Abstract
Background
This phase I/II study in patients with metastatic castration-resistant prostate cancer (mCRPC) explored ipilimumab as monotherapy and in combination with radiotherapy, based on the preclinical evidence of synergistic antitumor activity between anti-CTLA-4 antibody and radiotherapy.
Patients and methods
In dose escalation, 33 patients (≥6/cohort) received ipilimumab every 3 weeks × 4 doses at 3, 5, or 10 mg/kg or at 3 or 10 mg/kg + radiotherapy (8 Gy/lesion). The 10-mg/kg cohorts were expanded to 50 patients (ipilimumab monotherapy, 16; ipilimumab + radiotherapy, 34). Evaluations included adverse events (AEs), prostate-specific antigen (PSA) decline, and tumor response.
Results
Common immune-related AEs (irAEs) among the 50 patients receiving 10 mg/kg ± radiotherapy were diarrhea (54%), colitis (22%), rash (32%), and pruritus (20%); grade 3/4 irAEs included colitis (16%) and hepatitis (10%). One treatment-related death (5 mg/kg group) occurred. Among patients receiving 10 mg/kg ± radiotherapy, eight had PSA declines of ≥50% (duration: 3–13+ months), one had complete response (duration: 11.3+ months), and six had stable disease (duration: 2.8–6.1 months).
Conclusions
In mCRPC patients, ipilimumab 10 mg/kg ± radiotherapy suggested clinical antitumor activity with disease control and manageable AEs. Two phase III trials in mCRPC patients evaluating ipilimumab 10 mg/kg ± radiotherapy are ongoing.
ClinicalTrials.gov identifier: NCT00323882.
Keywords: ipilimumab, metastatic castration-resistant prostate cancer, phase I/II trial, prostate-specific antigen and radiotherapy, immunotherapy
introduction
Cancer immunotherapy, based on active immunization with tumor antigens, can induce antitumor immune responses and has been widely tested in prostate cancer [1]. Sipuleucel-T, an immunotherapy targeting prostatic acid phosphatase, demonstrated improvement in overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC), with no demonstrable effects on the serum prostate-specific antigen (PSA) level or tumor growth in phase III trials [2]. This and other treatments, including docetaxel, cabazitaxel, abiraterone, enzalutamide, and radium-223 chloride, showed only incremental OS improvements [3–7], so there is a need for novel therapeutic approaches providing durable disease control.
Cytotoxic T-lymphocyte antigen-4 (CTLA-4), a negative regulator of T-cell activation, has emerged as a target for cancer immunotherapy [8–10]. Ipilimumab, a fully human monoclonal antibody, specifically blocks the binding of CTLA-4 to its ligands (CD80/CD86) and thereby augments T-cell activation and proliferation and tumor regression [11–15]. Early clinical trials with ipilimumab showed clinical activity in several cancers including melanoma, renal cell carcinoma, non-Hodgkin lymphoma, pancreatic adenocarcinoma, lung and ovarian cancer [16–24]. Two randomized phase III trials demonstrated OS improvements and durable objective responses in patients with metastatic melanoma [25, 26]. Follow-up showed that 19%–36% of patients with metastatic melanoma treated with ipilimumab had long-term (4-year) OS [27–29]. Adverse events (AEs) associated with ipilimumab were often immune-related and occurred mainly within the skin, gastrointestinal tract, and liver; these were generally managed by established treatment guidelines [25, 26, 30].
Based on the antitumor immunity of the anti-CTLA-4 antibody in preclinical models of prostate cancer [31, 32], several studies of ipilimumab in mCRPC patients were carried out. These initial studies showed that ipilimumab 3 mg/kg given every 4 weeks for four doses had acceptable safety and preliminary antitumor activity [33–35]. We chose to evaluate ipilimumab 3, 5, or 10 mg/kg given every 3 weeks, because phase II data in melanoma patients showed a higher objective response rate (11.1%) at 10 mg/kg than at 3 mg/kg (4.2%) or 0.3 mg/kg (0%) [18].
Localized radiotherapy can cause immune-mediated tumor death and induce tumor regression at sites distant from the primary site of radiotherapy (abscopal effect) in an immune-mediated process [36, 37]. In murine models of breast and colon cancer, the combination of the anti-CTLA-4 antibody and localized tumor irradiation resulted in the synergistic inhibition of metastases [38, 39]. Furthermore, the abscopal effect involving immune response has been reported in two cases of metastatic melanoma treated with ipilimumab and localized radiotherapy [40, 41]. Therefore, we hypothesized that tumor antigens released during radiation-induced cell death may enhance the antitumor activity of ipilimumab in patients with mCRPC. Accordingly, we performed a phase I/II study in patients with mCRPC to systematically assess ipilimumab at various doses given alone or in combination with external-beam radiotherapy (XRT).
