Abstract
Infections caused by antibiotic-resistant bacteria, especially the “ESKAPE” pathogens, continue to increase in frequency and cause significant morbidity and mortality. New antimicrobial agents are greatly needed to treat infections caused by gram-negative bacilli (GNB) resistant to currently available agents. The Infectious Diseases Society of America (IDSA) continues to propose legislative, regulatory, and funding solutions to this continuing crisis. The current report updates the status of development and approval of systemic antibiotics in the United States as of early 2013. Only 2 new antibiotics have been approved since IDSA's 2009 pipeline status report, and the number of new antibiotics annually approved for marketing in the United States continues to decline. We identified 7 drugs in clinical development for treatment of infections caused by resistant GNB. None of these agents was included in our 2009 list of antibacterial compounds in phase 2 or later development, but unfortunately none addresses the entire spectrum of clinically relevant GNB resistance. Our survey demonstrates some progress in development of new antibacterial drugs that target infections caused by resistant GNB, but progress remains alarmingly elusive. IDSA stresses our conviction that the antibiotic pipeline problem can be solved by the collaboration of global leaders to develop creative incentives that will stimulate new antibacterial research and development. Our aim is the creation of a sustainable global antibacterial drug research and development enterprise with the power in the short term to develop 10 new, safe, and efficacious systemically administered antibiotics by 2020 as called for in IDSA's “10 × '20 Initiative.”
Keywords: antibacterial agents, antimicrobials, gram-negative bacilli, drug development, clinical trials, antibiotic pipeline
Infections caused by antibiotic-resistant bacteria, especially the “ESKAPE” pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species), cause significant morbidity and mortality [1, 2]. These and other drug-resistant gram-negative bacilli (GNB) infections impact not only hospitalized patients undergoing surgical and other procedures, but also otherwise healthy nonhospitalized patients in the United States and worldwide [3–7].
Since 2002, the Infectious Diseases Society of America (IDSA) has voiced concern with the absence of progress in developing novel therapeutics to treat multidrug-resistant (MDR) infections, including those caused by GNB. In our 2009 report, no antibacterial agent in development with a purely gram-negative spectrum had reached phase 2 of clinical study [2].
The need for new agents to treat infections caused by GNB resistant to currently available agents is even more urgent than at the time of our 2009 report [2]. Furthermore, the withdrawal of several large pharmaceutical companies from antibacterial research and development (R&D) has compromised the infrastructure for discovering and developing new antimicrobials, especially in the United States.
In its July 2004 policy report “Bad Bugs, No Drugs: As Antibiotic R&D Stagnates, a Public Health Crisis Brews,” IDSA proposed legislative, regulatory, and funding solutions to address this increasing public health problem [8]. Recognizing the need for new, creative approaches to address the problem of the dwindling antibiotic pipeline, IDSA launched the “10 × '20 Initiative” in 2010 [9]. This campaign calls for development and regulatory approval of 10 novel, efficacious, and safe systemically administered antibiotics by 2020 [9]. On World Health Day 2011, IDSA issued a policy statement titled “Combating Antimicrobial Resistance: Policy Recommendations to Save Lives,” which provides clear suggestions for addressing the “synergistic crises” of increasing antimicrobial resistance and decreasing availability of new antimicrobial therapies [10]. IDSA continues to work with Congress, the US Food and Drug Administration (FDA), US National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), and other stakeholder groups to ensure that the focus on the problem will not waver.
In this current communication, we report on the state of clinical development and regulatory approval of new, systemically administered antibacterials in the United States as of early 2013.
METHODS
As in our earlier report, we performed a literature review as well as an investigation of the clinical trials registry (www.clinicaltrials.gov). The following sources were utilized to identify antibiotic drug candidates in the development pipeline in the same manner as in our earlier report:
Abstracts from the 2010, 2011, and 2012 Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) were searched for investigational antimicrobials.
The website www.clinicaltrials.gov was accessed and searched by condition with a disease heading of “bacterial infections.” Compounds identified were confirmed by accessing the website of the innovator company. Given the high failure rate of compounds that have not successfully navigated phase 1 studies, only those compounds in phases 2 or 3 of development are discussed.
The PubMed database was searched for relevant English-language literature published between September 2009 and July 2012 by using the search terms antimicrobial drug development, investigational antimicrobials, and novel antimicrobials.
Interviews were conducted by the drafters of this report with leaders of the few remaining pharmaceutical and biotechnology companies identified in our earlier survey [2]; the websites of these companies were also accessed and data on drugs in development were reviewed.
We focus on new orally or intravenously administered antibiotics that have progressed to phase 2 or 3 studies, as these agents are more likely to reach the clinic and are associated with substantial investment by pharmaceutical sponsors. This update focuses on agents active against GNB as effective therapy, as these pathogens represent the most pressing medical needs. The focus is not to detract from the need for new orally active agents for treatment of methicillin-resistant Staphylococcus aureus (MRSA) infection and vancomycin-resistant enterococci, as well as agents for treatment of increasingly resistant gonococci. Nonabsorbable agents administered via the gastrointestinal tract (eg, rifaximin, fidaxomicin) were excluded. We do not include new indications of approved drugs or new indications for different formulations of approved drugs.
