Key Points
Perivascular cells maintain HSPCs ex vivo.
Abstract
Hematopoietic stem and progenitor cells (HSPCs) emerge and develop adjacent to blood vessel walls in the yolk sac, aorta-gonad-mesonephros region, embryonic liver, and fetal bone marrow. In adult mouse bone marrow, perivascular cells shape a “niche” for HSPCs. Mesenchymal stem/stromal cells (MSCs), which support hematopoiesis in culture, are themselves derived in part from perivascular cells. In order to define their direct role in hematopoiesis, we tested the ability of purified human CD146+ perivascular cells, as compared with unfractionated MSCs and CD146− cells, to sustain human HSPCs in coculture. CD146+ perivascular cells support the long-term persistence, through cell-to-cell contact and at least partly via Notch activation, of human myelolymphoid HSPCs able to engraft primary and secondary immunodeficient mice. Conversely, unfractionated MSCs and CD146− cells induce differentiation and compromise ex vivo maintenance of HSPCs. Moreover, CD146+ perivascular cells express, natively and in culture, molecular markers of the vascular hematopoietic niche. Unexpectedly, this dramatic, previously undocumented ability to support hematopoietic stem cells is present in CD146+ perivascular cells extracted from the nonhematopoietic adipose tissue.
Introduction
Blood and vasculature are indispensable to embryonic development, and are thus the first differentiated tissues produced in life. Incipient human hematopoiesis adapts to the rudimentary anatomy of the embryo and proceeds first in the yolk sac, then transiently in the placenta and liver before being stabilized in fetal bone marrow (FBM). Definitive hematopoietic stem and progenitor cells (HSPCs) first emerge in the aorta-gonad-mesonephros region of the embryo.1 Therefore, several organs of distinct germline origins, structures, and eventual roles converge functionally to produce blood cells during development. What remains however remarkably constant through pre- and postnatal life is the physical association of incipient hematopoietic cells with blood vessels. In the yolk sac, erythroid cells emerge within intravascular blood islands.2 It is now also well accepted that, from fish to humans, specialized blood-forming endothelial cells present in the dorsal aorta and possibly other organs supply the embryo with hematopoietic cells,3-7 an ontogenic transition that has been modeled in human embryonic stem cells.8 In addition to this direct developmental affiliation between embryonic endothelial cells and HSPCs, there is evidence that vascular cells nurture blood cells in pre- and postnatal life. The cellular and molecular mechanisms involved in this support can be analyzed in cocultures of stromal and hematopoietic cells.9-11 For instance, cultured endothelial cells use angiocrine factors to regulate HSPC differentiation or self-renewal.12-14 Mesenchymal stem/stromal cells (MSCs), the multilineage mesodermal progenitors spontaneously selected in long-term cultures of unfractionated cells from bone marrow and other tissues,15-18 can also, to some extent, sustain hematopoiesis in vitro.19-24 However, the relevance of this support to physiologic blood cell production in vivo has been unknown because MSCs have long eluded prospective identification.25 Similarities between MSCs and pericytes, which ensheath capillaries and microvessels in all organs, have been described.26-28 In an experimental approach combining stringent cell purification by flow cytometry and differentiation in culture and in vivo, we have demonstrated that human CD146+ perivascular cells represent ubiquitous ancestors of MSCs.29
Although hematopoietic stem cells (HSCs) were originally detected in the endosteal regions of the bone marrow,30 recent findings have suggested the existence of a distinct, perivascular niche for HSPCs.31-34 Perivascular reticular cells expressing CXCL12 were found to play a role in murine HSC maintenance.35 In a seminal study by Méndez-Ferrer et al,36 the function and identity of perivascular niche cells were further defined. The authors showed the existence in murine bone marrow of perivascular nestin+ MSCs associated with HSCs. Ablation of nestin+ MSCs led to a significant reduction in the number and homing ability of HSCs. The direct role for perivascular cells in hematopoiesis regulation was confirmed in a recent study by Ding et al.37 Selective shutoff of c-kit ligand expression in leptin receptor (Lep-R) positive cells surrounding murine bone marrow blood vessels significantly reduced the frequency of long-term reconstituting HSCs.37
In the present study, we demonstrate that CD146+ perivascular cells express in vivo nestin, CXCL12, and Lep-R in human FBM as well as in adult adipose tissue. We also report for the first time that human CD146+ perivascular cells are a subset of MSCs able to directly support the ex vivo maintenance of human HSPCs. We further demonstrate that cultured CD146+ perivascular support HSPCs through cell-to-cell contact and activation of Notch signaling. Conversely, conventional unfractionated MSCs or the CD146− subset of MSCs favor differentiation at the expense of stemness. CD146+ perivascular cells can therefore be considered as the bona fide human equivalents of the hematopoietic perivascular niche components recently described in the mouse.
Methods
Isolation of human primary stromal cells
Human stromal cells were derived from human lipoaspirate specimens (n = 4) and FBM (n = 2) as previously described.17,29 Lipoaspirates were obtained as discarded specimens without identifiable information, therefore no institutional review board approval was required. Fetal bones (16-18 weeks of pregnancy) were obtained from Novogenix. One hundred milliliters of lipoaspirate were incubated at 37°C for 30 minutes with digestion solution composed by RPMI 1640 (Cellgro), 3.5% bovine serum albumin (Sigma), and 1 mg/mL collagenase type II (Sigma). Adipocytes were discarded after centrifugation while the pellet was resuspended and incubated in red blood cell lysis (eBioscience) to obtain the stromal vascular fraction (SVF). Fetal bones were split open to flush the bone marrow cavity. The bones were placed in digestion solution for 30 minutes at 37°C. Mononuclear cells (MNCs) were isolated using Ficoll-Paque (GE Healthcare). Hematopoietic cells were excluded by magnetic immunodepletion of CD45+ cells as per manufacturer’s instructions (Miltenyi Biotec). An aliquot of SVF or CD45-depleted MNCs was plated in tissue-culture–treated flask for the expansion of conventional MSCs.17 Another aliquot of SVF or CD45-depleted MNCs was processed for fluorescence-activated cell sorting (FACS). Cells were incubated with the following antibodies: CD45–allophycocyanin (APC)–cy7 (BD Biosciences), CD34-APC (BD Biosciences), and CD146–fluorescein isothiocyanate (FITC; AbD Serotec). The viability dye 4,6 diamidino-2-phenylindole (DAPI; Sigma) was added before sorting, on a FACSAria III (BD Biosciences), DAPI−CD45−CD34−CD146+ perivascular cells, or DAPI−CD45−CD34+CD146− cells, as previously described.29,38 In some experiments, CD146− cells were purified from cultured MSCs.
For the animal studies, an animal care and use committee protocol (ARC no. 2008-175-11) was approved for the injection of human cells into immunodeficient mice and for the analysis of engraftment of transplanted cells.