patients and methods
patients
Men diagnosed with mCRPC (rising PSA or progression on scans with a serum testosterone concentration of <50 ng/dl) and the evidence of progression after the discontinuation of anti-androgen therapy who had no more than one prior chemotherapy were enrolled. Adenocarcinoma of the prostate was confirmed histologically, and the extent of disease was documented radiographically by bone scan and computed tomography. Patients had a life expectancy of >12 weeks, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate hematologic, hepatic, and renal functions. Patients with radiation-induced diarrhea within 12 months of study entry or with prior colitis or irritable bowel syndrome were excluded. Other key exclusion criteria were autoimmune disease (except for vitiligo) requiring systemic steroids or immunosuppressive agents, other prior malignancy within 5 years, active infection, bone pain severe enough to require routine narcotic analgesics, and prior treatment with anti-CTLA-4 therapies. All patients gave informed consent before enrollment. The study was conducted according to the principles of the Helsinki Declaration. The protocol was approved by Institutional Review Boards in all participating centers.
study design and treatment
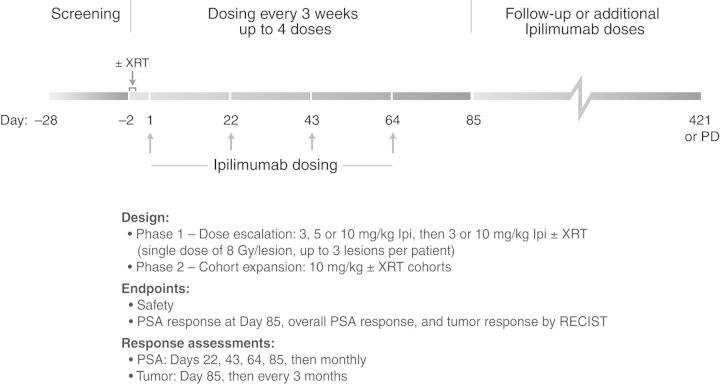
This was a phase I/II, non-randomized, open-label, multicenter study (ClinicalTrials.gov identifier: NCT00323882). In the dose-escalation phase, eligible patients (≥6 patients per cohort) received ipilimumab at 3, 5, or 10 mg/kg or at 3 or 10 mg/kg + XRT (Figure 1). Starting with the lowest monotherapy dose, ipilimumab was administered intravenously once every 3 weeks (days 1, 22, 43, and 64) in a cycle of up to four doses with a response assessment 3 weeks after the last dose (day 85). Dose escalation occurred after all six patients in the preceding cohort received at least two doses of ipilimumab and were observed for an additional 2 weeks with no more than one of the six patients experiencing a dose-limiting toxicity (DLT) during this 5-week period. The DLT was defined as a grade 3/4 immune-related AE (irAE) or other grade 3/4 treatment-related AE, which required surgical intervention or did not resolve to ≤grade 2 within 14 days of the start of immunosuppressive therapy. Dose escalation continued until the last monotherapy-dose cohort was enrolled or the maximum tolerated dose (MTD, defined as the highest dose at which no more than one of the six patients experienced a DLT) was identified.
Figure 1.
The study schema. XRT, external-beam radiotherapy; PD, progressive disease; PSA, prostate-specific antigen.
Once the monotherapy cohorts were fully enrolled, patients were assigned to 3 mg/kg + XRT, and after completion of accrual in this arm, to 10 mg/kg + XRT. Radiotherapy was given focally at a single dose of 8 Gy per target bone lesion for up to three bone lesions per patient at 24–48 h before the first ipilimumab dose. Single administration of 8 Gy has been shown to be therapeutically equivalent to fractionated regimens in terms of pain palliation and better tolerated [42]. Target lesions had to be ≥10 mm long in at least one direction when measured by radiologic imaging. The timing of XRT delivery was designed to provide CTLA-4 blockade at a time when antigen presentation due to radiation was expected to peak [43].
For the phase II portion, additional patients were assigned to monotherapy (at the MTD or at 10 mg/kg if the MTD was not reached), and after monotherapy accrual was completed, to combination therapy. Patients who progressed following an initial response or stable disease could have received up to three additional cycles of ipilimumab. No retreatment with XRT was allowed. After the initial treatment period (days 1–112), patients had follow-up visits every 4 weeks for 3 months and then every 12 weeks for 9 months or until disease progression, intolerance, or death. Patients who withdrew from the study due to disease progression or who completed all planned study visits were followed for survival every 3 months for up to 5 years.
assessments
AEs including irAEs were based on assessments by investigators of patients treated between the first dose and 70 days after the last ipilimumab dose. An irAE was defined as a treatment-related AE consistent with immune-mediated events. AEs, irAEs, and clinical laboratory tests were graded using the NCI Common Terminology Criteria for Adverse Events, version 3.0. The protocol defined guidelines for evaluation and treatment of irAEs of the gastrointestinal tract, liver, skin, eye, and endocrine glands; irAE management consisted of corticosteroids (e.g. prednisone or budesonide) given orally or intravenously. Additional immunosuppressive agents (e.g. infliximab for colitis and mycophenolate mofetil for hepatic irAEs) and hormone replacement therapy for endocrine irAEs were also used at the investigator's discretion. No dose reductions were allowed. For a grade 2 drug-related skin irAE or grade 3 skin irAE (regardless of causality), ipilimumab administration was delayed until its resolution to ≤grade 1. Ipilimumab administration was permanently discontinued for any non-skin-related AE of ≥grade 3 or any other AE of ≥grade 4.