Recent discovery and development efforts aimed at MDR GNB have focused largely on important mechanisms of resistance including β-lactamases and carpabenemases, as these are responsible for a large burden of drug-resistant infections reported globally. While a number of definitions exist, a proposed International Standard defines MDR as nonsusceptibility to at least 1 agent in 3 or more antimicrobial classes [11]. Enterobacteriaceae, P. aeruginosa, and A. baumannii that produce extended-spectrum β-lactamases (ESBLs) and/or carbapenemases have been increasingly reported. The β-lactamases act via enzymatic hydrolysis to break open the β-lactam ring and inactivate β-lactam antibiotics. The ESBLs confer resistance to most β-lactam antibiotics, including penicillins, cephalosporins, and the monobactam aztreonam, whereas carbapenemases confer additional resistance to carbapenem antibiotics (as well as some other β-lactam antibiotics) [12]. The β-lactamases are classified according to the Ambler classification (A–D) based on amino acid sequence structure or according to the Bush-Jacoby-Medeiros scheme [13]. Hydrolytic mechanisms in class A, C, and D β-lactamases all require an active-site serine at position 70; these are often called serine β-lactamases. Class B β-lactamases require zinc for activity and hence are also called metallo-β-lactamases; important examples include VIM, IMP, and NDM. The metallo-β-lactamases are inactivated by chelators, such as ethylenediaminetetraacetic acid, but not by β-lactamase inhibitors (eg, tazobactam) [12–14]. This report will classify what is known about activity of the drugs in development versus these important enzymes [15].
RESULTS
Despite ongoing efforts, only 2 new antibiotics—telavancin and ceftaroline fosamil—have been approved since our 2009 report (Table 1). We consider ceftaroline fosamil one of the hoped-for “10 × '20” drugs. The number of new antibiotics annually approved for marketing in the United States has continued to decline (Figure 1). Importantly, the number of large multinational pharmaceutical companies (ie, “Big Pharma”) actively developing antimicrobial drugs also continues to decline (Table 2).
Table 1.
Systemic Antibacterial Drug Approvals Since 1998a
| Antibacterial | Year Approved | Novel Mechanism? |
|---|---|---|
| Rifapentineb | 1998 | No |
| Quinupristin/dalfopristinc | 1999 | No |
| Moxifloxacin | 1999 | No |
| Gatifloxacind | 1999 | No |
| Linezolid | 2000 | Yes |
| Cefditoren pivoxil | 2001 | No |
| Ertapenem | 2001 | No |
| Gemifloxacind | 2003 | No |
| Daptomycin | 2003 | Yes |
| Telithromycind | 2004 | No |
| Tigecyclinee | 2005 | Yes |
| Doripenem | 2007 | No |
| Telavancin | 2009 | Yes |
| Ceftaroline fosamil | 2010 | No |
a Rifaxamin (Food and Drug Administration [FDA] approved in 2004) and fidaxomicin (FDA approved in 2011) are not systemically absorbed, and so are not included on this list.
b Antituberculous agent.
c Infrequently used due to adverse event profile.
d Withdrawn from market due to adverse event profile.
e Label warning regarding possible excess mortality.
Figure 1.
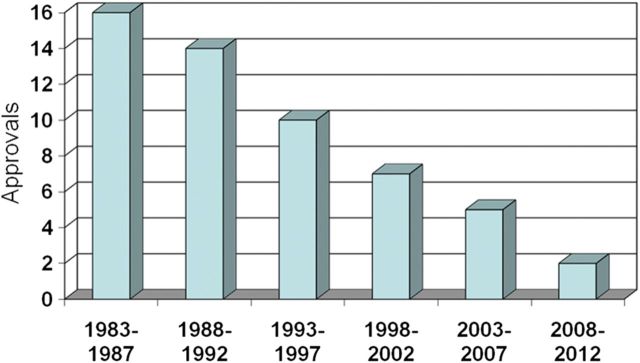
New systemic antibacterial agents approved by the US Food and Drug Administration per 5-year period, through 2012. Modified from Spellberg 2004 [23].
Table 2.
Antibacterial Pipeline (Anti–Gram Positive and Anti–Gram Negative), Big Pharma
| Company | Since 1998 | Phase 2/3 |
|---|---|---|
| Abbott Laboratories | 0 | 0 |
| AstraZeneca | 0 | 2 |
| Bayer | 0 | 0 |
| GlaxoSmithKline | 0 | 1 |
| Lilly | 0 | 0 |
| Merck/Schering-Plough | 1 | 1 |
| Novartis | 0 | 0 |
| Ortho McNeil/Johnson & Johnson | 1 | 0 |
| Pfizer/Wyeth | 2 | 0 |
| Roche | 0 | 0 |
| Sanofi | 0 | 0 |
We identified 7 parenteral drugs in clinical development for treatment of infections caused by MDR GNB (Table 3), and one whose phase 2 development program was recently halted [16–22]. As indicated in Table 3, complicated urinary tract infection (cUTI), complicated intraabdominal infection (cIAI), and acute bacterial skin and skin structure infection (ABSSSI) are the initially targeted regulatory indications. Of the 7 agents, 4 are β-lactam plus β-lactamase inhibitor combination drugs; 2 are protein synthesis inhibitors (one with a novel mechanism of action and one aminoglycoside); and one is a peptide mimetic. The antibiotic whose development was halted is a transfer RNA (tRNA) synthetase inhibitor. In addition, some promising compounds are in phase 1 development. The number of drugs in phase 1 development provide another, potentially useful metric of the pool of new drugs in development. However, in addition to the high phase 1 failure rates, the absence of publically available data led to our decision to exclude phase 1 candidate compounds.
Table 3.
