and how the cellular machinery used by MGB neurons contributes to the neural representations.
Overview of the anatomical and functional organization of the MGB
The MGB can be divided into three broad subdivisions, whose organization and properties are summarized in Table 1 and whose spatial arrangement can be seen in Fig. 1, going from rostral to caudal, using the marmoset as an example. The ventral division of the MGB (MGV) is the “core” subdivision for the rapid transmission of auditory information that is sharply tuned for frequency and able to respond to fast temporal modulations of sounds. For primates, this pathway also includes the anterodorsal nucleus of MGB (MGAD). These regions project to core regions in auditory cortex (AC), such as primary auditory cortex (A1), and stain heavily for parvalbumin, cytochrome oxidase and acetylcholinesterase (Hashikawa et al. 1991, Jones 2003, de la Mothe et al. 2006). The dorsal division of the MGB (MGD) is a slower, more integrative subdivision that is part of the “belt” pathway. Unlike MGV, auditory responses in MGD can be influenced strongly by non-auditory inputs, such as visual inputs or associating a sound with a reward. MGD neurons project mainly to belt AC and stain heavily for calbindin but relatively light for cytochrome oxidase and acetylcholinesterase (de la Mothe et al. 2006, Anderson et al. 2007, 2011). The most diverse region of the MGB is a collection of cell groups termed the caudal paralaminar nuclei (CPL) (Linke 1999, Linke et al. 2000), which includes the medial division of MGB (MGM), the suprageniculate nucleus, the posterior intralaminar nucleus and the peripedencular nucleus. These nuclei surround the ventral and medial aspects of MGB. The caudal paralaminar nuclei receive auditory and non-auditory inputs and has diverse responses to sound, with both very short-latency and longer-latency responses to sounds, as well as responses to visual and somatosensory inputs. CPL neurons have widespread projections to core and belt AC, as well as significant projections to subcortical regions such as the amygdala. These three main subdivisions will serve as a main way to understand the MGB. The human MGB is organized in largely the same way, except that the non-primary subdivisions (MGD, MGM, etc.) are relatively much larger in humans (Winer 1984), perhaps suggestive of an increased role in complex sound processing, such as for language.
Table 1.
Summary of properties of MGB subdivisions
| MGV | MGD | MGM | |
|---|---|---|---|
| Main cell type | Tufted | Stellate | Stellate/magnocellular |
| Tonotopic organization? | Yes | No | Yes, weaker than MGV |
| Calcium-binding proteins | Parvalbumin +++, Calbindin +,++ Cytochrome oxidase +++ | Calbindin +++, Parvalbumin + Cytochrome oxidase + | Calbindin ++,+++ Parvalbumin +,++ Cytochrome oxidase ++ |
| Main IC input | IC-central nucleus | IC-dorsal cortex | IC-external cortex |
| Major non-IC inputs (besides cortex) | Neuromodulators (acetylcholine, serotonin, etc.) | Lateral tegmentum, sagulum neuromodulators | Spinal cord, vestibular nuclei, hypothalamus, superior colliculus, neuromodulators |
| Cortical target | A1, non-A1 core, layers 3/4 | Belt and parabelt, weak core, layers 3/4 and some layers 1 and 6 | Core, belt, and parabelt, all layers, |
| Subcortical targets | None | Amygdala | Amygdala, striatum, IC |
| Cortical feedback | Layer 6, core | Layer 6, belt, layer 5, core and belt | Layer 6, core, belt |
| Tone frequency tuning | Narrow, usually single- peaked | Broad, inhibited, variable, multi-peaked | Heterogeneous, narrow and broad |
| Tone latency | Short | Longer | Heterogeneous, short and long |
| AM response | Synchronized to rapid modulation frequencies | Often non-synchronized or synchronized to low modulation frequencies | Heterogeneous, some synchronized to very rapid modulation frequencies |
| Receptive field plasticity | Sharply tuned near BF, short duration | Shift in BF to CS tone, lasts ≥ 24 hours | Shift in BF to CS tone, lasts ≥ 24 hours |
| Modulation of responses by non-auditory input | No | Yes, visual | Yes, visual and somatosensory |
| Modulation of responses by reward | No | Yes | Yes |
| IC EPSP properties | Large, stronger depression, and small, weak depression or facilitation | Small, weak depression or facilitation | Small, weak depression or facilitation |
| IC inhibition | GABAA and GABAB | GABAA and GABAB | GABAA only |
Abbreviations: MGV - medial geniculate, ventral division. MGD – medial geniculate, dorsal division. Includes posterodorsal division of primates. MGM – medial geniculate, medial division.
Figure 1. Marmoset MGB and calcium binding proteins.
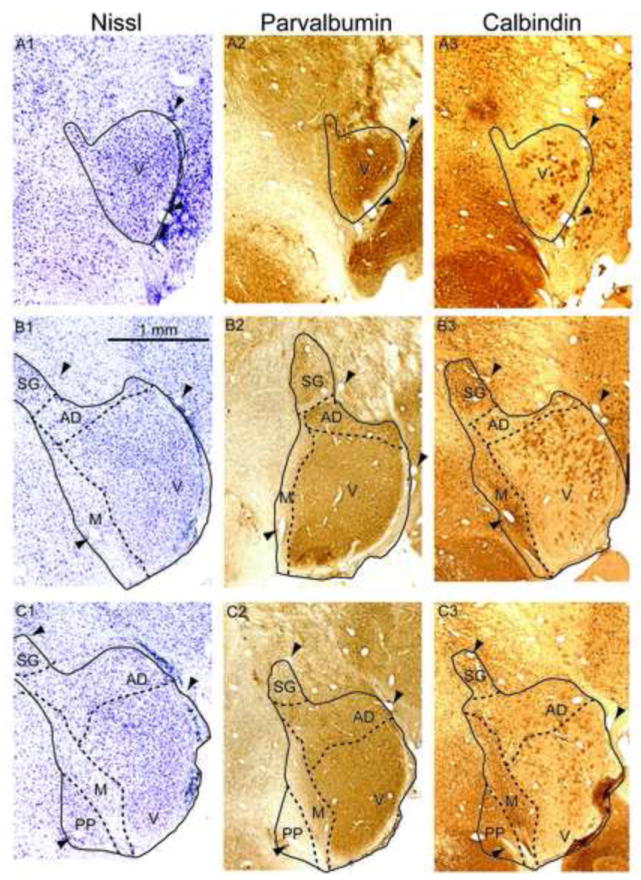
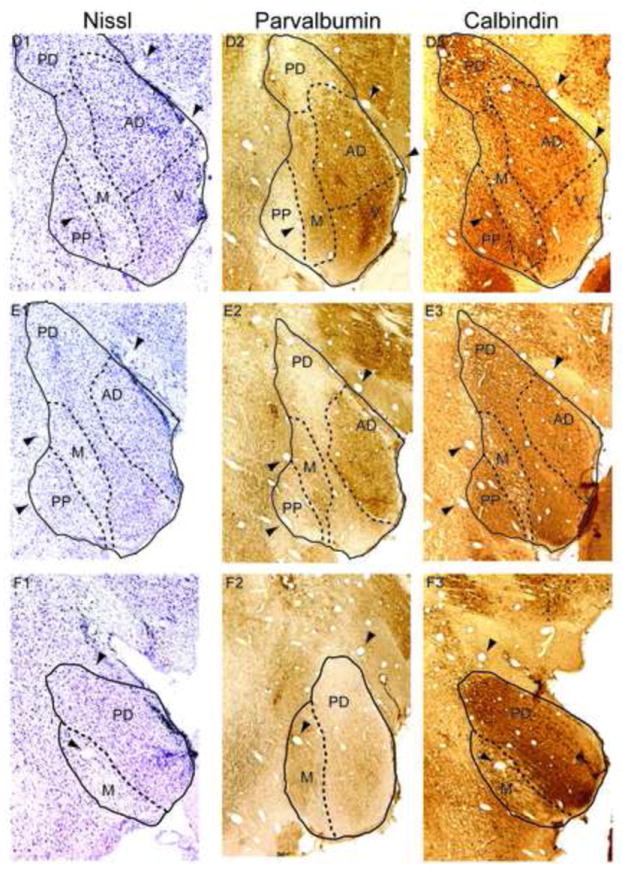
Left column: Nissl stain, with MGB subdivisions indicated by dashed lines. Arrowheads indicate blood vessels that are found in all three adjacent sections in a row. Middle column: Parvalbumin immunostaining. Right column: Calbindin immunostaining. The topmost row (A1-A3) is the most rostral portion of MGB. Subsequent rows progress caudally by approximately 240 m per row. Abbreviations: V, ventral division, PD, posterodorsal division, AD, anterodorsal division, M, medial division, SG, suprageniculate nucleus, PP, peripeduncular region.
Connectivity of the MGB- feedforward afferents
Across species, auditory inputs to the MGV arise mainly from the ipsilateral central nucleus of the IC (ICC, Fig. 2) (Oliver and Hall 1978, Calford and Aitkin 1983, Ledoux et al. 1985, Rouiller and de Ribaupierre 1985, Gonzalez-Hernandez et al. 1991). At the light microscopic level, many ICC axons ended as large terminals in MGV that are grouped within 50-100 m of each other (Malmierca et al. 1997; Pallas and Sur 1994; Bartlett et al. 2000), so that they may contact the dendritic arbors of one or more neighboring MGV neurons. IC axons in MGV also often ended as small or medium sized terminals (Malmierca et al. 1997; Pallas and Sur 1994; Bartlett et al. 2000). In species containing a significant proportion of interneurons (cat, primates), most IC axons have endings that contact MGB projection neurons and interneurons (Majorossy and Kiss 1976; Jones and Rockel 1973; Guillery 1995).
Figure 2. Connectivity of MGB subdivisions.
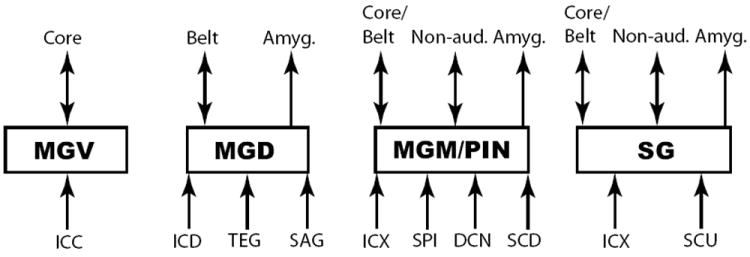
A diagram of the main inputs and projection targets of each main subdivision is shown. Core and belt refer to primary and non-primary auditory cortical regions, respectively. Double-headed arrows indicate bidirectional projections between two areas. Abbreviations: MGV, ventral division, MGD, dorsal division (includes posterodorsal division), MGM, medial division, SG, suprageniculate nucleus, PIN, posterior intralaminar nucleus, SCU, superior colliculus – upper layers, SCD, superior colliculus – deep layers ICC, central nucleus of the inferior colliculus, ICD, dorsal cortex of the inferior colliculus, ICX, external cortex of the inferior colliculus, SAG, sagulum, TEG, tegmentum, DCN, dorsal cochlear nuclei, SPI, spinal cord, Amyg., amygdala, Non-aud., non-auditory cortex, Ach, acetylcholine, Norad., noradrenaline, 5-HT, serotonin, TRN, thalamic reticular nucleus.
Neuromodulators (ACh, Norad., 5-HT) go to all MGB subdivisions.
All MGB subdivisions send collaterals to and receive inputs from TRN.
Injections of anterograde tracers into the IC dorsal cortex labels some terminals in MGV, but mainly labels terminals in the MGD, in the suprageniculate nucleus and in the medial division (Fig. 2) (Andersen et al. 1980, Kudo and Niimi 1980, Ledoux et al. 1985). The non-tonotopic IC dorsal cortex (ICD) receives mainly auditory cortical projections and ascending projections from the ICC central nucleus. The non-tonotopic external IC cortex receives auditory, intracollicular, somatosensory, and polymodal inputs (Coleman and Clerici 1987; Shi and Cassell 1997; Herbert et al. 1991; Herrera et al. 1994). IC external cortex injections label axons that project to MGD, MGM, the suprageniculate nucleus, the posterior intralaminar nucleus, and to a lesser degree in MGV (Fig. 2) (Ledoux et al. 1985, Calford and Aitkin, 1983). Besides the IC dorsal cortex input, there are also significant auditory inputs from the lateral tegmentum and nucleus sagulum to MGD (Morest 1965, Oliver and Hall 1978, Henkel and Schneiderman 1988). In addition to excitatory glutamatergic tegmental inputs, the tegmentum is a major source of neuromodulatory cholinergic inputs to MGB (Motts and Schofield 2011).
Anatomical studies have verified the multimodal nature of the CPL nuclei. These regions receive major auditory inputs primarily from the external and dorsal nuclei of the inferior colliculus, with a smaller contribution from the IC central nucleus (Kudo and Niimi 1980; LeDoux et al. 1985; Linke et al. 1999; Oliver and Hall 1978). MGM neurons receive an additional auditory input directly from the dorsal cochlear nuclei (Anderson et al. 2006, guinea pig). Unlike MGV, MGM and SG receive significant inputs from cells in all layers of the superior colliculus (Fig. 2) (Altman and Carpenter 1961; Graham 1977; Hicks et al. 1986; Holstege and Collewijn 1982; Linke et al. 1999; Tarlov and Moore 1966). Linke (1999) showed that inputs to SG neurons are mainly from the upper layers of SC where responses are purely visual and visuomotor, and inputs to MGM neurons are mainly from deep SC layers, which are multimodal. The MGM and PIN also receive a well-known input from spinal cord in the rat (Ledoux et al. 1987) which has been studied as part of an associative learning (fear conditioning) pathway that includes the IC, spinal cord, CPL MGB nuclei, and the amygdala (Rogan and Ledoux 1996). The paralaminar nuclei receive hypothalamus input that may be involved in mediating sound-induced (audiogenic) stress and anxiety (Campeau and Watson 2000). The rat MGM also receives sparse projections from the spinal vestibular nucleus (Doi et al. 1997), the globus pallidus (Shammah-Lagnado et al. 1996), the dorsal nucleus of the lateral lemniscus (Bajo et al. 1993), the lateral cervical nucleus (Giesler et al. 1988), cuneate and gracile nuclei (Massopust et al. 1985), the ventral funiculus (Bjorkeland and Boivie 1984), and the trigeminal nucleus (Peschanski 1984). From these studies, the CPL nuclei appear to integrate auditory, somatosensory and motor inputs while MGD might participate in multimodal networks to a lesser degree. The lemniscal MGV appears to be a purely auditory structure, based on its connectivity.
Connectivity of the MGB- efferents
Summarizing recent reviews (e.g Winer et al. 2005, Lee 2012), thalamocortical axons from MGV terminate mainly in cortical layers 3 and 4 of core auditory cortex. En route to layer 3/4, MGV axons emit collaterals that terminate in the auditory portion of the thalamic reticular nucleus (TRN) (Fig. 2) (Ojima 1994). Within the MGV, there is some segregation in the regions that give rise to projections to different core auditory cortical regions (Read et al. 2011), such that the caudal MGV projects to the ventral auditory field and rostral MGV projects to primary auditory cortex in the rat (Storace et al. 2010). Projections to A1 and the core region R also arise from separate populations of neurons in MGV (de la Mothe et al. 2006). Though it remains to be demonstrated clearly, these different MGV populations may have different properties that are correlated with their targets in cortex (Bendor and Wang 2008), such as longer latencies and poorer synchronization to temporal modulation in MGV neurons projecting to R compared to those projecting to A1. Similarly, the main projection of MGD is to layers 3 and 4 of non-primary belt auditory cortical regions (Fig. 2), but there are more projections to layers 1 and 6 than MGV projections (Mitani et al. 1987, Hackett et al. 1998, Kimura et al. 2003, de la Mothe et al. 2006, Lee and Winer 2008).