Isolation of human CD34+ cells from CB
Umbilical cord blood (CB) was collected from normal deliveries without individually identifiable information, therefore no institutional review board approval was required. MNCs were isolated by density gradient centrifugation using Ficoll-Paque (GE Healthcare). Enrichment of CD34+ cells was then performed using the magnetic-activated cell sorting system (Miltenyi Biotec) as per the manufacturer’s instructions.
Immunophenotype analysis of stromal cells
Cultured MSCs, CD146+ cells, and CD146− cells (between passages 3 and 10) were analyzed on an LSR II flow cytometer (Becton Dickinson). Cells were stained with monoclonal antibodies: CD146-FITC (AbD Serotec), CD31-APC (Biolegend), CD44–phycoerythrin (PE), CD73-PEcy7, CD105-PE, CD90-APC, and CD45-FITC (all from BD Biosciences). Unstained samples were used as negative controls. Data were analyzed using FlowJo software (Tree Star).
Mesodermal lineage differentiation assays
The ability of cells to differentiate into mesodermal lineages was tested in osteogenic or adipogenic differentiation medium (Hyclone). After 3 weeks of culture in differentiation conditions, cells were stained with Alizarin red or Oil red O (Sigma) for the detection of mineral deposits or lipids as previously described.29
Quantitative RT-PCR
Five hundred thousand cultured cells were processed for RNA extraction using a Qiagen micro kit. An Omniscript reverse transcriptase (RT) kit was used to make complementary DNA, which was subjected to quantitative polymerase chain reaction (qPCR) using Sybr green probe–based gene expression analysis (Applied Biosystems) for 2 housekeeping genes, TBP and GAPDH, and the target genes CD146, nestin, α-SMA, and NG2. A 7500 real-time PCR system was used (ABI). Data were analyzed using the comparative C(T) method.
Western blotting
Cells were lysed in denaturing cell extraction buffer (Invitrogen) containing protease inhibitor tablets (Roche). Proteins were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed using the XCell II western blot system (Invitrogen). Rat anti-human Jagged-1 (Abcam, 1:50) and monoclonal mouse anti-β actin (Sigma, 1:5000) antibodies were used. Donkey anti-rat horseradish peroxidase (HRP) and donkey anti-mouse HRP (Jackson Immunoresearch Inc; 1:5000) were used as secondary antibodies. The blots were developed using ECL Plus Western Blotting Substrate (Pierce).
Coculture of stromal cells and CB CD34+ cells
Cultured stromal cells (between passages 3 and 8) were irradiated (20 Gy) and plated on 96 multi-well plates at 1.5 × 104 cells per well. Twenty-four hours later, CB CD34+ cells (5-7 × 104 per well) were plated on top of the stromal layer. Stroma-free cultures were performed seeding CB CD34+ cells on recombinant retronectin (RN; Lonza) coated wells. Cocultures were performed in RPMI 1640, 5% fetal bovine serum, 1× penicillin/streptavidin. No supplemental cytokines were ever added. Cells were harvested after 1, 2, 4, and 6 weeks. Cocultures in the absence of cell-to-cell contact were performed in 96 multi-well transwell plates (Corning). For the inhibition of Notch, 10μM DAPT (N-[N-(3, 5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester; Sigma) or 10 μg/mL of anti-human Notch 1 neutralizing antibody (Biolegend) were added to each well every 48 hours. An equal volume of dimethylsulfoxide (DMSO; Sigma) or an equal concentration of mouse unrelated IgG (Biolegend) was added to wells as negative controls for DAPT and anti-Notch1 antibody, respectively.
Flow cytometric analysis of cultured CB CD34+ cells
After 1, 2, 4, and 6 weeks of coculture, cells were harvested and stained with the following antibodies: CD45-APC-cy7, CD34-PE-cy7, CD14-APC, CD10-APC, CD33-PE, CD19-FITC (all from BD Biosciences). Dead cells were identified with propidium iodide (PI; BD Biosciences).
Colony forming unit assay
After 1, 2, 4 and 6 weeks of coculture cells were harvested and 2.5 × 103 cells were plated in methylcellulose (Methocult; Stem Cell Technologies). Colonies, here reported as the sum of the progeny of colony forming unit (CFU) granulo-macrophage, burst-forming unit erythroid, and CFU mixed, were scored after 14 days.
In vivo repopulation assay
CB CD34+ cells were cocultured with MSCs or CD146+ cells for 2 weeks in RPMI 1640, 5% fetal bovine serum, 1× penicillin/streptavidin. An equal number of CD45+ cells (105) obtained from the cocultures was intratibially injected in sublethally irradiated (250 cGy), 6- to 8-week-old NSG mice (The Jackson Laboratory). Mice were sacrificed 6 weeks posttransplantation. Engraftment of human hematopoietic cells was evaluated by FACS analysis after staining with anti-human specific monoclonal antibodies: CD45-APC-cy7, HLA (A/B/C)–PE, CD34-PE-cy-7, CD19-FITC, CD14-APC, CD15-APC, CD33-APC (all from BD Biosciences). For secondary transplantation, bone marrows from 2 engrafted mice were pooled and intratibially injected into a secondary host (n = 4). Engraftment was evaluated 4 weeks posttransplantation.
Immunocytochemistry and immunohistochemistry
For immunofluorescence analysis, human adipose tissue-frozen sections, cells cultured in chamber slides (Millipore) or cytospun on microscope slides, were fixed with cold methanol/acetone (1:1) for 5 minutes at room temperature prior to incubation with blocking solution (phosphate-buffered saline 5% donkey serum) for 1 hour at room temperature. Overnight incubation at 4°C was performed with unconjugated primary antibodies: mouse anti-human CD146 (BD Biosciences), mouse anti-human CD45 (eBioscience), rat anti-human Jagged-1, rabbit anti-human N1ICD, mouse anti-human nestin, rabbit anti-human CXCL12, rabbit anti-human Lep-R, rabbit anti-human CD146 (all from Abcam). Tissue sections or cells were incubated for 2 hours at room temperature with FITC-conjugated mouse anti-human von Willebrand factor (VWF; US Biological). Tissue sections or cells were incubated for 1 hour at room temperature with the following conjugated antibodies: donkey anti-rabbit–Alexa 488, donkey anti-rabbit– Alexa 647, donkey anti-rat–Alexa 594 or donkey anti-mouse–Alexa 594 (all from Jackson Immunoresearch Inc). For immunohistochemistry on human FBM, fetal bones (16-18 weeks of pregnancy) were fixed in 4% paraformaldehyde (Sigma-Aldrich). Fixed tissues were embedded in paraffin and sections were stained with the same antibodies against nestin, CXCL12, Lep-R, and CD146. Secondary HRP-conjugated IMPRESS anti-rabbit and anti-mouse antibodies and 3, 3′-diaminobenzidine (Vector Laboratories) were used for revelation. As negative controls, tissue sections or cells were incubated only with secondary antibodies. Images were acquired on an Axiovision microscope (Carl Zeiss; software version 4.8) equipped with ApoTome.2 modules for Axio Imager.2 and Axio Observer, with 10×, 20×, 40×, and 63× (1.4 NA) objectives.