Intravenous Antimicrobials Active Against Gram-Negative Bacilli in Advanced (Phase 2 or 3) Clinical Developmenta
| Product | Class (Mechanism of Action) | Novel Mechanism of Action? | Status | Activity Targets |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enterobacteriaceae |
Psuedomonas aeruginosa |
Acinetobacter spp |
||||||||||
| ESBL | sCBP | mCBP | WT | MDR | mCBP | WT | MDR | |||||
| 1 | Ceftolozane/taxobactam (CXA-201; CXA-101/tazobactam) | Antipseudomonal cephalosporin/BLI combination (cell wall synthesis inhibitor) | No | Phase 3 (cUTI, cIAI) | Yes | No | No | Yes | IE | No | No | No |
| 2 | Ceftazidime-avibactam (ceftazidime/NXL104) | Antipseudomonal cephalosporin/BLI combination (cell wall synthesis inhibitor) | No | Phase 3 (cIAI) | Yes | Yes | No | Yes | IE | No | No | No |
| 3 | Ceftaroline-avibactam (CPT-avibactam; ceftaroline/NXL104) | Anti-MRSA cephalosporin/ BLI combination (cell wall synthesis inhibitor) | No | Phase 2 (cUTI, cIAI) | Yes | Yes | No | No | No | No | No | No |
| 4 | Imipenem/MK-7655 | Carbapenem/BLI combination (cell wall synthesis inhibitor) | No | Phase 2 (cUTI, cIAI) | Yes | Yes | No | Yes | IE | No | IE | No |
| 5 | Plazomicin (ACHN-490) | Aminoglycoside (protein synthesis inhibitor) | No | Phase 2 (cUTI) | Yesb | Yesb | IE | No | No | No | No | No |
| 6 | Eravacycline (TP-434) | Fluorocycline (protein synthesis inhibitor targeting the ribosome) | No | Phase 2 (cIAI) | Yesb | Yes | IE | No | No | No | IE | IE |
| 7 | Brilacidin (PMX-30063) | Peptide defense protein mimetic (cell membrane disruption) | Yes? | Phase 2 (ABSSSI) | Yes | IE | IE | IE | IE | IE | No | No |
Activity based on available information.
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; BLI, β-lactamase inhibitor; cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection; ESBL, extended-spectrum β-lactamase producers; IE, insufficient evidence available; mCPB, metallo-carbapenamase producers (eg, NDM, VIM, IMP); MDR, multidrug-resistant pattern including co-resistances to aminoglycosides (amikacin, gentamicin, tobramycin), fluoroquinolones, tetracyclines, and broad-spectrum β-lactams by various mechanisms carried on common genetic elements; MRSA, methicillin-resistant Staphylococcus aureus; sCBP, serine carbapenemase producers such as KPC; WT, wild-type pattern for species.
a Intravenous antimicrobials not listed in IDSA's 2009 drug status report [2].
b Incomplete coverage of some species (Proteus mirabilis and indole-positive Proteus species).
Ceftolozane/tazobactam, ceftazidime/avibactam, ceftaroline/avibactam, and MK-7655/imipenem are β-lactam/β-lactamase inhibitor combination products that act by inhibiting the β-lactamases (tazobactam, avibactam, MK-7655 are the inhibitors) so that the partner antibiotic (ceftolozane, ceftazidime, ceftaroline, and imipenem) can interfere with cell wall synthesis. Each of these drug combinations offers the potential to enhance β-lactam therapeutic options [16, 24].
Ceftolozane (CXA-101, Cubist) demonstrates enhanced affinity for P. aeruginosa penicillin-binding proteins, thus providing excellent intrinsic activity for P. aeruginosa. The combination with tazobactam as ceftolozane/tazobactam is being studied in clinical trials. Tazobactam is a “tried and true” β-lactamase inhibitor, whose spectrum extends to class A and some class C β-lactamases (eg, plasmid-borne cephalosporinase). Although not as broad a β-lactamase inhibitor as some new non–β-lactam β-lactamase inhibitors mentioned below, tazobactam provides activity against the important and increasingly common CTX-M-15 β-lactamase as well as other ESBLs. Ceftolozane alone or as the combination has limited activity against A. baumannii.
Ceftolozane/tazobactam is currently in phase 3 studies of cUTI and cIAI; this combination drug could become available in the next few years. In cUTI, including pyelonephritis, intravenous ceftolozane/tazobactam is being compared to parenteral levofloxacin [25]. In cIAI, the comparator agent is intravenous meropenem [26]. The sponsor recently announced plans to study ventilator-associated bacterial pneumonia (VABP) in a global trial. They plan an approximately 950-patient noninferiority study comparing a higher dose of ceftolozane/tazobactam, 3 g every 8 hours, to imipenem-cilastatin. This study will use a 28-day mortality endpoint [27].
Ceftazidime/avibactam (CAZ/avibactam; AstraZeneca/Forest Laboratories) demonstrates in vitro activity against most strains of P. aeruginosa, MDR Enterobacteriaceae (ESBL producers), and Klebsiella pneumoniae–producing serine carbapenemases (KPCs), but not metallo-β-lactamase producers (eg, VIM, NDM) [28–34]. Avibactam (previously NXL 104) is a potent, reversible, non–β-lactam β-lactamase inhibitor, the spectrum of which includes primarily class A and lass C β-lactamases. As is true for ceftolozane, the antimicrobial component of CAZ/avibactam has only very modest activity against Acinetobacter species. Currently in phase 3 development, trials are recruiting patients for a study of CAZ/avibactam plus metronidazole versus meropenem for cIAI, and one comparing ceftazadime/avibactam with doripenem in cUTI [35, 36]. In addition, AstraZeneca/Forest plans an open-label study of CAZ/avibactam in addition to the best available therapy (“standard of care”) for treatment of CAZ-resistant GNB infections (eg, caused by ESBL-producing organisms) [37].
Ceftaroline/avibactam (CPT/avibactam, ceftaroline fosamil [CPT] plus NXL 104) shows in vitro potency versus MRSA and Enterobacteriaceae (including those producing ESBLs and KPCs) but not P. aeruginosa or A. baumannii. This combination, also in development by AstraZeneca/Forest, is in phase 2 studies of cUTI and cIAI [33, 38, 39].