Across species, the cortical projections of MGM, SG, and PIN neurons distribute their multimodal information over a wide swath of primary auditory, non-primary auditory, and association cortical regions (Lee and Winer 2008, Lee 2012). Thalamocortical projections from CPL nuclei can terminate in layers 1 and 6 or layers 3 and 4 of all temporal cortical areas (Fig. 2, Table 1) (Mitani et al. 1984; Ledoux et al. 1985; Hashikawa et al. 1995, Shi and Cassell 1997; Huang and Winer 2000, Winer et al. 2005; Smith et al. 2010). Many MGM, PIN, and peripeduncular neurons send axons to targets in the lateral amygdala and amygdalostriatal transition zone (Ledoux et al. 1990, Linke et al. 2000) rather than to cortex. These are largely separate from the neurons that project to auditory cortex, but about 10% of MGM projections go to both cortex and amygdala (Doron and Ledoux 2000).
Connectivity of the MGB- feedback afferents
The basic organization of cortical feedback to MGB has been well-studied (see Lee et al. 2004, Lee 2012 for reviews). Primary auditory cortex is broadly reciprocally connected with MGV (Fig. 4A), such that regions in MGV that project to a given area of auditory cortex are likely to receive significant corticothalamic feedback from layer 6 neurons of that area of cortex (Winer et al. 2001, Kimura et al. 2005, Llano and Sherman 2008). Similar reciprocity holds for corticothalamic MGD and CPL inputs (Arnault and Roger 1990, Ojima 1994, Pandya et al. 1994).
Figure 4. Responses to sinusoidal amplitude modulation in different MGB subdivisions.
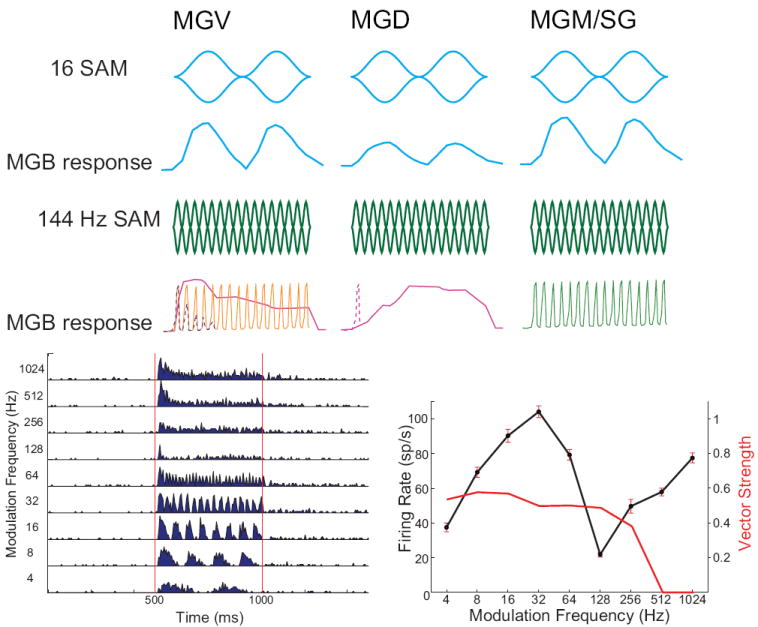
Top row: Modulation waveforms for 16 Hz sinusoidal AM (SAM), 100% modulation depth. Second row: MGB response to 16 Hz SAM. For low modulation frequencies (<32 Hz), MGV neurons (left) represent both periodicity and modulation waveform in their discharges. Firing rate curves represent peristimulus time histogram (PSTH) responses. MGD neurons (middle), if responsive, often have lower evoked firing rates but are often sometimes synchronized for low modulation frequencies. MGM neurons (right) respond similarly to MGV neurons at low modulation frequencies despite receiving IC inputs with different tuning and axon terminal sizes. Third row: Modulation waveforms for 144 Hz sinusoidal AM, 100% modulation depth. Fourth row: MGV neurons have three common responses to high frequency AM when recording from unanesthetized animals. Left: Some MGV neurons respond only phasically (dashed line). Others are able to maintain synchronized responses for the duration of the AM stimulus. Some MGV neurons respond with sustained increases in firing rate that are not synchronized with the AM modulation frequency. Middle: Most MGD neurons, when responsive, generate a long-latency sustained increase in firing rate for rapid AM stimuli (solid line) or just a brief onset (dashed line). Right: Many MGM neurons (guinea pig) and anterodorsal neurons (marmoset) are able to represent rapid temporal modulations with relatively precise synchrony compared to other MGB subdivisions. Fifth row, left: Example of a MGV neuron response to SAM. Same neuron as Fig. 6B. SAM tone, carrier frequency = 6.96 kHz, 70 dB SPL, 4-1024, 1 octave steps. Shown is the PSTH in response to SAM sounds. Red lines show when sound was playing. Fifth row, right: Mean firing rate (solid line) ± SEM for same unit. Also shown is vector strength (red line), where those that were not significantly synchronized were set to 0.
The layer 6 terminals are quite small and end on distal, small-caliber, higher-order MGB dendrites (Bartlett et al. 2000; Smith et al. 2007).
As described above, the TRN receives excitatory input from both thalamocortical MGB axons and corticothalamic axons from auditory cortex. GABAergic TRN axons then project back to the thalamus and end as inhibitory terminals on the dendrites and soma of MGB neurons (Montero 1985; Bartlett et al. 2000; Smith et al. 2007). Unlike the layer 6 corticothalamic feedback, the feedback from TRN is not necessarily reciprocal. Some projections from MGV to TRN are tonotopically matched (Kimura et al. 2009), but other projections are to different tonotopic regions or even to different subdivisions from the MGB area that it received input from (Crabtree 1998, Kimura et al. 2001, Kimura et al. 2009). In the cat, single auditory TRN neurons can project to MGV, to MGD, or to both regions (Crabtree 1998). Through these projections, TRN projections can flexibly and selectively determine MGB responses to complex, multi-frequency stimuli.
A second type of corticothalamic projection has also been observed in the MGD that is not observed in MGV. This projection arises from layer 5 neurons primary auditory cortex of the cat, rat and monkey and does not project to TRN (Fig. 4A,B) (Ojima 1994; Kelly and Wong 1981). Across sensory systems, the large terminals from sensory inputs (IC, retina) and from layer 5 of primary sensory cortex have been termed “driver” inputs (Sherman and Guillery 1998) because a small number of inputs are able to drive the thalamic neurons and determine what the neurons respond to. By contrast, the small corticothalamic terminals from layer 6 have been termed “modulators” because the individual influence of inputs is weak and a single input has only limited effect on the response properties of thalamic neurons (Lee 2012). In the following sections, I will discuss the physiology of MGB neurons and subdivisions while referring back to the anatomical organization as needed.
Responses to tone stimuli – frequency tuning
Accurate neural representations of frequencies are critical for accurate perceptions of speech sounds (Glasberg and Moore 1989, Carroll and Feng 2007), such as for discriminating between vowel formants, between speakers, and identifying gender. This is particularly true for speech reception in background noise (Horst 1987, Glasberg and Moore 1989), for hearing impaired listeners (Glasberg and Moore 1989, Carroll et al. 2011) and for listeners with specific language impairment (MacArthur and Bishop 2004, Hill et al. 2005). Tone stimuli, consisting of a single frequency, are the most commonly used sound stimuli to probe neural frequency tuning and its spatial arrangement within a region (tonotopy). MGV neurons across species are tonotopically organized, have the sharpest frequency tuning and short response latencies to tonal stimuli in the MGB (Bordi and Ledoux 1994; Anderson et al. 2007; Calford 1983; Bartlett et al. 2011) (Fig. 3A,B). For a given sound level, some neurons in MGM, MGD, and the anterodorsal division have multipeaked frequency responses areas (Fig. 3E) (Calford 1983, Rouiller et al. 1989, Anderson et al. 2007, Bartlett and Wang 2011).
Figure 3. Main tone frequency response area shapes in different MGB subdivisions.
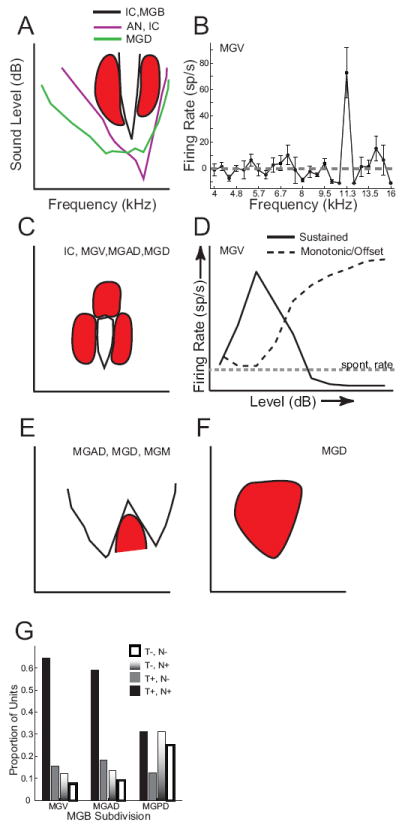
Schematic frequency response areas are shown with frequency along the x-axis and sound level along the y-axis. Axes in A apply to A, C, E, F. Responsive regions fall above (A,,E) or within the area (C) bounded by the solid lines and not within the red inhibited regions. A: In the auditory nerve (AN) and in many IC neurons, frequency tuning widens with sound level, especially on the low-frequency side. In MGV, MGAD, MGM and IC, many neurons have narrow excitatory tuning that is flanked by lateral inhibition (red shapes). If the rate-level function is monotonic or plateaus, the top of the FRA will be open. In MGD, frequency tuning for tones is often broad (green line). B: Example of a narrowly tuned MGV neuron (adapted from Bartlett and Wang 2011), showing firing rate as a function of tone frequency. With 1/12 octave spacing between tones, only one tone was strongly excitatory, and this was flanked on both the low and high frequency sides by strong inhibition that made the firing rate go below the spontaneous rate (gray dashed line). C: Similar narrow tuning as in A (solid black lines), but the rate-level function is non-monotonic, such that firing rates decrease for high sound levels. This is likely to be due to inhibition at high levels (red shape at top of FRA). This means that there is a restricted frequency and level range over which there is evoked excitation. D: Examples of sound level tuning in MGB neurons. The dashed line shows a monotonic increase in rate with increasing sound level, which can be found in all species to varying degrees and is found in lower auditory nuclei. The solid line shows a non-monotonic rate-level curve, where firing rate rises to a maximum at the neuron’s best level and then decreases for louder levels, sometimes becoming inhibited below the neuron’s spontaneous rate (gray dashed line). Non-monotonic responses are prevalent in primate MGB, and in these units, there is often an offset response at higher sound levels that rises monotonically, which is also represented by the black dashed line. In other neurons in MGD, tones generate inhibition that may or may not be followed by an offset response (not shown). E: MGAD, MGD, and MGM neurons have a moderate proportion of multi-peaked FRAs, with two or more excitatory peaks separated by elevated thresholds or inhibition. These multipeaked FRAs can also be non-monotonic (not shown) F: Some MGD neurons are only inhibited by F: Proportion of neurons responsive (T+) or unresponsive (T-) to tones and neurons responsive (N+) or unresponsive (N-) to noise for MGV, MGAD and MGPD (MGD). MGPD neurons had a much higher proportion of units that were inhibited or unresponsive to tones and noise (T-,N-) or only responsive to noise (T-,N+) compared to MGAD and MGV.
One of the main ways to measure how sharply tuned a neuron is for sound frequency is the quality factor, or Q value, calculated as the best frequency divided by the range of frequencies over which the neuron responds above some criterion (e.g. 50% maximal firing rate), measured at a given sound level. A comparison of Q10 values (Q value at 10 dB above response threshold) across studies demonstrates that MGV neurons are the most sharply tuned, with mean Q10 values of 2-3 in awake guinea pig (Edeline et al. 1999), anesthetized mouse (Anderson and Linden 2011), and awake squirrel monkey (Allon et al. 1981), 5.8 in anesthetized cat (Miller et al. 2002), 7.2 in the awake brown bat (Llano and Feng 1999), 15.9 in the awake marmoset monkey (Bartlett et al. 2011), and up to 200 in the specialized, hypersensitive 61 kHz region of the mustached bat that is used in echolocation (Suga and Tsuzuki 1985). Interestingly, the sharp frequency tuning observed in the bat and marmoset MGV is even sharper than frequency tuning typically observed in auditory nerve. The Q10 values in marmoset MGV are comparable to those observed for the onset response in marmoset auditory cortex, which then sharpens over time (Bartlett et al. 2011), and slightly less than that reported from awake human auditory cortex (Bitterman et al. 2008). This suggests that sequential sharpening of frequency tuning occurs in MGV and auditory cortex, though even this sharp single neuron tuning is less precise than human behavioral discrimination thresholds (Sinnott et al. 1985). The high frequency selectivity that humans exhibit behaviorally arises from a combination of narrow cochlear frequency tuning (Oxenham and Shera 2003) and mechanisms to maintain or sharpen frequency tuning that are still present in auditory cortex (Bitterman et al. 2008). Understanding frequency tuning mechanisms is important for speech and language processing, since deficits in frequency discrimination have been associated with specific language impairment (Hill et al. 2005, Rinker et al. 2007) and can persist (Hill et al. 2005).
Another characteristic that may be important for understanding frequency discriminability is the way that frequency tuning changes with sound level. In most neurons below the level of the inferior colliculus, frequency tuning becomes broader with increasing sound level (e.g. Evans 1972, cat auditory nerve), especially for frequencies lower than the best frequency (Fig. 3A, AN). This type of response is prevalent in the MGB of rodents (Anderson et al. 2007). In unanesthetized cat (Ramachandran et al. 1999) and marmoset monkey inferior colliculus (Nelson et al. 2009), the sharpness of frequency tuning is much more level-tolerant, which is important for maintaining high frequency selectivity at the suprathreshold sound levels during vocal exchanges (Fig. 3A, black lines). Similarly, frequency tuning is level-tolerant for both MGB neurons (Bartlett and Wang 2011) and for A1 neurons (Sadagopan and Wang 2008) in unanesthetized marmosets for the sound levels to which the neurons respond (see section on sound-level tuning for more detail).
Conversely, MGD neurons generally have longer response latencies and, in anesthetized animals, either broad or undefinable frequency tuning to tone stimuli (Fig. 3A, MGD). Some MGD neurons are unresponsive to tones (Bordi and Ledoux 1994; Calford 1983), habituate quickly to repeated stimuli (Bordi and Ledoux 1994; Calford 1983), or are inhibited by them (Fig. 3F) (Bartlett and Wang 2011). MGD neurons in most species are more broadly tuned on average when responsive (Calford 1983, Edeline et al. 1999, Bartlett and Wang 2011), though this does not appear to be the case in the mouse (Anderson and Linden 2011). Neurons in MGM have a wide range of response latencies and frequency tunings. Some MGM neurons are as sharply tuned as MGV neurons (Anderson and Linden 2011), but MGM neurons are often intermediate in tuning between MGV and MGD (Calford 1983, Edeline et al. 1999), but there is a wider range of variability of tuning in this region. Consistent with their connectivity patterns as a part of the core auditory pathway, the anterodorsal division of MGB in primates has sharply-tuned neurons with short-latency responses (Bartlett and Wang 2011).
Similar to the inferior colliculus, MGB neurons can respond to tone stimuli at the onset of the sound only (onset), strongly at onset and more weakly thereafter (primary-like, since this is similar to auditory nerve responses), at a similar level for the duration of the sound (sustained), or after a sound ends (offset). Onset and primary-like responses dominate in anesthetized animals (Calford 1983, Kvasnak et al. 2000, He 2001) and in awake rodents (Edeline et al. 1999, Anderson and Linden 2011). At the onset of a sound, a larger number of neurons are engaged, which then narrow to the population of neurons best tuned to the sound if the sound is maintained. Sustained responses to best frequency tones dominated in awake cats and primates (Aitkin and Prain 1974, Allon et al. 1981, Shamma and Symmes 1985, Bartlett and Wang 2011), regardless of location in MGB. In this way, the neurons can rapidly adjust their responses to changes in the sound around their best frequencies. Offset, variable, or habituating responses are most prevalent in MGD, where the offset response may occur due to the offset of inhibition followed by a rebound response (Bordi and Ledoux 1994, Calford 1983, He 2001, Bartlett and Wang 2011).