Statistical analysis
Mean and SDs were used to summarize continuous variables. Bivariate cross-sectional comparisons of continuous variables were performed using paired t tests. Continuous outcomes such as total numbers of CD45+ and CD34+ cells, frequency of CD34+Lin− cells and CFUs were collected over time. The experimental design involved 2 within-experiment factors, MSCs and pericytes, and time (week 1, 2, 4, 6), which corresponded to a strip-plot design. A mixed-model approach was used. Within the mixed-model framework, we performed hypothesis testing for the comparison of MSCs and pericytes at different time points. Pearson’s correlation (r) was reported to assess the linear correlation between CD34+lin− cells and CFUs. For the qPCR data, ΔCT values were calculated for each marker. A randomized block design model was fitted on ΔCT values. Donors were treated as random effects while stromal cells groups were treated as fixed effects. For all statistical investigations, tests for significance were 2-tailed. To account for type I error inflation due to multiple comparisons, P values were adjusted by Bonferroni correction. The Fisher exact test was performed to compare engraftment and not engraftment ability. Statistically significant threshold of P value was set at .05. Statistical analyses were carried out using SAS version 9.2 (SAS Institute).
Results
Human CD146+ perivascular cells express nestin, CXCL12, and Lep-R in hematopoietic and nonhematopoietic tissues
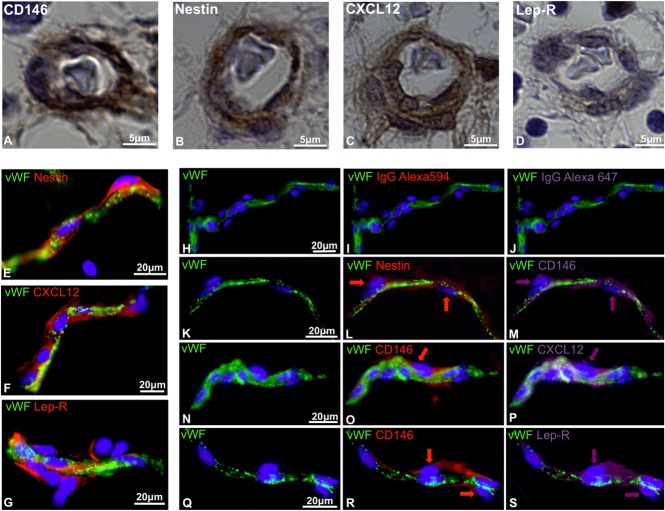
Recent studies have described murine perivascular cells as key players for the maintenance of HSPCs. Perivascular niche cells, displaying MSC features, have been identified based on the expression of CXCL12,34 nestin,36 and Lep-R.37 We have previously demonstrated that pericytes, surrounding microvessels, and capillaries, can be detected in multiple human tissues on expression of CD146.29 Consistent with our previous findings, immunohistochemistry performed on human FBM revealed the presence of CD146-expressing perivascular cells (Figure 1A). Nestin, CXCL12, and Lep-R, markers of the perivascular niche previously described in murine studies, were also expressed in human perivascular cells in FBM (Figure 1B-D). We further investigated the expression of the same stromal cell markers in human adult adipose tissue, considered as an abundant source of MSCs and recently suggested to also be a reservoir of HSCs.39 Nestin, CXCL12, and Lep-R were all expressed in cells immediately adjacent to VWF-positive endothelial cells (Figure 1E-G). Multicolor immunofluorescence showed that CD146+ pericytes, surrounding microvessels and capillaries, coexpress nestin, CXCL12, and Lep-R (Figure 1H-S). Thus, human CD146+ perivascular cells express in situ markers previously identified in murine studies to mark the perivascular hematopoietic niche.
Figure 1.
In situ expression of hematopoietic niche markers by human perivascular cells. (A-D) Immunohistochemistry performed on paraffin-embedded sections of 17-week-old human FBM. Pericytes surrounding microvessels express (A) CD146, (B) nestin, (C) CXCL12, and (D) leptin receptor (Lep-R) (original magnification, ×63). (E-S) Immunohistochemistry performed on human adipose tissue cryosections. (E-G) VWF-positive endothelial cells (green) are surrounded by perivascular cells expressing (E) nestin, (F) CXCL12, and (G) Lep-R. (H-S) Triple-staining immunohistochemistry performed on human adipose tissue cryosections shows coexpression of CD146 with (K-M) nestin, (N-P) CXCL12, and (Q-S) LepR. (H-J) Single staining with anti-VWF antibody followed by incubation with conjugated IgG controls revealed the lack of autofluorescence (original magnification, ×40). IgG, immunoglobulin G.
Purified and ex vivo–expanded CD146+ perivascular cells maintain expression of markers of the perivascular niche
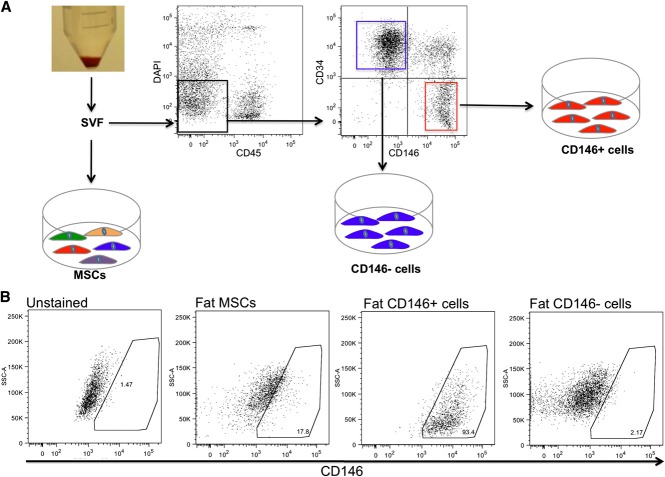
We then analyzed the expression of the perivascular niche markers in purified and ex vivo–expanded CD146+ perivascular cells as compared with unfractionated MSCs and CD146− cells. MSCs were conventionally derived from the adipose tissue SVF by plastic adherence, while CD146+ perivascular cells and CD146− cells were purified by FACS as previously described (Figure 2A).29,38 CD146+ perivascular cells demonstrated expression of cell-surface markers typical of unfractionated cultured MSCs, such as CD44, CD105, CD73, and CD90 and did not express the hematopoietic and endothelial cell markers CD45 and CD31 (supplemental Figure 1a-b, available on the Blood website). Also similar to unfractionated MSCs, cultured CD146+ cells were able to differentiate into osteoblasts and adipocytes in culture (supplemental Figure 1c-f). CD146+ perivascular cells retained uniform CD146 expression in culture, as did a small fraction of MSCs, while CD146− cells remained negative for CD146 expression in culture (Figure 2B). Quantitative RT-PCR analysis of established cultures confirmed that CD146+ cells expressed higher levels of the perivascular cell markers CD146, α-SMA, NG2, and nestin than did either unfractionated MSCs or CD146− cells derived from fat (Figure 3A) or FBM (Figure 3B). Furthermore, immunocytochemistry demonstrated that cultured CD146+ perivascular cells isolated from fat or FBM express higher levels of nestin and CXCL12 than CD146− cells do. No significant difference in the expression of Lep-R was observed between cultured CD146+ and CD146− cells (Figure 3C-N).