Imipenem/MK-7655 (Merck) is a combination of the carbapenem imipenem-cilastatin and a β-lactamase inhibitor similar in chemical structure to NXL104 (a class A and class C β-lactamase inhibitor). The combination demonstrates in vitro activity against P. aeruginosa and many ESBL producers, including carbapenem-resistant strains, but not against metallo-carbapenemases [40]. Activity against A. baumannii is limited. Two separate phase 2 studies of 2 doses (125 or 250 mg) of MK-7655 plus imipenem-cilastatin versus imipenem-cilastatin alone for treatment of cUTI or cIAI were initiated by Merck in early 2012 [41, 42].
Plazomicin (ACHN-490), a next-generation “neoglycoside” from Achaogen, demonstrates in vitro potency and in vivo activity against ESBL-producing pathogens, fluoroquinolone-resistant and aminoglycoside-resistant GNB, and GNB-expressing Amp C cephalosporinases, carbapenemases, and metallo-β-lactamases, but not Proteus species or strains with aminoglycoside-resistant methylase genes (eg, ArmA, RmtC) [16]. Activity against P. aeruginosa and A. baumannii remains limited. Data from a phase 2 study of intravenous plazomicin versus levofloxacin for treatment of cUTI were reported in September 2012 [43, 44].
Eravacycline (TP-434, Tetraphase) is a broad-spectrum fluorocycline antibiotic that—like other tetracycline agents—binds to bacterial ribosomes, thereby inhibiting protein synthesis [45]. In addition, TP-434 demonstrates stability to common tetracycline-resistance mechanisms, that is, tetracycline-specific efflux and ribosomal protection. This molecule demonstrates in vitro inhibitory activity against MRSA, vancomycin-resistant enterococci, and KPC-producing GNB, but not against Pseudomonas species or Acinetobacter species. Tetraphase is proceeding with development of the intravenous formulation; an oral formulation is also being investigated. Phase 1 studies of the latter are under way. Data from a phase 2, randomized, double-blind, double-dummy, multicenter, prospective study of 2-dose regimens of intravenous TP-434 compared with ertapenem in the treatment of adult community-acquired cIAI were recently reported [46, 47].
Brilacidin (PMX-30063, Polymedix), a peptide mimetic, disrupts bacterial membranes. In vitro studies of this molecule support activity against enteric GNB but uncertain activity against P. aeruginosa and no activity versus A. baumannii. In a phase 2 study of PMX-30063 versus daptomycin for treatment of ABSSSI due to methicillin-susceptible S. aureus, safety findings included sensory nerve symptoms and hypertensive episodes of unclear significance [48–50].
BAL30072, a siderophore sulfactam being developed by Basilea Pharmaceutica, has activity against Acinetobacter species, P. aeruginosa, Burkholderia cepacia, and some MDR Enterobacteriaceae but lower potency versus selected ESBL-producing Enterobacteriaceae [51–54]. However, this agent, which resembles aztreonam, is still in phase 1 study, and will likely be combined with meropenem in clinical development studies. BAL30072 targets >1 penicillin-binding protein and has stability against metallo-β-lactamase–producing GNBs. Another β-lactam/β-lactamase inhibitor combination, carbavance (biapenem/RPX7009), is being developed by Rempex Pharmaceuticals and is in late phase 1 study [55, 56]. Carbavance combines the activity of a well-studied carbapenem, biapenem, with a β-lactamase inhibitor potent against serine carbapenemases (class A). Enterobacteriaceae-producing ESBLs and KPC enzymes are inhibited as are P. aeruginosa and A. baumannii at minimum inhibitory concentration values similar to those of other carbapenems tested alone. Metallo-β-lactamase–producing isolates remain resistant to this combination. Clinical indications and study designs are pending.
GSK 052 (GlaxoSmithKline, previously AN3365 from Anacor) is a novel boron-based tRNA synthesis inhibitor that specifically targets the bacterial enzyme leucyl-tRNA synthetase, which is required for protein synthesis. This molecule is active in vitro against E. coli, K. pneumoniae, Citrobacter species, Serratia marcescens, Proteus vulgaris, Providencia species, P. aeruginosa, and Enterobacter species, although not against Acinetobacter species and some Pseudomonas species. Unfortunately, phase 2 studies in cIAI and cUTI were halted in February 2012, after discovery of an undefined “microbiologic finding” among cUTI patients [57]. GlaxoSmithKline announced discontinuation of clinical development of GSK 052 on 5 October 2012 [58].
None of the 7 drugs in full clinical development (phase 2 or 3) were included in our 2009 list of late-stage antibacterial compounds [2]. Unfortunately, none demonstrates activity against the entire spectrum of clinically relevant GNB resistance. Table 3 illustrates the glaring absence of β-lactam drugs able to withstand enzymatic attack by metallo-carbapenemases and the absence of drugs with predictable activity against A. baumannii. In addition, we were unable to identify any phase 2 or 3 clinical trials designed to address the important conditions of community-acquired bacterial pneumonia (CABP), hospital-acquired bacterial pneumonia (HABP), or bloodstream infection.
DISCUSSION
The number of antibacterial compounds in phase 2 or 3 development remains alarmingly low. The pace of R&D must accelerate to reach the goal of 10 new systemic drugs to treat infections caused by resistant bacteria by the year 2020 [9].
Of greatest concern is the near absence of drug candidates potentially active against GNB that produce metallo-β-lactamases, for example, IMP or VIM or NDM in Enterobacteriaceae, P. aeruginosa, and A. baumannii. In addition, the latter organism often possesses concomitant resistant mechanisms that result in resistance to virtually all other antimicrobial classes except the polymyxins, glycylcyclines (eg, tigecycline), and fosfomycin.
The number of novel compounds in development admittedly does not tell the whole story. Although truly novel compounds with a new mechanism of action provide substantive advances in treatment of infections compared with already available antibiotics, incremental improvements in existing classes can be very valuable and should not be dismissed [59].