Antitumor effects were assessed by serum PSA status using criteria consistent with guidelines of the Prostate Cancer Clinical Trials Working Group 1 (PCWG1) [44], the standard when the study was being designed, and by tumor status using Response Evaluation Criteria in Solid Tumors (RECIST) for soft tissue disease [45]. PSA assessments were performed on days 22, 43, 64, and 85 and every month thereafter. Tumor assessments were carried out on day 85 and every 3 months thereafter. This time schedule was chosen, because responses to ipilimumab have been observed weeks to months after therapy initiation [46]. Both the decline in PSA to ≥50% from baseline (PSA decline) and tumor response, as determined by investigators, were confirmed by repeat assessments at 4 weeks or later after the initial assessments. The PSA decline was calculated by comparing the greatest decline in post-therapy PSA concentration to baseline. End points included PSA decline by day 85, PSA decline at any time, tumor response at any time, time to and duration of tumor response, and OS.
statistical considerations
The primary objective of this study was to determine the safety of ipilimumab alone or in combination with a single dose of XRT. The secondary objective was to determine clinical antitumor activity based on PSA and radiologic responses. For the phase II portion of the study, the initial sample size of 30 patients was based on the design of the dose escalation for safety. For the phase II portion of the study, there were to be 16 assessable patients treated with ipilimumab monotherapy and 32 treated with the combination of ipilimumab + XRT (chemotherapy-naïve, 16; chemotherapy-experienced, 16). A sample size of 16 assessable patients was required to provide >80% power in a one-sample exact binomial test at the significance level of 0.05. Data were summarized using descriptive statistics. The Kaplan–Meier product limit method was used to estimate the median OS.
results
patients
Seventy-five patients were enrolled at nine sites in the United States: 71 were eligible and received treatment between January 2006 and September 2009. Forty-seven patients (66%) discontinued the study due to disease progression determined by either PCWG1 criteria or RECIST (Table 1). Of eight discontinuations due to AEs, five were caused by irAEs (colitis, 1; diarrhea, 3; hepatitis, 1). Of seven deaths causing discontinuation, one (5 mg/kg group) was due to aspergillosis, after 4 months of immunosuppressive therapy required to control grade 3 colitis, and was thus considered treatment-related. Three patients died from disease progression and one each from sepsis, pneumonia, and myocardial infarction; these deaths were deemed by investigators to be unrelated to treatment. The patient who died of sepsis (Clostridium difficile) received no immunosuppressives; no autopsy or endoscopy was carried out to rule out colitis, but the presentation did not include diarrhea above grade 2. Two deaths (10 mg/kg + XRT: pneumonia, 1; progression, 1) occurred within 30 days after the last ipilimumab dose.
Table 1.
Disposition of treated patients as of May 2012a
| Characteristic | Ipilimumab dose |
|||||
|---|---|---|---|---|---|---|
| 3 mg/kg |
5 mg/kg | 10 mg/kg |
||||
| −XRT (n = 8) | +XRT (n = 7) | −XRT (n = 6) | −XRT (n = 16; %) | +XRT (n = 34; %) | ±XRT (n = 50; %) | |
| Discontinued | 8 | 7 | 6 | 13 (91) | 32 (94) | 45 (90) |
| Progressive disease | 6 | 5 | 4 | 10 (63) | 22 (65) | 32 (64) |
| AE | 2 | 1 | 1 | 1 (6) | 3 (9) | 4 (8) |
| irAE | 1 | 1 | 1 | 1 (6) | 1 (3) | 2 (4) |
| Death | 0 | 0 | 1 | 1 (6) | 5 (15) | 6 (12) |
| Treatment-related | 0 | 0 | 1b | 0 | 0 | 0 |
| Unrelated to treatment | 0 | 0 | 0 | 1 (6)c | 5 (15)d | 6 (12) |
| Other | 0 | 1e | 0 | 0 | 1 (3) | 1 (2) |
| Lost to follow-up | 0 | 0 | 0 | 1 (6) | 1 (3) | 2 (4) |
| Completed scheduled follow-upf | 0 | 0 | 0 | 3 (19) | 2 (6) | 5 (10) |
aFour enrolled patients were not treated; reasons not known.
bDue to aspergillosis after prolonged immunosuppressive treatment of grade 3 colitis.
cDue to disease progression.
dOne death each due to sepsis (associated with malignant disease), pneumonia, and acute myocardial infarction (after discontinuation for grade 3 acute renal failure) and two deaths due to disease progression.
eReceived XRT, but no ipilimumab.
fPatients completing all scheduled visits during the initial treatment phase and the 12-month follow-up phase.