Figure 2.
Isolation and culture of MSCs and stromal subsets from lipoaspirate. (A) SVF was obtained from human lipoaspirate specimens (n = 4 donors). An aliquot of SVF was directly seeded in tissue-culture plates for the isolation of conventional MSCs by plastic adherence. Another aliquot of SVF was processed for FACS sorting of DAPI−CD45−CD34−CD146+ perivascular cells and DAPI−CD45−CD34+CD146− cells. (B) FACS analysis of cultured fat-derived MSCs, CD146+ perivascular cells, and CD146− cells. After 9 passages in culture, MSCs retain a low percentage of CD146+ cells, while purified CD146+ perivascular cells and CD146− cells retain a stable phenotype homogeneously positive and negative for CD146, respectively.
Figure 3.

Cultured CD146+ perivascular cells express markers of hematopoietic perivascular niche cells. (A-B) Ex vivo–expanded CD146+ perivascular cells purified from fat and FBM similarly express higher levels of mRNA of perivascular cell markers when compared with MSCs and CD146− cells (n = 2 donors for each tissue). (C-N) Fat and FBM-derived CD146+ perivascular cells similarly and almost exclusively express (C-F) nestin and (G-J) CXCL12 in culture compared with CD146− cells. (K-N) No difference in Lep-R expression was observed between CD146+ and CD146− cells from either fat and FBM (original magnification, ×20). mRNA, messenger RNA.
CD146+ perivascular cells support HSPCs ex vivo
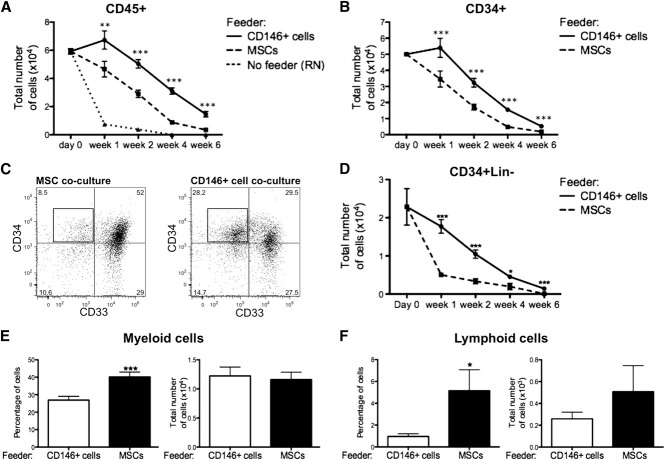
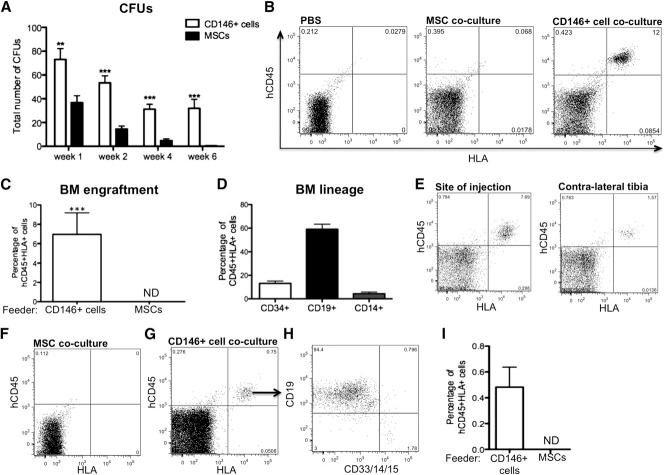
The ability of distinct stromal cells to support HSPCs ex vivo was assessed by coculturing CB-derived CD34+ cells (CB CD34+) in direct contact with either CD146+ perivascular cells, unfractionated MSCs, or CD146− cells all obtained from both lipoaspirate specimens and FBM. These cultures were performed in basal medium with a low concentration of serum (5%) and in the absence of any supplemental cytokines, so that the specific effect of each stromal cell subset could be assessed with minimal influence of exogenous factors. In the absence of any stromal cells or cytokines, hematopoietic cells cultured on RN died within the first 2 weeks, whereas CD45+ cells survived for up to 6 weeks in the presence of either MSCs or CD146+ perivascular cells (Figure 4A). The total number of CD45+ cells recovered from CD146+ cell cocultures remained significantly higher at any time of culture when compared with MSC cocultures (Figure 4A). A similar pattern was observed for the total number of CD34+ cells (Figure 4B). CD34 expression identifies human hematopoietic cells without discriminating between HSCs and lineage-committed progenitors. The most immature progenitors present in cocultures were further defined as CD34+Lin− cells based on expression of CD34 and lack of the early myeloid cell marker CD33 and lymphoid cell markers CD10 and CD19. CD146+ cell cocultures contained a significantly higher frequency and number of CD34+Lin− cells at all time points (Figure 4C-D). Consistent with these findings, culture in the presence of MSCs resulted in accelerated differentiation of CB CD34+ cells into CD14+ myeloid cells and CD10/CD19+ lymphoid cells, relative to coculture with CD146+ cells (Figure 4E-F). The increased frequency of myeloid and lymphoid cells was counterbalanced by the lower numbers of CD45+ cells in MSC cocultures, hence no significant difference in the absolute numbers of myeloid or lymphoid cells was observed (Figure 4E-F). Furthermore, the number of clonogenic cells detected after 1, 2, 4, and 6 weeks was significantly higher when CB CD34+ cells were cocultured with CD146+ perivascular cells compared with MSCs (Figure 5A).
Figure 4.
CD146+ perivascular cells promote ex vivo maintenance of undifferentiated HSPCs. (A) In the absence of cytokines and stromal cell feeder layer (No feeder), CD45+ hematopoietic cells cultured in RN-treated wells rapidly died within the first 2 weeks of culture. At any time of culture, the total number of CD45+ cells recovered from CD146+ cell cocultures was significantly higher when compared with MSC cocultures (n = at least 5 independent experiments for each time point, each experiment was performed in triplicate; **P < .01, ***P < .001). (B) A similar pattern was observed for the total number of CD34+ cells (n = at least 5 independent experiments for each time point, each experiment was performed in triplicate; ***P < .001). (C) Representative FACS analysis after 2 weeks of coculture of CB CD34+ cells with MSCs or CD146+ cell cocultures. After gating on CD45+CD10−CD19− cells, CD34+33− cells were defined as CD34+Lin− cells (black box). (D) The absolute number of CD34+Lin− cells was significantly higher in CD146+ cell cocultures, compared with MSC cocultures, at any time of culture (n=at least 5 independent experiments for each time point, each experiment was performed in triplicate; **P < .01, ***P < .001). (E-F) Coculture of CB CD34+ cells with MSCs led to a significantly higher frequency of CD14+ myeloid cells after 2 weeks (E) (40.24% ± 2.723% vs 26.67% ± 2.075%. n = 10 independent experiments, each experiment was performed in triplicate; ***P < .0001) and a higher frequency of CD10+/CD19+ lymphoid progenitors or mature cells after 4 weeks of coculture (F) (5.155% ± 1.918% vs 0.9541% ± 0.2564%, n = 8 independent experiments, each experiment was performed in triplicate; *P < .05). No difference in the absolute numbers of myeloid and lymphoid cells was observed between CD146+ cell and MSC cocultures. All data are presented as mean ± SEM.