Inhibition plays a key role in shaping responses in MGB. Use of single tones in a quiet background can reveal a neuron’s best frequency and can often demonstrate lateral inhibitory inputs that maintain sharp frequency tuning (Bartlett and Wang 2011). However, single tones do not predict well how a neuron will respond to sounds composed of more than one frequency, such as in speech, because single tones do not clearly reveal all inhibitory inputs or secondary excitatory inputs. To uncover facilitatory and suppressive interactions between frequencies, more complex stimuli such as two-tone stimuli (Horner et al. 1983, Wenstrup 1999), spectrotemporal ripple stimuli (Miller et al. 2002), or band-passed noise stimuli (Bartlett and Wang 2011) are used as probes. Use of band-passed noise stimuli can reveal the range over which a neuron integrates frequencies, and it can segregate neurons sensitive to broadband frequencies, which would respond best to sounds that often make up the background (e.g. oceans, air conditioners) from those sensitive to a small number of spectral peaks that often make up the foreground (e.g. speech). MGV neurons strongly prefer narrow bands of noise or single frequencies, whereas MGD neurons often respond better to noise stimuli or only to noise stimuli (Fig. 3G, Bartlett and Wang 2011). The selectivity of MGM neurons for tones and noise is heterogeneous, but follows more closely with MGV (Rodrigues-Dagaeff et al. 1989).
Responses to tone stimuli – sound level tuning
As discussed in the previous section, frequency selectivity can change as a function of sound level. This is even the case for a tone or narrow-band noise centered at a neuron’s best frequency. It can also affect how time-varying changes in modulation frequency and modulation depth are represented, particularly for relatively slow temporal modulations (<10 Hz, Malone et al. 2007). That is, the ability to detect and discriminate slow temporal modulations, which is important in speech perception, is related to the ability to detect and discriminate changes in sound level. Sound level tuning is often classified as monotonic, which occurs when the response to a sound increases as the sound intensity increases or when the firing rate rises to a maximum as sound level increases and then plateaus with increasing levels (Fig. 5A,C,E). Non-monotonic responses describe when the firing rate increases with increasing sound level up to a maximum, after which increasing sound level causes a decrease in the firing rate (Fig. 5B). Inferior colliculus neurons often have an approximately even mix of monotonic and non-monotonic responses (Ryan and Miller 1978; Aitkin 1991; Palombi and Caspary 1996; Ramachandran et al. 1999). The average decrease in firing rate for non-monotonic neurons ranged from about 25% in rat IC neurons (Palombi and Caspary 1996) to nearly 100% in decerebrated cats (Ramachandran et al. 1999). Similar findings have been observed in the MGB of awake guinea pigs (Edeline et al. 2000), with the decrease in firing rates for louder sounds (non-montonicity) comparable to the strong non-monotonicity observed in the Ramachandran et al. (1999) study. The proportion of non-monotonic units increases in cats and monkeys to become the dominant response such that nearly three-fourths of MGV neurons respond non-monotonically, with decreasing firing rates at the highest sound levels (Aitkin and Prain 1974; Rouiller et al. 1983, Bartlett and Wang 2011). This suggests that many of the non-monotonic responses observed in primate auditory cortex (Bartlett and Wang 2005, Malone et al. 2007, Bendor and Wang 2008) may be inherited from MGB inputs. The range over which non-monotonic units respond well is about 20 dB (Bartlett and Wang 2011), and in many neurons, louder sounds at BF completely inhibited responses, sometimes producing a response upon stimulus offset (Fig. 3D). The presence of a large proportion of non-monotonic responses suggests a different purpose for representing sound level, particularly in the presence of ongoing or simultaneous sounds. Whereas monotonic responses represent the absolute sound level within a receptive field, there is evidence in the auditory cortex suggesting that non-monotonic units adapt their response range to maintain sensitivity to a given sound level contrast in backgrounds of different sound levels (Ehret and Schreiner 2000, Watkins and Barbour 2008). Such precise level and contrast dependent processing of frequency selectivity may be critical for perception of complex sounds and in the presence of background noise. In support of this, it has been found in the marmoset MGB that strongly non-monotonic neurons had the highest frequency selectivity and the lowest thresholds in quiet (Bartlett and Wang 2011). An examples a non-monotonic rate-level (solid line) and its offset response is shown in Fig. 3D.
Figure 5. Neural representations of click stimuli by MGB subdivision.
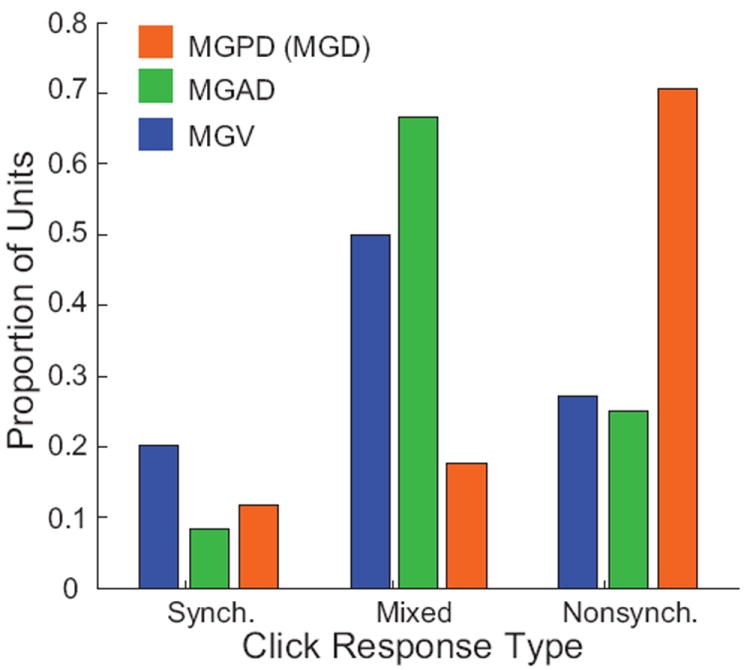
Adapted from Bartlett and Wang (2011). Proportion of response categories for responses to repetitive click trains in different MGB subdivisions. MGPD neurons had a much higher proportion of non-synchronized responses that MGV or MGAD neurons.
Responses to monaural and binaural stimulation and sound localization cues
In an auditory scene with multiple sources, spatial localization is a major cue for separating those sources (Drennan et al. 2003; Schimmel et al. 2008). Studies in the cat indicate that about 60% of MGB neurons can be excited by stimuli to either ear, but 20-30% can be excited only by contralateral ear stimulation (Calford 1983). About 10-20% are inhibited by ipsilateral stimulation, although few are only inhibited without excitation (Calford 1983). Many MGB neurons in the cat are responsive to sound localization cues such as interaural time differences and interaural level differences and modulate their response based on the azimuthal location of the sound source (Ivarsson et al. 1988; Clarey et al. 1995; Calford 1983). The sound location tuning of MGB neurons in anesthetized cats is approximately 60° for tone stimuli and 40° for noise stimuli (Clarey et al. 1995). This tuning is much less precise than the ability of cats to localize sounds (Tollin et al. 2005), suggesting that sound localization behavior results from the construction of more precise location tuning by single or multiple auditory cortex neurons or by brainstem projections to motor structures. The MGB does not appear to be strongly involved in simple localization tasks in rats (Kelly and Judge 1985), but this has not been tested in other species. When the receptive fields of MGB neurons are compared between contralateral, ipsilateral or binaural stimuli in the cat, there is strong overlap in tuning but the ipsilateral tuning is often opposite in sign to the contralateral tuning (Miller et al. 2002). In humans, fMRI studies have shown that the MGB is responsive during sound localization tasks or cues, with spatial overlap and similar activation to that found during sound recognition tasks (Maeder et al. 2001).
Temporal processing in the MGB
Most sound stimuli exhibit temporal modulations that are important for identifying and discriminating those sounds. Earlier, the idea of three different ranges of temporal modulations was introduced; 1-50 Hz for rudimentary speech recognition, mainly in quiet conditions, 50-500 Hz for speaker, gender and emotion identification, and >500 Hz for additional cues for speaker and emotion identification. Furthermore, modulations >50 Hz aid with understanding speech in noise.
For simple temporally modulated sounds such as sinusoidally amplitude modulated (SAM) sounds or repetitive click stimuli, inferior colliculus neurons represent modulation periodicity up to about 300-500 Hz with stimulus-synchronized discharges while modulation rate and envelope shape are represented mainly by firing rate (Krishna and Semple 2000, Zheng and Escabi 2008). These representations in IC then form the inputs to MGB neurons, with all IC subdivisions capable of phase-locking to modulations >100 Hz (Shaddock Palombi et al. 2001). MGB neurons in awake animals are able to respond robustly to amplitude or frequency modulated tones up to about 20-30 Hz, and many are able to generate synchronized spiking for modulations ≥ 100 Hz (Vernier and Galambos 1957, Creutzfeldt et al. 1980, Preuss and Muller-Preuss 1990, Bartlett and Wang 2007, 2011). The maximum synchronization frequency usually decreases under anesthesia, down to approximately 10-15 Hz for pentobarbital anesthesia in cats (Aitkin et al. 1966) and 60-70 Hz under ketamine anesthesia in cats (Miller et al. 2002). When different MGB subdivisions were compared in their responses to clicks, stimulus-synchronized discharges were the dominant response in cat and marmoset MGV (Fig. 4, Synch. population) (Rouiller and de Ribaupierre 1982, Bartlett and Wang 2011). Many MGV and MGAD neurons which generated synchronized responses for lower click rates in marmosets exhibited long-latency, sustained rate representations for high click rates, which is termed a Mixed response (Bartlett and Wang 2007). MGD neurons had mainly non-synchronized responses (Fig. 4, Nonsynch., described below) (Rouiller and de Ribaupierre 1982, Bartlett and Wang 2011). Rouiller and colleagues (Rouiller et al. 1981, Rouiller and de Ribaupierre 1982) found that synchronized responses were also the predominant response type in cat MGM neurons, comprising about 75% of the observed responses to clicks (Rouiller et al. 1981). Interestingly, a higher proportion of MGM neurons were able to synchronize to high modulation frequencies (>100 Hz) than MGV neurons (Fig. 4, right column) or phase-lock to low-frequency tones (Rouiller et al. 1981; Wallace et al. 2007). In addition to subdivisional differences, Rodrigues-Dagaeff et al. (1989) found there is a significant rostro-caudal gradient within cat MGV, such that neurons in the rostral half were much more likely to produce synchronized responses and at higher modulation frequencies.
In addition to the stimulus-synchronized responses in primate MGV, the MGAD has properties that suggest that it may be specialized for processing temporal modulations or other timing-dependent cues, such as those for sound localization. MGAD neurons responded to click and SAM stimuli with higher precision and with higher maximal synchronized frequencies than MGV neurons (Bartlett and Wang 2011). Furthermore, they were often highly responsive to either tone or broadband noise carriers, whereas MGV neurons were often preferentially responsive to tone or narrowband noise stimuli (Bartlett and Wang 2011), suggesting that MGAD neurons were specialized for temporal processing at the expense of fine spectral selectivity. Thus, MGB neurons are able to represent robustly periodic temporal modulations in the 1-50 Hz range with stimulus-synchronized responses, and some neurons are able to respond to higher temporal modulations up to about 200-300 Hz (Creutzfeldt et al. 1980, Bartlett and Wang 2007, 2011). In addition to simply representing the periodicity of a stimulus, representation of envelope shape is important for discriminating between sounds with similar periodicities (Malone et al. 2007; Zheng et al. 2008), and most speech sounds have non-sinusoidal envelopes. For slow modulations (~ 4-20 Hz), many MGB neurons represent envelope shape isomorphically, meaning that the firing rate is sinusoidally modulated over the entire stimulus period and generally follows the envelope contour (Bartlett and Wang 2008). This may change for very slow modulations (< 4 Hz), where the rate-level characteristics (monotonic or non-monotonic) become important determinants of the response (Malone et al. 2007). For faster modulations, when the number of spikes/period becomes <1, MGB neurons no longer follow the envelope contour closely, even though they still may be sensitive to differences in envelope shape (Bartlett and Wang 2008). An example of a synchronized MGV neuron with an isomorphic envelope representation from a marmoset is shown in Fig. 4 (bottom row).
Most studies of temporal processing in MGB have focused on the stimulus-synchronized responses to SAM or click stimuli that would be appropriate for speech sound recognition in quiet. This overlooks an interesting transformation that occurs in the MGB for rapid temporal modulations (>100 Hz), such that synchronized IC outputs are transformed to non-synchronized rate responses in MGB whose rates change with changing modulation frequency. Non-synchronized responses have been observed in the MGB of awake marmosets (Bartlett and Wang 2007) and anesthetized cats (Rouiller et al. 1981). This transformation from synchronized outputs to non-synchronized responses is then repeated in the auditory thalamocortical transformation (Lu et al. 2001) for a lower modulation frequency range (>50 Hz). The non-synchronized responses in MGB neurons comprise about 20-25% of the observed responses to click stimuli in MGV and the anterodorsal division. In the posterodorsal division (MGPD) of marmosets, non-synchronized responses are the main type of response (75% of responses) (Fig. 4, middle column, Fig. 5) (Bartlett and Wang 2011). These responses are high-pass, meaning that there is little or no sustained evoked response to low modulation frequencies, and they are typically longer-latency than synchronized responses (Bartlett and Wang 2007). This is in contrast to most neurons in MGV, which exhibit low-pass or band-pass rate responses. Similar to some IC neurons (Krishna and Semple 2000), a significant proportion of MGB neurons in all MGB subdivisions have synchronized responses for lower modulation frequencies and non-synchronized responses for high modulation frequencies, sometimes separated by a range of modulation frequencies that is inhibitory (Bartlett and Wang 2007). Although the cellular basis for this transformation is not fully known, a computational model of MGB neurons based on experimental synaptic data (Bartlett and Smith 2002) suggests that the non-synchronized response originates from the convergence of multiple IC excitatory terminals that exhibit weak, NMDA-receptor dependent facilitation at short intervals (Rabang and Bartlett 2011). In addition to the temporal to rate code transformation that occurs from the IC to the MGB, there may also be a modulation-frequency dependent latency shift in MGD (Abrams et al. 2011). These studies suggest that rapid modulations >200 Hz are typically represented by changes in firing rate, whereas modulations in the 50-200 Hz range are represented by a combination of stimulus-synchronized responses and rate responses in MGB. For speech sounds, the findings imply that the forms of neural representations for different speech components will be different and they will be conveyed by partially overlapping MGB populations.
Cellular bases for temporal processing - intrinsic properties of MGB neurons
In thinking about how animals and people perceive, process, and respond to sounds, it is important to understand that these complex, macroscopic processes occur due to a myriad of cellular and subcellular interactions between neurons in the auditory pathways. One way that MGB neurons may regulate how their responses to synaptic input are processed is through their membrane properties and intrinsic conductances, which can produce tradeoffs in sensitivity and fidelity.
It is necessary to measure subthreshold currents and potentials intracellularly, in contrast to the extracellular single-unit recordings that mainly measure a neuron’s spiking output. Nearly all intracellular recordings are obtained from brain slices that have been removed from an animal with the local neural circuitry mostly intact. The brain slice preparation has the advantages of more stable intracellular recordings, the ability to apply drugs specifically, and the ability to selectively stimulate inputs to a neuron, such as only IC inputs or only corticothalamic inputs.