In 2013, antibacterial drug development largely lies in the hands of small pharmaceutical or biotechnology companies, as well as some larger companies in Japan [60]. Our investigation found only 4 large multinational pharmaceutical companies engaged in antibacterial discovery. The “brain drain” that accompanies the loss of large pharmaceutical research organizations will surely be felt for years to come.
SIGNS OF HOPE
Recent focus on the problem of antimicrobial resistance from the World Health Organization, the US Congress, and the US FDA, NIH, and CDC support the idea that leaders in government now recognize the urgency of the current situation [5]. FDA leadership has publicly acknowledged the crisis [61, 62]. The FDA has invested in antibiotic-focused collaborations with the Brookings Institution, the Clinical Trials Transformation Initiative, and the Biomarkers Consortium of the Foundation for the NIH, which is assessing novel endpoints for antibacterial registrational trials in 3 clinical indications [63]. In addition, the European Commission initiated a landmark public–private collaboration. The pharmaceutical industry's Innovative Medicines Initiative has initial funding of €223.7 million and is aimed at the dual problem of antibiotic resistance and speeding the delivery of new antibiotics to patients [64]. Moreover, in June 2012, the US Congress enacted new incentives intended to advance antibiotic development.
IDSA'S ROLE
IDSA continues to support initiatives that create an R&D infrastructure that responds to current antimicrobial resistance and anticipates evolving resistance. Numerous professional and philanthropic societies have supported our efforts. Recent government actions are encouraging, and IDSA will continue to work with our partners to press for additional measures to ensure a sustainable antibacterial R&D infrastructure is in place [10].
Concomitantly, IDSA is committed to strengthened antibiotic public health and stewardship programs so as to preserve the current inventory of effective drugs. Optimizing use of currently available antibacterial drugs, via improved resistance data collection, surveillance, prevention, and control measures, including antimicrobial stewardship efforts, remains vital to combating bacterial resistance [9, 59, 65, 66].
REGULATORY GUIDANCE
IDSA will continue to support action on the regulatory front. The need for clear regulatory guidance remains greatest in studies of drugs for life-threatening infections and those caused by resistant GNB. Although the FDA has made progress recently, more is needed, especially for CABP and HAPB/VABP [62, 66–71]. IDSA also supports the urgent approval of FDA guidance on pathogen-specific clinical trials, which will help development of new antimicrobial drugs that target infections caused by drug-resistant pathogens. Creation of a new FDA approval pathway for limited-population antibacterial drugs would permit antibiotic approvals based on smaller clinical trials of the most serious bacterial infections, where insufficient therapeutic options exist [72]. Harmonization of FDA guidance and European Medicines Agency guidance is another critical need [73].
APPROPRIATE FINANCIAL INCENTIVES
Securing a solution will require ongoing investment by pharmaceutical sponsors. Both Big Pharma and smaller biotechnology companies require mitigation of the current disincentives to antibacterial R&D, as well as new incentives to make developing antibiotics a viable financial option. Notably, Congress’ recent passage of the FDA Safety and Innovation Act legislated an additional 5 years of exclusivity for antibacterial and antifungal drugs that treat serious and life-threatening infections.
IDSA also supports the adoption of “push” incentives that can facilitate investment in early-stage development, such as R&D tax credits and grants and contracts, as well as consideration of new reimbursement models [10, 72].
IDSA also enthusiastically favors increasing support for new and existing public–private partnerships that provide a supplemental means of support for antibacterial research and development [10]. Recent examples of successful partnerships include awards from the Biomedical Advanced Research and Development Authority at the Department of Health and Human Services for up to $94 million in US government funding to support development of GSK 052 in 2011 and up to $67 million for development of TP-434 [74, 75]. Advancing a sustainable solution also will require significantly increased commitments by the National Institute of Allergy and Infectious Diseases and the Department of Defense.
CONCLUSIONS
Our survey results demonstrate some tangible progress in the clinical development of new antibacterial drugs that target infections caused by drug-resistant GNB. However, progress remains alarmingly slow. The prognosis for sustainable R&D infrastructure depends upon clarification of FDA regulatory clinical trial guidance, and fair and appropriate economic incentives for small and large pharmaceutical companies.
In the meantime, the preservation of the miracle of antibacterials will not be possible without a determined focus on protecting our currently available antibacterial drugs via strong antibiotic stewardship and infection prevention.
Notes
Acknowledgments. The authors sincerely thank Drs Karen Bush, Carl Kraus, and Louis Rice for their insightful review of the manuscript. IDSA's Board of Directors thanks the drafters of this antibacterial pipeline status report for their ongoing commitment to solving the crisis in antibiotic drug development. In particular, the Board acknowledges the critical work of Drs Helen W. Boucher and George H. Talbot.
Financial support. IDSA has received no financial support from outside sources for its policy work and preparation of this report.