Figure 5.
CD146+ perivascular cells but not MSCs sustain functional HSPCs with engraftment potential and self-renewal ability. (A) CFU assay revealed significantly higher number of CFUs in CD146+ cell cocultures after 1, 2, 4, and 6 weeks of coculture as compared with MSC cocultures (n = 3 independent experiments, each experiment was performed in triplicate; *P < .05, ***P < .001). (B) Representative flow cytometry analysis for the detection of human CD45+HLA+ cells in bone marrow of NSG mice 6 weeks posttransplantation with phosphate-buffered saline, or with the same number of CD45+ cells (105) harvested after 2 weeks of CB CD34+ cell coculture with MSCs or CD146+ cells. (C) All mice injected with CD45+ cells obtained from CD146+ cell cocultures showed human engraftment whereas no engraftment was ever detected (ND) in mice that received MSC cocultures (n = 3 independent experiments, n = 11 mice per group; ***P < .0001). (D) Frequency of CD34+ progenitors, CD19+ lymphoid, and CD14+ myeloid cells within the CD45+HLA+ population of cells in the bone marrow of chimeric mice. (E) Human CD45+HLA+ hematopoietic cells were also detected 6 weeks posttransplantation in the contralateral tibia of mice injected with HSPCs cocultured with CD146+ perivascular cells. (F-I) Representative flow cytometry analysis of secondary host bone marrow. (F) Bone marrow from primary hosts transplanted with MSC coculture was injected in secondary hosts as a negative control. (G) Human engraftment was observed 4 weeks after secondary transplantation of bone marrow from chimeric mice transplanted with CD146+ cell coculture (n = 3 engrafted mice of 4). (H) Both CD19+ lymphoid and CD33/CD14/CD15+ myeloid cells were detectable within the human CD45+ engrafted hematopoietic cells in secondary hosts. (I) Quantification of the level of chimerism in secondary mice. All data are presented as mean ± SEM.
CD146+ perivascular cells isolated from either FBM or adipose tissue sustained significantly more CD34+Lin− cells and CFUs from CB CD34+ cells than CD146− stromal cells did (supplemental Figure 2a-d), thus confirming that within the heterogeneous MSC population, the ability to support HSPCs is confined to the subset of CD146+ perivascular cells, regardless of the tissue of origin.
CD146+ perivascular cells maintain human HSPCs with repopulating ability and self-renewal potential
We next investigated whether coculture with MSCs or CD146+ perivascular cells retains functional HSPCs. Sublethally irradiated NOD/SCID/IL-2 receptor γ-chain null (NSG) mice were injected with hematopoietic cells cocultured with CD146+ perivascular cells or MSCs for 2 weeks in low-serum concentration without added cytokines. Strikingly, all mice transplanted with hematopoietic cells cocultured with perivascular cells exhibited human hematopoietic cell engraftment 6 weeks posttransplantation, whereas no engraftment was observed in any of the mice transplanted with hematopoietic cells cocultured with MSCs (n = 11 mice per group, n = 3 individual experiments; Fisher exact test, P < .0001) (Figure 5B-C). Human CD34+ progenitors, CD19+ lymphoid cells and CD14+ myeloid cells were detected in the chimeric mice (Figure 5D). Human CD45+HLA+ cells were not only detected in the medullary site of injection, but also in the contralateral tibia, thus suggesting that HSPCs cocultured with CD146+ perivascular cells maintained the ability to migrate and home to distant sites after initial engraftment (Figure 5E). To assess the self-renewal potential of HSPCs cultured in the presence of CD146+ perivascular cells, bone marrow from chimeric mice was transplanted into secondary NSG mouse hosts. Lymphoid and myeloid engraftment of human cells was still detectable in secondary hosts (Figure 5F-I), demonstrating that the CD146+ cell fraction of MSCs is uniquely able to sustain human HSPCs with multilineage repopulating capacity and self-renewal ability.
Contact with CD146+ cells is required for HSPC maintenance
In addition to the phenotypic and functional differences described above (Figures 2 and 3), a different morphology and spatial distribution was observed between hematopoietic cells cocultured with MSCs or CD146+ perivascular cells. When CD146+ cells were used as a feeder layer, hematopoietic cells appeared small, rounded, and clustered (supplemental Figure 3a). In the presence of MSCs, hematopoietic cells were larger, less uniform in size, and scattered throughout the cultures, consistent with more vigorous hematopoietic differentiation (supplemental Figure 3c). Immunocytochemical analysis confirmed the presence of clusters of CD34+ cells in contact with underlying CD146+ cells in perivascular cell cocultures but not in MSC cocultures (supplemental Figure 3b,d). Based on these observations, we next investigated the role of cell-to-cell contact on HSPC maintenance. When direct contact between CD146+ cells and CB CD34+ cells was prevented in a transwell culture system, the total number of CD45+ cells was dramatically reduced after 1 week of coculture (supplemental Figure 3e). In these noncontact conditions, hematopoietic cells were barely detectable after 2 weeks and the limited number of cells recovered did not allow us to perform further immunophenotypic or functional analyses.
CD146+ perivascular cells express Notch ligands and activate Notch in hematopoietic cells
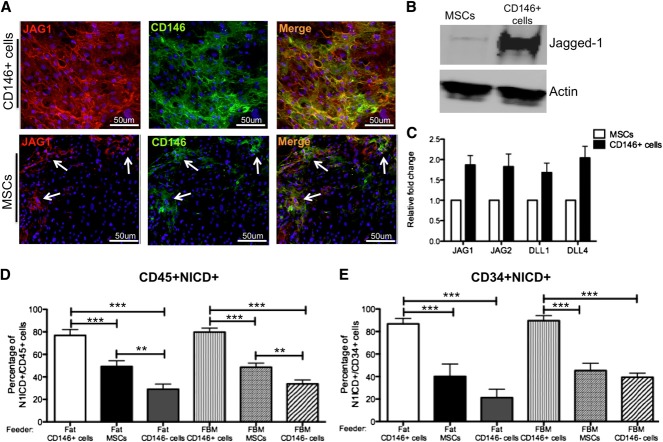
Transwell culture experiments suggested that CD146+ perivascular cells sustain hematopoietic cells through cell-to-cell contact rather than by secretion of soluble factors. As Notch signaling is one of the key pathways through which the microenvironment affects growth and differentiation of HSPCs during development,40 we investigated whether CD146+ perivascular cells sustain HSPCs through the activation of Notch. Immunocytochemistry revealed that all cultured CD146+ perivascular cells express high levels of the Notch ligand Jagged-1. In contrast, only rare MSCs expressed Jagged-1, the majority of which also expressed CD146 (Figure 6A). Western blot analysis detected Jagged-1 expression at high levels in CD146+ perivascular cells compared with unfractionated MSCs (Figure 6B). Expression of other Notch ligands (Jagged-2, DLL-1, and DLL-4) was also detected by qPCR in MSCs, albeit at a lower level compared with CD146+ perivascular cells (Figure 6C).