XRT, external-beam radiotherapy; AE, adverse event.
Patient demographics and baseline disease characteristics are listed in Table 2. Of 50 patients in the expanded 10 mg/kg ± XRT group, 27 received prior chemotherapy. Of 21 patients across other cohorts, 6 had prior chemotherapy.
Table 2.
Baseline Characteristics
| Characteristic | Ipilimumab dose |
|||||
|---|---|---|---|---|---|---|
| 3 mg/kg |
5 mg/kg | 10 mg/kg |
||||
| −XRT (n = 8) | +XRT (n = 7) | −XRT (n = 6) | −XRT (n = 16; %) | +XRT (n = 34; %) | ±XRT (n = 50; %) | |
| Age (years) | ||||||
| Median | 69 | 68 | 57 | 65 | 66 | 65 |
| Range | 55–78 | 54–81 | 51–68 | 53–76 | 50–83 | 50–83 |
| Race | ||||||
| Asian | 0 | 0 | 0 | 0 | 1 (3) | 1 (2) |
| Black | 0 | 1 | 0 | 0 | 3 (9) | 3 (6) |
| White | 8 | 6 | 6 | 16 (100) | 30 (88) | 46 (92) |
| ECOG performance status | ||||||
| 0 | 5 | 4 | 5 | 10 (63) | 9 (27) | 19 (38) |
| 1 | 3 | 2 | 1 | 6 (37) | 22 (65) | 28 (56) |
| 2 | 0 | 1 | 0 | 0 | 0 | 0 |
| Not reported | 0 | 0 | 0 | 0 | 3 (9) | 3 (6) |
| Time from diagnosis (months) | ||||||
| Median | 79 | 61 | 34 | 60 | 67 | 65 |
| Range | 41–135 | 20–198 | 10–98 | 22–204 | 10–239 | 10–239 |
| Selected tumor lesionsa | ||||||
| Lymph node | 3 | 2 | 2 | 7 (44) | 20 (59) | 27 (54) |
| Liver | 0 | 1 | 0 | 2 (13) | 5 (15) | 7 (14) |
| Lung | 0 | 0 | 2 | 0 | 6 (18) | 6 (12) |
| Bone lesions (no.) | ||||||
| Median | 4 | 6 | 5 | 2.5 | 8 | 6 |
| Range | 1–11 | 2–11 | 2–10 | 1–12 | 1–15 | 1–15 |
| Hemoglobin decreased | ||||||
| Grade 0–1 | 7 | 4 | 6 | 15 (94) | 25 (74) | 40 (80) |
| Grade 2–3 | 0 | 2 | 0 | 1 (6) | 5 (15) | 6 (12) |
| Not reported | 1 | 1 | 0 | 0 | 4 (12) | 4 (8) |
| Alkaline phosphatase increased | ||||||
| Grade 0–1 | 8 | 5 | 6 | 13 (81) | 25 (74) | 38 (76) |
| Grade 2–3 | 0 | 1 | 0 | 3 (19) | 5 (15) | 8 (16) |
| Grade 4 | 0 | 0 | 0 | 0 | 1 (3) | 1 (2) |
| Not reported | 0 | 1 | 0 | 0 | 3 (9) | 3 (6) |
| Serum PSA (ng/ml) | ||||||
| Median | 91 | 47 | 38 | 132 | 120 | 133 |
| Range | 7–449 | 14–197 | 3–111 | 13–2581 | 8–1314 | 8–2581 |
| Selected prior therapyb | ||||||
| Surgery | 7 | 5 | 6 | 16 (100) | 28 (82) | 44 (88) |
| Radiotherapy | 8 | 4 | 5 | 9 (56) | 20 (59) | 29 (58) |
| Goserelin | 1 | 2 | 0 | 3 (19) | 13 (38) | 16 (32) |
| Leuprolide | 7 | 7 | 6 | 14 (88) | 28 (82) | 42 (84) |
| Bicalutamide | 6 | 5 | 6 | 15 (94) | 29 (85) | 45 (90) |
| Flutamide | 0 | 1 | 0 | 3 (19) | 3 (9) | 6 (12) |
| Nilutamide | 4 | 1 | 0 | 3 (19) | 9 (27) | 12 (24) |
| Chemotherapyc | 0 | 4 | 2 | 6 (38) | 21 (62) | 27 (54) |
aReported in ≥10% of patients. Patients may have had lesions at more than one site.
bReported in ≥ 10% of patients. Patients may have received more than one therapy.
cDocetaxel.
XRT, external-beam radiotherapy; ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen.
exposure
There were 71 treated patients; 70 received at least one dose of ipilimumab (monotherapy, 29; combination therapy, 41) and one received XRT only (3 mg/kg + XRT group). In the 10 mg/kg ± XRT group, the median number of ipilimumab doses per patient was 3 (range: 1–8), with 22 (44%) patients receiving four or more doses. Patients in the 3- and 5-mg/kg cohorts received a median of 3.5–4 ipilimumab doses (range: 1–10). Of the 41 patients receiving combination therapy, 30, 8, and 3 had 1, 2, and 3 bone lesions irradiated, respectively.