The voltage responses of MGV and MGD neurons to positive current injections at depolarized and hyperpolarized membrane potential (Vmem) have been observed for thalamocortical neurons throughout the thalamus and are a hallmark of thalamocortical neurons across species (Jahnsen and Llinas 1984; Sherman and Guillery 1996; McCormick and Bal 1994). At resting potentials in the -55 to -60 mV range, depolarizing current pulses evoke a depolarization that can reach spiking threshold (Fig. 6) (Hu et al. 1994, Tennigkeit et al. 1996, Bartlett and Smith 1999). The trains of action potentials elicited at depolarized potentials have been termed the tonic, or sustained, firing mode (Jahnsen and Llinas 1984; Sherman and Guillery 1996; McCormick and Bal 1994; Sherman and Koch 1986), similar to other neurons throughout the brain. When the membrane potential becomes hyperpolarized to approximately −70 mV, depolarizing current injections lead to a large, long-lasting depolarization which generates one or more action potentials at frequencies >250 Hz (Fig. 6B) (Hu 1995; Tennigkeit et al. 1996, 1997; Bartlett and Smith 1999). The large calcium-dependent depolarization and high-frequency spikes are referred to as thalamic “bursts”, and the hyperpolarized state is referred to as the burst mode (Jahnsen and Llinas 1984, Tennigkeit et al. 1996, Sherman 2001). During sleep, bursts contribute to synchronizing the slow brain rhythms (Sherman 2001). Strong inhibition will hyperpolarize the neuron, and then the offset of inhibition will produce a burst response (Fig. 6C, offset responses in Fig. 3) (Yu et al. 2004), which is commonly seen with lateral inhibition or in non-monotonic MGB neurons following loud sounds (Bartlett and Wang 2011). This means that offset burst responses can be used to signal the cessation of a sound or sound feature. Second, bursts can amplify small inputs to MGB neurons (Hu et al. 1994; Bartlett and Smith 1999, Massaux et al. 2004) This may come at a cost of the ability to represent rapid acoustic changes, since it takes approximately 100 ms to recover from a burst and produce a second burst (Sherman 2001; Castro-Alamancos 2002). Given this, it is thought that bursts may contribute to signal detection while single spikes may be more important in maintaining the fidelity of a neuron’s inputs at higher modulation frequencies (Sherman 2001). If this is the case, one potential consequence would be that different weak stimuli could be detected by burst responses but could not be discriminated by their neural responses. In vivo recordings of MGB neurons have demonstrated that burst responses are more selective in their frequency tuning in response to tones than single spikes for awake and anesthetized animals (Massaux et al. 2004). Since most speech fluctuations important for intelligibility occur at <10 Hz (Elliott and Theunissen 2009), burst responses could potentially be important for robust signaling of syllabic onsets and offsets in speech, whereas tonic responses could be important for finer discrimination tasks that are more sensitive to acoustic degradation.
Figure 6. Intrinsic membrane properties of neurons in different MGB subdivisions.
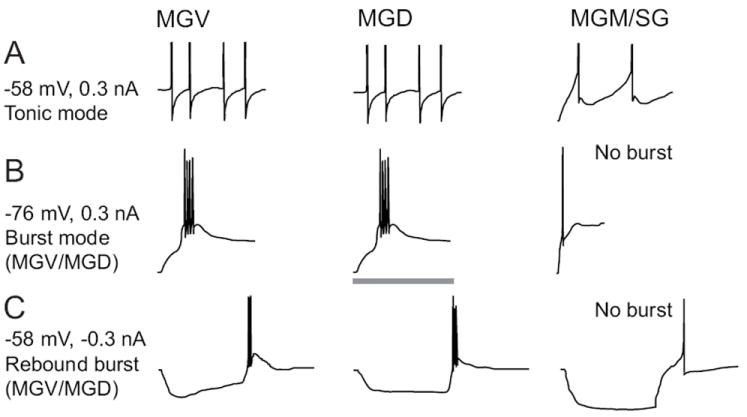
A: Intracellular voltage responses to injections of depolarizing currents. The resting membrane potential (-58 mV) and magnitude of injected current are shown to the left. Depolarization of MGV (left) and MGD (middle) neurons produces a series of single action potentials with monophasic afterhyperpolarizations (AHPs). MGM/SG (right) neurons produces single spikes that adapt and have biphasic AHPs due to activation of a small-conductance, calcium-activated potassium channel. B: Injection of depolarizing current starting from a hyperpolarized membrane potential (-76 mV) evokes burst responses in MGV and MGD neurons. The burst response consists of a large calcium-dependent depolarization evoked by activation of T-type calcium channels and 2-5 high-frequency action potentials (250-500 Hz) evoked at the peak of the calcium burst. About half of MGM/SG neurons produce calcium bursts, while about half have weak or absent calcium bursts, such as the example shown. C: Injection of hyperpolarizing current produces “rebound” burst responses in MGV and MGD neurons and about half of MGM/SG neurons. The other half of MGM/SG neurons fail to evoke a rebound burst, which is quite unusual for neurons in most thalamic nuclei. The gray bar represents 100 ms in A, 40 ms in B, and 200 ms in C.
Given the relative uniformity of thalamocortical neuron properties, it was surprising and interesting to find that many neurons in MGM, the SG, and the posterior intralaminar nucleus (Smith et al. 2006) differed in many key aspects from MGV, MGD and other thalamocortical neurons (Fig. 6, right column). Most notably, many MGM/SG neurons do not have different burst and tonic modes of response because they lack or nearly lack a calcium-mediated burst response at hyperpolarized potentials. This also implies that MGM/SG neurons may be independent from the sleep rhythms imposed by burst responses and interactions between thalamus and cortex, meaning that the MGM/SG neurons could potentially maintain responsiveness to behaviourally-relevant sounds during sleep (e.g. baby cries).
Cellular bases for temporal processing - synaptic properties of MGB neurons
It is clear from the previous sections that MGB neurons in different subdivisions represent sound stimuli quite differently. Between MGV and MGD neurons, there are differences in connectivity and cell morphology, but only minor differences in intrinsic properties. Therefore, significant differences in auditory responses are likely to originate mainly from the inherited tuning of their inputs and the way that afferent spike trains are transformed by the synapses. Previous studies of the IC input to MGB neurons have suggested that there are differences in these inputs to MGV and MGD neurons (Calford and Aitkin 1983, Hu et al. 1994, Bartlett and Smith 1999, 2002, Lee and Sherman 2010). The IC excitatory inputs to MGB neurons activate both faster AMPA and slower NMDA glutamate receptors on MGB neurons (Hu et al. 1994, Bartlett and Smith 1999, 2002; Smith et al. 2007), similar to other sensory inputs to thalamic nuclei (Turner and Salt 1998).
However, synaptic properties of the IC excitatory inputs differentiate the three main subdivisions in rodents and are consistent with their in vivo response properties. Using brain slice preparations, synaptic stimulation of IC inputs to MGV neurons often generates large, short-latency, all-or-none excitatory postsynaptic potentials (EPSPs) (Fig. 7A) or excitatory postsynaptic currents (EPSCs) (Hu et al. 1994, Bartlett and Smith 1999, Lee and Sherman 2010). These postsynaptic responses are similar to those observed in other sensory thalamic nuclei (Turner and Salt 1998, Reichova and Sherman 2004) and are referred to as “driver” inputs due to their ability to generate spiking activity with a small number of inputs (Sherman and Guillery 1998). In response to repetitive synaptic stimulation, the large EPSPs in MGB exhibit significant synaptic depression for stimulation >10 Hz, such that 40-50 Hz stimulation will depress EPSP or EPSC amplitudes by 40-65% (Bartlett and Smith 2002, Lee and Sherman 2010). In MGD, these driver-like responses are uncommon. Typically, EPSPs evoked in MGD neurons are smaller and occur at a longer latency than the large excitatory inputs in MGV (Fig. 7B) (Bartlett and Smith 1999, 2002). Repetitive stimulation of IC afferents to MGD yields weak depression, or in many cases, synaptic facilitation (Bartlett and Smith 2002, Lee and Sherman 2010). Surprisingly, nearly half of MGV neurons have EPSPs with similar characteristics to MGD neurons (Bartlett and Smith 1999, Lee and Sherman 2010). The dichotomy of large, depressing inputs and small, facilitating inputs has significant implications for the temporal representation of periodic or repetitive features of sound. Large inputs are able to generate stimulus-synchronized spiking responses in MGV neurons up to a cutoff frequency, after which the synaptic depression causes the responses to become subthreshold (Bartlett and Smith 2002), similar to the responses to repetitive click or SAM stimuli in the MGV in vivo (Bartlett and Wang 2007, 2011). Small inputs are subthreshold for low repetition rates or modulation frequencies, but frequency-dependent facilitation permits the inputs to generate longer-latency spikes at higher modulation frequencies, similar to the non-synchronized responses observed in MGD neurons (Bartlett and Wang 2011). Overall, the synaptic properties in MGV and MGD are consistent with their involvement in the generation of divergent responses to sounds, with slow and rapid temporal modulations represented by synchrony and rate codes, respectively.
Figure 7. Excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs) in MGB neurons.
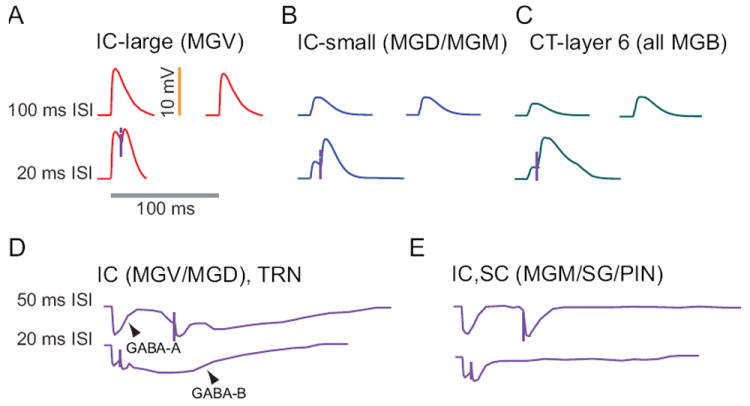
Top row, 100 ms interstimulus interval (ISI): Intracellular recordings of synaptic potentials evoked by sound or electrical stimulation of IC axons show that about half of MGV neurons produce large EPSPs (A). For 100 ms ISI, there is little difference between the first and second EPSPs. Small excitatory IC responses (B) are evoked in nearly all MGD neurons and about half of MGV neurons. Corticothalamic responses are similar throughout MGB, consisting of quite small EPSPs. Even for 100 ms ISI, corticothalamic responses (C) produce significant facilitation of their responses. Second row, 20 ms ISI. For briefer ISI, MGV neurons receiving large IC inputs (A) exhibit strong synaptic depression that limits the amount of depolarization produced by the second EPSP. By contrast, MGD and MGV neurons that receive small terminal IC input produce synaptic facilitation for short ISI (B). Corticothalamic inputs (C) demonstrate strong synaptic facilitation and show an enhanced NMDA component, especially for repetitive stimulation. Third row, 50 ms ISI: Inhibition from IC or TRN produces very similar IPSPs in MGV and MGD neurons (D). in MGV and MGD and IC and SC inhibition in MGM/SG/PIN (E) Electrical stimulation of their axons produces a short-latency GABAA receptor mediated response and a long-latency, metabotropic GABAB receptor mediated response that lasts for 200-400 ms. For 50 ms ISI, there is little depression of the GABAA response, and the GABAB hyperpolarization is just beginning when the second response is evoked. MGM neurons receive inhibitory inputs from IC and the superior colliculus. They differ from inputs to MGV and MGD in that there is no clear GABAB response (E). Bottom row, 20 ms ISI: For shorter ISI in MGV and MGD neurons, the GABAA induced hyperpolarization is slightly larger for the second pulse, indicating little or no depression of the early inhibitory response (D). The GABAB responses merge to produce a larger hyperpolarization than the GABAB IPSPs evoked by single stimuli. MGM responses are similar but lack the GABAB response (E).
MGM and SG neurons show similar EPSPs after IC stimulation to those in MGD, with clear convergence of numerous small IC inputs as stimulation strength increases. They exhibit little synaptic depression or facilitation (Smith et al. 2007). These neurons also receive inputs from the upper layers (SG) and deep layers (MGM) of the superior colliculus, which generate small EPSPs. Consistent with the local axon collaterals of MGM and SG neurons, stimulation of IC or superior colliculus inputs generates a second, longer-latency EPSP in over ¼ of MGM/SG neurons (Smith et al. 2007), which may sustain neural responses to longer-duration sounds.
As mentioned earlier, in the MGB of cats and primates, some IC axons terminate on inhibitory interneurons (Winer et al. 1996), similar to the organization of visual and somatosensory thalamus. Unlike the visual or somatosensory systems, the auditory system has an additional unique sensory projection to thalamus. Almost one-third of the ascending inputs from the IC to all subdivisions of MGB are inhibitory and GABAergic (Fig. 10D,E) (Peruzzi et al. 1997). These inhibitory inputs are conducted by large-caliber IC axons that are faster conducting than most excitatory IC inputs (Bartlett and Smith 1999). The functional role of this projection is not known, but the inhibition is able to control the ability of IC excitatory inputs to generate action potentials and to control the timing of them (Bartlett and Smith 1999). For processing of complex sounds, inhibition may suppress certain harmonics, enhance temporal sequence selectivity, or create the sharp frequency and sound level tuning found in MGB neurons (Fig. 3). Further work needs to be done to separate inhibitory contributions from the IC and those from MGB interneurons.
Neurons in all MGB subdivisions also receive excitatory feedback from auditory cortex and inhibitory feedback from thalamic reticular nucleus (TRN) neurons. The corticothalamic terminals originating from layer 6 auditory cortex neurons form the most profuse inputs to MGB neurons, decorating distal MGB neuron dendrites with numerous terminals (Bajo et al. 1995, Bartlett et al. 2000). Although these synapses are numerous, they are quite small and elicit only small EPSPs when stimulated. However, synchronous stimulation of corticothalamic axons can elicit robust spiking activity in thalamic neurons (Deschenes and Hu 1990; Turner and Salt 1998; Golshani et al. 1998, Bartlett and Smith 1999) via massive facilitatory responses (Fig. 7C), such that the second and subsequent EPSPs can be three times as large as the original EPSPs. These results suggest that under appropriate conditions where multiple corticothalamic axons are coactivated, the small inputs can collectively drive a large response in MGB neurons. In effect, the cortex potentially has the ability to “take over” thalamic firing under conditions where multiple cortical neurons are coactivated even at low frequencies (>5 Hz). As mentioned when discussing corticothalamic connectivity, layer 5 neurons in primary auditory cortex project to non-primary regions of auditory thalamus to end in large terminals (Rouiller and Welker 1991, Bajo et al. 1995, Bartlett et al. 2000). The sparsely distributed layer 5 terminals are considered “drivers” and are similar to the large IC inputs to MGV neurons in that they produce large EPSPs that show significant depression and lack a metabotropic component (Reichova and Sherman 2004).
Finally, there is an inhibitory feedback from thalamic reticular nucleus (TRN) neurons to MGB neurons that completes a short feedback loop by which collaterals of MGB thalamocortical projections excite TRN neurons and then TRN neurons project back to MGB. TRN neurons are also excited by collaterals of the small terminal layer 6 corticothalamic neurons (Lam and Sherman 2010). TRN inhibitory responses in MGB neurons are similar to the IC IPSPs in most respects tested thus far (Bartlett and Smith 2002).
Taken together, the responses are MGB neurons are dictated by excitatory and inhibitory sensory inputs mainly from IC, excitatory feedback from auditory cortex, and inhibitory feedback from TRN.