Figure 6.
CD146+ perivascular cells induce Notch activation in hematopoietic cells. (A) Immunocytochemical staining for Jagged-1 (JAG1, red), CD146 (green) and nuclei (DAPI, blue) performed on fat-derived CD146+ perivascular cells and MSCs (original magnification, ×20). White arrows indicate clusters of cells within the MSC that coexpress JAG1 and CD146. (B) Western blot analysis showing significantly higher expression of Jagged-1 in CD146+ perivascular cells compared with MSCs derived from fat. (C) qPCR analysis revealed that fat-derived MSCs and CD146+ perivascular cells express multiple Notch ligands. (D-E) Quantification of (D) CD45+ and (E) CD34+ hematopoietic and progenitor cells with activated Notch pathway (CD45+NICD+) after 1 week of coculture with fat or FBM-derived CD146+ perivascular cells, MSCs, and CD146− cells (n = 3 independent experiments, n = 40 random fields analyzed; ***P < .0001). Data are presented as mean ± SEM.
We used an antibody recognizing an epitope exclusively exposed after Notch 1 receptor cleavage (NICD) to measure the frequency of hematopoietic cells activating Notch in the presence of CD146+ cells or MSCs (supplemental Figure 4a-b). As expected, Notch activation was not observed when direct contact between CD146+ cells and hematopoietic cells was inhibited in transwell cocultures (supplemental Figure 4c-d). MSCs, which express all 4 Notch ligands tested, were able to activate Notch1 in ∼50% of hematopoietic cells and progenitors (Figure 6D-E). The percentage of NICD+CD45+ cells was significantly higher in CD146+ cell cocultures than in cocultures with total MSCs or CD146− cells, regardless of the tissue of origin (FBM or fat) (Figure 6D). Furthermore, Notch activation was significantly stronger in CD34+ progenitors cocultured with CD146+ cells compared with those cocultured with MSCs or CD146− cells (Figure 6E).
Notch inhibition in CD146+ cell/HSPC cocultures reduces progenitor cell numbers and stimulates B-cell differentiation
To further assess the functional role of Notch activation in HSPCs, CB CD34+ cells, and CD146+ perivascular cells were cocultured in the presence of the γ-secretase inhibitor DAPT. Notch inhibition resulted in significantly reduced total number of CD45+ cells, CD34+Lin− cells, and CFUs compared with control cocultures performed in the presence of the DMSO solvent alone (Figure 7A-C). A significantly higher frequency of PI+ dead cells was measured after Notch inhibition (Figure 7D). Of note, the frequency of PI+ cells was not increased when CD146+ perivascular cells or CB CD34+ cells were treated separately with DAPT in the absence of supplemental cytokines, thus excluding nonspecific cytotoxicity from DAPT (supplemental Figure 5a). Notch inhibition also resulted in a dramatic increase in B-cell differentiation (Figure 7E). A comparable decrease in output of total CD34+Lin− cells and increase in B-lymphoid cells (supplemental Figure 5b-d) was also observed when cocultures were performed in the presence of an antibody to specifically block the Notch-1 receptor. However, the effect was less pronounced when compared with DAPT treatment. To explain this difference, we determined the levels of Notch inhibition following DAPT or anti-Notch-1 blocking antibody treatment. Although Notch activation was totally abrogated by DAPT, low-level activation was still detected in a few cells after antibody treatment, confirming that the latter treatment is less efficient than chemical inactivation of Notch (supplemental Figure 4e-g).
Figure 7.

Notch inhibition affects survival and B-cell differentiation of HSPCs. Inhibition of Notch was achieved by addition of 10μM DAPT to CD146+ perivascular cells and CB CD34+ cell coculture every other day. Vehicle (DMSO) was added to control cocultures. (A-B) Total number of CD45+ cells and CD34+Lin− cells was significantly reduced after 2 weeks of coculture with DAPT (5.03 ± 0.54 × 104 vs 3.02 ± 0.37 × 104 CD45+ cells, n = 4 independent experiments, each experiment was performed in triplicate, **P < .01; 1.5 ± 0.16 × 104 vs 0.82 ± 0.12 × 104 CD34+Lin− cells, n = 4 independent experiments, each experiment was performed in triplicate, **P < .01). (C) Similarly, the total number of CFUs was significantly reduced after 4 weeks of coculture with DAPT (478.3 ± 112.4 vs 191.0 ± 43.28, n = 3 independent experiments, each experiment was performed in triplicate; * P < .05). (D) Flow cytometry viability analysis revealed a significantly higher frequency of PI+ dead cells in coculture performed in the presence of DAPT (13.08% ± 1.13% vs 19.94 ± 1.31, n = 4 independent experiments, each experiment was performed in triplicate, *** P < .0001). (E) Notch inhibition also significantly increased B-cell development (0.13 ± 0.04 × 103 vs 1.72 ± 0.55 × 103 of lymphoid cells, n = 3 individual experiments, each experiment performed in triplicate; **P < .01). Data are presented as mean ± SEM.
Altogether, these results show that CD146+ perivascular cells are a subset of MSCs able to support HSPCs and regulate lineage commitment in vitro through cell-to-cell interaction and partially through Notch activation.
Discussion
Blood formation in vertebrates is an opportunistic phenomenon that does not take place exclusively in specialized, hematopoiesis-restricted sites such as the bone marrow, thymus, spleen and avian bursa of Fabricius. Blood cells are also produced transiently in organs assuming other functions, such as the yolk sac, placenta, allantois and embryonic aorta-gonad-mesonephros, and liver. Moreover, extramedullary hematopoiesis can be resumed in pathologic conditions of the adult. Such anatomic diversity in blood-forming ability implies that developmentally and structurally distinct cellular environments can sustain hematopoiesis. Different blood-forming tissues may therefore share stromal cell subsets involved in blood formation. Although HSPCs have been characterized in detail and purified to homogeneity, the identity and function of the stromal cells involved in hematopoiesis have remained largely unknown. Although stroma-dependent hematopoiesis has been recapitulated in vitro for more than 3 decades using primary stromal cells or stromal cell lines,9-11 the nature of the stromal cells involved has been elusive. As an obstacle to characterization, native stromal cells involved in supporting hematopoiesis are infrequent: Wineman et al41 found that only a rare subpopulation of clonal fetal liver stromal cells is able to maintain HSPCs.