Eleven (15%) patients who showed an initial response or stable disease by PCWG1 criteria or RECIST and later progressed were retreated with the original ipilimumab dose. Eight patients (5 mg/kg, 2; 10 mg/kg, 2; 10 mg/kg + XRT, 4) received one additional cycle (one cycle: up to four doses once every 3 weeks), two (3 mg/kg) received two additional cycles, and one (3 mg/kg + XRT) received three additional cycles.
safety
Thirty-three patients were initially treated with escalating doses of ipilimumab at 3–10 mg/kg ± XRT. There were no DLTs during the 5-week assessment period. Since the MTD was not reached, ipilimumab 10 mg/kg ± XRT cohorts were expanded by 38 patients to 50 for phase II evaluation. Results for patients in the combined 10 mg/kg ± XRT group are emphasized in the following.
Treatment-related AEs were common, with the majority being grade 1/2 (Table 3). Most of the treatment-related AEs were irAEs in each cohort, and the majority of irAEs were grade 1/2 (Table 3). Common (≥15%) irAEs of any grade in the 10 mg/kg ± XRT group were diarrhea, colitis, rash, and pruritus. Other common treatment-treated AEs were fatigue, nausea, vomiting, and decreased appetite. Sixteen patients (32%) reported irAEs of grade 3/4, most commonly colitis (16%, all grade 3), diarrhea (8%, all grade 3), and hepatitis (4%, grade 3; 6%, grade 4). There were no reports of bowel perforation or neurologic irAEs.
Table 3.
Safety
| Ipilimumab dose |
||||||
|---|---|---|---|---|---|---|
| 3 mg/kg |
5 mg/kg | 10 mg/kg |
||||
| −XRT (n = 8; %) | +XRT (n = 7; %) | −XRT (n = 6; %) | −XRT (n = 16; %) | +XRT (n = 34; %) | ±XRT (n = 50; %) | |
| AEs, any gradea | ||||||
| Any treatment-related | 8 (100) | 6 (86) | 5 (83) | 16 (100) | 29 (85) | 45 (90) |
| Any immune-related | 6 (75) | 4 (57) | 5 (83) | 16 (100) | 24 (71) | 40 (80) |
| Diarrhea | 4 | 3 | 3 | 13 (81) | 14 (41) | 27 (54) |
| Rash | 1 | 1 | 2 | 9 (56) | 7 (21) | 16 (32) |
| Colitis | 1 | 1 | 2 | 7 (44) | 4 (12) | 11 (22) |
| Pruritus | 2 | 0 | 1 | 6 (38) | 4 (12) | 10 (20) |
| Fatigue | 6 | 3 | 3 | 8 (50) | 17 (50) | 25 (50) |
| Nausea | 3 | 3 | 2 | 3 (19) | 9 (27) | 12 (24) |
| Decreased appetite | 2 | 3 | 1 | 2 (13) | 9 (27) | 11 (22) |
| Vomiting | 2 | 2 | 0 | 2 (13) | 7 (21) | 9 (18) |
| AEs, grade 3/4b | ||||||
| Any treatment-related | 2 (25) | 3 (43) | 3 (50) | 10 (63) | 13 (38) | 23 (46) |
| Any immune-related | 1 (13) | 3 (43) | 3 (50) | 10 (63) | 6 (18) | 16 (32) |
| Colitis | 1 | 1 | 1 | 6 (38) | 2 (6) | 8 (16) |
| Hepatitis | 0 | 0 | 0 | 3 (19) | 2 (6) | 5 (10) |
| Diarrhea | 0 | 2 | 1 | 2 (13) | 2 (6) | 4 (8) |
| Fatigue | 0 | 1 | 0 | 0 | 3 (9) | 3 (6) |
| Laboratory abnormalities, any gradea,c | ||||||
| Evaluable patients | 8 | 6 | 5 | 15 (100) | 34 (100) | 49 (100) |
| Lymphopenia | 7 | 5 | 3 | 12 (80) | 31 (91) | 43 (88) |
| Hemoglobin decreased | 7 | 6 | 4 | 12 (80) | 28 (82) | 40 (82) |
| Alkaline phosphatase increased | 3 | 5 | 4 | 7 (47) | 21 (62) | 28 (57) |
| Alanine aminotransferase increased | 0 | 2 | 2 | 7 (47) | 10 (29) | 17 (35) |
| Aspartate aminotransferase increased | 0 | 2 | 2 | 6 (40) | 8 (24) | 14 (29) |
| Amylase increased | 2 | 0 | 3 | 4 (27) | 4 (12) | 8 (16) |
| Laboratory abnormalities, grade 3/4b,c | ||||||
| Evaluable patients | 8 | 6 | 5 | 15 (100) | 34 (100) | 49 (100) |
| Hemoglobin decreased | 0 | 0 | 0 | 1 (7) | 6 (18) | 7 (14) |
| Lymphopenia | 0 | 1 | 0 | 2 (13) | 3 (9) | 5 (10) |
| Alkaline phosphatase increased | 0 | 1 | 0 | 1 (7) | 5 (15) | 6 (12) |
| Alanine aminotransferase increased | 0 | 0 | 1 | 2 (13) | 1 (3) | 3 (6) |
| AEs leading to study therapy discontinuation | ||||||
| Any treatment-related AEs | 2 | 3 | 2 | 6 (38) | 8 (24) | 14 (28) |
| Any irAEs | 2 | 3 | 2 | 6 (38) | 5 (15) | 11 (22) |
| Diarrhea/colitis | 2 | 3 | 2 | 3 (19) | 3 (9) | 6 (12) |
| Hepatitis | 0 | 0 | 0 | 3 (19) | 1 (3) | 4 (8) |
| Diarrhea/colitis/hepatitis | 0 | 0 | 0 | 0 | 1 (3) | 1(2) |
| Death | 5 | 3 | 4 | 7 (44) | 18 (53) | 25 (50) |
| Within 30 days after last dose | 0 | 0 | 0 | 0 | 2 (6) | 2 (4) |
aListed were those AEs or laboratory abnormalities that occurred in ≥15% of patients in the 10 mg/kg ± XRT group.