MGB responses to vocalizations
Vocalizations are an important class of stimuli to examine for their neural representations, because they combine a high acoustic complexity with behavioral relevance. Human fMRI studies have shown significant speech-related modulation of MGB BOLD activity that is correlated with discriminating speech between different speakers (von Kriegstein et al. 2008) and speaker emotion (Ethofer et al. 2012). Neurons in the awake guinea pig and monkey MGB have been shown to respond to natural, species specific sounds that have complex frequency and amplitude modulated components (Creutzfeldt et al. 1980; Symmes et al. 1980, Allon et al. 1981). In these studies, which included neurons from all MGB subdivisions, the responses of neurons to natural sounds could often be predicted by their frequency tuning curves and by the temporal envelopes of the vocalizations, especially for rapid changes in amplitude. This was also the case for anesthetized guinea pigs (Suta et al. 2007), and in the case of some low-frequency MGV and MGM neurons, synchronizing to the fine structure of a guinea pig purr could be observed (Wallace et al. 2007). However, neurons in the primate MGD have less stable and less vigorous responses than the other regions (Allon et al. 1981). A recent study in anesthetized guinea pigs demonstrated that the temporal pattern of MGB firing rate changes measured over short time windows (10-50 ms) conveys much more information about call identity and characteristics than the average firing rate (Huetz et al. 2009). This indicates that the within-call changes in sound features can be detected. There was little or no difference in firing rates between natural and time-reversed calls in rats and guinea pigs (Philibert et al. 2005, Huetz et al. 2009), but there were clear differences in spike patterns in anesthetized animals. Therefore, contextual and sequence effects strongly influence the pattern of responses in MGB neurons. In the auditory cortex of marmosets (Wang and Kadia 2001), firing rates were significantly higher for natural (forward) calls and spike patterns were different. Fig. 8 shows an example of a neuron in marmoset MGV that demonstrates significant rate differences and spike pattern differences between forward and reversed calls for two call types. For the peep-trill vocalization (Fig. 8, top two panels), the firing rates are differ slightly but significantly, mainly owing to a greatly enhanced response to the onset of the trill component in the natural call (highlighted in red box). For the phee call, there was an overall enhanced response for the natural call, suggesting in both cases that the order of vocalization components matters substantially to magnitude and timing of the neural responses. While somewhat obvious, this further supports the idea that short-term changes in firing pattern will have a significant impact on discriminating between similar sounds.
Figure 8. Example of responses of neuron to natural and reversed marmoset vocalizations.
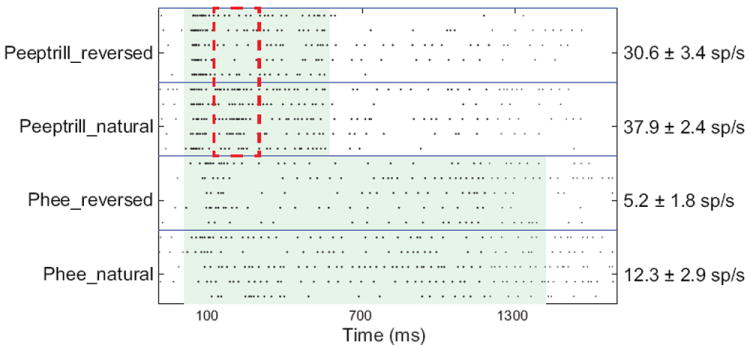
Dot raster representations. Each row represents a single sound presentation. Dots represent action potentials. Neuron was in MGV of marmoset. The sound level for all vocalizations was 50 dB SPL. Firing rates are mean ± SD. Red dashed lines in the top two boxes indicate a 200 ms time window over which there was the most significant difference in firing rates between the natural and reversed peeptrill calls.
MGB-effects of cortical and TRN feedback
Perception of sounds results not from the simple decomposition and transmission of sound features, but it is actively constructed by the interplay of ascending sensory input, intracortical processing, and feedback processing from auditory cortex to thalamus and other subcortical structures. MGB responses to acoustic stimuli are typically attributed to excitation by IC axons, but studies have indicated that corticothalamic feedback can alter MGB responses. This may be particularly important in language processing, where grammatical and semantic understanding can improve perceptual capabilities by predicting likely utterances. Cortical feedback can either be direct and excitatory from cortex or indirect and inhibitory due to auditory cortical excitation of inhibitory thalamic reticular nucleus (TRN) or interneuron inputs. During cortical desynchronization of the EEG, which indicates cortical arousal, cooling of the auditory cortex significantly reduces the spontaneous firing rate of MGB neurons in cats (Orman and Humphrey 1981), indicating a tonic excitation of MGB neurons by cortex. Corticothalamic inputs appear to facilitate MGB responses to noise in anesthetized cats or to tones in awake bats, but only when the cortical and thalamic regions are tuned to the same frequencies (Zhang et al. 1997; He 1997). This is referred to as “matched” selection or “egocentric” selection. For cortical and thalamic sites whose best response frequencies do not match, the corticothalamic input diminishes the MGB response (Zhang et al. 1997; He 1997) through activation of the inhibitory TRN. Furthermore, inactivation of the cortex in non-matched cortical and MGB areas can shape frequency tuning (Suga and Zhang 1997) and can shift the best responding frequency of the receptive field, but not the shape of the receptive field in bats (Zhang et al. 1997). He (2003) compared MGV and non-MGV responses to electrical stimulation of auditory cortex in anesthetized guinea pigs. MGV neurons are generally facilitated by auditory cortex stimulation, and this is strongest when the preferred frequencies of MGB and cortex neurons are similar (He et al. 2002, He 2003). Neurons in non-MGV nuclei (dorsal and shell nuclei in guinea pig) were inhibited in their onset responses to noise stimuli or had their offset responses enhanced by the cortical stimulation.
The functional data on the effect of matched and unmatched cortical inputs on MGB responses is supported by anatomical data on axonal projections of TRN to MGB, where many TRN axons project to lateral tonotopic regions from where they receive MGB and cortical input, while some TRN axons remain tonotopic (Kimura et al. 2007). Furthermore, auditory TRN neurons receive multimodal inputs (Kimura et al. 2007, Yu et al. 2011) and can project to multiple divisions within MGB (Crabtree et al. 1998, Kimura et al. 2007). These results suggest that the auditory cortex can provide a dynamic gain enhancement or suppression of MGB neurons through its influence on TRN. Such focal control over MGB excitability suggests that cortical feedback can enhance detectability of weak or degraded sounds and can enhance discriminability of closely related complex sounds. This gain control may be important in auditory attention, such as focusing on a single voice, and in enhancement of MGB representations by cortex through context and memory driven enhancements in cortical activity, such as predicting words in sentences. However, the functional influence of cortex on MGB neurons has mainly been demonstrated in cases of strong electrical stimulation, so the function of the profuse corticothalamic input during ongoing auditory activity or communication is still poorly understood.
Modulation of MGD and MGM/SG responses by adaptation, reward and non-auditory stimuli
The basic finding from multiple studies is that MGV neurons generally do not exhibit long-term changes in their receptive field properties and are not strongly influenced by non-auditory inputs or reward (Edeline and Weinberger 1991a, Komura et al. 2001, 2005). This makes sense if a main function of MGV is to provide auditory cortex with a reliable, context-independent representation of auditory stimuli. By contrast, MGD, SG, and MGM neurons have responses that are strongly modulated by adaptation, visual cues, reward cues, and behavioral context (Edeline and Weinberger 1991b, 1992, Lennartz and Weinberger 1992, Komura et al. 2001, 2005). Such complex integration of auditory and non-auditory information may be important in processes such as novelty detection, lip-reading or identifying a speaker or a noise source. Stimulus-specific adaptation, in which a stimulus that is repeated evokes progressively smaller responses, is present but weak in MGV (Bauerle et al 2011) and is strongest in MGD and MGM (Anderson et al. 2009, Antunes et al. 2010). Similar results have been seen using phonemic stimuli and measuring the evoked potentials in MGB, with little or no adaptation in MGV but significant adaptation in MGM (Kraus et al. 1994).
In experiments testing non-auditory cues affecting MGB, neurons were recorded while awake rats listened to sounds in the presence or absence of a light flash or a reward (Komura et al. 2001, 2005). Many neurons increased their responses when a sound was paired with a light or reward, but the facilitated responses went away when the association was no longer present. This implies that MGD and MGM neurons will integrate auditory and non-auditory inputs to provide a situation-dependent response to non-primary auditory cortices. For the sustained, reward-related increases in firing rate (Komura et al. 2001), this could be a neural correlate of a short-term memory built upon the stimulus-reward association. Similar results were found for the rhesus monkey during an auditory behavior task, during which two-thirds of MGB neurons were modulated by behavioral state, though the recorded subdivisions were not reported (Gilat and Perlman 1984). Another way to investigate the influence of non-auditory learned associations on MGB responses is to use a Pavlovian association protocol in which a sound (the conditioned stimulus, CS) is consistently paired with another stimulus, such as a light or a mild shock (the unconditioned stimulus, US). If this tone-shock pairing is done for MGV neurons, there are short-term changes in the best frequency of the recorded neurons towards the CS tone frequency, but only if the CS is within one-eighth octave from the neuron’s best frequency (Edeline and Weinberger 1991a). Performing the same associative tone-shock pairing produces in MGD and MGM neurons a significant decrease in the best frequency response and a corresponding increase in the CS frequency, and this altered receptive field can last for over 24 hours (Edeline and Weinberger 1991b, 1992, Lennartz and Weinberger 1992, O’Connor et al. 1997).
Changes in thalamic structure and function in neurological disorders
The primary roles of thalamocortical neurons, especially in the sensory pathways, are to control which information reaches the cerebral cortex and to shape the neural representations of that information. Similar to most brain regions, overall, there are very few physiological recordings of MGB in humans, so nearly all estimates of MGB neural representations in humans arise from non-invasive methods such as mid-latency auditory evoked potentials and fMRI. Activation of the MGB (fMRI BOLD signal) in response to rapidly varying sounds has been correlated with speech recognition scores (von Kriegstein et al. 2008). Disruption or alteration of the interplay between MGB and cortex by neurological disorders can significantly affect how sounds are processed in MGB and therefore can affect hearing and language abilities. Unilateral lesions of the MGB can largely abolish input to the ipsilateral AC (Fischer et al. 1995). In these cases, patients can still detect sounds easily and mainly exhibit perceptual deficits when different sounds are presented to the two ears (dichotic sounds) (Fututake and Hattori 1998; Wester et al. 2001). Similar to other central auditory nuclei, substantial auditory difficulties mainly occur when lesions are bilateral, preventing nearly all auditory input from reaching cerebral cortex (Hausler and Levine 2000).
FOXP2 and MGB
FOXP2 is a transcription factor whose proper function is critical for speech, language and other natural communication in humans, rodents, and birds. Mutations in FOXP2 lead to deficits both in speech production and linguistic perceptual processing in humans (Lai et al. 2003), aberrant vocalizations in mice (Fujita et al. 2008), and impairments in auditory learning (Kurt et al. 2012). In mammals, FOXP2 is enriched in the auditory thalamus (MGB), deep layers of auditory cortex, and sub-thalamic auditory structures including the inferior colliculus and nuclei of the lateral lemniscus (Campbell et al. 2009). This enrichment is nucleus-specific, since it is significantly less enriched in the visual thalamus (Horng et al. 2009, Campbell et al. 2009). In addition to its localization within the MGB, FOXP2 is also expressed in structures involved in vocal production, such as the cerebellum and basal ganglia (Lai et al. 2003, Ferland et al. 2003). Further work remains to be done to elucidate the regulatory targets of FOXP2 in the MGB.
Schizophrenia
Consistent with its role in dynamically filtering auditory input to the cerebral cortex, the MGB responds differently in schizophrenics versus non-schizophrenics. Many schizophrenics have difficulties ignoring competing sounds to focus on a target sound. In one study, urban noise consisting of multitalker speech, music, and other sounds produced a hyperactivation of MGB and prefrontal cortex blood oxygenation level dependent (BOLD) fMRI functional magnetic resonance imaging (fMRI) responses (Tregellas 2009), suggesting that the MGB cannot filter out irrelevant sounds as it normally would. Another common symptom in schizophrenia is the inability to distinguish easily between external speech, one’s own voice, and internally-generated speech. Control and schizophrenic patients both show activation of the thalamus (MGB and adjacent pulvinar nucleus) when attempting to distinguish distorted or undistorted versions of their own voice and another voice, but a greater magnitude of activation is correlated with the performance in this task and with not being schizophrenic (Kumari et al. 2010). This implies that the thalamus is involved in complex perceptual tasks such as voice recognition, and it implies that the thalamus is not properly engaged in tasks such as this in schizophrenia.
Dyslexia
Dyslexics have reading difficulties, often coupled with auditory and other sensory deficits (Fitch et al. 1994; Goswami et al. 2002; Shaywitz and Shaywitz 2005). A recent fMRI study found that impairments in phonological processing were correlated with deficiencies in MGB activation and with reading scores (Diaz et al. 2012). Auditory and reading deficits observed in people with dyslexia have been correlated with anatomical abnormalities in the MGB (Galaburda et al., 1985, 1994; Livingstone et al. 1991). Specifically, the brains of dyslexics often contain cortical malformations known as microgyria that lead to a decrease in the number of large cells in the MGB (Galaburda et al. 1994). Similar cortical malformations can be induced experimentally in rats by placing a freezing probe on the skull overlying somatosensory cortex in early postnatal rats (Fitch et al. 1994; Herman et. al 1997; Rosen et. al 2006; Escabi et al. 2007). Microgyric rats exhibit anatomical abnormalities in the auditory thalamus and auditory processing deficits similar to humans (Herman et al. 1997; Clark et. al 2000; Peiffer et al. 2002, 2004a,b). Specifically, there have been demonstrated reductions in temporal processing in both animals (Fitch et al. 1994, Peiffer et al. 2002) and humans (Goswami et al. 2002). These deficits can take multiple forms, such as difficulty in discriminating tone sequences when the tones or tone durations were brief or in discriminating between slowly versus rapidly rising envelopes. There currently remains a major knowledge gap regarding the cellular alterations of neurons in the auditory pathway that would underlie the behavioral deficits. One hypothesis regarding the origin of MGB alterations in microgyric rats is that the corticothalamic feedback is disrupted beginning in development, which is supported by studies showing a lack of corticothalamic feedback from the cortex within microgyria and showing altered corticothalamic neuron morphologies near microgyria (Rosen et al 2000; DiRocco et al. 2002).
Alzheimer’s disease
An early predictor of cognitive decline in Alzheimer’s disease is the presence of central auditory deficits, especially for complex signals such as speech in noise (Gates et al. 2002). There are changes in the IC and MGB even before the presence of the standard neuropathological markers of neurofibrillary tangles made of tau protein or senile plaques made of beta-amyloid protein (Baloyannis et al. 2009). When Golgi-labeling was used to label the neurons from recently deceased Alzheimer’s patients, it was found that the density of spines on the dendrites of IC and MGB neurons, which typically indicate where synapses are formed, were much lower in Alzheimer’s patients than age-matched patients (Baloyannis et al. 2009). In addition, the morphology of the mitochondria in auditory structures appear abnormal in a large proportion of the dementia patients. These findings suggest that subcortical auditory structures may be an early and sensitive predictor of dementia and that auditory testing may be a simple way to detect early signs of cognitive decline.
Conclusion
Speech perception involves complex, rapid and precise neural representations of relevant features such as spectral peaks, modulations on multiple timescales, contrast from noise, location of sound sources, and relationships to other sensory features and behavioural context. The MGB participates in the active shaping of these neural representations that then go to auditory cortex and to subcortical targets to shape our perceptions. MGB neurons do not act as a simple gate that may be open or shut to the ascending one-way transmission of sensory input, with only slight or long-term modulations by the cortex. Rather, there is a complex, bidirectional convergence of information that is evaluated by MGB neurons. To do this, MGB neurons have some region specific specialization of their sensory inputs, such as rapid feedforward inhibition and facilitating excitatory inputs, as well as subdivisions that are mainly involved in feature extraction (MGV), feature integration (MGD), and feature learning and sensory based action (MGM).
The auditory thalamus integrates sensory and cortical inputs for processing sounds
It has three main subdivisions with complementary responses to sounds and context.
It actively shapes auditory information necessary for processing complex sounds such as speech.