MSCs are cultured, multipotent adherent cells that can support hematopoiesis.19-24 We hypothesized that MSCs contain distinct subsets of cells with different roles in the regulation of HSPCs. Based on recent descriptions of (1) a key contribution of murine perivascular cells to the medullary hematopoietic “niche,”35-37 and (2) a pericyte ancestry for human MSCs,29 we directly addressed whether cultured human perivascular cells can sustain human HSPCs. Conventionally derived, heterogeneous MSCs and CD146+CD34−CD45− perivascular cells can be obtained from virtually all human vascularized tissues.29 In the present study, we derived MSCs and CD146+ perivascular cells from FBM and human adipose tissue, which is commonly used as a convenient and abundant source of MSCs. Interestingly, the sustained presence of hematopoietic cells within adipose tissue has been recently reported.42,43
CD146+ perivascular cells expressing nestin, CXCL12, and Lep-R were found in situ in the hematopoietic FBM as well as in adipose tissue. Sorted CD146+ perivascular cells homogeneously expressed in culture CD146 and higher levels of nestin, CXCL12, and Jagged-1 compared with unfractionated MSCs or to CD146− cells. CD146+ perivascular cells therefore appear to represent the human counterpart of the CAR cells or nestin+ cells recently described in the mouse.35-37 A similar cell population has been documented in human bone marrow, where CD146+ perivascular cells expressing CXCL12 and Jagged-1 can clonally recapitulate an ectopic hematopoietic microenvironment when implanted into mice.44 Human bone marrow reticular stromal cells, including CD146+nestin+VCAM+ cells, regulate HSPC homing through the secretion of CXCL12.45 Pericyte-like cells from the human placenta have been also suggested to support hematopoietic cells in culture.46 However, direct evidence for the ability of prospectively purified human perivascular cells to sustain primitive hematopoietic cells in long-term culture has not been provided. Several studies have investigated the ability of MSCs to maintain HSPCs in coculture systems, but these have routinely used cytokine supplementation either by direct addition or through transgene expression in MSCs.19-24,47,48 In most cases the decisive assays, primary and secondary transplantations of cocultured hematopoietic cells into immunodeficient mice, have not been used to document the maintenance of primitive self-renewing stem cells. Most importantly, the identity of the specific subset of MSCs directly involved in the interaction with HSPCs is still unknown. In the present study, culture of CD34+ cells with MSCs or CD146+ perivascular cells without the addition of exogenous cytokines allowed us to define the intrinsic properties of these stromal populations in terms of hematopoietic cell support. Remarkably, unfractionated MSCs and purified CD146+ perivascular cells derived from the same specimen exhibited profound differences in the ability to sustain HSPCs. Both stromal cell populations improved the survival of hematopoietic cells compared with stroma-free, cytokine-free cultures. However, the total number of recovered HSPCs was consistently and significantly higher in CD146+ cell cocultures. Furthermore, only CD146+ perivascular cells sustained primitive HSPCs able to establish multilineage hematopoiesis in immunodeficient mice. Conversely, MSCs promoted rapid HSPC differentiation with consequent loss of engraftment ability. Our results also demonstrate that cell-to-cell contact between HSPCs and CD146+ perivascular cells plays a key role in HSPCs maintenance in vitro. We show that CD146+ cells express Notch ligands and that Notch inhibition in cocultures results in decreased numbers of HSPCs and increased B-cell development, as previously described.49 Regulation of HSPCs by perivascular cells is very likely to be a multifaceted process, Notch signaling being only one of the mechanisms involved in HSPC maintenance. Although our results show an increase in PI+ dead cells in the whole coculture following Notch inhibition, suggesting a role for Notch in supporting cell survival, additional studies will be required to determine whether Notch activation prevents specifically apoptosis/death in HSPCs.
De Toni et al50 recently described the clinical-grade expansion of adipose MSCs able to support hematopoietic reconstitution in immunodeficient mice when co-injected with fresh CB CD34+ cells. The authors showed higher frequency of human CD45+ cells 3 weeks posttransplantation in mice that received CD34+ cells and MSCs as compared with mice injected with CD34+ cells alone. This difference was no longer observed 11 weeks posttransplantation, suggesting that fat MSCs support short-term progenitors. The lack of secondary transplantation assay did not allow the authors to establish whether fat MSCs can support maintenance of HSPC self-renewal. In our study, we demonstrate that unfractionated MSCs failed to ex vivo support HSPCs with reconstituting ability even in primary recipients. Conversely, we demonstrate for the first time that the CD146+ subset of MSCs was uniquely able to maintain self-renewing HSPCs after 2 weeks in culture without growth factors, as shown by reconstitution of primary and secondary hosts. The identification of CD146+ perivascular cells as a defined and specific subset of human stromal cells able to sustain HSPCs may therefore have a critical impact on future clinical applications based on ex vivo expansion and genetic manipulation of HSPCs. Butler et al14 recently described a cellular platform for the expansion of human HSPCs based on the coculture with transformed endothelial cells in the presence of defined growth factors. In the present work, cocultures were performed using nontransformed stromal cells in the complete absence of added growth factors. Having identified CD146+ perivascular cells as the specific subset of MSCs involved in HSPC maintenance, further studies aimed to define optimal conditions to promote HSPC expansion will be needed.
For the first time to our knowledge, we document the direct role of an anatomically and phenotypically defined subset of human stromal cells—the CD146+ perivascular cells—in maintaining cultured HSPCs. A fraction of native and all cultured pericytes express α-SMA,29 therefore these findings also support a myofibroblastic identity for human hematopoietic stromal cells.51 Besides a functional ability to support hematopoietic cells following dissociation, purification, and culture, human CD146+ perivascular cells from nonhematopoietic tissues share a similar phenotype with the perivascular niche cells recently described in murine bone marrow.35-37 Perivascular cells are ubiquitous52 and may therefore represent the key stem cell support shared by all blood-forming organs. It remains to be determined whether and how this ability to sustain HSCs is repressed in situ in nonhematopoietic tissues, and may be reactivated in pathologic conditions, as in the course of extramedullary hematopoiesis or leukemic dissemination.
Supplementary Material
Acknowledgments
The authors thank Yuhua Zhu and Rebecca Chan (Department of Pathology, UCLA) for their valuable technical assistance, Dr Ling Wu (Orthopaedic Hospital Department of Orthopaedic Surgery and the Orthopaedic Hospital Research Center) for the assistance with immunohistochemistry on FBM, and Dr David Stoker (Marina Plastic Surgery Associates, Marina del Rey, CA) for the procurement of human tissues. The authors are also grateful for the remarkable flow cytometry and sorting assistance provided by Jessica Scholes and Felicia Codrea (Eli and Edythe Broad Stem Cell Center, UCLA).