Potential conflicts of interest. H. W. B. serves or has served as an advisor or consultant to Basilea, Cerexa, Durata, Merck, Johnson & Johnson (J&J), Paratek, Rib X, Targanta/TMC, and Wyeth/Pfizer. G. H. T., through Talbot Advisors LLC, has received board compensation and/or consultancy fees from Achaogen, Actelion, Astellas, Basilea, Bayer, Cempra, Cerexa, Cubist-Calixa, Durata, FAB Pharma, J&J, Kalidex, Meiji, Merada, Merck, Nabriva, Paratek, Sanofi, Theravance, Toyama, and Wyeth/Pfizer (data safety monitoring board), and also has equity interests in Calixa, Cempra, Cerexa, Durata, Mpex, and Nabriva. D. K. B. receives support from the United States government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C) and the nonprofit organization Thrasher Research Foundation for his work in neonatal candidiasis (www.thrasherresearch.org); he also receives research support from Astellas Pharma US, AstraZeneca, J&J, Pfizer, Biosynexus, Cubist Pharma, Merck & Co, The Medicines Co, and UCB Pharma; consulting fees from Cerexa and Astellas Pharma US; and other support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). J. B.'s employer, the University of California, San Diego, has research or consulting contracts for the study of daptomycin (Cubist Pharmaceuticals), tedizolid (Trius), moxifloxacin (Bayer Healthcare), ceftaroline (Cerexa/Forest/AZ), and colistin (NIH), as well as grants from J&J, GlaxoSmithKline, and Merck Schering-Plough. R. J. G. is an employee of IDSA. R. N. J., through JMI Laboratories, Inc, has received research and educational grants in 2009–2012 from American Proficiency Institute, Anacor, Astellas, AstraZeneca, Bayer, Cempra, Cerexa, Contrafect, Cubist, Daiichi, Dipexium, Enanta, Furiex, GlaxoSmithKline, J&J (Ortho McNeil), LegoChem Biosciences Inc, Meiji Seika Kaisha, Merck, Nabriva, Novartis, Pfizer (Wyeth), Rempex, Rib-X Pharmaceuticals, Seachaid, Shionogi, The Medicines Co, Theravance, and ThermoFisher; in addition, some JMI employees are advisors/consultants for Astellas, Cubist, Pfizer, Cempra, Cerexa-Forest, J&J, and Theravance. B. E. M. has served as a consultant to or on an advisory board for Pfizer, Rib-X, Durata Therapeutics, Achaogen, The Medicines Co, GlaxoSmithKline, Theravance, IMS Consulting Group, and the European Food Safety Authority's Innovative Medicines Initiative Joint Undertaking; has received funding from the NIH, J&J, Intercell, Astellas, Cubist, Theravance, and Forest; has 1 patent for which there was no financial remuneration; has received royalties for chapters she wrote for ASM, UpToDate, and Harrison's Textbook of Internal Medicine; and has received honoraria and/or travel reimbursement for lectures and grand rounds from nonpharmaceutical organizations. R. A. B. receives support from Public Health Service grant R01AI072219 and R01AI063517 from the NIH and funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program, and the Geriatric Research Education and Clinical Center VISN 10, as well as grants from AstraZeneca, Merck, and Rib-X. D. G. serves or has served as an advisor or consultant for Achaogen, Cubist, and Pfizer.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079–81. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008. Infect Control Hosp Epidemiol. 2010;31:528–31. doi: 10.1086/652152. [DOI] [PubMed] [Google Scholar]
- 4.Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med. 2009;360:439–43. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. The evolving threat of antimicrobial resistance—options for action. 2012. Available at http://whqlibdoc.who.int/publications/2012/9789241503181_eng.pdf . Accessed 30 July 2012. [Google Scholar]
- 6.Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366:454–61. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6209a3.htm?s_cid=mm6209a3_w&mobile=noconten . Accessed 21 March 2013. Accessed 12 August 2012.
- 8.Bad bugs, no drugs: as antibiotic discovery stagnates, a public health crisis brews. Available at http://www.idsociety.org/uploadedFiles/IDSA/Policy_and_Advocacy/Current_Topics_and_Issues/Antimicrobial_Resistance/10%20/Images/Bad%20Bugs%20no%20Drugs.pdf .
- 9.Infectious Diseases Society of America. The 10 % '20 initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010;50:1081–3. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- 10.Spellberg B, Blaser M, et al. Infectious Diseases Society of America (IDSA) Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52(suppl 5):S397–428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon RH, Clark J. ESBLs: A clear and present danger? Crit Care Res Pract. 2012;2012:625170. doi: 10.1155/2012/625170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–33. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54:969–76. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talbot GH. β-Lactam antimicrobials: what have you done for me lately? Ann N Y Acad Sci. 2013;1277:76–83. doi: 10.1111/j.1749-6632.2012.06809.x. [DOI] [PubMed] [Google Scholar]
- 16.Bush K, Pucci MJ. New antimicrobial agents on the horizon. Biochem Pharmacol. 2011;82:1528–39. doi: 10.1016/j.bcp.2011.07.077. [DOI] [PubMed] [Google Scholar]
- 17.Butler MS, Cooper MA. Antibiotics in the clinical pipeline in 2011. J Antibiot (Tokyo) 2011;64:413–25. doi: 10.1038/ja.2011.44. [DOI] [PubMed] [Google Scholar]
- 18.Llarrull LI, Testero SA, Fisher JF, Mobashery S. The future of the beta-lactams. Curr Opin Microbiol. 2010;13:551–7. doi: 10.1016/j.mib.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pankuch GA, Lin G, Kubo A, Armstrong ES, Appelbaum PC, Kosowska-Shick K. Activity of ACHN-490 tested alone and in combination with other agents against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2011;55:2463–5. doi: 10.1128/AAC.01390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong ES, Miller GH. Combating evolution with intelligent design: the neoglycoside ACHN-490. Curr Opin Microbiol. 2010;13:565–73. doi: 10.1016/j.mib.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Mushtaq S, Warner M, Livermore D. Activity of the siderophore monobactam BAL30072 against multiresistant non-fermenters. J Antimicrob Chemother. 2010;65:266–70. doi: 10.1093/jac/dkp425. [DOI] [PubMed] [Google Scholar]
- 22.Bush K, Macielag MJ. New beta-lactam antibiotics and beta-lactamase inhibitors. Expert Opin Ther Pat. 2010;20:1277–93. doi: 10.1517/13543776.2010.515588. [DOI] [PubMed] [Google Scholar]
- 23.Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE., Jr Trends in antimicrobial drug development: implications for the future. Clin Infect Dis. 2004;38:1279–86. doi: 10.1086/420937. [DOI] [PubMed] [Google Scholar]
- 24.Perez F, Bonomo RA. Can we really use β-lactam/β-lactam inhibitor combinations for the treatment of infections caused by extended-spectrum β-lactamase-producing bacteria? Clin Infect Dis. 2012;54:175–7. doi: 10.1093/cid/cir793. [DOI] [PubMed] [Google Scholar]
- 25.National Institutes of Health. Study comparing the safety and efficacy of intravenous CXA-201 and intravenous levofloxacin in complicated urinary tract infection, including pyelonephritis. Available at http://clinicaltrials.gov/ct2/show/NCT01345955 . Accessed 30 July 2012.