Acknowledgments
Dr. Xiaoqin Wang provided facilities and mentorship for some of the work described here. Past and current work by Dr. Bartlett has been generously funded by the Hearing Health Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DA, Nicol T, Zecker S, Kraus N. A possible role for a paralemniscal auditory pathway in the coding of slow temporal information. Hear Res. 2011;272(1-2):125–134. doi: 10.1016/j.heares.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitkin LM, Dunlop CW, Webster WR. Click-evoked response patterns of single units in the medial geniculate body of the cat. Journal of Neurophysiology. 1966;29:109–123. doi: 10.1152/jn.1966.29.1.109. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Prain SM. Medial geniculate body: unit responses in the awake cat. Journal of Neurophysiology. 1974;37:512–521. doi: 10.1152/jn.1974.37.3.512. [DOI] [PubMed] [Google Scholar]
- Aitkin L. Rate-level functions of neurons in the inferior colliculus of cats measured with the use of free-field sound stimuli. Journal of Neurophysiology. 1991;65:383–92. doi: 10.1152/jn.1991.65.2.383. [DOI] [PubMed] [Google Scholar]
- Allon N, Yeshurun Y, Wollberg Z. Responses of single cells in the medial geniculate body of awake squirrel monkeys. Experimental Brain Research. 1981;41:222–232. doi: 10.1007/BF00238879. [DOI] [PubMed] [Google Scholar]
- Altman J, Carpenter MB. Fiber projections of the superior colliculus in the cat. Journal of Comparative Neurology. 1961;116:157–77. doi: 10.1002/cne.901160206. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Roth GL, Aitkin LM, Merzenich MM. The efferent projections of the central nucleus and the pericentral nucleus of the inferior colliculus in the cat. Journal of Comparative Neurology. 1980;194:649–662. doi: 10.1002/cne.901940311. [DOI] [PubMed] [Google Scholar]
- Anderson LA, Malmierca MS, et al. Evidence for a direct, short latency projection from the dorsal cochlear nucleus to the auditory thalamus in the guinea pig. Eur J Neurosci. 2006;24(2):491–8. doi: 10.1111/j.1460-9568.2006.04930.x. [DOI] [PubMed] [Google Scholar]
- Anderson LA, Wallace MN, et al. Identification of subdivisions in the medial geniculate body of the guinea pig. Hearing Research. 2007;228(1-2):156–167. doi: 10.1016/j.heares.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Anderson LA, Christianson GB, Linden JF. Stimulus-specific adaptation occurs in the auditory thalamus. J Neurosci. 2009;29(22):7359–7363. doi: 10.1523/JNEUROSCI.0793-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LA, Linden JF. Physiological differences between histologically defined subdivisions in the mouse auditory thalamus. Hearing Research. 2011;274:48–60. doi: 10.1016/j.heares.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnault P, Roger M. Ventral temporal cortex in the rat: connections of secondary auditory areas Te2 and Te3. Journal of Comparative Neurology. 1990;302:110–23. doi: 10.1002/cne.903020109. [DOI] [PubMed] [Google Scholar]
- Antunes FM, Nelken I, Covey E, Malmierca MS. Stimulus-specific adaptation in the auditory thalamus of the anesthetized rat. PLoS One. 2010;5(11):e14071. doi: 10.1371/journal.pone.0014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Merchán MA, López DE, Rouiller EM. Neuronal morphology and efferent projections of the dorsal nucleus of the lateral lemniscus in the rat. Journal of Comparative Neurology. 1993;334:241–62. doi: 10.1002/cne.903340207. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Rouiller EM, Welker E, Clarke S, Villa AE, de Ribaupierre Y, de Ribaupierre F. Morphology and spatial distribution of corticothalamic terminals originating from the cat auditory cortex. Hearing Research. 1995;83:161–174. doi: 10.1016/0378-5955(94)00199-z. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ. Dendritic pathology in Alzheimer’s disease. J Neurol Sci. 2009;283(1-2):153–157. doi: 10.1016/j.jns.2009.02.370. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Anatomic, intrinsic, and synaptic properties of dorsal and ventral division neurons in rat medial geniculate body. Journal of Neurophysiology. 1999;81(5):1999–2016. doi: 10.1152/jn.1999.81.5.1999. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Effects of paired-pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience. 2002;113(4):957–74. doi: 10.1016/s0306-4522(02)00240-3. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Stark JM, et al. Comparison of the fine structure of cortical and collicular terminals in the rat medial geniculate body. Neuroscience. 2000;100(4):811–28. doi: 10.1016/s0306-4522(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. Journal of Neurophysiology. 2007;97(2):1005–17. doi: 10.1152/jn.00593.2006. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Representation of amplitude modulation envelope in the marmoset auditory thalamus. Soc Neuro Abs. 2008:566.9. [Google Scholar]
- Bartlett EL, Wang X. Correlation of neural response properties with auditory thalamus subdivisions in the awake marmoset. Journal of Neurophysiology. 2011;105(6):2647–67. doi: 10.1152/jn.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskent D, Shannon RV. Frequency transposition around dead regions simulated with a noiseband vocoder. J Acoust Soc Am. 2006;119(2):1156–1163. doi: 10.1121/1.2151825. [DOI] [PubMed] [Google Scholar]
- Bauerle P, von der Behrens W, Kossl M, Gaese BH. Stimulus-specific adaptation in the gerbil primary auditory thalamus is the result of a fast frequency-specific habituation and is regulated by the corticofugal system. J Neurosci. 2011;31(26):9708–9722. doi: 10.1523/JNEUROSCI.5814-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor D, Wang X. Neural response properties of primary, rostral, and rostrotemporal core fields in the auditory cortex of marmoset monkeys. J Neurophysiol. 2008;100(2):888–906. doi: 10.1152/jn.00884.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkeland M, Boivie J. The termination of spinomesencephalic fibers in cat. An experimental anatomical study. Anatomy & Embryology. 1984;170:265–277. doi: 10.1007/BF00318730. [DOI] [PubMed] [Google Scholar]
- Bitterman Y, Mukamel R, Malach R, Fried I, Nelken I. Ultra-fine frequency tuning revealed in single neurons of human auditory cortex. Nature. 2008;451(7175):197–201. doi: 10.1038/nature06476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Experimental Brain Research. 1994;98:261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- Calford MB. The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci. 1983;3(11):2350–2364. doi: 10.1523/JNEUROSCI.03-11-02350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Aitkin LM. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. Journal of Neuroscience. 1983;3:2365–2380. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P, Reep RL, Stoll ML, Ophir AG, Phelps SM. Conservation and diversity of Foxp2 expression in muroid rodents: functional implications. J Comp Neurol. 2009;512(1):84–100. doi: 10.1002/cne.21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Watson SJ., Jr Connections of some auditory-responsive posterior thalamic nuclei putatively involved in activation of the hypothalamo-pituitary-adrenocortical axis in response to audiogenic stress in rats: an anterograde and retrograde tract tracing study combined with Fos expression. Journal of Comparative Neurology. 2000;423:474–91. [PubMed] [Google Scholar]
- Carroll J, Zeng FG. Fundamental frequency discrimination and speech perception in noise in cochlear implant simulations. Hear Res. 2007;231(1-2):42–53. doi: 10.1016/j.heares.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J, Tiaden S, Zeng FG. Fundamental frequency is critical to speech perception in noise in combined acoustic and electric hearing. J Acoust Soc Am. 2011;130(4):2054–62. doi: 10.1121/1.3631563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. Journal of Physiology. 2002;539:567–78. doi: 10.1113/jphysiol.2001.013283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarey JC, Barone P, Irons WA, Samson FK, Imig TJ. Comparison of noise and tone azimuth tuning of neurons in cat primary auditory cortex and medical geniculate body. Journal of Neurophysiology. 1995;74:961–980. doi: 10.1152/jn.1995.74.3.961. [DOI] [PubMed] [Google Scholar]
- Clark MG, Rosen GD, Tallal P, Fitch RH. Impaired processing of complex auditory stimuli in rats with induced cerebrocortical microgyria: An animal model of developmental language disabilities. Journal of Cognitive Neuroscience. 2000;12:828–39. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- Coleman JR, Clerici WJ. Sources of projections to subdivisions of the inferior colliculus in the rat. Journal of Comparative Neurology. 1987;262:215–226. doi: 10.1002/cne.902620204. [DOI] [PubMed] [Google Scholar]
- Crabtree JW. Organization in the auditory sector of the cat’s thalamic reticular nucleus. Journal of Comparative Neurology. 1998;390:167–182. [PubMed] [Google Scholar]
- Creutzfeldt O, Hellweg FC, Schreiner C. Thalamocortical transformation of responses to complex auditory stimuli. Experimental Brain Research. 1980;39:87–104. doi: 10.1007/BF00237072. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Killackey HP, et al. Parvalbumin and calbindin are differentially distributed within primary and secondary subregions of the mouse auditory forebrain. Neuroscience. 2001;105(3):553–569. doi: 10.1016/s0306-4522(01)00226-3. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Thalamic connections of the auditory cortex in marmoset monkeys: core and medial belt regions. Journal of Comparative Neurology. 2006;496:72–96. doi: 10.1002/cne.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Hu B. Electrophysiology and Pharmacology of the Corticothalamic Input to Lateral Thalamic Nuclei: an Intracelluar Study in the Cat. European. Journal of Neuroscience. 1990;2:140–152. doi: 10.1111/j.1460-9568.1990.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Díaz B, Hintz F, Kiebel SJ, von Kriegstein K. Dysfunction of the auditory thalamus in developmental dyslexia. Proc Natl Acad Sci U S A. 2012;109(34):13841–6. doi: 10.1073/pnas.1119828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rocco C, Iannelli A, Tamburrini G. Two anatomical specimens after hemispherectomy. Pediatric Neurosurgery. 2002;37:275. doi: 10.1159/000066207. [DOI] [PubMed] [Google Scholar]
- Doi K, Seki M, Kuroda Y, Okamura N, Ito H, Hayakawa T, Zyo K. Direct and indirect thalamic afferents arising from the vestibular nuclear complex of rats: medial and spinal vestibular nuclei. Okajimas Folia Anatomica Japonica. 1997;74:9–31. doi: 10.2535/ofaj1936.74.1_9. [DOI] [PubMed] [Google Scholar]
- Doron NN, Ledoux JE. Cells in the posterior thalamus project to both amygdala and temporal cortex: a quantitative retrograde double-labeling study in the rat. J Comp Neurol. 2000;425(2):257–274. [PubMed] [Google Scholar]
- Drennan WR, Gatehouse S, Lever C. Perceptual segregation of competing speech sounds: the role of spatial location. J Acoust Soc Am. 2000;114(4 Pt 1):2178–89. doi: 10.1121/1.1609994. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Thalamic short-term plasticity in the auditory system: associative returning of receptive fields in the ventral medial geniculate body. Behavavioral Neuroscience. 1991a;105:618–39. doi: 10.1037//0735-7044.105.5.618. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Subcortical adaptive filtering in the auditory system: associative receptive field plasticity in the dorsal medial geniculate body. Behavioral Neuroscience. 1991b;105:154–75. doi: 10.1037//0735-7044.105.1.154. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106(1):81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Manunta Y, Nodal FR, Bajo VM. Do auditory responses recorded from awake animals reflect the anatomical parcellation of the auditory thalamus? Hearing Research. 1999;131:135–52. doi: 10.1016/s0378-5955(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Manunta Y, Hennevin E. Auditory thalamus neurons during sleep: changes in frequency selectivity, threshold, and receptive field size. Journal of Neurophysiology. 2000;84:934–52. doi: 10.1152/jn.2000.84.2.934. [DOI] [PubMed] [Google Scholar]
- Ehret G, Schreiner CE. Regional variations of noise-induced changes in operating range in cat AI. Hear Res. 2000;141(1-2):107–16. doi: 10.1016/s0378-5955(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Elliott TM, Theunissen FE. The modulation transfer function for speech intelligibility. PLoS Comput Biol. 2009;5(3):e1000302. doi: 10.1371/journal.pcbi.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escabí MA, Higgins NC, Galaburda AM, Rosen GD, Read HL. Early cortical damage in rat somatosensory cortex alters acoustic feature representation in primary auditory cortex. Neuroscience. 2007;150:970–83. doi: 10.1016/j.neuroscience.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Gschwind M, Kreifelts B, Wildgruber D, Vuilleumier P. Emotional voice areas: anatomic location, functional properties, and structural connections revealed by combined fMRI/DTI. Cereb Cortex. 2012;22(1):191–200. doi: 10.1093/cercor/bhr113. [DOI] [PubMed] [Google Scholar]
- Evans EF. The frequency response and other properties of single fibres in the guinea-pig cochlear nerve. J Physiol. 1972;226(1):263–87. doi: 10.1113/jphysiol.1972.sp009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460(2):266–279. doi: 10.1002/cne.10654. [DOI] [PubMed] [Google Scholar]
- Fischer C, Bognar L, Turjman F, Lapras C. Auditory evoked potentials in a patient with a unilateral lesion of the inferior colliculus and medial geniculate body. Electroencephalography and Clinical Neurophysiology. 1995;96:261–7. doi: 10.1016/0168-5597(94)00273-h. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Tallal P, Brown CP, Galaburda AM, Rosen GD. Induced microgyria and auditory temporal processing in rats: a model for language impairment? Cerebral Cortex. 1994;4:260–70. doi: 10.1093/cercor/4.3.260. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110(2):1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Fujita E, Tanabe Y, Shiota A, Ueda M, Suwa K, Momoi MY, et al. Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proc Natl Acad Sci U S A. 2008;105(8):3117–3122. doi: 10.1073/pnas.0712298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Annals of Neurology. 1985;18:222–33. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Menard MT, Rosen GD. Evidence for aberrant auditory anatomy in developmental dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8010–8013. doi: 10.1073/pnas.91.17.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Beiser A, Rees TS, D’Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. Journal of the American Geriatric Society. 2002;50:482–8. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Jr, Björkeland M, Xu Q, Grant G. Organization of the spinocervicothalamic pathway in the rat. Journal of Comparative Neurology. 1988;268:223–33. doi: 10.1002/cne.902680207. [DOI] [PubMed] [Google Scholar]
- Gilat E, Perlman I. Single unit activity in the auditory cortex and the medial geniculate body of the rhesus monkey: behavioral modulation. Brain Research. 1984;324:323–33. doi: 10.1016/0006-8993(84)90043-x. [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BC. Psychoacoustic abilities of subjects with unilateral and bilateral cochlear hearing impairments and their relationship to the ability to understand speech. Scand Audiol Suppl. 1989;32:1–25. [PubMed] [Google Scholar]
- Golshani P, Warren RA, Jones EG. Progression of change in NMDA, non-NMDA, and metabotropic glutamate receptor function at the developing corticothalamic synapse. Journal of Neurophysiology. 1998;80:143–154. doi: 10.1152/jn.1998.80.1.143. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez TH, Galindo-Mireles D, Castaneyra-Perdomo A, Ferres-Torres R. Divergent projections of projecting neurons of the inferior colliculus to the medial geniculate body and the contralateral inferior colliculus in the rat. Hearing Research. 1991;52:17–21. doi: 10.1016/0378-5955(91)90184-b. [DOI] [PubMed] [Google Scholar]