This work was supported by funds from UCLA, Orthopaedic Hospital Department of Orthopaedic Surgery, and Eli and Edythe Broad Stem Cell Center at UCLA. This research was also made possible by a grant from the California Institute for Regenerative Medicine (grant no. RB3-05217) (G.M.C.). M.C. and C.P. acknowledge the support of a California Institute for Regenerative Medicine training grant (TG2-01169). The research was also partially founded by the European Community FP7 program, through the Reborne project (grant agreement no. 241879).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.C. designed and performed research, analyzed and interpreted data, and wrote the manuscript; C.P., A.S., W.W., S.G., D.E., and C.J.C. performed research; X.W. performed statistical analysis; E.M. and L.L. analyzed and interpreted data and contributed to writing the manuscript; and G.M.C and B.P. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruno Péault, 615 Charles E. Young Dr South, Room 410, Los Angeles, CA 90095; bpeault@mednet.ucla.edu.
References
- 1.Tavian M, Péault B. The changing cellular environments of hematopoiesis in human development in utero. Exp Hematol. 2005;33(9):1062–1069. doi: 10.1016/j.exphem.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. 1917. J Hematother Stem Cell Res. 2002;11(1):5–7. doi: 10.1089/152581602753448496. [DOI] [PubMed] [Google Scholar]
- 3.Oberlin E, Tavian M, Blazsek I, et al. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129(17):4147–4157. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- 4.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand JY, Chi NC, Santoso B, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boisset JC, van Cappellen W, Andrieu-Soler C, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464(7285):116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 7.Zovein AC, Hofmann JJ, Lynch M, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3(6):625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambidis ET, Park TS, Yu W, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112(9):3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dexter TM, Wright EG, Krizsa F, et al. Regulation of haemopoietic stem cell proliferation in long term bone marrow cultures. Biomedicine. 1977;27(9-10):344–349. [PubMed] [Google Scholar]
- 10.Whitlock CA, Witte ON. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987;138(4):1082–1087. [PubMed] [Google Scholar]
- 12.Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6(3):251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi H, Butler JM, O’Donnell R, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12(11):1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler JM, Gars EJ, James DJ, et al. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120(6):1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 16.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 17.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 19.Gan OI, Murdoch B, Larochelle A, et al. Differential maintenance of primitive human SCID-repopulating cells, clonogenic progenitors, and long-term culture-initiating cells after incubation on human bone marrow stromal cells. Blood. 1997;90(2):641–650. [PubMed] [Google Scholar]
- 20.Fei XM, Wu YJ, Chang Z, et al. Co-culture of cord blood CD34(+) cells with human BM mesenchymal stromal cells enhances short-term engraftment of cord blood cells in NOD/SCID mice. Cytotherapy. 2007;9(4):338–347. doi: 10.1080/14653240701291638. [DOI] [PubMed] [Google Scholar]
- 21.Huang GP, Pan ZJ, Jia BB, et al. Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood. Cell Transplant. 2007;16(6):579–585. doi: 10.3727/000000007783465073. [DOI] [PubMed] [Google Scholar]
- 22.Corre J, Barreau C, Cousin B, et al. Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J Cell Physiol. 2006;208(2):282–288. doi: 10.1002/jcp.20655. [DOI] [PubMed] [Google Scholar]
- 23.Flores-Guzmán P, Flores-Figueroa E, Montesinos JJ, et al. Individual and combined effects of mesenchymal stromal cells and recombinant stimulatory cytokines on the in vitro growth of primitive hematopoietic cells from human umbilical cord blood. Cytotherapy. 2009;11(7):886–896. doi: 10.3109/14653240903180076. [DOI] [PubMed] [Google Scholar]
- 24.Wagner W, Roderburg C, Wein F, et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25(10):2638–2647. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- 25.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 26.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 27.Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214(2):413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 28.Covas DT, Panepucci RA, Fontes AM, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36(5):642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 31.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Levesque JPN. N(o)-cadherin role for HSCs. Blood. 2012;120(2):237–238. doi: 10.1182/blood-2012-05-431148. [DOI] [PubMed] [Google Scholar]
- 33.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208(3):421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kincade PW. Plasticity of supporting cells in a stem cell factory. Immunity. 2010;33(3):291–293. doi: 10.1016/j.immuni.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corselli M, Chen CW, Sun B, et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21(8):1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poglio S, De Toni F, Lewandowski D, et al. In situ production of innate immune cells in murine white adipose tissue. Blood. 2012;120(25):4952–4962. doi: 10.1182/blood-2012-01-406959. [DOI] [PubMed] [Google Scholar]
- 40.Bigas A, Robert-Moreno A, Espinosa L. The Notch pathway in the developing hematopoietic system. Int J Dev Biol. 2010;54(6-7):1175–1188. doi: 10.1387/ijdb.093049ab. [DOI] [PubMed] [Google Scholar]
- 41.Wineman J, Moore K, Lemischka I, et al. Functional heterogeneity of the hematopoietic microenvironment: rare stromal elements maintain long-term repopulating stem cells. Blood. 1996;87(10):4082–4090. [PubMed] [Google Scholar]
- 42.Cousin B, André M, Arnaud E, et al. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun. 2003;301(4):1016–1022. doi: 10.1016/s0006-291x(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 43.Han J, Koh YJ, Moon HR, et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115(5):957–964. doi: 10.1182/blood-2009-05-219923. [DOI] [PubMed] [Google Scholar]
- 44.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 45.Schajnovitz A, Itkin T, D’Uva G, et al. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat Immunol. 2011;12(5):391–398. doi: 10.1038/ni.2017. [DOI] [PubMed] [Google Scholar]
- 46.Robin C, Bollerot K, Mendes S, et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell. 2009;5(4):385–395. doi: 10.1016/j.stem.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khoury M, Drake A, Chen Q, et al. Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential. Stem Cells Dev. 2011;20(8):1371–1381. doi: 10.1089/scd.2010.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie C, Jia B, Xiang Y, et al. Support of hMSCs transduced with TPO/FL genes to expansion of umbilical cord CD34+ cells in indirect co-culture. Cell Tissue Res. 2006;326(1):101–110. doi: 10.1007/s00441-006-0203-7. [DOI] [PubMed] [Google Scholar]
- 49.Nie L, Perry SS, Zhao Y, et al. Regulation of lymphocyte development by cell-type-specific interpretation of Notch signals. Mol Cell Biol. 2008;28(6):2078–2090. doi: 10.1128/MCB.00844-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Toni F, Poglio S, Youcef AB, et al. Human adipose-derived stromal cells efficiently support hematopoiesis in vitro and in vivo: a key step for therapeutic studies. Stem Cells Dev. 2011;20(12):2127–2138. doi: 10.1089/scd.2011.0044. [DOI] [PubMed] [Google Scholar]
- 51.Galmiche MC, Koteliansky VE, Brière J, et al. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82(1):66–76. [PubMed] [Google Scholar]
- 52.Andreeva ER, Pugach IM, Gordon D, et al. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell. 1998;30(1):127–135. doi: 10.1016/s0040-8166(98)80014-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.