bListed were those AEs or laboratory abnormalities that occurred in ≥5% of patients in the 10 mg/kg ± XRT group. All but three cases of hepatitis in the 10 mg/kg cohort were grade 3.
cCalculated from laboratory values.
XRT, external-beam radiotherapy; AEs, adverse events.
In the 10 mg/kg ± XRT group, of the 11 (22%) patients (12 events) who experienced grade 3 diarrhea/colitis, 8 had resolution to <grade 3 within 11 weeks, of whom 7 were treated with corticosteroids and/or infliximab; 4 patients needed no immunosuppressive therapy. Of the five (10%) patients (eight events) with grade 3/4 hepatic irAEs, four had resolution to <grade 3 between 3 and 13 weeks, with one patient having resolution after 61 weeks; all five were treated with corticosteroids and/or mycophenolate mofetil. All skin irAEs in this group were grade 1/2, except for one case of grade 3 pruritus. Endocrine irAEs were noted in nine patients, all of which were grade 1/2, and included adrenal insufficiency (n = 1), hyperthyroidism (n = 2), hypothyroidism (n = 2), hypophysitis (n = 1), and hypopituitarism (n = 3). AEs, consisting mainly of fatigue, diarrhea/colitis, and rash/pruritus, were observed in both chemotherapy-naive and chemotherapy-experienced patients (data not shown). Fourteen (28%) patients in the 10 mg/kg ± XRT group discontinued therapy due to treatment-related AEs, mostly diarrhea/colitis (Table 3). At the database lock of May 2010, 37 (52%) patients across all cohorts had died (Table 3), due mostly to disease progression (n = 24). None of the deaths, except for the one in the 5 mg/kg group described above, were treatment-related.
activity
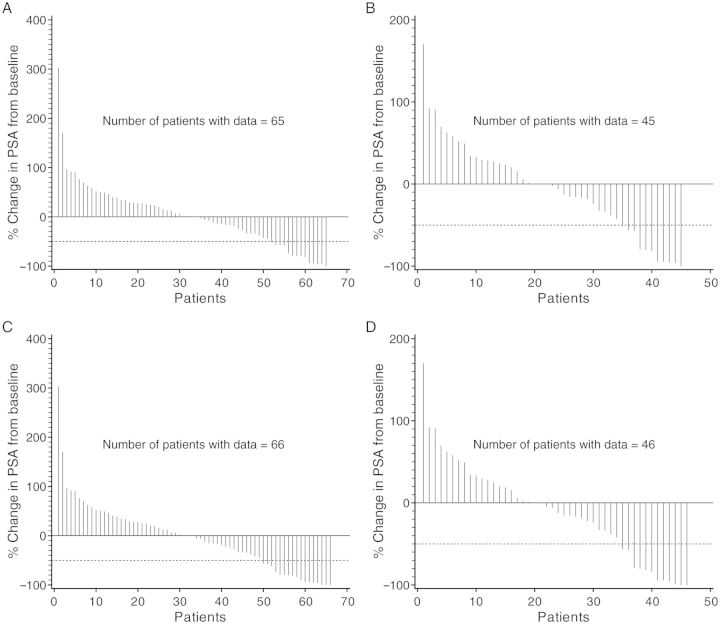
Activity by both PSA decline and tumor response was observed in all cohorts (Figure 2 and Table 4). In the 10 mg/kg ± XRT group, the confirmed PSA decline of ≥50% (at any time) was reported in eight patients (chemotherapy-naive, 6; chemotherapy-experienced, 2). The duration of response was 3–13+ months. Of the 11 patients who were retreated with ipilimumab, 6 had the confirmed PSA decline of ≥50% (at any time). One of the 28 tumor-evaluable patients in the 10 mg/kg ± XRT group (chemotherapy-naive) achieved confirmed complete response with time to response of 2.5 months and was censored at 11.3 months. Two patients (10 mg/kg, 1; 10 mg/kg + XRT, 1) had unconfirmed partial response. Six (chemotherapy-naive, 1; chemotherapy-experienced, 5) had stable disease lasting between 2.8 and 6.1 months.