- 26.National Institutes of Health. Safety and efficacy of intravenous CXA-201 and intravenous meropenem in complicated intraabdominal infections. Available at http://clinicaltrials.gov/ct2/show/NCT01445665?term=CXA-201&rank=3 . 30 July 2012.
- 27. Cubist Pharmaceuticals. Investors. Available at http://investors.cubist.com/GenPage.aspx?IID=4093793&GKP=1073747233 . Accessed 8 December 2012.
- 28.Citron DM, Tyrrell KL, Merriam V, Goldstein EJ. In vitro activity of ceftazidime-NXL104 against 396 strains of beta-lactamase-producing anaerobes. Antimicrob Agents Chemother. 2011;55:3616–20. doi: 10.1128/AAC.01682-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endimiani A, Hujer KM, Hujer AM, Pulse ME, Weiss WJ, Bonomo RA. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55:82–5. doi: 10.1128/AAC.01198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagace-Wiens PR, Tailor F, Simner P, et al. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases. Antimicrob Agents Chemother. 2011;55:2434–7. doi: 10.1128/AAC.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levasseur P, Girard AM, Claudon M, et al. In vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2012;56:1606–8. doi: 10.1128/AAC.06064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55:390–4. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mushtaq S, Warner M, Livermore DM. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother. 2010;65:2376–81. doi: 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 34.Walkty A, DeCorby M, Lagace-Wiens PR, Karlowsky JA, Hoban DJ, Zhanel GG. In vitro activity of ceftazidime combined with NXL104 versus Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals (CANWARD 2009 study) Antimicrob Agents Chemother. 2011;55:2992–4. doi: 10.1128/AAC.01696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institutes of Health. Compare ceftazidime-avibactam + metronidazole versus meropenem for hospitalized adults with complicated intra-abd infections. Available at http://clinicaltrials.gov/ct2/show/NCT01499290?term=avibactam&rank=7 . Accessed 30 July 2012.
- 36.National Institutes of Health. Ceftazidime-avibactam compared with doripenem followed by oral therapy for hospitalized adults with complicated UTIs (urinary tract infections) Available at http://clinicaltrials.gov/ct2/show/NCT01595438?term=avibactam&rank=5 .
- 37.National Institutes of Health. Ceftazidime-avibactam for the treatment of infections due to ceftazidime resistant pathogens. Available at http://clinicaltrials.gov/ct2/show/NCT01644643?term=avibactam&rank=4 . Accessed 30 July 2012.
- 38.Castanheira M, Sader HS, Farrell DJ, Mendes RE, Jones RN. Activity of ceftaroline-avibactam tested against gram-negative organism populations, including strains expressing one or more beta-lactamases and methicillin-resistant Staphylococcus aureus carrying various SCCmec types. Antimicrob Agents Chemother. 2012;56:4779–85. doi: 10.1128/AAC.00817-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiskirchen DE, Crandon JL, Furtado GH, Williams G, Nicolau DP. In vivo efficacy of a human-simulated regimen of ceftaroline combined with NXL104 against extended-spectrum-beta-lactamase (ESBL)-producing and non-ESBL-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55:3220–5. doi: 10.1128/AAC.00024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirsch EB, Ledesma KR, Chang KT, Schwartz MS, Motyl MR, Tam VH. In vitro activity of MK-7655, a novel beta-lactamase inhibitor, in combination with imipenem against carbapenem-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2012;56:3753–7. doi: 10.1128/AAC.05927-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institutes of Health. Study of the safety, tolerability, and efficacy of MK-7655 + imipenem/cilastatin vs imipenem/cilastatin alone for the treatment of complicated urinary tract infection (cUTI) (MK-7655-003 AM2) Available at http://www.clinicaltrials.gov/ct2/show/NCT01505634?term=MK-7655%5C&rank=2 . Accessed 30 July 2012.
- 42.National Institutes of Health. Study of the safety, tolerability, and efficacy of MK-7655 + imipenem/cilastatin versus imipenem/cilastatin alone to treat complicated intra-abdominal infection [cIAI] (MK-7655-004 AM2) Available at http://www.clinicaltrials.gov/ct2/show/NCT01506271?term=MK-7655%5C&rank=3 . Accessed 30 July 2012.
- 43.National Institutes of Health. Study for the treatment of complicated urinary tract infection and acute pyelonephritis. Available at http://www.clinicaltrials.gov/ct2/show/NCT01096849?term=achn+490&rank=5 . Accessed 30 July 2012.
- 44.Riddle VD, Cebrik E, Armstrong E, et al. Plazomicin safety and efficacy in patients with complicated urinary tract infection or acute pyelonephritis. In: 52nd ICAAC 2012, San Francisco, CA. Abstract L2-2118a. [Google Scholar]
- 45.Grossman TH, Starosta AL, Fyfe C, et al. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother. 2012;56:2559–64. doi: 10.1128/AAC.06187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Institutes of Health. Study to compare TP-434 and ertapenem in CA complicated intra-abdominal infections. Available at http://www.clinicaltrials.gov/ct2/show/NCT01265784?term=TP-434&rank=1 . Accessed 30 July 2012.