- Goswami U, Thomson J, Richardson U, Stainthorp R, Hughes D, Rosen S, Scott SK. Amplitude envelope onsets and developmental dyslexia: A new hypothesis. PNAS. 2002;99:10911–6. doi: 10.1073/pnas.122368599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. An autoradiographic study of the efferent connections of the superior colliculus in the cat. Journal of Comparative Neurology. 1977;173:629–54. doi: 10.1002/cne.901730403. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. Journal of Anatomy. 1995;187:583–592. [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. Journal of Comparative Neurology. 1998;400:271–86. doi: 10.1002/(sici)1096-9861(19981019)400:2<271::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hashikawa T, Rausell E, Molinari M, Jones EG. Parvalbumin- and calbindin-containing neurons in the monkey medial geniculate complex: differential distribution and cortical layer specific projections. Brain Res. 1991;544(2):335–341. doi: 10.1016/0006-8993(91)90076-8. [DOI] [PubMed] [Google Scholar]
- Hashikawa T, Molinari M, Rausell E, Jones EG. Patchy and laminar terminations of medial geniculate axons in monkey auditory cortex. Journal of Comparative Neurology. 1995;362:195–208. doi: 10.1002/cne.903620204. [DOI] [PubMed] [Google Scholar]
- Häusler R, Levine RA. Auditory dysfunction in stroke. Acta Otolaryngologica. 2000;120:689–703. doi: 10.1080/000164800750000207. [DOI] [PubMed] [Google Scholar]
- He J. On and off pathways segregated at the auditory thalamus of the guinea pig. Journal of Neuroscience. 2001;21:8672–9. doi: 10.1523/JNEUROSCI.21-21-08672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. Corticofugal modulation on both ON and OFF responses in the nonlemniscal auditory thalamus of the guinea pig. Journal of Neurophysiology. 2003;89:367–81. doi: 10.1152/jn.00593.2002. [DOI] [PubMed] [Google Scholar]
- He J, Yu YQ, Xiong Y, Hashikawa T, Chan YS. Modulatory effect of cortical activation on the lemniscal auditory thalamus of the Guinea pig. Journal of Neurophysiology. 2002;88:1040–50. doi: 10.1152/jn.2002.88.2.1040. [DOI] [PubMed] [Google Scholar]
- He J. Modulatory effects of regional cortical activation on the onset responses of the cat medial geniculate neurons. Journal of Neurophysiology. 1997;77:896–908. doi: 10.1152/jn.1997.77.2.896. [DOI] [PubMed] [Google Scholar]
- Henkel CK, Shneiderman A. Nucleus sagulum: projections of a lateral tegmental area to the inferior colliculus in the cat. Journal of Comparative Neurology. 1988;271:577–88. doi: 10.1002/cne.902710408. [DOI] [PubMed] [Google Scholar]
- Herbert H, Aschoff A, Ostwald J. Topography of projections from the auditory cortex to the inferior colliculus in the rat. Journal of Comparative Neurology. 1991;304:103–22. doi: 10.1002/cne.903040108. [DOI] [PubMed] [Google Scholar]
- Herman AE, Galaburda AM, Fitch RH, Carter AR, Rosen GD. Cerebral microgyria, thalamic cell size and auditory temporal processing in male and female rats. Cerebral Cortex. 1997;7:453–464. doi: 10.1093/cercor/7.5.453. [DOI] [PubMed] [Google Scholar]
- Herrera M, Hurtado-García JF, Collia F, Lanciego J. Projections from the primary auditory cortex onto the dorsal cortex of the inferior colliculus in albino rats. Archives of Italian Biology. 1994;132:147–64. [PubMed] [Google Scholar]
- Hicks TP, Stark CA, Fletcher WA. Origins of afferents to visual suprageniculate nucleus of the cat. Journal of Comparative Neurology. 1986;246:544–54. doi: 10.1002/cne.902460410. [DOI] [PubMed] [Google Scholar]
- Hill PR, Hogben JH, Bishop DM. Auditory frequency discrimination in children with specific language impairment: a longitudinal study. J Speech Lang Hear Res. 2005;48(5):1136–1146. doi: 10.1044/1092-4388(2005/080). [DOI] [PubMed] [Google Scholar]
- Holstege G, Collewijn H. The efferent connections of the nucleus of the optic tract and the superior colliculus in the rabbit. Journal of Comparative Neurology. 1982;209:139–75. doi: 10.1002/cne.902090204. [DOI] [PubMed] [Google Scholar]
- Horner K, de Ribaupierre Y, de Ribaupierre F. Neural correlates of cubic difference tones in the medial geniculate body of the cat. Hear Res. 1983;11(3):343–357. doi: 10.1016/0378-5955(83)90066-7. [DOI] [PubMed] [Google Scholar]
- Horng S, Kreiman G, Ellsworth C, Page D, Blank M, Millen K, et al. Differential gene expression in the developing lateral geniculate nucleus and medial geniculate nucleus reveals novel roles for Zic4 and Foxp2 in visual and auditory pathway development. J Neurosci. 2009;29(43):13672–13683. doi: 10.1523/JNEUROSCI.2127-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst JW. Frequency discrimination of complex signals, frequency selectivity, and speech perception in hearing-impaired subjects. J Acoust Soc Am. 1987;82(3):874–85. doi: 10.1121/1.395286. [DOI] [PubMed] [Google Scholar]
- Hu B, Senatorov V, Mooney D. Lemniscal and non-lemniscal synaptic transmission in rat auditory thalamus. Journal of Physiology. 1994;479:217–231. doi: 10.1113/jphysiol.1994.sp020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B. Cellular basis of temporal synaptic signalling: an in vitro electrophysiological study in rat auditory thalamus. Journal of Physiology. 1995;483:167–182. doi: 10.1113/jphysiol.1995.sp020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Larue DT, Winer JA. GABAergic organization of the cat medial geniculate body. J Comp Neurol. 1999;415(3):368–392. [PubMed] [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J Comp Neurol. 2000;427(2):302–331. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Integrative action in the cat’s lateral geniculate body. Journal of Physiology. 1961;155:385–98. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huetz C, Philibert B, Edeline JM. A spike-timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. Journal of Neuroscience. 2009;29:334–50. doi: 10.1523/JNEUROSCI.3269-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsson C, de Ribaupierre Y, de Ribaupierre F. Influence of auditory localization cues on neuronal activity in the auditory thalamus of the cat. Journal of Neurophysiology. 1988;59:586–606. doi: 10.1152/jn.1988.59.2.586. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. Journal of Physiology. 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Rockel AJ. Observations on complex vesicles, neurofilamentous hyperplasia and increased electron density during terminal degeneration in the inferior colliculus. J Comp Neurol. 1973;147(1):93–118. doi: 10.1002/cne.901470105. [DOI] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev. 2004;84(2):541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Judge PW. Effects of medial geniculate lesions on sound localization by the rat. Journal of Neurophysiology. 1985;53:361–372. doi: 10.1152/jn.1985.53.2.361. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wong D. Laminar connections of the cat’s auditory cortex. Brain Research. 1981;212:1–15. doi: 10.1016/0006-8993(81)90027-5. [DOI] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Okamoto K, Tamai Y. Topography of projections from the primary and non-primary auditory cortical areas to the medial geniculate body and thalamic reticular nucleus in the rat. Neuroscience. 2005;135:1325–42. doi: 10.1016/j.neuroscience.2005.06.089. [DOI] [PubMed] [Google Scholar]
- Kimura A, Imbe H, Donishi T. Axonal projections of auditory cells with short and long response latencies in the medial geniculate nucleus: distinct topographies in the connection with the thalamic reticular nucleus. European Journal of Neuroscience. 2009;30:783–99. doi: 10.1111/j.1460-9568.2009.06880.x. [DOI] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Sakoda T, Hazama M, Tamai Y. Auditory thalamic nuclei projections to the temporal cortex in the rat. Neuroscience. 2003;117:1003–16. doi: 10.1016/s0306-4522(02)00949-1. [DOI] [PubMed] [Google Scholar]
- Kimura A, Imbe H, Donishi T, Tamai Y. Axonal projections of single auditory neurons in the thalamic reticular nucleus: implications for tonotopy-related gating function and cross-modal modulation. Eur J Neurosci. 2007;26(12):3524–35. doi: 10.1111/j.1460-9568.2007.05925.x. [DOI] [PubMed] [Google Scholar]
- Komura Y, Tamura R, Uwano T, Nishijo H, Kaga K, Ono T. Retrospective and prospective coding for predicted reward in the sensory thalamus. Nature. 2001;412:546–9. doi: 10.1038/35087595. [DOI] [PubMed] [Google Scholar]
- Komura Y, Tamura R, Uwano T, Nishijo H, Ono T. Auditory thalamus integrates visual inputs into behavioral gains. Nature Neuroscience. 2005;8:1203–9. doi: 10.1038/nn1528. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Carrell T, King C, Littman T, Nicol T. Discrimination of speech-like contrasts in the auditory thalamus and cortex. J Acoust Soc Am. 1994;96(5 Pt 1):2758–2768. doi: 10.1121/1.411282. [DOI] [PubMed] [Google Scholar]
- Krishna BS, Semple MN. Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. Journal of Neurophysiology. 2000;84:255–73. doi: 10.1152/jn.2000.84.1.255. [DOI] [PubMed] [Google Scholar]
- Kudo M, Niimi K. Ascending projections of the inferior colliculus in the cat: an autoradiographic study. Journal of Comparative Neurology. 1980;191:545–556. doi: 10.1002/cne.901910403. [DOI] [PubMed] [Google Scholar]
- Kumari V, Fannon D, Ffytche DH, Raveendran V, Antonova E, Premkumar P, Cooke MA, Anilkumar AP, Williams SC, Andrew C, Johns LC, Fu CH, McGuire PK, Kuipers E. Functional MRI of verbal self-monitoring in schizophrenia: performance and illness-specific effects. Schizophrenia Bulletin. 2010;36:740–55. doi: 10.1093/schbul/sbn148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt S, Fisher SE, Ehret G. Foxp2 mutations impair auditory-motor association learning. PLoS One. 2012;7(3):e33130. doi: 10.1371/journal.pone.0033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvasnák E, Popelár J, Syka J. Discharge properties of neurons in subdivisions of the medial geniculate body of the guinea pig. Physiological Research. 2000;49:369–78. [PubMed] [Google Scholar]
- Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126(Pt 11):2455–2462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cerebral Cortex. 2010;20:13–24. doi: 10.1093/cercor/bhp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. Journal of Comparative Neurology. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990;10(4):1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol. 1987;264(1):123–146. doi: 10.1002/cne.902640110. [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Topography and physiology of ascending streams in the auditory tectothalamic pathway. PNAS. 2010;107(1):372–7. doi: 10.1073/pnas.0907873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: I. Thalamocortical system. Journal of Comparative Neurology. 2008;507:1879–900. doi: 10.1002/cne.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC. Thalamic and cortical pathways supporting auditory processing. Brain Lang. 2012 doi: 10.1016/j.bandl.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartz RC, Weinberger NM. Frequency selectivity is related to temporal processing in parallel thalamocortical auditory pathways. Brain Research. 1992;583:81–92. doi: 10.1016/s0006-8993(10)80011-3. [DOI] [PubMed] [Google Scholar]
- Linke R. Differential projection patterns of superior and inferior collicular neurons onto posterior paralaminar nuclei of the thalamus surrounding the medial geniculate body in the rat. European. Journal of Neuroscience. 1999;11:187–203. doi: 10.1046/j.1460-9568.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- Linke R, Braune G, Schwegler H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Experimental Brain Research. 2000;134:520–32. doi: 10.1007/s002210000475. Erratum in: Exp Brain Res (2001). 138, 135-8. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Rosen GD, Drislane FW, Galaburda AM. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. PNAS. 1991;88:7943–7. doi: 10.1073/pnas.88.18.7943. Erratum in: PNAS, 90, 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Feng AS. Response characteristics of neurons in the medial geniculate body of the little brown bat to simple and temporally-patterned sounds. Journal of Comparative Physiology A. 1999;184:371–85. doi: 10.1007/s003590050336. [DOI] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Evidence for nonreciprocal organization of the mouse auditory thalamocortical-corticothalamic projection systems. Journal of Comparative Neurology. 2008;507:1209–27. doi: 10.1002/cne.21602. [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, et al. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nature Neuroscience. 2001;4(11):1131–8. doi: 10.1038/nn737. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Bishop DV. Frequency discrimination deficits in people with specific language impairment: reliability, validity, and linguistic correlates. J Speech Lang Hear Res. 2004;47(3):527–41. doi: 10.1044/1092-4388(2004/041). [DOI] [PubMed] [Google Scholar]
- Maeder PP, Meuli RA, Adriani M, Bellmann A, Fornari E, Thiran JP, et al. Distinct pathways involved in sound recognition and localization: a human fMRI study. Neuroimage. 2001;14(4):802–816. doi: 10.1006/nimg.2001.0888. [DOI] [PubMed] [Google Scholar]
- Malone BJ, Scott BH, Semple MN. Dynamic amplitude coding in the auditory cortex of awake rhesus macaques. J Neurophysiol. 2007;98(3):1451–1474. doi: 10.1152/jn.01203.2006. [DOI] [PubMed] [Google Scholar]
- Majorossy K, Kiss A. Specific patterns of neuron arrangement and of synaptic articulation in the medial geniculate body. Experimental Brain Research. 1976;26:1–17. doi: 10.1007/BF00235246. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Rees A, LeBeau FEN. Ascending projections to the medial geniculate body from physiologically identified loci in the inferior colliculus. In: Syka J, editor. Acoustical Signal Processing in the Central Auditory System. New York: Plenum Press; 1997. [Google Scholar]
- Massaux A, Dutrieux G, Cotillon-Williams N, Manunta Y, Edeline JM. Auditory thalamus bursts in anesthetized and non-anesthetized states: contribution to functional properties. Journal of Neurophysiology. 2004;91:2117–34. doi: 10.1152/jn.00970.2003. [DOI] [PubMed] [Google Scholar]
- Massopust LC, Hauge DH, Ferneding JC, Doubek WG, Taylor JJ. Projection systems and terminal localization of dorsal column afferents: an autoradiographic and horseradish peroxidase study in the rat. Journal of Comparative Neurology. 1985;237:533–544. doi: 10.1002/cne.902370409. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Current Opinion in Neurobiology. 1994;4:550–556. doi: 10.1016/0959-4388(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Miller LM, Escabi MA, et al. Spectrotemporal receptive fields in the lemniscal auditory thalamus and cortex. Journal of Neurophysiology. 2002;87(1):516–27. doi: 10.1152/jn.00395.2001. [DOI] [PubMed] [Google Scholar]
- Mitani A, Itoh K, Mizuno N. Distribution and size of thalamic neurons projecting to layer I of the auditory cortical fields of the cat compared to those projecting to layer IV. J Comp Neurol. 1987;257(1):105–121. doi: 10.1002/cne.902570108. [DOI] [PubMed] [Google Scholar]
- Montero VM, Singer W. Ultrastructural identification of somata and neural processes immunoreactive to antibodies against glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the cat. Experimental Brain Research. 1985;59:151–165. doi: 10.1007/BF00237675. [DOI] [PubMed] [Google Scholar]
- Morest DK. The lateral tegmental system of the midbrain and the medial geniculate body: study with Golgi and Nauta methods in the cat. Journal of Anatomy. 1965;99:611–634. [PMC free article] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Cholinergic cells in the tegmentum send branching projections to the inferior colliculus and the medial geniculate body. Neuroscience. 2011;179:120–130. doi: 10.1016/j.neuroscience.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PC, Smith ZM, Young ED. Wide-dynamic-range forward suppression in marmoset inferior colliculus neurons is generated centrally and accounts for perceptual masking. J Neurosci. 2009;29(8):2553–62. doi: 10.1523/JNEUROSCI.5359-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KN, Allison TL, Rosenfield ME, Moore JW. Neural activity in the medial geniculate nucleus during auditory trace conditioning. Exp Brain Res. 1997;113(3):534–556. doi: 10.1007/pl00005605. [DOI] [PubMed] [Google Scholar]
- Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cerebral Cortex. 1994;4:646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Hall WC. The medial geniculate body of the tree shrew, Tupaia glis. I. Cytoarchitecture and midbrain connections. Journal of Comparative Neurology. 1978;182:423–58. doi: 10.1002/cne.901820305. [DOI] [PubMed] [Google Scholar]