Figure 2.
Waterfall plots of percent change in PSA from baseline. The greatest change at any time up to and including day 85 for patients (n = 65) across all cohorts (A) and for patients (n = 45) in the 10 mg/kg ± XRT group (B). The greatest change at any time during the entire study period for patients (n = 66) across all cohorts (C) and for patients (n = 46) in the 10 mg/kg ± XRT group (D). The number of patients with ≥50% PSA decline is higher in this figure than in Table 4 because the figure includes all declines, regardless of confirmation, and the table includes only confirmed declines.
Table 4.
PSA decline and tumor responsea
| Characteristics | Ipilimumab dose |
|||||
|---|---|---|---|---|---|---|
| 3 mg/kg |
5 mg/kg | 10 mg/kg |
||||
| −XRT (n = 8) | +XRT (n = 7) | −XRT (n = 6) | −XRT (n = 16; %) | +XRT (n = 34; %) | ±XRT (n = 50; %) | |
| PSA-evaluable patients | 8 | 6 | 6 | 16 (100) | 34 (100) | 50 (100) |
| PSA decline by day 85 | 1 | 0 | 1 | 3 (19) | 4 (12) | 7 (14) |
| PSA decline at any time | 2 | 2 | 1 | 4 (25) | 4 (12) | 8 (16) |
| Tumor-evaluable patients | 1 | 2 | 1 | 8 (100) | 20 (100) | 28 (100) |
| Complete response | 0 | 0 | 0 | 1 (13) | 0 | 1 (4) |
| Partial response | 0 | 0 | 0 | 0 | 0 | 0 |
| Partial response (unconfirmed) | 0 | 0 | 0 | 1 | 1 | 2 (4) |
| Stable disease | 1 | 1 | 1 | 1 (13) | 5 (25) | 6 (21) |
| Progressive disease | 0 | 1 | 0 | 3 (38) | 5 (25) | 8 (29) |
| Unknown | 0 | 0 | 0 | 2 (25) | 9 (45) | 11 (40) |
aPSA decline of ≥50% from baseline (day 85 and at any time) and tumor response (at any time) were confirmed by a second assessment at least 28 days after the initial assessment.
XRT, external-beam radiotherapy; PSA, prostate-specific antigen.
With a median follow-up of 15.7 months (range, 1.1–57.3) and 52 deaths reported across all cohorts (n = 71), the median OS by the Kaplan–Meier analysis was 17.4 months (95% CI: 11.5–24.7).
discussion
Ipilimumab is a cancer immunotherapy that has been shown to improve OS in patients with metastatic melanoma [25, 26], with 19%–36% of patients having long-term (4-year) OS [27–29]. As an immunotherapy, ipilimumab exerts its effect on the immune system instead of directly on the tumor as occurs for chemotherapy. In this study, we made a systematic evaluation of the safety and antitumor activity of ipilimumab as monotherapy or in combination with radiotherapy in mCRPC patients.
Patients in all cohorts, including 10 mg/kg with or without radiotherapy, experienced frequent (all grades, 80%; grade 3/4, 32%), but not unexpected, irAEs consisting mainly of diarrhea/colitis and rash/pruritus. Although no DLTs were noted within the 5-week assessment period, grade 3/4 AEs occurring beyond the DLT window were frequent and mostly immune-related, with some events having a long duration. These irAEs were generally managed with corticosteroids, endocrine hormone replacement therapy (e.g. hydrocortisone, levothyroxine), and supportive care. Five patients (5 mg/kg, 2; 10 mg/kg, 3) received additional immunosuppressives, including infliximab, tacrolimus, and mycophenolate mofetil. Early recognition and immediate intervention of irAEs, particularly diarrhea and colitis, are critical to their management but are not always adequate as seen in the patient with grade 3 colitis in this trial who died from aspergillosis after a 4-month immunosuppressive regimen.
Ipilimumab 10 mg/kg was the highest dose tested in this study and, since it showed a generally manageable safety profile, the 10 mg/kg ± XRT group was expanded to include 50 mCRPC patients. Clinical activity was assessed by both PCWG1 and RECIST guidelines. Among patients receiving 10 mg/kg ± XRT, there were PSA changes, irrespective of prior exposure to chemotherapy. These PSA data, taken together with the observation that, of 28 tumor-evaluable patients receiving 10 mg/kg ± XRT, 1 achieved complete response and 6 had stable disease, suggest potential clinical antitumor activity with disease control at ipilimumab 10 mg/kg. PSA declines further suggest a direct antitumor effect of ipilimumab in contrast to other biologics such as sipuleucel-T, which showed survival benefit in the absence of changes in PSA or progression in phase III trials [2].