- 47.Horn PG, Cesnauskas M, Ramesh M, et al. Efficacy and safety of TP-434 versus ertapenem in complicated intra-abdominal infection. In: 52nd ICAAC 2012, San Francisco, CA. Abstract L1-1647a.
- 48.National Institutes of Health. Initial treatment for acute bacterial skin infections (ABSSSI) caused by Staphylococcus aureus. Available at http://www.clinicaltrials.gov/ct2/show/NCT01211470?term=pmx-30063&rank=1 . Accessed 30 July 2012.
- 49.Polymedix. Brilacidin (PMX-30063) antibiotic fact sheet. Available at http://files.shareholder.com/downloads/ABEA-4ITCYZ/1993141727%0x580655/d3dd327f-5aa7–4079-b494-d692c1f1e362/Brilacidin_PMX-30063_Antibiotic_Fact_Sheet_July_2012_.pdf . Accessed July 30 2012.
- 50.Polymedix. Press release. Available at http://www.investors.polymedix.com/releasedetail.cfm?releaseid=600084 . Accessed 30 July 2012.
- 51.Higgins PG, Stefanik D, Page MG, Hackel M, Seifert H. In vitro activity of the siderophore monosulfactam BAL30072 against meropenem-non-susceptible Acinetobacter baumannii. J Antimicrob Chemother. 2012;67:1167–9. doi: 10.1093/jac/dks009. [DOI] [PubMed] [Google Scholar]
- 52.Mima T, Kvitko BH, Rholl DA, Page MG, Desarbre E, Schweizer HP. In vitro activity of BAL30072 against Burkholderia pseudomallei. Int J Antimicrob Agents. 2011;38:157–9. doi: 10.1016/j.ijantimicag.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page MG, Dantier C, Desarbre E. In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant gram-negative bacilli. Antimicrob Agents Chemother. 2010;54:2291–302. doi: 10.1128/AAC.01525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russo TA, Page MG, Beanan JM, et al. In vivo and in vitro activity of the siderophore monosulfactam BAL30072 against Acinetobacter baumannii. J Antimicrob Chemother. 2011;66:867–73. doi: 10.1093/jac/dkr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castanheira M, Becker HK, Rhomberg PR, Jones RN. Pre-clinical evaluation of a carbapenem/β-lactamase inhibitor combination (RPX2003/RPX7009) tested against serine-carbapenemase-producing pathogens. In: ICCAC 2012. Abstract F856.
- 56.Castanheira M, Konrardy ML, Rhomberg PR, Jones RN. In vitro activity of a carbapenem and novel-b-lactamase inhibitor combination (RPX2003/RPX7009) tested against contemporary populations of gram-negative organisms. In: IDWeek 2012. Abstract 1615.
- 57. National Institutes of Health. ClinicalTrials.gov. Available at http://www.clinicaltrials.gov/ct2/results?term=gsk2251052 . Accessed 30 July 2012.
- 58.Anacor. GSK 052. Available at http://www.anacor.com/gsk052.php . Accessed 6 November 2012.
- 59.Page MG, Heim J. New molecules from old classes: revisiting the development of beta-lactams. IDrugs. 2009;12:561–5. [PubMed] [Google Scholar]
- 60.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 61.FDA chief focuses on antibiotic resistance. The Wall Street Journal. October 2010 Available at http://online.wsj.com/article/SB10001424052748703735804575536411612671060.html . Accessed 13 August 2012.
- 62.Brookings Institution. Facilitating antibacterial drug development 2012. Available at http://www.brookings.edu/events/2012/05/09-antibacterial-drug-development . Accessed 30 July 2012.
- 63.Talbot GH, Powers JH, Fleming TR, et al. Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis. 2012;55:1114–21. doi: 10.1093/cid/cis566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.European Comission. Combating antibiotic resistance: NewDrugs4BadBugs (ND4BB) Available at http://www.imi.europa.eu/content/stage-1-4 . Accessed 30 July 2012.
- 65.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 66.Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS) Infect Control Hosp Epidemiol. 2012;33:322–7. doi: 10.1086/665010. [DOI] [PubMed] [Google Scholar]
- 67.US Food and Drug Administration. Guidance for industry: Acute bacterial skin and skin structure infections: developing drugs for treatment. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf . Accessed 30 July 2012.
- 68.US Food and Drug Administration. Guidance for industry: complicated urinary tract infections: developing drugs for treatment. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070981.pdf . Accessed 30 July 2012.
- 69.US Food and Drug Administration. Guidance for industry complicated intra-abdominal infections: developing drugs for treatment. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM321390.pdf . Accessed 8 December 2012.
- 70.US Food and Drug Administration. HABP/VABP guidance. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM234907.pdf . Accessed 30 July 2012.
- 71.Spellberg B, Talbot G Infectious Diseases Society of America (IDSA), American College of Chest Physicians (ACCP), American Thoracic Society (ATS), Society of Critical Care Medicine (SCCM) Recommended design features of future clinical trials of antibacterial agents for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(suppl 1):S150–70. doi: 10.1086/653065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spellberg B, Brass EP, Bradley JS, et al. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis. 2012;55:1031. doi: 10.1093/cid/cis688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.European Medicines Agency. Addendum to the note for guidance on evaluation of medicinal products indicated for treatment of bacterial infections (CPMP/EWP/558/95 REV 2) to address indication-specific clinical data. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129443.pdf . Accessed 30 July 2012.
- 74.US Department of Health and Human Services. BARDA partners to develop new class of antibiotic. Available at http://www.phe.gov/Preparedness/news/Pages/gramnegative-110906.aspx . Accessed 30 July 2012.
- 75.US Department of Health and Human Services. HHS aids development of next generation broad spectrum antibiotic. Available at http://www.phe.gov/Preparedness/news/Pages/broad-spectrum-120120.aspx . Accessed 30 July 2012.