- Orman SS, Humphrey GL. Effects of changes in cortical arousal and of auditory cortex cooling on neuronal activity in the medial geniculate body. Experimental Brain Research. 1981;42:475–482. doi: 10.1007/BF00237512. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Shera CA. Estimates of human cochlear tuning at low levels using forward and simultaneous masking. J Assoc Res Otolaryngol. 2003;4(4):541–54. doi: 10.1007/s10162-002-3058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. Physiology of the young adult Fischer 344 rat inferior colliculus: responses to contralateral monaural stimuli. Hearing Research. 1996;100:41–58. doi: 10.1016/0378-5955(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Pallas SL, Sur M. Morphology of retinal axon arbors induced to arborize in a novel target, the medial geniculate nucleus. II. Comparison with axons from the inferior colliculus. Journal of Comparative Neurology. 1994;349:363–376. doi: 10.1002/cne.903490304. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Rosene DL, Doolittle AM. Corticothalamic connections of auditory-related areas of the temporal lobe in the rhesus monkey. Journal of Comparative Neurology. 1994;345:447–71. doi: 10.1002/cne.903450311. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Friedman JT, Rosen GD, Fitch RH. Impaired gap detection in juvenile microgyric rats. Brain Research Developmental Brain Research. 2004a;152:93–8. doi: 10.1016/j.devbrainres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Severity of focal microgyria and associated rapid auditory processing deficits. Neuroreport. 2004b;15:1923–6. doi: 10.1097/00001756-200408260-00018. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Rosen GD, Fitch RH. Rapid auditory processing and MGN morphology in microgyric rats reared in varied acoustic environments. Brain Research Developmental Brain Research. 2002;138:187–93. doi: 10.1016/s0165-3806(02)00472-8. [DOI] [PubMed] [Google Scholar]
- Peschanski M, Lee CL, Ralston HJ., 3d The structural organization of the ventrobasal complex of the rat as revealed by the analysis of physiologically characterized neurons injected intracellularly with horseradish peroxidase. Brain Research. 1984;297:63–74. doi: 10.1016/0006-8993(84)90543-2. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Bartlett E, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. Journal of Neuroscience. 1997;17:3766–3777. doi: 10.1523/JNEUROSCI.17-10-03766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert B, Laudanski J, Edeline JM. Auditory thalamus responses to guinea-pig vocalizations: a comparison between rat and guinea-pig. Hear Res. 2005;209(1-2):97–103. doi: 10.1016/j.heares.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Preuss A, Muller-Preuss P. Processing of amplitude modulated sounds in the medial geniculate body of squirrel monkeys. Experimental Brain Research. 1990;79:207–211. doi: 10.1007/BF00228890. [DOI] [PubMed] [Google Scholar]
- Rabang CF, Bartlett EL. A computational model of cellular mechanisms of temporal coding in the medial geniculate body (MGB) PLoS One. 2011;6(12):e29375. doi: 10.1371/journal.pone.0029375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Davis KA, May BJ. Single-unit responses in the inferior colliculus of decerebrate cats. I. Classification based on frequency response maps. J Neurophysiol. 1999;82(1):152–63. doi: 10.1152/jn.1999.82.1.152. [DOI] [PubMed] [Google Scholar]
- Read HL, Nauen DW, et al. Distinct core thalamocortical pathways to central and dorsal primary auditory cortex. Hearing Research. 2011;274:95–104. doi: 10.1016/j.heares.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. Journal of Neurophysiology. 2004;92:2185–97. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Rinker T, Kohls G, Richter C, Maas V, Schulz E, Schecker M. Abnormal frequency discrimination in children with SLI as indexed by mismatch negativity (MMN) Neurosci Lett. 2007;413(2):99–104. doi: 10.1016/j.neulet.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Dagaeff C, Simm G, de Ribaupierre Y, Villa A, de Ribaupierre F, Rouiller EM. Functional organization of the ventral division of the medial geniculate body of the cat: evidence for a rostro-caudal gradient of response properties and cortical projections. Hearing Research. 1989;39:103–125. doi: 10.1016/0378-5955(89)90085-3. [DOI] [PubMed] [Google Scholar]
- Rogan MT, LeDoux JE. Emotion: systems, cells, synaptic plasticity. Cell. 1996;85:469–75. doi: 10.1016/s0092-8674(00)81247-7. [DOI] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond B Biol Sci. 1992;336(1278):367–373. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Mesples B, Hendriks M, Galaburda AM. Histometric changes and cell death in the thalamus after neonatal neocortical injury in the rat. Neuroscience. 2006;141:875–88. doi: 10.1016/j.neuroscience.2006.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GD, Burstein D, Galaburda AM. Changes in efferent and afferent connectivity in rats with induced cerebrocortical microgyria. Journal of Comparative Neurology. 2000;418:423–40. [PubMed] [Google Scholar]
- Rouiller E, de Ribaupierre Y, Toros-Morel A, de Ribaupierre F. Neural coding of repetitive clicks in the medial geniculate body of cat. Hearing Research. 1981;5:81–100. doi: 10.1016/0378-5955(81)90028-9. [DOI] [PubMed] [Google Scholar]
- Rouiller E, de Ribaupierre Y, Morel A, de Ribaupierre F. Intensity functions of single unit responses to tone in the medial geniculate body of cat. Hearing Research. 1983;11:235–247. doi: 10.1016/0378-5955(83)90081-3. [DOI] [PubMed] [Google Scholar]
- Rouiller E, de Ribaupierre F. Neurons sensitive to narrow ranges of repetitive acoustic transients in the medial geniculate body of the cat. Experimental Brain Research. 1982;48:323–326. doi: 10.1007/BF00238607. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Colomb E, Capt M, de Ribaupierre F. Projections of the reticular complex of the thalamus onto physiologically characterized regions of the medial geniculate body. Neuroscience Letters. 1985;53:227–232. doi: 10.1016/0304-3940(85)90190-9. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Capt M, Hornung JP, Streit P. Correlation between regional changes in the distributions of GABA-containing neurons and unit response properties in the medial geniculate body of the cat. Hearing Research. 1990;49:249–258. doi: 10.1016/0378-5955(90)90107-z. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Rodrigues-Dagaeff C, Simm G, de Ribaupierre Y, Villa A, de Ribaupierre F. Functional organization of the medial division of the medial geniculate body of the cat: tonotopic organization, spatial distribution of response properties and cortical connections. Hearing Research. 1989;39:127–142. doi: 10.1016/0378-5955(89)90086-5. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, de Ribaupierre F. Origin of afferents to physiologically defined regions of the medial geniculate body of the cat: ventral and dorsal divisions. Hearing Research. 1985;19:97–114. doi: 10.1016/0378-5955(85)90114-5. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. Morphology of corticothalamic terminals arising from the auditory cortex of the rat: a Phaseolus vulgaris-leucoagglutinin (PHA-L) tracing study. Hearing Research. 1991;56:179–190. doi: 10.1016/0378-5955(91)90168-9. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Capt M, Hornung JP, Streit P. Correlation between regional changes in the distributions of GABA-containing neurons and unit response properties in the medial geniculate body of the cat. Hearing Research. 1990;49:249–58. doi: 10.1016/0378-5955(90)90107-z. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, de Ribaupierre F. Arborization of corticothalamic axons in the auditory thalamus of the cat: a PHA-L tracing study. Neuroscience Letters. 1990;108:29–35. doi: 10.1016/0304-3940(90)90701-a. [DOI] [PubMed] [Google Scholar]
- Ryan A, Miller J. Single unit responses in the inferior colliculus of the awake and performing rhesus monkey. Experimental Brain Research. 1978;32:389–407. doi: 10.1007/BF00238710. [DOI] [PubMed] [Google Scholar]
- Sadagopan S, Wang X. Level invariant representation of sounds by populations of neurons in primary auditory cortex. J Neurosci. 2008;28(13):3415–26. doi: 10.1523/JNEUROSCI.2743-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel O, van de Par S, Breebaart J, Kohlrausch A. Sound segregation based on temporal envelope structure and binaural cues. J Acoust Soc Am. 2008;124(2):1130–45. doi: 10.1121/1.2945159. [DOI] [PubMed] [Google Scholar]
- Shaddock Palombi P, Backoff PM, Caspary DM. Responses of young and aged rat inferior colliculus neurons to sinusoidally amplitude modulated stimuli. Hearing Research. 2001;153:174–80. doi: 10.1016/s0378-5955(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Shamma SA, Symmes D. Patterns of inhibition in auditory cortical cells in awake squirrel monkeys. Hear Res. 1985;19(1):1–13. doi: 10.1016/0378-5955(85)90094-2. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Efferent connections of the caudal part of the globus pallidus in the rat. Journal of Comparative Neurology. 1996;376:489–507. doi: 10.1002/(SICI)1096-9861(19961216)376:3<489::AID-CNE10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Speech and music have different requirements for spectral resolution. Int Rev Neurobiol. 2005;70:121–134. doi: 10.1016/S0074-7742(05)70004-0. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270(5234):303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability) Biological Psychiatry. 2005;57:1301–9. doi: 10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. Journal of Neurophysiology. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Experimental Brain Research. 1986;63:1–20. doi: 10.1007/BF00235642. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2002;357:1695–708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends in Neuroscience. 2001;24:122–6. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid projections of rat temporal cortex. Journal of Comparative Neurology. 1997;382:153–175. [PubMed] [Google Scholar]
- Sinnott JM, Petersen MR, Hopp SL. Frequency and intensity discrimination in humans and monkeys. J Acoust Soc Am. 1985;78(6):1977–1985. doi: 10.1121/1.392654. [DOI] [PubMed] [Google Scholar]
- Smith PH, Bartlett EL, et al. Unique combination of anatomy and physiology in cells of the rat paralaminar thalamic nuclei adjacent to the medial geniculate body. Journal of Comparative Neurology. 2006;496(3):314–34. doi: 10.1002/cne.20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Bartlett EL, et al. Cortical and collicular inputs to cells in the rat paralaminar thalamic nuclei adjacent to the medial geniculate body. Journal of Neurophysiology. 2007;98(2):681–695. doi: 10.1152/jn.00235.2007. [DOI] [PubMed] [Google Scholar]
- Smith PH, Manning KA, Uhlrich DJ. Evaluation of inputs to rat primary auditory cortex from the suprageniculate nucleus and extrastriate visual cortex. J Comp Neurol. 2010;518(18):3679–3700. doi: 10.1002/cne.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storace FA, Higgins NC, Read HL. Thalamic label patterns suggest primary and ventral auditory fields are distinct core regions. Journal of Comparative Neurology. 2010;518(10):1630–46. doi: 10.1002/cne.22345. [DOI] [PubMed] [Google Scholar]
- Suga N, Tsuzuki K. Inhibition and level-tolerant frequency tuning in the auditory cortex of the mustached bat. J Neurophysiol. 1985;53(4):1109–1145. doi: 10.1152/jn.1985.53.4.1109. [DOI] [PubMed] [Google Scholar]
- Suga N, Zhang Y, et al. Sharpening of frequency tuning by inhibition in the thalamic auditory nucleus of the mustached bat. Journal of Neurophysiology. 1997;77(4):2098–114. doi: 10.1152/jn.1997.77.4.2098. [DOI] [PubMed] [Google Scholar]
- Suta D, Popelár J, Kvasnák E, Syka J. Representation of species-specific vocalizations in the medial geniculate body of the guinea pig. Experimental Brain Research. 2007;183:377–88. doi: 10.1007/s00221-007-1056-3. [DOI] [PubMed] [Google Scholar]
- Symmes D, Alexander GE, Newman JD. Neural processing of vocalizations and artificial stimuli in the medial geniculate body of squirrel monkey. Hearing Research. 1980;3:133–146. doi: 10.1016/0378-5955(80)90041-6. [DOI] [PubMed] [Google Scholar]
- Tarlov EC, Moore RY. The tecto-thalamic connections in the brain of the rabbit. Journal of Comparative Neurology. 1966;126:403–35. doi: 10.1002/cne.901260304. [DOI] [PubMed] [Google Scholar]
- Tennigkeit F, Schwarz DW, Puil E. Mechanisms for signal transformation in lemniscal auditory thalamus. Journal of Neurophysiology. 1996;76:3597–3608. doi: 10.1152/jn.1996.76.6.3597. [DOI] [PubMed] [Google Scholar]
- Tennigkeit F, Puil E, Schwarz DW. Firing modes and membrane properties in lemniscal auditory thalamus. Acta Oto-Laryngologica. 1997;117:254–257. doi: 10.3109/00016489709117782. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Populin LC, Moore JM, Ruhland JL, Yin TC. Sound-localization performance in the cat: the effect of restraining the head. Journal of Neurophysiology. 2005;93:1223–34. doi: 10.1152/jn.00747.2004. [DOI] [PubMed] [Google Scholar]
- Tregellas J. Connecting brain structure and function in schizophrenia. American Journal of Psychiatry. 2009;166:134–6. doi: 10.1176/appi.ajp.2008.08111685. [DOI] [PubMed] [Google Scholar]
- Turner JP, Salt TE. Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurones in vitro. Journal of Physiology. 1998;510:829–843. doi: 10.1111/j.1469-7793.1998.829bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velenovsky DS, Cetas JS, Price RO, Sinex DG, McMullen NT. Functional subregions in primary auditory cortex defined by thalamocortical terminal arbors: an electrophysiological and anterograde labeling study. Journal of Neuroscience. 2003;23:308–16. doi: 10.1523/JNEUROSCI.23-01-00308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernier VG, Galambos R. Response of single medial geniculate units to repetitive click stimuli. American. Journal of Physiology. 1957;188:233–237. doi: 10.1152/ajplegacy.1957.188.2.233. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Patterson RD, Griffiths TD. Task-dependent modulation of medial geniculate body is behaviorally relevant for speech recognition. Curr Biol. 2008;18(23):1855–1859. doi: 10.1016/j.cub.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MN, Anderson LA, et al. Phase-locked responses to pure tones in the auditory thalamus. Journal of Neurophysiology. 2007;98(4):1941–52. doi: 10.1152/jn.00697.2007. [DOI] [PubMed] [Google Scholar]
- Wang X, Kadia SC. Differential representation of species-specific primate vocalizations in the auditory cortices of marmoset and cat. J Neurophysiol. 2001;86(5):2616–2620. doi: 10.1152/jn.2001.86.5.2616. [DOI] [PubMed] [Google Scholar]
- Watkins PV, Barbour DL. Specialized neuronal adaptation for preserving input sensitivity. Nature Neuroscience. 2008;11:1259–61. doi: 10.1038/nn.2201. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ. Frequency organization and responses to complex sounds in the medial geniculate body of the mustached bat. J Neurophysiol. 1999;82(5):2528–2544. doi: 10.1152/jn.1999.82.5.2528. [DOI] [PubMed] [Google Scholar]
- Wester K, Irvine DR, Hugdahl K. Auditory laterality and attentional deficits after thalamic haemorrhage. J Neurol. 2001;248(8):676–683. doi: 10.1007/s004150170113. [DOI] [PubMed] [Google Scholar]
- Winer JA. The human medial geniculate body. Hearing Research. 1984;15:225–247. doi: 10.1016/0378-5955(84)90031-5. [DOI] [PubMed] [Google Scholar]
- Winer JA, Saint Marie RL, Larue DT, Oliver DL. GABAergic feedforward projections from the inferior colliculus to the medial geniculate body. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8005–8010. doi: 10.1073/pnas.93.15.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Miller LM, et al. Auditory thalamocortical transformation: structure and function. Trends in Neuroscience. 2005;28(5):255–263. doi: 10.1016/j.tins.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Winer JA, Diehl JJ, Larue DT. Projections of auditory cortex to the medial geniculate body of the cat. Journal of Comparative Neurology. 2001;430:27–55. [PubMed] [Google Scholar]
- Yu YQ, Xiong Y, Chan YS, He J. In vivo intracellular responses of the medial geniculate neurones to acoustic stimuli in anaesthetized guinea pigs. Journal of Physiology. 2004;560:191–205. doi: 10.1113/jphysiol.2004.067678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Suga N, Yan J. Corticofugal modulation of frequency processing in bat auditory system. Nature. 1997;387:900–903. doi: 10.1038/43180. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Escabí MA. Distinct roles for onset and sustained activity in the neuronal code for temporal periodicity and acoustic envelope shape. Journal of Neuroscience. 2008;28:14230–44. doi: 10.1523/JNEUROSCI.2882-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