Several lines of evidence suggest that ipilimumab 10 mg/kg ± XRT merits further evaluation in phase III trials of mCRPC. First, preclinical data and two recent case reports in metastatic melanoma showed synergistic antitumor activity between CTLA-4 blockade and radiotherapy [38–41]. Second, data from a dose-ranging phase II study in melanoma patients have further described the benefit/risk profile of ipilimumab 10 mg/kg [18], and long-term (4-year) OS has been seen in 19%–36% of melanoma patients treated with ipilimumab [27–29]. Third, ipilimumab 10 mg/kg ± XRT appeared to show activity in mCRPC patients, irrespective of prior chemotherapy exposure, with AEs that were managed by proactive intervention according to treatment guidelines (this study). Finally, recent phase I studies in mCRPC showed the combination of ipilimumab (1–10 mg/kg) and the granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells vaccine (GVAX) or PSA-targeted poxviral vaccine to be safe and tolerable with PSA declines relative to baseline [47, 48], supporting the view that ipilimumab has potential as a component of combination approaches for the treatment of mCRPC. Two confirmatory phase III trials of ipilimumab 10 mg/kg in mCRPC are underway. One trial (NCT01057810) is evaluating ipilimumab as monotherapy in patients who received no prior chemotherapy, and the other (NCT00861614) is evaluating ipilimumab and bone-directed XRT in patients who had prior chemotherapy.
funding
This work was supported by Bristol-Myers Squibb (no grant number) and NIH grant P50 CA092629 (PI: Howard I. Scher, MD).
disclosure
Employed by Bristol-Myers Squibb (BMS) and own BMS stock: KC, PG, and MBM; consultant or advisory role with BMS: CSH and HIS; research funding from BMS: HIS and TMB; honoraria from ECG and IMER and research funding from Progenics Bio Pharma, Inc: SFS; research funding from Medarex: CSH; consultant or advisory role with Roche, honoraria from Roche, and research funding from Roche and Genentech: OH; consultant or advisory role with Dendreon: HIS. All remaining authors have declared no conflicts of interest.
acknowledgements
We thank patients and investigators for their participation in the trial. We also thank Motasim Billah, PhD, of Bristol-Myers Squibb for writing and editorial support.
references
- 1.Slovin SF. Emerging role of immunotherapy in the management of prostate cancer. Oncology (Williston Park) 2007;21:326–333. [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de WR, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 7.Parker C, Nilsson S, Heinrich D. Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA) J Clin Oncol. 2012;30:LBA4512. [Google Scholar]
- 8.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 9.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 10.Page DB, Yuan J, Wolchok JD. Targeting cytotoxic T-lymphocyte antigen 4 in immunotherapies for melanoma and other cancers. Immunotherapy. 2010;2:367–379. doi: 10.2217/imt.10.21. [DOI] [PubMed] [Google Scholar]
- 11.Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein O, Ebert LM, Nicholaou T, et al. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res. 2009;15:2507–2513. doi: 10.1158/1078-0432.CCR-08-2424. [DOI] [PubMed] [Google Scholar]
- 16.O'Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 17.Weber JS, O'Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 18.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 19.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446–6453. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 23.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in extensive disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2012;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 24.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 27.Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maio M, Bondarenko I, Robert C, et al. Four-year survival update for metastatic melanoma (MM) patients (pts) treated with ipilimumab (IPI)+dacarbazine (DTIC) on phase 3 study CA184–024. Ann Oncol. 2012;23(Suppl 9):Abstr 1127P. [Google Scholar]
- 29.Lebbe C, Weber JS, Maio M, et al. Five-year survival rates for patients (pts) with metastatic melanoma (MM) treated with ipilimumab (IPI) in phase II trials. Ann Oncol. 2012;23(Suppl 9):Abstr 1116PD. doi: 10.1093/annonc/mdt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yervoy (Ipilimumab) US Full Prescribing Information. Princeton, NJ: Bristol-Myers Squibb; 2011. [Google Scholar]
- 31.Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon ED, Foster BA, Hurwitz AA, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci USA. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Small E, Higano C, Tchekmedyian N, et al. Randomized phase II study comparing 4 monthly doses of ipilimumab (MDX-010) as a single agent or in combination with a single dose of docetaxel in patients with hormone-refractory prostate cancer. J Clin Oncol. 2006;24:4609. [Google Scholar]
- 34.Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 35.Fong L, Kwek SS, O'Brien S, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2012;2:191. doi: 10.3389/fonc.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 39.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamell EF, Wolchok JD, Gnjatic S, et al. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 43.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 44.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 45.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 46.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- 47.van den Eertwegh AJ, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 48.Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]